
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 01 March 2024
Sec. Organic Chemistry
Volume 12 - 2024 | https://doi.org/10.3389/fchem.2024.1361266
This article is part of the Research Topic Recent Advances in Synthesizing and Utilizing Nitrogen-containing Heterocycles View all 12 articles
 Farzaneh Mohamadpour*
Farzaneh Mohamadpour* Ali Mohammad Amani*
Ali Mohammad Amani*Background: Organic dyes often have shorter lifetimes in the excited state, which is a major obstacle to the development of effective photoredox methods. The scientific community has shown a great deal of interest in a certain class of organic chromophores because of their unique characteristics and effectiveness. One characteristic of the molecules under research is thermally activated delayed fluorescence (TADF), which is only observed in molecules with a tiny energy gap (often less than 0.2 eV) between their lowest two excited states, i.e., singlet excited state (S1) and triplet excited state (T1). The extended singlet excited states arising from TADF and the simplicity with which their redox potentials may be altered make the isophthalonitrile family of chromophores an attractive option for organic photocatalyst applications.
Methods: The Biginelli reaction between β-ketoesters, arylaldehydes, and urea/thiourea has been used to build a sustainable technique for the production of 3,4-dihydropyrimidin-2-(1H)-one/thione derivatives. In the present study, the development of a green radical synthesis approach for this class of compounds is addressed in depth. As a photocatalyst, a new halogenated dicyanobenzene-based photosensitizer was employed in this study. As a renewable energy source activated by a blue LED, it was dissolved in ethanol, at room temperature in air atmosphere. The primary objective of this research is to employ a novel donor-acceptor (D-A) based on halogenated cyanoarene that is affordable, easily available, and innovative.
Findings: The 3DPAFIPN [2,4,6-tris(diphenylamino)-5-fluoroisophthalonitrile] photocatalyst, a thermally activated delayed fluorescence (TADF), induces single-electron transfer (SET) in response to visible light, offering a straightforward, eco-friendly, and highly efficient process. Additionally, we determined the 3,4-dihydropyrimidin-2-(1H)-one/thione derivatives turnover frequency (TOF) and turnover number (TON). It has also been demonstrated that gram-scale cyclization is a workable method for industrial purposes.
In recent literature, photoredox catalysis has served as a foundation for novel approaches in organic chemistry (Mohamadpour, 2021a; Mohamadpour, 2023a). The field of photoredox catalysis, which combines metal-promoted reactions with photoredox cycles, is gaining significant attention from both academia and industry (Pinosa et al., 2022). The main focus of research is the use of inexpensive, readily manufactured, and efficient organic dyes to help create novel, powerful, and selective metal-promoted reactions (Gualandi et al., 2021). In this sector, organic dyes must take the place of the commonly employed inorganic complexes that are dependent on Ir(III) and Ru(II). When compared to organic molecules, these compounds are known for their long excited state lifetimes, which may tend toward dynamic quenching. Organic dyes often have shorter lifetimes in the excited state, which is a major obstacle to the development of effective photoredox methods. The scientific community has shown a great deal of interest in a specific class of organic chromophores because of their unique characteristics and effectiveness (Bryden and Zysman-Colman, 2021). One characteristic of the molecules under study is thermally activated delayed fluorescence (TADF), which is only observed in molecules with a tiny energy gap (often less than 0.2 eV) between their lowest two excited states, i.e., S1 and T1. Under ambient conditions, the molecules under study undergo reverse intersystem crossing (RISC), aided by a thermally activated pathway from the triplet excited state (T1) to the singlet excited state (S1). This results in a delayed fluorescence phenomenon that is commonly observed in systems similar to this one. The present goal is to combine reduced instruction set computing’s (RISC) exceptional efficiency with fluorescence’s great quantum yield. 2012 saw a significant advancement in the field of organic light-emitting diodes (OLEDs) with the release of a basic work by Adachi (Uoyama et al., 2012). This approach covers the efficient usage of dicyanobenzene molecules with suitable photophysical properties as well as their demonstrated application in OLEDs. Similar TADF chromophores have been used in other domains, such as photocatalysis, since these initial discoveries (Yang et al., 2017; Bryden and Zysman-Colman, 2021). The extended singlet excited states arising from TADF and the simplicity with which their redox potentials may be altered make the isophthalonitrile family of chromophores a viable option for organic photocatalyst applications (Speckmeier et al., 2018). 2,4,6-tris(diphenylamino)-5-fluoroisophthalonitrile (3DPAFIPN) is a chemical that is widely used in a number of visible light-triggered synthetic procedures. Intramolecular cyclizations (Flynn et al., 2020; Wu et al., 2020) and the formation of C–C (Cardinale et al., 2020; Donabauer et al., 2020), N–C (Zhou et al., 2020), and P–C (Rothfelder et al., 2021) bonds (Pinosa et al., 2022) are a few examples of these processes.
