- 1Laser Research Centre, University of Johannesburg, Doornfontein, South Africa
- 2School of Bio Sciences and Technology, Vellore Institute of Technology, Vellore, TN, India
Photosensitizers with Aggregation-Induced Emission (AIE) can allow the efficient light-mediated generation of Reactive Oxygen Species (ROS) based on their complex molecular structure, while interacting with living cells. They achieve better tissue targeting and allow penetration of different wavelengths of Ultraviolet-Visible-Infrared irradiation. Not surprisingly, they are useful for fluorescence image-guided Photodynamic Therapy (PDT) against cancers of diverse origin. AIE-photosensitizers can also function as broad spectrum antimicrobials, capable of destroying the outer wall of microbes such as bacteria or fungi without the issues of drug resistance, and can also bind to viruses and deactivate them. Often, they exhibit poor solubility and cellular toxicity, which compromise their theranostic efficacy. This could be circumvented by using suitable nanomaterials for improved biological compatibility and cellular targeting. Such dual-function AIE-photosensitizers nanoparticles show unparalleled precision for image-guided detection of tumors as well as generation of ROS for targeted PDT in living systems, even while using low power visible light. In short, the development of AIE-photosensitizer nanoparticles could be a better solution for light-mediated destruction of unwanted eukaryotic cells and selective elimination of prokaryotic pathogens, although, there is a dearth of pre-clinical and clinical data in the literature.
Introduction
Natural products such as curcumin, hypericin, hypocrellin, riboflavin and many synthetic compounds such as, tetrapyrroles, phenothiaziniums, rose bengal and squaraine have been widely explored for their photodynamic activity in living tissues (Abrahamse and Hamblin, 2016). Further, photosensitizers such as Foscan, Fimaporfin, Hemoporfin, Redaporfin, Talaporfin sodium, Verteporfin, Photolon, Photosens and Tookad have been investigated in preclinical and clinical trials (Hamblin, 2020). These photosensitizers are characterized by excitation to a long-lived triplet state and transfer energy upon light illumination, thereby converting oxygen molecules to highly reactive singlet oxygen and free radicals. However, photosensitizers with Aggregation-Induced Emission (AIE) are able to replace conventional photosensitizer nanomaterials in theranostics due to their ease of synthesis and biological compatibility, enhanced permeability and retention effect, excellent fluorescence properties as well as their capacity for efficient photoacoustic imaging in living cells. They do undergo tissue aggregation with less autofluorescence making them very effective for Photodynamic Therapy (PDT) (Wang et al., 2021). Additionally, the ability of AIE-photosensitizers to ‘light-up' in response to an external stimuli or changes in their microenvironment makes them suitable for theranostic applications (Wang et al., 2018a).
Nanomaterials such as Au/Ag nanoparticles, mesoporous/magnetic/polymeric nanoparticles, carbon nanotubes/graphene, as well as quantum dots have been widely used as theranostic platforms (Kang et al., 2020). Several of these organic and inorganic nanomaterials coupled with AIE-photosensitizers may allow direct delivery into cellular organelles for studies of cellular processes and facilitate image-guided therapies. These nanoparticles have an increased surface area for drug delivery and they are responsive to near infrared wavelengths, just as AIE-photosensitizers. Currently, many of these AIE-photosensitizer nanoparticles have absorption maxima below 500 nm and emission spectra below 700 nm, although, it is desirable to have absorption spectra with a narrow band gap and emission maxima around 800 nm for the best biological effects (Korneev et al., 2019). These AIE-photosensitizers are quite useful for cancer therapy and can overcome resistance to antibiotics in microbes. This review explores the mechanisms of AIE-photosensitizers on cancer and also the photophysical properties against infectious agents.
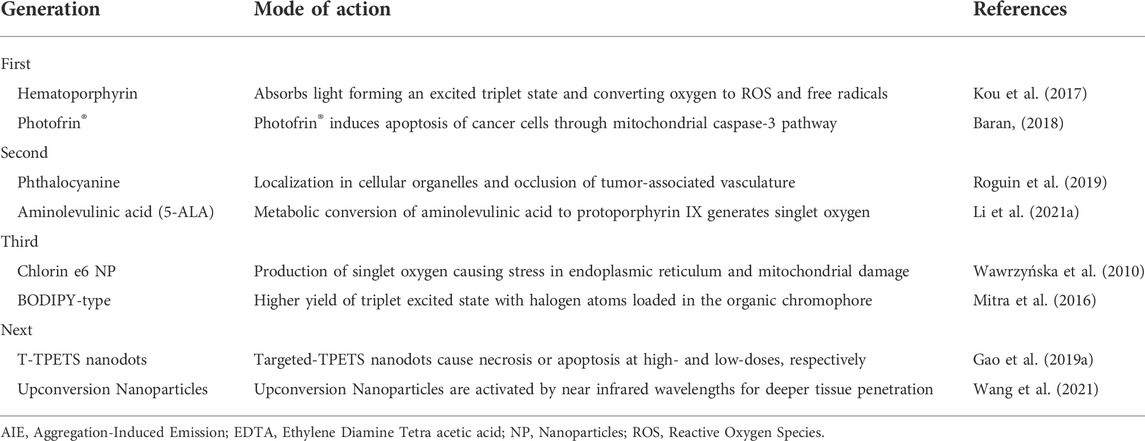
History of photosensitizers
The first generation photosensitizers examined for clinical use were porphyrin-based compounds such as hematoporphyrin derivative marketed as Photofrin®, which faced significant drawbacks in terms of skin photosensitization as well as a low molar absorption coefficient (Table 1). They were soon replaced by the second generation photosensitizers with significant modifications to the porphyrin core structure. The third generation non-porphyrin photosensitizers have the advantages of activation by longer wavelengths, shorter photosensitization periods and higher yield of singlet oxygen, although, they still have the tendency to aggregate in a polar environment and a lack of specificity (Zhang et al., 2018a). These drawbacks are attributed to the flat aromatic structures of photosensitizers resulting in the π-π stacking in the aggregated state, which is referred as Aggregation-Induced Quenching (AIQ). On the contrary, the molecular structures of AIE-photosensitizers take advantage of the aggregation of photosensitizers in concentrated solutions as well as in solid state to perform better than any of the conventional systems (Luo et al., 2001).
Mechanism of Aggregation-Induced Emission-photosensitization
PDT requires an optimal concentration of oxygen, duration and wavelength of light along with appropriate structural and photophysical properties and tissue distribution of the photosensitizer molecules (Silva et al., 2015). AIE-photosensitizer molecules have a propeller-like configuration, which allows the spatial orientation of conjugated units connected by single bonds. Their rotation will be accelerated in dilute solutions, especially during light irradiation. These rotations in dilute solution consume most of their absorbed energy while emitting weak or no luminescence. However, when these molecules are aggregated, the rotations of the conjugated units are inhibited due to the enhanced intermolecular interactions. This leads to the blockade of non-radiative decay and the energy released from the excited state of the molecule is seen as fluorescence or as phosphorescence (radiative decay) (Ni et al., 2021). In fact, the propeller like configuration of AIE-photosensitizers (referred to as rotors) is responsible for the intramolecular motion caused by the absorption of energy in dilute solutions. They emit only minimal or no light in dilute solutions while having large “Stokes Shift” upon aggregation. Their rotors possess intense non-radiative dissipation pathways in dilute solutions, but in the aggregated state they show radiative decay to emit fluorescence, and Intersystem Crossing (ISC) to the triplet state and photodynamic activity (Liu et al., 2021). In short, there will be restriction of their molecular motion during aggregation in concentrated or solid states, referred as Restricted-Intramolecular-Rotation (RIR) or Restricted-Intramolecular-Vibration (RIV), which will activate the radiative decay channel for emitting strong fluorescence (Wang et al., 2015).
The functionality of an AIE-photosensitizer commences with the absorption of a photon by its ground state (S0) to the electronic excitation states (S1 and S2). Subsequently, they can undergo ISC from their singlet (S1) state to the excited triplet state (T1) leading to the formation of free radicals by type I (electron transfer) or type II (energy transfer) resulting in the production of Reactive Oxygen Species (ROS) or reactive singlet oxygen (1O2) from ground state triplet oxygen (Figure 1). Since the advent of AIE-photosensitizers, several advances and modifications have been reported to increase the yield of fluorescence and free radicals including singlet oxygen. An increase in the production of oxide free radicals, especially singlet oxygen can be achieved by increasing the ISC from the lowest excited state (S1) to the lowest triplet state (T1). To accomplish this, the singlet high energy gap (ΔEST), which is the energy gap between singlet and triplet states and a large spin-orbit coupling, should be minimal. One of the ways to reduce ΔEST is by designing AIE-photosensitizers with a D-structure or with a higher ISC quantum yield (Hu et al., 2018). Further, ∆EST can be minimized for an efficient singlet oxygen production by inserting conjugated moieties as linkers or spacers (π) between the donor and acceptor moieties. Accordingly, the AIE-photosensitizer core structure consists of neutral, donor and acceptor. And, they are divided into 1) Donor-AIE (neutral)-acceptor and 2) AIE (donor)-acceptor.

