
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 11 December 2023
Sec. Photocatalysis and Photochemistry
Volume 11 - 2023 | https://doi.org/10.3389/fchem.2023.1330227
This article is part of the Research Topic Spotlight on Europe - Chemical Sciences 2023 View all 6 articles
The paper presents the fabrication and characterization of [CuI(L)]n thin films, where L represents various alkylpyridine ligands including 4-methylpyridine, 3-methylpyridine, 2-methylpyridine, 4-tbutylpyridine, 3,4-dimethylpyridine, and 3,5-dimethylpyridine. The thin films were synthesized by exposing the corresponding ligands to CuI thin films through vapor deposition. The coordination reactions occurring on the films were investigated using PXRD and time-dependent photoluminescence spectroscopy, and a comparison was made between the structures of the thin films and the corresponding powder phases. The films showed primarly blue emission (λem = 457–515 nm) and polymeric structures with excited state lifetimes ranging from 0.6 to 5.5 μs. Significantly, the studied compounds exhibited fast reversible luminescence quenching when exposed to vapors of dichloromethane and dibromomethane (15 and 30 min respectively), and the luminescence was restored upon re-exposure to the alkylpyridine ligand (after 20 min). These findings indicate that these thin films hold promise for applications as sensors (with sensitive and reversible detection capability) for volatile halogen-based compounds (VHC).
Copper iodide (CuI) has been a topic of interest for material scientists due to its unique optoelectronic properties and applications (Yu et al., 2017; Chen et al., 2018; Busch et al., 2019; Ki et al., 2021; Schlachter et al., 2021). CuI is a wide bandgap semiconductor (3.1 eV) which makes it an attractive component of optoelectronics, such as solar cells, photodetectors, and light-emitting diodes (LEDs) (RUSOP et al., 2004; Sankapal et al., 2004; Wang et al., 2017; Yin et al., 2021; Vora-ud et al., 2022). As well, CuI readily reacts with volatile organic compounds and may act as a reversible sensor for the detection of gases and monitoring temperature and pressure (Safko et al., 2012; Kondo et al., 2020; Dai et al., 2023; Jia et al., 2023).
Copper(I)-halide complexes exhibit structural diversity that is coupled to their photoluminescence (Boden et al., 2021; López et al., 2022; Murillo et al., 2022). Simple combinations of copper iodide and pyridine-based ligands in varying ratios yields compounds ranging from mononuclear [CuI(3-Methylpyridine)3] to blue emissive stair-step polymer [CuIpy]∞. The coordination sphere is largely determined by electronic and steric effects as well as crystallization solvent and concentration in solution. For instance, solutions of {CuIpy}n (where py = pyridine or pyridine derivatives) primarily exist as tetrahedral clusters, [CuIpy]4 unless it is in the presence of high concentrations of py leading to the formation of mononuclear or multinuclear structures. Conversely, crystalline solids isolated from such solutions may take the form of either tetranuclear or polymeric structures, depending mainly on the crystallization conditions, particularly solvent (Ford et al., 1999; Cariati et al., 2000; Näther and Jeß, 2002; Zhang et al., 2014).
Among the copper(I)-halide complexes, the compounds containing N-heteroaromatic ligands have garnered attention due to their versatility and impressive luminescent, photophysical, and electrochemical properties (Liu et al., 2015; Kirakci et al., 2017; Kobayashi et al., 2020; Egly et al., 2021). Their unique features render them optimal candidates and establishing them as a compelling research area for scientists. The cost-effectiveness and bright emissivity of copper(I)-halide complexes in the solid-state makes them a preferred choice. By judicious selection of the ligands, the emission wavelength of the complexes can be fine-tuned from 390–650 nm, underscoring their potential in sensing and identifying N-heteroaromatic compounds (Ohara et al., 2014; Ohara et al., 2017; Chen et al., 2023). Copper(I)-halide complexes are well known for their vapochromism, which could be applied for sensing molecules in the gas phase. Copper(I) materials undergo changes in their photoluminescence upon exposure to volatile compounds, which reversibly induce structural changes. The reversible structural changes and luminescent behavior make them candidates for sensor applications (Chai et al., 2015; Grupe et al., 2020; Tang et al., 2023).
The effectiveness of complexes in sensors relies heavily on producing thin films (Klochko et al., 2019). By reducing the density of the complex molecule through vapor exposure, changes in the complex can be more easily observed. Conversely, powdery samples require prolonged vapor exposure spanning several hours to exhibit changes in luminescence color (Touzani et al., 2011; Lee et al., 2022). This inconsistency is attributed to the difficult diffusion of vapor within densely packed crystal lattices.
The primary objective of this study is to explore the response of CuI films to various pyridine derivatives. To track any changes, we will employ time-dependent photoluminescence spectroscopy. Subsequently, we assess the responsivity of thin films to CH2Cl2 and CH2Br2 vapors to assess their effectiveness as sensors for halogenated organic molecules.
