
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 16 February 2024
Sec. Organic Chemistry
Volume 12 - 2024 | https://doi.org/10.3389/fchem.2024.1342784
This article is part of the Research TopicOrganic Chemistry Editor's Pick 2024View all 11 articles
 Israa Habeeb Naser1
Israa Habeeb Naser1 Hassan Thoulfikar A. Alamir2
Hassan Thoulfikar A. Alamir2 Ali Hisham Al-Shukarji3
Ali Hisham Al-Shukarji3 Batool Ali Ahmed4
Batool Ali Ahmed4 Talal Aziz Qassem5
Talal Aziz Qassem5 Maher Kamal6
Maher Kamal6 Tahani M. Almeleebia7
Tahani M. Almeleebia7 Enas R. Alwaily8
Enas R. Alwaily8 Eftikhaar Hasan Kadhum9
Eftikhaar Hasan Kadhum9 Ahmed Alawadi10,11,12*
Ahmed Alawadi10,11,12* Ali Alsalamy13
Ali Alsalamy13In this study, choline chloride/urea was used as a green deep eutectic solvent in the three-component reaction of hydrazine/phenylhydrazine, malononitrile, and aromatic aldehydes for synthesizing pyrazole derivatives, and in the four-component reaction of methyl/ethyl acetoacetate, hydrazine/phenylhydrazine, malononitrile, and aromatic aldehydes for synthesizing pyrano[2,3-c]pyrazole derivatives. Elemental analysis, 1H, and 13C NMR spectroscopy were used to confirm the structure of the synthesized pyrazole and pyrano[2,3-c] pyrazole derivatives. The antimicrobial effects of the synthesized pyrazole and pyrano[2,3-c] pyrazole derivatives were investigated. In antimicrobial tests, instructions from clinical and laboratory standards institutes were used. Antimicrobial study was done on pathogenic gram-positive and gram-negative species, and specialized aquatic strains and fungal species. Using choline chloride/urea, novel pyrazole derivatives and pyrano[2,3-c]pyrazole derivatives were synthesized, and other derivatives were synthesized with higher efficiency in less time than some previously reported methods. MIC (minimum inhibitory concentration) and MBC (minimum bactericidal concentration) obtained for derivatives were higher than some antibiotic drugs. Synthesis and reports of new derivatives of pyrazole and pyrano[2,3-c]pyrazole, and investigation and reports of their antimicrobial properties on gram-positive, gram-negative, and specialized aquatic and fungal species are among the novel and important findings of this study.
The use of green processes in synthesizing organic and heterocyclic compounds is noteworthy (Nishanth Rao et al., 2021). Green methods in organic synthesis are essential because they are environmentally friendly (Kumar et al., 2020). Among the green techniques in organic chemistry are the synthesis of organic and heterocyclic compounds during multicomponent reactions, the use of recyclable catalysts, and the use of deep eutectic solvents (Calvo-Flores and Mingorance-Sánchez, 2021; Javahershenas, 2023). Multi-component reactions, in which several reactants lead to the synthesis of the desired product in one pot and one step, in addition to being green, are important in the synthesis of organic and heterocyclic compounds due to their high efficiency, cost-effectiveness, and time-saving characteristics (Bhaskaruni et al., 2020; John et al., 2021). There have been several reports of the synthesis of five-membered heterocycles, such as pyrazoles, or two-ring heterocyclic compounds, such as pyranopyrazoles, using multicomponent reactions (Mamaghani and Hossein Nia, 2021; Becerra et al., 2022; Khazaal and Ibraheam, 2022). Pyrazole is a useful heterocyclic compound due to its agricultural and drug-discovery applications (Kumar et al., 2013). There have been reports of this heterocyclic compound in nature, for example, isolation from fungi such as Colletotrichum gloeosporioides (Cui et al., 2023). The biological properties of this heterocyclic compound that can be mentioned are its anxiolytic, antidepressant, anti-tubercular, analgesic properties, and anti-malarial activities, etc. (Ramadan et al., 2021). As there is a pyrazole structure in the structure of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1h-pyrazol-1-yl]benzene-sulfonamide, which is a drug with the brand name Celecoxib, this heterocyclic compound can be used as a medicinal and bioactive compound (Küçükgüzel and Şenkardeş, 2015). Pyrano[2,3-c]pyrazole derivatives are one of the pyrazole derivatives that contain two heterocyclic compounds of pyrazole and pyran, which are connected by one facet. Derivatives of pyrano[2,3-c] pyrazole can maintain the biological properties of both heterocyclic compounds of pyran and pyrazole. Biological properties, such as cytotoxic, mutagenicity, cancer therapy, and antiviral properties, have been reported for the derivatives of the 6-membered heterocyclic compound of pyran with one oxygen in its structure (Yahiaoui et al., 2022). Biological properties, such as anti-tubercular, anti-malarial, anti-microbial, and anti-cancer properties, have been reported for the derivatives of pyrano[2,3-c]pyrazole (Mokariya et al., 2022; Parikh et al., 2022). In addition to the green method of multicomponent reaction, by using other green techniques such as the use of deep eutectic solvents, pyrazole derivatives and pyrano[2,3-c]pyrazole derivatives can be synthesized, and reports have been made in this field (Nguyen et al., 2021; Sikandar and Zahoor, 2021). As mentioned, the use of the deep eutectic solvent is one of the important and green methods in synthesizing organic and heterocyclic compounds. With this method, there is no need to use dangerous organic solvents or catalysts in the synthesis of organic and heterocyclic compounds (Rushell et al., 2019). A deep eutectic solvent plays the role of solvent and catalyst in the synthesizing of heterocyclic and organic compounds (Fekri et al., 2020). Deep eutectic solvents can be used during multicomponent reactions (Calvo-Flores and Mingorance-Sánchez, 2021). In this regard, we can refer to reports such as the use of glycerol: potassium carbonate in the four-component reaction of malononitrile, carbon disulfide, carbonyl, and methylene compounds, which led to the synthesis of [1,3]dithiine derivatives (Moghaddam-Manesh and Hosseinzadegan, 2021). Another deep eutectic solvent system that has been reported so far for the synthesis of pyrazolopyridines derivatives (Vanegas et al., 2019); tetrahydrobenzo[b]pyran and pyrano[2, 3-d]pyrimidinone (thione) (Biglari et al., 2020); and pyrazolines, pyrimidines, and naphthyridines (Mohamed et al., 2022) is the use of choline chloride/urea. According to reports, using choline chloride/urea reduces the synthesis time of the desired derivatives and has led to higher efficiency to synthesis of new derivatives. Therefore, choline chloride/urea was used as a deep eutectic solvent to synthesize pyrazole derivatives and pyrano[2,3-c]pyrazole derivatives. The synthesis of new derivatives and the experiments of antimicrobial and antifungal properties of the synthesized derivatives were among the other investigations carried out in this study.
