- 1Department of Basic Courses, China Fire and Rescue Institute, Beijing, China
- 2Yangtze Delta Region Institute, University of Electronic Sciences and Technology of China, Huzhou, China
- 3Institute of Fundamental and Frontiers Sciences, University of Electronic Sciences and Technology of China, Chengdu, China
- 4Administration for Market Regulation of Zhengding, Shijiazhuang, China
1 Introduction
The progress of human civilization depends on the development of all kinds of materials. The establishment of modern science has led to the rapid development of synthetic materials. However, increasing energy demand and environmental pollution urgently require the search for new materials to solve the energy and environmental crisis.
Carbon is an extremely abundant element in nature and provides the basis for all life on Earth (Li et al., 2008; Toth et al., 2016). The carbon atom has six electrons outside the nucleus, and its outermost electron arrangement is 2s22p2, which shows a strong ability to form covalent bonds (Krueger, 2010). Porous carbon materials have advantages such as chemical stability, low density, high thermal conductivity, high electrical conductivity, and high mechanical strength (Gallo, 2017). Porous carbon materials also have a large specific surface area, adjustable pore size, and functional groups and can be prepared from a wide range of precursors at relatively low cost.
In recent years, a large number of researchers have been devoted to the synthesis and application of porous carbon (Ang, 2019; Liu, 2019; Liu, 2020a; Hwang, 2020; Raj, 2021). Depending on the pore size distribution, the pore structure of carbon materials can be divided into three categories, namely, micropores (pore size <2 nm), mesopores (2 nm < pore size <50 nm), and macropores (pore size >50 nm) (Vu, 2012). The size of the pore structure of porous carbon materials has a significant impact on their performance in practical applications.
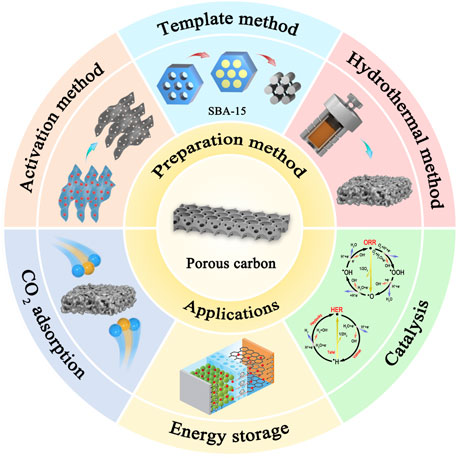
Due to these advantages, carbon materials are widely used in the fields of adsorption (He, 2019), catalysis (Dong et al., 2020), and energy storage (Peng, 2019). This paper mainly introduces the synthesis and application of carbon materials and describes the main improvement ideas for current carbon materials (Figure 1). Importantly, the future direction of carbon materials is further discussed.
2 Synthesis methods of carbon materials
2.1 Activation method
Many designs are currently focused on how to increase the specific surface area of carbon materials, which include heat treatment, physical activation, and chemical activation. The physical activation method refers to the use of CO2, water vapor, O2, and other gases as activation media for the preparation of activated carbon under high-temperature conditions (Sevilla, 2014; Rashidi, 2019; Ma, 2020). The CO2 molecules move at a slower rate thermally compared to water vapor activation, resulting in a larger specific surface area and a higher volume of microporous material. However, the small size and low activation of the activating molecules with a single activator result in a large number of blind pores and an ineffective specific surface area of the carbon material (Kim, 2017).
Chemical activation is the reaction of chemical reagents with the carbon source during pyrolysis, commonly known as KOH, NaOH, H3PO4, and ZnCl2 (Molina, 1995; Lillo, 2003). The main factors influencing the preparation of porous carbon materials by chemical activation include the composition of the precursor material, the activation temperature, the activator, and the impregnation ratio (Maia, 2018). A high impregnation ratio (raw material/activator) allows the formation of activated carbon with a high specific surface area, usually between 1:1 and 5:1 (Rashidi, 2016). KOH is the most commonly used activator, and the pore structure of the prepared material is well developed. Prasankumar converted Tasmanian blue gum tree bark into activated carbons through a simple KOH activationand carbonization method. The generated activated carbons have a hierarchically connected mesoporous structure and a surface area of 971 m2 g-1 with an average pore size of 2.2 nm (Thibeorchews, 2022).
