- 1Zhejiang Key Laboratory of Pathophysiology, Department of Cell Biology and Regenerative Medicine, Health Science Center, Ningbo University, Ningbo, China
- 2Research Institute for Medical and Biological Engineering, Ningbo University, Ningbo, China
- 3Department of Otorhinolaryngology, Lihuili Hospital of Ningbo University, Ningbo, China
Polyvinyl alcohol (PVA) hydrogel is favored by researchers due to its good biocompatibility, high mechanical strength, low friction coefficient, and suitable water content. The widely distributed hydroxyl side chains on the PVA molecule allow the hydrogels to be branched with various functional groups. By improving the synthesis method and changing the hydrogel structure, PVA-based hydrogels can obtain excellent cytocompatibility, flexibility, electrical conductivity, viscoelasticity, and antimicrobial properties, representing a good candidate for articular cartilage restoration, electronic skin, wound dressing, and other fields. This review introduces various preparation methods of PVA-based hydrogels and their wide applications in the biomedical field.
1 Introduction
Polyvinyl alcohol (PVA)-based hydrogels are attractive polymeric materials with great potential for biomedical applications. PVA, a synthetic macromolecular polymer, has relatively excellent mechanical properties and is biocompatible, inexpensive, and stable (Wang D. et al., 2023). These advantages make it a prevalent hydrogel preparation material in bioengineering; it is increasingly used to interface with living organisms and function in disease treatment, physiological signal monitoring, and others (Liu et al., 2019).
Benefiting from the numerous hydroxyl side chains on PVA, it can be grafted with different functional groups that may endow it with properties such as elasticity (Fang et al., 2020), antimicrobial properties (Li et al., 2022e), electrical conductivity (Su et al., 2020b), self-healing (Pan et al., 2020), environmental sensitivity (Montaser et al., 2019), and 3D printability (Lim et al., 2018; Abouzeid et al., 2020). Pure PVA hydrogels suffer from mismatched mechanical properties when used in biomedical applications. Therefore, much research has controlled and improved the PVA-based hydrogel structure and function by applying suitable cross-linking methods and adding different component materials to modify and change the hydrogel structure, allowing PVA-based hydrogels to be adapted to multiple applications and making them ideal candidates for cartilage scaffold materials (Liu J. et al., 2020; Yang et al., 2020), electronic sensing (Song et al., 2020; Ye et al., 2020), and wound dressings (Zhao H. et al., 2020; Zou et al., 2020).
A common problem of using synthetic hydrogels in biomedical applications is the mechanical strength and toughness insufficiency (Li et al., 2022b). Many scientists have investigated PVA-based hydrogels with simultaneously improved toughness and ductility by designing the hydrogel molecular structure by enhancing the crystallinity (Liu J. et al., 2020; Ye et al., 2020), filling nanoparticles (Jing et al., 2019; Liu et al., 2021), and building double networks (Li et al., 2019; Li L. et al., 2020). Accordingly, PVA-based hydrogels with the necessary properties and functions for each application can be synthesized through the rational selection and combination of toughening components. This paper reviews the different PVA-based hydrogel cross-linking methods and the latest research on their modification for biomedical applications; many superior mechanical properties of hydrogels are synthesized by combining multiple cross-linking methods. We also provide an outlook on further research directions and application prospects (Lu et al., 2020; Sun et al., 2020; Yang et al., 2020; Zhou et al., 2021).
2 Cross-linking methods of PVA-based hydrogels
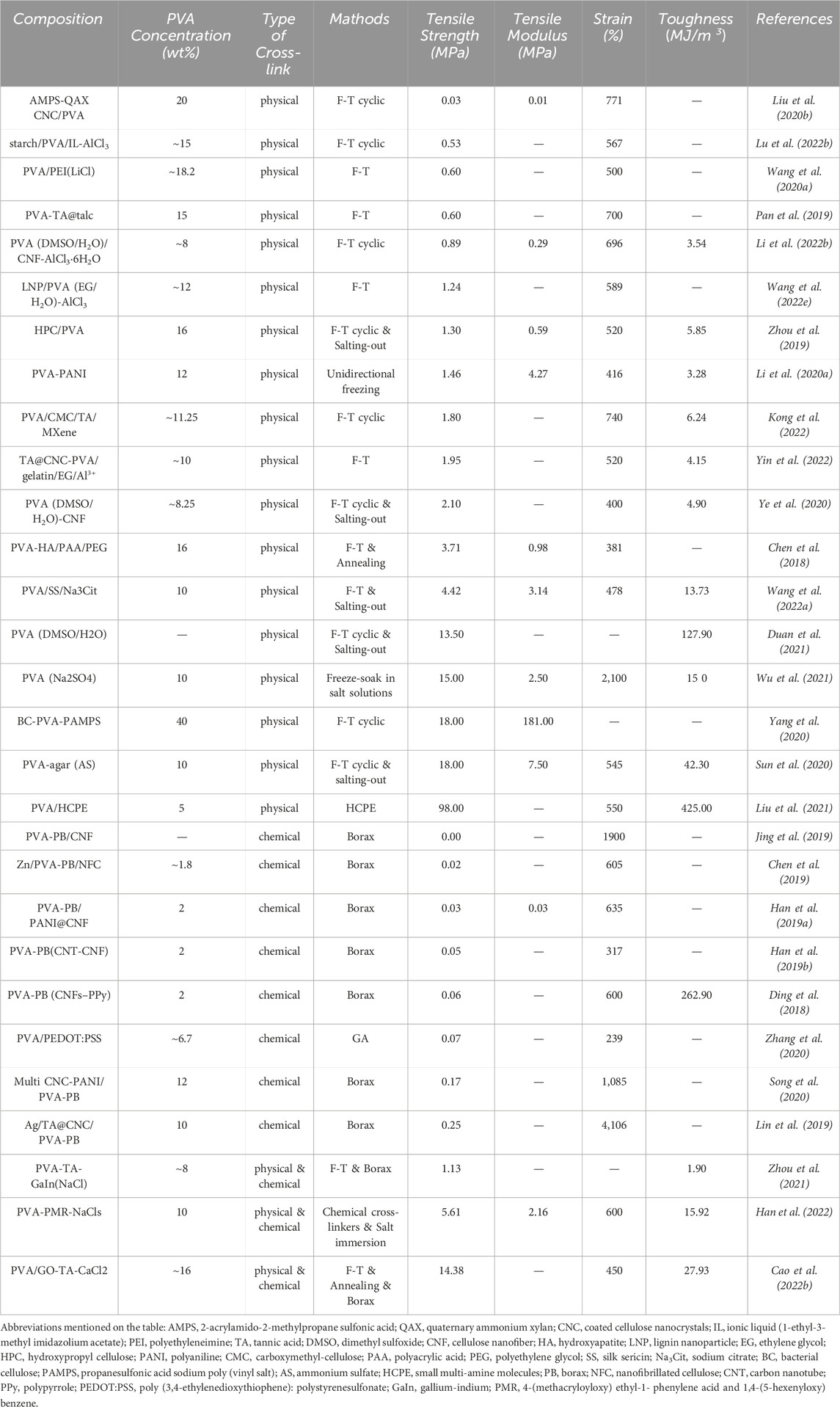
The cross-linking methods of PVA-based hydrogels can significantly impact the biological and mechanical properties. The main reason is the difference in the type and amount of interaction forces between PVA chains in hydrogels synthesized by different cross-linking methods. Cross-linking methods are divided into physical cross-linking when the PVA chains are connected by non-covalent interactions and chemical cross-linking when connected by covalent bonds. Many hydrogels with superior properties use both cross-linking methods in combination (Sun et al., 2020; Pei et al., 2021; Shao et al., 2023). Table 1 summarizes some attractive preparation methods for PVA-based hydrogels.
2.1 Physical cross-linking
Molecular linkages between physically cross-linked PVA-based hydrogels are mainly conducted by physical entanglement, hydrogen bonds, and hydrophobic and electrostatic interactions. Hydrogels synthesized through weak interaction forces are generally soft, flexible, and self-healing, with no toxic cross-linker residues; therefore, they are favored in the biomedical field. The common physical cross-linking methods of PVA-based hydrogels are mainly freeze-thawing (F-T) cyclic, salting-out, and gradient low-temperature.
2.1.1 F-T method
Since Stauffer and Peppast (1992) proposed the PVA-based hydrogel preparation by F-T cyclic in 1991, the method has been widely used in biomaterials, such as wound dressings (Kumar et al., 2020), electronic skin (Zhou et al., 2021), flexible sensors (Yin et al., 2022), and bio-capacitors (Li L. et al., 2020). When hydrogels are prepared with F-T cyclic, PVA chains are discharged due to water molecule crystallization during the freezing process, forming a high-concentration aggregation zone in which the PVA molecular chains are in close contact with each other, forming microcrystalline regions, with physical entanglement and hydrogen bonding between the PVA chains (Holloway et al., 2011). The loose water molecules between the PVA chains are precipitated, increasing the physical entanglement number and hydrogen bonds during thawing and refreezing. This process is repeated, forming a hydrogel filled with water molecules in a three-dimensional mesh structure. The number of F-T cycles, freezing temperature, and time affect the hydrogel molecular structure and mechanical properties (Stauffer and Peppast, 1992), allowing for the control of these properties. The increasing F-T cycle number increases the hydrogel crystallinity, toughness, and tensile properties while decreasing the swelling coefficient (Figure 1A). Our previous studies showed that after about six F-T cycles, the mechanical properties do not change with increasing the F-T cycle number (Hou et al., 2015).
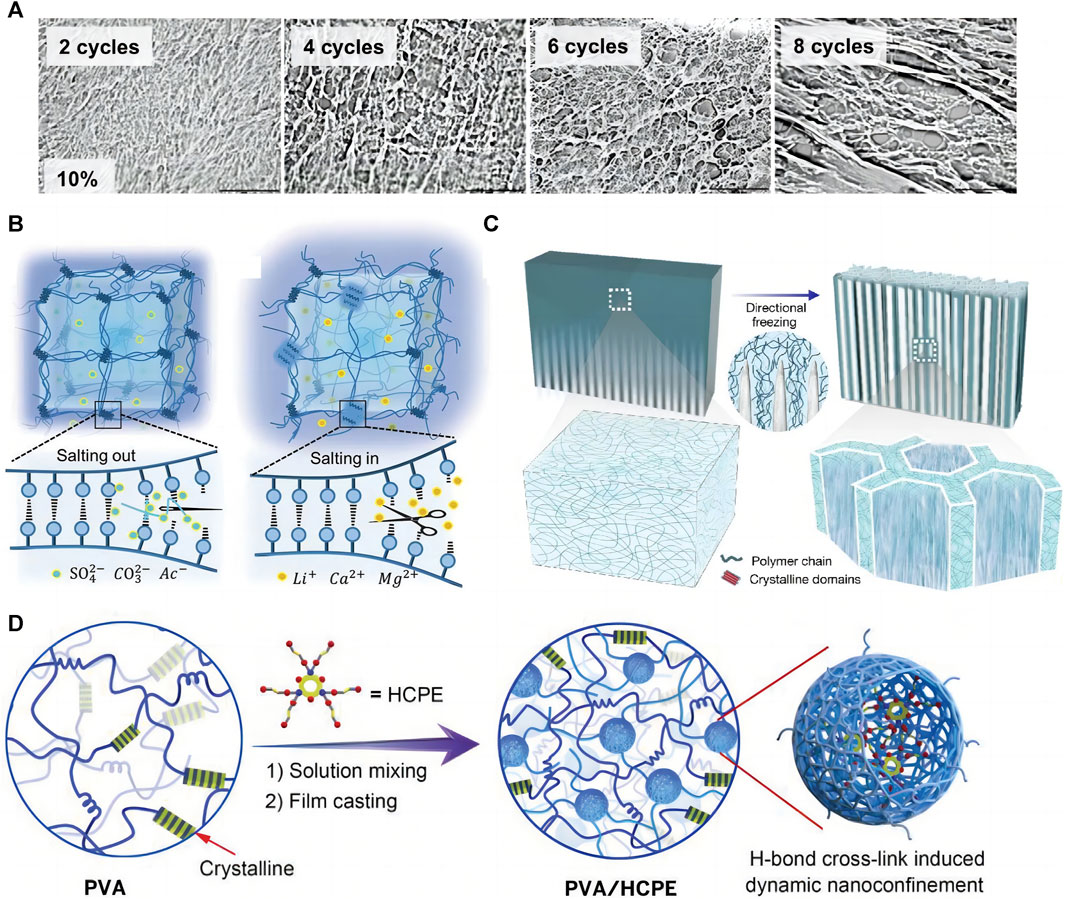
FIGURE 1. Physical cross-linking methods for PVA-based hydrogels. (A) PVA hydrogel prepared by F-T method, the number of F-T cycles can affect the structure and properties of the hydrogel (Stauffer and Peppast, 1992); (B) Principle of salting method (Wu et al., 2021); (C) Principle of directional freezing method (Hua et al., 2021); (D) Cross-linking by hydrogen bonding cross-linker (Liu et al., 2021).
2.1.2 Salting-out method
The salt precipitation method is based on the Hofmeister effect when the hydrogel precursor is immersed in salt solutions such as sodium chloride (Gao et al., 2020), sodium citrate (Duan et al., 2021; Yan G. H. et al., 2022), and ammonium sulfate (Sun et al., 2020), the solvent in the hydrogel precursor is replaced by the salt precipitation effect, aggregating the PVA chains and forming extensive physical cross-linking sites. The high ionic strength of the salt ions reduces the polymer solubility (Baldwin, 1996), further aggregating the PVA chains and causing more hydrogen bonds to form between the PVA chains. The approach enhances the hydrogel mechanical strength and fatigue fracture resistance due to the increased PVA-based hydrogel crystallinity that forms a dense polymer network (Gao et al., 2020). According to Wu et al. (2021), changing the salt type and concentration can adjust PVA hydrogel mechanical properties on a large scale. The higher the anion valence, the better the gelation and toughening effect promotion, while the opposite is true for the cation. The mechanical properties of PVA hydrogels after treatment with different salt solutions show a pattern that follows the Hofmeister effect. He et al. also demonstrated that PVA hydrogels treated with a saturated Na2SO4 solution showed highly tough and stretchable properties (Figure 1B).
Due to the increase in cross-linking density, the movement and crystallization of water molecules bound in the hydrogel are inhibited, so the hydrogel prepared by this method tends to have better anti-freezing and moisturizing ability. Furthermore, as salt ions are introduced into the PVA network, they can impart ionic conductivity to the hydrogel (Sun et al., 2020; Wang et al., 2021; Han et al., 2022). Many researchers have combined the application of binary solvents to overcome the limited use of ion-conductive hydrogels under low-temperature conditions, preparing cold-resistant electronic skins and sensors with superior performance (Ye et al., 2020; Duan et al., 2021).
2.1.3 Directional freezing
Directional freezing is a common and simple method to prepare anisotropic biomaterials (Hua et al., 2021; Mredha and Jeon, 2022) by applying a gradient low temperature to a hydrogel precursor in a certain way that causes the ice crystals to grow directionally along the temperature gradient; the PVA is discharged into the space between the ice crystals. The lamellar structure of ice crystals is used as a template to form an ordered microcrystalline structure (Zhao et al., 2017) (Figure 1C). Directional freezing facilitates PVA chain alignment and folding into the microcrystalline region, increasing PVA molecular structure ordering and forming a stronger hydrogel network, which can simultaneously improve the hydrogel mechanical strength and properties (Li L. et al., 2020). Most natural biomaterials exhibit anisotropic structures (Yan M. Z. et al., 2022), such as cellulose, lignin, etc. While artificial polymers are mostly isotropic. Therefore, increasing research aims to fabricate anisotropic hydrogels by various methods. Anisotropic hydrogels have excellent properties such as strong mechanical strength, cell guidance, tissue regeneration, and anisotropic mechanical/electrical/magnetic/thermal properties (Mredha and Jeon, 2022), allowing their applicability in many fields; stiffness gradient PVA hydrogels prepared with gradient cryogenics can be a tool for basic research on cell adhesion and migration behavior (Kim et al., 2015). The cyclic durability and specific capacitance of ordered polyaniline (PANI)-PVA capacitors prepared by directional freezing are much higher than those of the disordered version (Li W. et al., 2020; Chen Q. et al., 2021). Directional freezing is also important in tissue engineering, as it enables obtaining scaffold materials with unidirectional porous structures of controlled pore size (Yeon et al., 2022). Unidirectional micron-sized pores allow cells to grow inward and spread across the scaffold, while nanopores within the polymer walls of the scaffold allow cell signaling, nutrient delivery, excretion removal, and other vital activities. In addition, the stratified tissue porosity obtained by cryocasting the scaffold facilitates in vivo neovascularization and hydroxyapatite-rich cement line formation in the early osteoconduction stages (Zhao N. F. et al., 2020). Zhao et al. (2017) developed a bidirectional freezing scheme by introducing a low thermal conductivity polydimethylsiloxane wedge. This polymer forms a dual horizontal and vertical temperature gradient during cooling, controlling the ice crystal nucleation and growth toward the monolayer direction; the group prepared graphene oxide/PVA films with superb mechanical strength and toughness by this method. Directional freezing is widely used to prepare biomaterials due to the high operability and the obvious optimization of synthetic material properties. In addition, mechanical training was shown to enable hydrogels to acquire anisotropy (Yan M. Z. et al., 2022; Wang Q. et al., 2022).
