
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 10 May 2023
Sec. Medicinal and Pharmaceutical Chemistry
Volume 11 - 2023 | https://doi.org/10.3389/fchem.2023.1191498
This article is part of the Research Topic Advances in Natural Product Chemistry: Yunnan University 100th Anniversary View all 11 articles
 Meng Yin1†
Meng Yin1† Yongsheng Fang1†
Yongsheng Fang1† Xiaotong Sun1
Xiaotong Sun1 Minggao Xue1
Minggao Xue1 Caimei Zhang1
Caimei Zhang1 Zhiyun Zhu1
Zhiyun Zhu1 Yamiao Meng1
Yamiao Meng1 Lingmei Kong1
Lingmei Kong1 Yi Yi Myint2
Yi Yi Myint2 Yan Li1*
Yan Li1* Jingfeng Zhao1*
Jingfeng Zhao1* Xiaodong Yang1*
Xiaodong Yang1*Three series of podophyllotoxin derivatives with various nitrogen-containing heterocycles were designed and synthesized. The antitumor activity of these podophyllotoxin derivatives was evaluated in vitro against a panel of human tumor cell lines. The results showed that podophyllotoxin-imidazolium salts and podophyllotoxin-1,2,4-triazolium salts a1–a20 exhibited excellent cytotoxic activity. Among them, a6 was the most potent cytotoxic compound with IC50 values of 0.04–0.29 μM. Podophyllotoxin-1,2,3-triazole derivatives b1–b5 displayed medium cytotoxic activity, and podophyllotoxin-amine compounds c1–c3 has good cytotoxic activity with IC50 value of 0.04–0.58 μM. Furthermore, cell cycle and apoptosis experiments of compound a6 were carried out and the results exhibited that a6 could induce G2/M cell cycle arrest and apoptosis in HCT-116 cells.
According to the data from the International Agency for Cancer Research (IARC), there would be around 19.3 million new cancer diagnoses and nearly 10 million cancer-related deaths in 2020 (Sung et al., 2021). Therefore, the development of innovative anticancer agents and therapeutic strategies is essential (Boshuizen and Peeper, 2020). Medicinal chemists have increasingly viewed natural products as valuable resources for developing anticancer drug (Choi et al., 2017). About 84% of antitumor small molecule drugs approved between 1981 and 2019 were derived from natural products or structural units containing natural products (Newman and Cragg, 2020). The design and rational synthesis of natural product-like libraries, from which lead compounds with high efficiency, high selectivity, and low toxicity can be screened and discovered for preclinical studies, is one of the significant approaches for developing new drugs (Liu et al., 2017).
Podophyllotoxin is a natural product with anticancer activity belonging to the lignans cyclolignolide family (Dagenais et al., 2020). Podophyllotoxin and its semi-synthetic glycoside derivatives Etoposide, Teniposide and Etoposide Phosphate have been proved to be highly active antitumor agents with excellent clinical effects and are essential drugs for the treatment of small cell lung cancer, leukemia, testicular cancer and other types of tumors (Zhang et al., 2018; Li et al., 2019; Guo and Jiang, 2021; Zhao et al., 2021). Numerous structural and pharmacological studies have demonstrated that C-4 derivatization could enhance the biological activity of this family of drugs (Xiao et al., 2020).
On the other side, nitrogen-containing heterocycles are widely used in drug design and discovery (Xu et al., 2014a; Vitaku et al., 2014). The unique structural features of imidazoles and triazoles possess desirable electron rich properties, which are more favorable for conjugation with other molecules, and the molecular activity could be improved after hybridization (Verma et al., 2013; Gaba and Mohan, 2015; Bozorov et al., 2019; Xu et al., 2019; Dixit et al., 2021; Sharma et al., 2021). Among them, imidazolium salts have attracted much attention for their important and extensive biological and pharmacological activities, especially antitumor activity (Cui et al., 2003; Yang et al., 2009). In this context, our group has devoted to the synthesis of novel imidazolium salt derivatives and found a series of promising compounds with antitumor activity (Chen et al., 2013; Wang et al., 2013; Xu et al., 2014b; Xu et al., 2015; Zhou et al., 2016a; Zhou et al., 2016b). Further mechanistic studies confirmed that these imidazolium salt derivatives can induce cell cycle arrest and apoptosis in tumor cells (Liu et al., 2013; Liu et al., 2015; Huang et al., 2019). The representative examples are an effective antitumor active diosgenin-imidazolium salt and a new mTOR pathway inhibitor B591 (Figure 1) (Deng et al., 2019; Zhou et al., 2019).