Because visible light irradiation has a large energy reserve, is inexpensive, and can be used to access sustainable energy sources, it is considered a reliable method for creating organic compounds (Mohamadpour, 2021b; Mohamadpour, 2021c; Mohamadpour, 2022a).
It is expected that dihydropyrimidine structures have potent biological and pharmacological effects (Figure 1). Calcium channel blockers, antihypertensive effects (Sujatha et al., 2006), anticancer (Wisen et al., 2008), anti HIV agent (Heys et al., 2000), antibacterial and antifungal (Ashok et al., 2007), antiviral (Hurst and Hull, 1961), antioxidative (Magerramow et al., 2006), and anti-inflammatory (Bahekar and Shinde, 2004).
To produce 3,4-dihydropyrimidin-2-(1H)-one/thione derivatives, a number of catalysts are employed, including Na2 eosin Y (Mohamadpour, 2022b), copper (II)sulfamate (Liu et al., 2012), bakers, yeast (Kumar and Maurya, 2007), hydrotalcite (Lal et al., 2012), hexaaquaaluminium (III) tetrafluoroborate (Litvic et al., 2010), TBAB (Ahmed et al., 2009), copper (II) tetrafluoroborate (Kamal et al., 2007), [Btto][p-TSA] (Zhang et al., 2015), triethylammonium acetate (Attri et al., 2017), saccharin (Mohamadpour et al., 2016), caffeine (Mohamadpour and Lashkari, 2018), zirconium (IV)-salophen perfluorooctanesulfonate (Li et al., 2020), H3 [PW12O40] (Chopda and Dave, 2020), Dioxane-HCl (Choudhare et al., 2021), WSi/A15 (Bosica et al., 2021), H4 [W12SiO40] (V Chopda and Dave, 2020), Zr(H2PO4)2 (KÜÇÜKİSLAMOĞLU et al., 2010), and GO-chitosan (Maleki and Paydar, 2016). Complex procedures, lengthy reaction times, the use of costly chemicals, and lower yields are just a few of the variables that significantly impact the management and disposal of waste. Moreover, it might be challenging to extract homogeneous catalysts from reaction mixtures. Due to our interest in the development of photocatalytic reactions (Mohamadpour, 2022c; Mohamadpour, 2022d; Mohamadpour, 2023b; Mohamadpour, 2023c; Mohamadpour, 2023d), the current work discusses the use of photocatalysts in the synthesis of heterocyclic compounds, emphasizing the use of environmentally acceptable practices. The investigation indicates that using photo-redox catalysts for halogenated organic dyes is also financially feasible. A potent donor-acceptor (D-A) cyanoarene is employed as an efficient organo-photocatalyst by employing the previously outlined technique.
The primary focus of the investigation was 2,4,6-tris(diphenylamino)-5-fluoroisophthalonitrile (3DPAFIPN) because of its exceptional photophysical and photochemical properties. Organic chemists now have access to a wider range of photocatalysts thanks to the development of dicyanobenzene-based photosensitizers, which demonstrate exceptional photoelectric activity and thermally activated delayed fluorescence (TADF).
The current study has investigated 3DPAFIPN, a new halogenated cyanoarene-based donor-acceptor (D-A) photocatalyst that works by a sequence of visible-light-induced electron transfers. The three-condensation domino Biginelli reaction arylaldehydes with urea/thiourea and β-ketoesters are used in this procedure. In addition, this process employs blue LED, a sustainable and environmentally friendly energy source, in a room-temperature, ethanol medium as a green solvent. Regardless of the timely and effective completion of all obligations and adherence to the agreed-upon budget.
To find each compound’s melting point, an electrothermal instrument, a 9,100, was employed. 1HNMR spectra were collected using Bruker DRX-300 Avance equipment with DMSO-d6. Materials and reagents were acquired from Acros, Merck, and Fluka and utilized right away.
At room temperature in EtOH (3 mL), urea/thiourea (2, 1.5 mmol), ethyl/methyl acetoacetate (3, 1.0 mmol), and arylaldehyde derivatives (1, 1.0 mmol) were stirred with 3DPAFIPN (0.2 mol%) and blue light (5 W) (Scheme 1). We used thin-layer chromatography (TLC) to track the reaction’s development. Without requiring any additional purification procedures, the pure substance was obtained by screening, washing with water and ethanol, and crystallizing the crude solid from ethanol following the reaction. The Supplementary Material file contains a report on spectroscopic data.
