- Food and Pharmaceutical Engineering Institute, Guiyang University, Guiyang, China
Pitaya, or dragon fruit, is a typical tropical fruit with an appealing taste and diverse health benefits to humans. The plantation of pitaya in Guizhou province in China has greatly boosted the income of local farmers and alleviated poverty. However, the frequent occurrence of postharvest diseases has brought large economic loss. To find a solution, we set out to identify the postharvest disease-causing agents of Guizhou pitaya. Several fungi were isolated from diseased pitaya and identified as species based on the ITS1 sequence similarity. Of them, Penicillium spinulosum, Phoma herbarum, Nemania bipapillata, and Aspergillus oryzae were, for the first time, found to cause dragon fruit disease. In consideration of their prevalence in postharvest fruit diseases, Alternaria alternata H8 and Fusarium proliferatum H4 were chosen as representative pathogens for the drug susceptibility test. Among the tested drugs and plant extracts, 430 g/L tebuconazole and 45% prochloraz were found to be the most potent fungicides against H8 and H4, respectively. The research provides insights into the mechanism and control of postharvest diseases of dragon fruits in Guizhou, China, and thus could be of economic and social significance to local farmers and the government.
Introduction
Dragon fruit or pitaya (Hylocereus species) belongs to the family Cataceae, which is a typical tropical fruit. Since its introduction to Guizhou province in 2001 (Wang et al., 2018), the fruit has been shown to be quite adapted to the local climate and ecology. So far, three varieties, purple dragon, crystal red dragon, and pink dragon, have been introduced and cultivated in Guizhou, and the dragon fruit-planting area in Guizhou province has increased to be the third in China (Wang et al., 2018). With its renowned health benefits to consumers and appealing taste, the locally produced dragon fruit is widely accepted in the domestic market. Dragon fruit cultivation in Guizhou province has greatly boosted the local economy and been lifting local farmers out of poverty. With the planting history and area growing, the incidences of dragon fruit diseases are increasingly more frequent, especially the postharvest diseases, which lead to the decline of the yield and quality of marketable dragon fruits, affecting the economic benefits ultimately.
Due to the interruption of nutrient supply, the vitality of the postharvest dragon fruits was weakened, the disease resistance was reduced, and they could be infected easily by pathogenic microorganisms during storage and transportation, resulting in illness. At present, there are kinds of diseases caused by microorganisms such as anthracnose, soft rot, canker, black spot, and wilts in dragon fruit after harvest (Balendres and Bengoa, 2019). Among them, fungal diseases are more common and serious. Dragon fruit anthracnose was usually caused by Colletotrichum gloeosporioides (Masyahit et al., 2009) and C. truncatum (Guo et al., 2014). The pathogens that cause black spot disease of dragon fruit were reported to be Alternaria alternata (Castro et al., 2017) and Bipolaris cactivora (Tarnowski et al., 2010; Ben-Ze Ev et al., 2011). Dragon fruit soft rot is found to be caused by Neoscytalidium dimidiatum (Pan et al., 2021) and Gilbertella persicaria (Guo et al., 2012). However, there are few reports focusing on causative agents and control methods for postharvest diseases of dragon fruits in the Guizhou area.
In order to investigate the microbial species causing postharvest diseases on dragon fruits in Guizhou, samples of diseased dragon fruits were collected from the Luodian County of Guizhou province in China. Microbes were isolated from diseased fruit tissue and reinoculated on healthy fruits to confirm pathogenicity according to Koch’s rule. Pathogenic microorganisms were identified by rDNA-ITS sequence similarity analysis. Finally, a variety of prevention and control reagents were screened for inhibition efficacy against selected pathogens by an indoor experiment. This study provides a helpful understanding of the mechanism of postharvest diseases and control measures for dragon fruits in Guizhou province and the neighboring area.
Materials and Methods
Sample Collection and Microbial Isolation
Samples of diseased dragon fruits were collected in Luodian County in Guizhou Province. Pathogenic microorganisms were isolated by conventional tissue isolation methods in the laboratory. The potato dextrose agar (PDA) plates were used for separation and purification. The isolated strains were stored at −20°C in 40% glycerol.
Pathogenicity Test
Healthy dragon fruits were selected and soaked in 75% alcohol for 1 min, followed by repeated washes in sterile water, and surface-dried. Mycelia “cakes” (blank agar “cakes” as control) were chopped aseptically from the culture plate and placed onto the surface (non-injury inoculation) and the stabbing wound (stab inoculation) of dragon fruits, which was then allowed to incubate at 28°C, and the status of infection was observed along during incubation.
