- 1Instituto Gonçalo Moniz-IGM/FIOCRUZ, Salvador, Brazil
- 2Instituto de Microbiologia, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil
- 3Instituto de Investigação em Imunologia, São Paulo, Brazil
Leishmaniasis is an infectious disease caused by protozoans of the genus Leishmania. The macrophage is the resident cell in which the parasite replicates and it is important to identify new compounds that can aid in parasite elimination since the drugs used to treat leishmaniasis are toxic and present side effects. We have previously shown that treatment of Leishmania braziliensis-infected macrophages with DETC (Diethyldithiocarbamate) induces parasite killing, in vivo. Thus, the objective of this study was to further evaluate the effect of oxidants and antioxidants in L. braziliensis-infected macrophages, following treatment with either oxidizing Hydrogen Peroxide, Menadione, DETC, or antioxidant [NAC (N-Acetyl-Cyteine), Apocynin, and Tempol] compounds. We determined the percentage of infected macrophages and number of amastigotes. Promastigote survival was also evaluated. Both DETC (SOD-inhibitor) and Tempol (SOD-mimetic) decreased the percentage of infected cells and parasite load. Hydrogen peroxide did not interfere with parasite burden, while superoxide-generator Menadione had a reducing effect. On the other hand, NAC (GSH-replenisher) and Apocynin (NADPH-oxidase inhibitor) increased parasite burden. Tempol surfaces as an interesting candidate for the chemotherapy of CL with an IC50 of 0.66 ± 0.08 mM and selectivity index of 151. While it remains obscure how a SOD-mimetic may induce leishmanicidal effects, we suggest the possibility of developing Tempol-based topical applications for the treatment of cutaneous leishmaniasis caused by L. braziliensis.
Introduction
Leishmaniasis is zoonotic infection widely distributed from Asia to America which exhibits a high mortality rate. The clinical forms of leishmaniasis depend on the infecting organism and the general state of the host's immune response and are divided in visceral leishmaniasis (VL) and tegumentary leishmaniasis (TL). TL is characterized by cutaneous or mucosal lesions with low lethality, but with high morbidity. CL caused by Leishmania braziliensis is distinguished from other leishmaniasis by its chronicity, latency, and tendency to metastasize in the human host (Bittencourt et al., 2003). Brazil along with nine other countries account for 70–75% of the global CL incidence (Alvar et al., 2012). First choice drugs for leishmaniasis chemotherapy are pentavalent antimonials (Sbv) [Meglumine Antimoniate (Glucantime®) and Sodium Stibogluconate (Pentostam®) (Croft and Coombs, 2003)] which are significantly toxic and with reported drug resistance (Llanos-Cuentas et al., 2008). Amphotericin B (Annaloro et al., 2009) and Miltefosine (Machado et al., 2010) are also limited with regards to toxicity, cost, and/or time of treatment, reinforcing the need for new chemotherapeutic alternatives.
Leishmania promastigotes infect both resident macrophages and monocytes recruited to the infection site. Macrophages are the main host cell, where the parasite differentiates into replicating amastigotes. Upon macrophage activation by IFN-γ, NADPH oxidase generates O-•2 through the transfer of electrons from NADPH, coupling them to O2. In a phagosome where leishmania parasites reside, O-•2 may either undergo SOD degradation to form H2O2 or be used to generate other ROS, depending on expressed enzymes/cofactors availability and the imbalance between oxidants and antioxidants results in oxidative damage (Sies, 1993). ROS inhibits the growth of L. braziliensis amastigotes and contribute to parasite killing (Novais et al., 2014), while NO production alone does not suffice to control infection (Carneiro et al., 2016).
As an evasion strategy, Leishmania induces IFN-β production by infected macrophages, which on its turn induces the expression of the enzyme superoxide dismutase (SOD1). The enzyme SOD1 has an antioxidant function: it converts O-•2 into molecular oxygen (O2) and hydrogen peroxide (H2O2), the latter degraded by catalase. Survival of L. amazonensis and L. braziliensis in the host depends on this process (Khouri et al., 2009).