2.1.4 Hydrogen bonding cross-linker
Dynamic hydrogen bonding substantially connects PVA chains in physically cross-linked hydrogels; hydrogen bonding cross-linkers can increase the hydrogen bonding donors and acceptors in the hydrogel and act as bridging points between the PVA chains. Therefore, the PVA chains are connected and entangled with each other through the hydrogen bonds, resulting in a dense polymer chain network. Typical hydrogen bonding cross-linkers include carbon nanofibers (Li et al., 2022b), tannins (Li et al., 2022c), dopamine, and polyamine molecules (Liu et al., 2021); they are characterized by possessing high strength and abundant hydroxyl binding sites, which can enhance the interactions between the chains through many strong hydrogen bonds and lead to polymer chains with a high surface density. PVA-based hydrogels prepared with potent hydrogen-bonding cross-linkers provide high toughness, ductility, and tensile strength simultaneously (Liu et al., 2021; Li et al., 2022b) (Figure 1D) due to the dynamic fracture and reorganization of hydrogen bonds in the hydrogel network when they are subjected to tensile stress, which effectively dissipates energy and endows these hydrogels with good self-healing properties (Lin et al., 2020). The hydrogels prepared by this cross-linking method are biocompatible, only require simple mixing to form a gel, facilitate customizing and integrating special functions such as anti-freezing and electrical conductivity (Liu X. et al., 2022), mass production, and processing, besides being used in manufacturing electronic skin (Wen et al., 2021; Zhou et al., 2021), flexible sensors (Li et al., 2022c), wound dressings [35], and other tissue engineering materials (Pei et al., 2021).
2.2 Chemical cross-linking
Chemical cross-linking connects the molecular chains of PVA hydrogels by covalent or metal-ligand bonds; chemical hydrogels have better stability and toughness than physical hydrogels (Xiang et al., 2020). Cross-linking chemical agents facilitate the hydrogel insolubility in water (Ebhodaghe, 2020) and can be glued quickly in situ (Yuan et al., 2019; Chen Y. et al., 2021; Wang R. B. et al., 2022), enhancing hydrogel applications in tissue engineering. This is because in situ gel formation allows hydrogels to be used as filler materials and bioinks for fine surgical procedures and 3D printing of bionic scaffolds that need adaption to the wound surface (Abouzeid et al., 2020; Wu et al., 2022). Therefore, chemical cross-linking methods are favored by researchers despite the difficulty of completely removing the residual cross-linking agents (Peppas et al., 2006; Zhang et al., 2020; Chen Y. et al., 2021).
2.2.1 Chemical cross-linkers
Three main types are the most commonly used chemical cross-linking agents for preparing PVA-based hydrogels. The first is Aldol reaction initiation using aldehydes or ketones with α hydrogen atoms, such as glutaraldehyde, modified starch double aldehyde starch (DS) (Guo et al., 2020; Cao J. L. et al., 2022) (Figure 2A). The hydroxyl group (-OH) of PVA reacts with the aldehyde group (-CHO) of glutaraldehyde in acidic solutions [e.g., sulfuric (Guo et al., 2020) and phytic acids (Wei et al., 2021)] to form acetals or hemiacetals. This method can obtain a hydrogel with good structural stability under relatively mild conditions. Moreover, it possesses mechanical properties such as superior toughness and elasticity (Zhang et al., 2020) and has promising applications in flexible sensing and wound dressing (Hosseini and Nabid, 2020; Wei et al., 2021). However, glutaraldehyde affects cellular activity (Amiri et al., 2022); therefore, the non-toxic, biodegradable, and widely used chemically active modified double aldehyde starch (DS) is proposed as a substitute for glutaraldehyde (Cao J. L. et al., 2022).
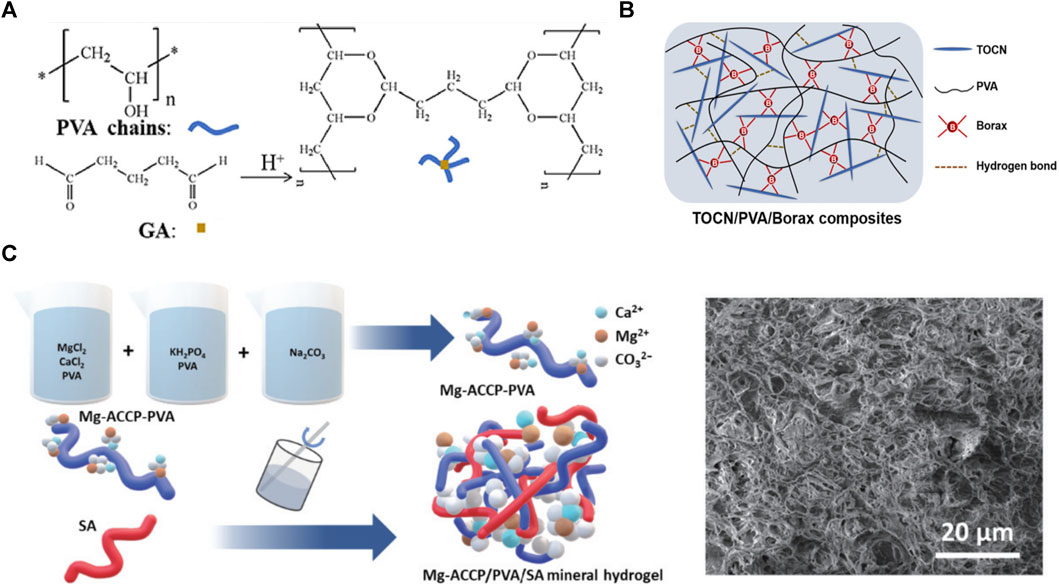
FIGURE 2. (A) PVA-based hydrogel cross-linked by glutaraldehyde (Pei et al., 2021); (B) PVA-based hydrogel cross-linked by borax (Songfeng et al., 2020); (C) PVA-SA copolymer hydrogel (Shen et al., 2022).
The second common cross-linking agent is borates, which polymerize PVA chains through borate ester bonds (Songfeng et al., 2020; Hamd-Ghadareh et al., 2022) (Figure 2B). Borax hydrolysis yields B(OH)4-, which forms diol-borate bonds with the widely present intermolecular hydroxyl groups of PVA. The reversible covalent bond can be broken and regenerated reversibly when damaged. Such dynamic bonds give PVA/borax hydrogels excellent self-healing properties, super-elongation, and plasticity (Ke et al., 2023). When the hydrogel pH value changes or in a sugar-containing environment (e.g., glucose and fructose), the borate ester bond is reversibly dissociated and reorganized. Monosaccharides such as glucose and fructose are rich in diol units that competitively break the borate ester bond between PVA and borate ions; sugar concentration regulates this destruction degree. Therefore, PVA hydrogels synthesized by this method possess a pH/sugar responsiveness (Yi et al., 2023). Finally, the hydrogel possesses underwater self-healing and conductive properties because the borate ions produced by borate ionization can move in the aqueous medium. These superior properties make PVA/borax hydrogel promising for wearable device applications (Zhang Z. et al., 2022; Li et al., 2022d).
In addition, multivalent metal ions such as Ca2+, Mg2+, Fe3+, etc., Have been used as cross-linkers for PVA-based hydrogels. The metal ions can form coordination bonds with the hydroxyl groups in PVA, constraining the PVA chains, and stabilize the network of PVA-based hydrogels (Shen et al., 2022). The mechanical strength and toughness of PVA-based hydrogels were increased due to the sacrificial breaking of the coordination bonds (Han et al., 2023; Li et al., 2023). In addition, the introduction of metal ions restricts the crystallization behavior of water molecules in the PVA-based hydrogel at low temperatures, thus the hydrogel acquires antifreeze properties (Shen et al., 2022).
2.2.2 Ultraviolet (UV) radiation, electron beam, and gamma irradiation
The cross-linking process by UV radiation, electron beam, or gamma irradiation generates free radicals and induces covalent bond formation between functional groups (Jin, 2022). Cross-linking in these ways requires no chemical cross-linking agent (Nasef et al., 2019), with the advantages of chemically cross-linked hydrogels, i.e., stable structure, superior mechanical properties, and controllable shape. The PVA-based hydrogels prepared by electron irradiation possess high fracture strength, but their cytocompatibility and tissue affinity are relatively lacking; therefore, it is commonly used to manufacture industrial conductive materials (Wang Z. Y. et al., 2023). The PVA-based hydrogels cross-linked by UV and gamma radiation possess significant advantages over the other chemical cross-linking methods mentioned above. Cross-linking is characterized by a mild reaction temperature, pH, and controlled reaction time and space (Eghbalifam et al., 2015; Vo et al., 2022). The properties of hydrogels synthesized by this method have a large scope for adjustment, and various parameters can be used to modify the hydrogel polymerization behavior (e.g., radiation dose, UV exposure, photo-initiator concentration, monomer chain length, and various biomolecule conjugation) (Montaser et al., 2019; Nasef et al., 2019). The PVA-based hydrogels prepared by this cross-linking method possess good mechanical toughness and biocompatibility. The tensile fracture strength, elastic modulus, and toughness reached 1.28 MPa, 426.4 kPa, and 2.53 MJ m–3. Hydrogel cross-linking increased the breaking stress, modulus of elasticity, and toughness of the hydrogel by 23, 20, and 25 times, respectively (Pei et al., 2021). PVA-based hydrogels can obtain controlled biodegradation rates by photopolymerization, enabling their wide use in various fields of tissue engineering.
2.3 Multi-network copolymerization
Multi-network copolymerization is an attractive strategy for hydrogel synthesis (Ding et al., 2018; Shen et al., 2022; Qin et al., 2023) (Figure 2C). The mechanical and biological features of a single PVA network are constrained by its loose structure, limiting its hydrogel capabilities. Cross-interconnection of multiple networks can enhance the hydrogel structure stability and mechanical properties (Wang et al., 2021; Li et al., 2022a). During macromolecular polymer copolymerization, the hydrogel structure is reinforced by electrostatic interactions, physical entanglement between chains, and extensive hydrogen bonding between functional groups (Wen et al., 2020). Preparing hydrogels by mixing with natural polysaccharides such as sodium alginate (SA) (Shen et al., 2022), chitosan (Shamloo et al., 2021), and starch (Lu L. et al., 2022) can overcome the natural polysaccharide hydrogel brittleness and improve the PVA hydrogel swelling properties and mechanical strength (Qing et al., 2021; Cao J. L. et al., 2022). This allows PVA-based hydrogels to have advantages over natural polysaccharide polymers, such as biocompatibility, biodegradability, and self-healing properties (Ounkaew et al., 2020; Cao J. L. et al., 2022). PVA-based hydrogels achieve excellent electrical conductivity when copolymerized with conductive materials such as acrylic acid (Dai et al., 2022) and PANI (Jing et al., 2019). Therefore, PVA-based hydrogels have potential applications in flexible sensing. Copolymerization with natural polysaccharide derivatives, such as modified double-formaldehyde starch and chitosan, can replace chemical cross-linking agents. In these methods, tough multi-network hydrogels are obtained by esterification or Schiff base reactions; such PVA-based hydrogels are more suitable for biomedical applications than cross-linking by glutaraldehyde (Altaf et al., 2020; Ounkaew et al., 2020; Takacs et al., 2022).
3 PVA-based hydrogels for biomedical applications and their construction
3.1 Artificial cartilage
Articular cartilage is a crucial lubricating and weight-bearing structure in the body. The lack of vascular tissue to transport nutrients makes it difficult for damaged articular cartilage to self-heal. Traditional treatments to promote cartilage self-healing, such as chondrocyte transplantation and microfracture, have low treatment efficiency and long recovery times (Ye et al., 2022; Zhao et al., 2022). Therefore, artificial joint cartilage replacement has become an important treatment modality. An alternative approach is localized articular surface replacement with conventional orthopedic materials (cobalt-chromium alloy, ultrahigh-molecular-weight polyethylene), but these implants have a high degree of stiffness that may ultimately result in abnormal stress and wear on the joints (Yang et al., 2020). The PVA hydrogel is an ideal candidate for preparing bionic articular cartilage (Hu et al., 2022; Oliveira et al., 2023) because of its similar porous structure to that of articular cartilage. Besides the advantages of non-toxicity and good biocompatibility (Figure 3G), it has good permeability and low friction (Figure 3D). The unique physical cross-linking method makes blending with other polymers or functional components possible by simple mixing, as it enables PVA-based hydrogels to have the mechanical strength and necessary biological properties as load-bearing materials.
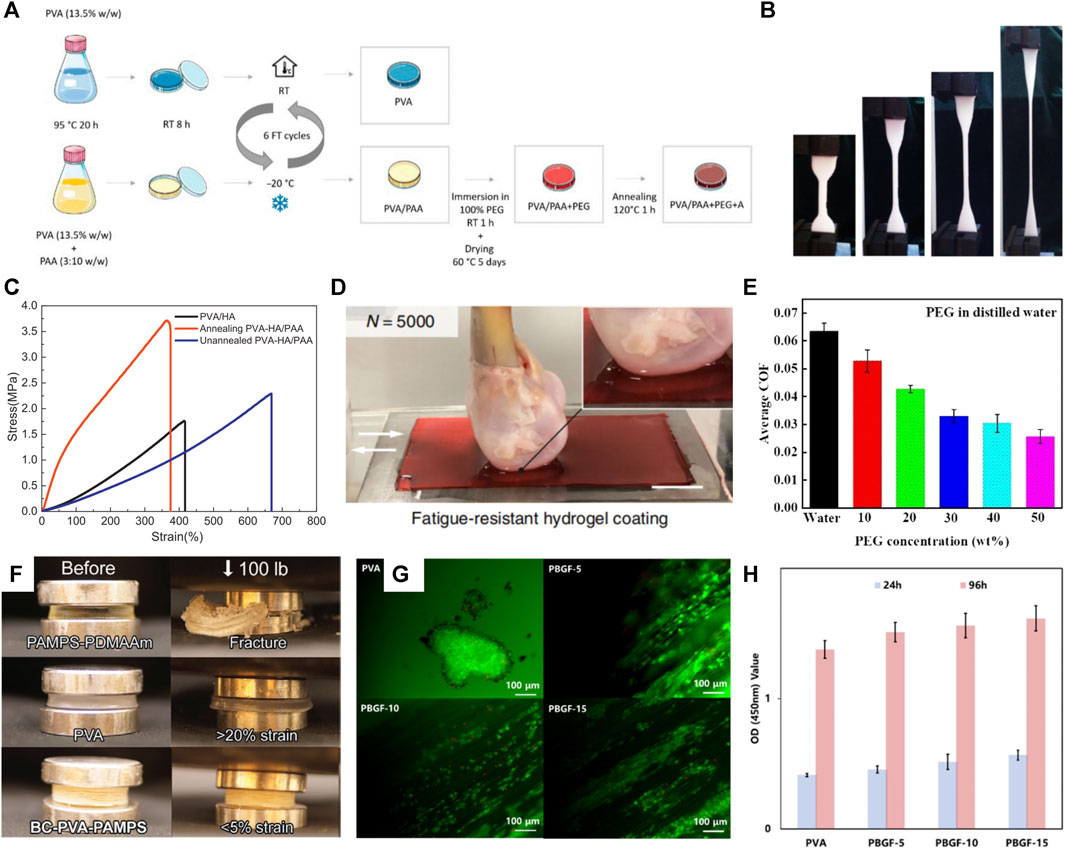
FIGURE 3. (A) Schematic diagram of PVA-based hydrogel synthesis (Branco et al., 2022); (B) PVA-based hydrogel has good flexibility; (C) Annealing can enhance the mechanical strength of hydrogel (Chen et al., 2018); (D) PVA-based hydrogel can be used as articular cartilage contact surface with good tribological properties (Liu J. et al., 2020); (E) Organic solvent impregnation can significantly reduce the friction coefficient of hydrogel (Hu et al., 2022); (F) PVA-based hydrogel has good compression resistance and has the potential to be used as a load-bearing joint (Yang et al., 2020); (G,H) PVA-based hydrogel has good biocompatibility (Zhu C. K. et al., 2022).
3.1.1 Mechanical properties enhancement
Articular cartilage has extreme strength (4–20 and 0.8–25 MPa compressive and tensile strengths, respectively (Li et al., 2022a)), toughness (fracture energy of 1,000–15,000 J m-2), and elasticity (fracture strain of 60%–120%), and can transmit 7–9 times the body weight, while the very low coefficient of friction (0.001–0.04) makes it possible to load the human body with flexible life activities for a long time (Cao J. L. et al., 2022). In contrast, the ultimate tensile strength of the pure PVA hydrogel was 0.6 MPa, the compressive strength was 24 MPa, and the strain at break was 400% (Ye et al., 2020). And the low compression modulus (0.31–0.8 MPa) of PVA hydrogels causes them to exhibit substantial deformation (>20%). This significant deformation suggests that using PVA alone as synthetic cartilage in the knee joint will cause forces to be transferred to the surrounding bone and cartilage (Yang et al., 2020). Consequently, insufficient mechanical properties are the main obstacle to applying pure PVA hydrogels in bionic joints. Therefore, many studies have developed PVA-based hydrogels with mechanical strength and low friction coefficient (Cao J. L. et al., 2022; Ye et al., 2022). Ye et al. (2022) introduced polyethylene glycol (PEG) into PVA hydrogel and prepared PVA/PEG composite hydrogel by blending the physical cross-linking method; the mechanical properties of the hydrogel were 21.2 MPa, and the friction coefficient was only 0.12.
