- 1Department of Natural Sciences and Mathematics, West Liberty University, West Liberty, WV, USA
- 2Department of Microbiology and Molecular Genetics, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
- 3Department of Medicine – Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
- 4Center for Vaccine Research, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
The highly infectious and deadly pathogen, Francisella tularensis, is classified by the CDC as a Category A bioterrorism agent. Inhalation of a single bacterium results in an acute pneumonia with a 30–60% mortality rate without treatment. Due to the prevalence of antibiotic resistance, there is a strong need for new types of antibacterial drugs. Resazurin is commonly used to measure bacterial and eukaryotic cell viability through its reduction to the fluorescent product resorufin. When tested on various bacterial taxa at the recommended concentration of 44 μM, a potent bactericidal effect was observed against various Francisella and Neisseria species, including the human pathogens type A F. tularensis (Schu S4) and N. gonorrhoeae. As low as 4.4 μM resazurin was sufficient for a 10-fold reduction in F. tularensis growth. In broth culture, resazurin was reduced to resorufin by F. tularensis. Resorufin also suppressed the growth of F. tularensis suggesting that this compound is the biologically active form responsible for decreasing the viability of F. tularensis LVS bacteria. Replication of F. tularensis in primary human macrophages and non-phagocytic cells was abolished following treatment with 44 μM resazurin indicating this compound could be an effective therapy for tularemia in vivo.
Introduction
Francisella tularensis is the causative agent of the zoonotic disease tularemia (Oyston et al., 2004). This disease is endemic in North America, Europe, and Asia with outbreaks often associated with the handling of infected animals or transmission by arthropod vectors (Sjostedt, 2007; Oyston, 2008). The Centers for Disease Control and Prevention has categorized F. tularensis as a Category A bioterrorism agent due to its ease of aerosolization, low infectious dose, and high mortality rate (McLendon et al., 2006). Inhalation of fewer than 10 bacteria results in an acute pneumonia that is lethal in 30–60% of individuals if left untreated (Dennis et al., 2001; McLendon et al., 2006).
When implemented early in infection, antibiotics are effective at reducing the case fatality rate for tularemia (Dennis et al., 2001; Barry et al., 2009). Aminoglycosides are commonly prescribed, specifically streptomycin or gentamicin, although tetracyclines and fluoroquinolones also have antimicrobial activity against F. tularensis (Nigrovic and Wingerter, 2008; Oyston, 2009). Tetracyclines, however, are associated with high relapse rates in tularemia patients (Thomas and Schaffner, 2010). Since this disease is often misdiagnosed due to its generic symptoms, antibiotic treatment may be delayed resulting in reduced survival (Barry et al., 2009). There is also a potential for the introduction of antibiotic-resistant strains (Oyston, 2009). While a tularemia vaccine is available (live vaccine strain, LVS), it is not currently licensed for use in the United States (Conlan and Oyston, 2007). Due to these concerns, there is an increased interest in developing alternative therapies for tularemia.
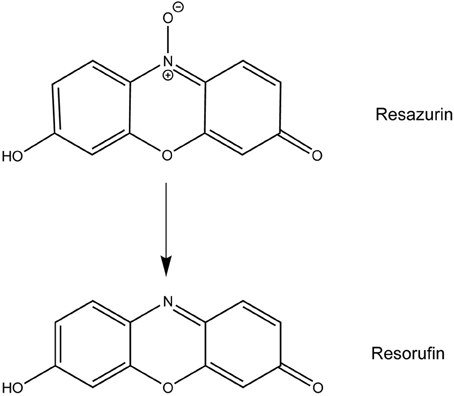
Resazurin, the active compound in alamarBlue®, has been used for decades to measure proliferation and cytotoxicity in prokaryotic and eukaryotic cells (Page et al., 1993; Ahmed et al., 1994; O'Brien et al., 2000). In metabolically active cells, this blue, non-fluorescent dye is reduced to the pink and highly fluorescent compound resorufin allowing for a quantitative measurement of cell viability (Figure 1) (O'Brien et al., 2000). Upon use of resazurin to monitor F. tularensis viability in culture at the recommended concentration of 44 μM, we discovered a novel antibacterial activity for this compound. Resazurin, and its reduced derivative resorufin, decreased the number of viable F. tularensis bacteria in broth culture by 100-fold after 1 day of cultivation. Growth of other bacterial genera was unaffected by this compound with the exception of Neisseria species, particularly the human pathogen N. gonorrhoaea. Resazurin also limited replication of F. tularensis in human macrophages and non-phagocytic cells highlighting the potential use of this compound as a novel antibacterial therapy in vivo.