Morphological Characterization of Pathogens
The pathogenic microorganisms were cultured on PDA and incubated at 28°C. The colony morphology was checked regularly, and the mycelia were observed under a Model EX30 inverted microscope (Ningbo Shunyu Tech. Co. Ltd., Zhejiang, China).
DNA Extraction and Molecular Identification
The fungus was cultured in potato dextrose broth (PDB) at 28°C for 3 days, and the mycelia were collected for genomic DNA (gDNA) extraction. Fungal gDNA was extracted according to the users’ instruction of the fungal genomic DNA rapid extraction kit (Sangon). The rDNA-ITS1 fragment of the gDNA was PCR-amplified using universal ITS1 primers and subjected to nucleotide sequencing. All the obtained sequences were searched for similarity against the NCBI nucleotide collection (nr) database with default parameters (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome). The phylogenetic tree was constructed using the neighbor-joining method to determine the taxonomic status.
Preparation of Tested Fungicides and Plant Extracts
All tested fungicides are commercially available. The plant extracts were prepared as such: the plant was air-dried and milled to powder. For the procedure, 10 g of the powder was extracted with 95% ethanol and heated for 4 h with refluxing. The extract was filtered and evaporated under reduced pressure. The residue was re-dissolved with hot water, cooled, and adjusted to a concentration of 500 mg/mL as stock solution.
Indoor Screening for Control Agents
Tested fungicide or plant extracts (Tween 20 as control) with appropriate quantity was added to melted PDA and cooled to make plates. The plates were then inoculated by placing an inoculum (4 mm disc from cultures of A. alternata and F. proliferatum) at the center and incubated at the ambient temperature of 28°C. Each test was performed in triplicate. After 6 days of incubation, the colony diameter was recorded, and the inhibition rate was calculated using the following formula:
Furthermore, the efficacy for the tested control agent was expressed as the half-maximal effective concentration (EC50, the concentration at which the tested fungicide reduced mycelial growth by 50%) determined by regression of the inhibition rate against the log10 values of the fungicide concentrations.
Results
Isolation and Purification of Pathogenic Fungi
A number of fungal isolates were separated from diseased dragon fruit tissue, and seven fungal strains were preliminarily established based on colony and mycelium morphology and labeled as H1, H2, H4, H6, H7, H8, and H9. Colony and conidia morphology are shown in Figure 1. The appearance of the H1 colony on PDA is brown with white slowly growing edges. The spores are elliptical to round with varying sizes but connected in tandem to each other. The H2 colony is whitish gray and grows fast. Its spores are rod-shaped under the microscope and distributed around the mycelia. The H4 strain colony exhibits a white appearance and grows fast with elliptical spores arranged in clusters around the top of the spore stalk. The colony of strain H6 is yellowish-brown with dense hyphae, and the spores appear round when observed under a microscope. The H7 colony is white in appearance and grows slowly on PDA. The spores are irregularly rod-shaped. The H8 colony is white with dense mycelia. The spores are oval under the microscope and densely clustered around spore stalks. The H9 colony is dark green and grows fast; the spores are spherical and connected in tandem to each other to form long chains.
