
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 04 April 2018
Sec. Clinical Microbiology
Volume 8 - 2018 | https://doi.org/10.3389/fcimb.2018.00102
This article is part of the Research Topic Cell Biology of Salmonella View all 10 articles
A correction has been applied to this article in:
Corrigendum: Organoid and Enteroid Modeling of Salmonella Infection
 Yuebang Yin1
Yuebang Yin1 Daoguo Zhou2,3*
Daoguo Zhou2,3*Salmonella are Gram-negative rod-shaped facultative anaerobic bacteria that are comprised of over 2,000 serovars. They cause gastroenteritis (salmonellosis) with headache, abdominal pain and diarrhea clinical symptoms. Salmonellosis brings a heavy burden for the public health in both developing and developed countries. Antibiotics are usually effective in treating the infected patients with severe gastroenteritis, although antibiotic resistance is on the rise. Understanding the molecular mechanisms of Salmonella infection is vital to combat the disease. In vitro immortalized 2-D cell lines, ex vivo tissues/organs and several animal models have been successfully utilized to study Salmonella infections. Although these infection models have contributed to uncovering the molecular virulence mechanisms, some intrinsic shortcomings have limited their wider applications. Notably, cell lines only contain a single cell type, which cannot reproduce some of the hallmarks of natural infections. While ex vivo tissues/organs alleviate some of these concerns, they are more difficult to maintain, in particular for long term experiments. In addition, non-human animal models are known to reflect only part of the human disease process. Enteroids and induced intestinal organoids are emerging as effective infection models due to their closeness in mimicking the infected tissues/organs. Induced intestinal organoids are derived from iPSCs and contain mesenchymal cells whereas enteroids are derive from intestinal stem cells and are comprised of epithelial cells only. Both enteroids and induced intestinal organoids mimic the villus and crypt domains comparable to the architectures of the in vivo intestine. We review here that enteroids and induced intestinal organoids are emerging as desired infection models to study bacterial-host interactions of Salmonella.
Salmonella are Gram-negative rod-shaped and facultative anaerobes belong to the family of Enterobacteriaceae (Coburn et al., 2007). According to the recent classification by the International Code of Nomenclature of Bacteria, the genus Salmonella are classified into two distinct species including Salmonella enterica and Salmonella bongori based on their 16S rRNA sequence relatedness (Popoff et al., 2003). Salmonella bongori (V) is treated as a separate species due to its unique clinical features (Fierer and Guiney, 2001). Salmonella enterica is further classified into six subspecies: enterica (I), salamae (II), arizonae (IIIa), diarizonae (IIIb), houtenae (IV), and indica (VI), mainly based on their genomic sequence and biochemical properties (Fierer and Guiney, 2001). Diverse biochemical properties of the flagellar, carbohydrate and lipopolysaccharide (LPS) of S. enterica divided them further into over 2,000 serovars (Eng et al., 2015). Over 50% of these serovars belong to S. enterica subspecies enterica which are responsible for most infections in human (Itri et al., 2017). Salmonella are also classified based on their somatic (O), capsular (K), and flagellar (H) antigenic determinants (Brenner et al., 2000). The commonly used Salmonella classification in clinical laboratories is based on simple agglutination reactions to antibodies or antisera specific to the somatic O antigens containing six serogroups designated A, B, C1, C2, D, and E (Eng et al., 2015).
Salmonella infections remain a big burden for the public health worldwide (Eng et al., 2015). There are three main types of Salmonellosis: (1) localized intestinal infection (gastroenteritis), (2) systemic infection of otherwise healthy hosts (typhoid), and (3) systemic infection of immune-compromised hosts (Griffin and McSorley, 2011; Hurley et al., 2014). Salmonella strains that cause these infections are separated into typhoid Salmonella and non-typhoid Salmonella (NTS) based on the clinical patterns in human salmonellosis (Crump et al., 2015; Eng et al., 2015). Typhoid Salmonella infection seems to be more severe, in which patients show prodromal symptoms such as headache, abdominal pain and diarrhea (or constipation), followed by the onset of fever, which might sustain an incubation period of 1 week or more (Eng et al., 2015). Typhoid Salmonella strains are normally restricted to humans causing typhoid fever (also called enteric fever), while NTS strains have a broader host-range and represent zoonotic features (Gordon, 2011). The typhoid Salmonella infections occur mostly in developing countries including many regions of the African and Asian continent. The illness causes 93.8 million foodborne cases and 155,000 deaths annually (Eng et al., 2015). In comparison, gastroenteritis is caused mainly by S. enterica Serovar Typhimurium (Salmonella Typhimurium) and Serovar Enteritidis, which are common even in developed countries (Eng et al., 2015). The clinical signs of gastroenteritis can be diarrhea, cramping, and most patients usually recover within 4–7 days without treatment (Griffin and McSorley, 2011). Salmonellosis is transmitted mainly via food or water contaminated with human or animal feces (Crump et al., 2015). NTS have been reported to transmit via contaminated animals and animal products (Crump et al., 2015). The mortality caused by typhoid Salmonella strains can be up to 7% even when antibiotics are used (Eng et al., 2015). The incidence of enteric fever in the USA and European countries is normally low, representing less than 10 per 100,000 each year (Eng et al., 2015). In contrast, the invasive infections caused by NTS are estimated to be 3.4 million with 681,000 deaths worldwide in 2010 (Crump et al., 2015). Up to 57% of these illnesses and deaths are in Africa (Crump et al., 2015). Most NTS infections occur in animals, while it can also happen in infants, young children, elderly people and immunocompromised patients (Eng et al., 2015). The actual cases of Salmonella infections are estimated to be much higher than the reported numbers because milder cases are likely not diagnosed or reported, especially in some developing countries (Hurley et al., 2014).
Many assays have been developed to diagnose Salmonella infections. Widal test is the most commonly used diagnostic assay based on agglutinating antibodies against Salmonella LPS (O) and flagella (H). The enzyme-linked immunosorbent assays (ELISAs) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) immunoblotting assays are useful in measuring antibodies in patients' sera. Recently, PCR, real-time PCR, and proteomic approaches have been used to analyze bacterial genes and proteins (Kumar et al., 2012; Nigro et al., 2016). Once the infection is diagnosed, antibiotics might be used to treat infected patients with severe gastroenteritis, while most milder infections do not require antibiotics treatment (Boyle et al., 2007; Hurley et al., 2014). Two typhoid vaccines have been licensed against enteric fever, both are only suitable for endemic situation (Griffin and McSorley, 2011; Crump et al., 2015; Eng et al., 2015). Vaccines against NTS infections have been developed recently (Griffin and McSorley, 2011; Eng et al., 2015). Currently, food safety from farm to fork and treatment of municipal water remain the most effective measures to control the transmission of Salmonella (Crump et al., 2015).
The virulence determinants needed for S. Typhimurium are similar to those of many other intestinal pathogens: First, it needs to successfully survive the hostile acidic environment in the stomach before making its way to colonize the small intestine. In the intestine, the bacteria must breach the barrier of intestinal epithelial cells and it has to survive inside the host cells. Pathogenic Salmonella spp. evolve complex systems, which enable the organisms to respond and survive in the stomach with low-pH (Foster, 1995), and to reach M cells and enterocytes in the small intestine (Takeuchi, 1967; Moulder, 1985; Lindquist et al., 1987; Nietfeld et al., 1992; Clark et al., 1994, 1996; Jones et al., 1994; Sansonetti and Phalipon, 1999). Salmonella Typhimurium has the ability to enter non-phagocytic eukaryotic cells and to exist as intracellular parasites inside enclosed vacuoles (Takeuchi, 1967; Moulder, 1985). The intracellular environment provides a unique niche for the bacteria to multiply and evade host immune responses. In addition, S. Typhimurium is capable of surviving and replicating within macrophages (Buchmeier and Heffron, 1989).
Several studies have led to the identification of genes that are required for Salmonella pathogenesis, in particular for Salmonella invasion into non-phagocytic cells (Galán and Curtiss, 1989; Ochman et al., 1996; Shea et al., 1996; Blanc-Potard and Groisman, 1997; Wong et al., 1998; Wood et al., 1998). Many of these virulence genes and operons are located in large genetic elements of the Salmonella chromosome. Since these large elements are absent from the chromosome of closely related Escherichia coli, they are termed pathogenicity islands. Virulence plasmids also contribute to Salmonella survival in macrophages and virulence (Gulig, 1990; Guiney et al., 1994; Wallis et al., 1995). At least five pathogenicity islands have been identified in Salmonella (Galán and Curtiss, 1989; Ochman et al., 1996; Shea et al., 1996; Blanc-Potard and Groisman, 1997; Wong et al., 1998; Wood et al., 1998) that contribute to virulence at defined stages of the infection process. Salmonella Pathogenicity Island I (SPI1) is the best studied one. It is located at centisome 63 on the Salmonella chromosome and is 43-kb in length. SPI1 is required for Salmonella entry into M cells (Clark et al., 1996) and epithelial cells (Galán and Curtiss, 1989) of the intestine. This is consistent with the fact that SPI1 mutants are defective in virulence when administered orally, but not if given systematically (Galán and Curtiss, 1989). Mutants that are defective in entry into epithelial cells were found to be avirulent in studies using the mouse-typhoid model (Galán and Curtiss, 1989) and in calves (Watson et al., 1998; Tsolis et al., 1999, 2000). SPI2, SPI3, and SPI4 are situated at centisome 31, 82, and 92 of the Salmonella chromosome, respectively. Genes in these three islands are essential for Salmonella survival and growth in the host (Hensel et al., 1997, 1998; Shea et al., 1999; Vazquez-Torres et al., 2000). SPI5 was originally found to be involved in inflammation and fluid secretion in the intestine (Norris et al., 1998; Wood et al., 1998). We have shown that at least one gene in this island (sopB) is also involved in the Salmonella invasion process (Galan and Zhou, 2000).