The PVA-based hydrogels applied to bionic cartilage are mainly formed by the physical cross-linking method through F-T cycles. Consequently, the hydrogel mechanical properties can be effectively enhanced by increasing the hydrogel crystallinity and constructing multi-network hydrogels.
3.1.1.1 Increasing crystallinity
Increasing the number of F-T cycles and annealing treatments can significantly enhance the PVA-based hydrogel mechanical strength (Cao J. L. et al., 2022). Chen et al. (2018) showed that annealing treatment can significantly increase PVA-based hydrogel crystallinity, reduce water content, and form a more dense and stable three-dimensional mesh structure. This treatment substantially increased the tensile strength and elastic modulus of the PVA hydrogel (Oliveira et al., 2023). Annealed PVA-based hydrogels have a low friction coefficient advantage due to dual-phase lubrication and fluid loading support (Chen et al., 2018; Branco et al., 2022). Solvent impregnation was used to enhance the crystallinity of PVA-based hydrogels. This method can effectively improve the mechanical and tribological properties of hydrogels; impregnation with acetone (Hu et al., 2022) and PEG (Branco et al., 2022) reinforced the structure of PVA-based hydrogels, bringing the hydrogels closer to the flexibility of natural cartilage.
3.1.1.2 Building a secondary network
Introducing a flexible second network enhances hydrogel mechanical strength primarily through electrostatic interactions and many hydrogen bond formations that increase the hydrogel cross-link density (Nie et al., 2020). Meanwhile, the sacrificial fracture of the second network can effectively dissipate energy and enhance hydrogel toughness (Yang et al., 2020; Branco et al., 2022) (Figure 3A). Biomaterials such as chitosan (Luo et al., 2022) and bacterial fibers (Yang et al., 2020; Zhu C. K. et al., 2022; Oliveira et al., 2023) are widely used in the second network of PVA-based hydrogels, as they have enhanced the hydrogel structure (Figure 3F). Ye et al. (Cao J. L. et al., 2022) introduced graphene oxide-tannic acid (TA) sub-network structure into PVA-based hydrogels. The tensile strength and fracture toughness of the constructed composite hydrogels reached 11–26 times that of pure PVA hydrogels (14.38 MPa/27.93 MJ m-3).
Additionally, a study combined hydrogel with titanium alloy to prepare a “soft (hydrogel)-hard (Ti6Al4V)" integrated material, revealing a very high interfacial toughness (3900 J m-2) with a high load-bearing capacity and excellent tribological properties (Ye et al., 2022).
3.1.2 Improvement of other performance
The effects of wear and tear are one of the main problems posed by artificial joints. To ensure the service life of artificial joint cartilage, a wear-resistant hydrogel with a low friction coefficient has become a current research direction. The main methods currently used to improve the tribological properties of hydrogels are lubricants, organic solvent impregnation, and hydrogel surface modification. Hu et al. (2022) encapsulated carbon quantum dots (CDs) in PVA hydrogels and significantly reduced the PVA-based hydrogel friction coefficient. Organic solvent dehydration treatment can also improve the hydrogel abrasion resistance. Ye et al. (2022) showed that the PVA/PEG composite gel could be made to have lubrication and high load-bearing capacity by acetone impregnation to maintain a low COF of 0.12 even under dry friction, and the wear rate was reduced to the original 27.4%. Chen et al. (2018) showed that annealing treatment could reduce the PVA-based hydrogel friction coefficient by about 40% (Figure 3C). Constructing microtextures by laser ablation improved the PVA-based hydrogel surface tribological properties (Zhou et al., 2022).
Introducing bioactive components (such as magnetic composite nanoparticles hydroxyapatite (Hou et al., 2015), hydroxyapatite (Abouzeid et al., 2020), and polysaccharides (Hou et al., 2013)) can promote cell adhesion and proliferation and improve the biological properties of PVA-based hydrogels (Nie et al., 2021). In previous studies, our group has designed nano-hydroxyapatite/PVA composite hydrogels, revealing that hydroxyapatite enhanced the nanocomposite hydrogel compressive strength and promoted cell adhesion and proliferation (Hou et al., 2015). Biodegradable glass (BGF) enhances the mechanical properties of PVA-based hydrogels and promotes cell proliferation and differentiation due to spontaneous BGF degradation, promoting cartilage repair (Zhu C. K. et al., 2022). Additionally, hybrid hydroxyapatite with high osteoconductive properties coated on the surface of PVA hydrogel promotes in situ osteogenesis (Luo et al., 2022).
3.2 Electronic skin
Wearable medical and health devices have received much attention recently. Polymer hydrogels with sensing properties are widely used in electronic skin and human-computer interaction; they work in health monitoring and disease diagnosis and treatment processes (Qin et al., 2022). PVA-based hydrogels are viable strain and temperature sensors (Chen S. Y. et al., 2022; Liu H. D. et al., 2022) (Figure 5A).
3.2.1 Sensing characteristics
The conductive hydrogel changes its resistance when it is deformed by pressure or when the temperature or environment changes, which is the basis of its function as a sensor. The main sensors designed based on PVA-based hydrogels are pressure, chemical (Qin et al., 2022) (Figure 5D), and temperature (Liu H. D. et al., 2022). Pure PVA hydrogels showed a low conductivity of 4.24 × 10−5 S cm–1 (Fan et al., 2020). To enhance the electrical conductivity of hydrogels, researchers have introduced conductive components such as carbon nanoparticles (graphene (Pan et al., 2020), carbon nanotubes (Song et al., 2020) and MXenes (Zhang et al., 2019; He et al., 2020)), nanometallic particles, charged ions (Zhou et al., 2019), and conductive active polymers (PANI (Li L. et al., 2020), and polypyrrole (Abodurexiti and Maimaitiyiming, 2022)) into the hydrogel matrix. Enhanced with improved conductivity sensitivity and stability, PVA-based hydrogels have emerged as exceptional candidates for sensor materials. However, their versatility surpasses expectations. By incorporating directional freezing and incorporating natural anisotropic elements, conductive hydrogels gain the ability to respond deliberately to the direction of stimuli, thus expanding the range of applications for these extraordinary materials.
3.2.1.1 Electrical conductivity
The current strategies for synthesizing conductive hydrogels include two main directions: Ionic conductive hydrogels prepared by adding electrolytes and electronic conductive hydrogels prepared by filling them with nanoparticles. PVA-based hydrogels possess a three-dimensional porous structure that provides a natural transport channel for the rapid migration of ions in the material. Therefore, introducing metal ions [e.g., Al3+(Fan et al., 2020; Lu L. et al., 2022), Ca2+ (Gan et al., 2021), Mg2+ (Guo et al., 2022), and Fe3+ (Merhebi et al., 2020)] in PVA-based hydrogels can form ion-conductive hydrogels (Figure 4A). The electrical conductivity of hydrogels can be improved by enhancing the crystal arrangement orderliness in the hydrogel and improving the migration efficiency of ions in the hydrogel. Ionic conductive hydrogels possess good electrical conductivity, water retention, and frost resistance (Wen et al., 2021). However, the conductive sensitivity (gauge factor value, GF) is relatively low (Lu et al., 2020; Pan et al., 2020). Introducing some metal ions, such as Al3+ and Mg2+, can enhance the electrical conductivity of hydrogels and make them possess antibacterial properties. The main principles are electrostatic interactions between ions and negatively charged surfaces of bacterial populations and Ph reduction due to Al3+ hydrolysis (Lu L. et al., 2022). Introducing various mineral ions can enhance the hydrogel conductivity; Shen et al. (2022) introduced colloidal and amorphous polyionic biominerals (Mg-ACCP, containing Mg2+, Ca2+, CO3 2- and PO4 3-) into a biocompatible PVA and sodium alginate network to construct novel hydrogels with abundant mineral ions. This hydrogel has high ionic conductivity and is highly sensitive even to small applied pressures and strains, and its conductivity reaches 1.7 S m-1 at a frequency of 105 Hz.

FIGURE 4. (A,B) PVA-based hydrogel doped with Al3+ or Ca2+, the metal ions can make the hydrogel have good ionic conductivity (Gan et al., 2021; Lu L. et al., 2022); (C) The application of organic solvents enables the hydrogel to maintain good electrical conductivity in low-temperature environments and underwater. The concentration of NaCl solution affects the conductivity, and this hydrogel possesses sustained conductivity (Li et al., 2022c).
Brine immersion is one of the main methods to impart ionic conductive properties to hydrogels (Han et al., 2022). The all-wood conductive hydrogel obtained by Yan M. Z. et al. (2022) by soaking in ammonium sulfate solution has excellent flexibility, electrical conductivity, and sensitivity. It can accurately distinguish macroscopic or subtle human movements, including finger flexion, pulse, and swallowing behavior, facilitating accurate human motion monitoring. Currently, ionic liquids are receiving great attention due to their high chemical and thermal stability, electrical conductivity, and solubility. Ionic liquids are used to prepare PVA-based hydrogels with high electrical conductivity and frost resistance (Lu L. et al., 2022). Furthermore, copolymerization with conductive polymers, including polyaniline (Qin et al., 2022) and polypyrrole (Abodurexiti and Maimaitiyiming, 2022), can yield excellent conductivity and sensitivity. For example, in 2020, Li et al. achieved an impressive conductivity of 20.5 S/m in a Polyvinyl Alcohol/Polyaniline hydrogel (Li L. et al., 2020).
Zhan et al. (2022) further designed a bilayer Janus conductive hydrogel with conductive and negative or weakly conductive layers, overcoming the mismatch between the mechanical properties of elastomer, the conductive hydrogel, and the lack of strong interfacial adhesion, eliminating the need for insulating elastomers, and making hydrogels more suitable for biomedical applications.
3.2.1.2 Anisotropy
Direction recognition is another important aspect of electronic skin that realistically mimics human skin, allowing a more accurate determination of the stimulus source. Anisotropic hydrogels are constructed to enable finer and more bionic reading of biosignals by electronic skin; directional freezing is one of the most prominent methods for forming anisotropic biomaterials. Another effective approach is introducing naturally anisotropic components such as cellulose nanofibrils (Yan M. Z. et al., 2022) and lignin (Yan G. H. et al., 2022). The currently proposed direction recognition still has limitations: it can only recognize motions in the same plane, including forward and reverse directions, and more complex motions are difficult to distinguish by electrical signals (Peng et al., 2020) (Figure 5E). Therefore, advanced materials with 3D orientation recognition capability still need to be further explored. It is possible to design the structure of anisotropic hydrogels to obtain three-dimensional woven receptors and then encode the readers to achieve three-dimensional perception.
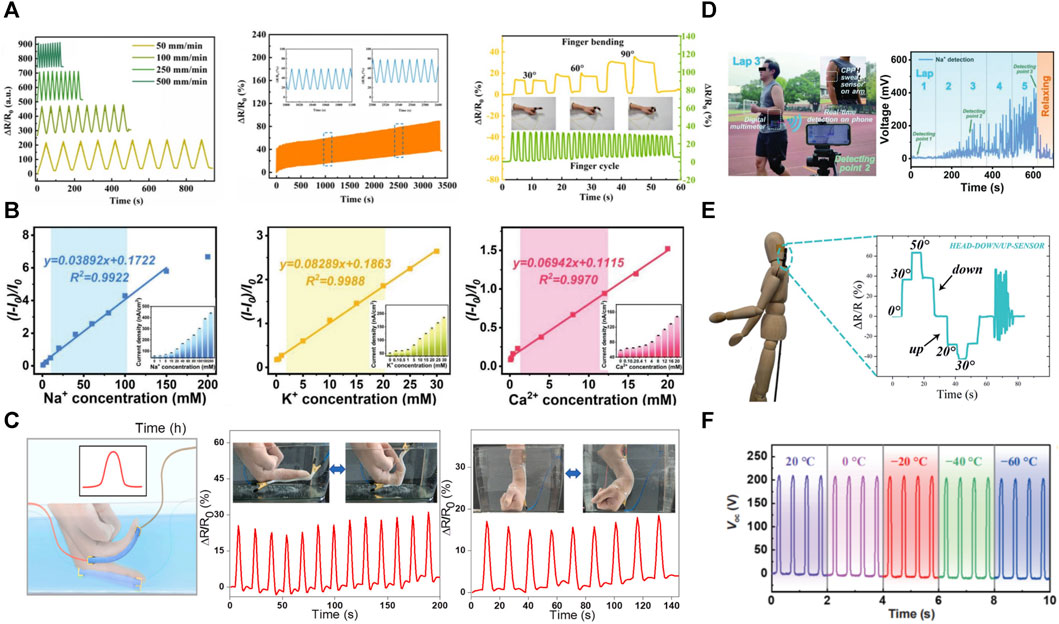
FIGURE 5. Properties and applications of PVA-based hydrogels in flexible sensing. (A) Ionically conductive PVA-based hydrogels can sensitively and reproducibly convert motion signals into electrical signals (Zhang L. et al., 2022); (B) Sweat sensors prepared using PVA-based hydrogels can respond to Na+, K+, Ca2+ and their concentrations in sweat (Qin et al., 2022); (C). Anti-freezing and water-preserving design of PVA-based hydrogel can in realizing sensing underwater (Lu L. et al., 2022); (D) PVA-based hydrogel monitors the movement process of human body (Qin et al., 2022); (E) Anisotropic PVA-based hydrogel can differentiate the movement signals in different directions (Peng et al., 2020); (F) PVA-composite hydrogel possesses excellent anti-freezing properties and can realize stable electrical signal conduction at −60°C (Dai et al., 2022).
3.2.2 Mechanical properties
PVA-based hydrogels offer significant versatility in terms of their mechanical properties, enabling adjustment over a wide range. By modifying factors such as the concentration of PVA, the composition of the hydrogel, and the synthesis methods, the mechanical performance of PVA-based hydrogels can be extensively tailored. Therefore, PVA-based hydrogels are well-suited for applications in electronic skin, as they exhibit flexibility, stretchability, and anti-fatigue characteristics. This adjustability allows these hydrogels to meet the diverse requirements of electronic skin (Wu et al., 2021). Progress has been made in toughening hydrogels by forming double networks (Zhang et al., 2023), adding nanofilms, and mechanical training. The main idea is to introduce an energy dissipation mechanism to enhance the fatigue fracture resistance of hydrogels using dynamic bonding, ligand bonding, or sacrificial fracture of the second network (Zheng et al., 2021).
3.2.2.1 Improving the mechanical strength of hydrogels through improved cross-linking methods
The first method to improving the mechanical strength of hydrogels uses a binary solvent. Water is used as a dispersant in conventional PVA-based hydrogels to create high water percentage hydrogels, which leads to loose cross-linking of hydrogels; therefore, reducing the water content enhances the mechanical strength of hydrogel. However, the inherent low solubility of PVA does not support preparing low-water-content hydrogels. Therefore, mixing organic solvents such as ethylene glycol (Wen et al., 2021; Dai et al., 2022), glycerol (He et al., 2020; Ma et al., 2020), and dimethyl sulfoxide with water in a certain ratio can overcome this issue by reducing the water content. Moreover, the strong hydrogen bonds formed between organic solvents and water molecules can enhance the hydrogel flexibility.
The second method Is to add a F-T cycle or a thermal annealing step. As mentioned above, increasing the number of F-T cycles increased physical entanglements and hydrogen bonds in PVA-based hydrogels, producing a stronger PVA-based hydrogel. Thermal annealing could also enhance the mechanical strength of hydrogels prepared by F-T cycles, significantly improving mechanical properties, including fracture toughness and tensile strength (Cao J. L. et al., 2022); hydrogels are subjected to 50°C–120°C and constant humidity for several hours to increase their crystallinity (Chen J. D. et al., 2022).
The third is the salting method that effectively enhances hydrogel mechanical strength and toughness (Wu et al., 2021; Yan G. H. et al., 2022) (Figure 6A). Higher concentrations of salt solutions would further enhance the effect on the mechanical properties of hydrogels. Accordingly, the mechanical properties of PVA hydrogels can be adjusted on a large scale by changing the salt type and concentration. The interaction between ions, water molecules, and polymer chains induces different degrees of aggregation of polymer chains, resulting in significantly different mechanical properties. The saturated Na2SO4 solution-treated PVA hydrogels exhibit considerable strength (15 ± 1 MPa), toughness (150 ± 20 MJ m-3), and elongation (2,100% ± 300%) (Wu et al., 2021).