FIGURE 1. Mechanism of AIE-photosensitization: AIE-photosensitizers at the ground state (S0) absorbs energy to become excited Singlet states (S1, S2), which then undergoes Intersystem Crossing (ISC) to the Triplet states for the transfer of electrons (T1) or energy (T2). Production of free radicals and singlet oxygen can be increased in T1 or T2 reactions by accelerating ISC from S1 to S2. Thus, it is ideal to have lower energy gap between S1 and S2 and a large spin-orbit coupling. Further, design of the AIE-photosensitizer molecules with a D-structure should allow aggregation-induced ISC. Unlike any other fluorescent dyes, AIE-photosensitizers can overcome Aggregation-Induced Quenching (AIQ) in their condensed state and are most suitable for theranostics. Abbreviations: O2, Oxygen; 1O2, Singlet oxygen; ROS, Reactive Oxygen Species.
Studies have shown that inserting conjugated moieties as linker(s) between the donor and acceptor moieties may lengthen the space between the Highest-energy Occupied Molecular Orbital (HOMO) and the Lowest-energy Unoccupied Molecular Orbital (LUMO), thereby minimizing the ∆EST and increasing singlet oxygen yield of the photosensitizers (Xu et al., 2015; Ni et al., 2021). An increased separation between the HUMO and LUMO reduces the electronic repulsion and the resultant lower ΔEST may improve the efficiency of ROS production. Thus, an effective separation of HOMO and LUMO results in less conjugation between acceptor and donor moieties leading to short wavelength absorption and emission. However, the combined acceptor moieties may results in a “red-shift” of absorption and emission due to the strong electron withdrawing ability of the two acceptors. The combined acceptor and donor moieties connected by a double bond give better conjugation resulting in a “red-shift” of absorption and emission (Liu et al., 2019a; Alam et al., 2020).
Cellular effects of Aggregation-Induced Emission-photosensitizers
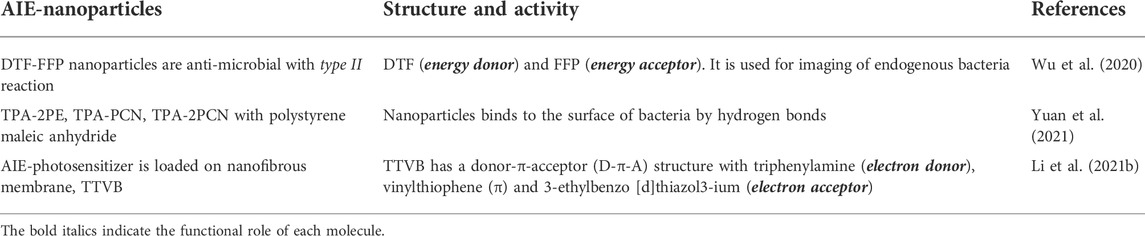
The activity of AIE-photosensitizers relies mainly on their retention in living tissues and their photophysical mechanisms. Accordingly, they are classified into three categories 1) electron transfer; 2) energy transfer; 3) combined mechanisms (Figure 2). Type I reaction involves transfer of the electrons at low oxygen concentrations (hypoxia) leading to the formation of superoxide radical anions. Usually, this is followed by the formation of hydroxyl radicals and peroxides in tumors in vivo as well as in spheroids in 3-dimensional cell cultures. Here, the electron transfer may happen in either directions, but usually, the excited photosensitizer will act as an oxidant. The type I photochemical mechanism can also occur where the electrons transfer from the AIE-photosensitizers to oxygen for the formation of superoxide and other free radicals. On the contrary, type II involves energy transfer from the triplet state of AIE-photosensitizer to the ground state of molecular oxygen (also a triplet) under normal oxygen concentrations (normoxia). The net result is the formation of reactive singlet oxygen, which is detrimental to both cancers as well as microbes. However, this hypothesis needs to be rigorously tested with various AIE-photosensitizers for proper validation.
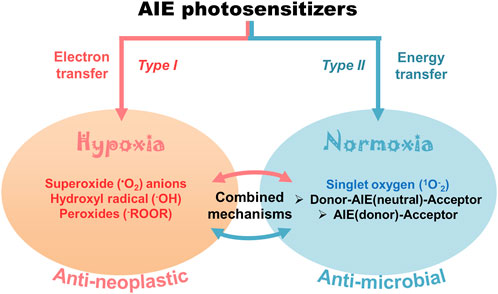
FIGURE 2. Cellular effects of AIE-photosensitizers: The AIE-photosensitizers are classified as type I and II according to their process of synthesis of ROS. Transfer of electrons at low oxygen concentration creates oxide free radicals (type I photosensitization), while transfer of energy to the molecular oxygen generates singlet oxygen (type II photosensitization). Often, electron transfer may happen from AIE-photosensitizer to oxygen in type II reactions forming superoxide anions. The type II photosensitization is subdivided into 1) Donor-AIE (neutral)-Acceptor 2) AIE (donor)-Acceptor, and 3) combined electron and energy transfer mechanisms. While the type I photosensitization has anti-neoplastic effects due to the robust oxidation of biomolecules, the latter is mostly anti-microbial. Paradoxically, type II reaction with electron transfer mechanisms has been widely used in anticancer theranostics.
PDT can be much more effective either by direct killing of tumor cells or by closing blood vessels immediately surrounding the tumor mass leading to hypoxia and necrosis. In general, AIE-photosensitizers are effective in metastatic tumors depending on the abundant supply of oxygen and blood for their rapid growth and spread (Wang et al., 2021). However, AIE-photosensitizers with type I ROS formation are adapted to the hypoxic microenviroment around the tumor tissues and, therefore be a better therapeutic choice. Rapidly growing tumors are metabolically active requiring more oxygen and nutrients, which is achieved by the process of neovascularization. For this reason, most of the photosensitizers synthesized and used for PDT are type II nowadays. These molecules require sufficient oxygen tension in the cellular microenvironment for their optimal activity. However, rapidly growing tumors as well as deep seated tumors fails to meet the demand of oxygen and nutrients due to their impedance in angiogenesis. Frequent exposure to PDT also creates an oxygen deprived condition leading to the ineffectiveness of type II reactions. Thus, there is an imminent need to identify and develop AIE-photosensitizers, which is much tolerant and effective in hypoxic environment with type I reaction for ROS formation (Zhou et al., 2016; Ni et al., 2021).
Theranostic applications of Aggregation-Induced Emission-photosensitizers
AIE-photosensitizers should have controllable excitation for absorbing many wavelengths of light, which will increase their activity on living cells, especially tumors and microbes. They need also to be excited using near infrared irradiation rendering them active against deep seated tumors. Often, the rational design of AIE-photosensitizers can result in long wavelength excitation peaks. The intramolecular charge transfer due to the strong electron D-A interaction as well as extended π-conjugation also results in the absorption and emission of longer wavelengths (Gao et al., 2019b; Wu et al., 2019). AIE-photosensitizers, TTPy and MeTTPy were synthesized using this approach and demonstrated excellent tumor targeting and ROS generation capabilities. They possess better absorption and emission at longer wavelengths, far red and near infrared regions. TTPy shows better penetration, less cytotoxicity as well as good anti-bacterial effects (Wang et al., 2018b; Wang et al., 2021).
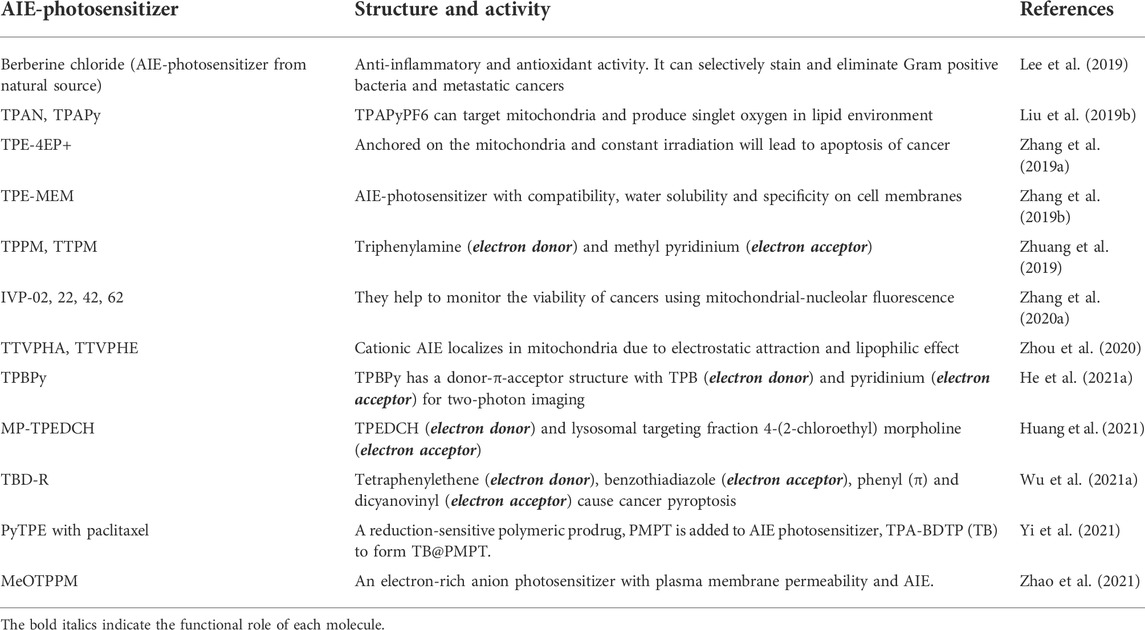
AIE-photosensitizers do not undergo AIQ due to their hydrophobic and intrinsically rigid planar structures in contrast to more traditional fluorophores (Liu et al., 2021). Quite often, they are accumulated in the cell membrane phospholipids, phosphatidylethanolamine intercalated with phosphatidylserine. Upon activation by light (photons) these AIE-photosensitizers with either electron transfer or energy transfer properties (classified as type I or type II) can produce a range of ROS. In fact, the appropriate donor and acceptor in the molecular skeleton of AIE is responsible for lowering the ΔEST by π-π stacking and a higher ISC, which results in higher ROS production (Wang et al., 2021). AIE-photosensitizers with type II reactivity are constructed as D-A or D-π-A to keep the ΔEST as low as possible and can either be configured as Donor-AIE (neutral)-acceptor or as AIE (donor)-acceptor. There are instances, when the AIE-photosensitizers may generate both free radicals and singlet oxygen by both type I and II reactions. These can be referred as “combined mechanisms” involving transfer of electrons as well as energy between the donor and acceptor molecules. Below we summarize the AIE-photosensitizers commonly used for cancer and microbial theranostics based on the information gleaned from available literature (Tables 2–5). Unfortunately, many of these findings from various sources fail to disclose the “structure-activity relationship” of AIE-photosensitizers, which make our classification incomplete.
