
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 22 June 2017
Sec. Parasite and Host
Volume 7 - 2017 | https://doi.org/10.3389/fcimb.2017.00281
This article is part of the Research Topic Tick-host-pathogen Interactions View all 39 articles
As long-term pool feeders, ticks have developed myriad strategies to remain discreetly but solidly attached to their hosts for the duration of their blood meal. The critical biological material that dampens host defenses and facilitates the flow of blood—thus assuring adequate feeding—is tick saliva. Saliva exhibits cytolytic, vasodilator, anticoagulant, anti-inflammatory, and immunosuppressive activity. This essential fluid is secreted by the salivary glands, which also mediate several other biological functions, including secretion of cement and hygroscopic components, as well as the watery component of blood as regards hard ticks. When salivary glands are invaded by tick-borne pathogens, pathogens may be transmitted via saliva, which is injected alternately with blood uptake during the tick bite. Both salivary glands and saliva thus play a key role in transmission of pathogenic microorganisms to vertebrate hosts. During their long co-evolution with ticks and vertebrate hosts, microorganisms have indeed developed various strategies to exploit tick salivary molecules to ensure both acquisition by ticks and transmission, local infection and systemic dissemination within the vertebrate host.
Ticks are obligate hematophagous arthropods and act as vectors of the greatest variety of pathogens including viruses, parasites, and bacteria (de la Fuente et al., 2008; Rizzoli et al., 2014). On a global scale, they represent the most important vectors of pathogens that affect animals, and are second only to mosquitoes where humans are concerned (Dantas-Torres et al., 2012). Their remarkable success as disease vectors is mainly related to their longevity, high reproductive potential and broad host spectrum for several species, as well as to their capacity to imbibe a very large quantity of blood over a relatively long period of time.
For most tick-borne pathogens (TBP), transmission to the vertebrate host occurs via the saliva, underscoring the importance of both salivary glands (SG) and saliva in the transmission process. During feeding, ticks inject saliva and absorb their meal in an alternating pattern through the same canal. They are pool feeders, ingurgitating all of the fluids that are exuded into the haemorrhagic pool generated by the bite. TBP are ingested by ticks during their feeding on infected hosts. From the midgut, TBP cross the digestive epithelium and invade the haemocoel, from which they can penetrate the SG epithelium to invade the SG. From there, TBP can be transmitted to a new host via saliva injected during a new blood meal (Figure 1).
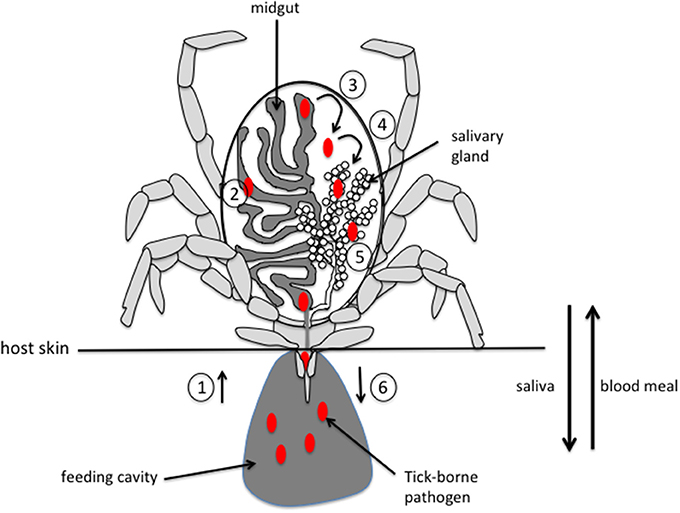
Figure 1. Schematic representation of pathogen acquisition, development and transmission by a tick. (1) pathogens are ingested by the tick along with the blood meal during the bite. (2) Pathogens invade the midgut and, depending on the species, stay in the midgut until the next feeding or immediately cross the epithelium of the digestive tract (3) to invade the tick body. (4) Pathogens move into the salivary glands by crossing the epithelium and invade the acini (5). (6) Pathogens are injected into a new host during feeding, along with saliva that counteracts host hemostasis, inflammation and immune responses, thus facilitating pathogen infection of host. Please note that, for clarity, only half of the digestive tract and a single salivary gland are represented.
Vertebrates react to skin injury inflicted by tick bites by the formation of a haemostatic plug, vasoconstriction, inflammation and tissue remodeling related to wound healing. If unchecked, these processes would cause tick rejection and/or disrupt tick feeding, and arrest their further development. To facilitate the flow of blood and assure feeding, however, ticks have evolved a complex and sophisticated pharmacological armament that blocks pain and itch, inhibits haemostasis, and modulates innate and adaptive immune responses, angiogenesis and wound healing in their hosts (Francischetti et al., 2009; Mans, 2011; Kazimirova and Stibraniova, 2013; Štibrániová et al., 2013; Wikel, 2013; Valdes, 2014; Kotal et al., 2015; Chmelar et al., 2016b). It has been demonstrated that these molecules create a favorable environment for transmission, survival, and propagation of TBP within the vertebrate host (Wikel, 1999; Kazimirova and Stibraniova, 2013). In addition, several studies have reported that tick SG differentially express transcripts and proteins in response to pathogen infection, but only a few SG factors have been identified as directly implicated in pathogen transmission (Liu and Bonnet, 2014). In this review, we will summarize the essential role of both SG and saliva in tick biology as well as in TBP acquisition and transmission.
The tick SG play multiple essential functions during both on- and off-host periods and represent a key route in transmission of TBP. The physiological activity and unique morphology of this tissue are intimately associated with adaptation of the tick to the parasitic lifestyle. Here we briefly describe the structure and function of SG in both ixodid and argasid ticks and discuss the most important findings with respect to the physiology of SG secretion in ixodid ticks.
In both argasid and ixodid ticks the female SG consist of a large number of acini (or otherwise called alveoli) of three different types (type I, II, and III) in ixodid and two different types (type I and II) in argasid ticks. In addition, ixodid males possess a fourth type (type IV) of acini in their SG. Agranular type I acini connect almost exclusively to the anterior part of the main salivary duct, while the granular type II and III acini are associated with more distally located secondary and tertiary ducts, respectively (Coons and Roshdy, 1973; Binnington, 1978; Fawcett et al., 1981a; Walker et al., 1985; Sonenshine, 1991). The agranular acini are morphologicaly similar in both argasid and ixodid ticks, and generally comprise four distinct cell types: a single central lamellate cell, multiple peripheral lamellate cells, peritubular cells, and one circumlumenal cell (Needham et al., 1990; Sonenshine, 1991). In type II and III acini, in addition to various agranular cell types (such as epithelial, adlumenal, ablumenal interstitial, and neck cells), 7–9 various glandular cells (divided into the a-f types depending on tick species), enclosing the secretory granules have generally been recognized (Fawcett et al., 1986; Sonenshine, 1991). The single adlumenal cell, also called the Cap or myoepithelial cell (Meredith and Kaufman, 1973; Krolak et al., 1982), lines the luminal surface of the type II and III acini in web-like fashion, and its contractions facilitate expulsion of the acinar contents into the connecting ducts during tick feeding (Krolak et al., 1982; Coons et al., 1994; Šimo et al., 2014b). During feeding of Ixodid females, the majority of the acinar cells of both type II and III acini undergo marked hypertrophy, resulting in overall increase in the mass of the SG (Binnington, 1978; Fawcett et al., 1986; Šimo et al., 2013). In particular, the lumen of type III acini greatly expands due to fluid uptake from the hemolymph (Meredith and Kaufman, 1973; Fawcett et al., 1981a; Kim et al., 2014), while in type II acini the cell bodies enlarge and the lumen remains proportionally smaller (Binnington, 1978; Walker et al., 1985).
Tick SG mediate diverse functions that ensure the tick's biological success during both on- and off-host periods. Here, we briefly discuss their role in absorption of moisture from unsaturated atmosphere, concentration of the nutrient portion of the blood meal by elimination of excess fluid and, finally, production of the cement that anchors the hypostome in the host skin. The crucial role of tick SG in secretion of biologically active molecules that facilitate acquisition of the blood meal and TBP development is elaborated later in this review.
During the fasting period off the host, the conservation of water is critical for ticks to avoid death due to desiccation. It is generally believed that the type I acini are actively involved in absorption of humidity from the surrounding environment. Under desiccating conditions these structures secrete a highly hygroscopic solution rich in Na+, K+, and Cl− onto the surface of the mouthparts, which is subsequently swallowed back along with absorbed moisture (Knulle and Rudolph, 1982; Needham and Teel, 1986; Needham et al., 1990; Sonenshine, 1991; Gaede and Knülle, 1997). The absorptive property of type I acini was recently confirmed by an elegant experiment in which a fluorescent trace dye (Rhodamine 123) imbibed by desiccated I. scapularis females was shown to accumulate exclusively in type I acini (Kim et al., 2016).
Among blood-feeding arthropods, ixodid ticks are unique in the long duration of attachment to the host, which varies from several days up to weeks depending on life stage and tick species. When feeding, female ticks are capable of increasing their weight more than 100-fold due to the blood-meal uptake. At the same time, a large amount of excess fluid (including ions) is excreted back to the feeding site via SG, thus maintaining homeostasis (Balashov, 1972; Sonenshine, 1991; Sauer et al., 1995). In particular, water and ions from the digested blood meal cross the wall of the midgut into the hemocoel where they are taken up by SG and subsequently secreted via the salivary ducts back into the host. In 1973, Kaufman and Philips reported that in Dermacentor andersoni females 74% of the water and 96% of the sodium are expelled back to the host via this route. Several studies have suggested that the epithelium of type III acini is the site at which water and electrolytes from surrounding haemolymph gain access into SG (Meredith and Kaufman, 1973; Fawcett et al., 1981b). This hypothesis has been reinforced by a recent study in which the sodium-potassium pump (Na/K-ATPase) involved in formation of the sodium-rich primary saliva was evidenced in the epithelial cells of all three types of SG acini (Kim et al., 2016). In argasid ticks, the mechanism by which excess water is eliminated is different, being accomplished by the coxal glands, which are unique to this tick family (Binnington, 1975).
When attached to a host, the mouthparts of most of the ixodid ticks are encased by a cement cone, allowing the ticks to anchor themselves firmly in the host skin and simultaneously protecting the mouthparts from the host immune system (Kemp et al., 1982). The production of the cement cone has been observed exclusively in the ixodid lineage, although not all of the species from the Ixodes genus produce cement (Kemp et al., 1982; Mans, 2014). The origin of this substance, composed of polymerized and hardened glycine-rich proteins, lipids and certain carbohydrates, are the cells of the type II and III SG acini (Chinery, 1973; Jaworski et al., 1992). In addition to glycine-rich proteins, recent proteomic analyses of the cement cone from Amblyomma americanum evidenced multiple serine protease inhibitors and metalloproteases (Bullard et al., 2016), some of them considered to be promising anti-tick vaccine candidates due to their antigenic properties (Shapiro et al., 1987; Mulenga et al., 1999; Bishop et al., 2002).
The tick SG is controlled by the nerves arising from the synganglion, the central nervous system of ticks (Kaufman and Harris, 1983; Fawcett et al., 1986; Šimo et al., 2009a,b, 2012, 2014a,b). Over the past four decades, pharmacological studies have revealed multiple chemical agents capable of inducing—either directly or indirectly—tick SG secretion (Kaufman, 1978; Sauer et al., 1995, 2000; Bowman and Sauer, 2004; Kim et al., 2014). Among these, catecholamines have been shown to be particularly effective activators of SG fluid secretion in both in vivo and in vitro assays (Kaufman, 1976; Lindsay and Kaufman, 1986; McSwain et al., 1992; Šimo et al., 2012). The downstream action of dopamine, the most potent activator of SG fluid secretion, has been studied in detail. It has been suggested that dopamine autocrine/paracrine signaling in tick SG (Kaufman, 1977, 1978; Šimo et al., 2011; Koči et al., 2014) activates two independent downstream pathways: cAMP-dependent transduction, which results in fluid secretion, and a calcium-dependent pathway leading to the secretion of prostaglandin E2 (PGE2) into the salivary cocktail. PGE2 may subsequently induce the exocytosis of anticoagulant proteins via paracrine signal in tick SG (Qian et al., 1998; Sauer et al., 2000). Dopamine acts via two different cognate receptors expressed in both type II and III SG acini: the D1 dopamine receptor and the invertebrate-specific D1-like dopamine receptor (InvD1L). Pharmacological study of these receptors has revealed that activation of the D1 receptor preferentially triggers the cAMP-dependent downstream pathway, while activation of the InvD1L exclusively causes mobilization of intracellular calcium (Šimo et al., 2011, 2014b). Based on immunohistochemical studies and subsequent physiological experiments performed on isolated SG, distinct structure-function relationships have been proposed for each of these receptors. In particular, the D1 receptor, which has been localized in cell junctions on the luminal surface of type II and III acini, may regulate the inward transport of fluid into the acini, while the InvD1L receptor, found to be expressed in the axon terminals in proximity to the myoepithelial cell in type II and III acini, is presumed to be involved in the expulsion of the acinar context into the connecting ducts (Šimo et al., 2011, 2014b; Kim et al., 2014).
In addition to dopamine, several other pharmacological agents, such as octopamine, norepinephrine, γ-aminobutyric acid (GABA), ergot alkaloids and pilocarpine, have been shown to exert either a direct or indirect effect on salivary secretion; their precise mode of action, however, remains enigmatic (Needham and Pannabecker, 1983; Pannabecker and Needham, 1985; Lindsay and Kaufman, 1986).
Based on recent studies, it appears that ticks are capable of selectively controlling particular types of acini (and likely individual cells within the acini) via the neuropeptidergic network arising from their synganglion and in which the SG is connected (Šimo et al., 2009a,b, 2012, 2013, 2014a; Roller et al., 2015). In particular, two neuropeptides, myoinhibitory peptide (MIP) and SIFamide, have been found to be co-expressed in the pair of giant neurons in the synganglion whose axonal projections reach the basal regions of type II and III acini (Šimo et al., 2009a,b, 2011, 2012, 2013, 2014a). The SIFamide receptor, as evidenced by immunostaining, was found in proximity to the acinar valve (basal region of acini), suggesting its role in control of this structure (Šimo et al., 2009b, 2013). Using immunohistochemical approaches, two other putative neuropeptides, orcokinin and pigment dispersing factor (PDF), were found in two pairs of neurons innervating exclusively type II SG acini (Šimo et al., 2009a, 2012; Roller et al., 2015). In summary, multiple axonal projections, expressing diverse signaling molecules or their receptors, reach the individual SG acini and regulate a variety of essential processes, presumably in response to the tick's fluctuating physiological requirements.
To understand the complex feeding biology of ticks, as well as the transmission of TBP, the composition of tick saliva must be molecularly resolved. Such resolution would underpin discovery of pharmacologically active compounds of clinical interest, protective antigens for anti-tick vaccines and antigens whose cognate antibody responses represent serological biomarkers of exposure to ticks. The first proteomic studies that addressed tick saliva date back to the first decade of the twenty-first century (Madden et al., 2004; Narasimhan et al., 2007a; Francischetti et al., 2008). Since then, transcript and protein profiling in tick SG have been applied to different developmental stages, sexes, and feeding stages of several species of hard and soft ticks. In addition, comparative analyses of tick SG have revealed that molecular expression varied according to tick life stage, sex, or behavior (Ribeiro et al., 2006; Anatriello et al., 2010; Diaz-Martin et al., 2013b), as well as according to the presence of pathogenic microorganism (Liu and Bonnet, 2014). It should be noted, however, that a minority of the salivary proteins have been functionally annotated, and that of these, the putative function has been verified for fewer than 5% (Francischetti et al., 2009). Nevertheless, these studies have already led to the discovery of multiple factors that contribute to successful tick feeding and evasion of the host immune and haemostatic defenses, which are reviewed below and presented in Table 1.
Ticks have developed strategies to block different arms of the haemostatic system of their hosts, and it has been suggested that these antihaemostatic strategies have evolved independently in argasid and ixodid ticks (Mans et al., 2008; Mans, 2011). Haemostasis refers to a set of processes—including vasoconstriction, formation of a platelet plug, blood coagulation, and fibrinolysis—that together control blood loss following vascular injury and ensure normal blood flow (Hoffman et al., 2009). After vascular injury, platelets adhere to exposed subendothelial tissue and are activated, principally owing to the engagement of platelet surface receptors by von Willebrand factor and collagen. Initial activation of platelets leads to release of soluble mediators—such as ADP, serotonin and thromboxane A2—which activate additional platelets, and to activation of platelet integrins. Integrin binding to multiple ligands in subendothelial tissue or on the surface of other platelets promotes further activation and aggregation of platelets, ultimately leading to the formation of a platelet plug. Moreover, serotonin and thromboxane A2 trigger vasoconstriction.
To counteract host-derived vasoconstrictors, ticks secrete vasodilators into the site of tissue injury, e.g., non-proteinaceous, lipid-derived substances, such as prostacyclin and prostaglandins (see Table 1). Certain salivary proteins, such as tick histamine release factor (tHRF) from Ixodes scapularis (Dai et al., 2010) and the serine proteinase inhibitor (serpin) IRS-2 from Ixodes ricinus, the latter of which inhibits chymase and cathepsin G (Chmelar et al., 2011), may modulate vascular permeability as well (Chmelar et al., 2012). An activity that counters vasoconstriction has also been evidenced in the salivary gland extracts (SGE) of Dermacentor reticulatus and Rhipicephalus appendiculatus, and while as yet unidentified, the active molecule(s) do not appear to belong to the prostaglandin family (Pekarikova et al., 2015).
Primary haemostasis, that is, platelet activation and aggregation at the site of vascular injury, is targeted by ticks in various manners (Francischetti, 2010). The tick adhesion inhibitor (TAI), found in Ornithodoros moubata, interferes with adhesion of platelets to soluble collagen and their ensuing activation and aggregation (Waxman and Connolly, 1993; Karczewski et al., 1995). In soft ticks (Ribeiro et al., 1991; Mans et al., 1998a, 2002a), as well as in certain hard tick species (Ribeiro et al., 1985; Liyou et al., 1999), salivary apyrase [an adenosine triphosphate (ATP)-diphosphohydrolase] has been found to degrade ADP. Salivary prostaglandins, such as PGI2 from I. scapularis (Ribeiro et al., 1988) or PGF2á from A. americanum (Aljamali et al., 2002) may induce increase in cAMP, an intracellular platelet aggregation inhibitor (Francischetti, 2010). Moubatin, a lipocalin derived from O. moubata, inhibits collagen-induced platelet aggregation by scavenging thromboxane A2 (Waxman and Connolly, 1993; Karczewski et al., 1995; Mans and Ribeiro, 2008). Longicornin, isolated from the SG of Haemapysalis longicornis, also inhibits collagen-mediated platelet aggregation, by a mechanism that remains to be defined (Cheng et al., 1999).
While playing an essential role in secondary haemostasis, as discussed below, the serine protease thrombin also activates platelets through cleavage of protein-activated receptors at the platelet surface. Tick salivary antithrombins can inhibit platelet aggregation induced by thrombin (Hoffmann et al., 1991; Nienaber et al., 1999; Kazimirova et al., 2002). The IRS-2 serpin from I. ricinus was found to inhibit platelet aggregation induced by both thrombin and cathepsin G (Chmelar et al., 2011), and the serpin IxscS-1E1 from I. scapularis to inhibit thrombin- and ADP-induced platelet aggregation (Ibelli et al., 2014).
