
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol., 24 July 2018
Sec. Microbiome in Health and Disease
Volume 8 - 2018 | https://doi.org/10.3389/fcimb.2018.00243
This article is part of the Research TopicInterplay of Infection and Microbiome View all 13 articles
Historically, Campylobacteriosis has been considered to be zoonotic; the Campylobacter species that cause human acute intestinal disease such as Campylobacter jejuni and Campylobacter coli originate from animals. Over the past decade, studies on human hosted Campylobacter species strongly suggest that Campylobacter concisus plays a role in the development of inflammatory bowel disease (IBD). C. concisus primarily colonizes the human oral cavity and some strains can be translocated to the intestinal tract. Genome analysis of C. concisus strains isolated from saliva samples has identified a bacterial marker that is associated with active Crohn's disease (one major form of IBD). In addition to C. concisus, humans are also colonized by a number of other Campylobacter species, most of which are in the oral cavity. Here we review the most recent advancements on C. concisus and other human hosted Campylobacter species including their clinical relevance, transmission, virulence factors, disease associated genes, interactions with the human immune system and pathogenic mechanisms.
Campylobacter, along with Arcobacter and Sulfurospirillum, are the three genera that belong to the family, Campylobacteraceae. Campylobacter species are Gram-negative, curved or spiral shaped, and most of them are motile with a single polar flagellum present at one or both ends of the bacteria, allowing them to have a corkscrew-like motion during movement (Lastovica et al., 2014). Campylobacter species have low G + C content in their genome, and the median G + C content for most of the Campylobacter species ranges from 28 to 40% (Pruitt et al., 2007). There are few Campylobacter species which have G + C content of more than 40% in their genomes, including Campylobacter curvus, Campylobacter rectus, Campylobacter showae and Campylobacter gracilis (Pruitt et al., 2007). A majority of the Campylobacter species are microaerophiles, while some require anaerobic conditions for their growth (Debruyne et al., 2008).
Most Campylobacter species live as normal flora in the gastrointestinal tract of various animals (Lastovica et al., 2014). Some of these animal hosted Campylobacter species, such as Campylobacter jejuni and Campylobacter coli, can cause acute bacterial gastroenteritis in humans through consumption of contaminated food or water (Galanis, 2007). In addition to gastroenteritis, C. jejuni also causes Guillain-Barré syndrome, due to molecular mimicry between its sialylated lipooligosaccharides and the human nerve gangliosides (Takahashi et al., 2005).
As C. jejuni and C. coli are the main Campylobacter pathogens which cause human acute intestinal disease and they originate from animal sources, Campylobacteriosis has historically been considered to be zoonotic. Several Campylobacter species utilize humans as their natural host and accumulated evidence supports their role in chronic inflammatory diseases of the human intestinal tract. Here we review recent advancements on human hosted Campylobacter species, their clinical relevance, transmission, virulence factors, disease associated genes, interactions with human immune system and pathogenic mechanisms. Most of the studies on the human hosted Campylobacter species in the past decade were on Campylobacter concisus, this bacterium is therefore the focus of this review. In addition, other human hosted Campylobacter species were also reviewed.
The natural host of a bacterium refers to the host that the bacterium normally lives and reproduces (Haydon et al., 2002; Control and Prevention, 2006). Bacterial species are usually not harmful to their hosts, although there are exceptions. For example, Helicobacter pylori is a human hosted bacterial species, causing gastritis and gastric ulcers and being a risk factor for gastric cancer (Roesler et al., 2014).
To date, 40 Campylobacter species and subspecies have been isolated from a wide variety of animal or human sources (Figure 1). Many Campylobacter species are naturally hosted by domesticated animals raised as food such as chicken, cattle and pigs (Lastovica et al., 2014). They survive as commensal bacteria in their hosts, and some species, such as C. jejuni and C. coli, can cause human diseases. The main human disease caused by animal hosted pathogenic Campylobacter species is acute gastroenteritis and clinical disorders can also arise if bacterial species colonizing sterile sites of the body (Table 1). A number of Campylobacter species are also able to cause diseases in animals. For example, Campylobacter fetus is known to cause abortion in bovine and ovine, and Campylobacter hepaticus is the causative agent of spotty liver disease in chicken (Campero et al., 2005; Van et al., 2016).
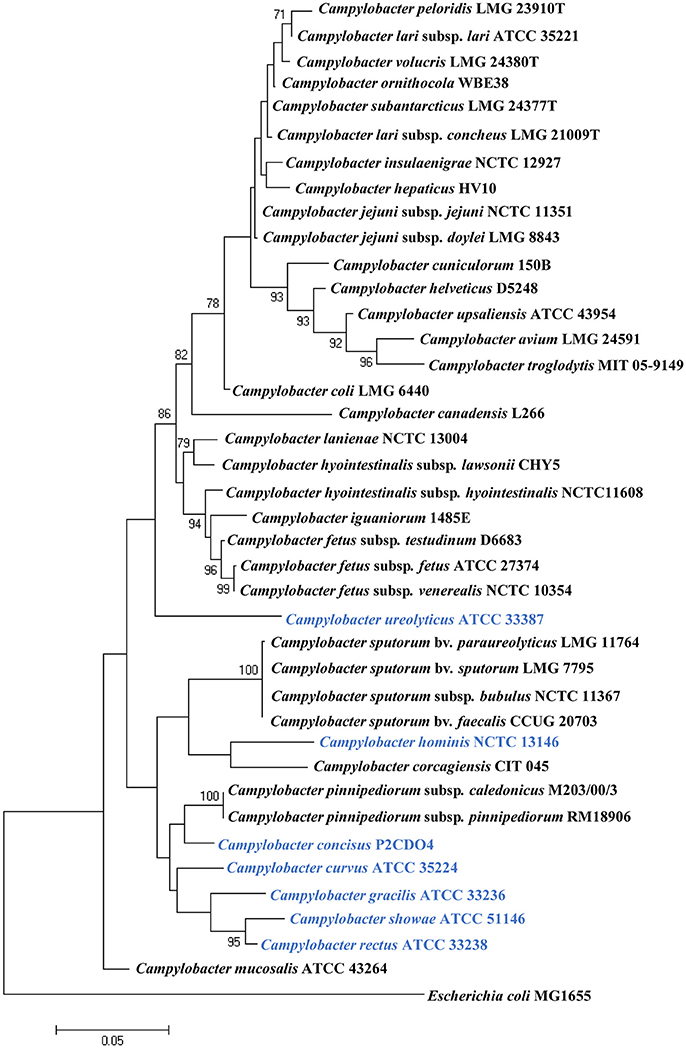
Figure 1. Phylogenetic tree based on the 16S rRNA gene of Campylobcter species. The tree was generated using the maximum likelihood method implemented in MEGA7. Bootstrap values were generated from 1000 replicates. Bootstrap values of more than 70 were indicated. Escherichia coli MG1655 was included as an outgroup. Human hosted Campylobacter species are in blue. Campylobacter geochelonis was not included because its 16S rRNA sequence was not available.
Campylobacter species hosted by humans include C. concisus, C. curvus, C. gracilis, Campylobacter hominis, C. rectus, and C. showae; to date these six Campylobacter species have only been isolated from humans (Hariharan et al., 1994; Zhang, 2015). Campylobacter ureolyticus was isolated from human samples in most cases, with only one study isolating C. ureolyticus from the endometria of healthy horses (Hariharan et al., 1994). Therefore, in this review, C. ureolyticus is considered as a human hosted Campylobacter species. In contrast to animal hosted Campylobacter pathogens, the human hosted Campylobacter pathogens are more often involved in chronic inflammatory conditions of the gastrointestinal tract (Table 2).
C. concisus has a curved or spiral shape and a single polar flagellum, with the size being 0.5–1 by 4 μm (Tanner et al., 1981). Its colony appears as convex shaped, translucent and is ~1 mm in diameter (Tanner et al., 1981). In early literature, C. concisus was described as a microaerophile, due to the growth of this bacterium under microaerophilic atmosphere enriched with hydrogen (H2) (Lastovica, 2006). Later, Lee et al. demonstrated that C. concisus is not a microaerophile, as they found that none of the 57 C. concisus strains grew in the microaerophilic conditions generated using the Oxoid BR56A and CN25A gas-generation systems (Lee et al., 2014). These C. concisus strains were able to grow under anaerobic conditions, with tiny colonies observed after 3 days of culture under anaerobic conditions, showing that C. concisus is an anaerobic bacterium. The anaerobic condition used in the study was generated using AN25A gas-generation system which absorbs oxygen with simultaneous production of CO2.
C. concisus is a chemolithotrophic bacterium, capable of using H2 as a source of energy to markedly increases its growth (Lee et al., 2014). C. concisus is able to oxidize H2 under both anaerobic and microaerobic conditions, although greater growth was observed under anaerobic conditions in the presence of 2.5–10% H2 (Lee et al., 2014). C. concisus is catalase negative, contributing to its inability to grow under microaerobic conditions (Tanner et al., 1981).
Currently, humans are the only known hosts of C. concisus with oral cavity being its natural colonization site (Zhang et al., 2010; Mahendran et al., 2013). C. concisus has been isolated from saliva samples of children as early as 3 years old, although the positive isolation rate was significantly lower than the other age groups (33 vs. 79–88%); and the highest C. concisus isolation rate was seen in the age group of 12–17 (Zhang et al., 2010) (Figure 2). When a PCR method was used for detection, the children from the 3 to 5 years old age bracket had a detection rate that was similar to other age groups (83 vs. 93–100%). These data show that although C. concisus colonize humans at the early stages of life, 3–5 year old children have lower bacterial loads of C. concisus in their saliva as compared to older children and the adults. Currently, no data are available regarding C. concisus colonization in children below 3 years old.
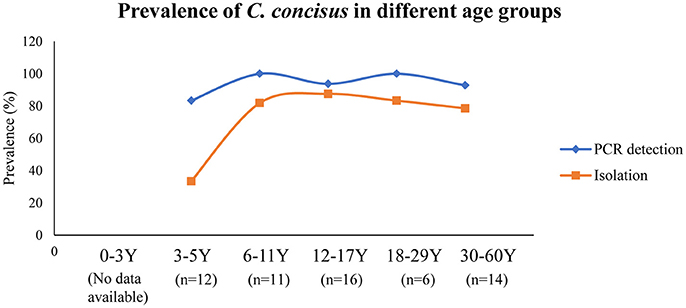
Figure 2. Detection and isolation rates of C. concisus from saliva samples in different age groups. The PCR detection rate of C. concisus from children of different age groups varied between 80 and 100%. However, the isolation rate of C. concisus from children at 3–5 years was only 33% (4/12), which was significantly lower than that in other age groups, indicating younger children were colonized with lower numbers of bacteria. Data are from Zhang et al. (2010).
Given that C. concisus colonizes the oral cavity, transmission would occur through saliva. The stability of C. concisus in saliva samples is related to sample storage. We were able to isolate C. concisus from saliva samples stored at 4°C for 3–6 days. However, we were unable to isolate C. concisus from the same saliva samples after storage at room temperature for 24 h. This suggests that in addition to direct contact, C. concisus contaminated food or drinks, particularly those stored in refrigerators, may also play a role in C. concisus transmission. Multiple strains of C. concisus have been isolated from saliva samples and enteric samples of given individuals, suggesting a possible dynamic colonization of new C. concicus strains in the human gastrointestinal tract (Ismail et al., 2012).
Gingivitis is a common bacterial disease which affects 90% of the population (Coventry et al., 2000). The oral mucosa consists of stratified squamous epithelial cells (Lumerman et al., 1995). Periodontitis develops when gingivitis is not well-treated (Pihlstrom et al., 2005). As disease progresses, a loss of attachment between the gingivae and the teeth leads to the formation of a periodontal pocket, which then allows extensive colonization by anaerobic bacteria causing further inflammation of the mucosa (Highfield, 2009). In periodontitis patients, higher proportions of Gram-negative and anaerobic bacterial species are present in the oral cavity as compared to healthy controls (Newman and Socransky, 1977). C. concisus was initially isolated from the oral cavity of patients with gingivitis and periodontitis (Tanner et al., 1981). However, studies have not revealed a clear association between C. concisus and gingivitis and periodontitis, its role in human oral inflammatory diseases remains unclear.
The esophagus is a muscular conduit with stratified squamous epithelial layers; its mucosa is colonized by microbes dominated by members of the genus Streptococcus (Di Pilato et al., 2016). Disturbances of the microbiota composition, such as the abnormal enrichment of some Gram-negative bacteria including Campylobacter spp. has been reported to be associated with gastroesophageal reflux disease (GERD), and is suggested to contribute to the development toward Barrett's esophagus and oesophageal adenocarcinoma (Di Pilato et al., 2016).
By analyzing the microbiome composition in biopsy samples of the distal esophagus collected from normal individuals and patients with esophagitis or Barrett's esophagus, Yang et al. found that type I microbiome (Gram-positive aerobic microbiome) was mainly associated with normal esophagus (11/12, 91.7%), while type II microbiome (Gram-negative anaerobic microbiome) was more closely associated with abnormal esophagus including esophagitis and Barrett's esophagus (13/22, 59.1%) (Yang et al., 2009). Furthermore, Campylobacter species was found to be one of the genera with increased abundance in type II microbiome (Yang et al., 2009).
By using bacterial cultivation methods, Macfarlane et al. found that high levels of C. concisus and C. rectus were present in oesophageal aspirate and mucosal samples of patients with Barrett's esophagus, but not in control subjects (Macfarlane et al., 2007). Similar findings were reported by another study, in which the Campylobacter genus dominated by C. concisus colonized patients with GERD and Barrett's esophagus with increased bacterial counts, accompanied by a significant decrease in bacterial counts for all other genera (Blackett et al., 2013). This relationship was not observed in patients with oesophageal adenocarcinoma. It is possible that C. concisus contributes to the inflammation associated with GERD and Barrett's esophagus.
Gastroenteritis is an inflammatory condition of the gastrointestinal tract which is characterized by diarrhea, abdominal pain, fever and vomiting (Galanis, 2007). It is usually self-limiting within 2–5 days (Galanis, 2007). Gastroenteritis can be caused by bacteria, viruses, parasites and fungi; the most common causes are rotavirus and bacterial species such as Escherichia coli, C. jejuni, and C. coli (Galanis, 2007).
A number of studies have reported the isolation of Campylobacter species other than C. jejuni and C. coli in diarrheal stool samples. Lindblom et al. found that in stool samples from diarrheal patients, Campylobacter upsaliensis, Campylobacter sputorum, and C. concisus were the most common species in addition to C. jejuni, of which C. concisus was only isolated from children (Lindblom et al., 1995). Similarly, Lastovica et al. reported that in addition to C. jejuni, C. concisus was the second most frequently isolated Campylobacter species from diarrheic stools of pediatric patients (Lastovica and Roux, 2000). Nielsen et al. also found that C. concisus was more frequently found in diarrheal stool samples of young children and elderly (Nielsen et al., 2013). The same group later reported a clinical study comparing clinical manifestations between adult patients infected with C. concisus and C. jejuni/C. coli, and they showed that although C. concisus infection seems to induce a milder course of acute gastroenteritis in comparison to that induced by C. jejuni/C. coli, it is associated with prolonged diarrhea (Nielsen et al., 2012). Recently, Serichantalergs et al. reported a significantly higher detection rate of C. concisus in traveller's diarrhea cases as compared to that in asymptomatic controls in Nepal (Serichantalergs et al., 2017). Another recent study by Tilmanne et al. showed that C. concisus had a similar prevalence in children with acute gastroenteritis as compared with the control group (Tilmanne et al., 2018). Unfortunately, most of the studies that reported the isolation of C. concisus from diarrheal stool samples did not have control fecal samples from healthy individuals, making it difficult to judge the role of this bacterium in gastroenteritis; and for those studies with control groups included, the results were controversial. Thus, whether C. concisus plays a role in gastroenteritis remains to be investigated.
