- 1Comprehensive Utilization of Edible and Medicinal Plant Resources Engineering Technology Research Center, Zhengzhou Key Laboratory of Synthetic Biology of Natural Products, Zhengzhou Key Laboratory of Medicinal Resources Research, Huanghe Science and Technology College, Zhengzhou, China
- 2Horticulture and Landscape College, Tianjin Agricultural University, Tianjin, China
- 3Key Laboratory of Agro-Products Quality and Safety Control in Storage and Transport Process, Ministry of Agriculture and Rural Affairs, Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences, Beijing, China
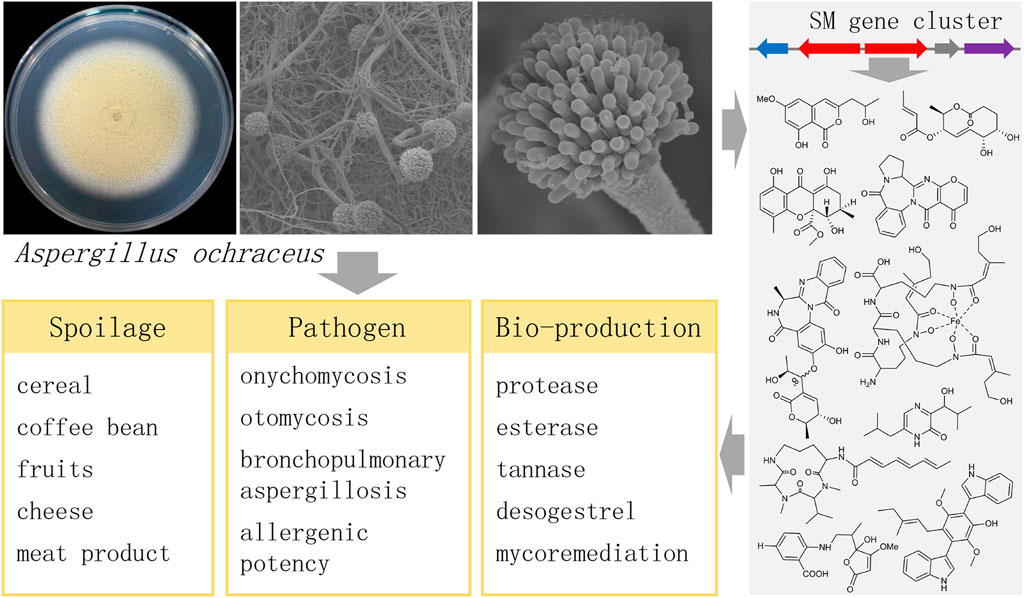
Aspergillus ochraceus, generally known as a food spoilage fungus, is the representative species in Aspergillus section Circumdati. A. ochraceus strains are widely distributed in nature, and usually isolated from cereal, coffee, fruit, and beverage. Increasing cases suggest A. ochraceus acts as human and animal pathogens due to producing the mycotoxins. However, in terms of benefits to mankind, A. ochraceus is the potential source of industrial enzymes, and has excellent capability to produce diverse structural products, including polyketides, nonribosomal peptides, diketopiperazine alkaloids, benzodiazepine alkaloids, pyrazines, bis-indolyl benzenoids, nitrobenzoyl sesquiterpenoids, and steroids. This review outlines recent discovery, chemical structure, biosynthetic pathway, and bio-activity of the natural compounds from A. ochraceus.
