- 1Qingdao Institute for Theoretical and Computational Sciences, Shandong University, Qingdao, China
- 2Max-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr, Germany
Open-shell molecules rarely fluoresce, due to their typically faster non-radiative relaxation rates compared to closed-shell ones. Even rarer is the fluorescence from states that have two more unpaired electrons than the open-shell ground state, since they involve excitations from closed-shell orbitals to vacant-shell orbitals, which are typically higher in energy compared to excitations from or out of open-shell orbitals. States that are dominated by the former type of excitations are known as tripdoublet states when they can be described as a triplet excitation antiferromagnetically coupled to a doublet state, and their description by unrestricted single-reference methods (e.g., U-TDDFT) is notoriously inaccurate due to large spin contamination. In this work, we applied our spin-adapted TDDFT method, X-TDDFT, and the efficient and accurate static-dynamic-static second order perturbation theory (SDSPT2), to the study of the excited states as well as their relaxation pathways of copper(II) porphyrin; previous experimental works suggested that the photoluminescence of some substituted copper(II) porphyrins originate from a tripdoublet state, formed by a triplet ligand π → π* excitation antiferromagnetically coupled with the unpaired d electron. Our results demonstrated favorable agreement between the X-TDDFT, SDSPT2 and experimental excitation energies, and revealed noticeable improvements of X-TDDFT compared to U-TDDFT, not only for vertical excitation energies but also for adiabatic energy differences. These suggest that X-TDDFT is a reliable tool for the study of tripdoublet state fluorescence. Intriguingly, we showed that the aforementioned tripdoublet state is only slightly above the lowest doublet excited state and lies only slightly higher than the lowest quartet state, which suggests that the tripdoublet of copper(II) porphyrin is long-lived enough to fluoresce due to a lack of efficient non-radiative relaxation pathways; an explanation for this unusual state ordering is given. Indeed, thermal vibration correlation function (TVCF)-based calculations of internal conversion, intersystem crossing, and radiative transition rates confirm that copper(II) porphyrin emits thermally activated delayed fluorescence (TADF) and a small amount of phosphorescence at low temperature (83 K), in accordance with experiment. The present contribution is concluded by a few possible approaches of designing new molecules that fluoresce from tripdoublet states.
1 Introduction
Fluorescence, while ubiquitous in organic and organometallic molecules, is in most cases observed in closed-shell systems. It is well-known that introducing an open-shell impurity, such as dioxygen (Schmidt, 2006), a stable organic radical (Green et al., 1990) or a transition metal ion (Varnes et al., 1972), frequently quenches the fluorescence of a closed-shell molecule (Evans, 1957). One reason of this phenomenon is that the addition of an unpaired electron to a system typically introduces additional low-lying states, in particular charge transfer states that involve an electron exciting from or out of the new open-shell orbital (O). Moreover, while spin-conserving single excitations of a singlet reference determinant from closed-shell (C) to vacant-shell (V) orbitals, hereafter termed CV excitations following our previous works (Li and Liu, 2010; Li et al., 2011; Li and Liu, 2011; Wang et al., 2020), give rise to nCnV singlet excited states and nCnV triplet excited states (where nC and nV are the number of closed-shell an vacant-shell orbitals, respectively), with an MS = 1/2 doublet determinant one obtains 2nCnV excitations that are mixtures of doublets and quartets (the
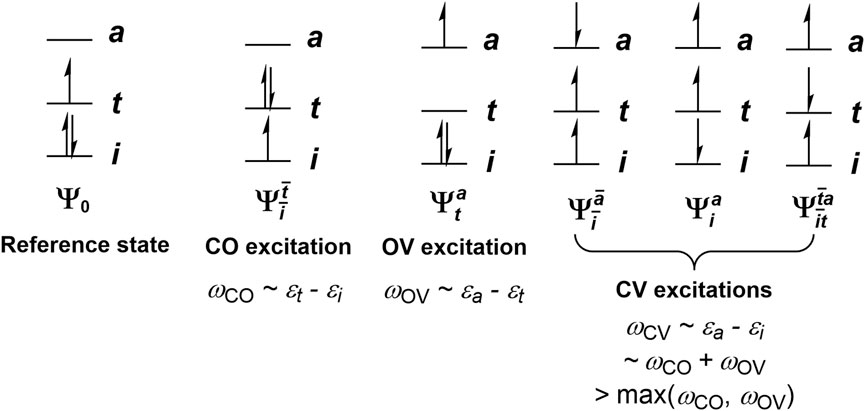
FIGURE 1. Schematic depictions of closed-open (CO), open-vacant (OV), and closed-vacant (CV) excitations, and their approximate excitation energies as predicted from restricted open-shell Kohn-Sham (ROKS) orbital energy differences.
In spin-adapted TDDFT methods, the latter are spin-adapted to give 2nCnV pure doublet states and nCnV quartet states, by mixing with the nCnV spin flip-up excitations from the MS = −1/2 component of the reference determinant, i.e., the
Note that both the “singdoublets” and “tripdoublets” are pure doublet states. While the singdoublets Eq. 1 (which we called the CV(0) states in our previous works (Li and Liu, 2010; Li et al., 2011; Li and Liu, 2011)) are direct analogs of singlet excited states out of a singlet reference, the tripdoublets Eq. 3 (CV(1) states) do not have analogs in closed-shell systems, and create extra spin-allowed non-radiative relaxation pathways compared to when the reference determinant is singlet. This further contributes to the short excited state lifetimes of doublet systems. As a consequence, doublet molecules (and open-shell molecules in general) are rarely fluorescent.
Still, there exist open-shell molecules that do fluoresce, which have found applications in, e.g., organic light-emitting diodes (OLEDs) (He et al., 2019; Gao et al., 2023). However, their fluorescence usually originates from an excited state that has only one unpaired electron, i.e., a CO or OV excited state (where CO stands for a single excitation from a closed-shell orbital to an open-shell one; similar for OV), instead of a CV excited state. This can be partly rationalized by approximating the excitation energies of the system by orbital energy differences. Under this approximation, there is at least one CO state and one OV state below any given CV state, since the lowest CV excitation energy is the sum of the excitation energies of a CO state and an OV state (Figure 1). Therefore, the lowest CV state tends to be a rather high excited state of the system, and thus usually has more energetically accessible non-radiative relaxation pathways than the low-lying CO and OV states do, rendering fluorescence from CV states especially hard to achieve. To counter this, one may try to inhibit the non-radiative relaxation of the CV state to lower excited states. However, the sheer number of non-radiative relaxation pathways that one would have to inhibit poses a great challenge for designing an open-shell molecule that fluoresces from a CV state. Alternatively, one may design a system where the orbital energy difference approximation fails dramatically, allowing the lowest CV state to become the first excited state, or only slightly higher than the first excited state. In this case, the fluorescence from the CV state only needs to compete with the intersystem crossings (ISCs) to the lowest quartet state(s) and the internal conversion (IC) to the ground state and lower doublet excited state(s), which are the only energy downhill non-radiative relaxation pathways available to the CV state. In particular, note that when the CV excitations shown in Figure 1 linearly combine to give singdoublets, tripdoublets and quartets via Eqs 3, 4, there is an energy splitting that usually places the quartet below the tripdoublet, and the tripdoublet below the singdoublet; while the former is a consequence of Hund’s rule, the latter can be rationalized by applying Hund’s rule after neglecting the coupling of the open-shell orbital to the closed-shell and vacant-shell ones. This gives tripdoublets a much greater chance than singdoublets for emitting fluorescence with an appreciable quantum yield. Nevertheless, the singdoublet-tripdoublet splitting appears to be small in general, compared to the orbital energy difference that one would have to overcome, which can amount to several eVs. Hence, even the fluorescence from tripdoublets proves to be scarce.