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the gastrointestinal tract with Crohn's disease (CD) and ulcerative colitis (UC) being two of its major forms (De Souza and Fiocchi, 2016). The two forms of IBD mainly differ in pathology. CD is characterized by having discontinuous “skip lesions” of transmural inflammation and the abnormalities can be found throughout the gastrointestinal tract (Walsh et al., 2011). In contrast to CD, the inflammation involvement for UC is usually confined to the mucosa and submucosa without skip lesions and mostly occurs in the large intestine (Walsh et al., 2011).
The etiology of IBD is not fully understood. Accumulated evidence has suggested that the mucosal immune system in genetically predisposed individuals has mounted responses to intestinal commensal bacterial species, which is believed to contribute to the pathogenesis of IBD (Knights et al., 2013). Intestinal commensal bacterial species have co-evolved with the intestinal mucosal immune system thus the immune responses to intestinal commensal bacterial species would need an external trigger. Microbes that have the ability to cause a prolonged primary intestinal barrier defect or alter the mucosal immune system are more likely to trigger IBD.
C. concisus is commonly present in the oral cavities of almost all individuals. However, patients with IBD have a significantly higher prevalence of C. concisus detected in their intestinal tissues as compared to healthy controls (Zhang et al., 2009, 2014; Man et al., 2010b; Mahendran et al., 2011; Mukhopadhya et al., 2011; Kirk et al., 2016). Comparison of the housekeeping genes and genomes of oral and enteric C. concisus strains suggests that enteric strains originate from oral C. concisus strains (Ismail et al., 2012; Chung et al., 2016).
C. concisus, C. hominis, C. showae, and C. ureolyticus have also been isolated from patients with CD. Additionally, C. homonis, C. showae, C. rectus, C. gracilis, and C. ureolyticus have been detected from fecal specimens of children with newly diagnosed CD (Zhang et al., 2009; Man et al., 2010b). However, no association has been found between the prevalence of these Campylobacter species in CD patients and healthy controls.
C. concisus strains can be separated into two major genomospecies (GS), consistently defined by the analysis of core genomes and housekeeping genes (Istivan, 2005; Miller et al., 2012; Mahendran et al., 2015; Chung et al., 2016; Nielsen et al., 2016). C. concisus 23S rRNA gene has polymorphisms, which was also used to define the genomospecies by comparison of the entire 23S rRNA gene sequence or PCR amplification of 23S rRNA gene fragments (Engberg et al., 2005; Kalischuk and Inglis, 2011; On et al., 2013; Huq et al., 2017; Wang et al., 2017).
Quantitative PCR methods targeting the polymorphisms of the 23S rRNA gene revealed that there were more GS2 than GS1 C. concisus in samples collected from the upper and lower gastrointestinal tract of both patients with IBD and healthy controls, suggesting that GS2 C. concisus is better adapted to the human gastrointestinal tract (Wang et al., 2017). A meta-analysis of the composition of the isolated GS1 and GS2 C. concisus strains showed similar findings except that in healthy individuals, a significantly lower number of GS2 C. concisus strains than GS1 C. concisus were isolated from fecal samples. This suggests a potential difference in the C. concisus strains or the enteric environment between patients with gastrointestinal diseases and healthy controls (Wang et al., 2017).
The two GS of C. concisus do not differ in morphology but have GS-specific genes and may have different pathogenic potentials. By examining C. concisus strains isolated from diarrheal fecal samples, Engberg et al. found that bloody diarrhea was only present in individuals infected with GS2 C. concisus strains (Engberg et al., 2005). Furthermore, Kalischuk and Inglis demonstrated that GS2 C. concisus exhibited higher levels of epithelial invasion and translocation (Kalischuk and Inglis, 2011). Similarly, Ismail et al. reported that the oral C. concisus strains that were more invasive to intestinal epithelial cells were GS2 strains (Ismail et al., 2012).
Recently, comparative genomic analyses have identified two novel genomic islands CON_PiiA and CON_PiiB that carry proteins homologous to the type IV secretion system, LepB-like and CagA-like effectors (Chung et al., 2016). CON_PiiA and CON_PiiB were found in strains from both GS1 and GS2 (Chung et al., 2016). The effects of the proteins possessed by CON_PiiA and CON_PiiB on human cells require further examination.
Both GS1 and GS2 contain diverse C. concisus strains. The isolation sources of C. concisus strains do not seem to contribute to the phylogenetic relatedness. C. concisus strains isolated from saliva, intestinal biopsies and feces are found in both GS1 and GS2, as are the strains isolated from patients with enteric disease and healthy controls. A recent study examining the genomes of 104 C. concisus strains isolated from saliva, mucosal biopsies and fecal samples of patients with IBD, gastroenteritis and healthy individuals showed that sampling site rather than disease phenotype was associated with the particular GS (Kirk et al., 2018). In that study, authors reported that genes involved in cell membrane synthesis were common in oral strains, while those related to cell transport, metabolism, and secretory pathways were more often found in enteric isolates. These results indicate that GS alone is unable to differentiate virulent C. concisus strains from commensal strains.
A recent study reported the identification of an exoprotein named C. concisus secreted protein 1 (Csep1) (Liu et al., 2018). The csep1 gene was found to be localized in the bacterial chromosome and the pICON plasmid. The chromosomally encoded csep1 gene was only found in GS2 C. concisus strains, while the pICON plasmid encoded csep1 gene was found in both GS1 and GS2 strains. Some csep1 genes contained a six-nucleotide insertion at the position 654–659 bp (csep1-6bpi). Importantly, the csep1-6bpi gene in oral C. consisus strains was found to be associated with active CD. So far, the csep1-6bpi gene is the only molecular marker in C. concisus reported to have an association with IBD.
Campylobacter movement through the mucus layer is driven by its polar flagellum, which is crucial for approaching, attaching, and invading the intestinal epithelial cells (Young et al., 2007). In C. jejuni, the extracellular flagella filament is composed of multimers of flagellin proteins with a major flagellin protein FlaA and a minor flagellin protein FlaB. FlaA has been shown to be essential for invasion of INT 407 cells and optimal colonization in chicken gut (Wassenaar et al., 1991, 1993). The flagellum also serves as a type III secretion system for transportation of Campylobacter invasion antigens to the host cells (Buelow et al., 2011; Neal-Mckinney and Konkel, 2012; Samuelson et al., 2013).
Both C. concisus and C. jejuni are spiral shaped. Unlike C. jejuni. which can have either single or bi-polar flagella, C. concisus only has a single polar flagellum. Although the flagellum in C. concisus has not been comprehensively investigated, studies have shown that C. concisus flagellum might be a virulence factor that contributes to its pathogenicity. Man et al. have observed flagellum mediated attachment and invasion of C. concisus to Caco-2 cells (Figure 3) (Man et al., 2010a).
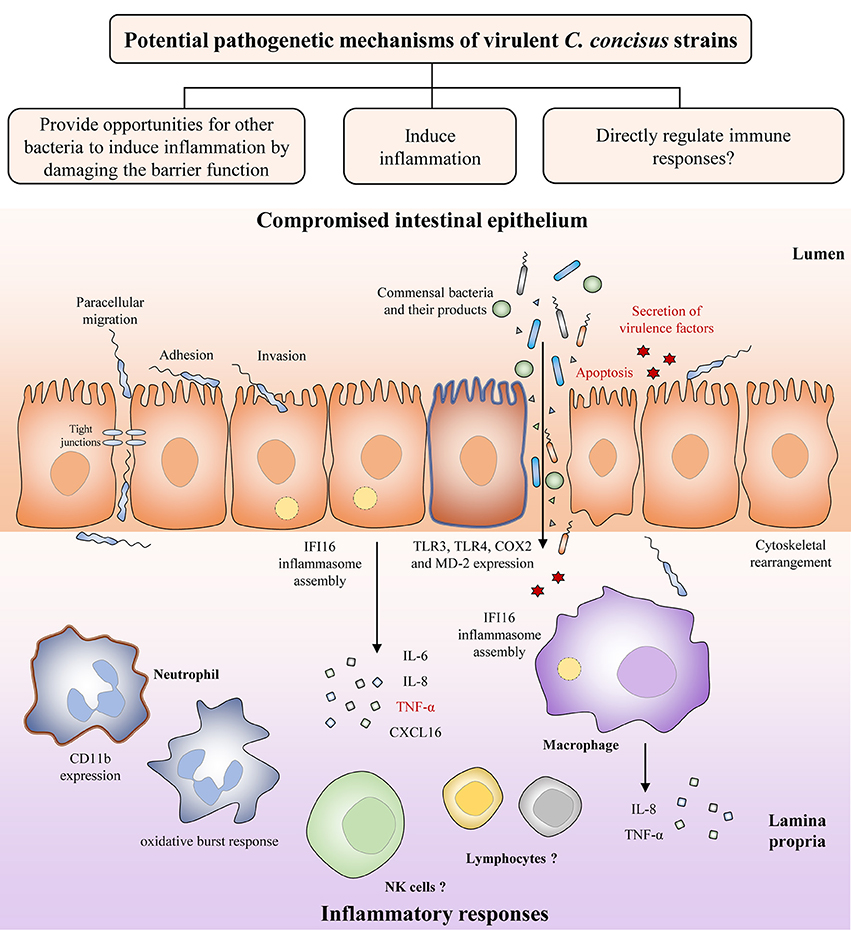
Figure 3. Potential pathogenetic mechanisms of virulent C. concisus strains. Once it reaches the intestine, C. concisus adheres and invades the epithelium with the help of its flagellin and spiral shape. This is followed by a number of host responses such as cytoskeletal rearrangement, inflammasome assembly, expression of toll-like receptors, and the release of proinflammatory cytokines. Expression of virulence factors such as the zonula occludens toxin also results in proinflammatory cytokine production, as well as apoptosis (colored in red). The resulting compromised intestinal epithelium allows the increased translocation of commensal bacteria and their products from the lumen to the lamina propria. Proinflammatory cytokines and chemokines released by intestinal epithelial cells recruit immune cells to the site of infection and contributes to inflammatory response development.
Several studies have examined the motility of C. concisus strains isolated from saliva, feces and intestinal biopsies of patients with IBD, gastroenteritis, and healthy individuals. The motility was shown to be strain dependent, and no statistically significant association was found between patients and healthy controls (Lavrencic et al., 2012; Ovesen et al., 2017). The authors also reported that the motility of C. concisus was lower than that of C. jejuni and C. fetus (Ovesen et al., 2017). Furthermore, comparative genomic analysis of C. concisus strains had identified proteins required for flagellin glycosylation pathway, which may affect bacterial flagellar filament assembly, autoagglutination, adhesion and invasion (Kaakoush et al., 2011).
Bacterial flagella are also involved in forming biofilm, a bacterial interaction that is important for its survival in the host (Reeser et al., 2007; Svensson et al., 2014). C. concisus was also shown to have the ability to form biofilms, and this ability did not differ between the strains examined (Lavrencic et al., 2012).
The intestinal epithelium is constructed by simple columnar epithelial cells (Clevers, 2013). Increased intestinal permeability is known to be associated with a number of chronic human diseases including IBD, being considered as a risk factor for its development (Hollander et al., 1986; Wyatt et al., 1993; Irvine and Marshall, 2000; D'Incà et al., 2006; Meddings, 2008). A compromised intestinal epithelial barrier may lead to loss of tolerance to the commensal enteric microbiota. Using in vitro cell culture models, several studies have indicated that C. concisus is able to increase the intestinal permeability. The intestinal epithelial permeability in Caco-2 cells was increased following C. concisus infection, and C. concisus also induced movement of tight junction proteins zonula occludens-1 and occludin from cell membrane into cytosol (Figure 3) (Man et al., 2010a). These changes in tight junction protein arrangement significantly increased the barrier permeability (Man et al., 2010a). These effects were also observed in HT-29/B6 intestinal epithelial cells, in which the cell monolayers infected with both oral and fecal C. concisus strains revealed epithelial barrier dysfunction (Nielsen et al., 2011).
Inflammatory cytokines are produced in enteric infections (Figure 3). C. concisus strains were able to stimulate the productions of interleukin (IL)-8 and tumor necrosis factor (TNF)-α in THP-1 macrophages, and the productions of IL-8 and cyclooxygenase (COX)-2 in HT-29 cells (Man et al., 2010a; Ismail et al., 2013). COX-2 is an enzyme responsible for producing prostaglandins and other inflammatory mediators (Williams et al., 1999). Some C. concisus strains, mostly those isolated from patients with IBD, have been shown to upregulate surface expression of lipopolysaccharides (LPS) receptors including Toll-like receptor (TLR) 4 and myeloid differentiation factor 2 in HT-29 cells (Ismail et al., 2013). Activation of neutrophil adherence molecule CD11b and oxidative burst response have been shown in neutrophils following C. concisus infection (Sørensen et al., 2013). Additionally, by using transcriptomics analysis, assembly of IFI16 inflammasome has been observed in both intestinal epithelial cells and macrophages following C. concisus infection (Kaakoush et al., 2015; Deshpande et al., 2016). The IFI16 inflammasome plays important role in the innate immune responses, acting as a nuclear pathogen sensor that promotes caspase-1 activation (Xiao, 2015). Production of mature proinflammatory cytokines, IL-1β, and 1L-18, is caspase-1 dependent (Xiao, 2015). A recent study has found that C. concisus was able to elevate the mRNA expression of p53, TNF-α, and IL-18 in Barrett's cell lines, however these effects have not been demonstrated at protein level (Namin et al., 2015).
Flagellin from various Gram-negative and Gram-positive bacteria are capable of triggering nuclear factor kappa light chain enhancer of activated B cells (NF-κB) signaling responses in intestinal epithelial cells, where it is recognized by TLR5 expressed on the surface of the host cells (Eaves-Pyles et al., 2001; Gewirtz et al., 2001). The conserved region on flagellin that is recognized by TLR5 has been studied in Salmonella typhimurium (Smith et al., 2003). Campylobacter flagellin shares limited sequence similarity with that of S. typhimurium, which explains why it is a poor stimulator of TLR5 (Watson and Galán, 2005). Studies have demonstrated that C. jejuni, C. coli, and C. concisus have limited potential to activate TLR5 (De Zoete et al., 2010; Ismail et al., 2013).