Introduction
Filamentous fungi in the genus Aspergillus are well known for their important roles in lifesaving drugs, devastating toxins, or mass-produced industrial enzymes. Aspergillus is currently subdivided into 27 sections by the physiologic, phenotypic, and DNA sequence data (Houbraken et al., 2020). A. ochraceus, the representative species in Aspergillus section Circumdati (Visagie et al., 2014), is generally known as a food spoilage fungus and is widely isolated from cereal, coffee, fruit, beverage, soil, and marine environments due to their environmental tolerance and fast growth. Ochratoxin A (OTA) makes A. ochraceus notorious for their role as contaminants in mycotoxigenic food and feed. Moreover, increasing cases have suggested A. ochraceus acts as human and animal pathogens causing onychomycosis (Xu et al., 2021), allergic bronchopulmonary aspergillosis (Hassanzad et al., 2019), and otomycosis (Ghibaudo and Peano, 2010). Some novel IgE-binding proteins have been identified from A. ochraceus, indicating the allergenic potency of mycelial proteins (Roy et al., 2021). Recently, A. ochraceus has been found in a SARS-CoV-2 positive immunocompetent patient in Iran (Koehler et al., 2020), and also can cause COVID-19 associated pulmonary aspergillosis (Hakamifard et al., 2021). On the other hand, the adaption of Aspergillus to shifting environments lead to the formation of a particular set of proteins (Day and Quinn, 2019; Wang et al., 2022b). A. ochraceus has been an important source of industrial enzymes like protease (El-Khonezy et al., 2021; Komarevtsev et al., 2021; Zhu et al., 2021), esterase (Romero-Borbón et al., 2018) and tannase (Aracri et al., 2019). Several medicinal metabolites, such as an intermediate for the synthesis of desogestrel and eplerenone, are characterized from A. ochraceus (Wang X. et al., 2020; Li et al., 2021). Some A. ochraceus strains have shown the mycoremediation potential to remove petroleum hydrocarbons (Bilen Ozyurek et al., 2021), and the remarkable capability for converting biodegradable waste to value-added end products for commercial applications (Jathanna and Rao, 2022).
Secondary metabolites (SMs) play important roles both as a food spoilage fungus and as an industrial strain for bio-production (Figure 1). A. ochraceus as a food spoilage fungus exhibits a remarkably versatile secondary metabolism. Most SMs are derived from polyketides synthases, non-ribosomal peptides synthases, and terpene synthases, and used to defend their habitat or inhibit the growth of competitors (Macheleidt et al., 2016). And these compounds are likely to remain in the food chain after the occurrence of A. ochraceus in food substrate. From the perspective of drug discovery, many compounds have been isolated from A. ochraceus and screened for bio-activities. In fungi, the genes required for the biosynthesis of SMs are generally clustered on the chromosome. A growing number of Aspergillus genomes impressively shows numbers of unknown biosynthetic gene clusters (BGCs) of SMs, which considerably exceed the number of identified SMs, indicating their potential production of novel structural compounds (Keller, 2019). It has been well summarized that the linkage between SMs with their BGCs in the different Aspergillus spp. (Frisvad and Larsen, 2015; Romsdahl and Wang, 2019; Yu et al., 2021). However, although lots of compounds have been isolated from A. ochraceus, no other BGCs have been identified except for the OTA BGC. Reviewing the SMs and biosynthetic diversity from A. ochraceus would give insights into the understanding and utilization of this fungus.
In this review, we have critically scrutinized the existing reports to provide an overview of the various SMs produced by A. ochraceus. Considering the structural characteristics and biogenetics, these compounds could be classified as polyketides, nonribosomal peptides, diketopiperazine alkaloids, benzodiazepine alkaloids, pyrazines, bis-indolyl benzenoids, nitrobenzoyl sesquiterpenoids, steroids, et al. In addition, we present the bioactivities and the possible biosynthetic pathway of some compounds, which are ignored due to the shading of mycotoxin OTA.
Secondary Metabolites From A. ochraceus
Ochratoxins
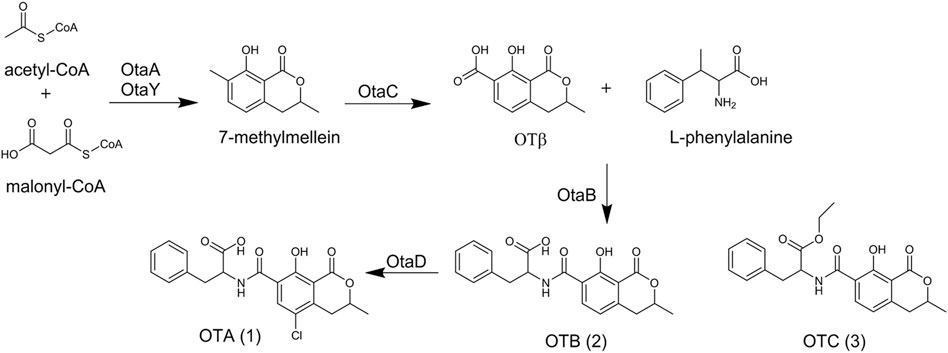
The most common A. ochraceus product described is ochratoxin A (1) (Figure 2). It was first discovered from A. ochraceus isolated from sorghum grain in South Africa in 1965 (van der Merwe et al., 1965b). Since then, more than 90 kinds of foodstuffs such as cereal, beer, coffee, cheese, and meat products have been found to contribute to OTA dietary exposure (Ostry et al., 2013). Recently, some A. ochraceus strains were re-classified as A. westerdijkiae based on the β-tubulin sequence and morphological identification (Cui et al.; Durand et al., 2019). Surprisingly, a genome mining study demonstrated the integral cluster of OTA was not found in the A. ochraceus genome, (Gil-Serna et al., 2020), while the strain A. ochraceus fc-1, re-classified as A. westerdijkiae, has been reported to contain an intact cluster (Wang et al., 2018; Wang et al., 2022a).
