
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Chem. , 07 March 2023
Sec. Nanoscience
Volume 11 - 2023 | https://doi.org/10.3389/fchem.2023.1107619
This article is part of the Research Topic Biofabrication of Nanostructures for Environmental, Agricultural, and Biomedical Applications View all 10 articles
The use of biological systems such as plants, bacteria, and fungi for the synthesis of nanomaterials has emerged to fill the gap in the development of sustainable methods that are non-toxic, pollution-free, environmentally friendly, and economical for synthesizing nanomaterials with potential in biomedicine, biotechnology, environmental science, and engineering. Current research focuses on understanding the characteristics of biogenic nanoparticles as these will form the basis for the biosynthesis of nanoparticles with multiple functions due to the physicochemical properties they possess. This review briefly describes the intrinsic enzymatic mimetic activity of biogenic metallic nanoparticles, the cytotoxic effects of nanoparticles due to their physicochemical properties and the use of capping agents, molecules acting as reducing and stability agents and which aid to alleviate toxicity. The review also summarizes recent green synthetic strategies for metallic nanoparticles.
Recent advances in nanotechnology have allowed researchers to develop devices with promising potential for use in a wide variety of applications in biomedicine, biotechnology, environmental science, and engineering (Bundschuh et al., 2018; Dan, 2020). Nanoparticles are the basic fundamental component in nanotechnology with sizes that range from 1 to 100 nm (Alavi and Karimi, 2018; Khan et al., 2019; Speranza, 2021). These structures offer major advantages due to their unique physicochemical properties such as their small sizes and diverse morphologies, large surface area to volume ratio, and in the case of metallic nanoparticles, their magnetization (Bundschuh et al., 2018). These physicochemical properties can be exploited for a broad spectrum of applications and present possible solutions to emerging global issues such as antimicrobial resistance, environmental pollution, and energy and food production (Ealias and Saravanakumar, 2017).
There is thus a need for more sustainable methods of synthesizing nanoparticles that are non-toxic, pollution free and more environmentally friendly when compared to the conventional chemical and physical methods for nanoparticle synthesis (Alavi and Karimi, 2018; Huynh et al., 2020; Bahrulolum et al., 2021; Ying et al., 2022). Recent studies focused on the use of biological organisms including plants, bacteria, yeast, fungi, lichens or algae to synthesize nanoparticles; in a method referred to as biological synthesis (Patil and Chandrasekaran, 2020; Nguyen et al., 2021; Ying et al., 2022). Proteins, enzymes, phenolic compounds, amines, alkaloids and pigments are some of the molecules in plants and microorganisms that can synthesize nanoparticles due to their reduction capability (Nadaroglu et al., 2017). The chemical and physical methods of synthesizing nanoparticles involve the use of reducing agents and stabilizing agents for the reduction of metal ions and to prevent agglomeration of the nanoparticles, however, these agents tend to be toxic to the environment and significantly contributes to nanoparticle toxicity which is highly unfavourable especially in the biomedical field (de Lima et al., 2012; Qu et al., 2019; Huynh et al., 2020; Nayak et al., 2021). In biological synthetic methods, biological organisms can produce biomolecules that act as reducing and stabilizing agents (Bahrulolum et al., 2021). These agents are not harmful to the environment, and maintain the stability of the synthesized nanoparticles thereby rendering them non-toxic (Nadaroglu et al., 2017).
Earlier reviews have highlighted the nanozyme activity of various types of nanoparticles (Ragg et al., 2015; Wu et al., 2019; Wang et al., 2020b; Ge et al., 2022). Some excellent review articles have also highlighted the biogenic strategies of metallic nanoparticles and advances in their role for biomedical application (Singh et al., 2021a; Nayak et al., 2021; Srivastava et al., 2021; Nayak et al., 2022). This mini review is different in that in provides an up to date overview of various biogenic strategies for metallic nanoparticle production, the role of biogenic synthesis as capping agents and up to date use of biogenic metallic nanoparticles as nanozymes.
Many different biological organisms have been found to have an ability to synthesize a variety of metallic nanoparticles, with the most recent (2018–2022) studies presented in Supplementary Table S1 in the Supplementary Information. Although Supplementary Table S1 covers plants, bacteria, fungi and lichen as systems that can be used for metallic nanoparticle production, it should be noted that Bryophytes also have an inherent ability to produce metallic nanoparticles. To the best of our knowledge, bryophytes have not been used to produce metallic nanoparticles from 2018, and thus a review by Srivastava et al. (2021) gives a good overview of the bryophytes used for metallic nanoparticle production (Srivastava et al., 2021).
Plants are promising candidates for nanoparticle synthesis because they detox and reduce the accumulation of metals as they alter the chemical composition of metals making them non-toxic and thus producing nanoparticles as a by-product (Nadaroglu et al., 2017; Zhang et al., 2020). Plant extracts such as sugars, flavonoids, sapogenins, proteins, enzymes, tannins, phenolics, alkaloids, steroids, and organic acids, can be obtained from plant parts, such as leaves, stems, roots, fruit, bark, flowers, seeds and buds (Moodley et al., 2018; Yulizar et al., 2020). The extracts act as reducing agents which result in the production of nanoparticles. Recently, plant extracts from Citrus sinensis, Lawsonia inermis, Artemisia haussknechtii, Cochlospermum gossypium and Juglans regia have been reported for their use in nanoparticle synthesis (Alavi and Karimi, 2018; Kredy, 2018; Srivastava et al., 2019).
Bacteria are also target candidates in nanoparticle production because of their rapid growth, cost-effectiveness, easy culturing, and since their growth conditions and environment can be easily controlled and manipulated (Nadaroglu et al., 2017). The emergence of resistance mechanisms in bacteria as a means of overcoming the harmful effects of metals also contributes to their ability to biosynthesize metallic nanoparticles. These mechanisms include transitions in the redox state, the operation of efflux systems, the buildup of metals inside the cell, intracellular precipitation, and extracellular creation of complexes (Figure 1A) (Moodley et al., 2021). These nanoparticles were believed to be formed through a method involving the NADH-dependent reductase enzyme, which goes through oxidation to create NAD+ and potentially, the lost free electron could turn Ag+ into AgNPs (Gurunathan et al., 2009; Sintubin et al., 2009).
