- 1Laboratoire d'Ingénierie des Systèmes Macromoléculaires (UMR7255), IMM, Centre National de la Recherche Scientifique and Aix-Marseille University, Marseille, France
- 2Department of Microbiology and Immunology, Stanford School of Medicine, Stanford University, Stanford, CA, USA
Pseudomonas aeruginosa is an opportunistic pathogen responsible for many diseases such as chronic lung colonization in cystic fibrosis patients and acute infections in hospitals. The capacity of P. aeruginosa to be pathogenic toward several hosts is notably due to different secretion systems. Amongst them, P. aeruginosa encodes three Type Six Secretion Systems (T6SS), named H1- to H3-T6SS, that act against either prokaryotes and/or eukaryotic cells. They are independent from each other and inject diverse toxins that interact with different components in the host cell. Here we summarize the roles of these T6SSs in the PAO1 strain, as well as the toxins injected and their targets. While H1-T6SS is only involved in antiprokaryotic activity through at least seven different toxins, H2-T6SS and H3-T6SS are also able to target prokaryotic as well as eukaryotic cells. Moreover, recent studies proposed that H2- and H3-T6SS have a role in epithelial cells invasion by injecting at least three different toxins. The diversity of T6SS effectors is astounding and other effectors still remain to be discovered. In this review, we present a table with other putative P. aeruginosa strain PAO1 T6SS-dependent effectors. Altogether, the T6SSs of P. aeruginosa are important systems that help fight other bacteria for their ecological niche, and are important in the pathogenicity process.
The Type Six Secretion system (T6SS) was discovered ten years ago in the laboratory of Pr. J. Mekalanos (Mougous et al., 2006; Pukatzki et al., 2006). It functions as a contractile molecular syringe consisting of a sheath and a puncturing device made of an Hcp tube terminated by a spike of VgrG and PAAR proteins. Contraction of the TssB/C sheath propels the puncturing device out of the cell into a target cell and leads to the injection of effector proteins (Alcoforado Diniz et al., 2015). The first studies focused on the phenotypes associated with T6SS mutations in different pathogens in the context of eukaryotic host infection. However in 2010, Dr. J. Mougous laboratory showed an unsuspected antibacterial activity mediated by the H1-T6SS from Pseudomonas aeruginosa, making T6SSs transkingdom machineries (Hood et al., 2010). Since this discovery, T6SSs have mainly been studied for their capacity to target prokaryotes and this would seem to be their primary function. But interestingly, several T6SSs are also known to target both prokaryotes and eukaryotes such as the T6SS of Vibrio cholera (Pukatzki et al., 2006; MacIntyre et al., 2010).
P. aeruginosa is one of the most virulent opportunistic human pathogens and is responsible for many diseases such as broncho-alveolar colonization in cystic fibrosis patients or acute infections of lungs and burned skin that can lead to septicemia. Its genome encodes many virulence factors including several secretion systems that help P. aeruginosa control its environment and the activity of host cells (Bleves et al., 2010). Among theses, P. aeruginosa harbors three independent Type Six Secretion Systems (T6SS). This review will aim to describe the roles, effectors, and targets of these three T6SSs.
P. aeruginosa Uses T6SSs as Antiprokaryotic Weapons