SPI1 and SPI2 encode specialized protein secretion and translocation systems termed type III secretion system. Genes in SPI1 can be divided into three groups: (1) one includes genes that encode the actual secretion/translocation apparatus; (2) a second group encodes proteins that are secreted and/or translocated into host cells; (3) a third group involves in gene regulation. The SPI1 secretion apparatus was shown by electron microscopy (Kubori et al., 1998) that appears to constitute a “needle complex” that is similar to the bacterial flagella system both biochemically and structurally. Purified needle complexes consist of at least three proteins encoded in SPI1 (PrgK, PrgH, and InvG). Mutations in prgK, prgH, or invG have been shown to abolish the secretion of a panel of S. Typhimurium proteins (SipA, SipB, SipC etc.). The translocation of these bacterial proteins into eukaryotic host cells is required for Salmonella invasion into non-phagocytic epithelial cells. Secretion has been reported to require host-cell contact (Zierler and Galán, 1995). However, these proteins are secreted under certain laboratory conditions in sufficient amounts to facilitate their studies in the absence of host cells. These secreted proteins can be visualized by SDS-PAGE from supernatants of S. Typhimurium cultures under such inducing conditions. At least nine secreted proteins have been identified using this approach, including: AvrA, SipA, SipB, SipC, SipD, SopE, SopE2, SopB, and SptP. During the infection process, these proteins are thought to be translocated inside the host cell, where they engage host cell components to promote bacterial uptake (Galán, 1998, 1999).
Successful Salmonella infection requires the bacteria to gain a growth advantage over the intestinal microflora while inducing intestinal inflammation. Although it remains a challenge to understand how Salmonella achieve this in the gut, several recent studies have shed light on the molecular mechanisms that Salmonella use. It has been reported that the long O-antigen chain in Salmonella conferred a growth advantage in the mouse colitis model (Crawford et al., 2012). It was proposed that Salmonella-induced colitis increased the luminal concentrations of total bile acids and fepE-mediated (O-antigen assembly) bile acid resistance is responsible for conferring a fitness advantage during luminal growth in the inflamed intestine (Crawford et al., 2012). Furthermore, in search for additional signals generated during Salmonella-induced inflammation, the methyl-accepting chemotaxis proteins (MCPs) including Trg, Tsr, and Aer, were identified to enhance the fitness of Salmonella in a mouse colitis model (Rivera-Chávez et al., 2013). Thus, it is becoming apparent that Salmonella utilize their virulence factors to induce inflammation and to generate inflammation-derived nutrients to edge out competing microbes in the inflamed intestine (Rivera-Chávez and Báumler, 2015). Furthermore, Salmonella are capable of using inflammation-derived nitrate to respire anaerobically and to compete with the commensal microbes in the gut using three nitrate reductases, encoded by the narGHI, narZYV, and napABC genes (Lopez et al., 2015). A recent study also demonstrated that the disturbance of the commensal Clostridia increased the susceptibility to Salmonella infection in a mouse model (Rivera-Chávez et al., 2016). This is largely due to the decreased butyrate levels, produced from the butyrate-producing Clostridia, led to increased oxygenation in the gut, promoting the aerobic expansion of Salmonella (Rivera-Chávez et al., 2016).
Many experimental models have been developed to study Salmonella infections including various in vitro, ex vivo, and in vivo models (Table 1). The most commonly used ones are the two-dimensional (2-D) immortalized cell line models including the Caucasian colon adenocarcinoma (Caco2) cells (Martinez-Argudo and Jepson, 2008), the immature human normal fetal intestinal epithelial cells (H4) (Newburg et al., 2016), the mature human metastatic colonic epithelial cells (T84) (Newburg et al., 2016), the human normal colon mucosal epithelial cells (NCM-460) (Newburg et al., 2016), the Microfold cells (or M cells) (Martinez-Argudo and Jepson, 2008), the RAW 264.7 murine macrophage cells (Tang et al., 2012), the cervical cancer (HeLa) cells (Fang et al., 2017), and the gut fermentation models (Le Blay et al., 2009). In addition, three-dimensional (3-D) organotypic models derived from the Int-407 cell line (later found to be a HeLa derivative) and the human colorectal adenocarcinoma cells (HT-29) (Nickerson et al., 2001; Höner Zu Bentrup et al., 2006). Salmonellosis in humans, monkeys, and calves are known to affect primarily the distal ileum and the proximal colon (Kinsey et al., 1976; Giannella et al., 1977; Wray and Sojka, 1978; McGovern and Slavutin, 1979; Samuel et al., 1980). These models have greatly aided the genetic, cell biological and biochemical analysis of the infection process.
In vitro cell culture lines are relatively easy to maintain and provide a more consistent environmental niche for evaluating bacterial survival and replication than most animal hosts (Finlay and Brumell, 2000). Genetic manipulations in these cell lines greatly aided the investigation of how Salmonella interact with host epithelial and macrophage cells (Finlay and Brumell, 2000; Zhou, 2001, 2006; Zhou and Galán, 2001). However, immortalized cell lines lack the complexity of cell types and the robust immune components, thus cannot closely mimic the natural infection process (Finlay and Brumell, 2000). For instance, the apoptosis process is regulated differently in immortalized cells comparing to that of healthy tissues (Finlay and Brumell, 2000). In addition, many mammalian cells cannot sustainably maintain their original characteristics during the long culturing process (Finlay and Brumell, 2000). For example, derivative cells may arise (Foulke-Abel et al., 2014). The 2-D cultures of immortalized cells only have one cell type, making it difficult to mimic complex architecture in the mucosa in vivo (Yin et al., 2015).
The lack of suitable models for testing effects of antimicrobials on enteropathogens hampers the development of novel antimicrobials combating Salmonella infections. Commonly used animal models cannot reproduce microbiota residing in the human intestine, and most continuous models for human intestinal microbiota have limitations on the microbial diversity, stability, cell density, and lack the ability to support long-term studies. An in vitro continuous colonic fermentation model has been developed to allow the bacteria to grow in biofilm structures, facilitating tests of new antimicrobials against Salmonella infections (Le Blay et al., 2009). This model uses immobilized child fecal microbiota and the introduction of Salmonella for the proximal colon to produce high bacterial density in gel beads and in reactor effluents. The growth conditions allow the protection of sensitive bacteria from shear, oxygen stress, and limit the washout and loss of less competitive bacteria. Le Blay et al. successfully used this model to examine the effects of two antibiotics on Salmonella and on the dynamic change of microbiota. Their result is consistent with in vivo data validating the fermentation model as a promising model platform for development of new antimicrobials against Salmonella.
Fully differentiated, functional intestinal epithelia in vivo possess unique organization of junctional, extracellular matrix, and brush border proteins, as well as highly localized mucin production (Höner Zu Bentrup et al., 2006). To mimic the 3-D architectural organization of the intestinal epithelia, a 3-D organotypic model has been developed to better recapitulate the characteristics associated with intestinal epithelia in vivo (Nickerson et al., 2001; Höner Zu Bentrup et al., 2006). This organotypic model uses RWV bioreactor based monolayer cultures of Int-407 or HT-29 cells. In contrast to the 2-D cells, the 3-D organotypic model has better organization of junctional, extracellular matrix, brush-border proteins, and highly localized mucin production (Höner Zu Bentrup et al., 2006). However, the 3-D organotypic model do not contain niches of the normal stem cells, which are responsible for renewing the intestinal tissues. To circumvent these shortcomings, ex vivo tissue culture models including the calf ileal epithelium model (Frost et al., 1997), the human intestinal in vitro organ culture (IVOC) model (Haque et al., 2004), the ex vivo intestinal mucosa model (Tsilingiri et al., 2012), and the ex vivo immature human intestinal tissue model (Newburg et al., 2016) have been developed to more closely mimic the surroundings of the organ during infection (Table 1). Despite the close resemblance of these ex vivo models to the clinical situation in the gut, their short lifetimes, laborious setups, wide experimental variabilities, limited availability of cells, and limited numbers of cells have hampered their potential use in the study of Salmonella infections (Höner Zu Bentrup et al., 2006).
