- School of Molecular Biosciences, Washington State University, Pullman, WA, USA
Campylobacter jejuni is a leading cause of bacterial gastroenteritis worldwide. Acute C. jejuni-mediated disease (campylobacteriosis) involves C. jejuni invasion of host epithelial cells using adhesins (e.g., CadF and FlpA) and secreted proteins [e.g., the Campylobacter invasion antigens (Cia)]. The genes encoding the Cia proteins are up-regulated upon co-culture of C. jejuni with epithelial cells. One of the Cia proteins, CiaC, is required for maximal invasion of host cells by C. jejuni. Previous work has also revealed that CiaC is, in part, responsible for host cell cytoskeletal rearrangements that result in membrane ruffling. This study was performed to test the hypothesis that CiaC is delivered to the cytosol of host cells. To detect the delivery of CiaC into cultured epithelial cells, we used the adenylate cyclase domain (ACD) of Bordetella pertussis CyaA as a reporter. In this study, we found that export and delivery of the C. jejuni Cia proteins into human INT 407 epithelial cells required a functional flagellar hook complex composed of FlgE, FlgK, and FlgL. Assays performed with bacterial culture supernatants supported the hypothesis that CiaC delivery requires bacteria-host cell contact. We also found that CiaC was delivered to host cells by cell-associated (bound) bacteria, as judged by experiments performed with inhibitors that specifically target the cell signaling pathways utilized by C. jejuni for cell invasion. Interestingly, the C. jejuni flgL mutant, which is incapable of exporting and delivering the Cia proteins, did not induce INT 407 cell membrane ruffles. Complementation of the flgL mutant with plasmid-encoded flgL restored the motility and membrane ruffling. These data support the hypothesis that the C. jejuni Cia proteins, which are exported from the flagellum, are delivered to the cytosol of host cells.
Introduction
Campylobacter jejuni remains a leading bacterial cause of gastroenteritis, with an estimated annual incidence of 2.4 million cases per year in the US (Mead et al., 1999), and 400–500 million cases worldwide (Konkel et al., 2001; Ruiz-Palacios, 2007). Most infections with C. jejuni are linked to the consumption of undercooked poultry or foods that have been contaminated with raw poultry (Konkel et al., 2001). This is due to the fact that C. jejuni is a commensal organism in chickens and is able to colonize the ceca of birds at levels greater than 108 CFU/gram of cecal contents (Sahin et al., 2002). Infection with C. jejuni (campylobacteriosis) is characterized by diarrhea containing blood and/or leukocytes, fever, and abdominal cramps. Although campylobacteriosis is usually self-limiting, some individuals require antibiotic treatment. Infection with C. jejuni can also lead to Guillain–Barré syndrome, an acute demyelinating neuropathy characterized by flaccid paralysis (Konkel et al., 2001).
Type III secretion systems (T3SS) are complex macromolecular structures that allow Gram-negative bacteria to secrete proteins across the inner and outer membranes without a periplasmic intermediate (Cornelis, 2006; Desvaux et al., 2006). Classical T3SS possessed by pathogenic bacteria such as Yersinia, Shigella, and Salmonella allow these organisms to deliver effector proteins from the bacterial cytosol directly to the cytosol of a target human cell (Sory et al., 1995; Cornelis, 2006; Galan and Wolf-Watz, 2006). The delivered effector proteins then modulate host cell behavior to facilitate uptake of the organism and subsequent disease. These classical T3SSs can be described as a “molecular syringe” and are composed of a protein complex spanning both bacterial membranes, which is attached to a needle complex that extends from the cell. The needle complex is a hollow conduit with a tip containing translocon proteins, which incorporate into the membrane of the host cell, allowing for delivery of effector proteins into the host cytosol (Cornelis, 2006; Galan and Wolf-Watz, 2006).
C. jejuni-mediated disease is dependent on many factors, including motility, cell adherence, and cell invasion (Konkel et al., 1999, 2001; Guerry, 2007; Flanagan et al., 2009). The flagellum, which is a T3SS with components homologous to the classical T3SS, plays a central role in C. jejuni pathogenesis. The flagellum is composed of a basal body, hook, and filament. The basal body spans both the inner and outer membranes. The hook is a short, curved conduit extending from the basal body. It is composed primarily of the protein FlgE, and is capped by the hook-filament junction proteins FlgK and FlgL. The components comprising the basal body and hook are assembled as they are exported (Ferris and Minamino, 2006). After the hook is completed by the incorporation of FlgK and FlgL, the export specificity of the flagellar T3SS changes to allow for secretion of FlaA and FlaB, which form the flagellar filament (Minamino et al., 1999; Ferris and Minamino, 2006). Deletion of flgK, flgL, or flgE abolishes the ability of the organism to form a flagellar filament (Konkel et al., 2004; Fernando et al., 2007). The flagellar filament is composed of FlaA and FlaB and extends approximately three microns from the bacterial cell (Grant et al., 1993; Guerry, 2007). The flagellar filament is capped by the FliD protein, which allows flagellin subunits to polymerize into the growing flagellum (Yokoseki et al., 1995).
Multiple research groups have concluded that proteins secreted from the flagellum play a role in both commensal colonization of poultry and C. jejuni invasion of human epithelial cells (Konkel et al., 1999; Ziprin et al., 2001; Fernando et al., 2007). Proteins found to be exported via the flagellum of C. jejuni include CiaB (Konkel et al., 1999), CiaC (Christensen et al., 2009), CiaI (Buelow et al., 2010), FlaC (Song et al., 2004), and FspA (Poly et al., 2007). The first report of a non-flagellar protein secreted through the C. jejuni flagellum was CiaB (Konkel et al., 1999). A mutation in the ciaB gene abolishes export of the other Cia proteins and reduces bacterial invasion into host cells. Complementation of the ciaB mutant restores host cell invasion in vitro and virulence in vivo (Rivera-Amill et al., 2001; Raphael et al., 2005a). FlaC binds to epithelial cells and is required for maximal cell invasion (Song et al., 2004). The FspA protein induces apoptosis of INT 407 cells (Poly et al., 2007). Konkel et al. demonstrated that the Cia proteins (i.e., CiaC and CiaI) modulate host cell signaling and alter C. jejuni intracellular trafficking (Konkel et al., 2004; Christensen et al., 2009; Buelow et al., 2010; Eucker and Konkel, 2011). As one might predict, the ciaB, ciaC, and ciaI genes up-regulated in response to conditions encountered in the gastrointestinal tract (i.e., bile salt deoxycholate), and have been shown to contain sequences for export through the flagellar T3SS (Malik-Kale et al., 2008; Christensen et al., 2009). Other research groups have also reported that the expression of ciaC and ciaI is linked to virulence (Carrillo et al., 2004; Stintzi et al., 2005). These findings are in agreement with the proposal that the Cia proteins contribute to C. jejuni virulence. Interestingly, flagellar proteins can be exported from the classical T3SS and vice versa (Lee and Galan, 2004; Badea et al., 2009). Moreover, proteins exported from the classical T3SS have been reported to ultimately result in the modulation of host cell signaling pathways (Sun et al., 2007). In bacteria that possess both flagellar and classical T3SS, the export of proteins is controlled via well-orchestrated gene regulatory mechanisms and chaperone specificity (Lee and Galan, 2004). The only T3SS present in C. jejuni is the flagellum (Parkhill et al., 2000).
C. jejuni invasion of host cells is a complex event that is initiated by C. jejuni binding to cells via several adhesins, including CadF, FlpA, Cj1349c, and CapA (Konkel et al., 1997; Ashgar et al., 2007; Flanagan et al., 2009; Konkel et al., 2010). While C. jejuni binding alone to host cells is sufficient to stimulate host cell signaling pathways, this event in itself is not sufficient to facilitate C. jejuni invasion of host cells. We have shown that the Cia proteins, which are synthesized and secreted by C. jejuni when co-cultured with epithelial cells, are required for maximal cell invasion (Christensen et al., 2009). The observation that chloramphenicol, an inhibitor of bacterial protein synthesis, retards C. jejuni invasion is consistent with the hypothesis that the Cia proteins contribute to cell invasion (Konkel and Cieplak, 1992; Oelschlaeger et al., 1993; Rivera-Amill et al., 2001). We propose the Cia proteins participate in cell invasion by triggering cell signaling events necessary for actin cytoskeletal rearrangement. Maximal C. jejuni invasion of host cells requires activation of the host cell integrin receptors, components of the focal complex, and Rac1 (Eucker and Konkel, 2011; Krause-Gruszczynska et al., 2011). Together, these events result in membrane ruffling and bacterial uptake.
The purpose of this study was to elucidate the mechanism of Cia protein delivery to host cells. Upon contact with epithelial cells, C. jejuni secrete ∼18 Cia proteins (Larson et al., 2008). It is not known if the Cia proteins act on the surface of the epithelial cell or if they are delivered to the cytosol of a target epithelial cell. We chose to use CiaC to investigate the mechanism of Cia protein delivery to host cells (Christensen et al., 2009). In this study, we present evidence that supports the hypothesis that CiaC is delivered to the cytosol of host cells by cell-associated bacteria.