Aggregation-Induced Emission-photosensitizers in anticancer theranostics
Most commonly synthesized and widely used AIE-photosensitizers are classified as Donor-AIE (neutral)-acceptor or AIE (donor)-acceptor, constructed on a tetraphenylethene (TPE), triphenylamine (TPA) and rarely on a triarylamine (TAA) to form a D-A or D-π-A structure (Figures 3, 4). These structures are efficient in the production of ROS and singlet oxygen upon interaction with living cells by type I and type II reactions respectively. Most of the newly synthesized AIE-photosensitizers exhibit type II reactions, although, they are more suitable on rapidly growing or metastatic cancers receiving abundant oxygen and nutrients.
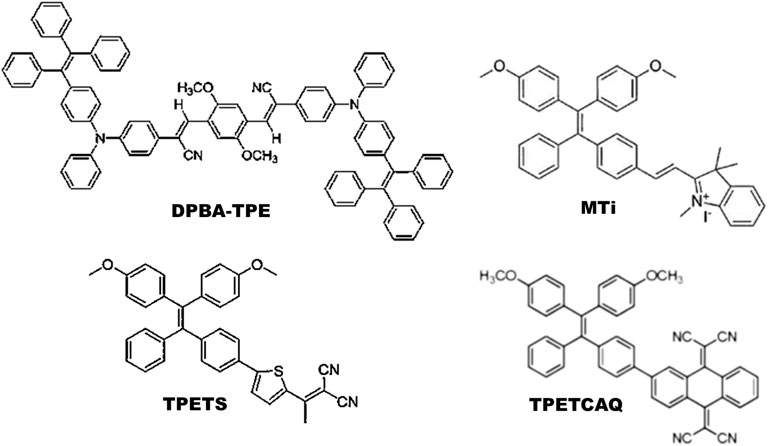
FIGURE 3. Illustrations for Donor-AIE(neutral)-Acceptor photosensitizers: In general, these are constructed on a tetraphenylethene (TPE) cytoskeleton. Alternatively, tetraphenylamine (TPA) can act as an electron donor and/or core of the AIE-photosensitizer. Source: DPBA-TPE (Feng et al., 2015a), MTi (Chen et al., 2019), TPETS (Gao et al., 2019a), TPETCAQ (Wu et al., 2017a). Please refer Tables 2–5 for details.
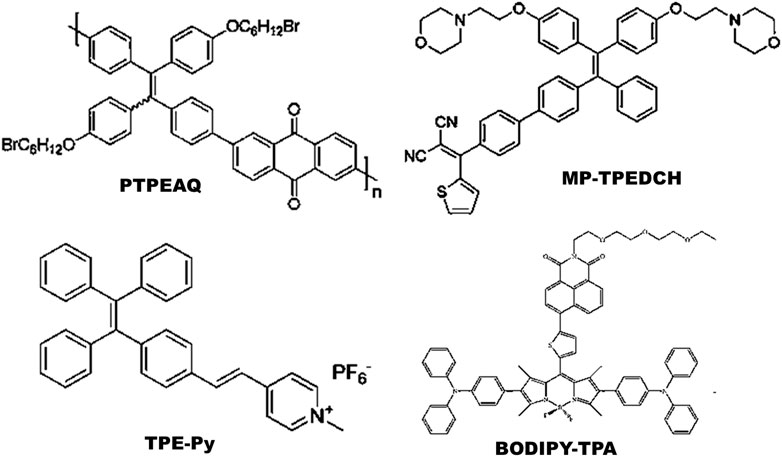
FIGURE 4. Illustrations for AIE(donor)-Acceptor photosensitizers: These are constructed on tetraphenylethene (TPE) cytoskeleton with a D-A or D-π-A structure. Addition of spacer (π) between donor (D) and acceptor (A) may decrease the singlet energy gap (ΔEST). Source: PTPEAQ (Wu et al., 2016), MP-TPEDCH (Huang et al., 2021), TPE-Py (Zhuang et al., 2019), BODIPY-TPA (Deng et al., 2021). Please refer Tables 2–5 for details.
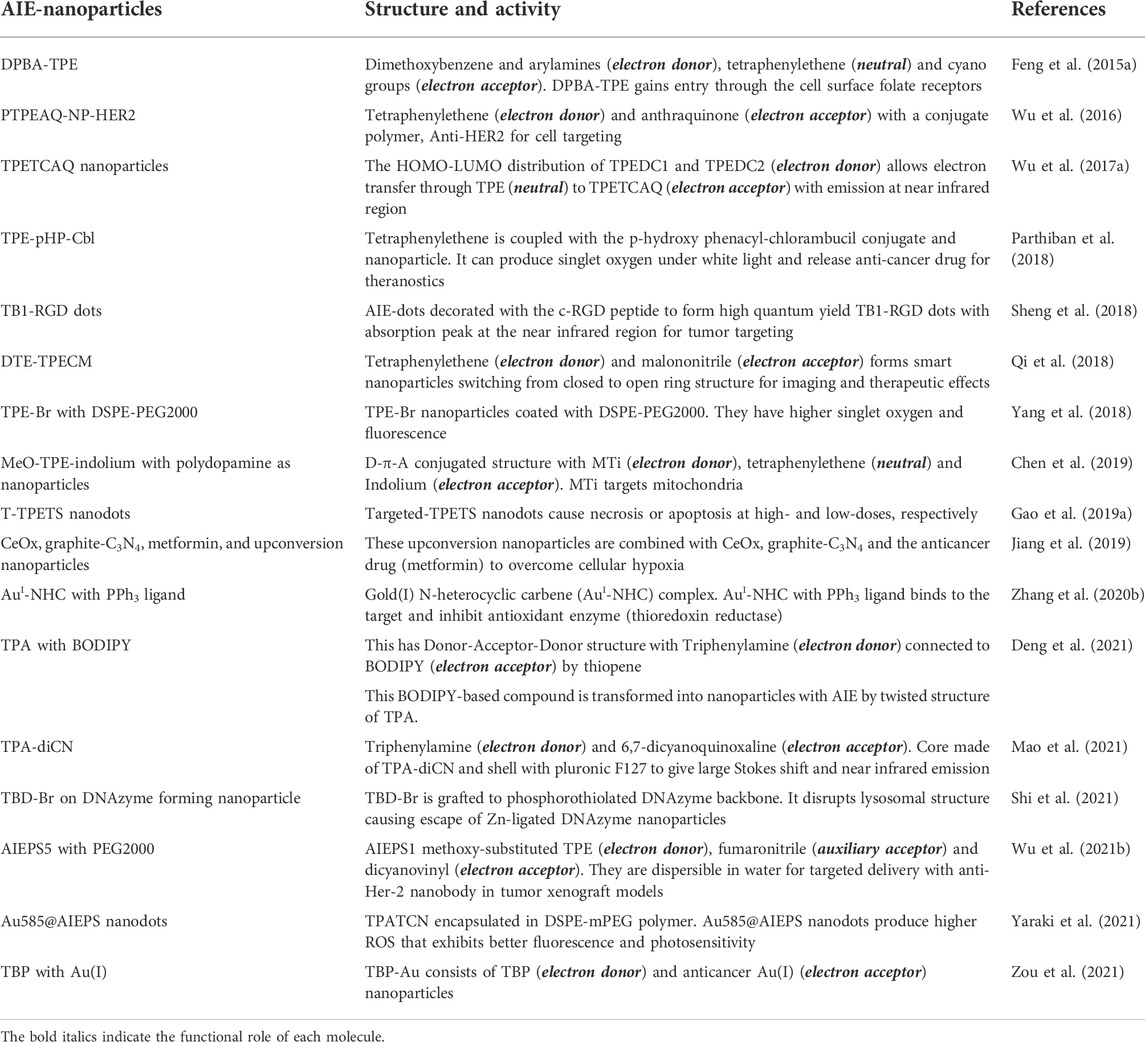
Aggregation-Induced Emission-photosensitizer nanoparticles for anticancer theranostics
Metallic nanoparticles containing heavy metals such as gold, silver, platinum or titanium have the ability to provide hydrophobicity in the cellular environment, which is necessary for the entry of photosensitizers and overcoming biological barriers (Crous and Abrahamse, 2020). The new generation nanoparticles such as fullerenes, titanium dioxide or quantum dots may improve the cellular delivery and photochemical internalization of genetically encoded protein photosensitizers (Tynga and Abrahamse, 2018). The AIE-photosensitizer nanoparticles possess effective biodistribution properties with strong optical absorption and scattering properties. Noble-metal nanoparticles can carry out localized surface plasmon resonance to increase their light absorption, while interacting with cellular molecules (He et al., 2021b). However, there is lack of evidence on the nature of ROS produced by various AIE-photosensitizers nanoparticles while interacting with the living cells in vitro and in vivo.