The Brookfield DV-II + Pro EXTRA viscometer was used to measure solvent viscosity, and the G LAB melting point apparatus was used to measure the melting point of solvent and derivatives. The 1H, and 13C NMR spectra, CHNS/O elemental analyzer, and mass analysis of compounds were prepared using the Varian Inova 500MHz, the EMA 502, and the Agilent technologies 5975C. The Thermo biomate 5 Spectrophotometer was used to prepare the suspension of bacteria. The suspension of bacteria was obtained from the American Type Culture Collection (ATCC). All the reagent materials used for the synthesis of deep eutectic solvent and the synthesis of pyrazole derivatives and pyrano[2,3-c] pyrazole derivatives such as choline chloride, urea, malononitrile, aldehyde derivatives, and hydrazine derivatives were obtained from Merck and Sigma.
To prepare the choline chloride/urea deep eutectic solvent, choline chloride and urea were weighed in different ratios, 1:1, 1:2, 1:3, and 1:4, and stirred at 80°C until a colorless homogeneous mixture was observed (Yadav and Pandey, 2014). The prepared mixture was cooled and used for other steps.
To 1 g choline chloride/urea (1:2) deep eutectic solvent, 1 mmol malononitrile and 1 mmol aldehyde derivatives were added and stirred at 80°C for 5 min. Then 1 mmol hydrazine derivatives was added and stirred at 80°C until the reaction was complete. The completion of the reaction was checked by TLC. Finally, after the completion of the reaction, the temperature was brought to the ambient temperature, and 5 mL of distilled water was added and stirred for 30 min. The sediments, which were the desired product, were separated with filter paper. The desired synthesized product was purified by recrystallization in a mixture of distilled water and ethanol (1:1).
To 1 g choline chloride/urea deep eutectic solvent, 1 mmol malononitrile and 1 mmol aldehyde derivatives were added and stirred at 70°C for 5 min. To 1 g choline chloride/urea deep eutectic solvent, 1 mmol ethyl acetoacetate and 1 mmol hydrazine derivatives were added and stirred at 80°C for 5 min. The mixtures were added together and stirred at 80°C until the reaction was complete. The completion of the reaction was checked by TLC. Finally, after the completion of the reaction, the temperature was brought to the ambient temperature, and 5 mL of distilled water was added and stirred for 30 min. The sediments, which were the desired product, were separated with filter paper. The desired synthesized product was purified by recrystallization in a mixture of distilled water and ethanol (1:1).
Based on previous studies reported according to relevant standards, the antimicrobial activity of synthetic derivatives on Streptomyces fradiae (10745), Staphylococcus aureus (29213), Streptococcus pyogenes (19615), Streptococcus agalactiae (12386), Yersinia enterocolitica (9610), Shigella dysenteriae (13313), Pseudomonas aeruginosa (15442), Acinetobacter baumannii (19606), Streptococcus iniae (29178), Loctococcus garvieae (43921), Yersinia ruckeri (29473), Edwardsiella tarda (15947), Candida albicans (10231), Cryptococcus neoformans (32045), Fusarium oxysporum (7601), and Aspergillus fumigatus Fresenius (1022) was investigated and evaluated. For this purpose, first, the half-McFarland concentration of the strains and the initial concentrations of 1, 2, 4, 8, 16, 32, 64, 128, 256, 512, 1024, and 2048 μg/mL of the derivatives were prepared separately. The minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), minimum fungicidal concentration (MFC), and inhibition zone diameter (IZD) were evaluated (Igei et al., 2016; Suksatan et al., 2022).
In this study, by using choline chloride/urea as a green and environmentally friendly deep eutectic solvent, in the three-component reaction of malononitrile, aldehyde derivatives, and hydrazine derivatives, novel pyrazole derivatives, and in the four-component reaction of malononitrile, aldehyde derivatives, ethyl acetoacetate, and hydrazine derivatives, novel pyrano[2,3-c]pyrazole derivatives were synthesized (Scheme 1).
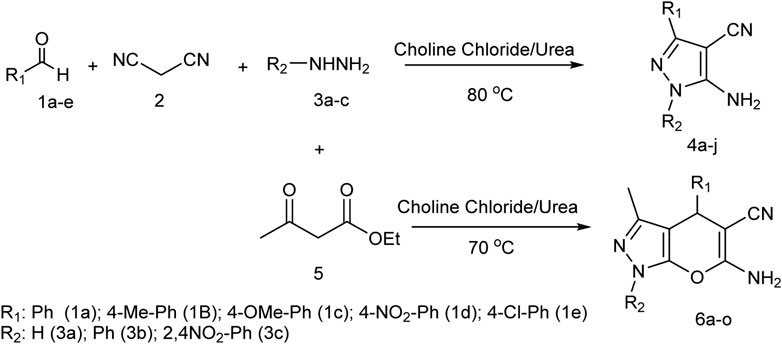
SCHEME 1. Using choline chloride/urea as green method for synthesis of pyrazole derivatives and pyrano[2,3-c]pyrazole derivatives.
In general, the greenness of the reported method, high efficiency, and shorter synthesis time were the advantages of using choline chloride/urea in synthesizing derivatives. Additional information about the synthesis of derivatives is given below.
The three-component synthesis of pyrazole derivatives used choline chloride/urea, malononitrile, aldehyde derivatives, and hydrazine derivatives were used as raw materials.
For this purpose, according to the method presented in Section 2.2, different ratios of choline chloride and urea were prepared. For the synthesis of 4a, they were tested according to Table 1.
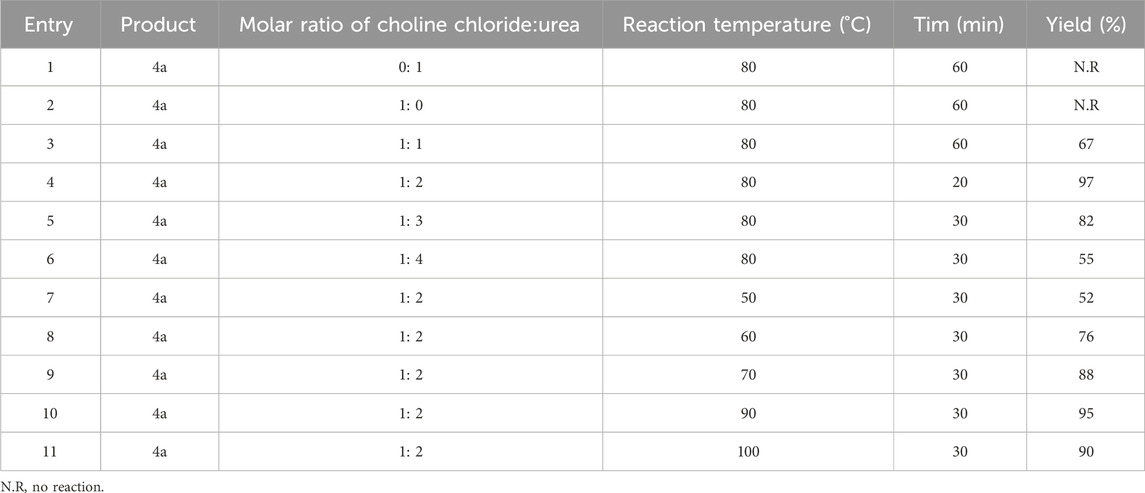
TABLE 1. Optimization of molar ratio of choline chloride:urea and reaction temperature in synthesis of pyrazole.