T6SSs are present in more than 200 Gram-negative bacteria including P. aeruginosa, whose genome encodes three different T6SS loci named H1-, H2-, and H3-T6SS (Table 1). Historically, H1-T6SS is the first T6SS machinery that was shown to display an antibacterial activity (Hood et al., 2010). H1-T6SS serves as a counter-attack weapon to outcompete other T6SS+ bacteria that coexist in a same ecological niche, and confers a growth advantage upon P. aeruginosa (Basler et al., 2013). More specifically, P. aeruginosa targets other bacteria through H1-T6SS dependent injection of effector Tse2 and also produces an anti-toxin Tsi2, protecting itself against the intrinsic effect of the toxin and from attack by sister-cells (Hood et al., 2010). It was recently shown that Tse2 induces quiescence in bacterial target cells, and that Tsi2 directly interacts with Tse2 in the cytoplasm to inactivate its lethal activity (Li et al., 2012). Recently, Tse2 toxicity was shown to be NAD-dependent and may involve an ADP-ribosyltransferase activity (Robb et al., 2016). Besides Tse2, which acts in the cytoplasm of prey cells, Tse1 and Tse3 are injected into the periplasm of target bacterial cells through H1-T6SS (Russell et al., 2011). Tse1 and Tse3 hydrolyse peptidoglycan, providing a fitness advantage for P. aeruginosa in competition with other bacteria. To protect from killing by sister-cells, P. aeruginosa uses the periplasmic immunity proteins Tsi1 and Tsi3 which counteract Tse1 and Tse3 toxicity (Russell et al., 2011). Later, X-ray studies revealed that Tse1 cleaves the γ-D-glutamyl-l-meso-diaminopimelic acid amide bond of crosslinked peptidoglycan (Benz et al., 2012; Chou et al., 2012). Moreover, the crystal structure of Tse1 in interaction with Tsi1 demonstrates that the immunity protein occludes the active site of Tse1 abolishing its enzyme activity (Benz et al., 2012). Tse3 functions as a muramidase, cleaving the β-1,4-linkage between N-acetylmuramic acid and N-acetylglucosamine in peptidoglycan (Lu et al., 2013). These three effectors were discovered in 2010 thanks to their coregulation with the H1-T6SS machinery (Table 1), and other H1-T6SS toxins were described later. Tse4 was identified as a H1-T6SS effector using quantitative cellular proteomics in interaction with Hcp (Whitney et al., 2014). Tse5, Tse6, and Tse7 were identified by their genetic association with VgrGs and the H1-T6SS (Hachani et al., 2014; Whitney et al., 2014). Those four effectors display antibacterial activity and are associated with cognate immunities (Table 1). Recently Tse6 was shown to degrade the essential dinucleotides NAD(+) and NADP(+) leading to bacteriostasis in the target bacterium (Whitney et al., 2015). Intriguingly Tse6 delivery into the host cytoplasm requires translation elongation factor Tu (EF-Tu). The interaction of a toxin with a house keeping protein may suggest that it can target phylogenetically diverse bacteria (Whitney et al., 2015). EF-Tu may facilitate Tse6 translocation into recipient cells or by driving the H1-T6SS needle at the cell surface of the preys either by favoring the passage of the toxin once delivered into the periplasm to the cytoplasm. Indeed EF-Tu is known as a moonlighting protein or anchorless multifunctional protein that is capable, when localized to the cell surface, of interfering with bacterial adherence (for a review see Henderson and Martin, 2011). Importantly work done on H1-T6SS toxins has revealed different conserved mechanisms for targeting T6SS effectors to the T6SS machinery (Table 1): (i) Hcp-dependent recruitment in the case of Tse1-4, (ii) direct VgrG-targeting for Tse5, (iii) VgrG-targeting for Tse7 through a PAAR motif and for Tse6 through an adaptator/chaperonne protein called EagT6. Altogether, H1-T6SS is a formidable antibacterial weapon, injecting many different effectors to compete bacterial cells, and allowing P. aeruginosa to overwhelm them during competition for the same ecological niche.
