
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Chem. , 20 January 2023
Sec. Nanoscience
Volume 11 - 2023 | https://doi.org/10.3389/fchem.2023.1119240
This article is part of the Research Topic Nanoscience Editor's Pick 2024 View all 12 articles
This mini-review summarizes the seminal exploration of aqueous supramolecular chemistry of crown ether macrocycles. In history, most research of crown ethers were focusing on their supramolecular chemistry in organic phase or in gas phase. In sharp contrast, the recent research evidently reveal that crown ethers are very suitable for studying abroad range of the properties and applications of water interactions, from: high water-solubility, control of Hofmeister series, “structural water”, and supramolecular adhesives. Key studies revealing more details about the properties of water and aqueous solutions are highlighted.
Supramolecular chemistry provides a powerful platform for achieving complex chemical/biological activities using non-covalent interactions. Water is significant for biomimetic chemistry and to achieve sustainability in both the natural environment and human societies. Thus, exploring aqueous supramolecular chemistry is essential for producing advanced functional materials for biomedical processing, energy, information technology, and environmental science applications (Zayed et al., 2010; Cremer et al., 2017; Zheng et al., 2018; Gatiatulin et al., 2019; Xiao et al., 2020a; Duan et al., 2020; WuXiao, 2020; Zhang et al., 2020; Zhang et al., 2021a; Zhang et al., 2021b; Ding et al., 2021; Liu et al., 2021; Zhu et al., 2021; Shi et al., 2022).
Crown ethers (CEs) are cyclic compounds comprising several ether linkages with a specifically sized cavity in their centers. The discovery of CEs was pivotal in the establishment of supramolecular chemistry. CE supramolecular chemistry is predominantly conducted in organic solvents (Qi et al., 2012; Qi et al., 2014; Qi and Schalley, 2014; Ali et al., 2019; Ge et al., 2019; Xiao et al., 2021) or partially in the gas phase (Weimann et al., 2009; Qi et al., 2013). The molecular structure of CEs indicates their great flexibility to adapt their conformation to interact with water molecules. In organic media, the lone electron pairs on the CE oxygen atoms create a region of high electron density in the ring cavity. CEs exhibit a rich conformational panorama and low energy barriers, and research in the past two decades has focused on fabricating CE-based threads or interlocking components for use in nanomachines. These reactions have primarily been conducted in organic solvents; however, in aqueous media, the polar surfaces of CE molecules are exposed, with a water-accessible surface area (ASA) originating from the ethylene glycol units. Therefore, it is reasonable to expect that CEs can be water-soluble macrocycles. However, the scientific community has long been skeptical of the solubility of CEs in water, resulting in few reported studies in the literature of water-soluble CEs (Zhang et al., 2022a; Wang et al., 2022) compared to those on other macrocycles, such as cucurbiturils (Das et al., 2019), calixarenes (Xu et al., 2019), cyclodextrins (Kwong et al., 2021; Li et al., 2021; Luo et al., 2021; Zhang et al., 2022b), and pillararenes (Yu et al., 2021). Recently, CEs have emerged as an intriguing host for studying water and aqueous supramolecular chemistry.
In this mini-review, we summarize applications that have capitalized on CE–water interactions to fabricate aqueous materials. Unexpectedly high water solubility, control of the Hofmeister cationic series, and “structural water” in supramolecular adhesives are highlighted.
In 2017, Qi et al. serendipitously discovered that benzo-21-crown-7 ether (C7) exhibits remarkably high water solubility (Dong et al., 2017a). The solubility of C7 in water at room temperature reaches 1,500 g/L (4.21 M), which is superior to those of many classic water-soluble supramolecular macrocycles, such as cucurbit [n]urils and α-, β-, and γ-cyclodextrins (see comparison in Figure 1). Concentration-dependent nuclear Overhauser effect spectra (NOESY) revealed no significant intermolecular interactions between the C7 molecules in solution. Diffusion-ordered NMR spectra (DOSY) indicated that the C7 diffusion constants at high and low concentrations were similar. The solution was prepared using the C7 monomer and no molecular aggregation was observed. The functional group on the benzo-moiety significantly affects the water-solubility of C7 (Figure 1). The solubility of the derivatives follows the sequence: C7 > C7NH2 > C7CN > C7COOH. Entropic desolvation of the CE ethylene glycol units is known to increase the hydrophobicity of the cyclic chain, which results in separation from the aqueous solution. Therefore, the thermo-responsivity of C7 and its derivatives in water is an intriguing topic of study (Zhang et al., 2021c). For example, low-molar-mass C7 and C7CN exhibit lower critical solution temperature (LCST) phenomena. Interestingly, C7COOH exhibits upper critical solution temperature (UCST) behavior followed by LCST phase behavior, implying that the benzo-group functionalization is instrumental in modulating the thermo-responsiveness of the CEs.