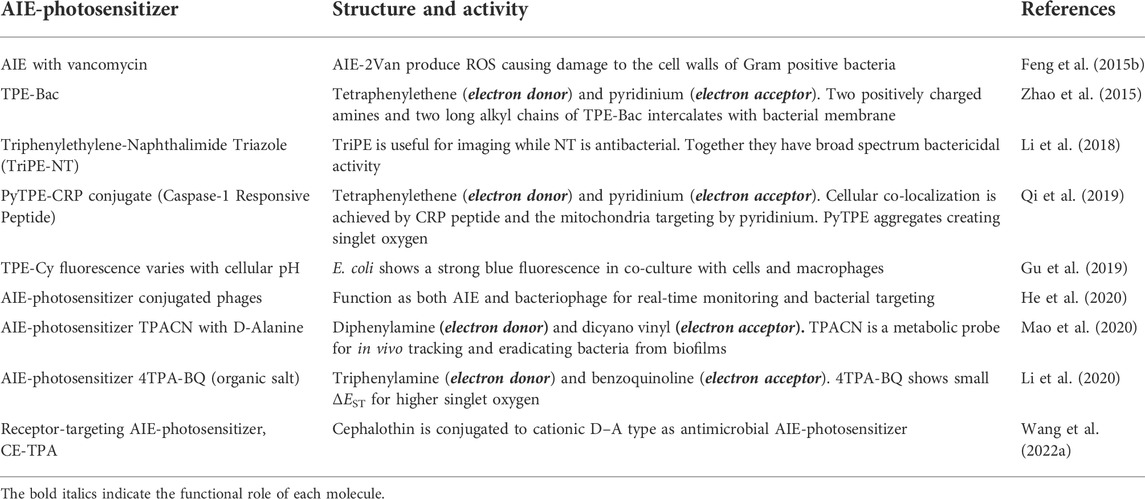
Aggregation-Induced Emission-photosensitizers in antimicrobial theranostics
AIE-photosensitizers can be constructed for microbial detection with precision from various environmental samples. Chen at al. (2014) used a fluorescent sensor array with five AIE probes to detect and differentiate eight different bacteria from water by flow cytometry and analysis. AIE-photosensitizers have ‘turn-on’ characteristics after aggregation and allow fluorescence imaging of infectious agents (Liu et al., 2021). They adhere more to the thick layer of peptidoglycan of Gram-positive bacteria than to the thin phospholipid membrane of Gram-negative bacteria (Bai et al., 2021). Yet, they may bind to Gram-negative bacteria by electrostatic action and destroy them. AIE-photosensitizers could also bind with the cell wall of drug-resistant fungi and their spores (Chen et al., 2022).
Aggregation-Induced Emission-photosensitizer nanoparticles for antimicrobial theranostics
AIE-phtotosensitizers were used to generate bacteriophage as AIE–PAP bioconjugate with capabilities for real-time tracking by fluorescence imaging and killing of antibiotic-sensitive or multi-drug-resistant bacteria (He et al., 2020). AIE-photosensitizer nanoparticles with an overall positive charge can act as broad spectrum antimicrobials activated by near infrared light to produce singlet oxygen and heat (Wang et al., 2022b). Glucose polymer-modified gold nanoparticles were incubated with diverse types of bacteria to be taken up through the ATP-binding cassette (ABC) transporter pathway before laser irradiation to achieve a three-fold increase in the microbicidal activity (Yang et al., 2022). Detailed studies are required to establish the sensitivity/specificity of nanoparticles on environmental and food pathogens.
Biological effects of Aggregation-Induced Emission-photosensitizer nanoparticles
Nano-drug delivery systems include dendrimers, liposomes, micelles, carbon nanotubes as well as various nanoparticles viz. gold, silver, zinc, magnetic, virus etc. All these make use of the gap junctions and breaches in the capillaries to selectively accumulate in tumors with an Enhanced Permeability and Retention (EPR) effect. Among these, gold nanoparticles have the highest cellular uptake as well as an increased production of singlet oxygen due to the surface plasmon resonance for enhancing the effects of PDT (Kruger and Abrahamse, 2018). Conventional photosensitizers are hydrophobic requiring any of these nano-drug delivery systems to gain entry into neoplasms by crossing gap junctions of cells. However, AIE-photosensitizers incorporated in organic nanoparticles show EPR, strong luminescence and good photostability without AIQ property. Moreover, nanomaterials as aggregates of AIE-photosensitizers (AIE-dots) have unique potential in theranostics (He et al., 2021b).
Nanoparticles can exert selective activity against tumors, providing increased bioavailability of photosensitizers to rapidly dividing cells with minimal side-effects. However, nanoparticles such as carbon black, carbon nanotubes, copper and zinc are not only toxic to hepatocytes but also sensitive to tissues, such as alveoli and neurons (Yao et al., 2019). They may cause endoplasmic reticulum stress, multiple organelle dysfunction and also affect mitochondrial dynamics (fusion-fission) resulting in cytochrome c-dependent apoptosis or mitophagy. Nanotoxicity refers to the capacity of the nanomaterials to alter cellular morphology and function, thereby reducing metabolic activity and cellular viability. Nanotoxicity can be a reason for either blockade or induction of autophagy, and can cause lysosomal rupture leading to oxidative stress and inflammation (Liu and Tang, 2020). On the contrary, AIE-photosensitizers encapsulated in organic nanomaterials or synthesized as AIE-dots can perform target-specific destruction of cancers and microbes with least toxicity (Figure 5).
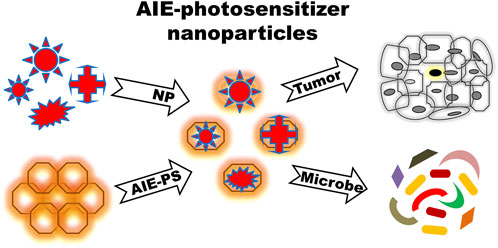
FIGURE 5. Mode of action of AIE-photosensitizer nanoparticles: A selected number of biocompatible nanoparticles can be incorporated into AIE-photosensitizers for an efficient drug delivery with desirable biological effects. AIE-photosensitizer nanoparticles with emission maxima in the far-red to near-infrared have high penetration depth into tumors, which is useful for bio-imaging as well as targeted therapy in PDT. Further, they are able to detect specifically and eliminate pathogens from various environment and food sources. Abbreviations: AIE-PS, Aggregation-Induced Emission-Photosensitizer; NP, Nanoparticles.
A suitable absorption/emission spectrum is essential for the use of AIE-photosensitizers in image-guided therapy. In a study, Yu et al. (2017) has shown that a mitochondria-anchored AIE-photosensitizer generated a high yield of singlet oxygen under white light leading to the apoptosis of cancer, while enabling its visualization under fluorescence “turn-on” mode. Similar functions were also shown by TPE-IQ-2O, which not only distinguishes cancers from healthy cells but also generates ROS upon irradiation with white light (Gui et al., 2017). The biotinylated AIE-photosensitizer, TPE-TETRAD, demonstrates differential staining of cancers from healthy cells and stains mitochondria with emission at the far red region under two/three-photon fluorescence microscopy (Nicol et al., 2017).
The dynamics of ROS production in living cells changes when using AIE-photosensitizers loaded into nanoparticles. Wu et al. (2017a, 2017b) reported an efficient yield of singlet oxygen by a newly synthesized AIE-photosensitizer (please refer Table 3) incorporated in nanoparticles capable of absorption in the ultraviolet-visible region and with stable emission in far red to near infrared region (700–1,000 nm). These AIE-photosensitizers demonstrated low dark toxicity, good photostability as well as good biocompatibility in BALB/c mice, which make them useful for image-guided anticancer PDT. In fact, the recent additions to the list of AIE-photosensitizers with emission in the far infrared region (1,000–1,500 nm) show superior tissue penetration and higher fluorescence emission suitable for photoacoustic dual-mode imaging as well as a tunable photothermal effect (Liu et al., 2020a). Interestingly, TBL dots with F127 emit chemiluminescence under infrared light (Liu et al., 2020b).
AIE-photosensitizers excited by near infrared irradiations can be modified by connecting tetraphenylethene with atypical AIE (donor)-acceptor molecules for the production of Upconversion Nanoparticles (UCNPs). They efficiently aggregate and generate ROS under near infrared irradiation in solutions with higher fractions of water (Dai et al., 2020). These UCNPs are efficient in converting near infrared to shorter wavelengths of visible light for the activation of photosensitizers. It is proposed that the combination of UCNPs and photosensitizers will improve the penetration depth and therapeutic efficacy of PDT (Zhang et al., 2007). Another attempt has been made using AIE-photosensitizers with amphiphilic polymers encapsulating hydrophobic UCNPs. This UCNP@AIE-cRGD formulation could maintain fluorescence intensity under near infrared illumination for a long period and induce apoptosis of tumors residing at a depth up to 6 mm in xenograft rodent models (Jin et al., 2019). The effect of near infrared irradiation on the mitochondrial energy transfer mechanism of living cells is detailed by George et al. (2022).
