- 1University Children's Hospital, University of Wuerzburg, Wuerzburg, Germany
- 2Department of Neonatology, Poznan University of Medical Sciences, Poznan, Poland
- 3Institute of Medical Microbiology and Hospital Hygiene, University Clinic of Heinrich-Heine University Duesseldorf, Duesseldorf, Germany
- 4Department of Surgery I, Molecular Oncology and Immunology, University of Wuerzburg, Wuerzburg, Germany
Background: Controversy remains concerning the impact of Ureaplasma on preterm neonatal morbidity.
Methods: Prospective single-center study in very low birth weight infants <30 weeks' gestation. Cord blood and initial nasopharyngeal swabs were screened for Ureaplasma parvum and U. urealyticum using culture technique and polymerase chain reaction. Neonatal outcomes were followed until death or discharge. Multi-analyte immunoassay provided cord blood levels of inflammatory markers. Using multivariate regression analyses, perinatal Ureaplasma exposure was evaluated as risk factor for the development of bronchopulmonary dysplasia (BPD), other neonatal morbidities until discharge and systemic inflammation at admission.
Results: 40/103 (39%) infants were positive for Ureaplasma in one or both specimens, with U. parvum being the predominant species. While exposure to Ureaplasma alone was not associated with BPD, we found an increased risk of BPD in Ureaplasma-positive infants ventilated ≥5 days (OR 1.64; 95% CI 0.12–22.98; p = 0.009). Presence of Ureaplasma was associated with a 7-fold risk of late onset sepsis (LOS) (95% CI 1.80–27.39; p = 0.014). Moreover, Ureaplasma-positive infants had higher I/T ratios (b 0.39; 95% CI 0.08–0.71; p = 0.014), increased levels of interleukin (IL)-17 (b 0.16; 95% CI 0.02–0.30; p = 0.025) and matrix metalloproteinase 8 (b 0.77; 95% CI 0.10–1.44; p = 0.020), decreased levels of IL-10 (b −0.77; 95% CI −1.58 to −0.01; p = 0.043) and increased ratios of Tumor necrosis factor-α, IL-8, and IL-17 to anti-inflammatory IL-10 (p = 0.003, p = 0.012, p < 0.001).
Conclusions: Positive Ureaplasma screening was not associated with BPD. However, exposure contributed to BPD in infants ventilated ≥5 days and conferred an increased risk of LOS and imbalanced inflammatory cytokine responses.
Introduction
Although substantial therapeutic advances have continously improved the survival of preterm infants, the incidence of neonatal morbidity and sequelae has not declined (Stoll et al., 2015). This is particularly due to the heightened susceptibility of very immature preterm infants to severe infections and major morbidities, such as bronchopulmonary dysplasia (BPD) (Stoll et al., 2015). Colonization with Ureaplasma species (spp.) has been associated with increased risk of BPD in preterm infants (Viscardi and Hasday, 2009; Lowe et al., 2014), and there is some evidence of additional implication in the pathogenesis of intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC) and retinopathy of prematurity (ROP) (Viscardi et al., 2008; Kasper et al., 2011; Silwedel et al., 2017). Ureaplasma spp. have been frequently isolated from amniotic fluid, cord blood and respiratory tract samples of preterm infants who later developed BPD (Viscardi and Hasday, 2009). Ureaplasma respiratory tract colonization appears to contribute to pulmonary inflammation and altered lung development (Viscardi and Hasday, 2009). Intraamniotic detection of Ureaplasma spp. was shown to be paralleled by intrauterine and fetal inflammatory response and increased risk of adverse pulmonary and neurologic outcome in preterm infants (Berger et al., 2009; Kasper et al., 2011; Glaser and Speer, 2015; Sweeney et al., 2017). However, the clinical relevance of detecting Ureaplasma spp. in microbiological specimens of preterm infants remains subject of discussion. Ureaplasma parvum (serovars 1, 3, 6, 14) and U. urealyticum (serovars 2, 4, 5, 7–13) are generally regarded as commensal bacteria being isolated from 40 to 80% of urogenital tract samples of women of reproductive age (Waites et al., 2005; Sweeney et al., 2017). For reasons of low pathogenicity in children and adults, the presence of Ureaplasma-driven inflammation and its impact on neonatal morbidity have been discussed controversially (Volgmann et al., 2005; Glaser and Speer, 2015). The present study aimed to investigate whether early life exposure to Ureaplasma spp. is associated with (i) the development of BPD, (ii) the development of other neonatal morbidities until discharge, and (iii) systemic inflammation at admission in a cohort of very low birth weight infants (VLBW, i.e., birth weight < 1,500 g) born at <30 weeks' gestational age (GA).
Methods
Study Outline
Preterm infants with GA <30 weeks and birth weight <1,500 g admitted to the tertiary neonatal intensive care unit at the Children's Hospital of Poznan, University of Medical Sciences, Poland, were eligible for this prospective study conducted between May 2014 and December 2015. Outborn infants as well as infants with major congenital malformations, lack of informed consent or absence of specimens for Ureaplasma screening and/or analysis of cord blood inflammatory markers were excluded. Among 202 infants eligible, 103 met the primary inclusion criteria (Figure 1). Infants were categorized as Ureaplasma-positive and negative depending on initial screening. Infant data were entered into a computerized data sheet, verified by two different investigators. Neonatal outcomes were followed until death or discharge.
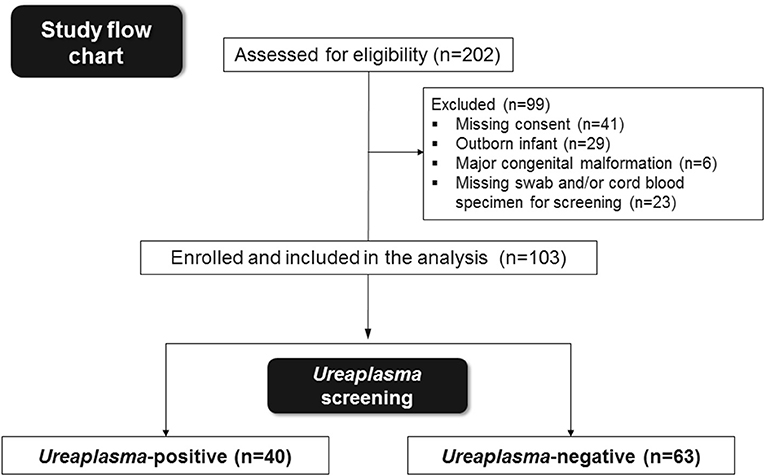
Figure 1. Summary of preterm infants eligible, enrolled in the study and included in the final analysis.
Outcome Definitions
BPD, the primary endpoint, was defined as requirement of oxygen therapy for at least 28 days and classified according to the need of supplemental oxygen at 36 weeks' postmenstrual age (Jobe and Bancalari, 2001). Predefined secondary endpoints comprised respiratory distress syndrome (RDS) ≥ grade 3, IVH ≥ grade 3, periventricular leukomalacia (PVL), NEC ≥ stage 2, ROP ≥ stage 3, late onset sepsis (LOS), meningitis and death prior to discharge. Diagnosis of RDS referred to clinical and radiographic features, and the requirement of supplemental oxygen. IVH was diagnosed by means of cranial ultrasound and graded according to the Papile classification (Papile et al., 1978). PVL was defined as bilateral cystic lesions in periventricular areas documented in coronal and parasagittal ultrasound scans performed > 28 days after birth (Perlman et al., 1996). NEC was diagnosed by clinical and radiological signs according to Bell's staging (Bell, 1978). ROP was determined following the international classification (International Committee for the Classification of Retinopathy of Prematurity, 2005). Diagnosis of LOS required clinical and laboratory signs of systemic infection at >72 h of age, positive blood culture and antibiotic treatment ≥ 5 days. Meningitis was defined by clinical and laboratory signs of systemic infection, abnormal cerebrospinal fluid parameters, and positive cerebrospinal fluid culture.
