
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 25 March 2024
Sec. Medicinal and Pharmaceutical Chemistry
Volume 12 - 2024 | https://doi.org/10.3389/fchem.2024.1353385
 Mohamed Radi1*
Mohamed Radi1* Zaina Eddardar2
Zaina Eddardar2 Aziz Drioiche1
Aziz Drioiche1 Firdaous Remok1
Firdaous Remok1 Md. Eram Hosen3
Md. Eram Hosen3 Khalid Zibouh1
Khalid Zibouh1 Brahim Ed-Damsyry1
Brahim Ed-Damsyry1 Amale Bouatkiout1,4
Amale Bouatkiout1,4 Sanae Amine1
Sanae Amine1 Hanane Touijer1
Hanane Touijer1 Ahmad Mohammad Salamatullah5
Ahmad Mohammad Salamatullah5 Mohammed Bourhia6*
Mohammed Bourhia6* Samir Ibenmoussa7
Samir Ibenmoussa7 Touriya Zair1
Touriya Zair1This work aims to add value to the Lavandula genus by identifying the chemical composition, antioxidant, and antimicrobial activities of two species lavender from Oulmès in Morocco; Lavandula abrialis and Lavandula stoechas. The uniqueness lies in the integrated approach that combines in vitro and in silico analyses to assess the biological properties of the essential oils (EO). The objective of this study is to enhance the significance of the Lavandula genus by analyzing the chemical composition, antioxidant properties, and antimicrobial effects of two lavender species found in Oulmès, Morocco: Lavandula abrialis and Lavandula stoechas. The distinctiveness is in the comprehensive methodology that merges in vitro and in silico investigations to evaluate the biological characteristics of the essential oils (EO). The extraction of essential oils (EO) by hydrodistillation from the aerial parts of Lavandula abrialis gave a high yield of essential oils (2.9%) compared to Lavandula stoechas (2.3%). A GC-MS analysis of the chemical composition revealed 56 chemical compounds, with some variation in the predominant components, representing between 99.98% and 100% of the EOs of the studied lavenders. Their antioxidant activity was assessed using the DPPH test. This method revealed that L. stoechas EO has a higher percentage of free radical inhibition than L. abrialis. The IC50 values demonstrate that the antioxidant activity of ascorbic acid is higher (1.62 g/mL) than the EOs of tested plants. Noteworthy, the EO of L. stoechas is more potent (12.94 g/mL) than that of Lavandula tibialis (34.71 g/mL). Regrading, the antibacterial tests, the EO of L. abrialis was particularly active against Staphylococcus aureus BLACT, which is inhibited at a concentration of 6.25 g/mL, while L. stoechas EO has a strong effect on Escherichia coli, with a MIC of 1.56 g/mL. Concerning the antifungal activity of the EOs, yeasts showed sensitivity toward EOs extracted from both L. tibialis and L. stoechas. Moreover, an in silico study was conducted targeting sarA protein of S. aureus (PDB ID: 2fnp) and NADPH oxidase from Lavandula sanfranciscensis (PDB: 2CDU) and results showed that Ishwarone and Selina-3,7 (11)-diene exhibited highest binding energy with −9.8 and −10.8 kcal/mol respectively. Therefore, these two compounds could be used as an antibacterial and antioxidant agents however more experimental and molecular study should be required.
Aromatic and medicinal plants (MAPs) hold an important place in modern and ancient civilizations (including ancient Egypt and China) and all continents (Fellah et al., 2006). They are used in daily life and consumed for their medicinal and nutritional properties. Increasing demand for MAPs as raw material has been noticed and their use for various purposes in many fields such as pharmacy, agri-food, and phytosanitary sectors (Raal, 2010).
With the increasing resistance of harmful microbes to traditional antibiotics, many scientific studies are now focusing on the use of plant extracts as antibiotic alternatives (Essawi and Srour, 2000; Boutahiri et al., 2022). They are also a rich source of compounds with high antioxidant potential. Indeed, these chemicals protect body cells from free radical damage, as well as maintain food quality and protect it from oxidation (Willcox et al., 2004). In this respect, essential oils have been the focus of various studies to identify new antioxidants of natural origin as an alternative to synthetic antioxidants, which have a certain toxicity (Atmani et al., 2009; Suhaj, 2006), in food preservation, and as a preventative agent for certain human diseases (Saidi et al., 2022; Khettal et al., 2017).
Morocco, due to its geographical area and climatic diversity, is a true phylogenetic reservoir, with approximately 4,200 species, nearly 400 of which belong to 116 families and 726 genera (Fakchich and Elachouri, 2021), allowing it to occupy a privileged position among Mediterranean countries with a long medical tradition and ancestral knowledge and experience on medicinal plants (Scherrer et al., 2005).
The Lamiaceae family, cited by Bachiri et al. (2015), is renowned in the field of herbal medicine for its extensive size, consisting of more than 250 genera and over 7,000 species, as reported by Gourich et al. (2022). Indeed, most of the plants in this family are rich in essential oils and are widely utilized in aromatherapy, perfumery, and the cosmetics industry (Fakchich and Elachouri, 2021; Cox-Georgian et al., 2019; Bekut et al., 2018). Furthermore, it includes the important genus Lavandula, which belongs to the subfamily Nepetoideae (Dupont and Guignard, 2012), and contains approximately 39 species, numerous hybrids, and nearly 400 registered cultivars (Upson and Andrews, 2004). This subshrub can grow to be 1 m tall and has blue-purple blooms. White and pink flowers can be found in other varieties (Mohammedi and Fawzia, 2011). In addition to cultivated species, most notably hybrid lavandin, several wild species abound in different regions (Bellakhdar, 1997).
They are aromatic perennials whose medicinal properties are widely used in herbalism and aromatherapy. These therapeutic properties are linked to their primary and secondary metabolites, particularly their essential oils. Indeed, the latter has antispasmodic, antibacterial, antifungal, antioxidant, and acaricidal properties (Mohammedi and Fawzia, 2011; Bachiri et al., 2017). Recent research on lavender essential oils emphasizes that the medicinal properties and fragrance of lavender essential oils are primarily due to their volatile organic compounds. Specifically, monoterpenes and sesquiterpenes are responsible for both the characteristic fragrance of lavender and the therapeutic properties of the essential oils. Moreover, the chemical makeup of the plants’ essential oils is influenced by various factors, including both inherent and external factors such as the genetic makeup, harvesting conditions, drying and preservation methods, and extraction techniques (Bachiri et al., 2016; Bachiri et al., 2017).
The territorial commune of Oulmès, which is part of the mid-Atlas in Morocco, contains several species of the genus Lavandula, which has attracted valuation attention due to its large geographic distribution, and represents a very important source of income for the local community, especially farmers and rural women. Lavandula has also offered promising prospects in contributing to the sustainable development of this marginal area by organizing producers and collectors into agricultural cooperatives. As well as more than a dozen cooperatives have participated in the improvement and diversification of lavender products, through the design of organic labels and certification of PGI (Protected Geographical Index) and PDO (Protected Designation of Origin) (Provincial Directorate of Agriculture DPA of Khémisset, Monograph of the province of Khémisset, 2022).
In this context, our work is related to the valorization of aromatic and medicinal plants in the plateau of Oulmès through a comparative analysis of the chemical composition, antioxidant activity, and antimicrobial activity of two species of lavender: cultivated lavender (Lavandula abrialis) and wild lavender (Lavandula stoechas).