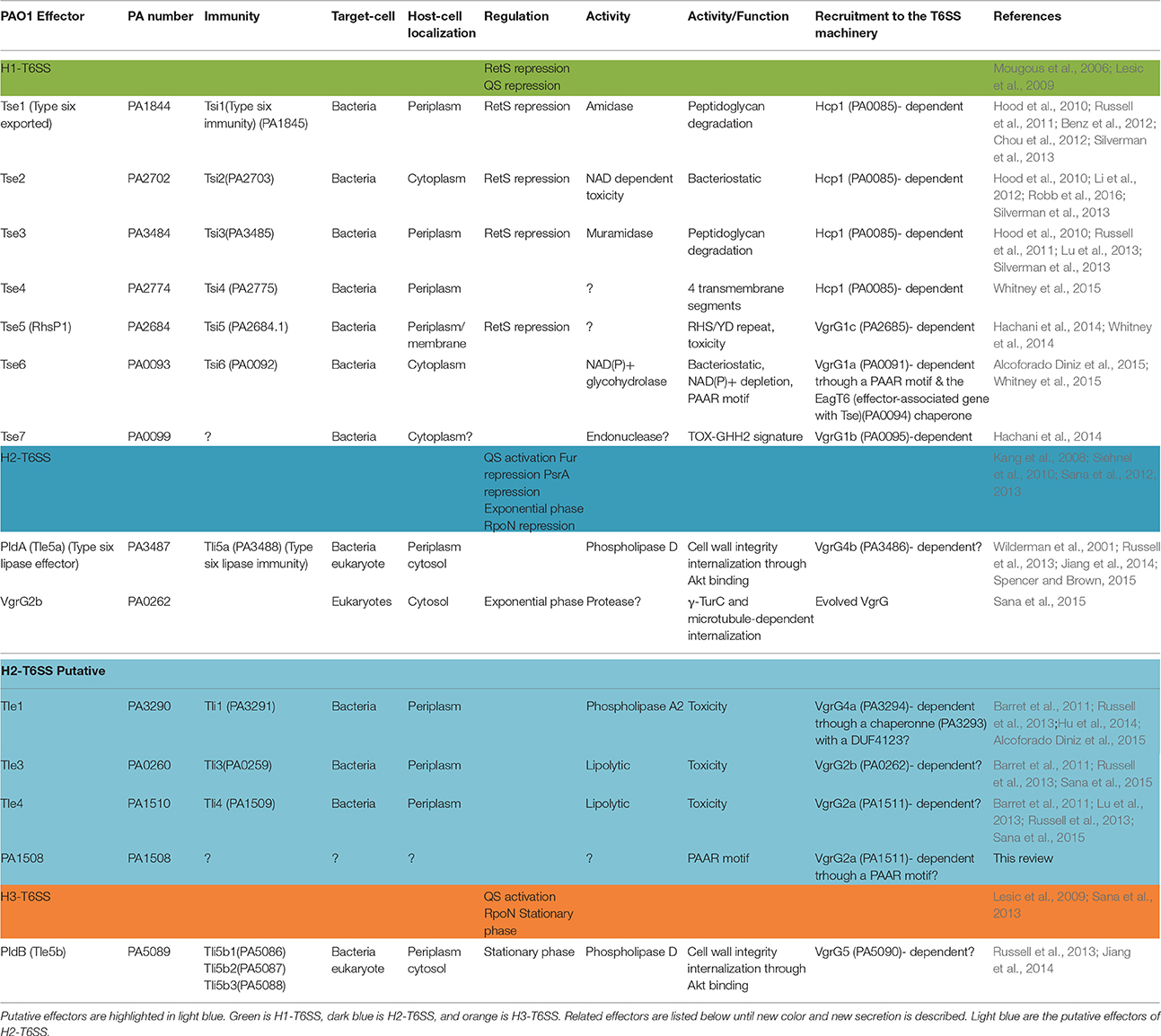
Table 1. Immunity proteins, enzymatic activities, targets, localizations, and recruitment of the T6SS effectors of the P. aeruginosa strain PAO1.
H2-T6SS and H3-T6SS also display antibacterial activity by injecting the phospholipase D enzymes PldA and PldB that belong to the Tle5 (type VI lipase effector) family into other bacterial cells (Russell et al., 2013; Jiang et al., 2014). The PldA toxin functions by degrading the major constituent of bacterial membranes, phosphatidylethanolamine (Russell et al., 2013). However, despite strong evidence that P. aeruginosa T6SSs participate widely in bacterial competition, several recent reports have focused on the ability of H2 and H3-T6SS to target epithelial cells.
P. aeruginosa T6SSs also Target Eukaryotic Cells
So far, H1-T6SS has never been shown to be directly involved in anti-eukaryotic activity. However, Hcp1 has been found in pulmonary secretions of cystic fibrosis patients as well as Hcp1-specific antibodies in their sera (Mougous et al., 2006), suggesting that the antiprokaryotic activity of H1-T6SS could be necessary for host colonization in a complex microbial community. Interestingly, several members of the gut microbiota actually encode T6SSs that could lead to the contact-dependent killing of other bacteria, including Bacteroidetes fragilis (Russell et al., 2014). This opens a new field of research at the interface between the pathogen, host and microbiota, giving a protective role for the commensal microbiota through T6SS-dependent killing of the pathogen. In support of this hypothesis, V. cholerae needs some of its antitoxins to establish in the host gut, strongly suggesting that it is subject to T6SS attacks from the microbiota (Fu et al., 2013). Moreover, it was shown that about half of the Bacteroidales genomes, the most prevalent Gram-negative bacterial order of the human gut, encode at least one T6SS (Coyne et al., 2016). Finally, a recent report shows that the antibacterial activity of Bacteroidetes fragilis is active in the mice gut and that it kills several members of the microbiota in vitro, suggesting a role in the gut colonization (Chatzidaki-Livanisa et al., 2016). Altogether, the T6SS antibacterial activity clearly has a role in the eukaryotic host as well, and should be studied into more details.
Despite being considered an extracellular pathogen, several reports demonstrate that P. aeruginosa actively invades non-phagocytic cells, such as the epithelial cells that line the mucosal barrier and the endothelial cells that form the vascular lumen (Chi et al., 1991; Engel and Eran, 2011). The entry step requires the actin network, most probably to allow membrane protrusion (Fleiszig et al., 1995). This is thought to help bacteria avoiding the immune system or to invade deeper tissues during the infection process. Although, bacteria are present in the lumen and therefore at the apical side of the epithelium, P. aeruginosa can only internalize through membrane that displays basolateral characteristics (Figure 1). To circumvent this, P. aeruginosa is able to transform apical membrane into basolateral membrane, creating a local microenvironment that facilitates colonization and entry into the epithelium (Kierbel et al., 2007). Interestingly, P. aeruginosa is also able to transmigrate through an epithelial barrier, taking advantage of cell division sites and senescent cell extrusion (Golovkine et al., 2016). Altogether, these convergent mechanisms for entering or crossing the epithelial barrier suggest that this ability is essential for successful colonization of the host by P. aeruginosa.