Sample Collection and Laboratory Methods
Umbilical cord blood samples were taken immediately after delivery using an aseptic technique. The needle puncture site was disinfected with a 0.1% aqueous solution of octenidine with phenoxyethanol (Octenisept®, Schuelke & Mayr GmbH, Germany) applied by means of a spray and saturated swabs thereafter. The site was allowed to dry for 1 min before cord blood was drawn using a sterile, unheparinized syringe. For Ureaplasma culture, 0.5 mL cord blood was immediately inoculated into 2 mL 10B broth (Thermo Fisher Scientific, Germany). Nasopharyngeal swabs obtained within 2 h after birth using sterile transport swabs (Copan, Italy) were also immediately placed in the commercially available ready-to-use tubes providing 2 mL of 10B broth. Swab and liquid cultures were incubated at 37°C in 5% CO2/air for 10 days and monitored daily. If color change occurred, inoculums of 0.2 mL were plated on A8 agar to confirm positive broth. All cord blood samples and swabs were additionally investigated for DNA of U. parvum and U. urealyticum using commercially available kits for DNA extraction and amplification according to the manufacturer's protocol (AmpliSens® DNA-sorb-B, AmpliSens® U. parvum/U. urealyticum-FRT; Ecoli Ltd., Slovak Republic). An mba-based real-time PCR approach was used to discriminate U. parvum serovars (Otgonjargal et al., 2018). Routine cord blood and nasopharyngeal swab cultures were assessed using the Bactec Peds Plus/F blood culture system (bioMérieux, Poland), selective agars and the Vitek® MS system (bioMérieux).
Cord blood leukocyte counts, immature/total neutrophil (I/T) ratios and C-reactive protein levels were analyzed within 2 h after birth. For quantification of TNF-α, IL-1β, IL-8, IL-12, IL-17, IL-10, IL-1ra, IFN-γ, IP-10, MMP-8/9, MIP-1α/β, MCP-1, VEGF, G-CSF, ICAM-1, and VCAM-1, serum aliquots of ≥0.2 mL were stored at −80°C until analysis. Multianalyte immunoassay was carried out using Luminex® multiplex kits and the xPonent® software (Merck group, Germany) as described before (Glaser et al., 2017, 2018a,b).
Maternal Screening and Placental Pathology
Maternal vaginal screening was assessed at admission as part of routine clinical care. Bacterial vaginosis was defined according to the Nugent criteria (Nugent et al., 1991). Placental examination was part of the research study and was conducted after written informed consent was obtained. Histopathological investigation was performed by 2 independent pathologists at the Institute of Pathology, Poznan University of Medical Sciences, following a published protocol (Rogers et al., 2002).
Statistical Analyses
Sample size was determined using an approximate sample size formula for studies with dichotomous outcomes (Schulz and Grimes, 2005). Based on preliminary data reporting on incidences of BPD in Ureaplasma colonized preterm infants (Lowe et al., 2014) and baseline BPD rates of about 25% in infants <32 weeks' GA in Poland (Fanaroff et al., 2007; Gortner et al., 2011), we calculated that minimum 38 infants per study group needed to be enrolled and included in the final analysis to detect a 2-fold risk of BPD with α = 0.05 and power.80. Infant characteristics were analyzed descriptively, and tested for differences using X2, Fisher's exact test and Cramér's V for qualitative variables, Q-Q plots, unpaired t, and Mann-Whitney U test for quantitative variables. Bivariate analyses using X2, Fisher's exact test and Cramér's V and multivariate logistic regressions were performed to test for associations between Ureaplasma exposure and primary and secondary outcomes. Covariates offered to the models were GA, birth weight, male sex, singleton status, antenatal corticosteroids, antenatal antibiotics, antenatal macrolides, preterm premature rupture of membranes >12 h, histologic chorioamnionitis, vaginal delivery, vaginal Ureaplasma/GBS/other bacteria/Candida, surfactant treatment, ventilation days, supplemental oxygen days, patent ductus arteriosus requiring treatment as well as presence of and duration of central venous lines. Covariates were included if significant at p < 0.10 in preceding analyses using X2, Fisher's exact test and Cramér's V, Q-Q plots, unpaired t and Mann-Whitney U. Subgroup analyses were performed on Ureaplasma-positive infants and Ureaplasma-negative infants, respectively, ventilated < or ≥5 days using the same approach. Corresponding bivariate and stepwise multivariate linear regression analyses examined the relationship between Ureaplasma exposure and cord blood inflammatory markers. A p-value < 0.05 was considered significant. Analyses were performed using the Statistical Package of Social Sciences (SPSS) software, version 24 (IBM, Germany).
Results
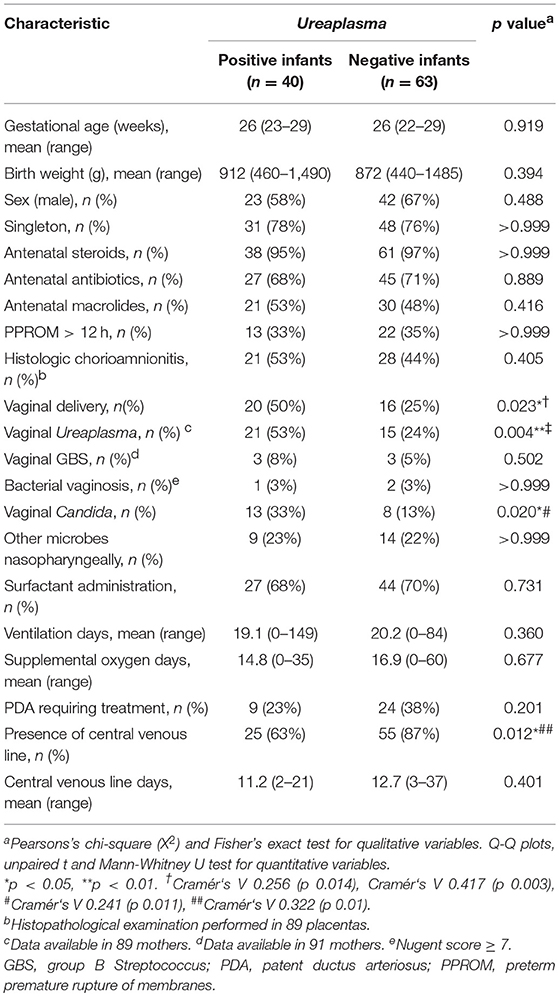
During the study period, 202 VLBW infants born at <30 weeks GA were admitted to the Poznan NICU. One hundred and three infants met the inclusion criteria and were included in the final analysis (Figure 1). Among these, 40 (39%) were culture/PCR positive for Ureaplasma spp. in one or both specimens and 63 (61%) were Ureaplasma-negative. The Ureaplasma-positive group comprised 32 infants with positive nasopharyngeal swabs, 16 infants with cord blood positive for Ureaplasma and 8 infants with detection of Ureaplasma spp. in both specimens. Quantitative real-time PCR detected Ureaplasma spp. in 40/40 Ureaplasma-positive study infants, culture technique spotted Ureaplasma in 11/40. Being detected in 33/40 (83%) Ureaplasma-positive infants, U. parvum was the predominant species. U. urealyticum was found in 7/40 infants, 3/40 infants carried both species. Differentiation of U. parvum serovars revealed different distribution patterns in swab and cord blood specimens (Figure 2). Serovar differentiation of U. urealyticum was not available. Detection of Ureaplasma was more frequent in infants with a history of vaginal delivery (p = 0.024) and maternal vaginal colonization with Ureaplasma (p = 0.003) and Candida spp. (p = 0.026) (Table 1). Additionally, Ureaplasma-positive and negative study infants differed in terms of central venous lines present, with a higher number of infants with a central venous catheter in the Ureaplasma-negative group (p = 0.006). Clinical characteristics otherwise did not differ (Table 1). None of the infants had a positive routine cord blood culture. Routine cultures of initial nasopharyngeal swabs detected Candida spp., Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, Streptococcus anginosus and Gardnerella vaginalis in 23 and 22% of infants, respectively (Table 1).