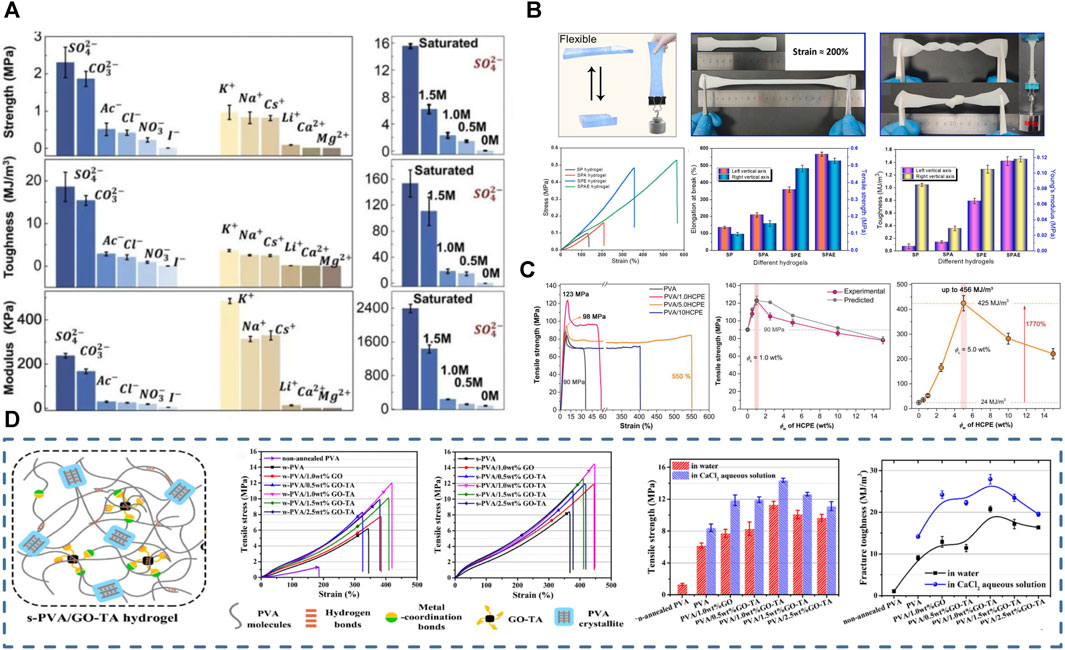
FIGURE 6. Methods to enhance the mechanical properties of conductive PVA-based hydrogels; (A) Salt precipitation, especially immersion in Na2SO4 solution can substantially enhance the toughness of hydrogels (Wu et al., 2021); (B) Starch/PVA dual-network hydrogels possess good tensile properties and tensile strength (Lu L. et al., 2022); (C) Small multi-amine molecules enable the formation of dynamic nanoconfinement in the hydrogel, allowing the hydrogel to have excellent mechanical strength (Liu et al., 2021); (D) Composite PVA-based hydrogels formed with the introduction of a large number of metal-ligand and reversible bonds possess good mechanical strength (Cao J. L. et al., 2022).
3.2.2.2 Improving the mechanical strength of hydrogels by enhancing PVA networks
The most common approach to improve the hydrogel mechanical strength is to build multiple networks (Figure 6B). The porous structure of PVA-based hydrogels allows them to act as a flexible backbone, and the interpenetrating network increases hydrogen bonding and electrostatic interactions in the material. The multi-network design can effectively improve the mechanical properties of hydrogels (Lu L. et al., 2022). For example, the double network of PVA and sodium alginate has excellent mechanical properties due to chelation between mineral ions and organic matrices (Shen et al., 2022). Additionally, the multi-network synergistic PVA/sodium carboxymethylcellulose/TA/MXene hydrogels show good mechanical strength (1.8 MPa/6.24 MJ m–3) (Yan G. H. et al., 2022) (Figure 6A).
Increasing the coordination or reversible bond type and number in the hydrogel network can also enhance its structural stability (Figure 6D). As mentioned previously, carbon nanofibers (Li et al., 2022b), tannins (Mredha and Jeon, 2022; Zhan et al., 2022), dopamine and polyamine molecules (Liu et al., 2021) are widely used as hydrogen bonding cross-linkers for PVA-based hydrogels. F-T-crosslinked hydrogels are more biocompatible than chemically crosslinked ones, although their structure is less stable. Hydrogen bonding is a physical interaction force that allows for tight junctions between PVA molecules–due to the abundant hydrogen bonds–and does not impair the bio-friendly properties of PVA materials. Therefore, the hydrogen bonding cross-linkers enhance the mechanical properties of physically cross-linked PVA-based hydrogels. Using hydrogen bonding cross-linkers can significantly enhance the strength and toughness of hydrogels. For example, Liu et al. (2021) prepared a PVA/HCPE (small multi-amine molecules) composite hydrogel using polyamine molecules, showing excellent fracture strength and strain to 98 MPa and 550% (Figure 6C).
3.2.3 Self-healing and self-adhesive
3.2.3.1 Self-healing
Self-healing materials have attracted a great deal of interest from researchers. The ideal self-healing material should be able to restore the mechanical properties and electrochemical functions relatively quickly. PVA-based hydrogels achieve self-healing mainly relying on the reversible breakage and reconstruction of dynamic covalent bonds and supramolecular interactions (Peng et al., 2020). So far, reversible chemical bonds (including disulfide bonds (Li et al., 2022e; Ling et al., 2022), borate ester bonds (Qin et al., 2022), Diels–Alder reactions, and reversible free radical reactions) and non-covalent interactions (including hydrophobic interactions, host-guest interactions, hydrogen bonds, and various weak molecular interactions) have been used to make self-healing hydrogels (Cao J. L. et al., 2022) (Figure 7A). When the material is stressed, dynamic bonds break and reorganize reversibly. Consequently, the material can have beneficial properties such as self-healing, shape memory, and better adaptability, leading to several applications of hydrogels in biomedicine, such as pressure sensors, wound dressings, and tissue adhesives (Takacs et al., 2022). Many polymers connected by non-covalent interactions can also achieve self-healing. However, high temperature or F-T are often required, which prolong self-healing time. Therefore, they can be used for material recycling but do not meet the need for immediate self-healing as electronic skin.
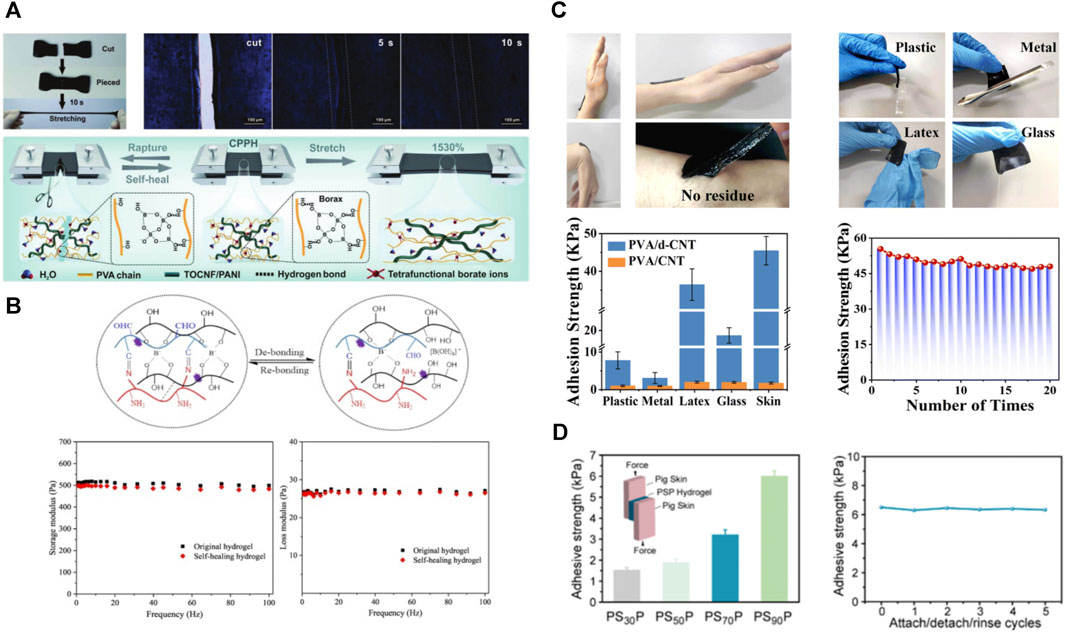
FIGURE 7. Reversible chemical bonding and non-covalent interactions can confer good self-healing properties to PVA-based hydrogels. (A) Self-healing photographs and pattern diagrams of PVA-based hydrogels enriched with borate ester bonding and hydrogen bonding (Qin et al., 2022); (B) Schematic illustration of the principle of self-healing in PVA-based hydrogels, and their energy storage modulus and loss modulus before and after self-healing (Cao J. L. et al., 2022); (C) Copolymerization with poly (dopamine) enabled the PVA-based hydrogel to obtain excellent adhesion properties, and showed a certain adhesion strength to different substrates (Zhu H. et al., 2022); (D) Repeatable interfacial adhesion of the PVA-based hydrogel to the pig skin exhibit (Huang et al., 2023).
3.2.3.2 Self-adhesive
The tissue adhesion ability of hydrogel is attributed to the chemical/physical bond formed between the hydrogel and the tissue. The amide bond is a usual chemical bond between bonding materials and tissues. The attachment between the aldehyde group of the hydrogel and the amino group of the surrounding tissue occurs because of the abundant presence of amino groups on the tissue surface (Gao et al., 2019). The main way to obtain tissue adhesion in PVA-based hydrogels is by introducing catechol groups or copolymerization with acrylic acid with abundant carboxyl groups (Su et al., 2020a; Zhu H. et al., 2022; Huang et al., 2023) (Figure 7C).
Some technical obstacles limit the development of underwater tissue adhesion hydrogels. First, current underwater adhesives rely mainly on surface patterning (Rao et al., 2018), dense interfacial physical bonding (Wang Z. et al., 2020), surface water absorption with nanocrystal formation or self-hydrophobicity to produce greater physical adhesion to various solid surfaces (Han et al., 2020). Most current underwater adhesives have a higher affinity for non-biological materials such as glass and metal. The second is the difficulty of rapid tissue adhesion and self-healing underwater. Unlike inorganic hydrophilic materials (e.g., glass and metals), animal tissues often contain oils or other hydrophobic substances, greatly reducing the adhesive capacity of most bonded hydrogels (Shao et al., 2018). Hydrophilic groups on the surface of hydrogels and tissues tend to form hydrogen, electrostatic bonds, or both with water molecules and organic components (e.g., glycine) underwater. This can further weaken the hydrogel-tissue and hydrogel-hydrogel interactions (Zhong et al., 2014). The third obstacle is the large swelling rate of most viscous hydrogels in water, which causes significant swelling and embrittlement of the hydrogel network in an aqueous environment. When swelling occurs, the adhesion usually decreases sharply due to the significant decreases in the bonding functional group density.
He et al. reported PVA/tannic acid/carbon nanotubes hydrogel had the ability to adhere pigskin underwater. Due to the presence of a large number of catechol and o-phenyltriol groups in tannic acid, it can endow hydrogel with self-adhesive properties through the formation of hydrogen bonds, electrostatic interactions, and hydrophobic interactions (He et al., 2022). The starch/PVA-borax/tea polyphenol/ethylene glycol hydrogel reported by Tao et al. also showed underwater adhesion properties. The adhesion of this hydrogel is mainly accomplished through the polyphenol chemistry of tea polyphenol, i.e., hydrogen bonding, hydrophobic interactions, metal coordination, and physical interactions (Ke et al., 2023). Other non PVA-based hydrogels, such as acrylamide composite hydrogels, also use hydrophobic properties to obtain underwater adhesion (Han et al., 2020).
3.2.4 Other characteristics
3.2.4.1 Frost resistance and durability
Conventional conductive hydrogels consist mainly of water and are susceptible to environmental factors due to the crystallization of water molecules below zero degrees, which results in a precipitous drop in the hydrogel conductivity at low temperatures. Conversely, hydrogels tend to dehydrate and become dry and hard when exposed to high temperatures. During the working process, the hydrogel gradually loses its original mechanical properties, flexibility, and ionic conductivity due to water loss. Applying binary solvents is one of the main methods to achieve freeze resistance and durability of PVA-based hydrogels. The organic solvents ethylene glycol, glycerol, and dimethyl sulfoxide were introduced into the gel to replace some water molecules (Li et al., 2022c) (Figure 4C). The formation of strong hydrogen bonding interactions between organic solvent and water molecules effectively inhibits forming ice crystals at low temperatures and dehydration at high temperatures. For example, GPPD (Graphene oxide–- PVA/polyacrylamide–- double network) hydrogel was prepared using a binary solvent system of ethylene glycol/water. The device possesses excellent cold resistance and moisture retention properties; it can work stably at very low temperatures of–50°C to–80°C and has a moisture retention duration of 100 days (Dai et al., 2022) (Figure 5F). Strong hydrogen bonding improves the gel stability for use in harsh environments and greatly slows the drying. Chelation between metal ions and organic matrices can also give hydrogels excellent antifreeze properties (Zhu X. Q. et al., 2022; Shen et al., 2022). For example, Mg2+ and Ca2+ composite hydrogels prepared by Shen et al. demonstrate excellent freeze resistance performance (Shen et al., 2022).
3.2.4.2 Self-powered capability
The self-powered capability allows hydrogel sensors to be adapted to multiple uses, acting as an intrinsic power source and a functional component (Han et al., 2022). Some interesting designs address the energy issues when applying PVA-based hydrogels as flexible sensors. The main designs for flexible wearable devices to achieve self-power are solar cells, friction nanogenerators (Xu et al., 2017; Xu et al., 2023), thermoelectric generators (Khan et al., 2016), and moisture generators (Hu et al., 2021). Among them, friction nanopower (Luo et al., 2021) and water vapor power (Hu et al., 2021) have been practiced in PVA-based hydrogels. Frictional nanogenerators use the friction between the hydrogel and the skin when the joint moves to convert the bending behavior into a voltage signal. Moisture generators are instead implemented by a polymer with ionization groups that spontaneously absorb water molecules from moist air and release positively/negatively charged ions (Luo et al., 2021). For example, Qin et al. (2022) designed a self-powered sweat sensor that enables real-time health monitoring by detecting soluble biomarkers such as electrolytes or metabolites.
3.3 Wound dressing
The ideal wound dressing must have passive and active protection to promote wound healing. Passive protection means it fits flexibly over the affected area, insulates the wound from contact with airborne dust and bacteria, and prevents secondary damage from external physical friction. The stretchability of human natural skin is 60%–75%, and the elastic modulus is 5–1,000 kPa in smaller strains (Chen, 2017; Qu et al., 2018), but it can even reach 4.1–18.8 MPa in larger strains (Joodaki and Panzer, 2018). Thus most of the PVA-based hydrogels are suitable for skin applications in terms of tensile properties and elastic modulus. Active protection means equipped with antibacterial and anti-inflammatory features that can slow-release drugs to kill bacteria or promote wound healing. The PVA-based hydrogel structure is similar to that of a natural extracellular matrix, with high water content, flexibility, loose porosity, and other characteristics, representing an ideal candidate for wound dressing materials. The high water content allows the hydrogel to provide a moist environment to the wound and prevent dehydration (Luo et al., 2021). The porous structure and biodegradability of hydrogels facilitate drug delivery, oxygen transport, and slow drug release (Khorasani et al., 2018; Montaser et al., 2019). Accordingly, PVA-based hydrogels are commonly used as wound dressings. The main research directions are to promote the antibacterial and anti-inflammatory effects and achieve conditionally responsive drug release.
3.3.1 Antibacterial and anti-inflammatory
Bacterial infection and inflammation play a crucial role in the development and persistence of chronic wounds. Consequently, it is essential to consider these factors when utilizing hydrogels as wet dressings. PVA-based hydrogels exhibit antimicrobial activity primarily through copolymerization with natural antimicrobial agents, incorporation of antimicrobial components, and application of near-infrared light (NIR) irradiation. The synergistic effect of these various antimicrobial mechanisms has demonstrated notable efficacy.
The first method is copolymerization with natural antibacterial ingredients. Natural polymers such as chitosan (Shamloo et al., 2021; Cao J. L. et al., 2022; Liu et al., 2023), silk protein (Eivazzadeh-Keihan et al., 2020), and sodium alginate (Abbasi et al., 2020) possess antibacterial activity. Its copolymerization with PVA can give the wound dressing a better healing promotion function and improve its weak mechanical properties (Figure 8B). For example, chitosan-PVA hydrogels have been widely used as trauma dressings, and this composite material possesses excellent antimicrobial activity, and promotes wound healing (Liu et al., 2018). The NH3+ in chitosan can interact with negatively charged cell membranes, leading to cytoplasmic extravasation. Low molecular weight chitosan can penetrate bacteria and destroy DNA. High molecular weight chitosan can cover the cell surface and inactivate bacteria by depriving them of nutrient supply (Khorasani et al., 2019).