Materials and Methods
Bacterial Strains and Growth Conditions
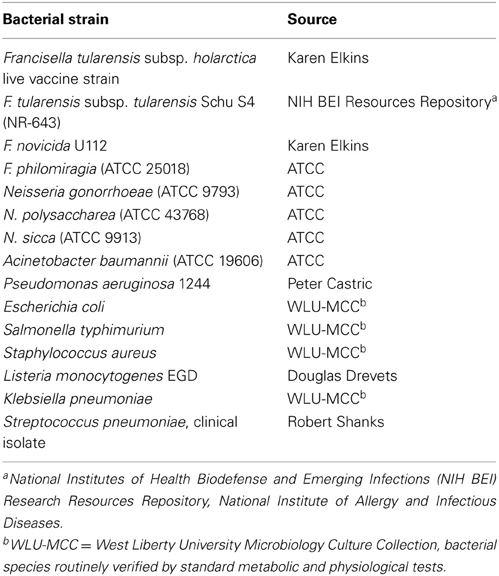
Bacterial strains used in this study are listed in Table 1. Bacteria grown on solid media were used to inoculate chocolate II agar plates or TSBc [trypticase soy broth (BD Biosciences) containing 0.1% L-cysteine hydrochloride monohydrate (Fisher)] supplemented with or without various concentrations of resazurin sodium salt (Acros Organics, dissolved in water) or resorufin (Tokyo Chemical Industries, dissolved in dimethyl sulfoxide). Chocolate II agar plates were incubated at 37°C with 5% CO2 (Francisella species, Neisseria species, and Listeria monocytogenes) or without CO2 (all other bacteria) for 1–3 days while broth cultures were incubated at 37°C with shaking. All work with Schu S4 was conducted under BSL-3 conditions at the University of Pittsburgh with approval from the CDC Select Agent Program.
Reduction of Resazurin to Resorufin by F. tularensis
F. tularensis was cultured in TSBc supplemented with 44 μM resazurin at 37°C with shaking for 24 h. At select timepoints, a Spectronic 200 Spectrophotometer was used to measure the absorbance at 600 nm and 570 nm to detect the presence of resazurin and resorufin, respectively. The ratio of these two optical densities was used to evaluate reduction of resazurin to resorufin over time.
Growth of F. tularensis in Human Macrophages and HEK293 Cells
Human monocytes purified from buffy coats from blood donations (New York Blood Center, Long Island City, NY and the Central Blood Bank, Pittsburgh, PA) were differentiated into macrophages as described previously (Carlson et al., 2007, 2009; Horzempa et al., 2008a,b, 2010; Robinson and Nau, 2008; Robinson et al., 2010, 2012; Russo et al., 2011; Schmitt et al., 2012). Macrophages were then washed and resuspended in Dulbecco's modified Eagle's medium (DMEM) supplemented with 1% human serum AB (Gemini Bio-Products), 25 mM HEPES (Cellgro), and 1% glutamine dipeptide (Fisher Scientific). HEK293 cells (ATCC CRL-1573), a non-phagocytic kidney epithelial cell line (Tachado et al., 2007), were cultured in DMEM supplemented with 10% fetal bovine serum (Gibco), 25 mM HEPES, and 1% glutamine dipeptide with 100 U/ml penicillin-streptomycin (Cellgro). HEK293 cells were passaged at least once without antibiotics prior to use. To assess intracellular growth, gentamicin protection assays were performed (Small et al., 1987). Macrophages and HEK293 cells were seeded in Primaria 96-well culture dishes (BD Biosciences) at a density of 5 × 104 cells/well. F. tularensis bacteria recovered from broth cultures described above were adjusted to an OD600 of 0.3 (approximately 1.5 × 109 CFU/ml) and diluted to achieve a multiplicity of infection (MOI) of 500. The actual MOI was measured by plating serial dilutions of the inoculum on chocolate II agar plates. Cells were incubated with this MOI for 2 h yielding an infection rate of >80% (Carlson et al., 2007; Horzempa et al., 2008a) either in the absence or presence of 44 μM resazurin. After this time period, cells were incubated with gentamicin (100 μg/ml) for 30 min to kill extracellular bacteria and then washed twice with warm Hanks balanced salt solution (Cellgro). Fresh culture media with or without resazurin (44 μM) was then added and cells were incubated for another 22 h at 37°C with 5% CO2. At the indicated timepoints, cells were lysed with 0.02% sodium dodecyl sulfate and viable CFU were measured as described below.
