
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 19 January 2018
Sec. Bacteria and Host
Volume 8 - 2018 | https://doi.org/10.3389/fcimb.2018.00003
This article is part of the Research Topic Biology and Pathogenesis of Legionella View all 15 articles
Legionella pneumophila is a gram-negative bacterium that inhabits freshwater ecosystems, where it is present in biofilm or as planktonic form. L. pneumophila is mainly found associated with protozoa, which serve as protection from hostile environments and as replication niche. If inhaled within aerosols, L. pneumophila is also able to infect and replicate in human alveolar macrophages, eventually causing the Legionnaires' disease. The transition between intracellular and extracellular environments triggers a differentiation program in which metabolic as well as morphogenetic changes occur. We here describe the current knowledge on how the different developmental states of this bacterium are regulated, with a particular emphasis on the stringent response activated during the transition from the replicative phase to the infectious phase and the metabolic features going in hand. We propose that the cellular differentiation of this intracellular pathogen is closely associated to key metabolic changes in the bacterium and the host cell, which together have a crucial role in the regulation of L. pneumophila virulence.
Whatever strategy microbial pathogens have evolved to successfully infect and replicate in their hosts, they have adapted in the course of evolution to face hostile environments. This adaptation allows them to benefit from the environment of the susceptible host cell and simultaneously to ensure their persistence for another infection cycle. A conspicuous group of bacteria, referred to as facultative and obligate intracellular pathogens, exploits a variety of different hosts to establish a cytosolic or vacuolar niche for replication. Thereby they face and learned to tolerate acidification, starvation, and changes in temperature, oxidative stress and many other host defense mechanisms. Most of these bacteria are also located in the extracellular space between intracellular infection cycles and display thus a dual intracellular/extracellular lifestyle. Among those, the intracellular pathogen Legionella pneumophila thrives in fresh water environments, where it either spreads planktonically as free-living microbe or it is associated with biofilm communities (Steinert et al., 2002; Hilbi et al., 2011), but it never has been demonstrated to replicate in these environments. In the environment Legionella replicate within eukaryotic phagocytic cells like the environmental amoeba Acanthamoeba castellanii, as well as in human monocytes and alveolar macrophages (Horwitz and Silverstein, 1980; Rowbotham, 1986). L. pneumophila has successfully adapted to new and challenging environments created by human activities, such as showers, air conditioning systems, water fountains, cooling towers or other artificial water systems facilitating access to humans and human infection, which can result in a severe pneumonia, called Legionnaire's disease or legionellosis (McDade et al., 1977). However, mainly the susceptible population like immunocompromised or elderly persons develop pneumonia caused by Legionella, as this bacterium evolved with aquatic protozoa and thus it has not evolved mechanisms to counteract the host defense in healthy humans. In addition to biofilm communities and protozoan predators, L. pneumophila has been found to colonize more extreme environmental niches, such as antarctic freshwater lakes at temperature at 0°C as well as extremely acidic habitats and water sources with temperature over 60°C (Hilbi et al., 2011). Therefore, L. pneumophila endures in disparate environmental conditions throughout its life cycle with respect to nutrient access and availability, pH, temperature, and host defenses during intracellular replication. The transition between intracellular and extracellular habitats triggers morphogenetic and metabolic changes during the bacterial lifecycle (Molofsky and Swanson, 2004). Accordingly, L. pneumophila alternates between different morphogenetic forms including a replicating form (RF), and a transmissive/virulent form that have many distinct features (Molofsky and Swanson, 2004; Brüggemann et al., 2006; Steinert et al., 2007). Starvation and environmental stress induce the transition from the metabolically active, replicating bacteria to motile, stress-resistant virulent bacteria (Molofsky and Swanson, 2004). Moreover, a mature intracellular form (MIF), characterized by bacteria that are highly infectious, motile and cyst-like was described (Garduño et al., 2002; Robertson et al., 2014) as well as viable but non-cultivable (VBNC) forms that develop in response to disparate conditions (Steinert et al., 1997; Al-Bana et al., 2014). The fine-tuned regulation of these different forms ensures the persistence and successful life cycle of this bacterium. Thus, L. pneumophila employs a multitude of regulatory elements allowing it to govern its multi-phasic life cycle.
In the environment, L. pneumophila preferentially establishes a parasitic relationship with protozoa, which provides not only a nutrition source for the persistence, replication and dissemination of Legionella, but also functions as shelter offering protection from adverse environmental conditions (Barker et al., 1995; Cunha et al., 2016). Interestingly, bacteria released from protozoa are more infectious, are highly motile and more efficient in surviving and multiplying within human monocytes in vitro compared to bacteria grown on agar (Cirillo et al., 1994; Brieland et al., 1997). The protozoan predators (amoebae and ciliates) are the natural hosts of L. pneumophila, and humans are accidental hosts as judged by the evidence that only a single and recent case of human-to-human transmission has been reported to date (Correia et al., 2016). Thus, L. pneumophila transmission to humans occurs primarily from man-made environmental sources (Hilbi et al., 2010; Newton et al., 2010). The dual host specificity of Legionella is thought to be derived from the fact that protozoa are primordial phagocytes and as such they share many similarity at both cellular and molecular level with macrophages. Therefore, the intracellular growth of L. pneumophila is very similar in both hosts (Fields et al., 2002; Hilbi et al., 2007) suggesting that the co-evolution of Legionella within protozoa had provided the bacterium with an effective strategy to colonize two evolutionally different host cells. Indeed, this co-evolution is reflected in its genome as sequence analyses revealed that L. pneumophila as well as L. longbeachae have acquired genes coding for proteins with eukaryotic-like properties from its protozoan predators (Cazalet et al., 2004, 2010; de Felipe et al., 2005; Gomez-Valero et al., 2011). These eukaryotic-like proteins were shown to be secreted effectors that mimic the functions of their host counterparts (Cazalet et al., 2004; de Felipe et al., 2005; Nora et al., 2009; Gomez-Valero et al., 2011; Escoll et al., 2016). Their translocation to the host cell is achieved by the Dot/Icm type 4B secretion system (T4BSS), which is indispensible for intracellular replication of this bacterium (Ninio and Roy, 2007; Isberg et al., 2009; Zhu et al., 2011). Thus, this intriguing feature of molecular mimicry is a major virulence strategy developed by this opportunistic bacterium due to a selective pressure from the natural environment (Nora et al., 2009; Escoll et al., 2016).
Adaptation of L. pneumophila to harsh environmental conditions allowed them to become ubiquitous in human-made aquatic systems where the temperature is higher than the ambient temperature. As consequence, thermally altered aquatic habitats may shift the availability of predators and bacterial preys, eventually promoting Legionella replication and the emergence of the disease by inhalation of infected aerosols. Potential sources of Legionella transmission include potable water sources, such as fountains, showers and taps, and non-potable sources such as spas, cooling towers and evaporative condensers (Steinert et al., 2007; Newton et al., 2010; Cunha et al., 2016). Upon inhalation of bacteria-contaminated aerosols, Legionella reach the lung and are engulfed by alveolar macrophages wherein they can actively replicate, causing a life-threatening pneumonia called Legionnaires' disease (Newton et al., 2010). As Legionella is an opportunistic pathogen, persons with chronic lung diseases, elderly, immune-compromised, male gender and smokers are mainly susceptible to contract the disease (Newton et al., 2010; Cunha et al., 2016). Interestingly, not all Legionella species seem to be able to cause human disease as among the 58 Legionella species currently identified, only about 20 have been associated to human disease. Among those, L. pneumophila serogroup 1 is responsible for over 85% of the Legionnaires' disease cases world-wide (Yu et al., 2002; Newton et al., 2010)
One of the striking features of L. pneumophila is its ability to replicate within a large number of different host cells. The intracellular lifestyle and the adaptation capacity require a series of temporally distinct events leading to the establishment of a successful infection cycle, many of which are provoked by the action of one or more of the over 300 effector proteins known to be secreted by the Dot/Icm secretion system. Following the uptake of L. pneumophila by phagocytic cells through conventional or coiling phagocytosis (Bozue and Johnson, 1996; Escoll et al., 2013), this bacterium avoids lysosome-mediated degradation and forms a unique replication-permissive compartment within its host cell (Figure 1). This single-membrane Legionella containing vacuole (LCV) differs from the cellular compartment containing non-pathogenic bacteria since it does not acidify and it has a distinct membrane identity that is achieved by the recruitment of vesicles rich in lipids and proteins on the cytoplasmic face. Four hours after entry into phagocytes, vesicles derived from rough endoplasmic reticulum (ER) cluster near the nascent LCV. Based on the localization of ER-associated proteins within the LCV, these vesicles, which exit the ER, are able to deliver their content into the vacuoles containing L. pneumophila (Robinson and Roy, 2006). In this compartment the bacteria are replicating intravacuolarly, but the LCV was found later in association with the late lysosomal compartment, suggesting that it may also play a role in the replication of the bacteria by providing a nutrient-rich environment (Sturgill-Koszycki and Swanson, 2000). Recent studies demonstrated that L. pneumophila evades the host-cell response and interferes also with the host autophagy machinery by modulating the host sphingolipid metabolism or autophagosome formation (Choy et al., 2012; Rolando et al., 2016). The question remains open, whether the manipulation of the host sphingolipd metabolism may not only modulate autophagy, but also provide L. pneumophila nutrients for replication. However, additional membrane trafficking events may occur and modulate the intracellular life cycle of this bacterium to manipulate the host response. Following intracellular multiplication, the depletion of nutrients triggers morphological changes and a switch from a replicative form, where bacteria are metabolically active but not infectious, to a transmissive form, which ensures that the bacteria activate the infectious traits for the escape and transmission into a new suitable host or the survival in the environment (Byrne and Swanson, 1998; Garduño et al., 2002; Molofsky and Swanson, 2004; Robertson et al., 2014; Figure 1).