The SOD1-inhibitor diethyldithiocarbamate (DETC) kills intracellular parasites in vitro and in vivo in a murine model of cutaneous leishmaniasis (Khouri et al., 2010). We have previously shown that DETC can be used as a topical treatment in the cutaneous lesions caused by L. braziliensis (Celes et al., 2016), suggesting that manipulation of the redox status during in vitro infection with L. braziliensis can contribute to the identification of novel therapeutic alternatives. To this purpose, we incubated promastigotes and infected macrophages with Glutahtione replenisher N-acetyl-cysteine (NAC) (Aldini et al., 2018), SOD-mimetic Tempol (Wilcox, 2010) and O-•2 -generator menadione (Hassan, 2013). Much to our surprise, we observed that Tempol, a SOD-mimetic, was as effective as DETC (SOD-inhibitor) and menadione (superoxide generator via redox cycling (Criddle et al., 2006) with regards to its ability to reduce macrophage infection by L. braziliensis, suggesting novel yet unexplained effects of antioxidants over Leishmania infection.
Materials and Methods
Ethics Statements
Female BALB/c mice, 6–8 weeks of age, were obtained from IGM/FIOCRUZ animal facility where they were maintained under pathogen-free conditions. All animal work was conducted according to the Guidelines for Animal Experimentation of the Colégio Brasileiro de Experimentação Animal and of the Conselho Nacional de Controle de Experimentação Animal. The local Ethics Committee on Animal Care and Utilization (CEUA) approved all procedures involving animals (CEUA L001/12 IGM/FIOCRUZ).
Parasites
Leishmania braziliensis (MHOM /BR/00/BA788/GFP) were grown in Schneider Insect medium (ThermoFisher Scientific) supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin and 10% inactivated FBS (ThermoFisher Scientific) at 26°C until the stationary phase.
Infection of Bone Marrow-Derived Macrophages (BMDM) With L. braziliensis and Treatment With Oxidants and Anti-oxidants
Bone marrow derived macrophages were obtained as described (Weischenfeldt and Porse, 2008) and were resuspended in DMEM medium (ThermoFisher Scientific) supplemented with 100 U/ml penicillin, 100 ug/ml streptomycin, and 10% inactivated FBS (ThermoFisher Scientific) and seeded at density of 3 × 105 cells per well in 24-well tissue plates. Monolayers received 3 × 106 L. braziliensis promastigotes and were incubated at 35 °C in supplemented DMEM medium for 24 h. Infected macrophages were washed to remove non-internalized parasites. Cultures were treated with Diethyldithiocarbamate (DETC) (1 or 2 mM) (Khouri et al., 2010; Celes et al., 2016), Hydrogen Peroxide (100 or 150 μM), N-acetyl cysteine (NAC) (1, 5, or 10 mM), Apocynin (APO) (20 mM) (Paiva et al., 2012), Tempol (4-Hydroxy-TEMPO) (0.5, 1, or 5 mM) (Hahn et al., 1997; Shilo and Tirosh, 2003; Kim et al., 2017) and Menadione (1, 10, or 20 μM) (Mittra et al., 2013), all from SIGMA. Compounds were diluted in DMSO (vehicle). Amphotericin B (0.25 μg/mL, Invitrogen) was used as positive control. After 48 h, cells were extensively washed, fixed, and stained with hematoxylin and eosin (Fischer et al., 2008). The number of infected cells and intracellular amastigotes were counted by optical microscopy in 200 macrophages. Cultures (control and infected macrophages) were performed in quintuplicate. Alternatively, the rate of infection was evaluated by flow cytometry. Briefly, cells were fixed in PBS with 2% paraformaldehyde for 10 min, and kept at 4°C in the dark until acquisition. Data were acquired in a Fortessa flow cytometer (BD Biosciences, USA), for analysis by using FlowJo software (Tree Star, Version 10.2).
Treatment of L. braziliensis Promastigotes With Tempol, DETC, and Menadione
Stationary-phase promastigotes (3 × 105) were cultured in supplemented Schneider in the presence of Tempol (1, 3, or 5 mM), DETC (0.1, 0.5, or 1 mM) and Menadione (2, 5, 10, or 20 μM), all from SIGMA. Promastigotes were cultured in 96-well plates for up to 3 days and the number of viable promastigotes was determined daily using hemocytometer. All assays were performed in quadruplicate and Schneider's medium alone or medium containing vehicle alone were used as a negative control. The half-maximal cytotoxic concentration (CC50) and half maximal effective concentration (EC50) values of Tempol were determined by a non-linear regression of the concentration-responses curves using GraphPad Software. The selectivity index (SI) was calculated as a ratio between CC50/EC50 obtained with murine macrophages and intracellular L. braziliensis amastigotes, respectively.
