
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 18 April 2023
Sec. Analytical Chemistry
Volume 11 - 2023 | https://doi.org/10.3389/fchem.2023.1171824
This article is part of the Research Topic Separation and Analytical Chemistry View all 7 articles
Introduction: Oligopeptides exhibit great prospects for clinical application and its separation is of great importance in new drug development.
Methods: To accurately predict the retention of pentapeptides with analogous structures in chromatography, the retention times of 57 pentapeptide derivatives in seven buffers at three temperatures and four mobile phase compositions were measured via reversed-phase high-performance liquid chromatography. The parameters (
Results: The results showed that
Discussion: In summary, the two six-parameter models were appropriate to characterize the chromatographic retention of amphoteric compounds, especially the acid or neutral pentapeptides, and could predict the chromatographic retention of pentapeptide compounds.
Active peptides are common small-molecule compounds in nature and generally possess invaluable medicinal value (Abdelhedi and Nasri, 2019; Suo et al., 2022). Peptides have a wide range of bioactivities and can be divided into two categories according to different sources: (1) endogenous peptides from precursor proteins and secreted cells and (2) exogenous peptides from enzymatic hydrolysis or synthesis (Wang et al., 2022). The oligopeptides produced by the enzymatic hydrolysis of animal proteins have been reported to exhibit outstanding hypotensive effects by inhibiting the angiotensin-I converting enzyme (Abdelhedi et al., 2017; Qiao et al., 2022). Moreover, the oligopeptides extracted from tea and brewer’s spent grain had excellent hypolipidemic activities (Ferreira et al., 2022; Ye et al., 2023), and the oligopeptides isolated from Siberian sturgeon cartilage could treat chronic diseases caused by oxidative stress (Sheng et al., 2022). More importantly, oligopeptides exhibit great prospects for clinical application due to their high degree of affinity and specificity and easy absorption (Zhang et al., 2020; Sitkov et al., 2021).
Oligopeptide separations by reversed-phase high-performance liquid chromatography (RP-HPLC) are extremely common (Ofosu et al., 2021; Samtiya et al., 2021; Waili et al., 2021), and the chromatographic retention of oligopeptides in RP-HPLC is driven by hydrophobic interactions (Sousa et al., 2021). Combining the molecular structure of compounds with the parameters describing the properties of chromatographic mobile and stationary phases, functional relationships could be obtained (Nie et al., 2022). These relationships can be used to analyze and predict the chromatographic behavior of other compounds (Janicka et al., 2020; Nie et al., 2022) and evaluate the pharmacokinetics and biochemical properties of drugs, such as absorption, distribution, metabolism, and excretion (ADME) in vivo (Langyan et al., 2021). It can also preliminarily determine the solubility, lipophilicity, bioaccumulation, and toxicity of compounds in vivo, which is of great significance in the field of new drug molecule development, especially in the analysis of the chemical properties of peptides in vivo.
The acid dissociation constant (pKa) is an elementary parameter in the analysis of drugs and strongly affects their pharmacokinetics and biochemical properties by characterizing the degree of ionization of drug molecules in solution at different pH values (Besleaga et al., 2021). pKa determines the existing form of compounds in the medium and their solubility, lipophilicity, permeability, bioaccumulation, and toxicity (Konçe et al., 2019; Xie et al., 2022); these characteristics play a particularly important role in the drug development process (Bergazin et al., 2021). Accurate prediction of the pKa value of organic compounds is highly important in numerous fields, especially in the development of new drugs (Xiong et al., 2022; Zhang et al., 2022). However, accurate prediction of the pKa for drug-like molecules is also a tremendous challenge in chemistry (Zhang et al., 2022).
Due to its high-resolution ratio, selectivity, and reproducibility, RP-HPLC is the most extensive and central technique in the analysis and separation of a wide range of compounds and the study of the pKa values of drug molecules (D'Archivio, 2019; Yılmaz Ortak and Cubuk Demiralay, 2019). Apart from molecular structure, numerous factors in chromatographic analysis programs have an important influence on retention time, such as the pH of the mobile phase, column temperature, mobile phase composition, and type of chromatographic column (Huang et al., 2019; Tsui et al., 2019; Annadi et al., 2022; Shi et al., 2022). The chromatographic conditions can be adjusted and optimized to achieve satisfactory separation of mixtures and symmetric peak shapes. Furthermore, an increasing number of studies have reported the combined effect of two or more factors on the retention time (Phyo et al., 2018; Biancolillo et al., 2020; Kaczmarski and Chutkowski, 2021; Yilmaz, 2021). Comprehensive models that consider the influence of different chromatographic conditions are more accurate in predicting the retention times of compounds. However, previous studies have generally predicted the chromatographic retention or lipophilicity by using the quantitative structure–retention relationship (QSRR) models (Yang X. et al., 2020; Fouad et al., 2022; Xu et al., 2023). The QSRR models mainly focus on the molecular descriptors of the solutes, with less emphasis on the influence of different chromatographic conditions. Recently, models based on empirical or semiempirical equations and thermodynamic properties have rarely been reported to investigate the simultaneous effect of diverse chromatographic conditions on retention.
Herein, this study aims to provide multiparameter models that combine the effects of pH, temperature (T), organic modifier composition (φ), and polarity (
RP-HPLC-grade methanol was purchased from Fisher Scientific, and trifluoroacetic acid (TFA) was purchased from Fluka (Buchs, Switzerland). All other reagents were from Kermel (Tianjin, China); these included citric acid, sodium citrate, disodium hydrogen phosphate, and sodium dihydrogen phosphate.
The pentapeptides (HGRFG and NPNPT) were isolated from Carapax Trionycis and showed high anti-fibrosis activity (Supplementary Figure S1). The C- or N-termini of the pentapeptides of HGRFG and NPNPT were replaced with the remaining 19 amino acids to obtain the sequences of the derived pentapeptides. Then, the derived pentapeptides were synthesized by solid-phase synthesis (SPPS) and purified by RPLC. In this study, the sequences of the 57 analyzed pentapeptides are as follows: NPNPA, NPNPC, NPNPD, NPNPE, NPNPG, NPNPH, NPNPI, NPNPK, NPNPM, NPNPN, NPNPP, NPNPQ, NPNPR, NPNPS, NPNPT, NPNPV, NPNPY, APNPT, CPNPT, DPNPT, EPNPT, GPNPT, HPNPT, IPNPT, KPNPT, LPNPT, MPNPT, PPNPT, QPNPT, RPNPT, SPNPT, TPNPT, VPNPT, YPNPT, HGRFA, HGRFD, HGRFE, HGRFG, HGRFH, HGRFK, HGRFN, HGRFQ, HGRFR, HGRFS, HGRFT, AGRFG, DGRFG, EGRFG, GGRFG, KGRFG, NGRFG, PGRFG, QGRFG, RGRFG, SGRFG, TGRFG, and VGRFG.
RP-HPLC was conducted via a Shimadzu Prominence LC-2030 Plus (Kyoto, Japan) instrument equipped with a SIL-20AC autosampler and two LC-20AD pumps. An SPD-20AV dual-wavelength detector at 215 nm and 254 nm was used to detect the pentapeptides. Instrument control, data acquisition, and processing were performed with LabSolutions software for RP-HPLC. A Shimadzu Shim-pack GIST C18 4.6 × 250 mm i. d., 5 μm particle size column was used as the stationary phase and was stable within the pH range of 1–10.