To date, only two virulence factors of C. concisus have been characterized, one of which is the zonula occludens toxin (Zot). Initially described in Vibrio cholerae, Zot is a known virulence factor that causes an increase in intestinal permeability. The zot genes in Campylobacter species are encoded by prophages and are divided into two clusters, which encode ZotCampyType_1 and ZotCampyType_2, respectively (Zhang et al., 2014; Liu et al., 2016). Although the two types of Zot toxins share common motifs, their overall sequence identities vary greatly, particularly at the C-terminal compartment (Liu et al., 2016). Mahendran et al. have detected a similar prevalence of the cluster 1 zot gene in the oral C. concisus strains isolated from patients with IBD and healthy controls (Mahendran et al., 2013). Later on, Mahendran and colleagues found that the ZotCampyType_1 caused prolonged damage on Caco-2 monolayers (Mahendran et al., 2016). The damaging effect is different from that of V. cholerae Zot which induces transient and reversible damage to the intestinal epithelium (Fasano et al., 1991). This prolonged damaging effect induced by C. concisus Zot is at least partially due to the induction of cell apoptosis and/or intestinal epithelial cell production of proinflammatory cytokines such as TNF-α and IL-8 (Mahendran et al., 2016). Furthermore, pre-exposure to Zot causes increased phagocytosis of E. coli K12 by THP-1 macrophages, suggesting a possible role for C. concisus in enhancing responses of macrophages to other enteric bacterial species (Mahendran et al., 2016). Additionally, transcriptomics analysis showed that C. concisus Zot was able to upregulate the expression of TLR3, proinflammatory cytokines IL-6, IL-8, and chemokine CXCL16 (Deshpande et al., 2016). The zot-containing prophages are also found in a number of other human and animal hosted Campylobacter species including C. ureolyticus, Campylobacter corcagiensis, C. gracilis, C. jejuni, Campylobacter hyointestinalis, and Campylobacter iguanorium, however their pathogenic effects on human cells have not been examined (Liu et al., 2016).
In additional to Zot, another C. concisus virulence factor characterized is the membrane protein phospholipase A. Istivan et al. have examined the activity of haemolytic phospholipase A2 (PLA2) from C. concisus strains isolated from children with gastroenteritis and found that PLA2 activity is detected in strains from both GS. Membrane extracts containing PLA2 exhibited cytolytic effects on CHO cells, supporting the idea that C. concisus may have potential to cause tissue destruction related to intestinal inflammation (Istivan et al., 2004).
To date, only one study has examined the effects of C. concisus in animal model (Aabenhus et al., 2008). Mice displayed a significant loss of body weight between day 2 and day 5 following C. concisus inoculation via gastric route. Signs of inflammation in the gut were not consistently found, but micro abscesses were found in liver of infected animals. Additionally, infiltration of lymphocytes was also observed in the jejunum and colon of infected mice. However, the details of the C. concisus strains used were not provided.
Environmental factors present in the gastrointestinal tract such as bile and pH have been found to affect the growth of C. concisus in vitro (Ma et al., 2015). Reduction in C. concisus growth was observed with lower pH. The gastric pH values in patients with CD and UC range between 1.5–4.1 and 1.55–4.4, respectively, which are significantly higher than those in healthy individuals (0.95–2.6) (Press et al., 1998). Under laboratory conditions, C. concisus strains were unable to grow following exposure to pH 2 for 30 min, while only 20% of the strains were able to survive following exposure to pH 3.5 for 30 min, and exposure to pH 5 for 120 min had minor effects on C. concisus growth (Ma et al., 2015). The sensitivity of C. concisus toward low pH suggests that the acidic environment may prevent C. concisus from colonizing the stomach and intestinal tract. The less acidic gastric environment observed in patients with IBD may provide a better condition for C. concisus colonization (Press et al., 1998). Recently, Ma et al. reported that the derivatives of the food additive, fumaric acid, were able to enhance the growth of C. concisus strains (Ma et al., 2018). It was found that C. concisus strains showed the greatest increase in growth when cultured in media containing 0.4% of neutralized fumaric acid, neutralized monosodium fumarate and sodium fumarate. These results imply that removal of fumaric acid and its salts from diet of patients with IBD may help to improve their condition.
Campylobacter species have different ability in bile resistance. Some oral C. concisus strains are able to grow in the presence of 2% bile, suggesting they are able to survive in the enteric environment. Enteric Campylobacter species such as C. jejuni and C. hominis are bile resistant. Fox et al. have demonstrated that although significant growth inhibition was observed when C. jejuni was cultured in the presence of 2.5% bile, C. jejuni was still able to proliferate in cultures containing 5% bile (Fox et al., 2007). Lawson et al. showed that C. hominis strains isolated from stool samples were able to tolerate 2% bile (Lawson et al., 2001).
There has been an increase in antibiotic resistance in Campylobacter isolates from both humans and animals worldwide (Luangtongkum et al., 2009; Iovine, 2013). Among antimicrobial therapies for treatment of Campylobacter enteritis, fluoroquinolones, and macrolides are most commonly used, while tetracyclines are rarely used (Alfredson and Korolik, 2007). Intravenous aminoglycosides are sometimes used to treat serious bacteraemia and other Campylobacter induced systemic infections (Alfredson and Korolik, 2007). Campylobacter species resist antibiotics by several mechanisms such as modification or occupation of target sites thus preventing the binding of antibiotic compounds; efflux pump systems that reduce intracellular antibiotic concentrations; changes in bacterial membrane permeability that prevents entering of antibiotic compounds; and hydrolysis of antibiotic compounds (Alfredson and Korolik, 2007; Iovine, 2013). The resistance determinants in C. jejuni and C. coli and their mechanisms of action have been widely studied, however the resistance determinants in C. concisus have not been investigated in detail. It is thought that antibiotics resistance has not developed in C. concisus as it is susceptible to a variety of antibiotics, of which tetracycline, erythromycin, ciprofloxacin, and macrolides are most commonly reported (Johnson et al., 1986; Aabenhus et al., 2005; Vandenberg et al., 2006; Nielsen et al., 2013). Ciprofloxacin, along with other antibiotics, have been shown to be effective in treating certain phenotypes of CD (Sartor, 2004).
Antibiotics are used as primary or adjuvant treatment along with anti-inflammatory and immunosuppressive drugs for the treatment of IBD (Sartor, 2004). Antibiotics are used to selectively reduce tissue invasion, decrease luminal, and mucosal bacterial loads and their translocation (Sartor, 2004). Some patients with IBD are colonized with multiple C. concisus strains, and a significantly higher prevalence of multiple oral C. concisus strains in patients with active IBD than healthy controls has been reported (Mahendran et al., 2013). Furthermore, for IBD patients who are in remission, those without antibiotic treatment usually have a higher prevalence of multiple oral C. concisus strains than those who were receiving antibiotics, indicating antibiotics were able to alleviate but not eradicate C. concisus growth in the intestinal tract (Mahendran et al., 2013).
In addition to antibiotics, the antimicrobial potentials of immunomodulating and anti-inflammatory drugs used for IBD treatments have not been widely tested. Immunosuppressive drugs such as azathioprine (AZA) and mercaptopurine (MP) used in the treatment of IBD have been reported to exhibit inhibitory effect on the growth of C. concisus strains, with the effect of AZA being more potent than MP (Liu F. et al., 2017). In their use as immunosuppressive drugs, both AZA and MP are eventually metabolized to purine analogs that interfere with DNA synthesis in immune cells (Nielsen et al., 2001). However, bioinformatics analysis has not identified all the enzymes required for AZA and MP metabolism in the C. concisus genome, indicating the inhibitory action of AZA and MP on C. concisus growth is not through the conventional pathway. AZA and MP can also reduce the growth of Mycobacterium avium subsp. paratuberculosis, another bacterium that is associated with human CD (Sanderson et al., 1992; Collins et al., 2000; Sieswerda and Bannatyne, 2006; Greenstein et al., 2007; Shin and Collins, 2008).
5-aminosalicylic acid (5-ASA) is an anti-inflammatory drug that is also commonly used to induce and maintain remission in IBD (Hanauer, 2006; Nikfar et al., 2009). Its effect on C. concisus growth varies between strains as it inhibits the growth of some C. concisus strains, while it promotes the growth of other strains, features which appears to be independent of C. concisus GS (Schwartz et al., 1982). Although 5-ASA is often used in the treatment of IBD, in some cases 5-ASA medications are found to cause exacerbations of colitis (Schwartz et al., 1982). The enhancement of C. concisus growth induced by 5-ASA may have implications in the deteriorated clinical conditions observed in patients with IBD following 5-ASA medications.
While antimicrobials may be effective in limiting the growth of C. concisus in the intestinal tract, continuous transportation of C. concisus from the oral cavity along with saliva and food makes eradication of this bacterium a challenge. Thus, antimicrobials targeting C. concisus in the oral cavity, particularly virulent strains, should be developed.
C. rectus (formerly named as Wolinella recta) is a small, straight, rod shaped, single polar flagellated bacterium with the size being 0.5 by 2–4 μm (Tanner et al., 1981). The colonies of C. rectus appear as convex shaped and spread or corrode blood agar plates (Tanner et al., 1981). C. rectus was initially identified using anaerobic condition consisting of 80% N2, 10% CO2, and 10% H2, although the study indicated that some stains can grow in the presence of 5% oxygen (Tanner et al., 1981). A number of studies have used anaerobic conditions for the isolation of C. rectus from different clinical samples such as dental plaques, feces, and oesophageal mucosal biopsies (Rams et al., 1993; Gmur and Guggenheim, 1994; Von Troil-Lindén et al., 1995; Lastovica and Roux, 2000; Macuch and Tanner, 2000; Macfarlane et al., 2007). A study comparing different atmospheric conditions on the growth of C. rectus demonstrated that its growth could be observed at 30, 35, and 42°C under anaerobic conditions; in contrast it did not grow in a conventional microaerophilic atmosphere consisting 5% O2, 10% CO2, and 85% N2 (Mahlen and Clarridge, 2009). Given all these findings, C. rectus is an anaerobic bacterium instead of microaerophilic bacterium.
C. rectus was first isolated from individuals with periodontal diseases (Tanner et al., 1981). It has since been isolated from various locations of the oral cavity including periodontal sulcus, tongue, cheek mucosa, and saliva (Könönen et al., 2007; Cortelli et al., 2008). C. rectus is predominantly localized in the middle and deep periodontal pocket zones, and tends to form clumps in tooth-attached and epithelium associated plaque areas (Noiri et al., 1997). A number of studies have reported the association between C. rectus and periodontal diseases. Macuch and Tanner detected C. rectus from 90% (42/47) of the subgingival sites of initial and established perondontitis individuals, which was signficianlty higher than that from gingivitis subjects (20%, 3/14) and healthy controls (10%, 2/18) (Macuch and Tanner, 2000). Dibart et al. also showed that C. rectus had a signfiicantly higher prevalence in supragingival plaque obtained from periodontally diseased subjects as comapred with healthy individuals (7/24 vs. 0/27) (Dibart et al., 1998). Furthermore, von Troil-Lindenl et al. reported that C. rectus was more frequently detected in saliva samples of subjects with advanced periondontitis (100%, 10/10) as comapred with individuals with initial or no periondontitis (40%, 4/10) (Von Troil-Lindén et al., 1995). Additionally, several studies have examined the prevalence of C. rectus in periondontitis patients with different disease status, however healthy subjects were not included. Several virulence factors of C. rectus including LPS, GroEL-like protein, and surface-layer protein have been characterized.
C. rectus acts as a stimulator of inflammation in the gingival tissue. It has been shown to enhance the production of IL-6 and IL-8 in human gingival fibroblasts (Dongari-Bagtzoglou and Ebersole, 1996). IL-6 is a multifunctional cytokine, as it can be both proinflammatory and anti-inflammatory, and its secretion is thought to drive the tissue damaging process in diseases such as periodontal disease (Irwin and Myrillas, 1998). C. rectus LPS is able to stimulate both plasmin activity and plasminogen activator activity in human gingival fibroblasts (Ogura et al., 1995). Plasmin is an enzyme that is converted from plasminogen via plasminogen activator catalysis (Martel-Pelletier et al., 1991). Plasmin is present in blood which degrades many blood plasma proteins such as fibrin clots (Martel-Pelletier et al., 1991). Significantly enhanced activities of plasmin and plasminogen activator activities have been observed in gingival fluid in periodontal disease (Hidaka et al., 1981; Talonpoika et al., 1990). Furthermore, C. rectus LPS also induces higher levels of IL-6 and prostaglandin E2 productions in aged human gingival fibroblasts as compared with those in younger cells, which may explain the increased susceptibility of periodontal disease in aged individuals (Ogura et al., 1996; Takiguchi et al., 1996, 1997).
The C. rectus GroEL-like protein is a 64 kDa protein with antigenic properties (Hinode et al., 1998). It was found to cross-react with antibodies against human heat shock protein 60, Helicobacter pylori whole cells and the GroEL-like protein from Actinobacillus actinomycetemcomitans, a bacterial species that is associated with localized aggressive periodontitis (Hinode et al., 1998, 2002; Tanabe et al., 2003; Henderson et al., 2010). The C. rectus GroEL-like protein is also able to stimulate the production of IL-6 and IL-8 from the human gingival fibroblast monolayer (Hinode et al., 1998). The C. rectus GroEL-like protein is also found to possess immunodominant epitopes within both amino and carboxyl termini, and it may share same carboxyl epitopes with the C. rectus surface-layer (S-layer) protein (Hinode et al., 2002).
The S-layer is a monomolecular layer of a single secreted protein surrounding the entire surface which is found in almost all archaea and some Gram-positive and Gram-negative bacteria (Konstantinov et al., 2008). The S-layer protein (SLP) in C. fetus and C. rectus have been previously characterized, and the gene encoding SLP is also carried by C. showae (Tay et al., 2013). The C. rectus SLP has a molecular weight ranging between 130 and 166 kDa (Kobayashi et al., 1993; Nitta et al., 1997). C. rectus strains expressing SLP are resistant to complement and phagocytic mediated killing in the absence of specific antibodies (Okuda et al., 1997). However, C. rectus SLP does not play a major role in bacterial adhesion. C. rectus strains lacking the crsA gene which encodes the SLP protein are less effective at adhering to Hep-2 oral epithelial cells, with CrsA+ cells only 30 to 50% more adherent than the CrsA- cells (Wang et al., 2000). The CrsA- cells exhibit no difference in inducing the production of IL-6, IL-8, and TNF-α in Hep-2 cells as compared with CrsA+ cells. However, CrsA- C. rectus strains induce higher levels of these cytokines at early time points of the infection, suggesting that the S-layer in C. rectus may facilitate bacterial survival at the site of infection by delaying the cytokine responses (Wang et al., 2000).
Macrolides are a class of antibiotics used to treat a wide variety of infections (Hirsch et al., 2012). It inhibits protein synthesis by acting on the P site of the 50S ribosomal subunit, thus preventing peptide elongation (Payot et al., 2006). The rRNA methylase enzyme encoded by the erythromycin ribosome methylase (erm) gene methylates a single adenine in the 23S component of the 50S subunit, which sterically hinders the proper interaction between the macrolide and the 50S subunit, providing antibiotic resistance (Weisblum, 1995). C. rectus is the only Campylobacter species that has the erm determinants described. These determinants include Erm B, Erm C, Erm F, and Erm Q, though their clinical implications have not been examined (Roe et al., 1995).