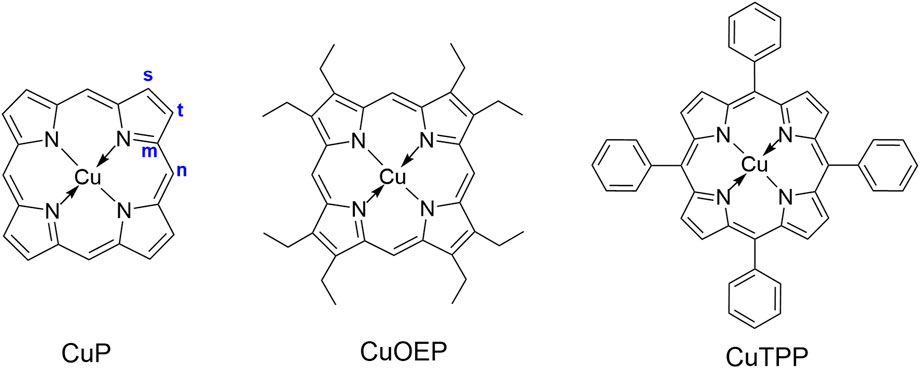
The present paper represents a preliminary attempt to unveil some of the factors that enable an open-shell molecule to fluoresce from a tripdoublet state, via a case study of copper(II) porphyrin complexes. Copper(II) porphyrin complexes (Figure 2), like most porphyrin complexes, show two intense visible absorption bands near 390–420 nm and 520–580 nm (Gouterman, 1959; Eastwood and Gouterman, 1969); they are conventionally termed the B and Q bands, respectively. Eastwood and Gouterman (1969) studied the luminescence of copper(II) porphyrin molecules in the solid state by exciting their Q bands, suggesting that the emission may originate from one of the two low-lying π → π* states, 2T or 4T (here the 2, 4 represent the overall spin multiplicity of the complex, and T denotes that the “local” spin multiplicity of the porphyrin ring is triplet). They speculated that a rapid equilibrium may exist between the 2T and 4T states. The equilibrium ratio of these two states is largely dependent on the energy gap (ΔEDQ) between them and the temperature, via the Boltzmann distribution. The radiative transition from the 2T state to the ground state is spin-allowed, making it much faster than the phosphorescence from the 4T state. Thus, when ΔEDQ is small and the temperature is high, the experimentally observed rapid emission is predominantly from the 2T state. Conversely, when ΔEDQ is large and the temperature is low, a slow emission attributed to the phosphorescence of the 4T state was observed instead, due to the concentration of the 4T state largely overwhelming that of the 2T state. Thus, molecules such as copper 2,3,7,8,12,13,17,18-octaalkylporphyrin (CuOAP), which possess small ΔEDQ values, exhibit luminescence primarily in the form of fluorescence from the 2T state at liquid nitrogen temperature, whereas copper 5,10,15,20-tetraphenylporphyrin (CuTPP) with a larger ΔEDQ mainly undergoes phosphorescence from the 4T state at the same temperatures. The unsubstituted copper porphyrin (CuP) is the most interesting of all, as pure phosphorescence was observed at low temperatures (35 K), which gradually gives way to fluorescence when the temperature was elevated, eventually giving pure fluorescence at 143 K (Bohandy and Kim, 1980). Similar results have been obtained by following works with different techniques and/or solvents (Magde et al., 1974; Kobayashi et al., 2008).
The simple and intuitive picture has since been supplemented by subsequent works, which also excited the B band, and proposed that charge transfer (CT) states may play an important role in the relaxation of the initial bright state to the essentially dark 2T state. Yan and Holten (1988) investigated the excited state relaxation processes of CuTPP and CuOEP at different temperatures and in different solvents, proposing possible pathways involving intermediate states that are probably ligand-to-metal CT (LMCT) states. This is supported by the gas-phase mass spectrometry experiments by Ha-Thi et al. (2013), although the precise composition of the CT state remains uncertain. Understanding the excited-state relaxation pathways of copper porphyrins is crucial for gaining insights into their photophysical processes and controlling their optical properties. In particular, whether any other excited state(s) lie below the 2T state may have a profound influence on whether the 2T state fluoresces or not, as follows from Kasha’s rule. Meanwhile, the energy gap of the 2T and 4T states is important for the relative concentration of the two states, and therefore the relative intensities of fluorescence from the 2T state and the phosphorescence from the 4T state, i.e., whether the experimentally observed luminescence should be attributed to fluorescence or phosphorescence, or both.
Despite the importance of tripdoublet fluorescence and the long history of experimental studies of copper porphyrins, accurate computational studies of this system prove to be difficult, as traditional unrestricted single-reference methods like U-TDDFT suffer from severe spin contamination issues, leading to systematically underestimated excitation energies. In particular, tripdoublet states are the worst scenario for U-TDDFT, as when the reference state itself is not spin-contaminated, the errors of the U-TDDFT ⟨S2⟩ values reach the theoretical maximum of singly excited states, i.e., 2, for tripdoublet states (Li and Liu, 2010; Li et al., 2011; Li and Liu, 2011; Li and Liu, 2016a; Wang et al., 2020). While multireference methods trivially solve the spin contamination problems, it is notoriously difficult to obtain an accurate multireference description of the electronic structure of metalloporphyrins, due to the complex interplay between static and dynamic correlation. In this study, we employed the methods developed by our group, namely, X-TDDFT (Li and Liu, 2011; Wang et al., 2020) and SDSPT2 (Liu and Hoffmann, 2014; Lei et al., 2017) (static-dynamic-static second-order perturbation theory), to address these challenges and provide a rational description of the photophysical processes in CuP. As the first rigorous spin-adapted TDDFT method (Li and Liu, 2011), X-TDDFT gives spin-adapted excited states even when the reference state is open-shell, thereby generally giving better excitation energies, as well as better transition matrix elements involving the excited states. The recent development of the analytic gradient of X-TDDFT (Wang et al., 2020) allowed us to use X-TDDFT for excited state geometry optimization and seminumerical Hessian calculations as well. For vertical excitation calculations, we could afford to use SDSPT2, which also served as a reference for benchmarking X-TDDFT and U-TDDFT.
2 Computational details
All DFT, TDDFT, and SDSPT2 calculations were performed using a development version of the Beijing Density Functional (BDF) package (Liu et al., 1997; Liu et al., 2003; Liu et al., 2004a; Liu et al., 2004b; Zhang et al., 2020). Geometry optimizations were conducted using the PBE0 (Adamo and Barone, 1999; Ernzerhof and Scuseria, 1999) functional and x2c-SVPall (Pollak and Weigend, 2017) basis set in the gas phase, including Grimme’s D3 dispersion correction (Grimme et al., 2010; Grimme et al., 2011), as implemented in the BDF software; relativistic effects were considered at the spin-free exact two component (sf-X2C) level (Liu et al., 2003; Li et al., 2014b; Liu et al., 1995; Liu et al., 2004b; Liu et al., 2004a). For transition metal complexes (especially when excited states are considered), the choice of the optimum functional may not be obvious. Herein, TDDFT calculations were performed using four different functionals [BP86 (Perdew, 1986a; Perdew, 1986b; Becke, 1988), B3LYP (Becke, 1993; Stephens et al., 1994), PBE0 and ωB97X (Chai and Head-Gordon, 2008)] in conjunction with the x2c-TZVPall (Pollak and Weigend, 2017) basis set, followed by benchmarking the results against SDSPT2 and experimental results, and the PBE0 functional was chosen based on its satisfactory and uniform accuracy (see Section 3.1 for details). The TDDFT/x2c-TZVPall energies differ from the TDDFT/x2c-SVPall ones by within 0.04 eV for ligand excited states (2T1, 2T2, 2S1, 2S2), within 0.14 eV for d-d excited states, and up to 0.29 eV for CT states. Based on this, we used TD-PBE0/x2c-SVPall in all rate constant calculations that involve only the ligand excited states; for rate constants that involve the d-d states (where even TDDFT/x2c-TZVPall predicts wrong state orderings), we calculated the adiabatic excitation energies of the corresponding states using SDSPT2 vertical absorption energies plus the adiabatic/vertical excitation energy differences calculated at the TD-PBE0/x2c-SVPall level, and calculated all the other requisite quantities with TD-PBE0/x2c-SVPall. The orbital diagrams were drawn and visualized with VMD v.1.9.4 (Humphrey et al., 1996), using cube files generated with the help of Multiwfn v.3.8(dev) (Lu and Chen, 2012).
The calculations of ISC rate constants were conducted by the ESD module of the ORCA program, version 5.0.4 (Neese, 2012; Neese, 2018; Neese et al., 2020; Neese, 2022), using the thermal vibration correlation function (TVCF) method based on a multimode harmonic oscillator model. Other rate constants involved in the excited state relaxation process were calculated by the MOMAP package, version 2022A (Peng et al., 2007; Niu et al., 2008; Niu et al., 2020), again using the TVCF method and a harmonic approximation of the potential energy surfaces. The default parameters of the two programs were used in all TVCF calculations, except for the “tmax” parameter in the MOMAP calculations (which controls the propagation time of the TVCF), which was set to 3,000 fs. For the 2T1 →2dd3 IC rate constant, the tmax = 3,000 fs calculation suffered from numerical instabilities, so we chose to use tmax = 1,500 fs instead. All necessary transition matrix elements, including the transition dipole moments, non-adiabatic coupling matrix elements (NACMEs) (Li et al., 2014a; Li and Liu, 2014; Wang et al., 2021), spin-orbit coupling matrix elements (SOCMEs) (Li et al., 2013; Li et al., 2014a; Li et al., 2014b), as well as the seminumerical Hessians necessary for the TVCF calculations, were calculated by BDF. Note however that all NACMEs were computed by U-TDDFT instead of X-TDDFT, since the theory of X-TDDFT NACMEs has not been developed yet; similarly, geometry optimization and frequency calculations of the 4T1 state were performed at the unrestricted Kohn-Sham (UKS) level, which is justified by the small spin contamination (⟨S2⟩ deviation
3 Results and discussion
3.1 Absorption process
As is well-known, density functionals generally have difficulties with simultaneously describing local excitation (LE) and CT states with good accuracy. Since we could only afford to do the geometry optimizations and frequency calculations under the DFT and TDDFT levels, a suitable functional that qualitatively reproduces the SDSPT2 excitation energies has to be chosen by comparing the TDDFT vertical absorption energies of a few common functionals with SDSPT2 data. B3LYP and PBE0 are generally common choices for the excited states of metalloporphyrins, and BP86 is often used to optimize their ground-state structures. Pure functionals usually tend to underestimate excitation energies, but empirically, their description of the Q band (an LE state) is better than many hybrid functionals, as will be confirmed by our calculation results. As CT states are involved in the relaxation process of the excited states of copper porphyrin, range-separated hybrid functionals (which provide good descriptions of CT states in general) may prove to be suitable as well. These considerations gave a list of four representative functionals, BP86, B3LYP, PBE0 and ωB97X, that were subjected to benchmark calculations.