Enumeration of Bacteria
At the indicated timepoints, a portion of the F. tularensis broth cultures or human cell lysates were serially diluted and plated onto chocolate II agar plates. Plates were incubated at 37°C at 5% CO2 for 2–3 days and individual colonies were enumerated. The limit of detection was 100 CFU per ml for broth culture or per 5 × 104 cells for intracellular growth assays.
Analytical Methods
Statistically significant differences in bacterial number were determined by a Student's t-test or ANOVA followed by a Dunnett's or Bonferroni post-hoc test (GraphPad Prism 5). The chemcial structures of resazurin, resorufin, and acridine were drawn using ChemDraw Pro 13.0 for comparative analysis.
Results
Resazurin Selectively Inhibits Growth of Francisella and Neisseria Species
Resazurin has been used previously as an indicator of cell growth for various bacterial species (Mendoza-Aguilar et al., 2012; Bassett et al., 2013; Bauer et al., 2013; Lall et al., 2013). We were interested in using this compound to monitor viability of F. tularensis in broth culture over time. Unexpectedly, no viable bacteria were detected 24 h post-inoculation following inclusion of resazurin in TSBc cultures of F. tularensis LVS at the concentration recommended by the manufacturer (44 μM) (data not shown). This concentration of resazurin had no effect on the growth of E. coli or P. aeruginosa cultivated in the same medium (data not shown). The antimicrobial activity of resazurin on F. tularensis LVS was not specific to TSBc as these bacteria were also unable to grow on chocolate II agar plates containing this compound (Table 2) as well as a chemically defined medium (data not shown). Lowering the resazurin concentration to as little as 4.4 μM still resulted in a 10-fold reduction in viable F. tularensis LVS compared to growth medium alone (Figure 2). These data suggest that resazurin exhibits bactericidal activity against F. tularensis.
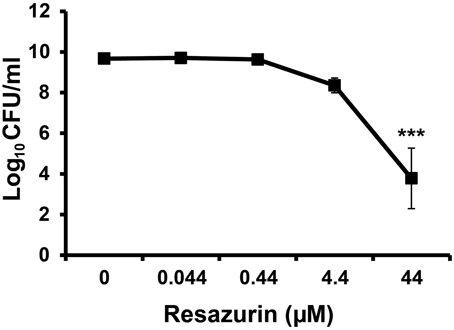
Figure 2. Resazurin has an antimicrobial effect on F. tularensis LVS. Bacteria were cultivated in tryptic soy broth supplemented with 0.1% cysteine HCl (TSBc) in the presence or absence of resazurin at the designated concentrations for 24 h. Cultures were then diluted and plated to determine the number of viable F. tularensis LVS bacteria 24 h post inoculation. Data shown are mean ± s.e.m. from three individual experiments. The limit of detection was 100 CFU per ml. Statistically significant differences in growth post-inoculation were determined by One-Way ANOVA followed by Dunnett's post-hoc test (***p < 0.001 compared to 0 μM resazurin).
To determine whether the antibacterial effect of resazurin was specific to this organism, an assortment of bacteria from diverse taxa were plated on chocolate II agar plates supplemented with resazurin. All bacterial species tested were able to grow in the presence of 44 μM resazurin except F. tularensis and Neisseria species (Table 2). These data suggest that resazurin is an antimicrobial compound with specificity for F. tularensis and Neisseria species bacteria.