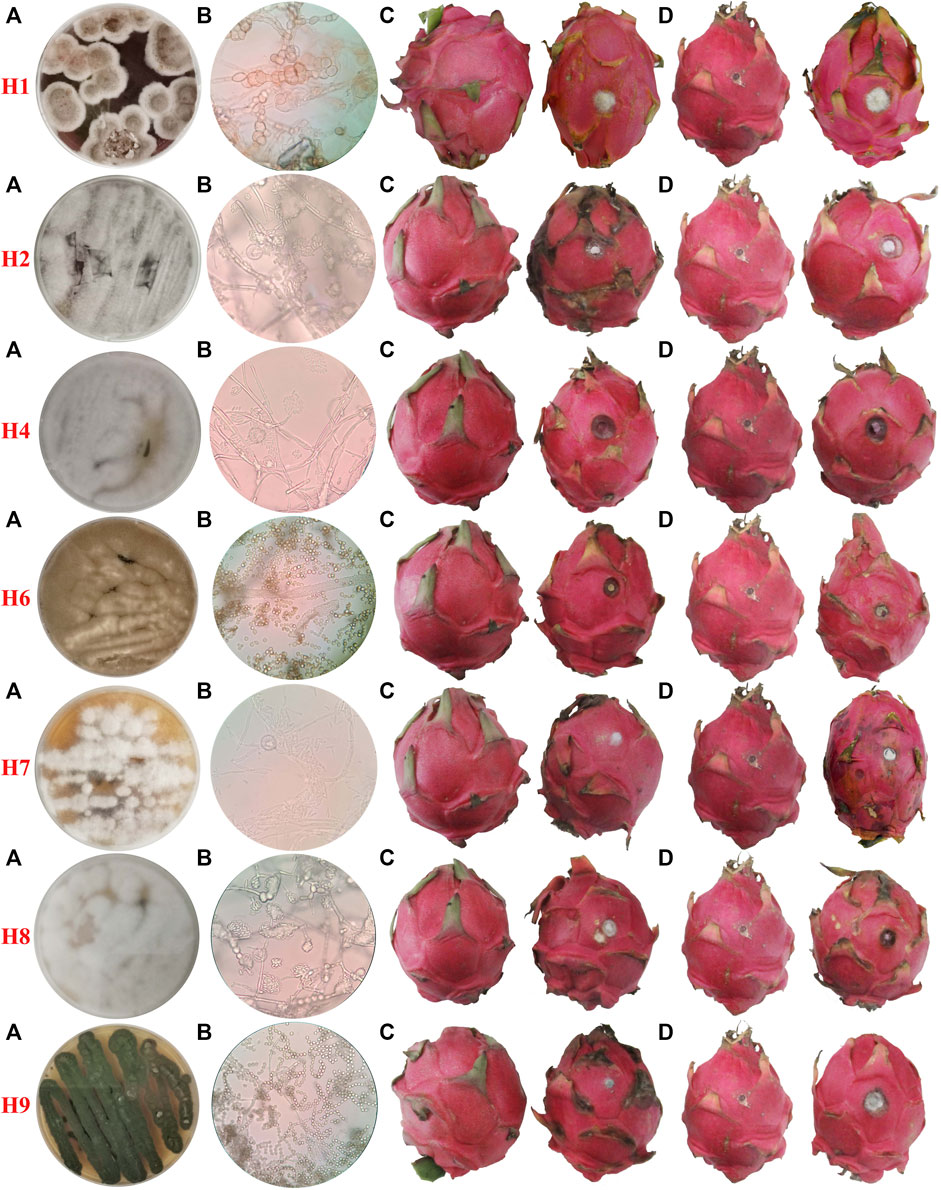
FIGURE 1. Morphological characteristics of pathogens and pathogenicity confirmation. (A) Colony morphology on the PDA plate. (B) Microscopic image of conidia taken with ×400 magnification, (C) non-injury inoculation, and (D) injury inoculation; fruits on the right were inoculated with mycelia “cakes,” and the ones on the left are blank controls.
Pathogenicity of Isolated Microorganisms
The isolated strains were re-inoculated onto fruits in two ways, a non-injured inoculation and a stab-injured inoculation. As shown in Figure 1, all the seven strains can infect dragon fruits with or without an injury. Despite the presence of a stabbing wound, no observable infection occurred in control experiments where blank agar was used for ‘inoculation’.
Taxonomic Identification
The ITS1 segments were PCR-amplified from corresponding genomic DNA extracted from the seven pathogenic fungi. Their nucleotide sequences were used as a query for blast searches, and top hits are listed in Table 1. According to the search results, the pathogenic strains were roughly assigned as Phoma herbarum H1, Colletotrichum nymphaeae H2, Alternaria alternata H4, Aspergillus oryzae H6, Penicillium spinulosum H7, Fusarium proliferatum H8, and Nemania bipapillata H9. Phylogenetic relationships based on the ITS1 sequence similarity between the strains and selected top hits are illustrated in Figure 2.
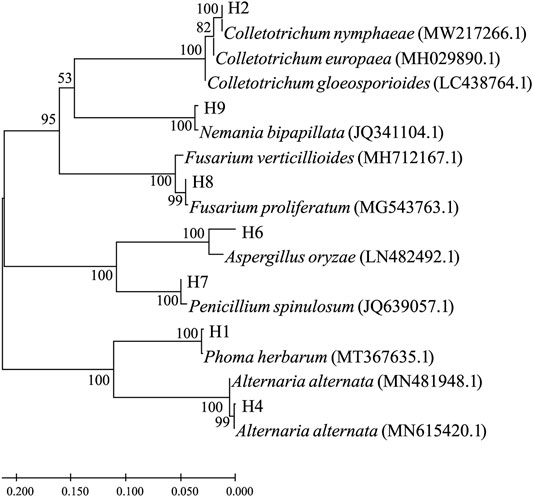
FIGURE 2. Molecular phylogenetic tree based on the rDNA-ITS sequence similarity. Sequence alignment and tree building were performed by MEGA5.0 using the neighbor-joining method, and phylogeny was tested by 500 bootstrap replications. The numbers on branches were calculated as bootstrap values. Accession numbers of ITS1 sequences from isolated strains were, namely, ON514545.1 (H1), ON514546.1 (H2), ON514547.1 (H4), ON514548.1 (H6), ON514549.1 (H7), ON514550.1 (H8), and ON514551.1 (H9).