Statistical Analysis
For non-parametric data, analyzes were performed using the Kruskal-Wallis test, followed by the Dunn's multiple comparisons test, for comparisons between three or more groups. For all the analyzes the confidence interval of 95% was established, being the values considered statistically significant when p<0.05. Three biological replicates were performed for each experiment. All analyzes were done using GraphPad Prism Software version 5.0. Flow cytometric analyzes were performed using FlowJo software version 10.
Results and Discussion
Herein, we tested a number of compounds for their ability to modulate the oxidative stress in macrophages infected with L. braziliensis. We hypothesized that compounds able to increase ROS induce parasite elimination, building on previous studies showing that such effect can be applied to the development of topical formulations for the treatment of cutaneous leishmaniasis (Celes et al., 2016).
BMDM were infected with L. braziliensis and treated with DETC. DETC significantly reduced the percentage of infected cells (Figure 1A) and the number of intracellular parasites (Figure 1B). DETC (2 mM) showed a leishmanicidal effect similar to that of Amphotericin B, used to treat human leishmaniasis and employed here as a positive control, corroborating our previous findings that the elevation of O-•2 levels by DETC-mediated inhibition of SOD1 induces L. braziliensis killing (Khouri et al., 2010; Celes et al., 2016). H2O2 significantly reduced the number of infected cells nor the number of amastigotes (Figures 1C,D, respectively), indicating that the ROS responsible for parasite killing induced by DETC is O-•2 itself or another species which uses O-•2 as a substrate. These results are in accordance with the killing of L. braziliensis amastigotes by EGCG (Inacio et al., 2014), shown to induce the production of superoxide anions, hydrogen peroxide, and other reactive oxygen species (ROS) (Suh et al., 2010), an effect inhibited by catalase-PEG. L. donovani was reported to evade oxidative conditions by removing H2O2 and allowing parasite survival (Channon and Blackwell, 1985). More recently, resistance of L. donovani-infected macrophages to H2O2− mediated apoptosis was shown to be due to upregulation of thioredoxin and SOCS (Srivastav et al., 2014).
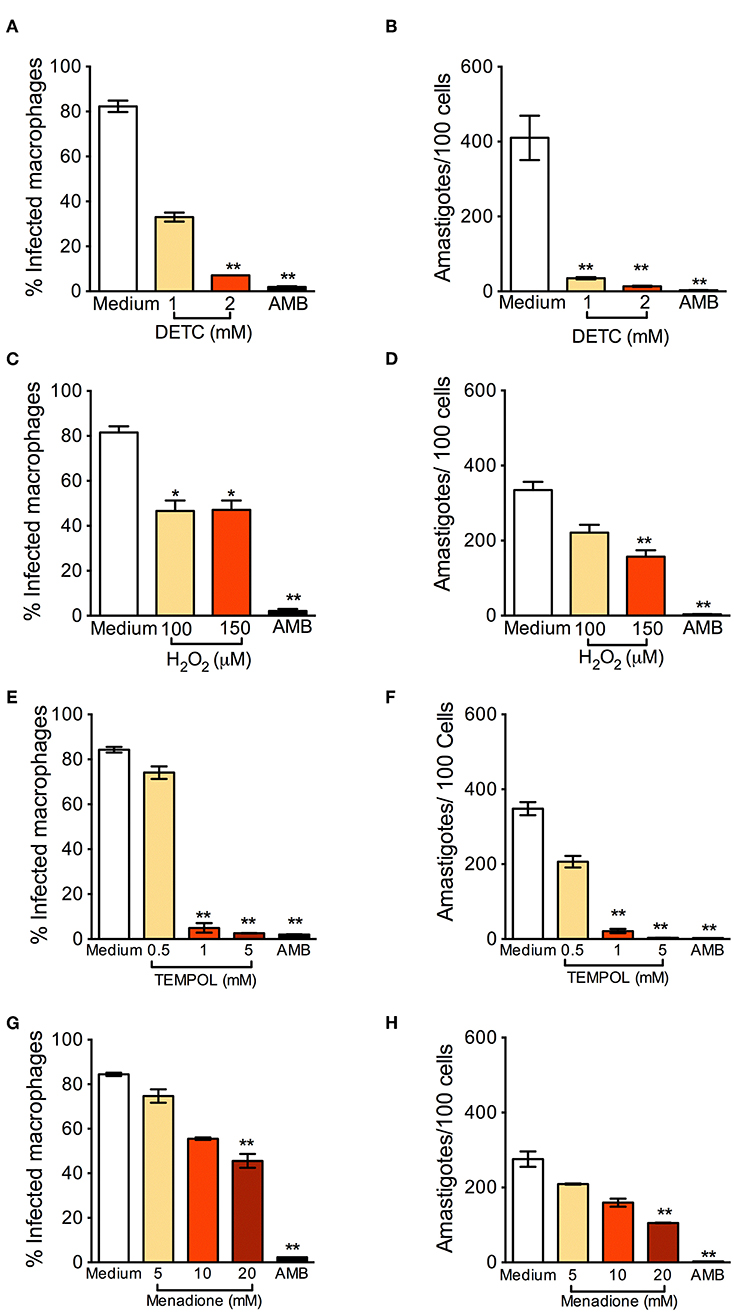
Figure 1. Oxidants reduce Leishmania braziliensis infection in vitro. Macrophages were infected with L. braziliensis for 24 h, and then exposed to different concentrations of DETC (A,B), H2O2 (C,D) for 24 h, Tempol (E,F), and Menadione (G,H) for 24 h. Cells were stained with H&E and assessed for the percentage of infection (A,C,E,F) and the number of amastigotes per 100 macrophages (B,D,G,H) by optical microscopy. Infected macrophages treated with Amphotericin B (AMB) were used as positive controls. Data are shown as mean ± SEM. *p < 0.05; **p < 0.01, ***p < 0.001, all comparisons were against negative control (medium).