The reaction was optimized in the current study using a 3 mL ethanol medium containing 1.0 mmol of benzaldehyde, 1.5 mmol of urea, and 1.0 mmol of ethyl acetoacetate. Without the aid of a photocatalyst, a trace quantity of 4j was produced for 20 min at room temperature in the presence of 3 mL of EtOH. The pace of reaction was enhanced by the inclusion of photocatalysts. The compounds include 3DPAFIPN, 3DPA2FBN, DCB, DCA, DCN, and diphenylamine, as indicated by the data in Figure 2
The present technique can create 4j with varying yields. The aforementioned data showed that 3DPAFIPN’s operational efficacy has increased. A reaction with 0.2 mol% 3DPAFIPN yielded a 97% yield, based on the data in Table 1, entry 2. Results for solvent-free conditions, EtOAc, DMSO, toluene, H2O, EtOH, MeOH, CHCl3, CH3CN, THF, and are displayed in Table 2. In the presence of EtOH, the reaction was demonstrated to have a notably enhanced rate and subsequent yield. Based on the data in Table 2, especially entry 5, a 97% yield was achieved. Table 2 lists the light sources that have been used in studies to assess the impact of blue light on production. In the assessment that was conducted without the use of an illumination tool, the 4j was discovered in extremely little amounts. The results of this investigation demonstrate that 3DPAFIPN and visible light are essential for the effective synthesis of product 4j. The top designs were determined using blue light-emitting diode (LED) intensities of 3 W, 5 W, and 7 W. The results of the investigation showed that the greatest results were obtained when blue light-emitting diodes (LEDs) with a 5 W power output were used. The outcomes of studies conducted on a range of substrates under optimal conditions are displayed in Table 3; Scheme 1 (and also Supplementary Table S1). The benzaldehyde substituent has no effect on the reaction’s result (Table 3). Both polar and halide substitutions were permitted in this reaction. In the current state of the reaction, reactions involving both electron-donating and electron-withdrawing functional groups are acceptable. Aromatic aldehydes that are ortho, meta, and para-substituted have a very high yield potential. The reactions of methyl and ethyl acetoacetate are comparable. Urea and thiourea have comparable reactivities.

TABLE 1. This table optimizes the photocatalyst for the synthesis of 4ja.

TABLE 2. The table for optimizing solvent and visible light in the synthesis of 4ja.

TABLE 3. Using 3DPAFIPN, a halogenated dicyanobenzene-based photosensitizer, 3,4-dihydropyrimidin-2-(1H)-one/thione derivatives be produced.
Table 4 presents the turnover number (TON) and turnover frequency (TOF) as objective value measures. Yield/Amount of Catalyst (mol) and Yield/Time/Amount of Catalyst (mol) are two distinct forms of yield that are commonly expressed as TON and TOF, respectively, in academic literature. The catalyst performance may be enhanced by higher turnover number (TON) and turnover frequency (TOF) values as they need less catalyst to provide the required yields. A TOF of 97 and a TON of 485 are considered high values for 4j. In relation to 4s, a TON of 480 is likewise regarded as high, although a TOF of 96 is deemed excessive. The aim of the inquiry was to reduce reaction times, increase production, and utilize the fewest amount of catalysts.

TABLE 4. The following calculated were used to determine the turnover frequency (TOF) and turnover number (TON).
The main emphasis of current research is whether the aforementioned chemicals can be produced on a gram-scale for use in pharmaceutical (R&D) procedures. In one experiment, 50 mmol of 4-methoxybenzaldehyde, 75 mmol of thiourea, and 50 mmol of ethyl acetoacetate were utilized. To retrieve the final product, a standard filtering process was applied after the 6-min reaction period. The 1HNMR spectroscopy results show that the chemical compound in question exhibits a high degree of spectroscopic purity.
Scheme 2 displays the outcomes of the control experiments used to elucidate the process utilizing the visible-light-induced. The synthesis of benzylideneurea (I) in the first stage and its condensation with ethyl acetoacetate (3) in the second are considered to be the two steps of the Biginelli reaction. Under standard circumstances (3DPAFIPN in EtOH under blue LED), benzaldehyde (1) and urea (2) were condensed by reducing H2O to produce benzylideneurea (I). As a consequence, in 97% of reactions between the iminium intermediate (I) and cation radical (II), under normal conditions, the expected product 4j was generated. Even though the reaction was carried out in total darkness, there was still a trace of product 4j created. The results of this experiment indicate that Scheme 3 presents a convincing and rational chemical process.