Activated carbon is mainly derived from various organic precursors rich in carbon (bitumen, coal, polymers, etc.). Due to the environmentally unfriendly nature of fossil fuels, a large number of activated carbons prepared from biomass as precursors have attracted a great deal of attention in recent years. They are prepared from natural substances such as walnut shell powder (Pang, 2016; Jiang, 2022), banana stem fibers (Taer, 2021), American poplar fruit (Kumar, 2020), bamboo (Khuong, 2022), castor seed (Neme, 2022), and lotus seed shell (Hu, 2018), synthesizing activated carbons with a high specific surface area.
2.2 Template method
Specific surface area and pore size distribution are key factors that affect the properties of carbon materials. The template method is considered to be an effective method for achieving controlled mesoporous structures. The template method can be divided into the hard template method and the soft-template method. The former involves thorough mixing of the hard template with the precursor materials, carbonization, and subsequent removal of the template. The synthesis of porous carbon materials using various structural silica templates, such as SBA-15 (Yan, 2020) and 3D cubic KIT-6 (Karthikeyan, 2022), has been reported in recent years. The pore size and pore volume of the obtained porous carbon materials can be systematically adjusted by changing the size of the template (Sang, 2011). In addition, porous carbon can also be prepared using CaCO3, MgO, Mg(OH)2, magnesium acetate, magnesium citrate, or magnesium gluconate as templates (Wei, 2019; Zhou, 2020), which can be subsequently removed with dilute hydrochloric acid. Liu found a new solvent-free method for the preparation of mesoporous carbon using mesoporous silica KIT-6 as a hard template for the preparation of mesoporous carbon, overcoming the disadvantages of the conventional filling process (Liu, 2019).
The soft-template method involves the self-assembly and co-condensation of a soft template with a precursor to produce a material with a specific structure (Zhang, 2009). The advantages of the soft-template method are that the template does not require subsequent processing and the experimental steps are simple and environmentally friendly. Soft templates include various triblock copolymers, such as F127 and F108. Peng prepared a unique hierarchical porous N-, O-, and S-enriched carbon foam via a combination of a soft-template method, freeze-drying, and chemical etching (Peng, 2019). The structure offers not only an ultra-high specific surface area but also a network of multiple-scale channels.
2.3 Hydrothermal carbonization method
Hydrothermal carbonization is a process in which carbon precursors are gradually hydrolyzed, dehydrated, condensed, and aromatized under high temperature and pressure using water as a solvent and eventually converted into carbon materials. This method is milder and allows for autonomous control of product morphology and better regulation of pore size distribution. In the hydrothermal carbonization process, there are many factors that influence the properties of the carbon material, such as the hydrothermal temperature, the rate of temperature increase, and the holding time. The specific surface area of the carbon material produced is generally low, and the pores are not well developed, so it is often used in combination with activation to obtain porous carbon materials with a high specific surface area.
Liu prepared nitrogen-doped porous carbon materials with a specific surface area of up to 2,864 m2 g-1 and a total pore volume of 1.6 cm3 g-1 by hydrothermal treatment of biomass raw materials and the addition of an activator, KOH, to the aqueous solution, followed by high-temperature pyrolysis and activation (Liu, 2016). Veltri prepared a nitrogen–oxygen co-doped biomass-based carbon material by hydrothermal charring of orange juice with a specific surface area of 1,725 m2 g-1 (Veltri, 2020). The pore structure tended to be reasonable, and the mass fractions of nitrogen and oxygen were as high as 5.65% and 5.38%
3 Applications
3.1 Application of porous carbons in adsorption
The International Panel on Climate Change (IPCC) report shows that the atmospheric concentrations of greenhouse gases (mainly CO2) continue to increase (Lunn, 2021). Porous carbon has a wide range of sources, stable physical and chemical properties, and fast adsorption and desorption rates. It is a kind of CO2 adsorption material with great potential for application.
