- 1College of Optoelectronic Engineering, Chengdu University of Information Technology, Chengdu, China
- 2School of Physics and Engineering Technology, Chengdu Normal University, Chengdu, China
According to Hund’s multiplicity rule, the energy of the lowest excited triplet state (T1) is always lower than that of the lowest excited singlet state (S1) in organic molecules, resulting in a positive singlet-triplet energy gap (ΔEST). Therefore, the up-converted reverse intersystem crossing (RISC) from T1 to S1 is an endothermic process, which may lead to the quenching of long-lived triplet excitons in electroluminescence, and subsequently the reduction of device efficiency. Interestingly, organic molecules with inverted singlet-triplet (INVEST) gaps in violation of Hund’s multiplicity rule have recently come into the limelight. The unique feature has attracted extensive attention in the fields of organic optoelectronics and photocatalysis over the past few years. For an INVEST molecule possessing a higher T1 with respect to S1, namely a negative ΔEST, the down-converted RISC from T1 to S1 does not require thermal activation, which is possibly conducive to solving the problems of fast efficiency roll-off and short lifetime of organic light-emitting devices. By virtue of this property, INVEST molecules are recently regarded as a new generation of organic light-emitting materials. In this review, we briefly summarized the significant progress of INVEST molecules in both theoretical calculations and experimental studies, and put forward suggestions and expectations for future research.
Introduction
Organic light-emitting diodes (OLEDs) based on organic molecules have shown great prospects in the fields of solid-illuminations and displays by virtue of a number of advantages, such as autoluminescence, flexibility, high color purity and low power consumption (Hong et al., 2021). Over the past few decades, several luminescence mechanisms have been proposed, including fluorescence (Friend et al., 1999; Huang et al., 2012), phosphorescence (Bernhard et al., 2002; Minaev et al., 2014; Zhou et al., 2014), thermally activated delayed fluorescence (TADF) (Endo et al., 2011; Uoyama et al., 2012; Zhang et al., 2012; Li et al., 2013; Peng et al., 2020; Hung et al., 2022; Lv et al., 2022) and hyperfluorescence (Nakanotani et al., 2014; Chan et al., 2021). Fluorescent materials are commonly derived from pure organic molecules with stable luminescence properties and rapid radiative decay from the lowest excited singlet states (S1) to the singlet ground state (S0) (Figure 1A). According to spin statistics, the ratio of singlet and triplet excitons is about 1:3 under electrical excitation (Rothberg and Lovinger, 1996). Therefore, the maximum internal quantum efficiency (IQE) of a fluorescent OLED is only 25%, and consequently the external quantum efficiency (EQE) is limited to about 5%. In turn, the IQEs of phosphorescent OLEDs can theoretically reach 100% by capturing both singlet and triplet excitons as a consequence of strong spin-orbit coupling (SOC) effect induced by heavy atoms (Figure 1B) (Bernhard et al., 2002; Chi and Chou, 2010; Zhou et al., 2014; Mao et al., 2021). Nonetheless, the utilization of precious metals brings problems of high cost and environmental pollution. In this regard, researchers have to turn attention back to pure organic molecules, and extensive efforts harvesting triplets have been carried out. Among them, TADF has received tremendous attention since Endo et al. applied a pure organic molecule with the TADF character into an OLED (Endo et al., 2011). For a TADF molecule, a small energy difference (ΔEST) between S1 and the lowest triplet excited state (T1) is required, which converts triplet excitons into singlet excitons through reverse intersystem crossing (RISC) (Figure 1C). Therefore, the IQEs of TADF emitters can also reach 100%. Meanwhile, ΔEST is proportional to the exchange integral between the spatial wave functions of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) (Endo et al., 2011; Yang et al., 2017). In this regard, separated Frontier orbital distributions are of significant importance during the molecular design of TADF materials (Fan et al., 2016).
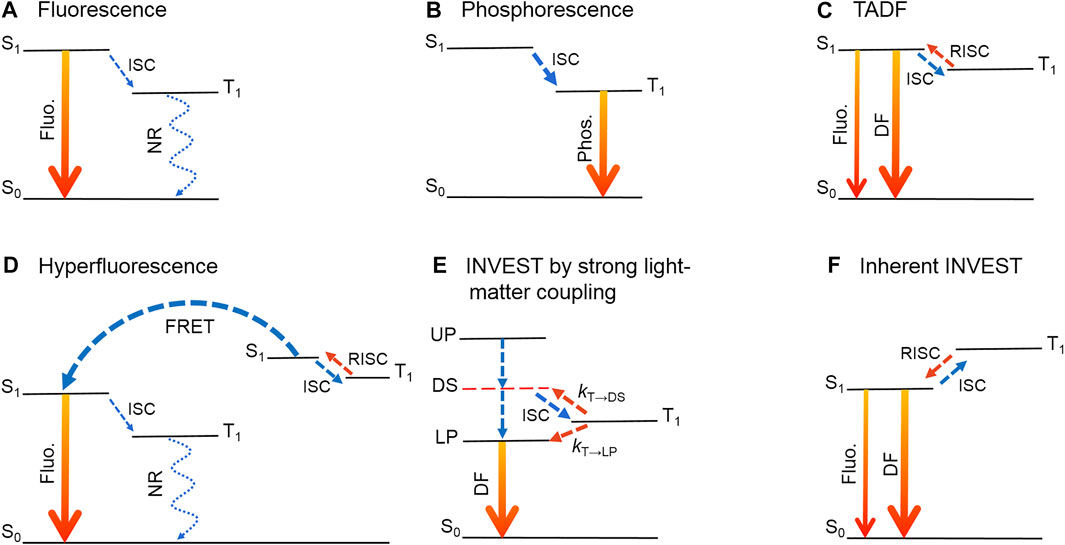
FIGURE 1. Luminescence mechanisms of organic light-emitting materials. (A) Fluorescence. (B) Phosphorescence. (C) Thermally activated delayed fluorescence (TADF). (D) Hyperfluorescence. (E) INVEST by strong light-matter coupling. (F) Inherent INVEST. Fluo., fluorescence; Phos., phosphorescence; DF, delayed fluorescence; ISC, intersystem crossing; RISC, reverse intersystem crossing; FRET, Förster resonance energy transfer; UP, upper polaritons; DS, dark singlet states; LP, lower polaritons; NR, nonradiative decay. kT→DS and kT→LP: RISC rate constants from T1 to DS and from T1 to LP, respectively.