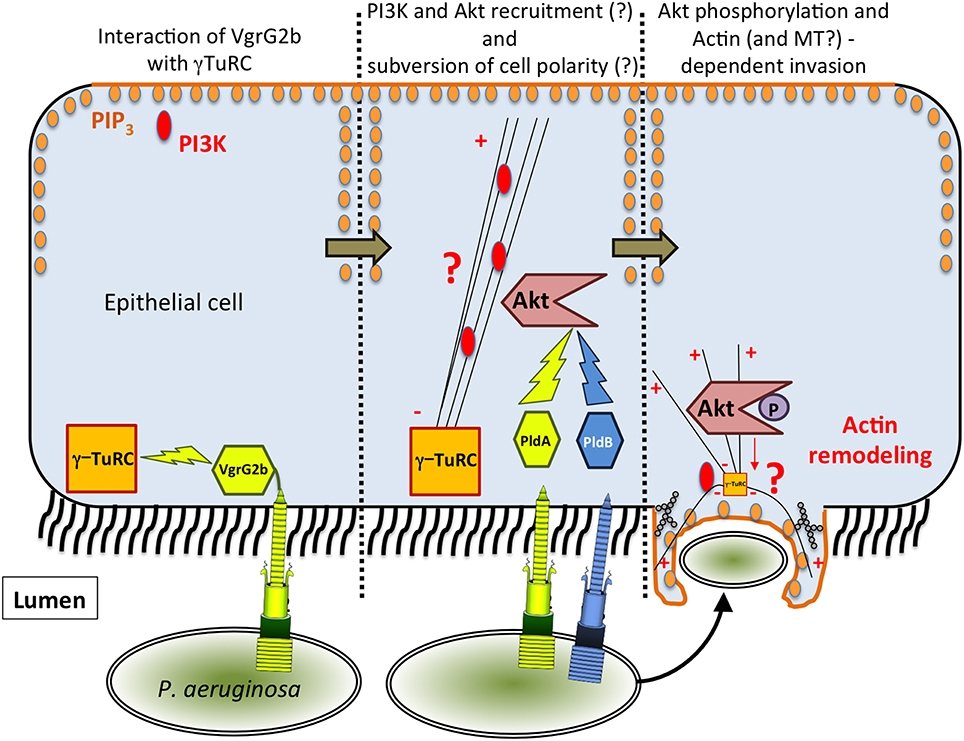
Figure 1. Model of T6SS-dependent internalization of P. aeruginosa into epithelial cells. In the proposed model, VgrG2b targets γ-TuRC, which could lead to the recruitment of PI3K at the apical membrane. Then, PldA/B both target Akt leading to actin remodeling and finally to the entry of P. aeruginosa.
The mechanism by which P. aeruginosa recruits host factors to internalize within non-phagocytic cells is still poorly understood. Among the various factors required for this process (for a review see Engel and Eran, 2011), we demonstrated that the H2-T6SS machinery (Figure 1) promotes the uptake of P. aeruginosa into pulmonary epithelial cells but, at the time, the identity of the cognate effector(s) involved remained to be discovered (Sana et al., 2012). Two recent reports have enabled key new insights into the T6SS-mediated invasion mechanism of P. aeruginosa. Host-cell invasion requires two phospholipase D enzymes, PldA and PldB, which are injected via the H2-T6SS or H3-T6SS machineries, respectively (Jiang et al., 2014; Table 1). The H3-T6SS machinery is thus required for P. aeruginosa internalization. PldA and PldB both target the host PI3K (phosphoinositide 3-kinase)/Akt pathway, which is hijacked during the internalization process (Kierbel et al., 2005; Engel and Eran, 2011). After injection into epithelial cells, the two T6SS effectors were shown to directly bind Akt, which may lead to activation of the PI3K-Akt signaling pathway. Indeed, Akt phosphorylation is thought to promote a profound remodeling of the apical membrane in which protrusions enriched in PIP3 (phosphatidylinositol-3,4,5-triphosphate) and actin form, facilitating further entry of P. aeruginosa (Bleves et al., 2014; Jiang et al., 2014). Interestingly, PldA and PldB are also known to target bacterial cells, making them trans-kingdom effectors (Bleves et al., 2014). Host-cell invasion also requires the evolved VgrG2b effector (Sana et al., 2015). VgrG2b is injected via the H2-T6SS into epithelial cells where it targets the microtubule network and more interestingly the gamma-tubulin ring complex components (γ-TuRC) of the microtubule nucleating-center (Kollman et al., 2011; Table 1). Remarkably this interaction is followed by a microtubule-dependent internalization of the pathogen since treatment of epithelial cells with drugs that disrupt the microtubule network decreases the number of internalized bacteria. Furthermore, injection of VgrG2b via the H2-T6SS machinery can be bypassed by directly producing VgrG2b in epithelial cells prior to infection. This can even lead to the internalization of H2-T6SS or vgrG2b mutants suggesting that VgrG2b is a central player in this process.