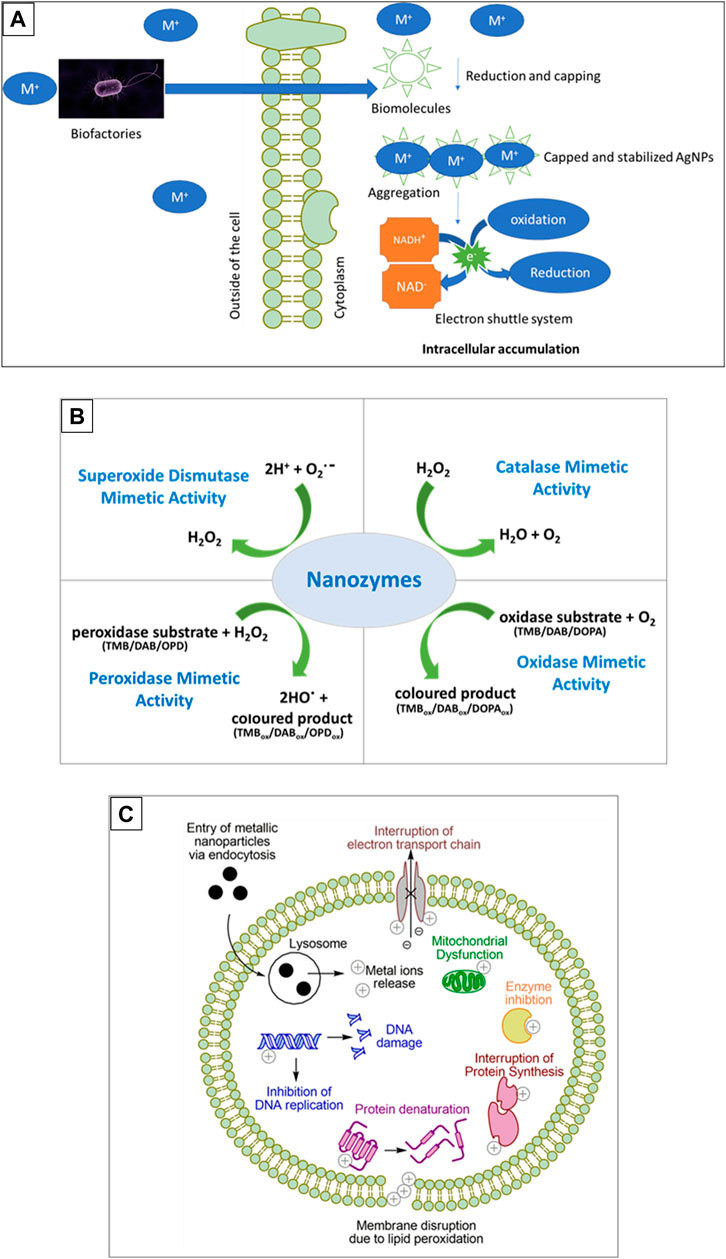
FIGURE 1. Synthesis, enzyme function and toxicity of metallic nanoparticles. (A) Is a schematic representation of biosynthesis of metal nanoparticles by microorganisms, (B) depicts the enzyme mimetic activity of biogenic nanoparticles and (C) shows the mechanisms of nanoparticle toxicity. Substrates used are 3, 3′, 5, 5′-Tetramethylbenzidine (TMB), 3, 3′-Diaminobenzidine (DAB), o-phenylenediamine (OPD), or 3,4-dihydroxyphenylalanine (DOPA).
Bacteria have the ability to convert heavy metal ions into nanoparticles by reducing them (Capuzzo, 2021). These advantages can therefore be exploited for nanoparticle synthesis. Bacteria including Pseudomonas stutzeri, Desulfovibrio alaskensis, Morganella psychrotolerans and Lactobacillus casei were recently reported to synthesize a variety of nanoparticles (Xu et al., 2018; Capeness et al., 2019).
Fungi are also ideal candidates for nanoparticle synthesis as their growth is easy and cost effective for laboratories and also at the industrial scale (Molnár et al., 2018). These organisms secrete a large number of enzymes and they have a large surface area due to their mycelia which play a vital role in rapidly forming nanoparticles as these characteristics causes metal precursor salts to be quickly converted to metallic nanoparticles (Khandel and Shahi, 2018; Li et al., 2021). Fungi such as Ganoderma lucidum, Lignosus rhinocerotis, Trichoderma longibrachiatum, and Penicillium corylophilum were recently used for the synthesis of metallic nanoparticles (Elamawi et al., 2018; Katas et al., 2019; Fouda et al., 2020; Nguyen et al., 2021).
Nanoparticles are known to be multifunctional and among the functions that they possess is the ability to catalyse reactions. Initially, the catalytic activity of nanoparticles was a result of the conjugation of catalysts or enzymes to the shell of the nanoparticles and therefore the nanoparticles would provide magnetic properties while the catalyst or enzyme on the surface of the nanoparticles provided the catalytic activity (Gao et al., 2007). This drew the interest of researchers to other possible intrinsic enzyme-like activities that nanoparticles may possess.
Nanoparticles exhibiting enzyme-like catalytic activities, referred to as nanozymes, act as mimic enzymes that can replace natural enzymes because natural enzymes have disadvantages in their catalytic functions due to the high cost of production, the time consuming process for production, denaturation in harsh environmental conditions and therefore must have suitable pH and temperature, and specific substrates (Ragg et al., 2015; Ahmed et al., 2019; Singh, 2019; Rastogi et al., 2021). Since nanozymes are easy to produce with low cost, have high stability, and good robustness; they are suitable candidates for applications requiring catalytic functions and were found to possess enzymatic activity identical to that of peroxidase, haloperoxidase, oxidase, catalase, hydrolase, and superoxide dismutase as summarized in Figure 1B (Ragg et al., 2015; Ahmed et al., 2019). To date, there are more than 300 types of nanomaterials that have been found to possess the intrinsic enzyme-like activity (Gao and Yan, 2016).