In obtaining the optimal conditions (Table 1), in addition to using different ratios of choline chloride and urea, temperatures of 50°C, 60°C, 70°C, 80°C, 90°C, and 100°C degrees Celsius were also tested. It was found that the ratio of 1:2 (choline chloride:urea) at 80°C had the highest efficiency in a shorter time for the synthesis.
Physical parameters such as viscosity (according to ASTM D445 testing method) and melting point for 1:2 choline chloride:urea mixture were obtained according to Table 2.
The above optimal conditions were used for synthesizing other derivatives, according to Table 3. Three of the derivatives reported in Table 3 are novel.

TABLE 3. Information on synthesized pyrazole derivatives using choline chloride/urea as green method.
The results of 1H NMR, 13C NMR, and CHNS/O elemental analyzer of the newly synthesized derivatives that confirm their structure are given in the Supplementary Material.
As the results show, in general, electron-donating substituents attached to hydrazine, such as phenyl, and electron-withdrawing groups attached to the aldehyde, such as nitro, increased the efficiency of the products. Therefore, the following mechanism (Scheme 2) can be proposed for this 3-component reaction, which corresponds to the difference in the results of the product’s efficiency.
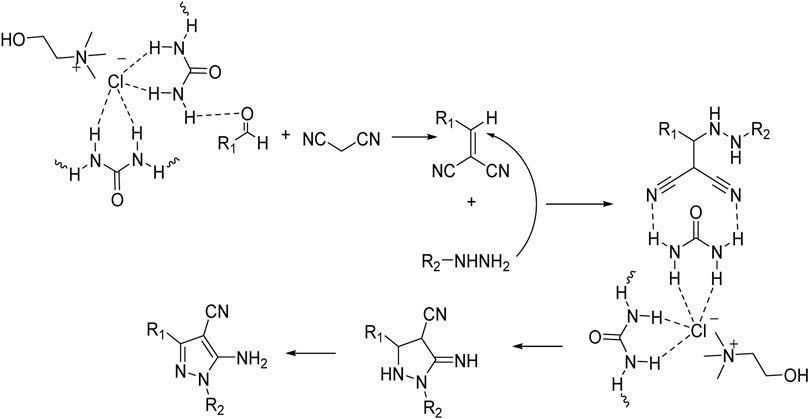
SCHEME 2. Mechanism for using choline chloride/urea as green method for synthesis of pyrazole derivatives (4a-j).
So far, many studies have been conducted on the three-component reaction of malononitrile, aldehyde derivatives, and hydrazine derivatives, which led to the synthesis of pyrazole derivatives. The conditions of some recent studies (from 2020 to 2023) compared to the conditions used in this study are given in Table 4.
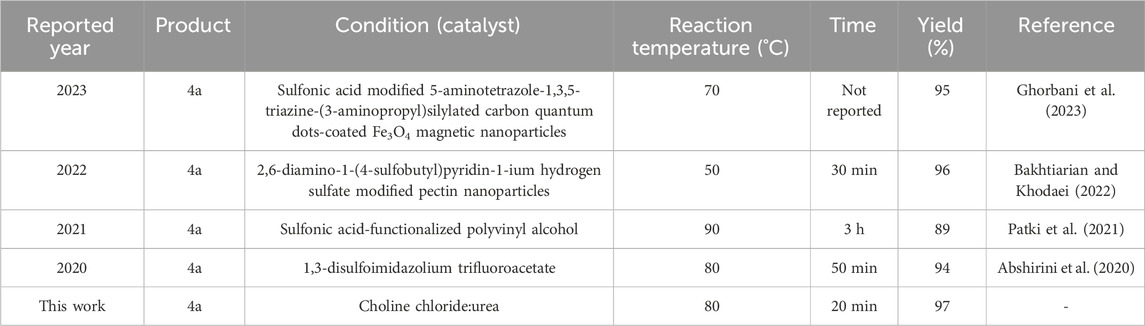
TABLE 4. The previously reported conditions and methods in the synthesis of pyrazole compared to the method presented in this study.
Ease of reaction conditions and no need for catalyst and solvent, reporting of newly synthesized derivatives, being green, and having high efficiency and shorter synthesis time can be mentioned as advantages of using choline chloride/urea to synthesize derivatives compared to recently reported methods.
In the continuation of this study, choline chloride/urea was also used in the 4-component reaction of malononitrile, aldehyde derivatives, hydrazine derivatives, and ethyl acetoacetate for the synthesis of pyrano[2,3-c]pyrazole derivatives.
The steps for synthesizing derivatives here were the first optimization according to Section 3.1, the results of which are given in Table 5.
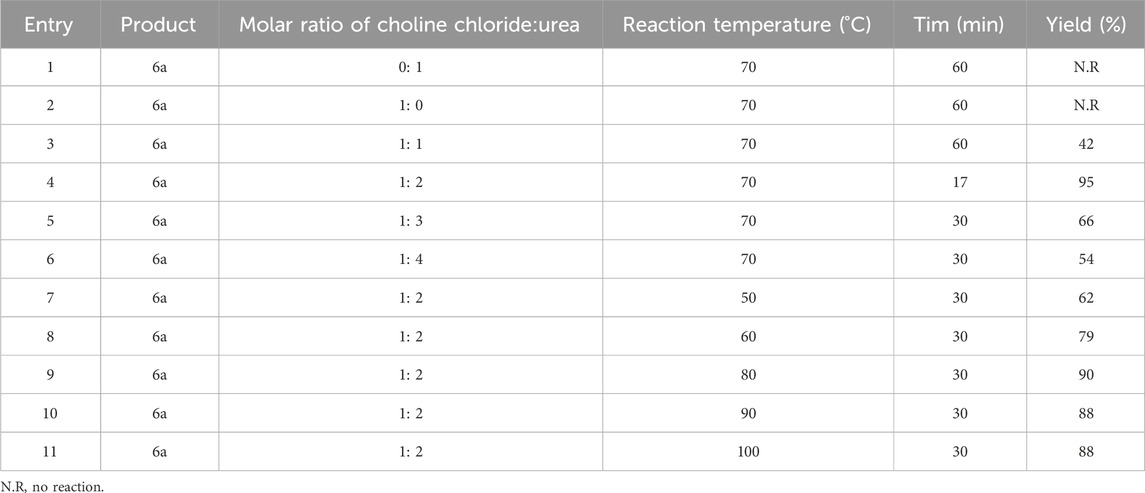
TABLE 5. Optimization of molar ratio of choline chloride:urea and reaction temperature in synthesis of pyrano[2,3-c] pyrazoles.