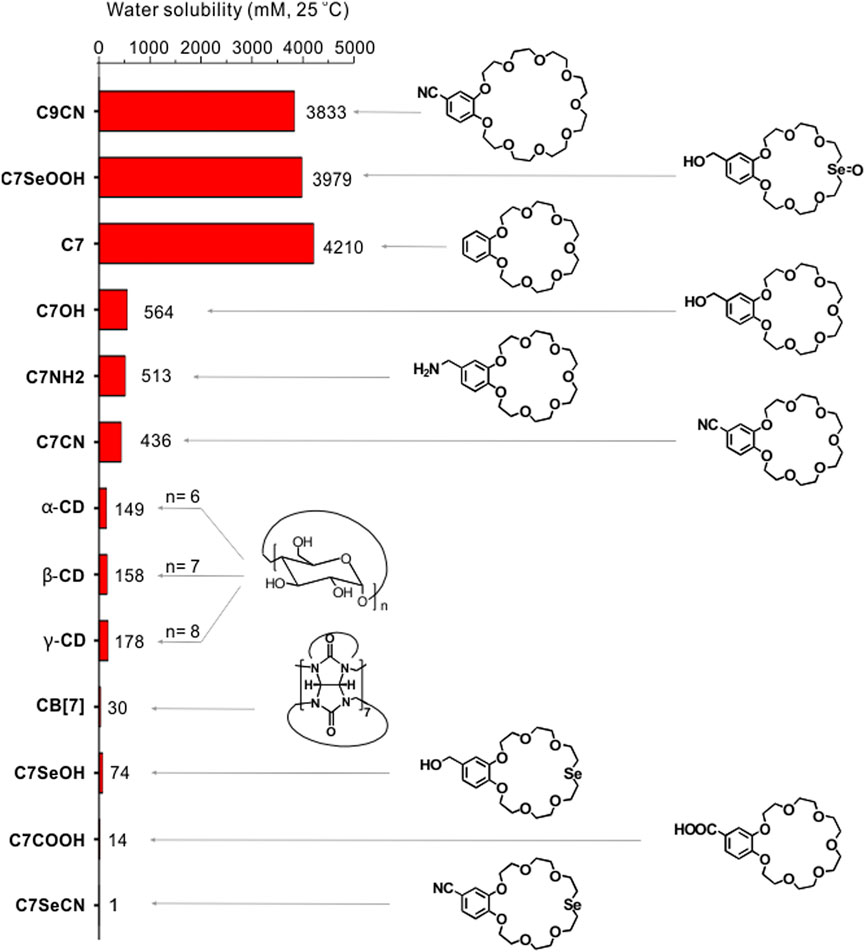
FIGURE 1. Comparison of the water-solubility of CEs and their derivatives with α-cyclodextrin, β-cyclodextrin, and γ-cyclodextrin and cucurbit[7]urils. Adapted and reproduced from Dong et al. (2017a), Jin et al. (2019), Xu et al. (2021) with kind permission from American Chemical Society and Elsevier.
Substituting the O atom opposite the benzo-moiety on the CE with an Se atom transformed the C7CN from hydrophilic to strongly hydrophobic, and the aqueous solubility of C7SeCN decreased to 1 mM (Figure 1) (Jin et al., 2019). Using detailed computer modeling, the solvation free energies (
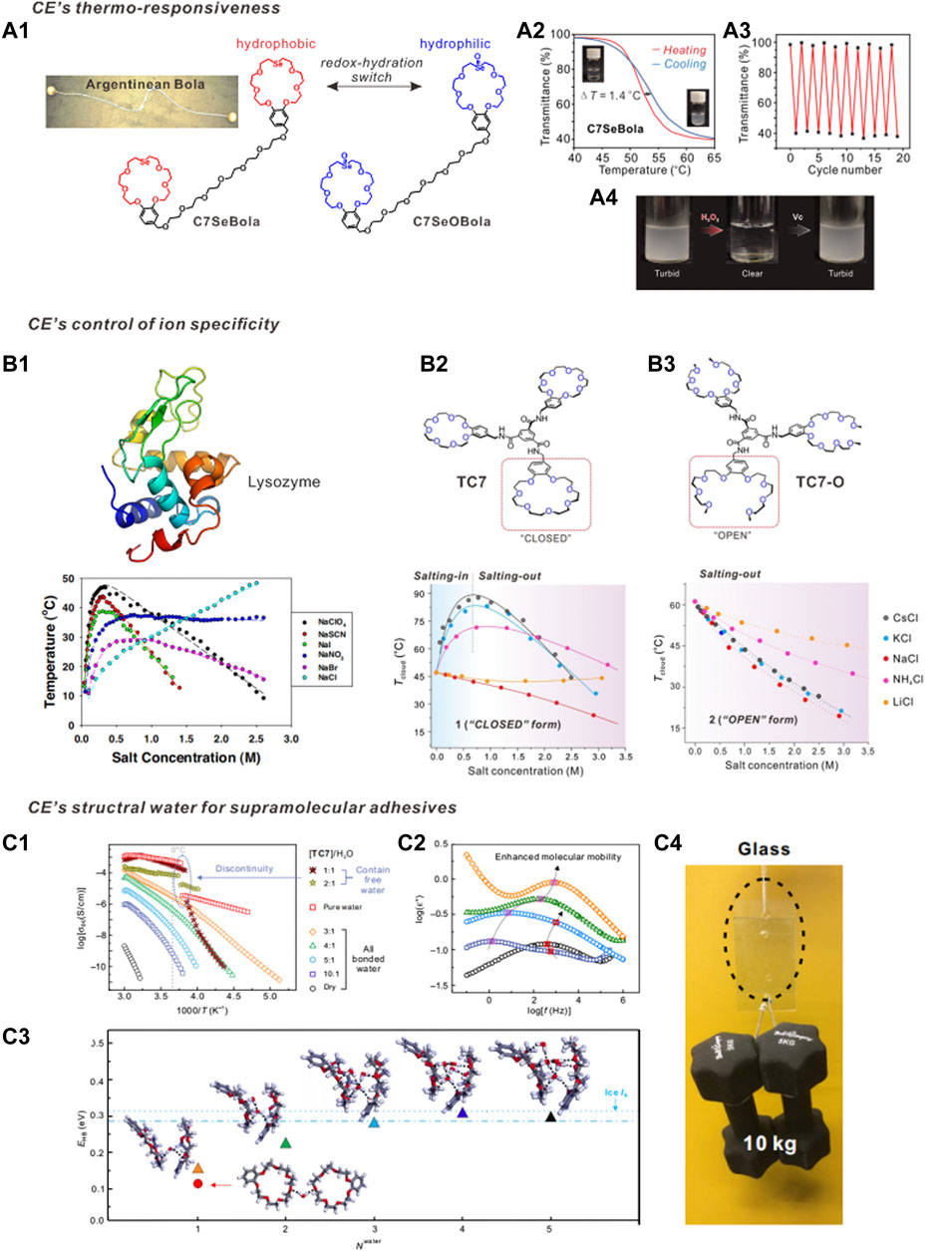
FIGURE 2. (A) The redox-controlled hydration of CE for design macrocyclic amphiphiles. (A1) the chemical structure of bolaamphiphile C7SeBola; (A2) C7SeBola exhibited rapid LCST behavior in water, (A3) The highly reversible LCST transition processes, (A4) turbid-to-clear and clear-to-turbid transitions can be controlled in an “ON-OFF” manner. (B) Hofmeister series reversal behavior. (B1) Hofmeister series reversal of anions for lysozyme reported by Cremer et al. The apparent association constants of anionic-lysozyme interaction for ClO4−, SCN−, I−, NO3−, Br− and Cl− are 0.10, 0.10, 0.09, 0.10, 0.18 and 0.12 M−1, respectively. (B2) Hofmeister series reversal of cation for a triple-CE-molecule (TC7). The inverse Hofmeister series at low salt concentration regimes should be the cation–solute interaction at wok. The charge density is initially dominant and then decays due to the increase of the ionic radius, then the enhanced “size-fitting” induced molecular recognition dominants the system. (B3) Ring-open counterpart (TC7-O) showed no Hofmeister series reversal. (C) TC7 generates structural water for fabricating supramolecular adhesive material. (C1) The dependence of DC conductivity