Different functionals display distinct behaviors for the excitation energies of CuP compared to the results obtained from SDSPT2, as shown in Figure 3. The two characteristic absorption bands of the porphyrin molecule correspond to the 2S1 (Q band) and 2S2 (B band) states, which are the only bright states of most porphyrin complexes in the visible region. They are also the only excited states for which accurate experimental vertical absorption energies are available: in benzene they have been measured as 2.25 and 3.15 eV, respectively (Eastwood and Gouterman, 1969). Moreover, the absorption energy of the 2T1 state has been measured by fluorescence excitation spectra experiments, but only for certain substituted porphyrins: for example, the 2T1 absorption energy of CuEtio (Etio = etioporphyrin I) was measured in n-octane as 1.81 eV, while the emission energy from the same state in the same solvent was 1.79 eV (Eastwood and Gouterman, 1969). Assuming that the Stokes shift of the 2T1 state is independent of the porphyrin substituents, and combined with the experimental emission energy of the 2T1 state of CuP in the same solvent (1.88 eV) (Eastwood and Gouterman, 1969), we obtain an estimate of the experimental 2T1 absorption energy of CuP as 1.90 eV. Gratifyingly, the SDSPT2 excitation energies of all three states agree with the experimental values to within 0.3 eV, which is typical of the accuracy of SDSPT2 (Song et al., 2022) and confirms the suitability of SDSPT2 as a benchmark reference for CuP. The B3LYP functional performs better for the two bright states 2S1 and 2S2 than the other functionals (Figure 3), with results closer to the SDSPT2 calculations, suggesting its suitability for localized excitations in the porphyrin system. However, it (as well as the pure functional BP86) performs poorly in describing the dark charge transfer (CT) states, significantly underestimating their energies, as expected. In contrast, the range-separated functional ωB97X shows better agreement with the CT states compared to SDSPT2 results, but its description of the 2S2 state is rather poor, with energies notably higher than the SDSPT2 results. The PBE0 functional represents a compromise between the two classes of functionals and provides more accurate overall descriptions of the LE and CT states, giving results closer to the SDSPT2 calculations. In particular, PBE0 gives the best predictions of all the d-d excitation energies, although a significant overestimation is still seen for the lowest d-d state, 2dd1. Considering the overall performance in describing different states, we chose to use PBE0 for the remaining part of the present study.

FIGURE 3. Errors of different excited states of CuP, computed by different functionals with the x2c-TZVPall basis set, with respect to SDSPT2 values. aTDA.
The 2S1 and 2S2 states are almost spin-adapted states with minimal spin contamination, even at the U-TDDFT level (Table 1), since they are dominated by singdoublet excitations. As shown in Figure 3, both X-TDDFT and U-TDDFT provide similar descriptions for these two states; note however that functionals with large amounts of HF exchange generally overestimate the excitation energies of these two states, especially 2S2. At the TDDFT levels, the CT states are dominated by CO-type excitations (from π to 3
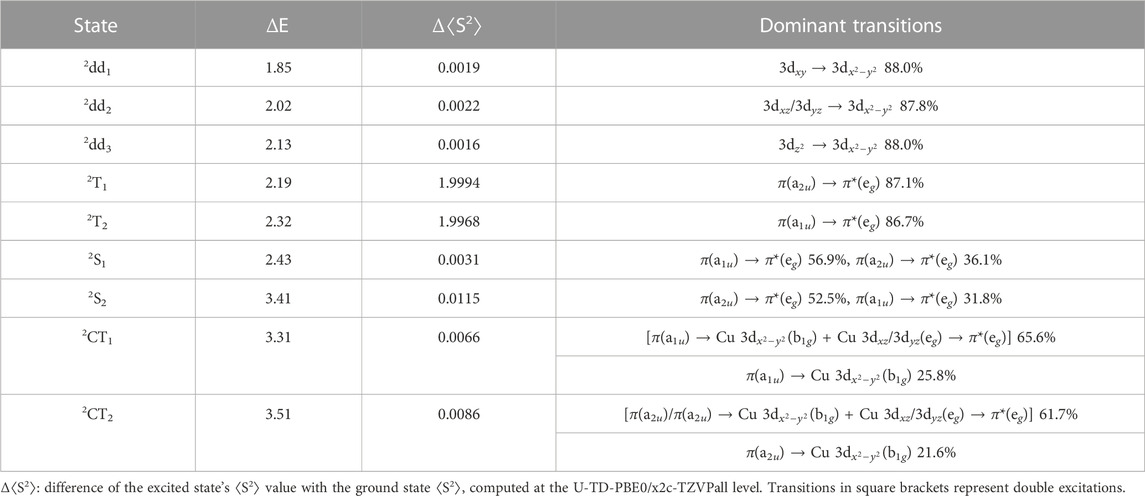
TABLE 1. The SDSPT2/x2c-TZVPall(Cu,N)/x2c-SVPall(C,H) excitation energies (in eV) computed at the sf-X2C-PBE0/x2c-SVPall ground state structure of CuP, along with the corresponding excited state compositions.
As follows from Eqs 2–4. U-TDDFT thus systematically underestimates the excitation energies of the 2T1 and 2T2 states, since the energies of quartets are in general lower than the corresponding tripdoublets, as discussed in the Introduction. In Section 3.3 we will also see that part of the underestimation is due to the failure of U-TDDFT to reproduce the energy degeneracy of
3.2 Analysis of the equilibrium geometry of the 2T1 state
Since all higher lying excited states are predicted to convert to 2T1 over a short timescale, to study the luminescence of CuP [and probably also other Cu(II) porphyrin complexes bearing alkyl or aryl substituents, given that these substituents do not change excitation energies drastically (Eastwood and Gouterman, 1969)], it should suffice to study the radiative and non-radiative processes starting from the 2T1 state. Unlike the three 2dd states, whose Franck-Condon emissions are symmetry forbidden, the emission from the 2T1 state is symmetry allowed, and existing experimental results unequivocally point to a ligand state luminescence, rather than luminescence from a d-d excited state. Therefore, accurately predicting the equilibrium geometry of the 2T1 state is crucial for subsequent studies.