Animal models are often used to explore the virulence mechanisms of Salmonella infections (Santos et al., 2001). Animals possess the complex cell types, architectural organizations, and specialized organ structures. More importantly, the intact immune systems of the animals have obvious advantages over all other models and therefore are considered the closest to clinical settings over in vitro cell or ex vivo organ and tissue models. C57BL/6 (Barthel et al., 2003; Ren et al., 2009) and BALB/c mice (Özkaya et al., 2012) are most commonly used for Salmonella infections. The rabbit ligated ileal loop model is used as an in vivo model for studying Salmonella infections (Giannella et al., 1973). These non-human animal models, including primates, only partially mirror the human disease process due to their inherent differences from humans (Hurley and McCormick, 2003; Firoz Mian et al., 2011). To circumvent this limitation, “humanized” mice have been developed as alternative platforms to study human infectious diseases (Legrand et al., 2009). These mice are transplanted with human cells or tissues representing confined human environments suitable for infectious agents. For example, humanized mice were generated by engrafting human hematopoietic stem cells into immunocompromised mice. These mice were used to study S. enterica Serovar Typhi which is usually restricted to infect only humans (Firoz Mian et al., 2011). However, the high cost of humanized mice hampers its wide application.
The term “enteroids” refer to multilobulated structures with a lumen that develops from intestinal stem cells (cycling crypt base columnar cells and quiescent stem cells) near the bottom of the intestinal crypts (also termed the intestinal stem cell niche), or single intestinal stem cells by formation of budding crypts (Stelzner et al., 2012). They generate the in vivo architecture and multi-lineage differentiation of the original intestinal epithelium in mammals (Dutta et al., 2017). They were first generated from mouse intestinal stem cells by Drs. Clevers and Sato at the Hubrecht institute (Utrecht, Netherlands) (Sato et al., 2009). Subsequently, human enteroids were successfully cultured by the same group (Sato et al., 2011). The growth of these intestinal stem cells is regulated mainly by Wnt, Notch, epidermal growth factors (EGFs), and the bone morphogenetic proteins (BMPs) signaling pathways in vivo (Sato and Clevers, 2013). Wnt and Notch signaling pathways play major roles in the proliferation of stem cells. EGF signals exert the robust mitogenic effects on stem cells via their corresponding receptors (EGFRs). BMP has an inhibitory effect on the stemness. Noggin promotes crypt like structures to form along the flanks of the villi (Sato and Clevers, 2013). To generate enteroids, the intestinal crypts containing the intestinal stem cell niches are separated from intestinal tissues by EDTA treatment. These crypts are then embedded in Matrigel, followed by supplementing with stemness supporting factor cocktails such as EGF, R-spondin-1, Noggin, and Wnt3a. The crypts will gradually develop into 3-D enteroids displaying many important organizations of the normal intestinal epithelium (Figures 1A,B).
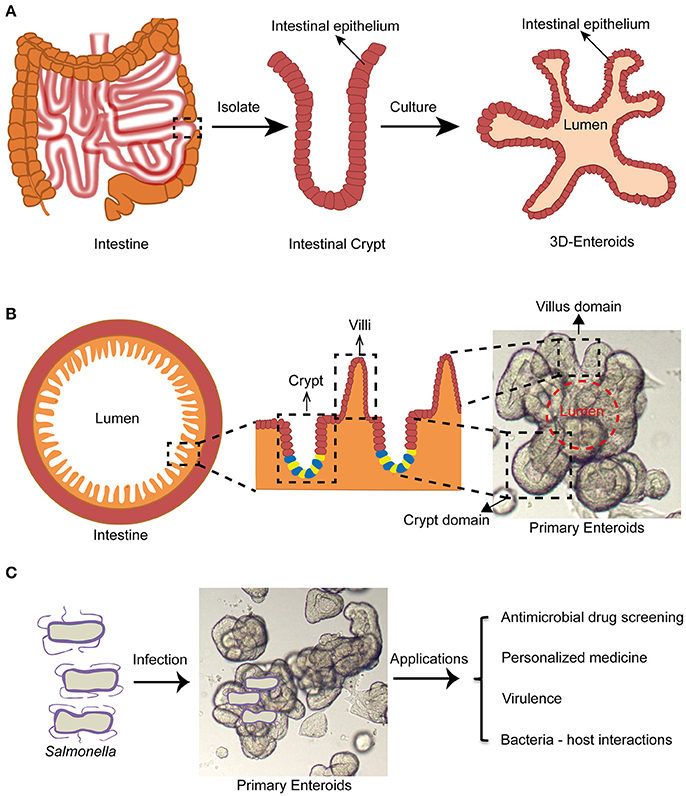
Figure 1. Enteroid model and its potential application in studying Salmonella infections. (A) Intestinal crypts can be isolated from surgery sections or biopsies, followed by culturing into 3-D enteroids. (B) Enteroids recapitulate architectures of healthy intestine containing villus and crypt domains. (C) Enteroids may be a promising experimental model for studying Salmonella infections for antimicrobial drug screening, personalized medicine, virulence mechanisms and bacterial-host interactions.
Enteroids have many advantages over traditional cell culture models. For example, they can be ever-expanding, and retain their original organ identity (Sato and Clevers, 2013). Karyotypings are usually done for long term cultures and demonstrated genetic stability of the enteroids after more than 15 generations (Yin et al., 2014). Enteroids also contain luminal layers with crypt and villus domains similar to the real intestine (Figure 1B). They contain almost all intestinal epithelial cell types including the intestinal stem cells, Paneth cells, Goblet cells, enteroendocrine cells, and enterocytes (Sato and Clevers, 2013). It was reported that certain specific intestinal cell types such as tuft cells or Peyer's patch M cells can be differentiated from intestinal stem cells in the enteroids by supplementing the media with rIL-4/rIL-13 or Tnfsf11 (RankL), respectively (de Lau et al., 2012; Gerbe et al., 2016).
Induced intestinal organoids with a lumen resembling the intestine could also be generated from pluripotent stem cells (PSCs) under specific culture conditions (Stelzner et al., 2012; Dutta et al., 2017). Induced intestinal organoids from PSCs have intestinal epithelium and mesenchyme (Figure 3). Meanwhile, induced intestinal organoids contain most cell types that are present in the human intestine (i.e., the polarized monolayer of epithelial cells with clear apical and basal sides, microvilli, Paneth, goblet, and enteroendocrine cells, etc.) with villi domain and crypt domain (Forbester et al., 2015). It takes a few weeks for induced intestinal organoids to “mature” (Yin et al., 2015). In contrast, enteroids derived from adult stem cells (ASCs) need only several days to grow into a 3-D structure with remarkable villi domains and crypt domains resembling the in vivo intestinal tissue (Yin et al., 2015). In addition, primary enteroids are often taken from individuals with underlying pathological conditions. Usually, the “healthy” tissues adjacent to the diseased tissue may carry pathological changes and influence the outcome of infections.
The 3-D architecture of enteroids is believed to mimic that of an intact tissue in vivo. Typical 3-D enteroids develop both the apical side and basolateral side situated properly toward the lumen and the outside of enteroids (Figure 2). This organization poses a challenge to deliver the infecting bacteria to the lumen from outside of enteroids (In et al., 2016). In most cases the main site of infection is the polarized epithelium lining the intestinal lumen (Martinez-Argudo and Jepson, 2008). However, the 3-D architecture of the enteroids limited access of pathogens to the luminal epithelial surface. To overcome this, microinjection has been used to deliver enteric pathogen (e.g., mouse adenovirus 2, MAdV-2) to the lumen of enteroids (Wilson et al., 2017). Despite its remarkable resemblance to tissues/organs, enteroids can be experimentally manipulated similar to classical cell lines. These include PCR, qPCR, Western blot, immunohistochemistry (IHC), lentivirus transduction, and CRISPR/Cas9 gene editing (Miyoshi and Stappenbeck, 2013; Van Lidth de Jeude et al., 2015; Yin et al., 2015; Driehuis and Clevers, 2017). This advantage has facilitated the rapid application of enteroids.
3-D enteroids can be substituted with 2-D monolayers on top of membranes inside transwells due to the obvious time and cost considerations. In this setup, transwells provide access to the apical surfaces of 2-D monolayers (Figure 2) (Wang et al., 2017). The 2-D monolayers have been widely used to study host-pathogen interactions in a more controllable and reproducible manner comparing to that of 3-D enteroids (Wang et al., 2017). However, 2-D cultures can be maintained for only a short period of time and are suitable for studying initial pathogen/host interactions that last a few hours. In contrast, 3D cultures are better suited for examining long-term host-pathogen interactions. In addition, 3D cultures are uniquely suited for modeling the contribution of the lumen (e.g., anti-microbial response, reactive oxygen species, etc.), since they have been shown to tolerate bacteria for days without obvious tissue damage (Wilson et al., 2017).