Post-activation inhibitors of platelet aggregation are known to target the platelet fibrinogen receptor. The disintegrin-like peptides savignygrin from O. savignyi (Mans et al., 2002b), monogrin from Argas monolakensis (Mans and Ribeiro, 2008), and variabilin from D. variabilis (Wang et al., 1996) display the integrin recognition motif RGD and prevent the binding of other ligands to the platelet receptor. By contrast, the fibrinogen receptor antagonist disaggregin, derived from O. moubata, lacks the RGD motif and prevents ligand binding and hence platelet aggregation by different means (Karczewski et al., 1994). Ixodegrins identified in Ixodes pacificus and I. scapularis are integrin antagonists that display sequence similarity to variabilin (Francischetti et al., 2005b; Francischetti, 2010). In addition, ticks have evolved strategies to disaggregate platelet aggregates, either by displacement of fibrinogen from its receptor by competitive binding, e.g., savignygrin (Mans et al., 2002c), or by fibrinolysis (Decrem et al., 2009; Anisuzzaman et al., 2011b; Diaz-Martin et al., 2013a).
Secondary haemostasis, which refers to blood coagulation, occurs concomitantly with primary haemostasis. Blood coagulation involves a series of enzymatic reactions during which a coagulation factor (inactive proenzyme) is converted to an active form, which then activates the next proenzyme. Thrombin is involved in the final (common) pathway of the blood coagulation cascade. It converts fibrinogen into fibrin and also regulates the activity of other coagulation factors. Different coagulation factors are countered by multiple tick salivary components, of which Kunitz-type proteinase inhibitors are the most abundant class (Koh and Kini, 2009; Chmelar et al., 2012). Tick-derived thrombin inhibitors target the enzyme at different sites and through various mechanisms (Koh and Kini, 2009). Ornithodorin (van de Locht et al., 1996), savignin (Nienaber et al., 1999), and monobin (Mans et al., 2008) from soft ticks are Kunitz-type antithrombins, while IRS-2 and IRIS from I. ricinus (Leboulle et al., 2002; Chmelar et al., 2011), IxscS-1E1 from I. scapularis (Ibelli et al., 2014) and RmS-15 from Rhipicephalus (Boophilus) microplus (Xu et al., 2016) are serpins. Antithrombins belonging to the hirudin-like/madanin/variegin superfamily include madanin 1 and 2 from H. longicornis (Iwanaga et al., 2003), variegin from Amblyomma variegatum (Koh et al., 2007) and hyalomin-1 from Hyalomma marginatum rufipes (Jablonka et al., 2015). The antithrombins microphilin (Ciprandi et al., 2006) and BmAP (Horn et al., 2000) from R. (B.) microplus and calcaratin from Boophilus calcaratus (Motoyashiki et al., 2003) cannot be classified in any of the previously mentioned groups.
TAP, the Kunitz-type tick anticoagulant peptide from O. moubata (Waxman et al., 1990), the TAP-like protein from O. savignyi (Joubert et al., 1998), amblyomin-X from Amblyomma cajennense (Batista et al., 2010) and Salp14 (a basic tail-secreted protein) from I. scapularis (Narasimhan et al., 2002) are all inhibitors of coagulation factor Xa. Kunitz-type inhibitors that display similarity to the tissue factor (TF) pathway inhibitor have been identified, e.g., in I. scapularis (Ixolaris and penthalaris) (Francischetti et al., 2002, 2004), and Rhipicephalus hemaphysaloides (Rhipilin-1 and -2) (Gao et al., 2011; Cao et al., 2013). SGE from D. andersoni was observed to inhibit factor V and factor VII (Gordon and Allen, 1991). TIX-5, found in SG of I. scapularis nymphs, was shown to inhibit the activation of factor V by factor Xa, and thus delay activation of the coagulation cascade (Schuijt et al., 2013). Haemaphysalin, a plasma kallikrein-kinin system inhibitor, and Ir-CPI, a contact phase inhibitor that impairs the intrinsic coagulation pathway, were identified in H. longicornis (Kato et al., 2005) and I. ricinus (Decrem et al., 2009), respectively. IRIS, an immunomodulatory serpin from SG of I. ricinus, was found to impair the contact phase-activated pathway of coagulation and prolong fibrinolysis (Prevot et al., 2006). Serpins AamS6 (Mulenga et al., 2013a), AamAV422 (Mulenga et al., 2013b), and serpin19 (Kim et al., 2015b), derived from A. americanum, and a 65 kDa protein from R. appendiculatus (Limo et al., 1991) all delay plasma clotting, although their mode of action remains to be elucidated. Tick-derived calcium-binding proteins with homology to the calreticulin (CRT) sequence may also modulate haemostasis by sequestration of calcium ions, which act as cofactors of the coagulation enzymes (Jaworski et al., 1995).
Finally, tick salivary components may display fibrinolytic activity. A metalloprotease mediating such activity was detected in saliva of I. scapularis (Francischetti et al., 2003). Longistatin from H. longicornis, an activator of plasminogen, was found to cause hydrolysis of fibrinogen and delay formation of the fibrin clot (Anisuzzaman et al., 2011a).
When skin integrity is compromised, processes required to repel microbial invasion and restore the barrier function of the skin are immediately deployed. Pre-positioned sentinel cells, such as mast cells (MC), macrophages and dendritic cells (DC), are activated by particular components released from damaged skin cells or expressed by microbial organisms. Soluble mediators released by MC, such as bradykinin and histamine, cause itch and pain. Sentinel cells release chemoattractants, including chemokines and leukotrienes, that recruit blood-borne innate immune cells, such as neutrophils and monocytes, to the site of damage, as well as pro-inflammatory cytokines, e.g., tumor necrosis factor alpha (TNFα) and interleukin (IL)-1, that enhance activation of local and infiltrating innate immune cells. Monocytes secrete growth hormones that induce proliferation of fibroblasts and deposition of extracellular matrix, thus contributing to wound healing. Activated DC that have acquired foreign antigen migrate via lymphatics to skin-draining lymph nodes, where antigen may be presented to naïve B and T lymphocytes, thereby initiating an adaptive immune response that culminates in the generation of antigen-specific antibodies and T lymphocytes.
Itch and pain—principally triggered by MC- and basophil-derived mediators—cause awareness of injury and, if unchecked, would arouse an alleviative behavioral response. Ticks mitigate itch and pain by means of salivary components that degrade bradykinin and sequester histamine. For example, salivary metalloproteases from I. scapularis (Ribeiro and Mather, 1998) and Amblyomma maculatum (Jelinski, 2016) hydrolyse bradykinin. Amine-binding proteins of the lipocalin family, e.g., the histamine-binding proteins RaHBP(M) and RaHBP(F)-1,2 from R. appendiculatus (Paesen et al., 1999) and the serotonin- and histamine-binding protein SHBP from D. reticulatus (Sangamnatdej et al., 2002), have been found to interfere with the activity of histamine and serotonin, the latter representing an important inflammatory mediator in rodents (Askenase et al., 1980). Moreover, the activity of human β-tryptases, which are MC-specific serine proteases involved in inflammation, is diminished by the tick-derived protease inhibitor (TdPI) from R. appendiculatus (Paesen et al., 2007).
Recruitment of blood-borne innate immune cells, and notably neutrophils, is also strongly suppressed by tick saliva. Salivary inhibitors of CXCL8 and of several CC chemokines (CCL-2, -3, -5, and -11) were evidenced in SGE of several ixodid species (Hajnicka et al., 2001). Three chemokine-binding proteins, called Evasin-1, -2, and -3, were subsequently purified from the SG of Rhipicephalus sanguineus (Frauenschuh et al., 2007) and shown to present selectivity for different chemokines (Frauenschuh et al., 2007; Deruaz et al., 2008). Later work has suggested that Evasin-3-like activity may be common among metastriate ixodid tick species (Vancova et al., 2010a). CXCL8-mediated chemotaxis of neutrophils is also inhibited by Salp16 Iper1 and Salp16 Iper2, salivary proteins from Ixodes persulcatus (Hidano et al., 2014). Ir-LBP, a lipocalin from I. ricinus, was found to bind leukotriene B4, an important inflammatory mediator, with high affinity, thereby interfering with neutrophil chemotaxis and activation (Beaufays et al., 2008). Several tick species express a homolog of the vertebrate macrophage migration inhibitory factor (MIF) (Wasala and Jaworski, 2012). For both A. americanum (Jaworski et al., 2001) and H. longicornis (Umemiya et al., 2007), MIF has been shown to inhibit migration of macrophages in in vitro assays, suggesting that MIF might diminish macrophage recruitment to the bite location in vivo.
Tick saliva quells inflammation at the bite location by diminishing or enhancing secretion of pro- and anti-inflammatory cytokines, respectively. D. andersoni SGE diminished production of IL-1 and TNFα by macrophages and IL-2 and interferon (IFN)-γ production by T lymphocytes (Ramachandra and Wikel, 1992). Hyalomin-A and -B from SG of Hyalomma asiaticum asiaticum were found to inhibit secretion of TNFα, CCL2, and IFN-γ, but to increase secretion of the immunosuppressive cytokine IL-10 (Wu et al., 2010). Amregulin from A. variegatum saliva was found to suppress the in vitro production of TNFα, IL-1, CXCL8, and IFN-γ (Tian et al., 2016). Moreover, non-proteinaceous substances such as PGE2 and Ado (purine nucleoside adenosine) from saliva of R. sanguineus have been found to impair the production of the pro-inflammatory cytokines IL-12p40 and TNFα and stimulate the production of IL-10 by murine DC (Oliveira et al., 2011). Beyond its impact on cytokine production, tick saliva counters multiple effector functions of innate immune cells, including the production of reactive oxygen species (ROS) by neutrophils (Ribeiro et al., 1990; Guo et al., 2009) and macrophages (Kopecký and Kuthejlová, 1998), phagocytosis by neutrophils (Ribeiro et al., 1990) and macrophages (Kramer et al., 2011) and cytotoxicity of NK cells (Kubes et al., 1994). IRS-2, an I. ricinus serpin, targets cathepsin G and chymase, enzymes produced by activated neutrophils and MC, respectively (Pekarikova et al., 2015).
Tick saliva also restricts wound healing and angiogenesis (Francischetti, 2010; Hajnicka et al., 2011). Salivary molecules from hard ticks are able to bind to the transforming growth factor (TGF)-β1, the platelet-derived growth factor (PDGF), the fibroblast growth factor (FGF)-2 and the hepatocyte growth factor (HGF), depending on the tick species (Hajnicka et al., 2011; Slovak et al., 2014). Dermacentor variabilis saliva was found to suppress basal and PDGF-stimulated fibroblast migration and reduce PDGF-stimulated activity of extracellular signal-regulated kinase (ERK) (Kramer et al., 2008). Tick compounds similar to disintegrin metalloproteases and thrombospondin can impair cell-matrix interactions and angiogenesis (Valenzuela et al., 2002; Francischetti et al., 2005a; Fukumoto et al., 2006). The I. scapularis proteins ISL 929 and ISL 1373, for example, impair both the expression of β2 integrins and the adherence of polymorphonuclear leukocytes (PMN) (Guo et al., 2009). A troponin I-like molecule (HLTnI) (Fukumoto et al., 2006) and a Kunitz-type protein (haemangin) (Islam et al., 2009) from the SG of H. longicornis also impair angiogenesis and wound healing.
The complement system links the host innate and adaptive immune responses and is activated through three pathways (alternative, classical, and lectin). The alternative pathway is the main line of defense against invading pathogens and is also involved in resistance to ticks (Wikel, 1979). Isac, Salp20 and Isac-1 from I. scapularis (Valenzuela et al., 2000; Tyson et al., 2007) and IRAC I and II from I. ricinus (Daix et al., 2007; Couvreur et al., 2008) inhibit formation of the C3 convertase of the alternative pathway by impeding the binding of complement factor B to complement C3b. The lipocalins OmCI (O. moubata complement inhibitor; Nunn et al., 2005) and TSGP2 and TSGP3 (Mans and Ribeiro, 2008) specifically target C5 activation. Inhibition of the classical complement pathway has also been reported for saliva and SGE of A. cajennense (Franco et al., 2016).
Tick saliva or SGE is also able to suppress the initiation of adaptive immunity, such as by interfering with the capacity of DC to present antigen to T cells and prime appropriate Th responses (Cavassani et al., 2005; Mejri and Brossard, 2007; Oliveira et al., 2008; Skallova et al., 2008; Carvalho-Costa et al., 2015). In some instances these activities have been assigned to molecularly-defined salivary components. Salivary cysteine protease inhibitors (cystatins) of I. scapularis were shown to possess inhibitory activity against certain cathepsins. Both Sialostatin L and L2 strongly inhibited cathepsin L, but Sialostatin L also inhibited cathepsin S, which is involved in antigen processing. Inhibition of cathepsin S by Sialostatin L diminished the capacity of DC to induce proliferation in antigen-specific CD4+ T cells (Kotsyfakis et al., 2006; Sa-Nunes et al., 2009), while Sialostatin L2 suppressed the type I IFN response in DC (Lieskovska et al., 2015b). Salp15 from I. scapularis, upon stimulation of DC by TLR-2 and -4 ligands, suppressed the production of pro-inflammatory cytokines IL-12p70, IL-6, and TNFα, and the capacity of DC to activate T cells (Hovius et al., 2008a). Japanin, a salivary gland lipocalin from R. appendiculatus, modifies the expression of co-stimulatory and co-inhibitory molecules, the production of diverse cytokines, and inhibits DC differentiation from monocytes (Preston et al., 2013). Not all immunomodulatory salivary mediators are proteins: in I. scapularis saliva, PGE2 was found to be a major inhibitor of DC maturation and TLR-ligand induced secretion of IL-12 and TNFα (Sá-Nunes et al., 2007).
In a number of studies, tick saliva or SGE has been observed to enhance production of T helper 2 (Th2) signature cytokines, such as IL-4, and diminish production of Th1 cytokines, e.g., IFN-γ (Ferreira and Silva, 1999; Mejri et al., 2001). T cell inhibitory salivary molecules include a secreted IL-2 binding protein in I. scapularis, which suppresses T cell proliferation and other IL-2 dependent activities (Gillespie et al., 2001), P36 in D. andersoni (Bergman et al., 2000), Iris in I. ricinus (Leboulle et al., 2002), and Salp15 in I. scapularis (Anguita et al., 2002). Iris was found to suppress T cell proliferation, promote a Th2 type response and inhibit the production of pro-inflammatory cytokines IL-6 and TNFα. Salp15 binds to CD4 molecules on the surface of CD4+ T (helper) cells, and consequently inhibits signaling mediated by T cell receptors, resulting in decreased IL-2 production and T cell proliferation (Anguita et al., 2002; Garg et al., 2006). Nevertheless, transcriptional profiling of the cutaneous response in mice to primary and secondary infestation by I. scapularis nymphs did not evidence a predominant Th subset in primary infestation and evidenced a mixed Th1/Th2 and possibly regulatory T cell response in secondary infestation (Heinze et al., 2012a,b). For secondary infestation with D. andersoni nymphs, the same authors observed an indeterminant Th profile in skin, but a pronounced upregulation of IL-4 expression in draining lymph nodes, suggesting that the systemic response to repeated infestation may display a marked Th2 bias (Heinze et al., 2014).
Tick salivary components also disarm the humoral arm of the adaptive immune response. BIP and BIF derived from I. ricinus and H. asiaticum asiaticum, respectively, were found to suppress B-cell responses (Hannier et al., 2004; Yu et al., 2006). Salivary immunoglobulin (IgG)-binding proteins bind ingested host IgG and facilitate their excretion in saliva during feeding, thus protecting ticks from ingested host IgG (Wang and Nuttall, 1999).
Owing—at least in part—to active interference by salivary compounds with development of an appropriate immune response, susceptible hosts, such as mice, may fail to develop acquired resistance to ticks despite repeated tick feeding (Schoeler et al., 1999). In tick resistant hosts (e.g. guinea pigs, rabbits), however, the presence of antibodies and effector T lymphocytes with specificity for tick antigens assures a rapid secondary response to infestation that impairs tick feeding. Significant diversity in resistance to tick infestation has been also observed among different breeds of cattle, some of which is related to immunity. In particular, T-cell-mediated responses directed against larval feeding, rather than IgG responses to R. (B.) microplus antigens, were shown to be protective (Jonsson et al., 2014). Microarray analysis of differentially expressed genes in bovine skin early after attachment of R. (B.) microplus larvae revealed an important role for lipid metabolism in control of inflammation and impairment of tick infestation in tick-resistant cattle, and an impairment of the acute phase response in susceptible animals (Carvalho et al., 2014). Variation among breeds in non-immune characteristics, such as the extracellular matrix of the skin, is also likely to contribute to the variation in resistance to ticks (Jonsson et al., 2014).
Host anti-tick immunity may have a major impact on both transmission and acquisition of TBP by ticks. For example, repeated infestation of resistant strains of laboratory animals with pathogen-free I. scapularis nymphs afforded protection against tick-transmitted B. burgdorferi, suggesting that immunity against tick salivary antigens can impair transmission of Borrelia (Wikel et al., 1997; Nazario et al., 1998). Moreover, immunization of guinea pigs with I. scapularis SG proteins produced during the first day of tick feeding interfered with Borrelia transmission from ticks to hosts, suggesting that at least some of the antigens involved in acquired resistance are secreted in saliva during the first 24 h of tick attachment (Narasimhan et al., 2007a). Indeed, the aforementioned Th2 polarization of the immune response by salivary immunomodulatory molecules has been proposed to enhance transmission of TBP (Gillespie et al., 2000; Schoeler and Wikel, 2001; Wikel and Alarcon-Chaidez, 2001; Brossard and Wikel, 2008).
Enhancement of pathogen transmission by tick saliva—called saliva-assisted transmission or SAT—has been documented for several tick-pathogen associations (Nuttall and Labuda, 2004). However, relatively few tick molecules implicated in pathogen transmission have been identified and characterized.
In the course of co-evolution with ticks and vertebrate hosts, tick-borne microorganisms have developed myriad strategies to subvert tick salivary molecules so as to ensure their transmission cycle (Brossard and Wikel, 2004; Nuttall and Labuda, 2004; Ramamoorthi et al., 2005; Wikel, 2013). Saliva not only provides the matrix in which TBP are inoculated, but also profoundly modifies the local environment at the bite location, with consequences for not only transmission of TBP from infected ticks to the uninfected vertebrate host but also acquisition of TBP by uninfected ticks.
The first observation of SAT concerned Thogoto virus (THOV). Acquisition of infection by virus-free R. appendiculatus nymphs was enhanced when they fed on guinea pigs injected with a mixture of THOV and SGE (Jones et al., 1989). Subsequent studies have demonstrated the SAT phenomenon for tick-borne encephalitis virus (TBEV) (Alekseev and Chunikhin, 1990; Labuda et al., 1993) and B. burgdorferi s.l. (Gern et al., 1993; Pechova et al., 2002; Zeidner et al., 2002; Machackova et al., 2006). Tick saliva has also been shown to enhance infection of vertebrate hosts, as documented for Powassan virus (Hermance and Thangamani, 2015), African swine fever virus (ASFV) (Bernard J. et al., 2016), B. burgdorferi s.l. (Pechova et al., 2002; Zeidner et al., 2002; Machackova et al., 2006), Francisella tularensis (Krocova et al., 2003), and Rickettsia conorii (Milhano et al., 2015).