Ferrihydrite nanoparticles synthesized from the bacteria Comamonas testosteroni exhibited peroxidase-like activity similar to that of horseradish peroxidase (HRP), and these nanoparticles were able to catalyze reactions of the peroxidase chromogenic agents 3, 3′, 5, 5′-Tetramethylbenzidine (TMB), 3, 3′-Diaminobenzidine (DAB), and o-phenylenediamine (OPD) in the presence of H2O2 (Ahmed et al., 2019). This peroxidase-like activity displayed by the bacteria was exploited to develop a colorimetric method for the detection of H2O2 and glucose which was used for successfully detecting glucose in human serum (Ahmed et al., 2019). Magnetic nanoparticles referred to as magnetosomes, synthesized from Magnetospirillum gryphiswaldense (magnetotactic bacteria) also exhibit intrinsic peroxidase-like activity indicated by their ability to catalyze TMB in vitro in the presence of H2O2 (Guo et al., 2012). The peroxidase-like activity of magnetosomes plays a role in reducing enhanced intracellular reactive oxygen species (ROS) levels generated under conditions having low oxygen and high iron concentration (Guo et al., 2012; Lin et al., 2019). ROS are very reactive chemical molecules containing oxygen, generated in cell organelles including the endoplasmic reticulum (ER), peroxisomes and the mitochondria (Yu et al., 2020b). This ROS elimination role is thought to be significant for the survival of magnetotactic bacteria growing under similar conditions (Guo et al., 2012; Lin et al., 2019). The peroxidase mimetic activity of magnetosomes has been used for the detection of H2O2 and glucose (Hu et al., 2010).
Plant extracts contain products that comprise functional groups including phenolic acids, proteins, polyphenol, bioactive alkaloids, terpenoids and sugars, which reduce metal ions in the synthetic mechanism for nanoparticles (Das et al., 2022a). These functional groups can stabilize the synthesized nanoparticles and improve their catalytic efficiency (Das et al., 2022a). Palladium nanoparticles synthesized using gum kondagogu, a tree gum from C. gossypium, were used for developing a colorimetric assay for quantifying cholesterol from human serum based on the peroxidase-like activity exhibited by the synthesized nanoparticles (Rastogi et al., 2021). This study showed the potential application of the intrinsic peroxidase mimicking properties of the palladium nanoparticles for diagnostic, detection and quantification purposes (Rastogi et al., 2021).
Prunus nepalensis fruit extract was used for synthesizing gold nanoparticles exhibiting peroxidase-like catalytic activity (Das et al., 2022a). The catalytic activity of the gold nanoparticles was confirmed by the ability to catalyze the oxidation of the substrate TMB in the presence of H2O2 (Das et al., 2022a). It was found that the gold nanoparticles exhibited a higher maximum reaction velocity and affinity for TMB compared to natural horse radish peroxidase (Das et al., 2022a). The improved catalytic efficiency of the gold nanoparticles is said to have been a result of the functional groups present in the fruit extract (Das et al., 2022a). This peroxidase-like activity of the gold nanoparticles was exploited for a potential colorimetric immuno-sensing assay for the detection of Mycobacterium bovis, a bovine tuberculosis transmitted from cattle to humans through the “consumption of unpasteurized milk” (Smith et al., 2004; Das et al., 2022a). Silver nanoparticles synthesized from Cucumis sativus and Aloe vera extracts, with the A. vera extract used as a capping agent, catalyzed the reduction of methyl orange dye and para-nitro-phenol (Riaz et al., 2022). This indicated the potential of these nanoparticles in the degradation of nitro-phenols that are found in industrial waste.
Ferrihydrite nanoparticles from Trichoderma guizhouense synthesized during interaction of the fungus with hematite, whereby fungi take up minerals to form nanoparticles, also exhibited peroxidase-like activity (Yu et al., 2020a). Fungi interact with minerals and biomineralize them into nanoparticles, and this interaction is known as fungus-mineral interaction. The interaction is important in the transformation of rocks, minerals and metals, the degradation of rhizospheric organic matter and phosphate fixation. The generation of ROS was observed during fungal growth and therefore, the concentration of these species must be maintained at sub-toxic levels (Yu et al., 2020a). The peroxidase-like activity of the synthesized ferrihydrite nanoparticles reduced the generated ROS, lowering their toxic effects. It was suggested that the production of the nanoparticles caused the ROS generated during fungal growth to decrease, therefore maintaining the concentration of the ROS at sub-toxic levels.
The enzymatic activity of biogenic nanoparticles similar to natural enzymes presents a platform whereby they can be exploited for developing several methods for diagnostic, detection and biosensing applications. To date, there are numerous patent applications and patents that have been granted for nanozyme production and nanozyme systems which highlights the importance of these small molecules. A list of these patents and patent applications are listed in Table 1, together with a summary of various applications of biogenic nanoparticles that have recently been evaluated, highlighting the versatility of these nano molecules.
Nanoparticle toxicity presents a challenge, especially in areas such as biomedicine, cosmetics, agriculture and the food science, and thus there is a need for the development of nanoparticles with low cytotoxicity. The main physicochemical properties that determine toxicity of nanoparticles are size, shape, surface chemistry, surface composition, surface area to volume ratio and stability (Aillon et al., 2009; Sukhanova et al., 2018). The primary mechanisms of toxicity of metallic nanoparticles stem from them entering the cell via endocytosis, which places them within an acidic lysosome resulting in oxidation and release of metal ions. The intracellular metal ions can then exert a myriad of toxic effects as summarised in Figure 1C (Repetto et al., 2010; Carrillo-Carrion et al., 2014).
Fortunately, encapsulation strategies have recently been on the radar of researchers in a bid to reduce the toxic effects of nanoparticles as it could provide stability to the nanoparticle and reduce its susceptibility to release metal ions.
Capping agents are molecules that play an essential role in the growth, aggregation, and physicochemical properties of nanoparticles by regulating their size, shape, geometry, and surface chemistry (Javed et al., 2022; Sidhu et al., 2022). Capping agents can firmly adsorb on the nanoparticle surface forming a single or multilayer protective coating thus providing long term nanoparticle stability (Javed et al., 2022). Capping agents can consist of proteins, carbohydrates, amino acids, and lipids (Bulgarini et al., 2021) and can prevent aggregation of nanoparticles (Sidhu et al., 2022).
Capping agents are found in biological organisms, and they can act as reducing and stability agents, an advantage that is noteworthy for biogenic nanoparticles (Guilger-Casagrande et al., 2021), (Javed et al., 2022). In addition, capping agents from biological organisms can be introduced to the chemical synthetic strategies and this has become an essential strategy in lowering the cytotoxic effects of chemically synthesized nanoparticles (Sidhu et al., 2022).