Materials and Methods
Bacterial Strains and Culture Methods
C. jejuni wild-type strains F38011 (Raphael et al., 2005b), 81116 (Black et al., 1988), and 81–176 (Black et al., 1988) were used in this study. Bacteria were grown on Mueller–Hinton agar plates supplemented with 5% citrated bovine blood (MHB) and in Mueller–Hinton (MH) broth with constant shaking. Deoxycholate was added to a concentration of 0.01% to broths where indicated. The bacteria were incubated in a microaerobic environment (85% N2, 10% CO2, 5% O2), at 37°C. The C. jejuni were passaged onto fresh media every 48 h. The E. coli Inv-α (Invitrogen) and S17–1 λ-pir (Tascon et al., 1993) strains were cultured on Luria–Bertani agar plates (LB) or in LB broth with shaking. The cultures were incubated at 37°C in an aerobic environment. All cloning steps were performed in E. coli TOP10 (Invitrogen), and the ACD fusion vectors were transformed into E. coli S-17 for conjugation into C. jejuni.
Cell Culture
INT 407 cells (ATCC #CCL-6) were grown in Minimal Essential Medium (MEM; Cellgro) supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories), 1% glutamine (Invitrogen), and 1% sodium pyruvate (Cellgro). Cultures were maintained at 37°C in a humidified 5% CO2 incubator. Cells were grown to confluency and passaged using 2.5% trypsin (Sigma). For ACD delivery assays, a 24-well plate was seeded with 1.5 × 105 cells/well 18 h prior to infection.
Generation of C. jejuni Mutants
The C. jejuni F38011 fliD, flaAflaB, fliD, ciaC, and ciaB mutants were generated as previously described (Konkel et al., 1999, 2004; Christensen et al., 2009; Buelow et al., 2010). The C. jejuni F38011 flgL, F38011 flgK, F38011 flgE, 81116 flaAflaB, and 81–176 flaAflaB mutants were generated by double-crossover recombination using the pBSK-Kan2 suicide vector. The pBSK-Kan2 plasmid is identical to the pBluescript vector (Stratagene), except that the original ampicillin resistance cassette was replaced with the apha-3 kanamycin resistance cassette, which functions in both E. coli and C. jejuni (Christensen et al., 2009). Approximately 1 kilobase of DNA flanking the target genes was amplified by PCR using the primers indicated in Table A1 and sequentially cloned into pBSK-Kan2 using SacI and SacII sites for the upstream fragment and SacII and XhoI sites for the downstream fragments. A tetracycline (Tet) resistance cassette was amplified using the primers indicated in Table A1 and inserted into the SacII site between the two flanking regions. The suicide vectors were introduced into C. jejuni via electroporation, and transformants were selected for on MHB plates supplemented with 2 μg/mL Tet. The suicide vectors described in this study were confirmed by nucleotide sequencing. Inactivation of the target genes in C. jejuni was confirmed by PCR (not shown).
The flgL mutant was complemented by PCR amplifying the native promoter and ORF using the primers listed in Table A1, and cloning the PCR product into the pRY111 vector using XhoI and EcoRI. The flgL complementation plasmid was electroporated into E. coli S-17, and subsequently conjugated into the C. jejuni F38011 flgL mutant.
Motility Assays
Overnight cultures of C. jejuni were harvested from MHB agar plates and re-suspended in MH broth to 1.0 OD540/mL. Ten μL of the bacterial suspension was spotted onto the center of a MH agar plate containing only 0.4% agar, and incubated for 48 h. The zone of motility was measured from the edge of the inoculum spot to the edge of growth. Each sample was assayed in triplicate. A separate motility plate was stab-inoculated with each strain for visual comparison of motility zones.
Transmission Electron Microscopy
Transmission electron microscopy was performed as previously described (Neal-Mckinney et al., 2010). Briefly, C. jejuni taken from MHB plates were re-suspended in water, added to formvar coated copper grids, and stained with 2% phosphotungstic acid. The grids were visualized using a FEI Tecnai G2 20 Twin microscope.
Cia Secretion Assay
Secretion of the Cia proteins into culture supernatants was assayed as previously described, with minor modifications (Konkel et al., 2004). Briefly, C. jejuni grown on MHB agar plates overnight were harvested and washed three times in MEM without methionine or FBS (Cellgro). Three mL of a 0.3 OD540/mL suspension was added to a T25 flask, followed by 10 μL of Express Protein Labeling Mix [β−35S]-methionine (Perkin Elmer) and 30 μL of dialyzed FBS, and incubated for 3 h at 37°C in a 5% CO2 incubator. After incubation, 1.5 mL of culture was centrifuged for 5 min in a benchtop centrifuge, and 1 mL of supernatant was filtered through a 0.2 μm filter and transferred to a new tube. The proteins in the supernatant were precipitated using 1 mM HCl-acetone and re-suspended in 100 μL (10-fold concentration). Concentrated supernatants were diluted 1:2 in 2X sample buffer, boiled, and separated by SDS-PAGE. The proteins were transferred to a PVDF membrane and dried overnight. The PVDF membrane was exposed to X-ray film (Kodak Biomax) for 48 h and developed. The secretion assay was repeated at least three times, and a representative image is shown.
Generation of Adenylate Cyclase Domain Fusion Vectors
The ACD (amino-terminus) of Bordetella pertussis cyaA was PCR amplified from the pJB2581 vector (Bardill et al., 2005), using the primers shown in Table A1 and cloned into the pRY111 vector using BglII and KpnI. Inverse PCR was then performed using the primers indicated in Table A1, to create a SacI-PstI-XhoI-BamHI-EcoRI-BglII multiple cloning site immediately upstream of the ACD to create the pRY111-ACD vector. The nucleotide sequences of ciaC, ciaI, flaA, flaG, flgB, and metK were amplified without the termination codon, using the primers shown in Table A1 and cloned into pRY111-ACD using SacI and EcoRI. The vectors were sequenced, and electroporated into E. coli S-17 for conjugation into C. jejuni.
Adenylate Cyclase Domain Immunoblots
Immunoblots were performed to detect ACD fusion proteins in whole-cell lysates (WCL) of C. jejuni. Bacteria were harvested after overnight growth in MH broth supplemented with 0.01% deoxycholate and suspended in PBS to a density of 10 OD540/mL, diluted 1:2 in 2X sample buffer, boiled, and separated by SDS-PAGE. The proteins were transferred to PVDF membranes and probed with a 1:1000 dilution of a mouse anti-ACD antibody (sc-13582, Santa Cruz Biotechnology). Bound mouse antibody was detected using 1:2000 diluted rabbit anti-mouse IgG conjugated to HRP (Sigma), and the blot was developed using Western Lightning ECL reagent (Perkin Elmer).
Adenylate Cyclase Domain Reporter Delivery Assays
INT 407 epithelial cells were seeded at a density of 1.5 × 105. C. jejuni were grown for 18 h in MH broth supplemented with 0.01% DOC, and harvested by centrifugation. The pellets were re-suspended and washed twice in PBS, then pelleted and re-suspended to a density of 0.3 OD540/mL in MEM with 1% FBS. The cell suspension was diluted 1:20, and one mL of the diluted suspension was used to infect a well of INT 407 cells in a 24-well plate. After incubation at 37°C in a humidified 5% CO2 incubator for 30 min, the cells were rinsed with PBS, and 300 μL of 0.1 M HCl was added to each well. The INT 407 cells were lysed by incubating the sealed 24-well plate atop boiling water for 15 min, and the lysates were collected. After centrifugation at 16,000 × g for 2 min to pellet cell debris, 100 μL of each sample was assayed for cAMP using an enzyme-linked immunosorbant assay cAMP kit (Enzo, New York, NY) according to the manufacturer's instructions. Each sample was tested in triplicate. The assays were performed at least three times to ensure reproducibility.
Supernatants from a C. jejuni wild-type strain harboring ciaC-ACD were collected by inoculating the strain to 0.6 OD540/mL in MEM with 1% FBS, and incubating for 1 h. The cultures were centrifuged and supernatants filtered through a 0.2 μm filter to remove any C. jejuni. The presence of CiaC-ACD in the supernatants was confirmed using an in vitro ACD activity assay and immunoblotting (not shown). For assays using a filter to block contact with host cells, a 6-well tissue culture plate was seeded with 3 × 105 cells/well and incubated overnight. The culture media was replaced with MEM with 1% FBS, and a Thincert (Greiner Bio-One) well insert with a 0.2 μm pore size was added to the wells. Two mL of C. jejuni (0.3 OD540/mL) in MEM with 1% FBS were used to inoculate the upper chamber of the inserts. TAE226 (Novartis) was dissolved in methanol. For assays using TAE226 to inhibit C. jejuni internalization, 10 μM TAE226 was added to the wells of a 24-well plate 30 min prior to infection with C. jejuni (0.3 OD540/mL). To increase the sensitivity of cAMP detection, we used a high inoculum (0.3 versus 0.015 OD540/mL used in the initial assays) in assays using filters to block C. jejuni contact with host cells and assays using TAE226 to inhibit invasion. The samples were assayed in triplicate, and the assay was repeated at least three times. A representative assay is shown.