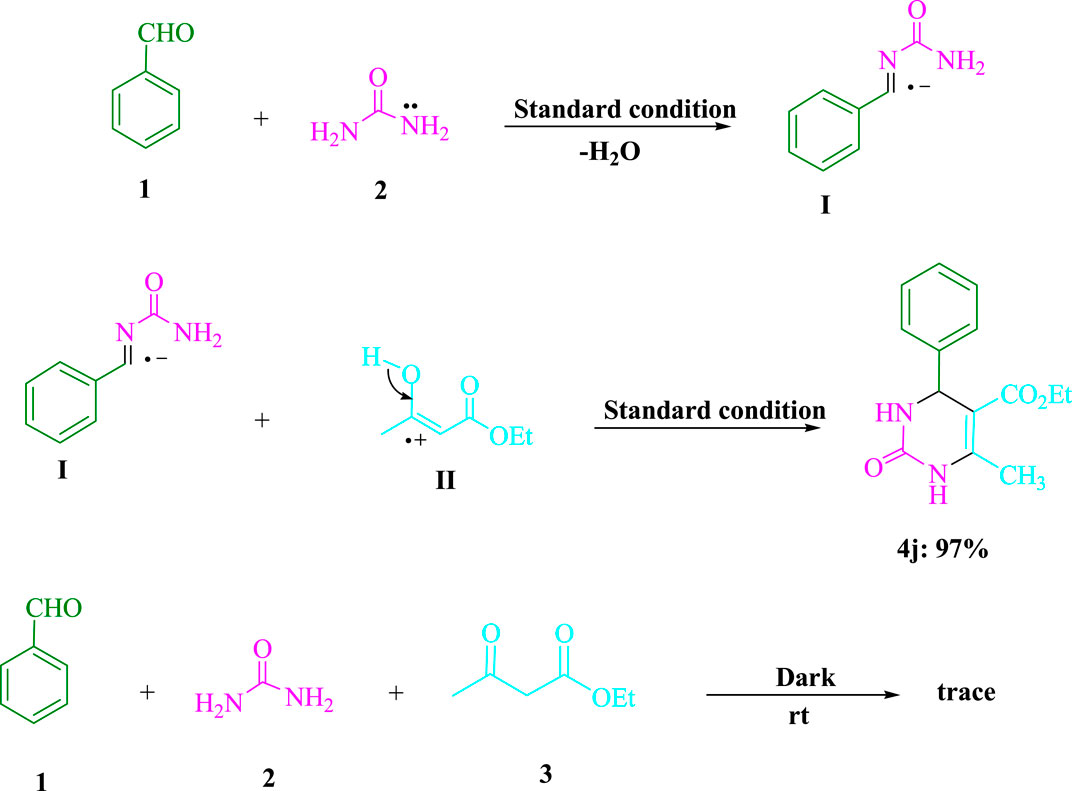
SCHEME 2. To comprehend the condensations of urea (2, 1.5 mmol), ethyl acetoacetate (3, 1.0 mmol), and benzaldehyde (1, 1.0 mmol), significant control tests are conducted.
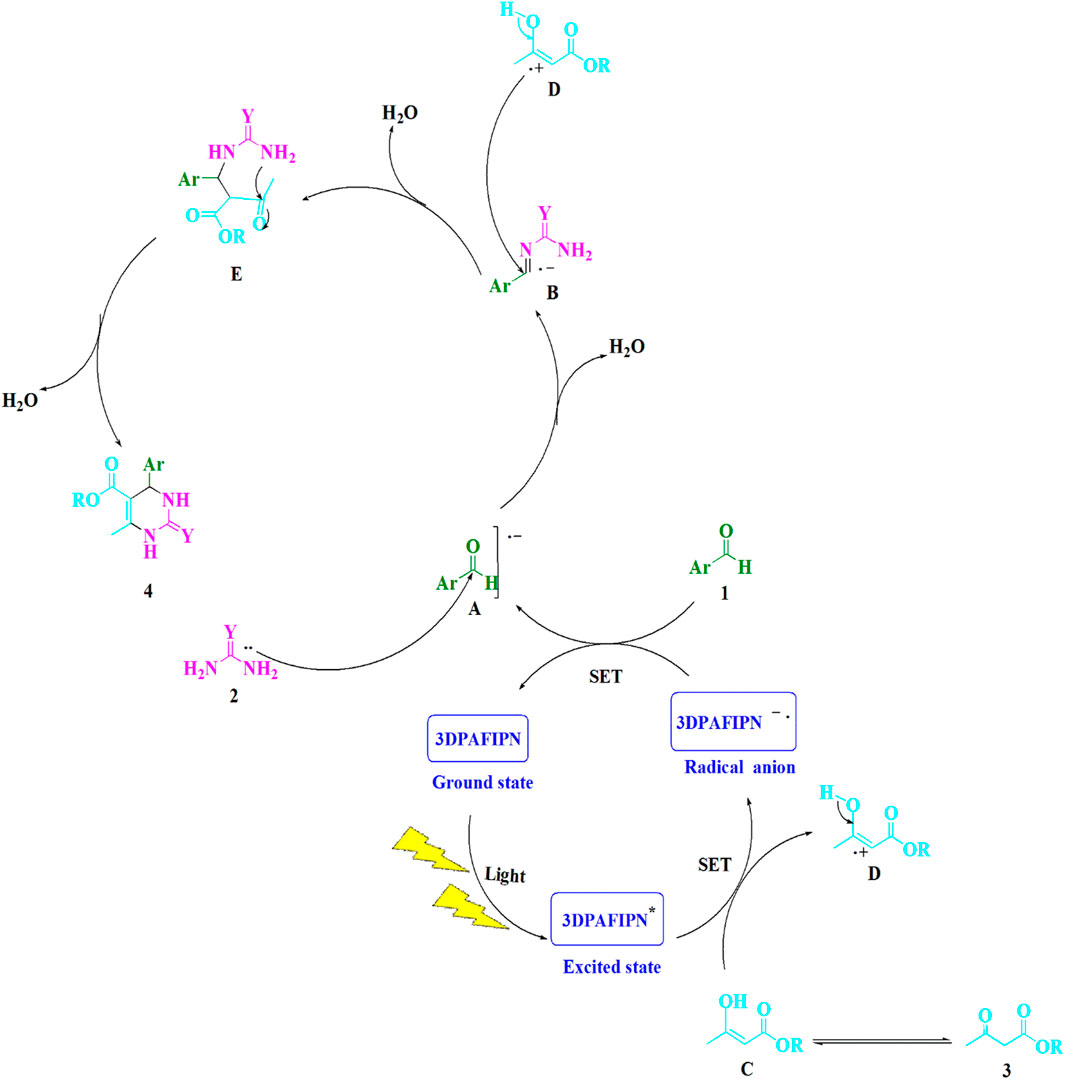
SCHEME 3. This is a detailed illustration of the synthetic procedure that yields 3,4-dihydropyrimidin-2-(1H)-one/thione derivatives.