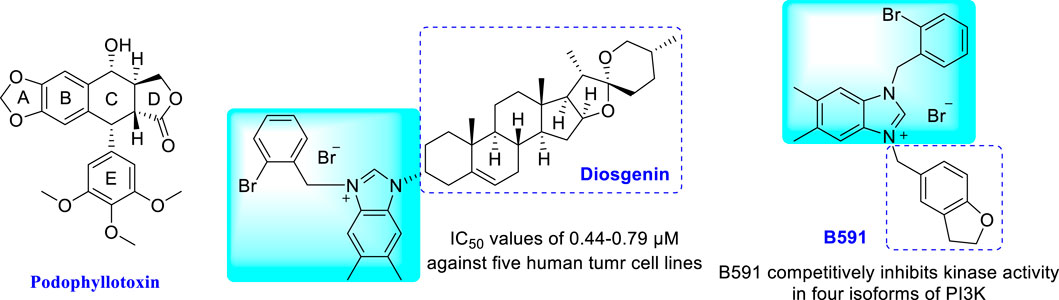
FIGURE 1. Structures of podophyllotoxin and representative imidazolium salts with antitumor activity.
In the past three decades, molecular hybridization has played an important role in drug discovery (Zhang et al., 2017; Yang et al., 2021). In view of the potential anticancer activity of podophyllotoxin and nitrogen-containing heterocycles, we launched the synthesis of hybrid compounds of natural product podophyllotoxin and imidazolium/triazolium salts. Although some nitrogen-containing heterocycles-podophyllotoxin derivatives were prepared and found to possess anticancer and neuroactive activities (Chen et al., 2011; Shang et al., 2012; Vishnuvardhan et al., 2017; Hou et al., 2019), to the best of our knowledge, there are no reports on the synthesis and bioactivity of imidazolium/triazolium salt hybrids of podophyllotoxin. With this in mind, we turned our attention to the synthesis and antitumor activity of a series of novel podophyllotoxin nitrogen-containing heterocycles, especially imidazolium and triazolium salts.
As shown in Scheme 1, firstly, to synthesize podophyllotoxin nitrogen-containing heterocycles, imidazole, 1,2,4-triazole, 2-methylimidazole, 1,2,3-triazole and amines were used for reaction. Using the commercial podophyllotoxin as starting material, the esterification reaction with 2-chloropropionyl chloride was carried out to obtain the ester S1. Next, S1 reacted with imidazole, 1,2,4-triazole and 2-methylimidazole to obtain the nitrogen-containing heterocycles a1–a3 (60%–70% yields, two steps). Then, treatment of a1–a3 with various bromides generated the podophyllotoxin imidazolium/triazolium salts a4–a21 (9%–91% yields). Secondly, as shown in Scheme 2, 4-chlorinated podophyllotoxin S2 was obtained by commercial podophyllotoxin reacting with thionyl chloride. Next, a nucleophilic substitution reaction with sodium azide was conducted to obtain compound S3 (46% yield, two steps). Then, azide S3 reacted with various terminal alkynes under Click reaction condition to get the podophyllotoxin-1,2,3-triazole derivatives b1–b5 (31%–47% yields). Finally, as shown in Scheme 3, using podophyllotoxin as the starting material, esterification reaction with 2-chloropropionyl chloride was performed to obtain the ester S1, which then underwent a nucleophilic substitution reaction with commercial cyclic amines (pyrrole, piperidine and morpholine) to furnish the podophyllotoxin-amines c1-c3 (48%–61% yields, two steps). To summarize, the structures and yields of all new podophyllotoxin nitrogen-containing heterocycle derivatives were shown in Table 1.