Tempol is an antioxidant able to promote O-•2 metabolism at rates similar to SOD and able to permeate membranes freely (Batinic-Haberle et al., 2010). It acts as an O-•2 scavenger that crosses cell membranes and therefore can be used to scavenge O-•2 in living phagocyte (Gariboldi et al., 1998). We thus expected that Tempol, as an antioxidant molecule, would also favor infection by L. braziliensis. Strinkingly, exposure to Tempol significantly reduced the percentage of infected cells (Figure 1E) and the number of amastigotes (Figure 1F), controlling L. braziliensis infection, as seen with DETC. Moreover, the combination DETC+Tempol also reduced the percentage of infected cells (Supplemental Figure 1), as seen individually with DETC (Figure 1). Tempol mimics superoxide dismutase activity thus generating hydrogen peroxide and water, destabilizing the oxidation. We can speculate that the presence of Tempol increased H2O2 levels, interfering with parasite viability. In this case, the concentrations of H2O2 reached inside the phagosome would need to be significantly higher than those obtained herein following macrophage incubation with 50 μM H2O2 (Figures 1C,D). Alternatively, Tempol may have off-target leishmanicidal effects superimposed to its anti-oxidant effects. Treatment with Menadione also significantly decreased the percentage of L. braziliensis-infected cells (Figure 1G) and the number of intracellular parasites (Figure 1H). We can speculate that, as seen with DETC (Celes et al., 2016), the presence of Menadione may have elevated superoxide levels, leading to parasite elimination.
In L. infantum-infected BMDM macrophages, addition of Tempol during phagocytosis increases intracellular infection (Gantt et al., 2001). In L. amazonensis-infected mice, SOD-mimetic Tempol exacerbated lesion development and increased parasite load after oral administration. This was associated with reduction of nitric oxide and sequestration of oxidizing molecules (Linares et al., 2008). Differences in the pathogenesis of CL caused by L. amazonensis and L. braziliensis have been reported regarding the role of neutrophils, for example (Novais et al., 2009; Roma et al., 2016; Carneiro et al., 2018). Therefore, we can speculate that the microbicidal effect of Tempol observed herein, in vitro, recapitulate such differences and thus warrant further in vivo experiments, especially given Tempol's ability to modulate H2O2 levels.