In addition to its role in periodontal disease, C. rectus has been implicated in the association between maternal periodontitis and adverse pregnancy outcomes. Madianos et al. reported that a significantly elevated level of fetal IgM to C. rectus was observed among premature infants as compared to full-term neonates, indicating that C. rectus, as a maternal oral pathogen, may act as a primary infectious agent in fetus that leads to prematurity (Madianos et al., 2001). Later study by the same group demonstrated that C. rectus mediated growth restriction in a pregnant mice model, as higher numbers of growth-restricted fetuses were observed in the groups subcutaneously challenged by C. rectus as compared to the non-challenged groups (Yeo et al., 2005). The same group also showed that maternal C. rectus infection increased fetal brain expression of proinflammatory cytokines IFN-γ (Offenbacher et al., 2005). Furthermore, Arce and colleagues demonstrated that C. rectus was more invasive to human trophoblasts as compared to C. jejuni, paralleled with significantly upregulated mRNA and protein expressions of IL-6 and TNF-α in a dose-dependent manner (Arce et al., 2010). Moreover, the study also showed that C. rectus was able to translocate in vivo from a distant site of infection to the fetoplacental unit (Arce et al., 2010). Taking together, these studies suggest that as a pathogen in the oral cavity, C. rectus has the potential to contribute to the adverse pregnancy outcomes.
C. ureolyticus (formally known as Bacteroides ureolyticus) was first described in 1978 by Jackson et al., the strain was isolated from amniotic fluid using anaerobic condition (Jackson and Goodman, 1978). A later study has isolated C. ureolyticus from 103 superficial necrotic or gangrenous lesions. However, C. ureolyticus was rarely the sole bacterial species isolated from the site of infections, suggesting other microorganisms may be responsible for the pathogenesis of these diseases (Duerden et al., 1982).
C. ureolyticus has been frequently isolated from the genital tracts of both male and female. By examining the whole cell proteins of C. ureolyticus strains, Akhtar and Eley showed that strains isolated from urethra of men with and without non-gonococcal urethritis showed no difference in SDS-PAGE patterns (Akhtar and Eley, 1992). Later studies by Bennett et al. reported that C. ureolyticus was isolated from healthy individuals at a similar prevalence as compared with that from males and females presenting non-gonococcal urethritis (Bennett et al., 1990, 1991). These results indicate that C. ureolyticus is a part of the normal flora in the genital tract of both male and female.
In the past decades, C. ureolyticus has been considered as an emergent Campylobacter species in gastroenteritis as it is frequently isolated from fecal samples of patients presenting with diarrheal illness. By using a multiplex-PCR systems, Bullman et al. first reported the isolation of C. ureolyticus from feces of patients with gastroenteritis. Among all the Campylobacter positive samples being screened, 24% (83/349) was found to be positive for C. ureolyticus, of which 64% (53/83) had C. ureolycitus as the sole Campylobacter species detected (Bullman et al., 2011). By using PCR methods, another study by Bullman et al. also found that C. ureolyticus was the second most common non-C. jejuni/C. coli species in fecal samples collected from patients presenting diarrheal illness (Bullman et al., 2012). However, healthy controls were not included in these studies.
By combining the bacterial isolation and molecular detection methods, Collado et al. reported that C. ureolyticus is the third most prevalent species of the Campylobacteria family in diarrheic stool samples in addition to C. concisus and C. jejuni, but no statistical difference was found between the diarrheic and healthy groups (Collado et al., 2013). A recent study has also reported the detection of C. ureolyticus from traveler's diarrhea cases (Serichantalergs et al., 2017). This study showed that C. ureolyticus was detected at a similar prevalence in patients presenting diarrhea and healthy individuals.
Despite being frequently isolated or detected from diarrheal stool samples, the association between C. ureolyticus and diarrheal diseases has not been established. However, the pathogenic potentials of C. ureolyticus have been examined by a number of in vitro studies. Through whole genome sequencing of C. ureolyticus strains, genes encoding known virulence factors have been identified. These factors were involved in bacterial adhesion, colonization, invasion, and toxin production (Bullman et al., 2013). By using an intestinal epithelial cell line model, C. ureolyticus was shown to be capable of adhering to Caco-2 cells but unable to invade, with the bacterial adhesion followed by cellular damage and microvillus degradation. Furthermore, secretome analysis had also detected release of putative virulence and colonization factors (Burgos-Portugal et al., 2012).
Hariharan et al. reported the isolation of C. ureolyticus from endometria of apparently normal mares under anaerobic condition (Hariharan et al., 1994). So far, this is the only study has reported isolation of C. ureolyticus from animal sources.
C. gracilis (formally known as Bacteroides gracilis) was first isolated by Tanner et al. in 1981 from patients with gingivitis and periodontitis (Tanner et al., 1981).
C. gracilis has been isolated from patients with periodontal and endodontic infections, however its pathogenetic role in these diseases is still controversial. Macuch et al. reported that in addition to C. rectus, C. gracilis was the most dominant Campylobacter species isolated from subgingival sites among eight Campylobacter species being examined. However, similar levels of C. gracilis were detected in healthy, gingivitis and periodontitis sites, suggesting that its prevalence or quantity was unrelated to periodontal health or disease (Macuch and Tanner, 2000). A study by Siqueira and Rocas examining the prevalence of C. gracilis in patients with primary endodontic infections also found that it was not associated with clinical symptoms of the diseases (Siqueira and Rocas, 2003).
The first complete genome of C. gracilis was sequenced by Miller et al., which lead to identification of genes encoding for virulence factors including haemagglutinins, Zot, immunity proteins and other putative pathogenic factors (Miller and Yee, 2015). Nevertheless, supporting evidence for the virulence of C. gracilis is still lacking.
C. showae was first isolated by Etoh et al. in 1993 from dental plaque of gingival crevices of healthy adults (Etoh et al., 1993). A study later by Macuch et al. examining the prevalence of oral Campylobacter species from subgingival sites showed that in addition to C. rectus, C. showae was also found more frequently and in higher levels from patients with periodontitis and gingivitis than from healthy controls (Macuch and Tanner, 2000).
C. hominis was first described in 1998 by Lawson et al. through Campylobacter specific PCR assays. C. hominis was detected from stool samples of 50% of the 20 healthy individuals, while it was absent in all the saliva samples of the same individuals (Lawson et al., 1998). Later in a study by Lawson et al., C. hominis was isolated from the fecal samples of healthy individuals (Lawson et al., 2001).
By using PCR methods targeting the Campylobacter 16S rRNA gene, C. showae and C. hominis have been detected from intestinal biopsy samples collected from macroscopically inflamed and noninflamed areas. Furthermore, this was the first study reported the isolation of C. concisus, C. showae, C ureolyticus, and C. hominis from intestinal biopsies (Zhang et al., 2009). A later study by Man et al. also detected C. showae and C. hominis from stool specimens collected from children with CD (Man et al., 2010b). However, no association was found between the prevalence of C. showae and C. hominis in patients with CD and healthy controls.
C. curvus (previously named as Wolinella curva) was first described in 1984 by Tanner et al. (Tanner et al., 1984). C. curvus is a rarely encountered Campylobacter species in humans. Lastovica et al. reported an isolation rate of C. curvus as low as 0.05% (2/4122) from diarrheic stools of pediatric patients (Lastovica and Roux, 2000). A study by Abbott et al. also isolated C curvus from stool samples and showed that the prevalence of C. curvus was associated with sporadic and outbreak of bloody gastroenteritis and Brainerd's diarrhea in Northern California (Abbott et al., 2005). There was one study which reported isolation of C. curvus and C. upsaliensis from stools of patients with Guillain-Barré syndrome and Fisher's syndrome (Koga et al., 1999). However, serological examination of the patient from whom C. curvus has been isolated did not detect antibodies to this bacterium.
Accumulated evidence from the past decade supports the role of human hosted C. concisus in the development of IBD and possibly Barrett's esophagus and GERD. Recently, a CD-associated C. concisus molecular marker csep1-6bpi has been identified, strongly suggests that csep1-6bpi positive C. concisus strains is a potential causative agent of human CD. In addition to C. concisus, the association between human hosted C. rectus and periodontal diseases has also been established.
FL played the major role in writing the review. LZ, RM, and YW provided critical feedback and helped in editing the manuscript.
This work is supported by a Faculty Research Grant awarded to LZ from the University of New South Wales (Grant No.: PS46772).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
5-ASA, 5-aminosalicylic acid; AZA, azathioprine; CD, Crohn's disease; CFU, colony forming unit; COX, cyclooxygenase; Erm, erythromycin ribosome methylase; GERD, gastroesophageal reflux disease; GS, genomospecies; IBD, inflammatory bowel disease; IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; MP, mercaptopurine; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PCR, polymerase chain reaction; PLA2, phospholipase A2; TLR, toll-like receptor; TNF, tumor necrosis factor; UC, ulcerative colitis; Zot, zonula occludes toxin.
Aabenhus, R., Permin, H., and Andersen, L. P. (2005). Characterization and subgrouping of Campylobacter concisus strains using protein profiles, conventional biochemical testing and antibiotic susceptibility. Eur. J. Gastroenterol. Hepatol. 17, 1019–1024. doi: 10.1097/00042737-200510000-00003
Aabenhus, R., Stenram, U., Andersen, L. P., Permin, H., and Ljungh, A. (2008). First attempt to produce experimental Campylobacter concisus infection in mice. World J. Gastroenterol. 14, 6954–6959. doi: 10.3748/wjg.14.6954
Abbott, S. L., Waddington, M., Lindquist, D., Ware, J., Cheung, W., Ely, J., et al. (2005). Description of Campylobacter curvus and C. curvus-like strains associated with sporadic episodes of bloody gastroenteritis and Brainerd's diarrhea. J. Clin. Microbiol. 43, 585–588. doi: 10.1128/JCM.43.2.585-588.2005
Akhtar, N., and Eley, A. (1992). Restriction endonuclease analysis and ribotyping differentiate genital and nongenital strains of Bacteroides ureolyticus. J. Clin. Microbiol. 30, 2408–2414.
Alfredson, D. A., and Korolik, V. (2007). Antibiotic resistance and resistance mechanisms in Campylobacter jejuni and Campylobacter coli. FEMS Microbiol. Lett. 277, 123–132. doi: 10.1111/j.1574-6968.2007.00935.x
Arce, R. Á., Diaz, P., Barros, S., Galloway, P., Bobetsis, Y., Threadgill, D., et al. (2010). Characterization of the invasive and inflammatory traits of oral Campylobacter rectus in a murine model of fetoplacental growth restriction and in trophoblast cultures. J. Reprod. Immunol. 84, 145–153. doi: 10.1016/j.jri.2009.11.003
Bennett, K., Eley, A., and Woolley, P. (1991). Isolation of Bacteroides ureolyticus from the female genital tract. Eur. J. Clin. Microbiol. Infect. Dis. 10, 593–594. doi: 10.1007/BF01967281
Bennett, K., Eley, A., Woolley, P., and Duerden, B. (1990). Isolation of Bacteroides ureolyticus from the genital tract of men with and without non-conococcal urethritis. Eur. J. Clin. Microbiol. Infect. Dis. 9, 825–826. doi: 10.1007/BF01967383
Bézian, M., Ribou, G., Barberis-Giletti, C., and Megraud, F. (1990). Isolation of a urease positive thermophilic variant of Campylobacter lari from a patient with urinary tract infection. Eur. J. Clin. Microbiol. Infect. Dis. 9, 895–897. doi: 10.1007/BF01967506
Blackett, K., Siddhi, S., Cleary, S., Steed, H., Miller, M., Macfarlane, S., et al. (2013). Oesophageal bacterial biofilm changes in gastro-oesophageal reflux disease, Barrett's and oesophageal carcinoma: association or causality? Aliment. Pharmacol. Ther. 37, 1084–1092. doi: 10.1111/apt.12317
Blaser, M. J., Glass, R. I., Huq, M. I., Stoll, B., Kibriya, G., and Alim, A. (1980a). Isolation of Campylobacter fetus subsp. jejuni from Bangladeshi children. J. Clin. Microbiol. 12, 744–747.
Blaser, M. J., Laforce, F. M., Wilson, N. A., and Wang, W. L. L. (1980b). Reservoirs for human Campylobacteriosis. J. Infect. Dis. 141, 665–669.
Blaser, M. J., Perez, G. P., Smith, P. F., Patton, C., Tenover, F. C., Lastovica, A. J., et al. (1986). Extraintestinal Campylobacter jejuni and Campylobacter coli infections: host factors and strain characteristics. J. Infect. Dis. 153, 552–559. doi: 10.1093/infdis/153.3.552
Buelow, D. R., Christensen, J. E., Neal-Mckinney, J. M., and Konkel, M. E. (2011). Campylobacter jejuni survival within human epithelial cells is enhanced by the secreted protein CiaI. ?Mol. Microbiol. 80, 1296–1312. doi: 10.1111/j.1365-2958.2011.07645.x
Bullman, S., Corcoran, D., O'leary, J., Lucey, B., Byrne, D., and Sleator, R. D. (2011). Campylobacter ureolyticus: an emerging gastrointestinal pathogen? Pathog. Dis. 61, 228–230. doi: 10.1111/j.1574-695X.2010.00760.x
Bullman, S., Lucid, A., Corcoran, D., Sleator, R. D., and Lucey, B. (2013). Genomic investigation into strain heterogeneity and pathogenic potential of the emerging gastrointestinal pathogen Campylobacter ureolyticus. PLoS ONE 8:e71515. doi: 10.1371/journal.pone.0071515
Bullman, S., O'leary, J., Corcoran, D., Sleator, R., and Lucey, B. (2012). Molecular-based detection of non-culturable and emerging campylobacteria in patients presenting with gastroenteritis. Epidemiol. Infect. 140, 684–688. doi: 10.1017/S0950268811000859
Burgos-Portugal, J. A., Kaakoush, N. O., Raftery, M. J., and Mitchell, H. M. (2012). Pathogenic potential of Campylobacter ureolyticus. Infect. Immun. 80, 883–890. doi: 10.1128/IAI.06031-11
Campero, C. M., Anderson, M., Walker, R., Blanchard, P., Barbano, L., Chiu, P., et al. (2005). Immunohistochemical identification of Campylobacter fetus in natural cases of bovine and ovine abortions. Zoonoses Public Health 52, 138–141. doi: 10.1111/j.1439-0450.2005.00834.x
Chan, F., Stringel, G., and Mackenzie, A. (1983). Isolation of Campylobacter jejuni from an appendix. J. Clin. Microbiol. 18, 422–424.