The chemistry of aqueous salt solutions is rich in ambiguities and a prime example is the Hofmeister effect, which is a prominent example of a multilateral interactive relationship (Jungwirth and Cremer, 2014). As early as 1888, Franz Hofmeister, a Czech protein scientist, noticed specific cationic and anionic effects on the solubility of proteins and discovered that the effectiveness of simple ions in protein precipitation followed a specific order (the Hofmeister series). The effectiveness of the influence of positive ions on precipitating proteins is smaller than negative charged ions (Aoki et al., 2016). Subsequently, a wide variety of phenomena, from protein folding and enzymatic activity to colloidal assembly and bacterial growth, have been shown to follow the Hofmeister series (Wang et al., 2021). Recent evidence suggests that Hofmeister effects arise from direct interactions between ions and solutes and between ions and the first solvation shells of those solutes. However, several observations have challenged these theoretical ideas, such as the Hofmeister series reversal. The reversal of the Hofmeister series between low and high salt concentration regimes was initially reported in 1911. However, explanation and prediction of such phenomena are not possible using existing theories on specific ion effects. The current prevailing theory, the law of matching water affinities (LMWA), can only qualitatively explain and rank ion–ion and ion–solute interactions on charged surfaces. The rules and parameters that govern the reversal phenomena on a neutral polar surface, such as the uncharged hydrophilic regions on proteins or synthetic materials, have not yet been determined.