CuI (99%), CuSO4·5H2O, (98%), Na2S2O3 (99.99%), KI (99%), 4-methylpyridine (99%), 3-methylpyridine (99%), 2-methylpyridine (98%), 4-tbutylpyridine (98%), 3,4-dimethylpyridine (98%), 3,5-dimethylpyridine (98%), dibromomethane (99%), acetonitrile (99.8%), and CDCl3 (99.8%) were purchased from Sigma Aldrich. Anhydrous dichloromethane (99.8%) and microscope slides were purchased from VWR Sweden. All chemicals were used as received without any further purification.
CuI was first deposited onto glass substrate by spin-coating. The solution for spin-coating was prepared by dissolving 0.10 g (0.525 mmol) of CuI into 10 mL of acetonitrile. Spinning condition was 3,000 rpm for 20 s. Subsequently, the thin film was heated using a hot plate set at 61°C to evaporate the solvents. Spin-coating was repeated 4 times for the same film to grow the thickness of the layer.
Another method for fabricating CuI thin films is the SILAR (successive ionic layer adsorption and reaction) method, in which a glass substrate is immersed separated cation and anion aqueous solutions with a water wash between steps, as described in the literature (Dhere et al., 2010). For the cation solution, 1.05 g (4.2 mmol) of CuSO4 5H2O was dissolved in 42 mL of water in a 50 mL beaker. Also, 94.8 mg (0.6 mmol) of Na2S2O3 was dissolved separately in 6 mL of water before mixing with the copper solution. The cation precursor solution was clear blue in color. The anion precursor, on the other hand, was a transparent KI solution in a 50 mL beaker that contained 199.2 mg (1.2 mmol) of KI dissolved in 48 mL water. Two beakers containing distilled water were placed between the cation and anion precursor for washing.
One SILAR cycle was completed by immersing the substrate into the cation precursor for 5 s and into the anion for 20 s. The washing steps each lasted for 3 s and removed excess ions from the substrate. The cycles were repeated 30 times to obtain a favorable film thickness.
Next, the CuI film was placed in an empty 2.0 mL vial, which was further placed in a 10.0 mL vial containing the ligand solutions. The 10.0 mL vials were sealed, which allowed the ligand solution in the container to vaporize and react with the CuI thin film and form the [CuI(L)]n compounds as thin films. The [CuI(L)]n thin films were emissive and further characterized by PXRD.
To test the vapochromism of the films in the presence of halogenated organics, thin films of copper(I) iodide complexes on glass slides were suspended in a quartz cell and a few drops of the desired CH2Cl2 or CH2Br2 solution was added inside the cell without any direct contact between the film and the solution.
X-ray diffraction (XRD) patterns were measured on X'Pert PRO, PANalytical using CuKα radiation (λ = 1.5406 Å) to analyze the purity of the prepared sample and to determine the phase and crystal structure of the prepared materials. 1H-NMR spectroscopic experiments were performed with a Bruker DMX-500 MHz spectrometer using CDCl3 as reference solvent.
Photoluminescence measurements were carried out on a Fluorolog FL 3-22 spectrometer (Horiba Jobin Yvon, Longjumeau, France), equipped with a double excitation monochromator, a single emission monochromator (HR320) and a R928P PMT detector. A continuous xenon lamp (450 W) was used for steady state measurements. A Delta Diode (λex 360 nm) was employed as the pulsed source for TCSPC lifetime acquisition. Luma 40 heat exchanger connected to TC1 temperature controller was used for temperature adjustment.
Synthesis of powder samples of [CuI(L)]n (n = 4 or ∞) where L = 4-methylpyridine (4-Mepy), 3-methylpyridine (3-Mepy), 2-methylpyridine (2-Mepy), 4-tbutylpyridine (4-tBupy), 3,4-dimethylpyridine (3,4-diMepy), and 3,5-dimethylpyridine (3,5-diMepy) were reported previously with their crystal structures (Die Strukturchemie der KupferI et al., 1980; Rath et al., 1986; Cariati et al., 2000; Cariati et al., 2005; Parmeggiani and Sacchetti, 2012; Kitada and Ishida, 2014). They exhibited strong blue emission to blue-green, yellow, or red according to their structures as shown in Figure 1.
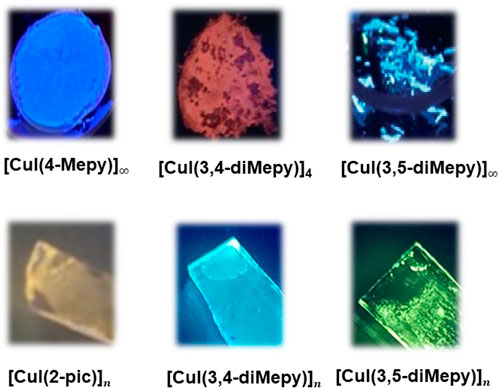
FIGURE 1. Photos of CuI complexes (top) in powder form and (bottom) on film under irradiation at 365 nm.