How can microtubule and actin cytoskeletons be integrated in a common invasion process? In Figure 1 we propose a working model that is restricted to the internalization mediated by the T6SS effector interplay. As mentioned above, the internalization of P. aeruginosa is a multifactorial process, and our goal here is to integrate and discuss the functions of the three anti-eukaryotic T6SS effectors encoded by the P. aeruginosa genome. H2-T6SS first injects VgrG2b which targets the microtubule network and in particular γ-TuRC. This interaction could subvert the polarization of epithelial cells by creating novel sites of non-radial microtubule nucleation along the apical-basal axis at the bacterium-binding site. These new sites would be enriched in microtubule-minus ends, which might interfere with the directional transport of microtubule-dependent cargoes in the cell among them the basal PI3K marker. Concomitantly, P. aeruginosa may also recruit Akt via the PldA and PldB effectors injected by the H2 and H3-T6SS machineries respectively. Indeed the apical PI3K may lead to PIP3 synthesis and recruitment of Akt creating a basolateral environment at the apical surface (Figure 1). This will activate the Akt signaling, allowing actin-dependent membrane protrusion, and ultimately the internalization of P. aeruginosa into epithelial cells (Figure 1). One can also propose that these protrusions may also contain microtubules. In this model, both H2 and H3-T6SS are essential components for the internalization process of P. aeruginosa into epithelial cells. We propose that H2-T6SS is active before H3-T6SS because (i) transcriptional studies show that it is expressed earlier in the growth phase (Sana et al., 2013), (ii) ectopic synthesis of VgrG2b inside epithelial cells trigger internalization of T6SS mutants (Sana et al., 2015), and (iii) PldA and PldB can compensate for each other during infection with stationary-phase grown bacteria (Jiang et al., 2014). However, the exact molecular mechanism by which VgrG2b acts on the γ-TuRC and the microtubule network has yet to be deciphered. Also, what is the nature of the effector domain of the evolved VgrG2b? How does the interaction of Akt with these two phospholipases D trigger its activation? Finally, the intracellular lifestyle of P. aeruginosa has to be studied in greater detail, particularly in light of very interesting reports which propose that P. aeruginosa creates its own bleb-niche in epithelial cells where it can replicate (Angus et al., 2008; Jolly et al., 2015).
More efforts have to be made to decipher this entire mechanism because it could lead to important biomedical applications. Indeed, P. aeruginosa is known to induce acute infection in patients with burned skin. Rationally, in this scenario, the first barrier P. aeruginosa will have to cross will be the skin, which is basically composed of epithelial cells. We also know that H2-T6SS and H3-T6SS are important for full virulence in worm models as well as in mouse models (Lesic et al., 2009; Sana et al., 2013). Therefore, it begs the question as to whether H2 or H3-T6SS are responsible for pathogen entry through the burned skin barrier. It will therefore be very interesting over the next years to study this invasion mechanism more deeply using for example a three dimensional model of burned skin (Shepherd et al., 2009). Thus, H2- and H3-T6SS of P. aeruginosa are potentially good candidates for new therapeutic targets. And finally, although most invasive bacteria manipulate host actin for entry (Cossart and Sansonetti, 2004) this T6SS-mediated entry mechanism could be common in other pathogens such as Campylobacter jejuni, and Citrobacter freundii, Neisseria gonorrhoeae, or Burkholderia cepacia that also appear to modulate the microtubule network to invade epithelial cells (Donnenberg et al., 1990; Oelschlaeger et al., 1993; Grassme et al., 1996; Yoshida and Sasakawa, 2003; Taylor et al., 2010).
Conclusions
The T6SS machineries of P. aeruginosa must be considered as versatile weapons that are able to target both prokaryotic and eukaryotic cells. In the future, studies should aim at determining the role of their antiprokaryotic activity in vivo because H1-T6SS is clearly active in cystic fibrosis patients. One could also ask whether this T6SS-driven antibacterial activity is a common weapon used by pathogens in vivo to outcompete either the commensal microbiota or other pathogens. As shown in Table 1 the repertoire of T6SS effectors in P. aeruginosa may not be complete and at least 4 H2-T6SS putative effectors can be proposed according to their genetic linkage with known effector genes (Table 1). Also, the exact mechanism of T6SS-dependent internalization within epithelial cells should be studied in more detail and its role in colonization and pathogenicity should be better understood.
Author Contributions
TS and SB wrote the review and created Figure 1. BB and SB created Table 1.
Funding
TS was financed by a Ph.D. fellowship from the French Research Ministry and with a “Teaching and Research” fellowship from AMU. This work is supported by a grant (N°RF20150501346/1/69) from “Association Gregory Lemarchal” and “Vaincre la Mucoviscidose” and by AMU and CNRS.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Chantal Soscia and Romé Voulhoux's team for constant support, and Theodore Hu and Ben Field for careful reading of the manuscript.