Some selected bond lengths for the optimized ground state and excited state structures are provided in Table 2. The difference in ground state bond lengths between the UKS and ROKS methods is extremely small (
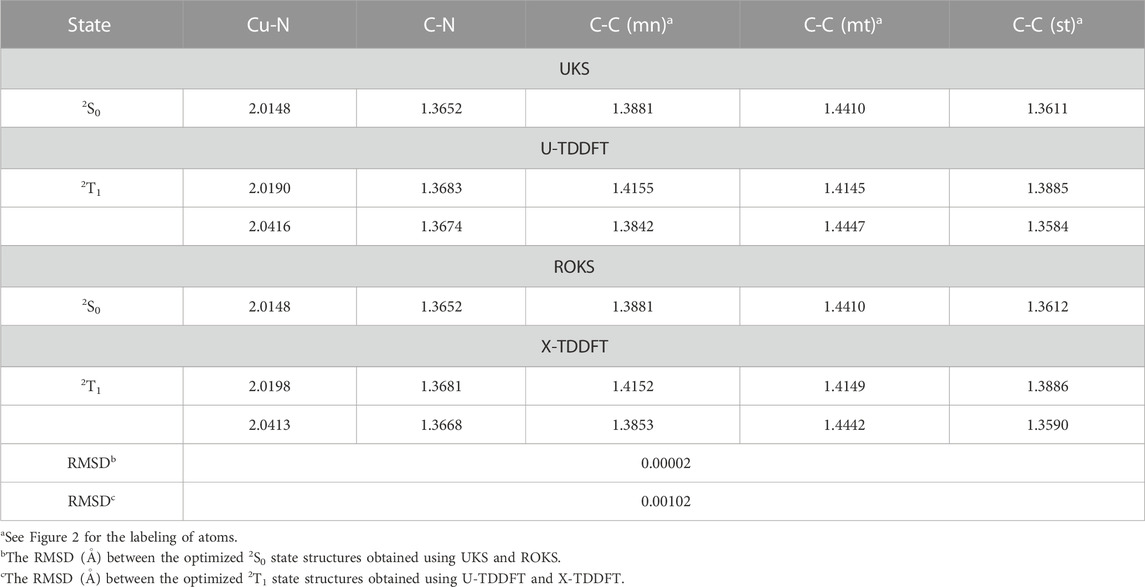
TABLE 2. The equilibrium bond lengths (in
3.3 Relaxation processes of the 2T1 state
As revealed by the above analyses, the relaxation process from high-lying excited states to the 2T1 state is rapid, and the only energetically accessible relaxation pathways are the radiative (fluorescence) and non-radiative (IC) relaxations from 2T1 to the ground state 2S0, the IC from 2T1 to one of the lower 2dd states, as well as the ISC from 2T1 to 4T1. The 4T1 state can furthermore convert back to the 2T1 state through reverse ISC (RISC), or relax to the ground state via radiative (phosphorescence) or non-radiative (ISC) pathways (Figure 4). While the RISC from 4T1 to the 2dd states is also possible, they are expected to be much slower than the spin-allowed 2T1 →2dd IC processes, and are therefore neglected in the present study.
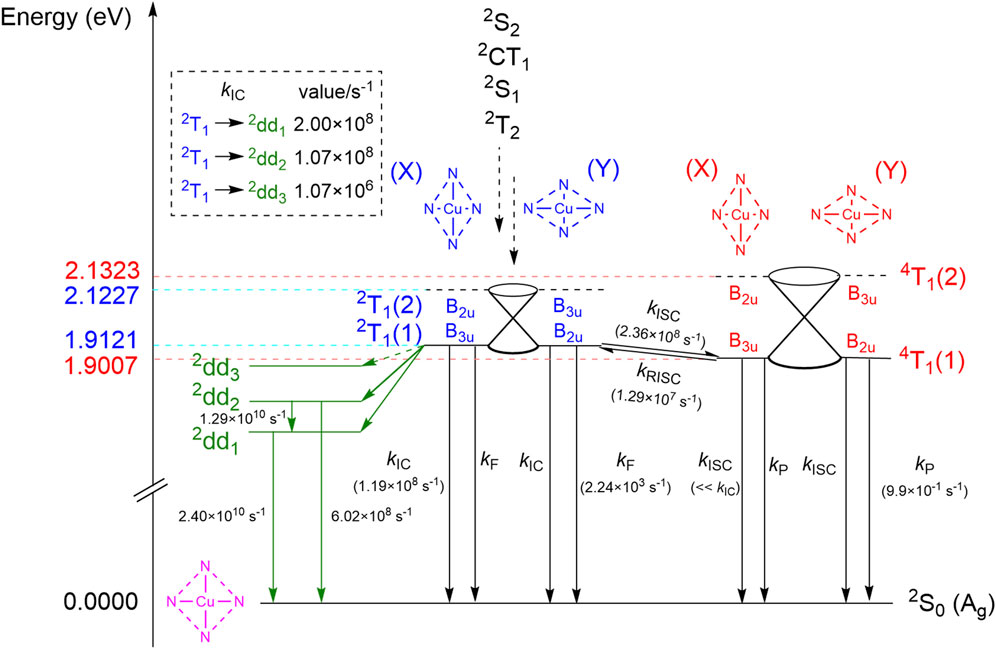
FIGURE 4. Radiative and non-radiative relaxation pathways of the 2T1 state. Both the 2T1 and 4T1 states are splitted by the Jahn-Teller effect to give two adiabatic states, labeled (1) and (2). Each of the (1) states have two equivalent D2h equilibrium structures, labeled (X) and (Y). The (2) states do not have equilibrium structures and are connected with the corresponding (1) states via conical intersections. The X-TDA(doublet)/U-TDA(quartet) adiabatic excitation energies of the (1) states, as well as the energies of the (2) states at the equilibrium geometries of their corresponding (1) states, are shown on the left. The transition rates are calculated at 83 K in the gas phase. The forward and reverse ISC rates between 2T1(1)(X) and 4T1(1)(Y) are equal to those between 2T1(1)(Y) and 4T1(1)(X) by symmetry, but the former ISC processes are omitted for clarity. Transition rates that are obviously equal by symmetry reasons are shown only once. Transitions out of the 2dd3 state are not shown since the quantum yield of the 2dd3 state is expected to be negligible based on the small 2T1 →2dd3 IC rate.
Before we discuss the quantitative values of transition rates, we first analyze the relevant electronic states from the viewpoint of point group symmetry. The equilibrium structures of the 2T1 and 4T1 states are both distorted owing to the Jahn-Teller effect, and possess only D2h symmetry, compared to the D4h symmetry of the ground state equilibrium structure. The implications are two-fold: the double degeneracy of the nT1(n = 2 or 4) state at the D4h geometry (where they both belong to the Eu irrep) is lifted to give two adiabatic states, hereafter termed the nT1(1) and nT1(2) states, respectively, where nT1(1) is the state with the lower energy; and the potential energy surface of the nT1(1) state has two chemically equivalent D2h minima, nT1(1)(X) and nT1(1)(Y), where different pairs of Cu-N bonds are lengthened and shortened (see the schematic depictions in Figure 4). Although nT1(1)(X) and nT1(1)(Y) are on the same adiabatic potential energy surface, their electronic wavefunctions represent different diabatic states, as they belong to the B3u and B2u irreps, respectively. The nT1(1)(X) structure is diabatically connected to nT1(2)(Y) (i.e., the nT1(2) state at the equilibrium structure of nT1(1)(Y)) via a D4h conical intersection, while nT1(1)(Y) is diabatically connected to nT1(2)(X) via the same conical intersection. Thus, the nT1(2)(X) and nT1(2)(Y) states are expected to undergo ultrafast IC from the D4h conical intersection, to give the nT1(1)(Y) and nT1(1)(X) states as the main products, respectively. The direct transition from nT1(2) to states other than nT1(1) can therefore be neglected. From the irreps of the electronic states, we conclude that certain ISC transitions are forbidden by spatial symmetry. These include the transitions between 2T1(1)(X) and 4T1(1)(X), between 2T1(1)(Y) and 4T1(1)(Y), and between any one of the 4T1(1) structures and 2S0. All IC and radiative transitions, plus the ISC transitions between 2T1(1)(X) and 4T1(1)(Y) as well as between 2T1(1)(Y) and 4T1(1)(X), are symmetry allowed. While symmetry forbidden ISC processes can still gain non-zero rates from the Herzberg-Teller effect, we deem that the rates are not large enough to have any noticeable consequences. On one hand, the two symmetry forbidden ISC pathways between the 2T1(1) and 4T1(1) states are overshadowed by the two symmetry allowed ones, so that the total ISC rate between 2T1(1) and 4T1(1) is undoubtedly determined by the latter alone. The ISC from 4T1(1) to 2S0, on the other hand, has to compete with the IC process from 2T1(1) to 2S0 in order to affect the quantum yield or the dominant relaxation pathway of the system noticeably, but the latter process is both spin-allowed and spatial symmetry-allowed, while the former is forbidden in both aspects. We therefore neglect all ISC rates whose Franck-Condon contributions are zero by spatial symmetry.