Salmonella infection is a dynamic process in which the bacteria encounter many cell types and organs. Proper in vitro models are essential for unraveling the pathogenic determinants functioning at various stages of the infection, and for developing new antimicrobials against Salmonella infections (Le Blay et al., 2009). Certain Salmonella serovars are restricted to human hosts or cause different diseases depending on whether infecting animals or humans. One such example is Salmonella Typhimurium (Forbester et al., 2015), which causes gastroenteritis in humans, but typhoid-like disease in mice, making it complicated to interpret data obtained from mouse experiments. Although many cell lines have been established to study Salmonella, the cancer-derived models cannot recapitulate the complex architecture of the intestine and might have different physiological characteristics compared to normal tissues.
The iHOs have been demonstrated to be a promising infection model for Salmonella, and microinjection is usually used to inoculate Salmonella into the lumen of iHOs (Forbester et al., 2015). The iHOs need 1–2 months to “mature” before it is suitable for experimentation and the 3-D epithelial structure is surrounded by mesenchyme (Figure 3; Yin et al., 2015). In contrast, enteroids are easier to setup and mature in 4–7 days (Figure 3).
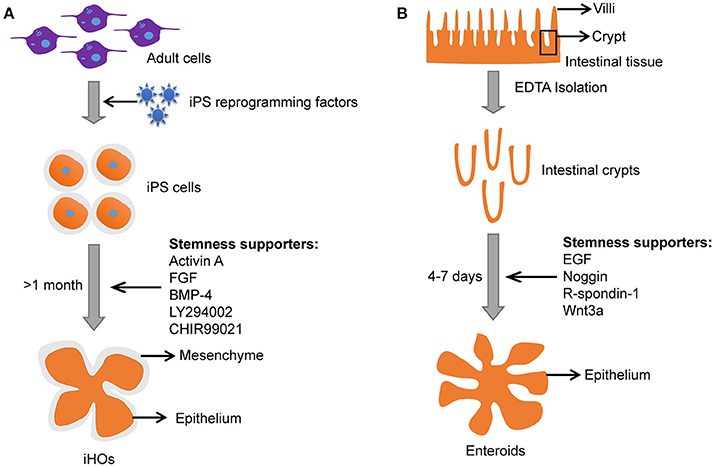
Figure 3. Comparison between iHOs and primary enteroids. (A) Culture process and features of iHOs. (B) Culture process and features of primary enteroids.
Salmonella infections induce intestinal inflammatory responses involving neutrophil infiltrations and the production of pro-inflammatory cytokines. It has been shown that cytokine secretions were enhanced in monolayer and 3-D organotypic models of human colonic epithelium (HT-29) as well as in the C57BL/6 mouse model upon Salmonella infection (Höner Zu Bentrup et al., 2006; Ren et al., 2009). Furthermore, patients infected by Salmonella display gamma interferon (IFN-γ) induction together with elevated tumor necrosis factor alpha (TNF-α) (Gal-Mor et al., 2012). Using crypt-derived mouse enteroids, Zhang et al. were able to reproduce the Salmonella-induced inflammatory responses (Zhang Y. G. et al., 2014). Furthermore, Salmonella infection induced the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway in the mouse enteroids accompanied by the expression of inflammatory cytokines including TNF-α and IFN-γ (Zhang K. et al., 2014). This is in line with results from Salmonella infection of iHOs where genes encoding proinflammatory cytokines were upregulated (Forbester et al., 2015). Moreover, Salmonella infection significantly decreased the expression of intestinal stem cell markers, Lgr5 and Bmi 1 (Forbester et al., 2015). The significance and mechanism behind this decrease require further investigation using the enteroids model.
Invasion of the intestinal epithelium is an essential step for Salmonella virulence (Galán and Curtiss, 1989; Galan and Zhou, 2000). A study using the crypt-derived mouse enteroids (6 days after passage) showed that Salmonella quickly attached and invaded the enteroids (Zhang Y. G. et al., 2014) accompanied by the typical morphologic changes of the host cells during Salmonella invasion as well as the disruption of epithelial tight junctions (Finlay et al., 1991; Galan and Zhou, 2000; Zhang Y. G. et al., 2014). It is further shown that wild type Salmonella strains microinjected into the lumen of iHOs are able to invade the epithelial layer, and continue to traffic inside the Salmonella-containing vacuoles. In contrast, a Salmonella invA mutant, defective in the Salmonella pathogenicity island 1 invasion apparatus, was less capable of invading the iHO epithelium (Forbester et al., 2015). Furthermore, mouse enteroids were utilized to study the survival and replication of Salmonella and found that naturally secreted α-defensins by Paneth cells in the lumen suppressed the growth of Salmonella (Wilson et al., 2015). This finding is in agreement with data obtained when using ex vivo intestinal crypts and villus (Ayabe et al., 2000).
Chronic Salmonella Typhi infections are one of the reported risk factors for Gallbladder carcinoma (GBC) (Wistuba and Gazdar, 2004). A recent study showed that Salmonella infections induced malignant transformation in murine gallbladder organoids (Scanu et al., 2015). Interestingly, Scanu et al. found that Salmonella-mediated activation of mitogen-activated protein kinase (MAPK) and protein kinase B (PKB or AKT) pathways is responsible for the transformation. Importantly, the result from murine gallbladder organoids is in agreement with observations in GBC patients (Scanu et al., 2015). Collectively, both iHOs and enteroids have been successfully used for dissecting Salmonella pathogenesis. Enteroids may provide a promising experimental platform to investigate Salmonella infections for antimicrobial drug screening and personalized medicine.
Conventional 2-D monolayer cultures have greatly aided the advancement of our understanding of host-pathogen interactions despite their limitations in single cell type and being tumor-derived (Duell et al., 2011). It is known that different pathogens may preferentially infect a subset of cell types and could exploit different host molecules to promote their infections. In addition, many biological processes that drive immune responses against pathogens are difficult if not impossible to mimic using just monolayer cell line cultures (Duell et al., 2011). Many infectious agents have been shown to infect enteroids (Table 2), including parasites (Gerbe et al., 2016), rotavirus (Yin et al., 2015), norovirus (Ettayebi et al., 2016), Enterohemorrhagic Escherichia coli (In et al., 2016), and Salmonella (Zhang Y. G. et al., 2014; Scanu et al., 2015; Wilson et al., 2015).
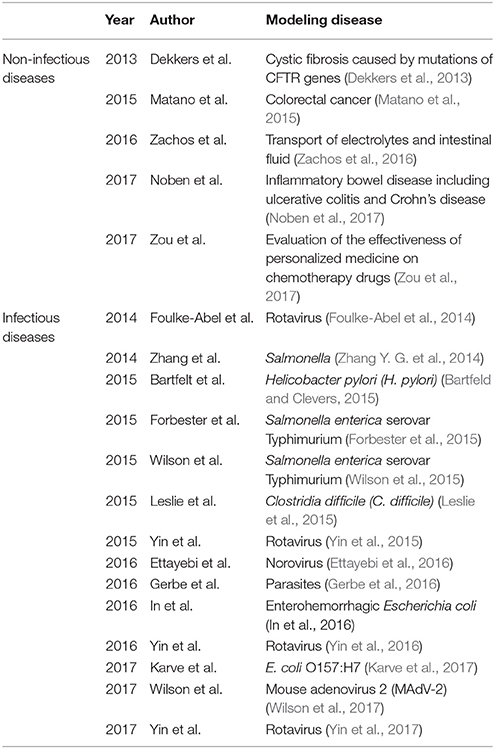
Table 2. Various intestinal physiology and disease processes have been modeled by organoids and enteroids.
Rotavirus is the leading cause of gastroenteritis and diarrhea in worldwide. Rotavirus is known to target the human intestinal epithelial cells and was shown to infect human enteroids (Foulke-Abel et al., 2014; Yin et al., 2015). This significant advance overcame the fact that human rotavirus replicates poorly in transformed cell lines. In addition, many animal models have limited use due to host range restrictions of rotavirus. It was shown that rotavirus infections led physiological lumenal expansion, a hallmark of rotavirus-induced diarrhea (Saxena et al., 2015). Laboratory adapted rotavirus strains and patient derived isolates were able to infect both mouse and human enteroids. Interestingly, human enteroids were more susceptible to human rotavirus infections than mouse enteroids (Yin et al., 2015). Moreover, patient derived rotavirus showed different infectivity in response to commonly used antiviral drugs including ribavirin and IFNα from that of the laboratory adapted rotavirus when infecting human enteroids (Yin et al., 2015). Rotavirus also induced less pronounced expression of genes involved in innate immune responses (interferon stimulated genes, ISGs) in enteroids than that in Caco2 cells (Yin et al., 2015). Consistent with clinical patient data, the antiviral effect of IFNα was less in enteroids as compared to that in Caco2 cells (Yin et al., 2015). Recently, enteroids developed from transgenic mice have been successfully used to characterize NOD-like receptor (NLR) Nlrp9b inflammasome-mediated rotavirus restriction in intestinal epithelial cells in mice (Zhu et al., 2017). Therefore, enteroids derived from a variety of transgenic mice highlight their potential to contribute to the understanding of molecular mechanisms during intestinal epithelial cell infections.