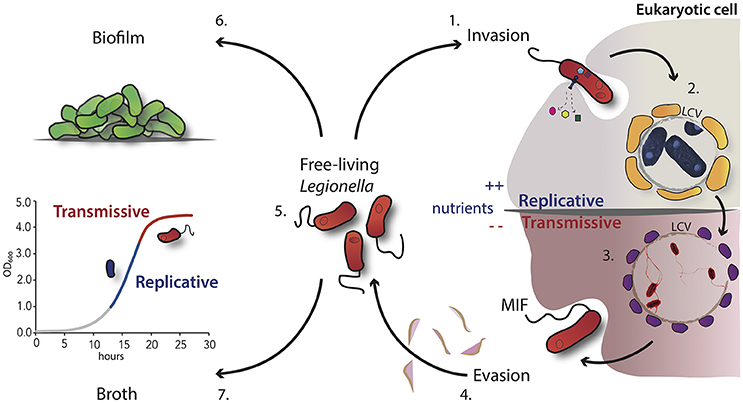
Figure 1. Schematic overview of the L. pneumophila morphological states during its growth cycle. 1. Uptake of virulent L. pneumophila by the host cell like protozoa or macrophages through convention or coiling phagocytosis. 2. After internalization, the bacteria evade the phagosome-lysosome fusion and start the intracellular multiplication within the LCV, which is surrounded by vesicles (in yellow) rich in lipids and proteins. 3. Nutrient starvation induces the activation of the stringent response and morphological changes. Bacteria express the transmissive traits such as motility (flagella) and become cytotoxic. 4. These infectious bacteria are able to lyse the vacuolar membrane and are released in the extracellular environment. 5. Free-living transmissive bacteria may start a new cycle or persist in the extracellular environment as planktonic form. 6. Alternatively, L. pneumophila may be associated within biofilms, either in natural fresh-water habitats or artificial ones. 7. In broth culture, L. pneumophila displays also a biphasic life cycle, which closely mimics the replicative and transmissive intracellular forms.
In a simple model one can describe the L. pneumophila life cycle as alternating between two distinct and reciprocal forms: a replicative and a transmissive form that was referred to as microbial differentiation (Molofsky and Swanson, 2004). This term implies physiological, morphogenetic and metabolic changes of the bacterium. Indeed, pronounced morphogenetic changes in the bacterial cell wall, the bacterial shape and in motility as well as the enrichment of the cell in energy-rich polymers are observed (Rowbotham, 1986). Within the LCV, acid-resistant, replicating bacteria exploit the nutrient-rich environment and actively inhibit the phagosome-lysosome fusion to be able to efficiently multiply. Therefore, bacterial density strongly increases whereas nutrient access dramatically decreases over time. Actively replicating bacteria appear as slender rods, are non-motile and display a wavy cell wall (Faulkner and Garduño, 2002). During this metabolically active state, traits related to virulence and transmission such as motility and cytotoxicity are not required thus the replicating bacteria either lack an activator of transmission and/or constitutively express a repressor of transmission traits (Byrne and Swanson, 1998; Molofsky and Swanson, 2003). As local nutrient levels become limiting and disadvantageous conditions are about to be faced, the bacteria convert into the infectious/transmissive variant. Interestingly, virulent bacteria appear as short, stubby rods with blunt ends containing cyst-like inclusions of poly-3-hydroxybutyrate (PHB), and display a smooth thick cell wall (Faulkner and Garduño, 2002). Those phenotypically distinct bacteria coordinately activate the expression of the so-called transmissive traits, which are required for lysosome evasion, escape from the spent host cell, survival in the extracellular environment and the invasion of a new suitable host. After successfully establishing a new intracellular niche, L. pneumophila reverts to its replicative form, starting a new cycle (Hammer and Swanson, 1999; Molofsky and Swanson, 2004). To limit costly energy levels, L. pneumophila has adopted a strategy employing a reciprocal biphasic expression pathway. Therefore, when conditions are favorable for multiplication, the virulence traits are neither required nor expressed. Conversely, in adverse conditions such as the nutrient deprivation, the bacteria do not replicate (Byrne and Swanson, 1998). Strikingly, the analyses of the global gene expression profiles of L. pneumophila in the in vivo infection model A. castellanii as well as in the in vitro broth culture model revealed that the pathogen's life cycle is very similar, as judged by the profound and similar changes in the gene expression program from the replicative/exponential growth phase to the transmissive/post-exponential growth phase of the bacteria (Brüggemann et al., 2006; Faucher et al., 2011). Thus, replicative and transmissive bacteria share a gene expression program similar to that of in vitro grown exponential (E) and post-exponential (PE) bacteria, respectively, suggesting that the biphasic life cycle is globally controlled by the bacterial growth phase and by nutrient availability. In addition, intracellular infection of the natural host A. castellanii revealed only few strain-specific differences, such as a shorter lag phase of strain L. pneumophila Paris and an earlier transition to transmissive form (Brüggemann et al., 2006). Interestingly, the global expression profiles of replicative and transmissive phases of three different L. pneumophila strains have been shown to be very similar (Brüggemann et al., 2006). In contrast, comparison of the gene expression program of the E and PE phases of the two major disease-associated species L. pneumophila and L. longbeachae, revealed clear differences. Particularly, the transition between the replicative and transmissive form is less pronounced in L. longbeachae, which seems to regulate this transition mainly by engaging secondary messenger molecules and less transcriptional and post- transcriptional regulators than L. pneumophila (Cazalet et al., 2010). Taken together, the transition from the exponential/replicative to the post-exponential/transmissive phase governs a common virulence program engaged of L. pneumophila within host cells (Brüggemann et al., 2006; Faucher et al., 2011).
In response to fluctuating intracellular environmental conditions, L. pneumophila certainly requires a well-balanced adaptation of its metabolism. Main questions are (i) what are the essential nutrients required for intracellular proliferation of L. pneumophila during infection, (ii) what is the nutrient availability in the LCV and (iii) what are the capacities of the bacterium to catabolize these compounds. The development of a chemical defined liquid medium gave first insights into the nature and physiology of this intracellular pathogen by suggesting that it utilizes only amino acids as energy and carbon sources (Pine et al., 1979; George et al., 1980; Ristroph et al., 1981; Tesh et al., 1983). While formulating this medium, it has been demonstrated that cysteine was an absolute requirement for the bacterial growth and that the addition of soluble ferric pyrophosphate had stimulatory effects. Unlike other microorganisms, L. pneumophila has been found to use serine and threonine as a primary supply for energy production rather than any other organic substrate (Pine et al., 1979; George et al., 1980; Ristroph et al., 1981; Fields, 1996). Accordingly, microarray analyses performed during replicative growth of L. pneumophila either in broth or upon infection of A. castellanii, revealed that while replicating, bacteria express genes indicating that an aerobic metabolism and amino acid catabolism, particularly for serine, threonine, glycine, tyrosine, alanine, and histidine is taking place (Sauer et al., 2005; Brüggemann et al., 2006). However, unexpectedly the up-regulation of genes encoding the Entner-Doudoroff (ED) pathway, as well as of a putative glucokinase, a sugar transporter and the myo-inositol catabolism indicated that L. pneumophila may be also able to exploit host carbohydrate-derivatives during the replicative phase of growth within amoebae (Brüggemann et al., 2006). Interestingly, these analyses suggested for the first time that intracellular L. pneumophila also may utilize starch or glycogen as judged by the expression of a eukaryotic-like glucoamylase (GamA) during exponential growth (Brüggemann et al., 2006). Indeed, later, it was shown that GamA is responsible for glycogen- and starch-degrading activities of L. pneumophila and that it is expressed and active during intracellular replication in A. castellanii, suggesting that L. pneumophila is degrading glycogen during intracellular replication (Herrmann et al., 2011). Hence, intracellular L. pneumophila not only uses amino acids but also diverse carbohydrates as carbon supply (Weiss et al., 1980; Eylert et al., 2010).