As a corollary of SAT, saliva is also presumed to enhance so-called non-viraemic transmission (NVT), i.e., transmission of TBP from infected to pathogen-free ticks that feed concomitantly on the same host, in the absence of systemic infection of the host. The pool of saliva produced by ticks that feed in close proximity is thought to enhance pathogen exchange between co-feeding ticks (Randolph, 2011; Voordouw, 2015). Indeed, transmission of THOV to uninfected R. appendiculatus ticks was observed to be more efficient while co-feeding with infected ticks on non-viraemic guinea pigs than while feeding on viraemic hamsters (Jones et al., 1987). NVT may assist TBP in circumventing the host immune response, such as by permitting viruses to evade virus-specific neutralizing antibodies (Labuda et al., 1997). Moreover, during NVT of TBEV between I. ricinus ticks, infectious virus was evidenced in the cellular infiltrate at the tick bite location, suggesting that migratory cells might transport the virus from infected to uninfected co-feeding ticks (Labuda et al., 1996). While NVT is thought to be most efficient for viruses, it has also been observed for various combinations of tick-borne bacteria and ticks, including B. burgdorferi s.l. and Ixodes spp (Gern and Rais, 1996; Piesman and Happ, 2001; Richter et al., 2002), R. conorii and R. sanguineus (Zemtsova et al., 2010). Rickettsia parkeri and A. maculatum (Banajee et al., 2015) or the Ehrlichia muris-like agent and I. scapularis (Karpathy et al., 2016).
In most instances SAT has been attributed to the immunomodulatory activity of salivary compounds, a paradigm that has been most extensively addressed for tick-borne bacteria, and more particularly B. burgdorferi. Indeed, when mice were infected with B. burgdorferi by syringe inoculation they developed a robust humoral response against the protective surface antigens Osp-A and -B, but failed to do so when infected by a tick bite (Gern et al., 1993). Moreover, BALB/c mice have been reported to develop a Th2-biased immune response against B. burgdorferi following an infectious tick bite, but a mixed Th1/Th2 response after injection (Christe et al., 2000). Furthermore, when Borrelia was injected into mice along with I. ricinus saliva, the number of leukocytes and T lymphocytes in the epidermis was diminished at early time-points after inoculation and the total number of cells in draining lymph nodes was reduced (Severinova et al., 2005). For Borrelia sp., the mechanisms that underlie SAT have been extensively addressed in relation to DC (for review see (Mason et al., 2014)). In vitro treatment of murine DC with I. ricinus saliva was found to inhibit DC maturation (Skallova et al., 2008), reduce phagocytosis of B. afzelii, reduce cytokine production, and impair proliferation and IL-2 production in Borrelia-specific CD4+ T cells (Slamova et al., 2011). Moreover, I. ricinus saliva modulated IFN-γ signaling pathways in DC (Lieskovska and Kopecky, 2012b), as well as pathways activated by a TLR2 ligand in Borrelia-stimulated DC (Lieskovska and Kopecky, 2012a). SGE of I. ricinus and saliva of I. scapularis inhibited in vitro killing of Borrelia by macrophages, through reduced production of ROS (Kuthejlova et al., 2001), and PMN (Montgomery et al., 2004), respectively. In the skin, expression of an anti-microbial peptide (AMP), cathelicidin, was induced by syringe inoculation of Borrelia but markedly suppressed when introduced by I. ricinus (Kern et al., 2011). Moreover, SGE derived from I. ricinus inhibited the in vitro inflammatory response of human primary keratinocytes induced by Borrelia or by OspC, a major surface antigen. In particular, chemokines (CXCL8 and CCL2) and AMPs (defensins, cathelicidin, psoriasin, and RNase 7) were down-regulated (Marchal et al., 2011). I. ricinus saliva also inhibited cytokine production by human primary keratinocytes in response to TLR2/TLR3 ligands during Borrelia transmission (Bernard Q. et al., 2016). Nevertheless, the effects of I. scapularis saliva on resident skin cells exposed to Borrelia were found to depend on the cell type (Scholl et al., 2016): tick saliva suppressed the production of the pro-inflammatory mediators IL-6, CXCL8, and TNFα by monocytes, but enhanced the production of CXCL8 and IL-6 by dermal fibroblasts.
Salivary mediators also appear to influence transmission of Rikettsiales bacteria. Saliva of I. scapularis was found to inhibit the pro-inflammatory cytokine response of murine macrophages to infection with the intracellular bacterium, Anaplasma phagocytophilum (Chen et al., 2012). For spotted group fever rickettsiae, immunomodulatory factors introduced during feeding of A. maculatum seemed to enhance the pathogenicity and dissemination of R. parkeri in rhesus macaques (Banajee et al., 2015). Mice experimentally inoculated with R. conorii and infested with R. sanguineus presented reduced levels of IL-1β and the transcription factor NF-κB and enhanced levels of IL-10 in the lung in comparison with mice inoculated with R. conori alone, suggesting that inflammation was inhibited (Milhano et al., 2015).
The mechanisms underlying SAT have less frequently been explored for viruses. Nevertheless, treatment of DC with I. ricinus saliva increased the proportion of TBEV-infected cells and decreased the production of TNFα and IL-6 and the induction of apoptosis elicited by the virus (Fialova et al., 2010).
The underlying molecular mechanisms implicated in TBP transmission have only begun to be elucidated, and only a few tick molecules directly or indirectly -through interaction with the host- involved in transmission, have been actually identified and functionally characterized (Table 2) (Nuttall and Labuda, 2004; Ramamoorthi et al., 2005; Kazimirova and Stibraniova, 2013; Wikel, 2013; Liu and Bonnet, 2014). Among these, some salivary compounds affect the acquisition of TBP by the vector, while others enhance the transmission of TBP to the host.
Expression of members of the 5.3-kDa family of salivary peptides that possess anti-microbial properties (Pichu et al., 2009; Liu et al., 2012) was found to be upregulated in SG of I. scapularis during infection with Langat virus (McNally et al., 2012), B. burgdorferi (Ribeiro et al., 2006), and A. phagocytophilum (Liu et al., 2012). RNAi knockdown of one member of the 5.3-kDa antimicrobial peptide family, encoded by gene-15, increased A. phagocytophilum burden in SG of I. scapularis and in blood of mice on which gene-15-deficient ticks fed (Liu et al., 2012). Thus, 5.3-kDa antimicrobial peptides are probably able to inhibit both acquisition and transmission of TBP. Moreover, the Janus kinase signaling transducer activator of transcription (JAK-STAT) pathway was implicated in the control of A. phagocytophilum infection in ticks by regulating the expression of antimicrobial peptides (Liu et al., 2012).
Distinct tick proteins promote the transmission of B. burgdorferi s.l. at different phases of infection, in relation to the phenotypic plasticity of this TBP (Radolf et al., 2012). In the infected tick, Borrelia spirochetes express outer surface protein OspA and bind to the midgut wall by means of a tick midgut protein (TROSPA) (Pal et al., 2004). Under the stimulus of a new blood meal and following tick attachment, spirochetes begin to express OspC and move from the midgut through the haemolymph to the SG, where they encounter Salp15. The secreted salivary protein Salp15, considered to be the first tick mediator of SAT to be discovered, was first identified in the SG of I. scapularis. Salp15 has been shown to bind to mammalian CD4 (Garg et al., 2006), inhibit activation of CD4+ T lymphocytes (Anguita et al., 2002), impair DC function by inhibiting TLR- and B. burgdorferi-induced production of pro-inflammatory cytokines by DCs, as well as DC-induced T cell activation (Hovius et al., 2008a). A Salp15 ortholog from I. ricinus inhibited the inflammatory response of human primary keratinocytes during transmission of Borrelia (Marchal et al., 2011). The activity of Salp15 has been shown to be critically important in pathogen transmission: RNAi silencing of Salp15 drastically reduced the capacity of ticks to transmit spirochetes to mice (Ramamoorthi et al., 2005), and immunization of mice with Salp15 afforded significant protection from I. scapularis-transmitted B. burgdorferi (Dai et al., 2009). In the tick SG, spirochetes bind to Salp15 via OspC, which protects them from antibody- and complement-mediated killing (Schuijt et al., 2008) and promotes their transmission and replication in the host skin (Ramamoorthi et al., 2005). Salp15 homologs Salp15 Iric-1 and IperSalp15 have been identified in I. ricinus (Hovius et al., 2008b) and I. persulcatus (Murase et al., 2015), respectively. These proteins have functions similar to those of Salp15 and appear to protect B. burgdorferi s.s., B. garinii, and B. afzelii from antibody-mediated killing in the host.
To evade complement-mediated killing, Borrelia also benefits from tick salivary proteins that inhibit complement activation at the tick bite location (de Taeye et al., 2013). The tick salivary lectin pathway (TSLP) inhibitor from SG of I. scapularis was found to interfere with the human lectin complement cascade and impair neutrophil phagocytosis and chemotaxis, thereby protecting Borrelia from killing by the lectin complement pathway (Schuijt et al., 2011). Moreover, silencing of TSLPI in Borrelia-infected ticks impaired experimental transmission of the spirochetes to mice. Salp20, a member of the I. scapularis anti-complement protein-like family of tick salivary proteins, inhibits the alternative complement pathway by binding properdin and causing dissociation of the C3 convertase (Tyson et al., 2007; Hourcade et al., 2016). Salp20 partially protects B. burgdorferi from lysis by normal human serum, suggesting that, together with the plasma activation factor H, Salp20 may protect Borrelia from components of the host complement system. Anti-complement proteins belonging to the Isac-like family, e.g., Isac from I. scapularis (Valenzuela et al., 2000) and its homologs IRAC I and II from I. ricinus (Daix et al., 2007) function very similarly to the previously described Salp20, but display different capacities to inhibit the alternative complement pathway depending upon the host species (Schroeder et al., 2007; Couvreur et al., 2008). Thus, proteins of the Isac-like family may potentially promote transmission of Borrelia to the vertebrate host (de Taeye et al., 2013).
BIP from I. ricinus SG was found to suppress B lymphocyte proliferation induced by the B. burgdorferi OspC, suggesting that BIP may enhance Borrelia transmission to the host (Hannier et al., 2003).
Salp25D is an immunodominant antioxidant salivary protein from I. scapularis. Silencing of Salp25D expression in SG impaired acquisition of B. burgdorferi. The protein is probably involved in acquisition of B. burgdorferi by ticks and acts as an antioxidant that promotes pathogen survival in the tick (Das et al., 2001; Narasimhan et al., 2007b).
Salivary tHRF has been shown capable of binding mammalian basophils and triggering release of histamine (Mulenga et al., 2003; Dai et al., 2010). Mulenga et al. (2003) proposed that tHRF was required for vasodilation during the rapid feeding phase of D. variabilis, when large volumes of blood are required. tHRF is up-regulated in I. scapularis SG during the rapid feeding phase and, by virtue of increasing blood flow to the tick bite location, may facilitate not only tick engorgement but also B. burgdorferi infection (Dai et al., 2010). Immunization of mice with the recombinant protein as well as silencing tHRF impaired tick feeding and decreased Borrelia burden in the host at 7 days after infection in skin and at 3 weeks in heart and joints (Dai et al., 2010). While reduced bacterial burden in mice may simply have been secondary to reduced burden in ticks, the authors suggested that the vasodilatory activity of histamine might also enhance systemic dissemination of Borrelia from the bite site (Dai et al., 2010).
IrSPI, a protein from I. ricinus SG belonging to the BPTI/Kunitz family of serine protease inhibitors, probably impairs host haemostasis and facilitates tick feeding and Bartonella henselae transmission, as RNAi silencing impaired tick feeding and diminished B. henselae load in the tick SG (Liu X. Y. et al., 2014).
Infection with A. phagocytophilum was reported to induce expression of the Salp16 gene in I. scapularis SG during feeding. Silencing of Salp16 gene expression interfered with trafficking of the bacteria ingested via the blood meal and infection of the tick SG, which demonstrated its role in persistence of this TBP within the tick (Sukumaran et al., 2006).
Silencing of P11, a secreted I. scapularis SG protein that was found to be upregulated in A. phagocytophilum-infected ticks, demonstrated that the protein enables infection of tick haemocytes and thus facilitates pathogen dissemination in the tick and its migration from the midgut to the SG through the haemolymph (Liu et al., 2011).
Sialostatin L and L2 from I. scapularis are potentially implicated in transmission of tick-borne viruses and bacteria. Sialostatin L2 was found to interfere with IFN-γ mediated immune responses in mouse splenic DC, resulting in enhanced replication of TBEV in DC (Lieskovska et al., 2015b) Sialostatin L and L2 modulated responses of murine bone marrow-derived DC exposed to Borrelia (Lieskovska et al., 2015a). Moreover, Sialostatin L2 stimulated proliferation of B. burgdorferi in murine skin (Kotsyfakis et al., 2010), possibly in relation to inhibition of the type I IFN response in DC (Lieskovska et al., 2015a,b). In addition, Sialostatin L2 inhibited inflammasome formation in mice during A. phagocytophilum infection and targeted caspase-1 activity (Chen et al., 2014).
Subolesin is a tick protective antigen with similarity to akirins, an evolutionarily conserved group of proteins in insects and vertebrates, and is suggested to control nuclear factor-kappa B (NF-kB)-dependent and independent gene expression and to play a role in tick immune responses to pathogens (Almazan et al., 2003; Naranjo et al., 2013). Upregulation of subolesin expression was observed in SG of D. variabilis ticks infected with A. marginale (Zivkovic et al., 2010). The impact of subolesin on transmission encompasses multiple vector-pathogen associations, as both gene silencing or immunization of hosts with recombinant subolesin protein resulted in decreased A. marginale, A. phagocytophilum, and Babesia bigemina burdens in their respective tick vectors (de la Fuente et al., 2006, 2010a; Merino et al., 2011). Moreover, vaccination of mice with vaccinia virus-expressed subolesin impaired engorgement of I. scapularis larvae and reduced acquisition of B. burgdorferi by tick larvae from infected mice and Borrelia transmission to uninfected mice (Bensaci et al., 2012). These findings suggest that subolesin may be involved in tick innate immunity to microbial agents by reducing their burden in SG while up-regulating factors facilitating pathogen acquisition by ticks.
Finally, a calcium-binding protein (CRT) has been found in the saliva of A. americanum and D. variabilis (Jaworski et al., 1995), in R. microplus (Ferreira et al., 2002) and I. ricinus (Cotté et al., 2014). Expression of the gene encoding CRT is up-regulated upon infection of R. annulatus with B. bigemina (Antunes et al., 2012) and CRT itself is up-regulated in the salivary proteome of I. ricinus upon infection with B. burgdorferi (Cotté et al., 2014). After immunization of rabbits with a fusion protein comprising the CRT of A. americanum, infestation with this tick caused necrotic feeding lesions (Jaworski et al., 1995). While salivary CRT might conceivably facilitate tick feeding and pathogen transmission through inhibition of thrombosis and complement, the precise role of CRT remains enigmatic. Indeed, while the CRT of A. americanum is able to bind to C1q, the first component of the classical complement pathway, it was not shown to inhibit activation of the complement cascade (Kim et al., 2015a).
Similar to vertebrates, ticks are protected against invading microorganisms by an innate immune system (Hajdusek et al., 2013; Hynes, 2014). During co-evolution, tick-borne microorganisms have developed various strategies to evade or suppress the immune responses of their vectors in order to ensure survival, persistence and transmission. As a countermeasure, ticks have developed means of maintaining pathogen burden at a level that preserves fitness and further development (Smith and Pal, 2014). Indeed, several studies have provided evidence that acquisition of TBP can exert a profound effect on gene expression in various tick organs, including SG. Multiple families of genes have been shown to be regulated in tick SG following infection, but in most cases, their precise role is unknown (Liu and Bonnet, 2014; Chmelar et al., 2016a).
The impact of infection on the SG transcriptome has been addressed for several species of ticks and TBP, including D. variabilis and Rickettsia montanensis (Macaluso et al., 2003), R. appendiculatus and Theileria parva (Nene et al., 2004), R. microplus and A. marginale (Zivkovic et al., 2010; Mercado-Curiel et al., 2011; Bifano et al., 2014), I. scapularis and A. phagocytophilum (Ayllon et al., 2015; Cabezas-Cruz et al., 2016, 2017) or Langat virus (McNally et al., 2012) and I. ricinus and B. henselae (Liu X. Y. et al., 2014) or B. afzelii (Valdes et al., 2016). Diverse technologies have been applied to the identification of differentially expressed genes, from seminal studies based on differential-display PCR (Macaluso et al., 2003) and sequencing of expressed sequence tags (Nene et al., 2004), to later studies that applied suppression subtractive hybridization (Zivkovic et al., 2010) and microarrays (Mercado-Curiel et al., 2011; McNally et al., 2012) and to recent studies that have exploited next-generation sequencing (Liu X. Y. et al., 2014; Ayllon et al., 2015). Moreover, the impact of infection on the proteome of the SG, or of saliva itself, has been investigated for infection of I. scapularis with A. phagocytophilum (Ayllon et al., 2015; Villar et al., 2016) or B. burgdorferi (Dai et al., 2009) and I. ricinus with B. burgdorferi (Cotté et al., 2014). Recently, a novel multidisciplinary approach involving structural analysis of the SG transcriptome and biophysical simulations was used to predict substrate specificity for uncharacterized lipocalins in the SG of I. ricinus that might play a role in transmission of B. afzelii (Valdes et al., 2016). Depending on the study, relatively modest or extensive modulation of gene expression has been observed following SG infection, possibly reflecting not only the experimental strategy employed, but also the type of relationship established by the tick/pathogen pair in question. It has, for example, been suggested that pathogens that are highly adapted to their tick, such as A. marginale for R. microplus, and whose presence imposes a minimal fitness cost, have little effect on the SG transcriptome (Zivkovic et al., 2010; Mercado-Curiel et al., 2011), whereas pathogens that have a dramatic effect on tick fitness, such as B. bovis for Rhipicephalus annulatus (Ouhelli et al., 1987), have a greater impact.
Anti-tick immunity was first described in the middle of the twentieth century (Trager, 1939), and many tick salivary proteins have since been shown to be immunogenic in vertebrate hosts (Wikel, 1996). Indeed, during feeding ticks inject multiple salivary proteins that elicit antibody responses, and it has been suggested, from the 1990s, that such responses could be used as biomarkers of host exposure to tick bites (Schwartz et al., 1990). Nevertheless, surveillance of TBD has largely relied on detection of host antibody responses to TBP or of TBP themselves in ticks or host, or on modeling/forecasting approaches (Hai et al., 2014), and evaluation of exposure to tick bites has only rarely been addressed. Biomarkers of exposure to ticks would be useful not only to assess host/vector contact, such as to evaluate the efficacy of anti-vector measures, but also to instruct diagnosis of TBD, by enabling documentation of an antecedent tick bite in patients for whom TBD is suspected. It is, in fact, well-known that self-reported tick exposure is a poor correlate of true tick exposure. The greatest challenge in this endeavor lies in identification of antigenic markers that allow discrimination among the different tick species to which the host has been exposed. Such specificity is particularly critical in areas with a high diversity of hematophagous arthropod species (Fontaine et al., 2011). Indeed, when antibodies against sonicated Ixodes dammini SG were first evaluated as markers of tick exposure in humans, cross-reactivity with other arthropods was shown to limit their epidemiologic utility (Schwartz et al., 1990). Since then, the use of a recombinant form of CRT has displayed higher specificity, albeit lower sensitivity, than whole SG for evaluation of exposure to I. scapularis bites (Sanders et al., 1999). Antibodies against CRT were thus sought in a longitudinal study that addressed the impact of educational interventions on tick exposure (Malouin et al., 2003), and further studies demonstrated that, in humans, such antibodies were found to persist for as long as a year and a half following tick exposure (Alarcon-Chaidez et al., 2006). Cross-reactivity, however, has been reported between recombinant CRT from I. scapularis and A. americanum (Sanders et al., 1998b), underscoring the challenge of developing specific ELISA tests. Efforts are thus still in progress to identify discriminant antigenic proteins in tick saliva that could be used to evaluate exposure to different tick species (Sanders et al., 1998a; Vu Hai et al., 2013a,b).