The commune of Oulmès (Figure 1) is part of the province of Khemisset (Region of Rabat-Sale Kenitra) and it covers an area of 1,001.66 km2. This area is dominated by rugged reliefs which represent more than 80% of the total surface area (Provincial Directorate of Agriculture DPA of Khémisset, Monograph of the province of Khémisset, 2022). The climate is subhumid, characterized by wet, cold winters and hot summers. Monthly rains are characterized by a rainfall regime that varies from 1 year to the next, reflecting the irregularity of precipitation with annual averages ranging between 280 and 400. Snowfall can occur from mid-November and the temperature varies between −2 and 40°C (Provincial Directorate of Agriculture DPA of Khémisset, Monograph of the province of Khémisset, 2022). This climate allows the region to be rich in aromatic and medicinal plants and it also contributes to the development of different varieties of lavender.
Lavandula abrialis RAB114629 and L. stoechas RAB114630 (Figures 2A, B) were collected in May 2022 in locations listed with their geographical coordinates in Table 1. The botanical identity of these two species was finalized (Table 2) at the Department of Botany, Scientific Institute in Rabat. The samples of leaves and flowers were collected in burlap bags and stored away from light and humidity until needed to preserve the integrity of the molecules as much as possible.
Figure 2. Photos of L. stoechas (A) Lavandula abrialis (B) taken by RADI Mohamed and ZAIR Touriya (2022).
The moisture content of the plant is measured by subjecting 5 g of each plant to a drying process in an oven at a temperature between 100°C and 104°C for a duration of 24 h, following the guidelines outlined in the AFNOR standard (NF - V03-402 1985) (AFNOR NF V03-402, 1985).
The following formula was used to determine the moisture content after each species underwent three repetitions (Equation 1):
With:
MC: moisture content of the dry plant in %.
mi: Initial mass of the plant before drying in (g).
mf: Final mass of the plant after drying in (g).
The pH of a specific product indicates its level of acidity or alkalinity. A volume of 10 mL of heated distilled water was introduced to 2 g of the experimental specimen. Following agitation, the liquid is subjected to filtration, then cooled, and the pH is determined using a pH meter.
Following the protocol, 10 g of the powder from each plant was mixed with 100 mL of distilled water and heated for 15 min while stirring. After the mixture had cooled, it was titrated with a solution of NaOH (N = 0.1) in the presence of phenolphthalein until a persistent pink color developed (AFNOR NF V03-402, 1985).
The acidity index is determined according to the following Equation (2):
With:
V: Volume of the KOH solution (0.1 mol/L) in ml.
N: Normality of the KOH solution (0.1 mol/L).
TS: mass of the test sample in g.
56.1: Constant FactorTa.
According to AFNOR NF V05-113 standard, 1972 (AFNOR NF V05-113,1972), the determination of the ash content is based on the destruction of any carbon particle of a 2 g sample of each plant, under a temperature of 550°C. The operation will only be completed when the color of the residue becomes grayish-white, which will turn into a white color after cooling.
Organic matter is calculated according to the following Equation (3):
With:
OM%: Organic matter.
W1: Weight of the capsule and the sample before calcination.
W2: Weight of the capsule and the sample after calcination.
TS: test sample.
The ash content (or mineral matter content: MM) is determined as follows: MM = 100-OM%
To determine the content of seven heavy metals (As, Cd, Cr, Fe, Pb, Sb, and Ti) in the plant matter of studied plants, the standardized mineralization protocol (NF EN, 1999-1-1/A1, 1999) was used.
This process consists of mixing the crushed plant material (0.1 g) with 3 mL of aqua regia prepared from 1 mL of nitric acid HNO3 (99%) and 2 mL of hydrochloric acid HCl (37%), The mixture refluxes at 200°C for 2 hours. Once liquid is chilled and separated from solid particles, it is collected and filtered through a 0.45 µm pore size filter. The mixture is diluted with distilled water to 15 mL. The concentrations of heavy metals are determined by the inductively coupled plasma atomic emission spectrophotometer ICP-AES (Ultima 2 Jobin Yvon) at the TSUSR laboratory (Technical Support Unit for Scientific Research) at CNRST in Rabat (Skujins, 1998).
The essential oils of the two lavenders were obtained using hydrodistillation using a Clevenger-type device (Clevenger, 1928). A mass of 100 g of plant material, of each dried and mixed lavender, is submerged in water (1 L) in a 2 L flask. This mixture was boiled for 3 h at a temperature of 90°C. Each plant material underwent three cycles. The oil was dehydrated using anhydrous sodium sulfate (Na2SO4) and then kept in an airtight brown glass container at a temperature of 4°C until it was ready to be used.
The EO Yield was calculated according to the following formula (Equation 4):
With:
V (EO): Volume of EO recovered (g).
M0: Mass of plant material (100 g).
The analysis of the lavender EO samples was carried out by a Thermo Electron type gas chromatograph (Trace GC Ultra) coupled to a Thermo Electron Trace MS system mass spectrometer (Thermo Electron: Trace GC Ultra; Polaris Q MS). Fragmentation is carried out by electronic impact with an intensity of 70 eV. The chromatograph is equipped with a DB-5 column (5% phenyl-methyl-siloxane) (30 m × 0.25 mm x 0.25 μm film thickness), and a flame ionization detector (FID) powered by a mixture of H2/Air gas. The column temperature is programmed at a rate of 4°C/min from 50°C to 200°C for 5 min. The device has a split–splitless PVT (Programmed Vaporization Temperature) injector. Split injection is employed, with a leakage ratio of 1/70 and a flow rate of 1 mL/min for the vector gas nitrogen.
The identification of the constituents of essential oils was made based on the determination and comparison of the Kovats indices (KI) of the compounds with those of the standard products known and described in the databases of Kovats (1965) and Adams (2007), By conducting a comparison of the peak retention periods with the known legitimate standards present in the authors’ laboratory, as well as comparing the stated KI and MS data with the mass spectral database standards of WILEY and NIST 14, along with published literature.
The density of an essential oil is determined by using a pycnometer at a temperature of 20°C. The term refers to the ratio between the density of the oil and the density of pure water at the same temperature. The determination was made based on the following formula (Equation 5).
With
m0: Mass in grams of the empty pycnometer.
m1: Mass in grams of the pycnometer filled with water.
m2: Mass in grams of the pycnometer filled with oil.
The acid value is the number of mg of KOH necessary for the neutralization of the free acids contained in 1 g of EO. The free acids are neutralized with a titrated ethanolic solution of potassium hydroxide (KOH). In our case, we used a 0.1 N KOH solution. Phenolphthalein is used as a color indicator.
The acidity result is expressed in % oleic acid by the following formula (Equation 6):
With:
V: Buret drop volume (in ml).
N: Normality of the KOH solution.
TS: Test sample in g.
MA: Molecular weight of oleic acid = 282 g/mol.
The Iodine value (IV) is a crucial analytical feature of oil that serves as an indication of its unsaturation. It is calculated according to the ISO 3961 method, by determining the number of grams of iodine attached to the double bonds present in 100 g of fat.
A 0.1 g quantity of the oil is added to a 100 mL Erlenmeyer flask along with 4 mL of chloroform and 5 mL of iodine monochloride (Wijs reagent). The solution is agitated gently and then stored in a dark location for 1 h at a temperature of 20°C. Subsequently, 4 mL of a potassium iodide solution with a concentration of 10% and 30 mL of distilled water are introduced.
The resulting mixture is titrated with sodium thiosulfate (0.1 N) using a few drops of starch solution as a colored indicator. The titration is continued until the blue color disappears. A blank test without oil is conducted at the same time and under the same circumstances.