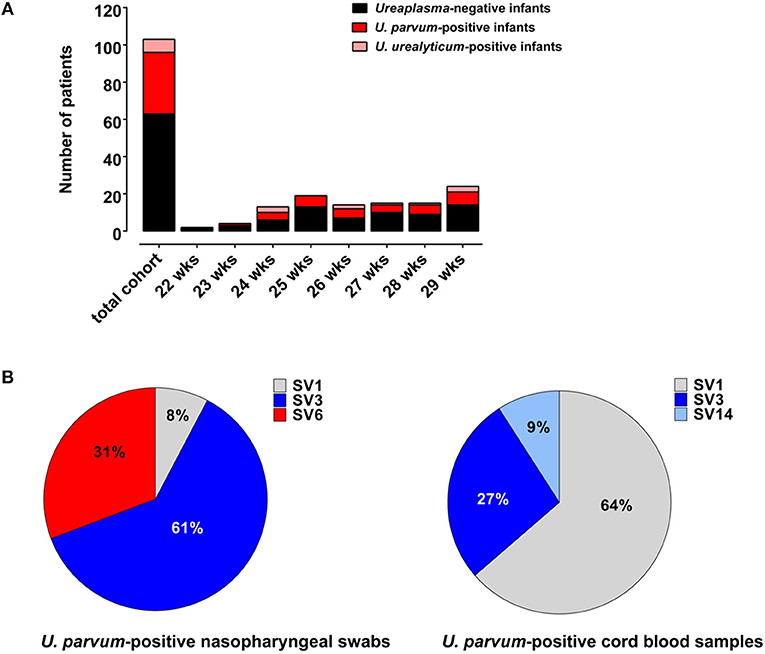
Figure 2. (A) Numbers of Ureaplasma-negative infants as well as U. parvum-positive and U. urealyticum-positive infants given for each week of gestation (assessed as completed weeks). (B) Distribution of U. parvum serovars in Ureaplasma-positive swab and cord blood specimens.
Bivariate and stepwise multivariate logistic regression analyses were used to test the primary hypothesis of increased BPD rates in Ureaplasma-positive infants and the association between Ureaplasma exposure and secondary endpoint measures. On bivariate analysis, BPD rates did not differ among both study groups (p = 0.218) (Table 2). Analyses including BPD severity (p = 0.629) and the compound measure moderate/severe BPD did not detect a significant correlation either (p = 0.374). Covariates independently associated with BPD were ventilation days (p = 0.039) and supplemental oxygen days (p = 0.032). Subgroup analysis was performed comparing Ureaplasma-positive infants with a history of < 5 days of mechanical ventilation (n = 20) with Ureaplasma-positive infants ventilated ≥5 days (n = 20), to examine whether perinatal Ureaplasma exposure interacts with mechanical ventilation. Bivariate analyses documented increased rates of BPD in Ureaplasma-positive infants ventilated ≥5 days (87 vs. 28%, p = 0.001), and adjusted analysis confirmed increased odds of BPD in infants with positive Ureaplasma screening (OR 1.64; 95% CI 0.12–22.98; p = 0.009). Covariates included in the model were GA, birth weight, male sex, vaginal delivery, antenatal antibiotics, vaginal Ureaplasma, RDS ≥grade 3, surfactant administration, ventilation and supplemental oxygen days. Birth weight was an independent predictor of BPD in this model (OR 0.99; 95% CI 0.98–1.00; p = 0.007). Analyses in the Ureaplasma-negative group documented BPD rates of 70% in infants ventilated ≥5 days compared to 48% in infants ventilated < 5 days (p = 0.156).

Table 2. Bivariate analyses testing for associations between perinatal exposure to Ureaplasma and primary and secondary outcome measures.
Testing the association between perinatal Ureaplasma exposure and secondary outcomes, we found a tendency toward increased rates of LOS in Ureaplasma-positive infants (Table 2). On multivariate regression adjusting for confounding variables, detection of Ureaplasma spp. was associated with a 7-fold risk of LOS (Table 3). There was no difference in the onset of sepsis among Ureaplasma-positive and negative study infants [mean day of life 17.0 (range 6–51) vs. 19.8 (7–52), p = 0.355]. Moreover, both groups did not differ in terms of exposure to a prior empiric antibiotic therapy at admission, that was mostly limited to the first 48–72 h of life. No infant had more than one episode of sepsis. Blood cultures of Ureaplasma-positive infants with LOS were positive for Escherichia coli (4 infants), Staphylococcus haemolyticus (3 infants), Staphylococcus aureus and Staphylococcus epidermidis. Infecting organisms in Ureaplasma-negative infants were Escherichia coli, Enterococcus faecalis, Acinetobacter baumanii, Streptococcus agalactiae, Staphylococcus haemolyticus, and Candida albicans. Ureaplasma-positive infants with and without LOS did not significantly differ in primary or secondary outcome measures or in terms of species and serovar isolated. U. parvum was present in 70% of infants with LOS (serovar 3: 57%, serovar 6: 28%, serovar 1: 14%). In one infant both species were detected.
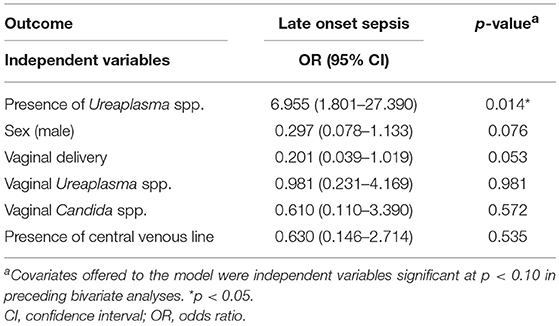
Table 3. Stepwise multivariate logistic regression analysis examining the correlation between Ureaplasma exposure and late onset sepsis.
Exposure to Ureaplasma spp. was not associated with the development of RDS ≥grade 3, IVH ≥grade 3, PVL, NEC ≥stage 2, ROP ≥stage 3, meningitis and death prior to discharge (Table 2; characteristics of all infants who died prior to discharge and causes of death given in Supplementary Table S1). However, subgroup analyses comparing Ureaplasma-positive infants ventilated <5 days with those ventilated ≥5 days revealed increased rates of IVH ≥grade 3 and PVL in the latter (IVH ≥grade 3: 0 vs. 55%, p < 0.001; PVL: 0 vs. 29%, p = 0.016). On multivariate regressions controlling for GA, birth weight, vaginal Ureaplasma, ventilation days, RDS ≥grade 3 and patent ductus arteriosus requiring treatment and GA, birth weight, surfactant administration and ventilation days, respectively, co-exposure to Ureaplasma and mechanical ventilation ≥5 days was associated with increased odds of IVH ≥grade 3 (OR 3.36; 95% CI 0.70–16.617; p = 0.030). The correlation with PVL was no longer significant (OR 0.1; 95% CI 0.01–1.09; p = 0.059). Low GA (OR 0.27; 95% CI 0.08–0.93; p = 0.037) and RDS ≥grade 3 (OR 25.53; 95% CI 1.18–554.41; p = 0.040) were independent risk factors for IVH ≥grade 3. Analyses in the Ureaplasma-negative group revealed rates of IVH ≥grade 3 of 18% in infants ventilated < 5 days and 32% in infants with a history of mechanical ventilation ≥5 days (p = 0.205) as well as similar rates of PVL in both subgroups (13 vs. 15%, p = 0.663).
Cord blood levels of inflammatory markers were compared among Ureaplasma-positive and Ureaplasma-negative infants. We found higher I/T ratios and increased levels of IL-17, but decreased levels of IL-10 and increased ratios of pro-inflammatory cytokines to anti-inflammatory IL-10 in the former group (Figure 3). Moreover, Ureaplasma-positive infants had higher levels of MMP-8 (p = 0.024) and ICAM-1 (p = 0.041) and showed a trend toward decreased levels of IL12p40 in cord blood (p = 0.089). Stepwise multivariate linear regressions adjusting for confounding confirmed an association between positive Ureaplasma screening and increased I/T ratios and levels of IL-17 and MMP-8, decreased levels of IL-10 and increased ratios of TNF-α, IL-8, and IL-17 to IL-10 (Table 4). Lower GA and histologic chorioamnionitis were independent predictors of increased cord blood levels of IL-17 and/or MMP-8, respectively. The impact of preterm premature rupture of membranes >12 h on IL-8/IL-10 ratios in cord blood was of borderline significance (Table 4).