We then calculated the rate constants for all transitions between 2T1, 4T1 and 2S0 whose rates are non-negligible, by the TVCF method. The rates (Figure 4) were calculated at 83 K, the temperature used in the quantum yield studies of Eastwood and Gouterman (1969); the latter studies gave a luminescence quantum yield of 0.09, in a solvent mixture of diethyl ether, isopentane, dimethylformamide and ethanol. The accurate treatment of solvation effects is however complicated and beyond the scope of the paper, so that all transition rates were computed in the gas phase. Our calculated kISC from 2T1 to 4T1 (2.36 × 108s−1) is slightly smaller than the total IC rate from 2T1 to 2S0 as well as to the three 2dd states (4.27 × 108s−1), suggesting that treating the 2T1 and 4T1 states as a rapid equilibrium [as in, e.g., Ake and Gouterman (1969) and Bohandy and Kim (1980)] is not justified at least in the gas phase. The IC from 2T1 to 2dd3 is two orders of magnitudes slower than other non-radiative relaxation pathways of 2T1, and is therefore not considered viable in the remaining discussions. At 83 K, the RISC from 4T1 to 2T1 is 11% of the forward ISC rate. Both rates are in favorable agreement with the experimental values of Cu(II) protoporphyrin IX dimethyl ester in benzene, kISC = 1.6 × 109s−1 and kRISC = 5.6 × 108s−1, at room temperature (Kobayashi et al., 2008). Our computed ISC and RISC rates give a 2T1-to-4T1 equilibrium concentration ratio of 1:9.0 when all IC processes are neglected, but our kinetic simulation shows that the steady state concentration ratio is 1:50.7 when the latter are considered, further illustrating that treating the 2T1-4T1 interconversion as a fast equilibrium can lead to noticeable error. Nevertheless, the fluorescence rate of 2T1 still exceeds the phosphorescence rate of 4T1 by three orders of magnitude, which more than compensates for the low steady state concentration of 2T1. Similar conclusions could be derived from the rates reported in Ake and Gouterman (1969) (3.6 × 103s−1 and 8.3 × 10−1s−1, respectively), calculated from semiempirical exchange and SOC integrals and experimental absorption oscillator strengths, which agree surprisingly well with the rates that we obtained here. Kinetic simulation suggests that 99.2% of the total luminescence at this temperature is contributed by fluorescence, and only 0.8% is due to phosphorescence. This can be compared with the experimental finding by Bohandy and Kim (1980) that the phosphorescence of CuP at 86 K is observable as a minor 0–0 peak besides the 0–0 fluorescence peak, with a fluorescence to phosphorescence ratio of about 5:1 to 10:1 [as estimated from Figure 5 of Bohandy and Kim (1980)]; however note that this study was performed in a triphenylene solid matrix.
The total luminescence quantum yield is predicted by our kinetic simulations to be 5.3 × 10−6, four orders of magnitude smaller than the experimental quantum yield (0.09) in solution. We believe one possible reason is that the 2T1–4T1 gap of CuP is larger in solution than in the gas phase. This can already be seen from the experimental 2T1–4T1 0–0 gaps of CuP in solid matrices with different polarities: the 0–0 gap was measured in polymethylmethacrylate as 500 cm−1 (Smith and Gouterman, 1968), but 310–320 cm−1 in n-octane (Noort et al., 1976) and 267 cm−1 in triphenylene (Bohandy and Kim, 1980). Therefore, the 0–0 gap in the gas phase is probably smaller than 267 cm−1, and indeed, our X-TDA calculations predict an adiabatic 2T1–4T1 gap of 92 cm−1 in the gas phase. The larger 2T1–4T1 gap in solution compared to the gas phase is expected to introduce a Boltzmann factor of
Interestingly, U-TDA completely fails to reproduce the qualitative picture of Figure 4 and predicts a 2T1–4T1 adiabatic gap of the wrong sign (−276 cm−1), violating Hund’s rule. At first sight, this may seem surprising: since the U-TDA “tripdoublet state” is a mixture of the true tripdoublet state and the quartet state, the U-TDA 2T1 energy should lie in between the energies of the true 2T1 state and the 4T1 state, which means that the U-TDA 2T1–4T1 gap should be smaller than the X-TDA gap but still have the correct sign. However, the U-TDA 2T1 state is contaminated by the MS = 1/2 component of the 4T1 state (Eq. 5), while a spin flip-up U-TDA calculation of the 4T1 state gives its MS = 3/2 component. The two spin components obviously have the same energy in the exact non-relativistic theory and in all rigorous spin-adapted methods, but not in U-TDA, even when the ground state is not spin-contaminated (Li and Liu, 2012; Li and Liu, 2016b). This shows that the restoration of the degeneracy of spin multiplets by the random phase approximation (RPA) correction in X-TDA (Li and Liu, 2011) indeed leads to qualitative improvement of the excitation energies, instead of being merely a solution to a conceptual problem. It also shows that estimating the tripdoublet energy by extrapolating from the energies of the spin-contaminated tripdoublet and the quartet by, e.g., the Yamaguchi method (Soda et al., 2000) does not necessarily give a qualitatively correct estimate of the spin-pure tripdoublet energy. The inverted doublet-quartet gap introduces qualitative defects to the computed photophysics of CuP. Already when the doublet-quartet gap is zero, the Boltzmann factor is expected to raise the kRISC to 9.24 × 107s−1, reducing the ratio of phosphorescence in the total luminescence to 0.11%. Further raising the quartet to reproduce the U-TDA doublet-quartet gap will reduce the kISC to 1.42 × 106s−1, which reduces the ratio of phosphorescence to 0.0007%. These values are obviously in much worse agreement with the experiments (Bohandy and Kim, 1980).
Finally, we briefly comment on the luminescence lifetimes. The luminescence of CuP is known to decay non-exponentially (Smith and Gouterman, 1968), so its luminescence lifetime can only be approximately determined. The luminescence lifetime of CuP has been determined as 400 μs (Smith and Gouterman, 1968) at 80 K in polymethylmethacrylate, and a biexponential decay with lifetimes 155 and 750 μs was reported (Eastwood and Gouterman, 1969) at 78 K in methylphthalylethylglycolate. The same references also reported that the luminescence lifetimes of CuOEP and CuTPP are also within the 50–800 μs range. However, in a room temperature toluene solution the luminescence lifetimes of CuOEP and CuTPP were reported to be 115 and 30 ns, respectively (Liu et al., 1995), and a few nanoseconds in the gas phase (Ha-Thi et al., 2013). If we define the luminescence lifetime as the time needed for 1–1/e ≈ 63.2% of the luminescence to be emitted, then kinetic simulations from our X-TDDFT rate constants give a gas-phase luminescence lifetime of 5.7 ns at 83 K, which is much shorter than the low-temperature condensed phase results, but in reasonable agreement with the room-temperature solution phase and especially the gas-phase experimental results. However, using the ISC and RISC rate constants consistent with the U-TDA doublet-quartet gap, one obtains a lifetime of 2.4 ns, differing noticeably from the X-TDDFT result. Thus, our results suggest that X-TDDFT/X-TDA gives non-negligible corrections upon the luminescence lifetime of U-TDDFT/U-TDA, and also confirm that the discrepancy of the experimental and calculated quantum yields is probably due to suppression of the IC of 2T1 by the low temperature solvent.
3.4 Discussions
As mentioned in the Introduction, the simple orbital energy difference model based on a restricted open-shell determinant (Figure 1) predicts that the excitation energy of the lowest tripdoublet of any doublet molecule is at least the sum of the excitation energies of the first two excited states (as long as the ROKS ground state satisfies the aufbau rule). It therefore comes as a surprise that the lowest tripdoublet state of CuP (2T1) is only barely higher than the lowest excited state (2dd1) by 0.34 eV (Table 1), even though the ROKS ground state of CuP is indeed an aufbau state (Figure 5). This suggests a failure of the ROKS orbital energy difference model.
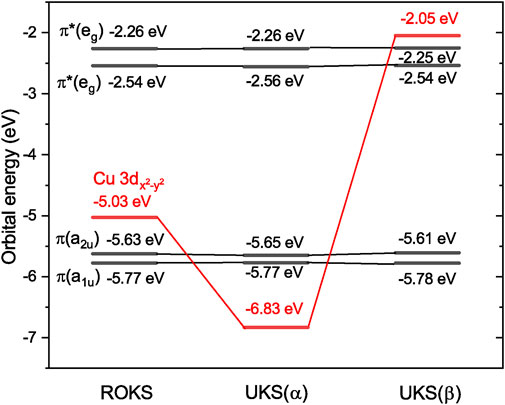
FIGURE 5. ROKS and UKS orbital energies of CuP at the X-TDDFT and U-TDDFT 2T1 equilibrium geometries, respectively, computed at the sf-X2C-PBE0/x2c-SVPall level of theory.
To understand why the ROKS orbital energies fail qualitatively for describing the excited state ordering of CuP, despite that the X-TDDFT method (which uses the ROKS determinant as the reference state) still gives reasonable excitation energies as compared to SDSPT2, we note that the α and β Fock matrices of an ROKS calculation are in general not diagonal under the canonical molecular orbital (CMO) basis. Only the unified coupling operator R, assembled from blocks of the CMO Fock matrices,
Is diagonal (Hirao and Nakatsuji, 1973). Note that herein we have used the Guest-Saunders parameterization (Guest and Saunders, 1974) of the diagonal blocks of R, which is the default choice of the BDF program, although our qualitative conclusions are unaffected by choosing other parameterizations. However, the leading term of the X-TDDFT calculation is not simply given by the eigenvalue differences of R,
but rather from the α and β Fock matrices themselves via
Here i, a represent occupied CMOs, j, b virtual CMOs, and σ, τ spin indices. For the diagonal matrix element of an arbitrary single excitation, Eqs 7, 8 differ by the following term:
where σ′ is the opposite spin of σ. For a general hybrid functional, the Fock matrix element differences in Eq. 9 are given by (where p is an arbitrary CMO, cx is the proportion of HF exchange, and vxc is the XC potential)
Assuming, for the sake of simplicity, that there is only one open-shell orbital t in the reference state. Assuming that the XC potential behaves similarly as the exact exchange potential, the difference Eq. 10 is positive, and should usually be the largest when p = t, while being small when p is spatially far from t. The corollary is that the orbital energy difference approximation Eq. 7 should agree well with the X-TDDFT leading term Eq. 8 for CV excitations (where the difference is proportional to the small exchange integral (pt|pt)), but underestimate the excitation energies of CO and OV excitations by a correction proportional to the large (tt|tt) integral.