Biological toxic studies performed on rats, trout, and mice demonstrated the carcinogenic potency of OTA (Kanisawa and Suzuki, 1978). The International Agency for Research on Cancer evaluated the experimental evidence for carcinogenicity as sufficient and classified OTA as a possible human carcinogen (group 2B) (IARC, 1993). Additionally, OTA was well documented in its nephrotoxicity, immunotoxicity, myelotoxicity, genotoxicity, embryotoxic, and teratogenicity in many species (Pfohl-Leszkowicz and Manderville, 2007; Malir et al., 2013a; Malir et al., 2013b).
OTA consists of a para-chlorophenolic moiety containing a dihydroiso-coumarin group that is amide-linked to l-phenylalanine. OTB (2) and OTC (3) were also isolated from A. ochraceus as the dechloro and ethyl ester derivatives of OTA. In similar toxicity tests, (2) and (3) were proved to be non-toxic at a thousand-fold higher dose level compared with (1) (Van der Merwe et al., 1965a). The metabolism of OTA has been extensively studied over the past decades. After in vitro incubation of OTA with the microsomes of human, rat, and pig, hydroxylated derivatives 4(R)-OH-OTA, 4(S)-OH-OTA (Størmer et al., 1981) and 10-OH-OTA (Størmer et al., 1983) have been detected. The α-chymotrypsine and carboxypeptidase from homogenates of the pancreas and small intestine led to the cleavage of the peptide bond in OTA and yield OTα (Hansen et al., 1982). Several derivatives occur naturally in the animal body by biotransformation, including OTA open lactone (OP-OA) (Gillman et al., 1999), OTA quinone (OTQ) (Gillman et al., 1999), OTA hydroquinone decarboxylated (DC-OTHQ) (Faucet-Marquis et al., 2006), conjugate OTA quinone-glutathion (OTQ-Glutathion) (Dai et al., 2002), OTA methyl ester (OTA-Me) (Li et al., 2000), Ethylamide OTA (OE-OA) (Xiao et al., 1995), tyrosine OTA (OTA-tyrosine) (Creppy et al., 1990) and so on (Malir et al., 2016).
The biosynthetic pathway of (1) was first investigated by exploring the related metabolites. Labeling study by the introduction of [1-14C] l-phenylalanine into the culture of A. ochraceus lead to the detection of radioactivity in the phenylalanine moiety of (1), indicating l-Phenylalanine was the precursor of (1) (Steyn et al., 1970). [2-14C]acetate and [2-14C]malonic acid radiolabeling experiments indicated malonic acid was involved in the isocoumarin moiety biosynthesis but not in the phenylalanine moiety biosynthesis (Ruhland et al., 1996), and the isocoumarin moiety most derived via acetate condensation.