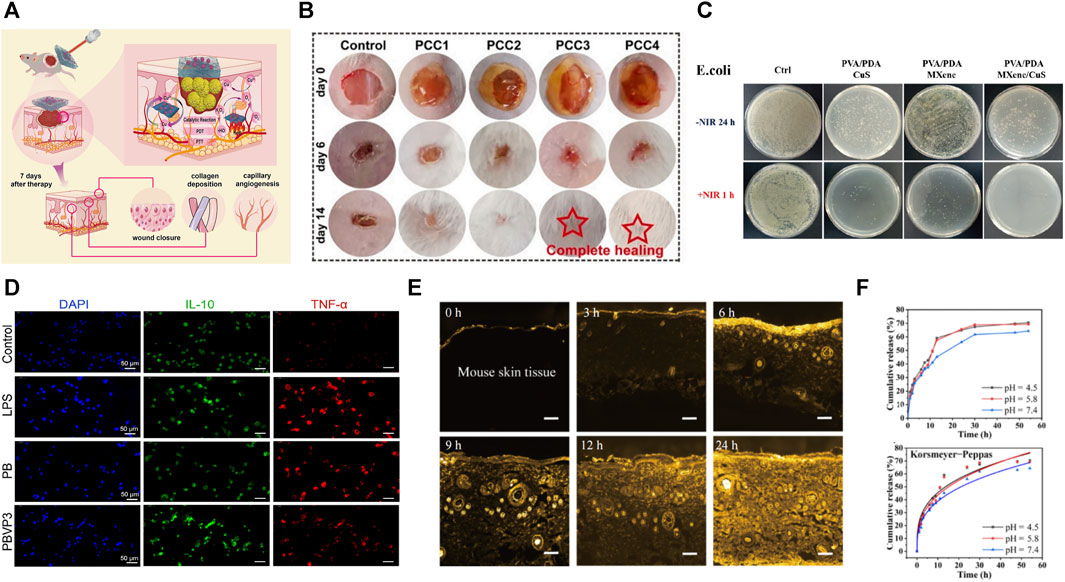
FIGURE 8. (A) PVA-based hydrogels can be affixed to chronic wounds and slowly release drugs under NIR light stimulation (Su et al., 2023); (B) PVA-based hydrogels can promote hydrogel wound healing (Liu et al., 2023); (C) PVA-based hydrogels can be copolymerized with antimicrobial active materials to obtain better antimicrobial properties (Su et al., 2023); (D) PVA-based hydrogels show better anti-inflammatory effects (Liu B. et al., 2022); (E,F) PVA-based hydrogels can achieve long-lasting drug release (Chen S. Y. et al., 2022).
The second method is to load inorganic antimicrobial ingredients. Many inorganic antimicrobial materials have been successfully incorporated into PVA-based hydrogels to synthesize various antimicrobial hydrogels (Wang et al., 2020). It mainly includes metal nanoparticles, metal ions, and carbon nanomaterials. These materials had three main antibacterial mechanisms: the first is that nanomaterials in photocatalysis can produce singlet oxygen (1O2) and hydroxyl radicals (·OH) to achieve sterilization through catalytic oxidation–photodynamic therapy (PDT) (Wang et al., 2019; Su et al., 2023); nanomaterial Ti3C2Tx (MXene) (Su et al., 2023), gold nanorods (Qin et al., 2023), and graphene oxide (Wang J. H. et al., 2022) have been widely used for preparing photocatalytic antibacterial dressings (Figure 8C). The second is the use of metal ions to disrupt cell membranes, resulting in the leakage of intracellular substances. Some of these smaller-sized metal elements can pass through the cell membrane and act on the cytoplasm, damaging the bacterial DNA and proteins. Many metallic materials [metallic nanoparticles such as silver (Song et al., 2021; Mojally et al., 2022; Qi et al., 2022; Ke et al., 2023), gold (Qin et al., 2023), cerium oxide (Kalantari et al., 2020), and zinc oxide (Khorasani et al., 2018; Raafat et al., 2018), as well as metal ions such as Cu2+ (Xia et al., 2022; Xia et al., 2022), Fe3+ (Qi et al., 2022), Al3+(Li et al., 2022b; Wang Y. et al., 2022), and Mg2+ (Eivazzadeh-Keihan et al., 2020; Albalwi et al., 2022)] have been successfully loaded into PVA-based hydrogels with good antibacterial effects. The third uses the photothermal effect the photothermal agent produces under NIR excitation–photothermal therapy; the principle is that during NIR irradiation, the photothermal agent generates heat, causing a local high temperature and irreversible damage to microorganism protein and enzymes, thus achieving sterilization (Su et al., 2023) (Figure 8A). Many materials with photothermal effects are used for photothermal therapy, such as carbide polymer dots (CPD) (Lu H. J. et al., 2022), graphene oxide (Wang J. H. et al., 2022), and molybdenum disulfide (MoS2) (Li et al., 2022e). Qin et al. (2023) designed a PAA/PVA-gold nanorod hydrogel that can rapidly and efficiently disrupt bacterial biofilms and promote infected wound healing under NIR irradiation.
Another method is to load organic antibacterial ingredients. For example, antibiotics (Qiao et al., 2020) [e.g., mupirocin (Zhao H. et al., 2020) and amikacin (Abbasi et al., 2020)], tannins (Li et al., 2022e; Su et al., 2023; Yi et al., 2023), antimicrobial peptides (Zou et al., 2020), and allicin (Wang J. H. et al., 2022) showed good broad-spectrum antibacterial activity in the PVA-based hydrogel bacteria. The design of loaded antimicrobial ingredients suffers from antimicrobial activity loss due to the active antimicrobial ingredient release. Non-releasing antimicrobial materials such as antimicrobial peptides, graphene oxide, chitosan, and ionic liquids can impart sustained antimicrobial activity to hydrogels. Liu B. et al. (2022) designed and synthesized a polymeric ionic liquid-PVA-borax tri-crosslinked hydrogel that achieved long-lasting antibacterial effects due to the non-releasing nature of ionic liquid (Figure 8D).
Furthermore, some studies have promoted wound healing by imparting hydrogel reactive oxygen scavenging (Zhao H. et al., 2020; Qiao et al., 2020) and oxygenation capacity (Li et al., 2022e; Ling et al., 2022; Nour et al., 2022) to slow the inflammatory response of wounds. The MoS2@TA/Fe enzyme-anchored multifunctional hydrogel has both advantages: MoS2 and TA can scavenge excess free radicals from the affected area, while catalase-like enzymes can decompose H2O2 to provide O2 to the affected area (Li et al., 2022e).
3.3.2 Drug delivery and conditional responsive release
The high water content, good biocompatibility, and the ability to easily encapsulate hydrophilic drugs make PVA-based hydrogels a desirable drug release system (Yao et al., 2022) (Figure 8E). The drug loading and delivery rate are mainly related to the hydrogel microstructure and swelling. Molecular imprinting is used to prepare hydrogels, which can be tailored to the hydrogel microstructure by controlling their polymerization behavior, facilitating hydrogel preparation for efficient and targeted drug release (Kakkar and Narula, 2022). The specific microstructure allows the hydrogel to respond to changes in the wound microenvironment or to the production of a certain substance. Hydrogels that exhibit responsiveness to pH, light, temperature, and other biological signals have garnered considerable attention (Qin et al., 2023).
PVA-based hydrogels can achieve environmentally responsive drug release through reactive cross-linking agents. For example, a ROS-scavenging hydrogel using ROS-responsive linker cross-linking was designed by Zhao H. et al. (2020), which downregulated pro-inflammatory cytokines, upregulated M2 phenotype macrophages, and promoted angiogenesis and collagen production. Meanwhile, due to the responsive cutting of the cross-linker, the hydrogel gradually degrades and releases loaded mupirocin and granulocyte-macrophage colony-stimulating factors, which inhibit bacterial infection and accelerate wound repair, respectively.
Part of the design achieves responsive release of the drug by copolymerizing PVA with a bio-signal responsive material. For example, sodium alginate, rich in carboxyl groups, can accept or release loaded anti-inflammatory drugs depending on the environmental pH. PVA/SA hydrogels have successfully controlled pH-sensitive drug release (Montaser et al., 2019; Abbasi et al., 2020). Qiao et al. (2020) developed a smart hydrogel-based trauma dressing by physically cross-linking PVA with an UV-cleavable polymer. The hydrogel can monitor bacterial infections by detecting specific substances they release and treating infections by stimulating antibiotic release through NIR. Qin et al. (2023) prepared a photothermal nanocomposite hydrogel that responds to NIR stimulation by exploiting the photothermal effect of gold nano-ions. The hydrogel could rapidly eliminate over 80% of Gram-negative/positive bacteria under NIR irradiation.
3.4 Other applications
PVA-based hydrogels are widely used in various biomedicine fields due to their good biocompatibility and suitable and tunable mechanical properties, showing great potential for applications in long-acting drug delivery, treatment, and chronic disease diagnosis.
Hydrogels have been designed as skin microneedle patches due to their water-absorbing properties and ease of transition between relatively rigid and flexible states (Wu et al., 2021). Microneedle (MN) based transdermal drug delivery systems are gaining interest as an alternative to traditional vaccine, drug, and cosmetic delivery methods. Chen S. Y. et al. (2022) designed a borate-PVA hydrogel for efficient skin penetration and sustained glucose-responsive insulin delivery (Figure 9A).
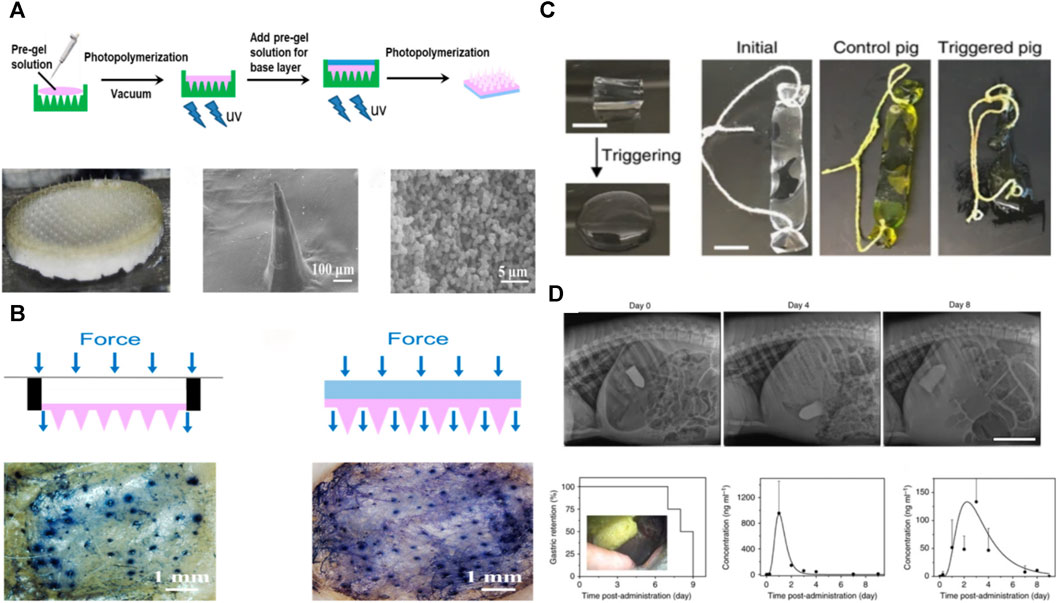
FIGURE 9. Other applications of PVA-based hydrogels; (A) Schematic and photographs of the synthesis of PVA-based hydrogels for microneedle patches; (B) Schematic and photographs of microneedle patch application (Chen S. Y. et al., 2022); (C) PVA-based hydrogel for gastric retention device, which can be rapidly dissolved in the stomach and retained for a long period of time up to 30 days (Liu et al., 2019); (D) Gastric retention device in porcine stomach with X-ray photographs and drug release profile (Liu et al., 2017).
Patients often need to take medications continuously for an extended period during chronic disease treatment; a carrier for a long-acting, sustained-release drug can simplify the treatment process, facilitate chronic disease management, and help improve patient compliance with medication. For example, Sunita et al. loaded PVA-based hydrogels with calcipotriol to treat psoriasis by adding polyvinylpyrrolidone to PVA to improve its hydrophilicity and water permeability (Thakur et al., 2023). The results show that the hydrogel can achieve prolonged drug release, promote epithelial tissue regeneration, and heal psoriatic lesions.
Hydrogels are ideal for gastric retention materials, enabling in vivo physiological monitoring, diagnostics, and extended drug delivery (Bellinger et al., 2016; Liu et al., 2017). A gastric retention device was prepared by Liu et al. (2019) that utilized polyacrylic acid particles with high water absorption to encapsulate a PVA-based hydrogel. It can rapidly absorb water and swell and reside in the stomach of the animal for a long time. The porous and loose structure of PVA-based hydrogels makes loading drugs easier, while adding nanocrystalline domains makes them robust, resilient, and fatigue-resistant. This is the core principle of the ability to perform therapeutically and remain robustly in the stomach under repetitive mechanical compression and gastric acid erosion (Figure 9C).
A hybrid coupling consisting of a PVA-coated Aβ probe has high sensitivity, selectivity, biocompatibility, and economy; this nanoprobe can enable the early diagnosis of Alzheimer’s disease by detecting Aβ peptides in human serum (Hamd-Ghadareh et al., 2022). In addition, PVA-based hydrogels have also been applied in areas such as crystalline lenses (Ran et al., 2023) and vascular pathways (Mannarino et al., 2020).
4 Summary and prospect
Due to the unique structure of PVA, PVA-based hydrogels have multiple promising applications in biomedicine. The researchers adopted different methods to make PVA-based hydrogels obtain various properties. These methods have their limitations and cannot adapt to all situations. For example, due to the low temperature and annealing, the physical cross-linking method for preparing hydrogel cannot be used for cell-loading research. Preparing the hydrogel by chemical cross-linking introduces a residual chemical reagent, which is not conducive to cell growth. The high UV radiation intensity is not conducive to cell loading and growth, and the low radiation intensity cannot form a high-strength hydrogel. Therefore, many problems need to be solved with PVA-based hydrogels. Accordingly, the best solution is to combine physical and chemical methods and explore innovative ways to prepare hydrogels.