A PHS-25 pH meter purchased from INESA (Shanghai, China) was used to measure the pH values, combined with an E-201F-type composite electrode. Potassium hydrogen phthalate, mixed phosphate, and sodium tetraborate from INESA (Shanghai, China) were used for electrode calibration.
Mobile phases were prepared with water (A)–methanol (B) components, degassed, and mixed online. The pentapeptides were analyzed under isocratic elution of organic solvent B. The analysis procedures were, respectively, as follows: a: 8–14 v/v (increment 2 v/v) (NPNPA, NPNPD, NPNPE, NPNPG, NPNPH, NPNPK, NPNPN, NPNPQ, NPNPR, NPNPS, NPNPT, APNPT, DPNPT, EPNPT, GPNPT, HPNPT, KPNPT, PPNPT, RPNPT, SPNPT, and TPNPT); b: 20–26 v/v (increment 2 v/v) (HGRFA, HGRFD, HGRFE, HGRFG, HGRFH, HGRFK, HGRFN, HGRFQ, HGRFR, HGRFS, HGRFT, AGRFG, DGRFG, EGRFG, GGRFG, KGRFG, NGRFG, PGRFG, QGRFG, RGRFG, SGRFG, TGRFG, VGRFG, NPNPC, NPNPI, NPNPM, NPNPP, NPNPV, NPNPY, CPNPT, IPNPT, LPNPT, MPNPT, QPNPT, VPNPT, and YPNPT). The retention times were separately obtained at temperatures of 25°C, 35°C, and 45°C. The aqueous phase was prepared at 25°C by diluting stock solutions of buffer salt.
The parameters
The solutes were initially dissolved in pure water at a concentration lower than 1 mg/ml and then filtered through a 0.45 µm nylon mobile phase filter. The flow rate of the chromatographic system was maintained at 1.0 ml/min, and the injection volume was 10 μL.
Both non-linear regressions of the chromatographic retention factor k with pH or other parameters in the multiparameter equation and linear regression were performed using MATLAB R2019a (Version 9.6.0; MathWorks, Natick, MA, USA).
The theoretical sigmoidal function of pH and retention factor k derived from chromatographic theory has been widely used for ionizable compounds (Konçe et al., 2019). Previous studies have verified the wide applicability of ionizable compounds in chromatographic analysis (Yang et al., 2018; Soriano-Meseguer et al., 2019). Thus, according to Equation 1, the acid–base equilibrium determined by the acidity constant
where
where the empirical formula could be used to estimate δ from solvent composition as follows:
where
For a reversible process of chromatographic analysis, the dissociation of the analyte and buffer and the solute migration during retention, which could be affected by the column temperature change, are applicable to the Van’t Hoff equation (Faisal et al., 2018; Marchetti et al., 2019; Yuan et al., 2020) as follows:
where
Similarly, for the reversible process
where
Introducing Eqs 4, and 5 into Eq. 1 produces the following equation:
where the fitting parameter includes the thermodynamic quantities related to the dissociation and transformation of the analyzed compound, i.e., the function composed of these quantities:
The composition of the mobile phase is the main variable used to optimize retention and selectivity in RP-HPLC. The Soczewiński–Wachtmeister equation is commonly used to describe the relationship between k and the change in mobile phase (Flieger et al., 2020; Lin et al., 2022).
where
Considering the influence of the polarity of the solute, stationary phase, and mobile phase on k, another linear model was proposed to accurately describe k, which represents the linear relationship between the retention rate and the polarity of the eluent (Gisbert-Alonso et al., 2021; Zhu et al., 2022); the relationship is as follows:
where p is the parameter describing the polarity of the solute,
For the water–methanol system, the relationship between
To eliminate the limit of all
where fitting parameters concerning the solute are twice those before, which improves the accuracy of model prediction.
The mobile phase composition affects not only the retention rate but also the ionization degree of the acid–base solute, and the addition of an organic solvent to the aqueous solution containing ionizable compounds changes the value of
Similarly, the relationship between
Based on the aforementioned analysis, combined with the model of pH and different mobile phase compositions, the six-parameter model is obtained as follows:
and
where X is the variable describing the change in the mobile phase, representing φ or
Small-molecular oligopeptides commonly participate in multiple physiological and pathological processes, including the transmission of signals and the regulation of immune and inflammatory responses (Yang J. et al., 2020; Gao et al., 2022). RP-HPLC is a common approach to separate small-molecular peptides by adjusting the chromatographic conditions (Liu et al., 2022). The retention factors of 57 ionizable solute derivatives of pentapeptides of NPNPT and HGRFG were determined at seven mobile pH values, four mobile phase compositions, and three column temperatures (84 data points for each solute). Some comparative chromatograms are shown in Supplementary Figure S2. We selected the pentapeptides with high polarity, similar retention, and a similar chemical structure for the model’s establishment and evaluation, and the 57 pentapeptides could be divided into five groups according to the acidity or basicity of the isoelectric points and the polarity of the pentapeptides. The groups included the following: (1) 8%–14% methanol acid pentapeptides: NPNPD, NPNPE, DPNPT, and EPNPT; (2) 8%–14% methanol basic pentapeptides: NPNPK, NPNPR, KPNPT, and RPNPT; (3) 8%–14% methanol neutral pentapeptides: NPNPA, NPNPG, NPNPH, NPNPN, NPNPQ, NPNPS, APNPT, GPNPT, HPNPT, NPNPT, PPNPT, SPNPT, and TPNPT; (4) 20%–26% methanol basic pentapeptides: HGRFA, HGRFG, HGRFH, HGRFK, HGRFN, HGRFQ, HGRFR, HGRFS, HGRFT, AGRFG, GGRFG, KGRFG, NGRFG, PGRFG, QGRFG, RGRFG, SGRFG, TGRFG, and VGRFG; and (5) 20%–26% methanol neutral pentapeptides: NPNPC, NPNPI, NPNPM, NPNPP, NPNPV, NPNPY, CPNPT, IPNPT, LPNPT, MPNPT, QPNPT, VPNPT, YPNPT, HGRFD, HGRFE, DGRFG, and EGRFG.
The pH of the mobile phase is one of the critical factors affecting the retention of compounds in chromatography due to its interference with the ionization efficiency and change in the protonation of analytes (Fan et al., 2022; Guo et al., 2023). Because the increased or decreased degree of chromatographic retention for compounds was different with the change in pH, adjusting the pH of the mobile phase was capable of separating the compounds with similar structures or confirming the absence of unrelated impurities (Fan et al., 2022; Tengattini et al., 2022). MATLAB R2019a was used to fit the S-curve of different pH values and the experimental retention factors (k) under the same temperature and the same mobile phase composition. From Eq. 1, we obtained the parameters
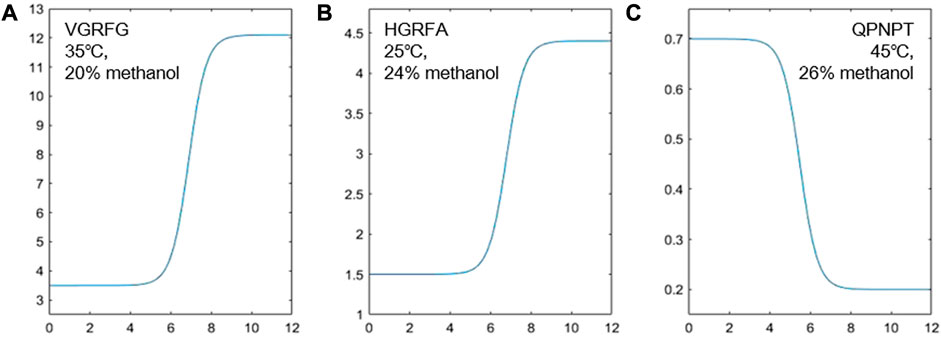
FIGURE 1. Fitting of the experimental retention and different pH values of VGRFG at 35°C and 20% methanol (A), HGRFA at 25°C and 24% methanol (B), and QPNPT at 45°C and 26% methanol (C).