We also verified the leishmanicidal effect of oxidants on L. braziliensis promastigotes: DETC significantly reduced L. braziliensis proliferation at all DETC concentrations tested (Supplemental Figure 2A). As seen with amastigotes (Figures 1E,F), SOD-mimetic Tempol also inhibited the proliferation of L. braziliensis promastigotes (Supplemental Figure 2B), similarly to SOD-inhibitor DETC, although we did not observe clear-cut dose-dependent effects. Menadione induced promastigote killing (Supplemental Figure 2C). Lastly, a combination of Tempol+DETC strongly reduced parasite survival as seen with combinations of Menadione + Tempol and Menadione + DETC combinations (Supplemental Figure 2D).
In parallel to the oxidants, we examined the effect of antioxidants: N-Acetyl-Cysteine (NAC) is a synthetic precursor of intracellular cysteine and glutathione, and its anti-ROS activity results from its ability to directly remove free radicals through the redox potential of thiols and indirectly by increasing levels of glutathione in cells (Sun, 2010). L. braziliensis-infected macrophages treated with NAC had a significantly higher percentage of infection (Figure 2A) and a significantly increased parasite load (Figure 2B). Similar results were reported in human monocytes infected with L. braziliensis and incubated with NAC (Novais et al., 2014). We believe that these effects are due to the neutralization of ROS, since NAC restores glutathione (GSH) and NAC may protect L. braziliensis from oxidative stress just as it does with human red blood cells (Grinberg et al., 2005). Alike NAC, exposure of L. braziliensis-infected macrophages to APO did not change the percentage of infected cells (Figure 2C), but induced an increase in the number of amastigotes in cells, indicating that the source of ROS in infected macrophages is indeed NADPH-oxidase respiratory burst (Figure 2D).
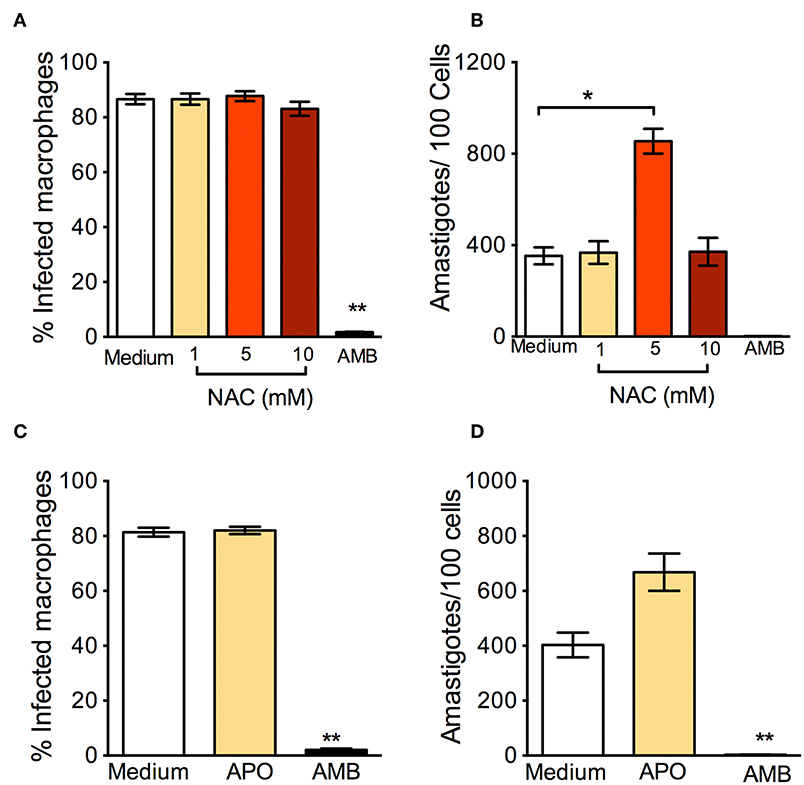
Figure 2. Anti-oxidants enhance in vitro infection with L. braziliensis. Macrophages were infected with L. braziliensis for 24 h, and then exposed to different concentrations of NAC (A,B) and Apocycin (C,D) for 48 h. Cells were stained with H&E and assessed for (A,C) the percentage of infected macrophages and (B,D) the number of amastigotes per 100 macrophages by optical microscopy. Infected macrophages treated with Amphotericin B (AMB) were used as positive controls. Data are shown as mean ± SEM. *p < 0.05, **p < 0.01, all comparisons were against negative control (medium).