Over the past decade, TADF molecules have been widely studied in light of the merits of high efficiency as well as low cost, and a number of TADF emitters have been developed (Jeon et al., 2019; Li et al., 2021c). Up to now, there are several pathways to realize TADF, such as traditional single molecule-based TADF (Uoyama et al., 2012; Zhang et al., 2012; Li et al., 2021d), exciplex-based TADF (Goushi et al., 2012; Li et al., 2014a; Oh et al., 2015; Li et al., 2021a; Li et al., 2021b; Xue and Xie, 2021; Li et al., 2022c; Gu et al., 2022), aggregation-induced emission (AIE)-based TADF (Zhao et al., 2018; Liu et al., 2020), excited-state intramolecular proton transfer (ESIPT)-based TADF (Mamada et al., 2017; Long et al., 2020) and multiple resonance-based TADF (MR-TADF) (Lee et al., 2020; Stavrou et al., 2021; Wu et al., 2021; Yang et al., 2022; Zou et al., 2022). Particularly, MR-TADF molecules have been considered as the most promising TADF materials on account of the attainment of both high efficiencies and high color purity. Nevertheless, the molecular design of MR-TADF is still rather limited in view of that almost all the MR-TADF molecules are B-, N-, S-, O-, carbonyl-, and/or sulfuryl-containing heterocyclic derivatives (Hatakeyama et al., 2016; Liang et al., 2018; Li et al., 2019; Yuan et al., 2019; Hall et al., 2020; Xu et al., 2021a; Huang et al., 2021; Meng et al., 2022). Additionally, for all the TADF molecules, there is always a problem that the RISC process is fairly slow, resulting in serious annihilation of triplet excitons and concomitantly serious efficiency roll-off at high current densities (Yersin et al., 2019; Hong et al., 2022).
Hyperfluorescence combines advantages of both fluorescence and TADF, in which fluorescent and TADF materials are introduced as emitters and host materials, respectively (Nakanotani et al., 2014). Under electrical excitation, almost all the singlet and triplet excitons are initially harvested by TADF molecules, and then triplet excitons can efficiently up-convert to be singlet excitons (Figure 1D). Subsequently, the energy can be transferred from S1 of TADF molecules to S1 of fluorescent molecules through Förster resonance energy transfer (FRET), and finally highly efficient fluorescence could be achieved (Chan et al., 2021). Notably, efficiency roll-off at high current densities could possibly occur in terms of a slow RISC in TADF molecules (Jakoby et al., 2019). Moreover, some other mechanisms, such as triplet-triplet annihilation (TTA) (Fukagawa et al., 2012; Jankus et al., 2013), pure organic room-temperature phosphorescence (RTP) (Zhou et al., 2018; Wen et al., 2021; Liu et al., 2022), utilization of higher excited states (Sato et al., 2015; Xu et al., 2021b), direct singlet harvesting (Yersin et al., 2019), doublet energy transfer with organic radicals (Li et al., 2022a) and radical-based emitters (Ai et al., 2018; Abdurahman et al., 2020; Cui et al., 2020), are proposed in recent years. However, current research suggests that these mechanisms have not yet shown a subversive improvement effect.
Recently, a mechanism of inverted singlet-triplet (INVEST) in violation of Hund’s multiplicity rule attracted much attention (Kollmar and Staemmler, 1978; Koseki et al., 1985; Segal et al., 2007; Difley et al., 2008; Sato et al., 2015; Ehrmaier et al., 2019; Gan et al., 2019; Mei et al., 2021; Pollice et al., 2021; Li et al., 2022b). For an INVEST molecule possessing a negative ΔEST, the intrinsic photophysics of RISC are thereby completely overturned from an endothermic process to an exothermic one. Therefore, the RISC process of INVEST molecules are mostly likely superior to the corresponding TADF molecules. Consequently, INVEST emitters can theoretically outperform all previous generations in terms of considerably lower triplet exciton populations, and potential applications in OLEDs, organic lasers and photocatalysis could be imagined (Hwang and Schlenker, 2021; Pollice et al., 2021). At present, there are mainly two INVEST mechanisms, INVEST by strong light-matter coupling and inherent INVEST.
Inverted singlet-triplet by strong light-matter coupling
INVEST by strong light-matter coupling is to build an optical microcavity to convert singlet excitons into two types of polaritons, namely lower polaritons (LPs) and upper polaritons (UPs). Meanwhile, an INVEST structure with a lower LP state relative to T1 can be realized by adjusting the microcavity structure (Figure 1E) (Eizner et al., 2019). Herein, polaritons are light-matter eigenstates forming when singlet electronic transitions are strongly coupled with the vacuum electromagnetic field in an optical cavity (Hopfield, 1958). In recent years, organic polaritons have been widely investigated in the areas of nonlinear interactions (Daskalakis et al., 2014), optoelectronic devices (Tischler et al., 2005; Ballarini et al., 2013; Sanvitto and Kena-Cohen, 2016; Eizner et al., 2018; Stranius et al., 2018) as well as chemical reactions (Feist et al., 2018; Ribeiro et al., 2018). Meanwhile, strong light-matter coupling has been regarded as an important tool to tailor molecular photophysical and photochemical properties without modifying chemical structures (Lidzey et al., 1998; Hertzog et al., 2019). As shown in Figure 1E, under optical excitation, the generation of delayed fluorescence for a TADF molecule in a polariton setup involves intersystem crossing (ISC) from the dark singlet state (DS) to T1, followed by two RISC processes from T1 to DS and from T1 to LP with rate constants of kT→DS and kT→LP, respectively. In this situation, it is anticipated that the down-converted RISC process from T1 to LP could be dominant and conductive to the reduction of efficient roll-off if the kT→LP can be much larger than kT→DS by modifying the microcavity structure.
In 2019, Eizner et al. demonstrated an inversion of the singlet LP and T1 based on a TADF molecule, 1,3,5-tris(4-(diphenylamino)phenyl)-2,4,6-tricyanobenzene (3DPA3CN) (Figure 2), and measured the RISC rate constant in strongly coupled organic microcavities (Eizner et al., 2019). Unexpectedly, the RISC rate constants were almost invariable regardless of the large energy level shifts under strong light-matter coupling. In 2021, Yu et al. (2021) demonstrated a barrier-free RISC from a molecular centered triplet state to a hybrid polaritonic state based on another TADF molecule, 9-([1,1′-biphenyl]-3-yl)-N,N,5,11-tetraphenyl-5,9-dihydro-5,9-diaza-13b-boranaphtho[3,2,1-de]anthracen-3-amine (DABNA-2) (Figure 2), in light of a good compromise on ΔEST and coupling strength (Yu et al., 2021). Interestingly, the connection between the uncoupled T1 and the polaritonic state was shown to depend on molecular constitution of the polaritons. As the photonic nature of LP increased, a gradual disconnection from T1 happened. By choosing an intermediate state, a system with both an energetic driving force and enough molecular constitution of the LP was achieved to maintain a barrier-free RISC directly from T1 to LP. Accordingly, strong light-matter coupling offers a new strategy to overcome the limit of Hund’s rule and to facilitate the harvest of triplet excitons. From these results, it is anticipated that more efforts on INVEST by strong light-matter coupling will be extensively explored.