Preliminary Screening of Agro-Agents for Fungicides Against Representative Pathogenic Fungi
In consideration of the prevalence of A. alternata and F. proliferatum in fruit disease, the two strains H4 and H8 were chosen as representative pathogenic microorganisms of dragon fruit and tested their susceptibility to a series of agricultural agents. As shown in Table 2, a total of 20 agro-agents were screened for indoor toxicity at concentrations of 50 μg/ml and 10 μg/ml on A. alternata H4 and F. proliferatum H8. For A. alternata H4, at 50 μg/ml concentration, 10% difenoconazole, 430 g/L tebuconazole, and 3% zhongshengmycin showed the highest inhibition rate, while at 10 μg/ml concentration, 10% difenoconazole, 430 g/L tebuconazole, 50% iprodione were the three most potent candidates. For F. proliferatum H8, 3% benziothiazolinone, 430 g/L tebuconazole, and 45% prochloraz exhibited the highest toxicity at a concentration of 50 μg/ml, while at the 10 μg/ml level, 10% difenoconazole, 50% iprodione, and 45% prochloraz were the top three candidates. Notably, three microbial preparations were included in the screening; the insecticidal Beauveria bassiana showed the highest inhibition rate at 50 μg/ml concentration, and the fungicidal Bacillus cereus was the most potent at 10 μg/ml.
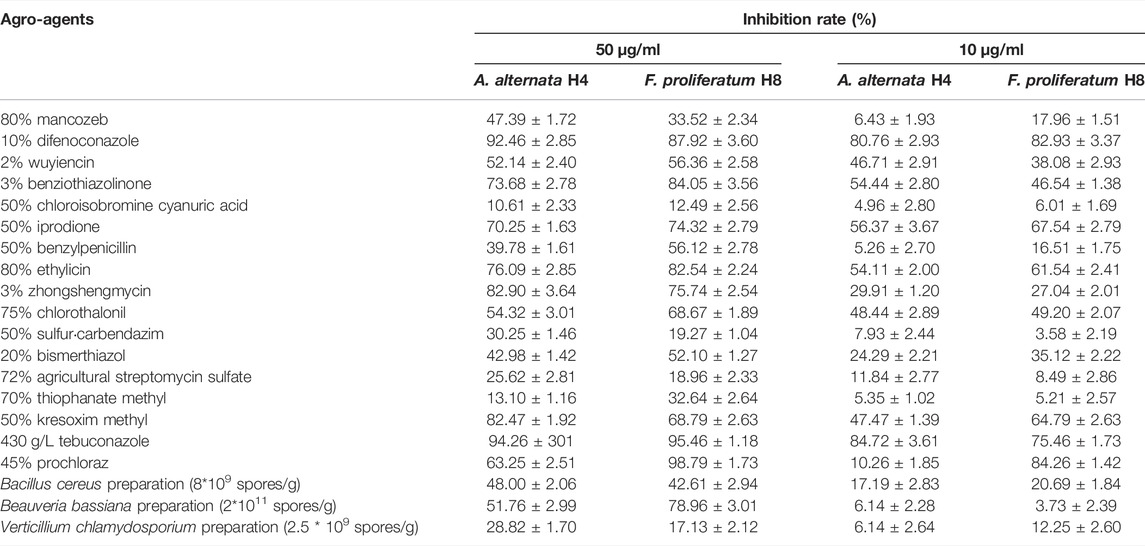
TABLE 2. Inhibitory effects of 20 agro-agents at the concentrations of 50 µg/ml and 10 µg/ml on A. alternata H4 and F. proliferatum H8.
