- 1Department of Mathematics, College of Sciences, University of Sharjah, Sharjah, United Arab Emirates
- 2Department of Mathematics, Loyola College, Chennai, India
- 3Department of Mathematics, Loyola College, University of Madras, Chennai, India
- 4Department of Mathematics, Women’s Christian College, Chennai, India
Covalent organic frameworks are a novel class of porous polymers, notable for their crystalline structure, intricate frameworks, defined pore sizes, and capacity for structural design, synthetic control, and functional customization. This paper provides a comprehensive analysis of graph entropies and hybrid topological descriptors, derived from geometric, harmonic, and Zagreb indices. These descriptors are applied to study two variations of Marta covalent organic frameworks based on contorted hexabenzocoronenes. We also conduct a comparative analysis using scaled entropies, offering refined tools for assessing the intrinsic topologies of these networks. Additionally, these hybrid descriptors are used to develop statistical models for predicting graph energy in higher-dimensional Marta-COFs.
1 Introduction
Reticular chemistry connects organic building blocks through strong covalent bonds, which have the capacity to regulate the pore sizes of frameworks by preserving their fundamental topology and varying the lengths of organic linkers, thus paving the way for the emergence of multiple classes of crystalline porous materials (Yaghi, 2016; Yaghi, 2019). Reticulated materials can be classified as metal organic frameworks (MOFs), created by the combination of organic linkers and metal atoms, and covalent organic frameworks (COFs), composed only of organic linkers (Gropp et al., 2020). COFs have drawn particular attention from researchers due to their regular pattern of organic building blocks, which allows for the creation of crystalline structures with extensive surface areas, stability, and customizable pores (El-Kaderi et al., 2007). COFs possess potential applicatiions in separation (Fan et al., 2023), luminescence (Haug et al., 2020), biomedicine (Shi et al., 2023), energy conversion (Sun et al., 2023), environmental remediation (Hou et al., 2023), seawater desalination (Jrad et al., 2023), photocatalysis (Gong et al., 2023), and electrocatalysis (Zhang et al., 2021). The COFs have predetermined structures based on their building blocks, allowing for highly ordered geometries (Huang et al., 2016; Chen et al., 2014). Their covalently crystalline structure gives them advantages over other porous materials such as molecular sieves, MOFs and zeolites (Yang et al., 2019; Jiao et al., 2019; Algieri and Drioli, 2021).
Covalent bonds within COFs can arise from a diverse range of functional groups. The methods for forming these bonds can be broadly classified into several categories, including boroxine-linked, boronate ester-linked, triazine-linked, imine-linked, hydrazone-linked,
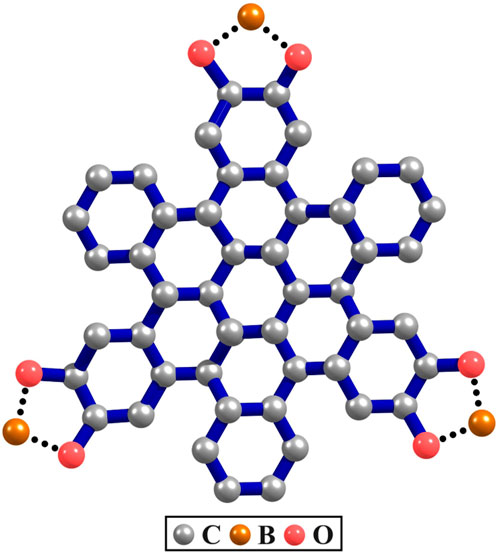
The COFs considered in this study are made up of polycyclic aromatic hydrocarbons (PAHs) with contorted hexabenzocoronene (c-HBC) serving as the core component for constructing the two Marta-COFs. The c-HBC adopts a doubly-concave structure, which sets it apart from the planar hexabenzocoronene. Its formation occurs when the aromatic core of HBC is distorted away from planarity due to steric congestion in its proximal carbon atoms. Structurally, c-HBC is the building block composed of six benzene rings attached to the periphery of a coronene molecule (Sepúlveda et al., 2017; Kim et al., 2022). The c-HBC unit, as shown in Figure 1, when copolymerized with pyrene-2,7-diboronic acid (PDBA), results in the formation of a highly crystalline two-dimensional COF known as Marta-COF-1 (Abadía et al., 2019). Notably, the c-HBC nodes and the PDBA display substantial
The two variations of highly crystalline Marta COFs can be evaluated through quantitative parameters called the topological descriptors that convert various structural attributes of the frameworks into measurable quantities. These quantifying functionals are essential for representing the molecular frameworks and are useful for QSPR and QSAR analyses (Jafari et al., 2024; Patil et al., 2024; Nath et al., 2023; Hayat et al., 2023). The incorporation of topological descriptors and graph-derived metrics in QSAR/QSPR studies has been extensively used in the domain of computational and material sciences. This amalgamation has provided robust tools for predicting structural behaviors and designing new materials with desired properties or functionalities. These approaches enable researchers to explore numerous applications, including drug discovery, material optimization, and the development of materials that can be tailored for specific applications or objectives (Balasubramanian and Saxena, 2021; Balasubramanian, 2022; Arockiaraj et al., 2023a; Hasani and Ghods, 2024; Abubakar et al., 2024; Meharban et al., 2024; Ullah et al., 2024; Shanmukha et al., 2023a; Gnanaraja et al., 2023; Zhang et al., 2023; Hassan et al., 2024).
The graph entropy measure enables the evaluation of the inherent complexity and diversity of COFs. This measure provides valuable insights into the arrangement and functioning of COF structures by associating fundamental graph components with appropriate weights (Junias and Clement, 2023; Arockiaraj et al., 2024a; Chu et al., 2023; Roy et al., 2023; Zhao et al., 2023; Lal et al., 2024). The applications of graph entropy continue to expand its relevance and significance across diverse domains due to its adaptable nature that surpasses disciplinary boundaries and facilitates the analysis of complex systems (Arockiaraj et al., 2023b; Junias et al., 2024; Huang et al., 2024). In recent years, there has been significant interest in the computation of topological expressions and entropies for COFs (Yang et al., 2024; Arockiaraj et al., 2023c; Augustine and Roy, 2022; Shanmukha et al., 2023b; Arockiaraj et al., 2024b). In this study, we provide hybrid topological characterizations and entropies for two variations of Marta COFs and conduct a comparative analysis of the bond-wise entropy of these frameworks. Furthermore, we construct regression models to predict the graph energy of these frameworks based on the calculated topological indices.
2 Computational methods
We consider the Marta-COF as a molecular graph where the sets
We now define the additive and multiplicative versions of topological descriptors related to the degree and degree-sum parameters of Marta-COF, involving the index function
When the index function
The index functions
These index functions, combined with the edge classes based on
In employing Shannon’s entropy method, defining a structural information function on the bonds of Marta-COFs is necessary. In our study, we adopt the index function
In a series of papers (Arockiaraj et al., 2023c; Mushtaq et al., 2022; Raza et al., 2023), the significance and implications of substituting the multiplicative factor have been comprehensively explored concerning the scalar multiplicative index. This leads to the formulation of the modified version of entropy as presented below.