Reduction of Resazurin to Resorufin does not alter its Antibacterial Activity
As previously mentioned, viable cells are capable of converting resazurin to resorufin. To determine whether this reduction was occurring in F. tularensis LVS cultures, the ratio of resorufin to resazurin was measured over time using the optical densities at 570 nm (resorufin) and 600 nm (resazurin). The ratio of resorufin to resazurin increased 3-fold within 2 h of inoculation with F. tularensis LVS, reaching a maximum ratio of 5 four hours post-inoculation which was maintained for the remainder of the 24 h period (Figure 3A). This suggested F. tularensis LVS was reducing resazurin to resorufin. Therefore, we determined if resorufin also exhibited antibacterial activity against F. tularensis. Following 24 h of culture in the presence of resorufin, the number of F. tularensis LVS bacteria was significantly reduced compared to growth medium supplemented with vehicle alone (Figure 3B). A similar decrease in bacterial number was observed following incubation of F. tularensis LVS with resazurin (Figure 3B). These data suggest resorufin is also bactericidal against F. tularensis. To evaluate whether resazurin must first be converted to resorufin to exhibit antibacterial activity, we measured viable F. tularensis LVS bacteria over time grown in the presence of resazurin and resorufin. In cultures treated with resazurin, reduction of this compound to resorufin was observed as early as 2 h post-inoculation (Figure 3A). However, a decline in viable bacteria occurred 8 h post-inoculation, reaching statistical significance by 12 h (Figure 3C). Moreover, a similar decrease in bacterial viability was observed in cultures initially treated with resorufin (Figure 3C). No significant differences in viable bacteria were observed between F. tularensis cultures treated with either resazurin or resorufin at any of the time points (Figure 3C). Because resazurin and resorufin both exhibit a similar antimicrobial effect on F. tularensis, this suggests that the redox reaction itself is not responsible for the observed bactericidal activity. However, the data indicating that resazurin is rapidly converted to resorufin, while the drop in viability occurs most drastically after 8 h, may suggest that resorufin is the biologically active form responsible for decreasing the viability of F. tularensis LVS bacteria.
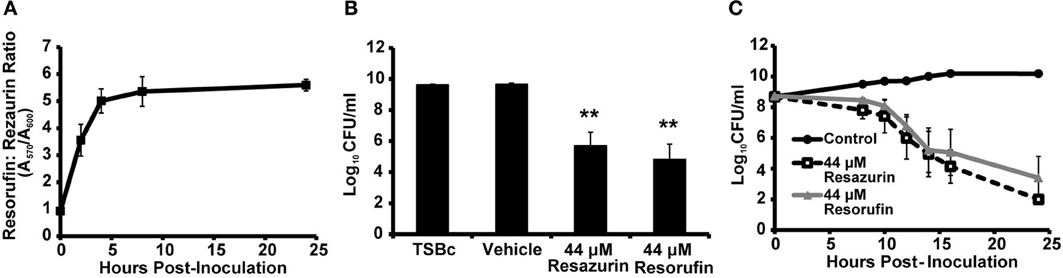
Figure 3. F. tularensis LVS reduces resazurin to resorufin which also inhibits bacterial growth. F. tularensis LVS bacteria were cultivated in TSBc that was treated with resazurin, resorufin, or the solvent vehicle (DMSO). (A) Conversion of resazurin (absorbance at 600 nm) to resorufin (absorbance at 570 nm) in F. tularensis LVS cultures over 24 h. (B,C) F. tularensis LVS cultures were diluted and plated to determine the number of viable bacteria at 24 h (B) or the indicated timepoints (C). Data shown are mean ± s.e.m. from three individual experiments. The limit of detection was 100 CFU per ml. Statistically significant differences in growth post-inoculation were determined by (B) One-Way ANOVA followed by Dunnett's post-hoc test (**p < 0.01 compared to TSBc) or (C) Two-Way ANOVA followed by Bonferroni comparison of means (p < 0.05 for Control vs. Resazurin and Control vs. Resorufin for 12–24 h).