The carbonized coconut shell was modified with urea at 350°C and activated with K2CO3 to produce a nitrogen-doped carbon material with good CO2 adsorption properties (Yue, 2018). He used polydopamine and melamine as carbon sources and CaCO3 nanoparticles as templating agents to synthesize porous carbon materials, which showed high CO2 adsorption capacity and selectivity at room temperature (He, 2019). Pluronic P123 as a soft template was polymerized with D-glucose by the hydrothermal method and activated by CO2 to produce a porous adsorbent with high microporous content (Nicolae, 2020). The CO2/N2 adsorption selectivity of 9 was achieved at 6.00 mmol g-1.
3.2 Application of porous carbon in energy storage
In order to mitigate climate change and environmental pollution caused by excessive use of fossil energy, clean and sustainable alternative energy sources are urgently needed around the world (Weigelt, 2016; Azcárate, 2017). In the past decades, a large number of researchers have been devoted to the development of new types of energy storage (Gondal, 2017; McKone, 2017). Carbon is widely used in energy storage and has the advantages of a large specific surface area, well-developed pores, good electrical conductivity, good electrolyte wetting, high chemical stability, and a wide potential window (Luo. 2020; Kim, 2021). The electrochemical properties of porous carbon electrode materials are a key factor affecting their energy storage properties.
Porous carbon materials are often used as anodes in batteries due to their good electrolyte wetting. The formation of uniform and elastic solid electrolyte interphase (SEI) or the use of tailored electrolytes can improve the stability of the SEI on the carbon surface, thereby increasing the safety and cyclability of the batteries (Fan, 2023; Gu, 2023). The porous carbon used as an electrode in a supercapacitor achieves a high specific capacitance and superior rate capability. Sun prepared nitrogen and sulfur co-doped carbon materials by chemical vapor deposition using magnesium hydroxide as a template, which had a large specific surface area (674 m2 g-1) and a porous interleaved network with a high level of heteroatom doping (Miao, 2019). As an anode material for Li-ion batteries, it shows a very high reversible capacity and excellent cycling stability.
3.3 Application of porous carbons in catalysis
The development of highly selective, catalytic, stable, green, and economically accessible catalysts is extremely important in industrial production. Carbon materials are often used as catalysts in CO2 electroreduction, oxygen reduction reactions (ORRs), and hydrogen evolution reactions (HERs) due to their abundance of sources and their excellent chemical properties.
Chen proposed an NH3 heat treatment strategy to completely remove pyrrole nitrogen and pyridine nitrogen dopants, and the prepared porous carbon material could efficiently electroreduce CO2, achieving a CO Faraday efficiency of 95.2% at a current density of −2.84 mA cm-2 (Dong et al., 2020). Saravanan treated peanut shells using a simple pyrolysis technique assisted by chemical activation and explored the HER properties of peanut shell-derived carbon nanosheets in acidic media (Saravanan, 2019). The nitrogen-doped carbon nanosheets with a high specific surface area and a large number of active sites showed excellent HER catalytic activity in aqueous electrolysis devices (Liu, 2020b). Lai synthesized two-dimensional porous disordered-layer carbon nanowebs with a large number of N-doped C defects using an aromatic ring as the carbon source and urea as the nitrogen source in a novel molecular design strategy (Lai, 2020). It was shown that carbon edge defects doped with graphitic N atoms could lead to materials exhibiting excellent ORR catalytic properties.