To address this issue, Qi and Dong developed a low-molecular-weight supramolecular system to reveal the underlying systematic relationships between ions, water, and solutes (Qi et al., 2018). A triple-CE-molecule (TC7) and its ring-opened counterpart (TC7-O) exhibit a distinctive topological effect (Figures 2B2, B3). When neutral polar groups are arranged in a crown geometry, the “size-fitting”-induced solute-cation recognition, though rather weak, efficiently tunes the Hofmeister series and results in a Hofmeister series reversal phenomenon with singly charged cations. The phase transition curve of TC7 with cations (Figure 2B2, bottom) is quite similar to that of lysozyme with anions (Figure 2B1, bottom) reported by Cremer et al. (Zhang and Cremer, 2009). Interestingly, isothermal titration calorimetry measurements revealed unexpectedly low cation-solute interactions in this Hofmeister series reversal, with an apparent equilibrium association constant Ka of ∼10 M−1. Similar low binding affinities, from a supramolecular chemistry viewpoint, are observed in the majority of reported supramolecular recognition motifs (Ka > 103 M−1). Therefore, CEs provide an elegant and simple model to control the Hofmeister series reversal behavior, which has long been a topic of debate in the physics community (Jungwirth and Cremer, 2014).
On-going research has further demonstrated the general principles of the spatial topology factor and site-fitting effect in CE-containing ionic systems (Huang et al., 2018; Luo et al., 2019; Pan et al., 2021). Chen et al. observed a similar coexisting salting-in and salting-out effect (Huang et al., 2018) when C7 was functionalized on a poly (vinyl alcohol) polymer system, demonstrating that CEs can serve as general building blocks to control ion specificity in aqueous media. A benzo-27-crown-9 ether (C9OH) system was used to explore the “size-fitting”-induced host-guest complexation between C9 and guanidinium. A similar coexisting salting-in and salting-out effect can be extended to guanidinium-type ions, such as guanidinium chloride and arginine (Pan et al., 2021).
Structural water molecules form strong HBs with the polar groups in proteins and are thought to tighten the protein matrix. These deeply-buried water molecules are considered an integral part of the folded protein structure. The concept of structural water bound within hydrophobic capsules that stabilize protein structures is well established in biology; however, the stabilization effects of water in materials science are rarely discussed. Supramolecular polymers are formed through non-covalent, directional interactions between monomeric building blocks. Assembly of these polymers is reversible, which enables functions such as coating, self-healing, and responsiveness (Wei et al., 2014; Xiao et al., 2020b; Lv et al., 2021). Water is typically regarded as a solvent in the assembly of supramolecular polymers; however, in 2017, Qi and Dong et al. reported a supramolecular polymer in which water acted as an indispensable comonomer in the supramolecular polymerization (Dong et al., 2017b).
In the dry state, TC7 is a fragile, slippery solid that has an amorphous glassy structure. However, when exposed to the ambient environment (25°C, 40% relative humidity), the dry TC7 quickly adsorbed the ambient water vapor, affording a TC7-water mixture with notable flexibility, elasticity, and adhesive properties (Figure 2C). Infrared spectra indicated that the TC7 molecules self-assembled into one-dimensional supramolecular aggregates via amido-HBs. Broadband dielectric spectra (BDS) provided key evidence of the existence of structural water molecules (Figures 2C1, C2). The frequency-dependence of the conductivity spectra of TC7n-water(H)1 (n = 1, 2, 3, 4, 5, 10; n denotes the weight ratio of TC7 to added water) presented the typical conductivity-frequency behavior observed in semiconducting materials (Zhang et al., 2021c). For weight ratios of TC7n-H1 above n > 2 (Figure 2C1), the
Inspired by this work, Dong et al. extended the structural water system to a pillararene-crown ether (PC) system (Li et al., 2020b) in which ten C7 units were decorated on the upper and lower rims of pillar[5]arene. The frequency-dependence of the BDS conductivity spectra for PC10-water (W)1 exhibited a similar discontinuity around the freezing point of water, indicating the existence of structural water. Intriguingly, the resulting CE-structural water supramolecular polymer system exhibited low-temperature-resistant adhesive properties. The resulting PC10-W1 complex exhibited adhesion strengths up to 2.49 MPa at −18°C. By contrast, the adhesion strengths of samples with a high water content were between 0.37 and 0.46 MPa, which is comparable to that of pure water (0.36 MPa). Notably, the structural water in the PC10-W1 sample remained liquid down to −80°C, indicating that structural water imparts anti-icing properties to the adhesive material. The macroscopic adhesive strength was maintained between 1.12 and 1.46 MPa at −80°C. By contrast, high-water-content PC samples became turbid solids at low temperatures because the free water in these samples froze. These studies elegantly demonstrate the potential of CEs with structural water as supramolecular adhesive materials (Zhang et al., 2019; Li et al., 2020c).
This mini-review has highlighted selected, preeminent examples from the growing field of aqueous supramolecular chemistry of CEs. These studies have revealed new and unusual properties of CEs arising from their unique ability to interact with water molecules. These studies demonstrated the correlation between the hydration of CEs and the cyclic topological effect and the implications of cation affinities for neutral polar surfaces in the Hofmeister series. There is still much to explore and accomplish in this field; 1) CEs decorated with diverse functional groups make it easier to create diverse functional building blocks (Shen et al., 2019), thus enabling numerous opportunities for designing novel supramolecular materials in water; 2) To date, investigations into the aqueous behaviors of Se- or Te-containing CEs are scarce. The intrinsic catalytic roles and redox-responsiveness of Se and Te compounds significantly expand the functional range of CEs via substitution of a chalcogen atom on the CE with Se or Te (Shang et al., 2021a; Shang et al., 2021b; Li et al., 2022); 3) Revealing the interactions between water, CEs, and ions can benefit applied areas such as anti-icing materials (Ge et al., 2022; Hu et al., 2022) and aqueous metal-ion batteries (Wang et al., 2020). Aqueous CE supramolecular chemistry is an emerging research field and further studies will broaden our understanding of the properties and potential applications of aqueous CE systems.