Norovirus is another enteric virus causes severe gastroenteritis in both infants and adults (Ettayebi et al., 2016). It is known that cultured cell lines do not support the replication of norovirus (Papafragkou et al., 2014). 3-D intestinal model derived from INT-407 cells and Caco2 cells also failed to facilitate norovirus replication (Papafragkou et al., 2014). In contrast, enteroids were shown to support norovirus infection and clear cytopathic effects (CPE) and viral particles were observed upon norovirus infection. The viral replication was even more pronounced by adding bile to the growth media. Human enteroids were also recently reported to be used to examine roles of secreted alpha-defensins during infection by mouse adenovirus 2 (Wilson et al., 2017).
In addition to viral studies, enteroids have been used successfully to explore bacterial pathogeneses besides Salmonella. Enterohemorrhagic Escherichia coli (EHEC) cause foodborne diseases in both developing and industrialized countries (In et al., 2016). Recently, human enteroids with the addition of human neutrophils were established to study E. coli O157:H7 infections (Karve et al., 2017). In the lumen of enteroids, pathogenic O157:H7 replicated rapidly while commensal E. coli did not (Karve et al., 2017). Interestingly, O157:H7 infections promoted the recruitment of human neutrophils (Karve et al., 2017). Furthermore, enteroids provided a unique model to study the interactions between the gut microbiota, enteric pathogens, and the host intestinal environment (Nigro et al., 2016).
Clostridium difficile (C. difficile), a Gram-positive obligate anaerobic bacteria ubiquitous in nature, infects the human colon in 2–5% of the adult population. Imbalance of the normal gut flora could increase the chances of Clostridium infection. The bacteria may produce diarrhea and inflammation in infected patients via the well characterized enterotoxin (C. difficile toxin A) and cytotoxin (C. difficile toxin B). IHIOs derived from human pluripotent stem cells were used to study C. difficile and the contribution of toxins. It was shown that the toxins played a major role in the colonization and disruption of the iHIO epithelium, and in the loss of the paracellular barrier function (Leslie et al., 2015).
Helicobacter pylori (H. pylori) colonization of the human stomach has been associated with chronic gastritis, ulceration, and adenocarcinoma. To study the pathogenesis of H. pylori infection, human gastric organoids were generated from surgical samples of human gastric corpus. The gastric organoids displayed the typical characteristics of their corresponding tissues, based on their histology, expression of markers, and euploidy (Bartfeld et al., 2015). This system has the potential to be used to study other gastric pathologies in addition to H. pylori infection.
One exciting potential use of patient-derived enteroids is to evaluate the effectiveness of personalized medicine, such as precision chemotherapy for cancer patients. Roy et al. successfully evaluated the effects of Mitomycin-C, 5-Fluorouracil (5-FU), Irinotecan, Oxaliplatin, Doxorubicin, and Paclitaxel on peritoneal metastases using enteroids (Roy et al., 2017). Enteroids have also been used to model various intestinal physiology and disease processes (Table 2). Human enteroids have been used to study the transport of electrolytes and intestinal fluid using luminal dilatation assay (Zachos et al., 2016). Enteroids have also contributed to the understanding of cystic fibrosis caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) using a swelling assay (Dekkers et al., 2013). Moreover, enteroids have been used to model colorectal cancer and inflammatory bowel disease including ulcerative colitis and Crohn's disease (Matano et al., 2015; Young and Reed, 2016; Noben et al., 2017). Finally, the potential transplantation of enteroids into patients with intestinal failure (IF), a life-threatening condition, further expanded the possibility of using enteroids to treat patients (Hong et al., 2017). Taken together, enteroids are emerging as robust infection models for study both viral and bacterial infections and may play a key role in the development of personalized medicine to aid the treatment of human infections and diseases.
Salmonellosis remains a major public health concern globally. The availability of various infection models has helped to identify bacterial virulence factors responsible for causing the diseases. Studies utilizing these infection models have advanced our understanding of how pathogens deploy their virulence factors to modulate host cell functions during infection. Classical infection models include various 2-D cultures of immortalized cells and animal models. Recent advances in stem cell research have helped to establish organoids and enteroids as viable alternatives to many established infection models. We anticipate that organoids and enteroid infection models will play a key role in advancing out understanding in antimicrobial drug screening, personalized medicine, virulence mechanisms and pathogen-host interactions.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ayabe, T., Satchell, D. P., Wilson, C. L., Parks, W. C., Selsted, M. E., and Ouellette, A. J. (2000). Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1, 113–118. doi: 10.1038/77783
Barrila, J., Radtke, A. L., Crabbe, A., Sarker, S. F., Herbst-Kralovetz, M. M., Ott, C. M., et al. (2010). Organotypic 3D cell culture models: using the rotating wall vessel to study host-pathogen interactions. Nat. Rev. Microbiol. 8, 791–801. doi: 10.1038/nrmicro2423
Bartfeld, S., Bayram, T., Van De Wetering, M., Huch, M., Begthel, H., Kujala, P., et al. (2015). In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148, 126–136 e126. doi: 10.1053/j.gastro.2014.09.042
Bartfeld, S., and Clevers, H. (2015). Organoids as model for infectious diseases: culture of human and murine stomach organoids and microinjection of Helicobacter pylori. J. Vis Exp. doi: 10.3791/53359. [Epub ahead of print].
Barthel, M., Hapfelmeier, S., Quintanilla-Martínez, L., Kremer, M., Rohde, M., Hogardt, M., et al. (2003). Pretreatment of mice with streptomycin provides a Salmonella enterica Serovar Typhimurium Colitis Model that allows analysis of both pathogen and host. Infect. Immun. 71, 2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003
Blanc-Potard, A. B., and Groisman, E. A. (1997). The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 16, 5376–5385. doi: 10.1093/emboj/16.17.5376
Boyle, E. C., Bishop, J. L., Grassl, G. A., and Finlay, B. B. (2007). Salmonella: from pathogenesis to therapeutics. J. Bacteriol. 189, 1489–1495. doi: 10.1128/JB.01730-06
Boyle, E. C., Dombrowsky, H., Sarau, J., Braun, J., Aepfelbacher, M., Lautenschläger, I., et al. (2015). Ex vivo perfusion of the isolated rat small intestine as a novel model of Salmonella enteritis. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G55–G63. doi: 10.1152/ajpgi.00444.2014
Brenner, F. W., Villar, R. G., Angulo, F. J., Tauxe, R., and Swaminathan, B. (2000). Salmonella nomenclature. J. Clin. Microbiol. 38, 2465–2467.
Buchmeier, N. A., and Heffron, F. (1989). Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect. Immun. 57, 1–7.
Clark, M. A., Jepson, M. A., Simmons, N. L., and Hirst, B. H. (1994). Preferential interaction of Salmonella typhimurium with mouse Peyer's patch M cells. Res. Microbiol. 145, 543–552. doi: 10.1016/0923-2508(94)90031-0
Clark, M. A., Reed, K. A., Lodge, J., Stephen, J., Hirst, B. H., and Jepson, M. A. (1996). Invasion of murine intestinal M cells by Salmonella typhimurium inv mutants severely deficient for invasion of cultured cells. Infect. Immun. 64, 4363–4368.