The underestimation of CO and OV excitation energies by ROKS orbital energy differences opens up the possibility of engineering a system to break the ωia = ωit + ωta constraint inherent in the ROKS orbital energy difference model, and make the tripdoublet state the lowest excited state or only slightly higher than the lowest excited state. Possible approaches include:
1. Increase the difference Eq. 9 for the CO and OV states, while keeping it small for the lowest CV state, so that all CO and OV states are pushed above the lowest CV state. This is most easily done by making the open-shell orbital t very compact, which naturally leads to a larger Fttβ − Fttα (due to a larger (tt|tt)) but a smaller Fppβ − Fppα, p ∈ {i, a} (due to a small absolute overlap between the p and t orbitals).
2. Reduce the orbital energy gap between the highest doubly occupied orbital and the lowest unoccupied orbital, which also helps to reduce the excitation energy of the lowest CV state. However, a too small orbital energy gap will favor the IC of the tripdoublet to the ground state, which may quench the fluorescence of the tripdoublet. As already mentioned in Section 3.3, the IC rate of CuP is already large enough to make CuP only barely fluorescent (quantum yield
Now, it becomes evident that CuP fits the above design principles very well. The unpaired electron in the ground state of CuP is on the Cu 3
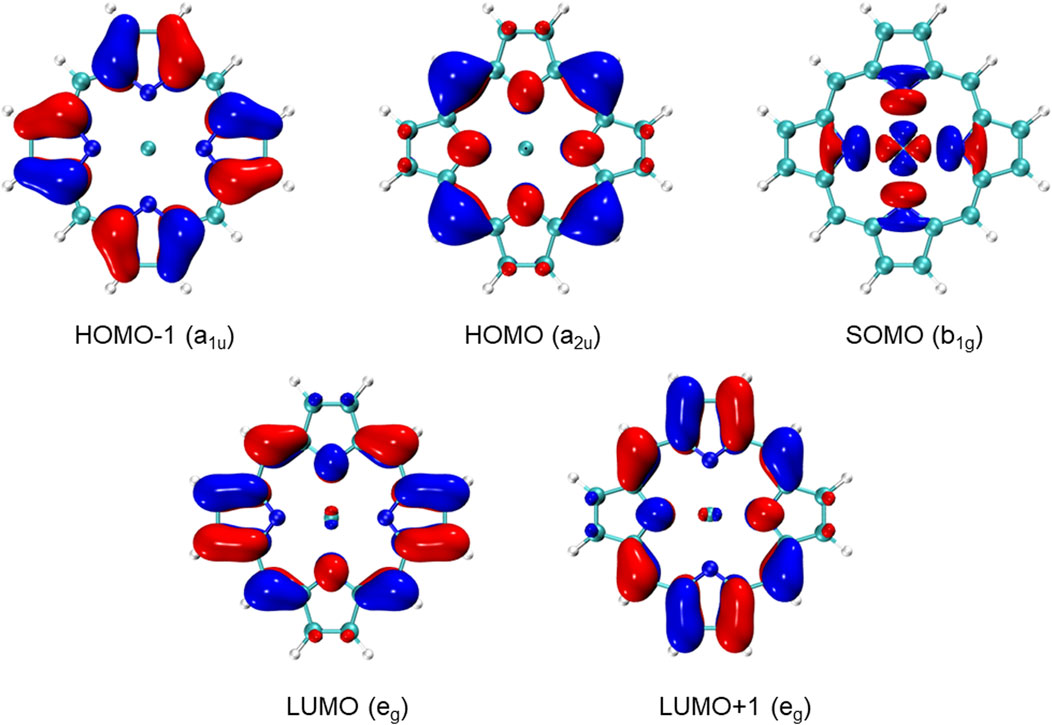
FIGURE 6. ROKS frontier molecular orbitals of CuP, computed at the sf-X2C-PBE0/x2c-SVPall level of theory.
Besides, we note that our SDSPT2 vertical absorption energy results suggest that three d-d excited states, 2dd1, 2dd2 and 2dd3, lie barely below the 2T1 state, although the actual energy ordering of 2dd3 and 2T1 cannot be said with full certainty. This means that CuP undergoes anti-Kasha fluorescence, which (as mentioned in the Introduction) is difficult to achieve especially for open-shell molecules. The 2dd states are very close in energy to the 2T1 state, strongly favoring an ultrafast IC process, while the 2dd states themselves relax radiationlessly to the ground state on the hundred picosecond timescale, mainly through the cascade 2dd2 →2dd1 →2S0 (Figure 4). In reality, however, our calculations suggest a 2T1 →2dd1 IC rate of only 2.00 × 108s−1 (with other 2T1 →2dd IC processes even slower), which although competitive with the 2T1 →2S0 IC rate, is very small for an IC process with an adiabatic gap of only 0.42 eV (the estimated SDSPT2 adiabatic excitation energies of the 2dd1, 2dd2, 2dd3 and 2T1 being 1.66, 1.90, 2.06 and 2.08 eV, respectively). The reason is that the 2T1 and 2dd states differ by a double excitation, while the nuclear derivative operator in the NACMEs is a one-electron operator, making the IC forbidden to first order. Moreover, to transform the 2T1 state to any of the 2dd states, one has to perform an LMCT excitation as well as an MLCT excitation (from α π*(eg) to α Cu 3
To conclude this section, we briefly note that even making the first doublet excited state a tripdoublet state still does not guarantee the realization of tripdoublet fluorescence. Two remaining potential obstacles are (1) the IC of the tripdoublet state to the ground state and (2) the ISC of the tripdoublet to the lowest quartet state (which is almost always lower than the lowest tripdoublet state owing to Hund’s rule). Both can be inhibited by making the molecule rigid, which is indeed satisfied by the porphyrin ligand in CuP. Alternatively, if the ISC from the first quartet state to the ground state is slow (as is the case of CuP, thanks to the spatial symmetry selection rules), and the gap between the first doublet and the first quartet is comparable to the thermal energy kT at the current temperature, then the quartet state can undergo RISC to regenerate the tripdoublet state, which can then fluoresce. This is well-known as the thermally activated delayed fluorescence (TADF) mechanism (Parker and Hatchard, 1961; Endo et al., 2011; Yang et al., 2017), although existing TADF molecules typically fluoresce from singlets and use a triplet “reservoir state” to achieve delayed fluorescence. In order for the TADF mechanism to outcompete the phosphorescence from the first quartet state, both the phosphorescence rate and the doublet-quartet gap have to be small. While the low phosphorescence rate of CuP can be explained by the fact that copper is a relatively light element, the small 2T1–4T1 gap of CuP can be attributed to the distributions of the frontier orbitals of CuP. Recall that the X-TDDFT gap between a tripdoublet excitation Eq. 3 and the associated quartet excitation Eq. 4 is exactly given by the X-RPA gap (Li and Liu, 2011), which is equal to
4 Conclusion
Fluorescence of open-shell molecules from tripdoublet states is a rare and underexplored phenomenon, for which traditional excited state methods such as U-TDDFT are unreliable due to severe spin contamination. In this work, we employed the high-precision method SDSPT2 to obtain accurate excitation energies of the CuP molecule, which suggests that the bright states obtained by light absorption relax to a low-lying doublet state, 2T1, via a cascade of ultrafast IC processes, in agreement with experiments. 2T1 is a tripdoublet state composed of a triplet ligand state antiferromagnetically coupled with the unpaired electron of Cu(II), and contrary to predictions from ROKS orbital energy differences, the only excited states lower than 2T1 are the d-d excited states 2dd1, 2dd2 and 2dd3, and the nonradiative relaxations from 2T1 to the 2dd states are slower than expected from their energy gaps, due to the large differences in excited state compositions. Using the SDSPT2 results as a benchmark, we found that the X-TDDFT method provides a more accurate description of the 2T1 state (which exhibits considerable spin contamination) compared to U-TDDFT, while for the CO excitations, U-TDDFT and X-TDDFT show similar performance.