Here, the optimal conditions for synthesizing pyrano[2,3-c]pyrazole derivatives with high efficiency and shorter time, were the ratio of 1:2 of choline chloride:urea and the reaction temperature of 70°C. With the obtained optimal conditions, other derivatives, including 15 derivatives, of which two were novel, were synthesized (Table 6).
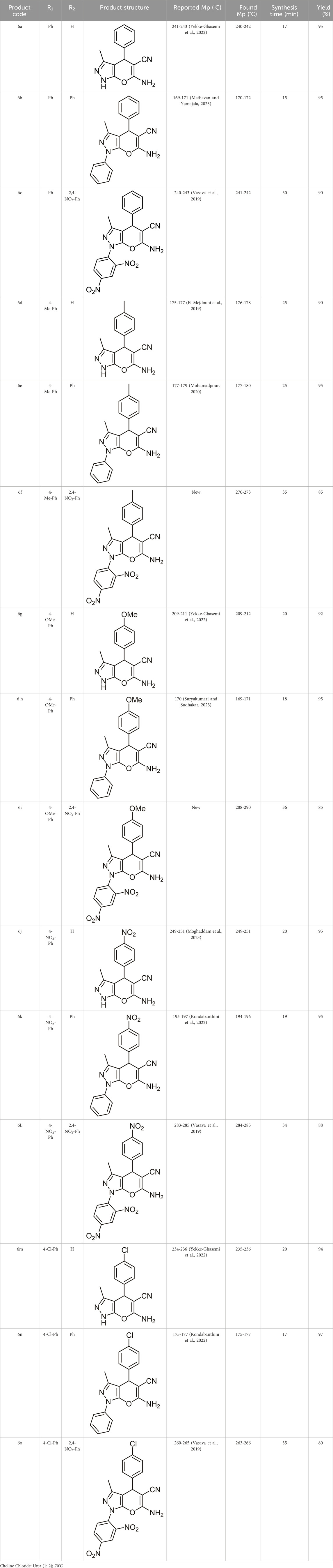
TABLE 6. Information on synthesized pyrano[2,3-c]pyrazole derivatives using choline chloride/urea as green method.
The results of 1H NMR, 13C NMR, and CHNS/O elemental analyzer of the newly synthesized derivatives that confirm their structure are given in the Supplementary Material.
Comparing the results of the efficiency with the structure of the raw materials here was also the same as above, and it was observed that electron-donating substituents attached to hydrazine, such as phenyl, and electron-withdrawing groups attached to the aldehyde, such as nitro, increased the efficiency of the products. Therefore, the Scheme 3 mechanism is proposed for this reaction.
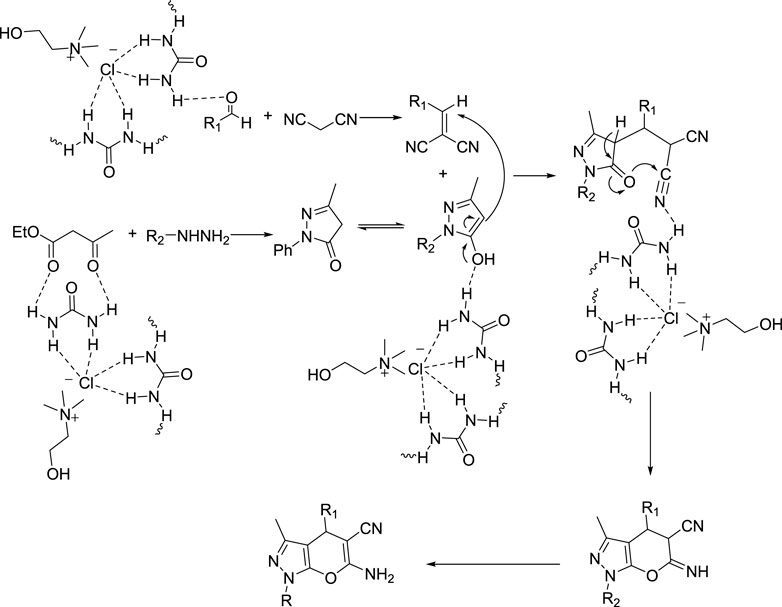
SCHEME 3. Mechanism for using choline chloride/urea as green method for synthesis of pyrazole pyrano[2,3-c]pyrazole derivatives (6a-o).
The result of the comparison of the method presented in this study to the recent studies in the synthesis of derivatives, as shown in Table 7, is similar to the conclusion for the synthesis of pyrazole derivatives. It can be stated here that ease of reaction conditions, catalyst and solvent not being necessary, newly synthesized derivatives being reported, being green, and having high efficiency and shorter synthesis time can be mentioned as advantages of using choline chloride/urea to synthesize derivatives compared to recently reported methods.

TABLE 7. The previously reported conditions and methods in the synthesis of pyrano[2,3-c]pyrazole compared to the method presented in this study.
In the investigation of antimicrobial and antifungal activity of derivatives, gram-positive, gram-negative, and specialized aquatic strains were used. First, the MIC with a concentration in the range of 1–2048 μg/mL of the derivatives was checked. After obtaining this parameter, its MBC and MFC were acquired. Finally, using the MIC, the IZD parameter of the derivatives on the used strains was obtained.
For further comparison of pyrazole derivatives and pyrano[2,3-c]pyrazole derivatives, the antibacterial properties of gram-positive, gram-negative, and specialized aquatic and fungal strains are shown separately in Tables 8–11.