Author contributions
YZ: Writing–original draft. QL: Writing–review and editing. HY: Writing–review and editing. LS: Writing–review and editing. XC: Writing–review and editing. QP: Writing–review and editing. YZ: Writing–review and editing. RH: Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (32000942), One health Interdisciplinary Research Project, Ningbo University (HY202209), the Natural Science Foundation of Ningbo (2023J082), the General Research Projects of Zhejiang Provincial Department of Education (Y202248671, Y202044155), the Natural Science Foundation of Zhejiang (LQ22C100001), Open Foundation of the State Key Laboratory of Fluid Power and Mechatronic Systems (GZKF-202024), Zhejiang Provincial Medical and Health Science and Technology Plan Project (2023KY241), National 111 Project of China (D16013).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbasi, A. R., Sohail, M., Minhas, M. U., Khaliq, T., Kousar, M., Khan, S., et al. (2020). Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 155, 751–765. doi:10.1016/j.ijbiomac.2020.03.248
Abodurexiti, A., and Maimaitiyiming, X. (2022). Self-healing, anti-freezing hydrogels and its application in diversified skin-like electronic sensors. Ieee Sensors J. 22, 12588–12594. doi:10.1109/jsen.2022.3157709
Abouzeid, R. E., Khiari, R., Salama, A., Diab, M., Beneventi, D., and Dufresne, A. (2020). In situ mineralization of nano-hydroxyapatite on bifunctional cellulose nanofiber/polyvinyl alcohol/sodium alginate hydrogel using 3D printing. Int. J. Biol. Macromol. 160, 538–547. doi:10.1016/j.ijbiomac.2020.05.181
Albalwi, H., Abou El Fadl, F. I., Ibrahim, M. M., and Abou Taleb, M. F. (2022). Antibacterial impact of acrylic acid/polyvinyl alcohol/MgO various nanocomposite hydrogels prepared by gamma radiation. Polym. Bull. 79, 7697–7709. doi:10.1007/s00289-021-03866-9
Altaf, F., Niazi, M. B. K., Jahan, Z., Ahmad, T., Akram, M. A., Safdar, A., et al. (2020). Synthesis and characterization of PVA/starch hydrogel membranes incorporating essential oils aimed to be used in wound dressing applications. J. Polym. Environ. 29, 156–174. doi:10.1007/s10924-020-01866-w
Amiri, S., Asghari, A., Harifi-Mood, A. R., Rajabi, M., He, T., and Vatanpour, V. (2022). Polyvinyl alcohol and sodium alginate hydrogel coating with different crosslinking procedures on a PSf support for fabricating high-flux NF membranes. Chemosphere 308, 136323. doi:10.1016/j.chemosphere.2022.136323
Baldwin, R. L. (1996). How Hofmeister ion interactions affect protein stability. Biophysical J. 71, 2056–2063. doi:10.1016/s0006-3495(96)79404-3
Bellinger, A. M., Jafari, M., Grant, T. M., Zhang, S. Y., Slater, H. C., Wenger, E. A., et al. (2016). Oral, ultra-long-lasting drug delivery: application toward malaria elimination goals. Sci. Transl. Med. 8, 365ra157. doi:10.1126/scitranslmed.aag2374
Branco, A. C., Oliveira, A. S., Monteiro, I., Nolasco, P., Silva, D. C., Figueiredo-Pina, C. G., et al. (2022). PVA-based hydrogels loaded with diclofenac for cartilage replacement. Gels 8, 143. doi:10.3390/gels8030143
Cao, J. L., He, G., Ning, X., Chen, X., Fan, L., Yang, M., et al. (2022a). Preparation and properties of O-chitosan quaternary ammonium salt/polyvinyl alcohol/graphene oxide dual self-healing hydrogel. Carbohydr. Polym. 287, 119318. doi:10.1016/j.carbpol.2022.119318
Cao, J. L., Zhao, X. W., and Ye, L. (2022b). Super-strong and anti-tearing poly(vinyl alcohol)/graphene oxide nano-composite hydrogels fabricated by formation of multiple crosslinking bonding network structure. J. Industrial Eng. Chem. 112, 366–378. doi:10.1016/j.jiec.2022.05.034
Chen, J. D., Dong, Z. C., Li, M., Li, X. P., Chen, K., and Yin, P. C. (2022a). Ultra-strong and proton conductive aqua-based adhesives from facile blending of polyvinyl alcohol and tungsten oxide clusters. Adv. Funct. Mater. 32, 2111892. doi:10.1002/adfm.202111892
Chen, K., Chen, G. Y., Wei, S., Yang, X. H., Zhang, D. K., and Xu, L. M. (2018). Preparation and property of high strength and low friction PVA-HA/PAA composite hydrogel using annealing treatment. Mater. Sci. Eng. C-Materials Biol. Appl. 91, 579–588. doi:10.1016/j.msec.2018.05.080
Chen, M., Chen, J., Zhou, W., Xu, J., and Wong, C.-P. (2019). High-performance flexible and self-healable quasi-solid-state zinc-ion hybrid supercapacitor based on borax-crosslinked polyvinyl alcohol/nanocellulose hydrogel electrolyte. J. Mater. Chem. A 7, 26524–26532. doi:10.1039/c9ta10944g
Chen, Q., Miao, X., Liu, Y., Zhang, X., Chen, S., Chen, Z., et al. (2021a). Polyaniline electropolymerized within template of vertically ordered polyvinyl alcohol as electrodes of flexible supercapacitors with long cycle life. Electrochimica Acta 390, 138819–138899. doi:10.1016/j.electacta.2021.138819
Chen, S. Y., Miyazaki, T., Itoh, M., Matsumoto, H., Moro-Oka, Y., Tanaka, M., et al. (2022b). A porous reservoir-backed boronate gel microneedle for efficient skin penetration and sustained glucose-responsive insulin delivery. Gels 8, 74. doi:10.3390/gels8020074
Chen, X. D. (2017). Making electrodes stretchable. Small Methods 1, 1600029. doi:10.1002/smtd.201600029
Chen, Y., Song, J., Wang, S., and Liu, W. (2021b). PVA-based hydrogels: promising candidates for articular cartilage repair. Macromol. Biosci. 21, e2100147. doi:10.1002/mabi.202100147
Dai, X. H., Long, Y., Jiang, B., Guo, W. B., Sha, W., Wang, J. W., et al. (2022). Ultra-antifreeze, ultra-stretchable, transparent, and conductive hydrogel for multi-functional flexible electronics as strain sensor and triboelectric nanogenerator. Nano Res. 15, 5461–5468. doi:10.1007/s12274-022-4153-5
Ding, Q. Q., Xu, X., Yue, Y., Mei, C., Huang, C., Jiang, S., et al. (2018). Nanocellulose-Mediated electroconductive self-healing hydrogels with high strength, plasticity, viscoelasticity, stretchability, and biocompatibility toward multifunctional applications. ACS Appl. Mater Interfaces 10, 27987–28002. doi:10.1021/acsami.8b09656
Duan, S. D., Wu, S. W., Hua, M. T., Wu, D., Yan, Y. C., Zhu, X. Y., et al. (2021). Tendon-inspired anti-freezing tough gels. Iscience 24, 102989. doi:10.1016/j.isci.2021.102989
Ebhodaghe, S. O. (2020). Hydrogel – based biopolymers for regenerative medicine applications: a critical review. Int. J. Polym. Mater. Polym. Biomaterials 71, 155–172. doi:10.1080/00914037.2020.1809409
Eghbalifam, N., Frounchi, M., and Dadbin, S. (2015). Antibacterial silver nanoparticles in polyvinyl alcohol/sodium alginate blend produced by gamma irradiation. Int. J. Biol. Macromol. 80, 170–176. doi:10.1016/j.ijbiomac.2015.06.042
Eivazzadeh-Keihan, R., Khalili, F., Aliabadi, H. A. M., Maleki, A., Madanchi, H., Ziabari, E. Z., et al. (2020). Alginate hydrogel-polyvinyl alcohol/silk fibroin/magnesium hydroxide nanorods: a novel scaffold with biological and antibacterial activity and improved mechanical properties. Int. J. Biol. Macromol. 162, 1959–1971. doi:10.1016/j.ijbiomac.2020.08.090
Fan, L., Xie, J., Zheng, Y., Wei, D., Yao, D., Zhang, J., et al. (2020). Antibacterial, self-adhesive, recyclable, and tough conductive composite hydrogels for ultrasensitive strain sensing. ACS Appl. Mater Interfaces 12, 22225–22236. doi:10.1021/acsami.0c06091
Fang, X., Li, Y., Li, X., Liu, W., Yu, X., Yan, F., et al. (2020). Dynamic hydrophobic domains enable the fabrication of mechanically robust and highly elastic poly(vinyl alcohol)-based hydrogels with excellent self-healing ability. ACS Mater. Lett. 2, 764–770. doi:10.1021/acsmaterialslett.0c00075
Gan, S. C., Bai, S. H., Chen, C., Zou, Y. L., Sun, Y. J., Zhao, J. H., et al. (2021). Hydroxypropyl cellulose enhanced ionic conductive double-network hydrogels. Int. J. Biol. Macromol. 181, 418–425. doi:10.1016/j.ijbiomac.2021.03.068
Gao, L., Zhou, Y., Peng, J., Xu, C., Xu, Q., Xing, M., et al. (2019). A novel dual-adhesive and bioactive hydrogel activated by bioglass for wound healing. NPG Asia Mater. 11, 66. doi:10.1038/s41427-019-0168-0
Gao, Y., Gu, S., Jia, F., and Gao, G. (2020). A skin-matchable, recyclable and biofriendly strain sensor based on a hydrolyzed keratin-containing hydrogel. J. Mater. Chem. A 8, 24175–24183. doi:10.1039/d0ta07883b
Guo, L., Ma, W.-B., Wang, Y., Song, X.-Z., Ma, J., Han, X.-D., et al. (2020). A chemically crosslinked hydrogel electrolyte based all-in-one flexible supercapacitor with superior performance. J. Alloys Compd. 843, 155895. doi:10.1016/j.jallcom.2020.155895
Guo, Z. Y., Zhang, Z. Z., Zhang, N., Gao, W. X., Li, J., Pu, Y. J., et al. (2022). A Mg2+/polydopamine composite hydrogel for the acceleration of infected wound healing. Bioact. Mater. 15, 203–213. doi:10.1016/j.bioactmat.2021.11.036
Hamd-Ghadareh, S., Salimi, A., Parsa, S., and Mowla, S. J. (2022). Development of three-dimensional semi-solid hydrogel matrices for ratiometric fluorescence sensing of Amyloid β peptide and imaging in SH-SY5 cells: improvement of point of care diagnosis of Alzheimer's disease biomarker. Biosens. Bioelectron. 199, 113895. doi:10.1016/j.bios.2021.113895
Han, J., Ding, Q., Mei, C., Wu, Q., Yue, Y., and Xu, X. (2019a). An intrinsically self-healing and biocompatible electroconductive hydrogel based on nanostructured nanocellulose-polyaniline complexes embedded in a viscoelastic polymer network towards flexible conductors and electrodes. Electrochimica Acta 318, 660–672. doi:10.1016/j.electacta.2019.06.132
Han, J., Wang, H., Yue, Y., Mei, C., Chen, J., Huang, C., et al. (2019b). A self-healable and highly flexible supercapacitor integrated by dynamically cross-linked electro-conductive hydrogels based on nanocellulose-templated carbon nanotubes embedded in a viscoelastic polymer network. Carbon 149, 1–18. doi:10.1016/j.carbon.2019.04.029
Han, L., Wang, M. H., Prieto-Lopez, L. O., Deng, X., and Cui, J. X. (2020). Self-hydrophobization in a dynamic hydrogel for creating nonspecific repeatable underwater adhesion. Adv. Funct. Mater. 30, 1907064. doi:10.1002/adfm.201907064
Han, L. B., Zhou, Q. F., Chen, D. S., Qu, R., Liu, L., Chen, Y. H., et al. (2022). Flexible sensitive hydrogel sensor with self-powered capability. Colloids Surfaces a-Physicochemical Eng. Aspects 639, 128381. doi:10.1016/j.colsurfa.2022.128381
Han, X. W., Wang, Z. X., Zhou, Z. J., Peng, Y. K., Zhang, T., Chen, H. Y., et al. (2023). Aldehyde modified cellulose-based dual stimuli responsive multiple cross-linked network ionic hydrogel toward ionic skin and aquatic environment communication sensors. Int. J. Biol. Macromol. 252, 126533. doi:10.1016/j.ijbiomac.2023.126533
He, P., Wu, J., Pan, X., Chen, L., Liu, K., Gao, H., et al. (2020). Anti-freezing and moisturizing conductive hydrogels for strain sensing and moist-electric generation applications. J. Mater. Chem. A 8, 3109–3118. doi:10.1039/c9ta12940e
He, Z., Liu, J. C., Fan, X., Song, B., and Gu, H. B. (2022). Tara tannin-cross-linked, underwater-adhesive, super self-healing, and recyclable gelatin-based conductive hydrogel as a strain sensor. Industrial Eng. Chem. Res. 61, 17915–17929. doi:10.1021/acs.iecr.2c03253
Holloway, J. L., Spiller, K. L., Lowman, A. M., and Palmese, G. R. (2011). Analysis of the in vitro swelling behavior of poly(vinyl alcohol) hydrogels in osmotic pressure solution for soft tissue replacement. Acta Biomater. 7, 2477–2482. doi:10.1016/j.actbio.2011.02.016
Hosseini, M. S., and Nabid, M. R. (2020). Synthesis of chemically cross-linked hydrogel films based on basil seed (Ocimum basilicum L.) mucilage for wound dressing drug delivery applications. Int. J. Biol. Macromol. 163, 336–347. doi:10.1016/j.ijbiomac.2020.06.252
Hou, R., Nie, L., Du, G., Xiong, X., and Fu, J. (2015). Natural polysaccharides promote chondrocyte adhesion and proliferation on magnetic nanoparticle/PVA composite hydrogels. Colloids Surf. B Biointerfaces 132, 146–154. doi:10.1016/j.colsurfb.2015.05.008
Hou, R., Zhang, G., Du, G., Zhan, D., Cong, Y., Cheng, Y., et al. (2013). Magnetic nanohydroxyapatite/PVA composite hydrogels for promoted osteoblast adhesion and proliferation. Colloids Surf. B Biointerfaces 103, 318–325. doi:10.1016/j.colsurfb.2012.10.067
Hu, F., Lu, H. L., Xu, G. S., Lv, L. F., Chen, L., and Shao, Z. L. (2022). Carbon quantum dots improve the mechanical behavior of polyvinyl alcohol/polyethylene glycol hydrogel. J. Appl. Polym. Sci. 139, e52805. doi:10.1002/app.52805
Hu, K., He, P., Zhao, Z., Huang, L., Liu, K., Lin, S., et al. (2021). Nature-inspired self-powered cellulose nanofibrils hydrogels with high sensitivity and mechanical adaptability. Carbohydr. Polym. 264, 117995. doi:10.1016/j.carbpol.2021.117995
Hua, M. T., Wu, S. W., Ma, Y. F., Zhao, Y. S., Chen, Z. L., Frenkel, I., et al. (2021). Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 590, 594–599. doi:10.1038/s41586-021-03212-z
Huang, X. X., Chen, C. W., Ma, X. H., Zhu, T. S., Ma, W. C., Jin, Q., et al. (2023). In situ forming dual-conductive hydrogels enable conformal, self-adhesive and antibacterial epidermal electrodes. Adv. Funct. Mater. 33. doi:10.1002/adfm.202302846
Jin, S. G. (2022). Production and application of biomaterials based on polyvinyl alcohol (PVA) as wound dressing. Chem. Asian J. 17, e202200595. doi:10.1002/asia.202200595
Jing, X., Li, H., Mi, H.-Y., Liu, Y.-J., Feng, P.-Y., Tan, Y.-M., et al. (2019). Highly transparent, stretchable, and rapid self-healing polyvinyl alcohol/cellulose nanofibril hydrogel sensors for sensitive pressure sensing and human motion detection. Sensors Actuators B Chem. 295, 159–167. doi:10.1016/j.snb.2019.05.082
Joodaki, H., and Panzer, M. B. (2018). Skin mechanical properties and modeling: a review. Proc. Institution Mech. Eng. Part H-Journal Eng. Med. 232, 323–343. doi:10.1177/0954411918759801
Kakkar, V., and Narula, P. (2022). Role of molecularly imprinted hydrogels in drug delivery-A current perspective. Int. J. Pharm. 625, 121883. doi:10.1016/j.ijpharm.2022.121883
Kalantari, K., Mostafavi, E., Saleh, B., Soltantabar, P., and Webster, T. J. (2020). Chitosan/PVA hydrogels incorporated with green synthesized cerium oxide nanoparticles for wound healing applications. Eur. Polym. J. 134, 109853. doi:10.1016/j.eurpolymj.2020.109853
Ke, T., Zhao, L., Fan, X., and Gu, H. B. (2023). Rapid self-healing, self-adhesive, anti-freezing, moisturizing, antibacterial and multi-stimuli-responsive PVA/starch/tea polyphenol-based composite conductive organohydrogel as flexible strain sensor. J. Mater. Sci. Technol. 135, 199–212. doi:10.1016/j.jmst.2022.06.032
Khan, Z. U., Edberg, J., Hamedi, M. M., Gabrielsson, R., Granberg, H., Wagberg, L., et al. (2016). Thermoelectric polymers and their elastic aerogels. Adv. Mater 28, 4556–4562. doi:10.1002/adma.201505364
Khorasani, M. T., Joorabloo, A., Adeli, H., Mansoori-Moghadam, Z., and Moghaddam, A. (2019). Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 207, 542–554. doi:10.1016/j.carbpol.2018.12.021
Khorasani, M. T., Joorabloo, A., Moghaddam, A., Shamsi, H., and Mansoorimoghadam, Z. (2018). Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 114, 1203–1215. doi:10.1016/j.ijbiomac.2018.04.010
Kim, T. H., An, D. B., Oh, S. H., Kang, M. K., Song, H. H., and Lee, J. H. (2015). Creating stiffness gradient polyvinyl alcohol hydrogel using a simple gradual freezing-thawing method to investigate stem cell differentiation behaviors. Biomaterials 40, 51–60. doi:10.1016/j.biomaterials.2014.11.017