Resazurin Limits Intracellular Replication of F. tularensis in Phagocytic and Non-Phagocytic Cells
In an infected host, F. tularensis resides and replicates inside macrophages (Elkins et al., 2007). Therefore, resazurin must be able to limit intracellular growth of this bacterium in these cells in order to be an effective therapeutic. To test this, primary human macrophages were infected with F. tularensis LVS, and viable intracellular bacteria were quantified using a gentamicin protection assay as described previously (Horzempa et al., 2008a, 2010). Cells were treated with 44 μM resazurin during the entire assay (+Rz), beginning 2 h post-infection (+2 h Rz), or left untreated. Both resazurin treatments resulted in a significant decrease in viable F. tularensis LVS bacteria over 22 h (Figure 4A). Visible observation of the macrophages 24 h post-infection indicated resazurin was reduced to resorufin, although this phenomenon was not quantified (data not shown). This suggested that the macrophages were still viable, and that the combination of resazurin and F. tularensis LVS did not culminate in undesirable toxicity. F. tularensis is also capable of infecting and replicating in non-phagocytic cells like epithelial cells and hepatocytes which is sufficient for pathogenesis (Horzempa et al., 2010). We next determined whether resazurin was also capable of inhibiting growth of F. tularensis LVS in non-phagocytic cells. To test this, a human kidney epithelial cell line HEK293 was used as a model for non-phagocytic cells. Similar to the results obtained with macrophages, treatment with resazurin significantly reduced the number of viable F. tularensis LVS bacteria in HEK293 cells 22 h post-infection (Figure 4B). In an additional experiment, no viable F. tularensis LVS bacteria were detected from HEK293 cells at 72 h post infection following treatment with 44 μM resazurin (data not shown). Based on these data, resazurin exhibits antimicrobial activity against intracellular F. tularensis bacteria.
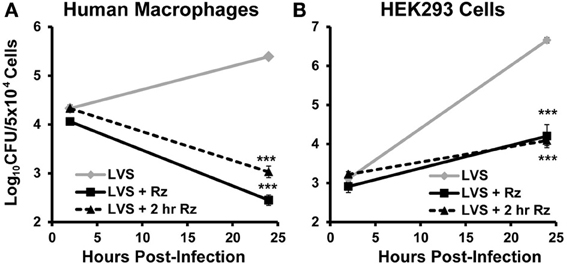
Figure 4. Resazurin exhibits antimicrobial activity on intracellular F. tularensis LVS. Primary human macrophages (A) or HEK293 (B) cells were infected with F. tularensis LVS bacteria using a gentamicin protection assay. Cells were treated with 44 μM resazurin during the entire assay (+ Rz), at 2 h post-infection (+ 2 h Rz), or were untreated. Data shown are mean ± s.e.m. and representative of three independent experiments. The limit of detection was 100 CFU. Statistically significant differences in growth at 24 h post-infection were determined by Two-Way ANOVA with Dunnett's post-hoc test (***p < 0.001).
Discussion
The prevalence of antibiotic resistance in today's society highlights the need for new classes of antibiotics (Bassetti et al., 2013). Here, we identified an unanticipated bactericidal activity for a compound commonly used to measure cellular viability. Resazurin inhibited growth of only F. tularensis and Neisseria species in vitro, notably the human pathogens, type A F. tularensis (Schu S4) and N. gonorrhoeae. The fact that resazurin targets such a limited array of pathogenic organisms is extremely desirable from the standpoint of limiting the potential of drug resistance in the future.
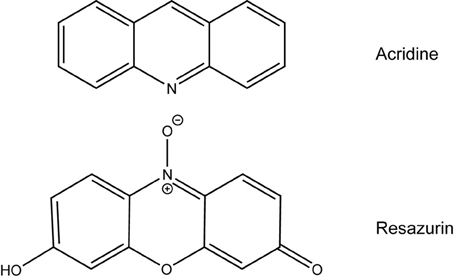
Most antibiotics target pathways that are conserved by numerous bacterial species like cell wall or protein synthesis (Lewis, 2013). Resazurin is unique in that it only exhibits antimicrobial activity against two types of bacteria tested in this work, F. tularensis and Neisseria (Table 2). Aside from their fastidious nature, there are no apparent similarities between these two groups of bacteria that distinguish them from the other bacterial genera tested to suggest a mechanism of action. In culture, resazurin is reduced to resorufin by F. tularensis LVS, however, this chemical reaction is not responsible for the decline in viability since both compounds are equivalently bactericidal (Figure 3). Examination of the chemical structure of resazurin elucidated similarities to acridine (Figure 5). Many acridine derivatives were used as antibacterial agents during World War II (Wainwright, 2001). The planar area of the tricyclic acridine nucleus allows for intercalation of DNA resulting in its bactericidal activity (Wainwright, 2001). The possibility that resazurin functions in a similar fashion is currently being investigated.