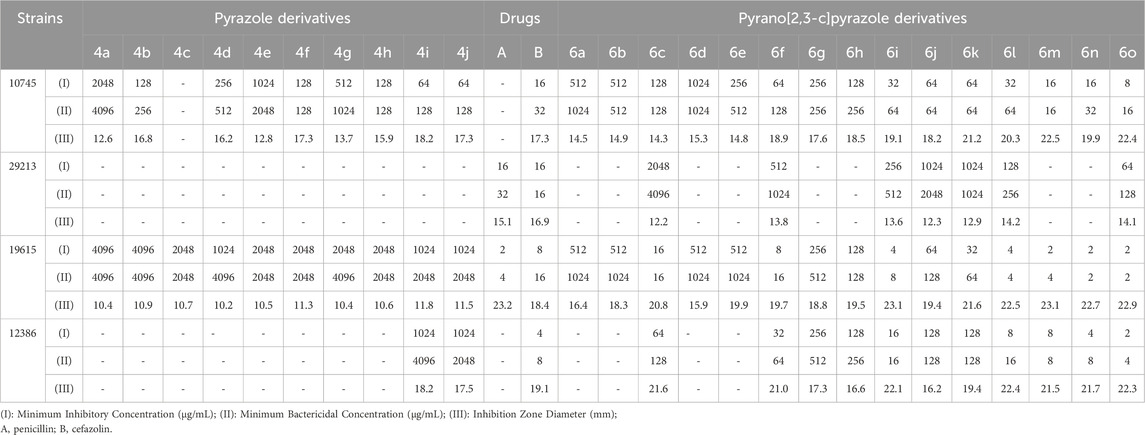
TABLE 8. Antibacterial activity of pyrazole derivatives (4a-j) and pyrazole pyrano[2,3-c]pyrazole derivatives (6a-o) against Gram-positive strains.
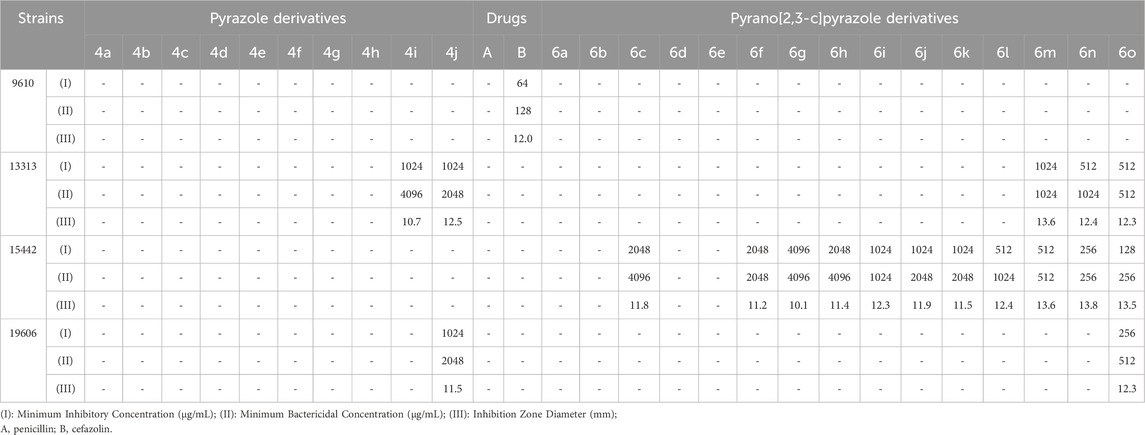
TABLE 9. Antibacterial activity of pyrazole derivatives (4a-j) and pyrazole pyrano[2,3-c]pyrazole derivatives (6a-o) against gram-negative strains.
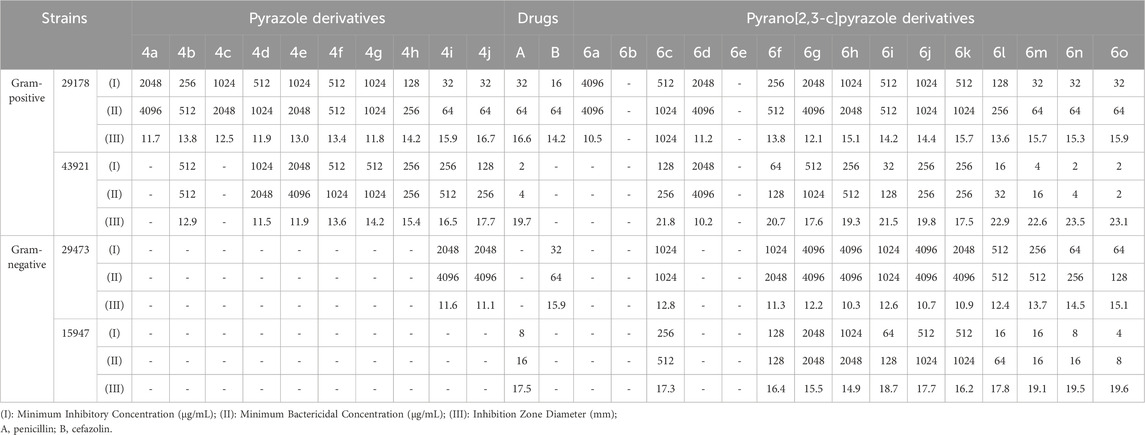
TABLE 10. Antibacterial activity of pyrazole derivatives (4a-j) and pyrazole pyrano[2,3-c]pyrazole derivatives (6a-o) against specialized aquatic strains.
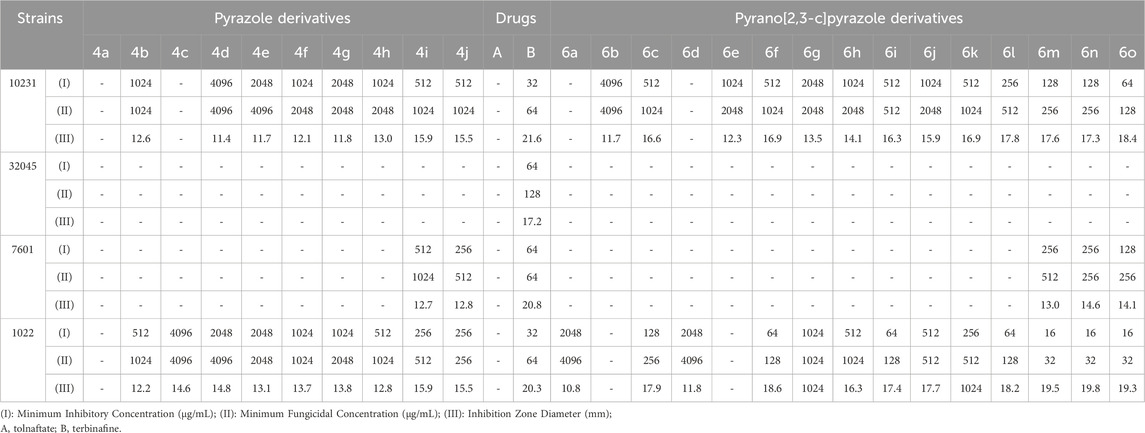
TABLE 11. Antifungal activity of pyrazole derivatives (4a-j) and pyrazole pyrano[2,3-c]pyrazole derivatives (6a-o).
Table 8 shows the results of investigating the antibacterial properties of the derivatives on the evaluated gram-positive strains.