In addition, the k-values calculated by the parameters
The temperature of the column could alter the density of the mobile phase, solute diffusion coefficients, and solute–stationary phase interactions and then affect the chromatographic retention (Nagase et al., 2021). The retention of solutes generally decreased as the column temperature was increased due to the accelerated molecular movement in RP-HPLC (Caltabiano et al., 2018; Idroes et al., 2020). Moreover, the
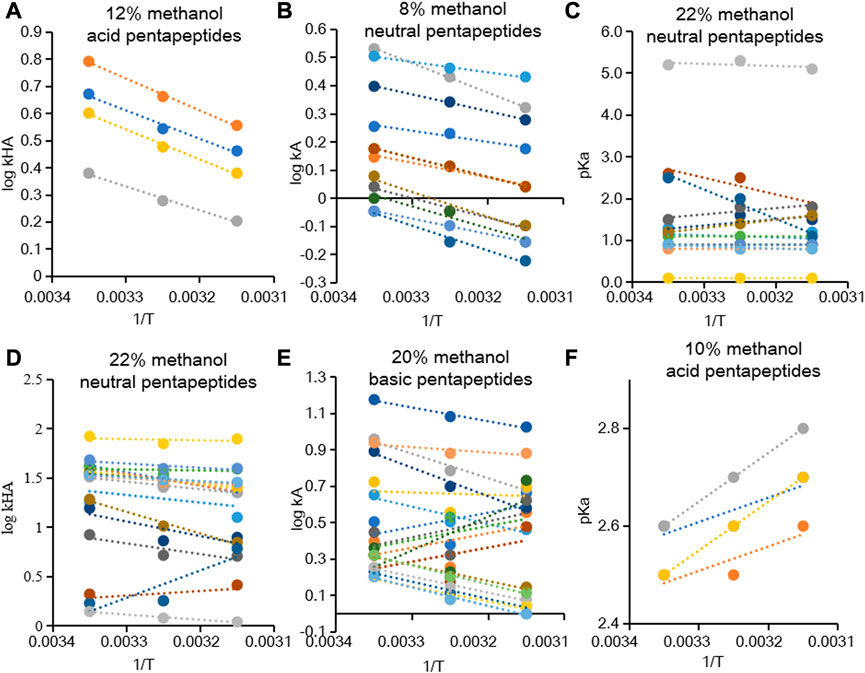
FIGURE 2. Dependence of
The linear relationship between
An appropriate composition of the mobile phase is beneficial for chromatographic separation and improving the chromatographic peak profile and efficiencies (Guo et al., 2018; Attwa et al., 2023). Adjusting the proportion of organic modifiers in the mobile phase is the most frequently used approach to achieve the separation of a series of compounds (Hong et al., 2020; Oney-Montalvo et al., 2022). Furthermore, the methanol volume fraction φ and polarity parameter
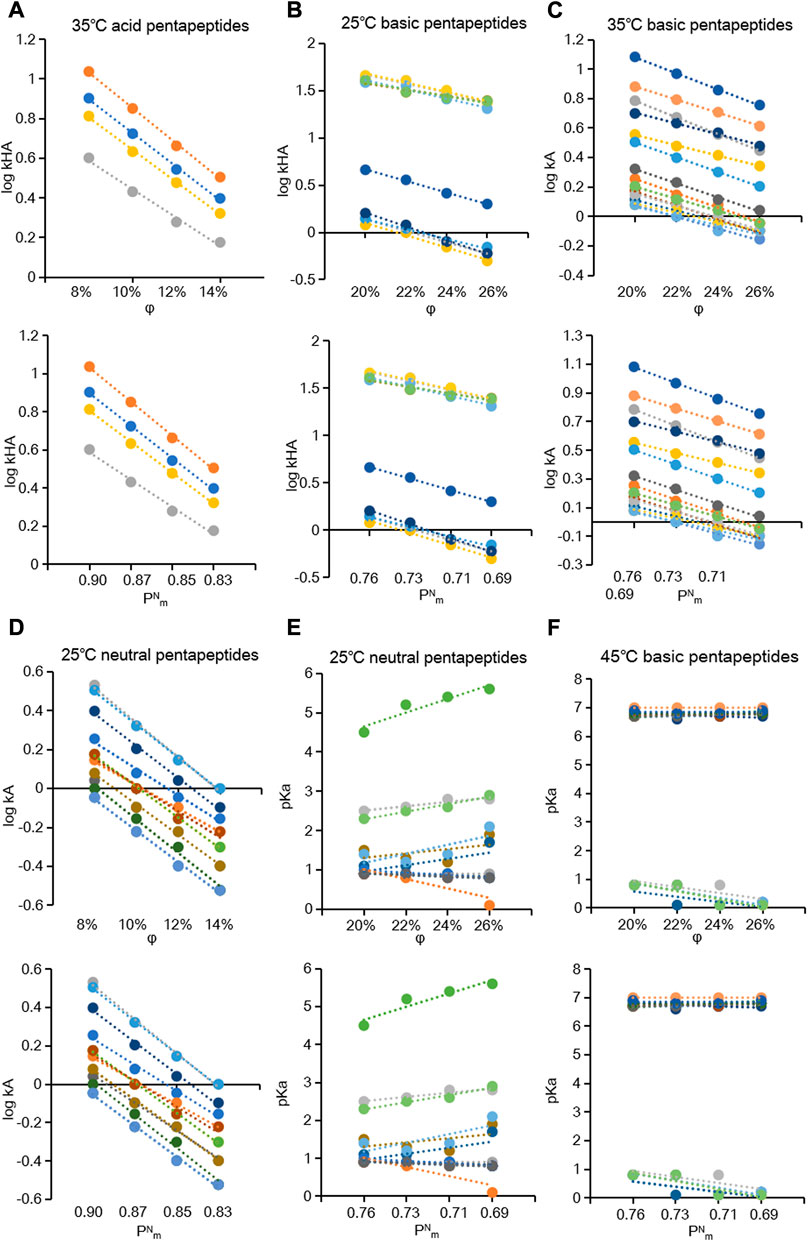
FIGURE 3. Plots of the
The coefficient
We combined the two variables of temperature and pH into a six-parameter model (as shown in Eq. 6) to explore the combined effect of temperature and pH on chromatographic retention. The fitting parameters a, b, c, d, e, and f (Table 5) of the 57 pentapeptides under the four mobile phase compositions were calculated by an established six-parameter model with pH and T as independent variables and k as the dependent variable to predict the k-value according to different pH and T values. All fitting parameters varied with the change in the mobile phase composition except pH and T. Moreover, a higher proportion of methanol in the mobile phase correlated to a smaller parameter of the acid pentapeptides. Only parameter c displayed an inversely proportional relationship with the proportion of methanol for neutral and basic pentapeptides, and there were no clear trends for other parameters in most cases.