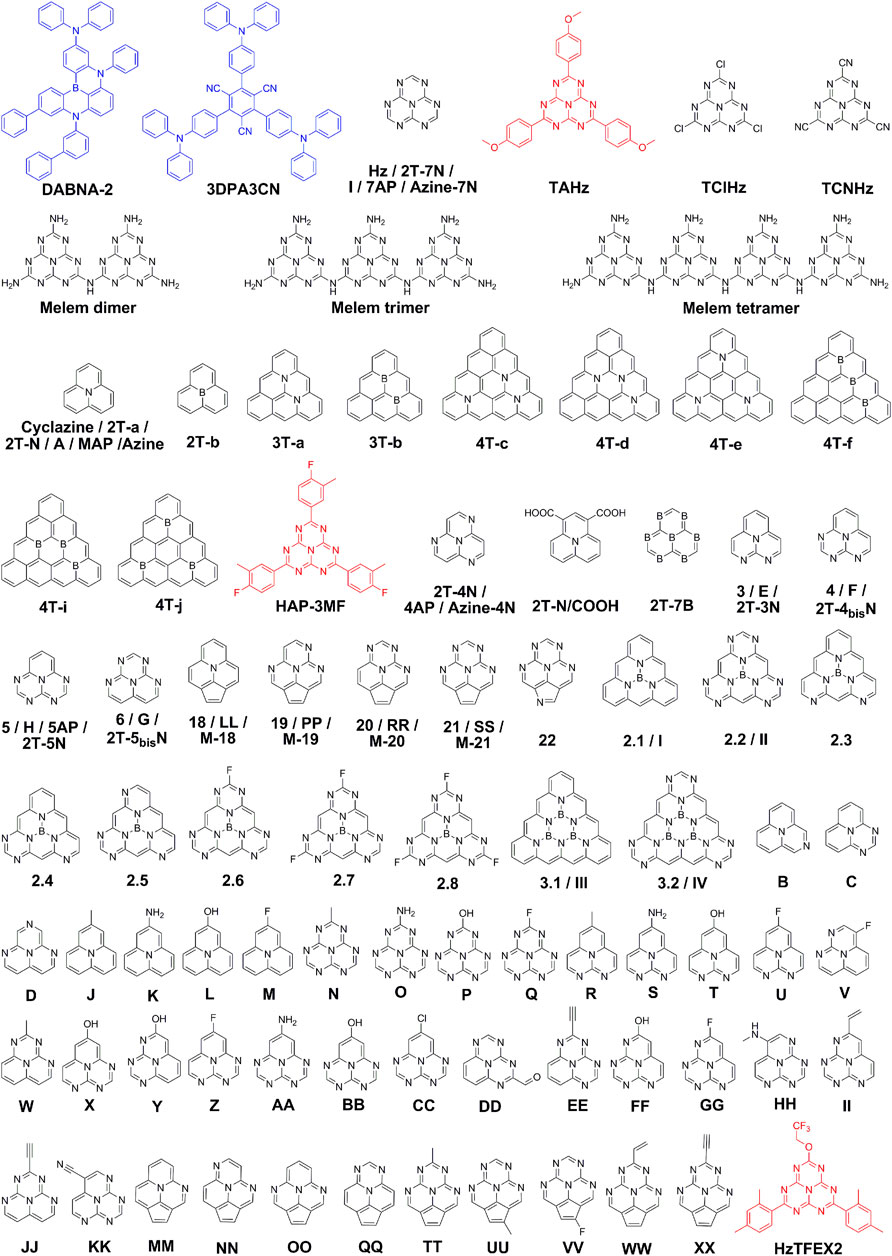
FIGURE 2. Chemical structures of INVEST molecules. The blue color represents INVEST molecules by strong light-matter coupling, while the black and red colors represent inherent INVEST molecules investigated by theoretical calculations and experimental verifications, respectively.
Inherent inverted singlet-triplet
In addition to INVEST by strong light-matter coupling, inherent INVEST is another important strategy to realize singlet-triplet inversion and has drawn growing interest in the fields of organic optoelectronics and photochemistry (Ehrmaier et al., 2019; Miyajima et al., 2021; Pollice et al., 2021). Contrary to the vast majority of known organic molecules, inherent INVEST molecules possess an inherent property of singlet-triplet inversion without any assistance of the environment. Thus, energy transition from T1 to S1 is a spontaneous down-conversion process, replacing the up-conversion in TADF emitters (Figure 1F) (Pollice et al., 2021). Therefore, inherent INVEST are most likely to have better performance and applications with respect to INVEST by strong light-matter coupling.
Up to now, a series of inherent INVEST molecules have been theoretically or experimentally investigated, and the related chemical structures mentioned below are depicted in Figure 2. In 2019, Ehrmaier et al. (2019) investigated the excited singlet and triplet states of a set of heptazine derivatives (Hz, TAHz, TClHz, TCNHz, melem dimer, melem trimer and melem tetramer, Figure 2), by correlated ADC(2), CC2, EOM-CCSD, and CASPT2 calculations. Remarkably, all the heptazine derivatives displayed negative ΔEST from −0.30 to −0.20 eV with the ADC(2) method, indicating that the singlet-triplet inversion characteristics of heptazine derivatives were extremely robust, being affected neither by substitutions nor by oligomerization. Almost at the same time, de Silva theoretically studied the excited-state energy inversion of S1 and T1 based on a N-containing heterocycle, cyclazine (Figure 2), from the perspective of electronic structure theory (de Silva, 2019). Through systematic calculations and analyses with different excited-state electronic structure methods, it was found that electron correlation in the form of double excitations would lead to the reduction of ΔEST and the emergence of negative ΔEST. The result indicates that popular electronic structure methods without the consideration of doubly excited configurations cannot accurately describe excited states of inherent INVEST molecules.
Notably, both abovementioned heptazine derivatives and cyclazine possess relatively small oscillator strengths, which are not qualified as emitters for highly efficient OLEDs. In 2021, Sanz-Rodrigo et al. (2021) calculated a set of N- and/or B-substituted triangle-shaped molecules by using popular time-dependent density functional theory (TD-DFT) and more sophisticated ab initio methods with correlation effects. Excitingly, molecules 2T-a, 2T-b, 3T-a, 3T-b, 4T-c, 4T-d, 4T-e, 4T-f, 4T-i and 4T-j (Figure 2) possess inherent INVEST characteristics and most of them showing nonvanishing oscillator strengths with highly correlated SA-CASSCF, SC-NEVPT2 and SCS-CC2 calculations (Sanz-Rodrigo et al., 2021). Sobolewski and Domcke investigated the electronic excitation energies of two previousy reported heptazine derivatives (HAP-3MF and HAP-3TPA) (Li et al., 2013; Li et al., 2014b) with the ADC(2) method, and HAP-3MF with a negative ΔEST of −0.24 eV was robustly verified (Sobolewski and Domcke, 2021). Dinkelbach et al. (2021) carried out a comprehensive theoretical study on the photophysics of Hz and HAP-3MF. Remarkably, they found that the ultimate luminescence efficiencies of these two inherent INVEST compounds were determined by not merely ISC/RISC processes but also the internal conversion from S1 to S0.