Table 9 shows the results of investigating the antibacterial properties of the derivatives on the evaluated gram-negative strains.
Table 10 shows the results of investigating the antibacterial properties of the derivatives on the evaluated specialized aquatic strains.
Table 11 shows the results of investigating the antibacterial properties of the derivatives on the evaluated fungal strains.
The highest effectiveness in antibacterial activity between pyrazole derivatives and pyrano[2,3-c]pyrazole derivatives was related to 6o.
The IZD value results of some pyrano[2,3-c]pyrazole derivatives compared to Yersinia ruckeri are given in Figure 1.
In general, the order of effectiveness of pyrazole derivatives was as follows:
In general, the order of effectiveness of pyrano[2,3-c]pyrazole derivatives was as follows:
By comparing the results of Tables 8–11, the pyrano[2,3-c]pyrazole derivatives, in general, are more effective than pyrazole derivatives because pyrano[2,3-c] pyrazoles contain two heterocyclic rings (pyran and pyrazole). Another general conclusion that can be drawn is that the order of effectiveness of derivatives is as described for compounds containing chlorine, nitrogen, and methoxy. These results can be justified based on previous studies on the effectiveness and antimicrobial properties of chlorine and nitrogen. The important point in determining antimicrobial activities is to compare them with commercial drugs, such as penicillin, cefazolin, Tolnaftate, and Terbinafine. As the results of Tables 8–11 show, some derivatives, such as 6o in many studied strains, are much more effective than tested drugs, which shows the value of synthetic compounds.
Choline chloride/urea was used as a deep eutectic solvent in the one-pot method and during the multicomponent reaction in synthesizing pyrazole derivatives and pyrano[2,3-c]pyrazole derivatives. The greenness of the synthesis method, the synthesis of new derivatives, and the characteristics of high efficiency and less time were among the advantages and novel findings of this study compared to previously reported methods. The effectiveness on gram-positive, gram-negative, and specialized aquatic strains and fungal species is one of the novel findings of this project. In the investigation of antimicrobial activity, the highest effectiveness was observed in derivative 6o, which contained chlorine and nitrogen, where MIC and MBC values between 2 and 512 μg/mL were observed. In addition, 6o showed higher effectiveness in some strains than drugs in the market, such as penicillin, cefazolin, Tolnaftate, and Terbinafine. Finally, the relationship between the antimicrobial properties of the derivatives and their structure were observed and reported.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
IH: Project administration, Writing–review and editing. HT: Conceptualization, Writing–review and editing. AHA-S: Methodology, Writing–original draft. BA: Validation, Writing–original draft. TQ: Formal Analysis, Writing–original draft. MK: Resources, Writing–review and editing. TA: Data curation, Writing–original draft. EA: Methodology, Writing–original draft. EH: Investigation, Writing–original draft. AhA: Supervision, Writing–original draft. AlA: Funding acquisition, Writing–original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the large research group program under grant number R.G.P.02/535/44.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abshirini, Z., Lotfifar, N., and Zare, A. (2020). A highly effectual and rapid protocol for the synthesis of 5-amino-1, 3-diaryl-1 H-pyrazole-4-carbonitriles using 1, 3-disulfonic acid imidazolium trifluoroacetate as a dual-functional catalyst. Org. Prep. Proced. Int. 52, 428–433. doi:10.1080/00304948.2020.1780884
Amirnejat, S., Nosrati, A., and Javanshir, S. (2020). Superparamagnetic Fe3O4@ Alginate supported L-arginine as a powerful hybrid inorganic–organic nanocatalyst for the one-pot synthesis of pyrazole derivatives. Appl. Organomet. Chem. 34, e5888. doi:10.1002/aoc.5888
Aryan, R., Beyzaei, H., Nojavan, M., and Rezaei, M. (2017). Novel biocompatible glucose-based deep eutectic solvent as recyclable medium and promoter for expedient multicomponent green synthesis of diverse three and four substituted pyrazole-4-carbonitrile derivatives. Res. Chem. Intermed. 43, 4731–4744. doi:10.1007/s11164-017-2908-5
Bakhtiarian, M., and Khodaei, M. M. (2022). Pyridinium-based dual acidic ionic liquid supported on the pectin for efficient synthesis of pyrazoles. J. Mol. Liq. 363, 119883. doi:10.1016/j.molliq.2022.119883
Becerra, D., Abonia, R., and Castillo, J.-C. (2022). Recent applications of the multicomponent synthesis for bioactive pyrazole derivatives. Molecules 27, 4723. doi:10.3390/molecules27154723
Bhaskaruni, S. V., Maddila, S., Gangu, K. K., and Jonnalagadda, S. B. (2020). A review on multi-component green synthesis of N-containing heterocycles using mixed oxides as heterogeneous catalysts. Arabian J. Chem. 13, 1142–1178. doi:10.1016/j.arabjc.2017.09.016
Biglari, M., Shirini, F., Mahmoodi, N. O., Zabihzadeh, M., and Mashhadinezhad, M. (2020). A choline chloride-based deep eutectic solvent promoted three-component synthesis of tetrahydrobenzo [b] pyran and pyrano [2, 3-d] pyrimidinone (thione) derivatives. J. Mol. Struct. 1205, 127652. doi:10.1016/j.molstruc.2019.127652
Calvo-Flores, F. G., and Mingorance-Sánchez, C. (2021). Deep eutectic solvents and multicomponent reactions: two convergent items to green chemistry strategies. ChemistryOpen 10, 815–829. doi:10.1002/open.202100137