Although news studies are needed to understand the mechanisms by which Tempol acts to eliminate L. braziliensis, we believe Tempol is an interesting candidate for the chemotherapy of CL. Of note in BMDM, the IC50 of Tempol was determined at 0.66 mM ± 0.08 mM, the CC50 was calculated as >100 mM and the selectivity index was established at 151. Tempol presents low toxicity and has successfully completed phase I clinical trials to be used topically against tissue damage (Metz et al., 2004). Given that treatment options for CL are currently limited and that the number of refractory cases has increased, Tempol surfaces as a viable alternative for further investigation.
Ethics Statement
All animal work was conducted according to the Guidelines for Animal Experimentation of the Colégio Brasileiro de Experimentação Animal and of the Conselho Nacional de Controle de Experimentação Animal. The local Ethics Committee on Animal Care and Utilization (CEUA) approved all procedures involving animals (CEUA L001/12 IGM/FIOCRUZ).
Author Contributions
LO and FC performed experiments. LO, CP, and CO drafted the manuscript. CP contributed reagents.
Funding
LO was the recipient of a CNPq fellowship (Iniciação Científica). FC was financed by the Coordenação de Aperfeiçoamento de pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. CO is a senior investigator from CNPq. This work was funded in part by Instituto Gonçalo Moniz(IGM)-Fiocruz.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the flow cytometery core.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2019.00237/full#supplementary-material
References
Aldini, G., Altomare, A., Baron, G., Vistoli, G., Carini, M., Borsani, L., et al. (2018). N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic. Res. 52, 751–762. doi: 10.1080/10715762.2018.1468564
Alvar, J., Velez, I. D., Bern, C., Herrero, M., Desjeux, P., Cano, J., et al. (2012). Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7:e35671. doi: 10.1371/journal.pone.0035671
Annaloro, C., Olivares, C., Usardi, P., Onida, F., Della Volpe, A., Tagliaferri, E., et al. (2009). Retrospective evaluation of amphotericin B deoxycholate toxicity in a single centre series of haematopoietic stem cell transplantation recipients. J. Antimicrob. Chemother. 63, 625–626. doi: 10.1093/jac/dkn549
Batinic-Haberle, I., Reboucas, J. S., and Spasojevic, I. (2010). Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid. Redox. Signal. 13, 877–918. doi: 10.1089/ars.2009.2876
Bittencourt, A., Silva, N., Straatmann, A., Nunes, V. L., Follador, I., and Badaro, R. (2003). Post-kala-azar dermal leishmaniasis associated with AIDS. Braz. J. Infect. Dis. 7, 229–233. doi: 10.1590/S1413-86702003000300009
Carneiro, M. B. H., Roma, E. H., Ranson, A. J., Doria, N. A., Debrabant, A., Sacks, D. L., et al. (2018). NOX2-derived reactive oxygen species control inflammation during Leishmania amazonensis infection by mediating infection-induced neutrophil apoptosis. J. Immunol. 200, 196–208. doi: 10.4049/jimmunol.1700899
Carneiro, P. P., Conceicao, J., Macedo, M., Magalhaes, V., Carvalho, E. M., and Bacellar, O. (2016). The role of nitric oxide and reactive oxygen species in the killing of Leishmania braziliensis by monocytes from patients with cutaneous leishmaniasis. PLoS ONE 11:e0148084. doi: 10.1371/journal.pone.0148084
Celes, F. S., Trovatti, E., Khouri, R., Van Weyenbergh, J., Ribeiro, S. J., Borges, V. M., et al. (2016). DETC-based bacterial cellulose bio-curatives for topical treatment of cutaneous leishmaniasis. Sci. Rep. 6:38330. doi: 10.1038/srep38330