Ricci et al. (2021) assessed the excited-state energy order of a set of N- or B-doped π-conjugated heterocycles by linear-response TD-DFT and correlated ab initio methods. Among these molecules, negative ΔEST could be realized for molecules 2T-N, 2T-4N, 2T-7N, 2T-N/COOH and 2T-7B (Figure 2) by CIS(D), SCS-CC2, SCS-ADC(2) or SC-NEVPT2 methods. Noteworthily, they found that negative ΔEST should be ascribed to an intricate interplay between the singlet-triplet exchange interaction, the influence of doubly-excited configurations, and the impact of dynamic correlation effects. The result is of significant importance for further molecular design of inherent INVEST molecules. Pollice et al. (2021) put forward that ideal emitters potentially surpassing TADF materials should have both negative ΔEST and substantial fluorescence rates. Based on computational studies on a series of N-substituted phenalene derivatives, molecules possessing both negative singlet-triplet gaps and considerable fluorescence rates, namely 3–6 and 18–22 (Figure 2), were obtained, suggesting that inherent INVEST molecules are more common than hypothesized previously and have the potential to become the next generation organic light-emitting materials. Pios et al. (2021) designed and characterized a number of triangular boron carbon nitrides (2.1–2.8 and 3.1–3.2, Figure 2) conceptually derived from cyclazine and heptazine by employing high-level ab initio electronic structure theory. As expected, these compounds showed robust inherent INVEST characteristics, exhibiting great potential as chromophores for organic optoelectronics.
In 2022, Li and coworkers theoretically investigated the response of INVEST behavior of cyclazine to a static electric-field as well as an unchirped and chirped laser pulse by using next-generation quantum theory of atoms in molecules (NG-QTAIM), demonstrating that NG-QTAIM is a useful tool for understanding the response to laser irradiation (Li et al., 2022d). Recently, Alipour and Izadkhast comprehensively calculated a series of inherent INVEST emitters (A-Z, AA-XX and I-IV, Figure 2) toward the development of modern double-hybrid density functionals for singlet-triplet inversion (Alipour and Izadkhast, 2022). They found that particular proportions among the nonlocal exchange and correlation contributions as well as the same-spin and opposite-spin parameters included in the direct and indirect terms are needed to achieve a reliable accuracy for the singlet-triplet inversion. Sancho-Garcia et al. assessed the singlet-triplet inversion feature of a set of azaphenalene compounds (2T-N, 2T-4N, 2T-7N, 2T-3N, 2T-4bisN, 2T-5N and 2T-5bisN, Figure 2) by TD-DFT employing a family of double-hybrid density functionals, and found that double-hybrid exchange-correlation functionals incorporating double excitations could be a good alternative to wavefunction methods (Sancho-Garcia et al., 2022). Subsequently, Sancho-García and San-Fabián investigated four azaphenalene derivatives (MAP, 4AP, 5AP and 7AP) to assess if methods going beyond standard TD-DFT could predict accurate excited-state energy inversion (Sancho-García and San-Fabián, 2022). Interestingly, negative ΔEST with high accuracy could be well realized by employing methods merging wavefunction and correlation functionals. Moreover, Ghosh and Bhattacharyya calculated seven azaphenalene derivatives (M-18 to M-21, Azine, Azine-4N and Azine-7N, Figure 2) by combining DFT and wave function methods, unveiling that inherent INVEST gaps could be obtained by using doubles-corrected TD-DFT with suitable double-hybrid functionals or excited-state DFT (Ghosh and Bhattacharyya, 2022). Overall, the molecular design of inherent INVEST emitters should consider both minimal exchange integrals leading to small singlet-triplet gaps, and significant double excitation character in electronic transitions in extended π-conjugated heteroatom-containing molecular systems. Particularly, current inherent INVEST molecules are derived from N- and/or B-containing fused heterocycles.
Notably, present research on inherent INVEST is mainly carried out by theoretical calculations, while experimental explorations are fairly rare, possibly due to the difficulty in synthesis of these N- and/or B-containing fused heterocycles. Excitingly, Miyajima and coworkers experimentally demonstrated the existence of highly efficient inherent INVEST emitters for OLEDs (Miyajima et al., 2021). Based on computational screening on a large quantity of heptazine derivatives initially by affordable standard linear-response TD-DFT calculations and then by high-cost correlated wave function theories including double excitation configurations, a heptazine derivative, HzTFEX2 (Figure 2) was chosen for experimental evaluation considering both the possibility to be an efficient blue inherent INVEST emitter and the synthetic feasibility. Expectedly, HzTFEX2 showed a negative ΔEST of −11 meV based on the fit of Arrhenius equation, and meanwhile the rate inversion of RISC and ISC. Ultimately, an OLED incorporating HzTFEX2 exhibited a fairly high EQE of 17.0% with a fast transient electroluminescence decay. Recently, Li et al. (2022b) experimentally investigated the photophysical properties of HAP-3MF which was previously theoretically evaluated as the first inherent INVEST emitter (Sobolewski and Domcke, 2021). Surprisingly, negative ΔEST of −0.22 eV in toluene and −0.19 eV in acetonitrile were directly obtained from the fluorescence and phosphorescence spectra. Moreover, to reveal the extremely weak delayed emission, a mixed solution system of HAP-3MF:1,3-di(9H-carbazol-9-yl)benzene (mCP) in toluene at various molar ratios was subtly designed. As expected, enhanced delayed emissions were achieved, and the efficient triplet-exciton harvesting process via a down-converted triplet-to-singlet channel was elucidated.
Conclusion and outlook
In summary, we have provided an overview of organic molecules with singlet-triplet inversion characteristics stemming from strong light-matter coupling and inherent INVEST. As compared to fluorescence, phosphorescence, TADF and hyperfluorescence, INVEST molecules possessing intriguing excited-state features have attracted great attention especially in the field of organic electroluminescence. Although numerous research results show that inherent INVEST molecules have great potential to become a new generation of high-performance organic light-emitting materials, there are still two main problems: 1) Most studies on INVEST molecules are merely based on theoretical calculations, whilst experimental results are currently sparse. 2) Present molecular design of inherent INVEST molecules is relatively limited, in view of that almost all these molecules are N- and/or B-containing heterocycles, especially heptazine derivatives. In this respect, it could be envisioned that more endeavors on experimental verifications and diverse molecular design will be carried out, and we believe that organic INVEST molecules will show bright prospects in organic optoelectronics and photochemistry in future.
Author contributions
JL and QG conceived the idea and supervised the whole work. ZL, HL, HG, JZ, and YY collected the articles and revised the manuscript. All authors contributed to the manuscript revision and approved the submitted version.