Scheme 3 provides a thorough description of the suggested methodology. Using single-electron transfer (SET) processes, the cyanoarene organic dye 3DPAFIPN has been utilized to develop photocatalytic reactions that use visible light energy as a sustainable resource. Utilizing visible light expedites the procedure. The ground-state 3DPAFIPN and the intermediate (A) regenerate as a result of the electron transfer (ET) activity between the arylaldehydes (1) and the 3DPAFIPN radical anion. A reactive iminium intermediate (B) is formed when this radical anion (A) is added nucleophilically to urea/thiourea (2). The single-electron transfer (SET) technique is utilized to enhance 3DPAFIPN*, which is produced by visible light, and produce the cation radical (D). The iminium intermediate (B) is attacked by the cation radical (D), leading to the formation of the cyclized dehydrated (4).
Table 5 compares how well various catalysts work to encourage the synthesis of 3,4-dihydropyrimidin-2-(1H)-one/thione derivatives. The process in question precipitates rapid chemical changes without generating any waste by using tiny quantities of photocatalyst. When there are quantifiable light wavelengths present, this approach can be used. At multigram scales, atom-economical processes are very efficient and have a big impact on the industrial domain.

TABLE 5. The results of assessing the various catalysts’ catalytic efficacy for the synthesis of 4ja.
We have green photosynthesized 3,4-dihydropyrimidin-2-(1H)-one/thione derivatives from arylaldehydes, β-ketoesters, and urea/thiourea by means of the radical-induced Biginelli reaction. In the current work, a new halogenated dicyanobenzene-based photosensitizer; 3DPAFIPN was employed as a donor-acceptor (D-A) photocatalyst. It works by causing a sequence of electron transfers that are triggered by visible light. Blue light-emitting diode (LED) technology has been demonstrated to generate a sustained energy-generating mechanism at room temperature and in an air environment when used in an ethanol medium. The suggested method has significant advantages for the field of chemical synthesis. Fast reaction times, the removal of hazardous solvents, higher product yields, streamlined reaction mechanisms, and the utilization of a sustainable energy source are some of these benefits. The separation method does not need chromatography. By preserving the result, it is possible to accelerate a multigram-scale reaction of model substrates. As a result, the method may be used in an environment that promotes long-term ecological and financial sustainability.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
FM: Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. AMA: Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
This work is financially supported by Iran National Science Foundation (INSF) (no. 4015618), financially supported by Iran’s National Elites Foundation (no. 4015618), and also, Shiraz University of Medical Sciences.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2024.1361266/full#supplementary-material
Ahmed, B., Khan, R. A., Habibullah, A., and Keshai, M. (2009). An improved synthesis of Biginelli-type compounds via phase-transfer catalysis. Tetrahydron Lett. 50, 2889–2892. doi:10.1016/j.tetlet.2009.03.177
Ashok, M., Holla, B. S., and Kumara, N. S. (2007). Convenient one pot synthesis of some novel derivatives of thiazolo [2,3-b] dihydropyrimidinone possessing 4-methylthiophenyl moiety and evaluation of their antibacterial and antifungal activities. Eur. J. Med. Chem. 42, 380–385. doi:10.1016/j.ejmech.2006.09.003
Attri, P., Bhatia, R., Gaur, J., Arora, B., Gupta, A., Kumar, N., et al. (2017). Triethylammonium acetate ionic liquid assisted one-pot synthesis of dihydropyrimidinones and evaluation of their antioxidant and antibacterial activities. Arabian J. Chem. 10, 206–214. doi:10.1016/j.arabjc.2014.05.007
Bahekar, S. S., and Shinde, D. B. (2004). Synthesis and anti-inflammatory activity of some 4,6-(4-substituted aryl)-2-thioxo-1. Bioorg. Med. Chem. Lett. 14 23. doi:10.1016/j.bmcl.2004.01.039
Bosica, G., Cachia, F., De Nittis, R., and Mariotti, N. (2021). Efficient one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones via a three-component Biginelli reaction. Molecules 26, 3753. doi:10.3390/molecules26123753