Cui, H., Wu, Z., Zhang, L., Ma, Q., Cai, D., Zhang, J., et al. (2023). Design, synthesis, antibacterial activity, and mechanism of novel mesoionic compounds based on natural pyrazole isolated from an endophytic fungus Colletotrichum gloeosporioides. J. Agric. Food Chem. 71, 10018–10027. doi:10.1021/acs.jafc.3c02908
El Mejdoubi, K., Sallek, B., Digua, K., Chaair, H., and Oudadesse, H. (2019). Natural phosphate K09 as a new reusable catalyst for the synthesis of dihydropyrano [2, 3-c] pyrazole derivatives at room temperature. Kinet. Catal. 60, 536–542. doi:10.1134/s0023158419040098
Fekri, L. Z., Nikpassand, M., Mostaghim, S., and Marvi, O. (2020). Green catalyst-free multi-component synthesis of aminobenzochromenes in deep eutectic solvents. Org. Prep. Proced. Int. 52, 81–90. doi:10.1080/00304948.2020.1714319
Ghorbani, S., Habibi, D., and Heydari, S. (2022). A phenylazophenylenediamine-based La-complex as a superb nanocatalyst for the synthesis of diverse pyrano [2, 3-c] pyrazoles. J. Mol. Struct. 1260, 132713. doi:10.1016/j.molstruc.2022.132713
Ghorbani, S., Habibi, D., Heydari, S., and Ebrahimiasl, H. (2023). The acid modified magnetic carbon quantum dots as a capable nano-catalyst for the synthesis of pyridines and pyrazoles. Inorg. Chem. Commun. 156, 111258. doi:10.1016/j.inoche.2023.111258
Igei, M., Bakavoli, M., Shiri, A., Ebrahimpour, Z., Azizollahi, H., Beyzaei, H., et al. (2016). Synthesis of some new pyrimido [4, 5-e] tetrazolo [5, 1-b] [1, 3, 4] thiadiazine derivatives via an S–N type Smiles rearrangement and their antibacterial evaluation. J. Chem. Res. 40, 628–632. doi:10.3184/174751916x14742893137631
Javahershenas, R. (2023). Recent advances in the application of deep eutectic solvents for the synthesis of Spiro heterocyclic scaffolds via multicomponent reactions. J. Mol. Liq. 385, 122398. doi:10.1016/j.molliq.2023.122398
John, S. E., Gulati, S., and Shankaraiah, N. (2021). Recent advances in multi-component reactions and their mechanistic insights: a triennium review. Org. Chem. Front. 8, 4237–4287. doi:10.1039/d0qo01480j
Karrabi, M., Malmir, M., Heravi, M. M., and Hosseinnejad, T. (2023). A theoretical and experimental study on ecofriendly-one-pot synthesis of pyrazolopyranopyrimidines catalysed by CuO functionalized montmorillonite. Inorg. Chem. Commun. 149, 110367. doi:10.1016/j.inoche.2022.110367
Khademi, S., Zahmatkesh, S., Aghili, A., and Badri, R. (2021). Tungstic acid (H4WO5) immobilized on magnetic-based zirconium amino acid metal–organic framework: an efficient heterogeneous Brønsted acid catalyst for l-(4-phenyl)-2,4-dihydropyrano[2,3c]pyrazole derivatives preparation. Appl. Organomet. Chem. 35, e6192. doi:10.1002/aoc.6192
Khazaal, S., and Ibraheam, S. Y. (2022). One-pot synthesis of new pyranopyrazoles via domino multicomponent reaction. Egypt. J. Chem. 65, 259–265. doi:10.21608/EJCHEM.2022.115337.5232
Kiyani, H., and Bamdad, M. (2018). Sodium ascorbate as an expedient catalyst for green synthesis of polysubstituted 5-aminopyrazole-4-carbonitriles and 6-amino-1, 4-dihydropyrano [2, 3-c] pyrazole-5-carbonitriles. Res. Chem. Intermed. 44, 2761–2778. doi:10.1007/s11164-018-3260-0
Kondabanthini, S., Katari, N. K., Srimannarayana, M., Gundla, R., Kapavarapu, R., and Pal, M. (2022). Wang resin catalyzed sonochemical synthesis of dihydropyrano [2, 3-c] pyrazole derivatives and their interactions with SIRT1. J. Mol. Struct. 1266, 133527. doi:10.1016/j.molstruc.2022.133527
Küçükgüzel, Ş. G., and Şenkardeş, S. (2015). Recent advances in bioactive pyrazoles. Eur. J. Med. Chem. 97, 786–815. doi:10.1016/j.ejmech.2014.11.059
Kumar, S., Jain, S., Nehra, M., Dilbaghi, N., Marrazza, G., and Kim, K.-H. (2020). Green synthesis of metal–organic frameworks: a state-of-the-art review of potential environmental and medical applications. Coord. Chem. Rev. 420, 213407. doi:10.1016/j.ccr.2020.213407
Kumar, V., Kaur, K., Gupta, G. K., and Sharma, A. K. (2013). Pyrazole containing natural products: synthetic preview and biological significance. Eur. J. Med. Chem. 69, 735–753. doi:10.1016/j.ejmech.2013.08.053
Mamaghani, M., and Hossein Nia, R. (2021). A review on the recent multicomponent synthesis of pyranopyrazoles. Polycycl. Aromat. Compd. 41, 223–291. doi:10.1080/10406638.2019.1584576
Mathavan, S., and Yamajala, R. B. (2023). Potassium dihydrogen phosphate-catalyzed synthesis of benzylpyrazolyl coumarin and pyrano [2, 3-c] pyrazole derivatives via cascade—one-pot—multicomponent reaction. J. Heterocycl. Chem. 60, 123–133. doi:10.1002/jhet.4569
Moghaddam, F. M., Aghili, S., Daneshfar, M., Moghimi, H., and Daneshfar, Z. (2023). Bread waste in the form of CoFe2O4@ TBW catalyst was used as a green biocatalyst to synthesize pyranopyrazole and tetraketone derivatives. Res. Chem. Intermed. 49, 1507–1543. doi:10.1007/s11164-022-04934-z
Moghaddam-Manesh, M., and Hosseinzadegan, S. (2021). Introducing new method for the synthesis of polycyclic compounds containing [1, 3] dithiine derivatives, with anticancer and antibacterial activities against common bacterial strains between aquatic and human. J. Heterocycl. Chem. 58, 2174–2180. doi:10.1002/jhet.4345
Mohamadpour, F. (2020). Catalyst-free green synthesis of dihydropyrano [2, 3-c] pyrazole scaffolds assisted by ethylene glycol (EG) as a reusable and biodegradable solvent medium. J. Chem. Sci. 132, 72–79. doi:10.1007/s12039-020-01775-4
Mohamed, E. A., Abdelmajeid, A., Behalo, M., Abel-Maaboud, A., and Hebaish, K. (2022). Green synthesis, cytotoxicity and antimicrobial activities of some new pyrazolines, pyrimidines and naphthyridines based on 1, 3-di (thien-2-yl) prop-2-en-1-one using choline chloride-urea mixture as A deep eutectic solvent. Egypt. J. Chem. 65, 651–663.