References
Alcoforado Diniz, J., Liu, Y. C., and Coulthurst, S. J. (2015). Molecular weaponry: diverse effectors delivered by the Type VI secretion system. Cell. Microbiol. 17, 1742–1751. doi: 10.1111/cmi.12532
Angus, A. A., Lee, A. A., Augustin, D. K., Lee, E. J., Evans, D. J., and Fleiszig, S. M. (2008). Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility. Infect. Immun. 76, 1992–2001. doi: 10.1128/IAI.01221-07
Barret, M., Egan, F., Fargier, E., Morrissey, J. P., and O'Gara, F. (2011). Genomic analysis of the type VI secretion systems in Pseudomonas spp.: novel clusters and putative effectors uncovered. Microbiology 157, 1726–1739. doi: 10.1099/mic.0.048645-0
Basler, M., Ho, B. T., and Mekalanos, J. J. (2013). Tit-for-tat: type VI secretion system counterattack during bacterial cell-cell interactions. Cell 152, 884–894. doi: 10.1016/j.cell.2013.01.042
Benz, J., Sendlmeier, C., Barends, T. R., and Meinhart, A. (2012). Structural insights into the effector-immunity system Tse1/Tsi1 from Pseudomonas aeruginosa. PLoS ONE 7:e40453. doi: 10.1371/journal.pone.0040453
Bleves, S., Sana, T. G., and Voulhoux, R. (2014). The target cell genus does not matter. Trends Microbiol. 22, 304–306. doi: 10.1016/j.tim.2014.04.011
Bleves, S., Viarre, V., Salacha, R., Michel, G. P., Filloux, A., and Voulhoux, R. (2010). Protein secretion systems in Pseudomonas aeruginosa: a wealth of pathogenic weapons. Int. J. Med. Microbiol. 300, 534–543. doi: 10.1016/j.ijmm.2010.08.005
Chatzidaki-Livanisa, M., Geva-Zatorsky, N., and Comstocka, L. E. (2016). Bacteroides fragilis type VI secretion systems use novel effector and immunity proteins to antagonize human gut Bacteroidales species. Proc. Natl. Acad. Sci. U.S.A. 113, 3627–3632. doi: 10.1073/pnas.1522510113
Chi, E., Mehl, T., Nunn, D., and Lory, S. (1991). Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect. Immun. 59, 822–828.
Chou, S., Bui, N. K., Russell, A. B., Lexa, K. W., Gardiner, T. E., LeRoux, M., et al. (2012). Structure of a peptidoglycan amidase effector targeted to Gram-negative bacteria by the type VI secretion system. Cell Rep. 1, 656–664. doi: 10.1016/j.celrep.2012.05.016
Cossart, P., and Sansonetti, P. J. (2004). Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304, 242–248. doi: 10.1126/science.1090124
Coyne, M. J., Roelofs, K. G., and Comstock, L. E. (2016). Type VI secretion systems of human gut Bacteroidales segregate into three genetic architectures, two of which are contained on mobile genetic elements. BMC Genomics 17, 58. doi: 10.1186/s12864-016-2377-z
Donnenberg, M. S., Donohue-Rolfe, A., and Keusch, G. T. (1990). A comparison of HEp-2 cell invasion by enteropathogenic and enteroinvasive Escherichia coli. FEMS Microbiol. Lett. 57, 83–86. doi: 10.1111/j.1574-6968.1990.tb04179.x
Engel, J., and Eran, Y. (2011). Subversion of mucosal barrier polarity by Pseudomonas aeruginosa. Front. Microbiol. 2:114. doi: 10.3389/fmicb.2011.00114
Fleiszig, S. M., Zaidi, T. S., and Pier, G. B. (1995). Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun. 63, 4072–4077.
Fu, Y., Waldor, M. K., and Mekalanos, J. J. (2013). Tn-Seq analysis of Vibrio cholerae intestinal colonization reveals a role for T6SS-mediated antibacterial activity in the host. Cell Host Microbe. 14, 652–663. doi: 10.1016/j.chom.2013.11.001
Golovkine, G., Faudry, E., Bouillot, S., Elsen, S., Attree, I., and Huber, P. (2016). Pseudomonas aeruginosa transmigrates at epithelial cell-cell junctions, exploiting sites of cell division and senescent cell extrusion. PLoS Pathog. 12:e1005377. doi: 10.1371/journal.ppat.1005377
Grassme, H. U., Ireland, R. M., and van Putten, J. P. (1996). Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect. Immun. 64, 1621–1630.