3 Results and discussion
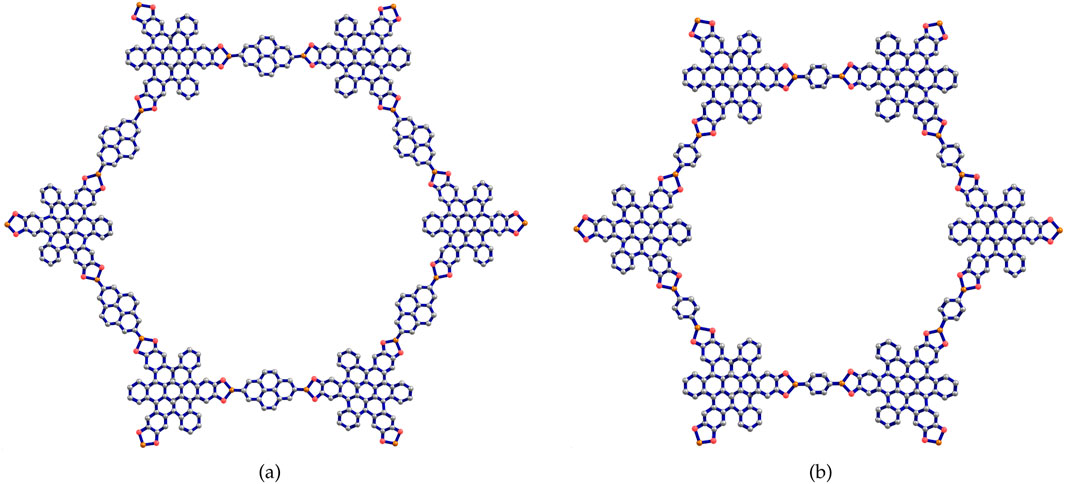
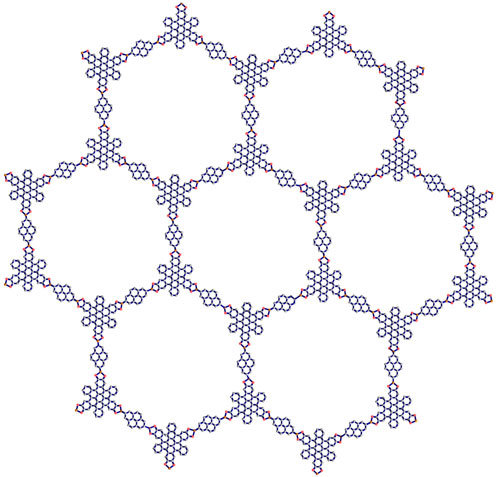
In this section, the two types of Marta-COFs are analyzed, and their structural properties are compared using topological descriptors and entropies. We consider the geometrical configuration of bi-trapezium (BT) shaped arrangements of Marta-COFs, which yield diverse configurations of Marta-COF layers. These Marta-COF structures are constructed using the unit cells as shown in Figure 2, which are the fundamental building blocks.
The Marta-COF-BT
Furthermore, the covalent organic framework Marta-COF-1-BT
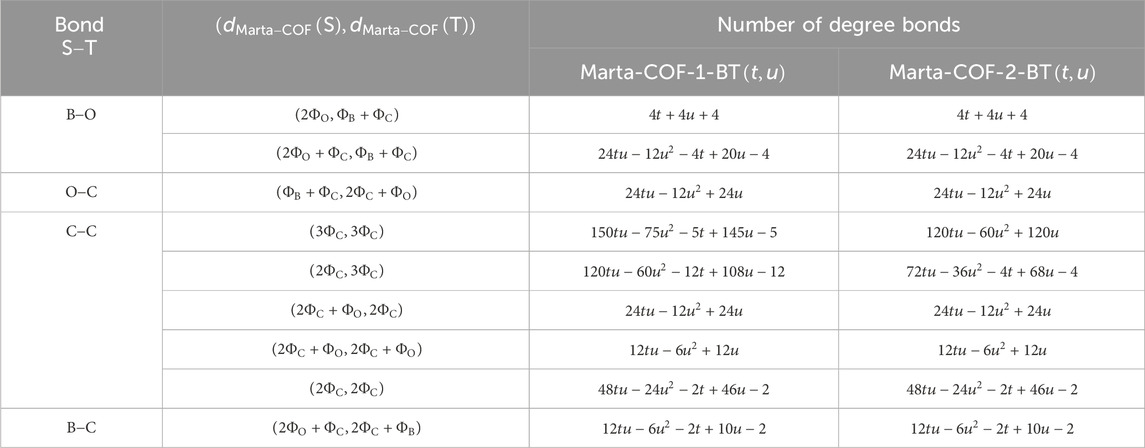
The degree based descriptors for Marta-COF-1-BT
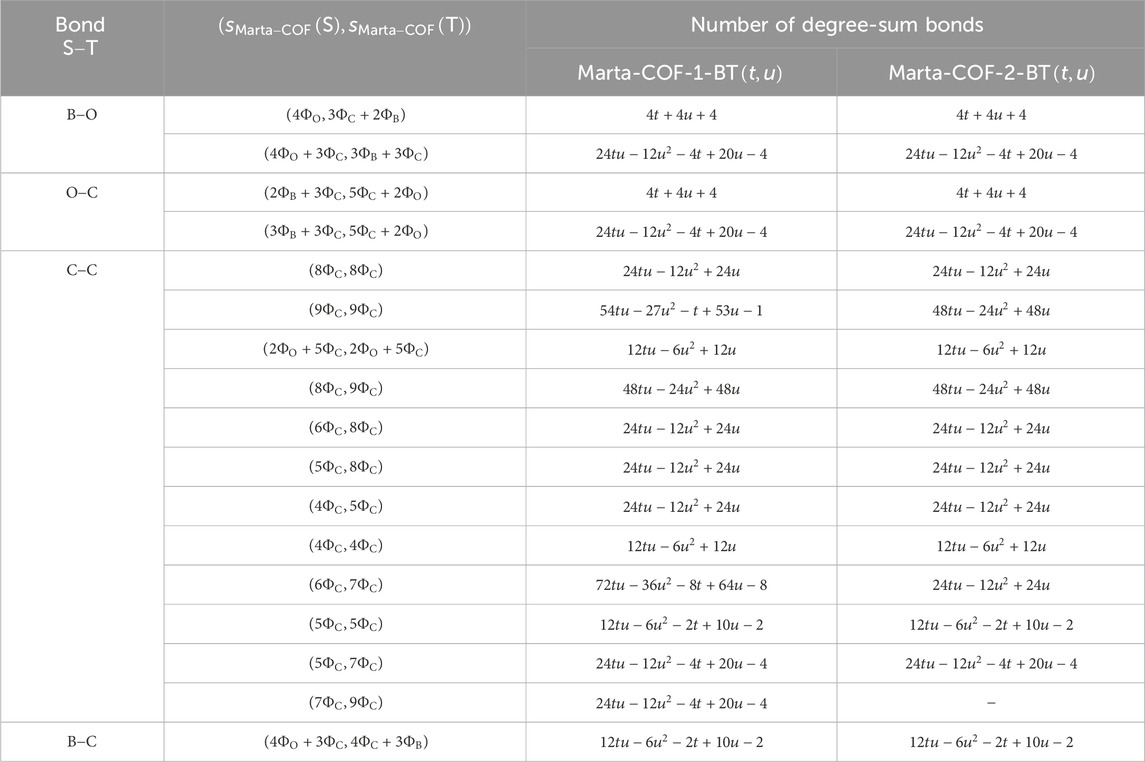
In computing the degree-sum descriptors of Marta-COF-1-BT
The resulting outcomes are given in the form,
Result 1. The quantitative expressions for Marta-COF-1-BT
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
The equations below generate the topological descriptors of Marta-COF-2-BT
Result 2. The quantitative expressions for Marta-COF-2-BT
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
To determine entropy values for the two variations of Marta-COFs, we use the quantitative expressions from the above derived results with the aid of scalar multiplicative self-powered descriptors. Let
1.