However, L. pneumophila is auxotrophic for the amino acids Arg, Cys, Ile, Leu, Met, Thr, Val, Ser, Pro, and Phe (Pine et al., 1979; George et al., 1980; Ristroph et al., 1981; Tesh et al., 1983). Moreover, 13C- isotopologue profiling revealed that L. pneumophila is able to perform gluconeogenesis and to use the pentose phosphate pathway (PPP), although not all the genes encoding canonical enzymes involved in these pathways are present (Eylert et al., 2010). Based on the presence of a glucose transporter protein and on 13C-tracer experiments, it was reported that glucose, metabolized through the ED and PPP pathways serves as co-substrate for L. pneumophila, although the addition of glucose in broth culture does not increase the bacterial growth rate (Tesh et al., 1983; Eylert et al., 2010; Eisenreich and Heuner, 2016; Häuslein et al., 2016). Isotopologue profiling of key metabolites of L. pneumophila unveiled a bi-partite metabolism, in which it preferentially uses serine as major carbon, nitrogen and energy supply, whereas glycerol and glucose are shuffled into anabolic processes (Eisenreich and Heuner, 2016; Häuslein et al., 2016; Figure 2). In addition, as expected from an intracellular bacterium, which engages a growth phase-dependent program to control its virulence, it was shown that also the carbon and energy sources are metabolized in dependence of the growth phase (Gillmaier et al., 2016; Häuslein et al., 2016). As such, Serine is mainly metabolized during the replicative phase for amino acid (Ser> Ala >Glu> Asp = Gly) and protein biosynthesis (>50 mol%) and for energy production. 13C-labeled serine was found to enter mainly the TCA cycle, generating pyruvate and then acetyl-CoA, and to produce PHB (Eylert et al., 2010; Gillmaier et al., 2016; Häuslein et al., 2016). Conversely, during the post-exponential phase, despite the availability of serine in the medium, serine-dependent protein biosynthesis appears to be reduced, whereas carbon from serine is still used for PHB and fatty acid biosynthesis until the post-exponential phase of growth. Hence, upon entry into the stationary phase and under nutrient starvation, the PHB produced is catabolized by L. pneumophila, serving as main carbon and energy storage (James et al., 1999; Eylert et al., 2010; Gillmaier et al., 2016; Häuslein et al., 2016; Figure 2).
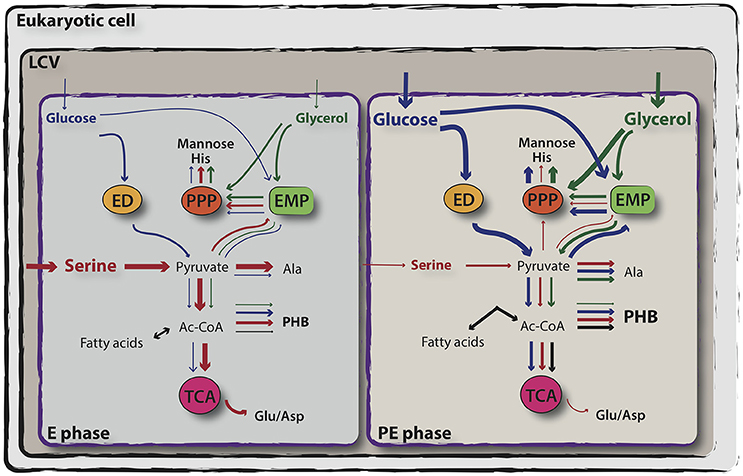
Figure 2. Simplified representation of exponential and post-exponential phase-dependent utilization of serine, glucose and glycerol by L. pneumophila. In vitro isotopologue labeling experiments using 13C-serine, 13C-glucose and 13C-glycerol revealed a bipartite metabolism in which serine (in red) is mainly used during the exponential phase of growth and enters primarily the TCA cycle, whereas glucose (in blue) and glycerol (in green) are shuffled into anabolic processes during the post-exponential growth phase of L. pneumophila. Relative carbon fluxes are depicted by the thickness of the arrows. For more detail, see the text. ED, Entner–Doudoroff pathway; PPP, pentose phosphate pathway; EMP, Embden-Meyerhof-Parnas pathway (glycolysis); TCA, tricarboxylic acid cycle (adapted from Eisenreich and Heuner, 2016).
The other player in this bi-partite metabolism is glucose, which in L. pneumophila predominantly enters into the PPP for the novo production of histidine and sugars (in particular mannose) and that is also used in lower amounts for the synthesis of other amino acids and PHB. Conversely to serine, glucose is mainly metabolized throughout the late exponential and post-exponential phase of growth (Figure 2). As previously mentioned, L. pneumophila uses mainly the ED pathway, the gluconeogenesis and the PPP, and to a minor extent the glycolysis to metabolize glucose (Harada et al., 2010; Häuslein et al., 2016). Furthermore, glucose metabolism through the ED pathway is necessary for full fitness of L. pneumophila during its biphasic life cycle (Eylert et al., 2010). Earlier studies provided the first indication that glycerol may be used by L. pneumophila as carbon source, as deduced from the upregulation of a glycerol-3-phospate dehydrogenase (glpD) during intracellular growth in human macrophages (Faucher et al., 2011). Indeed, glycerol is predominantly metabolized during the late and post-exponential phase of growth like the life stage dependent usage of other carbon sources. Similar to glucose, the carbon from glycerol is mainly shuffled into gluconeogenesis and PPP for histidine and mannose production, but only low flux rates of carbon from glycerol into the TCA cycle were reported (Häuslein et al., 2016). In contrast, saturated lipids like palmitate, another carbon source shown to be predominantly used after amino acid depletion, is used for energy production and PHB synthesis (Häuslein et al., 2017). Taken together, the results from 13C- isotopologue profiling and flux analyses suggested that the life cycle switch of Legionella is also reflected by a metabolic shift from amino acids usage during replication to glycerolipids and glucose when entering transmissive phase (Häuslein et al., 2017; Figure 2).
The acquisition of nutrients within host cells is an indispensable prerequisite for L. pneumophila multiplication and for a successful infection cycle. The presence and the up-regulation of genes encoding 12 different ABC transporters, amino acid permeases, proteases and phospholipases during intracellular multiplication within host cells suggested how L. pneumophila exploits host nutrient to support its intracellular growth (Brüggemann et al., 2006). Indeed, it was shown that intracellular replication of L. pneumophila depends on the host cell amino acid transporter SLC1A5 (Wieland et al., 2005) and that it employs the phagosomal transporter A (PhtA) to acquire threonine during growth (Sauer et al., 2005). Furthermore, 13C-Isotopologue compositions of amino acids from bacterial and amoebal proteins showed that L. pneumophila takes indeed amino acids up from its host (Schunder et al., 2014). In addition to the above mentioned transport systems, L. pneumophila was also reported to employ its effector proteins to generate nutrients for its growth. The L. pneumophila effector AnkB (Price et al., 2009; Lomma et al., 2010) is secreted in the host cell where it poly-ubiquitinates its targets leading to their proteolysis by the host proteasome. Price and colleagues suggested that in this way AnkB may generate short peptides and amino acids, which represent nutrients for intracellular bacterial multiplication as these free amino acids may be imported into the LCV via different host solute carriers and transporters, such as the glucose (Slc2a1, Slc2a6) and glycerol transporters (Slc37a1) (Price et al., 2011). A recent study reported opposing effects of two Dot/Icm secreted effector families, Lgt and SidE on the master regulator of host amino acid metabolism, the mechanistic target of rapamycin complex 1 (mTORC1). However, these two-effector families work synergistically to inhibit host translation and thereby liberate amino acids for L. pneumophila growth (De Leon et al., 2017). Thus, L. pneumophila not only employs its own transport systems for the uptake and to use amino acids but also seem to exploit the host proteasome machinery and mTORC1 to generate nutrients. Isoptopologue profiling during the replicative intracellular growth phase showed that L. pneumophila uses serine and other amino acids as main carbon and energy sources for protein biosynthesis, amino acid biosynthesis and PHB production (Price et al., 2011). Although less is known about the intracellular metabolism in the late phase of growth, it is likely that L. pneumophila gets access to carbohydrate sources such as glycogen, glucose and other polymers from the host upon LCV lysis (Lang and Flieger, 2011). In addition, the transmissive form of L. pneumophila contains high amounts of PHB, which serves as energy and carbon storage for the maintenance of the intracellular growth cycle (Gillmaier et al., 2016; Häuslein et al., 2017). Moreover, intracellular L. pneumophila metabolizes myo-inositol, which was reported to promote infection of A. castellanii and macrophages (Manske et al., 2016), and engages the translocated protein MavN, which once integrated in the host-derived LCV membrane, facilitates the acquisition of iron into its vacuole (Isaac et al., 2015). Once nutrients are limited in the LCV, this may be the signal for L. pneumophila to activate the infectious traits to escape the spent host. This transition from the replicative to the transmissive/virulent phase is highly regulated by a complex regulatory network, described in the following sections.