In accordance with the experimental section, we utilized the spin coating method to produce thin films of copper(I) iodide on glass substrates. Spin coating is widely recognized for rapidly generating uniform thin films with limited aggregation. Afterward, vapor diffusion as a direct technique for the preparation of copper(I) halide complexes as thin films were utilized. As seen in Figure 2, the X-ray diffractograms confirm the reaction of the ligands with the copper(I) films upon exposure to the ligand by vapor diffusion. No CuI peak is detected after the reactions and there are no broad peaks indicating the formation of amorphous material.
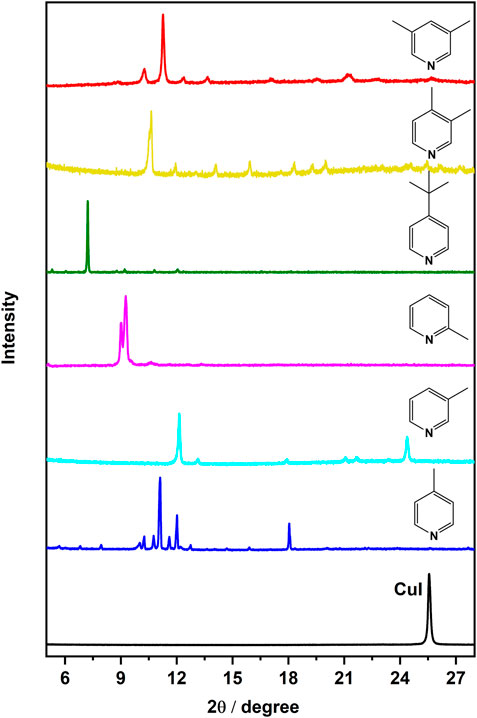
FIGURE 2. PXRD pattern of CuI film and complexes, [CuI(L)]n, on films shown with ligands (L) as prepared by the SILAR method.
By monitoring the photoluminescence spectra of copper(I) iodide films as a function of exposure time to ligand vapors the reaction coordination processes can be resolved throughout the entire reaction timeline. A detailed analysis of the photoluminescence spectra revealed distinct changes in emission characteristics as the reaction progressed. The emission intensity, peak position, and spectral shape exhibited notable variations over time, indicating the alteration of reaction coordination states on the film surface. Therefore, thin film of CuI was exposed to the vapor of L and the photoluminescence spectra and decay kinetics (lifetime, τ) were measured. The photophysical data and their comparison to the crystalline sample are shown in Table 1.

TABLE 1. Photoluminescence maxima and lifetimes of samples in powder and thin films at room temperature.
To prepare the thin films of the copper(I) iodide coordination complexes, one CuI film (2 × 0.5 cm2) was placed in an empty 2.0 mL vial, which was then placed into a 10.0 mL vial containing a ligand (L). Upon exposure to 4-Mepy vapor, the initially non-emissive CuI film exhibits a remarkable transformation in its emission. Figure 3 illustrates the time-dependent photoluminescence spectra, highlighting the evolution of emission characteristics. Within a few seconds of exposure, a vivid blue emission emerged, resembling the emission exhibited by the [CuI(4-Mepy)]∞ polymer as powder. The intensity of the blue emission reached its peak after three minutes, with an emission maximum (λem) at 498 nm.
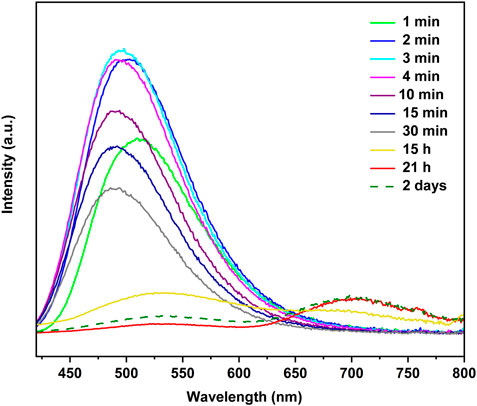
FIGURE 3. Time dependent photoluminescence for the reaction of a CuI film with 4-Mepy vapors at 25°C in a 10.0 mL vial.
Subsequently, the blue emission gradually diminished over a period of 15 h, accompanied by the appearance of a low-energy red emission band at 700 nm. This red emission became increasingly dominant after 21 h, suggesting the presence of a tetramer, [CuI(4-Mepy)]4, on the film’s surface. Notably, after 2 days, the high-energy band regained dominance, and it remained as the sole emission feature even after 33 days of storage in a sealed vial. The emission disappeared after 2 days of exposure to air, which shows the sensitivity of the stability of the film to environmental pressure.
The reaction of of 3-Mepy with CuI film led to the blue emission at 498 nm, which is characteristic of [CuI(3-Mepy)]∞ polymer (Supplementary Figure S1).
Based on the information presented in Figure 4, when CuI thin film is exposed to 2-Mepy vapor, a reaction occurs resulting in dual emission, with a dominant high energy peak observed from the initial moments of exposure. The peaks appear to originate from two distinct structures, possibly indicating the formation of two different stoichiometric structures (a tetramer and a polymer). Previous studies suggest that the enduring red emission, which remains stable even after two hours, can be attributed to [Cu2I2(2-Mepy)]n (Die Strukturchemie der KupferI et al., 1980). The X-ray diffraction patterns (PXRD) of both the film and the powder exhibit a prominent peak at 9.2°. However, when the blue-emitting [CuI(2-Mepy)]∞ film is placed in a sealed vial in contact with vapors at room temperature, a complete transformation to the yellow-emitting cubane [CuI(2-Mepy)]4 is achieved within a span of 2 days.