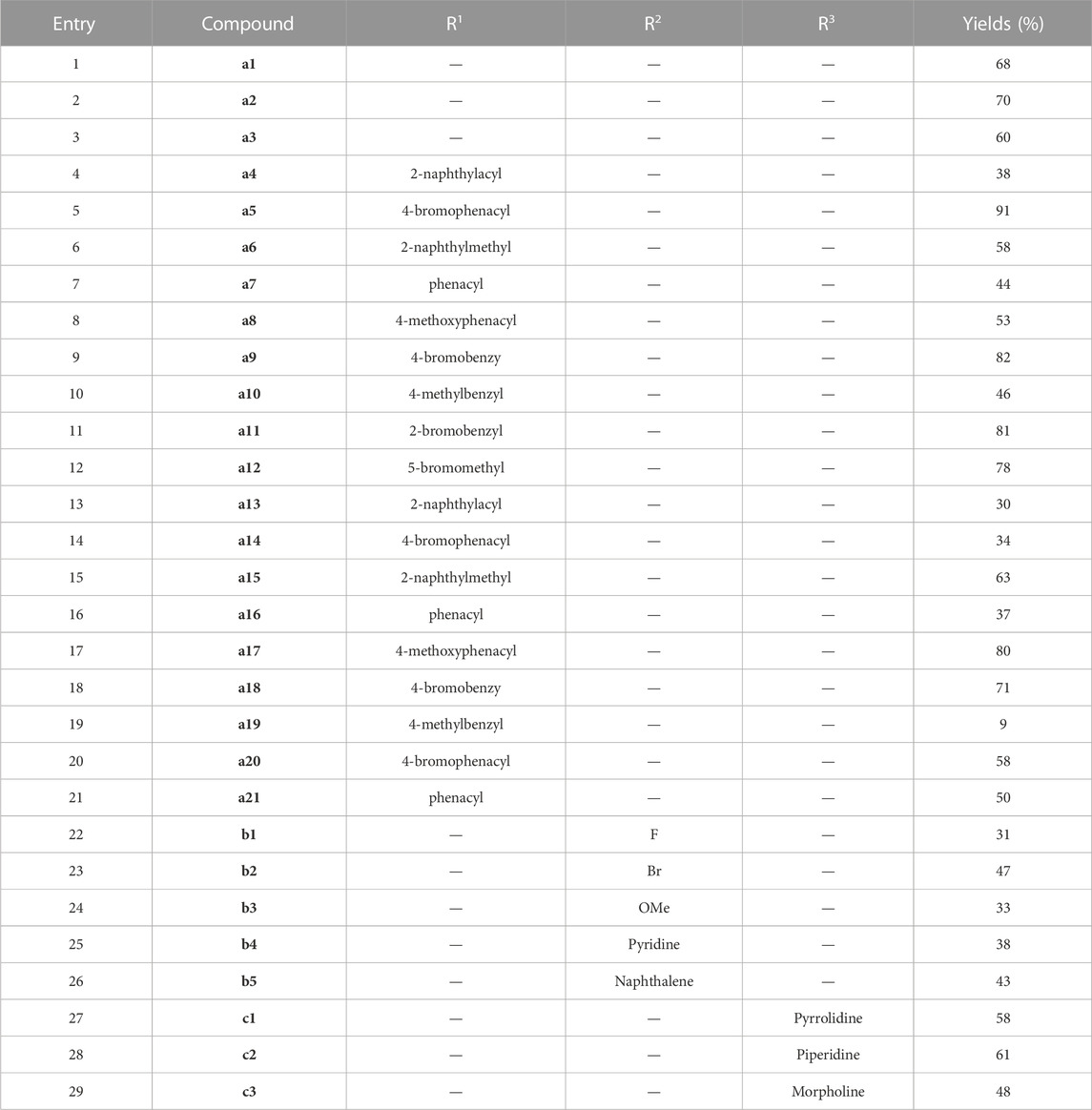
TABLE 1. Structures and yields of podophyllotoxin nitrogen-containing heterocycles a1–a21/b1–b5/c1–c3.
The synthesized twenty-nine podophyllotoxin nitrogen-containing derivatives were evaluated in vitro antitumor cytotoxic activity screening by MTS method (Perchellet et al., 2005). Four human cancer cell lines including hepatocellular carcinoma cells (HepG-2), non-small cell lung cancer cells (A-549), breast cancer cells (MDA-MB-231) and colon cancer cells (HCT-116) were selected to determine in vitro cytotoxic activity. DDP (Cisplatin), Etoposide, and Paclitaxel were chosen as positive controls. The results were listed in Table 2.