The production of antigen-specific antibodies directed against multiple tick salivary proteins (Vu Hai et al., 2013b), as well as the observation that vertebrates repeatedly exposed to tick bites develop immunity (Brossard and Wikel, 1997; Wikel and Alarcon-Chaidez, 2001) that affords a measure of protection against TBD (Bell et al., 1979; Wikel et al., 1997; Burke et al., 2005; Krause et al., 2009), has sparked interest in development of vaccinal strategies against tick bites. In contrast with conventional control measures, deployment of an anti-tick vaccine would not contaminate the environment and foodstuffs, nor have a deleterious impact on off-target species. Moreover, in light of the transmission of multiple TBP by the same tick species, vaccine strategies that target conserved processes in vector capacity would be expected to afford broad protection against multiple TBD transmitted by the same vector (Willadsen, 2004; Nuttall et al., 2006). Of note, such strategies hold the promise of protecting against uncharacterized TBP currently in circulation or as yet to emerge. The anti-tick vaccine approach is, moreover, compatible with the inclusion of multiple antigens, including those from TBP, so as to reinforce protection, and as demonstrated in the Borrelia sp./I. scapularis infection model for OsPA and Salp15 (Dai et al., 2009). The only ectoparasite vaccines that are commercially available (in Australia and Cuba) target Bm86, a midgut protein of R. microplus—a one-host tick species—and interfere with feeding and subsequent egg production (Willadsen et al., 1995). Diminution of transmission was thus secondary to reduction in the population of R. microplus ticks. Unfortunately, the efficacy of these vaccines is geographically variable due to species and strain specificity and they are no longer commercialized in Australia. Even if orthologs of Bm86 existed in other tick species, however, the strategy was not transposable to tick species that feed on multiple hosts, such as wildlife species, in addition to the host species for which the vaccine is intended. For TBP transmitted by ticks with a broad range of hosts, vaccines that exert a direct effect on tick blood meal acquisition or vector competence must be found. Nevertheless, the anti-Bm86 vaccines provide a powerful proof of principle for the feasibility of creating and deploying anti-tick vaccines.
Salivary proteins represent good vaccine candidates as, owing to their contact with the host immune system, they may allow natural boosting of the host response upon exposure to ticks, limiting the need for repeated administrations (Nuttall et al., 2006). Targeting salivary proteins playing a key role in tick feeding is expected to interfere with completion of the blood meal and subsequently affect their reproductive fitness. Moreover, this strategy may also cause tick rejection and thus abolish or limit pathogen transmission, which typically occurs many hours or even days after tick attachment for hard ticks. Indeed, encouraging results have been obtained for several tick species including H. longicornis (Mulenga et al., 1999; Imamura et al., 2005; Zhang et al., 2011; Anisuzzaman et al., 2012), R. appendiculatus (Imamura et al., 2006), O. moubata and O. erraticus (Astigarraga et al., 1995; Garcia-Varas et al., 2010), A. americanum (de la Fuente et al., 2010b), R. (B) microplus (Andreotti et al., 2002; Merino et al., 2013; Ali et al., 2015), and I. ricinus (Prevot et al., 2007; Decrem et al., 2008b). Finally, anti-tick vaccination may also target salivary proteins directly implicated in TBP transmission. Studies carried out to this end concern I. scapularis and the salivary antigens Salp15 (Dai et al., 2009), TSLPI (Schuijt et al., 2011), and tHRF (Dai et al., 2010), as regards the transmission of Borrelia sp. to mice, and the salivary antigen Salp25D as regards acquisition of Borrelia sp. by ticks (Wagemakers et al., 2016). Last, vaccination against the cement protein 64TRP of I. ricinus also afforded protection against TBE transmission to mice (Labuda et al., 2006).
The rapid evolution of tick distribution and density has created an urgent need for more effective methods for tick control and for surveillance and risk assessment for TBD (Heyman et al., 2010; Leger et al., 2013; Medlock et al., 2013). The deployment of anti-tick vaccines designed to reduce tick populations and/or transmission of TBP and reduce reliance on acaricides and repellents would represent a major improvement over current control measures, being environmentally safe and less likely than acaricides to select resistant strains. Risk assessment for human and animal populations will determine public and veterinary public health priorities, and instruct the implementation of appropriate countermeasures or complementary studies. These goals, however, require a better understanding of tick biology and of the tripartite relationship between ticks, TBP and vertebrate hosts, including the molecular interactions underlying TBP transmission. This includes the subversion of the host response mediated by saliva introduced into the host during tick feeding. Given the central role of tick SG and tick saliva both in tick biology and TBP transmission, their investigation may underpin the discovery of immunological markers for meaningful assessment of exposure to tick bites and as vaccinal candidates to protect against TBD. Ultimately, deciphering the physiology of the essential organ represented by tick SG may lead to the conception of hitherto unimagined strategies for controlling ticks and TBD.
LS, MK, JR, and SB conducted the literature research, wrote the paper and prepared the figures and tables. All authors provided critical review and revisions.
SB, JR, and LS were supported by the Institut National de la Recherche Agronomique (INRA). MK was supported by the Slovak Research and Development Agency (contract no. APVV-0737-12).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Alarcon-Chaidez, F., Ryan, R., Wikel, S., Dardick, K., Lawler, C., Foppa, I. M., et al. (2006). Confirmation of tick bite by detection of antibody to Ixodes calreticulin salivary protein. Clin. Vaccine Immunol. 13, 1217–1222. doi: 10.1128/CVI.00201-06
Alekseev, A. N., and Chunikhin, S. P. (1990). [The exchange of the tick-borne encephalitis virus between ixodid ticks feeding jointly on animals with a subthreshold level of viremia]. Med. Parazitol. (Mosk). 48–50.
Ali, A., Parizi, L. F., Guizzo, M. G., Tirloni, L., Seixas, A., Vaz Ida, S. Jr., et al. (2015). Immunoprotective potential of a Rhipicephalus (Boophilus) microplus metalloprotease. Vet. Parasitol. 207, 107–114. doi: 10.1016/j.vetpar.2014.11.007
Aljamali, M. N., Sauer, J. R., and Essenberg, R. C. (2002). RNA interference, applicability in tick research. Exp. Appl. Acarol. 28, 89–96. doi: 10.1023/A:1025346131903
Almazan, C., Kocan, K. M., Bergman, D. K., Garcia-Garcia, J. C., Blouin, E. F., and de la Fuente, J. (2003). Identification of protective antigens for the control of Ixodes scapularis infestations using cDNA expression library immunization. Vaccine 21, 1492–1501. doi: 10.1016/S0264-410X(02)00683-7
Anatriello, E., Ribeiro, J. M., de Miranda-Santos, I. K., Brandao, L. G., Anderson, J. M., Valenzuela, J. G., et al. (2010). An insight into the sialotranscriptome of the brown dog tick, Rhipicephalus sanguineus. BMC Genomics 11:450. doi: 10.1186/1471-2164-11-450
Andreotti, R., Gomes, A., Malavazi-Piza, K. C., Sasaki, S. D., Sampaio, C. A., and Tanaka, A. S. (2002). BmTI antigens induce a bovine protective immune response against Boophilus microplus tick. Int. Immunopharmacol. 2, 557–563. doi: 10.1016/S1567-5769(01)00203-X
Anguita, J., Ramamoorthi, N., Hovius, J. W. R., Das, S., Thomas, V., Persinski, R., et al. (2002). Salp15, an Ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity 16, 849–859. doi: 10.1016/S1074-7613(02)00325-4
Anisuzzaman, Islam, M. K., Alim, M. A., Miyoshi, T., Hatta, T., Yamaji, K., et al. (2011a). Longistatin, a plasminogen activator, is key to the availability of blood-meals for ixodid ticks. PLoS Pathog. 7:e1001312. doi: 10.1371/journal.ppat.1001312
Anisuzzaman, Islam, M. K., Alim, M. A., Miyoshi, T., Hatta, T., Yamaji, K., et al. (2012). Longistatin is an unconventional serine protease and induces protective immunity against tick infestation. Mol. Biochem. Parasitol. 182, 45–53. doi: 10.1016/j.molbiopara.2011.12.002
Anisuzzaman, Islam, M. K., Abdul Alim, M., Miyoshi, T., Hatta, T., Yamaji, K., et al. (2011b). Longistatin, a novel plasminogen activator from vector ticks, is resistant to plasminogen activator inhibitor-1. Biochem. Biophys. Res. Commun. 413, 599–604. doi: 10.1016/j.bbrc.2011.09.009
Antunes, S., Galindo, R. C., Almazan, C., Rudenko, N., Golovchenko, M., Grubhoffer, L., et al. (2012). Functional genomics studies of Rhipicephalus (Boophilus) annulatus ticks in response to infection with the cattle protozoan parasite, Babesia bigemina. Int. J. Parasitol. 42, 187–195. doi: 10.1016/j.ijpara.2011.12.003
Askenase, P. W., Bursztajn, S., Gershon, M. D., and Gershon, R. K. (1980). T cell-dependent mast cell degranulation and release of serotonin in murine delayed-type hypersensitivity. J. Exp. Med. 152, 1358–1374. doi: 10.1084/jem.152.5.1358
Astigarraga, A., Oleaga-Perez, A., Perez-Sanchez, R., and Encinas-Grandes, A. (1995). A study of the vaccinal value of various extracts of concealed antigens and salivary gland extracts against Ornithodoros erraticus and Ornithodoros moubata. Vet. Parasitol. 60, 133–147. doi: 10.1016/0304-4017(94)00772-5
Ayllon, N., Villar, M., Galindo, R. C., Kocan, K. M., Sima, R., Lopez, J. A., et al. (2015). Systems biology of tissue-specific response to Anaplasma phagocytophilum reveals differentiated apoptosis in the tick vector Ixodes scapularis. PLoS Genet. 11:e1005120. doi: 10.1371/journal.pgen.1005120
Balashov, Y. S. (1972). Bloodsucking Ticks (Ixodoidea) Vectors of Disease of Man and Animals. College Park, MD: Entomol Society of America.
Banajee, K. H., Embers, M. E., Langohr, I. M., Doyle, L. A., Hasenkampf, N. R., and Macaluso, K. R. (2015). Amblyomma maculatum feeding augments Rickettsia parkeri infection in a rhesus Macaque model: a pilot study. PLoS ONE 10:e0135175. doi: 10.1371/journal.pone.0135175
Batista, I. F., Ramos, O. H., Ventura, J. S., Junqueira-de-Azevedo, I. L., Ho, P. L., and Chudzinski-Tavassi, A. M. (2010). A new Factor Xa inhibitor from Amblyomma cajennense with a unique domain composition. Arch. Biochem. Biophys. 493, 151–156. doi: 10.1016/j.abb.2009.10.009
Beaufays, J., Adam, B., Menten-Dedoyart, C., Fievez, L., Grosjean, A., Decrem, Y., et al. (2008). Ir-LBP, an Ixodes ricinus tick salivary LTB4-binding lipocalin, interferes with host neutrophil function. PLoS ONE 3:e3987. doi: 10.1371/journal.pone.0003987
Bell, J. F., Stewart, S. J., and Wikel, S. K. (1979). Resistance to tick-borne Francisella tularensis by tick-sensitized rabbits: allergic klendusity. Am. J. Trop. Med. Hyg. 28, 876–880. doi: 10.4269/ajtmh.1979.28.876
Bensaci, M., Bhattacharya, D., Clark, R., and Hu, L. T. (2012). Oral vaccination with vaccinia virus expressing the tick antigen subolesin inhibits tick feeding and transmission of Borrelia burgdorferi. Vaccine 30, 6040–6046. doi: 10.1016/j.vaccine.2012.07.053
Bergman, D. K., Palmer, M. J., Caimano, M. J., Radolf, J. D., and Wikel, S. K. (2000). Isolation and molecular cloning of a secreted immunosuppressant protein from Dermacentor andersoni salivary gland. J. Parasitol. 86, 516–525. doi: 10.1645/0022-3395(2000)086[0516:IAMCOA]2.0.CO;2
Bernard, J., Hutet, E., Paboeuf, F., Randriamparany, T., Holzmuller, P., Lancelot, R., et al. (2016). Effect of O. porcinus tick salivary gland extract on the african swine fever virus infection in domestic pig. PLoS ONE 11:e0147869. doi: 10.1371/journal.pone.0147869
Bernard, Q., Gallo, R. L., Jaulhac, B., Nakatsuji, T., Luft, B., Yang, X., et al. (2016). Ixodes tick saliva suppresses the keratinocyte cytokine response to TLR2/TLR3 ligands during early exposure to Lyme borreliosis. Exp. Dermatol. 25, 26–31. doi: 10.1111/exd.12853
Bifano, T. D., Ueti, M. W., Esteves, E., Reif, K. E., Braz, G. R. C., Scoles, G. A., et al. (2014). Knockdown of the Rhipicephalus microplus cytochrome c oxidase subunit III gene is associated with a failure of Anaplasma marginale transmission. PLoS ONE 9:e98614. doi: 10.1371/journal.pone.0098614
Binnington, K. C. (1975). Secretory coxal gland, active during apolysis in ixodid and argasid ticks (Acarina). Int. J. Insect Morphol. Embryol. 4, 183–191. doi: 10.1016/0020-7322(75)90016-1
Binnington, K. C. (1978). Sequential changes in salivary gland structure during attachment and feeding of the cattle tick, Boophilus microplus. Int. J. Parasitol. 8, 97–115. doi: 10.1016/0020-7519(78)90004-8
Bishop, R., Lambson, B., Wells, C., Pandit, P., Osaso, J., Nkonge, C., et al. (2002). A cement protein of the tick Rhipicephalus appendiculatus, located in the secretory e cell granules of the type III salivary gland acini, induces strong antibody responses in cattle. Int. J. Parasitol. 32, 833–842. doi: 10.1016/S0020-7519(02)00027-9
Bowman, A. S., Dillwith, J. W., and Sauer, J. R. (1996). Tick salivary prostaglandins: presence, origin and significance. Parasitol. Today 12, 388–396. doi: 10.1016/0169-4758(96)10061-2
Bowman, A. S., and Sauer, J. R. (2004). Tick salivary glands: function, physiology and future. Parasitology 129(Suppl.), S67–S81. doi: 10.1017/S0031182004006468
Branco, V. G., Iqbal, A., Alvarez-Flores, M. P., Sciani, J. M., de Andrade, S. A., Iwai, L. K., et al. (2016). Amblyomin-X having a Kunitz-type homologous domain, is a noncompetitive inhibitor of FXa and induces anticoagulation in vitro and in vivo. Biochim. Biophys. Acta 1864, 1428–1435. doi: 10.1016/j.bbapap.2016.07.011
Brossard, M., and Wikel, S. K. (1997). Immunology of interactions between ticks and hosts. Med. Vet. Entomol. 11, 270–276. doi: 10.1111/j.1365-2915.1997.tb00406.x
Brossard, M., and Wikel, S. K. (2004). Tick immunobiology. Parasitology 129(Suppl.), S161–S176. doi: 10.1017/S0031182004004834
Brossard, M., and Wikel, S. K. (2008). “Tick immunobiology,” in Ticks: Biology, Disease and Control, eds A. S. Bowman and P. A. Nuttall (Cambridge: Cambridge University Press), 186–204.