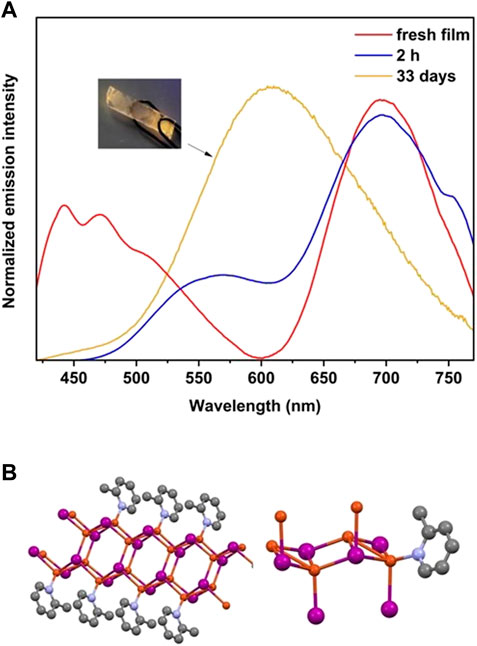
FIGURE 4. (A) Photoluminescence from the reaction of a CuI film with 2-Mepy after 30 min, 2 h and 33 days of exposure in a 10.0 mL vial. The inset shows a picture of the film under illumination after 33 days of exposure to the ligand. (B) Crystal structure of [Cu2I2(2-Mepy)]n, which shows red emission at 658 nm.
The photoluminescence spectra of the reaction with tBupy vapor exhibits time-dependent spectra that are characterized by a strong and blue emission at 500 nm and decay constant of 0.4 µs, accompanied by a broad and lower energy peak at 700 nm. Interestingly, this behavior contradicts what is observed in the solid state, indicating that the predominant species in the reaction are polymers, with only a small presence of clusters contributing to the excited state known as CC (charge-transfer) (Cariati et al., 2005).
The emission from 3,4-diMepy remains consistent even after being exposed to the ligand for a period of 2 days. The emission is characterized by a vivid blue color and shows high intensity. It appears that the reaction reaches completion within an hour, and even after 2 days of exposure to ambient air, there is no reduction in emission intensity or quenching observed (as depicted in Supplementary Figure S2A through emission spectra and color images). In contrast, when the same reaction is subjected to heating at 70°C, it achieves completion within a mere 2 min (as illustrated in Supplementary Figure S2B). On the other hand, for 3,5-diMepy, the reaction occurs at a significantly faster rate, resulting in the observation of a single green emission peak at 514 nm (as shown in Supplementary Figure S3).
Consequently, it appears that for di-substituted pyridine ligands and para-bulky substituted pyridine ligands such as tBupy (Figure 5), the presence of steric hindrance directs the reaction towards a single product. The literature and thermal gravimetry reports on these compounds suggest that degradation occurs as a result of the loss of 1 alkylpyridine ligand or in the case of two-stage degradation of half of the ligands. In such cases, fluorescence was not detectable either at room temperature or at low temperature (De Ahna and Hardt, 1972).
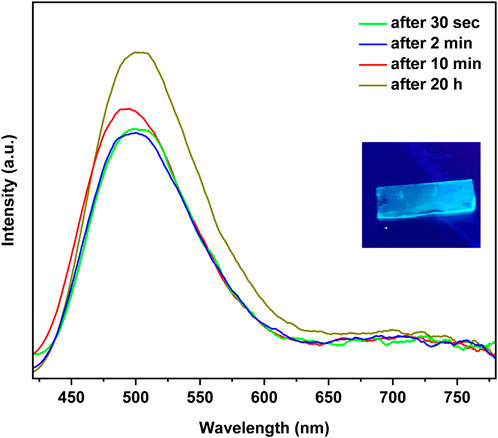
FIGURE 5. Time dependent photoluminescence of the reaction of CuI film with tBupy vapor at 25°C in a 10.0 mL vial.
Having sensors for halogens plays a crucial role in environmental monitoring, health and safety, industrial applications, and emergency response. These sensors help in detecting, quantifying, and tracking halogens, allowing for proactive measures and effective management of halogen-related risks and challenges. To test the viability of copper(I) iodide coordination complexes as sensors for volatile halogenated compounds, we investigated the photoluminescence response from [CuI(3,4-diMepy)]n and [CuI(3,5-diMepy)]n thin films upon exposure to CH2Cl2 and CH2Br2.
Figure 6 demonstrates that when the polymer film [CuI(3,4-diMepy)]n was exposed to a small quantity of dichloromethane vapor, the blue emission was gradually suppressed within a 15-min timeframe. This evidence indicates that the exposure to CH2Cl2 does not result in the conversion of the polymer to cubane, but rather leads to the emergence of a new non-emissive structure. Indeed, subsequent experiments confirmed this outcome. Conversely, the polymer could be transformed back to CuI(3,4-diMepy)]n by subjecting the sample to 3,4-diMepy vapors for 20 min, resulting in increasing photoluminescence intensity compared to the initial sample.