Advances in sequencing technology make scientists realize the dihydrocoumarin moiety of (1) is catalyzed by polyketide synthase (PKS) and the polyketide moiety of (1) is linked to l-phenylalanine catalyzed by non-ribosomal peptide synthetase (NRPS) (Huffman et al., 2010; Wang et al., 2015). In 2018, a consensus biosynthetic gene cluster was identified by comparative genomic analyses among OTA-producing fungi. And the biosynthetic pathway was clarified by discovering the intermediate metabolites in OTA gene disruption mutants. Briefly, OtaA (PKS) utilized acetyl-CoA and malonyl-CoA to synthesize 7-methylmellein by condensation, which was oxidized to OTβ by cytochrome P450 OtaC. Then, OtaB (NRPS) combined OTβ and l-phenylalanine to synthesize (2) by catalyzing the formation of an amide-bond. (2) was chlorinated by the halogenase OtaD to form (1). Recently, a cyclase gene otaY was proved to be involved in the biosynthesis of (1) (Figure 2). OtaY was speculated to catalyze the cyclization process of 7-methylmellein (Ferrara et al., 2020; Ferrara et al., 2021).
Polyketides
Polyketides occur in various organisms including fungi, bacteria, and plants. They are recognized as one of the most important categories of SMs. Polyketides have a common biosynthetic origin of small carboxylic acids such as acetate, propionate, and, rarely, butyrate, with diversity in structure (Palmer and Alper, 2019). Several polyketides have been isolated from A. ochraceus.
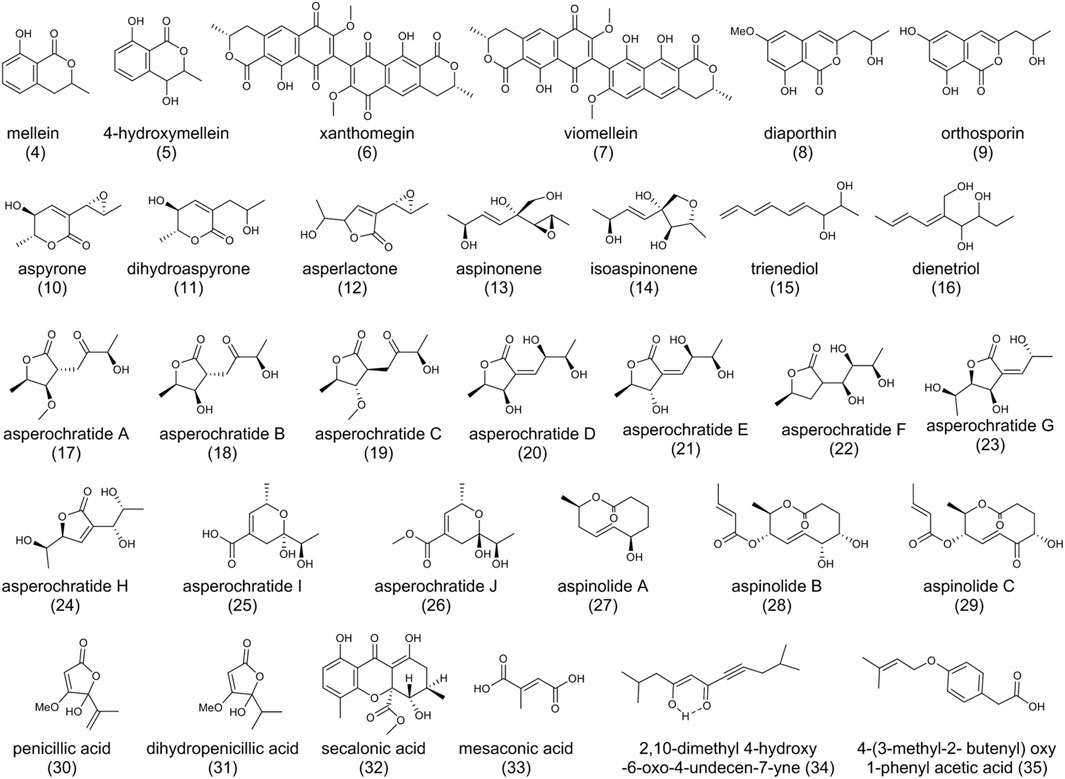
As shown in Figure 3, mellein (4) and 4-hydroxymellein (5), structurally similar to the dihydroisocoumarin moiety of (1), are also produced by A. ochraceus (Cole et al., 1971; Moore et al., 1972). The biosynthesis of (4) starts with the condensation of acetyl-CoA and malonyl-CoA, and acetyl units would be added until the pentaketide is formed (Huff and Hamilton, 1979), just like the biosynthesis 7-methylmellein in OTA. Compound (4) and its derivatives exhibit an array of bio-activities such as antitumor, antifungal, antibacterial, and anti-inflammatory (Hussain et al., 2015; Mdachi, 2016).