2.
Similarly for Marta-COF-2-BT
1.
2.
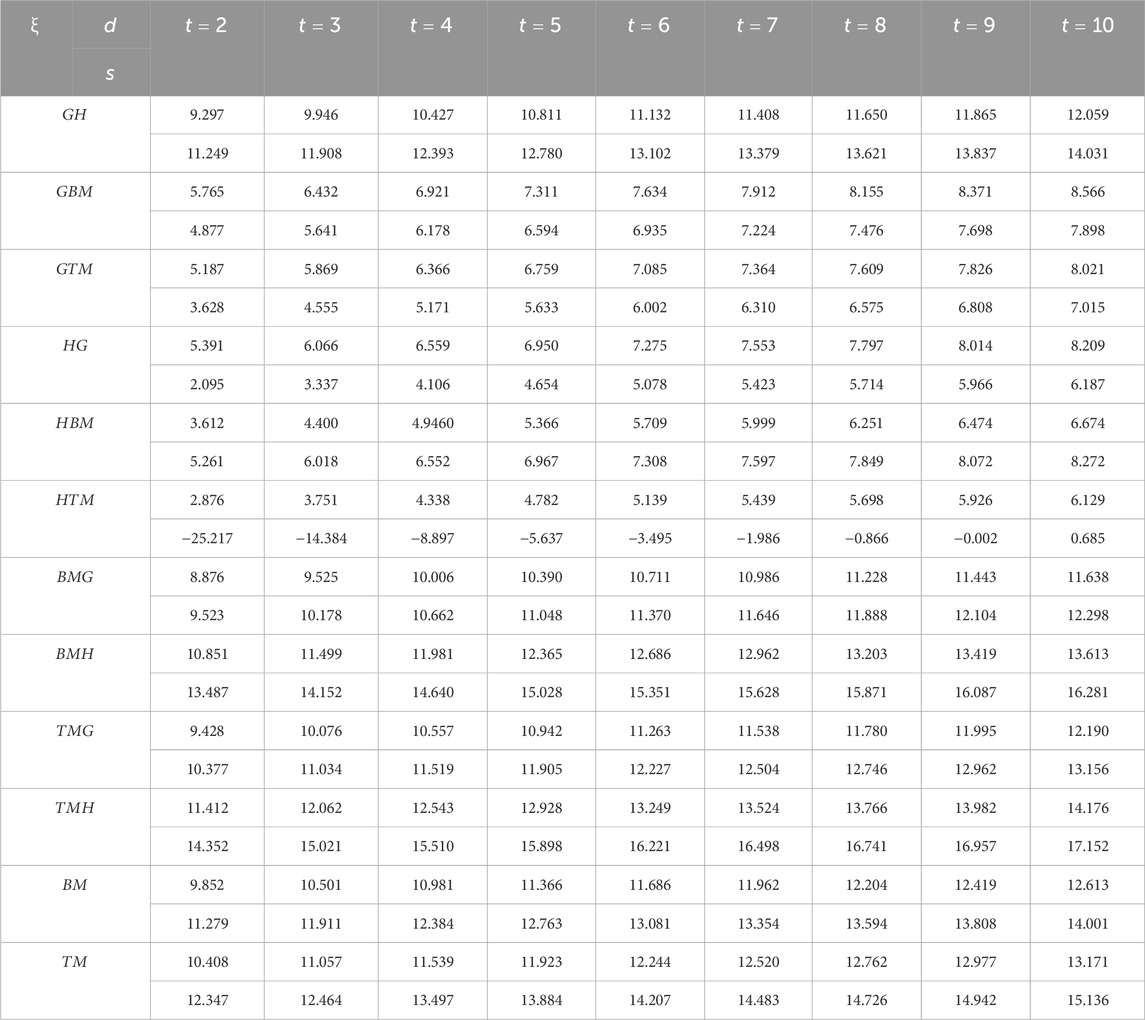
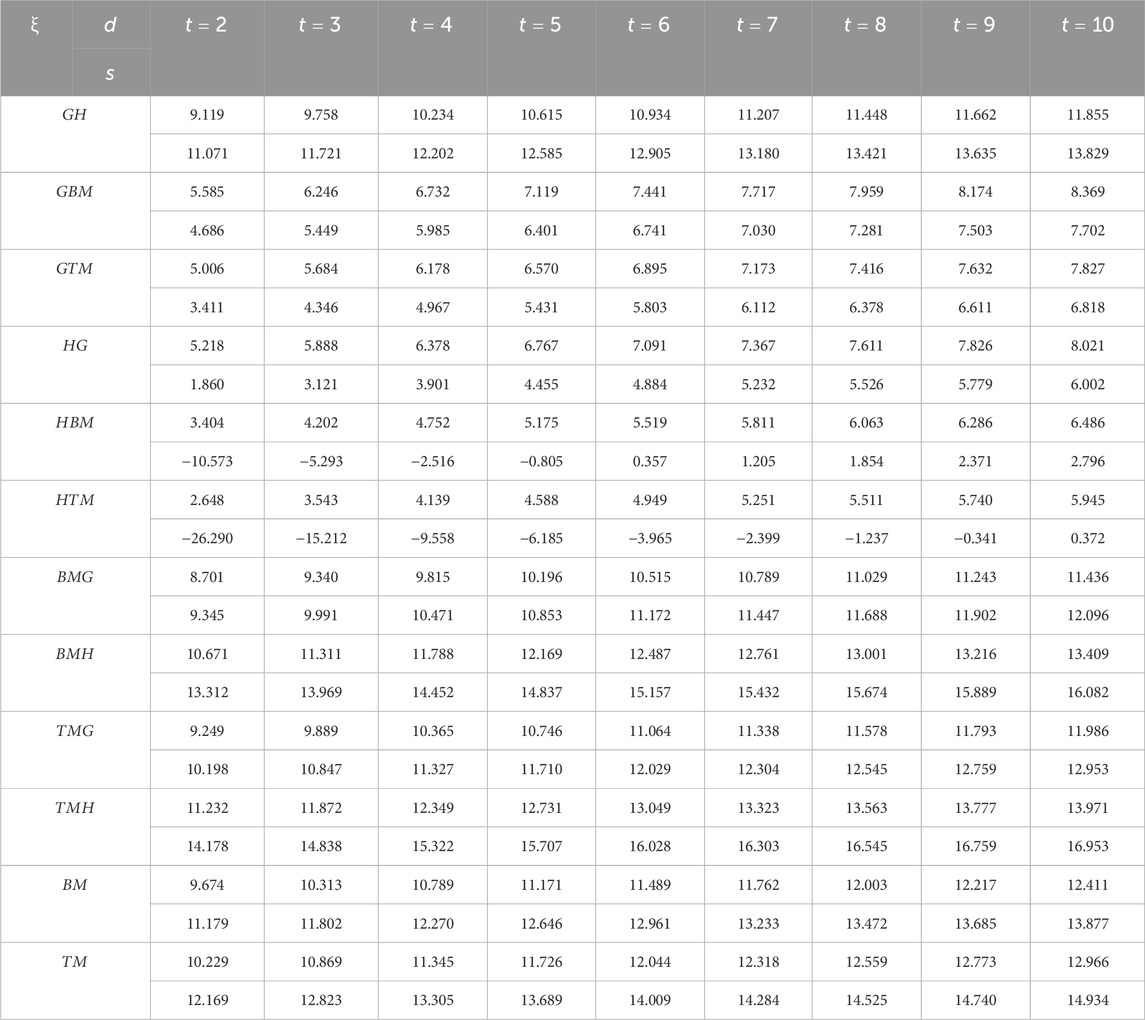
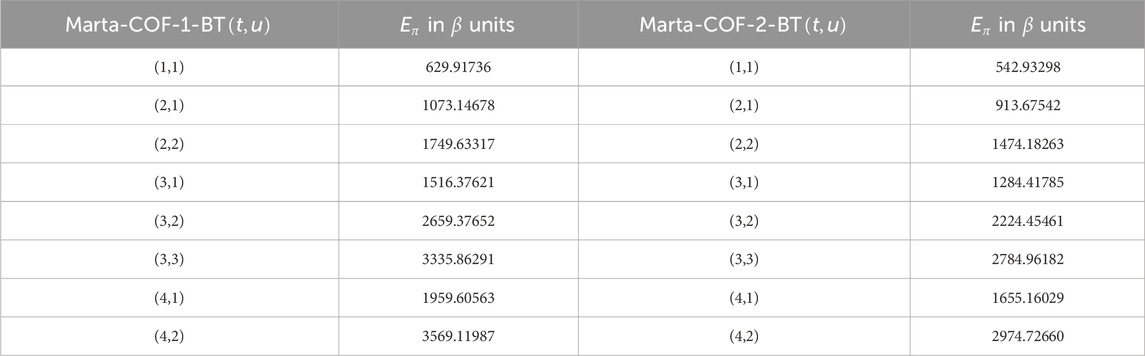
We are now ready to calculate the entropies of Marta-COFs using the provided mathematical expressions. Due to the complexity of these expressions, we determine the numerical values of Marta-COFs where the dimensions of the bi-trapezium configuration are set by BT
The entropies calculated for Marta-COF-1 and Marta-COF-2 primarily depend on their total number of bonds, which is unequal due to the fixed dimensions of these COFs. To compare their entropies effectively and investigate structural characteristics like bond energy and stability, we employ a scaling process. We perform scaling for the hexagonal and parallelogram configurations of Marta-COFs between two variations by calculating the ratio of total degree entropies to the total number of bonds. Table 5 clearly shows that the bond-wise entropies of the Marta-COF-2 framework are consistently higher than those of Marta-COF-1 across all hexagonal and