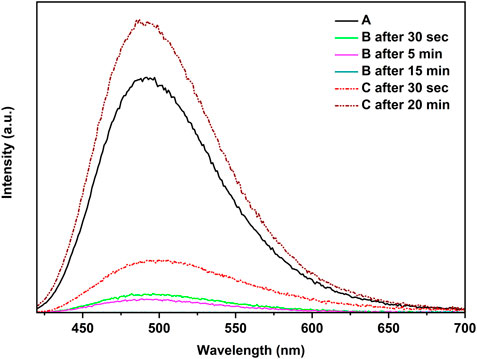
FIGURE 6. Reaction of [CuI(3,4-diMepy)]n, A, with CH2Cl2, B, and reverse reaction with 3,4-diMepy, C, at 25°C.
Although the PXRD pattern of [CuI(3,4-diMepy)]n samples remained relatively unchanged throughout the process (see Supplementary Figure S4), the PXRD analysis of [CuI(3,5-diMepy)]n (Figure 7A) displayed the appearance of a new peak at 8.7° and broadening of the peak at 11.3° (Figure 7B). Furthermore, after re-exposure to 3,5-diMepy vapor, the peak at 11.3° remained broadened, while the peak at 8.7° vanished (Figure 7C). A similar trend was observed following exposure to CH2Br2, albeit with a quenching time of approximately 30 min.
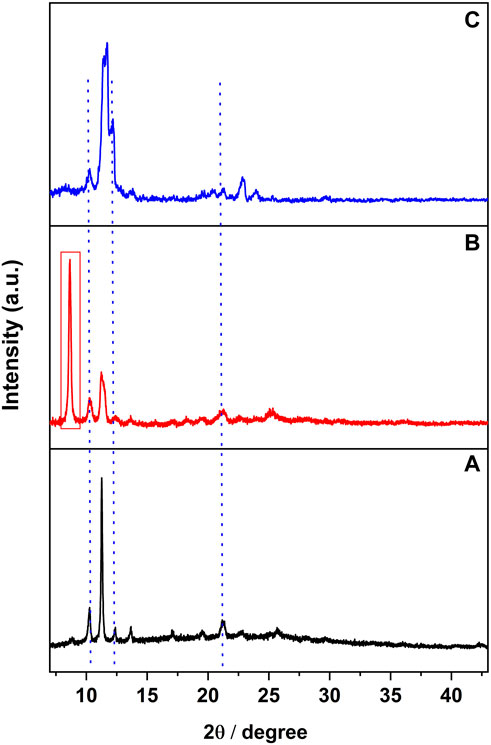
FIGURE 7. (A) PXRD pattern of complex 3,5-diMepy, and (B) that after CH2Cl2 vapor exposure. (C) PXRD pattern after exposing sample B to 3,5-diMepy vapor.
To conduct further analysis, the [CuI(3,4-diMepy)]n deposited on the substrate was dissolved in CDCl3 and subjected to 1H-NMR (Figure 8). The 1H-NMR spectrum of the ligand revealed the presence of three distinct hydrogen types in the aromatic region, specifically at chemical shifts of 6.9, 8.22, and 8.23 ppm. It was anticipated that these same peaks would be observed after nitrogen coordination to the copper. Surprisingly, the downfield signals were no longer detectable, and instead, a new broad signal emerged at 8.4 ppm. Additionally, a signal appeared at 7.1 ppm. We postulate that these observations may be indicative of hydrogen bond interactions occurring between the ortho hydrogens of the pyridine ring and the chloride present in CH2Cl2.
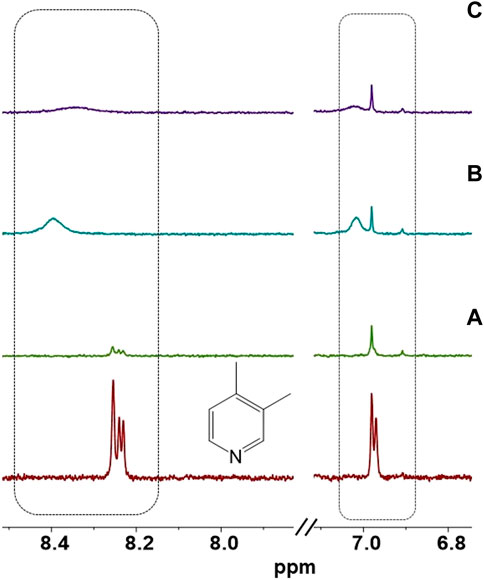
FIGURE 8. Aromatic region of 1H NMR of 3,4-diMepy ligand and (A) [CuI(3,5-diMepy)]n, and (B) after exposure to CH2Cl2 vapor. (C) After re-exposing the film to 3,4-diMepy vapor in CDCl3.