ZQ and YQ first conceived the idea, framework and writing direction of the article. YQ performed a literature search and summary, and wrote the first draft of the manuscript. ZQ, YQ, and YG scrutinized the manuscript for scientific terminology and made important contributions to the revision of the literature. All authors have read and checked the submitted version before uploading.
We gratefully acknowledge financial support from the National Natural Science Foundation of China (22071196, 22007078), Key R&D Program of Shaanxi Province (2021KWZ-18), Aeronautical Science Foundation of China (ASFC-2020Z061053001), Opening Project of State Key Laboratory of Polymer Materials Engineering (Sichuan University) (Grant No. klpme 2021-05-03), Student Innovation and Entrepreneurship Education Center of the Student Work Department of the Party Committee of NPU (2021-cxcy-012), Higher Education Research Fund of NPU (CJGZMS202202), National Innovation Training Program for College Students (202210699005), the Fundamental Research Funds for the Central Universities, and Fellowship from CSC Innovative Team Program (CXXM2110141862). We thank the Analytical & Testing Center of NPU for the characterization of materials. We thank Dong Shengyi’s scientific suggestion and assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ali, M. C., Liu, R., Chen, J., Cai, T., Zhang, H., Li, Z., et al. (2019). New deep eutectic solvents composed of crown ether, hydroxide and polyethylene glycol for extraction of non-basic N-compounds. Chin. Chem. Lett. 30, 871–874. doi:10.1016/j.cclet.2019.02.025
Aoki, K., Shiraki, K., and Hattori, T. (2016). Salt effects on the picosecond dynamics of lysozyme hydration water investigated by terahertz time-domain spectroscopy and an insight into the Hofmeister series for protein stability and solubility. Phys. Chem. Chem. Phys. 18, 15060–15069. doi:10.1039/C5CP06324H
Cremer, P. S., Flood, A. H., Gibb, B. C., and Mobley, D. L. (2017). Collaborative routes to clarifying the murky waters of aqueous supramolecular chemistry. Nat. Chem. 10, 8–16. doi:10.1038/nchem.2894
Das, D., Assaf, K. I., and Nau, W. M. (2019). Applications of cucurbiturils in medicinal chemistry and chemical biology. Front. Chem. 7, 619. doi:10.3389/fchem.2019.00619
Deng, Y., Li, X., Han, C., and Dong, S. (2020). Supramolecular control over LCST behavior of hybrid macrocyclic system based on pillar[5]arene and crown ether. Chin. Chem. Lett. 31, 3221–3224. doi:10.1016/j.cclet.2020.03.074
Ding, M. H., Liao, J., Tang, L. L., Ou, G. C., and Zeng, F. (2021). High-yield synthesis of a novel water-soluble macrocycle for selective recognition of naphthalene. Chin. Chem. Lett. 32, 1665–1668. doi:10.1016/j.cclet.2020.11.019
Dong, S., Leng, J., Feng, Y., Liu, M., Stackhouse, C. J., Schonhals, A., et al. (2017). Structural water as an essential comonomer in supramolecular polymerization. Sci. Adv. 3, eaao0900. doi:10.1126/sciadv.aao0900
Dong, S., Wang, L., Wu, J., Jin, L., Ge, Y., Qi, Z., et al. (2017). Thermosensitive phase behavior of benzo-21-crown-7 and its derivatives. Langmuir 33, 13861–13866. doi:10.1021/acs.langmuir.7b03431
Duan, Q., Wang, L., Wang, F., Zhang, H., and Lu, K. (2020). Calix[n]arene/Pillar[n]arene-Functionalized graphene nanocomposites and their applications. Front. Chem. 8, 504. doi:10.3389/fchem.2020.00504
Gatiatulin, A. K., Ziganshin, M. A., and Gorbatchuk, V. V. (2019). Smart molecular recognition: From key-to-lock principle to memory-based selectivity. Front. Chem. 7, 933. doi:10.3389/fchem.2019.00933
Ge, Y., Gong, H., Shang, J., Jin, L., Pan, T., Zhang, Q., et al. (2019). Supramolecular gel based on crown-ether-appended dynamic covalent macrocycles. Macromol. Rapid Commun. 40, e1800731. doi:10.1002/marc.201800731
Ge, Y., Xu, Q., Yu, Z., Wang, R., Shen, X., and Qi, Z. (2022). Research progress of bioinspried-antifreeze protein material. Chin. J. Chem. Edu 43, 11–17. doi:10.13884/j.1003-3807hxjy.2021110017