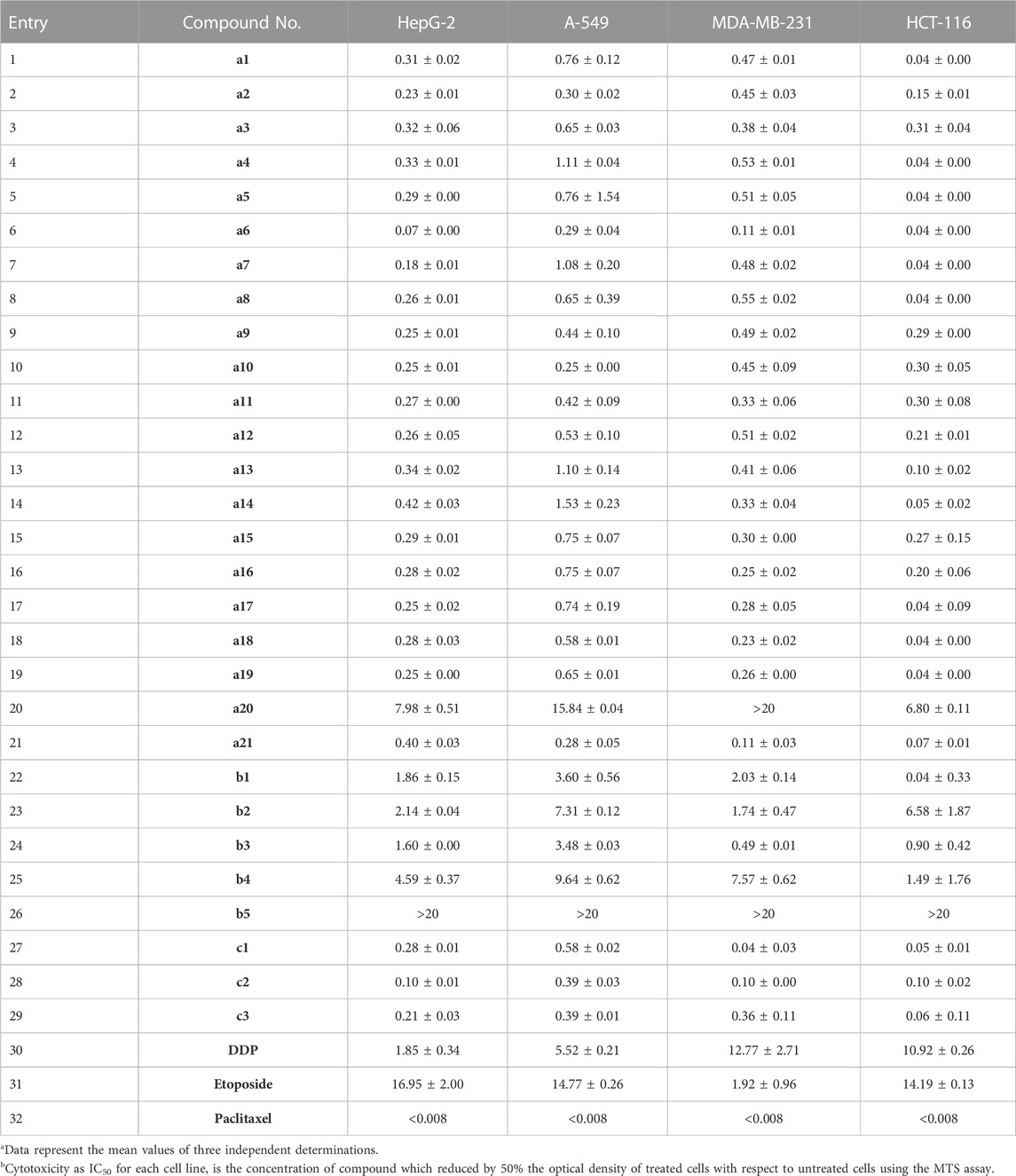
TABLE 2. Cytotoxic activities of podophyllotoxin nitrogen-containing heterocycles a1–a21/b1–b5/c1–c3 in vitroa (IC50, μMb).
As presented in Table 2, the majority of podophyllotoxin nitrogen-containing heterocycles showed potent inhibitory activity than positive controls Etoposide and DDP. Notably, these derivatives have obvious selective inhibitory against HCT-116 cell lines. The results showed that the structures of podophyllotoxin nitrogen-containing heterocycles plays a crucial role in regulating cytotoxic activity.
For pharmacophores of nitrogen-containing heterocycles, podophyllotoxin-imidazole and its salts (a1/a3/a4-a12/a20/a21) and podophyllotoxin-1,2,4-triazole and its salts (a2/a13–a19) exhibited excellent cytotoxic activity with IC50 values of 0.04–1.53 μM except a20. Among them, a6 was the most potent cytotoxic compound and its IC50 values for HepG2, A-549, MDA-MB-231 and HCT-116 were 0.07, 0.29, 0.11 and 0.04 μM, respectively. Secondly, the introduction of 1,2,3-triazole derivatives b1–b5 by Click reaction showed medium cytotoxic activity with IC50 values of 0.04–9.64 μM except b5. Finally, while compounds c1–c3 introduced with cyclic amines also showed excellent cytotoxic activity with IC50 values of 0.04–0.58 μM.
For the groups at position-3 of imidazolium and triazolium salts (a4–a21), the cytotoxic activities of most substituted benzyl groups were superior to those of substituted phenacyl groups. Among them, 2-naphthylmethyl substituent at position-3 of the imidazole ring (a6 and a15) showed excellent cytotoxic activity with IC50 values of 0.04–0.75 μM and a6 was the most powerful compound. Similarly, 4-bromobenzyl, 4-methylbenzyl, 4-methoxybenzoyl and 2-bromobenzyl groups at position-3 of the imidazole ring exhibited good cytotoxic activity with IC50 values of 0.04–0.65 μM.