According to the results of the six-parameter model, linear fitting of the experimental k-value and predicted k-value was conducted to assess the prediction capability of chromatographic retention. The R2 calculated by linear fitting was used as an evaluation criterion. A random error was present for all data, but the residuals were symmetrically distributed around the axis of y = 0 (Supplementary Figure S3). We then fitted the data from five groups of pentapeptides, and the R12 value was just 0.6055, showing the unsatisfactory capacity to predict the chromatographic retention of the studied pentapeptides (Figure 4A). Further classifying the data according to their acid–base properties and fittings, the R22 value was 0.8603 for the acid pentapeptides (Figure 4B), while both the R32 and R42 values were lower than 0.7 for the basic and neutral pentapeptides (Figures 4C, D). The results indicated that the six-parameter model had a certain prediction capability for the chromatographic retentions for the acid pentapeptides but was unable to characterize the chromatographic retentions for the basic or neutral pentapeptides. In addition, the R2 values fitted by the experimental k-value and predicted k-value under different chromatographic conditions in the six-parameter model of T and pH are shown in Supplementary Table S5. The R2 decreased with the increase in column temperature or the methanol volume fraction, indicating that this model was suitable for compounds with higher chromatographic retention.
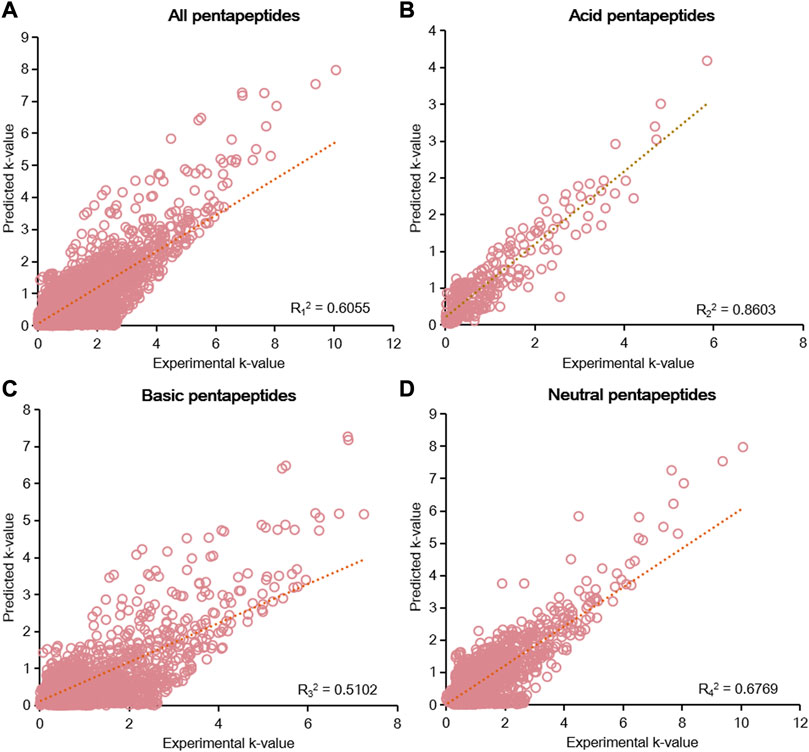
FIGURE 4. Linear fitting results of the predicted k-value and experimental k-value for all pentapeptides (A), acid pentapeptides (B), basic pentapeptides (C), and neutral pentapeptides (D) in the six-parameter model of T and pH.
Internal validation is a commonly used method for evaluating models free of experimental and environmental conditions’ limitations (Luo et al., 2020; Vasconcelos et al., 2023). In this study, we used 10-fold cross validation to conduct internal validation. The root mean squared error (RMSE) obtained from 10-fold cross validation was used to evaluate the prediction capability of the models in this study. The average RMSE from the 10 test sets was used to minimize the biased prediction results. The residuals of all pentapeptides and acid pentapeptides were randomly distributed around the y = 0 axis (Supplementary Figure S5). Moreover, the average RMSE of all pentapeptides and acid pentapeptides was 0.48 and 0.20 in the 10 tests (Supplementary Table S7), respectively, indicating that the six-parameter model had both random error and certain prediction capability.
We considered the combined influence of the mobile phase composition and pH on chromatographic retention by substituting pH and
The retention factors of the 57 pentapeptides under different elution conditions were predicted with φ or
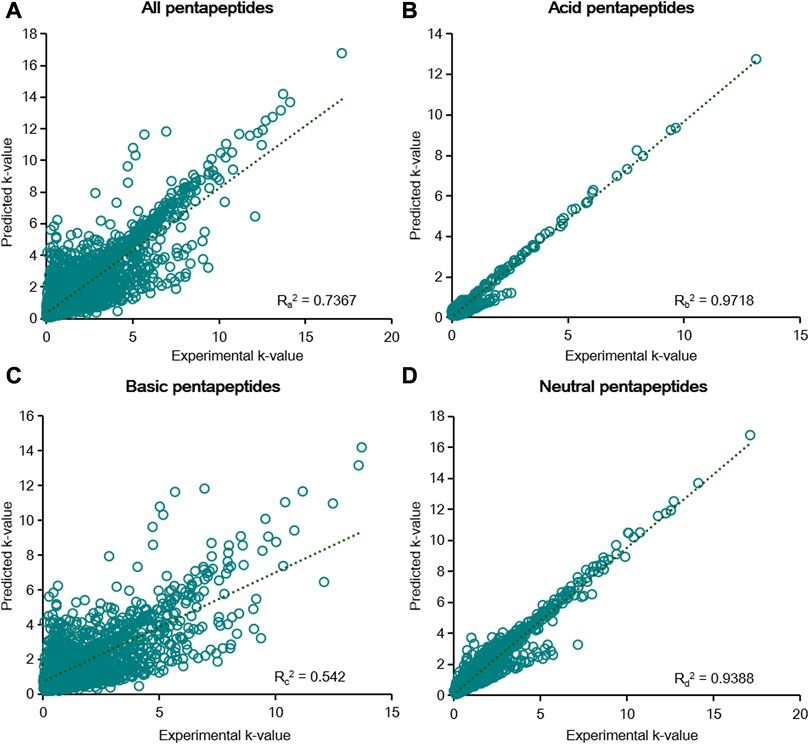
FIGURE 5. Linear fitting results of the predicted k-value and experimental k-value for all pentapeptides (A), acid pentapeptides (B), basic pentapeptides (C), and neutral pentapeptides (D) in the six-parameter model of φ and pH.