4 Discussions
Due to their high specific surface area, tunable physicochemical properties, low cost, and accessibility, porous carbon materials have shown a wide range of applications in areas such as catalysts, adsorbents, and energy storage. Previously, various methods were used to produce porous carbon materials with different pore structures, but most of the preparation was carried out in the laboratory with complex methods and processes, which made it difficult to meet the requirements of industrial preparation. Moreover, in order to regulate and optimize the structure of porous carbon materials at a more microscopic level and to point the way to the design and preparation of materials, further research on the mechanisms of action of carbon materials in their applications is needed. Thus, with the increasing energy shortage and environmental pollution, the further enhancement of the development of simple, clean, and cost-effective porous carbon materials could make a huge difference in a wider range of areas.
Author contributions
MN conceived and designed the entire review and wrote the manuscript. LZ, YL, and RN edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Sichuan Science and Technology Program (No. 2023NSFSC0972).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ang, F., Wang, C. Z., Pei, F., Cui, J. Q., Fang, X. L., and Zheng, N. F. (2019). Recent advances in hollow porous carbon materials for lithium-sulfur batteries. Small 15, 1804786. doi:10.1002/smll.201804786
Azcárate, C., Mallor, F., and Mateo, P. (2017). Tactical and operational management of wind energy systems with storage using a probabilistic forecast of the energy resource. Renew. Energy 102, 102445–102456. doi:10.1016/j.renene.2016.10.064
Dong, Y., Zhang, Q., Tian, Z., Li, B., Yan, W., Wang, S., et al. (2020). Ammonia thermal treatment toward topological defects in porous carbon for enhanced carbon dioxide electroreduction. Adv. Mater. 32 (28), 2001300. doi:10.1002/adma.202001300
Fan, L., Xie, H., Hu, Y., Cai, Z., Apparao, M. R., Zhou, J., et al. (2023). A tailored electrolyte for safe and durable potassium ion batteries. Energy Environ. Sci. 16, 305–315. doi:10.1039/D2EE03294E
Gallo, S. C., Li, X. Y., Fütterer, K., Charitidis, C. A., and Dong, H. (2017). Carbon nanofibers functionalized with active screen plasma-deposited metal nanoparticles for electrical energy storage devices. ACS Appl. Mater. Interfaces 9, 23195–23201. doi:10.1021/acsami.7b05567
Gondal, I. A., Masood, S. A., and Amjad, M. (2017). Review of geothermal energy development efforts in Pakistan and way forward. Renew. Sustain. Energy Rev. 71, 687–696. doi:10.1016/j.rser.2016.12.097
Gu, M., Apparao, M. R., Zhou, J., and Lu, B. (2023). In situ formed uniform and elastic SEI for high-performance batteries. Energy Environ. Sci. 16, 1166–1175. doi:10.1039/D2EE04148K
He, X., Liu, J., Jiang, Y., Yassen, M., Guan, H., Sun, J., et al. (2019). ‘Useful’template synthesis of N-doped acicular hollow porous carbon/carbon-nanotubes for enhanced capture and selectivity of CO2. Chem. Eng. J. 361, 278–285. doi:10.1016/j.cej.2018.12.031
Hu, L., Zhu, Q., Wu, Q., Li, D., An, Z., and Xu, B. (2018). Natural biomass-derived hierarchical porous carbon synthesized by an in situ hard template coupled with NaOH activation for ultrahigh rate supercapacitors. ACS Sustain. Chem. Eng. 6, 13949–13959. doi:10.1021/acssuschemeng.8b02299