Bryden, M. A., and Zysman-Colman, E. (2021). Organic thermally activated delayed fluorescence (TADF) compounds used in photocatalysis. Chem. Soc. Rev. 50, 7587–7680. doi:10.1039/D1CS00198A
Cardinale, L., Konev, M. O., and Jacobi von Wangelin, A. (2020). Photoredox-catalyzed addition of carbamoyl radicals to olefins: a 1,4-dihydropyridine approach. Chemistry–A Eur. J. 26, 8239–8243. doi:10.1002/chem.202002410
Chopda, L. V., and Dave, P. N. (2020). Heteropoly-12-tungstophosphoric acid H3[PW12O40] over natural bentonite as a heterogeneous catalyst for the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones. Arabian J. Chem. 13, 5911–5921. doi:10.1016/j.arabjc.2020.04.034
Choudhare, T. S., Wagare, D. S., Kadam, V. T., Kharpe, A. A., and Netankar, P. D. (2021). Rapid one-pot multicomponent dioxane-HCl complex catalyzed solvent-free synthesis of 3,4-dihydropyrimidine-2-one derivatives. Polycycl. Aromat. Compd. 42, 3865–3873. doi:10.1080/10406638.2021.1873808
Donabauer, K., Murugesan, K., Rozman, U., Crespi, S., and Konig, B. (2020). Photocatalytic reductive radical-polar crossover for a base-free corey-seebach reaction. Chemistry–A Eur. J. 26, 12945–12950. doi:10.1002/chem.202003000
Flynn, A. R., McDaniel, K. A., Hughes, M. E., Vogt, D. B., and Jui, N. T. (2020). Hydroarylation of arenes via reductive radical-polar crossover. J. Am. Chem. Soc. 142, 9163–9168. doi:10.1021/jacs.0c03926
Gualandi, A., Anselmi, M., Calogero, F., Potenti, S., Bassan, E., Ceroni, P., et al. (2021). Metallaphotoredox catalysis with organic dyes. Org. Biomol. Chem. 19, 3527–3550. doi:10.1039/D1OB00196E
Heys, L., Moore, C. G., and Murphy, P. (2000). The guanidine metabolites of Ptilocaulis spiculifer and related compounds; isolation and synthesis. Chem. Soc. Rev. 29, 57–67. doi:10.1039/A903712H
Hurst, E. W., and Hull, R. (1961). Two new synthetic substances active against viruses of the psittacosis-lymphogranuloma-trachoma group. J. Med. Chem. 3, 215–229. doi:10.1021/jm50015a002
Kamal, A., Krishnaji, T., and Azhar, M. A. (2007). Copper(II)tetrafluoroborate as a mild and efficient catalyst for the one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. Catal. Commun. 8, 1929–1933. doi:10.1016/j.catcom.2007.03.009
KÜÇÜKİSLAMOĞLU, M., BEŞOLUK, Ş., Zengin, M., Arslan, M., and Nebioğlu, M. (2010). An efficient one-pot synthesis of dihydropyrimidinones catalyzed by zirconium hydrogen phosphate under solvent-free conditions. Turkish J. Chem. 34, 411–416. doi:10.3906/kim-0912-357
Kumar, A., and Maurya, R. A. (2007). An efficient bakers’ yeast catalyzed synthesis of 3,4-dihydropyrimidin-2-(1H)-ones. Tetrahedron Lett. 48, 4569–4571. doi:10.1016/j.tetlet.2007.04.130
Lal, J., Sharma, M., Gupta, S., Parashar, P., Sahu, P., and Agarwal, D. D. (2012). Hydrotalcite: a novel and reusable solid catalyst for one-pot synthesis of 3,4-dihydropyrimidinones and mechanistic study under solvent free conditions. J. Mol. Catal. A Chem. 352, 31–37. doi:10.1016/j.molcata.2011.09.009
Li, N., Wang, Y., Liu, F., Zhao, X., Xu, X., An, Q., et al. (2020). Air-stable zirconium (IV)-salophen perfluorooctanesulfonate as a highly efficient and reusable catalyst for the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones under solvent-free conditions. Appl. Organomet. Chem. 34, e5454. doi:10.1002/aoc.5454
Litvic, M., Vecani, I., Ladisic, Z. M., Lovric, M., Voncovic, V., and Filipan-Litvic, M. (2010). First application of hexaaquaaluminium(III)tetrafluoroborate as a mild, recyclable, non-hygroscopic acid catalyst in organic synthesis: a simple and efficient protocol for the multigram scale synthesis of 3,4-dihydropyrimidinones by Biginelli reaction. Tetrahedron 66, 3463–3471. doi:10.1016/j.tet.2010.03.024
Liu, J. N., Li, J., Zhang, L., Song, L. P., Zhang, M., Cao, W. J., et al. (2012). Facile one-pot three-component reaction to synthesize trifluoromethylated cyclopenta[b]pyran derivatives and their further transformation. Tetrahedron Lett. 53, 2469–2472. doi:10.1016/j.tetlet.2012.03.023
Magerramow, A. M., Kurbanova, M. M., Abdinbekova, R. T., Rzaeva, I. A., Farzaliev, V. M., and Allokhverdiev, M. A. (2006). Synthesis and antioxidative properties of some 3,4-dihydropyrimidin-2(1H)ones (-thiones),Russian. J. Appl. Chem. 79, 787–790.