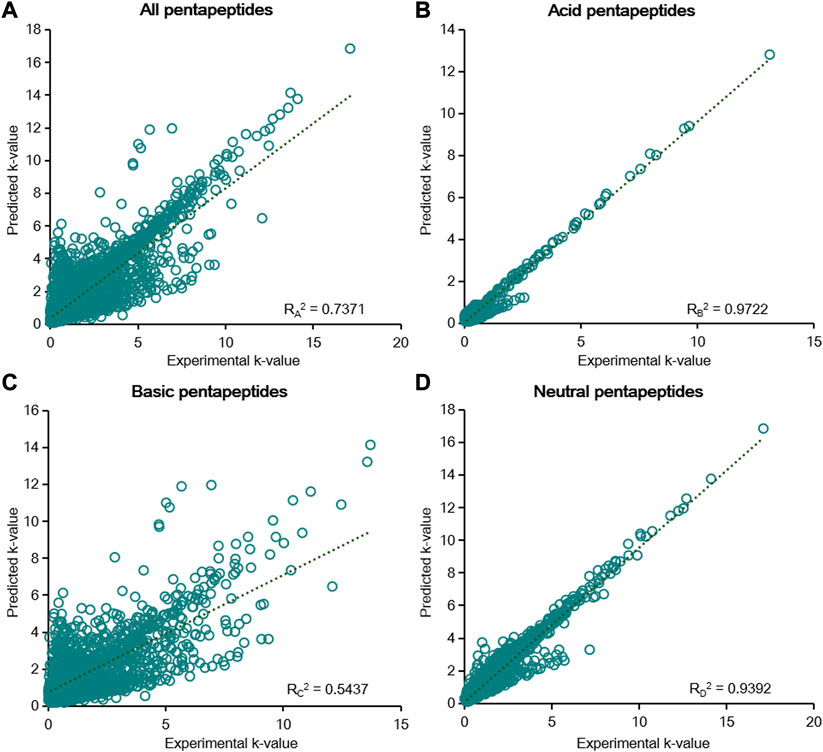
FIGURE 6. Linear fitting results of the predicted k-value and experimental k-value for all pentapeptides (A), acid pentapeptides (B), basic pentapeptides (C), and neutral pentapeptides (D) in the six-parameter model of
Retention behavior prediction of oligopeptides is valuable for efficient separation and purification. Previous studies have proposed various models to investigate the change in retention based on molecular descriptors or chromatographic theories (Park et al., 2020; Al Musaimi et al., 2023). There are five most commonly used models in studying the effect of mobile phase composition on retention behavior: (1) the linear-solvent-strength model, (2) the quadratic model, (3) the log–log (adsorption) model, (4) the mixed-mode model, and (5) the Neue–Kuss model (den Uijl et al., 2021). These models were able to predict the retention behavior at the first- and second-order levels. However, there was a clear deviation from linearity, especially in the lower organic modifier volume (Baeza-Baeza and García-Alvarez-Coque, 2020). Furthermore, the QSRR model displayed excellent prediction capacity for ionizable compounds but was limited to the type and calculation method of the molecular descriptors (Kumari et al., 2023). In addition, previous studies have reported the combined influence of temperature and mobile phase composition on chromatographic retention (Arkell et al., 2018; Caltabiano et al., 2018), and the simultaneous effect of pH and temperature or mobile phase composition has been less reported. The six-parameter model of pH and φ or
Herein, we established six-parameter models via RP-HPLC data for predicting the retention factors of pentapeptides under different chromatographic conditions. The relationships of the three parameters
In this study, there are also some limitations. First, the fitting results of the model would be more reliable with more temperature gradients of chromatographic conditions, but only 3 gradients of column temperature were used in this study. Second, higher chromatographic retention correlated to better fitting results. However, we cannot ensure evident retention results for all studied pentapeptides under other diverse elution conditions. Third, we selected a narrow range of methanol concentrations to produce symmetrical and sharp chromatographic peaks. The methanol concentrations outside this range were undefined as to whether they followed the Soczewiński–Wachtmeister equation. Finally, both six-parameter models showed unsatisfactory prediction capability for the basic pentapeptides, which needs further research.
In conclusion, our study determined that the six-parameter model of pH and φ or
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
HP completed the experiment, conducted data processing, and edited the manuscript. XY revised the manuscript and provided valuable input and suggestions. TZ offered important assistance in data processing. HF, ZZ, and JZ supplied the experimental platform and guide. JL and YL critically revised the paper and made amendments and corrections to the manuscript.
This work is supported by the National Natural Science Foundation of China (Nos. 92057111, 82071538), the Natural Science Foundation of Shaanxi Province (Nos. 2014JM4095, 2018JM7059) and the Xi’an City Science and Technology Project (No. 2017085CG/RC048 (XBDX001)).
The authors greatly appreciate Shaanxi Huikang Biotechnology Co., Ltd., and Xi’an Peihua University for providing the experimental platform.
ZZ and JZ were employed by the company Active Protein and Polypeptide Engineering Center of Shaanxi Huikang Biotechnology Co., Ltd. XY was employed by the company Kangya of Ningxia Pharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2023.1171824/full#supplementary-material
ADME, absorption, distribution, metabolism, and excretion; RP-HPLC, reversed-phase high-performance liquid chromatography; RPLC, reversed-phase liquid chromatography; SPPS, solid-phase synthesis; TFA, trifluoroacetic acid; pKa, acid dissociation constant; T, temperature; φ, organic modifier volume fraction;
Abdelhedi, O., and Nasri, M. (2019). Basic and recent advances in marine antihypertensive peptides: Production, structure-activity relationship and bioavailability. Trends Food Sci. Technol. 88, 543–557. doi:10.1016/j.tifs.2019.04.002
Abdelhedi, O., Nasri, R., Jridi, M., Mora, L., Oseguera-Toledo, M. E., Aristoy, M.-C., et al. (2017). In silico analysis and antihypertensive effect of ACE-inhibitory peptides from smooth-hound viscera protein hydrolysate: Enzyme-peptide interaction study using molecular docking simulation. Process Biochem. 58, 145–159. doi:10.1016/j.procbio.2017.04.032
Agrafiotou, P., Ràfols, C., Castells, C., Bosch, E., and Rosés, M. (2011). Simultaneous effect of pH, temperature and mobile phase composition in the chromatographic retention of ionizable compounds. J. Chromatogr. A 1218 (30), 4995–5009. doi:10.1016/j.chroma.2010.12.119
Al Musaimi, O., Valenzo, O. M. M., and Williams, D. R. (2023). Prediction of peptides retention behavior in reversed-phase liquid chromatography based on their hydrophobicity. J. Sep. Sci. 46 (2), 2200743. doi:10.1002/jssc.202200743
Alvarez-Segura, T., Subirats, X., and Rosés, M. (2019). Retention-pH profiles of acids and bases in hydrophilic interaction liquid chromatography. Anal. Chim. Acta 1050, 176–184. doi:10.1016/j.aca.2018.11.021
Annadi, A. M., El Zahar, N. M., El-Din, A. A.-S. N., Mohamed, E. H., Mahmoud, S. A., and Attia, M. S. (2022). Development and validation of molnupiravir assessment in bulk powder and pharmaceutical formulation by the RP-HPLC-UV method. RSC Adv. 12 (53), 34512–34519. doi:10.1039/D2RA05066H
Arkell, K., Breil, M. P., Frederiksen, S. S., and Nilsson, B. (2018). Mechanistic modeling of reversed-phase chromatography of insulins within the temperature range 10-40 °C. ACS Omega 3 (2), 1946–1954. doi:10.1021/acsomega.7b01527
Attwa, M. W., AlRabiah, H., Mostafa, G. A. E., and Kadi, A. A. (2023). Development of an LC-MS/MS method for quantification of sapitinib in human liver microsomes: In silico and in vitro metabolic stability evaluation. Molecules 28 (5), 2322. doi:10.3390/molecules28052322