Funding
This work was financially supported by the National Natural Science Foundation of China (61505015, 21801028), the Department of Science and Technology of Sichuan Province (2022NSFSC1200) and the Scientific Research Foundation of CUIT (KYTZ202174).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdurahman, A., Hele, T. J. H., Gu, Q., Zhang, J., Peng, Q., Zhang, M., et al. (2020). Understanding the luminescent nature of organic radicals for efficient doublet emitters and pure-red light-emitting diodes. Nat. Mat. 19, 1224–1229. doi:10.1038/s41563-020-0705-9
Ai, X., Evans, E. W., Dong, S., Gillett, A. J., Guo, H., Chen, Y., et al. (2018). Efficient radical-based light-emitting diodes with doublet emission. Nature 563, 536–540. doi:10.1038/s41586-018-0695-9
Alipour, M., and Izadkhast, T. (2022). Do any types of double-hybrid models render the correct order of excited state energies in inverted singlet-triplet emitters? J. Chem. Phys. 156, 064302. doi:10.1063/5.0077722
Ballarini, D., De Giorgi, M., Cancellieri, E., Houdré, R., Giacobino, E., Cingolani, R., et al. (2013). All-optical polariton transistor. Nat. Commun. 4, 1778. doi:10.1038/ncomms2734
Bernhard, S., Gao, X., Malliaras, G. G., and Abruña, H. D. (2002). Efficient electroluminescent devices based on a chelated osmium(II) complex. Adv. Mat. 14, 433–436. doi:10.1002/1521-4095(20020318)14:6<433::aid-adma433>3.0.co;2-w
Chan, C. Y., Tanaka, M., Lee, Y. T., Wong, Y. W., Nakanotani, H., Hatakeyama, T., et al. (2021). Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission. Nat. Photonics 15, 203–207. doi:10.1038/s41566-020-00745-z
Chi, Y., and Chou, P. T. (2010). Transition-metal phosphors with cyclometalating ligands: Fundamentals and applications. Chem. Soc. Rev. 39, 638–655. doi:10.1039/b916237b
Cui, Z., Abdurahman, A., Ai, X., and Li, F. (2020). Stable luminescent radicals and radical-based leds with doublet emission. CCS Chem. 2, 1129–1145. doi:10.31635/ccschem.020.202000210
Daskalakis, K. S., Maier, S. A., Murray, R., and Kena-Cohen, S. (2014). Nonlinear interactions in an organic polariton condensate. Nat. Mat. 13, 271–278. doi:10.1038/nmat3874
de Silva, P. (2019). Inverted singlet-triplet gaps and their relevance to thermally activated delayed fluorescence. J. Phys. Chem. Lett. 10, 5674–5679. doi:10.1021/acs.jpclett.9b02333
Difley, S., Beljonne, D., and Van Voorhis, T. (2008). On the singlet-triplet splitting of geminate electron-hole pairs in organic semiconductors. J. Am. Chem. Soc. 130, 3420–3427. doi:10.1021/ja076125m
Dinkelbach, F., Bracker, M., Kleinschmidt, M., and Marian, C. M. (2021). Large inverted singlet–triplet energy gaps are not always favorable for triplet harvesting: Vibronic coupling drives the (reverse) intersystem crossing in heptazine derivatives. J. Phys. Chem. A 125, 10044–10051. doi:10.1021/acs.jpca.1c09150
Ehrmaier, J., Rabe, E. J., Pristash, S. R., Corp, K. L., Schlenker, C. W., Sobolewski, A. L., et al. (2019). Singlet-triplet inversion in heptazine and in polymeric carbon nitrides. J. Phys. Chem. A 123, 8099–8108. doi:10.1021/acs.jpca.9b06215
Eizner, E., Brodeur, J., Barachati, F., Sridharan, A., and Kena-Cohen, S. (2018). Organic photodiodes with an extended responsivity using ultrastrong light-matter coupling. ACS Photonics 5, 2921–2927. doi:10.1021/acsphotonics.8b00254
Eizner, E., Martinez-Martinez, L. A., Yuen-Zhou, J., and Kena-Cohen, S. (2019). Inverting singlet and triplet excited states using strong light-matter coupling. Sci. Adv. 5, eaax4482. doi:10.1126/sciadv.aax4482
Endo, A., Sato, K., Yoshimura, K., Kai, T., Kawada, A., Miyazaki, H., et al. (2011). Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes. Appl. Phys. Lett. 98, 083302. doi:10.1063/1.3558906
Fan, J. Z., Lin, L. L., and Wang, C. K. (2016). Decreasing the singlet-triplet gap for thermally activated delayed fluorescence molecules by structural modification on the donor fragment: First-principles study. Chem. Phys. Lett. 652, 16–21. doi:10.1016/j.cplett.2016.04.027
Feist, J., Galego, J., and Garcia-Vidal, F. J. (2018). Polaritonic chemistry with organic molecules. ACS Photonics 5, 205–216. doi:10.1021/acsphotonics.7b00680
Friend, R. H., Gymer, R. W., Holmes, A. B., Burroughes, J. H., Marks, R. N., Taliani, C., et al. (1999). Electroluminescence in conjugated polymers. Nature 397, 121–128. doi:10.1038/16393
Fukagawa, H., Shimizu, T., Ohbe, N., Tokito, S., Tokumaru, K., and Fujikake, H. (2012). Anthracene derivatives as efficient emitting hosts for blue organic light-emitting diodes utilizing triplet-triplet annihilation. Org. Electron. 13, 1197–1203. doi:10.1016/j.orgel.2012.03.019
Gan, L., Xu, Z. D., Wang, Z. H., Li, B. B., Li, W., Cai, X. Y., et al. (2019). Utilizing a spiro TADF moiety as a functional electron donor in TADF molecular design toward efficient "multichannel" reverse intersystem crossing. Adv. Funct. Mat. 29, 1808088. doi:10.1002/adfm.201808088
Ghosh, S., and Bhattacharyya, K. (2022). Origin of the failure of density functional theories in predicting inverted singlet-triplet gaps. J. Phys. Chem. A 126, 1378–1385. doi:10.1021/acs.jpca.1c10492
Goushi, K., Yoshida, K., Sato, K., and Adachi, C. (2012). Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nat. Photonics 6, 253–258. doi:10.1038/nphoton.2012.31
Gu, J. N., Tang, Z. Y., Guo, H. Q., Chen, Y., Xiao, J., Chen, Z. J., et al. (2022). Intermolecular TADF: Bulk and interface exciplexes. J. Mat. Chem. C 10, 4521–4532. doi:10.1039/d1tc04950j
Hall, D., Suresh, S. M., dos Santos, P. L., Duda, E., Bagnich, S., Pershin, A., et al. (2020). Improving processability and efficiency of resonant TADF emitters: A design strategy. Adv. Opt. Mat. 8, 1901627. doi:10.1002/adom.201901627
Hatakeyama, T., Shiren, K., Nakajima, K., Nomura, S., Nakatsuka, S., Kinoshita, K., et al. (2016). Ultrapure blue thermally activated delayed fluorescence molecules: Efficient homo-lumo separation by the multiple resonance effect. Adv. Mat. 28, 2777–2781. doi:10.1002/adma.201505491