Bullard, R., Allen, P., Chao, C.-C., Douglas, J., Das, P., Morgan, S. E., et al. (2016). Structural characterization of tick cement cones collected from in vivo and artificial membrane blood-fed Lone Star ticks (Amblyomma americanum). Ticks Tick Borne Dis. 7, 880–892. doi: 10.1016/j.ttbdis.2016.04.006
Burke, G., Wikel, S. K., Spielman, A., Telford, S. R., McKay, K., Krause, P. J., et al. (2005). Hypersensitivity to ticks and Lyme disease risk. Emerg. Infect. Dis. 11, 36–41. doi: 10.3201/eid1101.040303
Cabezas-Cruz, A., Alberdi, P., Ayllon, N., Valdes, J. J., Pierce, R., Villar, M., et al. (2016). Anaplasma phagocytophilum increases the levels of histone modifying enzymes to inhibit cell apoptosis and facilitate pathogen infection in the tick vector Ixodes scapularis. Epigenetics 11, 303–319. doi: 10.1080/15592294.2016.1163460
Cabezas-Cruz, A., Alberdi, P., Valdes, J. J., Villar, M., and de la Fuente, J. (2017). Anaplasma phagocytophilum infection subverts carbohydrate metabolic pathways in the tick vector, Ixodes scapularis. Front. Cell. Infect. Microbiol. 7:23. doi: 10.3389/fcimb.2017.00023
Cao, J., Shi, L., Zhou, Y. Z., Gao, X., Zhang, H. S., Gong, H. Y., et al. (2013). CHARACTERIZATION OF A NEW KUNITZ-TYPE SERINE PROTEASE INHIBITOR FROM THE HARD TICK Rhipicephalus hemaphysaloides. Arch. Insect Biochem. Physiol. 84, 104–113. doi: 10.1002/arch.21118
Carvalho, W. A., Domingues, R., de Azevedo Prata, M. C., da Silva, M. V., de Oliveira, G. C., Guimaraes, S. E., et al. (2014). Microarray analysis of tick-infested skin in resistant and susceptible cattle confirms the role of inflammatory pathways in immune activation and larval rejection. Vet. Parasitol. 205, 307–317. doi: 10.1016/j.vetpar.2014.07.018
Carvalho-Costa, T. M., Mendes, M. T., da Silva, M. V., da Costa, T. A., Tiburcio, M. G. S., Anhê, A. C. B. M., et al. (2015). Immunosuppressive effects of Amblyomma cajennense tick saliva on murine bone marrow-derived dendritic cells. Parasit. Vectors 8:22. doi: 10.1186/s13071-015-0634-7
Cavassani, K. A., Aliberti, J. C., Dias, A. R. V., Silva, J. S., and Ferreira, B. R. (2005). Tick saliva inhibits differentiation, maturation and function of murine bone-marrow-derived dendritic cells. Immunology 114, 235–245. doi: 10.1111/j.1365-2567.2004.02079.x
Chen, G., Severo, M. S., Sohail, M., Sakhon, O. S., Wikel, S. K., Kotsyfakis, M., et al. (2012). Ixodes scapularis saliva mitigates inflammatory cytokine secretion during Anaplasma phagocytophilum stimulation of immune cells. Parasit. Vectors 5:229. doi: 10.1186/1756-3305-5-229
Chen, G., Wang, X. W., Severo, M. S., Sakhon, O. S., Sohail, M., Brown, L. J., et al. (2014). The tick salivary protein sialostatin L2 inhibits caspase-1-mediated inflammation during Anaplasma phagocytophilum infection. Infect. Immun. 82, 2553–2564. doi: 10.1128/IAI.01679-14
Cheng, Y., Wu, H., and Li, D. (1999). An inhibitor selective for collagen-stimulated platelet aggregation from the salivary glands of hard tick Haemaphysalis longicornis and its mechanism of action. Sci. China Life Sci. 42, 457–464. doi: 10.1007/BF02881768
Chinery, W. A. (1973). The nature and origin of the “Cement” substance at the site of attachment and feeding of adult Haemaphysalis Spinigera (Ixodidae). J. Med. Entomol. 10, 355–362. doi: 10.1093/jmedent/10.4.355
Chmelar, J., Calvo, E., Pedra, J. H., Francischetti, I. M., and Kotsyfakis, M. (2012). Tick salivary secretion as a source of antihemostatics. J. Proteomics 75, 3842–3854. doi: 10.1016/j.jprot.2012.04.026
Chmelar, J., Kotal, J., Karim, S., Kopacek, P., Francischetti, I. M., Pedra, J. H., et al. (2016a). Sialomes and mialomes: a systems-biology view of tick tissues and tick-host interactions. Trends Parasitol. 32, 242–254. doi: 10.1016/j.pt.2015.10.002
Chmelar, J., Kotal, J., Kopecky, J., Pedra, J. H., and Kotsyfakis, M. (2016b). All for one and one for all on the tick-host battlefield. Trends Parasitol. 32, 368–377. doi: 10.1016/j.pt.2016.01.004
Chmelar, J., Oliveira, C. J., Rezacova, P., Francischetti, I. M., Kovarova, Z., Pejler, G., et al. (2011). A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood 117, 736–744. doi: 10.1182/blood-2010-06-293241
Christe, M., Rutti, B., and Brossard, M. (2000). Cytokines (IL-4 and IFN-gamma) and antibodies (IgE and IgG2a) produced in mice infected with Borrelia burgdorferi sensu stricto via nymphs of Ixodes ricinus ticks or syringe inoculations. Parasitol. Res. 86, 491–496. doi: 10.1007/s004360050699
Ciprandi, A., de Oliveira, S. K., Masuda, A., Horn, F., and Termignoni, C. (2006). Boophilus microplus: its saliva contains microphilin, a small thrombin inhibitor. Exp. Parasitol. 114, 40–46. doi: 10.1016/j.exppara.2006.02.010
Coons, L. B., Lessman, C. A., Ward, M. W., Berg, R. H., and Lamoreaux, W. J. (1994). Evidence of a myoepithelial cell in tick salivary glands. Int. J. Parasitol. 24, 551–562. doi: 10.1016/0020-7519(94)90147-3
Coons, L. B., and Roshdy, M. A. (1973). Fine structure of the salivary glands of unfed male Dermacentor variabilis (Say) (Ixodoidea: Ixodidae). J. Parasitol. 59, 900–912. doi: 10.2307/3278433
Cotté, V., Sabatier, L., Schnell, G., Carmi-Leroy, A., Rousselle, J.-C., Arsène-Ploetze, F., et al. (2014). Differential expression of Ixodes ricinus salivary gland proteins in the presence of the Borrelia burgdorferi sensu lato complex. J. Proteomics 96, 29–43. doi: 10.1016/j.jprot.2013.10.033
Couvreur, B., Beaufays, J., Charon, C., Lahaye, K., Gensale, F., Denis, V., et al. (2008). Variability and action mechanism of a family of anticomplement proteins in Ixodes ricinus. PLoS ONE 3:e1400. doi: 10.1371/journal.pone.0001400
Dai, J., Narasimhan, S., Zhang, L., Liu, L., Wang, P., and Fikrig, E. (2010). Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the lyme disease agent. PLoS Pathog. 6:e1001205. doi: 10.1371/journal.ppat.1001205
Dai, J., Wang, P., Adusumilli, S., Booth, C. J., Narasimhan, S., Anguita, J., et al. (2009). Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host Microbe 6, 482–492. doi: 10.1016/j.chom.2009.10.006
Daix, V., Schroeder, H., Praet, N., Georgin, J. P., Chiappino, I., Gillet, L., et al. (2007). Ixodes ticks belonging to the Ixodes ricinus complex encode a family of anticomplement proteins. Insect Mol. Biol. 16, 155–166. doi: 10.1111/j.1365-2583.2006.00710.x
Dantas-Torres, F., Chomel, B. B., and Otranto, D. (2012). Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol. 28, 437–446. doi: 10.1016/j.pt.2012.07.003
Das, S., Banerjee, G., DePonte, K., Marcantonio, N., Kantor, F. S., and Fikrig, E. (2001). Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J. Infect. Dis. 184, 1056–1064. doi: 10.1086/323351
de la Fuente, J., Almazan, C., Blouin, E. F., Naranjo, V., and Kocan, K. M. (2006). Reduction of tick infections with Anaplasma marginale and A. phagocytophilum by targeting the tick protective antigen subolesin. Parasitol. Res. 100, 85–91. doi: 10.1007/s00436-006-0244-6
de la Fuente, J., Estrada-Pena, A., Venzal, J. M., Kocan, K. M., and Sonenshine, D. E. (2008). Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 13, 6938–6946. doi: 10.2741/3200
de la Fuente, J., Kocan, K. M., Blouin, E. F., Zivkovic, Z., Naranjo, V., Almazan, C., et al. (2010a). Functional genomics and evolution of tick-Anaplasma interactions and vaccine development. Vet. Parasitol. 167, 175–186. doi: 10.1016/j.vetpar.2009.09.019
de la Fuente, J., Manzano-Roman, R., Naranjo, V., Kocan, K. M., Zivkovic, Z., Blouin, E. F., et al. (2010b). Identification of protective antigens by RNA interference for control of the lone star tick, Amblyomma americanum. Vaccine 28, 1786–1795. doi: 10.1016/j.vaccine.2009.12.007
de Taeye, S. W., Kreuk, L., van Dam, A. P., Hovius, J. W., and Schuijt, T. J. (2013). Complement evasion by Borrelia burgdorferi: it takes three to tango. Trends Parasitol. 29, 119–128. doi: 10.1016/j.pt.2012.12.001
Decrem, Y., Beaufays, J., Blasioli, V., Lahaye, K., Brossard, M., Vanhamme, L., et al. (2008a). A family of putative metalloproteases in the salivary glands of the tick Ixodes ricinus. FEBS J. 275, 1485–1499. doi: 10.1111/j.1742-4658.2008.06308.x
Decrem, Y., Mariller, M., Lahaye, K., Blasioli, V., Beaufays, J., Zouaoui Boudjeltia, K., et al. (2008b). The impact of gene knock-down and vaccination against salivary metalloproteases on blood feeding and egg laying by Ixodes ricinus. Int. J. Parasitol. 38, 549–560. doi: 10.1016/j.ijpara.2007.09.003
Decrem, Y., Rath, G., Blasioli, V., Cauchie, P., Robert, S., Beaufays, J., et al. (2009). Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J. Exp. Med. 206, 2381–2395. doi: 10.1084/jem.20091007
Deruaz, M., Frauenschuh, A., Alessandri, A. L., Dias, J. M., Coelho, F. M., Russo, R. C., et al. (2008). Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J. Exp. Med. 205, 2019–2031. doi: 10.1084/jem.20072689
Diaz-Martin, V., Manzano-Roman, R., Oleaga, A., Encinas-Grandes, A., and Perez-Sanchez, R. (2013a). Cloning and characterization of a plasminogen-binding enolase from the saliva of the argasid tick Ornithodoros moubata. Vet. Parasitol. 191, 301–314. doi: 10.1016/j.vetpar.2012.09.019
Diaz-Martin, V., Manzano-Roman, R., Valero, L., Oleaga, A., Encinas-Grandes, A., and Perez-Sanchez, R. (2013b). An insight into the proteome of the saliva of the argasid tick Ornithodoros moubata reveals important differences in saliva protein composition between the sexes. J. Proteomics 80, 216–235. doi: 10.1016/j.jprot.2013.01.015
Fawcett, D. W., Binnington, K., and Voigt, W. P. (1986). The Cell Biology of the Ixodid Tick Salivary Gland. Chichester: Ellis Horwood.
Fawcett, D. W., Doxsey, S. J., and Buscher, G. (1981a). Salivary gland of the tick vector (R. appendiculaius) of East Coast Fever. I. Ultrastructure of the type III acinus. Tissue Cell 13, 209–230. doi: 10.1016/0040-8166(81)90002-1
Fawcett, D. W., Doxsey, S. J., and Buscher, G. (1981b). Salivary gland of the tick vector (R. appediculatus) of East Coast Fever. II. Cellular basis for fluid secretion in the type III acinus. Tissue Cell 13, 231–253. doi: 10.1016/0040-8166(81)90003-3
Ferreira, B. R., and Silva, J. S. (1999). Successive tick infestations selectively promote a T-helper 2 cytokine profile in mice. Immunology 96, 434–439. doi: 10.1046/j.1365-2567.1999.00683.x
Ferreira, C. A. S., Da Silva Vaz, I., da Silva, S. S., Haag, K. L., Valenzuela, J. G., and Masuda, A. (2002). Cloning and partial characterization of a Boophilus microplus (Acari: Ixodidae) calreticulin. Exp. Parasitol. 101, 25–34. doi: 10.1016/S0014-4894(02)00032-2
Fialova, A., Cimburek, Z., Iezzi, G., and Kopecky, J. (2010). Ixodes ricinus tick saliva modulates tick-borne encephalitis virus infection of dendritic cells. Microbes Infect. 12, 580–585. doi: 10.1016/j.micinf.2010.03.015
Fontaine, A., Diouf, I., Bakkali, N., Misse, D., Pages, F., Fusai, T., et al. (2011). Implication of haematophagous arthropod salivary proteins in host-vector interactions. Parasit. Vectors 4:187. doi: 10.1186/1756-3305-4-187
Francischetti, I. M. (2010). Platelet aggregation inhibitors from hematophagous animals. Toxicon 56, 1130–1144. doi: 10.1016/j.toxicon.2009.12.003
Francischetti, I. M., Mather, T. N., and Ribeiro, J. M. (2003). Cloning of a salivary gland metalloprotease and characterization of gelatinase and fibrin(ogen)lytic activities in the saliva of the Lyme disease tick vector Ixodes scapularis. Biochem. Biophys. Res. Commun. 305, 869–875. doi: 10.1016/S0006-291X(03)00857-X
Francischetti, I. M., Mather, T. N., and Ribeiro, J. M. (2004). Penthalaris, a novel recombinant five-Kunitz tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick vector of Lyme disease, Ixodes scapularis. Thromb. Haemost. 91, 886–898. doi: 10.1160/th03-11-0715
Francischetti, I. M., Mather, T. N., and Ribeiro, J. M. (2005a). Tick saliva is a potent inhibitor of endothelial cell proliferation and angiogenesis. Thromb. Haemost. 94, 167–174. doi: 10.1160/th04-09-0566
Francischetti, I. M., Meng, Z., Mans, B. J., Gudderra, N., Hall, M., Veenstra, T. D., et al. (2008). An insight into the salivary transcriptome and proteome of the soft tick and vector of epizootic bovine abortion, Ornithodoros coriaceus. J. Proteomics, 71, 493–512. doi: 10.1016/j.jprot.2008.07.006
Francischetti, I. M., My Pham, V., Mans, B. J., Andersen, J. F., Mather, T. N., Lane, R. S., et al. (2005b). The transcriptome of the salivary glands of the female western black-legged tick Ixodes pacificus (Acari: Ixodidae). Insect Biochem. Mol. Biol. 35, 1142–1161. doi: 10.1016/j.ibmb.2005.05.007
Francischetti, I. M., Sa-Nunes, A., Mans, B. J., Santos, I. M., and Ribeiro, J. M. (2009). The role of saliva in tick feeding. Front. Biosci. 14, 2051–2088. doi: 10.2741/3363
Francischetti, I. M., Valenzuela, J. G., Andersen, J. F., Mather, T. N., and Ribeiro, J. M. (2002). Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis: identification of factor X and factor Xa as scaffolds for the inhibition of factor VIIa/tissue factor complex. Blood 99, 3602–3612. doi: 10.1182/blood-2001-12-0237
Franco, P. F., Silva, N. C., Fazito do Vale, V., Abreu, J. F., Santos, V. C., Gontijo, N. F., et al. (2016). Inhibition of the classical pathway of the complement system by saliva of Amblyomma cajennense (Acari: Ixodidae). Exp. Parasitol. 164, 91–96. doi: 10.1016/j.exppara.2016.03.002
Frauenschuh, A., Power, C. A., Deruaz, M., Ferreira, B. R., Silva, J. S., Teixeira, M. M., et al. (2007). Molecular cloning and characterization of a highly selective chemokine-binding protein from the tick Rhipicephalus sanguineus. J. Biol. Chem. 282, 27250–27258. doi: 10.1074/jbc.M704706200
Fukumoto, S., Sakaguchi, T., You, M., Xuan, X., and Fujisaki, K. (2006). Tick troponin I-like molecule is a potent inhibitor for angiogenesis. Microvasc. Res. 71, 218–221. doi: 10.1016/j.mvr.2006.02.003
Gaede, K., and Knülle, W. (1997). On the mechanism of water vapour sorption from unsaturated atmospheres by ticks. J. Exp. Biol. 200, 1491–1498.
Gao, X., Shi, L., Zhou, Y., Cao, J., Zhang, H., and Zhou, J. (2011). Characterization of the anticoagulant protein Rhipilin-1 from the Rhipicephalus haemaphysaloides tick. J. Insect Physiol. 57, 339–343. doi: 10.1016/j.jinsphys.2010.12.001
Garcia-Varas, S., Manzano-Roman, R., Fernandez-Soto, P., Encinas-Grandes, A., Oleaga, A., and Perez-Sanchez, R. (2010). Purification and characterisation of a P-selectin-binding molecule from the salivary glands of Ornithodoros moubata that induces protective anti-tick immune responses in pigs. Int. J. Parasitol. 40, 313–326. doi: 10.1016/j.ijpara.2009.08.011
Garg, R., Juncadella, I. J., Ramamoorthi, N., Ashish, Ananthanarayanan, S. K., Thomas, V., et al. (2006). Cutting edge: CD4 is the receptor for the tick saliva immunosuppressor, Salp15. J. Immunol. 177, 6579–6583. doi: 10.4049/jimmunol.177.10.6579
Gern, L., and Rais, O. (1996). Efficient transmission of Borrelia burgdorferi between cofeeding Ixodes ricinus ticks (Acari: Ixodidae). J. Med. Entomol. 33, 189–192. doi: 10.1093/jmedent/33.1.189
Gern, L., Schaible, U. E., and Simon, M. M. (1993). Mode of inoculation of the Lyme disease agent Borrelia burgdorferi influences infection and immune responses in inbred strains of mice. J. Infect. Dis. 167, 971–975. doi: 10.1093/infdis/167.4.971
Gillespie, R. D., Dolan, M. C., Piesman, J., and Titus, R. G. (2001). Identification of an IL-2 binding protein in the saliva of the Lyme disease vector tick, Ixodes scapularis. J. Immunol. 166, 4319–4326. doi: 10.4049/jimmunol.166.7.4319
Gillespie, R. D., Mbow, M. L., and Titus, R. G. (2000). The immunomodulatory factors of bloodfeeding arthropod saliva. Parasite Immunol. 22, 319–331. doi: 10.1046/j.1365-3024.2000.00309.x
Gordon, J. R., and Allen, J. R. (1991). Factors, V., and VII anticoagulant activities in the salivary glands of feeding Dermacentor andersoni ticks. J. Parasitol. 77, 167–170. doi: 10.2307/3282577
Guo, X., Booth, C. J., Paley, M. A., Wang, X., DePonte, K., Fikrig, E., et al. (2009). Inhibition of neutrophil function by two tick salivary proteins. Infect. Immun. 77, 2320–2329. doi: 10.1128/IAI.01507-08
Hai, V. V., Almeras, L., Socolovschi, C., Raoult, D., Parola, P., and Pages, F. (2014). Monitoring human tick-borne disease risk and tick bite exposure in Europe: available tools and promising future methods. Ticks Tick Borne Dis. 5, 607–619. doi: 10.1016/j.ttbdis.2014.07.022
Hajdusek, O., Sima, R., Ayllon, N., Jalovecka, M., Perner, J., de la Fuente, J., et al. (2013). Interaction of the tick immune system with transmitted pathogens. Front. Cell. Infect. Microbiol. 3:26. doi: 10.3389/fcimb.2013.00026
Hajnicka, V., Kocakova, P., Slavikova, M., Slovak, M., Gasperik, J., Fuchsberger, N., et al. (2001). Anti-interleukin-8 activity of tick salivary gland extracts. Parasite Immunol. 23, 483–489. doi: 10.1046/j.1365-3024.2001.00403.x
Hajnicka, V., Vancova-Stibraniova, I., Slovak, M., Kocakova, P., and Nuttall, P. A. (2011). Ixodid tick salivary gland products target host wound healing growth factors. Int. J. Parasitol. 41, 213–223. doi: 10.1016/j.ijpara.2010.09.005
Hannier, S., Liversidge, J., Sternberg, J. M., and Bowman, A. S. (2003). Ixodes ricinus tick salivary gland extract inhibits IL-10 secretion and CD69 expression by mitogen-stimulated murine splenocytes and induces hyporesponsiveness in B lymphocytes. Parasite Immunol. 25, 27–37. doi: 10.1046/j.1365-3024.2003.00605.x
Hannier, S., Liversidge, J., Sternberg, J. M., and Bowman, A. S. (2004). Characterization of the B-cell inhibitory protein factor in Ixodes ricinus tick saliva: a potential role in enhanced Borrelia burgdoferi transmission. Immunology 113, 401–408. doi: 10.1111/j.1365-2567.2004.01975.x
Heinze, D. M., Carmical, J. R., Aronson, J. F., Alarcon-Chaidez, F., Wikel, S., and Thangamani, S. (2014). Murine cutaneous responses to the rocky mountain spotted fever vector, Dermacentor andersoni, feeding. Front. Microbiol. 5:198. doi: 10.3389/fmicb.2014.00198
Heinze, D. M., Carmical, J. R., Aronson, J. F., and Thangamani, S. (2012a). Early immunologic events at the tick-host interface. PLoS ONE 7:e47301. doi: 10.1371/journal.pone.0047301
Heinze, D. M., Wikel, S. K., Thangamani, S., and Alarcon-Chaidez, F. J. (2012b). Transcriptional profiling of the murine cutaneous response during initial and subsequent infestations with Ixodes scapularis nymphs. Parasit. Vectors 5:26. doi: 10.1186/1756-3305-5-26
Hermance, M. E., and Thangamani, S. (2015). Tick saliva enhances powassan virus transmission to the host, influencing its dissemination and the course of disease. J. Virol. 89, 7852–7860. doi: 10.1128/JVI.01056-15
Heyman, P., Cochez, C., Hofhuis, A., van der Giessen, J., Sprong, H., Porter, S. R., et al. (2010). A clear and present danger: tick-borne diseases in Europe. Expert Rev. Anti Infect. Ther. 8, 33–50. doi: 10.1586/eri.09.118
Hidano, A., Konnai, S., Yamada, S., Githaka, N., Isezaki, M., Higuchi, H., et al. (2014). Suppressive effects of neutrophil by Salp16-like salivary gland proteins from Ixodes persulcatus Schulze tick. Insect Mol. Biol. 23, 466–474. doi: 10.1111/imb.12101
Hoffman, R., Benz, E. J., Furie, B., and Shattil, S. J. (2009). Hematology: Basic Principles and Practice. Philadelphia, PA: Churchill Livingstone; Elsevier.
Hoffmann, A., Walsmann, P., Riesener, G., Paintz, M., and Markwardt, F. (1991). Isolation and characterization of a thrombin inhibitor from the tick Ixodes ricinus. Pharmazie 46, 209–212.
Horn, F., dos Santos, P. C., and Termignoni, C. (2000). Boophilus microplus anticoagulant protein: an antithrombin inhibitor isolated from the cattle tick saliva. Arch. Biochem. Biophys. 384, 68–73. doi: 10.1006/abbi.2000.2076
Hourcade, D. E., Akk, A. M., Mitchell, L. M., Zhou, H. F., Hauhart, R., and Pham, C. T. (2016). Anti-complement activity of the Ixodes scapularis salivary protein Salp20. Mol. Immunol. 69, 62–69. doi: 10.1016/j.molimm.2015.11.008
Hovius, J. W., de Jong, M. A., den Dunnen, J., Litjens, M., Fikrig, E., van der Poll, T., et al. (2008a). Salp15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and mRNA stabilization. PLoS Pathog. 4:e31. doi: 10.1371/journal.ppat.0040031
Hovius, J. W., Schuijt, T. J., de Groot, K. A., Roelofs, J. J., Oei, G. A., Marquart, J. A., et al. (2008b). Preferential protection of Borrelia burgdorferi sensu stricto by a Salp15 homologue in Ixodes ricinus saliva. J. Infect. Dis. 198, 1189–1197. doi: 10.1086/591917
Hynes, W. L. (2014). “How ticks control microbes: innate immune responses,” in Biology of Ticks, eds D. Sonnenshine and R. M. Roe (Oxford; New York, NY: Oxford University Press), 129–146.