Baeza-Baeza, J. J., and García-Alvarez-Coque, M. C. (2020). Extension of the linear solvent strength retention model including a parameter that describes the elution strength changes in liquid chromatography. J. Chromatogr. A 1615, 460757. doi:10.1016/j.chroma.2019.460757
Bergazin, T. D., Tielker, N., Zhang, Y., Mao, J., Gunner, M. R., Francisco, K., et al. (2021). Evaluation of log P, pK(a), and log D predictions from the SAMPL7 blind challenge. J. Comput. Aided. Mol. Des. 35 (7), 771–802. doi:10.1007/s10822-021-00397-3
Besleaga, I., Stepanenko, I., Petrasheuskaya, T. V., Darvasiova, D., Breza, M., Hammerstad, M., et al. (2021). Triapine analogues and their copper(II) complexes: Synthesis, characterization, solution speciation, redox activity, cytotoxicity, and mR2 RNR inhibition. Inorg. Chem. 60 (15), 11297–11319. doi:10.1021/acs.inorgchem.1c01275
Biancolillo, A., Maggi, M. A., Bassi, S., Marini, F., and D'Archivio, A. A. (2020). Retention modelling of phenoxy acid herbicides in reversed-phase HPLC under gradient elution. Molecules 25 (6), 1262. doi:10.3390/molecules25061262
Caltabiano, A. M., Foley, J. P., and Striegel, A. M. (2018). Organic solvent modifier and temperature effects in non-aqueous size-exclusion chromatography on reversed-phase columns. J. Chromatogr. A 1531, 83–103. doi:10.1016/j.chroma.2017.11.027
D'Archivio, A. A. (2019). Artificial neural network prediction of retention of amino acids in reversed-phase HPLC under application of linear organic modifier gradients and/or pH gradients. Molecules 24 (3), 632. doi:10.3390/molecules24030632
D’Archivio, A. A., and Maggi, M. A. (2017). Investigation by response surface methodology of the combined effect of pH and composition of water-methanol mixtures on the stability of curcuminoids. Food Chem. 219, 414–418. doi:10.1016/j.foodchem.2016.09.167
den Uijl, M. J., Schoenmakers, P. J., Pirok, B. W. J., and van Bommel, M. R. (2021). Recent applications of retention modelling in liquid chromatography. J. Sep. Sci. 44 (1), 88–114. doi:10.1002/jssc.202000905
Elmansi, H., Nasr, J. J., Rageh, A. H., El-Awady, M. I., Hassan, G. S., Abdel-Aziz, H. A., et al. (2019). Assessment of lipophilicity of newly synthesized celecoxib analogues using reversed-phase HPLC. BMC Chem. [Online] 13 (1), 84. [Accessed 2019/12//]. doi:10.1186/s13065-019-0607-6
Faisal, Z., Derdák, D., Lemli, B., Kunsági-Máté, S., Bálint, M., Hetényi, C., et al. (2018). Interaction of 2'R-ochratoxin A with serum albumins: Binding site, effects of site markers, thermodynamics, species differences of albumin-binding, and influence of albumin on its toxicity in mdck cells. Toxins (Basel) 10 (9), 353. doi:10.3390/toxins10090353
Fan, K., Peng, J., Peng, H., Zhang, Z., Chen, J., Luo, P., et al. (2022). Effect of spacer alkyl chain length on retention among three imidazolium stationary phases under various modes in high performance liquid chromatography. J. Chromatogr. A 1685, 463646. doi:10.1016/j.chroma.2022.463646
Ferreira, M. R., Garzón, A. G., Oliva, M. E., Cian, R. E., Drago, S. R., and D'Alessandro, M. E. (2022). Lipid-lowering effect of microencapsulated peptides from brewer's spent grain in high-sucrose diet-fed rats. Food Biosci. 49, 101981. doi:10.1016/j.fbio.2022.101981
Flieger, J., Orzeł, A., Kowalska-Kępczyńska, A., Pizoń, M., Trębacz, H., Majerek, D., et al. (2020). Teicoplanin-Modified HPLC column as a source of experimental parameters for prediction of the anticonvulsant activity of 1,2,4-triazole-3-thiones by the regression models. Mater. (Basel) 13 (11), 2650. doi:10.3390/ma13112650
Flieger, J., Trębacz, H., Pizoń, M., Plazińska, A., Plaziński, W., Kowalska, A., et al. (2019). Thermodynamic study of new antiepileptic compounds by combining chromatography on the phosphatidylcholine biomimetic stationary phase and differential scanning calorimetry. J. Sep. Sci. 42 (16), 201900248, doi:10.1002/jssc.201900248
Fouad, M. A., Serag, A., Tolba, E. H., El-Shal, M. A., and El Kerdawy, A. M. (2022). QSRR modeling of the chromatographic retention behavior of some quinolone and sulfonamide antibacterial agents using firefly algorithm coupled to support vector machine. BMC Chem. 16 (1), 85. doi:10.1186/s13065-022-00874-2
Galaon, T., and David, V. (2011). Deviation from van't Hoff dependence in RP-LC induced by tautomeric interconversion observed for four compounds. J. Sep. Sci. 34 (12), 1423–1428. doi:10.1002/jssc.201100029
Gao, X., Fang, D., Liang, Y., Deng, X., Chen, N., Zeng, M., et al. (2022). Circular RNAs as emerging regulators in COVID-19 pathogenesis and progression. Front. Immunol. 13, 980231. doi:10.3389/fimmu.2022.980231
Gisbert-Alonso, A., Navarro-Huerta, J. A., Torres-Lapasió, J. R., and García-Alvarez-Coque, M. C. (2021). Global retention models and their application to the prediction of chromatographic fingerprints. J. Chromatogr. A 1637, 461845. doi:10.1016/j.chroma.2020.461845
Guo, H., Li, L., and Gao, L. (2023). Paraquat and diquat: Recent updates on their pretreatment and analysis methods since 2010 in biological samples. Molecules 28 (2), 684. doi:10.3390/molecules28020684
Guo, H., Wahab, M. F., Berthod, A., and Armstrong, D. W. (2018). Mass spectrometry detection of basic drugs in fast chiral analyses with vancomycin stationary phases. J. Pharm. Anal. 8 (5), 324–332. doi:10.1016/j.jpha.2018.08.001
Hong, X., Zhao, Y., Zhuang, R., Liu, J., Guo, G., Chen, J., et al. (2020). Bioremediation of tetracycline antibiotics-contaminated soil by bioaugmentation. RSC Adv. 10 (55), 33086–33102. doi:10.1039/D0RA04705H
Huang, W. W., Hong, B. H., Sun, J. P., Tan, R., Bai, K. K., Yang, T., et al. (2019). Comparing the simultaneous determination of cis- and trans-palmitoleic acid in fish oil using HPLC and GC. Lipids Health Dis. 18 (1), 86. doi:10.1186/s12944-019-1033-4
Idroes, R., Muslem, M. S., Idroes, G. M., Suhendra, R., Suhendra, R., et al. (2020). The effect of column and temperature variation on the determination of the dead time in gas chromatographic systems using indirect methods. Heliyon 6 (2), e03302. doi:10.1016/j.heliyon.2020.e03302