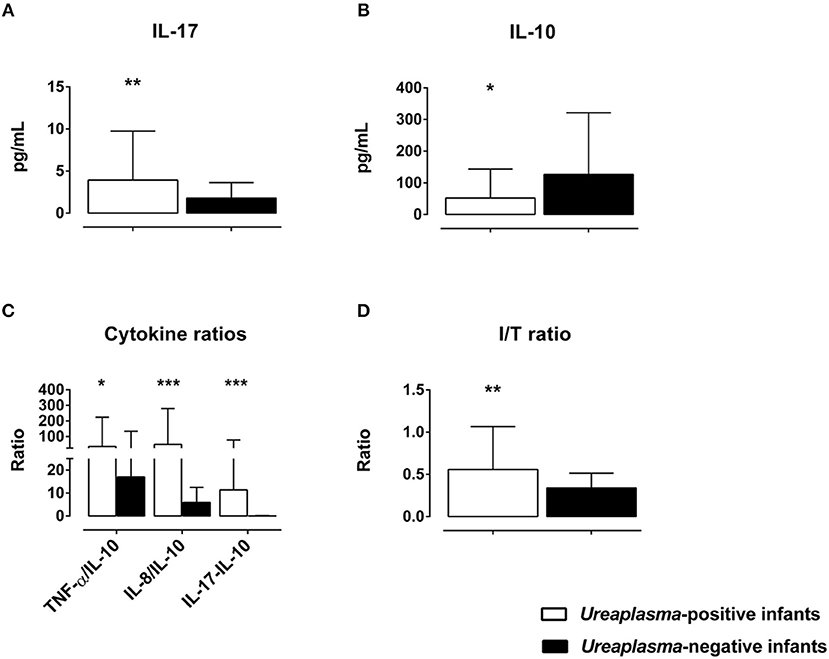
Figure 3. Differences in cord blood serum levels of IL-17 (A) and IL-10 (B), ratios of pro-inflammatory cytokines to anti-inflammatory IL-10 (C) and I/T ratios (D) among Ureaplasma-positive and Ureaplasma-negative infants on bivariate analysis (*p < 0.05, **p < 0.01, ***p < 0.001).
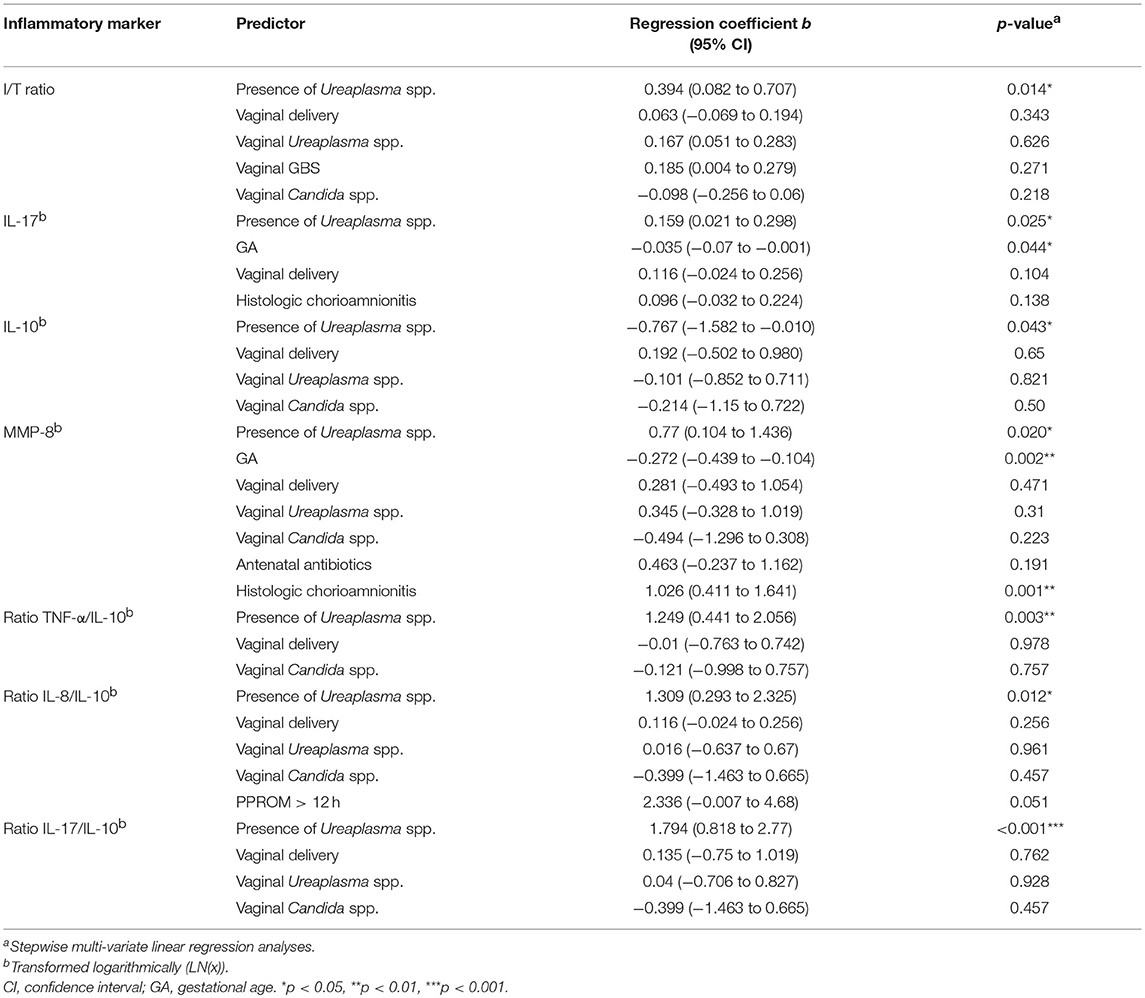
Table 4. Inflammatory markers associated with positive Ureaplasma screening adjusted for confounding variables.
Discussion
With improving care options but enduring occurrence of relevant complications in very immature preterm infants, it is of considerable importance to elucidate whether Ureaplasma spp. are harmless commensals or true pathogens in this cohort (Volgmann et al., 2005; Glaser and Speer, 2015). In the present study, 39% of infants <30 weeks' GA and <1,500 g were positive for Ureaplasma at initial screening. Detection of Ureaplasma spp., however, was not associated with BPD development, rejecting our primary hypothesis. In contrast, we found an increased risk of BPD in Ureaplasma-positive infants co-exposed to mechanical ventilation ≥5 days. Positive screening for Ureaplasma, moreover, was associated with increased odds of LOS and imbalanced cytokine ratios pushed toward pro-inflammation.
There is a number of epidemiologic data indicating an association between Ureaplasma respiratory tract colonization and the development of BPD (Viscardi and Hasday, 2009; Lowe et al., 2014). In contrast, several other studies did not confirm this correlation on adjusted analyses (van Waarde et al., 1997; Patterson et al., 1998; Sung et al., 2011; Payne et al., 2012). In the present investigation, we observed high overall rates of BPD, and we can only speculate on the reasons. With low GA and low birth weight being strong predictors of BPD (Stoll et al., 2010; Isayama et al., 2012; Jobe, 2016), enrolled infants were at increased risk of developing BPD per se. The observed BPD rates may further owe to the inclusion of those infants who received supplemental oxygen for ≥28 days but were breathing room air at 36 weeks' postmenstrual age. Depending on the characteristics of a study cohort and the underlying definition of BPD, reported incidences may vary significantly (Lowe et al., 2014). Rates of BPD, defined as oxygen supplementation at 36 weeks' postmenstrual age, ranged from 10 to 25% among preterm infants < 32 weeks' GA born in different regions of Central Europe (Gortner et al., 2011), and ranged from 12 to 15% among VLBW infants followed in the Canadian, Israeli and Japanese neonatal networks (Isayama et al., 2012; Klinger et al., 2013). In contrast, BPD rates were as high as 68% in the NICHD Neonatal Research Network, when including infants who were breathing room air at 36 weeks' postmenstrual age (Stoll et al., 2010). It is noteworthy, however, that we observed a high number of ventilation days in the present study, most likely, due to the restricted use of early non-invasive respiratory support. Mechanical ventilation has been acknowledged as a major postnatal determinant of BPD (Jobe and Bancalari, 2001; Jobe, 2016), and was an independent predictor of BPD in our study. Although both groups did not differ in terms of ventilation days, a particularly strong impact of mechanical ventilation may have masked the effects of less strong predictors.