The encapsulation of nanoparticles with capping agents forms a barrier between the inner core of the nanoparticle and the surrounding environment, improving nanoparticle solubility, reactivity, interactions with biomolecules, preventing aggregation and inducing their biological functions (Weingart et al., 2013). A study by Guilger-Casagrande et al. (2021) evaluated the effects and functions of capping agents on the stability of silver nanoparticles synthesized by the fungal strain Trichoderma harzianum by comparing the stability of the nanoparticles with and without capping agents. It was found that when capping agents were removed from nanoparticles, the diameter of the nanoparticles increased and it was proposed that the reason for this may be “subsequent aggregation of the nanoparticles” (Guilger-Casagrande et al., 2021).
Soliman et al. (2022) suggested that extracellular enzymes and proteins from Trichoderma saturnisporum acted as capping agents in the synthesis of silver and gold nanoparticles. Puri and Patil (2022) confirmed the presence of phytochemicals by screening Tinospora cordifolia extract used for synthesizing selenium nanoparticles. Among the phytochemicals present in the extract were phenolics and flavonoids and the hydroxyl groups of these biomolecules were proposed to act as capping agents (Puri and Patil, 2022). Highly stable, negatively charged, spherically shaped and nano-sized selenium nanoparticles were synthesized from the plant extracts and it was suggested that the stability of the nanoparticles was due to the phytochemicals detected in the extract (Puri and Patil, 2022). Silver nanoparticles synthesized using yeast extract were capped by biomolecules from the extract, and the resulting silver nanoparticles exhibited shapes, sizes and surface chemistry that exhibited good long-term stability (Shu et al., 2020).
Biogenic nanoparticles thus present a promising potential for a variety of applications with the added advantage of low toxicity due to their inherent capping potential, a feature that is lacking in physicochemical synthetic strategies that are reliant on addition of synthetic capping agents resulting in labor intensive multi-step processes (de Lima et al., 2012).
The emergence of nanotechnology has seen nanoparticles having widespread application and being the molecule of choice, resulting in them being in high demand. Although there are numerous synthetic strategies for nanoparticle production, the physical and chemical routes pose many challenges such as the need for expensive equipment, capping agents and harmful chemicals, and production of monodisperse nanoparticles with similar morphology is not so straight forward. Green synthetic methods, using inherent biological machinery and phytochemicals as capping and stabilization agents, is therefore seen as the preferred method for nanoparticle production. However, large scale production is still seen as a challenge and researchers are daily evaluating new strategies for scaling up. One of the novel methods that could be explored for large scale production of nanoparticles is investigating the role of soil microbes in influencing plant growth and uptake of nutrients. This could have importance in nanoparticle production as soil microbes could directly affect how metals and nutrients are taken up by plants before being packaged into nanoparticles (Das et al., 2022b).
The use of biological organisms for synthesizing nanoparticles is increasing as these organisms produce their own reducing and stabilizing agents that are involved in reducing metal ions to nanoparticles and also providing encapsulation. The biogenic nanoparticles thus have the important benefit of reduced toxicity and have immense application in a wide variety of biotechnology and biomedical fields such as drug delivery systems, scanning techniques, cosmetics, and assay systems. There is thus huge potential for exploiting biogenic nanoparticles for our advancement, however the scaling up of nanoparticle production is still an area that requires more research. An exciting area of research that could possibly result in an effective scaling up mechanism could be the role of soil microbes in plant growth as a symbiotic relationship could potentially be manipulated to allow improved metal and nutrient uptake by the plants thereby resulting in increased nanoparticle production.
KN and KP have conceptualized and prepared the manuscript. SBNK edited and critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This work is based on the research supported by the National Research Foundation of South Africa (Grant number 107539). The opinions, findings and conclusions or recommendations is that of the authors and the NRF accepts no liability whatsoever in this regard.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2023.1107619/full#supplementary-material
Abdullah, S. M., Kolo, K., and Sajadi, S. M. (2020). Greener pathway toward the synthesis of lichen-based ZnO@TiO2@SiO(2)and Fe3O4@SiO(2)nanocomposites and investigation of their biological activities. Food Sci. Nutr. 8 (8), 4044–4054. doi:10.1002/fsn3.1661
Abolhasani Zadeh, F., Abdalkareem Jasim, S., Atakhanova, N. E., Majdi, H. S., Abed Jawad, M., Khudair Hasan, M., et al. (2022). Drug delivery and anticancer activity of biosynthesised mesoporous Fe(2) O(3) nanoparticles. IET Nanobiotechnol 16 (3), 85–91. doi:10.1049/nbt2.12080
Ahmed, A., Abagana, A., Cui, D., and Zhao, M. (2019). De novo iron oxide hydroxide, ferrihydrite produced by Comamonas testosteroni exhibiting intrinsic peroxidase-like activity and their analytical applications. BioMed Res. Int. 2019, 1–14. doi:10.1155/2019/7127869
Aillon, K. L., Xie, Y., El-Gendy, N., Berkland, C. J., and Forrest, M. L. (2009). Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 61 (6), 457–466. doi:10.1016/j.addr.2009.03.010
Akpinar, I., Unal, M., and Sar, T. (2021). Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum f. sp. radicis-lycopersici (FORL) strains. SN Appl. Sci. 3 (4), 506–514. doi:10.1007/s42452-021-04524-5
Al-Sheddi, E. S., Farshori, N. N., Al-Oqail, M. M., Al-Massarani, S. M., Saquib, Q., Wahab, R., et al. (2018). Anticancer potential of green synthesized silver nanoparticles using extract of nepeta deflersiana against human cervical cancer cells (HeLA). Bioinorg. Chem. Appl. 2018, 1–12. doi:10.1155/2018/9390784
Alavi, M., and Karimi, N. (2018). Characterization, antibacterial, total antioxidant, scavenging, reducing power and ion chelating activities of green synthesized silver, copper and titanium dioxide nanoparticles using Artemisia haussknechtii leaf extract. Artif. Cells Nanomed Biotechnol. 46 (8), 2066–2081. doi:10.1080/21691401.2017.1408121
Alavi, M., Karimi, N., and Valadbeigi, T. (2019). Antibacterial, antibiofilm, antiquorum sensing, antimotility, and antioxidant activities of green fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via protoparmeliopsis muralis lichen aqueous extract against multi-drug-resistant bacteria. Acs Biomaterials Sci. Eng. 5 (9), 4228–4243. doi:10.1021/acsbiomaterials.9b00274
Bahrulolum, H., Nooraei, S., Javanshir, N., Tarrahimofrad, H., Mirbagheri, V. S., Easton, A. J., et al. (2021). Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnology 19 (1), 86–26. doi:10.1186/s12951-021-00834-3
Bulgarini, A., Lampis, S., Turner, R. J., and Vallini, G. (2021). Biomolecular composition of capping layer and stability of biogenic selenium nanoparticles synthesized by five bacterial species. Microb. Biotechnol. 14 (1), 198–212. doi:10.1111/1751-7915.13666
Bundschuh, M., Filser, J., Lüderwald, S., McKee, M. S., Metreveli, G., Schaumann, G. E., et al. (2018). Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 30 (1), 6–17. doi:10.1186/s12302-018-0132-6
Cai, X., Zhu, Q., Zeng, Y., Zeng, Q., Chen, X., and Zhan, Y. (2019). <p>Manganese oxide nanoparticles as MRI contrast agents in tumor multimodal imaging and therapy</p>. Int. J. nanomedicine 14, 8321–8344. doi:10.2147/ijn.s218085
Cao, Y. C., Liu, C., Liu, H., Wang, Z., and Yang, S. H. (2011). Nanozymes, methods of making nanozymes, and methods of using nanozymes. PCT/US2011/032980.