Janicka, M., Sztanke, M., and Sztanke, K. (2020). Predicting the blood-brain barrier permeability of new drug-like compounds via HPLC with various stationary phases. Molecules 25 (3), 487. doi:10.3390/molecules25030487
Kaczmarski, K., and Chutkowski, M. (2021). Impact of changes in physicochemical parameters of the mobile phase along the column on the retention time in gradient liquid chromatography. Part A – temperature gradient. J. Chromatogr. A 1655, 462509. doi:10.1016/j.chroma.2021.462509
Konçe, İ., Demiralay, E. Ç., and Ortak, H. Y. (2019). Chromatographic determination of thermodynamic acid dissociation constants of tetracycline antibiotics and their epimers. J. Chromatogr. Sci. 57 (8), 745–750. doi:10.1093/chromsci/bmz051
Kumari, P., Van Laethem, T., Hubert, P., Fillet, M., Sacré, P. Y., and Hubert, C. (2023). Quantitative structure retention-relationship modeling: Towards an innovative general-purpose strategy. Molecules 28 (4), 1696. doi:10.3390/molecules28041696
Langyan, S., Khan, F. N., Yadava, P., Alhazmi, A., Mahmoud, S. F., Saleh, D. I., et al. (2021). In silico proteolysis and analysis of bioactive peptides from sequences of fatty acid desaturase 3 (FAD3) of flaxseed protein. Saudi. J. Biol. Sci. 28 (10), 5480–5489. doi:10.1016/j.sjbs.2021.08.027
Lin, T., Chen, B., Fang, L., You, H., Chu, C., Shao, Q., et al. (2022). Solvent strength of organic phase for two biphasic solvent systems in high speed countercurrent chromatography. J. Chromatogr. A 1680, 463422. doi:10.1016/j.chroma.2022.463422
Liu, C., Wang, W., Zhang, K., Liu, Q., Ma, T., Tan, L., et al. (2022). Protective effects of polydatin from grapes and reynoutria japonica houtt. On damaged macrophages treated with acetaminophen. Nutrients 14 (10), 2077. doi:10.3390/nu14102077
Luo, Y., Chalkou, K., Yamada, R., Funada, S., Salanti, G., and Furukawa, T. A. (2020). Predicting the treatment response of certolizumab for individual adult patients with rheumatoid arthritis: Protocol for an individual participant data meta-analysis. Syst. Rev. 9 (1), 140. doi:10.1186/s13643-020-01401-x
Marchetti, N., Giovannini, P. P., Catani, M., Pasti, L., and Cavazzini, A. (2019). Thermodynamic insights into the separation of carotenoids in reversed-phase liquid chromatography. Int. J. Anal. Chem. 2019, 1. 7. doi:10.1155/2019/7535813
Nagase, K., Umemoto, Y., and Kanazawa, H. (2021). Effect of pore diameter on the elution behavior of analytes from thermoresponsive polymer grafted beads packed columns. Sci. Rep. 11 (1), 9976. doi:10.1038/s41598-021-89165-9
Nie, Y., Li, J., Yang, X., Hou, X., and Fang, H. (2022). Development of QSRR model for hydroxamic acids using PCA-GA-BP algorithm incorporated with molecular interaction-based features. Front. Chem. 10, 1056701. doi:10.3389/fchem.2022.1056701
Numviyimana, C., Chmiel, T., Kot-Wasik, A., and Namieśnik, J. (2019). Study of pH and temperature effect on lipophilicity of catechol-containing antioxidants by reversed phase liquid chromatography. Microchem. J. 145, 380–387. doi:10.1016/j.microc.2018.10.048
Ofosu, F. K., Mensah, D. F., Daliri, E. B., and Oh, D. H. (2021). Exploring molecular insights of cereal peptidic antioxidants in metabolic syndrome prevention. Antioxidants (Basel) 10 (4), 518. doi:10.3390/antiox10040518
Oney-Montalvo, J. E., Morozova, K., Ramírez-Sucre, M. O., Scampicchio, M., and Rodríguez-Buenfil, I. M. (2022). Determination of peak purity in HPLC by coupling coulometric array detection and two-dimensional correlation analysis. Sensors (Basel) 22 (5), 1794. doi:10.3390/s22051794
Park, S. H., De Pra, M., Haddad, P. R., Grosse, S., Pohl, C. A., and Steiner, F. (2020). Localised quantitative structure–retention relationship modelling for rapid method development in reversed-phase high performance liquid chromatography. J. Chromatogr. A 1609, 460508. doi:10.1016/j.chroma.2019.460508
Phyo, Y. Z., Cravo, S., Palmeira, A., Tiritan, M. E., Kijjoa, A., Pinto, M. M. M., et al. (2018). Enantiomeric resolution and docking studies of chiral xanthonic derivatives on chirobiotic columns. Molecules 23 (1), 142. doi:10.3390/molecules23010142
Qiao, Q. Q., Luo, Q. B., Suo, S. K., Zhao, Y. Q., Chi, C. F., and Wang, B. (2022). Preparation, characterization, and cytoprotective effects on HUVECs of fourteen novel angiotensin-I-converting enzyme inhibitory peptides from protein hydrolysate of tuna processing by-products. Front. Nutr. 9, 868681. doi:10.3389/fnut.2022.868681
Rook, M. L., Musgaard, M., and MacLean, D. M. (2021). Coupling structure with function in acid-sensing ion channels: Challenges in pursuit of proton sensors. J. Physiol. 599 (2), 417–430. doi:10.1113/JP278707
Samtiya, M., Acharya, S., Pandey, K. K., Aluko, R. E., Udenigwe, C. C., and Dhewa, T. (2021). Production, purification, and potential health applications of edible seeds' bioactive peptides: A concise review. Foods 10 (11), 2696. doi:10.3390/foods10112696
Sheng, Y., Qiu, Y. T., Wang, Y. M., Chi, C. F., and Wang, B. (2022). Novel antioxidant collagen peptides of siberian sturgeon (acipenserbaerii) cartilages: The preparation, characterization, and cytoprotection of H(2)O(2)-damaged human umbilical vein endothelial cells (HUVECs). Mar. Drugs 20 (5), 325. doi:10.3390/md20050325
Shi, Y., Hu, J., Wang, H., Yan, Z., Zhao, G., Gao, X., et al. (2022). Establishing a UHPLC-MS/MS method for evaluation of the influence of stir-frying on the pharmacokinetics of seven compounds in Arctii Fructus. RSC Adv. 12 (42), 27525–27533. doi:10.1039/D2RA03637A
Sitkov, N., Zimina, T., Kolobov, A., Sevostyanov, E., Trushlyakova, V., Luchinin, V., et al. (2021). Study of the fabrication Technology of hybrid microfluidic biochips for label-free detection of proteins. Micromachines (Basel) 13 (1), 20. doi:10.3390/mi13010020
Soriano-Meseguer, S., Fuguet, E., Port, A., and Rosés, M. (2019). Influence of the acid-base ionization of drugs in their retention in reversed-phase liquid chromatography. Anal. Chim. Acta 1078, 200–211. doi:10.1016/j.aca.2019.05.063
Sousa, H. B. A., Martins, C. S. M., and Prior, J. A. V. (2021). You don't learn that in school: An updated practical guide to carbon quantum dots. Nanomater. (Basel) 11 (3), 611. doi:10.3390/nano11030611