In contrast to Ureaplasma exposure alone, co-exposure to Ureaplasma spp. and mechanical ventilation ≥5 days was associated with BPD outcome. These data support previous reports of increased risk of moderate/severe BPD in preterm infants with Ureaplasma-positive tracheal aspirates or gastric fluid specimens and mechanical ventilation for any duration and mechanical ventilation ≥14 days, respectively (Sung et al., 2011; Inatomi et al., 2012). Data from a preterm baboon model suggest that antenatal Ureaplasma exposure contributes to early lung fibrosis in conjunction with postnatal mechanical ventilation (Viscardi et al., 2006). There is substantial evidence that mechanical ventilation may interact with antenatal and/or postnatal infection to further increase the risk of BPD in preterm infants (Van Marter et al., 2002; Lahra et al., 2009; Balany and Bhandari, 2015). Our findings suggest that early life exposure to Ureaplasma spp. and prolonged mechanical ventilation may synergistically contribute to the development of BPD. Of note, according to the present data, co-exposure to Ureaplasma spp. and mechanical ventilation ≥5 days may also increase the risk of high-grade IVH in very immature preterm infants. Several case reports and some former studies suggested an implication of Ureaplasma spp. in the pathogenesis of IVH and adverse neurologic outcome (Viscardi et al., 2008; Berger et al., 2009; Kasper et al., 2011; Glaser and Speer, 2015), with neonatal systemic and central nervous system inflammation being potential underlying mechanisms (Viscardi et al., 2008; Glaser and Speer, 2015). We confirm and extend those works and discuss that Ureaplasma-driven inflammation may modulate neonatal respiratory and neurologic outcome in conjunction with a second postnatal injurious hit.
Inflammation has been acknowledged as a common pathway and principle mechanism in the multifactorial pathogenesis of BPD (Groneck et al., 1994; Jobe and Bancalari, 2001; Balany and Bhandari, 2015). While it remains debated whether or not chorioamnionitis represents a risk factor for BPD (Thomas and Speer, 2011; Hartling et al., 2012), persistent perinatal lung inflammation and postnatal infection have been identified as independent determinants (Groneck et al., 1996; Van Marter et al., 2002; Lahra et al., 2009; Balany and Bhandari, 2015). The present study indicates that Ureaplasma spp. effectively induce pro-inflammatory immune responses, but might not stimulate anti-inflammatory responses in VLBW infants and, thus, promote cytokine imbalances pushed toward a pro-inflammatory state. Our findings support data from previous studies documenting increased expression of pro-inflammatory mediators in airway secretions, serum and amniotic fluid specimens of preterm infants colonized with Ureaplasma spp. (Groneck et al., 1996; Patterson et al., 1998; Viscardi and Hasday, 2009), and data from in vitro models documenting Ureaplasma-driven pro-inflammatory responses in macrophages and neonatal monocytes (Manimtim et al., 2001; Viscardi and Hasday, 2009; Glaser et al., 2017, 2018a,b). Of note, bronchoalveolar lavage samples of preterm infants with BPD revealed diminished levels of IL-10, indicating a role for imbalanced inflammatory responses in the pathogenesis of BPD (Jones et al., 1996). In vitro studies reported missing IL-10 expression and imbalanced cytokine ratios pushed toward pro-inflammation in Ureaplasma-stimulated neonatal monocytes (Manimtim et al., 2001; Glaser et al., 2017). The current data may provide additional evidence of a role for Ureaplasma in dysregulated neonatal inflammation.
It is of particular interest, that preterm infants positive for Ureaplasma were more likely to develop LOS in the present study. In general, very immature preterm infants are at increased risk of LOS for reasons of immature immunity as well as exposure to a variety of partly interwoven risk factors, such as invasive interventions like central venous lines and mechanical ventilation, parenteral nutrition and delayed initiation of enteral feeding, colonization with LOS-associated microorganisms as well as prior antibiotic treatment or comorbidities of prematurity like PDA and NEC (Boghossian et al., 2013; Dong et al., 2019). The present analysis controlled for the majority of these risk factors. Central venous lines, of note, were less present in infants stratified to the Ureaplasma-positive study group. Study groups did not differ in terms of initial empiric antibiotic therapy. However, data on antibiotic treatment in the course of hospital stay and results of weekly routine microbiological screening were not assessed. Despite these limitations, the present results may indicate immunomodulatory features of Ureaplasma spp. in the event of secondary bacterial infection, that might drive adverse outcome. This hypothesis adds to data from a preterm sheep model and in vitro studies. While monocytes derived from animals with acute (7 day) Ureaplasma infection showed augmented cytokine responses to secondary stimulation with E. coli lipopolysaccharide (LPS) in vitro, monocytes isolated from animals with chronic (70 day) infection displayed decreased responsiveness to secondary LPS stimulation (Kallapur et al., 2011). In vitro studies of acute Ureaplasma infection in neonatal and adult monocytes documented a differential modulation of cytokine responses in the event of co-infection with E. coli LPS (Manimtim et al., 2001; Glaser et al., 2017, 2018a). Moreover, U. parvum and U. urealyticum were shown to alter Toll-like receptor 2 and 4 expression and to suppress antimicrobial peptide expression in human monocytes in vitro (Xiao et al., 2014; Glaser et al., 2017). Given our current findings, we speculate that immunomodulatory capacities of Ureaplasma may affect preterm immune homeostasis in specific ways, promoting imbalanced neonatal inflammation on the one hand and increasing susceptibility to secondary bacterial infection on the other hand. Time and duration of Ureaplasma exposure might be key to the nature of immune alteration. In fact, positive Ureaplasma screening at admission may reflect chronic prenatal challenge to the organism in one infant and rather acute exposure in another. Moreover, a phase of Ureaplasma-driven inflammation might be followed by a refractory stage. This sequence is known from a transiently altered immune state referred to as “endotoxin tolerance” (López-Collazo and Del Fresno, 2013). Pathogen-pathogen or polymicrobial interaction as well as host genetics or maturity-related host immune function may additionally contribute to individual outcomes.
Detection rates of Ureaplasma in the present study are in accordance with colonization rates of 24–52% reported in preterm infants <32 weeks' GA (van Waarde et al., 1997; Waites et al., 2005; Viscardi and Hasday, 2009; Sung et al., 2011; Payne et al., 2012). Discrepancy to clinical studies reporting lower detection rates in preterm infants may be due to differences in study design and study cohorts, site of sampling and, in particular, the diagnostic and statistical methods applied. It was not until the introduction of molecular techniques that detection of Ureaplasma spp. improved and detection frequency increased (Oh et al., 2010; Xiao et al., 2010). Sensitivity and specificity has been shown to be much higher for PCR analysis. Culture technique alone often misses a significant amount of Ureaplasma-positive samples (Oh et al., 2010). However, given that the screening for Ureaplasma was limited to cord blood and nasopharyngeal swabs obtained at admission, this study still may have missed a certain amount of Ureaplasma-positive infants. A very recent investigation reported on increased yield of Ureaplasma detection by collecting specimens at different time points (Brand et al., 2018).
Few previous studies on Ureaplasma colonization in preterm infants indicated an inverse relationship between colonization and GA, with highest detection rates in infants 25–27 weeks and <26 weeks, respectively (van Waarde et al., 1997; Sung et al., 2011). In our cohort, we found similar detection rates independent of GA. Exclusion of infants >30 weeks' GA might have accounted for this discrepancy. Reports on vertical transmission rates of Ureaplasma spp. in pregnancy have documented discrepant percentages, ranging from 15 to 88%, and have also pointed toward an inverse relationship with GA (Sánchez and Regan, 1990; Alfa et al., 1995; Chua et al., 1999; Kafetzis et al., 2004). In the present study, maternal cultures were not subject of speciation and serovar differentiation. Conclusions on potential transmission rates could not be provided.