In addition to vertical absorption calculations and structural analyses, we conducted a detailed analysis of the relaxation rate constants of the excited states of CuP. Our results suggest that, in the gas phase and at low temperature (83 K), CuP emits fluorescence from the lowest tripdoublet state 2T1 with a very small quantum yield (
Based on the computational results, we proposed a few possible approaches that can be used to design new doublet molecules that fluoresce from tripdoublets: 1) keep the open-shell orbital of the molecule spatially compact, to open up a gap between the α and β UKS orbital energies of the open-shell orbital; 2) make the gap between the highest doubly occupied orbital and the lowest vacant orbital small enough so that both orbitals fit into the gap between the α and β open-shell orbitals, but not overly small as to encourage IC of the lowest tripdoublet state to the ground state; 3) make the molecule rigid to minimize unwanted non-radiative relaxation processes; 4) avoid introducing heavy elements in order to suppress unwanted ISC and phosphorescence processes; 5) localize the open-shell orbital and the frontier π/π* orbitals onto different molecular fragments, and (if possible) make them belong to different irreps, to minimize the doublet-quartet gap; and 6) when the presence of doublet excited states below the tripdoublet is unavoidable, make those states differ significantly in composition from the tripdoublet state, to avoid the quenching of tripdoublet fluorescence by excited state-excited state IC. We hope that the present work will facilitate the discovery of novel molecules that fluoresce from tripdoublet states. Moreover, we expect that the success of the X-TDDFT and SDSPT2 methods will encourage the use of these two methods in the excited state studies of other systems.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Author contributions
XW: Data curation, Investigation, Writing–original draft, Software, Visualization. CW: Software, Funding acquisition, Methodology, Validation, Writing–review and editing. ZW: Methodology, Validation, Writing–review and editing, Conceptualization, Data curation, Investigation, Supervision, Writing–original draft. WL: Conceptualization, Funding acquisition, Resources, Supervision, Writing–review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant Nos 21833001, 21973054, 22101155), Mountain Tai Climbing Program of Shandong Province, and Key-Area Research and Development Program of Guangdong Province (Grant No. 2020B0101350001). ZW gratefully acknowledges generous financial support by the Max Planck society.
Acknowledgments
The authors acknowledge the computational software provided by the Institute of Scientific Computing Software in Shandong University and Qingdao BDF Software Technology Co., Ltd. The authors also acknowledge that an earlier version of this manuscript was submitted as a preprint on arXiv.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adamo, C., and Barone, V. (1999). Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170. doi:10.1063/1.478522
Ake, R. L., and Gouterman, M. (1969). Porphyrins XIV. Theory for the luminescent state in VO, Co, Cu complexes. Theor. Chim. Acta 15, 20–42. doi:10.1007/BF00526463
Becke, A. (1988). Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A At., Mol. Opt. Phys. 38, 3098–3100. doi:10.1103/PhysRevA.38.3098
Becke, A. (1993). Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652. doi:10.1063/1.464913
Bohandy, J., and Kim, B. F. (1980). Temperature dependence of Mg porphin, Cu porphin, and Pd porphin luminescence. J. Chem. Phys. 73, 5477–5481. doi:10.1063/1.440093
Bräm, O., Cannizzo, A., and Chergui, M. (2019). Ultrafast broadband fluorescence up-conversion study of the electronic relaxation of metalloporphyrins. J. Phys. Chem. A 123, 1461–1468. doi:10.1021/acs.jpca.9b00007
Chai, J.-D., and Head-Gordon, M. (2008). Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620. doi:10.1039/B810189B
Eastwood, D., and Gouterman, M. (1969). Porphyrins: XII. luminescence of copper complexes at liquid nitrogen temperature. J. Mol. Spectrosc. 30, 437–458. doi:10.1016/0022-2852(69)90276-8
Endo, A., Sato, K., Yoshimura, K., Kai, T., Kawada, A., Miyazaki, H., et al. (2011). Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes. Appl. Phys. Lett. 98, 083302. doi:10.1063/1.3558906
Ernzerhof, M., and Scuseria, G. E. (1999). Assessment of the perdew–burke–ernzerhof exchange-correlation functional. J. Chem. Phys. 110, 5029–5036. doi:10.1063/1.478401
Evans, D. F. (1957). 772. Magnetic perturbation of singlet–triplet transitions. Part II. J. Chem. Soc., 3885–3888. doi:10.1039/JR9570003885
Gao, S., Cui, Z., and Li, F. (2023). Doublet-emissive materials for organic light-emitting diodes: exciton formation and emission processes. Chem. Soc. Rev. 52, 2875–2885. doi:10.1039/D2CS00772J
Gouterman, M. (1959). Study of the effects of substitution on the absorption spectra of porphin. J. Chem. Phys. 30, 1139–1161. doi:10.1063/1.1730148
Gouterman, M., Mathies, R. A., Smith, B. E., and Caughey, W. S. (1970). Porphyrins. XIX. Tripdoublet and quartet luminescence in Cu and VO complexes. J. Chem. Phys. 52, 3795–3802. doi:10.1063/1.1673560
Green, S. A., Simpson, D. J., Zhou, G., Ho, P. S., and Blough, N. V. (1990). Intramolecular quenching of excited singlet states by stable nitroxyl radicals. J. Am. Chem. Soc. 112, 7337–7346. doi:10.1021/ja00176a038
Grimme, S., Antony, J., Ehrlich, S., and Krieg, H. (2010). A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104. doi:10.1063/1.3382344
Grimme, S., Ehrlich, S., and Goerigk, L. (2011). Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465. doi:10.1002/jcc.21759
Guest, M., and Saunders, V. R. (1974). On methods for converging open-shell Hartree-Fock wave-functions. Mol. Phys. 28, 819–828. doi:10.1080/00268977400102171
Guo, Y., Zhang, N., Lei, Y., and Liu, W. (2021). iCISCF: an iterative configuration interaction-based multiconfigurational self-consistent field theory for large active spaces. J. Chem. Theory Comput. 17, 7545–7561. doi:10.1021/acs.jctc.1c00781
Ha-Thi, M.-H., Shafizadeh, N., Poisson, L., and Soep, B. (2013). An efficient indirect mechanism for the ultrafast intersystem crossing in copper porphyrins. J. Phys. Chem. A 117, 8111–8118. doi:10.1021/jp4008015
He, C., Li, Z., Lei, Y., Zou, W., and Suo, B. (2019). Unraveling the emission mechanism of radical-based organic light-emitting diodes. J. Phys. Chem. Lett. 10, 574–580. doi:10.1021/acs.jpclett.8b03864
Hirao, K., and Nakatsuji, H. (1973). General scf operator satisfying correct variational condition. J. Chem. Phys. 59, 1457–1462. doi:10.1063/1.1680203
Humphrey, W., Dalke, A., and Schulten, K. (1996). VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38. doi:10.1016/0263-7855(96)00018-5
Kobayashi, T., Huppert, D., Straub, K. D., and Rentzepis, P. M. (2008). Picosecond kinetics of copper and silver protoporphyrins. J. Chem. Phys. 70, 1720–1726. doi:10.1063/1.437689
Lei, Y., Liu, W., and Hoffmann, M. R. (2017). Further development of SDSPT2 for strongly correlated electrons. Mol. Phys. 115, 2696–2707. doi:10.1080/00268976.2017.1308029
Lei, Y., Suo, B., and Liu, W. (2021). iCAS: imposed automatic selection and localization of complete active spaces. J. Chem. Theory Comput. 17, 4846–4859. doi:10.1021/acs.jctc.1c00456
Li, Z., and Liu, W. (2010). Spin-adapted open-shell random phase approximation and time-dependent density functional theory. I. Theory. J. Chem. Phys. 133, 064106. doi:10.1063/1.3463799
Li, Z., and Liu, W. (2011). Spin-adapted open-shell time-dependent density functional theory. III. An even better and simpler formulation. J. Chem. Phys. 135, 194106. doi:10.1063/1.3660688
Li, Z., and Liu, W. (2012). Theoretical and numerical assessments of spin-flip time-dependent density functional theory. J. Chem. Phys. 136, 024107. doi:10.1063/1.3676736
Li, Z., and Liu, W. (2014). First-order nonadiabatic coupling matrix elements between excited states: a Lagrangian formulation at the CIS, RPA, TD-HF, and TD-DFT levels. J. Chem. Phys. 141, 014110. doi:10.1063/1.4885817
Li, Z., and Liu, W. (2016a). Critical assessment of td-dft for excited states of open-shell systems: I. doublet-doublet transitions. J. Chem. Theory Comput. 12, 238–260. doi:10.1021/acs.jctc.5b01158
Li, Z., and Liu, W. (2016b). Critical assessment of time-dependent density functional theory for excited states of open-shell systems: II. doublet-quartet transitions. J. Chem. Theory Comput. 12, 2517–2527. doi:10.1021/acs.jctc.5b01219
Li, Z., Liu, W., Zhang, Y., and Suo, B. (2011). Spin-adapted open-shell time-dependent density functional theory. II. Theory and pilot application. J. Chem. Phys. 134, 134101. doi:10.1063/1.3573374
Li, Z., Suo, B., and Liu, W. (2014a). First order nonadiabatic coupling matrix elements between excited states: implementation and application at the TD-DFT and pp-TDA levels. J. Chem. Phys. 141, 244105. doi:10.1063/1.4903986
Li, Z., Suo, B., Zhang, Y., Xiao, Y., and Liu, W. (2013). Combining spin-adapted open-shell TD-DFT with spin-orbit coupling. Mol. Phys. 111, 3741–3755. doi:10.1080/00268976.2013.785611
Li, Z., Xiao, Y., and Liu, W. (2014b). On the spin separation of algebraic two-component relativistic Hamiltonians: molecular properties. J. Chem. Phys. 141, 054111. doi:10.1063/1.4891567
Liu, F., Cunningham, K. L., Uphues, W., Fink, G. W., Schmolt, J., and McMillin, D. R. (1995). Luminescence quenching of copper (ii) porphyrins with Lewis bases. Inorg. Chem. 34, 2015–2018. doi:10.1021/ic00112a011
Liu, W., and Hoffmann, M. R. (2014). SDS: the ’static-dynamic-static’ framework for strongly correlated electrons. Theor. Chem. Acc. 1, 12–1512. doi:10.1007/s00214-014-1481-x
Liu, W., Hong, G., Dai, D., Li, L., and Dolg, M. (1997). The Beijing four-component density functional program package (BDF) and its application to EuO, EuS, YbO and YbS. Theor. Chem. Acc. 96, 75–83. doi:10.1007/s002140050207
Liu, W., Wang, F., and Li, L. (2003). The Beijing density functional (BDF) program package: methodologies and applications. J. Theor. Comput. Chem. 2, 257–272. doi:10.1142/S0219633603000471
Liu, W., Wang, F., and Li, L. (2004a). “Recent advances in relativistic density functional methods,” in Encyclopedia of computational Chemistry. Editors P. von Ragué Schleyer, N. L. Allinger, T. Clark, J. Gasteiger, P. A. Kollman, and H. F. Schaefer (Chichester, UK: Wiley).