parallelogram configurations, as depicted in Figure 5. As a result, the Marta-COF-2 frameworks exhibit a higher degree of information disorder than the Marta-COF-1 frameworks.

Table 5. Scaled entropy values for parallelogram and hexagonal configurations between Marta-COF-1 and Marta-COF-2.
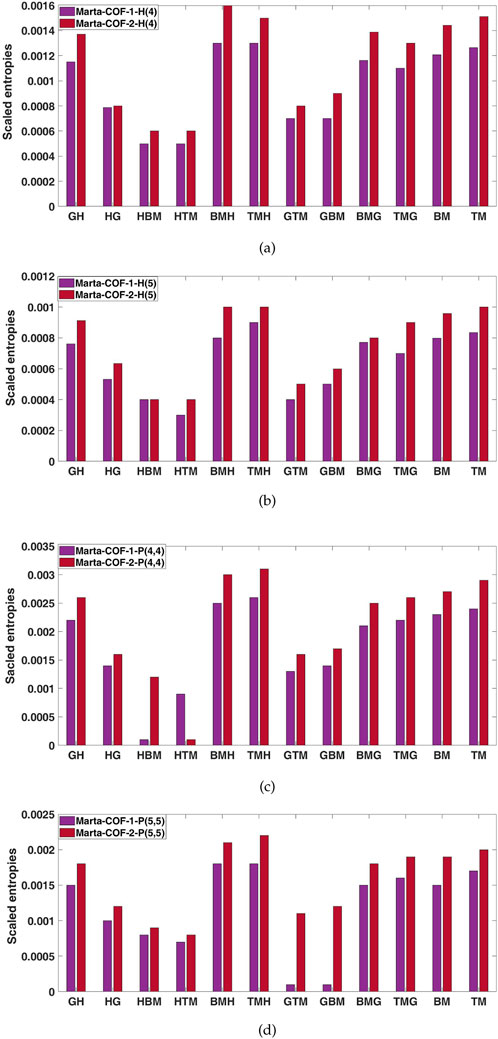
Figure 5. Bar diagrams of scaled entropies (A, B) Marta-COF-1-H
4 Prediction of graph energy
A prominent application of spectral graph theory is its ability to relate graph spectrum to the molecular orbital energy levels of
Evaluating the graph energy of Marta covalent organic frameworks in higher-order dimensions
We conducted a correlation analysis to explore the relationship between topological descriptors and graph energy in two Marta-COFs. Next, we applied simple linear regression to examine the relationship between these two quantitative variables, providing a clear representation of the link between the predictor and the dependent variable. The proposed equation relating graph energy to topological descriptors is presented below.