Hu, B., Li, G., Ai, G., Zhang, M., Su, S., He, X., et al. (2022). Macrocycle molecule-based cryoprotectants for ice recrystallization inhibition and cell cryopreservation. J. Mater Chem. B 10, 6922–6927. doi:10.1039/d2tb01083f
Huang, D., Zhang, Q., Deng, Y., Luo, Z., Li, B., Shen, X., et al. (2018). Polymeric crown ethers: LCST behavior in water and stimuli-responsiveness. Polym. Chem. 9, 2574–2579. doi:10.1039/c8py00412a
Jin, L., Li, B., Cui, Z., Shang, J., Wang, Y., Shao, C., et al. (2019). Selenium substitution-induced hydration changes of crown ethers as tools for probing water interactions with supramolecular macrocycles in aqueous solutions. J. Phys. Chem. B 123, 9692–9698. doi:10.1021/acs.jpcb.9b09618
Jungwirth, P., and Cremer, P. S. (2014). Beyond hofmeister. Nat. Chem. 6, 261–263. doi:10.1038/nchem.1899
Kwong, C. H. T., Mu, J., Li, S., Fang, Y., Liu, Q., Zhang, X., et al. (2021). Reviving chloroquine for anti-SARS-CoV-2 treatment with cucurbit[7]uril-based supramolecular formulation. Chin. Chem. Lett. 32, 3019–3022. doi:10.1016/j.cclet.2021.04.008
Li, B., Xu, Q., Shen, X., Pan, T., Shang, J., Ge, Y., et al. (2022). Atom-economic macrocyclic amphiphile based on guanidinium-functionalized selenacrown ether acting as redox-responsive nanozyme. Chin. Chem. Lett., 108015. doi:10.1016/j.cclet.2022.108015
Li, D., Zhang, Q., Zhao, W., Dong, S., Li, T., and Stang, P. J. (2020). Thermo/Anion dual-responsive supramolecular organoplatinum-crown ether complex. Org. Lett. 22, 4289–4293. doi:10.1021/acs.orglett.0c01333
Li, S., Gao, Y., Ding, Y., Xu, A., and Tan, H. (2021). Supramolecular nano drug delivery systems mediated via host-guest chemistry of cucurbit[n]uril (n = 6 and 7). Chin. Chem. Lett. 32, 313–318. doi:10.1016/j.cclet.2020.04.049
Li, T., Zhang, Q., Li, D., Dong, S., Zhao, W., and Stang, P. J. (2020). Rational design and bulk synthesis of water-containing supramolecular polymers. ACS Appl. Mater Interfaces 12, 38700–38707. doi:10.1021/acsami.0c11546
Li, X., Lai, J., Deng, Y., Song, J., Zhao, G., and Dong, S. (2020). Supramolecular adhesion at extremely low temperatures: A combined experimental and theoretical investigation. J. Am. Chem. Soc. 142, 21522–21529. doi:10.1021/jacs.0c10786
Liu, Y., Wang, H., Shangguan, L., Liu, P., Shi, B., Hong, X., et al. (2021). Selective separation of phenanthrene from aromatic isomer mixtures by a water-soluble azobenzene-based macrocycle. J. Am. Chem. Soc. 143, 3081–3085. doi:10.1021/jacs.1c01204
Luo, Y., Zhang, W., Liu, M., Zhao, J., Fan, Y., Bian, B., et al. (2021). A supramolecular fluorescent probe based on cucurbit[10]uril for sensing the pesticide dodine. Chin. Chem. Lett. 32, 367–370. doi:10.1016/j.cclet.2020.02.023
Luo, Z., Deng, Y., Li, X., Zhang, Q., Wu, J., Qi, Z., et al. (2019). LCST behavior controlled by size-matching selectivity from low molecular weight monomer systems. New J. Chem. 43, 6890–6896. doi:10.1039/c9nj00846b
Lv, P., Shen, X., Cui, Z., Li, B., Xu, Q., Yu, Z., et al. (2021). Mechanically strong and stiff supramolecular polymers enabled by fiber reinforced long-chain alkane matrix. J. Polym. Sci. 59, 3001–3008. doi:10.1002/pol.20210454
Pan, T., Li, J., Li, B., Xu, Q., Cui, Z., Shang, J., et al. (2021). Guanidinium-responsive crown ether-based macrocyclic amphiphile in aqueous medium. J. Phys. Chem. Lett. 12, 7418–7422. doi:10.1021/acs.jpclett.0c02994
Qi, Z., Chiappisi, L., Gong, H., Pan, R., Cui, N., Ge, Y., et al. (2018). Ion selectivity in nonpolymeric thermosensitive systems induced by water-attenuated supramolecular recognition. Chem. Eur. J. 24, 3854–3861. doi:10.1002/chem.201705838
Qi, Z., Malo de Molina, P., Jiang, W., Wang, Q., Nowosinski, K., Schulz, A., et al. (2012). Systems chemistry: Logic gates based on the stimuli-responsive gel–sol transition of a crown ether-functionalized bis(urea) gelator. Chem. Sci. 3, 2073–2082. doi:10.1039/C2SC01018F