In the human host, F. tularensis and N. gonorrhoeae reside inside host cells (Post et al., 2002; Oyston, 2008; Horzempa et al., 2011). Therefore, resazurin must be able to penetrate infected host cells to maintain its antibacterial activity in vivo. During the in vitro infection assays, resazurin was capable of limiting intracellular replication of F. tularensis in both phagocytic and non-phagocytic cells (Figure 4). While resazurin was converted to resorufin in these assays, the reduction of this compound by the host cells did not decrease the potency of the drug, which is consistent with the data indicating that both derivatives are bactericidal (Figures 3, 4). Resazurin has been tested for toxicity in mice at a dose 100-fold higher than that used in this study and shown to be relatively well-tolerated (Lutty, 1978). Another recently developed antibacterial compound, fosmidomycin that was effective at killing intracellular Francisella bacteria in vitro, also prolonged survival following a lethal challenge in an animal model (McKenney et al., 2012). The data presented here strongly suggest that resazurin will be an effective therapeutic for use during an in vivo F. tularensis or Neisseria infection, and substantiate the need for pre-clinical animal trials using mouse models of infection.
Author Contributions
Deanna M. Schmitt, Gerard J. Nau, and Joseph Horzempa conceived and designed the experiments. Joseph Horzempa, Deanna M. Schmitt, Dawn M. O'Dee, Brianna N. Cowan, James W.-M. Birch, and Leanne K. Mazzella performed the experiments. Deanna M. Schmitt, Joseph Horzempa, Gerard J. Nau, and Dawn M. O'Dee analyzed the data. Deanna M. Schmitt and Joseph Horzempa wrote the paper.
Conflict of Interest Statement
The authors acknowledge that Joseph Horzempa, Dawn M. O'Dee, and Gerard J. Nau have submitted a patent on the antimicrobial activity of the compounds used in this manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Karen Elkins for providing the F. tularensis LVS and F. novicida U112 strains, Dr. Peter Castric for providing the P. aeruginosa 1244 strain, Dr. Douglas Drevets for providing L. monocytogenes EGD, and Dr. Robert Shanks for providing S. pneumoniae used in this study. This work was funded by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (P20GM103434) and a grant from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (5K22AI087703).
References
Ahmed, S. A., Gogal, R. M. Jr., and Walsh, J. E. (1994). A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170, 211–224. doi: 10.1016/0022-1759(94)90396-4
Barry, E. M., Cole, L. E., and Santiago, A. E. (2009). Vaccines against tularemia. Hum. Vaccin. 5, 832–838. Available online at: https://www.landesbioscience.com/journals/vaccines/article/10297/ or https://www.landesbioscience.com/journals/vaccines/BarryHV5-12.pdf
Bassett, I. M., Lun, S., Bishai, W. R., Guo, H., Kirman, J. R., Altaf, M., et al. (2013). Detection of inhibitors of phenotypically drug-tolerant Mycobacterium tuberculosis using an in vitro bactericidal screen. J. Microbiol. 51, 651–658. doi: 10.1007/s12275-013-3099-4
Bassetti, M., Merelli, M., Temperoni, C., and Astilean, A. (2013). New antibiotics for bad bugs: where are we? Ann. Clin. Microbiol. Antimicrob. 12, 22. doi: 10.1186/1476-0711-12-22
Bauer, J., Siala, W., Tulkens, P. M., and Van Bambeke, F. (2013). A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 57, 2726–2737. doi: 10.1128/AAC.00181-13
Carlson, P. E. Jr., Carroll, J. A., O'Dee, D. M., and Nau, G. J. (2007). Modulation of virulence factors in Francisella tularensis determines human macrophage responses. Microb. Pathog. 42, 204–214. doi: 10.1016/j.micpath.2007.02.001
Carlson, P. E. Jr., Horzempa, J., O'Dee, D. M., Robinson, C. M., Neophytou, P., Labrinidis, A., et al. (2009). Global transcriptional response to spermine, a component of the intramacrophage environment, reveals regulation of Francisella gene expression through insertion sequence elements. J. Bacteriol. 191, 6855–6864. doi: 10.1128/JB.00995-09