Biomedical applications of Aggregation-Induced Emission-photosensitizer nanoparticles
Organic nanoparticle t-BPITBT-TPE aggregates encapsulated with DSPE-mPEG micelles shows good biocompatibility and biodistribution in the zebra fish embryo. The in vitro grown cancer cell lines tagged with t-BPITBT-TPE in polymeric nanoparticles efficiently track them for their growth and metastasis in zebra fish larvae and in Balb/c mice (Lin et al., 2017). These fluorescent photosensitizer nanoparticles with AIE properties when tested in a transparent zebra fish larvae with inducible liver hyperplasia gave an understanding of the biodistribution of nanoparticles helpful for the screening of various AIE-photosensitizer nanoparticle in vivo (Manghnani, et al., 2018). Furthermore, this study shows a correlation between the uptake of nanoparticles, dosage of light and the duration of trigger activation.
Gu et al. (2018) found that cancer theranostics can be boosted by restricting intra-particle microenvironment using corannulene-incorporated AIE-photosensitizer nanoparticles. Corannulene limited the intramolecular rotation thereby facilitating the fluorescence pathway and ISC of the AIE-photosensitizers in tumor xenograft models. Similarly, aptamer-AIE organic nanomaterials precisely targets cancer cells using their aptamer-cholesteryl and PEG-lipid moieties for biosensing as well as imaging (Zhang et al., 2018b). Organic nanodots targeted to integrin ανβ3 and bearing AIE-photosensitizers activated by red light are also developed for image-guided PDT and theranostics. These targeted PETS nanodots demonstrates a high yield of singlet oxygen causing dose- and time-dependent apoptosis resulting in the ablation of hepatocellular carcinoma (Gao et al., 2019a).
Nanocarriers are useful for tumor targeting of photosensitizers and reduce the toxicity to normal healthy cells. In one particular study, a pH-responsive AIE-photosensitizer without a nanocarrier was tested in a tumor-bearing mouse model for efficacy and safety. These AIE nano-photosensitizers are self-assembled from amphiphilic AIE-photosensitizers, and avoided the protonation and deprotonation of the carboxyl groups resulting in a higher ROS yield and good treatment efficiency under white light (Cheng et al., 2020). These changes in the redox potential are quite sensitive, and often an increase in the level of oxidative stress hinders cancer (George and Abrahamse, 2020). In another study, three photosensitizer nanostructures made up of 2,3-bis(4′-(diphenylamino)-[1,1′-biphenyl]-4-yl) fumaronitrile (BDBF) are encapsulated in Pluronic F-127 nanoparticles, which shows better ability to generate ROS with AIE characteristics for imaging and tumor regression. The encapsulation in Pluronic F-127 nanoparticles improves the ultrastructure of BDBF, which could self-assemble as nanorods or spherical structures for therapeutic benefits (Han et al., 2020).
Redox-sensitive AIE nanoparticles are developed as micelles of poly (ethylene glycol) (PEG) and cholesterol (CE) conjugated disulfide-containing polyamido amines are found to be useful for fluorescence imaging. These TPE-MI encapsulated micelles show a red shift and an increased fluorescence emission according to the concentration of cellular glutathione, thus mimicking the redox potential of the cells (Cheng et al., 2014). AIE dye-loaded polymer nanoparticle formulations are developed using Pluronic F127 and PEGylated phospholipid with deep-red emission for siRNA delivery, and could be used as nanovectors for gene silencing of mutant K-ras in pancreatic cancer (Hu et al., 2014). Imaging of cells is also accomplished using AIE nanoparticles with nucleic acid induced peptide co-assembly, which emitted fluorescence in response to an increasing concentration of nucleic acids. This allows real time monitoring of drug release from peptide-based nanocarriers in vivo and in vitro (Li et al., 2021c). AIE-photosensitizer nanoparticles are also useful tools for tracking the metastasis of cancers as well as differentiating neurons with high penetration and retention in neuronal cells (Jang et al., 2021).
Conclusion
AIE-photosensitizers are useful for detecting and eliminating tumors and microbes in the tissue environment. After PDT there will be an additional restriction of nutrients and oxygen, especially due to the photochemical consumption of oxygen during the continuous PDT process. Type I AIE-photosensitizers are active even in hypoxic environments rendering them suitable for use in poorly-perfused solid tumors. This property of type I AIE-photosensitizers makes them useful for in vitro PDT assays using 3-dimensional spheroids. On the contrary, other AIE-photosensitizers are type II, and their optimal activity may require oxygen supply to living cells. Thus, the latter may be more effective as microbicidal agents.
Aggregates of AIE-photosensitizers with restricted intermolecular motion reduce the loss of energy by radiative decay, while, relaxation of the excited state results in fluorescence and ISC. Aggregation also results in the increased energy transfer from the excited singlet to triplet states thereby reducing the energy gap (ΔEST) and resulting in the higher production of ROS (Yang et al., 2016). Another strategy to improve the yield of ROS at the triplet state is by modifying the donor and acceptor (D-A) structures and perhaps, adding a spacer (D-π-A) to stabilize the incoming electrons towards generating free radial anions (Zhou et al., 2016). These modifications may also extend the excitation/emission of AIE-photosensitizers to wavelengths in the infrared region, making them ideal choice for theranostics. Furthermore, characteristics of the two/three-photon excitation can be achieved by extending conjugation length of the π-system, which will increase their cross-sectional distance. This will make AIE-photosensitizers suitable for two/three-photon excitation for 3-dimensional imaging in greater depth and detail with least amount of photobleaching and autofluorescence.
AIE-photosensitizers and AIE-dots can be tailored with controllable excitation wavelengths, which may increase their penetration depth in tissues affected with tumors or infections. ROS generation is found to be higher in stimulus-responsive AIE-photosensitizers, which also have improved water solubility and can carry out photothermal effects for sustained photosensitization (Wang et al., 2021). Some AIE-active nanomaterials such as graphene, quantum dots etc. have been proven useful for drug delivery, optoelectronics, as well as theranostics. However, more efficient methods for the qualitative and quantitative detection of ROS in cells, and the use of two/three-photon excitation PDT should be investigated for future development of AIE-photosensitizers and AIE-dots in theranostics.
Author contributions
HA wrote the manuscript after discussion with MH. Figures and tables were created by SG.
Fundings
This research is supported by the South African Research Chairs Initiative (SARCHI) of the Department of Science and Technology and National Research Foundation of South Africa (Grant No. 98337). SG is supported by the Vellore Institute of Technology (VIT), Vellore, TN, India. MH was supported by US NIH Grants R01AI050875 and R21AI121700.
Conflict of interest
MH declares possible conflicts of interests with Scientific Advisory Boards: Transdermal Cap Inc., Cleveland, OH; BeWell Global Inc., Wan Chai, Hong Kong; Hologenix Inc. Santa Monica, CA; LumiThera Inc., Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon Inc., Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV Inc., Minneapolis-St. Paul MN; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV Inc., Lansing MI; Illumiheal & Petthera, Shoreline, WA; MB Laser therapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp. Incline Village, NV; Niraxx Light Therapeutics, Inc., Boston, MA. Consulting; Lexington Int, Boca Raton, FL; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland BV Eindhoven, Netherlands; Johnson & Johnson Inc., Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholdings: Global Photon Inc., Bee Cave, TX; Mitonix, Newark, DE.