The results are expressed as follows (Equation 7):
With:
IV: Iodine value.
V0: Volume of sodium thiosulfate used for the blank titration.
V1: Volume of the sodium thiosulfate solution.
N: Normality of the thiosulfate solution.
TS: Test sample.
Peroxide value (PV) is the most widely used chemical technique for evaluating the oxidative degradation of unsaturated fatty acids in essential oils.
According to regulation CEE/2568/9, the prescribed amounts for the combination are as follows: 0.5 g of oil in a container with a volume of 100 mL, 4 mL of dichloromethane, 6 mL of acetic acid, and 0.1 mL of an aqueous solution containing saturated potassium iodide (15 g of potassium iodide diluted in 10 mL of distilled water). Afterward, the bottle is tightly sealed and forcefully shaken for a period of 1 min. Afterwards, the balloon is left undisturbed in darkness for 5 min, and then 10 cc of distilled water is added.
The liberated iodine is quantitatively determined by aggressively swirling it with a 0.1 N thiosulfate solution in the presence of starch as a chromogenic indicator. A control experiment, devoid of any oil, is conducted simultaneously and under identical circumstances.
The peroxide index (PI) is expressed in milliequivalents of oxygen per kg of oil (Equation 8).
With:
V: Volume poured with sodium thiosulfate (in ml).
TS: Oil sample to be analyzed in g.
N: Normality of sodium thiosulfate solution.
This test aims to evaluate the anti-radical effect of EOs from the plants studied using a stable free radical which is 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Wong et al., 2006).
The DPPH solution is prepared by solubilizing 2.4 mg of DPPH• in 100 mL of pure methanol. The test is carried out by mixing 100 µL of each EO with 3.9 mL of DPPH• solution. The standard used is ascorbic acid at different concentrations. At the same time, a negative control (blank) was carried out with pure methanol alone. All tests are repeated three times. The samples are then left in the dark for 30 min, and the discoloration compared to the negative control containing only the DPPH• solution is measured at 517 nm.
The results were expressed as percentage reduction or inhibition of DPPH•(AA%) using the following formula (Equation 9):
With:
AA%: Percentage of antioxidant activity.
A control: Absorbance of the solution containing only the solution of the DPPH• radical.
A sample: Absorbance of the solution of the samples to be tested in the presence of DPPH•.
The study of the variation in absorbance as a function of the concentration of ascorbic acid made it possible to determine the inhibitory concentrations of EOs (IC50), the values of which were determined graphically. It should be noted that since there is no absolute measure of the antioxidant capacity of a compound, results are often compared to a reference antioxidant, such as ascorbic acid.
The assessment of the antibacterial efficacy of the essential oils (EOs) derived from two types of lavender was conducted on a total of 10 microorganisms (Table 3). All strains (bacterial and fungal) were isolated from the hospital environment: Mohamed V Provincial Hospital-Meknes. These specific microbes are pathogenic, renowned for their robust antibiotic resistance, as well as their ability to invade and produce toxins in humans. These strains were taken from a 20% glycerol stock at −80°C, rejuvenated on Mueller Hinton and Sabouraud broths, and subcultured before use (Ailli et al., 2023).
The minimum inhibitory concentration (MIC) is defined as the lowest concentration of essential oil capable of inhibiting the growth of a microorganism (Kuete,2 004). The determination of the MIC was carried out using the microdilution method (Balouiri et al., 2016; Ailli et al., 2023). The MIC value of each extract was determined using a sterile 96-well microtiter plate. A microtiter plate was prepared by transferring 100 µL of Mueller-Hinton broth for bacteria and Sabouraud for fungi to all wells. From a stock solution of the essential oil prepared in 10% DMSO, a dilution series was carried out to obtain concentrations of 5 to 0.93 10−2 mg/mL of each EO. And then, 10 μL of inoculum 106 CFU/mL (bacteria) or 104 CFU/mL (fungi) was added to all wells. The microplates were incubated for 24–48 h at 37°C. After incubation, 10 µL of resazurin (5 mg/mL) is added to each well as an indicator of microbial growth. After a second incubation for 2 h at 37°C, microbial growth was revealed by the change in color from purple to pink. The MIC value is determined as the lowest concentration that prevents a color change of resazurin. To determine the minimum bactericidal concentration (MBC)/minimum fungicidal concentration (MFC), 10 µL was taken from each well without visible growth and inoculated in Mueller Hinton (MH) agar for bacteria or in Sabouraud for fungi. The boxes are incubated for 24 h at 37°C. The MBC and MFC were defined as the lowest concentrations of the analyzed samples that produced a 99.99% reduction in UFC/mL compared to the control (Bachiri et al., 2016). The MBC/MIC or MFC/MIC ratio is calculated to evaluate the antimicrobial potency. If this ratio is less than 4, the effect of the EO is bactericidal/fungicidal, if the ratio is greater than 4, the sample has a bacteriostatic/fungistatic effect.
The GC-MS analysis detected total 56 phytochemicals from L. stoechas and L. abrialis were used as a ligand for docking analysis. For virtual screening, all ligands are downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/) in SDF format.
For in silico antibacterial and antioxidant analysis, the x-ray crystal structure of sarA protein of Staphylococcus aureus (PDB ID: 2fnp) and NADPH oxidase from Lavandula sanfranciscensis (PDB: 2CDU) were retrieved from the protein data bank (https://www.rcsb.org/). The Discovery Studio software was employed to clean the structure of the selected proteins obtained from the Protein Data Bank. Heteroatoms were eliminated during this process. Subsequently, utilizing the GROMOS96 43b1 force field and the SwissPDB Viewer software, the energy of the refined protein structures was subjected to minimization and optimization procedures.
The molecular docking analysis involving the interaction of phytochemicals derived from L. stoechas and L. abrialis with target proteins was performed using the PyRx software through the autodock wizard. The protein structure underwent conversion into a macromolecule, while the ligands were transformed into the PDBQT format. For the docked complexes, the center and grid box size were as on their binding pocket. The final docking calculations were executed using PyRx, and the selection of top molecules was based on lower binding energy. Subsequently, Discovery Studio software was employed to investigate the binding interactions and poses of the docked complexes.
Results were presented using the mean ± standard mean error. Statistical significance was set at
The collected samples underwent quality control by assessing several distinguishing factors, including moisture content (MC), pH, acidity, ash, and heavy metal content. The results are presented in Tables 4 and 5.
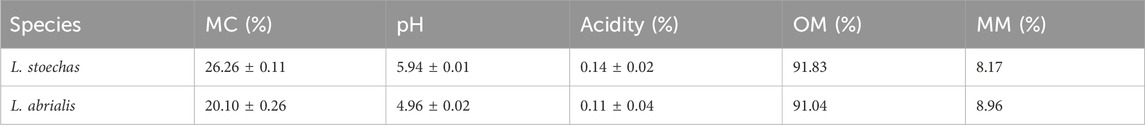
Table 4. Quality control of plant matter: moisture content (MC), pH, acidity, organic material (OM), and mineral matter (MM).
The water content of L. stoechas and L. abrialis varied between 26.26% and 20.10% respectively. Several factors could influence the moisture content of plants such as the nature of the fibers, the age of the plants, the condition of the soil, and the shelf life of the plant after harvest (Bachiri et al., 2016).