Hwang, J., Aleksander, E., Ralph, F., Joanna, G., Bernhard, V. K., and Schmidt, J. (2020). Controlling the morphology of metal–organic frameworks and porous carbon materials: Metal oxides as primary architecture-directing agents. Chem. Soc. Rev. 49, 3348–3422. doi:10.1039/c9cs00871c
Jiang, Y., Chen, J., Zeng, Q., Zou, Z., Li, J., Zeng, L., et al. (2022). Facile method to produce sub-1 nm pore-rich carbon from biomass wastes for high performance supercapacitors. J. Colloid Interface Sci. 612, 213–222. doi:10.1016/j.jcis.2021.12.144
Karthikeyan, G. G., Ramani, V., Muthusankar, E., and Pandurangan, A. (2022). Simple and efficient CVD synthesis of graphitic P-doped 3D cubic ordered mesoporous carbon at low temperature with excellent supercapacitor performance. Adv. Powder Technol. 33 (3), 103439. doi:10.1016/j.apt.2022.103439
Khuong, D. A., Trinh, K. T., Nakaoka, Y., Tsubota, T., Tashima, D., Nguyen, H. N., et al. (2022). The investigation of activated carbon by K2CO3 activation: Micropores-and macropores-dominated structure. Chemosphere 299, 134365. doi:10.1016/j.chemosphere.2022.134365
Kim, D., Kil, H., Nakabayashi, K., Yoon, S., and Miyawaki, J. (2017). Structural elucidation of physical and chemical activation mechanisms based on the microdomain structure model. Carbon 114, 98–105. doi:10.1016/j.carbon.2016.11.082
Kim, J., Park, J., Lee, J., Lim, W., Jo, C., and Lee, J. (2021). Biomass-derived P,N self doped hard carbon as bifunctional oxygen electrocatalyst and anode material for seawater batteries. Adv. Funct. Mater. 31 (22), 2010882. doi:10.1002/adfm.202010882
Krueger, A. (2010). Carbon material and nanotechnology. Hoboken, New Jersey, United States: John Wiley & Sons.
Kumar, T. R., Raja, A., Pan, Z., Pan, J., and Sun, Y. A. (2020). Tubular-like porous carbon derived from waste American poplar fruit asadvanced electrode material for high-performance supercapacitor. J. Energy Storage 32, 101903–101914. doi:10.1016/j.est.2020.101903
Lai, Q. X., Zheng, J., Tang, Z. M., Bi, D., Zhao, J. X., and Liang, Y. Y. (2020). Optimal configuration of N-doped carbon defects in 2D turbostratic carbon nanomesh for advanced oxygen reduction electrocatalysis. Angew. Chem. Int. Ed. 59, 11999–12006. doi:10.1002/ange.202000936
Li, X., Zhang, G., Bai, X., Sun, X., Wang, X., Wang, E., et al. (2008). Highly conducting graphene sheets and Langmuir-Blodgett films. Nat. Nanotech 3, 538–542. doi:10.1038/nnano.2008.210
Lillo, R. M., Cazorla, A. D., and Linares, S. A. (2003). Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon 41 (2), 267–275. doi:10.1016/S0008-6223(02)00279-8
Liu, B., Zhou, X. H., Chen, H. B., Liu, Y. J., and Li, H. M. (2016). Promising porous carbons derived from lotus seedpods with outstanding supercapacitance performance. Electrochimica Acta 208, 55. doi:10.1016/j.electacta.2016.05.020
Liu, H. G., Wu, S. Q., Tian, N., Yan, F. X., You, C. Y., and Yang, Y. (2020a). Carbon foams: 3D porous carbon materials holding immense potential. J. Mater. Chem. A 8, 23699–23723. doi:10.1039/D0TA08749A
Liu, S., Wang, Z., Han, T., Fei, T., Zhang, T., and Zhang, H. Y. (2019). Solvent-free synthesis of mesoporous carbon employing KIT-6 as hard template for removal of aqueous Rhodamine B. J. Porous Mater. 26 (4), 941–950. doi:10.1007/s10934-018-0692-2