Internalization Assay (Gentamicin Protection Assay)
Binding and internalization assays were performed as described elsewhere (Christensen et al., 2009). Briefly, each well of a 24-well plate was inoculated with MEM 1% FBS containing 0.03 OD540/mL of C. jejuni wild-type strain harboring pRY107 (kanamycin resistant), ciaC mutant (chloramphenicol resistant), or a mixture of the two strains. The concentration of the ciaC mutant strain was held constant at 0.03 OD540/mL, while increasing ratios (i.e., 1:2, 1:5, 1:10) of the wild-type strain were added. Gentamicin was added to the wells 3 h post-infection at a concentration 250 μg/mL, a concentration that has been shown previously to efficiently kill extracellular bacteria without affecting intracellular bacteria (Monteville et al., 2003). INT 407 cell lysates were serially diluted and plated on both MHB Kan and MHB Cm plates to select for the wild-type strain and ciaC mutant, respectively. Where indicated, TAE226 was added to cells 30 min prior to infection. Statistical significance was evaluated using a two-tailed Student's t-test (P < 0.05). The samples were assayed in quadruplicate wells, and the assay was performed four times.
Scanning Electron Microscopy
Scanning electron microscopy (SEM) was performed to observe membrane ruffling, as described elsewhere (Eucker and Konkel, 2011). Two independent, blinded observers quantified the number of INT 407 cells with membrane ruffling in four fields of view at 1000× magnification.
Results
Secretion of the Cia Proteins Requires the Flagellar Hook
Flagellar gene deletions were generated in C. jejuni F38011 by homologous recombination, resulting in the deletion of the fliD, flaAflaB, flgL, flgK, and flgE loci. Motility assays were performed to ensure that the motility phenotypes of the flagellar mutant were in agreement with previously published data (Konkel et al., 2004; Fernando et al., 2007; Neal-Mckinney et al., 2010). Inactivation of the flaAflaB, flgL, flgK, or flgE loci completely abolished motility compared to the C. jejuni F38011 wild-type strain and isogenic fliD mutant (Figure 1). The reduced motility of the C. jejuni fliD mutant in motility agar compared to the C. jejuni wild-type strain is in agreement with the hypothesis that FliD helps incorporate exported FlaA and FlaB monomers into the growing flagellar filament. The flagellin monomers can diffuse away from the flagellar filament when the C. jejunifliD mutant is grown in broth, reducing flagellum length, and bacterial motility (Yokoseki et al., 1995). Based on the results of the Cia secretion assays (see below), experiments were performed to specifically complement the flgL mutant by transforming the mutant with a plasmid harboring a wild-type copy of the gene. In contrast to the flgL mutant, motility was restored in the transformed isolate, although not to the same amount as the wild-type strain. This is due possibly to differences in transcriptional regulation of the flgL gene in the wild-type strain (chromosomal) and flgL complemented strain (multi-copy plasmid). Transmission electron microscopy was used to confirm the results of the motility assays, and revealed that the wild-type strain and flgL mutant transformed with the flgL plasmid produced complete flagella, while the flgL mutant did not produce a flagellar filament (Figure A1). This finding demonstrates that the phenotype displayed by the mutant was due solely to the absence of the FlgL protein.
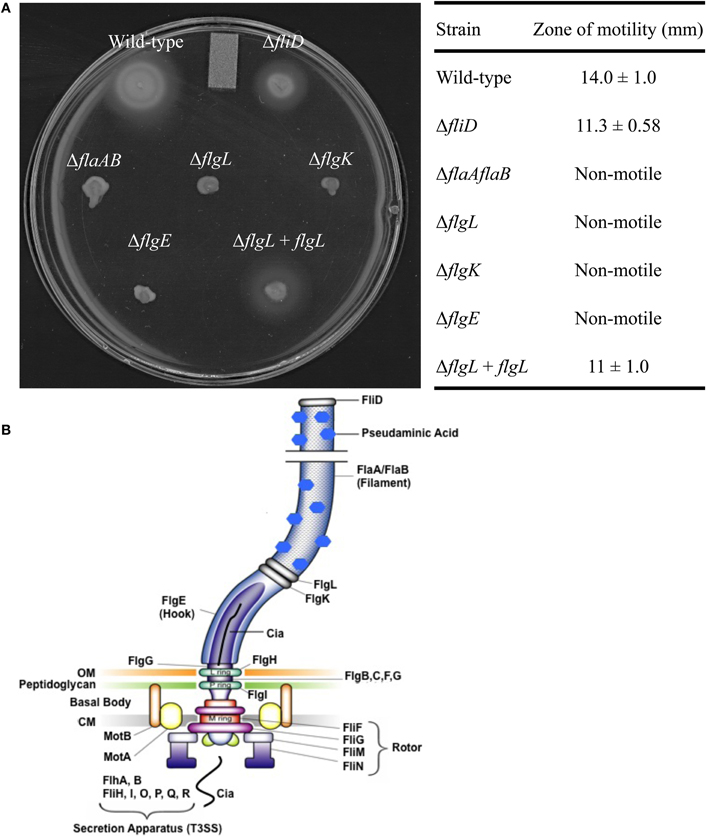
Figure 1. Motility of the C. jejuni flagellar mutants. Motility assays were performed using C. jejuni F38011 wild-type strain, the isogenic fliD, flaAflaB, flgL, flgK, and flgE mutants, and the flgL mutant complemented with flgL(A). The table contains zones of motility ± one standard deviation obtained from a quantitative motility assay performed in triplicate. The zone of motility was determined by measuring from the edge of the inoculum spot to the edge of growth. A diagram of the C. jejuni flagellum is also included, and was adopted from [Larson et al. (2008)] (B). OM, outermembrane; CM, cytoplasmic membrane.
Secretion of the Cia proteins from the C. jejunifliD, flaAflaB, flgK, flgL, and flgE mutants was assayed by autoradiography of protein supernatants from C. jejuni grown in the presence of 1% FBS to stimulate Cia secretion (Figure 2A) (Konkel et al., 2001, 2004). The proteins in the supernatant were concentrated, and probed with antibody reactive against the ACD (Figure 2B). Supernatants and whole cell lysates (Figures 2C,D, respectively) of each of the isolates were probed with an anti-CysM serum to ensure that bacterial cell lysis was not responsible for the proteins observed in the supernatants by autoradiography. The Cia proteins were secreted from the fliD and flaAflaB mutants but not the flgL, flgK, and flgE mutants, as judged by autoradiography. The ∼66 kDa band visible in the flgL, flgK, and flgE mutant secretion profiles is background from the FBS used to stimulate Cia secretion, and was not observed in samples without 1% FBS (not shown). In agreement with the results of the autoradiograph, CiaC-ACD was detected in the supernatant of the wild-type strain, as well as the fliD and flaAB mutants (the flgL mutant transformed with the flgL plasmid did not contain the ciaC-ACD gene). Interestingly, the fliD and flaAB mutants had a more intense secretion profile than wild-type.
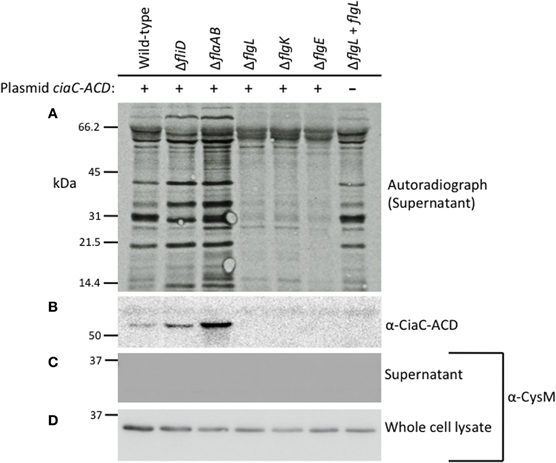
Figure 2. The Cia proteins are secreted from the C. jejuni F38011 wild-type strain and fliD and flaAflaB mutants, but not flgL, flgK, and flgE mutants. The C. jejuni F38011 wild-type strain and isogenic fliD, flaAB, flgL, flgK, and flgE mutants were assayed for secretion of the Cia proteins as well as for secretion of plasmid-encoded CiaC-ACD. Newly synthesized proteins were labeled with [35S]-methionine and Cia secretion was stimulated by the addition of 1% FBS. Proteins in the supernatants were concentrated 10-fold, separated by SDS-PAGE, transferred to PVDF membranes, and exposed to X-ray film (A) or probed with an anti-ACD antibody (B). Faint bands present in all lanes represent bovine serum albumin (66.5 kDa) and degradation products. The molecular mass of the pre-stained protein standards are listed in kDa. Supernatants (C) and whole-cell lysates (D) were probed with anti-CysM antibody to confirm that cell lysis did not occur.
The results obtained for the secretion assay performed with the newly generated C. jejuni F38011 flaAflaB mutant were not consistent with our previous findings using a C. jejuni 81116 mutant with a partial flaAflaB gene deletion (Grant et al., 1993). We regenerated new C. jejuni 81116 and 81–176 flaAflaB mutants whereby the entire flaA and flaB ORFs were deleted. All three of the C. jejuniflaAflaB mutants (F38011, 81116, and 81–176) were non-motile, and exported the Cia proteins into the supernatants as judged by the secretion assay (Figure A2).