The hydrogen potential provides information regarding the assimilation of nutrients by plants (Saidi et al., 2023). In the present work, the
The analyses performed on studied samples showed that the amounts of organic matter in L. stoechas and L. abrialis were approximately 91.83% and 91.04%, respectively. However, the amounts of mineral matter (or ash) were low, coming in at only 8.17% and 8.96%, respectively (Table 4). These findings showed that organic matter represented most of the weight of these plants.
Atomic absorption spectrophotometry is used to determine the concentration of heavy metals (Cr, Sb, Pb, Cd, Fe, and Ti) in two studied lavender plants. According to the results shown in Table 5, several metals were undetectable and had very low and fluctuating quantities. Additionally, the analyzed samples had values that were below the FAO/WHO (2009) limit threshold.
Hydrodistillation was used to extract the essential oils from the aerial parts of L. stoechas and L. abrialis. The essential oils produced ranged in color from light yellow to clear yellow but were always extremely strongly scented.
The yield of essential oils varies greatly from one species to another, according to the results obtained in Table 6, we noted that Lavandula abrialisa gave a good yield of essential oil (2.9% ± 0.13%) and L. stoechas revealed almost 2.3% ± 0.11%.
These findings corroborate those of Bachiri et al. (2016) and N'Dédianhoua et al. (2014), who also used hydrodistillation to recover the aerial parts of lavender plants from Morocco’s Mid-Atlas region. However, research on L. stoechas from Turkey (Ahmet et al., 2002) and Algeria (Mohammedi and Fawzia, 2011). found that their respective EO yields were low at 1.33% and 1.2%. The essential oil content of L. abrialis was slightly higher than the findings of Zrira (2007). Various factors, such as the environment, genotype, geographical origin of the plant, cultural practices, and extraction method, may contribute to differences in essential oil production between species (Aberchane et al., 2001; Bourkhis et al., 2009).
The GC/MS analysis of the essential oil of L. abrialis revealed 56 chemical compounds, totaling 99.98% (Table 7), including 72.13% oxygenated monoterpenes, 18.59% oxygenated sesquiterpenes, 7.7% hydrocarbon monoterpenes, and 1.56% sesquiterpenes (Figure 5A).
The main volatile compounds of the essential oil extracted from the flowers and leaves of L. abrialis (Figure 3) were linalool (19.16%), linalool acetate (13.41%), camphor (11.24%), borneol (9.88%), and cineole <1.8->(9.28%). This EO also contained other compounds such as terpineol<α-> (5.99%), lavandulylacetate (2.88%), terpinen-4-ol (2.63%), geranylacetate (2.45%) and geraniol (2.12%).
Our results corroborate with studies carried out in Mediterranean areas with small differences in the percentages. N’Dédianhoua et al. (2014) showed that linalool (25.86%), linalool acetate (13.66%), camphor (16.06%), borneol (11.94%), and Cineole<1.8-> (16.04%) were the main components of L. abrialis EO collected in the Mid-Atlas of Morocco. Zrira (2007) mentioned that the EO of this species is dominated by linalool (33.7%), camphor (17.6%), cineole <1.8> (14.5%) and linalylacetate (13.5%).
The analysis of L. stoechas (Figure 4) EO also showed 56 compounds. The latter represents 100% of its total composition (Table 7). The main components of this oil were camphor (24.35%), cubenol (15.54%), fenchone (9.58%), Pinene<α-> (7.19%), camphene (6.07%) and borneol (4.45%). The compounds identified were classified into four chemical families, namely, oxygenated monoterpenes (49.93%), oxygenated sesquiterpenes (26.42%), monoterpenes (16.71%) and sesquiterpenes (6.94%) (Figure 5B).
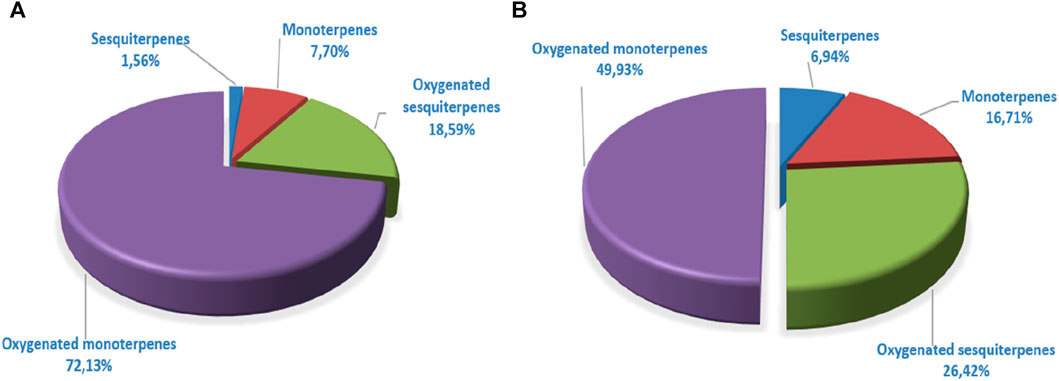
Figure 5. Distribution of terpene families of Lavandula abrialis (A) and Lavandula stoechas (B) Eos.
Previous studies have noted a distinct chemical composition. According to Mohammedi and Fawzia (2011), the EO of L. stoechas collected in Telmsen, Algeria, is distinguished by the presence of 21 compounds, including camphor (18.1%), cineole (18.9%), and fenchone (27.6%). Our sample had different contents, according to the chemical analysis than those examined by Ahmet et al. (2002) for Turkish L. stoechas, where they discovered high concentrations of pulegone (40.37%), hexahydrothymol (menthol), and menthone (12.57%).
A comparison of the chemical composition of the EOs demonstrated that these two lavenders contained variable proportions of oxygenated monoterpenes and oxygenated sesquiterpenes. The EO of L. abrialis was rich in alcohol (47.1%) followed by esters (22.72%) and ketones (11.24%) (Figure 6A), while the EO of L. stoechas was dominated mainly by ketones (38.19%), alcohols (34.05%), and hydrocarbons (23.65%) (Figure 6B). In addition, other chemical families were found in the EOs of these plants but with low percentages, specifically ethers, aldehydes, and epoxides.
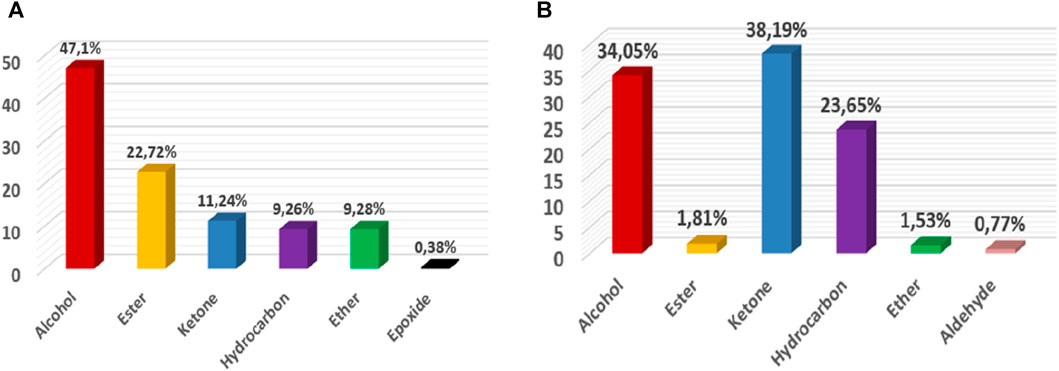
Figure 6. Distribution of families of chemical compounds of EOs of Lavandula abrialis (A) and Lavandula stoechas (B).