Hertzog, M., Wang, M., Mony, J., and Borjesson, K. (2019). Strong light-matter interactions: A new direction within chemistry. Chem. Soc. Rev. 48, 937–961. doi:10.1039/c8cs00193f
Hong, G., Gan, X., Leonhardt, C., Zhang, Z., Seibert, J., Busch, J. M., et al. (2021). A brief history of OLEDs-emitter development and industry milestones. Adv. Mat. 33, 2005630. doi:10.1002/adma.202005630
Hong, X., Zhang, D., Yin, C., Wang, Q., Zhang, Y., Huang, T., et al. (2022). TADF molecules with π-extended acceptors for simplified high-efficiency blue and white organic light-emitting diodes. Chem 8, 1705–1719. doi:10.1016/j.chempr.2022.02.017
Hopfield, J. J. (1958). Theory of the contribution of excitons to the complex dielectric constant of crystals. Phys. Rev. 112, 1555–1567. doi:10.1103/physrev.112.1555
Huang, F., Wang, K., Shi, Y. Z., Fan, X. C., Zhang, X., Yu, J., et al. (2021). Approaching efficient and narrow rgb electroluminescence from D-A-type TADF emitters containing an identical multiple resonance backbone as the acceptor. ACS Appl. Mat. Interfaces 13, 36089–36097. doi:10.1021/acsami.1c09743
Huang, J. H., Su, J. H., and Tian, H. (2012). The development of anthracene derivatives for organic light-emitting diodes. J. Mat. Chem. 22, 10977–10989. doi:10.1039/c2jm16855c
Hung, Y. T., Luo, D. A., Chen, L. M., Huang, D. C., Wu, J. Z., Chen, Y. S., et al. (2022). Harnessing bipolar acceptors for highly efficient exciplex-forming systems. J. Mat. Chem. C 10, 4748–4756. doi:10.1039/d1tc04700k
Hwang, D., and Schlenker, C. W. (2021). Photochemistry of carbon nitrides and heptazine derivatives. Chem. Commun. 57, 9330–9353. doi:10.1039/d1cc02745j
Jakoby, M., Richards, B. S., Lemmer, U., and Howard, I. A. (2019). Investigations of singlet and triplet diffusion in thermally activated delayed-fluorescence emitters: Implications for hyperfluorescence. Phys. Rev. B 100, 045303. doi:10.1103/physrevb.100.045303
Jankus, V., Chiang, C. J., Dias, F., and Monkman, A. P. (2013). Deep blue exciplex organic light-emitting diodes with enhanced efficiency; p-type or e-type triplet conversion to singlet excitons? Adv. Mat. 25, 1455–1459. doi:10.1002/adma.201203615
Jeon, S. K., Lee, H. L., Yook, K. S., and Lee, J. Y. (2019). Recent progress of the lifetime of organic light-emitting diodes based on thermally activated delayed fluorescent material. Adv. Mat. 31, 1803524. doi:10.1002/adma.201803524
Kollmar, H., and Staemmler, V. (1978). Violation of hund's rule by spin polarization in molecules. Theor. Chim. Acta 48, 223–239. doi:10.1007/bf00549021
Koseki, S., Nakajima, T., and Toyota, A. (1985). Violation of hund's multiplicity rule in the electronically excited states of conjugated hydrocarbons. Can. J. Chem. 63, 1572–1579. doi:10.1139/v85-267
Lee, H., Karthik, D., Lampande, R., Ryu, J. H., and Kwon, J. H. (2020). Recent advancement in boron-based efficient and pure blue thermally activated delayed fluorescence materials for organic light-emitting diodes. Front. Chem. 8, 373. doi:10.3389/fchem.2020.00373
Li, F., Gillett, A. J., Gu, Q., Ding, J., Chen, Z., Hele, T. J. H., et al. (2022a). Singlet and triplet to doublet energy transfer: Improving organic light-emitting diodes with radicals. Nat. Commun. 13, 2744. doi:10.1038/s41467-022-29759-7
Li, J., Gong, H., Zhang, J., Liu, H., Tao, L., Wang, Y., et al. (2021a). Efficient exciplex-based deep-blue organic light-emitting diodes employing a bis(4-fluorophenyl)amine-substituted heptazine acceptor. Molecules 26, 5568. doi:10.3390/molecules26185568
Li, J., Gong, H., Zhang, J., Zhou, S., Tao, L., Jiang, L., et al. (2021b). Enhanced electroluminescence based on a pi-conjugated heptazine derivative by exploiting thermally activated delayed fluorescence. Front. Chem. 9, 693813. doi:10.3389/fchem.2021.693813
Li, J., Li, Z., Liu, H., Gong, H. Q., Zhang, J. C., Li, X. Y., et al. (2022b). Down-conversion-induced delayed fluorescence via an inverted singlet-triplet channel. Dyes Pigm. 203, 110366. doi:10.1016/j.dyepig.2022.110366
Li, J., Li, Z., Liu, H., Gong, H. Q., Zhang, J. C., Yao, Y., et al. (2022c). Advances in blue exciplex-based organic light-emitting materials and devices. Front. Chem. 10, 952116. doi:10.3389/fchem.2022.952116
Li, J., Nakagawa, T., MacDonald, J., Zhang, Q., Nomura, H., Miyazaki, H., et al. (2013). Highly efficient organic light-emitting diode based on a hidden thermally activated delayed fluorescence channel in a heptazine derivative. Adv. Mat. 25, 3319–3323. doi:10.1002/adma.201300575
Li, J., Nomura, H., Miyazaki, H., and Adachi, C. (2014a). Highly efficient exciplex organic light-emitting diodes incorporating a heptazine derivative as an electron acceptor. Chem. Commun. 50, 6174–6176. doi:10.1039/c4cc01590h
Li, J., Tao, L., Wang, Y., Yao, Y., and Guo, Q. (2021c). Heptazine-based pi-conjugated materials for light-emitting. Front. Chem. 9, 717569. doi:10.3389/fchem.2021.717569
Li, J., Zhang, J. C., Gong, H. Q., Tao, L., Wang, Y. Q., and Guo, Q. (2021d). Efficient deep-blue electroluminescence employing heptazine-based thermally activated delayed fluorescence. Photonics 8, 293. doi:10.3390/photonics8080293
Li, J., Zhang, Q. S., Nomura, H., Miyazaki, H., and Adachi, C. (2014b). Thermally activated delayed fluorescence from 3nπ* to 1nπ* up-conversion and its application to organic light-emitting diodes. Appl. Phys. Lett. 105, 013301. doi:10.1063/1.4887346
Li, X., Shi, Y.-Z., Wang, K., Zhang, M., Zheng, C.-J., Sun, D.-M., et al. (2019). Thermally activated delayed fluorescence carbonyl derivatives for organic light-emitting diodes with extremely narrow full width at half-maximum. ACS Appl. Mat. Interfaces 11, 13472–13480. doi:10.1021/acsami.8b19635