In conclusion, the fabrication process of thin films of copper iodide alkylpyridine compounds was carried out in a straightforward manner. The characteristics of the resulting structures, their photoluminescence, and stability were found to be influenced by the steric hinderance of the alkylpyridine ligand. It was observed that bulkier ligands led to the formation of a polymer blue emitter structure, which exhibited enhanced stability at ambient temperature.
Additionally, the sensitivity of the films to environmental pressure was demonstrated by the rapid loss of emission within just 2 days of exposure to air. This highlights the crucial importance of considering stability factors when utilizing CuI films in practical applications.
Furthermore, the compounds [CuI(3,4-diMepy)]n and [CuI(3,5-diMepy)]n were specifically investigated as detectors for volatile halogenated compounds. The emission of these compounds was found to be quenched due to structural changes induced by the presence of the volatile compounds. Importantly, the emission could be restored upon re-exposure to the ligand, indicating a reversible behavior.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
MJ: Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. JB: Formal Analysis, Investigation, Writing–original draft. JG: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Swedish Energy Agency (Energimyngidgheten) supported this research through a research grant. The fund were used to purchase chemicals and pay the salary of the individuals involved in this article. The Swedish government also funded this research through the strategic research area STandUP for ENERGY and helped pay for JG’s efforts in the article.
We gratefully acknowledge the Swedish Energy Agency for financial support (Grant number: 49278-1). We would like to thank KTH’s Department of Chemistry for supplying the caffeine required to complete this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2023.1330227/full#supplementary-material
Boden, P., Di Martino-Fumo, P., Busch, J. M., Rehak, F. R., Steiger, S., Fuhr, O., et al. (2021). Investigation of luminescent triplet states in tetranuclear CuI complexes: thermochromism and structural characterization. Chem. – A Eur. J. 27, 5439–5452. doi:10.1002/chem.202004539
Busch, J. M., Zink, D. M., Di Martino-Fumo, P., Rehak, F. R., Boden, P., Steiger, S., et al. (2019). Highly soluble fluorine containing Cu(i) AlkylPyrPhos TADF complexes. Dalton Trans. 48, 15687–15698. doi:10.1039/c9dt02447f
Cariati, E., Bu, X., and Ford, P. C. (2000). Solvent- and vapor-induced isomerization between the luminescent solids [CuI(4-pic)]4 and [CuI(4-pic)]∞ (pic = methylpyridine). The structural basis for the observed luminescence vapochromism. Chem. Mater. 12, 3385–3391. doi:10.1021/cm0010708
Cariati, E., Roberto, D., Ugo, R., Ford, P. C., Galli, S., and Sironi, A. (2005). New structural motifs, unusual quenching of the emission, and second harmonic generation of copper(I) iodide polymeric or oligomeric adducts with para-substituted pyridines or trans-stilbazoles. Inorg. Chem. 44, 4077–4085. doi:10.1021/ic050143s
Chai, W., Hong, M., Song, L., Jia, G., Shi, H., Guo, J., et al. (2015). Three reversible polymorphic copper(I) complexes triggered by ligand conformation: insights into polymorphic crystal habit and luminescent properties. Inorg. Chem. 54, 4200–4207. doi:10.1021/ic502709b
Chen, C., Li, R.-H., Zhu, B.-S., Wang, K.-H., Yao, J.-S., Yin, Y.-C., et al. (2018). Highly luminescent inks: aggregation-induced emission of copper–iodine hybrid clusters. Angew. Chem. Int. Ed. 57, 7106–7110. doi:10.1002/anie.201802932
Chen, M., Ye, C., Dai, C., Li, R., Qi, R., Luo, C., et al. (2023). Self-assembly formation of CuI hybrid micron phosphors with tunable emission for multifunctional applications. J. Colloid Interface Sci. 638, 834–841. doi:10.1016/j.jcis.2023.01.083
Dai, Z.-Q., Song, L., Wang, Y.-Y., Wang, J.-T., Jia, Y.-F., Zhang, D.-Q., et al. (2023). Two luminescent materials of CuI clusters based on mono-phosphine ligands and their fluorescence sensing properties. J. Clust. Sci. 34, 2497–2507. doi:10.1007/s10876-022-02401-8
De Ahna, H. D., and Hardt, H. D. (1972). Fluoreszenz und Fluoreszenz-Thermochromie bei Alkyl-pyridinokupfer(I)-Halogeniden. Z. für Anorg. Allg. Chem. 387, 61–71. doi:10.1002/zaac.19723870108