Chua, K., Gürtler, V., Montgomery, J., Fraenkel, M., Mayall, B. C., and Grayson, M. L. (2007). Campylobacter insulaenigrae causing septicaemia and enteritis. J. Med. Microbiol. 56, 1565–1567. doi: 10.1099/jmm.0.47366-0
Chung, H. K., Tay, A., Octavia, S., Chen, J., Liu, F., Ma, R., et al. (2016). Genome analysis of Campylobacter concisus strains from patients with inflammatory bowel disease and gastroenteritis provides new insights into pathogenicity. Sci. Rep. 6:38442. doi: 10.1038/srep38442
Clevers, H. (2013). The intestinal crypt, a prototype stem cell compartment. Cell 154, 274–284. doi: 10.1016/j.cell.2013.07.004
Collado, L., Gutiérrez, M., González, M., and Fernández, H. (2013). Assessment of the prevalence and diversity of emergent Campylobacteria in human stool samples using a combination of traditional and molecular methods. DMID 75, 434–436. doi: 10.1016/j.diagmicrobio.2012.12.006
Collins, M. T., Lisby, G., Moser, C., Chicks, D., Christensen, S., Reichelderfer, M., et al. (2000). Results of multiple diagnostic tests for Mycobacterium avium subsp. paratuberculosis in patients with inflammatory bowel disease and in controls. J. Clin. Microbiol. 38, 4373–4381.
Control and Prevention (2006). Principles of Epidemiology in Public Health Practice: An Introduction to Applied Epidemiology and Biostatistics. Atlanta, GA: US Dept. of Health and Human Services, Centers for Disease Control and Prevention(CDC), Office of Workforce and Career Development.
Cortelli, J. R., Aquino, D. R., Cortelli, S. C., Fernandes, C. B., De Carvalho-Filho, J., Franco, G. C. N., et al. (2008). Etiological analysis of initial colonization of periodontal pathogens in oral cavity. J. Clin. Microbiol. 46, 1322–1329. doi: 10.1128/JCM.02051-07
Coventry, J., Griffiths, G., Scully, C., and Tonetti, M. (2000). ABC of oral health: periodontal disease. BMJ 321:36. doi: 10.1136/bmj.321.7252.36
Cunningham, C., and Lee, C. H. (2003). Myocarditis related to Campylobacter jejuni infection: a case report. BMC Infect. Dis. 3:16. doi: 10.1186/1471-2334-3-16
De Souza, H. S., and Fiocchi, C. (2016). Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13:13. doi: 10.1038/nrgastro.2015.186
De Vries, J., Arents, N., and Manson, W. (2008). Campylobacter species isolated from extra-oro-intestinal abscesses: a report of four cases and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 27, 1119–1123. doi: 10.1007/s10096-008-0550-2
De Zoete, M. R., Keestra, A. M., Roszczenko, P., and Van Putten, J. P. (2010). Activation of human and chicken toll-like receptors by Campylobacter spp. Infect. Immun. 78, 1229–1238. doi: 10.1128/IAI.00897-09
Debruyne, L., Gevers, D., and Vandamme, P. (2008). “Taxonomy of the family Campylobacteraceae,” in Campylobacter, 3rd Edn, eds I. Nachamkin, M. J. Blaser, and C. M. Szymanski (Washington, DC: American Society of Microbiology), 3–25.
Debruyne, L., On, S. L., De Brandt, E., and Vandamme, P. (2009). Novel Campylobacter lari-like bacteria from humans and molluscs: description of Campylobacter peloridis sp. nov., Campylobacter lari subsp. concheus subsp. nov. and Campylobacter lari subsp. lari subsp. nov. Int. J. Syst. Evol. Microbiol. 59, 1126–1132. doi: 10.1099/ijs.0.000851-0
Deshpande, N. P., Wilkins, M. R., Castano-Rodriguez, N., Bainbridge, E., Sodhi, N., Riordan, S. M., et al. (2016). Campylobacter concisus pathotypes induce distinct global responses in intestinal epithelial cells. Sci Rep 6:34288. doi: 10.1038/srep34288
Dhawan, V. K., Ulmer, D. D., Rao, B., See, R. C., and Nachum, R. (1986). Campylobacter jejuni septicemia-epidemiology, clinical features and outcome. West. J. Med. 144:324.
Di Pilato, V., Freschi, G., Ringressi, M. N., Pallecchi, L., Rossolini, G. M., and Bechi, P. (2016). The esophageal microbiota in health and disease. Ann. N. Y. Acad. Sci. 1381, 21–33. doi: 10.1111/nyas.13127
Dibart, S., Skobe, Z., Snapp, K., Socransky, S., Smith, C., and Kent, R. (1998). Identification of bacterial species on or in crevicular epithelial cells from healthy and periodontally diseased patients using DNA-DNA hybridization. Mol. Oral Microbiol. 13, 30–35. doi: 10.1111/j.1399-302X.1998.tb00747.x
D'Incà, R., Annese, V., Di Leo, V., Latiano, A., Quaino, V., Abazia, C., et al. (2006). Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn's disease. Aliment. Pharmacol. Ther. 23, 1455–1461. doi: 10.1111/j.1365-2036.2006.02916.x
Dongari-Bagtzoglou, A., and Ebersole, J. (1996). Production of inflammatory mediators and cytokines by human gingival fibroblasts following bacterial challenge. J. Periodont. Res. 31, 90–98. doi: 10.1111/j.1600-0765.1996.tb00469.x
Duerden, B., Bennet, K., and Faulkner, J. (1982). Isolation of Bacteroides ureolyticus (B corrodens) from clinical infections. J. Clin. Pathol. 35, 309–312. doi: 10.1136/jcp.35.3.309
Duerden, B., Eley, A., Goodwin, L., Magee, J., Hindmarch, J., and Bennett, K. (1989). A comparison of Bacteroides ureolyticus isolates from different clinical sources. J. Med. Microbiol. 29, 63–73. doi: 10.1099/00222615-29-1-63
Duerden, B., Goodwin, L., and O'neil, T. (1987). Identification of Bacteroides species from adult periodontal disease. J. Med. Microbiol. 24, 133–137. doi: 10.1099/00222615-24-2-133
Eaves-Pyles, T., Murthy, K., Liaudet, L., Virág, L., Ross, G., Soriano, F. G., et al. (2001). Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: IκBα degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J. Immunol. 166, 1248–1260. doi: 10.4049/jimmunol.166.2.1248
Edmonds, P., Patton, C., Barrett, T., Morris, G., Steigerwalt, A., and Brenner, D. (1985). Biochemical and genetic characteristics of atypical Campylobacter fetus subsp. fetus strains isolated from humans in the United States. J. Clin. Microbiol. 21, 936–940.
Edmonds, P., Patton, C., Griffin, P., Barrett, T., Schmid, G., Baker, C., et al. (1987). Campylobacter hyointestinalis associated with human gastrointestinal disease in the United States. J. Clin. Microbiol. 25, 685–691.
Engberg, J., Bang, D. D., Aabenhus, R., Aarestrup, F. M., Fussing, V., and Gerner-Smidt, P. (2005). Campylobacter concisus: an evaluation of certain phenotypic and genotypic characteristics. Clin. Microbiol. Infect. 11, 288–295. doi: 10.1111/j.1469-0691.2005.01111.x
Etoh, Y., Dewhirst, F., Paster, B., Yamamoto, A., and Goto, N. (1993). Campylobacter showae sp. nov., isolated from the human oral cavity. Int. J. Syst. Evol. Microbiol. 43, 631–639. doi: 10.1099/00207713-43-4-631
Fasano, A., Baudry, B., Pumplin, D. W., Wasserman, S. S., Tall, B. D., Ketley, J. M., et al. (1991). Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. U.S.A. 88, 5242–5246. doi: 10.1073/pnas.88.12.5242
Fennell, C., Rompalo, A., Totten, P., Bruch, K., Flores, B., and Stamm, W. (1986). Isolation of “Campylobacter hyointestinalis” from a human. J. Clin. Microbiol. 24, 146–148.
Figura, N., Guglielmetti, P., Zanchi, A., Partini, N., Armellini, D., Bayeli, P. F., et al. (1993). Two cases of Campylobacter mucosalis enteritis in children. J. Clin. Microbiol. 31, 727–728.
Fox, E. M., Raftery, M., Goodchild, A., and Mendz, G. L. (2007). Campylobacter jejuni response to ox-bile stress. FEMS Immunol. Med. Microbiol. 49, 165–172. doi: 10.1111/j.1574-695X.2006.00190.x
Francioli, P., Herzstein, J., Grob, J.-P., Vallotton, J.-J., Mombelli, G., and Glauser, M. P. (1985). Campylobacter fetus subspecies fetus bacteremia. Arch. Intern. Med. 145, 289–292. doi: 10.1001/archinte.1985.00360020125020
Fujihara, N., Takakura, S., Saito, T., Iinuma, Y., and Ichiyama, S. (2006). A case of perinatal sepsis by Campylobacter fetus subsp. fetus infection successfully treated with carbapenem–case report and literature review. J. Infect. 53, e199–e202. doi: 10.1016/j.jinf.2006.01.009
Galanis, E. (2007). Campylobacter and bacterial gastroenteritis. CMAJ 177, 570–571. doi: 10.1503/cmaj.070660
Garcia, M. M., Lutze-Wallace, C. L., Denes, A. S., Eaglesome, M. D., Holst, E., and Blaser, M. J. (1995). Protein shift and antigenic variation in the S-layer of Campylobacter fetus subsp. venerealis during bovine infection accompanied by genomic rearrangement of sapA homologs. J. Bacteriol. 177, 1976–1980. doi: 10.1128/jb.177.8.1976-1980.1995
Gewirtz, A. T., Navas, T. A., Lyons, S., Godowski, P. J., and Madara, J. L. (2001). Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167, 1882–1885. doi: 10.4049/jimmunol.167.4.1882
Gilbert, G., Davoren, R., Cole, M., and Radford, N. (1981). Midtrimester abortion associated with septicaemia caused by Campylobacter jejuni. Med. J. Aust. 1, 585–586.
Gmur, R., and Guggenheim, B. (1994). Interdental supragingival plaque—a natural habitat of Actinobacillus actinomycetemcomitans, Bacteroides forsythus, Campylobacter rectus, and Prevotella nigrescens. J. Dent. Res. 73, 1421–1428. doi: 10.1177/00220345940730080501
Goossens, H., Kremp, L., Boury, R., Vlaes, L., Van Den Borre, C., Henocque, G., et al. (1986). Nosocomial outbreak of Campylobacter jejuni meningitis in newborn infants. Lancet 328, 146–149. doi: 10.1016/S0140-6736(86)91956-2
Gorkiewicz, G., Feierl, G., Zechner, R., and Zechner, E. L. (2002). Transmission of Campylobacter hyointestinalis from a pig to a human. J. Clin. Microbiol. 40, 2601–2605. doi: 10.1128/JCM.40.7.2601-2605.2002
Greenstein, R. J., Su, L., Haroutunian, V., Shahidi, A., and Brown, S. T. (2007). On the action of methotrexate and 6-mercaptopurine on M. avium subspecies paratuberculosis. PLoS ONE 2:e161. doi: 10.1371/journal.pone.0000161
Gurgan, T., and Diker, K. S. (1994). Abortion associated with Campylobacter upsaliensis. J. Clin. Microbiol. 32, 3093–3094.
Hagiya, H., Ogawa, H., Takahashi, Y., Hasegawa, K., Hanayama, Y., and Otsuka, F. (2015). Infective internal iliac artery aneurysm caused by Campylobacter fetus. Intern. Med. 54, 2021–2024. doi: 10.2169/internalmedicine.54.4845
Han, X. Y., Tarrand, J. J., Rice, D. C., Han, X. Y., Tarrand, J. J., and Rice, D. C. (2005). Oral Campylobacter species involved in extraoral abscess : a report of three cases. J. Clin. Microbiol. 43, 2513–2515. doi: 10.1128/JCM.43.5.2513-2515.2005
Hanauer, S. B. (2006). Review article: high-dose aminosalicylates to induce and maintain remissions in ulcerative colitis. Aliment. Pharmacol. Ther. 24(Suppl. 3), 37–40. doi: 10.1111/j.1365-2036.2006.03058.x
Hannu, T., Kauppi, M., Tuomala, M., Laaksonen, I., Klemets, P., and Kuusi, M. (2004). Reactive arthritis following an outbreak of Campylobacter jejuni infection. J. Rheumatol. 31, 528–530.
Hannu, T., Mattila, L., Rautelin, H., Pelkonen, P., Lahdenne, P., Siitonen, A., et al. (2002). Campylobacter-triggered reactive arthritis: a population-based study. Rheumatology 41, 312–318. doi: 10.1093/rheumatology/41.3.312
Hariharan, H., Richardson, G., Homey, B., Heaney, S., Bryenton, J., and Moore, I. (1994). Isolation of Bacteroides ureolyticus from the equine endometrium. J. Vet. Diagn. Investig. 6, 127–130. doi: 10.1177/104063879400600130
Haydon, D. T., Cleaveland, S., Taylor, L. H., and Laurenson, M. K. (2002). Identifying reservoirs of infection: a conceptual and practical challenge. Emerg. Infect. Dis. 8, 1468–1473. doi: 10.3201/eid0812.010317
Henderson, B., Ward, J. M., and Ready, D. (2010). Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen? Periodontol 2000 54, 78–105. doi: 10.1111/j.1600-0757.2009.00331.x
Hidaka, N., Maeda, K., Kawakami, C., Aono, M., and Okada, H. (1981). Fibrinolytic activity in periodontal disease: the relationship between fibrinolytic activity and severity of periodontal disease. J. Periodontol. 52, 181–186. doi: 10.1902/jop.1981.52.4.181
Highfield, J. (2009). Diagnosis and classification of periodontal disease. Aust. Dent. J. 54, S11–S26. doi: 10.1111/j.1834-7819.2009.01140.x
Hinode, D., Yokoyama, M., Tanabe, S., Yoshioka, M., and Nakamura, R. (2002). Antigenic properties of the GroEL-like protein of Campylobacter rectus. Mol. Oral Microbiol. 17, 16–21. doi: 10.1046/j.0902-0055.2001.00086.x
Hinode, D., Yoshioka, M., Tanabe, S.-I., Miki, O., Masuda, K., and Nakamura, R. (1998). The GroEL-like protein from Campylobacter rectus: immunological characterization and interleukin-6 and-8 induction in human gingival fibroblast. FEMS Microbiol. Lett. 167, 1–6. doi: 10.1111/j.1574-6968.1998.tb13199.x
Hirsch, R., Deng, H., and Laohachai, M. (2012). Azithromycin in periodontal treatment: more than an antibiotic. J. Periodont. Res. 47, 137–148. doi: 10.1111/j.1600-0765.2011.01418.x
Hollander, D., Vadheim, C. M., Brettholz, E., Petersen, G. M., Delahunty, T., and Rotter, J. I. (1986). Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann. Intern. Med. 105, 883–885. doi: 10.7326/0003-4819-105-6-883
Horio, Y., Shiraishi, Y., Watanabe, N., Inoue, S., Imanishi, T., and Asano, K. (2017). Empyema associated with Campylobacter curvus infection. Respirol Case Rep 5:e00234. doi: 10.1002/rcr2.234
Huq, M., Van, T. T. H., Gurtler, V., Elshagmani, E., Allemailem, K. S., Smooker, P. M., et al. (2017). The ribosomal RNA operon (rrn) of Campylobacter concisus supports molecular typing to genomospecies level. Gene Rep. 6, 8–14. doi: 10.1016/j.genrep.2016.10.008
Ichiyama, S., Hirai, S., Minami, T., Nishiyama, Y., Shimizu, S., Shimokata, K., et al. (1998). Campylobacter fetus subspecies fetus cellulitis associated with bacteremia in debilitated hosts. Clin. Infect. Dis. 27, 252–255. doi: 10.1086/514654
Iovine, N. M. (2013). Resistance mechanisms in Campylobacter jejuni. Virulence 4, 230–240. doi: 10.4161/viru.23753
Irvine, E. J., and Marshall, J. K. (2000). Increased intestinal permeability precedes the onset of Crohn's disease in a subject with familial risk. Gastroenterology 119, 1740–1744. doi: 10.1053/gast.2000.20231
Irwin, C., and Myrillas, T. (1998). The role of IL-6 in the pathogenesis of periodontal disease. Oral Dis. 4, 43–47. doi: 10.1111/j.1601-0825.1998.tb00255.x
Ismail, Y., Lee, H., Riordan, S. M., Grimm, M. C., and Zhang, L. (2013). The effects of oral and enteric Campylobacter concisus strains on expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 cells. PLoS ONE 8:e56888. doi: 10.1371/journal.pone.0056888
Ismail, Y., Mahendran, V., Octavia, S., Day, A. S., Riordan, S. M., Grimm, M. C., et al. (2012). Investigation of the enteric pathogenic potential of oral Campylobacter concisus strains isolated from patients with inflammatory bowel disease. PLoS ONE 7:e38217. doi: 10.1371/journal.pone.0038217
Istivan, T. (2005). Molecular Characterisation of Campylobacter concisus: A Potential Etiological Agent of Gastroenteritis in Children. School of Applied Sciences, RMIT University.