Maleki, A., and Paydar, R. (2016). Bionanostructure-catalyzed one-pot three-component synthesis of 3, 4-dihydropyrimidin-2 (1H)-one derivatives under solvent-free conditions. React. Funct. Polym. 109, 120–124. doi:10.1016/j.reactfunctpolym.2016.10.013
Mohamadpour, F. (2021a). New role for photoexcited organic dye, Na2 eosin Y via the direct hydrogen atom transfer (HAT) process in photochemical visible-light-induced synthesis of spiroacenaphthylenes and 1H-pyrazolo[1,2-b]phthalazine-5,10-diones under air atmosphere. Dyes Pigments 194, 109628. doi:10.1016/j.dyepig.2021.109628
Mohamadpour, F. (2021b). Catalyst-free, visible light irradiation promoted synthesis of spiroacenaphthylenes and 1H-pyrazolo[1,2-b]phthalazine-5,10-diones in aqueous ethyl lactate. J. Photochem. Photobiol. A Chem. 407, 113041. doi:10.1016/j.jphotochem.2020.113041
Mohamadpour, F. (2021c). Catalyst-free and solvent-free visible light irradiation-assisted Knoevenagel–Michael cyclocondensation of aryl aldehydes, malononitrile, and resorcinol at room temperature. Monatsh. für Chemie-Chemical Mon. 152, 507–512. doi:10.1007/s00706-021-02763-1
Mohamadpour, F. (2022a). Catalyst-free and solvent-free visible light assisted synthesis of tetrahydrobenzo[b]Pyran scaffolds at room temperature. Polycycl. Aromat. Compd. 42, 7607–7615. doi:10.1080/10406638.2021.2006244
Mohamadpour, F. (2022b). Visible-light-induced radical condensation cyclization to synthesize 3,4-dihydropyrimidin-2-(1H)-ones/thiones using photoexcited Na2 eosin Y as a direct hydrogen atom transfer (HAT) catalyst. ACS Omega 7, 8429–8436. doi:10.1021/acsomega.1c05808
Mohamadpour, F. (2022c). The development of imin-based tandem Michael–Mannich cyclocondensation through a single-electron transfer (SET)/energy transfer (EnT) pathway in the use of methylene blue (MB+) as a photo-redox catalyst. RSC Adv. 12, 10701–10710. doi:10.1039/D2RA01190E
Mohamadpour, F. (2022d). Visible-light-driven radical Friedländer hetero-annulation of 2-aminoaryl ketone and α-methylene carbonyl compound via organic dye fluorescein through a single-electron transfer (SET) pathway. BMC Chem. 16, 116. doi:10.1186/s13065-022-00910-1
Mohamadpour, F. (2023a). Acridine yellow G-catalyzed visible-light-promoted synthesis of 2-amino-4H-chromene scaffolds via a photo-induced electron transfer process in an aqueous media. Catal. Surv. Asia 27, 306–317. doi:10.1007/s10563-023-09397-9
Mohamadpour, F. (2023b). 3DPAFIPN as a halogenated dicyanobenzene-based photosensitizer catalyzed gram-scale photosynthesis of pyrano[2,3-d]pyrimidine scaffolds. Sci. Rep. 13, 13142. doi:10.1038/s41598-023-40360-w
Mohamadpour, F. (2023c). Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media. Curr. Res. Green Sustain. Chem. 6, 100356. doi:10.1016/j.crgsc.2023.100356
Mohamadpour, F. (2023d). Carbazole-based photocatalyst (4CzIPN) as a novel donor-acceptor fluorophore-catalyzed visible-light-induced photosynthesis of dihydropyrano[2,3-c]pyrazole scaffolds via a proton-coupled electron transfer process. J. Chem. Sci. 135, 74. doi:10.1007/s12039-023-02204-y
Mohamadpour, F., and Lashkari, M. (2018). Three-component reaction of β-keto esters, aromatic aldehydes and urea/thiourea promoted by caffeine, a green and natural, biodegradable catalyst for eco-safe Biginelli synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones derivatives under solvent-free conditions. J. Serbian Chem. Soc. 83, 673–684. doi:10.2298/JSC170712041M
Mohamadpour, F., Maghsoodlou, M. T., Heydari, R., and Lashkari, M. (2016). Saccharin: a green, economical and efficient catalyst for the one-pot, multi-component synthesis of 3,4-dihydropyrimidin-2-(1H)-one derivatives and 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives and substituted dihydro-2-oxypyrrole. J. Iran. Chem. Soc. 13, 1549–1560. doi:10.1007/s13738-016-0871-5