Coburn, B., Grassl, G. A., and Finlay, B. B. (2007). Salmonella, the host and disease: a brief review. Immunol. Cell Biol. 85, 112–118. doi: 10.1038/sj.icb.7100007
Crawford, R. W., Keestra, A. M., Winter, S. E., Xavier, M. N., Tsolis, R. M., Tolstikov, V., et al. (2012). Very long O-antigen chains enhance fitness during Salmonella-induced colitis by increasing bile resistance. PLoS Pathog. 8:e1002918. doi: 10.1371/journal.ppat.1002918
Crump, J. A., Sjölund-Karlsson, M., Gordon, M. A., and Parry, C. M. (2015). Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin. Microbiol. Rev. 28, 901–937. doi: 10.1128/CMR.00002-15
de Lau, W., Kujala, P., Schneeberger, K., Middendorp, S., Li, V. S., Barker, N., et al. (2012). Peyer's patch M cells derived from Lgr5(+) stem cells require SpiB and are induced by RankL in cultured “miniguts”. Mol. Cell. Biol. 32, 3639–3647. doi: 10.1128/MCB.00434-12
Dekkers, J. F., Wiegerinck, C. L., De Jonge, H. R., Bronsveld, I., Janssens, H. M., De Winter-De Groot, K. M., et al. (2013). A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 19, 939–945. doi: 10.1038/nm.3201
Dostal, A., Gagnon, M., Chassard, C., Zimmermann, M. B., O'mahony, L., and Lacroix, C. (2014). Salmonella adhesion, invasion and cellular immune responses are differentially affected by iron concentrations in a combined in vitro gut fermentation-cell model. PLoS ONE 9:e93549. doi: 10.1371/journal.pone.0093549
Driehuis, E., and Clevers, H. (2017). CRISPR/Cas 9 genome editing and its applications in organoids. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G257–G265. doi: 10.1152/ajpgi.00410.2016
Duell, B. L., Cripps, A. W., Schembri, M. A., and Ulett, G. C. (2011). Epithelial cell coculture models for studying infectious diseases: benefits and limitations. J. Biomed. Biotechnol. 2011:852419. doi: 10.1155/2011/852419
Dutta, D., Heo, I., and Clevers, H. (2017). Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med. 23, 393–410. doi: 10.1016/j.molmed.2017.02.007
Eng, S.-K., Pusparajah, P., Ab Mutalib, N.-S., Ser, H.-L., Chan, K.-G., and Lee, L.-H. (2015). Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 8, 284–293. doi: 10.1080/21553769.2015.1051243
Ettayebi, K., Crawford, S. E., Murakami, K., Broughman, J. R., Karandikar, U., Tenge, V. R., et al. (2016). Replication of human noroviruses in stem cell-derived human enteroids. Science 353, 1387–1393. doi: 10.1126/science.aaf5211
Fang, S. B., Huang, C. J., Huang, C. H., Wang, K. C., Chang, N. W., Pan, H. Y., et al. (2017). speG Is required for intracellular replication of Salmonella in various human cells and affects its polyamine metabolism and global transcriptomes. Front. Microbiol. 8:2245. doi: 10.3389/fmicb.2017.02245
Fierer, J., and Guiney, D. G. (2001). Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J. Clin. Invest. 107, 775–780. doi: 10.1172/JCI12561
Finlay, B. B., and Brumell, J. H. (2000). Salmonella interactions with host cells: in vitro to in vivo. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 355, 623–631. doi: 10.1098/rstb.2000.0603
Finlay, B. B., Ruschkowski, S., and Dedhar, S. (1991). Cytoskeletal rearrangements accompanying Salmonella entry into epithelial-cells. J. Cell Sci. 99, 283–293.
Firoz Mian, M., Pek, E. A., Chenoweth, M. J., and Ashkar, A. A. (2011). Humanized mice are susceptible to Salmonella typhi infection. Cell. Mol. Immunol. 8, 83–87. doi: 10.1038/cmi.2010.52
Forbester, J. L., Goulding, D., Vallier, L., Hannan, N., Hale, C., Pickard, D., et al. (2015). Interaction of Salmonella enterica Serovar Typhimurium with intestinal organoids derived from human induced pluripotent stem cells. Infect. Immun. 83, 2926–2934. doi: 10.1128/IAI.00161-15
Foster, J. W. (1995). Low pH adaptation and the acid tolerance response of Salmonella typhimurium. Crit. Rev. Microbiol. 21, 215–237. doi: 10.3109/10408419509113541
Foulke-Abel, J., In, J., Kovbasnjuk, O., Zachos, N. C., Ettayebi, K., Blutt, S. E., et al. (2014). Human enteroids as an ex-vivo model of host-pathogen interactions in the gastrointestinal tract. Exp. Biol. Med. (Maywood) 239, 1124–1134. doi: 10.1177/1535370214529398
Frost, A. J., Bland, A. P., and Wallis, T. S. (1997). The early dynamic response of the calf ileal epithelium to Salmonella typhimurium. Vet. Pathol. 34, 369–386. doi: 10.1177/030098589703400501
Galán, J. E. (1998). Interactions of Salmonella with host cells: encounters of the closest kind. Proc. Natl. Acad. Sci. U. S. A. 95, 14006–14008. doi: 10.1073/pnas.95.24.14006
Galán, J. E. (1999). Interaction of Salmonella with host cells through the centisome 63 type III secretion system. Curr. Opin. Microbiol. 2, 46–50. doi: 10.1016/S1369-5274(99)80008-3
Galán, J. E., and Curtiss, R. III. (1989). Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. U.S.A. 86, 6383–6387. doi: 10.1073/pnas.86.16.6383
Galan, J. E., and Zhou, D. (2000). Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc. Natl. Acad. Sci. U.S.A. 97, 8754–8761. doi: 10.1073/pnas.97.16.8754
Gal-Mor, O., Suez, J., Elhadad, D., Porwollik, S., Leshem, E., Valinsky, L., et al. (2012). Molecular and cellular characterization of a Salmonella enterica serovar Paratyphi a outbreak strain and the human immune response to infection. Clin. Vaccine Immunol. 19, 146–156. doi: 10.1128/CVI.05468-11
Gerbe, F., Sidot, E., Smyth, D. J., Ohmoto, M., Matsumoto, I., Dardalhon, V., et al. (2016). Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230. doi: 10.1038/nature16527
Giannella, R. A., Formal, S. B., Dammin, G. J., and Collins, H. (1973). Pathogenesis of salmonellosis. Studies of fluid secretion, mucosal invasion, and morphologic reaction in the rabbit ileum. J. Clin. Invest. 52, 441–453. doi: 10.1172/JCI107201
Giannella, R. A., Rout, W. R., and Formal, S. B. (1977). Effect of indomethacin on intestinal water transport in salmonella-infected rhesus monkeys. Infect. Immun. 17, 136–139.
Gordon, M. A. (2011). Invasive Non-typhoidal Salmonella Disease–epidemiology, pathogenesis and diagnosis. Curr. Opin. Infect. Dis. 24:484. doi: 10.1097/QCO.0b013e32834a9980
Griffin, A. J., and McSorley, S. J. (2011). Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol. 4, 371–382. doi: 10.1038/mi.2011.2
Guiney, D. G., Fang, F. C., Krause, M., and Libby, S. (1994). Plasmid-mediated virulence genes in non-typhoid Salmonella serovars. FEMS Microbiol. Lett. 124, 1–9. doi: 10.1111/j.1574-6968.1994.tb07253.x
Gulig, P. A. (1990). Virulence plasmids of Salmonella typhimurium and other salmonellae. Microb. Pathog. 8, 3–11. doi: 10.1016/0882-4010(90)90003-9
Haque, A., Bowe, F., Fitzhenry, R. J., Frankel, G., Thomson, M., Heuschkel, R., et al. (2004). Early interactions of Salmonella enterica serovar typhimurium with human small intestinal epithelial explants. Gut 53, 1424–1430. doi: 10.1136/gut.2003.037382
Hensel, M., Shea, J. E., Raupach, B., Monack, D., Falkow, S., Gleeson, C., et al. (1997). Functional analysis of ssaJ and the ssaK/U operon, 13 genes encoding components of the type III secretion apparatus of Salmonella Pathogenicity Island 2. Mol. Microbiol. 24, 155–167. doi: 10.1046/j.1365-2958.1997.3271699.x
Hensel, M., Shea, J. E., Waterman, S. R., Mundy, R., Nikolaus, T., Banks, G., et al. (1998). Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30, 163–174. doi: 10.1046/j.1365-2958.1998.01047.x
Höner Zu Bentrup, K., Ramamurthy, R., Ott, C. M., Emami, K., Nelman-Gonzalez, M., Wilson, J. W., et al. (2006). Three-dimensional organotypic models of human colonic epithelium to study the early stages of enteric salmonellosis. Microbes Infect. 8, 1813–1825. doi: 10.1016/j.micinf.2006.02.020
Hong, S. N., Dunn, J. C., Stelzner, M., and Martín, M. G. (2017). Concise review: the potential use of intestinal stem cells to treat patients with intestinal failure. Stem Cells Transl. Med. 6, 666–676. doi: 10.5966/sctm.2016-0153
Hurley, B. P., and McCormick, B. A. (2003). Translating tissue culture results into animal models: the case of Salmonella typhimurium. Trends Microbiol. 11, 562–569. doi: 10.1016/j.tim.2003.10.002
Hurley, D., Mccusker, M. P., Fanning, S., and Martins, M. (2014). Salmonella-host interactions - modulation of the host innate immune system. Front Immunol 5:481. doi: 10.3389/fimmu.2014.00481
In, J., Foulke-Abel, J., Zachos, N. C., Hansen, A. M., Kaper, J. B., Bernstein, H. D., et al. (2016). Enterohemorrhagic Escherichia coli reduce mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell. Mol. Gastroenterol. Hepatol. 2, 48–62.e43. doi: 10.1016/j.jcmgh.2015.10.001
Itri, F., Monti, D. M., Chino, M., Vinciguerra, R., Altucci, C., Lombardi, A., et al. (2017). Identification of novel direct protein-protein interactions by irradiating living cells with femtosecond UV laser pulses. Biochem. Biophys. Res. Commun. 492, 67–73. doi: 10.1016/j.bbrc.2017.08.037
Jones, B. D., Ghori, N., and Falkow, S. (1994). Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180, 15–23. doi: 10.1084/jem.180.1.15
Karve, S. S., Pradhan, S., Ward, D. V., and Weiss, A. A. (2017). Intestinal organoids model human responses to infection by commensal and Shiga toxin producing Escherichia coli. PLoS ONE 12:e0178966. doi: 10.1371/journal.pone.0178966
Kinsey, M. D., Dammin, G. J., Formal, S. B., and Giannella, R. A. (1976). The role of altered intestinal permeability in the pathogenesis of salmonella diarrhea in the rhesus monkey. Gastroenterology 71, 429–434.