For the groups at position-4 of 1,2,3-triazole ring (b1–b5), when the substituent was replaced with electron donating groups (R2 = OMe), b3 exhibits higher inhibitory activity with IC50 values of 0.49–3.48 μM. In contrast, when the substituent was charged with electron-withdrawing groups (R2 = F, Br), b1 and b2 were decreased slightly with IC50 values of 0.04–7.31 μM. When the substituent group was pyridine, b4 exhibited poor inhibitory activity with IC50 values of 1.49–9.64 μM, due to the electron-withdrawing effect of pyridine. When the substituent was a naphthalene ring, b5 did not exhibit any inhibitory activity.
For the cyclic amines (c1–c3), piperidine derivative of podophyllotoxin (c2) displayed excellent cytotoxic activity with IC50 values of 0.10–0.39 μM, which was superior to pyrrole derivative (0.04–0.58 μM) and morpholine derivative (0.06–0.39 μM). Notably, compound c1 has selective inhibitory against MDA-MB-231 cell lines with an IC50 value of 0.04 μM.
The results demonstrated that the introduction of imidazole ring into podophyllotoxin and a 2-naphthyl methyl substituent at the imidazolium salt’s 3-position play a critical role in enhancing cytotoxic activity. The preliminary structure activity relationships (SARs) of the derivatives were summarized in Scheme 4.
To determine the possible mechanism of compound a6 induced proliferation inhibition, cell cycle and apoptosis analysis were performed with flow cytometry. Firstly, HCT-116 cells were treated with indicated concentrations of compound a6 for 24 h and the cell cycle phase distribution of a6-treated cells was determined with propidium iodide (PI) staining. As shown in Figure 2, a6 exposure caused G2/M phase arrest in HCT-116 cells when compared with the control group, indicating that compound a6 inhibited cell proliferation through inducing G2/M cell cycle arrest.
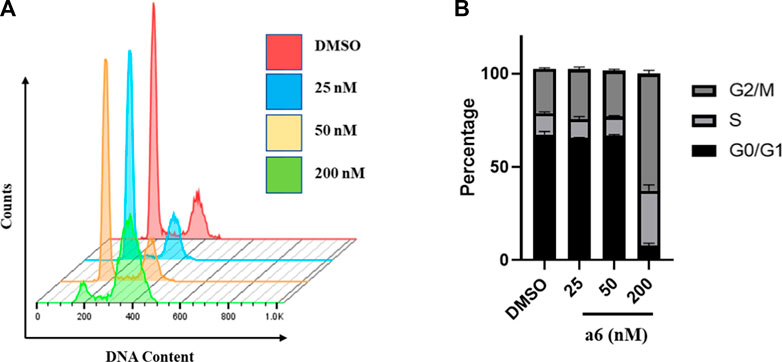
FIGURE 2. Compound a6 induced G2/M phase arrest in HCT-116 cells. (A) Cells were treated with different concentrations of compound a6 (25, 50 and 200 nM) for 24 h, and cell cycle was determined by cell cytometry with PI staining. (B) The percentages of cells in different phases were quantified.
The compound a6 induced cell apoptosis was also determined with Annexin V-FITC/PI staining. As shown in Figure 3, after treated with compound a6 at 25, 50 and 200 nM for 48 h, the apoptotic rate of HCT-116 cells remarkably elevated to 5.37 ± 0.37%, 10.45 ± 0.20% and 64.98 ± 2.40%, respectively. The results suggested that compound a6 inhibited cell proliferation through induction of G2/M cell cycle arrest and apoptosis of HCT-116 cells.
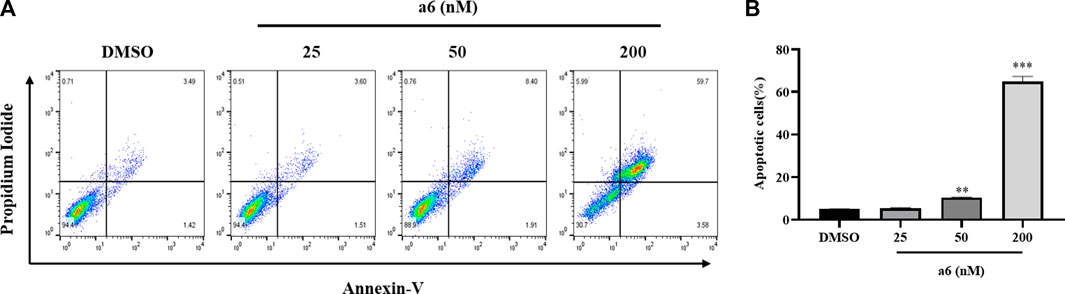
FIGURE 3. Compound a6 induced apoptosis of HCT-116 cells. (A) Cells were treated with 25, 50 and 200 nM compound a6 for 48 h. Cell apoptosis was determined by Annexin V-FITC/PI staining analysis. (B) The quantification of apoptotic cells.