After replicating within the LCV to high numbers, nutrients become limited, which triggers complex and coordinated regulation to allow the expression of transmissive traits, which provide L. pneumophila with the ability of leaving the host cell, of long-term survival under hostile extracellular conditions and of re-infecting a new host cell. By analogy to E. coli, it was proposed that L. pneumophila, when starved for amino acids, initiates a stringent response by synthesizing the second messenger guanosine tetraphosphate (p)ppGpp via the synthetase enzyme RelA (Hammer and Swanson, 1999). Indeed, a L. pneumophila relA mutant replicates efficiently within either amoebae or macrophages however upon entry into the post-exponential phase of growth, the mutant strain does not produce the second messenger (Zusman et al., 2002). Additionally, virulence traits are poorly expressed when L. pneumophila lacks RelA and consequently the alarmone (p)ppGpp (Hammer and Swanson, 1999; Zusman et al., 2002; Dalebroux et al., 2010a). The mild effects displayed by the lack of RelA on the expression of the virulent traits suggested that additional clues and redundant strategies are employed by L. pneumophila to govern its virulence (Hammer and Swanson, 1999; Zusman et al., 2002). Indeed L. pneumophila is equipped with two ppGpp synthetases, which respond to two distinct metabolic cues. Whereas, RelA synthesizes (p)ppGpp is following fluctuations in amino acid availability, the bifunctional enzyme SpoT leads to the accumulation of the alarmone (p)ppGpp in response to fatty acid depletion. By analogy to E. coli, a L. pneumophila strain depleted of relA and spoT lacks (p)ppGpp completely, however whether it results in rRNA transcriptional activation and/or in the synthesis of stable RNA remains unclear (Dalebroux et al., 2009; Dalebroux and Swanson, 2012; Trigui et al., 2015). Thus, the L. pneumophila biphasic life cycle requires the fine tuning of the levels of alarmone (p)ppGpp present in the bacteria. When nutrients are abundant, virulent bacteria hydrolyze (p)ppGpp in a SpoT-dependent manner, allowing the bacteria to actively multiply and repress the transmission traits (Molofsky and Swanson, 2003; Dalebroux et al., 2009, 2010a; Trigui et al., 2015). Conversely, as replicating bacteria exhaust the available nutrients within the LCV, (p)ppGpp is produced by RelA and additionally, the equilibrium of SpoT is shifted more toward synthesis instead degradation. This leads to a massive accumulation of the alarmone and triggers the entry into the transmissive state (Hammer and Swanson, 1999; Molofsky and Swanson, 2004; Dalebroux et al., 2009). SpoT is required throughout the entire infection cycle to mediate (p)ppGpp turnover via its hydrolase and synthase activities (Xiao et al., 1991; Potrykus and Cashel, 2008; Dalebroux et al., 2009, 2010a).
In L. pneumophila the signaling alarmone (p)ppGpp is a key player for the reorganization of the bacterial transcriptome by recruiting sigma factors, allowing the activation of genes necessary for the adaptation to the new condition and the repression of the ones that are no longer required (Dalebroux et al., 2010a). Particularly, the accumulation of (p)ppGpp increases the amount of the alternative sigma factor RpoS (σS/38), which results in the regulation of multiple pathways associated with motility and pathogenic functions as well as the activity of transcriptional regulators and Dot/Icm effectors (Hales and Shuman, 1999; Bachman and Swanson, 2004; Trigui et al., 2015). The mechanism that links the accumulation of (p)ppGpp with the expression of RpoS remains to be elucidated, however (p)ppGpp is suggested to destabilize the binding of the vegetative sigma factor σD/70 to the core and endorses the recruitment of alternative sigma factors and the expression of their targets, as demonstrated for E.coli (Jishage et al., 2002). An additional regulatory element, which acts as cofactor for (p)ppGpp-dependent transcriptional regulation is the RNA polymerase (RNAP) secondary channel interacting protein DksA (Haugen et al., 2008; Potrykus and Cashel, 2008). L. pneumophila DksA function may be dependent on bacterial stimuli. In particular, DksA seems to respond to fatty acid stress by inducing bacterial differentiation in a (p)ppGpp-independent manner, as judged by the expression of certain transmissive traits within macrophages (Dalebroux et al., 2010b). However, upon (p)ppGpp accumulation, DksA and (p)ppGpp coordinately regulate the hierarchical cascade for flagellar expression. Therefore, L. pneumophila employs both (p)ppGpp and DksA to act independently or cooperatively during bacterial differentiation (Dalebroux et al., 2010b). At the bottom of the hierarchical cascade governing L. pneumophila differentiation one can find the flagellar regulon, composed of four different classes of genes, whose coordinated expression is crucial for efficient and maximal virulence of the bacterium (Heuner et al., 1997; Dietrich et al., 2001; Brüggemann et al., 2006; Appelt and Heuner, 2017). Class I genes, which include the genes encoding the flagellar master regulator and the σ54 activator protein FleQ together with the alternative sigma factor RpoN (σ54), are required for the expression of the class II genes, leading to the formation of the flagellar basal body, hook and the activation of the regulatory proteins (Jacobi et al., 2004; Steinert et al., 2007; Albert-Weissenberger et al., 2010). Finally, the flagellar sigma factor FliA (σ28) (encoded by a class III gene and regulated by DksA) is directly controlling the flagellar class IV genes such as flaA and fliDS, encoding the flagellin and the filament cap respectively, leading to the complete formation of the flagellum (Heuner and Steinert, 2003; Jacobi et al., 2004; Brüggemann et al., 2006; Albert-Weissenberger et al., 2007, 2010; Dalebroux et al., 2010b). Interestingly, the flagellar sigma factor FliA is not only implicated in the regulation of the flagellum production but also acts as regulator of virulence genes that are required for the expression of pathways important for cytotoxicity, lysosome evasion, and replication of L. pneumophila (Heuner et al., 2002; Molofsky and Swanson, 2004; Brüggemann et al., 2006).
As in many other bacterial pathogens, L. pneumophila post-transcriptional regulation is controlled by two-component systems (TCS), which use protein phosphorylation cascades for signal transduction (Padilla-Vaca et al., 2017). L. pneumophila employs at least four distinct TCSs LetA/S, PmrA/B, LsqR/ST, and CpxR/A that govern its differentiation from the replicative to the transmissive state (Gal-Mor and Segal, 2003a; Tiaden et al., 2007; Zusman et al., 2007; Altman and Segal, 2008). Particularly, the TCS LetA/LetS (Legionella transmission activator and sensor, respectively) of L. pneumophila is an important regulator system for the activation of a large set of virulence phenotypes and the control of the progression into the transmissive state (Hammer et al., 2002; Gal-Mor and Segal, 2003b; Lynch et al., 2003). Probably directly activated by the accumulation of the alarmone (p)ppGpp, LetA is regulating the expression of the small ncRNAs RmsX,Y,Z, which are required to relieve the repression exerted by the global regulator CsrA, an RNA-binding protein, on many virulence genes, thereby ensuring the expression of the transmissive traits (Hovel-Miner et al., 2009; Rasis and Segal, 2009; Sahr et al., 2009; Edwards et al., 2010). The carbon storage regulator protein CsrA was reported to bind more than 450 mRNA targets in L. pneumophila, altering their translation, transcription and/or their stability (Sahr et al., 2009, 2017). Among those targets, CsrA affects the expression of the previously mentioned regulators FleQ, RpoS, the quorum sensing regulator LqsR and it also control the expression of over 40 Dot/Icm substrates (Sahr et al., 2017). Moreover, CsrA controls its own expression and the relA mRNA in a regulatory feedback loop. This in turn makes the CsrA protein indispensable for L. pneumophila thus only conditional or partial mutants could be obtained, which are all however strongly attenuated for intracellular multiplication, underlining its essential role in the life cycle of L. pneumophila (Molofsky and Swanson, 2003; Sahr et al., 2017). Another TCS important for virulence gene expression is PmrA/B (Zusman et al., 2007). L. pneumophila PmrA/B not only activates the expression of 43 effector-encoding genes but also positively regulates CsrA and consequently post-transcriptional repression of the CsrA- regulated effectors (Zusman et al., 2007; Al-Khodor et al., 2009; Rasis and Segal, 2009) (Figure 3). It is likely that a regulatory switch between at least two sets of effectors occurs: one set of effectors, activated by PmrA/B and expressed in the replicative state and the second group of effectors which is regulated by the LetA/S TCS upon entry into the transmissive phase of L. pneumophila. Another player in this complex regulatory network, is the TCS LqsRS (Legionella quorum sensing), whose role in the regulation of gene expression during the transmissive phase has been extensively studied (Hochstrasser and Hilbi, 2017). Importantly, the production of LqsR is regulated at the post-transcriptional level by the global repressor CsrA (Sahr et al., 2009, 2017). Finally, the CpxR/A TCS, which acts as dual regulator and thus as an activator and repressor, was shown to control the expression of at least 27 Dot/Icm substrates as well as type II- secreted virulence factors, playing a important role in L. pneumophila virulence gene regulation (Gal-Mor and Segal, 2003a; Altman and Segal, 2008).
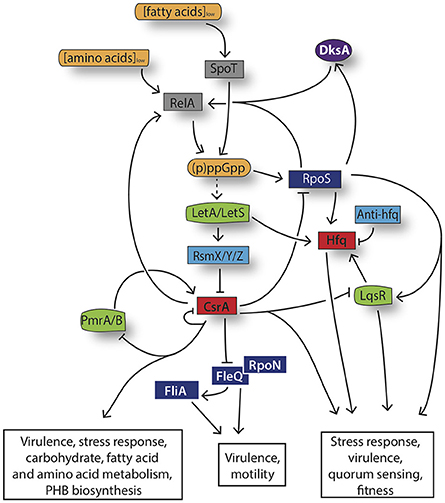
Figure 3. Model the stringent response network governing L. pneumophila differentiation. Amino acid and fatty acid starvation triggers RelA and SpoT to produce the alarmone (p)ppGpp. Its accumulation induces the activation of the stress sigma factor RpoS, the LetA/LetS TCS and consequently an increased transcription of the RsmZ, RsmX, and RsmY sRNAs. The three sRNAs act as “sponge,” sequestering CsrA and leading thereby to the activation of the infectious traits and to changes in the metabolism. The dashed arrows indicate suggested but not yet confirmed direct interactions.