Mokariya, J. A., Rajani, D. P., and Patel, M. P. (2022). Anomaly of pyrano [2, 3-c] pyrazole synthesis towards pyrazolyl-aryl-methyl-malononitrile derivatives and their antimicrobial activity. ChemistrySelect 7, e202201341. doi:10.1002/slct.202201341
Nemati, R., Elhamifar, D., Zarnegaryan, A., and Shaker, M. (2021). Core-shell structured magnetite silica-supported hexatungstate: a novel and powerful nanocatalyst for the synthesis of biologically active pyrazole derivatives. Appl. Organomet. Chem. 35, e6409. doi:10.1002/aoc.6409
Nguyen, H. T., Van Le, T., and Tran, P. H. (2021). AC-SO3H/[CholineCl] [Urea] 2 as a green catalytic system for the synthesis of pyrano [2, 3-c] pyrazole scaffolds. J. Environ. Chem. Eng. 9, 105228. doi:10.1016/j.jece.2021.105228
Nishanth Rao, R., Jena, S., Mukherjee, M., Maiti, B., and Chanda, K. (2021). Green synthesis of biologically active heterocycles of medicinal importance: a review. Environ. Chem. Lett. 19, 3315–3358. doi:10.1007/s10311-021-01232-9
Pandey, A., Kumar, A., and Shrivastava, S. (2021). Sugarcane bagasse ash-based silica-supported boric acid (SBA-SiO2-H3BO3): a versatile and reusable catalyst for the synthesis of 1, 4Dihydropyrano [2, 3c] pyrazole derivatives. Russ. J. Org. Chem. 57, 653–660. doi:10.1134/s1070428021040229
Parikh, P. H., Timaniya, J. B., Patel, M. J., and Patel, K. P. (2022). Microwave-assisted synthesis of pyrano [2, 3-c]-pyrazole derivatives and their anti-microbial, anti-malarial, anti-tubercular, and anti-cancer activities. J. Mol. Struct. 1249, 131605. doi:10.1016/j.molstruc.2021.131605
Patki, A. S., Patil, K. N., Kusuma, S., Muley, D. B., and Jadhav, A. H. (2021). One-pot synthesis of multicomponent pyrazole-4-carbonitrile derivatives under solvent-free condition by using engineered polyvinyl alcohol catalyst. Res. Chem. Intermed. 47, 2751–2773. doi:10.1007/s11164-021-04450-6
Poonam, S., and Singh, R. (2019). Facile one-pot synthesis of 5-amino-1 H-pyrazole-4-carbonitriles using alumina–silica-supported MnO 2 as recyclable catalyst in water. Res. Chem. Intermed. 45, 4531–4542. doi:10.1007/s11164-019-03847-8
Ramadan, M., Aly, A. A., El-Haleem, L. E. A., Alshammari, M. B., and Bräse, S. (2021). Substituted pyrazoles and their heteroannulated analogs—recent syntheses and biological activities. Molecules 26, 4995. doi:10.3390/molecules26164995
Rushell, E., Tailor, Y. K., Khandewal, S., Verma, K., Agarwal, M., and Kumar, M. (2019). Deep eutectic solvent promoted synthesis of structurally diverse hybrid molecules with privileged heterocyclic substructures. New J. Chem. 43, 12462–12467. doi:10.1039/c9nj02694k
Shinde, S. K., Patil, M. U., Damate, S. A., and Patil, S. S. (2018). Synergetic effects of naturally sourced metal oxides in organic synthesis: a greener approach for the synthesis of pyrano [2, 3-c] pyrazoles and pyrazolyl-4 H-chromenes. Res. Chem. Intermed. 44, 1775–1795. doi:10.1007/s11164-017-3197-8
Sikandar, S., and Zahoor, A. F. (2021). Synthesis of pyrano [2, 3-c] pyrazoles: a review. J. Heterocycl. Chem. 58, 685–705. doi:10.1002/jhet.4191
Suksatan, W., Kazemzadeh, P., Afzali, D., Moghaddam-Manesh, M., Chauhan, N. P. S., and Sargazi, G. (2022). A controllable study on ultrasound assisted synthesis of a novel Ni/Zn based hybrid MOF nanostructures for Dextranase immobilization. Inorg. Chem. Commun. 139, 109410. doi:10.1016/j.inoche.2022.109410
Suryakumari, A., and Sudhakar, C. (2023). One-pot synthesis of 1, 4 dihydro pyrano [2, 3-c] pyrazole using chitosan-p-toluene sulfonate as a biodegradable catalyst in an aqueous medium. Lett. Org. Chem. 20, 596–602. doi:10.2174/1570178620666221216144752
Vanegas, S., Rodríguez, D., and Ochoa-Puentes, C. (2019). An efficient and eco-friendly one-pot synthesis of pyrazolopyridines mediated by choline chloride/urea eutectic mixture. ChemistrySelect 4, 3131–3134. doi:10.1002/slct.201900314
Vasava, M. S., Bhoi, M. N., Rathwa, S. K., Shetty, S. S., Patel, R. D., Rajani, D. P., et al. (2019). Novel 1, 4-dihydropyrano [2, 3-c] pyrazole derivatives: synthesis, characterization, biological evaluation and in silico study. J. Mol. Struct. 1181, 383–402. doi:10.1016/j.molstruc.2018.12.053
Yadav, A., and Pandey, S. (2014). Densities and viscosities of (choline chloride+ urea) deep eutectic solvent and its aqueous mixtures in the temperature range 293.15 K to 363.15 K. J. Chem. Eng. Data 59, 2221–2229. doi:10.1021/je5001796
Yahiaoui, A. A., Ghichi, N., Hannachi, D., Djedouani, A., Meskaldji, S., Merazig, H., et al. (2022). Synthesis, XRD/HSA-interactions, biological activity, optical and nonlinear optical responses studies of new pyran derivative. J. Mol. Struct. 1263, 133161. doi:10.1016/j.molstruc.2022.133161
Yekke-Ghasemi, Z., Heravi, M. M., Malmir, M., Jahani, G., Bisafar, M. B., and Mirzaei, M. (2022). Fabrication of heterogeneous-based lacunary polyoxometalates as efficient catalysts for the multicomponent and clean synthesis of pyrazolopyranopyrimidines. Inorg. Chem. Commun. 140, 109456. doi:10.1016/j.inoche.2022.109456
Keywords: choline chloride/urea, deep eutectic solvent, pyrazole, pyrano[2,3-c]pyrazole, biological evaluation, antimicrobial agent
Citation: Habeeb Naser I, Thoulfikar A. Alamir H, Al-Shukarji AH, Ahmed BA, Qassem TA, Kamal M, Almeleebia TM, Alwaily ER, Hasan Kadhum E, Alawadi A and Alsalamy A (2024) Choline chloride/urea as a green and efficient deep eutectic solvent in three-component and four-component synthesis of novel pyrazole and pyrano[2,3-c] pyrazole derivatives with antibacterial and antifungal activity. Front. Chem. 12:1342784. doi: 10.3389/fchem.2024.1342784
Received: 22 November 2023; Accepted: 15 January 2024;
Published: 16 February 2024.
Edited by:
Mohamed Saleh Abdelfattah, Helwan University, EgyptReviewed by:
Ajay Kumar Chinnam, University of Idaho, United StatesCopyright © 2024 Habeeb Naser, Thoulfikar A. Alamir, Al-Shukarji, Ahmed, Qassem, Kamal, Almeleebia, Alwaily, Hasan Kadhum, Alawadi and Alsalamy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed Alawadi, YWhtZWRhbGF3YWRpODVAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.