Kubori, T., Matsushima, Y., Nakamura, D., Uralil, J., Lara-Tejero, M., Sukhan, A., et al. (1998). Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280, 602–605. doi: 10.1126/science.280.5363.602
Kumar, G., Pratap, C. B., Mishra, O. P., Kumar, K., and Nath, G. (2012). Use of urine with nested PCR targeting the flagellin gene (fliC) for diagnosis of typhoid fever. J. Clin. Microbiol. 50, 1964–1967. doi: 10.1128/JCM.00031-12
Le Blay, G., Rytka, J., Zihler, A., and Lacroix, C. (2009). New in vitro colonic fermentation model for Salmonella infection in the child gut. FEMS Microbiol. Ecol. 67, 198–207. doi: 10.1111/j.1574-6941.2008.00625.x
Legrand, N., Ploss, A., Balling, R., Becker, P. D., Borsotti, C., Brezillon, N., et al. (2009). Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell Host Microbe 6, 5–9. doi: 10.1016/j.chom.2009.06.006
Leslie, J. L., Huang, S., Opp, J. S., Nagy, M. S., Kobayashi, M., Young, V. B., et al. (2015). Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect. Immun. 83, 138–145. doi: 10.1128/IAI.02561-14
Lindquist, B. L., Lebenthal, E., Lee, P. C., Stinson, M. W., and Merrick, J. M. (1987). Adherence of Salmonella typhimurium to small-intestinal enterocytes of the rat. Infect. Immun. 55, 3044–3050.
Lopez, C. A., Rivera-Chávez, F., Byndloss, M. X., and Báumler, A. J. (2015). The Periplasmic Nitrate Reductase NapABC supports luminal growth of Salmonella enterica Serovar Typhimurium during Colitis. Infect. Immun. 83, 3470–3478. doi: 10.1128/IAI.00351-15
Martinez-Argudo, I., and Jepson, M. A. (2008). Salmonella translocates across an in vitro M cell model independently of SPI-1 and SPI-2. Microbiology 154, 3887–3894. doi: 10.1099/mic.0.2008/021162-0
Matano, M., Date, S., Shimokawa, M., Takano, A., Fujii, M., Ohta, Y., et al. (2015). Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med. 21, 256–262. doi: 10.1038/nm.3802
Mathur, R., Oh, H., Zhang, D., Park, S. G., Seo, J., Koblansky, A., et al. (2012). A mouse model of salmonella typhi infection. Cell 151, 590–602. doi: 10.1016/j.cell.2012.08.042
McGovern, V. J., and Slavutin, L. J. (1979). Pathology of salmonella colitis. Am. J. Surg. Pathol. 3, 483–490. doi: 10.1097/00000478-197912000-00001
Miyoshi, H., and Stappenbeck, T. S. (2013). In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protocols 8, 2471–2482. doi: 10.1038/nprot.2013.153
Moulder, J. W. (1985). Comparative biology of intracellular parasitism. Microbiol. Rev. 49, 298–337.
Newburg, D. S., Ko, J. S., Leone, S., and Nanthakumar, N. N. (2016). Human Milk Oligosaccharides and Synthetic Galactosyloligosaccharides Contain 3′-, 4-, and 6′-Galactosyllactose and Attenuate Inflammation in Human T84, NCM-460, and H4 Cells and Intestinal Tissue Ex Vivo. J. Nutr. 146, 358–367. doi: 10.3945/jn.115.220749
Nickerson, C. A., Goodwin, T. J., Terlonge, J., Ott, C. M., Buchanan, K. L., Uicker, W. C., et al. (2001). Three-dimensional tissue assemblies: novel models for the study of Salmonella enterica serovar Typhimurium pathogenesis. Infect. Immun. 69, 7106–7120. doi: 10.1128/IAI.69.11.7106-7120.2001
Nietfeld, J. C., Tyler, D. E., Harrison, L. R., Cole, J. R., Latimer, K. S., and Crowell, W. A. (1992). Invasion of enterocytes in cultured porcine small intestinal mucosal explants by Salmonella choleraesuis. Am. J. Vet. Res. 53, 1493–1499.
Nigro, G., Hanson, M., Fevre, C., Lecuit, M., and Sansonetti, P. J. (2016). Intestinal organoids as a novel tool to study microbes-epithelium interactions. Methods Mol. Biol. doi: 10.1007/7651_2016_12. [Epub ahead of print].
Noben, M., Verstockt, B., De Bruyn, M., Hendriks, N., Van Assche, G., Vermeire, S., et al. (2017). Epithelial organoid cultures from patients with ulcerative colitis and Crohn's disease: a truly long-term model to study the molecular basis for inflammatory bowel disease? Gut 66, 2193–2195. doi: 10.1136/gutjnl-2016-313667
Norris, F. A., Wilson, M. P., Wallis, T. S., Galyov, E. E., and Majerus, P. W. (1998). SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc. Natl. Acad. Sci. U.S.A. 95, 14057–14059. doi: 10.1073/pnas.95.24.14057
Ochman, H., Soncini, F. C., Solomon, F., and Groisman, E. A. (1996). Identification of a pathogenicity island required for Salmonella survival in host cells. Proc. Natl. Acad. Sci. U.S.A. 93, 7800–7804. doi: 10.1073/pnas.93.15.7800
Özkaya, H., Akcan, A. B., Aydemir, G., AydinÖz, S., Razia, Y., Gammon, S. T., et al. (2012). Salmonella typhimurium infections in BALB/c mice: a comparison of tissue bioluminescence, tissue cultures and mice clinical scores. New Microbiol. 35, 53–59.
Papafragkou, E., Hewitt, J., Park, G. W., Greening, G., and Vinjé, J. (2014). Challenges of culturing human norovirus in three-dimensional organoid intestinal cell culture models. PLoS ONE 8:e63485. doi: 10.1371/journal.pone.0063485
Popoff, M. Y., Bockemühl, J., and Gheesling, L. L. (2003). Supplement 2001 (no. 45) to the Kauffmann-White scheme. Res. Microbiol. 154, 173–174. doi: 10.1016/S0923-2508(03)00025-1
Ren, Z., Gay, R., Thomas, A., Pae, M., Wu, D., Logsdon, L., et al. (2009). Effect of age on susceptibility to Salmonella Typhimurium infection in C57BL/6 mice. J. Med. Microbiol. 58, 1559–1567. doi: 10.1099/jmm.0.013250-0
Rivera-Chávez, F., and Báumler, A. J. (2015). The pyromaniac inside you: Salmonella metabolism in the host gut. Annu. Rev. Microbiol. 69, 31–48. doi: 10.1146/annurev-micro-091014-104108
Rivera-Chávez, F., Winter, S. E., Lopez, C. A., Xavier, M. N., Winter, M. G., Nuccio, S. P., et al. (2013). Salmonella uses energy taxis to benefit from intestinal inflammation. PLoS Pathog. 9:e1003267. doi: 10.1371/journal.ppat.1003267
Rivera-Chávez, F., Zhang, L. F., Faber, F., Lopez, C. A., Byndloss, M. X., Olsan, E. E., et al. (2016). Depletion of butyrate-producing clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe 19, 443–454. doi: 10.1016/j.chom.2016.03.004
Roy, P., Canet-Jourdan, C., Annereau, M., Zajac, O., Gelli, M., Broutin, S., et al. (2017). Organoids as preclinical models to improve intraperitoneal chemotherapy effectiveness for colorectal cancer patients with peritoneal metastases: preclinical models to improve HIPEC. Int. J. Pharm. 531, 143–152. doi: 10.1016/j.ijpharm.2017.07.084
Samuel, J. L., O'boyle, D. A., Mathers, W. J., and Frost, A. J. (1980). Distribution of Salmonella in the carcases of normal cattle at slaughter. Res. Vet. Sci. 28, 368–372.
Sansonetti, P. J., and Phalipon, A. (1999). M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin. Immunol. 11, 193–203. doi: 10.1006/smim.1999.0175
Santos, R. L., Zhang, S., Tsolis, R. M., Kingsley, R. A., Adams, L. G., and Bäumler, A. J. (2001). Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 3, 1335–1344. doi: 10.1016/S1286-4579(01)01495-2
Sato, T., and Clevers, H. (2013). Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194. doi: 10.1126/science.1234852
Sato, T., Stange, D. E., Ferrante, M., Vries, R. G., Van Es, J. H., Van Den Brink, S., et al. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772. doi: 10.1053/j.gastro.2011.07.050
Sato, T., Vries, R. G., Snippert, H. J., Van De Wetering, M., Barker, N., Stange, D. E., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. doi: 10.1038/nature07935
Saxena, K., Blutt, S. E., Ettayebi, K., Zeng, X. L., Broughman, J. R., Crawford, S. E., et al. (2015). Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J. Virol. 90, 43–56. doi: 10.1128/JVI.01930-15
Scanu, T., Spaapen, R. M., Bakker, J. M., Pratap, C. B., Wu, L. E., Hofland, I., et al. (2015). Salmonella manipulation of host signaling pathways provokes cellular transformation associated with gallbladder carcinoma. Cell Host Microbe 17, 763–774. doi: 10.1016/j.chom.2015.05.002
Shea, J. E., Beuzon, C. R., Gleeson, C., Mundy, R., and Holden, D. W. (1999). Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect. Immun. 67, 213–219.