Hachani, A., Allsopp, L. P., Oduko, Y., and Filloux, A. (2014). The VgrG proteins are “a la carte” delivery systems for bacterial type VI effectors. J. Biol. Chem. 289, 17872–17884. doi: 10.1074/jbc.M114.563429
Henderson, B., and Martin, A. (2011). Bacterial virulence in the moonlight: multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect. Immun. 79, 3476–3491. doi: 10.1128/IAI.00179-11
Hood, R. D., Singh, P., Hsu, F., Guvener, T., Carl, M. A., Trinidad, R. R., et al. (2010). A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe. 7, 25–37. doi: 10.1016/j.chom.2009.12.007
Hu, H., Zhang, H., Gao, Z., Wang, D., Liu, G., Xu, J., et al. (2014). Structure of the type VI secretion phospholipase effector Tle1 provides insight into its hydrolysis and membrane targeting. Acta Crystallogr. D Biol. Crystallog. 70, 2175–2185. doi: 10.1107/S1399004714012899
Jiang, F., Waterfield, N. R., Yang, J., Yang, G., and Jin, Q. (2014). A Pseudomonas aeruginosa type VI secretion phospholipase D effector targets both prokaryotic and eukaryotic cells. Cell Host Microbe. 15, 600–610. doi: 10.1016/j.chom.2014.04.010
Jolly, A. L., Takawira, D., Oke, O. O., Whiteside, S. A., Chang, S. W., Wen, E. R., et al. (2015). Pseudomonas aeruginosa-induced bleb-niche formation in epithelial cells is independent of actinomyosin contraction and enhanced by loss of cystic fibrosis transmembrane-conductance regulator osmoregulatory function. MBio 6, e02533. doi: 10.1128/mBio.02533-14
Kang, Y., Nguyen, D. T., Son, M. S., and Hoang, T. T. (2008). The Pseudomonas aeruginosa PsrA responds to long-chain fatty acid signals to regulate the fadBA5 beta-oxidation operon. Microbiology 154, 1584–1598. doi: 10.1099/mic.0.2008/018135-0
Kierbel, A., Gassama-Diagne, A., Mostov, K., and Engel, J. N. (2005). The phosphoinositol-3-kinase-protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol. Biol. Cell. 16, 2577–2585. doi: 10.1091/mbc.E04-08-0717
Kierbel, A., Gassama-Diagne, A., Rocha, C., Radoshevich, L., Olson, J., Mostov, K., et al. (2007). Pseudomonas aeruginosa exploits a PIP3-dependent pathway to transform apical into basolateral membrane. J. Cell Biol. 177, 21–27. doi: 10.1083/jcb.200605142
Kollman, J. M., Merdes, A., Mourey, L., and Agard, D. A. (2011). Microtubule nucleation by gamma-tubulin complexes. Nat. Rev. Mol. Cell Biol. 12, 709–721. doi: 10.1038/nrm3209
Lesic, B., Starkey, M., He, J., Hazan, R., and Rahme, L. G. (2009). Quorum sensing differentially regulates Pseudomonas aeruginosa type VI secretion locus I and homologous loci II and III, which are required for pathogenesis. Microbiology 155, 2845–2855. doi: 10.1099/mic.0.029082-0
Li, M., Le Trong, I., Carl, M. A., Larson, E. T., Chou, S., De Leon, J. A., et al. (2012). Structural basis for type VI secretion effector recognition by a cognate immunity protein. PLoS Pathog. 8:e1002613. doi: 10.1371/journal.ppat.1002613
Lu, D., Shang, G., Yu, Q., Zhang, H., Zhao, Y., Cang, H., et al. (2013). Expression, purification and preliminary crystallographic analysis of the T6SS effector protein Tse3 from Pseudomonas aeruginosa. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 69, 524–527. doi: 10.1107/S1744309113007148
MacIntyre, D. L., Miyata, S. T., Kitaoka, M., and Pukatzki, S. (2010). The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl. Acad. Sci. U.S.A. 107, 19520–19524. doi: 10.1073/pnas.1012931107
Mougous, J. D., Cuff, M. E., Raunser, S., Shen, A., Zhou, M., Gifford, C. A., et al. (2006). A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312, 1526–1530. doi: 10.1126/science.1128393
Oelschlaeger, T. A., Guerry, P., and Kopecko, D. J. (1993). Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. U.S.A. 90, 6884–6888. doi: 10.1073/pnas.90.14.6884
Pukatzki, S., Ma, A. T., Sturtevant, D., Krastins, B., Sarracino, D., Nelson, W. C., et al. (2006). Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. U.S.A. 103, 1528–1533. doi: 10.1073/pnas.0510322103
Robb, C. S., Robb, M., Nano, F. E., and Boraston, A. B. (2016). The structure of the Toxin and Type Six Secretion System Substrate Tse2 in complex with its immunity protein. Structure 24, 277–284. doi: 10.1016/j.str.2015.11.012
Russell, A. B., Hood, R. D., Bui, N. K., LeRoux, M., Vollmer, W., and Mougous, J. D. (2011). Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475, 343–347. doi: 10.1038/nature10244