Based on the results of the secretion assay and the fact that FlgL was the most distal component of the flagellar apparatus that appeared necessary for Cia protein secretion, we tested whether the flgL complement strain could secrete the Cia proteins. Complementation of the flgL mutant with a plasmid encoding flgL restored secretion of the Cia proteins. Based on these data, we concluded that the hook and hook-associated proteins, but not the flagellar filament, are required for Cia protein secretion.
C. jejuni CiaC is Delivered to Host Cells
We hypothesized that CiaC, which is a secreted from the flagellar apparatus and required for maximal invasion of host cells, is delivered to the cytosol of epithelial cells. To test this hypothesis, we used the adenylate cyclase domain (ACD) of the Bordetella pertussis CyaA protein as a reporter for host cell delivery (Glaser et al., 1988; Sory and Cornelis, 1994; Sory et al., 1995). The ACD, which is activated only in the presence of calmodulin found in eukaryotic cells, catalyzes the production of cAMP from ATP. We generated a pRY111-based (Yao et al., 1993) shuttle plasmid whereby a number of C. jejuni genes, including ciaC, were cloned in-frame with the ACD. As a negative control, the metK gene driven from its native promoter was also cloned in-frame with the ACD. The C. jejuni MetK protein, which is an S-adenosylmethionine synthetase, is localized in the cytoplasm (Parkhill et al., 2000). The ciaC-ACD and metK-ACD plasmids were introduced into the C. jejuni F38011 wild-type strain and isogenic flagellar mutants, and ACD delivery assays were performed.
INT 407 cells were infected at a MOI of ∼500, similar to that used in our gentamicin protection assays. The amount of intracellular cAMP produced by the INT 407 cells was assayed at 30 min post-infection, as preliminary experiments revealed this time-point resulted in the greatest level of cAMP (data not shown). Infection of INT 407 cells with the C. jejuni F38011 wild-type strain, fliD mutant, and flaAflaB mutant harboring ciaC-ACD resulted in a significant increase in intracellular cAMP compared to the amount of cAMP produced by cells inoculated with the flgL, flgK, and flgE mutants harboring ciaC-ACD (Figure 3). Only minimal levels of cAMP were detected in INT 407 cells inoculated with the C. jejuni F38011 wild-type strain and flgE mutant harboring metK-ACD. Noteworthy is that the delivery of CiaC to INT 407 cells was also demonstrated using MOI of ∼5000 and ∼100, whereas a significant increase in cAMP levels (as compared to the flgE mutant negative control) was not detected in INT 407 cells for MetK regardless of the MOI (data not shown). These results are consistent with the hypothesis that the CiaC protein is delivered to the cytosol of host cells. Further, these results indicate that a flagellar hook and hook-associated proteins (FlgE, FlgK, and FlgL) are required for Cia delivery.
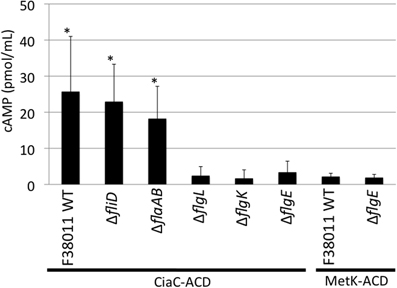
Figure 3. The CiaC virulence proteins is delivered to the cytosol of INT 407 cells from the C. jejuni F38011 wild-type strain, fliD mutant, and flaAflaB mutant, but not flgL, flgK, and flgE mutants. Delivery of the CiaC-ACD fusion protein from the cytosol of C. jejuni to the cytosol of INT 407 cells was assayed in the C. jejuni F38011 wild-type strain and isogenic fliD, flaAflaB, flgL, flgK, and flgE mutants, and delivery of MetK-ACD was assayed in the C. jejuni F38011 wild-type strain and flgE mutant. The concentration of cAMP within the INT 407 cells was assayed 30 min post-infection. The error bars represent one standard deviation from mean. The asterisk indicates values that are significantly different from the flgE mutant harboring ciaC-ACD as judged by the Student's t-test (P < 0.05). Data from two biological replicates were combined.
C. jejuni CiaI is Delivered to Host Cells
Previous studies have suggested that multiple flagellar and Cia proteins are secreted upon cultivation with epithelial cells, some of which function to modulate cell behavior and facilitate pathogenesis (Konkel et al., 1999; Christensen et al., 2009; Buelow et al., 2010). To test whether other flagellar exported proteins are delivered to host cells, the ACD reporter assay was used to examine delivery of a known Cia protein (i.e., CiaI) (Buelow et al., 2010), as well as secreted flagellar components (i.e., FlaA, FlaG, and FlgB) (Hendrixson and Dirita, 2003; Larson et al., 2008; Christensen et al., 2009) (Figure 4). Each ACD construct was transformed into the C. jejuni wild-type strain and flgE mutant and assayed for cAMP production in INT 407 cells after 30 min infection. Infection with C. jejuni F38011 wild-type harboring ciaI-ACD resulted in a significant increase in cAMP, compared to the flgE mutant harboring ciaI-ACD. The flaA-ACD, flaG-ACD, and flgB-ACD plasmids in the C. jejuni F38011 wild-type strain did not result in an increase in cAMP levels compared to the flgE mutant. The CiaI-ACD, FlgB-ACD, FlaA-ACD, and FlaG-ACD proteins were exported, as judged by immunoblot analysis of supernatants using anti-ACD and anti-CysM antibodies (Figure A3). Collectively, these results demonstrate that the CiaC and CiaI proteins are exported and delivered to host cells in a flagellar T3SS dependent manner. The flagellar proteins FlaA, FlaG, and FlgB are either not delivered to host cells or delivered in low concentrations undetectable by our ACD reporter assay.
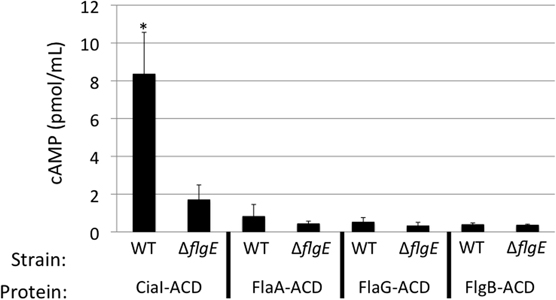
Figure 4. CiaI is delivered from the C. jejuni F38011 wild-type strain to INT 407 cells. Delivery of the CiaI-ACD, FlaA-ACD, FlaG-ACD, and FlgB-ACD fusion proteins was assayed in the C. jejuni F38011 wild-type strain and isogenic flgE mutant. INT 407 cells were inoculated with the C. jejuni F38011 wild-type strain and flgE mutant harboring the various ACD plasmids. The concentration of cAMP within the INT 407 cells was assayed 30 min post-infection. The error bars represent one standard deviation from mean. The asterisk indicates that the amount of cAMP produced in the wild-type strain was significantly greater than the value obtained from the flgE mutant, as judged by the Student's t-test (P < 0.05).
To ensure that each of the ACD fusion proteins were synthesized at equivalent levels in the C. jejuni wild-type strain and flgE mutant, whole cell lysates of each strain were probed by immunoblot, using an anti-ACD antibody (Figure 5). The intensity of the reactive bands in the wild-type strain and flgE mutant were similar, indicating that production of the ACD fusion proteins was not significantly affected by mutation of flgE.
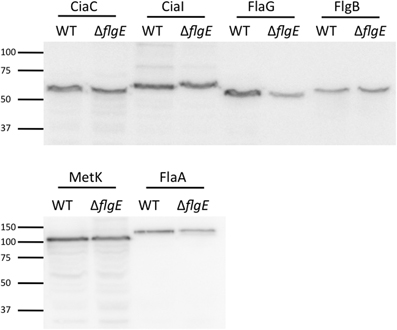
Figure 5. Immunoblot using anti-ACD antibody reveals equivalent protein synthesis of ACD fusion proteins in C. jejuni F38011 wild-type strain and flgE mutant. The amount of ACD fusion proteins produced in the C. jejuni F38011 wild-type strain and isogenic flgE mutant was examined by immunoblot. Whole cell lysates were prepared from bacteria grown overnight in MH broth supplemented with 0.01% deoxycholate, transferred to PVDF membranes, and probed with an anti-ACD antibody. The molecular mass of the pre-stained protein standards are listed in kDa.