Ibelli, A. M., Kim, T. K., Hill, C. C., Lewis, L. A., Bakshi, M., Miller, S., et al. (2014). A blood meal-induced Ixodes scapularis tick saliva serpin inhibits trypsin and thrombin, and interferes with platelet aggregation and blood clotting. Int. J. Parasitol. 44, 369–379. doi: 10.1016/j.ijpara.2014.01.010
Imamura, S., da Silva Vaz Junior, I., Sugino, M., Ohashi, K., and Onuma, M. (2005). A serine protease inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine 23, 1301–1311. doi: 10.1016/j.vaccine.2004.08.041
Imamura, S., Namangala, B., Tajima, T., Tembo, M. E., Yasuda, J., Ohashi, K., et al. (2006). Two serine protease inhibitors (serpins) that induce a bovine protective immune response against Rhipicephalus appendiculatus ticks. Vaccine 24, 2230–2237. doi: 10.1016/j.vaccine.2005.10.055
Islam, M. K., Tsuji, N., Miyoshi, T., Alim, M. A., Huang, X., Hatta, T., et al. (2009). The Kunitz-like modulatory protein haemangin is vital for hard tick blood-feeding success. PLoS Pathog. 5:e1000497. doi: 10.1371/journal.ppat.1000497
Iwanaga, S., Okada, M., Isawa, H., Morita, A., Yuda, M., and Chinzei, Y. (2003). Identification and characterization of novel salivary thrombin inhibitors from the ixodidae tick, Haemaphysalis longicornis. Eur. J. Biochem. 270, 1926–1934. doi: 10.1046/j.1432-1033.2003.03560.x
Jablonka, W., Kotsyfakis, M., Mizurini, D. M., Monteiro, R. Q., Lukszo, J., Drake, S. K., et al. (2015). Identification and mechanistic analysis of a novel tick-derived inhibitor of thrombin. PLoS ONE 10:e0133991. doi: 10.1371/journal.pone.0133991
Jaworski, D. C., Jasinskas, A., Metz, C. N., Bucala, R., and Barbour, A. G. (2001). Identification and characterization of a homologue of the pro-inflammatory cytokine Macrophage Migration Inhibitory Factor in the tick, Amblyomma americanum. Insect Mol. Biol. 10, 323–331. doi: 10.1046/j.0962-1075.2001.00271.x
Jaworski, D. C., Rosell, R., Coons, L. B., and Needham, G. R. (1992). Tick (Acari: Ixodidae) attachment cement and salivary gland cells contain similar immunoreactive polypeptides. J. Med. Entomol. 29, 305–309. doi: 10.1093/jmedent/29.2.305
Jaworski, D. C., Simmen, F. A., Lamoreaux, W., Coons, L. B., Muller, M. T., and Needham, G. R. (1995). A secreted calreticulin protein in ixodid tick (Amblyomma-Americanum) saliva. J. Insect Physiol. 41, 369–375. doi: 10.1016/0022-1910(94)00107-R
Jelinski, J. W (2016). Painless Hematophagy: The Functional Role of Novel Tick Metalloproteases in Pain Suppression. Honors Theses 401, The University of Southern Mississippi. Available online at: http://aquila.usm.edu/honors_theses/401
Jones, L. D., Davies, C. R., Steele, G. M., and Nuttall, P. A. (1987). A novel mode of arbovirus transmission involving a nonviremic host. Science 237, 775–777. doi: 10.1126/science.3616608
Jones, L. D., Hodgson, E., and Nuttall, P. A. (1989). Enhancement of virus transmission by tick salivary glands. J. Gen. Virol. 70(Pt 7), 1895–1898. doi: 10.1099/0022-1317-70-7-1895
Jonsson, N. N., Piper, E. K., and Constantinoiu, C. C. (2014). Host resistance in cattle to infestation with the cattle tick Rhipicephalus microplus. Parasite Immunol. 36, 553–559. doi: 10.1111/pim.12140
Joubert, A. M., Louw, A. I., Joubert, F., and Neitz, A. W. (1998). Cloning, nucleotide sequence and expression of the gene encoding factor Xa inhibitor from the salivary glands of the tick, Ornithodoros savignyi. Exp. Appl. Acarol. 22, 603–619. doi: 10.1023/A:1006198713791
Karczewski, J., Endris, R., and Connolly, T. M. (1994). Disagregin is a fibrinogen receptor antagonist lacking the Arg-Gly-Asp sequence from the tick, Ornithodoros moubata. J. Biol. Chem. 269, 6702–6708.
Karczewski, J., Waxman, L., Endris, R. G., and Connolly, T. M. (1995). An inhibitor from the argasid tick Ornithodoros moubata of cell adhesion to collagen. Biochem. Biophys. Res. Commun. 208, 532–541. doi: 10.1006/bbrc.1995.1371
Karpathy, S. E., Allerdice, M. E., Sheth, M., Dasch, G. A., and Levin, M. L. (2016). Co-feeding transmission of the ehrlichia muris-like agent to mice (Mus musculus). Vector-Borne Zoonotic Dis. 16, 145–150. doi: 10.1089/vbz.2015.1878
Kato, N., Iwanaga, S., Okayama, T., Isawa, H., Yuda, M., and Chinzei, Y. (2005). Identification and characterization of the plasma kallikrein-kinin system inhibitor, haemaphysalin, from hard tick, Haemaphysalis longicornis. Thromb. Haemost. 93, 359–367. doi: 10.1160/th04-05-0319
Kaufman, W. (1976). The influence of various factors on fluid secretion by in vitro salivary glands of ixodid Ticks. J. Exp. Biol. 64, 727–742.
Kaufman, W. R. (1977). The influence of adrenergic agonists and their antagonists on isolated salivary glands of ixodid ticks. Eur. J. Pharmacol. 45, 61–68. doi: 10.1016/0014-2999(77)90058-9
Kaufman, W. R. (1978). Actions of some transmitters and their antagonists on salivary secretion in a tick. Am. J. Physiol. 235, R76–R81.
Kaufman, W. R., and Harris, R. A. (1983). Neural pathways mediating salivary fluid secretion in the ixodid tick Amblyomma hebraeum. Can. J. Zool. 61, 1976–1980. doi: 10.1139/z83-260
Kazimirova, M., and Stibraniova, I. (2013). Tick salivary compounds: their role in modulation of host defences and pathogen transmission. Front. Cell. Infect. Microbiol. 3:43. doi: 10.3389/fcimb.2013.00043
Kazimirova, M., Jancinova, V., Petrikova, M., Takac, P., Labuda, M., and Nosal, R. (2002). An inhibitor of thrombin-stimulated blood platelet aggregation from the salivary glands of the hard tick Amblyomma variegatum (Acari: Ixodidae). Exp. Appl. Acarol. 28, 97–105. doi: 10.1023/A:1025398100044
Kemp, D. H., Stone, B. F., and Binnington, K. C. (1982). “Tick attachment and feeding: role of the mouthparts, feeding apparatus, salivary gland secretions and the host response,” in Physiology of Ticks, eds F. D. Obenchain and R. Galun (Oxford; New York, NY; Toronto, ON; Sydney, NSW; Paris; Frankfurt: Pergamon Press), 119–168.
Kern, A., Collin, E., Barthel, C., Michel, C., Jaulhac, B., and Boulanger, N. (2011). Tick saliva represses innate immunity and cutaneous inflammation in a murine model of Lyme disease. Vector Borne Zoonotic Dis. 11, 1343–1350. doi: 10.1089/vbz.2010.0197
Kim, D., Šimo, L., and Park, Y. (2014). Orchestration of salivary secretion mediated by two different dopamine receptors in the blacklegged tick Ixodes scapularis. J. Exp. Biol. 217, 3656–3663. doi: 10.1242/jeb.109462
Kim, D., Urban, J., Boyle, D. L., and Park, Y. (2016). Multiple functions of Na/K-ATPase in dopamine-induced salivation of the Blacklegged tick, Ixodes scapularis. Sci. Rep. 6:21047. doi: 10.1038/srep21047
Kim, T. K., Ibelli, A. M. G., and Mulenga, A. (2015a). Amblyomma americanum tick calreticulin binds C1q but does not inhibit activation of the classical complement cascade. Ticks Tick Borne Dis. 6, 91–101. doi: 10.1016/j.ttbdis.2014.10.002
Kim, T. K., Tirloni, L., Radulovic, Z., Lewis, L., Bakshi, M., Hill, C., et al. (2015b). Conserved Amblyomma americanum tick Serpin19, an inhibitor of blood clotting factors Xa and XIa, trypsin and plasmin, has anti-haemostatic functions. Int. J. Parasitol. 45, 613–627. doi: 10.1016/j.ijpara.2015.03.009
Knulle, W., and Rudolph, D. (1982). Humidity Relationships and Water Balance of Ticks. Oxford: Pergamon Press.
Koči, J., Šimo, L., and Park, Y. (2014). Autocrine/paracrine dopamine in the salivary glands of the blacklegged tick Ixodes scapularis. J. Insect Physiol. 62, 39–45. doi: 10.1016/j.jinsphys.2014.01.007
Koh, C. Y., and Kini, R. M. (2009). Molecular diversity of anticoagulants from haematophagous animals. Thromb. Haemost. 102, 437–453. doi: 10.1160/th09-04-0221
Koh, C. Y., Kazimirova, M., Trimnell, A., Takac, P., Labuda, M., Nuttall, P. A., et al. (2007). Variegin, a novel fast and tight binding thrombin inhibitor from the tropical bont tick. J. Biol. Chem. 282, 29101–29113. doi: 10.1074/jbc.M705600200
Kopecký, J., and Kuthejlová, M. (1998). Suppressive effect of Ixodes ricinus salivary gland extract on mechanisms of natural immunity in vitro. Parasite Immunol. 20, 169–174.
Kotal, J., Langhansova, H., Lieskovska, J., Andersen, J. F., Francischetti, I. M., Chavakis, T., et al. (2015). Modulation of host immunity by tick saliva. J. Proteomics 128, 58–68. doi: 10.1016/j.jprot.2015.07.005
Kotsyfakis, M., Horka, H., Salat, J., and Andersen, J. F. (2010). The crystal structures of two salivary cystatins from the tick Ixodes scapularis and the effect of these inhibitors on the establishment of Borrelia burgdorferi infection in a murine model. Mol. Microbiol. 77, 456–470. doi: 10.1111/j.1365-2958.2010.07220.x
Kotsyfakis, M., Sa-Nunes, A., Francischetti, I. M., Mather, T. N., Andersen, J. F., and Ribeiro, J. M. (2006). Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J. Biol. Chem. 281, 26298–26307. doi: 10.1074/jbc.M513010200
Kramer, C., Nahmias, Z., Norman, D. D., Mulvihill, T. A., Coons, L. B., and Cole, J. A. (2008). Dermacentor variabilis: regulation of fibroblast migration by tick salivary gland extract and saliva. Exp. Parasitol. 119, 391–397. doi: 10.1016/j.exppara.2008.04.005
Kramer, C. D., Poole, N. M., Coons, L. B., and Cole, J. A. (2011). Tick saliva regulates migration, phagocytosis, and gene expression in the macrophage-like cell line, IC-21. Exp. Parasitol. 127, 665–671. doi: 10.1016/j.exppara.2010.11.012
Krause, P. J., Grant-Kels, J. M., Tahan, S. R., Dardick, K. R., Alarcon-Chaidez, F., Bouchard, K., et al. (2009). Dermatologic changes induced by repeated Ixodes scapularis bites and implications for prevention of tick-borne infection. Vector Borne Zoonotic Dis. 9, 603–610. doi: 10.1089/vbz.2008.0091
Krocova, Z., Macela, A., Hernychova, L., Kroca, M., Pechova, J., and Kopecky, J. (2003). Tick salivary gland extract accelerates proliferation of Francisella tularensis in the host. J. Parasitol. 89, 14–20. doi: 10.1645/0022-3395(2003)089[0014:TSGEAP]2.0.CO;2
Krolak, J. M., Ownby, C. L., and Sauer, J. R. (1982). Alveolar structure of salivary glands of the lone star tick, Amblyomma americanum (L.): unfed females. J. Parasitol. 68, 61–82. doi: 10.2307/3281326
Kubes, M., Fuchsberger, N., Labuda, M., Zuffova, E., and Nuttall, P. A. (1994). Salivary gland extracts of partially fed Dermacentor reticulatus ticks decrease natural killer cell activity in vitro. Immunology 82, 113–116.
Kuthejlova, M., Kopecky, J., Stepanova, G., and Macela, A. (2001). Tick salivary gland extract inhibits killing of Borrelia afzelii spirochetes by mouse macrophages. Infect. Immun. 69, 575–578. doi: 10.1128/IAI.69.1.575-578.2001
Labuda, M., Austyn, J. M., Zuffova, E., Kozuch, O., Fuchsberger, N., Lysy, J., et al. (1996). Importance of localized skin infection in tick-borne encephalitis virus transmission. Virology 219, 357–366. doi: 10.1006/viro.1996.0261
Labuda, M., Jones, L. D., Williams, T., Danielova, V., and Nuttall, P. A. (1993). Efficient transmission of tick-borne encephalitis virus between cofeeding ticks. J. Med. Entomol. 30, 295–299. doi: 10.1093/jmedent/30.1.295
Labuda, M., Kozuch, O., Zuffova, E., Eleckova, E., Hails, R. S., and Nuttall, P. A. (1997). Tick-borne encephalitis virus transmission between ticks cofeeding on specific immune natural rodent hosts. Virology 235, 138–143. doi: 10.1006/viro.1997.8622
Labuda, M., Trimnell, A. R., Lickova, M., Kazimirova, M., Davies, G. M., Lissina, O., et al. (2006). An antivector vaccine protects against a lethal vector-borne pathogen. PLoS Pathog. 2:e27. doi: 10.1371/journal.ppat.0020027
Leboulle, G., Crippa, M., Decrem, Y., Mejri, N., Brossard, M., Bollen, A., et al. (2002). Characterization of a novel salivary immunosuppressive protein from Ixodes ricinus ticks. J. Biol. Chem. 277, 10083–10089. doi: 10.1074/jbc.M111391200
Leger, E., Vourc'h, G., Vial, L., Chevillon, C., and McCoy, K. D. (2013). Changing distributions of ticks: causes and consequences. Exp. Appl. Acarol. 59, 219–244. doi: 10.1007/s10493-012-9615-0
Lieskovska, J., and Kopecky, J. (2012a). Effect of tick saliva on signalling pathways activated by TLR-2 ligand and Borrelia afzelii in dendritic cells. Parasite Immunol. 34, 421–429. doi: 10.1111/j.1365-3024.2012.01375.x
Lieskovska, J., and Kopecky, J. (2012b). Tick saliva suppresses IFN signalling in dendritic cells upon Borrelia afzelii infection. Parasite Immunol. 34, 32–39. doi: 10.1111/j.1365-3024.2011.01345.x
Lieskovska, J., Palenikova, J., Langhansova, H., Chagas, A. C., Calvo, E., Kotsyfakis, M., et al. (2015a). Tick sialostatins L and L2 differentially influence dendritic cell responses to Borrelia spirochetes. Parasit. Vectors 8:275. doi: 10.1186/s13071-015-0887-1
Lieskovska, J., Palenikova, J., Sirmarova, J., Elsterova, J., Kotsyfakis, M., Chagas, A. C., et al. (2015b). Tick salivary cystatin sialostatin L2 suppresses IFN responses in mouse dendritic cells. Parasite Immunol. 37, 70–78. doi: 10.1111/pim.12162
Limo, M. K., Voigt, W. P., Tumbo-Oeri, A. G., Njogu, R. M., and ole-MoiYoi, O. K. (1991). Purification and characterization of an anticoagulant from the salivary glands of the ixodid tick Rhipicephalus appendiculatus. Exp. Parasitol. 72, 418–429. doi: 10.1016/0014-4894(91)90088-E
Lindsay, P. J., and Kaufman, W. R. (1986). Potentiation of salivary fluid secretion in ixodid ticks: a new receptor system for gamma-aminobutyric acid. Can. J. Physiol. Pharmacol. 64, 1119–1126. doi: 10.1139/y86-191
Liu, J., Renneker, S., Beyer, D., Kullmann, B., Seitzer, U., Ahmed, J., et al. (2014). Identification and partial characterization of a Salp15 homolog from Ixodes ricinus. Ticks Tick Borne Dis. 5, 318–322. doi: 10.1016/j.ttbdis.2013.12.004
Liu, L., Dai, J., Zhao, Y. O., Narasimhan, S., Yang, Y., Zhang, L., et al. (2012). Ixodes scapularis JAK-STAT pathway regulates tick antimicrobial peptides, thereby controlling the agent of human granulocytic anaplasmosis. J. Infect. Dis. 206, 1233–1241. doi: 10.1093/infdis/jis484
Liu, L., Narasimhan, S., Dai, J., Zhang, L., Cheng, G., and Fikrig, E. (2011). Ixodes scapularis salivary gland protein P11 facilitates migration of Anaplasma phagocytophilum from the tick gut to salivary glands. EMBO Rep. 12, 1196–1203. doi: 10.1038/embor.2011.177
Liu, X. Y., and Bonnet, S. I. (2014). Hard tick factors implicated in pathogen transmission. PLoS Negl. Trop. Dis. 8:e2566. doi: 10.1371/journal.pntd.0002566
Liu, X. Y., de la Fuente, J., Cote, M., Galindo, R. C., Moutailler, S., Vayssier-Taussat, M., et al. (2014). IrSPI, a tick serine protease inhibitor involved in tick feeding and Bartonella henselae infection. PLoS Negl. Trop. Dis. 8:e2993. doi: 10.1371/journal.pntd.0002993
Liyou, N., Hamilton, S., Elvin, C., and Willadsen, P. (1999). Cloning and expression of ecto 5-nucleotidase from the cattle tick Boophilus microplus. Insect Mol. Biol. 8, 257–266. doi: 10.1046/j.1365-2583.1999.820257.x
Macaluso, K. R., Mulenga, A., Simser, J. A., and Azad, A. F. (2003). Differential expression of genes in uninfected and rickettsia-infected Dermacentor variabilis ticks as assessed by differential-display PCR. Infect. Immun. 71, 6165–6170. doi: 10.1128/IAI.71.11.6165-6170.2003
Machackova, M., Obornik, M., and Kopecky, J. (2006). Effect of salivary gland extract from Ixodes ricinus ticks on the proliferation of Borrelia burgdorferi sensu stricto in vivo. Folia Parasitol. 53, 153–158. doi: 10.14411/fp.2006.020
Madden, R. D., Sauer, J. R., and Dillwith, J. W. (2004). A proteomics approach to characterizing tick salivary secretions. Exp. Appl. Acarol. 32, 77–87. doi: 10.1023/B:APPA.0000018316.80224.54
Malouin, R., Winch, P., Leontsini, E., Glass, G., Simon, D., Hayes, E. B., et al. (2003). Longitudinal evaluation of an educational intervention for preventing tick bites in an area with endemic lyme disease in Baltimore County, Maryland. Am. J. Epidemiol. 157, 1039–1051. doi: 10.1093/aje/kwg076
Mans, B. J. (2011). Evolution of vertebrate hemostatic and inflammatory control mechanisms in blood-feeding arthropods. J. Innate Immun. 3, 41–51. doi: 10.1159/000321599
Mans, B. J. (2014). “Heme processing and the evolution of hematophagy,” in Biology of Ticks, eds E. Daniel and M. R. R. Sonenshine (New York, NY: Oxford University Press), 220–239.