Russell, A. B., LeRoux, M., Hathazi, K., Agnello, D. M., Ishikawa, T., Wiggins, P. A., et al. (2013). Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature 496, 508–512. doi: 10.1038/nature12074
Russell, A. B., Wexler, A. G., Harding, B. N., Whitney, J. C., Bohn, A. J., Goo, Y. A., et al. (2014). A type VI secretion-related pathway in Bacteroidetes mediates interbacterial antagonism. Cell Host Microbe. 16, 227–236. doi: 10.1016/j.chom.2014.07.007
Sana, T. G., Baumann, C., Merdes, A., Soscia, C., Rattei, T., Hachani, A., et al. (2015). Internalization of Pseudomonas aeruginosa strain PAO1 into epithelial cells is promoted by interaction of a T6SS effector with the microtubule network. MBio 6:e00712. doi: 10.1128/mBio.00712-15
Sana, T. G., Hachani, A., Bucior, I., Soscia, C., Garvis, S., Termine, E., et al. (2012). The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and Fur and modulates internalization in epithelial cells. J. Biol. Chem. 287, 27095–27105. doi: 10.1074/jbc.M112.376368
Sana, T. G., Soscia, C., Tonglet, C. M., Garvis, S., and Bleves, S. (2013). Divergent control of two type VI secretion systems by RpoN in Pseudomonas aeruginosa. PLoS ONE 8:e76030. doi: 10.1371/journal.pone.0076030
Shepherd, J., Douglas, I., Rimmer, S., Swanson, L., and MacNeil, S. (2009). Development of three-dimensional tissue-engineered models of bacterial infected human skin wounds. Tissue Eng. Part C Methods 15, 475–484. doi: 10.1089/ten.tec.2008.0614
Siehnel, R., Traxler, B., An, D. D., Parsek, M. R., Schaefer, A. L., and Singh, P. K. (2010). A unique regulator controls the activation threshold of quorum-regulated genes in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 107, 7916–7921. doi: 10.1073/pnas.0908511107
Silverman, J. M., Agnello, D. M., Zheng, H., Andrews, B. T., Li, M., Catalano, C. E., et al. (2013). Haemolysin coregulated protein is an exported receptor and chaperone of type VI secretion substrates. Mol. Cell. 51, 584–593. doi: 10.1016/j.molcel.2013.07.025
Spencer, C., and Brown, H. A. (2015). Biochemical characterization of a Pseudomonas aeruginosa phospholipase D. Biochemistry 54, 1208–1218. doi: 10.1021/bi501291t
Taylor, J. B., Hogue, L. A., LiPuma, J. J., Walter, M. J., Brody, S. L., and Cannon, C. L. (2010). Entry of Burkholderia organisms into respiratory epithelium: CFTR, microfilament and microtubule dependence. J. Cyst. Fibros. 9, 36–43. doi: 10.1016/j.jcf.2009.10.002
Whitney, J. C., Beck, C. M., Goo, Y. A., Russell, A. B., Harding, B. N., De Leon, J. A., et al. (2014). Genetically distinct pathways guide effector export through the type VI secretion system. Mol. Microbiol. 92, 529–542. doi: 10.1111/mmi.12571
Whitney, J. C., Quentin, D., Sawai, S., LeRoux, M., Harding, B. N., Ledvina, H. E., et al. (2015). An interbacterial NAD(P)(+) glycohydrolase toxin requires elongation factor Tu for delivery to target cells. Cell 163, 607–619. doi: 10.1016/j.cell.2015.09.027
Wilderman, P. J., Vasil, A. I., Johnson, Z., and Vasil, M. L. (2001). Genetic and biochemical analyses of a eukaryotic-like phospholipase D of Pseudomonas aeruginosa suggest horizontal acquisition and a role for persistence in a chronic pulmonary infection model. Mol. Microbiol. 39, 291–303. doi: 10.1046/j.1365-2958.2001.02282.x
Keywords: Pseudomonas aeruginosa, Type Six Secretion System, invasion mechanism, epithelial cells, gamma-tubulin complex, microtubules, PI3K Akt pathway, antibacterial activity
Citation: Sana TG, Berni B and Bleves S (2016) The T6SSs of Pseudomonas aeruginosa Strain PAO1 and Their Effectors: Beyond Bacterial-Cell Targeting. Front. Cell. Infect. Microbiol. 6:61. doi: 10.3389/fcimb.2016.00061
Received: 08 March 2016; Accepted: 23 May 2016;
Published: 09 June 2016.
Edited by:
Damien F. Meyer, CIRAD, FranceReviewed by:
Suzanne Mariane Janete Fleiszig, University of California, Berkeley, USASusu M. Zughaier, Emory University, USA
Copyright © 2016 Sana, Berni and Bleves. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thibault G. Sana, dHNhbmFAc3RhbmZvcmQuZWR1;
Sophie Bleves, YmxldmVzQGltbS5jbnJzLmZy
†Present Address: Thibault G. Sana Department of Microbiology and Immunology, Stanford School of Medicine, Stanford University, Stanford, CA, USA
 Thibault G. Sana
Thibault G. Sana Benjamin Berni
Benjamin Berni Sophie Bleves
Sophie Bleves