where
Based on the correlation analysis, we identified the optimal predictive models for Marta-COF-1 and Marta-COF-2 based on degree descriptors. The geometric-bi-Zagreb index yielded a perfect correlation for both frameworks, with the lowest standard error (Se) and the highest
In the same way, the linear regression equations derived from degree-sum descriptors particularly the bi-Zagreb harmonic index for Marta-COF-1-BT
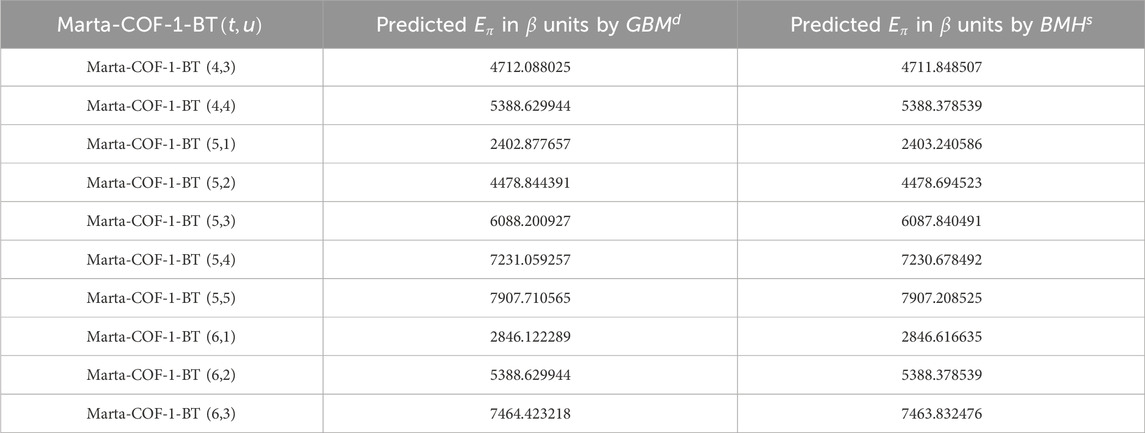
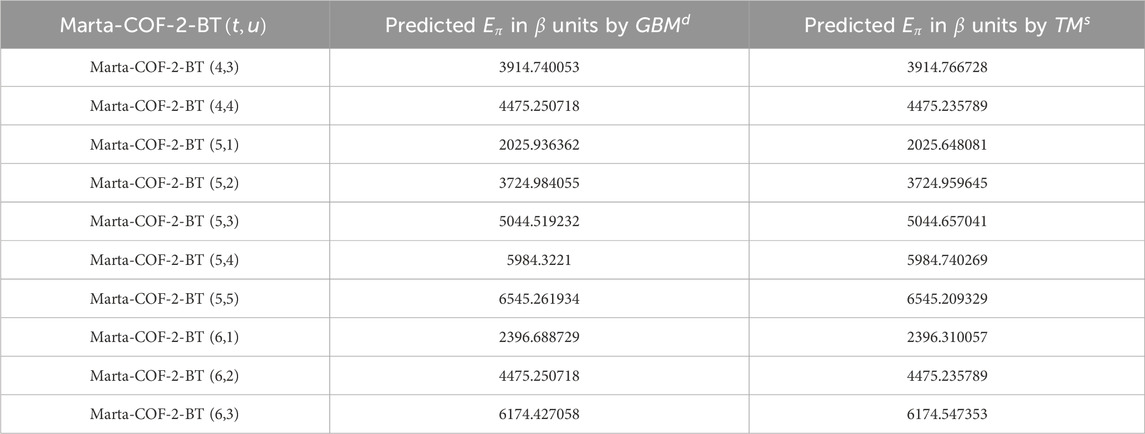
Using the regression equations mentioned above, we estimated the graph energy of Marta-COF-1 and Marta-COF-2 based on both degree and degree-sum descriptors in higher dimensions. The resulting predictions are presented in Tables 7, 8 and visually depicted in Figure 6. The predicted energy of Marta-COFs based on degree descriptors shows a perfect correlation compared to degree-sum descriptors, making these predictive models useful for estimating graph energy values in higher-dimensional Marta-COFs.
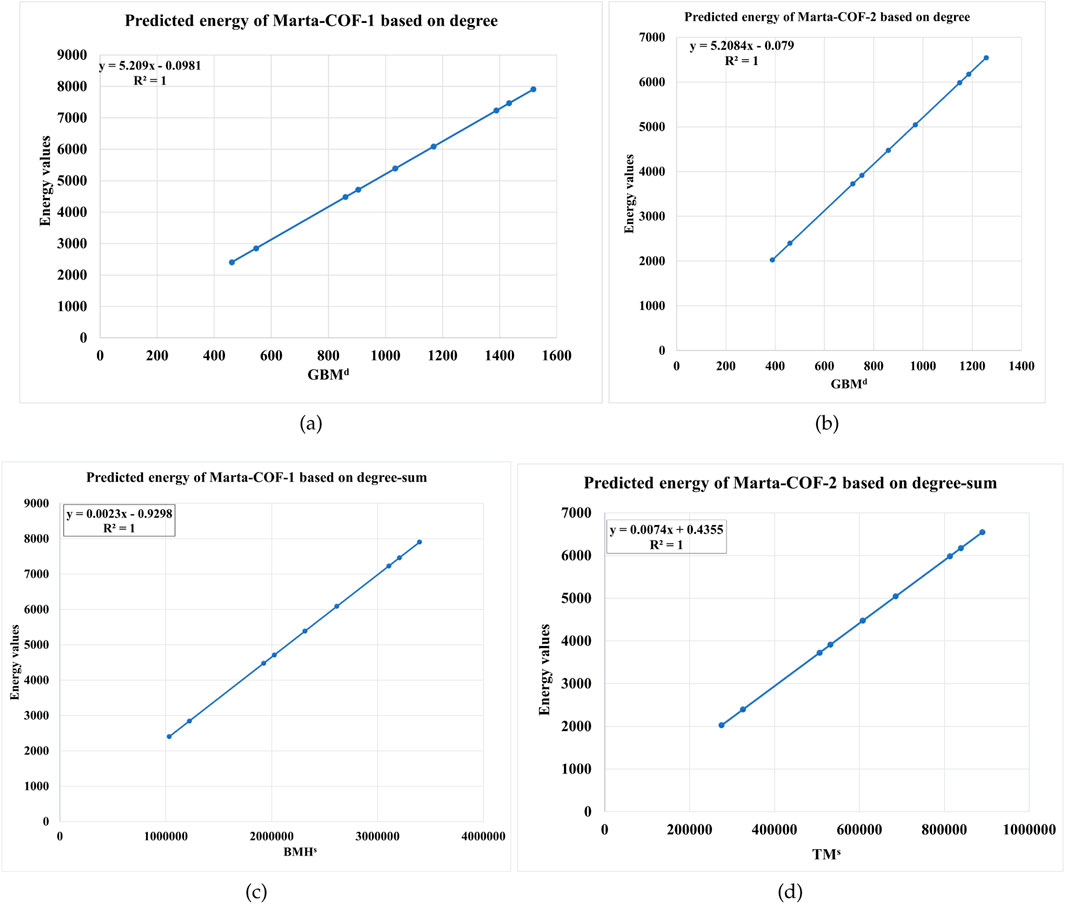
Figure 6. Comparison of predicted graph energy based on degree/degree-sum (A, B) Marta-COF-1-BT
5 Conclusion
The mathematical expressions for topological descriptors have been formulated, and entropy quantities for two variations of Marta-COFs have been derived. A refined edge partition technique has been employed, involving the use of innovative hybrid descriptors that combine geometric, harmonic, and Zagreb descriptors. Furthermore, a comparative analysis between Marta-COF-1 and Marta-COF-2 has been conducted, revealing that higher entropy values were consistently displayed by Marta-COF-2 in both hexagonal and parallelogram frameworks compared to Marta-COF-1. Optimal linear regression models to predict graph energy across different dimensional Marta frameworks have also been developed, significantly reducing computational complexity. These findings and techniques can be applied to link properties such as mechanical stability, solubility, hardness, and electrophilicity, provided that experimental data are available.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZR: Formal Analysis, Funding acquisition, Methodology, Validation, Writing–review and editing. MA: Conceptualization, Investigation, Methodology, Supervision, Writing–review and editing. AM: Conceptualization, Methodology, Validation, Visualization, Writing–original draft. AS: Conceptualization, Formal Analysis, Methodology, Validation, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. ZR is supported by the University of Sharjah Research Grant No. 23021440148 and MASEP Research Group.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abadía, M. M., Stoppiello, C. T., Strutynski, K., Berlanga, B. L., Gastaldo, C. M., Saeki, A., et al. (2019). A wavy two-dimensional covalent organic framework from core-twisted polycyclic aromatic hydrocarbons. J. Am. Chem. Soc. 141 (36), 14403–14410. doi:10.1021/jacs.9b07383
Abadía, M. M., Strutynski, K., Stoppiello, C. T., Berlanga, B. L., Gastaldo, C. M., Khlobystov, A. N., et al. (2021). Understanding charge transport in wavy 2D covalent organic frameworks. Nanoscale 13 (14), 6829–6833. doi:10.1039/d0nr08962a
Abubakar, M. S., Aremu, K. O., Aphane, M., and Amusa, L. B. (2024). A QSPR analysis of physical properties of antituberculosis drugs using neighbourhood degree-based topological indices and support vector regression. Heliyon 10, e28260. doi:10.1016/j.heliyon.2024.e28260
Algieri, C., and Drioli, E. (2021). Zeolite membranes: synthesis and applications. Sep. Purif. Technol. 278, 119295. doi:10.1016/j.seppur.2021.119295
Arockiaraj, M., Fiona, J. C., Abraham, J., Klavžar, S., and Balasubramanian, K. (2024a). Guanidinium and hydrogen carbonate rosette layers: distance and degree topological indices, szeged-type indices, entropies, and NMR spectral patterns. Heliyon 10, e24814. doi:10.1016/j.heliyon.2024.e24814
Arockiaraj, M., Fiona, J. C., and Shalini, A. J. (2024c). Comparative study of entropies in silicate and oxide frameworks. Silicon 16, 3205–3216. doi:10.1007/s12633-024-02892-2
Arockiaraj, M., Greeni, A. B., and Kalaam, A. R. A. (2023a). Linear versus cubic regression models for analyzing generalized reverse degree based topological indices of certain latest corona treatment drug molecules. Int. J. Quantum Chem. 123 (16), e27136. doi:10.1002/qua.27136
Arockiaraj, M., Jency, J., Maaran, A., Abraham, J., and Balasubramanian, K. (2023d). Refined degree bond partitions, topological indices, graph entropies and machine-generated boron NMR spectral patterns of borophene nanoribbons. J. Mol. Struct. 1295 (11), 136524. doi:10.1016/j.molstruc.2023.136524
Arockiaraj, M., Jency, J., Mushtaq, S., Shalini, A. J., and Balasubramanian, K. (2023c). Covalent organic frameworks: topological characterizations, spectral patterns and graph entropies. J. Math. Chem. 61, 1633–1664. doi:10.1007/s10910-023-01477-5