Mans, B. J., Andersen, J. F., Francischetti, I. M., Valenzuela, J. G., Schwan, T. G., Pham, V. M., et al. (2008). Comparative sialomics between hard and soft ticks: implications for the evolution of blood-feeding behavior. Insect Biochem. Mol. Biol. 38, 42–58. doi: 10.1016/j.ibmb.2007.09.003
Mans, B. J., Gaspar, A. R., Louw, A. I., and Neitz, A. W. (1998a). Apyrase activity and platelet aggregation inhibitors in the tick Ornithodoros savignyi (Acari: Argasidae). Exp. Appl. Acarol. 22, 353–366. doi: 10.1023/A:1024517209621
Mans, B. J., Louw, A. I., and Neitz, A. W. (2002a). Evolution of hematophagy in ticks: common origins for blood coagulation and platelet aggregation inhibitors from soft ticks of the genus Ornithodoros. Mol. Biol. Evol. 19, 1695–1705. doi: 10.1093/oxfordjournals.molbev.a003992
Mans, B. J., Louw, A. I., and Neitz, A. W. (2002b). Savignygrin, a platelet aggregation inhibitor from the soft tick Ornithodoros savignyi, presents the RGD integrin recognition motif on the Kunitz-BPTI fold. J. Biol. Chem. 277, 21371–21378. doi: 10.1074/jbc.M112060200
Mans, B. J., Louw, A. I., and Neitz, A. W. (2002c). Disaggregation of aggregated platelets by savignygrin, a alphaIIbeta3 antagonist from Ornithodoros savignyi. Exp. Appl. Acarol. 27, 231–239. doi: 10.1023/A:1021613001297
Mans, B. J., and Ribeiro, J. M. (2008). Function, mechanism and evolution of the moubatin-clade of soft tick lipocalins. Insect Biochem. Mol. Biol. 38, 841–852. doi: 10.1016/j.ibmb.2008.06.007
Marchal, C., Schramm, F., Kern, A., Luft, B. J., Yang, X., Schuijt, T. J., et al. (2011). Antialarmin effect of tick saliva during the transmission of Lyme disease. Infect. Immun. 79, 774–785. doi: 10.1128/IAI.00482-10
Mason, L. M., Veerman, C. C., Geijtenbeek, T. B., and Hovius, J. W. (2014). Menage a trois: borrelia, dendritic cells, and tick saliva interactions. Trends Parasitol. 30, 95–103. doi: 10.1016/j.pt.2013.12.003
McNally, K. L., Mitzel, D. N., Anderson, J. M., Ribeiro, J. M., Valenzuela, J. G., Myers, T. G., et al. (2012). Differential salivary gland transcript expression profile in Ixodes scapularis nymphs upon feeding or flavivirus infection. Ticks Tick Borne Dis. 3, 18–26. doi: 10.1016/j.ttbdis.2011.09.003
McSwain, J. L., Essenberg, R. C., and Sauer, J. R. (1992). Oral secretion elicited by effectors of signal transduction pathways in the salivary glands of Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 29, 41–48. doi: 10.1093/jmedent/29.1.41
Medlock, J. M., Hansford, K. M., Bormane, A., Derdakova, M., Estrada-Pena, A., George, J. C., et al. (2013). Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors 6:1. doi: 10.1186/1756-3305-6-1
Mejri, N., and Brossard, M. (2007). Splenic dendritic cells pulsed with Ixodes ricinus tick saliva prime naive CD4+T to induce Th2 cell differentiation in vitro and in vivo. Int. Immunol. 19, 535–543. doi: 10.1093/intimm/dxm019
Mejri, N., Franscini, N., Rutti, B., and Brossard, M. (2001). Th2 polarization of the immune response of BALB/c mice to Ixodes ricinus instars, importance of several antigens in activation of specific Th2 subpopulations. Parasite Immunol. 23, 61–69.
Mercado-Curiel, R. F., Palmer, G. H., Guerrero, F. D., and Brayton, K. A. (2011). Temporal characterisation of the organ-specific Rhipicephalus microplus transcriptional response to Anaplasma marginale infection. Int. J. Parasitol. 41, 851–860. doi: 10.1016/j.ijpara.2011.03.003
Meredith, J., and Kaufman, W. R. (1973). A proposed site of fluid secretion in the salivary gland of the ixodid tick, Dermacentor andersoni. Parasitology 67, 205–217. doi: 10.1017/S0031182000046424
Merino, O., Almazan, C., Canales, M., Villar, M., Moreno-Cid, J. A., Galindo, R. C., et al. (2011). Targeting the tick protective antigen subolesin reduces vector infestations and pathogen infection by Anaplasma marginale and Babesia bigemina. Vaccine 29, 8575–8579. doi: 10.1016/j.vaccine.2011.09.023
Merino, O., Antunes, S., Mosqueda, J., Moreno-Cid, J. A., Perez de la Lastra, J. M., Rosario-Cruz, R., et al. (2013). Vaccination with proteins involved in tick-pathogen interactions reduces vector infestations and pathogen infection. Vaccine 31, 5889–5896. doi: 10.1016/j.vaccine.2013.09.037
Milhano, N., Saito, T. B., Bechelli, J., Fang, R., Vilhena, M., De Sousa, R., et al. (2015). The role of Rhipicephalus sanguineus sensu lato saliva in the dissemination of Rickettsia conorii in C3H/HeJ mice. Med. Vet. Entomol. 29, 225–229. doi: 10.1111/mve.12118
Montgomery, R. R., Lusitani, D., De Boisfleury Chevance, A., and Malawista, S. E. (2004). Tick saliva reduces adherence and area of human neutrophils. Infect. Immun. 72, 2989–2994. doi: 10.1128/IAI.72.5.2989-2994.2004
Mori, A., Konnai, S., Yamada, S., Hidano, A., Murase, Y., Ito, T., et al. (2010). Two novel Salp15-like immunosuppressant genes from salivary glands of Ixodes persulcatus Schulze tick. Insect Mol. Biol. 19, 359–365. doi: 10.1111/j.1365-2583.2010.00994.x
Motoyashiki, T., Tu, A. T., Azimov, D. A., and Ibragim, K. (2003). Isolation of anticoagulant from the venom of tick, Boophilus calcaratus, from Uzbekistan. Thromb. Res. 110, 235–241. doi: 10.1016/S0049-3848(03)00409-2
Mulenga, A., Kim, T., and Ibelli, A. M. G. (2013a). Amblyomma americanum tick saliva serine protease inhibitor 6 is a cross-class inhibitor of serine proteases and papain-like cysteine proteases that delays plasma clotting and inhibits platelet aggregation. Insect Mol. Biol. 22, 306–319. doi: 10.1111/imb.12024
Mulenga, A., Kim, T. K., and Ibelli, A. M. G. (2013b). Deorphanization and target validation of cross-tick species conserved novel Amblyomma americanum tick saliva protein. Int. J. Parasitol. 43, 439–451. doi: 10.1016/j.ijpara.2012.12.012
Mulenga, A., Macaluso, K. R., Simser, J. A., and Azad, A. F. (2003). The American dog tick, Dermacentor variabilis, encodes a functional histamine release factor homolog. Insect Biochem. Mol. Biol. 33, 911–919. doi: 10.1016/S0965-1748(03)00097-3
Mulenga, A., Sugimoto, C., Sako, Y., Ohashi, K., Musoke, A., Shubash, M., et al. (1999). Molecular characterization of a Haemaphysalis longicornis tick salivary gland-associated 29-kilodalton protein and its effect as a vaccine against tick infestation in rabbits. Infect. Immun. 67, 1652–1658.
Murase, Y., Konnai, S., Yamada, S., Githaka, N., Isezaki, M., Ito, T., et al. (2015). An investigation of binding ability of Ixodes persulcatus Schulze Salp15 with Lyme disease spirochetes. Insect Biochem. Mol. Biol. 60, 59–67. doi: 10.1016/j.ibmb.2015.01.010
Nakajima, C., Imamura, S., Konnai, S., Yamada, S., Nishikado, H., Ohashi, K., et al. (2006). A novel gene encoding a thrombin inhibitory protein in a cDNA library from Haemaphysalis longicornis salivary gland. J. Vet. Med. Sci. 68, 447–452. doi: 10.1292/jvms.68.447
Naranjo, V., Ayllon, N., Perez de la Lastra, J. M., Galindo, R. C., Kocan, K. M., Blouin, E. F., et al. (2013). Reciprocal regulation of NF-kB (Relish) and Subolesin in the tick vector, Ixodes scapularis. PLoS ONE 8:e65915. doi: 10.1371/journal.pone.0065915
Narasimhan, S., Deponte, K., Marcantonio, N., Liang, X., Royce, T. E., Nelson, K. F., et al. (2007a). Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS ONE 2:e451. doi: 10.1371/journal.pone.0000451
Narasimhan, S., Koski, R. A., Beaulieu, B., Anderson, J. F., Ramamoorthi, N., Kantor, F., et al. (2002). A novel family of anticoagulants from the saliva of Ixodes scapularis. Insect Mol. Biol. 11, 641–650. doi: 10.1046/j.1365-2583.2002.00375.x
Narasimhan, S., Sukumaran, B., Bozdogan, U., Thomas, V., Liang, X., DePonte, K., et al. (2007b). A tick antioxidant facilitates the Lyme disease agent's successful migration from the mammalian host to the arthropod vector. Cell Host Microbe 2, 7–18. doi: 10.1016/j.chom.2007.06.001
Nazario, S., Das, S., de Silva, A. M., Deponte, K., Marcantonio, N., Anderson, J. F., et al. (1998). Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. Am. J. Trop. Med. Hyg. 58, 780–785. doi: 10.4269/ajtmh.1998.58.780
Needham, G. R., and Pannabecker, T. L. (1983). Effects of octopamine, chlordimeform, and demethylchlordimeform on amine-controlled tick salivary glands isolated from feeding Amblyomma americanum (L.). Pestic. Biochem. Physiol. 19, 133–140. doi: 10.1016/0048-3575(83)90132-3
Needham, G. R., Rosell, R., and Greenwald, L. (1990). Ultrastructure of type-I salivary-gland acini in four species of ticks and the influence of hydration states on the type-I acini of Amblyomma americanum. Exp. Appl. Acarol. 10, 83–104. doi: 10.1007/BF01194085
Needham, G. R., and Teel, P. D. (1986). “Water balance by ticks between bloodmeals,” in Morphology, Physiology, and Behavioral Biology of Ticks, eds J. R. Sauer and J. Alexander Hair (Chichester: Ellis Horwood Limited), 100–151.
Nene, V., Lee, D., Kang'a, S., Skilton, R., Shah, T., de Villiers, E., et al. (2004). Genes transcribed in the salivary glands of female Rhipicephalus appendiculatus ticks infected with Theileria parva. Insect Biochem. Mol. Biol. 34, 1117–1128. doi: 10.1016/j.ibmb.2004.07.002
Nienaber, J., Gaspar, A. R., and Neitz, A. W. (1999). Savignin, a potent thrombin inhibitor isolated from the salivary glands of the tick Ornithodoros savignyi (Acari: Argasidae). Exp. Parasitol. 93, 82–91. doi: 10.1006/expr.1999.4448
Nunn, M. A., Sharma, A., Paesen, G. C., Adamson, S., Lissina, O., Willis, A. C., et al. (2005). Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J. Immunol. 174, 2084–2091. doi: 10.4049/jimmunol.174.4.2084
Nuttall, P. A., and Labuda, M. (2004). Tick-host interactions: saliva-activated transmission. Parasitology 129(Suppl.), S177–S189. doi: 10.1017/S0031182004005633
Nuttall, P. A., Trimnell, A. R., Kazimirova, M., and Labuda, M. (2006). Exposed and concealed antigens as vaccine targets for controlling ticks and tick-borne diseases. Parasite Immunol. 28, 155–163. doi: 10.1111/j.1365-3024.2006.00806.x
Oliveira, C. J. F., Cavassani, K. A., Moré, D. D., Garlet, G. P., Aliberti, J. C., Silva, J. S., et al. (2008). Tick saliva inhibits the chemotactic function of MIP-1 α and selectively impairs chemotaxis of immature dendritic cells by down-regulating cell-1410 surface CCR5. Int. J. Parasitol. 38, 705–716. doi: 10.1016/j.ijpara.2007.10.006
Oliveira, C. J., Sa-Nunes, A., Francischetti, I. M., Carregaro, V., Anatriello, E., Silva, J. S., et al. (2011). Deconstructing tick saliva: non-protein molecules with potent immunomodulatory properties. J. Biol. Chem. 286, 10960–10969. doi: 10.1074/jbc.M110.205047
Ouhelli, H., Pandey, V. S., and Aboughal, A. (1987). Effect of infection by Babesia spp. on the development and survival of free-living stages of Boophilus annulatus. Vet. Parasitol. 23, 147–154. doi: 10.1016/0304-4017(87)90033-1
Paesen, G. C., Adams, P. L., Harlos, K., Nuttall, P. A., and Stuart, D. I. (1999). Tick histamine-binding proteins: isolation, cloning, and three-dimensional structure. Mol. Cell 3, 661–671. doi: 10.1016/S1097-2765(00)80359-7
Paesen, G. C., Siebold, C., Harlos, K., Peacey, M. F., Nuttall, P. A., and Stuart, D. I. (2007). A tick protein with a modified Kunitz fold inhibits human tryptase. J. Mol. Biol. 368, 1172–1186. doi: 10.1016/j.jmb.2007.03.011
Pal, U., Li, X., Wang, T., Montgomery, R. R., Ramamoorthi, N., Desilva, A. M., et al. (2004). TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119, 457–468. doi: 10.1016/j.cell.2004.10.027
Palenikova, J., Lieskovska, J., Langhansova, H., Kotsyfakis, M., Chmelar, J., and Kopecky, J. (2015). Ixodes ricinus salivary serpin IRS-2 affects Th17 differentiation via inhibition of the interleukin-6/STAT-3 signaling pathway. Infect. Immun. 83, 1949–1956. doi: 10.1128/IAI.03065-14
Pannabecker, T., and Needham, G. R. (1985). Effects of octopamine on fluid secretion by isolated salivary glands of a feeding ixodid tick. Arch. Insect Biochem. Physiol. 2, 217–226. doi: 10.1002/arch.940020209
Pechova, J., Stepanova, G., Kovar, L., and Kopecky, J. (2002). Tick salivary gland extract-activated transmission of Borrelia afzelii spirochaetes. Folia Parasitol. 49, 153–159. doi: 10.14411/fp.2002.027
Pekarikova, D., Rajska, P., Kazimirova, M., Pechanova, O., Takac, P., and Nuttall, P. A. (2015). Vasoconstriction induced by salivary gland extracts from ixodid ticks. Int. J. Parasitol. 45, 879–883. doi: 10.1016/j.ijpara.2015.08.006
Pichu, S., Ribeiro, J. M. C., and Mather, T. N. (2009). Purification and characterization of a novel salivary antimicrobial peptide from the tick, Ixodes scapularis. Biochem. Biophys. Res. Commun. 390, 511–515. doi: 10.1016/j.bbrc.2009.09.127
Piesman, J., and Happ, C. M. (2001). The efficacy of co-feeding as a means of maintaining Borrelia burgdorferi: a North American model system. J. Vector Ecol. 26, 216–220.