Suo, S. K., Zhao, Y. Q., Wang, Y. M., Pan, X. Y., Chi, C. F., and Wang, B. (2022). Seventeen novel angiotensin converting enzyme (ACE) inhibitory peptides from the protein hydrolysate of Mytilus edulis: Isolation, identification, molecular docking study, and protective function on HUVECs. Food Funct. 13 (14), 7831–7846. doi:10.1039/d2fo00275b
Tanase, M., Soare, A., David, V., and Moldoveanu, S. C. (2019). Sources of nonlinear van't Hoff temperature dependence in high-performance liquid chromatography. ACS Omega 4 (22), 19808–19817. doi:10.1021/acsomega.9b02689
Tengattini, S., Rimaroli, C., Galmozzi, M. R., Furlanetto, S., Massolini, G., and Temporini, C. (2022). Effect of mobile phase pH on liquid chromatography retention of mepartricin related compounds and impurities as support to the structural investigation by liquid chromatography–mass spectrometry. J. Pharm. Biomed. 220, 114971. doi:10.1016/j.jpba.2022.114971
Tsui, H.-W., Kuo, C.-H., and Huang, Y.-C. (2019). Elucidation of retention behaviors in reversed-phase liquid chromatography as a function of mobile phase composition. J. Chromatogr. A 1595, 127–135. doi:10.1016/j.chroma.2019.02.049
Vasconcelos, L., Dias, L. G., Leite, A., Ferreira, I., Pereira, E., Silva, S., et al. (2023). SVM regression to assess meat characteristics of bísaro pig loins using NIRS methodology. Foods 12 (3), 470. doi:10.3390/foods12030470
Waili, Y., Gahafu, Y., Aobulitalifu, A., Chang, Z., Xie, X., and Kawuli, G. (2021). Isolation, purification, and characterization of antioxidant peptides from fresh mare's milk. Food Sci. Nutr. 9 (7), 4018–4027. doi:10.1002/fsn3.2292
Wang, J., Wu, Y., Chen, Z., Chen, Y., Lin, Q., and Liang, Y. (2022). Exogenous bioactive peptides have a potential therapeutic role in delaying aging in rodent models. Int. J. Mol. Sci. 23 (3), 1421. doi:10.3390/ijms23031421
Xie, Y., Yuan, P., Heng, T., Du, L., An, Q., Zhang, B., et al. (2022). Insight into the formation of cocrystal and salt of tenoxicam from the isomer and conformation. Pharmaceutics 14 (9), 1968. doi:10.3390/pharmaceutics14091968
Xiong, J., Li, Z., Wang, G., Fu, Z., Zhong, F., Xu, T., et al. (2022). Multi-instance learning of graph neural networks for aqueous pKa prediction. Bioinformatics 38 (3), 792–798. doi:10.1093/bioinformatics/btab714
Xu, Z., Chughtai, H., Tian, L., Liu, L., Roy, J. F., and Bayen, S. (2023). Development of quantitative structure-retention relationship models to improve the identification of leachables in food packaging using non-targeted analysis. Talanta 253, 123861. doi:10.1016/j.talanta.2022.123861
Yang, J., Moraga, A., Xu, J., Zhao, Y., Luo, P., Lao, K. H., et al. (2020a). A histone deacetylase 7-derived peptide promotes vascular regeneration via facilitating 14-3-3γ phosphorylation. Stem Cells 38 (4), 556–573. doi:10.1002/stem.3122
Yang, X., Peng, H., Han, N., Zhang, Z., Bai, X., Zhao, T., et al. (2020b). Quantitative structure–chromatographic retention relationship of synthesized peptides (HGRFG, NPNPT) and their derivatives. Anal. Biochem. 597, 113653. doi:10.1016/j.ab.2020.113653
Yang, Y.-X., Zhang, Q., Li, Q.-Q., Xia, Z.-N., Chen, H., Zhou, K., et al. (2018). pH-dependent surface electrostatic effects in retention on immobilized artificial membrane chromatography: Determination of the intrinsic phospholipid-water sorption coefficients of diverse analytes. J. Chromatogr. A 1570, 172–182. doi:10.1016/j.chroma.2018.07.081
Ye, H., Xu, Y., Sun, Y., Liu, B., Chen, B., Liu, G., et al. (2023). Purification, identification and hypolipidemic activities of three novel hypolipidemic peptides from tea protein. Food Res. Int. 165, 112450. doi:10.1016/j.foodres.2022.112450
Yılmaz Ortak, H., and Cubuk Demiralay, E. (2019). Effect of temperature on the retention of Janus kinase 3 inhibitor in different mobile phase compositions using reversed-phase liquid chromatography. J. Pharm. Biomed. Anal. 164, 706–712. doi:10.1016/j.jpba.2018.11.032
Yuan, N., Chen, J., Cai, T., Li, Z., Guan, M., Zhao, L., et al. (2020). Glucose-based carbon dots-modified silica stationary phase for hydrophilic interaction chromatography. J. Chromatogr. A 1619, 460930. doi:10.1016/j.chroma.2020.460930
Żesławska, E., Zakrzewski, R., Nowicki, A., Korona-Głowniak, I., Lyčka, A., Kania, A., et al. (2022). Synthesis, crystal structures, lipophilic properties and antimicrobial activity of 5-Pyridylmethylidene-3-rhodanine-carboxyalkyl acids derivatives. Molecules 27 (13), 3975. doi:10.3390/molecules27133975
Zhang, Y., Liang, R., Xie, A., Shi, W., Huang, H., and Zhong, Y. (2020). Antagonistic peptides that specifically bind to the first and second extracellular loops of CCR5 and anti-IL-23p19 antibody reduce airway inflammation by suppressing the IL-23/Th17 signaling pathway. Mediat. Inflamm. 2020, 1–13. doi:10.1155/2020/1719467
Zhang, Y., Zhao, Z., Wang, K., Lyu, K., Yao, C., Li, L., et al. (2022). Molecular docking assisted exploration on solubilization of poorly soluble drug remdesivir in sulfobutyl ether-tycyclodextrin. AAPS Open 8 (1), 9. doi:10.1186/s41120-022-00054-5
Zhu, X., Li, P., Tang, J., Su, Y., Xiao, M., Xue, H., et al. (2022). A simple and practical solvent system selection strategy for high-speed countercurrent chromatography based on the HPLC polarity parameter model. Anal. Methods 14 (46), 4822–4831. doi:10.1039/D2AY01377K
Keywords: chromatographic retention, pentapeptides, six-parameter model, retention factor, prediction capacity
Citation: Peng H, Yang X, Fang H, Zhang Z, Zhao J, Zhao T, Liu J and Li Y (2023) Simultaneous effect of different chromatographic conditions on the chromatographic retention of pentapeptide derivatives (HGRFG and NPNPT). Front. Chem. 11:1171824. doi: 10.3389/fchem.2023.1171824
Received: 22 February 2023; Accepted: 29 March 2023;
Published: 18 April 2023.
Edited by:
Janardhan Reddy Koduru, Kwangwoon University, Republic of KoreaReviewed by:
Chang-Feng Chi, Zhejiang Ocean University, ChinaCopyright © 2023 Peng, Yang, Fang, Zhang, Zhao, Zhao, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Li, bGl5YW54anR1QHhqdHUuZWR1LmNu; Jianli Liu, amxsaXVAbnd1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.