The remaining authors declare that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrahamse, H., and Hamblin, M. R. (2016). New photosensitizers for photodynamic therapy. Biochem. J. 473, 347–364. doi:10.1042/bj20150942
Alam, P., He, W., Leung, N., Chao, M., Kwok, R., Jacky, W., et al. (2020). Red AIE‐active fluorescent probes with tunable organelle‐specific targeting. Adv. Funct. Mat. 30, 1909268. doi:10.1002/adfm.201909268
Bai, H., He, W., Chau, J. H. C., Zheng, Z., Kwok, R. T. K., Lam, J. W. Y., et al. (2021). AIEgens for microbial detection and antimicrobial therapy. Biomaterials 268, 120598. doi:10.1016/j.biomaterials.2020.120598
Baran, T. M. (2018). Photofrin® photodynamic therapy with intratumor photosensitizer injection provides similar tumor response while reducing systemic skin photosensitivity: Pilot murine study. Lasers Surg. Med. 50, 476–482. doi:10.1002/lsm.22774
Chen, L., Wang, X., Yuan, Y., Hu, R., Chen, Q., Zhu, L., et al. (2022). Photosensitizers with aggregation-induced emission and their biomedical applications. Eng. Regen. 3, 59–72. doi:10.1016/j.engreg.2022.01.005
Chen, W., Li, Q., Zheng, W., Hu, F., Zhang, G., Wang, Z., et al. (2014). Identification of bacteria in water by a fluorescent array. Angew. Chem. Int. Ed. 53, 13734–13739. doi:10.1002/anie.201407606
Chen, Y., Ai, W., Guo, X., Li, Y., Ma, Y., Chen, L., et al. (2019). Mitochondria-targeted polydopamine nanocomposite with AIE photosensitizer for image-guided photodynamic and photothermal tumor ablation. Small (Weinheim der Bergstrasse, Ger. 15, e1902352. doi:10.1002/smll.201902352
Cheng, G., Wang, H., Zhang, C., Hao, Y., Wang, T., Zhang, Y., et al. (2020). Multifunctional nano-photosensitizer: A carrier-free aggregation-induced emission nanoparticle with efficient photosensitization and pH-responsibility. Chem. Eng. J. 390, 124447. doi:10.1016/j.cej.2020.124447
Cheng, W., Wang, G., Pan, X., Zhang, Y., Tang, B. Z., and Liu, Y. (2014). Redox-responsive nanoparticles with aggregation-induced emission (AIE) characteristic for fluorescence imaging. Macromol. Biosci. 14, 1059–1066. doi:10.1002/mabi.201400076
Crous, A., and Abrahamse, H. (2020). Effective gold nanoparticle-antibody-mediated drug delivery for photodynamic therapy of lung cancer stem cells. Int. J. Mol. Sci. 21, 3742. doi:10.3390/ijms21113742
Dai, J., Li, Y., Long, Z., Jiang, R., Zhuang, Z., Wang, Z., et al. (2020). Efficient near-infrared photosensitizer with aggregation-induced emission for imaging-guided photodynamic therapy in multiple xenograft tumor models. ACS Nano 14, 854–866. doi:10.1021/acsnano.9b07972
Deng, J., Yang, M., Li, C., Liu, G., Sun, Q., Luo, X., et al. (2021). Single molecular-based nanoparticles with aggregation-induced emission characteristics for fluorescence imaging and efficient cancer phototherapy. Dyes Pigm. 187, 109130. doi:10.1016/j.dyepig.2020.109130
Feng, G., Qin, W., Hu, Q., Tang, B. Z., and Liu, B. (2015a). Cellular and mitochondrial dual-targeted organic dots with aggregation-induced emission characteristics for image-guided photodynamic therapy. Adv. Healthc. Mat. 4, 2667–2676. doi:10.1002/adhm.201500431
Feng, G., Yuan, Y., Fang, H., Zhang, R., Xing, B., Zhang, G., et al. (2015b). A light-up probe with aggregation-induced emission characteristics (AIE) for selective imaging, naked-eye detection and photodynamic killing of Gram-positive bacteria. Chem. Commun. 51, 12490–12493. doi:10.1039/c5cc03807c
Gao, X., Mao, D., Zuo, X., Hu, F., Cao, J., Zhang, P., et al. (2019b). Specific targeting, imaging, and ablation of tumor-associated macrophages by theranostic mannose-AIEgen conjugates. Anal. Chem. 91, 6836–6843. doi:10.1021/acs.analchem.9b01053
Gao, Y., Zheng, Q. C., Xu, S., Yuan, Y., Cheng, X., Jiang, S., et al. (2019a). Theranostic nanodots with aggregation-induced emission characteristic for targeted and image-guided photodynamic therapy of hepatocellular carcinoma. Theranostics 9, 1264–1279. doi:10.7150/thno.29101
George, S., and Abrahamse, H. (2020). Redox potential of antioxidants in cancer progression and prevention. Antioxidants (Basel, Switz. 9, 1156. doi:10.3390/antiox9111156
George, S., Hamblin, M. R., and Abrahamse, H. (2022). Neuronal differentiation potential of primary and immortalized adipose stem cells by photobiomodulation. J. Photochem. Photobiol. B Biol. 230, 112445. doi:10.1016/j.jphotobiol.2022.112445
Gu, M., Zeng, Z., Wu, M. Y., Leung, J. K., Zhao, E., Wang, S., et al. (2019). Imaging macrophage phagocytosis using AIE luminogen-labeled E. coli. Chem. Asian J. 14, 775–780. doi:10.1002/asia.201801859
Gu, X., Zhang, X., Ma, H., Jia, S., Zhang, P., Zhao, Y., et al. (2018). Corannulene-incorporated AIE nanodots with highly suppressed nonradiative decay for boosted cancer phototheranostics in vivo. Adv. Mat.Deerf. Beach, Fla.) 30, e1801065. doi:10.1002/adma.201801065
Gui, C., Zhao, E., Kwok, R., Leung, A., Lam, J., Jiang, M., et al. (2017). AIE-Active theranostic system: Selective staining and killing of cancer cells. Chem. Sci. 8, 1822–1830. doi:10.1039/c6sc04947h
Hamblin, M. R. (2020). Photodynamic Therapy for Cancer: What's past is prologue. Photochem. Photobiol. 96, 506–516. doi:10.1111/php.13190
Han, W., Zhang, S., Deng, R., Deng, R., Du, Y., Qian, J., et al. (2020). Self-assembled nanostructured photosensitizer with aggregation-induced emission for enhanced photodynamic anticancer therapy. Sci. China Mat. 63, 136–146. doi:10.1007/s40843-019-9477-3
He, X., Yang, Y., Guo, Y., Lu, S., Du, Y., Li, J. J., et al. (2020). Phage-guided targeting, discriminative Imaging, and synergistic killing of bacteria by AIE bioconjugates. J. Am. Chem. Soc. 142 (8), 3959–3969. doi:10.1021/jacs.9b12936
He, Z., Gao, Y., Zhang, H., Xue, Y., Meng, F., and Luo, L. (2021a). Mitochondrion‐anchored photosensitizer with near infrared‐I aggregation‐induced emission for near infrared‐II two‐photon photodynamic therapy. Adv. Healthc. Mater 10 (24), e2101056. doi:10.1002/adhm.202101056
He, Z., Tian, S., Gao, Y., Meng, F., and Luo, L. (2021b). Luminescent AIE dots for anticancer photodynamic therapy. Front. Chem. 9, 672917. doi:10.3389/fchem.2021.672917
Hu, F., Xu, S., and Liu, B. (2018). Photosensitizers with aggregation-induced emission: Materials and biomedical applications. Adv. Mat.Deerf. Beach, Fla.) 30 (45), e1801350. doi:10.1002/adma.201801350
Hu, R., Yang, C., Wang, Y., Lin, G., Qin, W., Ouyan, Q., et al. (2014). Aggregation-induced emission (AIE) dye loaded polymer nanoparticles for gene silencing in pancreatic cancer and their in vitro and in vivo biocompatibility evaluation. Nano Res. 8, 1563–1576. doi:10.1007/s12274-014-0642-5
Huang, W., Zhang, Y., Tan, X., Wang, N., Wang, J., He, M., et al. (2021). An AIEgen-based photosensitizer for lysosome imaging and photodynamic therapy in tumor. Sensors Actuators B Chem. 335, 129698. doi:10.1016/j.snb.2021.129698
Jang, S. E., Qiu, L., Cai, X., Lee, J., Zhang, W., Tan, E. K., et al. (2021). Aggregation-induced emission (AIE) nanoparticles labeled human embryonic stem cells (hESCs)-derived neurons for transplantation. Biomaterials 271, 120747. doi:10.1016/j.biomaterials.2021.120747
Jiang, W., Zhang, C., Ahmed, A., Zhao, Y., Deng, Y., Ding, Y., et al. (2019). H2 O2 ‐Sensitive upconversion nanocluster bomb for tri‐mode imaging‐guided photodynamic therapy in deep tumor tissue. Adv. Healthc. Mat. 8, 1900972. doi:10.1002/adhm.201900972
Jin, G., He, R., Liu, Q., Lin, M., Dong, Y., Li, K., et al. (2019). Near-infrared light-regulated cancer theranostic nanoplatform based on aggregation-induced emission luminogen encapsulated upconversion nanoparticles. Theranostics 9, 246–264. doi:10.7150/thno.30174
Kang, M., Zhang, Z., Song, N., Li, M., Sun, P., Chen, X., et al. (2020). Aggregation‐enhanced theranostics: AIE sparkles in biomedical field. Aggregate 1, 80–106. doi:10.1002/agt2.7
Korneev, O. V., Sakhno, T. V., and Korotkova, I. V. (2019). Nanoparticles-based photosensitizers with effect of aggregation-induced emission. Biopolym. Cell 35, 249–267.