Poole, N. M., Mamidanna, G., Smith, R. A., Coons, L. B., and Cole, J. A. (2013a). Prostaglandin E2 in tick saliva regulates host cell migration and cytokine profile. Parasit. Vectors 6:261. doi: 10.1186/1756-3305-6-261
Poole, N. M., Nyindodo-Ogari, L., Kramer, C., Coons, L. B., and Cole, J. A. (2013b). Effects of tick saliva on the migratory and invasive activity of Saos-2 osteosarcoma and MDA-MB-231 breast cancer cells. Ticks Tick Borne Dis. 4, 120–127. doi: 10.1016/j.ttbdis.2012.09.003
Preston, S. G., Majtan, J., Kouremenou, C., Rysnik, O., Burger, L. F., Cabezas Cruz, A., et al. (2013). Novel immunomodulators from hard ticks selectively reprogramme human dendritic cell responses. PLoS Pathog. 9:e1003450. doi: 10.1371/journal.ppat.1003450
Prevot, P. P., Adam, B., Boudjeltia, K. Z., Brossard, M., Lins, L., Cauchie, P., et al. (2006). Anti-hemostatic effects of a serpin from the saliva of the tick Ixodes ricinus. J. Biol. Chem. 281, 26361–26369. doi: 10.1074/jbc.M604197200
Prevot, P. P., Couvreur, B., Denis, V., Brossard, M., Vanhamme, L., and Godfroid, E. (2007). Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine 25, 3284–3292. doi: 10.1016/j.vaccine.2007.01.008
Qian, Y., Yuan, J., Essenberg, R. C., Bowman, A. S., Shook, A. L., Dillwith, J. W., et al. (1998). Prostaglandin E2 in the salivary glands of the female tick, Amblyomma americanum (L.): calcium mobilization and exocytosis. Insect Biochem. Mol. Biol. 28, 221–228. doi: 10.1016/S0965-1748(98)00018-6
Radolf, J. D., Caimano, M. J., Stevenson, B., and Hu, L. T. (2012). Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10, 87–99. doi: 10.1038/nrmicro2714
Ramachandra, R. N., and Wikel, S. K. (1992). Modulation of host-immune responses by ticks (Acari: Ixodidae): effect of salivary gland extracts on host macrophages and lymphocyte cytokine production. J. Med. Entomol. 29, 818–826. doi: 10.1093/jmedent/29.5.818
Ramamoorthi, N., Narasimhan, S., Pal, U., Bao, F., Yang, X. F., Fish, D., et al. (2005). The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436, 573–577. doi: 10.1038/nature03812
Randolph, S. E. (2011). Transmission of tick-borne pathogens between co-feeding ticks: Milan Labuda's enduring paradigm. Ticks Tick Borne Dis. 2, 179–182. doi: 10.1016/j.ttbdis.2011.07.004
Ribeiro, J. M., Alarcon-Chaidez, F., Francischetti, I. M., Mans, B. J., Mather, T. N., Valenzuela, J. G., et al. (2006). An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem. Mol. Biol. 36, 111–129. doi: 10.1016/j.ibmb.2005.11.005
Ribeiro, J. M., Endris, T. M., and Endris, R. (1991). Saliva of the soft tick, Ornithodoros moubata, contains anti-platelet and apyrase activities. Comp. Biochem. Physiol. A Comp. Physiol. 100, 109–112. doi: 10.1016/0300-9629(91)90190-N
Ribeiro, J. M., Makoul, G. T., and Robinson, D. R. (1988). Ixodes dammini: evidence for salivary prostacyclin secretion. J. Parasitol. 74, 1068–1069. doi: 10.2307/3282240
Ribeiro, J. M., Makoul, G. T., Levine, J., Robinson, D. R., and Spielman, A. (1985). Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J. Exp. Med. 161, 332–344. doi: 10.1084/jem.161.2.332
Ribeiro, J. M., and Mather, T. N. (1998). Ixodes scapularis: salivary kininase activity is a metallo dipeptidyl carboxypeptidase. Exp. Parasitol. 89, 213–221. doi: 10.1006/expr.1998.4296
Ribeiro, J. M., Weis, J. J., and Telford, S. R. III. (1990). Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp. Parasitol. 70, 382–388. doi: 10.1016/0014-4894(90)90121-R
Richter, D., Allgöwer, R., and Matuschka, F.-R. (2002). Co-feeding transmission and its contribution to the perpetuation of the lyme disease spirochete Borrelia afzelii. Emerg. Infect. Dis. 8, 1421–1425. doi: 10.3201/eid0812.010519
Rizzoli, A., Silaghi, C., Obiegala, A., Rudolf, I., Hubalek, Z., Foldvari, G., et al. (2014). Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front. Public Health 2:251. doi: 10.3389/fpubh.2014.00251
Roller, L., Šimo, L., Mizoguchi, L., Slovák, M., Park, Y., and Žitňan, D. (2015). Orcokinin-like immunoreactivity in central neurons innervating the salivary glands and hindgut of ixodid ticks. Cell Tissue Res. 360, 209–222. doi: 10.1007/s00441-015-2121-z
Sanders, M. L., Glass, G. E., Nadelman, R. B., Wormser, G. P., Scott, A. L., Raha, S., et al. (1999). Antibody levels to recombinant tick calreticulin increase in humans after exposure to Ixodes scapularis (Say) and are correlated with tick engorgement indices. Am. J. Epidemiol. 149, 777–784. doi: 10.1093/oxfordjournals.aje.a009887
Sanders, M. L., Glass, G. E., Scott, A. L., and Schwartz, B. S. (1998a). Kinetics and cross-species comparisons of host antibody responses to lone star ticks and American dog ticks (Acari: Ixodidae). J. Med. Entomol. 35, 849–856. doi: 10.1093/jmedent/35.5.849
Sanders, M. L., Jaworski, D. C., Sanchez, J. L., DeFraites, R. F., Glass, G. E., Scott, A. L., et al. (1998b). Antibody to a cDNA-derived calreticulin protein from Amblyomma americanum as a biomarker of tick exposure in humans. Am. J. Trop. Med. Hyg. 59, 279–285. doi: 10.4269/ajtmh.1998.59.279
Sangamnatdej, S., Paesen, G. C., Slovak, M., and Nuttall, P. A. (2002). A high affinity serotonin- and histamine-binding lipocalin from tick saliva. Insect Mol. Biol. 11, 79–86. doi: 10.1046/j.0962-1075.2001.00311.x
Sa-Nunes, A., Bafica, A., Antonelli, L. R., Choi, E. Y., Francischetti, I. M., Andersen, J. F., et al. (2009). The immunomodulatory action of sialostatin L on dendritic cells reveals its potential to interfere with autoimmunity. J. Immunol. 182, 7422–7429. doi: 10.4049/jimmunol.0900075
Sá-Nunes, A., Bafica, A., Lucas, D. A., Conrads, T. P., Veenstra, T. D., Andersen, J. F., et al. (2007). Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J. Immunol. 179, 1497–1505. doi: 10.1016/j.cyto.2007.07.138
Sauer, J. R., Essenberg, R. C., and Bowman, A. C. (2000). Salivary glands in ixodid ticks: control and mechanism of secretion. J. Insect Physiol. 46, 1069–1078. doi: 10.1016/S0022-1910(99)00210-3
Sauer, J. R., McSwain, J. L., Bowman, A. S., and Essenberg, R. C. (1995). Tick salivary gland physiology. Annu. Rev. Entomol. 40, 245–267. doi: 10.1146/annurev.en.40.010195.001333
Schoeler, G. B., Manweiler, S. A., and Wikel, S. K. (1999). Ixodes scapularis: effects of repeated infestations with pathogen-free nymphs on macrophage and T lymphocyte cytokine responses of BALB/c and C3H/HeN mice. Exp. Parasitol. 92, 239–248. doi: 10.1006/expr.1999.4426
Schoeler, G. B., and Wikel, S. K. (2001). Modulation of host immunity by haematophagous arthropods. Ann. Trop. Med. Parasitol. 95, 755–771. doi: 10.1080/00034983.2001.11813695
Scholl, D. C., Embers, M. E., Caskey, J. R., Kaushal, D., Mather, T. N., Buck, W. R., et al. (2016). Immunomodulatory effects of tick saliva on dermal cells exposed to Borrelia burgdorferi, the agent of Lyme disease. Parasit. Vectors 9:394. doi: 10.1186/s13071-016-1638-7
Schroeder, H., Daix, V., Gillet, L., Renauld, J. C., and Vanderplasschen, A. (2007). The paralogous salivary anti-complement proteins IRAC I and IRAC II encoded by Ixodes ricinus ticks have broad and complementary inhibitory activities against the complement of different host species. Microbes Infect. 9, 247–250. doi: 10.1016/j.micinf.2006.10.020
Schuijt, T. J., Bakhtiari, K., Daffre, S., Deponte, K., Wielders, S. J., Marquart, J. A., et al. (2013). Factor Xa activation of factor V is of paramount importance in initiating the coagulation system: lessons from a tick salivary protein. Circulation 128, 254–266. doi: 10.1161/CIRCULATIONAHA.113.003191
Schuijt, T. J., Coumou, J., Narasimhan, S., Dai, J., Deponte, K., Wouters, D., et al. (2011). A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell Host Microbe 10, 136–146. doi: 10.1016/j.chom.2011.06.010
Schuijt, T. J., Hovius, J. W. R., van Burgel, N. D., Ramamoorthi, N., Fikrig, E., and van Dam, A. P. (2008). The tick salivary protein Salp15 inhibits the killing of serum-sensitive Borrelia burgdorferi sensu lato isolates. Infect. Immun. 76, 2888–2894. doi: 10.1128/IAI.00232-08
Schwartz, B. S., Ribeiro, J. M., and Goldstein, M. D. (1990). Anti-tick antibodies: an epidemiologic tool in Lyme disease research. Am. J. Epidemiol. 132, 58–66. doi: 10.1093/oxfordjournals.aje.a115643
Severinova, J., Salat, J., Krocova, Z., Reznickova, J., Demova, H., Horka, H., et al. (2005). Co-inoculation of Borrelia afzelii with tick salivary gland extract influences distribution of immunocompetent cells in the skin and lymph nodes of mice. Folia Microbiol. (Praha). 50, 457–463. doi: 10.1007/BF02931430
Shapiro, S. Z., Buscher, G., and Dobbelaere, D. A. (1987). Acquired resistance to Rhipicephalus appendiculatus (Acari: Ixodidae): identification of an antigen eliciting resistance in rabbits. J. Med. Entomol. 24, 147–154. doi: 10.1093/jmedent/24.2.147
Šimo, L., Daniel, S. E., Park, Y., and Žitňan, D. (2014a). “The nervous and sensory systems: structure, function, proteomics and genomics,” in Biology of Ticks, eds E. Daniel and M. R. R. Sonenshine (New York, NY: Oxford University Press), 309–367.
Šimo, L., Koči, J., Kim, D., and Park, Y. (2014b). Invertebrate specific D1-like dopamine receptor in control of salivary glands in the black-legged tick Ixodes scapularis. J. Comp. Neurol. 522, 2038–2052. doi: 10.1002/cne.23515
Šimo, L., Koči, J., and Park, Y. (2013). Receptors for the neuropeptides, myoinhibitory peptide and SIFamide, in control of the salivary glands of the blacklegged tick Ixodes scapularis. Insect Biochem. Mol. Biol. 43, 376–387. doi: 10.1016/j.ibmb.2013.01.002
Šimo, L., Koči, J., Žitňan, D., and Park, Y. (2011). Evidence for D1 dopamine receptor activation by a paracrine signal of dopamine in tick salivary glands. PLoS ONE 6:e16158. doi: 10.1371/journal.pone.0016158
Šimo, L., Žitňan, D., and Park, Y. (2012). Neural control of salivary glands in ixodid ticks. J. Insect Physiol. 58, 459–466. doi: 10.1016/j.jinsphys.2011.11.006
Šimo, L., Slovak, M., Park, Y., and Žitňan, D. (2009a). Identification of a complex peptidergic neuroendocrine network in the hard tick, Rhipicephalus appendiculatus. Cell Tissue Res. 335, 639–655. doi: 10.1007/s00441-008-0731-4
Šimo, L., Žitňan, D., and Park, Y. (2009b). Two novel neuropeptides in innervation of the salivary glands of the black-legged tick, Ixodes scapularis: myoinhibitory peptide and SIFamide. J. Comp. Neurol. 517, 551–563. doi: 10.1002/cne.22182
Skallova, A., Iezzi, G., Ampenberger, F., Kopf, M., and Kopecky, J. (2008). Tick saliva inhibits dendritic cell migration, maturation, and function while promoting development of Th2 responses. J. Immunol. 180, 6186–6192. doi: 10.4049/jimmunol.180.9.6186
Slamova, M., Skallova, A., Palenikova, J., and Kopecky, J. (2011). Effect of tick saliva on immune interactions between Borrelia afzelii and murine dendritic cells. Parasite Immunol. 33, 654–660. doi: 10.1111/j.1365-3024.2011.01332.x
Slovak, M., Stibraniova, I., Hajnicka, V., and Nuttall, P. A. (2014). Antiplatelet-derived growth factor (PDGF) activity in the saliva of ixodid ticks is linked with their long mouthparts. Parasite Immunol. 36, 32–42. doi: 10.1111/pim.12075
Smith, A. A., and Pal, U. (2014). Immunity-related genes in Ixodes scapularis. perspectives from genome information. Front. Cell. Infect. Microbiol. 4:116. doi: 10.3389/fcimb.2014.00116
Štibrániová, I., Lahová, M., and Bartíková, P. (2013). Immunomodulators in tick saliva and their benefits. Acta Virol. 57, 200–216. doi: 10.4149/av_2013_02_200
Sukumaran, B., Narasimhan, S., Anderson, J. F., DePonte, K., Marcantonio, N., Krishnan, M. N., et al. (2006). An Ixodes scapularis protein required for survival of Anaplasma phagocytophilum in tick salivary glands. J. Exp. Med. 203, 1507–1517. doi: 10.1084/jem.20060208
Tian, Y., Chen, W., Mo, G., Chen, R., Fang, M., Yedid, G., et al. (2016). An immunosuppressant peptide from the hard tick Amblyomma variegatum. Toxins 8:133. doi: 10.3390/toxins8050133
Tyson, K., Elkins, C., Patterson, H., Fikrig, E., and de Silva, A. (2007). Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol. Biol. 16, 469–479. doi: 10.1111/j.1365-2583.2007.00742.x
Umemiya, R., Hatta, T., Liao, M., Tanaka, M., Zhou, J., Inoue, N., et al. (2007). Haemaphysalis longicornis: molecular characterization of a homologue of the macrophage migration inhibitory factor from the partially fed ticks. Exp. Parasitol. 115, 135–142. doi: 10.1016/j.exppara.2006.07.006
Valdes, J. J. (2014). Antihistamine response: a dynamically refined function at the host-tick interface. Parasit. Vectors 7:491. doi: 10.1186/s13071-014-0491-9
Valdes, J. J., Cabezas-Cruz, A., Sima, R., Butterill, P. T., Ruzek, D., and Nuttall, P. A. (2016). Substrate prediction of Ixodes ricinus salivary lipocalins differentially expressed during Borrelia afzelii infection. Sci. Rep. 6:32372. doi: 10.1038/srep32372
Valenzuela, J. G., Charlab, R., Mather, T. N., and Ribeiro, J. M. (2000). Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J. Biol. Chem. 275, 18717–18723. doi: 10.1074/jbc.M001486200
Valenzuela, J. G., Francischetti, I. M., Pham, V. M., Garfield, M. K., Mather, T. N., and Ribeiro, J. M. (2002). Exploring the sialome of the tick Ixodes scapularis. J. Exp. Biol. 205, 2843–2864.
van de Locht, A., Stubbs, M. T., Bode, W., Friedrich, T., Bollschweiler, C., Hoffken, W., et al. (1996). The ornithodorin-thrombin crystal structure, a key to the TAP enigma? EMBO J. 15, 6011–6017.
Vancova, I., Hajnicka, V., Slovak, M., Kocakova, P., Paesen, G. C., and Nuttall, P. A. (2010a). Evasin-3-like anti-chemokine activity in salivary gland extracts of ixodid ticks during blood-feeding: a new target for tick control. Parasite Immunol. 32, 460–463. doi: 10.1111/j.1365-3024.2010.01203.x
Villar, M., Lopez, V., Ayllon, N., Cabezas-Cruz, A., Lopez, J. A., Vazquez, J., et al. (2016). The intracellular bacterium Anaplasma phagocytophilum selectively manipulates the levels of vertebrate host proteins in the tick vector Ixodes scapularis. Parasit. Vectors 9:467. doi: 10.1186/s13071-016-1747-3
Voordouw, M. J. (2015). Co-feeding transmission in Lyme disease pathogens. Parasitology 142, 290–302. doi: 10.1017/S0031182014001486
Vu Hai, V., Almeras, L., Audebert, S., Pophillat, M., Boulanger, N., Parola, P., et al. (2013a). Identification of salivary antigenic markers discriminating host exposition between two European ticks: Rhipicephalus sanguineus and Dermacentor reticulatus. Comp. Immunol. Microbiol. Infect. Dis. 36, 39–53. doi: 10.1016/j.cimid.2012.09.003
Vu Hai, V., Pages, F., Boulanger, N., Audebert, S., Parola, P., and Almeras, L. (2013b). Immunoproteomic identification of antigenic salivary biomarkers detected by Ixodes ricinus-exposed rabbit sera. Ticks Tick Borne Dis. 4, 459–468. doi: 10.1016/j.ttbdis.2013.06.001
Wagemakers, A., Coumou, J., Schuijt, T. J., Oei, A., Nijhof, A. M., van 't Veer, C., et al. (2016). An Ixodes ricinus tick salivary lectin pathway inhibitor protects Borrelia burgdorferi sensu lato from human complement. Vector Borne Zoonot. Dis. 16, 223–228. doi: 10.1089/vbz.2015.1901
Walker, A. R., Fletcher, J. D., and Gill, H. S. (1985). Structural and histochemical changes in the salivary glands in Rhipicephalus appendiculatus during feeding. Int. J. Parasitol. 15, 80–100. doi: 10.1016/0020-7519(85)90106-7
Wang, H., and Nuttall, P. A. (1999). Immunoglobulin-binding proteins in ticks: new target for vaccine development against a blood-feeding parasite. Cell. Mol. Life Sci. 56, 286–295. doi: 10.1007/s000180050430
Wang, X., Coons, L. B., Taylor, D. B., Stevens, S. E. Jr., and Gartner, T. K. (1996). Variabilin, a novel RGD-containing antagonist of glycoprotein IIb-IIIa and platelet aggregation inhibitor from the hard tick Dermacentor variabilis. J. Biol. Chem. 271, 17785–17790. doi: 10.1074/jbc.271.30.17785
Wasala, N. B., and Jaworski, D. C. (2012). Dermacentor variabilis: characterization and modeling of macrophage migration inhibitory factor with phylogenetic comparisons to other ticks, insects and parasitic nematodes. Exp. Parasitol. 130, 232–238. doi: 10.1016/j.exppara.2011.12.010
Waxman, L., and Connolly, T. M. (1993). Isolation of an inhibitor selective for collagen-stimulated platelet aggregation from the soft tick Ornithodoros moubata. J. Biol. Chem. 268, 5445–5449.
Waxman, L., Smith, D. E., Arcuri, K. E., and Vlasuk, G. P. (1990). Tick anticoagulant peptide (TAP) is a novel inhibitor of blood coagulation factor Xa. Science 248, 593–596. doi: 10.1126/science.2333510
Wikel, S. (2013). Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front. Microbiol. 4:337. doi: 10.3389/fmicb.2013.00337
Wikel, S. K. (1979). Acquired resistance to ticks. Expression of resistance by C4-deficient guinea pigs. Am. J. Trop. Med. Hyg, 28, 586–590. doi: 10.4269/ajtmh.1979.28.586
Wikel, S. K. (1996). Host immunity to ticks. Annu. Rev. Entomol. 41, 1–22. doi: 10.1146/annurev.en.41.010196.000245
Wikel, S. K. (1999). Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 29, 851–859. doi: 10.1016/S0020-7519(99)00042-9
Wikel, S. K., and Alarcon-Chaidez, F. J. (2001). Progress toward molecular characterization of ectoparasite modulation of host immunity. Vet. Parasitol. 101, 275–287. doi: 10.1016/S0304-4017(01)00556-8
Wikel, S. K., Ramachandra, R. N., Bergman, D. K., Burkot, T. R., and Piesman, J. (1997). Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect. Immun. 65, 335–338.
Willadsen, P. (2004). Anti-tick vaccines. Parasitology 129(Suppl.), S367–S387. doi: 10.1017/S0031182003004657
Willadsen, P., Bird, P., Cobon, G. S., and Hungerford, J. (1995). Commercialisation of a recombinant vaccine against Boophilus microplus. Parasitology 110(Suppl.), S43–S50. doi: 10.1017/S0031182000001487
Wu, J., Wang, Y., Liu, H., Yang, H., Ma, D., Li, J., et al. (2010). Two immunoregulatory peptides with antioxidant activity from tick salivary glands. J. Biol. Chem. 285, 16606–16613. doi: 10.1074/jbc.M109.094615
Xu, T., Lew-Tabor, A., and Rodriguez-Valle, M. (2016). Effective inhibition of thrombin by Rhipicephalus microplus serpin-15 (RmS-15) obtained in the yeast Pichia pastoris. Ticks Tick Borne Dis. 7, 180–187. doi: 10.1016/j.ttbdis.2015.09.007
Yu, D., Liang, J., Yu, H., Wu, H., Xu, C., Liu, J., et al. (2006). A tick B-cell inhibitory protein from salivary glands of the hard tick, Hyalomma asiaticum asiaticum. Biochem. Biophys. Res. Commun. 343, 585–590. doi: 10.1016/j.bbrc.2006.02.188
Zeidner, N. S., Schneider, B. S., Nuncio, M. S., Gern, L., and Piesman, J. (2002). Coinoculation of Borrelia spp. with tick salivary gland lysate enhances spirochete load in mice and is tick species-specific. J. Parasitol. 88, 1276–1278. doi: 10.1645/0022-3395(2002)088[1276:COBSWT]2.0.CO;2
Zemtsova, G., Killmaster, L. F., Mumcuoglu, K. Y., and Levin, M. L. (2010). Co-feeding as a route for transmission of Rickettsia conorii israelensis between Rhipicephalus sanguineus ticks. Exp. Appl. Acarol. 52, 383–392. doi: 10.1007/s10493-010-9375-7
Zhang, P., Tian, Z., Liu, G., Xie, J., Luo, J., Zhang, L., et al. (2011). Characterization of acid phosphatase from the tick Haemaphysalis longicornis. Vet. Parasitol. 182, 287–296. doi: 10.1016/j.vetpar.2011.05.053
Zhu, K., Bowman, A. S., Brigham, D. L., Essenberg, R. C., Dillwith, J. W., and Sauer, J. R. (1997). Isolation and characterization of americanin, a specific inhibitor of thrombin, from the salivary glands of the lone star tick Amblyomma americanum (L.). Exp. Parasitol. 87, 30–38. doi: 10.1006/expr.1997.4175
Keywords: ticks, tick saliva, tick-borne pathogens, tick salivary glands
Citation: Šimo L, Kazimirova M, Richardson J and Bonnet SI (2017) The Essential Role of Tick Salivary Glands and Saliva in Tick Feeding and Pathogen Transmission. Front. Cell. Infect. Microbiol. 7:281. doi: 10.3389/fcimb.2017.00281
Received: 21 April 2017; Accepted: 08 June 2017;
Published: 22 June 2017.
Edited by:
Margaret E. Bauer, Indiana University School of Medicine, United StatesReviewed by:
Jianfeng Dai, Soochow University, ChinaCopyright © 2017 Šimo, Kazimirova, Richardson and Bonnet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah I. Bonnet, c2FyYWguYm9ubmV0QHZldC1hbGZvcnQuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.