So far, little is known about differences in virulence at the level of Ureaplasma species or among individual serovars (Viscardi and Hasday, 2009; Silwedel et al., 2017; Sweeney et al., 2017). Moreover, there is only scarce data on virulence factors potentially related to disease manifestation (Abele-Horn et al., 1997; Sung et al., 2011; Xiao et al., 2011a; Paralanov et al., 2012; Uchida et al., 2013; Payne et al., 2014; Sweeney et al., 2017). While U. parvum has been associated with chorioamniotis and preterm labor in some investigations (Robertson et al., 1986; Kim et al., 2003; Cox et al., 2016; Sweeney et al., 2017), other studies failed to identify serovars more often associated with adverse pregnancy outcome and invasive urogenital tract diseases (Xiao et al., 2011a; Paralanov et al., 2012). A number of studies in preterm infants at risk for BPD documented a predominance of U. parvum in clinical isolates (Katz et al., 2005; Oue et al., 2009; Payne et al., 2010, 2012, 2014; Sung et al., 2011; Winters et al., 2013). Predominance of U. parvum in the current study is in accordance with these previous observations. There is only one study reporting on a predominance of U. urealyticum and an increased risk of BPD in colonized infants (Abele-Horn et al., 1997). In another study, simultaneous colonization with both species was more frequent in preterm infants with the later development of BPD (Katz et al., 2005). So far, no other study has identified an association between neonatal outcome and isolation of a particular Ureaplasma species or serovar. The small number of U. urealyticum-positive infants in our cohort did not allow to test for differences in neonatal outcome at the species level. However, serovar 3 and 6 accounted for >90% of U. parvum swab and serovar 1 for >60% of cord blood isolates. While serovar 6 was not isolated from cord blood, serovar 14 was exclusively found in those specimens. This study is the first to report on potential differences of U. parvum serovar distribution in swab and blood samples. In one previous study in preterm infants assessing respiratory tract specimens, serovar 3 and 6 accounted for 96% of U. parvum respiratory isolates (Sung et al., 2011). Further data are essential to gain a better understanding of individual serovars and potential associations with colonization and/or invasive infection. As far as maternal colonization with U. parvum serovars is concerned, serovar 3 and serovar 6 have been associated with adverse pregnancy outcome, in particular (Robertson et al., 1986; Xiao et al., 2011b). Comparative genome analysis approaches, so far, have been complicated by the limited knowledge of virulence factors in Ureaplasma per se, phenomens of size and phase variation in the major virulence factor known, the so-called multiple banded antigen, and a horizontal gene transfer oberserved in Ureaplasma (Xiao et al., 2011a; Paralanov et al., 2012; Silwedel et al., 2017; Sweeney et al., 2017).
Strengths of this study include its prospective character and its focus on very immature preterm infants, the assessment of Ureaplasma screening by means of culture and PCR technique and the differentiation of species and serovars. To the best of our knowledge, this is the first study to describe the distribution of U. parvum serovars in a cohort of VLBW infants in Central Europe and Poland, respectively. Limitations of the present study are given by its monocentric character. Multicentre studies are warranted to confirm our current findings. Further limitations are related to restrictions of secondary endpoint and subgroup analyses. These findings need confirmation by larger studies powered to assess the individual outcome measures.
In conclusion, our data may add considerably to the understanding of Ureaplasma-driven neonatal morbidity, suggesting that perinatal Ureaplasma exposure may be a driver in the development of neonatal inflammation and infection as well as lung injury. Key might be the duration and intensity of exposure and the addition of a second postnatal injurious hit.
Data Availability
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Ethics Statement
Prior to enrollment, parents were informed about all details of the study and written parental consent was obtained. The study was approved by the ethics committees of the Poznan University of Medical Sciences (#328/14) and the Medical Faculty of Wuerzburg (#2014081001) and was conducted in accordance with the World Medical Association Declaration of Helsinki.
Author Contributions
All authors have contributed significantly. KG, AG-L, MS-B, CS: study conception and design; KG, AG-L, NK-L, BH, AW-G: acquisition and analysis of data; KG, AG-L, MS-B, BH, CS: interpretation of data; KG, AG-L, MS-B, NK-L, BH, AW-G, CS: drafting and critical revision; KG, AG-L, MS-B, NK-L, BH, AW-G, CS: final approval; KG, AG-L, MS-B, NK-L, BH, AW-G, CS: agreement to be accountable for all aspects of the work. No assistance was used in the preparation of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all parents who participated in this study, and our medical and nursing colleagues who assisted in patient enrollment and sample collection. We thank Mariola Dragan for excellent technical support.
Publication of this manuscript was supported by the German Research Foundation (DFG) and the University of Wuerzburg in the funding programme Open Access Publishing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2019.00068/full#supplementary-material
References
Abele-Horn, M., Wolff, C., Dressel, P., Pfaff, F., and Zimmermann, A. (1997). Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetric patients, and gynecological patients with pelvic inflammatory disease. J. Clin. Microbiol. 35, 1199–1202.
Alfa, M. J., Embree, J. E., Degagne, P., Olson, N., Lertzman, J., Macdonald, K. S., et al. (1995). Transmission of Ureaplasma urealyticum from mothers to full and preterm infants. Pediatr. Infect. Dis. J. 14, 341–345. doi: 10.1097/00006454-199505000-00001
Balany, J., and Bhandari, V. (2015). Understanding the impact of infection, inflammation, and their persistence in the pathogenesis of bronchopulmonary dysplasia. Front. Med. 2:90. doi: 10.3389/fmed.2015.00090
Bell, M. J. (1978). Neonatal necrotizing enterocolitis. N. Engl. J. Med. 298, 281–282. doi: 10.1056/NEJM197802022980519
Berger, A., Witt, A., Haiden, N., Kaider, A., Klebermasz, K., Fuiko, R., et al. (2009). Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. J. Perinat. Med. 37, 72–78. doi: 10.1515/JPM.2009.016
Boghossian, N. S., Page, G. P., Bell, E. F., Stoll, B. J., Murray, J. C., Cotten, C. M., et al. (2013). Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. J. Pediatr. 162, 1120–1124, 1124.e1. doi: 10.1016/j.jpeds.2012.11.089
Brand, M. C., Mandy, G. T., Arora, S., Castro, E. C., Gordon, P. S., Hagan, J. L., et al. (2018). Optimum detection of Ureaplasma in premature infants. Pediatr. Infect. Dis. J. 37, 1294–1298. doi: 10.1097/INF.0000000000001994
Chua, K. B., Ngeow, Y. F., Lim, C. T., Ng, K. B., and Chye, J. K. (1999). Colonization and transmission of Ureaplasma urealyticum and Mycoplasma hominis from mothers to full and preterm babies by normal vaginal delivery. Med. J. Malaysia 54, 242–246.
Cox, C., Saxena, N., Watt, A. P., Gannon, C., Mckenna, J. P., Fairley, D. J., et al. (2016). The common vaginal commensal bacterium Ureaplasma parvum is associated with chorioamnionitis in extreme preterm labor. J. Matern. Fetal Neonatal. Med. 29, 3646–3651. doi: 10.3109/14767058.2016.1140734
Dong, Y., Glaser, K., and Speer, C. P. (2019). Late-onset sepsis caused by gram-negative bacteria in very low birth weight infants: a systematic review. Expert Rev. Anti Infect. Ther. 17, 177–188. doi: 10.1080/14787210.2019.1568871
Fanaroff, A. A., Stoll, B. J., Wright, L. L., Carlo, W. A., Ehrenkranz, R. A., Stark, A. R., et al. (2007). Trends in neonatal morbidity and mortality for very low birthweight infants. Am. J. Obstet. Gynecol. 196, 147.e1–147.e8. doi: 10.1016/j.ajog.2006.09.014
Glaser, K., Silwedel, C., Fehrholz, M., Henrich, B., Waaga-Gasser, A. M., Claus, H., et al. (2018a). Ureaplasma isolates stimulate pro-inflammatory CC chemokines and matrix metalloproteinase-9 in neonatal and adult monocytes. PLoS ONE 13:e0194514. doi: 10.1371/journal.pone.0194514
Glaser, K., Silwedel, C., Fehrholz, M., Waaga-Gasser, A. M., Henrich, B., Claus, H., et al. (2017). Ureaplasma species differentially modulate pro- and anti-inflammatory cytokine responses in newborn and adult human monocytes pushing the state toward pro-inflammation. Front. Cell. Infect. Microbiol. 7:484. doi: 10.3389/fcimb.2017.00484
Glaser, K., Silwedel, C., Waaga-Gasser, A. M., Henrich, B., Fehrholz, M., Claus, H., et al. (2018b). Ureaplasma isolates differentially modulate growth factors and cell adhesion molecules in human neonatal and adult monocytes. Cytokine 105, 45–48. doi: 10.1016/j.cyto.2018.01.026
Glaser, K., and Speer, C. P. (2015). Neonatal CNS infection and inflammation caused by Ureaplasma species: rare or relevant? Expert Rev. Anti Infect. Ther. 13, 233–248. doi: 10.1586/14787210.2015.999670
Gortner, L., Misselwitz, B., Milligan, D., Zeitlin, J., Kollée, L., Boerch, K., et al. (2011). Rates of bronchopulmonary dysplasia in very preterm neonates in Europe: results from the MOSAIC cohort. Neonatology 99, 112–117. doi: 10.1159/000313024
Groneck, P., Goetze-Speer, B., and Speer, C. P. (1996). Inflammatory bronchopulmonary response of preterm infants with microbial colonisation of the airways at birth. Arch. Dis. Child. Fetal Neonatal Ed. 74, F51–F55. doi: 10.1136/fn.74.1.F51
Groneck, P., Götze-Speer, B., Oppermann, M., Eiffert, H., and Speer, C. P. (1994). Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics 93, 712–718.