Liu, Z., Zhou, Q. L., Zha, B., Li, S. L., Xiong, Y. Q., and Xu, W. J. (2020b). Few-layer N-doped porous carbon nanosheets derived from corn stalks as a bi-functional electrocatalyst for overall water splitting. Fuel 280, 118567. doi:10.1016/j.fuel.2020.118567
Lunn, J., and Peeva, N. (2021). Communications in the IPCC’s sixth assessment report cycle. Clim. Change 169 (1), 1–10. doi:10.1007/s10584-021-03233-7
Luo, X., Yang, Q., Dong, Y., Huang, X., Kong, D., Wang, B., et al. (2020). Maximizing pore and heteroatom utilization within N, P codoped polypyrrole-derived carbon nanotubes for high performance supercapacitors. J. Mater. Chem. A 8 (34), 17558–17567. doi:10.1039/D0TA06238C
Ma, M., Ying, H., Cao, F., Wang, Q., and Ai, N. (2020). Adsorption of Congo red on mesoporous activated carbon prepared by CO2 physical activation. Chin. J. Chem. Eng. 28 (4), 1069–1076. doi:10.1016/j.cjche.2020.01.016
Maia, D., Oliveira, D., Nazzarro, M., Sapag, K., Lopez, R., Lucena, S., et al. (2018). CO2 gas-adsorption calorimetry applied to the study of chemically activated carbons. Chem. Eng. Res. Des. 136, 753–760. doi:10.1016/j.cherd.2018.06.034
McKone, J. R., DiSalvo, F. J., and Abruña, H. D. (2017). Solar energy conversion, storage, and release using an integrated solar-driven redox flow battery. J. Mater. Chem. A 5, 5362–5372. doi:10.1039/C7TA00555E
Miao, X., Sun, D. F., Zhou, X. Z., and Lei, Z. Q. (2019). Designed formation of nitrogen and sulfur dual-doped hierarchically porous carbon for long-life lithium and sodium ion batteries. Chem. Eng. J. 364, 208–216. doi:10.1016/j.cej.2019.01.158
Molina, S. M., Rodriguez, R. F., Caturla, F., and Selles, M. (1995). Porosity in granular carbons activated with phosphoric acid. Carbon 33 (8), 1105–1113. doi:10.1016/0008-6223(95)00059-M
Neme, I., Gonfa, G., and Masi, C. (2022). Preparation and characterization of activated carbon from castor seed hull by chemical activation with H3PO4. Results Mater 15, 100304. doi:10.1016/j.rinma.2022.100304
Nicolae, S. A., Szilágyi, P. Á., and Titirici, M. M. (2020). Soft templating production of porous carbon adsorbents for CO2 and H2S capture. Carbon 169, 193–204. doi:10.1016/j.carbon.2020.07.064
Pang, L. Y., Zou, B., Han, X., Cao, L. Y., Wang, W., and Guo, Y. P. (2016). One-step synthesis of high-performance porous carbon from corn starch for supercapacitor. Mater. Lett. 184, 88–91. doi:10.1016/j.matlet.2016.07.147
Peng, H., Yao, B., Wei, X., Liu, T., Kou, T., Xiao, P., et al. (2019). Pore and heteroatom engineered carbon foams for supercapacitors. Adv. Energy Mater. 9, 1803665. doi:10.1002/aenm.201803665
Raj, C., Manikandan, R., Rajesh, M., Sivakumar, P., Jung, H., Das, S., et al. (2021). Corn husk mesoporous activated carbon electrodes and seawater electrolyte: The sustainable sources for assembling retainable supercapacitor module. J. Power Sources 490, 229518. doi:10.1016/j.jpowsour.2021.229518
Rashidi, N. A., and Yusup, S. (2019). Production of palm kernel shell-based activated carbon by direct physical activation for carbon dioxide adsorption. Environ. Sci. Pollut. Res. 26 (33), 33732–33746. doi:10.1007/s11356-018-1903-8
Rashidi, N., and Yusup, S. (2016). An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J. CO2 Util. 13, 1–16. doi:10.1016/j.jcou.2015.11.002
Sang, L. C., Vinu, A., and Coppens, M. O. (2011). Ordered mesoporous carbon with tunable, unusually large pore size and well-controlled particle morphology. J. Mater. Chem. 21 (20), 7410–7417. doi:10.1039/C1JM10683J