Dhere, S. L., Latthe, S. S., Kappenstein, C., Mukherjee, S. K., and Rao, A. V. (2010). Comparative studies on p-type CuI grown on glass and copper substrate by SILAR method. Appl. Surf. Sci. 256, 3967–3971. doi:10.1016/j.apsusc.2010.01.058
Die Strukturchemie der Kupfer(I), , Hiller, W., Eitel, E., and Oelkrug, D. (1980). Die Strukturchemie der Kupfer(I)Halogenid-Lewisbase Addukte: darstellung und Kristallstruktur von (CuI)2 · (2-Methylpyridin). Z. für Kristallogr. - Cryst. Mater. 153, 277–284. doi:10.1524/zkri.1980.153.14.277
Egly, J., Bissessar, D., Achard, T., Heinrich, B., Steffanut, P., Mauro, M., et al. (2021). Copper(I) complexes with remotely functionalized phosphine ligands: synthesis, structural variety, photophysics and effect onto the optical properties. Inorganica Chim. Acta 514, 119971. doi:10.1016/j.ica.2020.119971
Ford, P. C., Cariati, E., and Bourassa, J. (1999). Photoluminescence properties of multinuclear copper(I) compounds. Chem. Rev. 99, 3625–3648. doi:10.1021/cr960109i
Grupe, M., Bäppler, F., Theiß, M., Busch, J. M., Dietrich, F., Volz, D., et al. (2020). Real-time observation of molecular flattening and intersystem crossing in [(DPEPhos)Cu(i)(PyrTet)] via ultrafast UV/Vis- and mid-IR spectroscopy on solution and solid samples. Phys. Chem. Chem. Phys. 22, 14187–14200. doi:10.1039/c9cp05749h
Jia, Y.-F., Song, L., Xu, W.-Z., Wu, J.-T., Jin, H.-X., Zhu, Y.-F., et al. (2023). A three-coordinate cuprous complex exhibiting both photochromic and fluorescent sensing properties, and some TD-DFT calculations. J. Mol. Struct. 1292, 136134. doi:10.1016/j.molstruc.2023.136134
Ki, W., Hei, X., Yi, H. T., Liu, W., Teat, S. J., Li, M., et al. (2021). Two-Dimensional copper iodide-based inorganic–organic hybrid semiconductors: synthesis, structures, and optical and transport properties. Chem. Mater. 33, 5317–5325. doi:10.1021/acs.chemmater.1c01421
Kirakci, K., Fejfarová, K., Martinčík, J., Nikl, M., and Lang, K. (2017). Tetranuclear copper(I) iodide complexes: a new class of X-ray phosphors. Inorg. Chem. 56, 4609–4614. doi:10.1021/acs.inorgchem.7b00240
Kitada, N., and Ishida, T. (2014). Polymeric one- and two-dimensional copper(i) iodide complexes showing photoluminescence tunable by azaaromatic ligands. CrystEngComm 16, 8035–8040. doi:10.1039/c4ce01231c
Klochko, N. P., Klepikova, K. S., Kopach, V. R., Zhadan, D. O., Starikov, V. V., Sofronov, D. S., et al. (2019). Solution-produced copper iodide thin films for photosensor and for vertical thermoelectric nanogenerator, which uses a spontaneous temperature gradient. J. Mater. Sci. Mater. Electron. 30, 17514–17524. doi:10.1007/s10854-019-02103-4
Kobayashi, R., Imoto, H., and Naka, K. (2020). Stimuli-responsive emission of dinuclear rhombic copper(I) iodide complexes having triphenylarsine and N-heteroaromatic Co-ligands. Eur. J. Inorg. Chem. 2020, 3548–3553. doi:10.1002/ejic.202000644
Kondo, S., Yoshimura, N., Yoshida, M., Kobayashi, A., and Kato, M. (2020). Vapochromic luminescence of a spin-coated copper(i) complex thin film by the direct coordination of vapour molecules. Dalton Trans. 49, 16946–16953. doi:10.1039/d0dt03167d
Lee, H. J., Park, M.-S., Lee, S., Kim, B.-J., and Hong, K. (2022). Solution-processed copper iodide film as a p-type electrical conductor and their applications. ACS Appl. Electron. Mater. 4, 1232–1237. doi:10.1021/acsaelm.1c01282
Liu, W., Fang, Y., Wei, G. Z., Teat, S. J., Xiong, K., Hu, Z., et al. (2015). A family of highly efficient CuI-based lighting phosphors prepared by a systematic, bottom-up synthetic approach. J. Am. Chem. Soc. 137, 9400–9408. doi:10.1021/jacs.5b04840
López, J., Murillo, M., Lifante-Pedrola, G., Cantelar, E., Gonzalez-Platas, J., Rodríguez-Mendoza, U. R., et al. (2022). Multi-stimulus semiconductor Cu(i)–I-pyrimidine coordination polymer with thermo- and mechanochromic sensing. CrystEngComm 24, 341–349. doi:10.1039/d1ce01315g
Murillo, M., Álvarez-Conde, J., Wannemacher, R., Cabanillas-González, J., González-Platas, J., Rodríguez-Mendoza, U. R., et al. (2022). A 1D Cu(i)–I-pyrazine coordination polymer with controlled pressure-induced phase transition and opto-electronic response depending on mechanical stimuli, temperature, and CuI content. J. Mater. Chem. C 10, 18004–18016. doi:10.1039/d2tc04127h