Qi, Z., and Schalley, C. A. (2014). Exploring macrocycles in functional supramolecular gels: From stimuli responsiveness to systems chemistry. Acc. Chem. Res. 47, 2222–2233. doi:10.1021/ar500193z
Qi, Z., Schlaich, C., and Schalley, C. A. (2013). Multivalency in the gas phase: H/D exchange reactions unravel the dynamic "rock 'n' roll" motion in dendrimer-dendrimer complexes. Chem. Eur. J. 19, 14867–14875. doi:10.1002/chem.201301951
Qi, Z., Traulsen, N. L., Malo de Molina, P., Schlaich, C., Gradzielski, M., and Schalley, C. A. (2014). Self-recovering stimuli-responsive macrocycle-equipped supramolecular ionogels with unusual mechanical properties. Org. Biomol. Chem. 12, 503–510. doi:10.1039/c3ob41523f
Shang, J., Gong, H., Zhang, Q., Cui, Z., Li, S., Lv, P., et al. (2021). The dynamic covalent reaction based on diselenide-containing crown ether irradiated by visible light. Chin. Chem. Lett. 32, 2005–2008. doi:10.1016/j.cclet.2020.11.043
Shang, J., Li, B., Shen, X., Pan, T., Cui, Z., Wang, Y., et al. (2021). Selenacrown macrocycle in aqueous medium: Synthesis, redox-responsive self-assembly, and enhanced disulfide formation reaction. J. Org. Chem. 86, 1430–1436. doi:10.1021/acs.joc.0c02083
Shen, X., Li, B., Pan, T., Wu, J., Wang, Y., Shang, J., et al. (2019). Self-assembly behaviors of perylene- and naphthalene-crown macrocycle conjugates in aqueous medium. Beilstein J. Org. Chem. 15, 1203–1209. doi:10.3762/bjoc.15.117
Shi, B., Chai, Y., Qin, P., Zhao, X-X., Li, W., Zhang, Y-M., et al. (2022). Detection of aliphatic aldehydes by a pillar[5]arene-based fluorescent supramolecular polymer with vaporchromic behavior. Chem. Asian. J. 17, e202101421. doi:10.1002/asia.202101421
Wang, C., Zhang, Y. M., Li, H., Zhang, J., Zhou, Y., Liu, G., et al. (2022). Synergistic activation of photoswitchable supramolecular assembly based on sulfonated crown ether and dithienylethene derivative. Chin. Chem. Lett. 33, 2447–2450. doi:10.1016/j.cclet.2021.09.106
Wang, H., He, J., Liu, J., Qi, S., Wu, M., Wen, J., et al. (2020). Electrolytes enriched by crown ethers for lithium metal batteries. Adv. Funct. Mater 31, 2002578. doi:10.1002/adfm.202002578
Wang, P., Cao, S., Yin, T., and Ni, X. L. (2021). Unprecedented tunable hydrophobic effect and anion recognition triggered by AIE with Hofmeister series in water. Chin. Chem. Lett. 32, 1679–1682. doi:10.1016/j.cclet.2020.11.068
Wei, Q., Schlaich, C., Prevost, S., Schulz, A., Bottcher, C., Gradzielski, M., et al. (2014). Supramolecular polymers as surface coatings: Rapid fabrication of healable superhydrophobic and slippery surfaces. Adv. Mater 26, 7358–7364. doi:10.1002/adma.201401366
Weimann, D. P., Winkler, H. D. F., Falenski, J. A., Koksch, B., and Schalley, C. A. (2009). Highly dynamic motion of crown ethers along oligolysine peptide chains. Nat. Chem. 1, 573–577. doi:10.1038/nchem.352
Wu, S., Zhang, Q., Deng, Y., Li, X., Luo, Z., Zheng, B., et al. (2020). Assembly pattern of supramolecular hydrogel induced by lower critical solution temperature behavior of low-molecular-weight gelator. J. Am. Chem. Soc. 142, 448–455. doi:10.1021/jacs.9b11290
WuXiao, H. T. (2020). Supramolecular polymers with AIE property fabricated from a cyanostilbene motif-derived ditopic benzo-21-crown-7 and a ditopic dialkylammonium salt. Front. Chem. 8, 610093. doi:10.3389/fchem.2020.610093
Xiao, T., Elmes, R., and Yao, Y. (2020). Editorial: Host-Guest chemistry of macrocycles. Front. Chem. 8, 628200. doi:10.3389/fchem.2020.628200
Xiao, T., Wang, J., Shen, Y., Bao, C., Li, Z. Y., Sun, X. Q., et al. (2021). Preparation of a fixed-tetraphenylethylene motif bridged ditopic benzo-21-crown-7 and its application for constructing AIE supramolecular polymers. Chin. Chem. Lett. 32, 1377–1380. doi:10.1016/j.cclet.2020.10.037
Xiao, T., Zhou, L., Sun, X. Q., Huang, F., Lin, C., and Wang, L. (2020). Supramolecular polymers fabricated by orthogonal self-assembly based on multiple hydrogen bonding and macrocyclic host–guest interactions. Chin. Chem. Lett. 31, 1–9. doi:10.1016/j.cclet.2019.05.011