Liu, W., Wang, F., and Li, L. (2004b). “Relativistic density functional theory: the BDF program package,” in Recent advances in relativistic molecular theory. Editors K. Hirao, and Y. Ishikawa (Singapore: World Scientific), 257–282.
Lu, T., and Chen, F. (2012). Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592. doi:10.1002/jcc.22885
Magde, D., Windsor, M. W., Holten, D., and Gouterman, M. (1974). Picosecond flash photolysis: transient absorption in Sn(IV), Pd(II), and Cu(II) porphyrins. Chem. Phys. Lett. 29, 183–188. doi:10.1016/0009-2614(74)85008-6
Neese, F. (2018). Software update: the ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 8, e1327. doi:10.1002/wcms.1327
Neese, F. (2022). Software update: the ORCA program system—version 5.0. WIREs Comput. Mol. Sci. 12, e1606. doi:10.1002/wcms.1606
Neese, F., Wennmohs, F., Becker, U., and Riplinger, C. (2020). The ORCA quantum chemistry program package. J. Chem. Phys. 152, 224108. doi:10.1063/5.0004608
Niu, Y., Li, W., Peng, Q., Geng, H., Yi, Y., Wang, L., et al. (2020). Molecular materials property prediction package (MOMAP) 1.0: a software package for predicting the luminescent properties and mobility of organic functional materials. Mol. Phys. 116, 1078–1090. doi:10.1080/00268976.2017.1402966
Niu, Y., Peng, Q., and Shuai, Z. (2008). Promoting-mode free formalism for excited state radiationless decay process with Duschinsky rotation effect. Sci. China Chem. B 51, 1153–1158. doi:10.1007/s11426-008-0130-4
Noort, M., Jansen, G., Canters, G., and van der Waals, J. (1976). High resolution spectra of palladium, platinum and copper porphins in n-octane crystals. Spectrochim. Acta A Mol. Spectrosc. 32, 1371–1375. doi:10.1016/0584-8539(76)80183-3
Parker, C. A., and Hatchard, C. G. (1961). Triplet-singlet emission in fluid solutions. phosphorescence of eosin. Trans. Faraday Soc. 57, 1894–1904. doi:10.1039/TF9615701894
Peng, Q., Yi, Y., Shuai, Z., and Shao, J. (2007). Excited state radiationless decay process with Duschinsky rotation effect: formalism and implementation. J. Chem. Phys. 126, 114302. doi:10.1063/1.2710274
Perdew, J. (1986a). Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B Condens. Matter Mater. Phys. 33, 8822–8824. doi:10.1103/physrevb.33.8822
Perdew, J. (1986b). Erratum: density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B Condens. Matter Mater. Phys. 34, 7406. doi:10.1103/PhysRevB.34.7406
Pollak, P., and Weigend, F. (2017). Segmented contracted error-consistent basis sets of double-and triple-ζ valence quality for one-and two-component relativistic all-electron calculations. J. Chem. Theory Comput. 13, 3696–3705. doi:10.1021/acs.jctc.7b00593
Schmidt, R. (2006). Photosensitized generation of singlet oxygen. Photochem. Photobiol. 82, 1161–1177. doi:10.1562/2006-03-03-lR-833
Smith, B., and Gouterman, M. (1968). Quartet luminescence from copper porphyrins. Chem. Phys. Lett. 2, 517–519. doi:10.1016/0009-2614(63)80001-9
Soda, T., Kitagawa, Y., Onishi, T., Takano, Y., Shigeta, Y., Nagao, H., et al. (2000). Ab initio computations of effective exchange integrals for H–H, H–He–H and Mn2O2 complex: comparison of broken-symmetry approaches. Chem. Phys. Lett. 319, 223–230. doi:10.1016/s0009-2614(00)00166-4
Song, Y., Guo, Y., Lei, Y., Zhang, N., Liu, W., and Qi, J. (2022). The static–dynamic–static family of methods for strongly correlated electrons: methodology and benchmarking. Top. Curr. Chem. 19, 181–187. doi:10.2174/1567202619666220614153209
Stephens, P. J., Devlin, F. J., Chabalowski, C. F., and Frisch, M. J. (1994). Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627. doi:10.1021/j100096a001
Varnes, A. W., Dodson, R. B., and Wehry, E. (1972). Interactions of transition-metal ions with photoexcited states of flavines. fluorescence quenching studies. J. Am. Chem. Soc. 94, 946–950. doi:10.1021/ja00758a037
Wang, Z., Li, Z., Zhang, Y., and Liu, W. (2020). Analytic energy gradients of spin-adapted open-shell time-dependent density functional theory. J. Chem. Phys. 153, 164109. doi:10.1063/5.0025428
Wang, Z., Wu, C., and Liu, W. (2021). NAC-TDDFT: time-dependent density functional theory for nonadiabatic couplings. Acc. Chem. Res. 54, 3288–3297. doi:10.1021/acs.accounts.1c00312
Yan, X., and Holten, D. (1988). Effects of temperature and solvent on excited-state deactivation of copper (II) octaethyl-and tetraphenylporphyrin: relaxation via a ring-to-metal charge-transfer excited state. J. Phys. Chem. 92, 5982–5986. doi:10.1021/j100332a029
Yang, Z., Mao, Z., Xie, Z., Zhang, Y., Liu, S., Zhao, J., et al. (2017). Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 46, 915–1016. doi:10.1039/C6CS00368K
Keywords: tripdoublet, fluorescence, photophysics, spin-adapted TDDFT, SDSPT2
Citation: Wang X, Wu C, Wang Z and Liu W (2023) When do tripdoublet states fluoresce? A theoretical study of copper(II) porphyrin. Front. Chem. 11:1259016. doi: 10.3389/fchem.2023.1259016
Received: 14 July 2023; Accepted: 23 October 2023;
Published: 10 November 2023.
Edited by:
Jose Luis Cabellos, Polytechnic University of Tapachula, MexicoReviewed by:
Christof Holzer, Karlsruhe Institute of Technology (KIT), GermanyGerardo Martinez-Guajardo, Universidad Autónoma de Zacatecas, Mexico
Copyright © 2023 Wang, Wu, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zikuan Wang, endhbmdAa29mby5tcGcuZGU=; Wenjian Liu, bGl1d2pAc2R1LmVkdS5jbg==
 Xingwen Wang1
Xingwen Wang1 Chenyu Wu
Chenyu Wu Zikuan Wang
Zikuan Wang