Cao, Y. C., Liu, C., Liu, H., Wang, Z., and Yang, S. H. (2018). Nanozymes, methods of making nanozymes, and methods of using nanozymes. United States of America patent application US10081542B2.
Cao, Y. C., and Liu, C. (2015). Nanozymes, methods of making nanozymes, and methods of using nanozymes. WO2015023715A1.
Cao, Y. C., and Liu, C. (2016). Nanozymes, methods of making nanozymes, and methods of using nanozymes. United States of America patent application US20160215279A1. Florida.
Capeness, M. J., Echavarri-Bravo, V., and Horsfall, L. E. (2019). Production of biogenic nanoparticles for the reduction of 4-nitrophenol and oxidative laccase-like reactions. Front. Microbiol. 10, 997–999. doi:10.3389/fmicb.2019.00997
Capuzzo, A. (2021). Bacterial synthesis of nanoparticles: Current trends in biotechnology and biomedical fields. Ann. Adv. Biomed. Sci. 4, 1–18. doi:10.20944/preprints202103.0358.v1
Carrillo-Carrion, C., Nazarenus, M., Paradinas, S. S., Carregal-Romero, S., Almendral, M. J., Fuentes, M., et al. (2014). Metal ions in the context of nanoparticles toward biological applications. Curr. Opin. Chem. Eng. 4, 88–96. doi:10.1016/j.coche.2013.11.006
Chaudhari, A., Kaida, T., Desai, H. B., Ghosh, S., Bhatt, R. P., and Tanna, A. R. (2022). Dye degradation and antimicrobial applications of manganese ferrite nanoparticles synthesized by plant extracts. Chem. Phys. Impact 5, 1–6. doi:10.1016/j.chphi.2022.100098
Ciplak, Z., Gokalp, C., Getiren, B., Yildiz, A., and Yildiz, N. (2018). Catalytic performance of Ag, Au and Ag-Au nanoparticles synthesized by lichen extract. Green Process. Synthesis 7 (5), 433–440. doi:10.1515/gps-2017-0074
Dan, D. (2020). Nanotechnology, nanoparticles and nanoscience: A new approach in chemistry and life sciences. Soft Nanosci. Lett. 10, 17–26. doi:10.4236/snl.2020.102002
Das, B., Lou-Franco, J., Gilbride, B., Ellis, M. G., Stewart, L. D., Grant, I. R., et al. (2022a). Peroxidase-mimicking activity of biogenic gold nanoparticles produced from Prunus nepalensis fruit extract: Characterizations and application for the detection of Mycobacterium bovis. ACS Appl. Bio Mater. 5 (6), 2712–2725. doi:10.1021/acsabm.2c00180
Das, P. P., Singh, K. R., Nagpure, G., Mansoori, A., Singh, R. P., Ghazi, I. A., et al. (2022b). Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 214 (1), 113821. doi:10.1016/j.envres.2022.113821
de Lima, R., Seabra, A. B., and Duran, N. (2012). Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 32 (11), 867–879. doi:10.1002/jat.2780
Dong, Y., Zhu, H., Shen, Y., Zhang, W., and Zhang, L. (2019). Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLOS ONE 14 (9), 1–12. doi:10.1371/journal.pone.0222322
Ealias, A. M., and Saravanakumar, M. P. (2017). A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 263, 1–16. doi:10.1088/1757-899X/263/3/032019
Elamawi, R. M., Al-Harbi, R. E., and Hendi, A. A. (2018). Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control 28 (1), 28–11. doi:10.1186/s41938-018-0028-1
Fotiadou, R., Chatzikonstantinou, A. V., Hammami, M. A., Chalmpes, N., Moschovas, D., Spyrou, K., et al. (2021). Green synthesized magnetic nanoparticles as effective nanosupport for the immobilization of lipase: Application for the synthesis of lipophenols. Nanomater. (Basel) 11 (2), 1–20. doi:10.3390/nano11020458
Fouda, A., Salem, S. S., Wassel, A. R., Hamza, M. F., and Shaheen, T. I. (2020). Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon 6 (9), 1–13. doi:10.1016/j.heliyon.2020.e04896
Gami, B., Bloch, K., Mohammed, S. M., Karmakar, S., Shukla, S., Asok, A., et al. (2022). Leucophyllum frutescens mediated synthesis of silver and gold nanoparticles for catalytic dye degradation. Front. Chem. 10, 1–14. doi:10.3389/fchem.2022.932416
Gandhi, A. D., Murugan, K., Umamahesh, K., Babujanarthanam, R., Kavitha, P., and Selvi, A. (2019). Lichen parmelia sulcata mediated synthesis of gold nanoparticles: An eco-friendly tool against Anopheles stephensi and Aedes aegypti. Environ. Sci. Pollut. Res. 26 (23), 23886–23898. doi:10.1007/s11356-019-05726-6
Gandhi, A. D., Miraclin, P. A., Abilash, D., Sathiyaraj, S., Velmurugan, R., Zhang, Y., et al. (2021). Nanosilver reinforced Parmelia sulcata extract efficiently induces apoptosis and inhibits proliferative signalling in MCF-7 cells. Environ. Res. 199, 111375. doi:10.1016/j.envres.2021.111375
Gao, L., and Yan, X. (2016). Nanozymes: An emerging field bridging nanotechnology and biology. Sci. China Life Sci. 59 (4), 400–402. doi:10.1007/s11427-016-5044-3
Gao, L., Zhuang, J., Nie, L., Zhang, J., Zhang, Y., Ning, G., et al. (2007). Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2, 577–583. doi:10.1038/nnano.2007.260