In conclusion, a series of novel podophyllotoxin nitrogen-containing heterocycle derivatives with potential antitumor activity were prepared using a straightforward synthetic approach. The results showed that the imidazole-substituted derivatives demonstrated more effective inhibitory activity than 1,2,4-triazole-substituted and 1,2,3-triazole-substituted equivalents. The biological activity was significantly improved when the imidazole or imidazolium salt group was introduced into the structure of podophyllotoxin. Among them, imidazolium salt a6 was the most potent cytotoxic activity with IC50 values of 0.04–0.29 μM. It has an obvious selective inhibitory against HCT-116 cell lines with an IC50 value of 0.04 μM and could induce G2/M cell cycle arrest and apoptosis in HCT-116 cells. Podophyllotoxin-imidazolium salt a6 could be employed as a promising lead compound for further structural modification and in-depth activity research to identify new starting points for more effective anticancer agents.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
XY, JZ, and YL conceived and designed the experiments. MY, YF, XS, MX, CZ, and YaM performed the experiments. MY, YF, ZZ, LK, and YiM analyzed the data. XY, YF, and LK wrote the article.
This work was supported by grants from the National Key R&D Program of China (2019YFE0109200), the Central Government Guides Local Science and Technology Development Fund (202207AA110007, 202207AB110002), Yunnan Science and Technology Department and Yunnan University Joint Fund Project (2019FY003010), Program for Xingdian Talents (Yun-Ling Scholars) and IRTSTYN, and the Project of Yunnan Characteristic Plant Screening and R&D Service CXO Platform (2022YKZY001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2023.1191498/full#supplementary-material
Boshuizen, J., and Peeper, D. S. (2020). Rational cancer treatment combinations: An urgent clinical need. Mol. Cell. 78 (6), 1002–1018. doi:10.1016/j.molcel.2020.05.031
Bozorov, K., Zhao, J., and Aisa, H. A. (2019). 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 27 (16), 3511–3531. doi:10.1016/j.bmc.2019.07.005
Chen, H., Zuo, S., Wang, X., Tang, X., Zhao, M., Lu, Y., et al. (2011). Synthesis of 4β-triazole-podophyllotoxin derivatives by azide–alkyne cycloaddition and biological evaluation as potential antitumor agents. Eur. J. Med. Chem. 46 (9), 4709–4714. doi:10.1016/j.ejmech.2011.07.024
Chen, W., Deng, X. Y., Li, Y., Yang, L. J., Wan, W. C., Wang, X. Q., et al. (2013). Synthesis and cytotoxic activities of novel hybrid 2-phenyl-3-alkylbenzofuran and imidazole/triazole compounds. Bioorg. Med. Chem. Lett. 23 (15), 4297–4302. doi:10.1016/j.bmcl.2013.06.001
Choi, H., Cho, S. Y., Pak, H. J., Kim, Y., Choi, J.-y., Lee, Y. J., et al. (2017). Npcare: Database of natural products and fractional extracts for cancer regulation. J. Cheminformatics 9 (1), 2. doi:10.1186/s13321-016-0188-5
Cui, B. L., Zheng, B. L., He, K., and Zheng, Q. Y. (2003). Imidazole alkaloids from lepidium meyenii. J. Nat. Prod. 66, 1101–1103. doi:10.1021/np030031i
Dagenais, G. R., Leong, D. P., Rangarajan, S., Lanas, F., Lopez-Jaramillo, P., Gupta, R., et al. (2020). Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): A prospective cohort study. Lancet 395 (10226), 785–794. doi:10.1016/s0140-6736(19)32007-0
Deng, G., Zhou, B., Wang, J., Chen, Z., Gong, L., Gong, Y., et al. (2019). Synthesis and antitumor activity of novel steroidal imidazolium salt derivatives. Eur. J. Med. Chem. 168, 232–252. doi:10.1016/j.ejmech.2019.02.025
Dixit, D., Verma, P. K., and Marwaha, R. K. (2021). A review on ‘triazoles’: Their chemistry, synthesis and pharmacological potentials. J. Iran. Chem. Soc. 18 (10), 2535–2565. doi:10.1007/s13738-021-02231-x