Xu, Q., Cui, Z., Yao, J., Li, B., Lv, P., Shen, X., et al. (2021). Constitutionally adaptive crown ether-based macrocyclic bolaamphiphile with redox-responsive switching of lower critical solution temperature behaviors. Chin. Chem. Lett. 32, 4024–4028. doi:10.1016/j.cclet.2021.05.058
Xu, Z., Jia, S., Wang, W., Yuan, Z., Jan Ravoo, B., and Guo, D. S. (2019). Heteromultivalent peptide recognition by co-assembly of cyclodextrin and calixarene amphiphiles enables inhibition of amyloid fibrillation. Nat. Chem. 11, 86–93. doi:10.1038/s41557-018-0164-y
Yu, S., Wang, Y., Chatterjee, S., Liang, F., Zhu, F., and Li, H. (2021). Pillar[5]arene-functionalized nanochannel platform for detecting chiral drugs. Chin. Chem. Lett. 32, 179–183. doi:10.1016/j.cclet.2020.11.055
Zayed, J. M., Nouvel, N., Rauwald, U., and Scherman, O. A. (2010). Chemical complexity—Supramolecular self-assembly of synthetic and biological building blocks in water. Chem. Soc. Rev. 39, 2806–2816. doi:10.1039/B922348G
Zhang, J., Qiu, H., He, T., Li, Y., and Yin, S. (2020). Fluorescent supramolecular polymers formed by crown ether-based host-guest interaction. Front. Chem. 8, 560. doi:10.3389/fchem.2020.00560
Zhang, K. R., Hu, M., Luo, J., Ye, F., Zhou, T. T., Yuan, Y. X., et al. (2022). Pseudo-crown ether having AIE and PET effects from a TPE-CD conjugate for highly selective detection of mercury ions. Chin. Chem. Lett. 33, 1505–1510. doi:10.1016/j.cclet.2021.08.072
Zhang, Q., Dong, S., Zhang, M., and Huang, F. (2021). Supramolecular control over thermo-responsive systems with lower critical solution temperature behavior. Aggregate 2, 35–47. doi:10.1002/agt2.12
Zhang, Q., Li, T., Duan, A., Dong, S., Zhao, W., and Stang, P. J. (2019). Formation of a supramolecular polymeric adhesive via water-participant hydrogen bond formation. J. Am. Chem. Soc. 141, 8058–8063. doi:10.1021/jacs.9b02677
Zhang, S., Boussouar, I., and Li, H. (2021). Selective sensing and transport in bionic nanochannel based on macrocyclic host-guest chemistry. Chin. Chem. Lett. 32, 642–648. doi:10.1016/j.cclet.2020.06.035
Zhang, W., Xiao, P., Lin, L., Guo, F., Wang, Q., Piao, Y., et al. (2022). Study of a water-soluble supramolecular complex of curcumin and β-cyclodextrin polymer with electrochemical property and potential anti-cancer activity. Chin. Chem. Lett. 33, 4043–4047. doi:10.1016/j.cclet.2021.12.037
Zhang, Y., and Cremer, P. S. (2009). The inverse and direct Hofmeister series for lysozyme. Proc. Natl. Acad. Sci. U.S.A. 106, 15249–15253. doi:10.1073/pnas.0907616106
Zhang, Y., Wang, L., Wang, J., Xin, S., and Sheng, X. (2021). Enzyme-responsive polysaccharide supramolecular nanoassembly for enhanced DNA encapsulation and controlled release. Chin. Chem. Lett. 32, 1902–1906. doi:10.1016/j.cclet.2021.01.032
Zheng, B., Luo, Z., Deng, Y., Zhang, Q., Gao, L., and Dong, S. (2019). A degradable low molecular weight monomer system with lower critical solution temperature behaviour in water. Chem. Commun. 55, 782–785. doi:10.1039/c8cc09160a
Zheng, X., Miao, Q., Wang, W., and Qu, D. H. (2018). Constructing supramolecular polymers from phototrigger containing monomer. Chin. Chem. Lett. 29, 1621–1624. doi:10.1016/j.cclet.2018.04.002
Keywords: crown ethers, selenium, responsive materials, hofmeister series, structural water
Citation: Qi Z, Qin Y, Wang J, Zhao M, Yu Z, Xu Q, Nie H, Yan Q and Ge Y (2023) The aqueous supramolecular chemistry of crown ethers. Front. Chem. 11:1119240. doi: 10.3389/fchem.2023.1119240
Received: 08 December 2022; Accepted: 10 January 2023;
Published: 20 January 2023.
Edited by:
Maria Vamvakaki, University of Crete, GreeceReviewed by:
Tangxin Xiao, Changzhou University, ChinaCopyright © 2023 Qi, Qin, Wang, Zhao, Yu, Xu, Nie, Yan and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhui Qi, cWlAbndwdS5lZHUuY24=; Qiangqiang Xu, c2t5ZWVAbWFpbC5ud3B1LmVkdS5jbg==; Yan Ge, Z2VAbndwdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.