Inhibition Effect of 10 Edible and Medicinal Plant Extracts on the Tested Fungus
The ethanol extract was obtained from 10 edible and medicinal plants, namely, Houttuynia cordata Thunb, Mentha haplocalyx, Zanthoxylum bungeanum Maxim, Lonicera japonica Thunb, Dendrobium officinale Kimura et Migo, Piper nigrum, Zingiber officinale Roscoe, Gastrodia elata, Schisandra chinensis, and Illicium verum. Some of the plants are renowned for their microbe-inhibitory activity. The inhibition rate results in this study are shown in Table 3. All 10 ethanolic extracts prepared at a final concentration of 50 mg/ml showed an inhibitory effect on the two fungal pathogens, A. alternata H4 and F. proliferatum H8. In comparison, 50 mg/ml and 80% ethylicin was included in the test. The inhibition rates of Zanthoxylum bungeanum Maxim against A. alternata H4 and F. proliferatum H8 were 68.75 and 75.12%, those of Zingiber officinale Roscoe were 70.14 and 60.48%, and those of Piper nigrum were 69.84 and 60.93%, respectively. The three extracts showed the highest inhibitory effect against both tested pathogens of dragon fruit. Notably, 80% ethylicin at the same concentration exhibited a 100% inhibition rate in both strains.
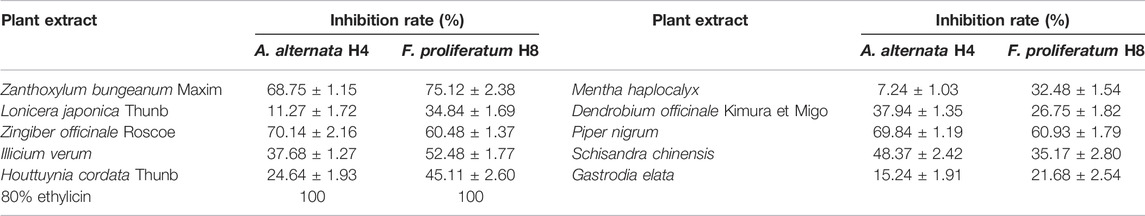
TABLE 3. Determination of the inhibitory effect of 10 Chinese edible and medicinal plant extracts on A. alternata H4 and F. proliferatum H8.
Fungicidal Efficacy Test on Representative Fungi With Promising Candidates
Based on previousscreening results, several fungicides were selected for the efficacy test. As shown in Table 4, 430 g/L tebuconazole exhibited the smallest EC50 at 0.0133 μg/ml, suggesting the highest potency toward A. alternata H4, while 50% iprodione with EC50 at 3.6840 μg/ml showed the second highest potency. For the pathogen F. proliferatum H8, 45% prochloraz showed the smallest EC50 at 0.0122 μg/ml, and 430 g/L tebuconazole is the second smallest with EC50 at 0.0307 μg/ml. It is worth mentioning that the efficacy of the biological agent Bacillus cereus is also tested, which exhibited EC50 at 81.3915 μg/ml toward F. proliferatum H8.
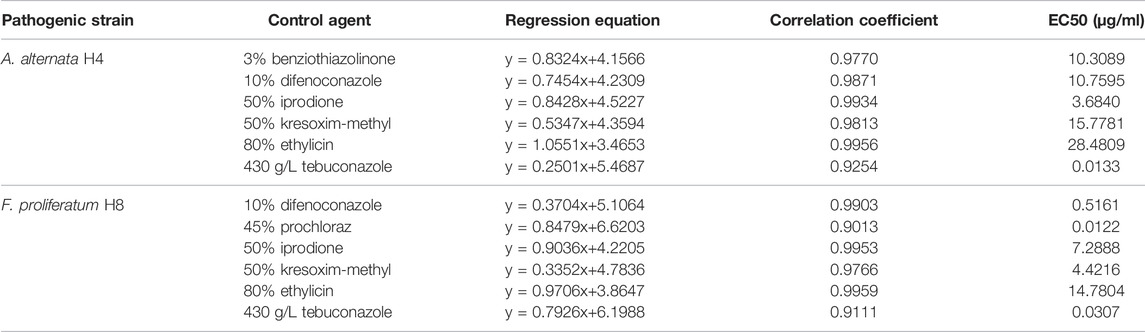
TABLE 4. Determination of the efficacy of screened fungicides to A. alternata H4 and F. proliferatum H8.