Gaba, M., and Mohan, C. (2015). Development of drugs based on imidazole and benzimidazole bioactive heterocycles: Recent advances and future directions. Med. Chem. Res. 25 (2), 173–210. doi:10.1007/s00044-015-1495-5
Guo, Q., and Jiang, E. (2021). Recent advances in the application of podophyllotoxin derivatives to fight against multidrug-resistant cancer cells. Curr. Top. Med. Chem. 21 (19), 1712–1724. doi:10.2174/1568026621666210113163327
Hou, W., Zhang, G., Luo, Z., Su, L., and Xu, H. (2019). Click chemistry-based synthesis and cytotoxic activity evaluation of 4α-triazole acetate podophyllotoxin derivatives. Chem. Biol. Drug Des. 93 (4), 473–483. doi:10.1111/cbdd.13436
Huang, M., Duan, S., Ma, X., Cai, B., Wu, D., Li, Y., et al. (2019). Synthesis and antitumor activity of aza-brazilan derivatives containing imidazolium salt pharmacophores. Med. Chem. Commun. 10 (6), 1027–1036. doi:10.1039/c9md00112c
Li, Y., Chen, M., Yao, B., Lu, X., Zhang, X., He, P., et al. (2019). Transferrin receptor-targeted redox/pH-sensitive podophyllotoxin prodrug micelles for multidrug-resistant breast cancer therapy. J. Mat. Chem. B 7 (38), 5814–5824. doi:10.1039/c9tb00651f
Liu, L.-X., Wang, X.-Q., Yan, J.-M., Li, Y., Sun, C.-J., Chen, W., et al. (2013). Synthesis and antitumor activities of novel dibenzo[b,d]furan–imidazole hybrid compounds. Eur. J. Med. Chem. 66, 423–437. doi:10.1016/j.ejmech.2013.06.011
Liu, L. X., Wang, X. Q., Zhou, B., Yang, L. J., Li, Y., Zhang, H. B., et al. (2015). Synthesis and antitumor activity of novel N-substituted carbazole imidazolium salt derivatives. Sci. Rep. 5, 13101. doi:10.1038/srep13101
Liu, Z., Zhang, C., Duan, S., Liu, Y., Chen, W., Li, Y., et al. (2017). Synthesis and cytotoxic activity of novel hybrid compounds between indolo[b]tetrahydrofuran and imidazolium salts. Chin. J. Org. Chem. 37 (6), 1506–1515. doi:10.6023/cjoc201610043
Newman, D. J., and Cragg, G. M. (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83 (3), 770–803. doi:10.1021/acs.jnatprod.9b01285
Perchellet, E. M., Perchellet, J.-P., and Baures, P. W. (2005). Imidazole-4,5-dicarboxamide derivatives with antiproliferative activity against HL-60 cells. J. Med. Chem. 48 (19), 5955–5965. doi:10.1021/jm050160r
Shang, H., Chen, H., Zhao, D., Tang, X., Liu, Y., Pan, L., et al. (2012). Synthesis and biological evaluation of 4α/4β-imidazolyl podophyllotoxin analogues as antitumor agents. Arch. Pharm. Chem. Life Sci. 345 (1), 43–48. doi:10.1002/ardp.201100094
Sharma, P., LaRosa, C., Antwi, J., Govindarajan, R., and Werbovetz, K. A. (2021). Imidazoles as potential anticancer agents: An update on recent studies. Molecules 26 (14), 4213. doi:10.3390/molecules26144213
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Verma, A., Joshi, S., and Singh, D. (2013). Imidazole: Having versatile biological activities. J. Chem. 2013, 1–12. doi:10.1155/2013/329412
Vishnuvardhan, M., V, S. R., Chandrasekhar, K., Lakshma Nayak, V., Sayeed, I. B., Alarifi, A., et al. (2017). Click chemistry-assisted synthesis of triazolo linked podophyllotoxin conjugates as tubulin polymerization inhibitors. Med. Chem. Commun. 8 (9), 1817–1823. doi:10.1039/c7md00273d
Vitaku, E., Smith, D. T., and Njardarson, J. T. (2014). Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57 (24), 10257–10274. doi:10.1021/jm501100b
Wang, X. Q., Liu, L. X., Li, Y., Sun, C. J., Chen, W., Li, L., et al. (2013). Design, synthesis and biological evaluation of novel hybrid compounds of imidazole scaffold-based 2-benzylbenzofuran as potent anticancer agents. Eur. J. Med. Chem. 62, 111–121. doi:10.1016/j.ejmech.2012.12.040