Ge, X., Cao, Z., and Chu, L. (2022). The antioxidant effect of the metal and metal-oxide nanoparticles. Antioxidants 11 (4), 791. doi:10.3390/antiox11040791
Guilger-Casagrande, M., Germano-Costa, T., Bilesky-José, N., Pasquoto-Stigliani, T., Carvalho, L., Fraceto, L. F., et al. (2021). Influence of the capping of biogenic silver nanoparticles on their toxicity and mechanism of action towards Sclerotinia sclerotiorum. J. Nanobiotechnology 19 (1), 53–18. doi:10.1186/s12951-021-00797-5
Guo, F. F., Yang, W., Jiang, W., Geng, S., Peng, T., and Li, J. L. (2012). Magnetosomes eliminate intracellular reactive oxygen species in Magnetospirillum gryphiswaldense MSR-1. Environ. Microbiol. 14 (7), 1722–1729. doi:10.1111/j.1462-2920.2012.02707.x
Gurunathan, S., Kalishwaralal, K., Vaidyanathan, R., Venkataraman, D., Pandian, S. R., Muniyandi, J., et al. (2009). Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B Biointerfaces 74 (1), 328–335. doi:10.1016/j.colsurfb.2009.07.048
Hu, L., Song, T., Ma, Q., Chen, C., Pan, W., Xie, C., et al. (2010). in Bacterial magnetic nanoparticles as peroxidase mimetics and application in immunoassay. Editors U. Häfeli, W. Schütt, and M. Zborowski, 369–374.
Huynh, K. H., Pham, X. H., Kim, J., Lee, S. H., Chang, H., Rho, W. Y., et al. (2020). Synthesis, properties, and biological applications of metallic alloy nanoparticles. Int. J. Mol. Sci. 21 (14), 1–29. doi:10.3390/ijms21145174
Jang, J., Lee, S., and Lee, J. (2014). Synthesis method of enzyme-mimic magnetic nanocatalysts, and enzyme-mimic magnetic nanocatalysts thereby. South Korea patent application. KR1020120002401A.
Javed, R., Sajjad, A., Naz, S., Sajjad, H., and Ao, Q. (2022). Significance of capping agents of colloidal nanoparticles from the perspective of drug and gene delivery, bioimaging, and biosensing: An insight. Int. J. Mol. Sci. 23 (18), 10521–10528. doi:10.3390/ijms231810521
Katas, H., Lim, C. S., Nor Azlan, A. Y. H., Buang, F., and Mh Busra, M. F. (2019). Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm. J. SPJ official Publ. Saudi Pharm. Soc. 27 (2), 283–292. doi:10.1016/j.jsps.2018.11.010
Khan, I., Saeed, K., and Khan, I. (2019). Nanoparticles: Properties, applications and toxicities. Arabian J. Chem. 12 (7), 908–931. doi:10.1016/j.arabjc.2017.05.011
Khandel, P., and Shahi, S. K. (2018). Mycogenic nanoparticles and their bio-prospective applications: Current status and future challenges. J. Nanostructure Chem. 8 (4), 369–391. doi:10.1007/s40097-018-0285-2
Khorrami, S., Zarrabi, A., Khaleghi, M., Danaei, M., and Mozafari, M. R. (2018). Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomedicine 13, 8013–8024. doi:10.2147/ijn.s189295
Kredy, H. (2018). The effect of pH, temperature on the green synthesis and biochemical activities of silver nanoparticles from Lawsonia inermis extract. J. Pharm. Sci. Res. 10, 2022–2026.
Lee, S., Lee, S., Kim, M., Shin, S., Lee, J., and Kim, M. (2018). Ig e detection and allergy diagnostic method using enzyme-mimetic nanozyme-based immunoassay. WO2018084340A1.
Li, Q., Liu, F., Li, M., Chen, C., and Gadd, G. M. (2021). Nanoparticle and nanomineral production by fungi. Fungal Biol. Rev. 41, 31–44. doi:10.1016/j.fbr.2021.07.003
Lin, W., Kirschvink, J. L., Paterson, G. A., Bazylinski, D. A., and Pan, Y. (2019). On the origin of microbial magnetoreception. Natl. Sci. Rev. 7 (2), 472–479. doi:10.1093/nsr/nwz065
López-Miranda, J. L., Esparza, R., Rosas, G., Perez, R., and Estevez-Gonzalez, M. (2019). Catalytic and antibacterial properties of gold nanoparticles synthesized by a green approach for bioremediation applications. 3 Biotech. 9 (4), 135–139. doi:10.1007/s13205-019-1666-z
Medintz, I. L., Vranish, J. N., Ancona, M., Susumu, K., and Diaz, S. A. (2017). Nanoparticle-attached enzyme cascades for accelerated multistep biocatalysis. US20180171325A1.
Molnár, Z., Bodai, V., Szakacs, G., Erdelyi, B., Fogarassy, Z., Safran, G., et al. (2018). Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci. Rep. 8 (1), 1–12. doi:10.1038/s41598-018-22112-3
Moodley, J. S., Krishna, S. B. N., Pillay, K., Sershen, N., and Govender, P. (2018). Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 9 (1), 015011–015019. doi:10.1088/2043-6254/aaabb2
Moodley, J. S., Krishna, S. B. N., Pillay, K., and Govender, P. (2021). “Green synthesis of metal nanoparticles for antimicrobial activity,” in Novel nanomaterials. Editor K. Krishnamoorthy (London: IntechOpen), 253–278.
Nadaroglu, H., Alayli, A., and Ince, S. (2017). Synthesis of nanoparticles by green synthesis method. Int. J. Innovative Res. Rev. 1, 6–9.