L.stoechas and L. abrialis both produced EOs with nearly identical densities. It fluctuated between 0.925 ± 0.001 and 0.929 ± 0.002 g/mL, respectively. These two EOs can be characterized as light oils because of their lower density (0.9982 g/mL) than water. The density of these oils complied with the AFNOR standard 2005 which sets a density of 0.906–0.990 as quality references (AFNOR, 2005; De Cliff and Harerimana, 2013).
The acid value indicates the content of free fatty acids in studied EOs. A low acidity value characterizes the purity and stability of an oil at room temperature [14]. The EOs of L. stoechas and L. abrialis had acid values between 10.09 ± 0.01 and 4.49 ± 0.003 respectively. These values indicate that L. stoechas oils were more susceptible to undergo alterations than L. abrialis EO.
The iodine value indicates the overall degree of unsaturation of the oils. The more unsaturated an oil is, the higher its iodine value (Wolff, 1968). The values of the iodine values of the EOs of the studied species (0.647 ± 0.01 for L. abrialis and 0.546 ± 0.01 for L. stoechas) were lower than those provided by the Codex Alimentarius standard (87–111 g of iodine/100 g) (Codex Alimentarius, 2017), and thus the conservation of these EOs could be done without running a significant risk of auto-oxidation.
The peroxide value is studied to assess the oxidation state of essential oils. The peroxide value of L. stoechas EO (18 ± 0.2) was higher than that of L. abrialis essential oil (14 ± 0.1). Comparing these values with those of the commercial standards CODEX STAN 210-1999, the two oils were categorized as good since they match the standards.
The antioxidant activity of the essential oils of the two lavenders was evaluated using the DPPH (2,2-diphenyl-1-picrylhydrazyl) test. The obtained results are displayed in Figure 7. The capacity of DPPH radicals to be scavenged was measured in terms of the percentage of DPPH free radicals that were inhibited by the antioxidants found in the various essential oils under study. The best antioxidant activity was indicated by the highest percentage of inhibition. Concentrations that provide 50% inhibition (IC50) were calculated from the curve in Figure 7 and are presented in Figure 8.
According to the present findings, the percentage of inhibition of free radicals increases with the increase in the concentration of ascorbic acid or EOs of the two lavenders. Furthermore, L. stoechas essential oil exhibited greater antioxidant activity than L. abrialis (61.03% and 53.93%, respectively) and lower than ascorbic acid (80.95%). The IC50 values showed that ascorbic acid has a higher antioxidant power (1.62 μg/mL) than EOs of the two studied lavenders and that L. stoechas EO was more effective (IC50 = 12.94 μg/mL) than L. abrialis EO (IC50 = 34.71 μg/mL). This antioxidant activity of extracted oils may have a connection with the chemical constituents that compose them. Indeed, camphor which is a major compound of the two studied EOs (24.35% for L. stoechas and 11.24% for L. abrialis), presented a strong correlation with the antioxidant activity, which was confirmed by the results of the heat map. Previous studies have confirmed that this compound also has strong antioxidant activity (Radi et al., 2022; Laib, 2012). Additionally, to camphor’s particular antioxidant properties, the synergistic interactions that can be established between the different constituents of essential oil were also behind its considerable antioxidant power (Laib and Barkat, 2011; Radi et al., 2022; Laib, 2012; Fatiha et al., 2011).
The assessment of the antibacterial activity of the studied lavender EOs was carried out at different concentrations on five bacterial strains (Table 8) (three Gram-negative bacteria and two Gram-positive bacteria). Table 9 shows that the EOs extracted from studied plants exhibit significant bactericidal activities against all bacteria tested. Indeed, these EOs inhibited the growth of the strains; Wild Escherichia coli, S. aureus BLACT, and Enterobacter cloacae at an amount of 1.56–12.5 μg/mL. For the two other strains (Klebsiella pneumoniae and Staphylococcus epidermidis), growth was inhibited at a concentration of 25 μg/mL.
The antifungal activity of the lavender EOs studied was also tested against five fungal strains. The results in Table 10 showed that all fungal strains were inhibited (100%) with concentrations less than or equal to 3.13 μg/mL, except for the yeast Candida dubliniensis which showed remarkable resistance to L. stoechas EO where inhibition was achieved at a MIC of 6.25 μg/mL.
The Heat map is one of the most often used graphical representations, especially in the sciences, for illustrating the influence of phenomena, including organic ones (Ailli et al., 2023). This map was used in this case to ascertain the relationship between the chemical composition of the studied lavender EOs, the antioxidant activity, and the antimicrobial activity (Figure 9).
Oxygenated monoterpenes had a positive effect on bacterial and fungal strains. Camphor, as their major component, was more effective against K. pneumoniae and S. epidermidis while fenchone, pinene, and camphene have a stronger effect on E. coli. Regarding the inhibition of strain growth of E. cloacae and S. aureus BLACT, linalool displayed a very high positive correlation while the other compounds of the oxygenated monoterpene family have a moderate correlation with studied fungal strains. Borneol and cineol have a strong correlation with C. dubliniensis.
Oxygenated sesquiterpenes are more effective against bacterial strains except for cadinol which has a moderate effect against fungal strains. Indeed, cubenol which was the principal ingredient of L. stoechas essential oil had a strong correlation against E. coli. Likewise, linaool acetate, a primary constituent of L. abrialis EO, has a very substantial correlation with strain growth inhibition of E. cloacae and S. aureus. These results corroborate with previous studies including Radi et al. (2022) who showed that L. stoechas EOs demonstrated significant antibacterial activity against several strains, such as S. aureus, Staphylococcus typhi, E. coli, A. baumanii, E. Cloacae and Staphylococcus dysenteria and antifungal activity against Candida glabrata, Candida albicans, Candida spp., Aspergillus fisheri, and Fusarum solani. According to Dorman and Deans, (2000), the antibacterial activity was brought on by the presence of phenol (1,8 cineole), alcohols (cubenol, borneol, terpineol, etc.), aldehydes, and ketones (camphor, linalool acetate, etc.). Bachiri et al. (2016) reported that L. stoechas EOs showed an antibacterial action against the four pathogens E. coli, K. pneumoniae, S. aureus, and P. mirabilis. Moreover, N'Dédianhoua et al. (2014) discovered that L. abrialis EO has an antibacterial effect on K. pneumoniae and E. coli.
The in vitro analysis revealed that the extracts showed strong antibacterial activity against S. aureus, therefore we selected SarA protein of S. aureus for molecular docking analysis. The sarA locus regulates over 100 genes on the S. aureus chromosome, playing a role in the intrinsic multidrug resistance mechanism by regulating drug accumulation (O'Leary et al., 2004). This protein is considered a master regulator of biofilm formation in S. aureus, and targeting this protein has been explored by several researchers as a potential strategy for drug development (Arya et al., 2015; Hosen et al., 2023). Among the 56 phytochemicals from two extracts the top five best ligands were selected based on their lowest binding energy, interaction with target protein, pose and RMSD value shown in Table 11.
Whereas, the compounds CID: 15559789 and CID: 10398656 exhibited maximum binding energy with −9.8 kcal/mol and 9.3 kcal/mol respectively (Table 1). The complex between 2fnp + CID: 15559789 was stabilized with highest four hydrogen bonds at A:Phe110, A:Leu113, B:Tyr142, and B:His159 (Figure 10A) while complex 2fnp + CID: 10398656 exhibited three hydrogen bond at A:Phe110, A:Leu113, and B:His159 (Figure 10B). The binding of these compounds at the active site of SarA protein could inhibit the pathogenesis of S. aureus (EF1).