Discussion
This study descripted the isolation and identification of several pathogenic fungal strains from diseased dragon fruits suffering from soft rot, anthracnose, and black spot based on field observation. The capability of the pathogens to infect healthy dragon fruits was confirmed by re-inoculation. Based on the morphological and molecular characteristics, the isolates were roughly identified to be P. herbarum H1, C. nymphaeae H2, A. alternata H4, A. oryzae H6, P. spinulosum H7, F. proliferatum H8, and N. bipapillata H9. Previous studies have suggested that a variety of Fusarium can cause postharvest soft rot disease to dragon fruit (Oeurn et al., 2015), but few studies report on F. proliferatum, which is found for the first time in the study in Guizhou province, suggesting that F. proliferatum could be a regional pathogen. C. gloeosporioides (Bordoh et al., 2020) and C. truncatum (Guo et al., 2014; Vijaya et al., 2015) of Colletotrichum genera were recognized as the pathogenic microorganisms of pitaya anthracnose. C. nymphaeae was reported to cause anthrax in other fruits such as plum (Chang et al., 2018) and blueberry (Tomoo et al., 2015), and this is the first time found in diseased pitaya. A. alternata is the main pathogen causing postharvest disease, which can cause black spot disease in dragon fruit (Castro et al., 2017), pears (Tian et al., 2006), peaches (Inoue and Nasu, 2000), and other fruits (Prusky et al., 1997; Prusky et al., 1999; Zhao and Liu, 2012) in the post-harvest preservation. This study found first that A. oryzae can cause infection on dragon fruit. Since A. oryzae is a ubiquitous environmental fungus, the infection observed in this study could be opportunistic. This research reported for the first time several pathogenic microorganisms of dragon fruit in Guizhou, indicating that the distribution of pathogenic microorganisms in dragon fruit varies with a geographical environment which on the other hand signifies the importance of the geography-specific plan for prevention and control measures.
A. alternata and F. proliferatum are two typical postharvest pathogenic microorganisms that can cause postharvest diseases in a variety of fruits (Konstantinou et al., 2011; Abd Murad et al., 2017; Castro et al., 2017; Wang et al., 2021), so we selected these two to test their susceptibility to the control agents, respectively. The results showed that A. alternata H4 exhibited the highest sensitivity to 430 g/L tebuconazole and the lowest sensitivity to 80% ethylicin, while F. proliferatum H8 showed the highest sensitivity to 45% prochloraz and the lowest sensitivity to Bacillus cereus. The results showed that 10% difenoconazole, 50% iprodione, 50% kresoxim-methyl, 80% ethylicin, and 430 g/L tebuconazole all had inhibitory effects on the two postharvest pathogenic microorganisms of pitaya to a certain degree. Natural antimicrobials obtained from plants can provide alternative materials instead of commonly used fungicides in a more sustainable and environment-friendly way (Romanazzi et al., 2012). This study demonstrated that the 10 edible and medicinal plant extracts all showed some inhibitory effects on the two representative pathogenic fungi, although they are much less potent than chemically synthesized fungicides. Chemical fungicides showing the most efficacy to tested pathogens can be good choices for field application; however, long-term single use of control agents will likely cause drug resistance of pathogenic microorganisms. Therefore, it is recommended to carry out field experiments using a combination of agents which would improve the control effect, but the study only conducted a sensitivity test indoors which may not represent the field effect.
Pathogenic microorganisms can infect fruits in many ways. For example, fungal spores can spread with the help of wind or insects to infect fruits. Therefore, cultivation and management should be strengthened at regular times, and attention should be paid to ventilation and light transmission to reduce the reproduction of fungi since the reproduction and accumulation of pathogenic microorganisms would cause more fruits to rot, aggravating microbial infections. At present, the postharvest preservation methods of dragon fruit mainly include low-temperature preservation, film preservation, chemical preservation, thermal treatments, and irradiation (Jalgaonkar et al., 2020). These preservation measures have problems such as being time-consuming and labor-intensive, high cost, chemical residues, environmental pollution, and food safety hazards. Plant extracts, especially those from edible or medicinal plants, with fungal inhibitory effects can find their use in postharvest disease prevention.
Conclusion
The study isolated seven strains from diseased dragon fruits, and their ability to infect healthy dragon fruits was confirmed. Of them, Penicillium spinulosum, Phoma herbarum, Nemania bipapillata and Aspergillus oryzae were for the first time found to cause dragon fruit disease. In consideration of their prevalence in postharvest fruit diseases, A. alternata H8 and F. proliferatum H4 were chosen as representative pathogens for the drug susceptibility test. Among the tested agro-agents and plant extracts, 430 g/L tebuconazole and 45% prochloraz were found to be the most potent fungicides against H8 and H4, respectively.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author Contributions
WW designed and devised the study, and YL and HC analyzed the data and prepared the original manuscript. HC supervised YA and LM for pathogen isolation and the drug sensitivity test. HW revised the manuscript. All authors discussed, edited, and approved the final version.