Shea, J. E., Hensel, M., Gleeson, C., and Holden, D. W. (1996). Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc. Natl. Acad. Sci. U.S.A. 93, 2593–2597. doi: 10.1073/pnas.93.6.2593
Stelzner, M., Helmrath, M., Dunn, J. C., Henning, S. J., Houchen, C. W., Kuo, C., et al. (2012). A nomenclature for intestinal in vitro cultures. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1359–G1363. doi: 10.1152/ajpgi.00493.2011
Takeuchi, A. (1967). Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am. J. Pathol. 50, 109–136.
Tang, H. J., Chen, C. C., Zhang, C. C., Cheng, K. C., Chiang, S. R., Chiu, Y. H., et al. (2012). Use of Carbapenems against clinical, nontyphoid Salmonella isolates: results from in vitro and in vivo animal studies. Antimicrob. Agents Chemother. 56, 2916–2922. doi: 10.1128/AAC.00110-12
Tsilingiri, K., Barbosa, T., Penna, G., Caprioli, F., Sonzogni, A., Viale, G., et al. (2012). Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut 61, 1007–1015. doi: 10.1136/gutjnl-2011-300971
Tsolis, R. M., Adams, L. G., Ficht, T. A., and Bäumler, A. J. (1999). Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 67, 4879–4885.
Tsolis, R. M., Adams, L. G., Hantman, M. J., Scherer, C. A., Kimbrough, T., Kingsley, R. A., et al. (2000). SspA is required for lethal Salmonella enterica serovar Typhimurium infections in calves but is not essential for diarrhea. Infect. Immun. 68, 3158–3163. doi: 10.1128/IAI.68.6.3158-3163.2000
Van Lidth de Jeude, J. F., Vermeulen, J. L. M., Montenegro-Miranda, P. S., Van Den Brink, G. R., and Heijmans, J. (2015). A protocol for lentiviral transduction and downstream analysis of intestinal organoids. J. Vis. Exp. e52531. doi: 10.3791/52531
Vazquez-Torres, A., Xu, Y., Jones-Carson, J., Holden, D. W., Lucia, S. M., Dinauer, M. C., et al. (2000). Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287, 1655–1658. doi: 10.1126/science.287.5458.1655
Wallis, T. S., Paulin, S. M., Plested, J. S., Watson, P. R., and Jones, P. W. (1995). The Salmonella dublin virulence plasmid mediates systemic but not enteric phases of salmonellosis in cattle. Infect. Immun. 63, 2755–2761.
Wang, Y., Disalvo, M., Gunasekara, D. B., Dutton, J., Proctor, A., Lebhar, M. S., et al. (2017). Self-renewing monolayer of primary colonic or rectal epithelial cells. Cell. Mol. Gastroenterol. Hepatol. 4, 165–182.e167. doi: 10.1016/j.jcmgh.2017.02.011
Watson, P. R., Galyov, E. E., Paulin, S. M., Jones, P. W., and Wallis, T. S. (1998). Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect. Immun. 66, 1432–1438.
Wilson, S. S., Bromme, B. A., Holly, M. K., Wiens, M. E., Gounder, A. P., Sul, Y., et al. (2017). Alpha-defensin-dependent enhancement of enteric viral infection. PLoS Pathog. 13:e1006446. doi: 10.1371/journal.ppat.1006446
Wilson, S. S., Tocchi, A., Holly, M. K., Parks, W. C., and Smith, J. G. (2015). A small intestinal organoid model of non-invasive enteric pathogen-epithelial cell interactions. Mucosal. Immunol. 8, 352–361. doi: 10.1038/mi.2014.72
Wistuba, I. I., and Gazdar, A. F. (2004). Gallbladder cancer: lessons from a rare tumour. Nat. Rev. Cancer 4, 695–706. doi: 10.1038/nrc1429
Wong, K. K., Mcclelland, M., Stillwell, L. C., Sisk, E. C., Thurston, S. J., and Saffer, J. D. (1998). Identification and sequence analysis of a 27-kilobase chromosomal fragment containing a Salmonella pathogenicity island located at 92 minutes on the chromosome map of Salmonella enterica serovar typhimurium LT2. Infect. Immun. 66, 3365–3371.
Woo, J. L., and Berk, A. J. (2007). Adenovirus ubiquitin-protein ligase stimulates viral late mRNA nuclear export. J. Virol. 81, 575–587. doi: 10.1128/JVI.01725-06
Wood, M. W., Jones, M. A., Watson, P. R., Hedges, S., Wallis, T. S., and Galyov, E. E. (1998). Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol. Microbiol. 29, 883–891. doi: 10.1046/j.1365-2958.1998.00984.x
Wray, C., and Sojka, W. J. (1978). Experimental Salmonella typhimurium infection in calves. Res. Vet. Sci. 25, 139–143.
Yin, X., Farin, H. F., Van Es, J. H., Clevers, H., Langer, R., and Karp, J. M. (2014). Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat. Methods 11, 106–112. doi: 10.1038/nmeth.2737
Yin, Y., Bijvelds, M., Dang, W., Xu, L., Van Der Eijk, A. A., Knipping, K., et al. (2015). Modeling rotavirus infection and antiviral therapy using primary intestinal organoids. Antiviral Res.123, 120–131. doi: 10.1016/j.antiviral.2015.09.010
Yin, Y., Dang, W., Zhou, X., Xu, L., Wang, W., Cao, W., et al. (2017). PI3K-Akt-mTOR axis sustains rotavirus infection via the 4E-BP1 mediated autophagy pathway and represents an antiviral target. Virulence 9, 83–98. doi: 10.1080/21505594.2017.1326443
Yin, Y., Wang, Y., Dang, W., Xu, L., Su, J., Zhou, X., et al. (2016). Mycophenolic acid potently inhibits rotavirus infection with a high barrier to resistance development. Antiviral Res. 133, 41–49. doi: 10.1016/j.antiviral.2016.07.017
Young, M., and Reed, K. R. (2016). Organoids as a model for colorectal cancer. Curr. Colorectal Cancer Rep. 12, 281–287. doi: 10.1007/s11888-016-0335-4
Zachos, N. C., Kovbasnjuk, O., Foulke-Abel, J., In, J., Blutt, S. E., De Jonge, H. R., et al. (2016). Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J. Biol. Chem. 291, 3759–3766. doi: 10.1074/jbc.R114.635995
Zhang, K., Dupont, A., Torow, N., Gohde, F., Leschner, S., Lienenklaus, S., et al. (2014). Age-dependent enterocyte invasion and microcolony formation by Salmonella. PLoS Pathog. 10:e1004385. doi: 10.1371/journal.ppat.1004385
Zhang, Y. G., Wu, S., Xia, Y., and Sun, J. (2014). Salmonella-infected crypt-derived intestinal organoid culture system for host-bacterial interactions. Physiol. Rep. 2:e12147. doi: 10.14814/phy2.12147
Zhou, D. (2001). Collective efforts to modulate the host actin cytoskeleton by Salmonella type III-secreted effector proteins. Trends Microbiol. 9, 567–569; discussion 569–570. doi: 10.1016/S0966-842X(01)02227-2
Zhou, D. (2006). “Bacterial invasion into non-phagocytic cells,” in Molecular Paradigms of Infectious Disease, eds C. A. Nickerson and M. J. Schurr ( New York, NY: Springer Ecience+Business Media, LLC), 247–273.
Zhou, D., and Galán, J. (2001). Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 3, 1293–1298. doi: 10.1016/S1286-4579(01)01489-7
Zhu, S., Ding, S., Wang, P., Wei, Z., Pan, W., Palm, N. W., et al. (2017). Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature 546, 667–670. doi: 10.1038/nature22967
Zierler, M. K., and Galán, J. E. (1995). Contact with cultured epithelial cells stimulates secretion of Salmonella typhimurium invasion protein InvJ. Infect. Immun. 63, 4024–4028.
Keywords: Salmonella, infection models, enteroids, intestine, organoids
Citation: Yin Y and Zhou D (2018) Organoid and Enteroid Modeling of Salmonella Infection. Front. Cell. Infect. Microbiol. 8:102. doi: 10.3389/fcimb.2018.00102
Received: 14 July 2017; Accepted: 16 March 2018;
Published: 04 April 2018.
Edited by:
Leigh A. Knodler, Washington State University, United StatesReviewed by:
Bruce Vallance, University of British Columbia, CanadaCopyright © 2018 Yin and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daoguo Zhou, emhvdWRAcHVyZHVlLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.