Protein Delivery Requires Bacteria-Host Cell Contact
To address whether the Cia proteins are delivered to host cells from outer membrane vesicles released into the culture supernatants from C. jejuni or via another mechanism not requiring bacteria-host cell contact, we cultured C. jejuni in medium containing 1% FBS for 1 h and then prepared bacterial cell-free supernatants. The purpose of adding FBS to the medium was to stimulate the synthesis and export of the Cia proteins into the supernatants (Rivera-Amill et al., 2001). For these assays, C. jejuni were suspended in medium at a level that was 40-fold greater (0.6 OD540/mL) than used for the previous C. jejuni-cell infection assays, in order to increase the amount of Cia proteins in the supernatants. Using this procedure, we were readily able to detect CiaC-ACD in the cell-free supernatant by immunoblots and by an in vitro activity assay whereby the supernatants were mixed with activity buffer containing ATP and calmodulin (data not shown). The cell-free supernatant was then added to INT 407 epithelial cells and cAMP levels of the cells were measured after 30 min (Figure 6). The results were compared to those obtained for cells infected with a wild-type strain (positive control) or flgE mutant (negative control) harboring ciaC-ACD (Panel A). No cAMP production was observed in cells treated with CiaC-ACD containing supernatants, indicating that the secreted CiaC-ACD protein was not delivered to the host cell cytosol. An additional experiment was performed using a 0.2 μm pore filter to block physical contact of C. jejuni with the epithelial cells (Panel B). The filter used created two chambers within the tissue culture well, a bottom chamber containing the epithelial cells and an apical chamber containing a C. jejuni wild-type strain harboring ciaC-ACD. While proteins were secreted from C. jejuni and entered the bottom chamber, no cAMP production was observed. These results suggest that C. jejuni binding to host cells is necessary for CiaC protein delivery.
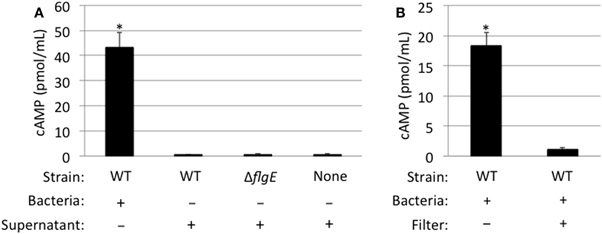
Figure 6. CiaC-ACD delivery into INT 407 cells requires bacteria-host cell contact. The delivery of CiaC-ACD into INT 407 cells assayed using whole bacteria and CiaC-ACD containing supernatants (A), as well as a filter to block physical contact of bacteria with INT 407 cells (B). (A) Cell-free supernatants of C. jejuni F38011 wild-type strain and isogenic flgE mutant were harvested from bacteria incubated in MEM supplemented with 1% FBS for 1 h. The C. jejuni F38011 strain harboring the ciaC-ACD plasmid or cell-free supernatants collected from this strain were added to INT 407 cells and cAMP was measured 30 min post-inoculation. (B) A delivery assay was performed in the presence and absence of a 0.2 μm pore filter. To accommodate the 0.2 μm pore filter, six-well tissue culture plates (rather than 24-well) were used. The value shown for C. jejuni F38011 wild-type strain was normalized by subtracting the background cAMP value obtained for the flgE mutant, with or without a 0.2 μm pore filter. The error bars represent one standard deviation from mean. The asterisk indicates the amount of cAMP produced in the wild-type strain was significantly greater than the value obtained from the flgE mutant as judged by the Student's t-test (P < 0.05)
Cia Protein Delivery does not Require Bacterial Internalization
It had previously been shown that effector proteins can be exported by pathogens located both inside and outside of the target cell (Coburn et al., 2007). To determine whether Cia protein delivery was occurring by cell-associated (bound) bacteria, the delivery of CiaC-ACD to host cells was assayed in the presence of TAE226 to inhibit bacterial invasion (Figure 7). TAE226 is a specific inhibitor of Focal Adhesion Kinase, which is required for maximal C. jejuni invasion of INT 407 cells (Eucker and Konkel, 2011). The viability of INT 407 cells treated with TAE226 or methanol (vehicle control) was assessed using trypan blue (Sigma) staining, and neither treatment affected the viability of the cells (data not shown). Treatment of cells with TAE226 significantly reduced invasion of INT 407 cells by C. jejuni to a level below that of the ciaB mutant (invasion deficient control) in a dose-dependent manner (Figure 7A). The addition of TAE226 did not significantly reduce the level of cAMP produced in response to delivery of CiaC-ACD (Figure 7B). These data demonstrate that C. jejuni delivers proteins to epithelial cells prior to internalization.
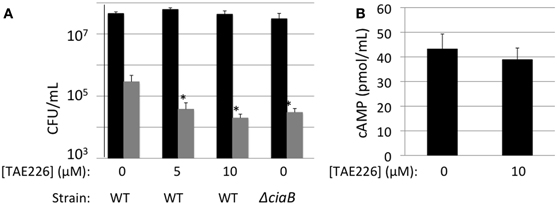
Figure 7. CiaC-ACD is delivered into INT 407 cells by extracellular bacteria. A bacterial invasion assay (A) and delivery assay (B) were performed in the presence of TAE226, an inhibitor of Focal Adhesion Kinase. Focal adhesion kinase is required for maximal invasion of host cells by C. jejuni. (A) The gentamicin protection assay was used to determine the number of cells of C. jejuni F38011 wild-type strain and its isogenic ciaB mutant internalized in the presence of 0, 5, or 10 μM TAE226. Gentamicin was added 3 h post-infection to kill extracellular bacteria. The INT 407 cells were then lysed and the bacteria were enumerated by serial dilution and plating. Shown are the number of cell-associated bacteria (black bars) and internalized bacteria (gray bars). The number of bacteria internalized in the presence of TAE226 was significantly reduced, when compared to C. jejuni infected cells in the absence of inhibitor, as judged by the Student's t-test (P < 0.05). (B) Delivery of the CiaC-ACD fusion protein was assayed at 30 min post-infection in the presence of 0 or 10 μM TAE226. The values shown were normalized by subtracting the background cAMP value obtained for the flgE mutant (i.e., Cia secretion negative) containing each ACD fusion construct from the values obtained for the wild-type strain. The error bars represent one standard deviation from the mean. The amount of cAMP produced in the presence of TAE226 was not significantly different from the value obtained in the absence of TAE226, as judged by the Student's t-test (P < 0.05). Cells were treated with methanol (vehicle control), and trypan blue staining was performed to confirm that the solvent used for TAE226 did not affect cell viability (not shown).
A Functional Flagellar Export Apparatus is Required for C. jejuni to Induce Membrane Ruffling of INT 407 Cells
Delivery of the Cia virulence proteins modulates signaling pathways in host cells, leading to cytoskeletal rearrangement mediated in part by the Rho GTPase Rac1 (Eucker and Konkel, 2011). Rearrangement of the host cell cytoskeleton causes the formation of membrane ruffles that can be visualized and quantified using SEM. We performed SEM of cells infected with the C. jejuni F38011 wild-type strain, flgL mutant, and flgL mutant complemented with flgL to confirm that the Cia virulence proteins were delivered to host cells in a flagellar T3SS dependent manner (Figure 8). In the absence of bacteria, membrane ruffling was observed in 33.5% (# of cells examined = 212) of the INT 407 cells examined (Panel H). In contrast, 63.7% (# of cells examined = 216) of the INT 407 cells infected with the C. jejuni wild-type strain exhibited membrane ruffling (Panels A and B). Consistent with our previous findings demonstrating that FlgL is required for CiaC export and delivery, the amount of membrane ruffling observed in cells inoculated with the flgL mutant (Panels C and D) was indistinguishable from that of non-infected INT 407 cells (membrane ruffling was observed in 35% of the cells, # of cells examined = 356), whereas the complemented flgL mutant (Panels E and F) caused membrane ruffling in 58.5% of cells (# of cells examined = 232). The addition of cell-free supernatants containing the Cia proteins to INT 407 cells (Panel G) did not stimulate membrane ruffling (27.3% of cells, # of cells examined = 157). Importantly, bacteria-host cell contact was promoted by centrifugation of the tissue culture plates to eliminate the effects of motility on adherence. Despite the fact that the flgL mutant was in contact with the epithelial cells, contact alone did not appear sufficient to induce membrane ruffling. These results support the hypothesis that the delivery of the Cia proteins induces cytoskeletal rearrangement, and the delivery of the Cia proteins depends on a functional flagellar export apparatus.
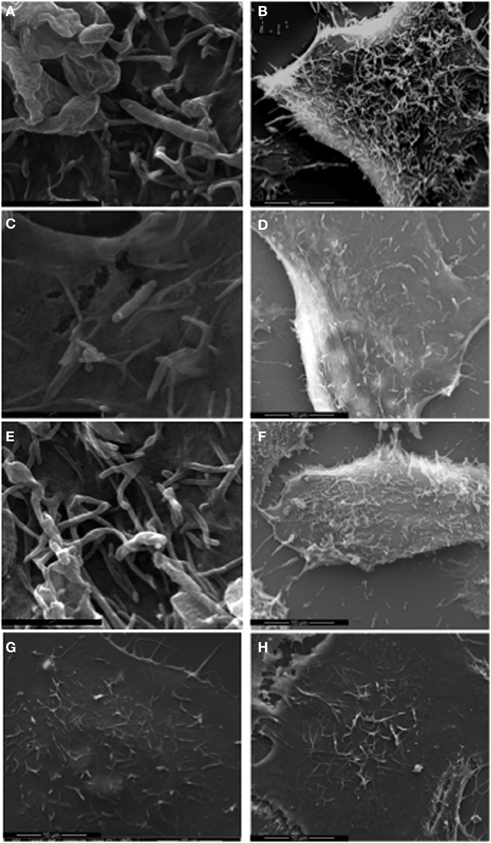
Figure 8. C. jejuni F38011 wild-type induces membrane ruffling. INT 407 cells were infected with the C. jejuni F38011 wild-type strain (A and B), flgL mutant (C and D), flgL mutant harboring a plasmid containing native flgL(E and F), cell-free supernatants containing the Cia proteins (G), or were uninfected (H). The cells were fixed and removed at 15 min post-infection, and visualized by SEM at 50,000× (A, C, and E) and 7000× (B, D, F, G, and H) magnification.