Conlan, J. W., and Oyston, P. C. (2007). Vaccines against Francisella tularensis. Ann. N.Y. Acad. Sci. 1105, 325–350. doi: 10.1196/annals.1409.012
Dennis, D. T., Inglesby, T. V., Henderson, D. A., Bartlett, J. G., Ascher, M. S., Eitzen, E., et al. (2001). Tularemia as a biological weapon: medical and public health management. JAMA 285, 2763–2773. doi: 10.1001/jama.285.21.2763
Elkins, K. L., Cowley, S. C., and Bosio, C. M. (2007). Innate and adaptive immunity to Francisella. Ann. N.Y. Acad. Sci. 1105, 284–324. doi: 10.1196/annals.1409.014
Horzempa, J., Carlson, P. E. Jr., O'Dee, D. M., Shanks, R. M., and Nau, G. J. (2008a). Global transcriptional response to mammalian temperature provides new insight into Francisella tularensis pathogenesis. BMC Microbiol. 8:172. doi: 10.1186/1471-2180-8-172
Horzempa, J., Tarwacki, D. M., Carlson, P. E. Jr., Robinson, C. M., and Nau, G. J. (2008b). Characterization and application of a glucose-repressible promoter in Francisella tularensis. Appl. Environ. Microbiol. 74, 2161–2170. doi: 10.1128/AEM.02360-07
Horzempa, J., O'Dee, D. M., Shanks, R. M., and Nau, G. J. (2010). Francisella tularensis DeltapyrF mutants show that replication in nonmacrophages is sufficient for pathogenesis in vivo. Infect. Immun. 78, 2607–2619. doi: 10.1128/IAI.00134-10
Horzempa, J., O'Dee, D. M., Stolz, D. B., Franks, J. M., Clay, D., and Nau, G. J. (2011). Invasion of erythrocytes by Francisella tularensis. J. Infect. Dis. 204, 51–59. doi: 10.1093/infdis/jir221
Lall, N., Henley-Smith, C. J., De Canha, M. N., Oosthuizen, C. B., and Berrington, D. (2013). Viability reagent, prestoblue, in comparison with other available reagents, utilized in cytotoxicity and antimicrobial assays. Int. J. Microbiol. 2013:420601. doi: 10.1155/2013/420601
Lewis, K. (2013). Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 12, 371–387. doi: 10.1038/nrd3975
Lutty, G. A. (1978). The acute intravenous toxicity of biological stains, dyes, and other fluorescent substances. Toxicol. Appl. Pharmacol. 44, 225–249. doi: 10.1016/0041-008X(78)90185-0
McKenney, E. S., Sargent, M., Khan, H., Uh, E., Jackson, E. R., San Jose, G., et al. (2012). Lipophilic prodrugs of FR900098 are antimicrobial against Francisella novicida in vivo and in vitro and show GlpT independent efficacy. PLoS ONE 7:e38167. doi: 10.1371/journal.pone.0038167
McLendon, M. K., Apicella, M. A., and Allen, L. A. (2006). Francisella tularensis: taxonomy, genetics, and Immunopathogenesis of a potential agent of biowarfare. Annu. Rev. Microbiol. 60, 167–185. doi: 10.1146/annurev.micro.60.080805.142126
Mendoza-Aguilar, M., Almaguer-Villagran, L., Jimenez-Arellanes, A., Arce-Paredes, P., Cid-Gutierrez, J. L., and Rojas-Espinosa, O. (2012). The use of the microplate alamar blue assay (MABA) to assess the susceptibility of Mycobacterium lepraemurium to anti-leprosy and other drugs. J. Infect. Chemother. 18, 652–661. doi: 10.1007/s10156-012-0387-6
Nigrovic, L. E., and Wingerter, S. L. (2008). Tularemia. Infect. Dis. Clin. North Am. 22, 489–504, ix. doi: 10.1016/j.idc.2008.03.004
O'Brien, J., Wilson, I., Orton, T., and Pognan, F. (2000). Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267, 5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x
Oyston, P. C. (2008). Francisella tularensis: unravelling the secrets of an intracellular pathogen. J. Med. Microbiol. 57, 921–930. doi: 10.1099/jmm.0.2008/000653-0
Oyston, P. C. (2009). Francisella tularensis vaccines. Vaccine 27(Suppl. 4), D48–D51. doi: 10.1016/j.vaccine.2009.07.090
Oyston, P. C., Sjostedt, A., and Titball, R. W. (2004). Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2, 967–978. doi: 10.1038/nrmicro1045
Page, B., Page, M., and Noel, C. (1993). A new fluorometric assay for cytotoxicity measurements in-vitro. Int. J. Oncol. 3, 473–476.