Xanthomegnin (6) and viomellein (7) are mycotoxins produced by Penicillium viridicatum, A. melleus, A. sulphureus, as well as A. ochraceus (Stack and Mislivec, 1978; Kamiya et al., 2017). It was reported that the toxicity of P. viridicatum strain 66-68-2 was due to (6), (7), rubrosulphin, viopurpurin, and brevianamide A, instead of (1) or citrinin (Stack et al., 1977). Gene inactivation experiments suggested (6) and (7) originated from the same polyketide pathway (Nicolaisen et al., 1996; Kandemir et al., 2015).
Diaporthin (8) and orthosporin (9) were also characterized from A. ochraceus (Harris and Mantle, 2001). (8) was reported to reproduce symptoms of canker in Chestnut trees and (9) could cause irregular brown spots on the leaves of oats (Hallock et al., 1988). The difference between the two compounds is the replacement of the methoxy by hydroxyl moiety on the benzene ring. Structurally, we hypothesize that one PKS could be responsible for the biosynthesis of these compounds.
Another array of SMs found in A. ochraceus was aspyrone (10), dihydroaspyrone (11), asperlactone (12), aspinonene (13), isoaspinonene (14), trienediol (15), and dienetriol (16), with the bioactivities of anti-microbial and anti-nematode (Fuchser et al., 1994; Kimura et al., 1996; Fuchser and Zeeck, 1997; Yurchenko et al., 2019). (10) and (13) have structural similarities and belong to the growing family of fungal epoxides. They contain a C9 carbon skeleton and one oxirane ring at a similar position and share the same biosynthetic pathway. The unknown PKS catalyzes the biosynthesis of an intermediate metabolite β-hydroxy acid, followed by modification of post-polyketide enzymes. A rearrangement of the carbon skeleton forms a branched pentaketide, and the aldehyde intermediate is either oxidized or reduced to yield (10) and (13), respectively (Fuchser et al., 1995; Fuchser and Zeeck, 1997). Recently, asperochratides A-J (17-26), which belong to aspyrone co-metabolites, have been isolated from A. ochraceus and found to exert significant cytotoxic effects on BV-2 cell line (Zou et al., 2020). Aspinolides A-C (27-29) are pentaketides with different precursors from (10)/(13), indicating the different PKS pathways. Generally, PKS catalyzes the biosynthesis of an intermediate metabolite hydroxy acid, followed by cyclization by a thioesterase to form a 10-membered lactone. The intermediate lactone is further modified following two pathways (reductase and acylase) to form (28) and (29) (Fuchser and Zeeck, 1997). Given their interesting structure and bioactivity, total synthesis by different approaches has been explored (Pilli et al., 2000; Ghosh and Rao, 2007; Chowdhury et al., 2009).
Organic acids derived from PKS pathways such as penicillic acid (30) (Frank et al., 2019), dihydropenicillic acid (31), secalonic acid (32) (Yamazaki et al., 1971), and mesaconic acid (33) (Zou et al., 2017) were isolated from A. ochraceus stains. (30) caused significant problems in animal and human health, and (32) showed antimicrobial activity against Bacillus subtilis and Piricularia oryzae. 2, 10-dimethyl 4-hydroxy-6-oxo-4-undecen-7-yne (34) and 4-(3-methyl-2-butenyl) oxy 1-phenyl acetic acid (35) were actively produced in an A. ochraceus mutant strain by UV irradiation (Awad et al., 2005).
Nonribosomal Peptides
Non-ribosomal peptides are synthesized by multi-modular NRPSs from building blocks of 20 kinds of proteinogenic amino acids and non-proteinogenic amino acids such as ornithine and β-alanine (Wang et al., 2016; Stevenson et al., 2019). They have been optimized for a certain function in the native producer during years of evolution, as well as represent a promising basis for the development of substances with excellent activities. Generally, most of the non-ribosomal peptides described from A. ochraceus are cyclic peptides.