A C. jejuni Wild-Type Strain cannot Rescue the Invasion of A ciaC Mutant
As we observed intense membrane ruffling in response to delivery of the Cia proteins, we sought to determine whether the Cia proteins delivered by a wild-type bacterium could induce global changes in the cell architecture to facilitate the internalization of an invasion deficient bacterium. We performed internalization assays with various ratios of a C. jejuni wild-type strain and the ciaC mutant (Figure 9). In this experiment, the amount of the ciaC mutant added to each well was kept constant while increasing amounts of the C. jejuni wild-type strain were added. Consistent with previous work (Christensen et al., 2009), a significant reduction in invasion was noted for the C. jejuniciaC mutant when compared to the wild-type strain. However, the number of internalized ciaC mutant bacteria did not change in response to additional wild-type bacteria. These results suggest that the Cia proteins act in a localized manner within the cell, and that the membrane ruffling induced by wild-type C. jejuni is not sufficient to facilitate the uptake of a bacterium attached to another location on the host cell.
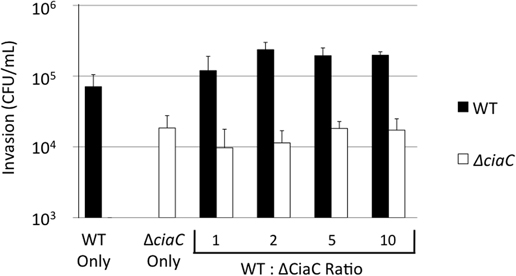
Figure 9. Cia delivery by the C. jejuni F38011 wild-type strain cannot rescue the invasion of a ciaC mutant. A bacterial invasion assay was performed using the C. jejuni F38011 wild-type strain, the isogenic ciaC mutant, or a mixture of both strains. The numbers of internalized C. jejuni F38011 wild-type (black bars) and ciaC mutant (white bars) bacteria were determined at 3 h post-infection using the gentamicin protection assay. The numbers below the graph indicate the quantity of C. jejuni F38011 wild-type and ciaC mutant bacteria in each well, in units of 0.03 OD540. The error bars represent one standard deviation. The number of C. jejuniciaC mutant bacteria internalized did not change in response to increasing amounts of wild-type bacteria, as judged by the Student's t-test (P < 0.05).
Discussion
While the precise roles of virulence proteins in facilitating bacterial invasion of host epithelial cells remain to be elucidated, it is clear that virulence proteins are secreted from the cell in a flagellar dependent manner (Konkel et al., 1999, 2004; Song et al., 2004; Poly et al., 2007). While some secreted virulence proteins likely act on the surface of the cell, others are presumably delivered to the cytosol, based on their biological functions. Prior to this study, it was not known whether the Cia proteins: (1) modulate host cell activities from the extracellular environment; (2) are delivered to the cytosol of host cells from the flagellum; or (3) are exported to the extracellular environment and then taken into the cytosol via a mechanism independent of the flagellum. Although we are unable to elucidate the precise mechanism of CiaC delivery, we demonstrate that cell-associated C. jejuni are necessary for delivery of CiaC to the cytosol of host cells. The delivery of CiaC is dependent on a functional flagellar T3SS. The flagellar proteins FlaA, FlaG, and FlgB were not delivered to host cells, suggesting that the mechanism of delivery of some Cia proteins to a host cell is specific. The addition of cell-free supernatants containing CiaC-ACD did not result in cAMP production, indicating that CiaC is delivered by a mechanism without an extracellular intermediate.
In a previous study, it was found that inactivation of the flaAflaB locus in C. jejuni 81116 (GRK7 strain) abolished secretion of the Cia proteins (Grant et al., 1993). Noteworthy is that the C. jejuni 81116 GRK7 flaAflaB mutant was constructed via a different method than the C. jejuni F38011 flaAflaB mutant, such that the C. jejuni 81116 flaAflaB mutant chromosome still possessed a 5′ portion of the flaA gene. Surprisingly, when we completely deleted the flaA and flaB locus in the C. jejuni F38011 strain, the Cia proteins were still secreted. To determine whether this phenotype was strain-specific, we generated identical mutations in C. jejuni strains F38011, 81116, and 81–176. When the flaAflaB locus was completely deleted, the three mutant strains were still able to secrete the Cia proteins. Our results demonstrate that FlaA and FlaB are not required for secretion of the Cia proteins. Based on this finding, it may be useful to re-examine previous studies that have examined the role of FlaA and FlaB in motility, human disease, and commensal colonization of chickens, to ensure that the observed phenotypes are due solely to loss of motility and not loss of protein secretion through the T3SS (Grant et al., 1993; Konkel et al., 2004).
One of the first questions we sought to address in this study is whether the Cia proteins were actually delivered to the cytosol of host cells. Using the ACD reporter assay, we found that Cia secretion competent C. jejuni strains (i.e., C. jejuni F38011 wild-type, fliD mutant, and flaAB mutant) were able to deliver CiaC-ACD and CiaI-ACD to host cells, resulting in an increase in cAMP levels. C. jejuni strains that could not secrete the Cia proteins (i.e., C. jejuni flgL mutant, flgK mutant, and flgE mutant) were unable to deliver CiaC-ACD to host cells. Other proteins secreted through the flagella (i.e., FlaA, FlgB, and FlaG) are either not delivered to the host cell, or delivered in minute quantities compared to CiaC or CiaI, as the FlaA-ACD, FlgB-ACD, and FlaG-ACD proteins did not result in significant increases in cAMP. Noteworthy is that CiaI-ACD, FlaA-ACD, and FlaG-ACD were secreted from C. jejuni at similar levels as judged by immunoblot (Figure A3), while CiaI-ACD was the only protein delivered to host cells. While the precise mechanism of Cia delivery via the flagellum remains to be elucidated, it is clear that the Cia proteins are delivered to the cytosol of host cells.
To our knowledge, C. jejuni is the only organism that uses a flagellar T3SS to deliver effector proteins to the cytosol of target cells. While it had been hypothesized that the Cia proteins could be delivered to host cells via the flagellar filament, a critique of this hypothesis was the inequality of the injectisome needle and flagellar filament. The injectisome needle complex is typically ∼1 μm in length, rigid, and stationary (Sory et al., 1995; Cornelis, 2006). In contrast, the flagellar filament of C. jejuni can be longer than 3 μm, is flexible, and is rapidly rotating during bacterial movement (Grant et al., 1993). Interestingly, many components of the flagellar T3SS have orthologs in injectisome T3SS (Desvaux et al., 2006; Erhardt et al., 2010). In fact, the basal body and hook structure of the flagellum is similar in structure to the basal body and needle of the injectisome (Cornelis, 2006; Erhardt et al., 2010). This study revealed that the flagellar cap protein FliD and filament proteins FlaA and FlaB are dispensable for Cia protein secretion, while the hook protein FlgE and hook-filament junction proteins FlgK and FlgL are required. The secretion negative phenotype of the flgK mutant is in agreement with a previous study by Fernando et al. (Fernando et al., 2007). Thus, it is possible that the C. jejuni flagellar T3SS without a filament could function analogous to an injectisome. We hypothesize that there may be additional proteins that interact with the flagellar hook (i.e., FlgE, FlgK, and/or FlgL) to enable direct delivery of CiaC, similar to a translocon complex that interacts with the tip of the injectisome needle.
Treatment of INT 407 cells with the FAK inhibitor TAE226 inhibits C. jejuni internalization in a dose-dependent manner. In this study, we found that delivery of CiaC-ACD was not affected by TAE226 at concentrations that reduced invasion ∼10-fold, demonstrating that CiaC can be delivered without invasion. In the Yersinia Ysc T3SS, protein delivery to host cells requires intimate contact with host cells (Sory et al., 1995). In this study, when the bacteria-host cells contact was prevented (i.e., supernatants only or filter to block contact), no protein delivery was detected. These results suggest that the delivery of the Cia proteins from C. jejuni to a host cell requires intimate contact. Additionally, the results of the mixed infection internalization assay (Figure 9) suggest that the Cia proteins act in a localized fashion within the host cell. While these findings are in agreement with the hypothesis that the flagellar T3SS of C. jejuni directly delivers proteins to the host cell cytosol, they do not eliminate the possibility that the Cia proteins are exported by the flagellar T3SS and taken into the host cell indirectly by a mechanism dependent on bacteria host-cell contact. However, the finding that not all flagellar exported proteins are delivered to the host cell cytosol suggests a specific mechanism for the delivery of CiaC and CiaI by the flagellum. Regardless, identification of a flagellar-associated translocon complex is necessary to conclude that the flagellar T3SS of C. jejuni also functions as an injectisome.