Post, D. M., Phillips, N. J., Shao, J. Q., Entz, D. D., Gibson, B. W., and Apicella, M. A. (2002). Intracellular survival of Neisseria gonorrhoeae in male urethral epithelial cells: importance of a hexaacyl lipid A. Infect. Immun. 70, 909–920. doi: 10.1128/IAI.70.2.909-920.2002
Robinson, C. M., Jung, J. Y., and Nau, G. J. (2012). Interferon-gamma, tumor necrosis factor, and interleukin-18 cooperate to control growth of Mycobacterium tuberculosis in human macrophages. Cytokine 60, 233–241. doi: 10.1016/j.cyto.2012.06.012
Robinson, C. M., and Nau, G. J. (2008). Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J. Infect. Dis. 198, 359–366. doi: 10.1086/589774
Robinson, C. M., O'Dee, D., Hamilton, T., and Nau, G. J. (2010). Cytokines involved in interferon-gamma production by human macrophages. J. Innate Immun. 2, 56–65. doi: 10.1159/000247156
Russo, B. C., Horzempa, J., O'Dee, D. M., Schmitt, D. M., Brown, M. J., Carlson, P. E., et al. (2011). A Francisella tularensis locus required for spermine responsiveness is necessary for virulence. Infect. Immun. 79, 3665–3676. doi: 10.1128/IAI.00135-11
Schmitt, D. M., O'Dee, D. M., Horzempa, J., Carlson, P. E. Jr., Russo, B. C., Bales, J. M., et al. (2012). A Francisella tularensis live vaccine strain that improves stimulation of antigen-presenting cells does not enhance vaccine efficacy. PLoS ONE 7:e31172. doi: 10.1371/journal.pone.0031172
Sjostedt, A. (2007). Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann. N.Y. Acad. Sci. 1105, 1–29. doi: 10.1196/annals.1409.009
Small, P. L., Isberg, R. R., and Falkow, S. (1987). Comparison of the ability of enteroinvasive Escherichia coli, Salmonella typhimurium, Yersinia pseudotuberculosis, and Yersinia enterocolitica to enter and replicate within HEp-2 cells. Infect. Immun. 55, 1674–1679.
Tachado, S. D., Zhang, J., Zhu, J., Patel, N., Cushion, M., and Koziel, H. (2007). Pneumocystis-mediated IL-8 release by macrophages requires coexpression of mannose receptors and TLR2. J. Leukoc. Biol. 81, 205–211. doi: 10.1189/jlb.1005580
Thomas, L. D., and Schaffner, W. (2010). Tularemia pneumonia. Infect. Dis. Clin. North Am. 24, 43–55. doi: 10.1016/j.idc.2009.10.012
Keywords: Francisella, resazurin, antibiotic, Neisseria, resorufin, tularemia, antibacterial, macrophages
Citation: Schmitt DM, O'Dee DM, Cowan BN, Birch JW-M, Mazzella LK, Nau GJ and Horzempa J (2013) The use of resazurin as a novel antimicrobial agent against Francisella tularensis. Front. Cell. Infect. Microbiol. 3:93. doi: 10.3389/fcimb.2013.00093
Received: 30 October 2013; Paper pending published: 14 November 2013;
Accepted: 20 November 2013; Published online: 06 December 2013.
Edited by:
Max Maurin, Université Aix-Marseille II, FranceReviewed by:
Anders Sjostedt, Umeå University, SwedenMax Maurin, Université Aix-Marseille II, France
Copyright © 2013 Schmitt, O'Dee, Cowan, Birch, Mazzella, Nau and Horzempa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph Horzempa, Department of Natural Sciences and Mathematics, West Liberty University, 207 Arnett Hall, 208 University Drive, College Union Box #139, West Liberty, WV 26074, USA e-mail:am9zZXBoLmhvcnplbXBhQHdlc3RsaWJlcnR5LmVkdQ==
 Deanna M. Schmitt
Deanna M. Schmitt Dawn M. O'Dee2
Dawn M. O'Dee2 James W.-M. Birch
James W.-M. Birch Gerard J. Nau
Gerard J. Nau Joseph Horzempa
Joseph Horzempa