Istivan, T. S., Coloe, P. J., Fry, B. N., Ward, P., and Smith, S. C. (2004). Characterization of a haemolytic phospholipase A(2) activity in clinical isolates of Campylobacter concisus. J. Med. Microbiol. 53, 483–493. doi: 10.1099/jmm.0.45554-0
Jackson, F. L., and Goodman, Y. E. (1978). Bacteroides ureolyticus, a new species to accommodate strains previously identified as “Bacteroides corrodens, anaerobic”. Int. J. Syst. Bacteriol. 28, 197–200. doi: 10.1099/00207713-28-2-197
Jimenez, S. G., Heine, R. G., Ward, P. B., and Robins-Browne, R. M. (1999). Campylobacter upsaliensis gastroenteritis in childhood. Pediatr. Infect. Dis. J. 18, 988–992. doi: 10.1097/00006454-199911000-00011
Johnson, C. C., Reinhardt, J. F., Mulligan, M. E., George, W. L., and Finegold, S. M. (1986). In vitro activities of 17 antimicrobial agents against the formate/fumarate-requiring, anaerobic gram-negative bacilli. DMID 5, 269–272. doi: 10.1016/0732-8893(86)90011-8
Johnson, C. C., Reinhardt, J., Edelstein, M., Mulligan, M., George, W., and Finegold, S. (1985). Bacteroides gracilis, an important anaerobic bacterial pathogen. J. Clin. Microbiol. 22, 799–802.
Kaakoush, N. O., Deshpande, N. P., Man, S. M., Burgos-Portugal, J. A., Khattak, F. A., Raftery, M. J., et al. (2015). Transcriptomic and proteomic analyses reveal key innate immune signatures in the host response to the gastrointestinal pathogen Campylobacter concisus. Infect. Immun. 83, 832–845. doi: 10.1128/IAI.03012-14
Kaakoush, N. O., Deshpande, N. P., Wilkins, M. R., Raftery, M. J., Janitz, K., and Mitchell, H. (2011). Comparative analyses of Campylobacter concisus strains reveal the genome of the reference strain BAA-1457 is not representative of the species. Gut Pathog. 3, 15–15. doi: 10.1186/1757-4749-3-15
Kalischuk, L., and Inglis, G. (2011). Comparative genotypic and pathogenic examination of Campylobacter concisus isolates from diarrheic and non-diarrheic humans. BMC Microbiol. 11:53. doi: 10.1186/1471-2180-11-53
Kirk, K. F., Méric, G., Nielsen, H. L., Pascoe, B., Sheppard, S. K., Thorlacius-Ussing, O., et al. (2018). Molecular epidemiology and comparative genomics of Campylobacter concisus strains from saliva, faeces and gut mucosal biopsies in inflammatory bowel disease. Sci. Rep. 8:1902. doi: 10.1038/s41598-018-20135-4
Kirk, K. F., Nielsen, H. L., Thorlacius-Ussing, O., and Nielsen, H. (2016). Optimized cultivation of Campylobacter concisus from gut mucosal biopsies in inflammatory bowel disease. Gut Pathog. 8:27. doi: 10.1186/s13099-016-0111-7
Kist, M., Keller, K.-M., Niebling, W., and Kilching, W. (1984). Campylobacter coli septicaemia associated with septic abortion. Infection 12, 88–90. doi: 10.1007/BF01641678
Klein, B. S., Vergeront, J. M., Blaser, M. J., Edmonds, P., Brenner, D. J., Janssen, D., et al. (1986). Campylobacter infection associated with raw milk: an outbreak of gastroenteritis due to Campylobacter jejuni and thermotolerant Campylobacter fetus subsp fetus. JAMA 255, 361–364. doi: 10.1001/jama.1986.03370030081032
Knights, D., Lassen, K. G., and Xavier, R. J. (2013). Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut 62, 1505–1510. doi: 10.1136/gutjnl-2012-303954
Kobayashi, Y., Ohta, H., Kokeguchi, S., Murayama, Y., Kato, K., Kurihara, H., et al. (1993). Antigenic properties of Campylobacter rectus (Wolinella recta) major S-layer proteins. FEMS Microbiol. Lett. 108, 275–280. doi: 10.1111/j.1574-6968.1993.tb06115.x
Koga, M., Yuki, N., Takahashi, M., Saito, K., and Hirata, K. (1999). Are Campylobacter curvus and Campylobacter upsaliensis antecedent infectious agents in Guillain–Barré and Fisher's syndromes? J. Neurol. Sci. 163, 53–57.
Kohler, A., De Torrenté, A., and Inderwildi, B. (1988). Fisher's syndrome associated with Campylobacter jejuni infection. Eur. Neurol. 28, 150–151.
Könönen, E., Paju, S., Pussinen, P. J., Hyvönen, M., Di Tella, P., Suominen-Taipale, L., et al. (2007). Population-based study of salivary carriage of periodontal pathogens in adults. J. Clin. Microbiol. 45, 2446–2451. doi: 10.1128/JCM.02560-06
Konstantinov, S. R., Smidt, H., De Vos, W. M., Bruijns, S. C., Singh, S. K., Valence, F., et al. (2008). S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. U.S.A. 105, 19474–19479. doi: 10.1073/pnas.0810305105
Korman, T., Varley, C., and Spelman, D. (1997). Acute hepatitis associated with Campylobacter jejuni bacteraemia. Eur. J. Clin. Microbiol. Infect. Dis. 16, 678–681. doi: 10.1007/BF01708559
Krause, R., Ramschak-Schwarzer, S., Gorkiewicz, G., Schnedl, W., Feierl, G., Wenisch, C., et al. (2002). Recurrent septicemia due to Campylobacter fetus and Campylobacter lari in an immunocompetent patient. Infection 30, 171–174. doi: 10.1007/s15010-002-2115-0
Kwon, S. Y., Cho, D. H., Lee, S. Y., Lee, K., and Chong, Y. (1994). Antimicrobial susceptibility of Campylobacter fetus subsp. fetus isolated from blood and synovial fluid. Yonsei Med. J. 35, 314–319. doi: 10.3349/ymj.1994.35.3.314
La Scolea, L. J. (1985). Campylobacter fetus subsp. fetus meningitis in a neonate. Clin. Microbiol. Newsl. 7, 125–126. doi: 10.1016/S0196-4399(85)80024-6
Lastovica, A. J. (2006). Emerging Campylobacter spp.: the tip of the iceberg. Clin. Microbiol. Newsl. 28, 49–56. doi: 10.1016/j.clinmicnews.2006.03.004
Lastovica, A. J., and Le Roux, E. (2001). Efficient isolation of Campylobacter upsaliensis from stools. J. Clin. Microbiol. 39, 4222–4223. doi: 10.1128/JCM.39.11.4222-4223.2001
Lastovica, A. J., and Roux, E. (2000). Efficient isolation of Campylobacteria from stools. J. Clin. Microbiol. 38, 2798–2800.
Lastovica, A. J., On, S. L., and Zhang, L. (2014). “The family Campylobacteraceae,” in The Prokaryotes, eds E. Rosenberg, E. F. DeLong, S. Lory, E. Stackebrandt, and F. Thompson (Berlin, Heidelberg: Springer), 307–335. doi: 10.1007/978-3-642-39044-9_274
Lastovica, A., Le Roux, E., and Penner, J. (1989). “Campylobacter upsaliensis” isolated from blood cultures of pediatric patients. J. Clin. Microbiol. 27, 657–659.
Lavrencic, P., Kaakoush, N. O., Huinao, K. D., Kain, N., and Mitchell, H. M. (2012). Investigation of motility and biofilm formation by intestinal Campylobacter concisus strains. Gut Pathog. 4, 22–22. doi: 10.1186/1757-4749-4-22
Lawson, A. J., Linton, D., and Stanley, J. (1998). 16S rRNA gene sequences of ‘Candidatus Campylobacter hominis’, a novel uncultivated species, are found in the gastrointestinal tract of healthy humans. Microbiology 144, 2063–2071.
Lawson, A. J., On, S., Logan, J., and Stanley, J. (2001). Campylobacter hominis sp. nov., from the human gastrointestinal tract. Int. J. Syst. Evol. Microbiol. 51, 651–660. doi: 10.1099/00207713-51-2-651
Lawson, A., Linton, D., Stanley, J., and Owen, R. (1997). Polymerase chain reaction detection and speciation of Campylobacter upsaliensis and C. helveticus in human faeces and comparison with culture techniques. J. Appl. Microbiol. 83, 375–380. doi: 10.1046/j.1365-2672.1997.00240.x
Lee, H., Ma, R., Grimm, M. C., Riordan, S. M., Lan, R., Zhong, L., et al. (2014). Examination of the anaerobic growth of Campylobacter concisus strains. Int. J. Microbiol. 2014:476047. doi: 10.1155/2014/476047
Lee, H.-J., Kim, J.-K., Cho, J.-Y., Lee, J.-M., and Hong, S.-H. (2012). Quantification of subgingival bacterial pathogens at different stages of periodontal diseases. Curr. Microbiol. 65, 22–27. doi: 10.1007/s00284-012-0121-8
Leo, Q. J. N., and Bolger, D. T. (2014). Septic cavernous sinus thrombosis due to Campylobacter rectus infection. BMJ Case Rep. 2014:bcr2013203351. doi: 10.1136/bcr-2013-203351
Lindblom, G., Sjogren, E., Hansson-Westerberg, J., and Kaijser, B. (1995). Campylobacter upsaliensis, C. sputorum sputorum and C. concisus as common causes of diarrhoea in Swedish children. Scand. J. Infect. Dis. 27, 187–188. doi: 10.3109/00365549509019006
Linscott, A. J., Flamholtz, R. B., Shukla, D., Song, Y., Liu, C., and Finegold, S. M. (2005). Fatal septicemia due to Clostridium hathewayi and Campylobacter hominis. Anaerobe 11, 97–98. doi: 10.1016/j.anaerobe.2004.10.002
Liu, F., Lee, H., Lan, R., and Zhang, L. (2016). Zonula occludens toxins and their prophages in Campylobacter species. Gut Pathog. 8:43. doi: 10.1186/s13099-016-0125-1
Liu, F., Ma, R., Riordan, S. M., Grimm, M. C., Liu, L., Wang, Y., et al. (2017). Azathioprine, mercaptopurine, and 5-aminosalicylic acid affect the growth of IBD-associated Campylobacter species and other enteric microbes. Front. Microbiol. 8:527. doi: 10.3389/fmicb.2017.00527
Liu, F., Ma, R., Tay, C. Y. A., Octavia, S., Lan, R., Chung, H. K. L., et al. (2018). Genomic analysis of oral Campylobacter concisus strains identified a potential bacterial molecular marker associated with active Crohn's disease. Emerg. Microbes Infect. 7:64. doi: 10.1038/s41426-018-0065-6
Liu, Y.-H., Yamazaki, W., Huang, Y.-T., Liao, C.-H., Sheng, W.-H., and Hsueh, P.-R. (2017). Clinical and microbiological characteristics of patients with bacteremia caused by Campylobacter species with an emphasis on the subspecies of C. fetus. J. Microbiol. Immunol. Infect. doi: 10.1016/j.jmii.2017.07.009. [Epub ahead of print].