Näther, C., and Jeß, I. (2002). Synthesis, crystal structures and thermal properties of new copper(I)halide 2-ethylpyrazine coordination polymers: the influence of solid-state kinetics on product formation. J. Solid State Chem. 169, 103–112. doi:10.1016/s0022-4596(02)00049-x
Ohara, H., Kobayashi, A., and Kato, M. (2014). Simple and extremely efficient blue emitters based on mononuclear Cu(i)-halide complexes with delayed fluorescence. Dalton Trans. 43, 17317–17323. doi:10.1039/c4dt02709d
Ohara, H., Ogawa, T., Yoshida, M., Kobayashi, A., and Kato, M. (2017). Reversible luminescent colour changes of mononuclear copper(i) complexes based on ligand exchange reactions by N-heteroaromatic vapours. Dalton Trans. 46, 3755–3760. doi:10.1039/c7dt00532f
Parmeggiani, F., and Sacchetti, A. (2012). Preparation and luminescence thermochromism of tetranuclear copper(I)–Pyridine–Iodide clusters. J. Chem. Educ. 89, 946–949. doi:10.1021/ed200736b
Rath, N. P., Holt, E. M., and tanimura, K. (1986). Fluorescent copper(I) complexes: correlation of structural and emission characteristics of [{Cul(quin)2}2] and [Cu4I4(quin)4](quin = quinoline). J. Chem. Soc. Dalton Trans., 2303–2310. doi:10.1039/dt9860002303
Rusop, M., Soga, T., Jimbo, T., and Umeno, M. (2004). COPPER IODIDE THIN FILMS AS A p-TYPE ELECTRICAL CONDUCTIVITY IN DYE-SENSITIZED p-CuI|Dye|n-TiO2 HETEROJUNCTION SOLID STATE SOLAR CELLS. Surf. Rev. Lett. 11, 577–583. doi:10.1142/s0218625x04006554
Safko, J. P., Kuperstock, J. E., McCullough, S. M., Noviello, A. M., Li, X., Killarney, J. P., et al. (2012). Network formation and photoluminescence in copper(i) halide complexes with substituted piperazine ligands. Dalton Trans. 41, 11663–11674. doi:10.1039/c2dt31241g
Sankapal, B. R., Goncalves, E., Ennaoui, A., and Lux-Steiner, M. C. (2004). Wide band gap p-type windows by CBD and SILAR methods. Thin Solid Films 451-452, 128–132. doi:10.1016/j.tsf.2003.11.002
Schlachter, A., Tanner, K., and Harvey, P. D. (2021). Copper halide-chalcogenoether and -chalcogenone networks: chain and cluster motifs, polymer dimensionality and photophysical properties. Coord. Chem. Rev. 448, 214176. doi:10.1016/j.ccr.2021.214176
Tang, S.-Y., Song, L., Jia, Y.-F., Xu, W.-Z., Yang, Y.-X., Sun, L.-J., et al. (2023). Three heteroleptic copper(I) complexes with [Cu(P˄P)N2]+ structure and their fluorescence sensing for VOCs. Appl. Organomet. Chem. 37, e7242. doi:10.1002/aoc.7242
Touzani, R., Vasapollo, G., Scorrano, S., Del Sole, R., Manera, M. G., Rella, R., et al. (2011). New complexes based on tridentate bispyrazole ligand for optical gas sensing. Mater. Chem. Phys. 126, 375–380. doi:10.1016/j.matchemphys.2010.11.008
Vora-ud, A., Chaarmart, K., Kasemsin, W., Boonkirdram, S., and Seetawan, T. (2022). Transparent thermoelectric properties of copper iodide thin films. Phys. B Condens. Matter 625, 413527. doi:10.1016/j.physb.2021.413527
Wang, H., Yu, Z., Jiang, X., Li, J., Cai, B., Yang, X., et al. (2017). Efficient and stable inverted planar perovskite solar cells employing CuI as hole-transporting layer prepared by solid–gas transformation. Energy Technol. 5, 1836–1843. doi:10.1002/ente.201700422
Yin, J., Lei, Q., Han, Y., Bakr, O. M., and Mohammed, O. F. (2021). Luminescent copper(I) halides for optoelectronic applications. Phys. status solidi (RRL) – Rapid Res. Lett. 15, 2100138. doi:10.1002/pssr.202100138
Yu, M., Chen, L., Jiang, F., Zhou, K., Liu, C., Sun, C., et al. (2017). Cation-induced strategy toward an hourglass-shaped Cu6I7– cluster and its color-tunable luminescence. Chem. Mater. 29, 8093–8099. doi:10.1021/acs.chemmater.7b01790
Keywords: copper(I) iodide, N-donor ligand, thin film, sensor, time-dependent photoluminescence
Citation: Jamshidi M, Bouheriche J and Gardner JM (2023) Photoluminescent copper(I) iodide alkylpyridine thin films as sensors for volatile halogenated compounds. Front. Chem. 11:1330227. doi: 10.3389/fchem.2023.1330227
Received: 30 October 2023; Accepted: 01 December 2023;
Published: 11 December 2023.
Edited by:
Clara S. B. Gomes, New University of Lisbon, PortugalReviewed by:
Wen-Xiang Chai, China Jiliang University, ChinaCopyright © 2023 Jamshidi, Bouheriche and Gardner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James M. Gardner, amdhcmRuZXJAa3RoLnNl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.