In addition to TCSs and the RNA-binding protein CsrA, another major player in the regulation of the transition from replicative to transmissive L. pneumophila is the RNA binding protein and chaperone Hfq (McNealy et al., 2005; Trigui et al., 2013). This pleiotropic regulatory element is known to modulate gene expression by facilitating the interaction between sRNA and their mRNA targets in diverse bacterial pathogens, controlling pathways related to metabolism, transport, energy production and conversion or membrane proteins (Boudry et al., 2014). In L. pneumophila, Hfq expression is influenced by RpoS and LetA regulatory elements as both directly or indirectly turn on hfq transcription upon onset of the late post-exponential phase. Furthermore, L. pneumophila Hfq regulates its own expression in an auto-regulatory loop (Oliva et al., 2017). Although, only two direct targets (hfq mRNA and Anti-hfq sRNA) of L. pneumophila Hfq have been identified to date by in vitro assays, Hfq was reported to regulate the bacterium's virulence, as judged by the findings that this post-transcriptional regulator promotes motility and is required for efficient multiplication of L. pneumophila within A. castellanii at environmental temperatures (McNealy et al., 2005; Oliva et al., 2017) (Figure 3).
The complex and hierarchical regulation of the L. pneumophila life cycle includes also the recruitment of small RNAs, which ensure a fast and more cost-effective regulation. Previous evidences in E. coli showed that the BarA/UvrY TCS (the L. pneumophila LetA/S homolog) controls the expression of two sRNAs, named CsrB and CsrC, whose sequences contain GGA motifs, which are the characteristic binding sequences for CsrA (Liu et al., 1997). A first bioinformatics search revealed in L. pneumophila the presence of two homologs of CsrB, named RsmY and RsmZ (Kulkarni et al., 2006). Functional analyses confirmed that these sRNAs were the missing regulatory elements linking the LetA/S TCS and the RNA binding protein CsrA in L. pneumophila (Rasis and Segal, 2009; Sahr et al., 2009). In detail, LetA binds directly to a conserved consensus sequence upstream the rsmY/Z genes, leading to their expression. These sRNAs contain multiple CsrA binding motifs and act as sponge to bind and sequester CsrA from their target mRNAs, leading to the expression of virulence traits. RsmY and RsmZ were the first characterized sRNAs implicated in the regulation of L. pneumophila virulence. However, deep RNA sequencing from exponentially (replicative) and post exponentially (virulent) in vitro grown L. pneumophila have identified more than 700 sRNAs, 60% of which are growth-phase dependently regulated, including a third LetA-dependent sRNA, named RsmX, suggesting that a set of these yet uncharacterized sRNAs, might influence the expression of replication or virulence determinants in L. pneumophila (Sahr et al., 2012). Recently, we characterized one of these ncRNAs, a cis-encoded sRNA for which we showed that it is implicated in the regulation of the RNA binding protein Hfq (Oliva et al., 2017). This sRNA, named Anti-hfq, is transcribed antisense to the hfq transcript and controls the expression of Hfq through a base pairing mechanism during the exponential phase of L. pneumophila growth (Oliva et al., 2017). Moreover, it is important to mention that Hfq was reported to influence L. pneumophila differentiation by interacting with the major regulatory elements of the cascade. Thus, it is expected that Hfq, acting as RNA chaperone and RNA binder might regulate a number of still unknown sRNAs implicated in bacterial virulence.
Taken together, L. pneumophila is equipped with a sophisticated regulatory network, including transcriptional and post-transcriptional regulatory elements, including small non-coding sense and antisense RNAs to control the reciprocal expression of distinct sets of genes under different environmental conditions (Figure 3).
Similarly to what has been described in other bacterial pathogens, many regulatory factors implicated in virulence gene expression are also major regulators of metabolic pathways. Indeed, L. pneumophila exhibits a bipartite metabolism, which requires a fine-tuned regulation. An intriguing example of a regulator that is important for the expression of virulence and the regulation of metabolic traits is the RNA binding protein CsrA. Interestingly, L. pneumophila harbors some of the key genes encoding enzymes of the glycolysis/gluconeogenesis (glyceraldeyde-3-phosphate dehydrogenase or Gap, phosphoglycerate kinase, and pyruvate kinase) and the PPP (transketolase) in one single operon. The combined or individual regulation of these two pathways is under the control of the RNA binding protein CsrA, whose presence ensures the efficient expression of the both parts of this operon (Sahr et al., 2017). When nutrients are abundant CsrA binds within the gap transcript, and stabilizes the alternative secondary structure that covers the Rho-dependent transcription termination site. Consequently, this leads to a CsrA-dependent transcription of the glycolysis part of the operon toward gluconeogenesis, which under starvation or stress is not expressed. Another example of how CsrA influences metabolism, is that this regulatory element affects the production of secondary metabolites, in particular thiamine pyrophosphate, ensuring the effecting functioning of central enzymes of the carbohydrate metabolism when required (Sahr et al., 2017).
Indeed, using 13C-isotopologue profiling and carbon-flux analyses of a wild-type and a csrA mutant strain confirmed that CsrA plays a major role in regulating the carbon flux between the PPP and the glycolysis (Häuslein et al., 2017). Furthermore, this study highlighted the impact of CsrA on the bipartite metabolism of L. pneumophila, as the absence of CsrA induces a reduction of the carbon flux from serine via gluconeogenesis into the PPP. By contrast, CsrA has a negative impact on the incorporation and the metabolism of glycerol and glucose. As such, the absence of CsrA results in the increase of the carbon flux from glucose into the PPP and ED pathways and the carbon flux from glycerol into the PPP and the gluconeogenesis (Häuslein et al., 2017). These studies also showed the important influence of CsrA on the production of the storage molecule PHB suggesting that CsrA is a major player in the utilization of the different carbon sources during the biphasic life cycle of L. pneumophila (Häuslein et al., 2017). The biphasic life cycle of L. pneumophila within the host supports the usage of amino acids as main carbon and energy source during multiplication due to the expression of CsrA that is simultaneously repressing the usage of alternative carbon sources, such as glycerol. Conversely, upon onset of the post-exponential phase of growth, the stress response induces the sRNA RsmX, Y, and Z that sequester CsrA, resulting in an increased utilization of glycerolipids, which along with glucose, mostly trigger the synthesis of lipopolysaccharide sugars through the PPP and in addition, the production of the energy and carbon storage polymer PHB (Häuslein et al., 2017). Hence, CsrA is a major organizer of the biphasic life cycle of Legionella pneumophila integrating and coordinating the metabolic carbon switch and the transition between replicative and transmissive traits.
L. pneumophila is an intracellular opportunistic pathogen, which exploits amoebae and other protozoa as environmental hosts, but that is also able to infect human macrophages, eventually causing Legionnaires' disease, a severe pneumonia that is often fatal when not treated promptly. L. pneumophila is ubiquitously found in fresh water habitats, as planktonic form or forming biofilm. In response to diverse and hostile environmental conditions encountered during its life cycle, L. pneumophila has evolved sophisticated mechanisms to successfully replicate within different host niches and to also survive in extracellular environments. As such, this intracellular bacterium displays at least two reciprocal stages: a replicative and a transmissive form. The transition between the non-virulent replicative and the virulent non-replicative phase is governed by a complex regulatory network, in which transcriptional and post-transcriptional regulatory elements are engaged to insure an efficient infection cycle. The trigger of this morphological stress response is mainly mediated by metabolic changes and therefore the availability of nutrients in the surroundings. Thus, within the LCV the usage of serine as carbon and energy source supports the multiplication of the bacteria in which the replicating bacteria show a high metabolic activity. Upon amino acid depletion, the stringent response mediates the expression of the virulent traits but in parallel also enables the bacteria to survive for long term under stress and starving condition. This is ensured amongst others by the expression of stress and virulence related genes and an overall metabolic shift leading to the usage of alternative carbon sources like glucose and glycerolipids and an increased production of the storage molecule PHB. Under these conditions, L. pneumophila is optimally equipped to escape the spent host, survive for an uncertain period in the extracellular environment and eventually start a new infection cycle.
Taken together, the biphasic life cycle of L. pneumophila results in distinct morphological changes and a bipartite carbon metabolism. Thus, during the biphasic life cycle the metabolism influences the transition between replicative and transmissive phase as well as the reciprocal expression of virulence factors and their regulators, in particular CsrA, which is implicated in the regulation of virulence and the metabolism. A comprehensive analysis of L. pneumophila adaptation to metabolic cues during the transmissive phase in vivo either in amoebae or macrophages is still missing and would provide additional information about the utilization of diverse carbohydrates, and the cross-talk of the regulatory elements which govern L. pneumophila virulence. Continuous unrevealing of this complex interplay between metabolism and virulence of L. pneumophila may teach us also about host-pathogen interaction in general.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Work in the CB laboratory is financed by the Institut Pasteur, the grant n°ANR-10-LABX-62-IBEID, the Fondation pour la Recherche Médicale (FRM) grant N° DEQ20120323697 and the Pasteur-Weizmann consortium ≪ The roles of non-coding RNAs in regulation of microbial life styles and virulence ≫. GO was supported by a stipend from the Pasteur—Paris University (PPU) International PhD Program.