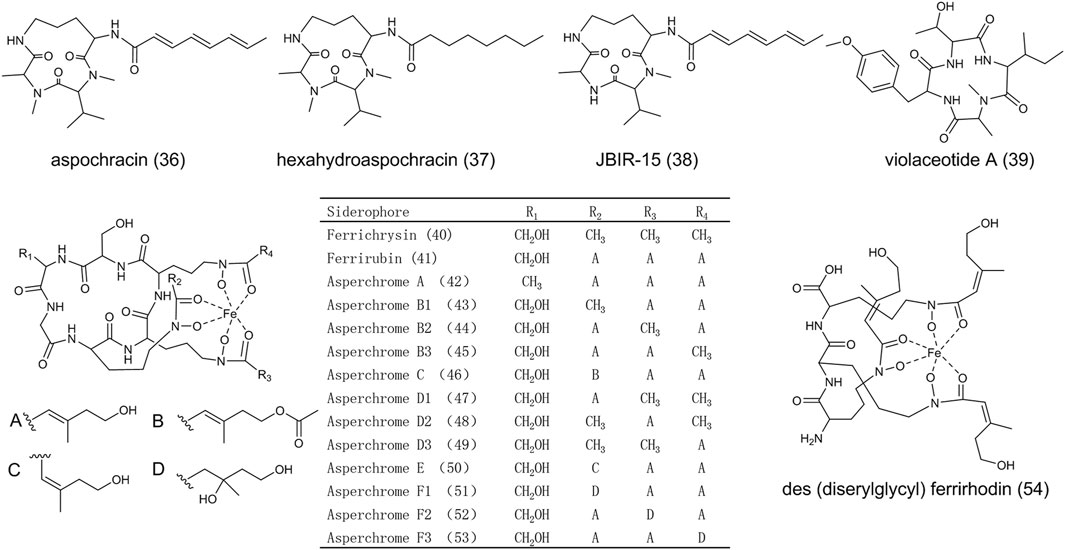
As shown in Figure 4, aspochracin (36) is a cyclotripeptide composed of N-methy-l-alanine, N-methy-l-valine and l-ornithine (Chang et al., 1969). (36) demonstrated contact toxicity to the first instar larvae and eggs of silkworm. However, the insecticidal activity completely diminished in hexahydroaspochracin (37), indicating the triene in the side chain has been involved in molecular bioactivity. JBIR-15 (38), of which the N-methyl alanine is replaced by alanine compared to (36), was isolated from A. sclerotiorum (Motohashi et al., 2009). Violaceotide A (39) was extracted from a solid rice medium of A. ochraceus (Frank et al., 2019) and its structure was elucidated as cyclic tetrapeptide with l-threonine, L-O-methy-tyrosine, N-methy-l-alanine and l-lsoleucine. (39) showed anti-inflammatory activity with a high inhibitory rate (Liu et al., 2018).
Most fungi can produce siderophores under iron deficiency or other iron-related conditions. Hydroxamate-type siderophores, classified into fusarinines, coprogens, or ferrichromes, were mostly found and characterized in fungi (Garnerin et al., 2017). A large number of ferrichromes were isolated from iron-deficient cultures of A. ochraceus (Jalal et al., 1984). Ferrichromes are cyclic hexapeptides composed of three Nδ-acyl-Nδ-hydroxy-l-ornithine, one glycine, and two variable amino acids (alanine, serine, or glycine) linked by peptide bonds. The structures of ferrichrysin (40), ferrirubin (41), and asperchromes (42-53) were shown in Figure 4. Serine and alanine participate in the formation of these molecules as the variable amino acids; the acetyl and anhydromevalonyl are acyl groups linked to ornithine. Des (diserylglycyl)ferrirhodin (54) does not follow the typical ferrichrome structure. It is a linear siderophore consisting of three Nδ-cis-anhydromevalonic acid-Nδ-hydroxy- l-ornithine moieties linked by peptide bonds. The absence of a cyclic hexapeptide ring in des (diserylglycyl)ferrirhodin leads to a bathochromic shift withpH value decreasing from 2.0 to 1.7, indicating the change of iron-binding property (Jalal et al., 1984). It is rare among microorganisms that A. ochraceus produces various siderophores derived from a common cyclic hexapeptide ring with the N-acyl side chain surrounding the iron atom. Their diversity of function and structure is worthy to be further explored.
