
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Chem., 25 June 2024
Sec. Medicinal and Pharmaceutical Chemistry
Volume 12 - 2024 | https://doi.org/10.3389/fchem.2024.1445671
This article is part of the Research TopicFive-membered Heterocycles: Synthesis and ApplicationsView all 5 articles
Editorial on the Research Topic
Five-membered heterocycles: synthesis and applications
Small synthetic molecules remain prevalent as drugs both in development pipelines and on the market despite of new and innovative therapeutic approaches emerging over the years. About 90% of approved drugs are small molecules and most of these drugs contain at least one nitrogen heterocycle. These molecules easily diffuse across cell membranes to interact with the cellular organelles and proteins (Roberts et al., 2013). Several five-membered azaheterocycles are the cornerstones of various privileged scaffolds in drug discovery (Alam, 2023; Alam et al., 2023). The 1,2,3-triazole (a five-membered azaheterocycle) ring has emerged as a major pharmacophore in drug discovery. In addition to a core structure, this heterocycle is being extensively used as a “linker” to connect different pharmacophore units by using “click” chemistry (Bozorov et al., 2019). The structure of some of the most recently approved drugs containing five-membered azaheterocycle nuclei are shown in the Figure 1.
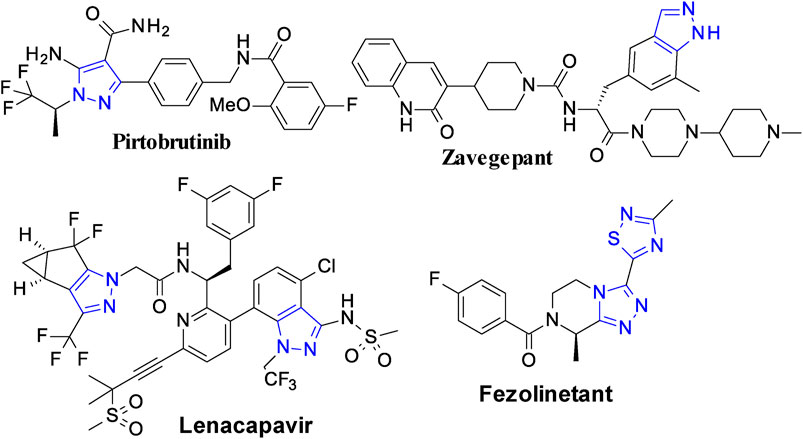
Figure 1. Representative examples of recently approved drugs containing five-membered azaheterocycles.
In this Research Topic, experts have contributed four articles related to the five-membered azaheterocycle small molecules.
Mangal et al. illustrated the use of ‘click chemistry’ to conjugate a bioactive natural product, zingerone with catechol, a siderophore of Gram-negative bacteria, particularly Pseudomonas aeruginosa. The triazole moiety acted as a linker for targeted drug delivery exploiting the bacterial iron scavenging pathway. Docking studies suggested that the hybrid compound interacted with a membrane receptor, PirA. In another research article, (Saeed et al.) reported the synthesis of theophylline-linked 1,2,4-triazole compounds under ultrasonic irradiation. Several of these chimeric compounds showed potent serine protease inhibitory activity and inhibited the growth of bacteria with minimum inhibitory concentration as low as 0.28 μg/mL.
Wang et al. have comprehensively reviewed the design, synthesis, and various biological effects of new analogs of XRP44X, an aryl pyrazole derivative developed by (Wang et al.). XRP44X is a colchicine binding site inhibitor (CBSI). The review article compiled five-membered azaheterocycles: thiadiazole, triazole, tetrazole, thiazole, oxazole, and isoxazole analogs of the pyrazole nucleus of XRP44X. The volume and lipophilicity of the N-aryl group determine the inhibitory property of the colchicine binding site. Sunbal et al. contributed a mini-review transition metal–catalyzed syntheses of saturated five-membered N-heterocycles along with other oxygen and sulfur heterocycles. Different reactions such as cycloaddition, ring-closing metathesis, and coupling are being used to synthesize these five-membered heterocycles. Cobalt, copper, iron, palladium, rhodium, and ruthenium are the most used metals for the synthesis of these bioactive compounds. Gold and iridium are also gaining attention for the synthesis of five-membered heterocycles.
MA: Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alam, M. A. (2023). “Chapter 1 - thiazole, a privileged scaffold in drug discovery,” in Privileged scaffolds in drug discovery. Editors B. Yu, N. Li, and C. Fu (China: Academic Press), 1–19.
Alam, M. A. (2023). Pyrazole: an emerging privileged scaffold in drug discovery. Future Med. Chem. 15 (21), 2011–2023. doi:10.4155/fmc-2023-0207
Bozorov, K., Zhao, J., and Aisa, H. A. (2019). 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: a recent overview. Bioorg Med. Chem. 27 (16), 3511–3531. doi:10.1016/j.bmc.2019.07.005
Keywords: heterocycle, pyrazole, imidazole, five-member ring, thiazole
Citation: Alam MA (2024) Editorial: Five-membered heterocycles: synthesis and applications. Front. Chem. 12:1445671. doi: 10.3389/fchem.2024.1445671
Received: 07 June 2024; Accepted: 11 June 2024;
Published: 25 June 2024.
Edited and reviewed by:
Michael Kassiou, The University of Sydney, AustraliaCopyright © 2024 Alam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Abrar Alam, bWFsYW1AYXN0YXRlLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.