Funding
We acknowledge the funds from the Science and TechnologyFund Project of Guizhou [QKHJC(2020)1Y089], the Young Sci-Tech Talents Growth Program from the Department of Education of Guizhou Province [No. QJHKYZ(2018)297], the Guiyang Science and Technology Bureau and Guiyang University [GYU-KYZ(2019∼2020)PT10-06], the Research Foundation of Guiyang University [GYU-KY-(2022)], and the UndergraduateInnovation and Entrepreneurship Training Program (No. 2019520371).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd Murad, N. B., Mohamed Nor, N. M. I., Shohaimi, S., and Mohd Zainudin, N. A. I. (2017). Genetic Diversity and Pathogenicity of Fusarium Species Associated with Fruit Rot Disease in Banana across Peninsular Malaysia. J. Appl. Microbiol. 123, 1533–1546. doi:10.1111/jam.13582
Balendres, M. A., and Bengoa, J. C. (2019). Diseases of Dragon Fruit (Hylocereus Species): Etiology and Current Management Options. Crop Prot. 126, 104920. doi:10.1016/j.cropro.2019.104920
Ben-Ze Ev, I. S., Assouline, I., Levy, E., and Elkind, G. (2011). First Report of Bipolaris Cactivora Causing Fruit Blotch and Stem Rot of Dragon Fruit (Pitaya) in israel. Phytoparasitica 39, 195–197. doi:10.1007/s12600-011-0143-y
Bordoh, P. K., Ali, A., Dickinson, M., Siddiqui, Y., and Romanazzi, G. (2020). A Review on the Management of Postharvest Anthracnose in Dragon Fruits Caused by Colletotrichum Spp. Crop Prot. 130, 105067. doi:10.1016/j.cropro.2019.105067
Castro, J. C., Endo, E. H., de Souza, M. R., Zanqueta, E. B., Polonio, J. C., Pamphile, J. A., et al. (2017). Bioactivity of Essential Oils in the Control of Alternaria alternata in Dragon Fruit (Hylocereus Undatus Haw.). Industrial Crops Prod. 97, 101–109. doi:10.1016/j.indcrop.2016.12.007
Chang, T. H., Lee, Y. S., and Hassan, O. (2018). First Report of Anthracnose of Japanese Plum (Prunus Salicina) Caused by Colletotrichum Nymphaeae in Korea. Plant Dis. 102, 1461. doi:10.1094/PDIS-01-18-0018-PDN
Guo, L. W., Wu, Y. X., Ho, H. H., Su, Y. Y., Mao, Z. C., He, P. F., et al. (2014). First Report of Dragon Fruit (Hylocereus Undatus) Anthracnose Caused by Colletotrichum Truncatum in China. J. Phytopathol. 162, 272–275. doi:10.1111/jph.12183
Guo, L. W., Wu, Y. X., Mao, Z. C., Ho, H. H., and He, Y. Q. (2012). Storage Rot of Dragon Fruit Caused by Gilbertella Persicaria. Plant Dis. 96, 1826. doi:10.1094/PDIS-07-12-0635-PDN
Inoue, K., and Nasu, H. (2000). Black Spot of Peach Caused by Alternaria Alternata (fr.) Keissler. J. Gen. Plant Pathol. 66, 18–22. doi:10.1007/PL00012916
Jalgaonkar, K., Mahawar, M. K., Bibwe, B., and Kannaujia, P. (2020). Postharvest Profile, Processing and Waste Utilization of Dragon Fruit (Hylocereus spp.): A Review. Food Rev. Int. 1, 1–27. doi:10.1080/87559129.2020.1742152
Konstantinou, S., Karaoglanidis, G. S., Bardas, G. A., Minas, I. S., Doukas, E., and Markoglou, A. N. (2011). Postharvest Fruit Rots of Apple in greece: Pathogen Incidence and Relationships between Fruit Quality Parameters, Cultivar Susceptibility, and Patulin Production. Plant Dis. 95, 666–672. doi:10.1094/PDIS-11-10-0856
Masyahit, M., Sijam, K., Awang, Y., and Mohd Satar, M. G. (2009). The First Report of the Occurrence of Anthracnose Disease Caused by Colletotrichum Gloeosporioides (penz.) Penz. & Sacc. On Dragon Fruit (Hylocereus spp.) in Peninsular malaysia. Am. J. Appl. Sci. 6, 902–912. doi:10.3844/ajas.2009.902.912