Channon, J. Y., and Blackwell, J. M. (1985). A study of the sensitivity of Leishmania donovani promastigotes and amastigotes to hydrogen peroxide. II. Possible mechanisms involved in protective H2O2 scavenging. Parasitology 91 (Pt 2), 207–217. doi: 10.1017/S0031182000057310
Criddle, D. N., Gillies, S., Baumgartner-Wilson, H. K., Jaffar, M., Chinje, E. C., Passmore, S., et al. (2006). Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J. Biol. Chem. 281, 40485–40492. doi: 10.1074/jbc.M607704200
Croft, S. L., and Coombs, G. H. (2003). Leishmaniasis–current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 19, 502–508. doi: 10.1016/j.pt.2003.09.008
Fischer, A. H., Jacobson, K. A., Rose, J., and Zeller, R. (2008). Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008:pdb prot4986. doi: 10.1101/pdb.prot4986
Gantt, K. R., Goldman, T. L., Mccormick, M. L., Miller, M. A., Jeronimo, S. M., Nascimento, E. T., et al. (2001). Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J. Immunol. 167, 893–901. doi: 10.4049/jimmunol.167.2.893
Gariboldi, M. B., Lucchi, S., Caserini, C., Supino, R., Oliva, C., and Monti, E. (1998). Antiproliferative effect of the piperidine nitroxide TEMPOL on neoplastic and nonneoplastic mammalian cell lines. Free Radic. Biol. Med. 24, 913–923. doi: 10.1016/S0891-5849(97)00372-9
Grinberg, L., Fibach, E., Amer, J., and Atlas, D. (2005). N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radic. Biol. Med. 38, 136–145. doi: 10.1016/j.freeradbiomed.2004.09.025
Hahn, S. M., Mitchell, J. B., and Shacter, E. (1997). Tempol inhibits neutrophil and hydrogen peroxide-mediated DNA damage. Free Radic. Biol. Med. 23, 879–884. doi: 10.1016/S0891-5849(97)00079-8
Hassan, G. S. (2013). Menadione. Profiles Drug. Subst. Excip. Relat. Methodol. 38, 227–313. doi: 10.1016/B978-0-12-407691-4.00006-X
Inacio, J. D., Gervazoni, L., Canto-Cavalheiro, M. M., and Almeida-Amaral, E. E. (2014). The effect of (-)-epigallocatechin 3-O–gallate in vitro and in vivo in Leishmania braziliensis: involvement of reactive oxygen species as a mechanism of action. PLoS Negl. Trop Dis. 8:e3093. doi: 10.1371/journal.pntd.0003093
Khouri, R., Bafica, A., Silva Mda, P., Noronha, A., Kolb, J. P., Wietzerbin, J., et al. (2009). IFN-beta impairs superoxide-dependent parasite killing in human macrophages: evidence for a deleterious role of SOD1 in cutaneous leishmaniasis. J. Immunol. 182, 2525–2531. doi: 10.4049/jimmunol.0802860
Khouri, R., Novais, F., Santana, G., De Oliveira, C. I., Vannier Dos Santos, M. A., Barral, A., et al. (2010). DETC induces Leishmania parasite killing in human in vitro and murine in vivo models: a promising therapeutic alternative in Leishmaniasis. PLoS ONE 5:e14394. doi: 10.1371/journal.pone.0014394
Kim, J. Y., Choi, G. E., Yoo, H. J., and Kim, H. S. (2017). Interferon potentiates toll-like receptor-induced prostaglandin D2 production through positive feedback regulation between signal transducer and activators of transcription 1 and reactive oxygen species. Front. Immunol. 8:1720. doi: 10.3389/fimmu.2017.01720
Linares, E., Giorgio, S., and Augusto, O. (2008). Inhibition of in vivo leishmanicidal mechanisms by tempol: nitric oxide down-regulation and oxidant scavenging. Free Radic. Biol. Med. 44, 1668–1676. doi: 10.1016/j.freeradbiomed.2008.01.027
Llanos-Cuentas, A., Tulliano, G., Araujo-Castillo, R., Miranda-Verastegui, C., Santamaria-Castrellon, G., Ramirez, L., et al. (2008). Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin. Infect. Dis. 46, 223–231. doi: 10.1086/524042