Kou, J., Dou, D., and Yang, L. (2017). Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 8, 81591–81603. doi:10.18632/oncotarget.20189
Kruger, C. A., and Abrahamse, H. (2018). Utilisation of targeted nanoparticle photosensitiser drug delivery systems for the enhancement of photodynamic therapy. Mol. (Basel, Switz. 23, 2628. doi:10.3390/molecules23102628
Lee, M., Zheng, L., Yu, B., Xu, W., Kwok, R., Lam, J., et al. (2019). A highly efficient and AIE-active theranostic agent from natural herbs. Mat. Chem. Front. 3, 1454–1461. doi:10.1039/c9qm00242a
Li, M., Wen, H., Li, H., Yan, Z. C., Li, Y., Wang, L., et al. (2021b). AIEgen-loaded nanofibrous membrane as photodynamic/photothermal antimicrobial surface for sunlight-triggered bioprotection. Biomaterials 276, 121007. doi:10.1016/j.biomaterials.2021.121007
Li, Q., Li, Y., Min, T., Gong, J., Du, L., Phillips, D. L., et al. (2020). Time-dependent photodynamic therapy for multiple targets: A highly efficient AIE-active photosensitizer for selective bacterial elimination and cancer cell ablation. Angew. Chem. Int. Ed. 59 (24), 9470–9477. doi:10.1002/anie.201909706
Li, W., Zhang, Y., Wang, Y., Ma, Y., Wang, D., Li, H., et al. (2021c). Nucleic acids induced peptide-based AIE nanoparticles for fast cell imaging. Chin. Chem. Lett. 32, 1571–1574. doi:10.1016/j.cclet.2020.09.054
Li, Y., Zhao, Z., Zhang, J., Kwok, R., Xie, S., Tang, R., et al. (2018). A bifunctional aggregation-induced emission luminogen for monitoring and killing of multidrug resistant bacteria. Adv. Funct. Mat. 28, 1804632. doi:10.1002/adfm.201804632
Li, Z., Teng, M., Wang, Y., Wang, Q., Feng, Y., Xiao, Z., et al. (2021a). The mechanism of 5-aminolevulinic acid photodynamic therapy in promoting endoplasmic reticulum stress in the treatment of HR-HPV-infected HeLa cells. Photodermatol. Photoimmunol. Photomed. 37, 348–359. doi:10.1111/phpp.12663
Lin, G., Manghnani, P., Mao, D., Teh, C., Li, Y., Zhao, Z., et al. (2017). Robust red organic nanoparticles for in vivo fluorescence imaging of cancer cell progression in xenografted zebrafish. Adv. Funct. Mat. 27, 1701418. doi:10.1002/adfm.201701418
Liu, C., Wang, X., Liu, J., Yue, Q., Chen, S., Lam, J., et al. (2020b). Near-Infrared AIE dots with chemiluminescence for deep-tissue imaging. Adv. Mat. 32, e2004685. doi:10.1002/adma.202004685
Liu, N., and Tang, M. (2020). Toxic effects and involved molecular pathways of nanoparticles on cells and subcellular organelles. J. Appl. Toxicol. 40, 16–36. doi:10.1002/jat.3817
Liu, S., Li, Y., Kwok, R., Lam, J., and Tang, B. Z. (2020a). Structural and process controls of AIEgens for NIR-II theranostics. Chem. Sci. 12, 3427–3436. doi:10.1039/d0sc02911d
Liu, X., Yang, Z., Xu, W., Chu, Y., Yang, J., Yan, Y., et al. (2019a). Fine tuning of pyridinium-functionalized dibenzo[a, c]phenazine near-infrared AIE fluorescent biosensors for the detection of lipopolysaccharide, bacterial imaging and photodynamic antibacterial therapy. J. Mat. Chem. C Mat. 7, 12509–12517. doi:10.1039/C9TC04427B
Liu, Y., Chen, Q., Sun, Y., Chen, L., Yuan, Y., and Gu, M. (2021). Aggregation-induced emission shining in the biomedical field: From bench to bedside. Eng. Regen. 2, 206–218. doi:10.1016/j.engreg.2021.11.001
Liu, Z., Zou, H., Zhao, Z., Zhang, P., Shan, G. G., Kwok, R., et al. (2019b). Tuning organelle specificity and photodynamic therapy efficiency by molecular function design. ACS Nano 13 (10), 11283–11293. doi:10.1021/acsnano.9b04430
Luo, J., Xie, Z., Lam, J. W., Cheng, L., Chen, H., Qiu, C., et al. (2001). Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Comm. 18, 1740–1741. doi:10.1039/b105159h
Manghnani, P. N., Wu, W., Xu, S., Hu, F., Teh, C., and Liu, B. (2018). Visualizing photodynamic therapy in transgenic zebra fish using organic nanoparticles with aggregation-induced emission. Nano-Micro Lett. 10, 61–70. doi:10.1007/s40820-018-0214-4
Mao, D., Hu, F., Kenry, K., Qi, G., ji, S., Wu, W., et al. (2020). One-step in-vivo metabolic labeling as a theranostic approach for overcoming drug-resistant bacterial infections. Mat. Horiz. 7, 1138–1143. doi:10.1039/c9mh01675a
Mao, L., Huang, H., Hu, D., Ma, H., Tian, M., Zhang, X., et al. (2021). A near-infrared bioprobe with aggregation-induced emission feature for in vitro photodynamic therapy. Dyes Pigm. 194, 109521. doi:10.1016/j.dyepig.2021.109521
Mitra, K., Gautam, S., Kondaiah, P., and Chakravarty, A. R. (2016). BODIPY-appended 2-(2-Pyridyl) benzimidazole Platinum(II) catecholates for mitochondria-targeted photocytotoxicity. ChemMedChem 11, 1956–1967. doi:10.1002/cmdc.201600320
Ni, J., Wang, Y., Zhang, H., Sun, J. Z., and Tang, B. Z. (2021). Aggregation-induced generation of reactive oxygen species: Mechanism and photosensitizer construction. Molecules 26, 268–290. doi:10.3390/molecules26020268
Nicol, A., Qin, W., Kwok, R., Burkhartsmeyer, J., Zhu, Z., Su, H., et al. (2017). Functionalized AIE nanoparticles with efficient deep-red emission, mitochondrial specificity, cancer cell selectivity and multiphoton susceptibility. Chem. Sci. 8, 4634–4643. doi:10.1039/c7sc00908a
Parthiban, C., Pavithra, M., Reddy, V., Sen, D., Samuel, M., and Singh, P. (2018). Visible-light triggered fluorescent organic nanoparticles for chemo-photodynamic therapy with real-time cellular imaging. ACS Appl. Nano Mat. 1 (11), 6281–6288. doi:10.1021/acsanm.8b01495
Qi, G., Hu, F., KenryShi, L., Wu, M., and Liu, B. (2019). An AIEgen-peptide conjugate as a phototheranostic agent for phagosome-entrapped bacteria. Angew. Chem. Int. Ed. 58, 16229–16235. in English). doi:10.1002/anie.201906099
Qi, J., Chen, C., Zhang, X., Hu, X., Ji, S., Kwok, R., et al. (2018). Light-driven transformable optical agent with adaptive functions for boosting cancer surgery outcomes. Nat. Commun. 9, 1848. doi:10.1038/s41467-018-04222-8
Roguin, L. P., Chiarante, N., García Vior, M. C., and Marino, J. (2019). Zinc(II) phthalocyanines as photosensitizers for antitumor photodynamic therapy. Int. J. Biochem. Cell Biol. 114, 105575. doi:10.1016/j.biocel.2019.105575
Sheng, Z., Guo, B., Hu, D., Xu, S., Wu, W., Liew, W. H., et al. (2018). Bright aggregation-induced-emission dots for targeted synergetic NIR-II fluorescence and NIR-I photoacoustic imaging of orthotopic brain tumors. Adv. Mat. 28, e1800766. doi:10.1002/adma.201800766
Shi, L., Wu, W., Duan, Y., Xu, L., Li, S., Gao, X., et al. (2021). Carrier-free hybrid DNA nanoparticles for light-induced self-delivery of functional nucleic acid enzymes. ACS Nano 15, 1841–1849. doi:10.1021/acsnano.0c10045
Silva, Z. S., Bussadori, S. K., Fernandes, K. P., Huang, Y. Y., and Hamblin, M. R. (2015). Animal models for photodynamic therapy (PDT). Biosci. Rep. 35, e00265. doi:10.1042/BSR20150188
Tynga, I. M., and Abrahamse, H. (2018). Nano-mediated photodynamic therapy for cancer: Enhancement of cancer specificity and therapeutic effects. Nanomater. (Basel). 8, 923–937. doi:10.3390/nano8110923
Wang, C., Wang, J., Xue, K., Xiao, M., Sun, Z., and Zhu, C. (2022a). A receptor-targeting AIE photosensitizer for selective bacterial killing and real-time monitoring of photodynamic therapy outcome. Chem. Commun. 58, 7058–7061. doi:10.1039/d2cc02230c
Wang, D., Lee, M., Shan, G., Kwok, R., Lam, J., Su, H., et al. (2018b). Highly efficient photosensitizers with Far-Red/Near-Infrared aggregation-induced emission for in vitro and in vivo cancer theranostics. Adv. Mat. 30 (39), e1802105. doi:10.1002/adma.201802105
Wang, D., Lee, M., Xu, W., Kwok, R., Lam, J., and Tang, B. (2018a). Theranostics based on AIEgens. Theranostics 8, 4925–4956. doi:10.7150/thno.27787
Wang, H., Zhao, E., Jacky, L., and Tang, B. (2015). AIE luminogens: Emission brightened by aggregation. Mat. TodayKidlingt. 18, 365–377. doi:10.1016/j.mattod.2015.03.004
Wang, S., Wang, X., Yu, L., and Sun, M. (2021). Progress and trends of photodynamic therapy: From traditional photosensitizers to AIE-based photosensitizers. Photodiagnosis Photodyn. Ther. 34, 102254. doi:10.1016/j.pdpdt.2021.102254
Wang, W., Wu, F., Zhang, Q., Zhou, N., Zhang, M., Zheng, T., et al. (2022b). Aggregation-induced emission nanoparticles for single near-infrared light-triggered photodynamic and photothermal antibacterial therapy. ACS Nano 16, 7961–7970. doi:10.1021/acsnano.2c0073410.1021/acsnano.2c00734