Xiao, J., Gao, M., Sun, Z., Diao, Q., Wang, P., and Gao, F. (2020). Recent advances of podophyllotoxin/epipodophyllotoxin hybrids in anticancer activity, mode of action, and structure-activity relationship: An update (2010-2020). Eur. J. Med. Chem. 208, 112830. doi:10.1016/j.ejmech.2020.112830
Xu, H., Tang, H., Feng, H., and Li, Y. (2014a). Design, synthesis and anticancer activity evaluation of novel C14 heterocycle substituted epi-triptolide. Eur. J. Med. Chem. 73, 46–55. doi:10.1016/j.ejmech.2013.11.044
Xu, X. L., Wang, J., Yu, C. L., Chen, W., Li, Y. C., Li, Y., et al. (2014b). Synthesis and cytotoxic activity of novel 1-((indol-3-yl)methyl)-1H-imidazolium salts. Bioorg. Med. Chem. Lett. 24 (21), 4926–4930. doi:10.1016/j.bmcl.2014.09.045
Xu, X. L., Yu, C. L., Chen, W., Li, Y. C., Yang, L. J., Li, Y., et al. (2015). Synthesis and antitumor activity of novel 2-substituted indoline imidazolium salt derivatives. Org. Biomol. Chem. 13 (5), 1550–1557. doi:10.1039/c4ob02385d
Xu, Z., Zhao, S. J., and Liu, Y. (2019). 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 183, 111700. doi:10.1016/j.ejmech.2019.111700
Yang, X. D., Zeng, X. H., Zhang, Y. L., Qing, C., Song, W. J., Li, L., et al. (2009). Synthesis and cytotoxic activities of novel phenacylimidazolium bromides. Bioorg. Med. Chem. Lett. 19 (7), 1892–1895. doi:10.1016/j.bmcl.2009.02.065
Yang, Z., Zhou, Z., Luo, X., Luo, X., Luo, H., Luo, L., et al. (2021). Design and synthesis of novel podophyllotoxins hybrids and the effects of different functional groups on cytotoxicity. Molecules 27 (1), 220. doi:10.3390/molecules27010220
Zhang, H., Tian, Y., Kang, D., Huo, Z., Zhou, Z., Liu, H., et al. (2017). Discovery of uracil-bearing DAPYs derivatives as novel HIV-1 NNRTIs via crystallographic overlay-based molecular hybridization. Eur. J. Med. Chem. 130, 209–222. doi:10.1016/j.ejmech.2017.02.047
Zhang, X., Rakesh, K. P., Shantharam, C. S., Manukumar, H. M., Asiri, A. M., Marwani, H. M., et al. (2018). Podophyllotoxin derivatives as an excellent anticancer aspirant for future chemotherapy: A key current imminent needs. Bioorg. Med. Chem. 26 (2), 340–355. doi:10.1016/j.bmc.2017.11.026
Zhao, W., Cong, Y., Li, H. M., Li, S., Shen, Y., Qi, Q., et al. (2021). Challenges and potential for improving the druggability of podophyllotoxin-derived drugs in cancer chemotherapy. Nat. Prod. Rep. 38 (3), 470–488. doi:10.1039/d0np00041h
Zhou, B., Liu, Z. F., Deng, G. G., Chen, W., Li, M. Y., Yang, L. J., et al. (2016a). Synthesis and antitumor activity of novel N-substituted tetrahydro-beta-carboline-imidazolium salt derivatives. Org. Biomol. Chem. 14 (39), 9423–9430. doi:10.1039/c6ob01495j
Zhou, H., Yu, C., Kong, L., Xu, X., Yan, J., Li, Y., et al. (2019). B591, a novel specific pan-PI3K inhibitor, preferentially targets cancer stem cells. Oncogene 38 (18), 3371–3386. doi:10.1038/s41388-018-0674-5
Keywords: podophyllotoxin, imidazolium salts, triazoles, antitumor activity, structure-activity relationships
Citation: Yin M, Fang Y, Sun X, Xue M, Zhang C, Zhu Z, Meng Y, Kong L, Myint YY, Li Y, Zhao J and Yang X (2023) Synthesis and anticancer activity of podophyllotoxin derivatives with nitrogen-containing heterocycles. Front. Chem. 11:1191498. doi: 10.3389/fchem.2023.1191498
Received: 22 March 2023; Accepted: 21 April 2023;
Published: 10 May 2023.
Edited by:
Siva S. Panda, Augusta University, United StatesReviewed by:
Cheng-xue Pan, Guangxi Normal University, ChinaCopyright © 2023 Yin, Fang, Sun, Xue, Zhang, Zhu, Meng, Kong, Myint, Li, Zhao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Li, eWFuLmxpQHludS5lZHUuY24=; Jingfeng Zhao, amZ6aGFvQHludS5lZHUuY24=; Xiaodong Yang, eGR5YW5nQHludS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.