Machado, P. R., Ampuero, J., Guimaraes, L. H., Villasboas, L., Rocha, A. T., Schriefer, A., et al. (2010). Miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis in Brazil: a randomized and controlled trial. PLoS Negl. Trop. Dis. 4:e912. doi: 10.1371/journal.pntd.0000912
Metz, J. M., Smith, D., Mick, R., Lustig, R., Mitchell, J., Cherakuri, M., et al. (2004). A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin. Cancer Res. 10, 6411–6417. doi: 10.1158/1078-0432.CCR-04-0658
Mittra, B., Cortez, M., Haydock, A., Ramasamy, G., Myler, P. J., and Andrews, N. W. (2013). Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. J. Exp. Med. 210, 401–416. doi: 10.1084/jem.20121368
Novais, F. O., Nguyen, B. T., Beiting, D. P., Carvalho, L. P., Glennie, N. D., Passos, S., et al. (2014). Human classical monocytes control the intracellular stage of Leishmania braziliensis by reactive oxygen species. J. Infect. Dis. 209, 1288–1296. doi: 10.1093/infdis/jiu013
Novais, F. O., Santiago, R. C., Bafica, A., Khouri, R., Afonso, L., Borges, V. M., et al. (2009). Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. J. Immunol. 183, 8088–8098. doi: 10.4049/jimmunol.0803720
Paiva, C. N., Feijo, D. F., Dutra, F. F., Carneiro, V. C., Freitas, G. B., Alves, L. S., et al. (2012). Oxidative stress fuels Trypanosoma cruzi infection in mice. J. Clin. Invest. 122, 2531–2542. doi: 10.1172/JCI58525
Roma, E. H., Macedo, J. P., Goes, G. R., Goncalves, J. L., Castro, W., Cisalpino, D., et al. (2016). Impact of reactive oxygen species (ROS) on the control of parasite loads and inflammation in Leishmania amazonensis infection. Parasit. Vectors 9:193. doi: 10.1186/s13071-016-1472-y
Shilo, S., and Tirosh, O. (2003). Selenite activates caspase-independent necrotic cell death in Jurkat T cells and J774.2 macrophages by affecting mitochondrial oxidant generation. Antioxid. Redox Signal 5, 273–279. doi: 10.1089/152308603322110850
Sies, H. (1993). Strategies of antioxidant defense. Eur. J. Biochem. 215, 213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x
Srivastav, S., Basu Ball, W., Gupta, P., Giri, J., Ukil, A., and Das, P. K. (2014). Leishmania donovani prevents oxidative burst-mediated apoptosis of host macrophages through selective induction of suppressors of cytokine signaling (SOCS) proteins. J. Biol. Chem. 289, 1092–1105. doi: 10.1074/jbc.M113.496323
Suh, K. S., Chon, S., Oh, S., Kim, S. W., Kim, J. W., Kim, Y. S., et al. (2010). Prooxidative effects of green tea polyphenol (-)-epigallocatechin-3-gallate on the HIT-T15 pancreatic beta cell line. Cell Biol. Toxicol. 26, 189–199. doi: 10.1007/s10565-009-9137-7
Sun, S. Y. (2010). N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol. Ther. 9, 109–110. doi: 10.4161/cbt.9.2.10583
Weischenfeldt, J., and Porse, B. (2008). Bone Marrow-Derived Macrophages (BMM): isolation and applications. Cold Spring Harbor. Protocols 2008:pdb.prot5080-pdb.prot5080. doi: 10.1101/pdb.prot5080
Keywords: L. braziliensis, oxidants, anti-oxidants, leishmaniasis, chemotherapy
Citation: Oliveira LB, Celes FS, Paiva CN and de Oliveira CI (2019) The Paradoxical Leishmanicidal Effects of Superoxide Dismutase (SOD)-Mimetic Tempol in Leishmania braziliensis Infection in vitro. Front. Cell. Infect. Microbiol. 9:237. doi: 10.3389/fcimb.2019.00237
Received: 01 December 2018; Accepted: 14 June 2019;
Published: 26 June 2019.
Edited by:
Alexandre Barbosa Reis, Universidade Federal de Ouro Preto, BrazilReviewed by:
Isabel Mauricio, New University of Lisbon, PortugalRodrigo Soares, Oswaldo Cruz Foundation (Fiocruz), Brazil
Copyright © 2019 Oliveira, Celes, Paiva and de Oliveira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Camila I. de Oliveira, camila@bahia.fiocruz.br
 Laíse B. Oliveira1
Laíse B. Oliveira1