Pinosa, E., Bassan, E., Cetin, S., Villa, M., Potenti, S., Calogero, F., et al. (2022). Light-induced access to carbazole-1, 3-dicarbonitrile: a thermally activated delayed fluorescent (TADF) photocatalyst for cobalt-mediated allylations. J. Org. Chem. 88, 6390–6400. doi:10.1021/acs.joc.2c01825
Rothfelder, V., Streitferdt, U., Lennert, J., Cammarata, D., Scott, J., Zeitler, K., et al. (2021). Photocatalytic arylation of P4 and PH3: reaction development through mechanistic insight. Angew. Chem. 60, 24855–24863. doi:10.1002/ange.202110619
Speckmeier, E., Fischer, T. G., and Zeitler, K., A toolbox approach to construct broadly applicable metal-free catalysts for photoredox chemistry: deliberate tuning of redox potentials and importance of halogens in donor-acceptor cyanoarenes, J. Am. Chem. Soc. 140 (2018) 15353 −15365. doi:10.1021/jacs.8b08933
Sujatha, K., Shanmugam, P., Perumal, P. T., Muralidharan, D., and Rajendran, M. (2006). Synthesis and cardiac effects of 3,4-dihydropyrimidin-2(1H)-one-5carboxylates. Bioorg. Med. Chem. Lett. 16, 4893–4897. doi:10.1016/j.bmcl.2006.06.059
Uoyama, H., Goushi, K., Shizu, K., Nomura, H., and Adachi, C. (2012). Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238. doi:10.1038/nature11687
V Chopda, L., and Dave, P. N. (2020). 12-Tungstosilicic acid H4[W12SiO40] over natural bentonite as a heterogeneous catalyst for the synthesis of 3,4-dihydropyrimidin-2(1H)-Ones. ChemistrySelect 5, 2395–2400. doi:10.1002/slct.201904962
Wisen, S., Androsavich, J., Evans, C. G., Chang, L., and Gestwi cki, J. E. (2008). Chemical modulators of heat shock protein 70 (Hsp70) by sequential, microwave-accelerated reactions on solid phase. Bioorg. Med. Chem. Lett. 18, 60–65. doi:10.1016/j.bmcl.2007.11.027
Wu, Q. A., Chen, F., Ren, C. C., Liu, X. F., Chen, H., Xu, L. X., et al. (2020). Donor-acceptor fluorophores as efficient energy transfer photocatalysts for [2 + 2] photodimerization. Org. Biomol. Chem. 18, 3707–3716. doi:10.1039/C9OB02735A
Yang, Z., Mao, Z., Xie, Z., Zhang, Y., Liu, S., Zhao, J., et al. (2017). Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 46, 915–1016. doi:10.1039/C6CS00368K
Zhang, Y., Wang, B., Zhang, X., Huang, J., and Liu, C. (2015). An efficient synthesis of 3,4-dihydropyrimidin-2(1H)-Ones and thiones catalyzed by a novel brønsted acidic ionic liquid under solvent-free conditions. Molecules 20, 3811–3820. doi:10.3390/molecules20033811
Keywords: dicyanobenzene-based photosensitizer (3DPAFIPN), renewable energy source, visible-light-induced electron-transfer, photosynthesis, 3, 4-dihydropyrimidin-2-(1H)-one/thione derivatives
Citation: Mohamadpour F and Amani AM (2024) Halogenated dicyanobenzene-based photosensitizer (3DPAFIPN) as a thermally activated delayed fluorescence (TADF) used in gram-scale photosynthesis 3,4-dihydropyrimidin-2-(1H)-one/thione derivatives via a consecutive visible-light-induced electron-transfer pathway. Front. Chem. 12:1361266. doi: 10.3389/fchem.2024.1361266
Received: 25 December 2023; Accepted: 15 February 2024;
Published: 01 March 2024.
Edited by:
Takashi Ohshima, Kyushu University, JapanReviewed by:
Vinay S. Sharma, Gujarat University, IndiaCopyright © 2024 Mohamadpour and Amani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farzaneh Mohamadpour, Zl9tb2hhbWFkcG91ckBzdW1zLmFjLmly, bW9oYW1hZHBvdXIuZi43QGdtYWlsLmNvbQ==; Ali Mohammad Amani, YW1hbmlfYUBzdW1zLmFjLmly, YWxpYW1hbmlAc3Vtcy5hYy5pcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.