Li, Z., Yang, Y., Xu, T., Fruchtl, H., van Mourik, T., Paterson, M. J., et al. (2022d). Next generation quantum theory of atoms in molecules for the design of emitters exhibiting thermally activated delayed fluorescence with laser irradiation. J. Comput. Chem. 43, 206–214. doi:10.1002/jcc.26783
Liang, X., Yan, Z. P., Han, H. B., Wu, Z. G., Zheng, Y. X., Meng, H., et al. (2018). Peripheral amplification of multi-resonance induced thermally activated delayed fluorescence for highly efficient OLEDs. Angew. Chem. Int. Ed. 57, 11316–11320. doi:10.1002/anie.201806323
Lidzey, D. G., Bradley, D. D. C., Skolnick, M. S., Virgili, T., Walker, S., and Whittaker, D. M. (1998). Strong exciton-photon coupling in an organic semiconductor microcavity. Nature 395, 53–55. doi:10.1038/25692
Liu, D., Wei, J. Y., Tian, W. W., Jiang, W., Sun, Y. M., Zhao, Z., et al. (2020). Endowing TADF luminophors with aie properties through adjusting flexible dendrons for highly efficient solution-processed nondoped OLEDs. Chem. Sci. 11, 7194–7203. doi:10.1039/d0sc02194f
Liu, H., Pan, G., Yang, Z., Wen, Y., Zhang, X., Zhang, S.-T., et al. (2022). Dual-emission of fluorescence and room-temperature phosphorescence for ratiometric and colorimetric oxygen sensing and detection based on dispersion of pure organic thianthrene dimer in polymer host. Adv. Opt. Mat. 10, 2102814. doi:10.1002/adom.202102814
Long, Y., Mamada, M., Li, C., dos Santos, P. L., Colella, M., Danos, A., et al. (2020). Excited state dynamics of thermally activated delayed fluorescence from an excited state intramolecular proton transfer system. J. Phys. Chem. Lett. 11, 3305–3312. doi:10.1021/acs.jpclett.0c00498
Lv, X., Miao, J., Liu, M., Peng, Q., Zhong, C., Hu, Y., et al. (2022). Extending the pi-skeleton of multi-resonance TADF materials towards high-efficiency narrowband deep-blue emission. Angew. Chem. Int. Ed. Engl. 61, e202201588. doi:10.1002/anie.202201588
Mamada, M., Inada, K., Komino, T., Potscavage, W. J., Nakanotani, H., and Adachi, C. (2017). Highly efficient thermally activated delayed fluorescence from an excited-state intramolecular proton transfer system. ACS Cent. Sci. 3, 769–777. doi:10.1021/acscentsci.7b00183
Mao, M. X., Li, F. L., Shen, Y., Liu, Q. M., Xing, S., Luo, X. F., et al. (2021). Simple synthesis of red iridium(III) complexes with sulfur-contained four-membered ancillary ligands for OLEDs. Molecules 26, 2599. doi:10.3390/molecules26092599
Mei, Y. Q., Liu, D., Li, J. Y., Li, H. T., and Wei, W. K. (2021). Acridin-9(10h)-one based thermally activated delayed fluorescence material: Simultaneous optimization of risc and radiation processes to boost luminescence efficiency. J. Mat. Chem. C 9, 5885–5892. doi:10.1039/d1tc00592h
Meng, G., Liu, L., He, Z., Hall, D., Wang, X., Peng, T., et al. (2022). Multi-resonant thermally activated delayed fluorescence emitters based on tetracoordinate boron-containing pahs: Colour tuning based on the nature of chelates. Chem. Sci. 13, 1665–1674. doi:10.1039/d1sc05692a
Minaev, B., Baryshnikov, G., and Agren, H. (2014). Principles of phosphorescent organic light emitting devices. Phys. Chem. Chem. Phys. 16, 1719–1758. doi:10.1039/c3cp53806k
Miyajima, D., Aizawa, N., Pu, Y. J., Nihonyanagi, A., Ibuka, R., Inuzuka, H., et al. (2021). Delayed fluorescence from inverted singlet and triplet excited states for efficient organic light-emitting diodes. Res. Square (Preprint). doi:10.21203/rs.3.rs-478258/v1
Nakanotani, H., Higuchi, T., Furukawa, T., Masui, K., Morimoto, K., Numata, M., et al. (2014). High-efficiency organic light-emitting diodes with fluorescent emitters. Nat. Commun. 5, 4016. doi:10.1038/ncomms5016
Oh, C. S., Kang, Y. J., Jeon, S. K., and Lee, J. Y. (2015). High efficiency exciplex emitters using donor-acceptor type acceptor material. J. Phys. Chem. C 119, 22618–22624. doi:10.1021/acs.jpcc.5b05292
Peng, C. C., Yang, S. Y., Li, H. C., Xie, G. H., Cui, L. S., Zou, S. N., et al. (2020). Highly efficient thermally activated delayed fluorescence via an unconjugated donor–acceptor system realizing EQE of over 30%. Adv. Mat. 32, 2003885. doi:10.1002/adma.202003885
Pios, S., Huang, X., Sobolewski, A. L., and Domcke, W. (2021). Triangular boron carbon nitrides: An unexplored family of chromophores with unique properties for photocatalysis and optoelectronics. Phys. Chem. Chem. Phys. 23, 12968–12975. doi:10.1039/d1cp02026a
Pollice, R., Friederich, P., Lavigne, C., Gomes, G. D., and Aspuru-Guzik, A. (2021). Organic molecules with inverted gaps between first excited singlet and triplet states and appreciable fluorescence rates. Matter 4, 1654–1682. doi:10.1016/j.matt.2021.02.017
Ribeiro, R. F., Martinez-Martinez, L. A., Du, M., Campos-Gonzalez-Angulo, J., and Yuen-Zhou, J. (2018). Polariton chemistry: Controlling molecular dynamics with optical cavities. Chem. Sci. 9, 6325–6339. doi:10.1039/c8sc01043a
Ricci, G., San-Fabian, E., Olivier, Y., and Sancho-Garcia, J. C. (2021). Singlet-triplet excited-state inversion in heptazine and related molecules: Assessment of TD-DFT and ab initio methods. ChemPhysChem 22, 553–560. doi:10.1002/cphc.202000926
Rothberg, L. J., and Lovinger, A. J. (1996). Status of and prospects for organic electroluminescence. J. Mat. Res. 11, 3174–3187. doi:10.1557/jmr.1996.0403
Sancho-Garcia, J. C., Bremond, E., Ricci, G., Perez-Jimenez, A. J., Olivier, Y., and Adamo, C. (2022). Violation of hund's rule in molecules: Predicting the excited-state energy inversion by TD-DFT with double-hybrid methods. J. Chem. Phys. 156, 034105. doi:10.1063/5.0076545
Sancho-García, J. C., and San-Fabián, E. (2022). Organic emitters showing excited-states energy inversion: An assessment of MC-PDFT and correlation energy functionals beyond TD-DFT. Computation 10, 13. doi:10.3390/computation10020013
Sanvitto, D., and Kena-Cohen, S. (2016). The road towards polaritonic devices. Nat. Mat. 15, 1061–1073. doi:10.1038/nmat4668