Arockiaraj, M., Paul, D., Clement, J., Tigga, S., Jacob, K., and Balasubramanian, K. (2023e). Novel molecular hybrid geometric-harmonic-Zagreb degree based descriptors and their efficacy in QSPR studies of polycyclic aromatic hydrocarbons. Sar. QSAR Environ. Res. 34 (7), 569–589. doi:10.1080/1062936x.2023.2239149
Arockiaraj, M., Paul, D., Ghani, M. U., Tigga, S., and Chu, Y. M. (2023b). Entropy structural characterization of zeolites BCT and DFT with bond-wise scaled comparison. Sci. Rep. 13, 10874. doi:10.1038/s41598-023-37931-2
Arockiaraj, M., Paul, D., Klavžar, S., Clement, J., Tigga, S., and Balasubramanian, K. (2022). Relativistic distance based and bond additive topological descriptors of zeolite RHO materials. J. Mol. Struct. 1250 (2), 131798. doi:10.1016/j.molstruc.2021.131798
Arockiaraj, M., Raza, Z., Maaran, A., Abraham, J., and Balasubramanian, K. (2024b). Comparative analysis of scaled entropies and topological properties of triphenylene-based metal and covalent organic frameworks. Chem. Pap. 78, 4095–4118. doi:10.1007/s11696-023-03295-0
Augustine, T., and Roy, S. (2022). Topological study on triazine-based covalent-organic frameworks. Symmetry 14 (8), 1590. doi:10.3390/sym14081590
Balasubramanian, K. (2021). “Relativistic quantum chemical and molecular dynamics techniques for medicinal chemistry of bioinorganic compounds,” in Biophysical and computational tools in drug discovery, topics in medicinal chemistry. Editor A. K. Saxena (Cham: Springer), 37, 133–193. doi:10.1007/7355_2020_109
Balasubramanian, K. (2022). Computational and artificial intelligence techniques for drug discovery and administration, Compr. Adv. Pharmacol. 2, 553–616. doi:10.1016/B978-0-12-820472-6.00015-3
Chen, X., Huang, N., Gao, J., Xu, H., Xu, F., and Jiang, D. (2014). Towards covalent organic frameworks with predesignable and aligned open docking sites. Chem. Commun. 50 (46), 6161–6163. doi:10.1039/c4cc01825g
Chu, Z.-Q., Siddiqui, M. K., Manzoor, S., Kirmani, S. A. K., Hanif, M. F., and Muhammad, M. H. (2023). On rational curve fitting between topological indices and entropy measures for graphite carbon nitride. Polycycl. Aromat. Compd. 43 (3), 2553–2570. doi:10.1080/10406638.2022.2048034
Cot̂é, A. P., Benin, A. I., Ockwing, N. W., O’keeffe, M., Matzger, A. J., and Yaghi, O. M. (2005). Porous, crystalline, covalent organic frameworks. Science 310 (5751), 1166–1170. doi:10.1126/science.1120411
El-Kaderi, H. M., Hunt, J. R., Cortés, J. L. M., Cot̂é, A. P., Taylor, R. E., O’keeffe, M., et al. (2007). Designed synthesis of 3D covalent organic frameworks. Science 316 (5822), 268–272. doi:10.1126/science.1139915
Fan, H., Wang, H., Peng, M., Meng, H., Mundstock, A., Knebel, A., et al. (2023). Pore-in-pore engineering in a covalent organic framework membrane for gas separation. ACS Nano 17 (8), 7584–7594. doi:10.1021/acsnano.2c12774
Geng, K., He, T., Liu, R., Dalapati, S., Tan, K. T., Li, Z., et al. (2020). Covalent organic frameworks: design, synthesis, and functions. Chem. Rev. 120 (16), 8814–8933. doi:10.1021/acs.chemrev.9b00550
Gnanaraja, L. R. M., Ganesana, D., and Siddiqui, M. K. (2023). Topological indices and QSPR analysis of NSAID drugs. Polycycl. Aromat. Compd. 43 (10), 9479–9495. doi:10.1080/10406638.2022.2164315
Gong, Y.-N., Guan, X., and Jiang, H.-L. (2023). Covalent organic frameworks for photocatalysis: synthesis, structural features, fundamentals and performance. Coord. Chem. Rev. 475, 214889. doi:10.1016/j.ccr.2022.214889
Graovac, A., Gutman, I., and Trianjistić, N. (1975). On the coulson integral formula for total π-electron energy. Chem. Phys. Lett. 35 (4), 555–557. doi:10.1016/0009-2614(75)85666-1
Gropp, C., Canossa, S., Wuttke, S., Gándara, F., Li, Q., Gagliardi, L., et al. (2020). Standard practices of reticular chemistry. ACS Cent. Sci. 6 (8), 1255–1273. doi:10.1021/acscentsci.0c00592
Gutman, I., and Furtula, B. (2017). The total π-electron energy saga. Croat. Chem. Acta 90, 359–368. doi:10.5562/cca3189
Hakeem, A., Ullah, A., and Zaman, S. (2023). Computation of some important degree-based topological indices for γ - graphyne and Zigzag graphyne nanoribbon. Mol. Phys. 121 (14), e2211403. doi:10.1080/00268976.2023.2211403
Hasani, M., and Ghods, M. (2024). Topological indices and QSPR analysis of some chemical structures applied for the treatment of heart patients. Int. J. Quantum Chem. 124 (1), e27234. doi:10.1002/qua.27234
Hassan, M. M., Waqar, A., Ali, H., and Ali, P. (2024). Computation of connection-based Zagreb indices in chain graphs and triangular sheets. J. Coord. Chem. 77, 896–919. doi:10.1080/00958972.2024.2305819
Haug, W. K., Moscarello, E. M., Wolfson, E. R., and McGrier, P. L. (2020). The luminescent and photophysical properties of covalent organic frameworks. Chem. Soc. Rev. 49 (3), 839–864. doi:10.1039/c9cs00807a