Hartling, L., Liang, Y., and Lacaze-Masmonteil, T. (2012). Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 97, F8–F17. doi: 10.1136/adc.2010.210187
Inatomi, T., Oue, S., Ogihara, T., Hira, S., Hasegawa, M., Yamaoka, S., et al. (2012). Antenatal exposure to Ureaplasma species exacerbates bronchopulmonary dysplasia synergistically with subsequent prolonged mechanical ventilation in preterm infants. Pediatr. Res. 71, 267–273. doi: 10.1038/pr.2011.47
International Committee for the Classification of Retinopathy of Prematurity (2005). The International classification of retinopathy of prematurity revisited. Arch. Ophthalmol. 123, 991–999. doi: 10.1001/archopht.123.7.991
Isayama, T., Lee, S. K., Mori, R., Kusuda, S., Fujimura, M., Ye, X. Y., et al. (2012). Comparison of mortality and morbidity of very low birth weight infants between Canada and Japan. Pediatrics 130, e957–e965. doi: 10.1542/peds.2012-0336
Jobe, A. H. (2016). Mechanisms of lung injury and bronchopulmonary dysplasia. Am. J. Perinatol. 33, 1076–1078. doi: 10.1055/s-0036-1586107
Jobe, A. H., and Bancalari, E. (2001). Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729. doi: 10.1164/ajrccm.163.7.2011060
Jones, C. A., Cayabyab, R. G., Kwong, K. Y., Stotts, C., Wong, B., Hamdan, H., et al. (1996). Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr. Res. 39, 966–975. doi: 10.1203/00006450-199606000-00007
Kafetzis, D. A., Skevaki, C. L., Skouteri, V., Gavrili, S., Peppa, K., Kostalos, C., et al. (2004). Maternal genital colonization with Ureaplasma urealyticum promotes preterm delivery: association of the respiratory colonization of premature infants with chronic lung disease and increased mortality. Clin. Infect. Dis. 39, 1113–1122. doi: 10.1086/424505
Kallapur, S. G., Kramer, B. W., Knox, C. L., Berry, C. A., Collins, J. J., Kemp, M. W., et al. (2011). Chronic fetal exposure to Ureaplasma parvum suppresses innate immune responses in sheep. J. Immunol. 187, 2688–2695. doi: 10.4049/jimmunol.1100779
Kasper, D. C., Mechtler, T. P., Bohm, J., Petricevic, L., Gleiss, A., Spergser, J., et al. (2011). In utero exposure to Ureaplasma spp. is associated with increased rate of bronchopulmonary dysplasia and intraventricular hemorrhage in preterm infants. J. Perinat. Med. 39, 331–336. doi: 10.1515/jpm.2011.022
Katz, B., Patel, P., Duffy, L., Schelonka, R. L., Dimmitt, R. A., and Waites, K. B. (2005). Characterization of ureaplasmas isolated from preterm infants with and without bronchopulmonary dysplasia. J. Clin. Microbiol. 43, 4852–4854. doi: 10.1128/JCM.43.9.4852-4854.2005
Kim, M., Kim, G., Romero, R., Shim, S. S., Kim, E. C., and Yoon, B. H. (2003). Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J. Perinat. Med. 31, 146–152. doi: 10.1515/JPM.2003.020
Klinger, G., Sokolover, N., Boyko, V., Sirota, L., Lerner-Geva, L., Reichman, B., et al. (2013). Perinatal risk factors for bronchopulmonary dysplasia in a national cohort of very-low-birthweight infants. Am. J. Obstet. Gynecol. 208, 115e1–115e9. doi: 10.1016/j.ajog.2012.11.026
Lahra, M. M., Beeby, P. J., and Jeffery, H. E. (2009). Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics 123, 1314–1319. doi: 10.1542/peds.2008-0656
López-Collazo, E., and Del Fresno, C. (2013). Pathophysiology of endotoxin tolerance: mechanisms and clinical consequences. Crit. Care 17:242. doi: 10.1186/cc13110
Lowe, J., Watkins, W. J., Edwards, M. O., Spiller, O. B., Jacqz-Aigrain, E., Kotecha, S. J., et al. (2014). Association between pulmonary ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: updated systematic review and meta-analysis. Pediatr. Infect. Dis. J. 33, 697–702. doi: 10.1097/INF.0000000000000239
Manimtim, W. M., Hasday, J. D., Hester, L., Fairchild, K. D., Lovchik, J. C., and Viscardi, R. M. (2001). Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect. Immun. 69, 3906–3915. doi: 10.1128/IAI.69.6.3906-3915.2001
Nugent, R. P., Krohn, M. A., and Hillier, S. L. (1991). Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 29, 297–301.
Oh, K. J., Lee, S. E., Jung, H., Kim, G., Romero, R., and Yoon, B. H. (2010). Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J. Perinat. Med. 38, 261–268. doi: 10.1515/jpm.2010.040
Otgonjargal, B., Batbaatar, G., Pfeffer, K., Bruhn, T., Battogtokh, C., and Henrich, B. (2018). A novel mba-based Real time PCR approach for genotyping of Ureaplasma parvum validated in a cohort of Mongolian mothers and offspring. Int. J. Med. Microbiol. 308, 865–871. doi: 10.1016/j.ijmm.2018.08.001
Oue, S., Hiroi, M., Ogawa, S., Hira, S., Hasegawa, M., Yamaoka, S., et al. (2009). Association of gastric fluid microbes at birth with severe bronchopulmonary dysplasia. Arch. Dis. Child. Fetal Neonatal Ed. 94, F17–F22. doi: 10.1136/adc.2008.138321
Papile, L. A., Burstein, J., Burstein, R., and Koffler, H. (1978). Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534. doi: 10.1016/S0022-3476(78)80282-0
Paralanov, V., Lu, J., Duffy, L. B., Crabb, D. M., Shrivastava, S., Methé, B. A., et al. (2012). Comparative genome analysis of 19 Ureaplasma urealyticum and Ureaplasma parvum strains. BMC Microbiol. 12:88. doi: 10.1186/1471-2180-12-88
Patterson, A. M., Taciak, V., Lovchik, J., Fox, R. E., Campbell, A. B., and Viscardi, R. M. (1998). Ureaplasma urealyticum respiratory tract colonization is associated with an increase in interleukin 1-beta and tumor necrosis factor alpha relative to interleukin 6 in tracheal aspirates of preterm infants. Pediatr. Infect. Dis. J. 17, 321–328. doi: 10.1097/00006454-199804000-00011
Payne, M. S., Goss, K. C., Connett, G. J., Kollamparambil, T., Legg, J. P., Thwaites, R., et al. (2010). Molecular microbiological characterization of preterm neonates at risk of bronchopulmonary dysplasia. Pediatr. Res. 67, 412–418. doi: 10.1203/PDR.0b013e3181d026c3
Payne, M. S., Goss, K. C., Connett, G. J., Legg, J. P., Bruce, K. D., and Chalker, V. (2012). A quantitative analysis of Ureaplasma urealyticum and Ureaplasma parvum compared with host immune response in preterm neonates at risk of developing bronchopulmonary dysplasia. J. Clin. Microbiol. 50, 909–914. doi: 10.1128/JCM.06625-11
Payne, M. S., Tabone, T., Kemp, M. W., Keelan, J. A., Spiller, O. B., and Newnham, J. P. (2014). High-resolution melt PCR analysis for genotyping of Ureaplasma parvum isolates directly from clinical samples. J. Clin. Microbiol. 52, 599–606. doi: 10.1128/JCM.03036-13
Perlman, J. M., Risser, R., and Broyles, R. S. (1996). Bilateral cystic periventricular leukomalacia in the premature infant: associated risk factors. Pediatrics 97, 822–827.