Oeurn, S., Jitjak, W., and Sanoamuang, N. (2015). Fungi on Dragon Fruit in Loei Province, thailand and the Ability of Bipolaris Cactivora to Cause Post-harvest Fruit Rot. Asia-Pacific J. Sci. Technol. 20, 405–418. doi:10.14456/kkurj.2015.34
Pan, L., Zhang, R., Lu, F., Liu, C., and Fu, J. (2021). Comparative Proteomic Analysis of the Fungal Pathogen Neoscytalidium Dimidiatum Infection in the Pitaya. Hortic. Environ. Biotechnol. 62, 649–659. doi:10.1007/s13580-021-00341-2
Prusky, D., Fuchs, Y., Kobiler, I., Roth, I., Weksler, A., Shalom, Y., et al. (1999). Effect of Hot Water Brushing, Prochloraz Treatment and Waxing on the Incidence of Black Spot Decay Caused by Alternaria Alternata in Mango Fruits. Postharvest Biol. Technol. 15, 165–174. doi:10.1016/S0925-5214(98)00082-9
Prusky, D., Perez, A., Zutkhi, Y., and Ben-Arie, R. (1997). Effect of Modified Atmosphere for Control of Black Spot, Caused by Alternaria Alternata, on Stored Persimmon Fruits. Phytopathology 87, 203–208. doi:10.1094/PHYTO.1997.87.2.203
Romanazzi, G., Lichter, A., Gabler, F. M., and Smilanick, J. L. (2012). Recent Advances on the Use of Natural and Safe Alternatives to Conventional Methods to Control Postharvest Gray Mold of Table Grapes. Postharvest Biol. Technol. 63, 141–147. doi:10.1016/j.postharvbio.2011.06.013
Tarnowski, T. L. B., Palmateer, A. J., and Crane, J. H. (2010). First Report of Fruit Rot on Hylocereus Undatus Caused by Bipolaris Cactivora in South florida. Plant Dis. 94, 1506. doi:10.1094/PDIS-06-10-0406
Tian, S., Wan, Y., Qin, G., and Xu, Y. (2006). Induction of Defense Responses against Alternaria Rot by Different Elicitors in Harvested Pear Fruit. Appl. Microbiol. Biotechnol. 70, 729–734. doi:10.1007/s00253-005-0125-4
Tomoo, M., Harukuni, H., and Toyozo, S. (2015). First Occurrence of Blueberry Anthracnose Caused by Colletotrichum Nymphaeae (An Additional Pathogen) and Colletotrichum Fioriniae in Hokkaido. Annu. Rep. Soc. Plant Prot. North Jpn. 2015, 101–105. doi:10.11455/kitanihon.2015.66_101
Vijaya, S. I., Anuar, I. S. M., and Zakaria, L. (2015). Characterization and Pathogenicity ofColletotrichum truncatumCausing Stem Anthracnose of Red-Fleshed Dragon Fruit (Hylocereus Polyrhizus) in Malaysia. J. Phytopathol. 163, 67–71. doi:10.1111/jph.12261
Wang, C., Wang, Y., Wang, L., Li, X., Wang, M., and Wang, J. (2021). Fusarium Species Causing Postharvest Rot on Chinese Cherry in China. Crop Prot. 141, 105496. doi:10.1016/j.cropro.2020.105496
Wang, L., Zhang, X., Chen, W., Xiao, T., Zhao, X., Ma, Y., et al. (2018). Shading Reduced the Injury Caused by Winter Chill on Pitaya Plant. Not. Bot. Horti Agrobo 47, 470–477. doi:10.15835/nbha47111364
Keywords: pitaya, postharvest disease, pathogen identification, drug sensitivity test, plant extracts
Citation: Li Y, Chen H, Ma L, An Y, Wang H and Wu W (2022) Laboratory Screening of Control Agents Against Isolated Fungal Pathogens Causing Postharvest Diseases of Pitaya in Guizhou, China. Front. Chem. 10:942185. doi: 10.3389/fchem.2022.942185
Received: 12 May 2022; Accepted: 24 May 2022;
Published: 30 June 2022.
Edited by:
Pei Li, Kaili University, ChinaCopyright © 2022 Li, Chen, Ma, An, Wang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haijiang Chen, YjA1Y2hlbmhqQDEyNi5jb20=; Wenneng Wu, d3V3ZW5uZW5nMTIzQDEyNi5jb20=
†These authors have contributed equally to this work
 Yong Li
Yong Li Haijiang Chen
Haijiang Chen Lan Ma
Lan Ma Wenneng Wu
Wenneng Wu