Logan, J., Burnens, A., Linton, D., Lawson, A., and Stanley, J. (2000). Campylobacter lanienae sp. nov., a new species isolated from workers in an abattoir. Int. J. Syst. Evol. Microbiol. 50, 865–872. doi: 10.1099/00207713-50-2-865
López, R., Dahlén, G., Retamales, C., and Baelum, V. (2011). Clustering of subgingival microbial species in adolescents with periodontitis. Eur. J. Oral Sci. 119, 141–150. doi: 10.1111/j.1600-0722.2011.00808.x
Luangtongkum, T., Jeon, B., Han, J., Plummer, P., Logue, C. M., and Zhang, Q. (2009). Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol. 4, 189–200. doi: 10.2217/17460913.4.2.189
Lumerman, H., Freedman, P., and Kerpel, S. (1995). Oral epithelial dysplasia and the development of invasive squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 79, 321–329. doi: 10.1016/S1079-2104(05)80226-4
Ma, R., Liu, F., Yap, S. F., Lee, H., Leong, R. W., Riordan, S., et al. (2018). The growth and protein expression of inflammatory bowel disease-associated Campylobacter concisus is affected by the derivatives of the food additive fumaric acid. Front. Microbiol. 9:896. doi: 10.3389/fmicb.2018.00896
Ma, R., Sapwell, N., Chung, H. K., Lee, H., Mahendran, V., Leong, R. W., et al. (2015). Investigation of the effects of pH and bile on the growth of oral Campylobacter concisus strains isolated from patients with inflammatory bowel disease and controls. J. Med. Microbiol. 64, 438–445. doi: 10.1099/jmm.0.000013
Macfarlane, S., Furrie, E., Macfarlane, G. T., and Dillon, J. F. (2007). Microbial colonization of the upper gastrointestinal tract in patients with Barrett's esophagus. Clin. Infect. Dis. 45, 29–38. doi: 10.1086/518578
Macuch, P., and Tanner, A. (2000). Campylobacter species in health, gingivitis, and periodontitis. J. Dent. Res. 79, 785–792. doi: 10.1177/00220345000790021301
Madianos, P., Lieff, S., Murtha, A., Boggess, K., Auten, R., Beck, J., et al. (2001). Maternal periodontitis and prematurity. Part II: maternal infection and fetal exposure. Ann. Periodontol. 6, 175–182. doi: 10.1902/annals.2001.6.1.175
Mahendran, V., Liu, F., Riordan, S., Grimm, M., Tanaka, M., and Zhang, L. (2016). Examination of the effects of Campylobacter concisus zonula occludens toxin on intestinal epithelial cells and macrophages. Gut Pathog. 8:18. doi: 10.1186/s13099-016-0101-9
Mahendran, V., Octavia, S., Demirbas, O. F., Sabrina, S., Ma, R., Lan, R., et al. (2015). Delineation of genetic relatedness and population structure of oral and enteric Campylobacter concisus strains by analysis of housekeeping genes. Microbiology 161, 1600–1612. doi: 10.1099/mic.0.000112
Mahendran, V., Riordan, S. M., Grimm, M. C., Tran, T. A., Major, J., Kaakoush, N. O., et al. (2011). Prevalence of Campylobacter species in adult Crohn's disease and the preferential colonization sites of Campylobacter species in the human intestine. PLoS ONE 6:e25417. doi: 10.1371/journal.pone.0025417
Mahendran, V., Tan, Y. S., Riordan, S. M., Grimm, M. C., Day, A. S., Lemberg, D. A., et al. (2013). The prevalence and polymorphisms of zonula occluden toxin gene in multiple Campylobacter concisus strains isolated from saliva of patients with inflammatory bowel disease and controls. PLoS ONE 8:e75525. doi: 10.1371/journal.pone.0075525
Mahlen, S. D., and Clarridge, J. E. (2009). Oral abscess caused by Campylobacter rectus: case report and literature review. J. Clin. Microbiol. 47, 848–851. doi: 10.1128/JCM.01590-08
Man, S. M., Kaakoush, N. O., Leach, S. T., Nahidi, L., Lu, H. K., Norman, J., et al. (2010a). Host attachment, invasion, and stimulation of proinflammatory cytokines by Campylobacter concisus and other non-Campylobacter jejuni Campylobacter species. J. Infect. Dis. 202, 1855–1865. doi: 10.1086/657316
Man, S. M., Zhang, L., Day, A. S., Leach, S. T., Lemberg, D. A., and Mitchell, H. (2010b). Campylobacter concisus and other Campylobacter species in children with newly diagnosed Crohn's disease. Inflamm. Bowel Dis. 16, 1008–1016. doi: 10.1002/ibd.21157
Manfredi, R., Nanetti, A., Ferri, M., and Chiodo, F. (1999). Fatal Campylobacter jejuni bacteraemia in patients with AIDS. J. Med. Microbiol. 48, 601–603. doi: 10.1099/00222615-48-6-601
Martel-Pelletier, J., Faure, M., Mccollum, R., Mineau, F., Cloutier, J., and Pelletier, J. (1991). Plasmin, plasminogen activators and inhibitor in human osteoarthritic cartilage. J. Rheumatol. 18, 1863–1871.
Martinot, M., Jaulhac, B., Moog, R., De Martino, S., Kehrli, P., Monteil, H., et al. (2001). Campylobacter lari bacteremia. Clin. Microbiol. Infect. 7, 96–97. doi: 10.1046/j.1469-0691.2001.00212.x
Meddings, J. (2008). What role does intestinal permeability have in IBD pathogenesis? Inflamm. Bowel Dis. 14(Suppl. 2), S138–S139. doi: 10.1002/ibd.20719
Megraud, F., Tachoire, C., Latrille, J., and Bondonny, J. (1982). Appendicitis due to Campylobacter jejuni. Br. Med. J. 285:1165. doi: 10.1136/bmj.285.6349.1165-a
Mendz, G. L., Petersen, R., Quinlivan, J. A., and Kaakoush, N. O. (2014). Potential involvement of Campylobacter curvus and Haemophilus parainfluenzae in preterm birth. BMJ Case Rep. 2014:bcr2014205282. doi: 10.1136/bcr-2014-205282
Meyer, A., Stallmach, T., Goldenberger, D., and Altwegg, M. (1997). Lethal Maternal Sepsis Caused by Campylobacter jejuni: pathogen Preserved in Placenta and Identified by Molecular Method. Mod. Pathol. 10, 1253–1256.
Miller, W. G., and Yee, E. (2015). Complete Genome Sequence of Campylobacter gracilis ATCC 33236T. Genome Announc. 3:e01087-15. doi: 10.1128/genomeA.01087-15
Miller, W. G., Chapman, M. H., Yee, E., On, S. L., Mcnulty, D. K., Lastovica, A. J., et al. (2012). Multilocus sequence typing methods for the emerging Campylobacter species, C. hyointestinalis, C. lanienae, C. sputorum, C. concisus, and C. curvus. Front. Cell. Infect. Microbiol. 2:45. doi: 10.3389/fcimb.2012.00045
Møller Nielsen, E., Engberg, J., and Madsen, M. (1997). Distribution of serotypes of Campylobacter jejuni and C. coli from Danish patients, poultry, cattle and swine. FEMS Immunol. Med. Microbiol. 19, 47–56. doi: 10.1111/j.1574-695X.1997.tb01071.x
Morooka, T., Umeda, A., Fujita, M., Matano, H., Fujimoto, S., Yukitake, K., et al. (1996). Epidemiologic application of pulsed-field gel electrophoresis to an outbreak of Campylobacter fetus meningitis in a neonatal intensive care unit. Scand. J. Infect. Dis. 28, 269–270. doi: 10.3109/00365549609027170
Morris, C. N., Scully, B., and Garvey, G. J. (1998). Campylobacter lari Associated with Permanent Infection and Bacteremia. Clin. Infect. Dis. 27, 220–221. doi: 10.1086/517683
Morrison, V. A., Lloyd, B. K., Chia, J. K., and Tuazon, C. U. (1990). Cardiovascular and bacteremic manifestations of Campylobacter fetus infection: case report and review. Clin. Infect. Dis. 12, 387–392. doi: 10.1093/clinids/12.3.387
Mortensen, N. P., Kuijf, M. L., Ang, C. W., Schiellerup, P., Krogfelt, K. A., Jacobs, B. C., et al. (2009). Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microb Infect 11, 988–994. doi: 10.1016/j.micinf.2009.07.004
Mukhopadhya, I., Thomson, J. M., Hansen, R., Berry, S. H., El-Omar, E. M., and Hold, G. L. (2011). Detection of Campylobacter concisus and other Campylobacter species in colonic biopsies from adults with ulcerative colitis. PLoS ONE 6:e21490. doi: 10.1371/journal.pone.0021490
Namin, B. M., Dallal, M. M. S., and Daryani, N. E. (2015). The Effect of Campylobacter concisus on expression of IL-18, TNF-α and p53 in Barrett's cell lines. Jundishapur J. Microbiol. 8:e26393. doi: 10.5812/jjm.26393t
Neal-Mckinney, J. M., and Konkel, M. E. (2012). The Campylobacter jejuni CiaC virulence protein is secreted from the flagellum and delivered to the cytosol of host cells. Front. Cell Infect. Microbiol. 2:31. doi: 10.3389/fcimb.2012.00031
Neuzil, K. M., Wang, E., Haas, D. W., and Blaser, M. J. (1994). Persistence of Campylobacter fetus bacteremia associated with absence of opsonizing antibodies. J. Clin. Microbiol. 32, 1718–1720.
Newman, M., and Socransky, S. (1977). Predominant cultivable microbiota in periodontosis. J. Periodont. Res. 12, 120–128. doi: 10.1111/j.1600-0765.1977.tb00114.x
Nielsen, H. L., Engberg, J., Ejlertsen, T., Bücker, R., and Nielsen, H. (2012). Short-term and medium-term clinical outcomes of Campylobacter concisus infection. Clin. Microbiol. Infect. 18, E459–465. doi: 10.1111/j.1469-0691.2012.03990.x
Nielsen, H. L., Nielsen, H., and Torpdahl, M. (2016). Multilocus sequence typing of Campylobacter concisus from Danish diarrheic patients. Gut Pathog. 8:44. doi: 10.1186/s13099-016-0126-0
Nielsen, H. L., Nielsen, H., Ejlertsen, T., Engberg, J., Günzel, D., Zeitz, M., et al. (2011). Oral and fecal Campylobacter concisus strains perturb barrier function by apoptosis induction in HT-29/B6 intestinal epithelial cells. PLoS ONE 6:e23858. doi: 10.1371/journal.pone.0023858
Nielsen, H., Ejlertsen, T., Engberg, J., and Nielsen, H. (2013). High incidence of Campylobacter concisus in gastroenteritis in North Jutland, Denmark: a population-based study. Clin. Microbiol. Infect. 19, 445–450. doi: 10.1111/j.1469-0691.2012.03852.x
Nielsen, O. H., Vainer, B., and Rask-Madsen, J. (2001). Review article: the treatment of inflammatory bowel disease with 6-mercaptopurine or azathioprine. Aliment. Pharmacol. Ther. 15, 1699–1708. doi: 10.1046/j.1365-2036.2001.01102.x
Nikfar, S., Rahimi, R., Rezaie, A., and Abdollahi, M. (2009). A meta-analysis of the efficacy of sulfasalazine in comparison with 5-aminosalicylates in the induction of improvement and maintenance of remission in patients with ulcerative colitis. Dig. Dis. Sci. 54, 1157–1170. doi: 10.1007/s10620-008-0481-x
Nitta, H., Holt, S. C., and Ebersole, J. L. (1997). Purification and characterization of Campylobacter rectus surface layer proteins. Infect. Immun. 65, 478–483.
Noël, A., Verroken, A., Belkhir, L., and Rodriguez-Villalobos, H. (2018). Fatal thoracic empyema involving Campylobacter rectus: a case report. Anaerobe 49, 95–98. doi: 10.1016/j.anaerobe.2017.12.014
Noiri, Y., Ozaki, K., Nakae, H., Matsuo, T., and Ebisu, S. (1997). An immunohistochemical study on the localization of Porphyromonas gingivalis, Campylobacter rectus and Actinomyces viscosus in human periodontal pockets. J. Periodont. Res. 32, 598–607. doi: 10.1111/j.1600-0765.1997.tb00937.x
O'doherty, A., Koziel, M., De Barra, L., Corcoran, D., Bullman, S., Lucey, B., et al. (2014). Development of nalidixic acid amphotericin B vancomycin (NAV) medium for the isolation of Campylobacter ureolyticus from the stools of patients presenting with acute gastroenteritis. Br. J. Biomed. Sci. 71, 6–12. doi: 10.1080/09674845.2014.11669956
Offenbacher, S., Riche, E., Barros, S., Bobetsis, Y., Lin, D., and Beck, J. (2005). Effects of maternal Campylobacter rectus infection on murine placenta, fetal and neonatal survival, and brain development. J. Periodontol. 76, 2133–2143. doi: 10.1902/jop.2005.76.11-S.2133
Ogura, N., Matsuda, U., Tanaka, F., Shibata, Y., Takiguchi, H., and Abiko, Y. (1996). In vitro senescence enhances IL-6 production in human gingival fibroblasts induced by lipopolysaccharide from Campylobacter rectus. Mech. Ageing Dev. 87, 47–59. doi: 10.1016/0047-6374(96)01701-0
Ogura, N., Shibata, Y., Matsuda, U., Oikawa, T., Takiguchi, H., Izumi, H., et al. (1995). Effect of Campylobacter rectus LPS on plasminogen activator-plasmin system in human gingival fibroblast cells. J. Periodont. Res. 30, 132–140. doi: 10.1111/j.1600-0765.1995.tb01262.x
Okuda, K., Kigure, T., Yamada, S., Kaneko, T., Ishihara, K., Miura, T., et al. (1997). Role for the S-layer of Campylobacter rectus ATCC33238 in complement mediated killing and phagocytic killing by leukocytes from guinea pig and human peripheral blood. Oral Dis. 3, 113–120. doi: 10.1111/j.1601-0825.1997.tb00022.x
On, S., Siemer, B., Chung, P., and Lastovica, A. (2013). Characterisation of Campylobacter concisus strains from South Africa using Amplified Fragment Length Polymorphism (AFLP) profiling and a Genomospecies-specific Polymerase Chain Reaction (PCR) assay: Identification of novel genomospecies and correlation with clinical data. Afr. J. Microbiol. Res. 7, 1845–1851. doi: 10.5897/AJMR12.2182
Ovesen, S., Kirk, K. F., Nielsen, H. L., and Nielsen, H. (2017). Motility of Campylobacter concisus isolated from saliva, feces, and gut mucosal biopsies. APMIS 125, 230–235. doi: 10.1111/apm.12655
Patrick, M. E., Gilbert, M. J., Blaser, M. J., Tauxe, R. V., Wagenaar, J. A., and Fitzgerald, C. (2013). Human infections with new subspecies of Campylobacter fetus. Emerg. Infect. Dis. 19, 1678. doi: 10.3201/eid1910.130883
Payot, S., Bolla, J.-M., Corcoran, D., Fanning, S., Mégraud, F., and Zhang, Q. (2006). Mechanisms of fluoroquinolone and macrolide resistance in Campylobacter spp. Microb. Infect. 8, 1967–1971. doi: 10.1016/j.micinf.2005.12.032
Pena, L. A., and Fishbein, M. C. (2007). Fatal myocarditis related to Campylobacter jejuni infection: a case report. Cardiovasc. Pathol. 16, 119–121. doi: 10.1016/j.carpath.2006.09.007
Petersen, R. F., Harrington, C. S., Kortegaard, H. E., and On, S. L. (2007). A PCR-DGGE method for detection and identification of Campylobacter, Helicobacter, Arcobacter and related Epsilobacteria and its application to saliva samples from humans and domestic pets. J. Appl. Microbiol. 103, 2601–2615. doi: 10.1111/j.1365-2672.2007.03515.x
Pihlstrom, B. L., Michalowicz, B. S., and Johnson, N. W. (2005). Periodontal diseases. Lancet 366, 1809–1820. doi: 10.1016/S0140-6736(05)67728-8
Platts-Mills, J. A., Liu, J., Gratz, J., Mduma, E., Amour, C., Swai, N., et al. (2014). Detection of Campylobacter in stool and determination of significance by culture, enzyme immunoassay, and PCR in developing countries. J. Clin. Microbiol. 52, 1074–1080. doi: 10.1128/JCM.02935-13
Press, A., Hauptmann, I., Hauptmann, L., Fuchs, B., Fuchs, M., Ewe, K., et al. (1998). Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 12, 673–678. doi: 10.1046/j.1365-2036.1998.00358.x
Pruitt, K. D., Tatusova, T., and Maglott, D. R. (2007). NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 35, D61–D65. doi: 10.1093/nar/gkl842
Rams, T., Feik, D., and Slots, J. (1993). Campylobacter rectus in human periodontitis. Mol. Oral Microbiol. 8, 230–235. doi: 10.1111/j.1399-302X.1993.tb00565.x
Reeser, R. J., Medler, R. T., Billington, S. J., Jost, B. H., and Joens, L. A. (2007). Characterization of Campylobacter jejuni biofilms under defined growth conditions. Appl. Environ. Microbiol. 73, 1908–1913. doi: 10.1128/AEM.00740-06
Roe, D. E., Weinberg, A., and Roberts, M. C. (1995). Mobile rRNA methylase genes in Campylobacter (Wotinella) rectus. J. Antimicrob. Chemother. 36, 738–740. doi: 10.1093/jac/36.4.738
Roesler, B. M., Rabelo-Gonçalves, E. M., and Zeitune, J. M. (2014). Virulence factors of Helicobacter pylori: a review. Clin. Med. Insights Gastroenterol. 7:9. doi: 10.4137/CGast.S13760
Roop Ii, R. M., Smibert, R. M., Johnson, J. L., and Krieg, N. R. (1985). DNA homology studies of the catalase-negative campylobacters and “Campylobacter fecalis,” an emended description of Campylobacter sputorum, and proposal of the neotype strain of Campylobacter sputorum. Can. J. Microbiol. 31, 823–831. doi: 10.1139/m85-154
Samuelson, D. R., Eucker, T. P., Bell, J. A., Dybas, L., Mansfield, L. S., and Konkel, M. E. (2013). The Campylobacter jejuni CiaD effector protein activates MAP kinase signaling pathways and is required for the development of disease. J. Cell Commun. Signal. 11:79. doi: 10.1186/1478-811X-11-79
Sanderson, J., Moss, M., Tizard, M., and Hermon-Taylor, J. (1992). Mycobacterium paratuberculosis DNA in Crohn's disease tissue. Gut 33, 890–896. doi: 10.1136/gut.33.7.890
Sartor, R. B. (2004). Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 126, 1620–1633. doi: 10.1053/j.gastro.2004.03.024
Sauerwein, R., Horrevorts, A., and Bisseling, J. (1993). Septic abortion associated with Campylobacter fetus subspecies fetus infection: case report and review of the literature. Infection 21, 331–333. doi: 10.1007/BF01712458
Schwartz, A. G., Targan, S. R., Saxon, A., and Weinstein, W. M. (1982). Sulfasalazine-induced exacerbation of ulcerative colitis. N. Engl. J. Med. 306, 409–412. doi: 10.1056/NEJM198202183060708
Serichantalergs, O., Ruekit, S., Pandey, P., Anuras, S., Mason, C., Bodhidatta, L., et al. (2017). Incidence of Campylobacter concisus and C. ureolyticus in traveler's diarrhea cases and asymptomatic controls in Nepal and Thailand. Gut Pathog. 9:47. doi: 10.1186/s13099-017-0197-6
Shin, S. J., and Collins, M. T. (2008). Thiopurine drugs azathioprine and 6-mercaptopurine inhibit Mycobacterium paratuberculosis growth in vitro. Antimicrob. Agents Chemother. 52, 418–426. doi: 10.1128/AAC.00678-07
Shinha, T. (2015). Fatal bacteremia caused by Campylobacter gracilis, United States. Emerg. Infect. Dis. 21, 1084. doi: 10.3201/eid2106.142043
Sieswerda, L. E., and Bannatyne, R. M. (2006). Mapping the effects of genetic susceptibility and Mycobacterium avium subsp. paratuberculosis infection on Crohn's disease: strong but independent. J. Clin. Microbiol. 44, 1204–1205. doi: 10.1128/JCM.44.3.1204-1205.2006
Simor, A., Karmali, M., Jadavji, T., and Roscoe, M. (1986). Abortion and perinatal sepsis associated with Campylobacter infection. Rev. Infect. Dis. 8, 397–402. doi: 10.1093/clinids/8.3.397
Siqueira, J. F. Jr., and Rocas, I. N. (2003). Campylobacter gracilis and Campylobacter rectus in primary endodontic infections. Int. Endod. J. 36, 174–180. doi: 10.1046/j.1365-2591.2003.00636.x
Skirrow, M. (1977). Campylobacter enteritis: a “new” disease. Br. Med. J. 2, 9–11. doi: 10.1136/bmj.2.6078.9
Smith, K. D., Andersen-Nissen, E., Hayashi, F., Strobe, K., Bergman, M. A., Barrett, S. L. R., et al. (2003). Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol 4:1247. doi: 10.1038/ni1011
Sørensen, N. B., Nielsen, H. L., Varming, K., and Nielsen, H. (2013). Neutrophil activation by Campylobacter concisus. Gut Pathog. 5:17. doi: 10.1186/1757-4749-5-17
Steele, T. W., Sangster, N., and Lanser, J. A. (1985). DNA relatedness and biochemical features of Campylobacter spp. isolated in central and South Australia. J. Clin. Microbiol. 22, 71–74.