Nadhe, S. B., Singh, R., Wadhwani, S. A., and Chopade, B. A. (2019). Acinetobacter sp. mediated synthesis of AgNPs, its optimization, characterization and synergistic antifungal activity against C. albicans. J. Appl. Microbiol. 127 (2), 445–458. doi:10.1111/jam.14305
Nan, X., Lai, W., Li, D., Tian, J., Hu, Z., and Fang, Q. (2021). Biocompatibility of bacterial magnetosomes as mri contrast agent: A long-term in vivo follow-up study. Nanomater. (Basel) 11 (5), 1235. doi:10.3390/nano11051235
Nayak, V., Singh, K. R., Singh, A. K., and Singh, R. P. (2021). Potentialities of selenium nanoparticles in biomedical science. New J. Chem. 45 (6), 2849–2878. doi:10.1039/d0nj05884j
Nayak, V., Singh, K. R. B., Verma, R., Pandey, M. D., Singh, J., and Singh, R. P. (2022). Recent advancements of biogenic iron nanoparticles in cancer theranostics. Mater. Lett. 313, 131769. doi:10.1016/j.matlet.2022.131769
Nguyen, V. P., Le Trung, H., Nguyen, T. H., Hoang, D., and Tran, T. H. (2021). Synthesis of biogenic silver nanoparticles with eco-friendly processes using Ganoderma lucidum extract and evaluation of their theranostic applications. J. Nanomater. 2021, 1–11. doi:10.1155/2021/6135920
Ortac, I., Esener, S. C., Yayla, I. G., and Messmer, B. (2019). Enzyme-encapsulated nanoparticle platform. US10300152B2.
Patil, S., and Chandrasekaran, R. (2020). Biogenic nanoparticles: A comprehensive perspective in synthesis, characterization, application and its challenges. J. Genet. Eng. Biotechnol. 18 (1), 67–23. doi:10.1186/s43141-020-00081-3
Pugazhendhi, A., Prabhu, R., Muruganantham, K., Shanmuganathan, R., and Natarajan, S. (2019). Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem Photobiol. B 190, 86–97. doi:10.1016/j.jphotobiol.2018.11.014
Puri, A., and Patil, S. (2022). Tinospora cordifolia stem extract-mediated green synthesis of selenium nanoparticles and its biological applications. Pharmacogn. Res. 14 (3), 289–296. doi:10.5530/pres.14.3.42
Qu, Y., Li, X., Lian, S., Dai, C., Jv, Z., Zhao, B., et al. (2019). Biosynthesis of gold nanoparticles using fungus Trichoderma sp. WL-Go and their catalysis in degradation of aromatic pollutants. IET Nanobiotechnol 13 (1), 12–17. doi:10.1049/iet-nbt.2018.5177
Ragg, R., Tahir, M., and Tremel, W. (2015). Solids go bio: Inorganic nanoparticles as enzyme mimics. Eur. J. Inorg. Chem. 2016, 1896–1915. doi:10.1002/ejic.201600408
Rastogi, L., Dash, K., and Sashidhar, R. B. (2021). Selective and sensitive detection of cholesterol using intrinsic peroxidase-like activity of biogenic palladium nanoparticles. Curr. Res. Biotechnol. 3, 42–48. doi:10.1016/j.crbiot.2021.02.001
Repetto, M. G., Ferrarotti, N. F., and Boveris, A. (2010). The involvement of transition metal ions on iron-dependent lipid peroxidation. Archives Toxicol. 84 (4), 255–262. doi:10.1007/s00204-009-0487-y
Riaz, M., Sharafat, U., Zahid, N., Ismail, M., Park, J., Ahmad, B., et al. (2022). Synthesis of biogenic silver nanocatalyst and their antibacterial and organic pollutants reduction ability. ACS Omega 7 (17), 14723–14734. doi:10.1021/acsomega.1c07365
Rotello, V. M., Landis, R. F., Gupta, A., and Lee, Y. W. (2017). Stabilized polymeric nanocapsules, dispersions comprising the nanocapsules, and methods for the treatment of bacterial biofilms. WO2017040024A1.
Salama, A. M., Behaery, M. S., Elaal, A. E. A., and Abdelaal, A. (2022). Influence of cerium oxide nanoparticles on dairy effluent nitrate and phosphate bioremediation. Environ. Monit. Assess. 194 (5), 326. doi:10.1007/s10661-022-10003-0
Saleh, M., and Alwan, S. (2020). Bio-synthesis of silver nanoparticles from bacteria Klebsiella pneumonia: Their characterization and antibacterial studies. J. Phys. Conf. Ser. 1664, 012115. doi:10.1088/1742-6596/1664/1/012115
Sarwer, Q., Amjad, M. S., Mehmood, A., Binish, Z., Mustafa, G., Farooq, A., et al. (2022). Green synthesis and characterization of silver nanoparticles using myrsine africana leaf extract for their antibacterial, antioxidant and phytotoxic activities. Molecules 27 (21), 7612. doi:10.3390/molecules27217612
Shu, M., He, F., Li, Z., Zhu, X., Ma, Y., Zhou, Z., et al. (2020). Biosynthesis and antibacterial activity of silver nanoparticles using yeast extract as reducing and capping agents. Nanoscale Res. Lett. 15 (1), 14–19. doi:10.1186/s11671-019-3244-z
Siddiqi, K. S., Rashid, M., Rahman, A., TajuddinHusen, A., and Rehman, S. (2018). Biogenic fabrication and characterization of silver nanoparticles using aqueous-ethanolic extract of lichen (Usnea longissima) and their antimicrobial activity. Biomater. Res. 22, 23. doi:10.1186/s40824-018-0135-9
Sidhu, A. K., Verma, N., and Kaushal, P. (2022). Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front. Nanotechnol. 3, 1–17. doi:10.3389/fnano.2021.801620
Singh, K. R., Nayak, V., Singh, J., Singh, A. K., and Singh, R. P. (2021a). Potentialities of bioinspired metal and metal oxide nanoparticles in biomedical sciences. RSC Adv. 11 (40), 24722–24746. doi:10.1039/d1ra04273d
Singh, P., Singh, K. R., Singh, J., Das, S. N., and Singh, R. P. (2021b). Tunable electrochemistry and efficient antibacterial activity of plant-mediated copper oxide nanoparticles synthesized by Annona squamosa seed extract for agricultural utility. RSC Adv. 11 (29), 18050–18060. doi:10.1039/d1ra02382a