Wawrzyńska, M., Kałas, W., Biały, D., Zioło, E., Arkowski, J., Mazurek, W., et al. (2010). In vitro photodynamic therapy with chlorin e6 leads to apoptosis of human vascular smooth muscle cells. Arch. Immunol. Ther. Exp. Warsz. 58, 67–75. doi:10.1007/s00005-009-0054-5
Wu, M., Liu, X., Chen, H., Duan, Y., Liu, J., Pan, Y., et al. (2021a9093–9098). Activation of pyroptosis by membrane-anchoring AIE photosensitizer design: New prospect for photodynamic cancer cell ablation. Angew. Chem. Int. Ed. 60, 9093–9098. doi:10.1002/anie.202016399
Wu, M., Wu, W., Duan, Y., Liu, X., Wang, M., Phan, C. U., et al. (2020). HClO-activated fluorescence and photosensitization from an AIE nanoprobe for image-guided bacterial ablation in phagocytes. Adv. Mat. 32, e2005222. doi:10.1002/adma.202005222
Wu, W., Feng, G., Xu, S., and Liu, B. (2016). A photostable far-red/near-infrared conjugated polymer photosensitizer with aggregation-induced emission for image-guided cancer cell ablation. Macromolecules 49, 5017–5025. doi:10.1021/acs.macromol.6b00958
Wu, W., Mao, D., Hu, F., Xu, S., Chen, C., Zhang, C. J., et al. (2017a). A highly efficient and photostable photosensitizer with near-infrared aggregation-induced emission for image-guided photodynamic anticancer therapy. Adv. Mat.Deerf. Beach, Fla.) 29, 1700548. doi:10.1002/adma.201700548
Wu, W., Mao, D., Xu, S., Fard, M., Duan, Y., Hu, F., et al. (2019). Precise molecular engineering of photosensitizers with aggregation‐induced emission over 800 nm for photodynamic therapy. Adv. Funct. Mat. 29, 1901791. doi:10.1002/adfm.201901791
Wu, W., Mao, D., Xu, S., Ji, S., Hu, F., Ding, D., et al. (2017b). High performance photosensitizers with aggregation-induced emission for image-guided photodynamic anticancer therapy. Mat. Horiz. 4, 1110–1114. doi:10.1039/C7MH00469A
Wu, W., Shi, L., Duan, Y., Xu, S., Shen, L., Zhu, T., et al. (2021b). Nanobody modified high-performance AIE photosensitizer nanoparticles for precise photodynamic oral cancer therapy of patient-derived tumor xenograft. Biomaterials 274, 120870. doi:10.1016/j.biomaterials.2021.120870
Xu, S., Yuan, Y., Cai, X., Zhang, C. J., Hu, F., Liang, J., et al. (2015). Tuning the singlet-triplet energy gap: A unique approach to efficient photosensitizers with aggregation-induced emission (AIE) characteristics. Chem. Sci. 6, 5824–5830. doi:10.1039/c5sc01733e
Yang, J., Gu, X., Su, W., Hao, X., Shi, Y., Zhao, L., et al. (2018). (2-(4-Bromophenyl)ethene-1, 1, 2-triyl)tribenzene with aggregation induced emission for ablation of HeLa cells. Mat. Chem. Front. 2, 1842–1846. doi:10.1039/C8QM00304A,
Yang, L., Wang, X., Zhang, G., Chen, X., Zhang, G., and Jiang, J. (2016). Aggregation-induced intersystem crossing: A novel strategy for efficient molecular phosphorescence. Nanoscale 8, 17422–17426. doi:10.1039/c6nr03656b
Yang, Y., Chu, B., Cheng, J., Tang, J., Song, B., Wang, H., et al. (2022). Bacteria eat nanoprobes for aggregation-enhanced imaging and killing diverse microorganisms. Nat. Commun. 13, 1255. doi:10.1038/s41467-022-28920-6)
Yao, Y., Zang, Y., Qu, J., Tang, M., and Zhang, T. (2019). The toxicity of metallic nanoparticles on liver: The subcellular damages, mechanisms and outcomes. Int. J. Nanomedicine 14, 8787–8804. doi:10.2147/ijn.s212907
Yaraki, M., Wu, M., Middha, E., Wu, W., Daqiqeh, S., Liu, B., et al. (2021). Gold nanostars-AIE theranostic nanodots with enhanced fluorescence and photosensitization towards effective image-guided photodynamic therapy. Nano-Micro Lett. 13, 58. doi:10.1007/s40820-020-00583-2
Yi, X., Hu, J., Dai, J., Lou, X., Zhao, Z., Xia, F., et al. (2021). Self-guiding polymeric prodrug micelles with two aggregation-induced emission photosensitizers for enhanced chemo-photodynamic therapy. ACS Nano 15, 3026–3037. doi:10.1021/acsnano.0c0940710.1021/acsnano.0c09407
Yu, C. Y., Xu, H., Ji, S., Kwok, R. T., Lam, J. W., Li, X., et al. (2017). Mitochondrion-anchoring photosensitizer with aggregation-induced emission characteristics synergistically boosts the radiosensitivity of cancer cells to ionizing radiation. Adv. Mat.Deerf. Beach, Fla.) 29, 1606167. doi:10.1002/adma.201606167
Yuan, H., Li, Z., Bai, H., Chen, Z., Yao, C., Jia, S., et al. (2021). Aggregation-induced emission nanoparticles with NIR and photosensitizing characteristics for resistant bacteria elimination and real-time tracking. Mat. Chem. Front. 5, 6611–6617. doi:10.1039/d1qm00752a
Zhang, J., Jiang, C., Figueiró Longo, J. P., Azevedo, R. B., Zhang, H., and Muehlmann, L. A. (2018a). An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 8, 137–146. doi:10.1016/j.apsb.2017.09.003
Zhang, J., Zou, H., Lei, J., He, B., He, X., Sung, H., et al. (2020b). Multifunctional AuI -based AIEgens: Manipulating molecular structures and boosting specific cancer cell imaging and theranostics. Angew. Chem. Int. Ed. 59, 7097–7105. doi:10.1002/anie.202000048
Zhang, P., Steelant, W., Kumar, M., and Scholfield, M. (2007). Versatile photosensitizers for photodynamic therapy at infrared excitation. J. Am. Chem. Soc. 129, 4526–4527. doi:10.1021/ja0700707
Zhang, P., Zhao, Z., Li, C., Su, H., Wu, Y., Kwok, R., et al. (2018b). Aptamer-decorated self-assembled aggregation-induced emission organic dots for cancer cell targeting and imaging. Anal. Chem. 90, 1063–1067. doi:10.1021/acs.analchem.7b03933
Zhang, R., Niu, G., Lu, Q., Huang, X., Chau, J., Kwok, R., et al. (2020a). Cancer cell discrimination and dynamic viability monitoring through wash-free bioimaging using AIEgens. Chem. Sci. 11, 7676–7684. doi:10.1039/d0sc01213k
Zhang, T., Li, Y., Zheng, Z., Ye, R., Zhang, Y., Kwok, R., et al. (2019a). In situ monitoring apoptosis process by a self-reporting photosensitizer. J. Am. Chem. Soc. 141, 5612–5616. doi:10.1021/jacs.9b00636
Zhang, W., Huang, Y., Chen, Y., Zhao, E., Hong, Y., Chen, S., et al. (2019b). Amphiphilic tetraphenylethene-based pyridinium salt for selective cell-membrane imaging and room-light-induced special Reactive Oxygen Species generation. ACS Appl. Mat. Interfaces 11, 10567–10577. doi:10.1021/acsami.9b00643
Zhao, E., Chen, Y., Wang, H., Chen, S., Lam, J. W., Leung, C. W., et al. (2015). Light-enhanced bacterial killing and wash-free imaging based on AIE fluorogen. ACS Appl. Mat. Interfaces 7, 7180–7188. doi:10.1021/am509142k
Zhao, X., Dai, Y., Ma, F., Misal, S., Hasrat, K., Zhu, H., et al. (2021). Molecular engineering to accelerate cancer cell discrimination and boost AIE-active type I photosensitizer for photodynamic therapy under hypoxia. Chem. Eng. J. 410, 128133. doi:10.1016/j.cej.2020.128133
Zhou, T., Zhu, J., Shang, D., Chai, C., Li, Y., Sun, H., et al. (2020). Mitochondria-anchoring and AIE-active photosensitizer for self-monitored cholangiocarcinoma therapy. Mat. Chem. Front. 4, 3201–3208. doi:10.1039/d0qm00503g
Zhou, Z., Song, J., Nie, L., and Chen, X. (2016). Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 45, 6597–6626. doi:10.1039/c6cs00271d
Zhuang, W., Yang, L., Ma, B., Kong, Q., Li, G., Wang, Y., et al. (2019). Multifunctional two-photon AIE luminogens for highly mitochondria-specific bioimaging and efficient photodynamic therapy. ACS Appl. Mat. Interfaces 11 (23), 20715–20724. doi:10.1021/acsami.9b04813
Keywords: aggregation-induced emission, laser, light, nanoparticles, photosensitizers, theranostics
Citation: Abrahamse H, Hamblin MR and George S (2022) Structure and functions of Aggregation-Induced Emission-Photosensitizers in anticancer and antimicrobial theranostics. Front. Chem. 10:984268. doi: 10.3389/fchem.2022.984268
Received: 01 July 2022; Accepted: 08 August 2022;
Published: 30 August 2022.
Edited by:
Yong Fan, Fudan University, ChinaReviewed by:
Jing Mu, Shenzhen Hospital, Peking University, ChinaKang-Nan Wang, Shandong University, China
Copyright © 2022 Abrahamse, Hamblin and George. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sajan George, c2FqYW5nZW9yZ2VnQHVqLmFjLnph, c2FqYW4uZ2VvcmdlQHZpdC5hYy5pbg==
†ORCID: Heidi Abrahamse, orcid.org/0000-0001-5002-827X; Michael R. Hamblin, orcid.org/0000-0001-6431-4605; Sajan George, orcid.org/0000-0002-8214-4563
 Heidi Abrahamse
Heidi Abrahamse Michael R. Hamblin1†
Michael R. Hamblin1† Sajan George
Sajan George