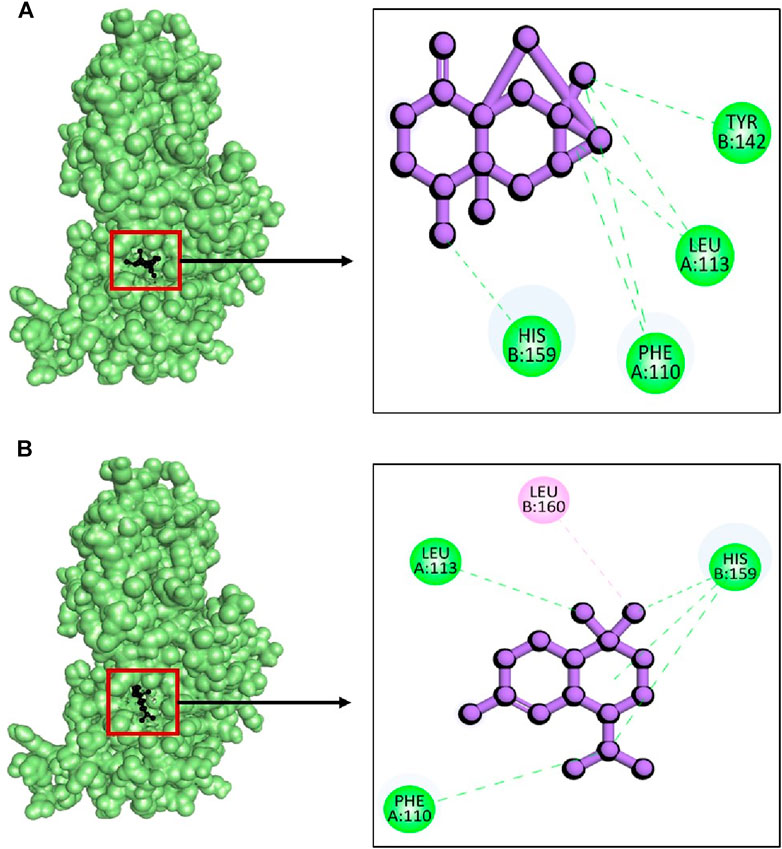
Figure 10. The interactions between phytochemicals and target SarA protein. Where (A) 2fnp + CID 15559789 and (B) 2fnp + CID 10398656.
This study also involved molecular docking study of NADPH oxidase (PDB: 2CDU) from L. sanfranciscensis to screen phytochemicals with the goal of identifying the most specific and potent inhibitors. NADPH oxidase is recognized for generating reactive oxygen species (ROS) at normal physiological levels. However, under certain disease conditions, it can become excessively activated, leading to the production of an excess of ROS. Several compounds have demonstrated the capacity to inhibit the overactivity of the enzyme (Kumar Deb et al., 2022; Kandsi et al., 2022). Interestingly, there limited published study has been reported on the inhibitory activity of L. stoechas and L. abrialis phytochemicals on NADPH oxidase. In the molecular docking study, from 56 selected phytochemical compounds of L. stoechas and L. abrialis, top 5 compounds were chosen based on the lowest binding energy (Table 12).

Table 12. The molecular interaction of plants phytochemicals against target NADPH oxidase (PDB: 2CDU).
In this case, against the target 2cdu, the highest binding energy was observed in the complex 2cdu + CID: 522296 with −10.8 kcal/mol followed by complex 2cdu + CID: 9855795 with binding energy −10.1 kcal/mol. The docking results revealed that to inhibit the 2cdu, the compound CID: 522296 showed five hydrogen bond at A:Pro432, B:His10, B:Phe14, B: Lys17, and B:Tyr62 (Figure 11A). Similarly, the complex 2cdu + CID: 9855795 stabilized with target protein with three hydrogen bond at A:Phe14, A:Lys17, B:Phe433, and B:Ile438 (Figure 11B).
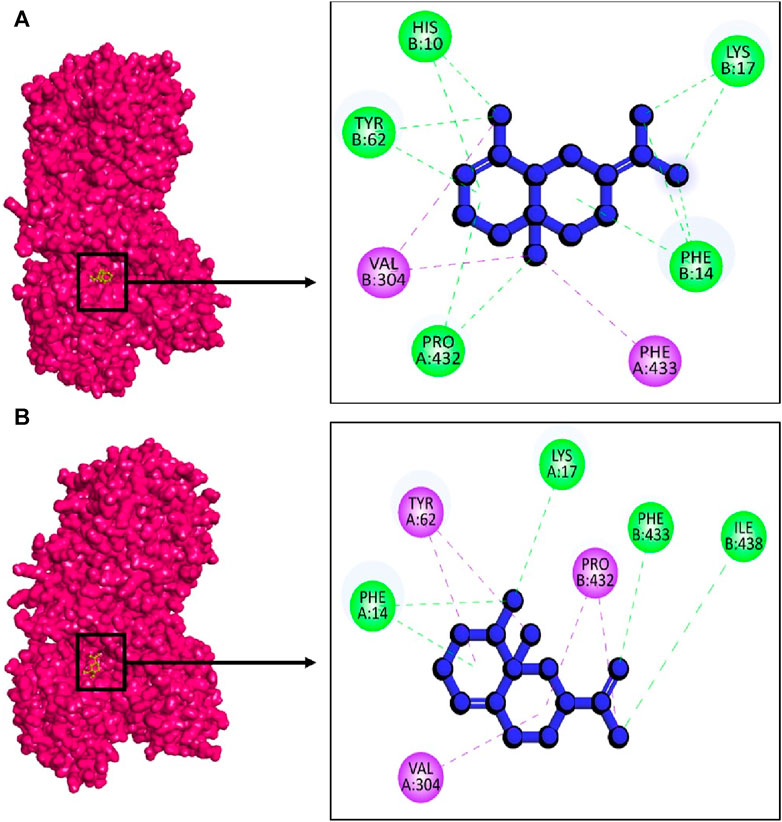
Figure 11. The interactions between phytochemicals and target NADPH oxidase. Where (A) 2CDU + CID 522296 and (B) 2CDU + CID 9855795.
The purpose of this study was to compare the chemical profile, antioxidant activity, and antimicrobial activity of essential oils extracted from two species: cultivated lavender (L. abrialis) and wild lavender (L.stoechas) as part of the valorization of aromatic and medicinal plants in the commune of Oulmès in Morocco.
Results of hydrodistillation extractions from the aerial parts of the two lavender species under study revealed that L. abrialis produced more essential oil than L. stoechas. According to the analysis of the EOs’ chemical composition, these two lavenders were particularly high in terpenes and contained a variety of typical chemical components, albeit in varying amounts. Linalool, linalyl acetate, and camphor represented most of the chemical constituents of L. abrialis, whereas cubenol, fenchone, and camphor make up the principal components of L. stoechas.