Singh, S. (2019). Nanomaterials exhibiting enzyme-like properties (nanozymes): Current advances and future perspectives. Front. Chem. 7, 46–10. doi:10.3389/fchem.2019.00046
Sintubin, L., De Windt, W., Dick, J., Mast, J., van der Ha, D., Verstraete, W., et al. (2009). Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl. Microbiol. Biotechnol. 84 (4), 741–749. doi:10.1007/s00253-009-2032-6
Smith, R. M., Drobniewski, F., Gibson, A., Montague, J. D., Logan, M. N., Hunt, D., et al. (2004). Mycobacterium bovis infection, United Kingdom. Emerg. Infect. Dis. 10 (3), 539–541. doi:10.3201/eid1003.020819
Soliman, M. K. Y., Salem, S. S., Abu-Elghait, M., and Azab, M. S. (2022). Biosynthesis of silver and gold nanoparticles and their efficacy towards antibacterial, antibiofilm, cytotoxicity, and antioxidant activities. Appl. Biochem. Biotechnol. 195, 1158–1183. doi:10.1007/s12010-022-04199-7
Speranza, G. (2021). Carbon nanomaterials: Synthesis, functionalization and sensing applications. Nanomater. (Basel, Switz. 11 (4), 967–999. doi:10.3390/nano11040967
Srivastava, V., Pandey, S., Mishra, A., and Choubey, A. K. (2019). Green synthesis of biogenic silver particles, process parameter optimization and application as photocatalyst in dye degradation. SN Appl. Sci. 1 (12), 1722. doi:10.1007/s42452-019-1762-z
Srivastava, S., Usmani, Z., Atanasov, A. G., Singh, V. K., Singh, N. P., Abdel-Azeem, A. M., et al. (2021). Biological nanofactories: Using living forms for metal nanoparticle synthesis. Mini Rev. Med. Chem. 21 (2), 245–265. doi:10.2174/1389557520999201116163012
Sukhanova, A., Bozrova, S., Sokolov, P., Berestovoy, M., Karaulov, A., and Nabiev, I. (2018). Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 13 (1), 44–21. doi:10.1186/s11671-018-2457-x
Tripathi, S., Sanjeevi, R., Jayaraman, A., Chauhan, D., and Rathoure, D. A. (2018). Nano-Bioremediation: Nanotechnology and bioremediation, 202–219.
Wang, D., Cui, L., Chang, X., and Guan, D. (2020a). Biosynthesis and characterization of zinc oxide nanoparticles from Artemisia annua and investigate their effect on proliferation, osteogenic differentiation and mineralization in human osteoblast-like MG-63 Cells. J. Photochem Photobiol. B 202, 111652. doi:10.1016/j.jphotobiol.2019.111652
Wang, P., Wang, T., Hong, J., Yan, X., and Liang, M. (2020b). Nanozymes: A new disease imaging strategy. Front. Bioeng. Biotechnol. 8, 15–10. doi:10.3389/fbioe.2020.00015
Wei, H., Wiśniowska, A., Fan, J., Harvey, P., Li, Y., Wu, V., et al. (2021). Single-nanometer iron oxide nanoparticles as tissue-permeable MRI contrast agents. Proc. Natl. Acad. Sci. U. S. A. 118 (42), e2102340118–8. doi:10.1073/pnas.2102340118
Weingart, J., Vabbilisetty, P., and Sun, X.-L. (2013). Membrane mimetic surface functionalization of nanoparticles: Methods and applications. Adv. colloid interface Sci. 197-198, 68–84. doi:10.1016/j.cis.2013.04.003
Wu, J., Wang, X., Wang, Q., Lou, Z., Li, S., Zhu, Y., et al. (2019). Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 48, 1004–1076. doi:10.1039/C8CS00457A
Xu, C., Guo, Y., Qiao, L., Ma, L., Cheng, Y., and Roman, A. (2018). Biogenic synthesis of novel functionalized selenium nanoparticles by Lactobacillus casei ATCC 393 and its protective effects on intestinal barrier dysfunction caused by enterotoxigenic Escherichia coli K88. Front. Microbiol. 9, 1–13. doi:10.3389/fmicb.2018.01129
Yassin, M. A., Elgorban, A. M., El-Samawaty, A., and Almunqedhi, B. M. A. (2021). Biosynthesis of silver nanoparticles using Penicillium verrucosum and analysis of their antifungal activity. Saudi J. Biol. Sci. 28 (4), 2123–2127. doi:10.1016/j.sjbs.2021.01.063
Ying, S., Guan, Z., Ofoegbu, P. C., Clubb, P., Rico, C., He, F., et al. (2022). Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innovation 26, 1–20. doi:10.1016/j.eti.2022.102336
Yu, G.-H., Chi, Z.-L., Kappler, A., Sun, F.-S., Liu, C.-Q., Teng, H. H., et al. (2020a). Fungal nanophase particles catalyze iron transformation for oxidative stress removal and iron acquisition. Curr. Biol. 30 (15), 2943–2950.e4. doi:10.1016/j.cub.2020.05.058
Yu, Z., Li, Q., Wang, J., Yu, Y., Wang, Y., Zhou, Q., et al. (2020b). Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res. Lett. 15 (1), 115. doi:10.1186/s11671-020-03344-7
Yulizar, Y., Kusrini, E., Apriandanu, D. O. B., and Nurdini, N. (2020). Datura metel L. Leaves extract mediated CeO2 nanoparticles: Synthesis, characterizations, and degradation activity of DPPH radical. Surfaces Interfaces 19, 1–6. doi:10.1016/j.surfin.2020.100437
Keywords: biogenic metallic nanoparticles, nanozymes, capping agents, toxicity, green synthesis
Citation: Ngcongco K, Krishna SBN and Pillay K (2023) Biogenic metallic nanoparticles as enzyme mimicking agents. Front. Chem. 11:1107619. doi: 10.3389/fchem.2023.1107619
Received: 25 November 2022; Accepted: 22 February 2023;
Published: 07 March 2023.
Edited by:
Sougata Ghosh, RK University, IndiaReviewed by:
Ravindra Pratap Singh, Indira Gandhi National Tribal University, IndiaCopyright © 2023 Ngcongco, Krishna and Pillay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karen Pillay, bXV0aHVzYW15QHVrem4uYWMuemE=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.