Kong, D. S., El-Bahy, Z. M., Algadi, H., Li, T., El-Bahy, S. M., Nassan, M. A., et al. (2022). Highly sensitive strain sensors with wide operation range from strong MXene-composited polyvinyl alcohol/sodium carboxymethylcellulose double network hydrogel. Adv. Compos. Hybrid Mater. 5, 1976–1987. doi:10.1007/s42114-022-00531-1
Kumar, A., Behl, T., and Chadha, S. (2020). Synthesis of physically crosslinked PVA/Chitosan loaded silver nanoparticles hydrogels with tunable mechanical properties and antibacterial effects. Int. J. Biol. Macromol. 149, 1262–1274. doi:10.1016/j.ijbiomac.2020.02.048
Li, H., Lv, T., Sun, H., Qian, G., Li, N., Yao, Y., et al. (2019). Ultrastretchable and superior healable supercapacitors based on a double cross-linked hydrogel electrolyte. Nat. Commun. 10, 536. doi:10.1038/s41467-019-08320-z
Li, L., Zhang, Y., Lu, H., Wang, Y., Xu, J., Zhu, J., et al. (2020a). Cryopolymerization enables anisotropic polyaniline hybrid hydrogels with superelasticity and highly deformation-tolerant electrochemical energy storage. Nat. Commun. 11 (62), 62. doi:10.1038/s41467-019-13959-9
Li, M., Chen, D., Sun, X., Xu, Z., Yang, Y., Song, Y., et al. (2022b). An environmentally tolerant, highly stable, cellulose nanofiber-reinforced, conductive hydrogel multifunctional sensor. Carbohydr. Polym. 284, 119199. doi:10.1016/j.carbpol.2022.119199
Li, M., Fu, R., Duan, Z., Zhu, C., and Fan, D. (2022e). Construction of multifunctional hydrogel based on the tannic acid-metal coating decorated MoS2 dual nanozyme for bacteria-infected wound healing. Bioact. Mater 9, 461–474. doi:10.1016/j.bioactmat.2021.07.023
Li, M., Li, H., Wu, C. W., and Zhang, W. (2022a). PVA-AAm-AG multi-network hydrogel with high mechanical strength and cell adhesion. Polymer 247, 124786. doi:10.1016/j.polymer.2022.124786
Li, M., Ma, Y., Li, D., Lu, S., Li, Y., and Li, Z. (2022d). Highly stretchable, self-healing, and degradable ionic conductive cellulose hydrogel for human motion monitoring. Int. J. Biol. Macromol. 223, 1530–1538. doi:10.1016/j.ijbiomac.2022.11.014
Li, M., Yang, Y., Yue, C., Song, Y., Manzo, M., Huang, Z., et al. (2022c). Stretchable, sensitive, and environment-tolerant ionic conductive organohydrogel reinforced with cellulose nanofibers for human motion monitoring. Cellulose 29, 1897–1909. doi:10.1007/s10570-022-04418-8
Li, W., Li, X., Zhang, X., Wu, J., Tian, X., Zeng, M.-J., et al. (2020b). Flexible poly(vinyl alcohol)–polyaniline hydrogel film with vertically aligned channels for an integrated and self-healable supercapacitor. ACS Appl. Energy Mater. 3, 9408–9416. doi:10.1021/acsaem.0c01794
Li, X. F., Jiang, M., Du, Y. M., Ding, X., Xiao, C., Wang, Y. Y., et al. (2023). Self-healing liquid metal hydrogel for human-computer interaction and infrared camouflage. Mater. Horizons 10, 2945–2957. doi:10.1039/d3mh00341h
Lim, K. S., Levato, R., Costa, P. F., Castilho, M. D., Alcala-Orozco, C. R., Van Dorenmalen, K. M. A., et al. (2018). Bio-resin for high resolution lithography-based biofabrication of complex cell-laden constructs. Biofabrication 10, 034101. doi:10.1088/1758-5090/aac00c
Lin, F., Wang, Z., Chen, J., Lu, B., Tang, L., Chen, X., et al. (2020). A bioinspired hydrogen bond crosslink strategy toward toughening ultrastrong and multifunctional nanocomposite hydrogels. J. Mater Chem. B 8, 4002–4015. doi:10.1039/d0tb00424c
Lin, F., Wang, Z., Shen, Y., Tang, L., Zhang, P., Wang, Y., et al. (2019). Natural skin-inspired versatile cellulose biomimetic hydrogels. J. Mater. Chem. A 7, 26442–26455. doi:10.1039/c9ta10502f
Ling, H., Shen, Y., Xu, L., Pan, H., Shen, N., Li, K., et al. (2022). Preparation and characterization of dual-network interpenetrating structure hydrogels with shape memory and self-healing properties. Colloids Surfaces A Physicochem. Eng. Aspects 636, 128061. doi:10.1016/j.colsurfa.2021.128061
Liu, B., Fu, R. Z., Duan, Z. G., Zhu, C. H., Deng, J. J., and Fan, D. D. (2022a). Ionic liquid-based non-releasing antibacterial, anti-inflammatory, high-transparency hydrogel coupled with electrical stimulation for infected diabetic wound healing. Compos. Part B-Engineering 236, 109804. doi:10.1016/j.compositesb.2022.109804
Liu, H., Wang, C., Li, C., Qin, Y., Wang, Z., Yang, F., et al. (2018). A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 8, 7533–7549. doi:10.1039/c7ra13510f
Liu, H. D., Du, C. F., Liao, L. L., Zhang, H. J., Zhou, H. Q., Zhou, W. C., et al. (2022b). Approaching intrinsic dynamics of MXenes hybrid hydrogel for 3D printed multimodal intelligent devices with ultrahigh superelasticity and temperature sensitivity. Nat. Commun. 13, 3420. doi:10.1038/s41467-022-31051-7
Liu, J., Lin, S., Liu, X., Qin, Z., Yang, Y., Zang, J., et al. (2020a). Fatigue-resistant adhesion of hydrogels. Nat. Commun. 11, 1071. doi:10.1038/s41467-020-14871-3
Liu, J. Y., Pang, Y., Zhang, S. Y., Cleveland, C., Yin, X. L., Booth, L., et al. (2017). Triggerable tough hydrogels for gastric resident dosage forms. Nat. Commun. 8, 124. doi:10.1038/s41467-017-00144-z
Liu, L., Zhu, M., Xu, X., Li, X., Ma, Z., Jiang, Z., et al. (2021). Dynamic nanoconfinement enabled highly stretchable and supratough polymeric materials with desirable healability and biocompatibility. Adv. Mater 33, e2105829. doi:10.1002/adma.202105829
Liu, S., Li, D., Wang, Y., Zhou, G., Ge, K., and Jiang, L. (2023). Adhesive, antibacterial and double crosslinked carboxylated polyvinyl alcohol/chitosan hydrogel to enhance dynamic skin wound healing. Int. J. Biol. Macromol. 228, 744–753. doi:10.1016/j.ijbiomac.2022.12.169
Liu, X., Qin, J., Wang, J., Chen, Y., Miao, G., Qi, P., et al. (2022c). Robust conductive organohydrogel strain sensors with wide range linear sensing, UV filtering, anti-freezing and water-retention properties. Colloids Surfaces A Physicochem. Eng. Aspects 632, 127823. doi:10.1016/j.colsurfa.2021.127823
Liu, X., Steiger, C., Lin, S., Parada, G. A., Liu, J., Chan, H. F., et al. (2019). Ingestible hydrogel device. Nat. Commun. 10, 493. doi:10.1038/s41467-019-08355-2
Liu, X., Yang, K., Chang, M., Wang, X., and Ren, J. (2020b). Fabrication of cellulose nanocrystal reinforced nanocomposite hydrogel with self-healing properties. Carbohydr. Polym. 240, 116289. doi:10.1016/j.carbpol.2020.116289
Lu, H. J., Liu, J., Yu, M. Z., Li, P. L., Huang, R. B., Wu, W. Z., et al. (2022a). Photothermal-enhanced antibacterial and antioxidant hydrogel dressings based on catechol-modified chitosan-derived carbonized polymer dots for effective treatment of wound infections. Biomaterials Sci. 10, 2692–2705. doi:10.1039/d2bm00221c
Lu, L., Huang, Z., Li, X., Li, X., Cui, B., Yuan, C., et al. (2022b). A high-conductive, anti-freezing, antibacterial and anti-swelling starch-based physical hydrogel for multifunctional flexible wearable sensors. Int. J. Biol. Macromol. 213, 791–803. doi:10.1016/j.ijbiomac.2022.06.011
Lu, Y., Qu, X., Zhao, W., Ren, Y., Si, W., Wang, W., et al. (2020). Highly stretchable, elastic, and sensitive MXene-based hydrogel for flexible strain and pressure sensors. Res. (Wash D C) 2020, 2038560. doi:10.34133/2020/2038560
Luo, C. H., Guo, A. D., Li, J., Tang, Z. Q., and Luo, F. L. (2022). Janus hydrogel to mimic the structure and property of articular cartilage. Acs Appl. Mater. Interfaces 14, 35434–35443. doi:10.1021/acsami.2c09706
Luo, X. X., Zhu, L. P., Wang, Y. C., Li, J. Y., Nie, J. J., and Wang, Z. L. (2021). A flexible multifunctional triboelectric nanogenerator based on MXene/PVA hydrogel. Adv. Funct. Mater. 31, 2104928. doi:10.1002/adfm.202104928
Ma, Y., Gao, Y., Liu, L., Ren, X., and Gao, G. (2020). Skin-contactable and antifreezing strain sensors based on bilayer hydrogels. Chem. Mater. 32, 8938–8946. doi:10.1021/acs.chemmater.0c02919
Mannarino, M. M., Bassett, M., Donahue, D. T., and Biggins, J. F. (2020). Novel high-strength thromboresistant poly(vinyl alcohol)-based hydrogel for vascular access applications. J. Biomaterials Science-Polymer Ed. 31, 601–621. doi:10.1080/09205063.2019.1706148
Merhebi, S., Mayyas, M., Abbasi, R., Christoe, M. J., Han, J., Tang, J., et al. (2020). Magnetic and conductive liquid metal gels. ACS Appl. Mater Interfaces 12, 20119–20128. doi:10.1021/acsami.0c03166
Mojally, M., Sharmin, E., Obaid, N. A., Alhindi, Y., and Abdalla, A. N. (2022). Polyvinyl alcohol/corn starch/castor oil hydrogel films, loaded with silver nanoparticles biosynthesized in Mentha piperita leaves' extract. J. King Saud Univ. Sci. 34, 101879. doi:10.1016/j.jksus.2022.101879
Montaser, A. S., Rehan, M., and El-Naggar, M. E. (2019). pH-Thermosensitive hydrogel based on polyvinyl alcohol/sodium alginate/N-isopropyl acrylamide composite for treating re-infected wounds. Int. J. Biol. Macromol. 124, 1016–1024. doi:10.1016/j.ijbiomac.2018.11.252
Mredha, M. T. I., and Jeon, I. (2022). Biomimetic anisotropic hydrogels: advanced fabrication strategies, extraordinary functionalities, and broad applications. Prog. Mater. Sci. 124, 100870. doi:10.1016/j.pmatsci.2021.100870
Nasef, S. M., Khozemy, E. E., Kamoun, E. A., and El-Gendi, H. (2019). Gamma radiation-induced crosslinked composite membranes based on polyvinyl alcohol/chitosan/AgNO3/vitamin E for biomedical applications. Int. J. Biol. Macromol. 137, 878–885. doi:10.1016/j.ijbiomac.2019.07.033
Nie, L., Deng, Y., Li, P., Hou, R., Shavandi, A., and Yang, S. (2020). Hydroxyethyl chitosan-reinforced polyvinyl alcohol/biphasic calcium phosphate hydrogels for bone regeneration. ACS Omega. 5, 10948–10957. doi:10.1021/acsomega.0c00727
Nie, L., Li, X. C., Chang, P. B., Liu, S., Wei, Q. Q., Guo, Q. P., et al. (2021). A fast method for in vitro biomineralization of PVA/alginate/biphasic calcium phosphate hydrogel. Mater Lett. 308, 131182. doi:10.1016/j.matlet.2021.131182
Nour, S., Imani, R., and Shari, A. M. (2022). Angiogenic effect of a nanoniosomal deferoxamine-loaded poly(vinyl alcohol)-egg white film as a promising wound dressing. Acs Biomaterials Sci. Eng. 8, 3485–3497. doi:10.1021/acsbiomaterials.2c00046
Oliveira, A. S., Silva, J. C., Loureiro, M. V., Marques, A. C., Kotov, N. A., Colaco, R., et al. (2023). Super-strong hydrogel composites reinforced with PBO nanofibers for cartilage replacement. Macromol. Biosci. 23, e2200240. doi:10.1002/mabi.202200240
Ounkaew, A., Kasemsiri, P., Jetsrisuparb, K., Uyama, H., Hsu, Y. I., Boonmars, T., et al. (2020). Synthesis of nanocomposite hydrogel based carboxymethyl starch/polyvinyl alcohol/nanosilver for biomedical materials. Carbohydr. Polym. 248, 116767. doi:10.1016/j.carbpol.2020.116767
Pan, X., Wang, Q., Guo, R., Ni, Y., Liu, K., Ouyang, X., et al. (2019). An integrated transparent, UV-filtering organohydrogel sensor via molecular-level ion conductive channels. J. Mater. Chem. A 7, 4525–4535. doi:10.1039/c8ta12360h
Pan, X., Wang, Q., He, P., Liu, K., Ni, Y., Chen, L., et al. (2020). A bionic tactile plastic hydrogel-based electronic skin constructed by a nerve-like nanonetwork combining stretchable, compliant, and self-healing properties. Chem. Eng. J. 379, 122271. doi:10.1016/j.cej.2019.122271
Pei, M., Peng, X., Wan, T., Fan, P., Yang, H., Liu, X., et al. (2021). Double cross-linked poly(vinyl alcohol) microcomposite hydrogels with high strength and cell compatibility. Eur. Polym. J. 160, 110786. doi:10.1016/j.eurpolymj.2021.110786
Peng, W., Han, L., Huang, H., Xuan, X., Pan, G., Wan, L., et al. (2020). A direction-aware and ultrafast self-healing dual network hydrogel for a flexible electronic skin strain sensor. J. Mater. Chem. A 8, 26109–26118. doi:10.1039/d0ta08987g
Peppas, N. A., Hilt, J. Z., Khademhosseini, A., and Langer, R. (2006). Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 18, 1345–1360. doi:10.1002/adma.200501612
Qi, J. R., Zhang, J., Jia, H., Guo, X. Y., Yue, Y., Yuan, Y. H., et al. (2022). Synthesis of silver/Fe3O4@chitosan@polyvinyl alcohol magnetic nanoparticles as an antibacterial agent for accelerating wound healing. Int. J. Biol. Macromol. 221, 1404–1414. doi:10.1016/j.ijbiomac.2022.09.030
Qiao, B., Pang, Q., Yuan, P., Luo, Y., and Ma, L. (2020). Smart wound dressing for infection monitoring and NIR-triggered antibacterial treatment. Biomater. Sci. 8, 1649–1657. doi:10.1039/c9bm02060h
Qin, M. M., Guo, Y. Q., Su, F. F., Huang, X. P., Qian, Q. P., Zhou, Y. L., et al. (2023). High-strength, fatigue-resistant, and fast self-healing antibacterial nanocomposite hydrogels for wound healing. Chem. Eng. J. 455, 140854. doi:10.1016/j.cej.2022.140854
Qin, Y., Mo, J. L., Liu, Y. H., Zhang, S., Wang, J. L., Fu, Q., et al. (2022). Stretchable triboelectric self-powered sweat sensor fabricated from self-healing nanocellulose hydrogels. Adv. Funct. Mater. 32, 2201846. doi:10.1002/adfm.202201846
Qing, X. Y., He, G. H., Liu, Z. D., Yin, Y. H., Cai, W. Q., Fan, L. H., et al. (2021). Preparation and properties of polyvinyl alcohol/N-succinyl chitosan/lincomycin composite antibacterial hydrogels for wound dressing. Carbohydr. Polym. 261, 117875. doi:10.1016/j.carbpol.2021.117875
Qu, J., Zhao, X., Liang, Y., Zhang, T., Ma, P. X., and Guo, B. (2018). Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 183, 185–199. doi:10.1016/j.biomaterials.2018.08.044
Raafat, A. I., El-Sawy, N. M., Badawy, N. A., Mousa, E. A., and Mohamed, A. M. (2018). Radiation fabrication of Xanthan-based wound dressing hydrogels embedded ZnO nanoparticles: in vitro evaluation. Int. J. Biol. Macromol. 118, 1892–1902. doi:10.1016/j.ijbiomac.2018.07.031
Ran, R. J., Wang, T., Tao, M. Y., Gou, Y. Q., and Zhang, M. (2023). A novel approach for 25-gauge transconjunctival sutureless vitrectomy to evaluate vitreous substitutes in rabbits. Int. J. Ophthalmol. 16, 1568–1573. doi:10.18240/ijo.2023.10.02
Rao, P., Sun, T. L., Chen, L., Takahashi, R., Shinohara, G., Guo, H., et al. (2018). Tough hydrogels with fast, strong, and reversible underwater adhesion based on a multiscale design. Adv. Mater. 30, 1801884. doi:10.1002/adma.201801884
Shamloo, A., Aghababaie, Z., Afjoul, H., Jami, M., Bidgoli, M. R., Vossoughi, M., et al. (2021). Fabrication and evaluation of chitosan/gelatin/PVA hydrogel incorporating honey for wound healing applications: an in vitro, in vivo study. Int. J. Pharm. 592, 120068. doi:10.1016/j.ijpharm.2020.120068
Shao, C., Wang, M., Meng, L., Chang, H., Wang, B., Xu, F., et al. (2018). Mussel-Inspired cellulose nanocomposite tough hydrogels with synergistic self-healing, adhesive, and strain-sensitive properties. Chem. Mater. 30, 3110–3121. doi:10.1021/acs.chemmater.8b01172
Shao, Z., Yin, T., Jiang, J., He, Y., Xiang, T., and Zhou, S. (2023). Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioact. Mater. 20, 561–573. doi:10.1016/j.bioactmat.2022.06.018
Shen, J. D., Du, P., Zhou, B. B., Zhang, G. B., Tang, X. X., Pan, J., et al. (2022). An anti-freezing biomineral hydrogel of high strain sensitivity for artificial skin applications. Nano Res. 15, 6655–6661. doi:10.1007/s12274-022-4213-x
Song, M., Yu, H., Zhu, J., Ouyang, Z., Abdalkarim, S. Y. H., Tam, K. C., et al. (2020). Constructing stimuli-free self-healing, robust and ultrasensitive biocompatible hydrogel sensors with conductive cellulose nanocrystals. Chem. Eng. J. 398, 125547. doi:10.1016/j.cej.2020.125547
Song, S., Liu, Z., Abubaker, M. A., Ding, L., Zhang, J., Yang, S. R., et al. (2021). Antibacterial polyvinyl alcohol/bacterial cellulose/nano-silver hydrogels that effectively promote wound healing. Mater. Sci. Eng. C-Materials Biol. Appl. 126, 112171. doi:10.1016/j.msec.2021.112171