Hayat, S., Suhaili, N., and Jamil, H. (2023). Statistical significance of valency-based topological descriptors for correlating thermodynamic properties of benzenoid hydrocarbons with applications. Comput. Theor. Chem. 1227, 114259. doi:10.1016/j.comptc.2023.114259
Hou, K., Gu, H., Yang, Y., Lam, S. S., Li, H., Sonne, C., et al. (2023). Recent progress in advanced covalent organic framework composites for environmental remediation. Adv. Compos. Hybrid. Mater. 6, 199. doi:10.1007/s42114-023-00776-4
Huang, N., Wang, P., and Jiang, D. (2016). Covalent organic frameworks: a materials platform for structural and functional designs. Nat. Rev. Mater 1, 16068. doi:10.1038/natrevmats.2016.68
Huang, R., Hanif, M. F., Siddiqui, M. K., and Hanif, M. F. (2024). On analysis of entropy measure via logarithmic regression model and Pearson correlation for Tri-s-triazine. Comput. Mater. Sci. 240, 112994. doi:10.1016/j.commatsci.2024.112994
Jafari, M., Isfahani, T. M., Shafiei, F., and Senejani, M. A. (2024). QSPR study to predict some of quantum chemical properties of anticancer imidazo[4,5-b]pyridine derivatives using genetic algorithm multiple linear regression and molecular descriptors. Int. J. Quantum Chem. 124 (1), e27259. doi:10.1002/qua.27259
Jiao, L., Seow, J. Y. R., Skinner, W. S., Wang, Z. U., and Jiang, H.-L. (2019). Metal-organic frameworks: structures and functional applications. Mater. Today 27, 43–68. doi:10.1016/j.mattod.2018.10.038
Jrad, A., Olson, M. A., and Trabolis, A. (2023). Molecular design of covalent organic frameworks for seawater desalination: a state-of-the-art review. Chem 9 (6), 1413–1451. doi:10.1016/j.chempr.2023.04.012
Junias, J. S., and Clement, J. (2023). Predictive analytics of conductance and HOMO-LUMO gaps with topological descriptors of porphyrin nanosheets. Phys. Scr. 99 (1), 015208. doi:10.1088/1402-4896/ad0c1b
Junias, J. S., Clement, J., Rahul, M. P., and Arockiaraj, M. (2024). Two-dimensional phthalocyanine frameworks: topological descriptors, predictive models for physical properties and comparative analysis of entropies with different computational methods. Comput. Mater. Sci. 235, 112844. doi:10.1016/j.commatsci.2024.112844
Kalaam, A. R. A., Greeni, A. B., and Arockiaraj, M. (2024). Modified reverse degree descriptors for combined topological and entropy characterizations of 2D metal organic frameworks: applications in graph energy prediction. Front. Chem. 12, 1470231. doi:10.3389/fchem.2024.1470231
Kim, Y. J., Kang, M., Kim, Y. H., Suh, E.-K., Yang, M., Cho, S. Y., et al. (2022). Contorted hexabenzocoronene derivatives as a universal organic precursor for dimension-customized carbonization. Carbon 200, 21–27. doi:10.1016/j.carbon.2022.08.049
Kuhn, P., Antonietti, M., and Thomas, A. (2008). Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 47 (18), 3450–3453. doi:10.1002/anie.200705710
Lal, S., Bhat, V. K., and Sharma, S. (2024). Topological indices and graph entropies for carbon nanotube Y-junctions. J. Math. Chem. 62, 73–108. doi:10.1007/s10910-023-01520-5
Li, H., Li, H., Dai, Q., Li, H., and Brédas, J.-L. (2018). Hydrolytic stability of boronate ester-linked covalent organic frameworks. Adv. Theory Simul. 1 (2), 1700015. doi:10.1002/adts.201700015
Meharban, S., Ullah, A., Zaman, S., Hamraz, A., and Razaq, A. (2024). Molecular structural modeling and physical characteristics of anti-breast cancer drugs via some novel topological descriptors and regression models. Curr. Res. Struct. Biol. 7, 100134. doi:10.1016/j.crstbi.2024.100134
Mondal, S., De, N., and Pal, A. (2022). Topological indices of some chemical structures applied for the treatment of COVID-19 patients. Polycycl. Aromat. Compd. 42 (4), 1220–1234. doi:10.1080/10406638.2020.1770306
Mushtaq, S., Arockiaraj, M., Fiona, J. C., Jency, J., and Balasubramanian, K. (2022). Topological properties, entropies, stabilities and spectra of armchair versus zigzag coronene-like nanoribbons. Mol. Phys. 120 (17), e2108518. doi:10.1080/00268976.2022.2108518
Nath, A., Ojha, P. K., and Roy, K. (2023). QSAR assessment of aquatic toxicity potential of diverse agrochemicals. Sar. QSAR Environ. Res. 34 (11), 923–942. doi:10.1080/1062936x.2023.2278074
Patil, V. M., Gupta, S. P., Masand, N., and Balasubramanian, K. (2024). Experimental and computational models to understand protein-ligand, metal-ligand and metal-DNA interactions pertinent to targeted cancer and other therapies. Eur. J. Med. Chem. Rep. 10, 100133. doi:10.1016/j.ejmcr.2024.100133
Paul, D., Arockiaraj, M., Jacob, K., and Clement, J. (2023). Multiplicative versus scalar multiplicative degree based descriptors in QSAR/QSPR studies and their comparative analysis in entropy measures. Eur. Phys. J. Plus 138, 323. doi:10.1140/epjp/s13360-023-03920-7
Ramane, H. S., Pise, K. S., Jummannaver, R. B., and Patil, D. D. (2021). Applications of neighbors degree sum of a vertex on zagreb indices, MATCH Commun. Math. Comput. Chem. 85, 329–348.