Al-Bana, B. H., Haddad, M. T., and Garduño, R. A. (2014). Stationary phase and mature infectious forms of Legionella pneumophila produce distinct viable but non-culturable cells. Environ. Microbiol. 16, 382–395. doi: 10.1111/1462-2920.12219
Albert-Weissenberger, C., Cazalet, C., and Buchrieser, C. (2007). Legionella pneumophila- a human pathogen that co-evolved with fresh water protozoa. Cell Mol. Life Sci. 64, 432–448. doi: 10.1007/s00018-006-6391-1
Albert-Weissenberger, C., Sahr, T., Sismeiro, O., Hacker, J., Heuner, K., and Buchrieser, C. (2010). Control of flagellar gene regulation in Legionella pneumophila and its relation to growth phase. J. Bacteriol. 192, 446–455. doi: 10.1128/JB.00610-09
Al-Khodor, S., Kalachikov, S., Morozova, I., Price, C. T., and Abu Kwaik, Y. (2009). The PmrA/PmrB two-component system of Legionella pneumophila is a global regulator required for intracellular replication within macrophages and protozoa. Infect. Immun. 77, 374–386. doi: 10.1128/IAI.01081-08
Altman, E., and Segal, G. (2008). The response regulator CpxR directly regulates expression of several Legionella pneumophila icm/dot components as well as new translocated substrates. J. Bacteriol. 90, 1985–1996. doi: 10.1128/JB.01493-07
Appelt, S., and Heuner, K. (2017). The flagellar regulon of Legionella-a review. Front. Cell. Infect. Microbiol. 7:454. doi: 10.3389/fcimb.2017.00454
Bachman, M. A., and Swanson, M. S. (2004). Genetic evidence that Legionella pneumophila RpoS modulates expression of the transmission phenotype in both the exponential phase and the stationary phase. Infect. Immun. 72, 2468–2476. doi: 10.1128/IAI.72.5.2468-2476.2004
Barker, J., Scaife, H., and Brown, M. R. (1995). Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob. Agents Chemother. 39, 2684–2688. doi: 10.1128/AAC.39.12.2684
Boudry, P., Gracia, C., Monot, M., Caillet, J., Saujet, L., Hajnsdorf, E., et al. (2014). Pleiotropic role of the RNA chaperone protein Hfq in the human pathogen Clostridium difficile. J. Bacteriol. 196, 3234–3248. doi: 10.1128/JB.01923-14
Bozue, J. A., and Johnson, W. (1996). Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect. Immun. 64, 668–673.
Brieland, J. K., Fantone, J. C., Remick, D. G., LeGendre, M., McClain, M., and Engleberg, N. C. (1997). The role of Legionella pneumophila-infected Hartmannella vermiformis as an infectious particle in a murine model of Legionnaire's disease. Infect. Immun. 65, 5330–5333.
Brüggemann, H., Hagman, A., Jules, M., Sismeiro, O., Dillies, M. A., Gouyette, C., et al. (2006). Virulence strategies for infecting phagocytes deduced from the in vivo transcriptional program of Legionella pneumophila. Cell. Microbiol. 8, 1228–1240. doi: 10.1111/j.1462-5822.2006.00703.x
Byrne, B., and Swanson, M. S. (1998). Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66, 3029–3034.
Cazalet, C., Gomez-Valero, L., Rusniok, C., Lomma, M., Dervins-Ravault, D., Newton, H. J., et al. (2010). Analysis of the Legionella longbeachae genome and transcriptome uncovers unique strategies to cause Legionnaires' disease. PLoS Genet. 6:e1000851. doi: 10.1371/journal.pgen.1000851
Cazalet, C., Rusniok, C., Brüggemann, H., Zidane, N., Magnier, A., Ma, L., et al. (2004). Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36, 1165–1173. doi: 10.1038/ng1447
Choy, A., Dancourt, J., Mugo, B., O'Connor, T. J., Isberg, R. R., Melia, T. J., et al. (2012). The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science 338, 1072–1076. doi: 10.1126/science.1227026
Cirillo, J. D., Falkow, S., and Tompkins, L. S. (1994). Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect. Immun. 62, 3254–3261.
Correia, A. M., Ferreira, J. S., Borges, V., Nunes, A., Gomes, B., Capucho, R., et al. (2016). Probable person-to-person transmission of Legionnaires' disease. N. Engl. J. Med. 374, 497–498. doi: 10.1056/NEJMc1505356
Cunha, B. A., Burillo, A., and Bouza, E. (2016). Legionnaires' disease. Lancet 387, 376–385. doi: 10.1016/S0140-6736(15)60078-2
Dalebroux, Z. D., Edwards, R. L., and Swanson, M. S. (2009). SpoT governs Legionella pneumophila differentiation in host macrophages. Mol. Microbiol. 71, 640–658. doi: 10.1111/j.1365-2958.2008.06555.x
Dalebroux, Z. D., Svensson, S. L., Gaynor, E. C., and Swanson, M. S. (2010a). ppGpp conjures bacterial virulence. Microbiol. Mol. Biol. Rev. 74, 171–199. doi: 10.1128/MMBR.00046-09
Dalebroux, Z. D., and Swanson, M. S. (2012). ppGpp: magic beyond RNA polymerase. Nat. Rev. Microbiol. 10, 203–212. doi: 10.1038/nrmicro2720
Dalebroux, Z. D., Yagi, B. F., Sahr, T., Buchrieser, C., and Swanson, M. S. (2010b). Distinct roles of ppGpp and DksA in Legionella pneumophila differentiation. Mol. Microbiol. 76, 200–219. doi: 10.1111/j.1365-2958.2010.07094.x
de Felipe, K. S., Pampou, S., Jovanovic, O. S., Pericone, C. D., Ye, S. F., Kalachikov, S., et al. (2005). Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J. Bacteriol. 187, 7716–7726. doi: 10.1128/JB.187.22.7716-7726.2005
De Leon, J. A., Qiu, J., Nicolai, C. J., Counihan, J. L., Barry, K. C., Xu, L., et al. (2017). Positive and negative regulation of the master metabolic regulator mTORC1 by two families of Legionella pneumophila effectors. Cell Rep. 21, 2031–2038. doi: 10.1016/j.celrep.2017.10.088
Dietrich, C., Heuner, K., Brand, B. C., Hacker, J., and Steinert, M. (2001). Flagellum of Legionella pneumophila positively affects the early phase of infection of eukaryotic host cells. Infect. Immun. 69, 2116–2122. doi: 10.1128/IAI.69.4.2116-2122.2001
Edwards, R., Jules, M., Sahr, T., Buchrieser, C., and Swanson, M. (2010). The multi-step design of the LetA/LetS two-component system increases Legionella pneumophila versatility. Infect. Immun. 78, 2571–2583. doi: 10.1128/IAI.01107-09
Eisenreich, W., and Heuner, K. (2016). The life stage-specific pathometabolism of Legionella pneumophila. FEBS Lett. 590, 3868–3886. doi: 10.1002/1873-3468.12326
Escoll, P., Mondino, S., Rolando, M., and Buchrieser, C. (2016). Targeting of host organelles by pathogenic bacteria: a sophisticated subversion strategy. Nat. Rev. Microbiol. 14, 5–19. doi: 10.1038/nrmicro.2015.1
Escoll, P., Rolando, M., Gomez-Valero, L., and Buchrieser, C. (2013). From amoeba to macrophages: exploring the molecular mechanisms of Legionella pneumophila infection in both hosts. Curr. Top. Microbiol. Immunol. 376, 1–34. doi: 10.1007/82_2013_351
Eylert, E., Herrmann, V., Jules, M., Gillmaier, N., Lautner, M., Buchrieser, C., et al. (2010). Isotopologue profiling of Legionella pneumophila: role of serine and glucose as carbon substrates. J. Biol. Chem. 285, 22232–22243. doi: 10.1074/jbc.M110.128678
Faucher, S. P., Mueller, C. A., and Shuman, H. A. (2011). Legionella pneumophila transcriptome during intracellular multiplication in human macrophages. Front. Microbiol. 2:60. doi: 10.3389/fmicb.2011.00060
Faulkner, G., and Garduño, R. A. (2002). Ultrastructural analysis of differentiation in Legionella pneumophila. J. Bacteriol. 184, 7025–7041. doi: 10.1128/JB.184.24.7025-7041.2002
Fields, B. S. (1996). The molecular ecology of Legionellae. Trends Microbiol. 4, 286–290. doi: 10.1016/0966-842X(96)10041-X
Fields, B. S., Benson, R. F., and Besser, R. E. (2002). Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15, 506–526. doi: 10.1128/CMR.15.3.506-526.2002
Gal-Mor, O., and Segal, G. (2003a). Identification of CpxR as a positive regulator of icm and dot virulence genes of Legionella pneumophila. J. Bacteriol. 185, 4908–4919. doi: 10.1128/JB.185.16.4908-4919.2003
Gal-Mor, O., and Segal, G. (2003b). The Legionella pneumophila GacA homolog (LetA) is involved in the regulation of icm virulence genes and is required for intracellular multiplication in Acanthamoeba castellanii. Microb. Pathog. 34, 187–194. doi: 10.1016/S0882-4010(03)00027-5
Garduño, R. A., Garduño, E., Hiltz, M., and Hoffman, P. S. (2002). Intracellular growth of Legionella pneumophila gives rise to a differentiated form dissimilar to stationary-phase forms. Infect. Immun. 70, 6273–6283. doi: 10.1128/IAI.70.11.6273-6283.2002
George, J. R., Pine, L., Reeves, M. W., and Harrell, W. K. (1980). Amino acid requirements of Legionella pneumophila. J. Clin. Microbiol. 11, 286–291.