Robertson, J. A., Honore, L. H., and Stemke, G. W. (1986). Serotypes of Ureaplasma urealyticum in spontaneous abortion. Pediatr. Infect. Dis. 5, S270–S272. doi: 10.1097/00006454-198611010-00014
Rogers, B. B., Alexander, J. M., Head, J., Mcintire, D., and Leveno, K. J. (2002). Umbilical vein interleukin-6 levels correlate with the severity of placental inflammation and gestational age. Hum. Pathol. 33, 335–340. doi: 10.1053/hupa.2002.32214
Sánchez, P. J., and Regan, J. A. (1990). Vertical transmission of Ureaplasma urealyticum from mothers to preterm infants. Pediatr. Infect. Dis. J. 9, 398–401. doi: 10.1097/00006454-199006000-00006
Schulz, K. F., and Grimes, D. A. (2005). Sample size calculations in randomised trials: mandatory and mystical. Lancet 365, 1348–1353. doi: 10.1016/S0140-6736(05)61034-3
Silwedel, C., Speer, C. P., and Glaser, K. (2017). Ureaplasma-associated prenatal, perinatal, and neonatal morbidities. Expert Rev. Clin. Immunol. 13, 1073–1087. doi: 10.1080/1744666X.2017.1381559
Stoll, B. J., Hansen, N. I., Bell, E. F., Shankaran, S., Laptook, A. R., Walsh, M. C., et al. (2010). Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics 126, 443–456. doi: 10.1542/peds.2009-2959
Stoll, B. J., Hansen, N. I., Bell, E. F., Walsh, M. C., Carlo, W. A., Shankaran, S., et al. (2015). Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314, 1039–1051. doi: 10.1001/jama.2015.10244
Sung, T. J., Xiao, L., Duffy, L., Waites, K. B., Chesko, K. L., and Viscardi, R. M. (2011). Frequency of ureaplasma serovars in respiratory secretions of preterm infants at risk for bronchopulmonary dysplasia. Pediatr. Infect. Dis. J. 30, 379–383. doi: 10.1097/INF.0b013e318202ac3a
Sweeney, E. L., Dando, S. J., Kallapur, S. G., and Knox, C. L. (2017). The human Ureaplasma species as causative agents of chorioamnionitis. Clin. Microbiol. Rev. 30, 349–379. doi: 10.1128/CMR.00091-16
Thomas, W., and Speer, C. P. (2011). Chorioamnionitis: important risk factor or innocent bystander for neonatal outcome? Neonatology 99, 177–187. doi: 10.1159/000320170
Uchida, K., Nakahira, K., Mimura, K., Shimizu, T., De Seta, F., Wakimoto, T., et al. (2013). Effects of Ureaplasma parvum lipoprotein multiple-banded antigen on pregnancy outcome in mice. J. Reprod. Immunol. 100, 118–127. doi: 10.1016/j.jri.2013.10.001
Van Marter, L. J., Dammann, O., Allred, E. N., Leviton, A., Pagano, M., Moore, M., et al. (2002). Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J. Pediatr. 140, 171–176. doi: 10.1067/mpd.2002.121381
van Waarde, W. M., Brus, F., Okken, A., and Kimpen, J. L. (1997). Ureaplasma urealyticum colonization, prematurity and bronchopulmonary dysplasia. Eur. Respir. J. 10, 886–890.
Viscardi, R. M., Atamas, S. P., Luzina, I. G., Hasday, J. D., He, J. R., Sime, P. J., et al. (2006). Antenatal Ureaplasma urealyticum respiratory tract infection stimulates proinflammatory, profibrotic responses in the preterm baboon lung. Pediatr. Res. 60, 141–146. doi: 10.1203/01.pdr.0000228322.73777.05
Viscardi, R. M., and Hasday, J. D. (2009). Role of Ureaplasma species in neonatal chronic lung disease: epidemiologic and experimental evidence. Pediatr. Res. 65, 84R−90R. doi: 10.1203/PDR.0b013e31819dc2f9
Viscardi, R. M., Hashmi, N., Gross, G. W., Sun, C. C., Rodriguez, A., and Fairchild, K. D. (2008). Incidence of invasive ureaplasma in VLBW infants: relationship to severe intraventricular hemorrhage. J. Perinatol. 28, 759–765. doi: 10.1038/jp.2008.98
Volgmann, T., Ohlinger, R., and Panzig, B. (2005). Ureaplasma urealyticum-harmless commensal or underestimated enemy of human reproduction? A review. Arch. Gynecol. Obstet. 273, 133–139. doi: 10.1007/s00404-005-0030-1
Waites, K. B., Katz, B., and Schelonka, R. L. (2005). Mycoplasmas and ureaplasmas as neonatal pathogens. Clin. Microbiol. Rev. 18, 757–789. doi: 10.1128/CMR.18.4.757-789.2005
Winters, A. H., Levan, T. D., Vogel, S. N., Chesko, K. L., Pollin, T. I., and Viscardi, R. M. (2013). Single nucleotide polymorphism in toll-like receptor 6 is associated with a decreased risk for ureaplasma respiratory tract colonization and bronchopulmonary dysplasia in preterm infants. Pediatr. Infect. Dis. J. 32, 898–904. doi: 10.1097/INF.0b013e31828fc693
Xiao, L., Crabb, D. M., Dai, Y., Chen, Y., Waites, K. B., and Atkinson, T. P. (2014). Suppression of antimicrobial peptide expression by ureaplasma species. Infect. Immun. 82, 1657–1665. doi: 10.1128/IAI.01231-13
Xiao, L., Crabb, D. M., Moser, S. A., Duffy, L. B., Glass, J. I., Paralanov, V., et al. (2011a). Genotypic characterization of ureaplasma serovars from clinical isolates by pulsed-field gel electrophoresis. J. Clin. Microbiol. 49, 3325–3328. doi: 10.1128/JCM.01235-11
Xiao, L., Glass, J. I., Paralanov, V., Yooseph, S., Cassell, G. H., Duffy, L. B., et al. (2010). Detection and characterization of human Ureaplasma species and serovars by real-time PCR. J. Clin. Microbiol. 48, 2715–2723. doi: 10.1128/JCM.01877-09
Keywords: Ureaplasma parvum, Ureaplasma urealyticum, preterm infants, VLBW, bronchopulmonary dysplasia, late onset sepsis, neonatal outcome, inflammation
Citation: Glaser K, Gradzka-Luczewska A, Szymankiewicz-Breborowicz M, Kawczynska-Leda N, Henrich B, Waaga-Gasser AM and Speer CP (2019) Perinatal Ureaplasma Exposure Is Associated With Increased Risk of Late Onset Sepsis and Imbalanced Inflammation in Preterm Infants and May Add to Lung Injury. Front. Cell. Infect. Microbiol. 9:68. doi: 10.3389/fcimb.2019.00068
Received: 20 December 2018; Accepted: 04 March 2019;
Published: 02 April 2019.
Edited by:
Ran Nir-Paz, Hebrew University of Jerusalem, IsraelReviewed by:
Rose M. Viscardi, School of Medicine, University of Maryland, United StatesSmadar Eventov-Friedman, Hebrew University of Jerusalem, Israel
Copyright © 2019 Glaser, Gradzka-Luczewska, Szymankiewicz-Breborowicz, Kawczynska-Leda, Henrich, Waaga-Gasser and Speer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kirsten Glaser, R2xhc2VyX0tAdWt3LmRl
 Kirsten Glaser
Kirsten Glaser Anna Gradzka-Luczewska
Anna Gradzka-Luczewska Marta Szymankiewicz-Breborowicz2
Marta Szymankiewicz-Breborowicz2