Steinkraus, G. E., and Wright, B. D. (1994). Septic abortion with intact fetal membranes caused by Campylobacter fetus subsp. fetus. J. Clin. Microbiol. 32, 1608–1609.
Suzuki, J., Sugiyama, T., Ito, K., Hadano, Y., Kawamura, I., Okinaka, K., et al. (2013). Campylobacter showae bacteremia with cholangitis. J. Infect. Chemother. 19, 960–963. doi: 10.1007/s10156-012-0524-2
Svensson, S. L., Pryjma, M., and Gaynor, E. C. (2014). Flagella-mediated adhesion and extracellular DNA release contribute to biofilm formation and stress tolerance of Campylobacter jejuni. PLoS ONE 9:e106063. doi: 10.1371/journal.pone.0106063
Takahashi, M., Koga, M., Yokoyama, K., and Yuki, N. (2005). Epidemiology of Campylobacter jejuni isolated from patients with Guillain-Barré and Fisher syndromes in Japan. J. Clin. Microbiol. 43, 335–339. doi: 10.1128/JCM.43.1.335-339.2005
Takiguchi, H., Yamaguchi, M., Mochizuki, K., and Abiko, Y. (1996). Effect of in vitro aging on Campylobacter rectus lipopolysaccharide-stimulated PGE2 release from human gingival fibroblasts. Oral Dis. 2, 202–209. doi: 10.1111/j.1601-0825.1996.tb00225.x
Takiguchi, H., Yamaguchi, M., Okamura, H., and Abiko, Y. (1997). Contribution of IL-1β to the enhancement of Campylobacter rectus lipopolysaccharide-stimulated PGE 2 production in old gingival fibroblasts in vitro. Mech. Ageing Dev. 98, 75–90. doi: 10.1016/S0047-6374(97)00068-7
Talonpoika, J., Söderling, E., Tiekso, J., and Paunio, K. (1990). Gingival crevicular fluid plasmin activity in different clinical conditions and after periodontal treatment. Proc. Finn. Dent. Soc. 87, 329–337.
Tanabe, S., Hinode, D., Yokoyama, M., Fukui, M., Nakamura, R., Yoshioka, M., et al. (2003). Helicobacter pylori and Campylobacter rectus share a common antigen. Oral Microbiol. Immunol. 18, 79–87. doi: 10.1034/j.1399-302X.2003.00049.x
Tanner, A. C. R., Badger, S., Lai, C., Listgarten, M. A., Visconti, R. A., and Socransky, S. S. (1981). Wolinella gen. nov., WoZinella succinogenes (Vibrio succinogenes Wolin et al.) comb. nov., and Description of Bacteroides gracilis sp. nov., Wolinella recta sp. nov., Campylobacter concisus sp. nov., and Eikenella corrodens from Humans with Periodontal Disease. Int. J. Syst. Bacteriol. 4, 432–445. doi: 10.1099/00207713-31-4-432
Tanner, A., Listgarten, M., and Ebersole, J. (1984). Wolinella curva sp. nov.:“Vibrio succinogenes” of human origin. Int. J. Syst. Evol. Microbiol. 34, 275–282.
Tay, A. P., Kaakoush, N. O., Deshpande, N. P., Chen, Z., Mitchell, H., and Wilkins, M. R. (2013). Genome sequence of Campylobacter showae UNSWCD, isolated from a patient with Crohn's disease. Genome Announc. 1:e00193-12. doi: 10.1128/genomeA.00193-12
Tee, W., Luppino, M., and Rambaldo, S. (1998). Bacteremia due to Campylobacter sputorum Biovar sputorum. Clin. Infect. Dis. 27, 1544–1545. doi: 10.1086/517748
Thomas, K., Chan, K. N., and Ribeiro, C. (1980). Campylobacter jejuni/coli meningitis in a neonate. BMJ 280:1301. doi: 10.1136/bmj.280.6227.1301
Tilmanne, A., Martiny, D., Hallin, M., Cornelius, A., Wautier, M., Quach, C., et al. (2018). Campylobacter concisus and acute gastroenteritis in children: lack of association. Pediatr. Infect. Dis. J. doi: 10.1097/INF.0000000000002028. [Epub ahead of print].
Tu, Z.-C., Dewhirst, F. E., and Blaser, M. J. (2001). Evidence that the Campylobacter fetus sap locus is an ancient genomic constituent with origins before mammals and reptiles diverged. Infect. Immun. 69, 2237–2244. doi: 10.1128/IAI.69.4.2237-2244.2001
Tu, Z.-C., Zeitlin, G., Gagner, J.-P., Keo, T., Hanna, B. A., and Blaser, M. J. (2004). Campylobacter fetus of reptile origin as a human pathogen. J. Clin. Microbiol. 42, 4405–4407. doi: 10.1128/JCM.42.9.4405-4407.2004
Van, T. T. H., Elshagmani, E., Gor, M. C., Scott, P. C., and Moore, R. J. (2016). Campylobacter hepaticus sp. nov., isolated from chickens with spotty liver disease. Int. J. Syst. Evol. Microbiol. 66, 4518–4524. doi: 10.1099/ijsem.0.001383
Vandenberg, O., Houf, K., Douat, N., Vlaes, L., Retore, P., Butzler, J.-P., et al. (2006). Antimicrobial susceptibility of clinical isolates of non-jejuni/coli campylobacters and arcobacters from Belgium. J. Antimicrob. Chemother. 57, 908–913. doi: 10.1093/jac/dkl080
Viejo, G., Gomez, B., De Miguel, D., Del Valle, A., Otero, L., and De La Iglesia, P. (2001). Campylobacter fetus subspecies fetus bacteremia associated with chorioamnionitis and intact fetal membranes. Scand. J. Infect. Dis. 33, 126–127. doi: 10.1080/003655401750065517
Von Troil-Lindén, B., Torkko, H., Alaluusua, S., Jousimies-Somer, H., and Asikainen, S. (1995). Salivary levels of suspected periodontal pathogens in relation to periodontal status and treatment. J. Dent. Res. 74, 1789–1795. doi: 10.1177/00220345950740111201
Walsh, A., Mabee, J., and Trivedi, K. (2011). Inflammatory bowel disease. Prim. Care 38, 415–432. doi: 10.1016/j.pop.2011.06.001
Wang, B., Kraig, E., and Kolodrubetz, D. (2000). Use of defined mutants to assess the role of the Campylobacter rectus S-layer in bacterium-epithelial cell interactions. Infect. Immun. 68, 1465–1473. doi: 10.1128/IAI.68.3.1465-1473.2000
Wang, Y., Liu, F., Zhang, X., Chung, H. K., Riordan, S. M., Grimm, M. C., et al. (2017). Campylobacter concisus genomospecies 2 is better adapted to the human gastrointestinal tract as compared with Campylobacter concisus genomospecies 1. Front. Physiol. 8:543. doi: 10.3389/fphys.2017.00543
Wassenaar, T. M., Bleumink-Pluym, N., and Van Der Zeijst, B. (1991). Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 10:2055.
Wassenaar, T. M., Van Der Zeijst, B. A., Ayling, R., and Newell, D. G. (1993). Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. Microbiology 139, 1171–1175. doi: 10.1099/00221287-139-6-1171
Watson, R. O., and Galán, J. E. (2005). Signal transduction in Campylobacter jejuni-induced cytokine production. Cell. Microbiol. 7, 655–665. doi: 10.1111/j.1462-5822.2004.00498.x
Weisblum, B. (1995). Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 39:577. doi: 10.1128/AAC.39.3.577
Werno, A. M., Klena, J. D., Shaw, G. M., and Murdoch, D. R. (2002). Fatal case of Campylobacter lari prosthetic joint infection and bacteremia in an immunocompetent patient. J. Clin. Microbiol. 40, 1053–1055. doi: 10.1128/JCM.40.3.1053-1055.2002
Williams, C. S., Mann, M., and Dubois, R. N. (1999). The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18. doi: 10.1038/sj.onc.1203286
Wolfs, T. F., Duim, B., Geelen, S. P., Rigter, A., Thomson-Carter, F., Fleer, A., et al. (2001). Neonatal sepsis by Campylobacter jejuni: genetically proven transmission from a household puppy. Clin. Infect. Dis. 32, e97–e99. doi: 10.1086/319224
Wyatt, J., Vogelsang, H., Hubl, W., Waldhoer, T., and Lochs, H. (1993). Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 341, 1437–1439. doi: 10.1016/0140-6736(93)90882-H
Xiao, T. S. (2015). The nucleic acid-sensing inflammasomes. Immunol. Rev. 265, 103–111. doi: 10.1111/imr.12281
Yang, L., Lu, X., Nossa, C. W., Francois, F., Peek, R. M., and Pei, Z. (2009). Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 137, 588–597. doi: 10.1053/j.gastro.2009.04.046
Yeo, A., Smith, M. A., Lin, D., Riché, E. L., Moore, A., Elter, J., et al. (2005). Campylobacter rectus mediates growth restriction in pregnant mice. J. Periodontol. 76, 551–557. doi: 10.1902/jop.2005.76.4.551
Young, K. T., Davis, L. M., and Dirita, V. J. (2007). Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 5, 665–679. doi: 10.1038/nrmicro1718
Yu, W.-L., and Chen, W.-Y. (1997). Tubo-ovarian abscess caused by multidrug resistant Bacteroides gracilis. J. Formos. Med. Assoc. 96, 457–460.
Zhang, L. (2015). Oral Campylobacter species: Initiators of a subgroup of inflammatory bowel disease? World J. Gastroenterol. 21, 9239–9244. doi: 10.3748/wjg.v21.i31.9239
Zhang, L., Budiman, V., Day, A. S., Mitchell, H., Lemberg, D. A., Riordan, S. M., et al. (2010). Isolation and detection of Campylobacter concisus from saliva of healthy individuals and patients with inflammatory bowel disease. J. Clin. Microbiol. 48, 2965–2967. doi: 10.1128/JCM.02391-09
Zhang, L., Lee, H., Grimm, M. C., Riordan, S. M., Day, A. S., and Lemberg, D. A. (2014). Campylobacter concisus and inflammatory bowel disease. World J. Gastroenterol. 20, 1259–1267. doi: 10.3748/wjg.v20.i5.1259
Keywords: Campylobacter concisus, inflammatory bowel disease, human hosted Campylobacter, virulence factor, bacterial marker, transmission, oral Campylobacter, pathogenic mechanism
Citation: Liu F, Ma R, Wang Y and Zhang L (2018) The Clinical Importance of Campylobacter concisus and Other Human Hosted Campylobacter Species. Front. Cell. Infect. Microbiol. 8:243. doi: 10.3389/fcimb.2018.00243
Received: 13 March 2018; Accepted: 25 June 2018;
Published: 24 July 2018.
Edited by:
Wilhelmina May Huston, University of Technology Sydney, AustraliaReviewed by:
Deborah Threadgill, Texas A&M University, United StatesCopyright © 2018 Liu, Ma, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhang, bC56aGFuZ0B1bnN3LmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.