Saravanan, K., Peabu, N., Sasidharanm, , and Maduraiveeran, G. (2019). Nitrogen-self doped activated carbon nanosheets derived from peanut shells for enhanced hydrogen evolution reaction. Appl. Surf. Sci. 489, 725–733. doi:10.1016/j.apsusc.2019.06.040
Sevilla, M., and Mokaya, R. (2014). Energy storage applications of activated carbons: Supercapacitors and hydrogen strorage. Energy & Environ. Sci. 7 (4), 1250–1280. doi:10.1039/C3EE43525C
Taer, E., Afdal, Y. D., Amri, A., Awitdrus., Rika, T., Apriwandi, , et al. (2021). The synthesis of activated carbon made from banana stem ffbers as the supercapacitor electrodes. Mater. Today Proc. 44, 3346–3349. doi:10.1016/j.matpr.2020.11.645
Thibeorchews, P., Devashish, S., Sohini, B., Kaaviah, M., Ram, M., Mata, M., et al. (2022). Biomass derived hierarchical porous carbon for supercapacitor application and dilute stream CO2 capture. Carbon 199 (31), 249–257. doi:10.1016/j.carbon.2022.07.057
Toth, P. S., Velický, M., Bissett, M. A., Slater, T. J. A., Savjani, N., Aminu, K. R., et al. (2016). Asymmetric MoS2/graphene/metal sandwiches: Preparation, characterization, and application. Adv. Mater 28, 8256–8264. doi:10.1002/adma.201600484
Veltri, F., Alessandro, F., Scarcello, A., Beneduci, A., Polanco, M., Perez, D., et al. (2020). Porous carbon materials obtained by the hydrothermal carbonization of orange juice. Nanomaterials 10 (4), 655. doi:10.3390/nano10040655
Vu, A., Qian, Y., and Stein, A. (2012). Porous electrode materials for lithium-ion batteries-how to prepare them and what makes them special. Adv. Energy Mater. 2, 1056–1085. doi:10.1002/aenm.201200320
Wei, F., Zhang, H., He, X., Ma, H., Dong, S., Xie, X., et al. (2019). Synthesis of porous carbons from coal tar pitch for high-performance supercapacitors. New Carbon Mater. 34 (2), 132–138. doi:10.1016/S1872-5805(19)60006-5
Weigelt, C., and Shittu, E. (2016). Competition, regulatory policy, and firms' resource investments: The case of renewable energy technologies. Acad. Manag. J. 59 (2), 678–704. doi:10.5465/amj.2013.0661
Yan, S., Tang, C., Yang, Z., Wang, X., Zhang, H., Liu, S., et al. (2020). Hierarchical porous electrospun carbon nanofibers with nitrogen doping as binder-free electrode for supercapacitor. J. Mater. Sci. Mater. Electron. 31 (19), 16247–16259. doi:10.1007/s10854-020-04173-1
Yue, L. M., Xia, Q. Z., Wang, L. W., Wang, L. L., Dacosta, H., Yang, J., et al. (2018). CO2 adsorption at nitrogen doped carbons prepared by K2CO3 activation of urea-modified coconut shell. J. Colloid Interface Sci. 511, 259–267. doi:10.1016/j.jcis.2017.09.040
Zhang, L. L., and Zhao, X. S. (2009). Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 38 (9), 2520–2531. doi:10.1039/B813846J
Keywords: porous carbon materials, synthesis, adsorption, energy storage, catalysis
Citation: Ni M, Zhou L, Liu Y and Ni R (2023) Advances in the synthesis and applications of porous carbon materials. Front. Chem. 11:1205280. doi: 10.3389/fchem.2023.1205280
Received: 14 April 2023; Accepted: 26 June 2023;
Published: 11 July 2023.
Edited by:
Aleksandar Kondinski, University of Cambridge, United KingdomReviewed by:
Bingan Lu, Hunan University, ChinaCopyright © 2023 Ni, Zhou, Liu and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mei Ni, bmltZWlAY2ZyaS5lZHUuY24=
 Mei Ni
Mei Ni Lei Zhou2,3
Lei Zhou2,3