Gillmaier, N., Schunder, E., Kutzner, E., Tlapák, H., Rydzewski, K., Herrmann, V., et al. (2016). Growth-related metabolism of the carbon storage Poly-3-hydroxybutyrate in Legionella pneumophila. J. Biol. Chem. 291, 6471–6482. doi: 10.1074/jbc.M115.693481
Gomez-Valero, L., Rusniok, C., Cazalet, C., and Buchrieser, C. (2011). Comparative and functional genomics of Legionella identified eukaryotic like proteins as key players in host-pathogen interactions. Front. Microbiol. 2:208. doi: 10.3389/fmicb.2011.00208
Hales, L. M., and Shuman, H. A. (1999). The Legionella pneumophila rpoS gene is required for growth within Acanthamoeba castellanii. J. Bacteriol. 181, 4879–4889.
Hammer, B. K., and Swanson, M. S. (1999). Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33, 721–731. doi: 10.1046/j.1365-2958.1999.01519.x
Hammer, B. K., Tateda, E. S., and Swanson, M. S. (2002). A two-component regulator induces the transmission phenotype of stationary-phase Legionella pneumophila. Mol. Microbiol. 44, 107–118. doi: 10.1046/j.1365-2958.2002.02884.x
Harada, E., Iida, K., Shiota, S., Nakayama, H., and Yoshida, S. (2010). Glucose metabolism in Legionella pneumophila: dependence on the Entner-Doudoroff pathway and connection with intracellular bacterial growth. J. Bacteriol. 192, 2892–2899. doi: 10.1128/JB.01535-09
Haugen, S. P., Ross, W., and Gourse, R. L. (2008). Advances in bacterial promoter recognition and its control by factors that do not bind DNA. Nat. Rev. Microbiol. 6, 507–519. doi: 10.1038/nrmicro1912
Häuslein, I., Manske, C., Goebel, W., Eisenreich, W., and Hilbi, H. (2016). Pathway analysis using (13) C-glycerol and other carbon tracers reveals a bipartite metabolism of Legionella pneumophila. Mol. Microbiol. 100, 229–246. doi: 10.1111/mmi.13313
Häuslein, I., Sahr, T., Escoll, P., Klausner, N., Eisenreich, W., and Buchrieser, C. (2017). Legionella pneumophila CsrA regulates a metabolic switch from amino acid to glycerolipid metabolism. Open Biol 7:170149. doi: 10.1098/rsob.170149
Herrmann, V., Eidner, A., Rydzewski, K., Blädel, I., Jules, M., Buchrieser, C., et al. (2011). GamA is a eukaryotic-like glucoamylase responsible for glycogen- and starch-degrading activity of Legionella pneumophila. Int. J. Med. Microbiol. 301, 133–139. doi: 10.1016/j.ijmm.2010.08.016
Heuner, K., Dietrich, C., Skriwan, C., Steinert, M., and Hacker, J. (2002). Influence of the alternative sigma(28) factor on virulence and flagellum expression of Legionella pneumophila. Infect. Immun. 70, 1604–1608. doi: 10.1128/IAI.70.3.1604-1608.2002
Heuner, K., Hacker, J., and Brand, B. C. (1997). The alternative sigma factor sigma28 of Legionella pneumophila restores flagellation and motility to an Escherichia coli fliA mutant. J. Bacteriol. 179, 17–23. doi: 10.1128/jb.179.1.17-23.1997
Heuner, K., and Steinert, M. (2003). The flagellum of Legionella pneumophila and its link to the expression of the virulent phenotype. Int. J. Med. Microbiol. 293, 133–143. doi: 10.1078/1438-4221-00259
Hilbi, H., Hoffmann, C., and Harrison, C. F. (2011). Legionella spp. outdoors: colonization, communication and persistence. Environ. Microbiol. Rep. 3, 286–296. doi: 10.1111/j.1758-2229.2011.00247.x
Hilbi, H., Jarraud, S., Hartland, E., and Buchrieser, C. (2010). Update on Legionnaires' disease: pathogenesis, epidemiology, detection and control. Mol. Microbiol. 76, 1–11 doi: 10.1111/j.1365-2958.2010.07086.x
Hilbi, H., Weber, S. S., Ragaz, C., Nyfeler, Y., and Urwyler, S. (2007). Environmental predators as models for bacterial pathogenesis. Environ. Microbiol. 9, 563–575. doi: 10.1111/j.1462-2920.2007.01238.x
Hochstrasser, R., and Hilbi, H. (2017). Intra-species and inter-kingdom signaling of Legionella pneumophila. Front. Microbiol. 8:79. doi: 10.3389/fmicb.2017.00079
Horwitz, M. A., and Silverstein, S. C. (1980). Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J. Clin. Invest. 66, 441–450. doi: 10.1172/JCI109874
Hovel-Miner, G., Pampou, S., Faucher, S. P., Clarke, M., Morozova, I., Morozov, P., et al. (2009). SigmaS controls multiple pathways associated with intracellular multiplication of Legionella pneumophila. J. Bacteriol. 191, 2461–2473. doi: 10.1128/JB.01578-08
Isaac, D. T., Laguna, R. K., Valtz, N., and Isberg, R. R. (2015). MavN is a Legionella pneumophila vacuole-associated protein required for efficient iron acquisition during intracellular growth. Proc. Natl. Acad. Sci. U.S.A. 112, E5208–E5217. doi: 10.1073/pnas.1511389112
Isberg, R. R., O'Connor, T. J., and Heidtman, M. (2009). The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7, 13–24. doi: 10.1038/nrmicro1967
Jacobi, S., Schade, R., and Heuner, K. (2004). Characterization of the alternative sigma factor sigma54 and the transcriptional regulator FleQ of Legionella pneumophila, which are both involved in the regulation cascade of flagellar gene expression. J. Bacteriol. 186, 2540–2547. doi: 10.1128/JB.186.9.2540-2547.2004
James, B. W., Mauchline, W. S., Dennis, P. J., Keevil, C. W., and Wait, R. (1999). Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low-nutrient environments. Appl. Environ. Microbiol. 65, 822–827.
Jishage, M., Kvint, K., Shingler, V., and Nyström, T. (2002). Regulation of sigma factor competition by the alarmone ppGpp. Genes Dev. 16, 1260–1270. doi: 10.1101/gad.227902
Kulkarni, P. R., Cui, X., Williams, J. W., Stevens, A. M., and Kulkarni, R. V. (2006). Prediction of CsrA-regulating small RNAs in bacteria and their experimental verification in Vibrio fischeri. Nucleic Acids Res. 34, 3361–3369. doi: 10.1093/nar/gkl439
Lang, C., and Flieger, A. (2011). Characterisation of Legionella pneumophila phospholipases and their impact on host cells. Eur. J. Cell Biol. 90, 903–912. doi: 10.1016/j.ejcb.2010.12.003
Liu, M. Y., Gui, G., Wei, B., Preston, J. F. R., Oakford, L., Yüksel, U., et al. (1997). The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J. Biol. Chem. 272, 17502–17510. doi: 10.1074/jbc.272.28.17502
Lomma, M., Dervins-Ravault, D., Rolando, M., Nora, T., Newton, H. J., Sansom, F. M., et al. (2010). The Legionella pneumophila F-box protein Lpp2082 (AnkB) modulates ubiquitination of the host protein parvin B and promotes intracellular replication. Cell. Microbiol. 12, 1272–1291. doi: 10.1111/j.1462-5822.2010.01467.x
Lynch, D., Fieser, N., Glöggler, K., Forsbach-Birk, V., and Marre, R. (2003). The response regulator LetA regulates the stationary-phase stress response in Legionella pneumophila and is required for efficient infection of Acanthamoeba castellanii. FEMS Microbiol. Lett. 219, 241–248. doi: 10.1016/S0378-1097(03)00050-8
Manske, C., Schell, U., and Hilbi, H. (2016). Metabolism of myo-inositol by Legionella pneumophila promotes infection of amoeba and macrophages. Appl. Environ. Microbiol. 82, 5000–5014. doi: 10.1128/AEM.01018-16
McDade, J. E., Shepard, C. C., Fraser, D. W., Tsai, T. R., Redus, M. A., and Dowdle, W. R. (1977). Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N. Engl. J. Med. 297, 1197–1203. doi: 10.1056/NEJM197712012972202
McNealy, T. L., Forsbach-Birk, V., Shi, C., and Marre, R. (2005). The Hfq homolog in Legionella pneumophila demonstrates regulation by LetA and RpoS and interacts with the global regulator CsrA. J. Bacteriol. 187, 1527–1532. doi: 10.1128/JB.187.4.1527-1532.2005
Molofsky, A. B., and Swanson, M. S. (2003). Legionella pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. Mol. Microbiol. 50, 445–461. doi: 10.1046/j.1365-2958.2003.03706.x
Molofsky, A. B., and Swanson, M. S. (2004). Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53, 29–40. doi: 10.1111/j.1365-2958.2004.04129.x
Newton, H. J., Ang, D. K., van Driel, I. R., and Hartland, E. L. (2010). Molecular pathogenesis of infections caused by Legionella pneumophila. Clin. Microbiol. Rev. 23, 274–298. doi: 10.1128/CMR.00052-09
Ninio, S., and Roy, C. R. (2007). Effector proteins translocated by Legionella pneumophila: strength in numbers. Trends Microbiol. 15, 372–380. doi: 10.1016/j.tim.2007.06.006
Nora, T., Lomma, M., Gomez-Valero, L., and Buchrieser, C. (2009). Molecular mimicry: an important virulence strategy employed by Legionella pneumophila to subvert host functions. Future Microbiol. 4, 691–701. doi: 10.2217/fmb.09.47
Oliva, G., Sahr, T., Rolando, M., Knoth, M., and Buchrieser, C. (2017). A unique cis-encoded small noncoding RNA is regulating Legionella pneumophila Hfq expression in a life cycle-dependent manner. MBio 8:e02182-16. doi: 10.1128/mBio.02182-16
Padilla-Vaca, F., Mondragón-Jaimes, V., and Franco, B. (2017). General aspects of two-component regulatory circuits in bacteria: domains, signals and roles. Curr. Protein Pept. Sci. 18, 990–1004. doi: 10.2174/1389203717666160809154809
Pine, L., George, J. R., Reeves, M. W., and Harrell, W. K. (1979). Development of a chemically defined liquid medium for growth of Legionella pneumophila. J. Clin. Microbiol. 9, 615–626.