These terpene molecules in EOs contribute to their intriguing antioxidant and antibacterial effects. In this present work, L. stoechas was found to have greater antioxidant activity than L. abrialis. Furthermore, the investigated EOs had strong antibacterial activity against yeast, mold, and bacteria strains (both Gram-positive and Gram-negative). Because of these proprieties as natural antibacterial and antifungal agents, these oils are extremely valuable as a source of phytopharmaceutical ingredients that can be used to treat both bacterial and fungal diseases. The acquired results allowed us to conduct future experiments to develop formulations for using these bioactive natural compounds for their antioxidant, antibacterial, and antifungal characteristics in the food, pharmaceutical, and cosmetic industries.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
MR: Conceptualization, Writing–original draft. ZE: Conceptualization, Writing–original draft. AD: Data curation, Writing–original draft. FR: Methodology, Writing–original draft. MEH: Formal Analysis, Writing–original draft. KZ: Data curation, Writing–original draft. BE-D: Writing–original draft, Writing–review and editing. AB: Validation, Writing–original draft. SA: Visualization, Writing–original draft. HT: Writing–original draft, Investigation. AS: Writing–original draft, Supervision. MB: Writing–original draft, Writing–review and editing. SI: Writing–original draft, Methodology. TZ: Formal Analysis, Writing–original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is financially supported by the Researchers Supporting Project number (RSP-2024R437), King Saud University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aberchane, M., Fechtal, M., Chaouch, A., and Bouayoune, T. (2001). Influence de la durée et de la technique d’extraction sur le rendement et la qualité des huiles essentielles du cèdre de l’Atlas. Ann. Rech. For. Maroc 34, 110–118.
Adams, R. P. (2007). Allured publishing corporation carol stream, IL. Identif. Essent. oil components by gas Chromatogr. Spectrom. 456.
AFNOR (2005). Norme française NF ISO 3063: huile essentielle d’ylang-ylang [Cananga odorata (Lamarck) J.D. Hooker et Thomson forma genuina]. Paris: France. AFNOR.
Ahmet, C. G., Gülaci, T., Gökhan, B., Mine, B., Zeynep, A., and John, M. P. (2002). The chemical constituents and biological activity of essential oil of Lavandula stoechas ssp. Stoechas. Z Naturforsch C J. Biosci. 57, 797–800. doi:10.1515/znc-2002-9-1007
Ailli, A., Handaq, N., Touijer, H., Gourich, A. A., Drioiche, A., Zibouh, K., et al. (2023). Phytochemistry and biological activities of essential oils from six aromatic medicinal plants with cosmetic properties. Antibiotics 12, 721. doi:10.3390/antibiotics12040721
Arya, R., Ravikumar, R., Santhosh, R. S., and Princy, S. A. (2015). SarA based novel therapeutic candidate against Staphylococcus aureus associated with vascular graft infections. Front. Microbiol. 6, 416. doi:10.3389/fmicb.2015.00416
Atmani, D., Chaher, N., Berboucha, M., Ayouni, K., Lounis, H., Boudaoud, H., et al. (2009). Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chem. 112, 303–309. doi:10.1016/j.foodchem.2008.05.077
Bachiri, L., Bammou, M., Echchegadda, G., and Ibijbijen, J. (2017). El Rhaffari, L., Haloui, Z., Nassiri, L. Composition Chimique Et Activité Antimicrobienne Des Huiles Essentielles De Deux Espèces De Lavande: Lavandula dentata spp. dentata et Lavandula peduncultata spp. pedunculata. Eur. Sci. J. 21, 256.
Bachiri, L., Echchegadda, G., Ibijbijen, J., and Nassiri, L. (2016). Étude Phytochimique et activité antibactérienne de deux espèces de Lavande Autochtones au Maroc: «Lavandula stoechas L. et Lavandula dentata L. Eur. Sci. J. ESJ 12, 313. doi:10.19044/esj.2016.v12n30p313
Bachiri, L., Labazi, N., Daoudi, A., Ibijbijen, J., Nassiri, L., Echchegadda, G., et al. (2015). Étude ethnobotanique de quelques lavandes marocaines spontanées. Int. J. Biol. Chem. Sci. 9, 1308–1318. doi:10.4314/ijbcs.v9i3.16
Balouiri, M., Sadiki, M., and Ibnsouda, S. K. (2016). Methods for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal. 6, 71–79. doi:10.1016/j.jpha.2015.11.005
Bekut, M., Brkić, S., Kladar, N., Dragović, G., Gavarić, N., and Božin, B. (2018). Potential of selected Lamiaceae plants in anti(retro)viral therapy. Pharmacol. Res. 133, 301–314. doi:10.1016/j.phrs.2017.12.016
Bourkhis, M., Hnach, M., Bourkhis, B., Ouhssine, M., Chaouch, A., and Satrani, B. (2009). Effet de séchage sur la teneur et la composition chimique des huiles essentielles de Tetraclinis articulate (Vahl) Masters. Agrosolution 20, 44–48.
Boutahiri, S., Eto, B., Bouhrim, M., Mechchate, H., Saleh, A., Al kamaly, O., et al. (2022). Lavandula pedunculata (mill.) cav. Aqueous extract antibacterial activity improved by the addition of Salvia rosmarinus spenn., salvia lavandulifolia vahl and Origanum compactum benth. Life 12, 328. doi:10.3390/life12030328
Clevenger, J. F. (1928). Apparatus for the determination of volatile oil. J. Am. Pharm. Assoc. 1928 (17), 345–349. doi:10.1002/jps.3080170407
Codex Alimentarius (2017). Standard for named vegetable oils, CXS 210-1999 adopted in 1999. Revised in 2001, 2003, 2009, 2017. Amended in 2005, 2011, 2013, 2015. Codex Stand., 1–13.
Cox-Georgian, D., Ramadoss, N., Dona, C., and Basu, C. (2019). Therapeutic and medicinal uses of terpenes. Med. Plants 12, 333–359. doi:10.1007/978-3-030-31269-5_15
De Cliff, S., and Harerimana, P. C. (2013). Extraction de l’huile essentielle complète des fleurs de Cananga odorata de la plaine de l’Imbo: vers la vulgarisation d’une nouvelle filière de plantes industrielles au Burundi. Revue de l’Université de Burundi. J. Série Sci. exactes 28, 1–17.
Dorman, H. J., and Deans, S. G. (2000). Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 88, 308–316. doi:10.1046/j.1365-2672.2000.00969.x
Dupont, F., and Guignard, J. L. (2012). Abrégés de pharmacie. Botanique, les familles de plantes. 15e édn. Paris.France: Elsevier Masson.
Essawi, T., and Srour, M. (2000). Screening of some Palestinian medicinal plants for antibacterial activity. J. Ethnopharmacol. 70, 343–349. doi:10.1016/s0378-8741(99)00187-7
Fakchich, J., and Elachouri, M. (2021). An overview on ethnobotanico-pharmacological studies carried out in Morocco, from 1991 to 2015: systematic review (part 1). J. Ethnopharmacol. 267, 113200. doi:10.1016/j.jep.2020.113200
Fatiha, A., Satrani, B., Ghanmi, M., Abderrahman, A., Farah, A., Lotfi, A., et al. (2011). Activité antioxydante et composition chimique des huiles essentielles de quatre espèces de thym du Maroc. Acta Bot. Gallica 158, 513–523. doi:10.1080/12538078.2011.10516292
Fellah, S., Romdhane, M., and Abderraba, M. (2006). Extraction et étude des huiles essentielles de la Salvia officinalis L. cueillie dans deux régions différentes de la Tunisie. Journal-Societe Algerienne De. Chim. 16 (2), 193.