Songfeng, E., Ning, D., Wang, Y., Huang, J., Jin, Z., Ma, Q., et al. (2020). Ternary synergistic strengthening and toughening of bio-inspired TEMPO-oxidized cellulose nanofibers/borax/polyvinyl alcohol composite film with high transparency. Acs Sustain. Chem. Eng. 8, 15661–15669. doi:10.1021/acssuschemeng.0c05292
Stauffer, S. R., and Peppast, N. A. (1992). Poly(vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer 33, 3932–3936. doi:10.1016/0032-3861(92)90385-a
Su, X., Luo, Y., Tian, Z. L., Yuan, Z. Y., Han, Y. M., Dong, R. F., et al. (2020a). Ctenophore-inspired hydrogels for efficient and repeatable underwater specific adhesion to biotic surfaces. Mater. Horizons 7, 2651–2661. doi:10.1039/d0mh01344g
Su, X., Wang, H., Tian, Z., Duan, X., Chai, Z., Feng, Y., et al. (2020b). A solvent Co-cross-linked organogel with fast self-healing capability and reversible adhesiveness at extreme temperatures. ACS Appl. Mater Interfaces 12, 29757–29766. doi:10.1021/acsami.0c04933
Su, Y. M., Zhang, X. M., Gu, Y., Xu, H. L., Liao, Z. M., Zhao, L. Q., et al. (2023). Nanocatalytic hydrogel with rapid photodisinfection and robust adhesion for fortified cutaneous regeneration. ACS Appl. Mater. Interfaces 15, 6354–6370. doi:10.1021/acsami.2c17366
Sun, X., Luo, C., and Luo, F. (2020). Preparation and properties of self-healable and conductive PVA-agar hydrogel with ultra-high mechanical strength. Eur. Polym. J. 124, 109465. doi:10.1016/j.eurpolymj.2019.109465
Takacs, T., Abdelghafour, M. M., Lamch, L., Szenti, I., Sebok, D., Janovak, L., et al. (2022). Facile modification of hydroxyl group containing macromolecules provides autonomously self-healing polymers through the formation of dynamic Schiff base linkages. Eur. Polym. J. 168, 111086. doi:10.1016/j.eurpolymj.2022.111086
Thakur, S., Anjum, M. M., Jaiswal, S., Kumar, A., Deepak, P., Anand, S., et al. (2023). Novel synergistic approach: tazarotene-calcipotriol-loaded-PVA/PVP-nanofibers incorporated in hydrogel film for management and treatment of psoriasis. Mol. Pharm. 20, 997–1014. doi:10.1021/acs.molpharmaceut.2c00713
Vo, T. M. T., Piroonpan, T., Preuksarattanawut, C., Kobayashi, T., and Potiyaraj, P. (2022). Characterization of pH-responsive high molecular-weight chitosan/poly (vinyl alcohol) hydrogel prepared by gamma irradiation for localizing drug release. Bioresour. Bioprocess. 9, 89. doi:10.1186/s40643-022-00576-6
Wang, C., Hu, K., Zhao, C., Zou, Y., Liu, Y., Qu, X., et al. (2020a). Customization of conductive elastomer based on PVA/PEI for stretchable sensors. Small 16, e1904758. doi:10.1002/smll.201904758
Wang, D., Cui, F., Xi, L., Tan, X., Li, J., and Li, T. (2023a). Preparation of a multifunctional non-stick tamarind polysaccharide-polyvinyl alcohol hydrogel immobilized with a quorum quenching enzyme for maintaining fish freshness. Carbohydr. Polym. 302, 120382. doi:10.1016/j.carbpol.2022.120382
Wang, F. F., Li, Z., Guo, J. Q., Liu, L., Fu, H., Yao, J. M., et al. (2022a). Highly strong, tough, and stretchable conductive hydrogels based on silk sericin-mediated multiple physical interactions for flexible sensors. Acs Appl. Polym. Mater. 4, 618–626. doi:10.1021/acsapm.1c01553
Wang, J. H., Liu, X. Y., Wang, Y. Q., An, M. W., and Fan, Y. B. (2022b). Casein micelles embedded composite organohydrogel as potential wound dressing. Int. J. Biol. Macromol. 211, 678–688. doi:10.1016/j.ijbiomac.2022.05.081
Wang, Q., Zhang, Q., Wang, G. Y., Wang, Y. R., Ren, X. Y., and Gao, G. H. (2022c). Muscle-Inspired anisotropic hydrogel strain sensors. Acs Appl. Mater. Interfaces 14, 1921–1928. doi:10.1021/acsami.1c18758
Wang, R. B., Jovanska, L., Tsai, Y. T., Yeh, Y. Y., and Yeh, Y. C. (2022d). Fabrication of water-resistant, thermally stable, and antibacterial fibers through in situ multivalent crosslinking. J. Appl. Polym. Sci. 139, 11. doi:10.1002/app.52100
Wang, T., Zhang, F., Zhao, R., Wang, C., Hu, K., Sun, Y., et al. (2020). Polyvinyl alcohol/sodium alginate hydrogels incorporated with silver nanoclusters via green tea extract for antibacterial applications. Des. Monomers. Polym. 23, 118–133. doi:10.1080/15685551.2020.1804183
Wang, Y., Liu, S., Wang, Q., Ji, X., An, X., Liu, H., et al. (2022e). Nanolignin filled conductive hydrogel with improved mechanical, anti-freezing, UV-shielding and transparent properties for strain sensing application. Int. J. Biol. Macromol. 205, 442–451. doi:10.1016/j.ijbiomac.2022.02.088
Wang, Y. Q., Zhu, Y., Wang, J. H., Li, X. N., Wu, X. G., Qin, Y. X., et al. (2021). Fe3+, NIR light and thermal responsive triple network composite hydrogel with multi-shape memory effect. Compos. Sci. Technol. 206, 108653. doi:10.1016/j.compscitech.2021.108653
Wang, Z., Guo, L. F., Xiao, H. Y., Cong, H., and Wang, S. T. (2020b). A reversible underwater glue based on photo- and thermo-responsive dynamic covalent bonds. Mater. Horizons 7, 282–288. doi:10.1039/c9mh01148j
Wang, Z. C., Wang, Y. X., Peng, X. Y., He, Y. L., Wei, L., Su, W. H., et al. (2019). Photocatalytic antibacterial agent incorporated double-network hydrogel for wound healing. Colloids Surfaces B-Biointerfaces 180, 237–244. doi:10.1016/j.colsurfb.2019.04.043
Wang, Z. Y., Sheng, K. Y., Zhou, Z. J., Li, H. Z., Zhang, Z. Y., Xiong, M. Z., et al. (2023b). High ionic conductivity of Room Temperature Molten Salt-based PVA hydrogel electrolyte prepared by electron beam irradiation. Radiat. Phys. Chem. 204, 110682. doi:10.1016/j.radphyschem.2022.110682
Wei, H., Kong, D., Li, T., Xue, Q., Wang, S., Cui, D., et al. (2021). Solution-processable conductive composite hydrogels with multiple synergetic networks toward wearable pressure/strain sensors. ACS Sens. 6, 2938–2951. doi:10.1021/acssensors.1c00699
Wen, J., Tang, J., Ning, H., Hu, N., Zhu, Y., Gong, Y., et al. (2021). Multifunctional ionic skin with sensing, UV-filtering, water-retaining, and anti-freezing capabilities. Adv. Funct. Mater. 31, 2011176. doi:10.1002/adfm.202011176
Wen, N., Jiang, B. J., Wang, X. J., Shang, Z. F., Jiang, D. W., Zhang, L., et al. (2020). Overview of polyvinyl alcohol nanocomposite hydrogels for electro-skin, actuator, supercapacitor and fuel cell. Chem. Rec. 20, 773–792. doi:10.1002/tcr.202000001
Wu, S., Hua, M., Alsaid, Y., Du, Y., Ma, Y., Zhao, Y., et al. (2021). Poly(vinyl alcohol) hydrogels with broad-range tunable mechanical properties via the hofmeister effect. Adv. Mater 33, e2007829. doi:10.1002/adma.202007829
Wu, Y., Chee, A. J. Y., Golzar, H., Yu, A. C. H., and Tang, X. (2022). Embedded 3D printing of ultrasound-compatible arterial phantoms with biomimetic elasticity. Adv. Funct. Mater. 14, 2110153. doi:10.1002/adfm.202110153
Xia, H., Zhang, Y., Xin, H., Yan, D., Li, G., and Chen, R. (2022). Metal–phenolic network-based polydopamine@Cu within a polyvinyl alcohol hydrogel film for improved infected wound healing through antibacterial and pro-angiogenesis activity. Mater. Des. 221, 110904. doi:10.1016/j.matdes.2022.110904
Xiang, J., Shen, L., and Hong, Y. (2020). Status and future scope of hydrogels in wound healing: synthesis, materials and evaluation. Eur. Polym. J. 130, 109609. doi:10.1016/j.eurpolymj.2020.109609
Xu, L. J., Chen, Y., Yu, M. L., Hou, M. J., Gong, G., Tan, H. H., et al. (2023). NIR light-induced rapid self-healing hydrogel toward multifunctional applications in sensing. Nano Energy 107, 108119. doi:10.1016/j.nanoen.2022.108119
Xu, W., Huang, L. B., Wong, M. C., Chen, L., Bai, G. X., and Hao, J. H. (2017). Environmentally friendly hydrogel-based triboelectric nanogenerators for versatile energy harvesting and self-powered sensors. Adv. Energy Mater. 7, 1601529. doi:10.1002/aenm.201601529
Yan, G. H., He, S. M., Chen, G. F., Ma, S., Zeng, A. Q., Chen, B. L., et al. (2022a). Highly flexible and broad-range mechanically tunable all-wood hydrogels with nanoscale channels via the hofmeister effect for human motion monitoring. Nano-Micro Lett. 14, 84. doi:10.1007/s40820-022-00827-3
Yan, M. Z., Cai, J. Q., Fang, Z. Q., Wang, H., Qiu, X. Q., and Liu, W. F. (2022b). Anisotropic muscle-like conductive composite hydrogel reinforced by lignin and cellulose nanofibrils. Acs Sustain. Chem. Eng. 10, 12993–13003. doi:10.1021/acssuschemeng.2c02506
Yang, F., Zhao, J., Koshut, W. J., Watt, J., Riboh, J. C., Gall, K., et al. (2020). A synthetic hydrogel composite with the mechanical behavior and durability of cartilage. Adv. Funct. Mater. 30, 2003451. doi:10.1002/adfm.202003451
Yao, J. L., Hui, J. F., Yang, J., Yao, J. X., Hu, C. Q., and Fan, D. D. (2022). Sprayable nanodrug-loaded hydrogels with enzyme-catalyzed semi-inter penetrating polymer network (Semi-IPN) for solar dermatitis. Nano Res. 15, 6266–6277. doi:10.1007/s12274-022-4236-3
Ye, Y., Zhang, Y., Chen, Y., Han, X., and Jiang, F. (2020). Cellulose nanofibrils enhanced, strong, stretchable, freezing-tolerant ionic conductive organohydrogel for multi-functional sensors. Adv. Funct. Mater. 30, 2003430. doi:10.1002/adfm.202003430
Ye, Z. S., Lu, H. L., Jia, E. D., Chen, J., and Fu, L. F. (2022). Organic solvents enhance polyvinyl alcohol/polyethylene glycol self-healing hydrogels for artificial cartilage. Polym. Adv. Technol. 33, 3455–3469. doi:10.1002/pat.5799
Yeon, J. S., Gupta, N., Bhattacharya, P., and Park, H. S. (2022). A new era of integrative ice frozen assembly into multiscale architecturing of energy materials. Adv. Funct. Mater. 32, 2112509. doi:10.1002/adfm.202112509
Yi, X. T., Cheng, F., Wei, X. J., Li, H. B., Qian, J. T., and He, J. M. (2023). Bioinspired adhesive and self-healing bacterial cellulose hydrogels formed by a multiple dynamic crosslinking strategy for sealing hemostasis. Cellulose 30, 397–411. doi:10.1007/s10570-022-04909-8
Yin, J. Y., Lu, C. C., Li, C. H., Yu, Z. K., Shen, C., Yang, Y. Y., et al. (2022). A UV-filtering, environmentally stable, healable and recyclable ionic hydrogel towards multifunctional flexible strain sensor. Compos. Part B-Engineering 230, 109528. doi:10.1016/j.compositesb.2021.109528
Yuan, T., Qu, X. X., Cui, X. M., and Sun, J. Q. (2019). Self-healing and recyclable hydrogels reinforced with in situ-Formed organic nanofibrils exhibit simultaneously enhanced mechanical strength and stretchability. Acs Appl. Mater. Interfaces 11, 32346–32353. doi:10.1021/acsami.9b08208
Zhan, Y. W., Xing, Y. C., Ji, Q., Ma, X. M., and Xia, Y. Z. (2022). Strain-sensitive alginate/polyvinyl alcohol composite hydrogels with Janus hierarchy and conductivity mediated by tannic acid. Int. J. Biol. Macromol. 212, 202–210. doi:10.1016/j.ijbiomac.2022.05.071
Zhang, H., Qin, L., Chen, Y., Long, T., Guan, R., Cheng, X., et al. (2023). Silicone-enhanced polyvinyl alcohol hydrogels for high performance wearable strain sensors. Mater. Des. 229, 111911. doi:10.1016/j.matdes.2023.111911
Zhang, J., Wan, L., Gao, Y., Fang, X., Lu, T., Pan, L., et al. (2019). Highly stretchable and self-healable MXene/polyvinyl alcohol hydrogel electrode for wearable capacitive electronic skin. Adv. Electron. Mater. 5, 1900285. doi:10.1002/aelm.201900285
Zhang, L., Hu, O., Zhang, J., Hou, L., Ye, D., Jiang, X., et al. (2022a). Preparation of tough and ionic conductive PVA/carboxymethyl chitosan bio-based organohydrogels with long-term stability for strain sensor. Cellulose 29, 9323–9339. doi:10.1007/s10570-022-04844-8
Zhang, Y.-F., Guo, M.-M., Zhang, Y., Tang, C. Y., Jiang, C., Dong, Y., et al. (2020). Flexible, stretchable and conductive PVA/PEDOT:PSS composite hydrogels prepared by SIPN strategy. Polym. Test. 81, 106213. doi:10.1016/j.polymertesting.2019.106213
Zhang, Z., Abidi, N., and Lucia, L. A. (2022b). Dual crosslinked-network self-healing composite hydrogels exhibit enhanced water adaptivity and reinforcement. Industrial Eng. Chem. Res. 61, 17876–17884. doi:10.1021/acs.iecr.2c02783
Zhao, H., Huang, J., Li, Y., Lv, X., Zhou, H., Wang, H., et al. (2020a). ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 258, 120286. doi:10.1016/j.biomaterials.2020.120286
Zhao, J. C., Tong, H. Y., Kirillova, A., Koshut, W. J., Malek, A., Brigham, N. C., et al. (2022). A synthetic hydrogel composite with a strength and wear resistance greater than cartilage. Adv. Funct. Mater. 32, 2205662. doi:10.1002/adfm.202205662
Zhao, N. F., Li, M., Gong, H. X., and Bai, H. (2020b). Controlling ice formation on gradient wettability surface for high-performance bioinspired materials. Sci. Adv. 6, eabb4712. doi:10.1126/sciadv.abb4712
Zhao, N. F., Yang, M., Zhao, Q., Gao, W. W., Xie, T., and Bai, H. (2017). Superstretchable nacre-mimetic graphene/poly(vinyl alcohol) composite film based on interfacial architectural engineering. Acs Nano 11, 4777–4784. doi:10.1021/acsnano.7b01089
Zheng, H. Y., Lin, N., He, Y. Y., and Zuo, B. Q. (2021). Self-healing, self-adhesive silk fibroin conductive hydrogel as a flexible strain sensor. Acs Appl. Mater. Interfaces 13, 40013–40031. doi:10.1021/acsami.1c08395
Zhong, C., Gurry, T., Cheng, A. A., Downey, J., Deng, Z., Stultz, C. M., et al. (2014). Strong underwater adhesives made by self-assembling multi-protein nanofibres. Nat. Nanotechnol. 9, 858–866. doi:10.1038/nnano.2014.199
Zhou, Y., Wan, C., Yang, Y., Yang, H., Wang, S., Dai, Z., et al. (2019). Highly stretchable, elastic, and ionic conductive hydrogel for artificial soft electronics. Adv. Funct. Mater. 29, 1806220. doi:10.1002/adfm.201806220
Zhou, Z., Qian, C., and Yuan, W. (2021). Self-healing, anti-freezing, adhesive and remoldable hydrogel sensor with ion-liquid metal dual conductivity for biomimetic skin. Compos. Sci. Technol. 203, 108608. doi:10.1016/j.compscitech.2020.108608
Zhou, Z. Z., Chu, Y. H., Hou, Z. S., Zhou, X. P., and Cao, Y. (2022). Modification of frictional properties of hydrogel surface via laser ablated topographical micro-textures. Nanomaterials 12, 4103. doi:10.3390/nano12224103
Zhu, C. K., Huang, C. Y., Zhang, W. X., Ding, X. L., and Yang, Y. (2022a). Biodegradable-glass-Fiber reinforced hydrogel composite with enhanced mechanical performance and cell proliferation for potential cartilage repair. Int. J. Mol. Sci. 23, 8717. doi:10.3390/ijms23158717
Zhu, H., Xu, J., Sun, X., Guo, Q., Guo, Q., Jiang, M., et al. (2022b). Wearable, fast-healing, and self-adhesive multifunctional photoactive hydrogel for strain and temperature sensing. J. Mater. Chem. A 10, 23366–23374. doi:10.1039/d2ta06072h
Zhu, X. Q., Ji, C. C., Meng, Q. Q., Mi, H. Y., Yang, Q., Li, Z. X., et al. (2022c). Freeze-Tolerant hydrogel electrolyte with high strength for stable operation of flexible zinc-ion hybrid supercapacitors. Small 18, 2200055. doi:10.1002/smll.202200055
Keywords: PVA, hydrogels, articular cartilage restoration, electronic skin, wound dressing
Citation: Zhong Y, Lin Q, Yu H, Shao L, Cui X, Pang Q, Zhu Y and Hou R (2024) Construction methods and biomedical applications of PVA-based hydrogels. Front. Chem. 12:1376799. doi: 10.3389/fchem.2024.1376799
Received: 26 January 2024; Accepted: 05 February 2024;
Published: 15 February 2024.
Edited by:
Lei Nie, Xinyang Normal University, ChinaCopyright © 2024 Zhong, Lin, Yu, Shao, Cui, Pang, Zhu and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Pang, cGFuZ3FpYW5AbmJ1LmVkdS5jbg==; Ruixia Hou, aG91cnVpeGlhQG5idS5lZHUuY24=
 Yi Zhong1
Yi Zhong1 Xiang Cui
Xiang Cui Yabin Zhu
Yabin Zhu Ruixia Hou
Ruixia Hou