Raza, Z., Arockiaraj, M., Maaran, A., Kavitha, S. R. J., and Balasubramanian, K. (2023). Topological entropy characterization, NMR and ESR spectral patterns of coronene-based transition metal organic frameworks. ACS Omega 8 (14), 13371–13383. doi:10.1021/acsomega.3c00825
Roy, S. G. S., Balasubramanian, K., and Prabhu, S. (2023). Topological indices and entropies of triangular and rhomboidal tessellations of kekulenes with applications to NMR and ESR spectroscopies. J. Math. Chem. 61, 1477–1490. doi:10.1007/s10910-023-01465-9
Sepúlveda, D., Guan, Y., Rangel, U., and Wheeler, S. E. (2017). Stacked homodimers of substituted contorted hexabenzocoronenes and their complexes with C60 fullerene. Org. Biomol. Chem. 15 (28), 6042–6049. doi:10.1039/c7ob01333g
Shanmukha, M. C., Ismail, R., Gowtham, K. J., Usha, A., Azeem, M., and Sabri, E. H. A. A. (2023b). Chemical applicability and computation of K-Banhatti indices for benzenoid hydrocarbons and triazine-based covalent organic frameworks. Sci. Rep. 13, 17743. doi:10.1038/s41598-023-45061-y
Shanmukha, M. C., Usha, A., Basavarajappa, N. S., and Shilpa, K. C. (2023a). Comparative study of multilayered graphene using numerical descriptors through M-polynomial. Phys. Scr. 98 (7), 075205. doi:10.1088/1402-4896/acd820
Shi, Y., Yang, J., Gao, F., and Zhang, Q. (2023). Covalent organic frameworks: recent progress in biomedical applications. ACS Nano 17 (3), 1879–1905. doi:10.1021/acsnano.2c11346
Stevanović, L., Brankov, V., and Cvetković, D. (2021). 21S. Simić, newGRAPH: a fully integrated environment used for research process in graph theory. Available at: http://www.mi.sanu.ac.rs/newgraph/index.html.
Sun, J., Xu, Y., Lv, Y., Zhang, Q., and Zhou, X. (2023). Recent advances in covalent organic framework electrode materials for alkali metal-ion batteries. CCS Chem. 5 (6), 1259–1276. doi:10.31635/ccschem.023.202302808
Ullah, A., Jama, M., and Zaman, S. (2024). Shamsudin, Connection based novel AL topological descriptors and structural property of the zinc oxide metal organic frameworks. Phys. Scr. 99 (5), 055202. doi:10.1088/1402-4896/ad350c
Wang, H., Wang, H., Wang, Z., Tang, L., Zeng, G., Xu, P., et al. (2020). Covalent organic framework photocatalysts: structures and applications. Chem. Soc. Rev. 49 (12), 4135–4165. doi:10.1039/d0cs00278j
Yaghi, O. M. (2016). Reticular chemistry construction, properties, and precision reactions of frameworks. J. Am. Chem. Soc. 138 (48), 15507–15509. doi:10.1021/jacs.6b11821
Yaghi, O. M. (2019). Reticular chemistry in all dimensions. ACS Cent. Sci. 5 (8), 1295–1300. doi:10.1021/acscentsci.9b00750
Yang, H., Hanif, M. F., Siddiqui, M. K., Hussain, M., Hussain, N., and Fufa, S. A. (2024). On topological analysis of two-dimensional covalent organic frameworks via M-polynomial. Sci. Rep. 14, 6931. doi:10.1038/s41598-024-57291-9
Yang, Z., Wang, D., Meng, Z., and Li, Y. (2019). Adsorption separation of CH4/N2 on modified coal-based carbon molecular sieve. Sep. Purif. Technol. 218, 130–137. doi:10.1016/j.seppur.2019.02.048
Yu, Y., Khalid, A., Aamir, M., Siddiqui, M. K., Muhammad, M. H., and Bashir, Y. (2023). On some topological indices of metal-organic frameworks. Polycycl. Aromat. Compd. 43 (6), 5607–5628. doi:10.1080/10406638.2022.2105909
Zaman, S., Jalani, M., Ullah, A., Ali, M., and Shahzadi, T. (2023). On the topological descriptors and structural analysis of cerium oxide nanostructures. Chem. Pap. 77, 2917–2922. doi:10.1007/s11696-023-02675-w
Zhang, H., Zhu, M., Schmidt, O. G., Chen, S., and Zhang, K. (2021). Covalent organic frameworks for efficient energy electrocatalysis: rational design and progress. Adv. Energy Sustain. Res. 2 (4), 2000090. doi:10.1002/aesr.202000090
Zhang, X., Saif, M. J., Idrees, N., Kanwal, S., Parveen, S., and Saeed, F. (2023). QSPR analysis of drugs for treatment of schizophrenia using topological indices. ACS Omega 8 (44), 41417–41426. doi:10.1021/acsomega.3c05000
Keywords: hexabenzocoronenes, covalent organic frameworks, vertex degree indices, entropies, graph energy
Citation: Raza Z, Arockiaraj M, Maaran A and Shalini AJ (2024) A comparative study of topological entropy characterization and graph energy prediction for Marta variants of covalent organic frameworks. Front. Chem. 12:1511678. doi: 10.3389/fchem.2024.1511678
Received: 15 October 2024; Accepted: 11 November 2024;
Published: 20 December 2024.
Edited by:
Pranab Sarkar, Visva-Bharati University, IndiaReviewed by:
Bholanath Mandal, University of Burdwan, IndiaEugeny Alexandrov, Samara State Medical University, Russia
Copyright © 2024 Raza, Arockiaraj, Maaran and Shalini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zahid Raza, enJhemFAc2hhcmphaC5hYy5hZQ==; Micheal Arockiaraj, bWFyb2NraWFyYWpAZ21haWwuY29t
 Zahid Raza
Zahid Raza Micheal Arockiaraj
Micheal Arockiaraj Aravindan Maaran
Aravindan Maaran Arul Jeya Shalini
Arul Jeya Shalini