Sanz-Rodrigo, J., Ricci, G., Olivier, Y., and Sancho-Garcia, J. C. (2021). Negative singlet-triplet excitation energy gap in triangle-shaped molecular emitters for efficient triplet harvesting. J. Phys. Chem. A 125, 513–522. doi:10.1021/acs.jpca.0c08029
Sato, T., Uejima, M., Tanaka, K., Kaji, H., and Adachi, C. (2015). A light-emitting mechanism for organic light-emitting diodes: molecular design for inverted singlet-triplet structure and symmetry-controlled thermally activated delayed fluorescence. J. Mat. Chem. C 3, 870–878. doi:10.1039/c4tc02320j
Segal, M., Singh, M., Rivoire, K., Difley, S., Van Voorhis, T., and Baldo, M. A. (2007). Extrafluorescent electroluminescence in organic light-emitting devices. Nat. Mat. 6, 374–378. doi:10.1038/nmat1885
Sobolewski, A. L., and Domcke, W. (2021). Are heptazine-based organic light-emitting diode chromophores thermally activated delayed fluorescence or inverted singlet-triplet systems? J. Phys. Chem. Lett. 12, 6852–6860. doi:10.1021/acs.jpclett.1c01926
Stavrou, K., Danos, A., Hama, T., Hatakeyama, T., and Monkman, A. (2021). Hot vibrational states in a high-performance multiple resonance emitter and the effect of excimer quenching on organic light-emitting diodes. ACS Appl. Mat. Interfaces 13, 8643–8655. doi:10.1021/acsami.0c20619
Stranius, K., Hertzog, M., and Borjesson, K. (2018). Selective manipulation of electronically excited states through strong light-matter interactions. Nat. Commun. 9, 2273. doi:10.1038/s41467-018-04736-1
Tischler, J. R., Bradley, M. S., Bulovic, V., Song, J. H., and Nurmikko, A. (2005). Strong coupling in a microcavity led. Phys. Rev. Lett. 95, 036401. doi:10.1103/physrevlett.95.036401
Uoyama, H., Goushi, K., Shizu, K., Nomura, H., and Adachi, C. (2012). Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238. doi:10.1038/nature11687
Wen, Y., Liu, H., Zhang, S.-T., Pan, G., Yang, Z., Lu, T., et al. (2021). Modulating room temperature phosphorescence by oxidation of thianthrene to achieve pure organic single-molecule white-light emission. CCS Chem. 3, 1940–1948. doi:10.31635/ccschem.020.202000433
Wu, X. G., Su, B. K., Chen, D. G., Liu, D. H., Wu, C. C., Huang, Z. X., et al. (2021). The role of host-guest interactions in organic emitters employing mr-TADF. Nat. Photonics 15, 780–786. doi:10.1038/s41566-021-00870-3
Xu, Y., Wang, Q., Cai, X., Li, C., and Wang, Y. (2021a). Highly efficient electroluminescence from narrowband green circularly polarized multiple resonance thermally activated delayed fluorescence enantiomers. Adv. Mat. 33, 2100652. doi:10.1002/adma.202100652
Xu, Y., Xu, P., Hu, D., and Ma, Y. (2021b). Recent progress in hot exciton materials for organic light-emitting diodes. Chem. Soc. Rev. 50, 1030–1069. doi:10.1039/d0cs00391c
Xue, Q., and Xie, G. H. (2021). Thermally activated delayed fluorescence beyond through-bond charge transfer for high-performance OLEDs. Adv. Opt. Mat. 9, 2002204. doi:10.1002/adom.202002204
Yang, Y., Li, N., Miao, J., Cao, X., Ying, A., Pan, K., et al. (2022). Chiral multi-resonance TADF emitters exhibiting narrowband circularly polarized electroluminescence with an EQE of 37.2 . Angew. Chem. Int. Ed. Engl. 61, e202202227. doi:10.1002/anie.202202227
Yang, Z., Mao, Z., Xie, Z., Zhang, Y., Liu, S., Zhao, J., et al. (2017). Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 46, 915–1016. doi:10.1039/c6cs00368k
Yersin, H., Mataranga-Popa, L., Czerwieniec, R., and Dovbii, Y. (2019). Design of a new mechanism beyond thermally activated delayed fluorescence toward fourth generation organic light emitting diodes. Chem. Mat. 31, 6110–6116. doi:10.1021/acs.chemmater.9b01168
Yu, Y., Mallick, S., Wang, M., and Borjesson, K. (2021). Barrier-free reverse-intersystem crossing in organic molecules by strong light-matter coupling. Nat. Commun. 12, 3255. doi:10.1038/s41467-021-23481-6
Yuan, Y., Tang, X., Du, X.-Y., Hu, Y., Yu, Y.-J., Jiang, Z.-Q., et al. (2019). The design of fused amine/carbonyl system for efficient thermally activated delayed fluorescence: novel multiple resonance core and electron acceptor. Adv. Opt. Mat. 7, 1801536. doi:10.1002/adom.201801536
Zhang, Q. S., Li, J., Shizu, K., Huang, S. P., Hirata, S., Miyazaki, H., et al. (2012). Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes. J. Am. Chem. Soc. 134, 14706–14709. doi:10.1021/ja306538w
Zhao, Y. D., Wang, W. G., Gui, C., Fang, L., Zhang, X. L., Wang, S. J., et al. (2018). Thermally activated delayed fluorescence material with aggregation-induced emission properties for highly efficient organic light-emitting diodes. J. Mat. Chem. C 6, 2873–2881. doi:10.1039/c7tc04934j
Zhou, C., Zhang, S., Gao, Y., Liu, H., Shan, T., Liang, X., et al. (2018). Ternary emission of fluorescence and dual phosphorescence at room temperature: A single-molecule white light emitter based on pure organic aza-aromatic material. Adv. Funct. Mat. 28, 1802407. doi:10.1002/adfm.201802407
Zhou, L., Kwong, C. L., Kwok, C. C., Cheng, G., Zhang, H., and Che, C. M. (2014). Efficient red electroluminescent devices with sterically hindered phosphorescent platinum(II) schiff base complexes and iridium complex codopant. Chem. Asian J. 9, 2984–2994. doi:10.1002/asia.201402618
Keywords: inverted singlet-triplet, down conversion, organic light-emitting materials, reverse intersystem crossing, thermal activated delayed fluorescence
Citation: Li J, Li Z, Liu H, Gong H, Zhang J, Yao Y and Guo Q (2022) Organic molecules with inverted singlet-triplet gaps. Front. Chem. 10:999856. doi: 10.3389/fchem.2022.999856
Received: 21 July 2022; Accepted: 01 August 2022;
Published: 24 August 2022.
Edited by:
Haichang Zhang, Qingdao University of Science and Technology, ChinaReviewed by:
Xing Feng, Guangdong University of Technology, ChinaHaichao Liu, Jilin University, China
Copyright © 2022 Li, Li, Liu, Gong, Zhang, Yao and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Guo, cWlhbmdndW9AY3VpdC5lZHUuY24=
 Jie Li
Jie Li Zhi Li1
Zhi Li1 Qiang Guo
Qiang Guo