Potrykus, K., and Cashel, M. (2008). (p)ppGpp: still magical? Annu. Rev. Microbiol. 62, 35–51. doi: 10.1146/annurev.micro.62.081307.162903
Price, C. T., Al-Khodor, S., Al-Quadan, T., Santic, M., Habyarimana, F., Kalia, A., et al. (2009). Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa. PLoS Pathog. 5:e1000704. doi: 10.1371/journal.ppat.1000704
Price, C. T., Al-Quadan, T., Santic, M., Rosenshine, I., and Abu Kwaik, Y. (2011). Host proteasomal degradation generates amino acids essential for intracellular bacterial growth. Science 334, 1553–1557. doi: 10.1126/science.1212868
Rasis, M., and Segal, G. (2009). The LetA-RsmYZ-CsrA regulatory cascade, together with RpoS and PmrA, post-transcriptionally regulates stationary phase activation of Legionella pneumophila Icm/Dot effectors. Mol. Microbiol. 72, 995–1010. doi: 10.1111/j.1365-2958.2009.06705.x
Ristroph, J. D., Hedlund, K. W., and Gowda, S. (1981). Chemically defined medium for Legionella pneumophila growth. J. Clin. Microbiol 13, 115–119.
Robertson, P., Abdelhady, H., and Garduño, R. A. (2014). The many forms of a pleomorphic bacterial pathogen-the developmental network of Legionella pneumophila. Front. Microbiol. 5:670. doi: 10.3389/fmicb.2014.00670
Robinson, C. G., and Roy, C. R. (2006). Attachment and fusion of endoplasmic reticulum with vacuoles containing Legionella pneumophila. Cell. Microbiol. 8, 793–805. doi: 10.1111/j.1462-5822.2005.00666.x
Rolando, M., Escoll, P., Nora, T., Botti, J., Boitez, V., Bedia, C., et al. (2016). Legionella pneumophila S1P-lyase targets host sphingolipid metabolism and restrains autophagy. Proc. Natl. Acad. Sci. U.S.A. 113, 1901–1906. doi: 10.1073/pnas.1522067113
Rowbotham, T. J. (1986). Current views on the relationships between amoebae, legionellae and man. Isr. J. Med. Sci. 22, 678–689.
Sahr, T., Bruggemann, H., Jules, M., Lomma, M., Albert-Weissenberger, C., Cazalet, C., et al. (2009). Two small ncRNAs jointly govern virulence and transmission in Legionella pneumophila. Mol. Microbiol. 72, 741–762. doi: 10.1111/j.1365-2958.2009.06677.x
Sahr, T., Rusniok, C., Dervins-Ravault, D., Sismeiro, O., Coppee, J. Y., and Buchrieser, C. (2012). Deep sequencing defines the transcriptional map of L. pneumophila and identifies growth phase-dependent regulated ncRNAs implicated in virulence. RNA Biol. 9, 503–519. doi: 10.4161/rna.20270
Sahr, T., Rusniok, C., Impens, F., Oliva, G., Sismeiro, O., Coppee, J. Y., et al. (2017). The Legionella pneumophila genome evolved to accommodate multiple regulatory mechanisms controlled by the CsrA-system. PLoS Genet. 13:e1006629. doi: 10.1371/journal.pgen.1006629
Sauer, J. D., Bachman, M. A., and Swanson, M. S. (2005). The phagosomal transporter A couples threonine acquisition to differentiation and replication of Legionella pneumophila in macrophages. Proc. Natl. Acad. Sci. U.S.A. 102, 9924–9929. doi: 10.1073/pnas.0502767102
Schunder, E., Gillmaier, N., Kutzner, E., Eisenreich, W., Herrmann, V., Lautner, M., et al. (2014). Amino acid uptake and metabolism of Legionella pneumophila hosted by Acanthamoeba castellanii. J. Biol. Chem. 289, 21040–21054. doi: 10.1074/jbc.M114.570085
Steinert, M., Emody, L., Amann, R., and Hacker, J. (1997). Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl. Environ. Microbiol. 63, 2047–2053.
Steinert, M., Hentschel, U., and Hacker, J. (2002). Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol. Rev. 26, 149–162. doi: 10.1111/j.1574-6976.2002.tb00607.x
Steinert, M., Heuner, K., Buchrieser, C., Albert-Weissenberger, C., and Glockner, G. (2007). Legionella pathogenicity: genome structure, regulatory networks and the host cell response. Int. J. Med. Microbiol. 297, 577–587. doi: 10.1016/j.ijmm.2007.03.009
Sturgill-Koszycki, S., and Swanson, M. S. (2000). Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 192, 1261–1272. doi: 10.1084/jem.192.9.1261
Tesh, M. J., Morse, S. A., and Miller, R. D. (1983). Intermediary metabolism in Legionella pneumophila: utilization of amino acids and other compounds as energy sources. J. Bacteriol. 154, 1104–1109.
Tiaden, A., Spirig, T., Weber, S. S., Bruggemann, H., Bosshard, R., Buchrieser, C., et al. (2007). The Legionella pneumophila response regulator LqsR promotes host cell interactions as an element of the virulence regulatory network controlled by RpoS and LetA. Cell. Microbiol. 9, 2903–2920. doi: 10.1111/j.1462-5822.2007.01005.x
Trigui, H., Dudyk, P., Oh, J., Hong, J. I., and Faucher, S. P. (2015). A regulatory feedback loop between RpoS and SpoT supports the survival of Legionella pneumophila in water. Appl. Environ. Microbiol. 81, 918–928. doi: 10.1128/AEM.03132-14
Trigui, H., Dudyk, P., Sum, J., Shuman, H. A., and Faucher, S. P. (2013). Analysis of the transcriptome of Legionella pneumophila hfq mutant reveals a new mobile genetic element. Microbiology 159(Pt 8), 1649–1660. doi: 10.1099/mic.0.067983-0
Weiss, E., Peacock, E. M., and Williams, J. C. (1980). Glucose and glutamate metabolism of Legionella pneumophila. Curr. Microbiol. 4, 1–6. doi: 10.1007/BF02602882
Wieland, H., Ullrich, S., Lang, F., and Neumeister, B. (2005). Intracellular multiplication of Legionella pneumophila depends on host cell amino acid transporter SLC1A5. Mol. Microbiol. 55, 1528–1537. doi: 10.1111/j.1365-2958.2005.04490.x
Xiao, H., Kalman, M., Ikehara, K., Zemel, S., Glaser, G., and Cashel, M. (1991). Residual guanosine 3',5'-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266, 5980–5990.
Yu, V. L., Plouffe, J. F., Pastoris, M. C., Stout, J. E., Schousboe, M., Widmer, A., et al. (2002). Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J. Infect. Dis. 186, 127–128. doi: 10.1086/341087
Zhu, W., Banga, S., Tan, Y., Zheng, C., Stephenson, R., Gately, J., et al. (2011). Comprehensive identification of protein substrates of the Dot/Icm type iv transporter of Legionella pneumophila. PLoS ONE 6:e17638. doi: 10.1371/journal.pone.0017638
Zusman, T., Aloni, G., Halperin, E., Kotzer, H., Degtyar, E., Feldman, M., et al. (2007). The response regulator PmrA is a major regulator of the icm/dot type IV secretion system in Legionella pneumophila and Coxiella burnetii. Mol. Microbiol. 63, 1508–1523. doi: 10.1111/j.1365-2958.2007.05604.x
Keywords: Legionella pneumophila, regulation, virulence, metabolism, life cycle
Citation: Oliva G, Sahr T and Buchrieser C (2018) The Life Cycle of L. pneumophila: Cellular Differentiation Is Linked to Virulence and Metabolism. Front. Cell. Infect. Microbiol. 8:3. doi: 10.3389/fcimb.2018.00003
Received: 19 November 2017; Accepted: 05 January 2018;
Published: 19 January 2018.
Edited by:
Matthias P. Machner, National Institutes of Health (NIH), United StatesReviewed by:
Klaus Heuner, Robert Koch Institut, GermanyCopyright © 2018 Oliva, Sahr and Buchrieser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carmen Buchrieser, Y2J1Y2hAcGFzdGV1ci5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.