In our current model of disease, C. jejuni enters the intestine where it encounters environmental stimuli (i.e., deoxycholate) that up-regulate the transcription of virulence genes, including the genes encoding the Cia proteins (Stintzi et al., 2005; Malik-Kale et al., 2008). C. jejuni then bind to host cells via adhesins (e.g., CadF, CapA, and FlpA) (Konkel et al., 1997, 2010; Ashgar et al., 2007; Flanagan et al., 2009). The binding of C. jejuni to host cells allows the Cia proteins to be delivered to the host cell cytosol, where they modulate host cell signaling events (Eucker and Konkel, 2011). The observation that chloramphenicol reduces C. jejuni internalization into host cells by preventing de novo synthesis of the Cia proteins supports the proposal that C. jejuni binding to host cells via the constitutively synthesized adhesins is not sufficient for host internalization, but rather sets the stage for delivery of the Cia proteins (Konkel and Cieplak, 1992). Our model of Cia protein delivery is comparable to the canonical T3SS-dependent virulence strategy used by other intestinal pathogens (Gauthier and Finlay, 1998). Pathogens that utilize T3SS often secrete and deliver multiple proteins that contribute to the processes of colonization, invasion, modulation of immune function, cytotoxicity, and tight junction disruption (Coburn et al., 2007). C. jejuni secretes ∼18 Cia proteins, and the majority have yet to be identified and characterized. It is likely that these proteins contribute to different stages of virulence. The results of this study demonstrate for the first time that CiaC and CiaI are delivered to the cytosol of host cells, and provide a framework for the dissection of C. jejuni pathogenesis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Jeffrey Christensen for the initial development of the ACD reporter delivery assay for C. jejuni, and Derrick Samuelson for performing SEM. We also thank Dr. Bob Heinzen for kindly providing the pJB2581 plasmid used in this study and Novartis Pharma AG (Switzerland) for providing TAE226. The diagram of the flagellum was adopted from (Larson et al., 2008), with permission from the publisher, ASM Press.
Funding
This study was supported from funds provided to MEK by the School of Molecular Biosciences at Washington State University and the National Institutes of Health (R56 AI088518–01A1). Jason Neal-McKinney was supported by Award Number T32GM083864 from the National Institute of General Medical Sciences. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the NIH.
References
Ashgar, S. S., Oldfield, N. J., Wooldridge, K. G., Jones, M. A., Irving, G. J., Turner, D. P., and Ala'Aldeen, D. A. (2007). CapA, an autotransporter protein of Campylobacter jejuni, mediates association with human epithelial cells and colonization of the chicken gut. J. Bacteriol. 189, 1856–1865.
Badea, L., Beatson, S. A., Kaparakis, M., Ferrero, R. L., and Hartland, E. L. (2009). Secretion of flagellin by the LEE-encoded type III secretion system of enteropathogenic Escherichia coli. BMC Microbiol. 9, 30.
Bardill, J. P., Miller, J. L., and Vogel, J. P. (2005). IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol. Microbiol. 56, 90–103.
Black, R. E., Levine, M. M., Clements, M. L., Hughes, T. P., and Blaser, M. J. (1988). Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157, 472–479.
Buelow, D. R., Christensen, J. E., Neal-Mckinney, J. M., and Konkel, M. E. (2010). Campylobacter jejuni survival within human epithelial cells is enhanced by the secreted protein CiaI. Mol. Microbiol. 80, 1296–1312.
Carrillo, C. D., Taboada, E., Nash, J. H., Lanthier, P., Kelly, J., Lau, P. C., Verhulp, R., Mykytczuk, O., Sy, J., Findlay, W. A., Amoako, K., Gomis, S., Willson, P., Austin, J. W., Potter, A., Babiuk, L., Allan, B., and Szymanski, C. M. (2004). Genome-wide expression analyses of Campylobacter jejuni NCTC11168 reveals coordinate regulation of motility and virulence by flhA. J. Biol. Chem. 279, 20327–20338.
Christensen, J., Pacheco, S., and Konkel, M. (2009). Identification of a Campylobacter jejuni virulence protein that is secreted from the flagellum and is required for maximal invasion of host cells. Mol. Microbiol. 73, 650–662.
Coburn, B., Sekirov, I., and Finlay, B. B. (2007). Type III secretion systems and disease. Clin. Microbiol. Rev. 20, 535–549.
Desvaux, M., Hebraud, M., Henderson, I. R., and Pallen, M. J. (2006). Type III secretion: what's in a name? Trends Microbiol. 14, 157–160.
Erhardt, M., Namba, K., and Hughes, K. T. (2010). Bacterial nanomachines: the flagellum and type III injectisome. Cold Spring Harb. Perspect. Biol. 2, a000299.
Eucker, T. P., and Konkel, M. E. (2011). The cooperative action of bacterial fibronectin-binding proteins and secreted proteins promote maximal Campylobacter jejuni invasion of host cells by stimulating membrane ruffling. Cell. Microbiol. 14, 226–238.
Fernando, U., Biswas, D., Allan, B., Willson, P., and Potter, A. A. (2007). Influence of Campylobacter jejunifliA, rpoN and flgK genes on colonization of the chicken gut. Int. J. Food Microbiol. 118, 194–200.
Ferris, H. U., and Minamino, T. (2006). Flipping the switch: bringing order to flagellar assembly. Trends Microbiol. 14, 519–526.
Flanagan, R. C., Neal-Mckinney, J. M., Dhillon, A. S., Miller, W. G., and Konkel, M. E. (2009). Examination of Campylobacter jejuni putative adhesins leads to the identification of a new protein, designated FlpA, required for chicken colonization. Infect. Immun. 77, 2399–2407.
Galan, J. E., and Wolf-Watz, H. (2006). Protein delivery into eukaryotic cells by type III secretion machines. Nature 444, 567–573.
Gauthier, A., and Finlay, B. B. (1998). Protein translocation: delivering virulence into the host cell. Curr. Biol. 8, R768–R770.
Glaser, P., Danchin, A., Ladant, D., Barzu, O., and Ullmann, A. (1988). Bordetella pertussis adenylate cyclase: the gene and the protein. Tokai J. Exp. Clin. Med. 13 Suppl., 239–252.
Grant, C. C., Konkel, M. E., Cieplak, W. Jr., and Tompkins, L. S. (1993). Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect. Immun. 61, 1764–1771.
Hendrixson, D. R., and Dirita, V. J. (2003). Transcription of sigma54-dependent but not sigma28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol. Microbiol. 50, 687–702.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | CrossRef Full Text
Konkel, M. E., and Cieplak, W. Jr. (1992). Altered synthetic response of Campylobacter jejuni to cocultivation with human epithelial cells is associated with enhanced internalization. Infect. Immun. 60, 4945–4949.
Konkel, M. E., Garvis, S. G., Tipton, S. L., Anderson, D. E. Jr., and Cieplak, W. Jr. (1997). Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol. Microbiol. 24, 953–963.
Konkel, M. E., Kim, B. J., Rivera-Amill, V., and Garvis, S. G. (1999). Bacterial secreted proteins are required for the internalization of Campylobacter jejuni into cultured mammalian cells. Mol. Microbiol. 32, 691–701.
Konkel, M. E., Klena, J. D., Rivera-Amill, V., Monteville, M. R., Biswas, D., Raphael, B., and Mickelson, J. (2004). Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J. Bacteriol. 186, 3296–3303.
Konkel, M. E., Larson, C. L., and Flanagan, R. C. (2010). Campylobacter jejuni FlpA binds fibronectin and is required for maximal host cell adherence. J. Bacteriol. 192, 68–76.
Konkel, M. E., Monteville, M. R., Rivera-Amill, V., and Joens, L. A. (2001). The pathogenesis of Campylobacter jejuni-mediated enteritis. Curr. Issues Intest. Microbiol. 2, 55–71.
Krause-Gruszczynska, M., Boehm, M., Rohde, M., Tegtmeyer, N., Takahashi, S., Buday, L., Oyarzabal, O. A., and Backert, S. (2011). The signaling pathway of Campylobacter jejuni-induced Cdc42 activation: role of fibronectin, integrin beta1, tyrosine kinases and guanine exchange factor Vav2. Cell Commun. Signal. 9, 32.
Larson, C. L., Christensen, J. E., Pacheco, S. A., Minnich, S. A., and Konkel, M. (2008). “Campylobacter jejuni secretes proteins via the flagellar type III secretion system that contribute to host cell invasion and gastroenteritis,” in Campylobacter, eds I. Nachamkin, C. M. Szymanski, and M. J. Blaser (Washington, DC: ASM Press), 315–332.
Lee, S. H., and Galan, J. E. (2004). Salmonella type III secretion-associated chaperones confer secretion-pathway specificity. Mol. Microbiol. 51, 483–495.
Malik-Kale, P., Parker, C. T., and Konkel, M. E. (2008). Culture of Campylobacter jejuni with sodium deoxycholate induces virulence gene expression. J. Bacteriol. 190, 2286–2297.
Mead, P. S., Slutsker, L., Dietz, V., McCaig, L. F., Bresee, J. S., Shapiro, C., Griffin, P. M., and Tauxe, R. V. (1999). Food-related illness and death in the United States. Emerg. Infect. Dis. 5, 607–625.