Gourich, A. A., Bencheikh, N., Bouhrim, M., Regragui, M., Rhafouri, R., Drioiche, A., et al. (2022). Comparative analysis of the chemical composition and antimicrobial activity of four Moroccan north middle atlas medicinal plants’ essential oils: rosmarinus officinalis L., Mentha pulegium L., Salvia officinalis L., and Thymus zygis subsp. gracilis (boiss.) R. Morales. Chemistry 4, 1775–1788. doi:10.3390/chemistry4040115
Hosen, M. E., Supti, S. J., Akash, S., Rahman, M. E., Faruqe, M. O., Manirujjaman, M., et al. (2023). Mechanistic insight of Staphylococcus aureus associated skin cancer in humans by Santalum album derived phytochemicals: an extensive computational and experimental approaches. Front. Chem. 11, 1273408. doi:10.3389/fchem.2023.1273408
Kandsi, F., Elbouzidi, A., Lafdil, F. Z., Meskali, N., Azghar, A., Addi, M., et al. (2022). Antibacterial and antioxidant activity of Dysphania ambrosioides (L.) mosyakin and clemants essential oils: experimental and computational approaches. Antibiotics 11 (4), 482. doi:10.3390/antibiotics11040482
Khettal, B., Kadri, N., Tighilet, K., Adjebli, A., Dahmoune, F., and Maiza-Benabdeslam, F. (2017). Phenolic compounds from Citrus leaves: antioxidant activity and enzymatic browning inhibition. J. Complement. Integr. Med. 1, 14. doi:10.1515/jcim-2016-0030
Kovats, E. S. (1965). « Gas chromatographic characterization of organic substances in the retention index system. ». Adv. Chromatogr. 1, 229–247.
Kuete, V. (2004). PenlapBeng, V., Etoa,f-X., Modjo, S.L., Bogne, P., Assob, J.C., et Lontsi, D. Activité antimicrobienne de l'extrait total et des fractions de jus de fruits de Citrus medical in (Rutaceaa). J. Pharm. Méd. Trad. Afr. 13, 91–101.
Kumar Deb, P., Shilkar, D., and Sarkar, B. (2022). UHPLC-ESI-QTOF-MS/MS based identification, quantification, and assessment of in silico molecular interactions of major phytochemicals from bioactive fractions of clerodendrum glandulosum lindl. Leaves. Chem. Biodivers. 19 (10), e202200617. doi:10.1002/cbdv.202200617
Laib, I. (2012). Étude des activités antioxydante et antifongique de l’huile essentielle des fleurs sèches de Lavandulaofficinalis: application aux moisissures des légumes secs. J. Nature& Technol. 7, 44–52.
Laib, I., and Barkat, M. (2011). Composition chimique et activite antioxydante de l’huile essentielle des fleurs sèches de Lavandula officinalis. J. Agric. 2, 89–101.
Mohammedi, Z., and Fawzia, A. (2011). Pouvoir antifongique et antioxydant de l’huile essentielle de Lavandula stoechas L. J. Nat. Technol. 2011 (6), 34–39.
N’Dédianhoua, K. S., Majdouli, K., Khabbal, Y., and Zair, T. (2014). Chemical composition and antibacterialactivity of LavandulaspeciesL.dentata L., L. pedunculata Mill, and Lavandula abrialis are essential oils from Morocco against foodborne and nosocomial pathogens. Int. J. Innovation AppliedStudies 2014, 774–781.
NF EN 1999-1-1/A1 (1999). NF EN 1999-1-1/A1; eurocode 9—design of aluminum structures—Part 1-1: general rules. AFNOR Ed. La Plaine Saint-Denis, Fr.
NF V03-402 (1985). NF V03-402; spices and HERBS—determination of water content—entrainment method. Spices and aromatics—determination of water content—entrainment method. AFNOR Ed. La Plaine Saint-Denis, Fr.
NF V05-113 (1972). NF V05-113; fruits, vegetables and derived products—mineralization of OrganicMatter—method by incineration. AFNOR Ed. La Plaine Saint-Denis, Fr., 64.
O'Leary, J. O., Langevin, M. J., Price, C. T., Blevins, J. S., Smeltzer, M. S., and Gustafson, J. E. (2004). Effects of sarA inactivation on the intrinsic multidrug resistance mechanism of Staphylococcus aureus. FEMS Microbiol. Lett. 237 (2), 297–302. doi:10.1111/j.1574-6968.2004.tb09710.x
Provincial Directorate of Agriculture (DPA) of Khémisset, Monograph of the province of Khémisset. 2022.
Raal, A. (2010). Maailma ravimtaimede entsüklopeedia; Estonian encyclopaedia publishers. Tallinn: Estonia. ISBN 978-9985-70-313-7.
Radi, F., Zekri, N., Drioiche, A., Zerkani, H., Boutakiout, A., Bouzoubaa, A., et al. (2022). Volatile and non-volatile chemical compounds and biological power of the genus Lavandula: case of two Moroccan lavenders Lavandula angustifolia mill. (Cultivated lavander) and Lavandula pedunculata (mill.) cav. (Spontaneous lavander). Egypt. J. Chem. 2022 (65), 0–294. doi:10.21608/ejchem.2021.82036.4053
Saidi, S., Handaq, N., Remok, F., Drioiche, A., Bouissane, L., and Zair, T. (2022). Citrus ×clementina (leaf). Total phenolic, flavonoid contents, antioxidant activity, and chemical composition of their essential oil. Arabian J. Med. Aromatic Plants 8, 87–107.
Saidi, S., Remok, F., Handaq, N., Drioiche, A., Gourich, A. A., Menyiy, N. E., et al. (2023). Phytochemical profile, antioxidant, antimicrobial, and antidiabetic activities of Ajuga iva (L.). Life 13, 1165. doi:10.3390/life13051165
Scherrer, A. M., Motti, R., and Weckerle, C. S. (2005). Traditional plant use in the areas of monte vesole and ascea, cilento national park (campania, southern Italy). J. Ethnopharmacol. 97, 129–143. doi:10.1016/j.jep.2004.11.002
Skujins, S. (1998). Handbook for ICP-aes (Varian-Vista). Switzerland. A Short Guide To Vista Series ICP-AES Operation. Varian Int. AG, Zug, Version 1.0.
Suhaj, M. (2006). Spice antioxidants isolation and their antiradical activity: a review. J. Food Compos. Analysis 19, 531–537. doi:10.1016/j.jfca.2004.11.005
Upson, T., and Andrews, S. (2004). “The genus Lavandula,” in Portland and Oregon (USA: Timber Press).
Willcox, J. K., Ash, S. L., and Catignani, G. L. (2004). Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 44, 275–295. doi:10.1080/10408690490468489
Wong, C. C., Li, H. B., Cheng, K. W., and Chen, F. (2006). A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 97, 705–711. doi:10.1016/j.foodchem.2005.05.049
Keywords: lavender, quality control, phytochemicals, antioxidant/antimicrobial activities, oulmès -Morocco, molecular docking
Citation: Radi M, Eddardar Z, Drioiche A, Remok F, Hosen ME, Zibouh K, Ed-Damsyry B, Bouatkiout A, Amine S, Touijer H, Salamatullah AM, Bourhia M, Ibenmoussa S and Zair T (2024) Comparative study of the chemical composition, antioxidant, and antimicrobial activity of the essential oils extracted from Lavandula abrialis and Lavandula stoechas: in vitro and in silico analysis. Front. Chem. 12:1353385. doi: 10.3389/fchem.2024.1353385
Received: 10 December 2023; Accepted: 19 February 2024;
Published: 25 March 2024.
Edited by:
Mohamed Shaaban, National Research Centre, EgyptCopyright © 2024 Radi, Eddardar, Drioiche, Remok, Hosen, Zibouh, Ed-Damsyry, Bouatkiout, Amine, Touijer, Salamatullah, Bourhia, Ibenmoussa and Zair. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Radi, bW9oLnJhZGlAZWR1LnVtaS5hYy5tYQ==; Mohammed Bourhia, Ym91cmhpYW1vaGFtbWVkQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.