Minamino, T., Gonzalez-Pedrajo, B., Yamaguchi, K., Aizawa, S. I., and Macnab, R. M. (1999). FliK, the protein responsible for flagellar hook length control in Salmonella, is exported during hook assembly. Mol. Microbiol. 34, 295–304.
Monteville, M. R., Yoon, J. E., and Konkel, M. E. (2003). Maximal adherence and invasion of INT 407 cells by Campylobacter jejuni requires the CadF outer-membrane protein and microfilament reorganization. Microbiology 149, 153–165.
Neal-Mckinney, J. M., Christensen, J. E., and Konkel, M. E. (2010). Amino-terminal residues dictate the export efficiency of the Campylobacter jejuni filament proteins via the flagellum. Mol. Microbiol. 76, 918–931.
Oelschlaeger, T. A., Guerry, P., and Kopecko, D. J. (1993). Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. U.S.A. 90, 6884–6888.
Parkhill, J., Wren, B. W., Mungall, K., Ketley, J. M., Churcher, C., Basham, D., Chillingworth, T., Davies, R. M., Feltwell, T., Holroyd, S., Jagels, K., Karlyshev, A. V., Moule, S., Pallen, M. J., Penn, C. W., Quail, M. A., Rajandream, M. A., Rutherford, K. M., van Vliet, A. H., Whitehead, S., and Barrell, B. G. (2000). The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403, 665–668.
Poly, F., Ewing, C., Goon, S., Hickey, T. E., Rockabrand, D., Majam, G., Lee, L., Phan, J., Savarino, N. J., and Guerry, P. (2007). Heterogeneity of a Campylobacter jejuni protein that is secreted through the flagellar filament. Infect. Immun. 75, 3859–3867.
Raphael, B., Monteville, M. R., Klena, J. D., Joens, L. A., and Konkel, M. E. (2005a). “Interactions of Campylobacter jejuni with non-professional phagocytic cells,” in Campylobacter: Molecular and Cellular Biology, eds J. M. Ketley and M. E. Konkel (Horizon Scientific Press: Wymondham, UK), 397–413.
Raphael, B. H., Pereira, S., Flom, G. A., Zhang, Q., Ketley, J. M., and Konkel, M. E. (2005b). The Campylobacter jejuni response regulator, CbrR, modulates sodium deoxycholate resistance and chicken colonization. J. Bacteriol. 187, 3662–3670.
Rivera-Amill, V., Kim, B. J., Seshu, J., and Konkel, M. E. (2001). Secretion of the virulence-associated Campylobacter invasion antigens from Campylobacter jejuni requires a stimulatory signal. J. Infect. Dis. 183, 1607–1616.
Ruiz-Palacios, G. M. (2007). The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin. Infect. Dis. 44, 701–703.
Sahin, O., Morishita, T. Y., and Zhang, Q. (2002). Campylobacter colonization in poultry: sources of infection and modes of transmission. Anim. Health Res. Rev. 3, 95–105.
Song, Y. C., Jin, S., Louie, H., Ng, D., Lau, R., Zhang, Y., Weerasekera, R., Al Rashid, S., Ward, L. A., Der, S. D., and Chan, V. L. (2004). FlaC, a protein of Campylobacter jejuni TGH9011 (ATCC43431) secreted through the flagellar apparatus, binds epithelial cells and influences cell invasion. Mol. Microbiol. 53, 541–553.
Sory, M. P., Boland, A., Lambermont, I., and Cornelis, G. R. (1995). Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl. Acad. Sci. U.S.A. 92, 11998–12002.
Sory, M. P., and Cornelis, G. R. (1994). Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14, 583–594.
Stintzi, A., Marlow, D., Palyada, K., Naikare, H., Panciera, R., Whitworth, L., and Clarke, C. (2005). Use of genome-wide expression profiling and mutagenesis to study the intestinal lifestyle of Campylobacter jejuni. Infect. Immun. 73, 1797–1810.
Sun, Y. H., Rolan, H. G., and Tsolis, R. M. (2007). Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J. Biol. Chem. 282, 33897–33901.
Tascon, R. I., Rodriguez-Ferri, E. F., Gutierrez-Martin, C. B., Rodriguez-Barbosa, I., Berche, P., and Vazquez-Boland, J. A. (1993). Transposon mutagenesis in Actinobacillus pleuropneumoniae with a Tn10 derivative. J. Bacteriol. 175, 5717–5722.
Yao, R., Alm, R. A., Trust, T. J., and Guerry, P. (1993). Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130, 127–130.
Yokoseki, T., Kutsukake, K., Ohnishi, K., and Iino, T. (1995). Functional analysis of the flagellar genes in the fliD operon of Salmonella typhimurium. Microbiology 141 (Pt 7), 1715–1722.
Ziprin, R. L., Young, C. R., Byrd, J. A., Stanker, L. H., Hume, M. E., Gray, S. A., Kim, B. J., and Konkel, M. E. (2001). Role of Campylobacter jejuni potential virulence genes in cecal colonization. Avian Dis. 45, 549–557.
Appendix
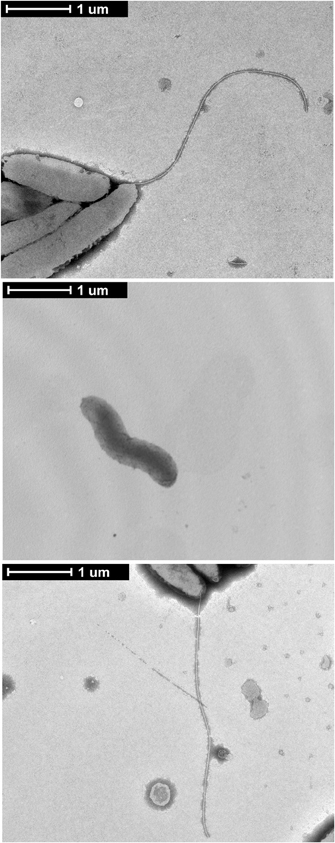
Figure A1. Complementation of the C. jejuni flgL mutant with a plasmid harboring flgL restores the flagellar filament.C. jejuni wild-type strain, the flgL mutant, and flgL mutant harboring plasmid-encoded flgL were examined by TEM. C. jejuni were spotted onto formvar coated copper grids, stained with 2% phosphotungstic acid, and visualized at 6500× magnification. The scale bar represents one micron.
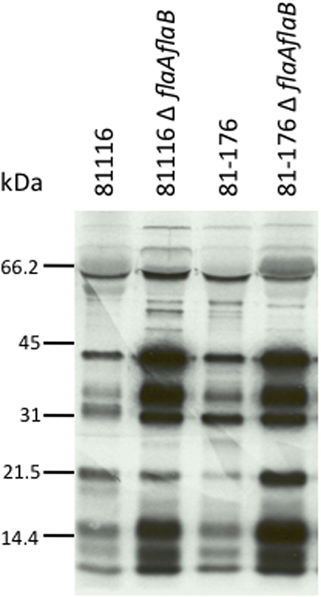
Figure A2. C. jejuni 81116 and 81–176 flaAflaB mutants secrete the Cia proteins. Secretion of the Cia proteins from 81116 and 81–176 wild-type strains and isogenic flaAflaB mutants was stimulated by incubation with 1% FBS, and the secreted proteins were analyzed by autoradiograph.
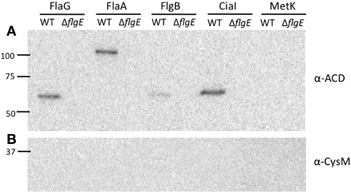
Figure A3. FlaG, FlaA, FlgB, and CiaI are secreted through the flagellar T3SS. Secretion of the FlaG-, FlaA-, FlgB-, CiaI-, and MetK-ACD fusion proteins from the C. jejuni wild-type strain and flgE mutant was examined. The C. jejuni strains were transformed with the various ACD plasmids, and Cia secretion was stimulated by the addition of FBS. After 3 h, the proteins in the supernatant fractions were precipitated and an immunoblot was performed using an anti-ACD (A) and anti-CysM (B) antibodies. MetK-ACD was included as a negative control, as it is not secreted from C. jejuni. The molecular mass of the pre-stained protein standards are listed in kDa.
Keywords: T3SS, flagellum, effector proteins, adenylate cyclase, membrane ruffling
Citation: Neal-McKinney JM and Konkel ME (2012) The Campylobacter jejuni CiaC virulence protein is secreted from the flagellum and delivered to the cytosol of host cells. Front. Cell. Inf. Microbio. 2:31. doi: 10.3389/fcimb.2012.00031
Received: 02 November 2011; Accepted: 29 February 2012;
Published online: 20 March 2012.
Edited by:
Alain Stintzi, Ottawa Institute of Systems Biology, CanadaReviewed by:
Stacey Gilk, National Institutes of Health, USASusan Logan, National Research Council of Canada, Canada
Copyright: © 2012 Neal-McKinney and Konkel. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Michael E. Konkel, School of Molecular Biosciences, Washington State University, 1770 NE Stadium Road, Biotechnology Life Sciences Building, Room 302c, Pullman, WA 99164–7520, USA. e-mail:a29ua2VsQHZldG1lZC53c3UuZWR1