Diketopiperazine Alkaloids
Diketopiperazine alkaloids are commonly isolated from fungi with excellent biological activities such as anticancer, antimicrobial, antiviral, antioxidant, and immunomodulatory (Hu et al., 2019; Wang M.-H. et al., 2020). Diketopiperazine alkaloids, with a stable six-membered ring backbone, are cyclic dipeptides formed by the condensation of two amino acids through peptide bonds by NRPS (Jia et al., 2019). A. ochraceus is capable of producing abundant diketopiperazine alkaloids with structural diversity and biological activity (Figure 5).
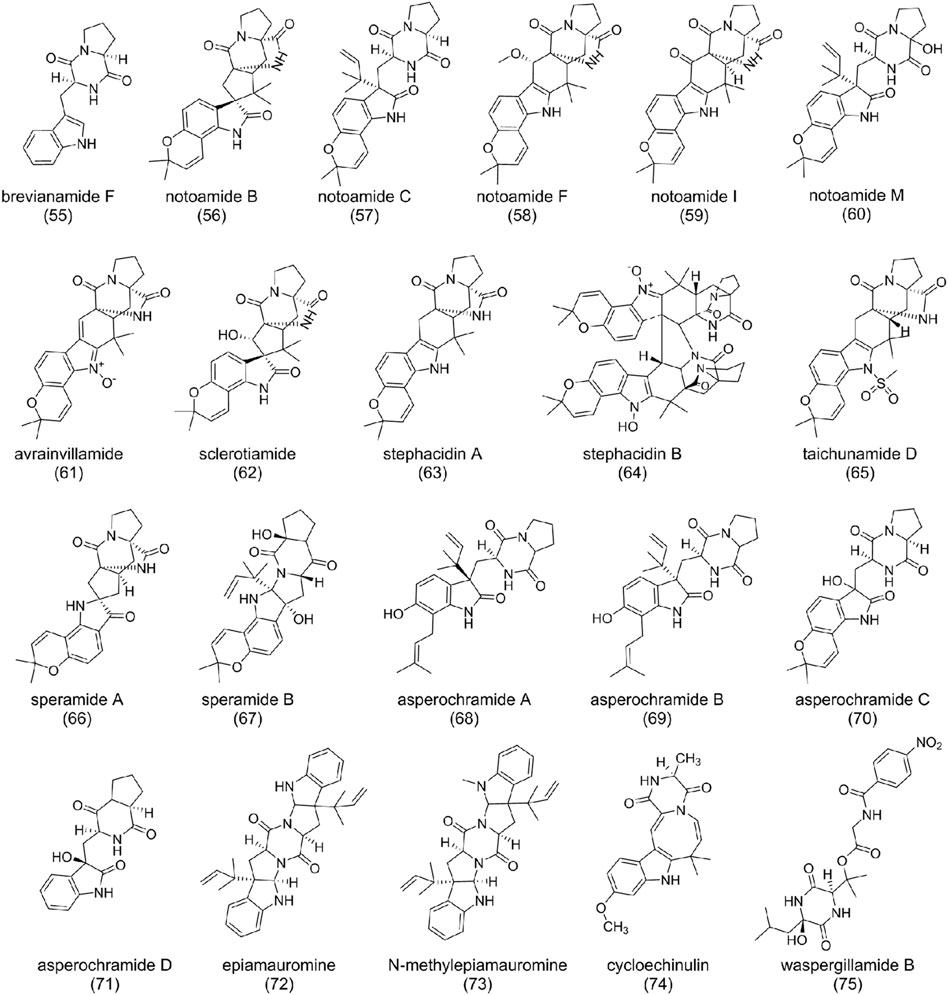
Indole diketopiperazine alkaloids are the main compounds in A. ochraceus, which were characterized by condensation of a complete tryptophan and other amino acids such as tryptophan, proline, and alanine (Ma et al., 2016). Brevianamide F (55), formed by condensation of a tryptophan and a proline without any modification, is the common precursor of many indole diketopiperazines. It was first isolated and characterized from P. piscarium and subsequently found in A. ochraceus (Vinokurova et al., 2003; Liu et al., 2018). Notoamide family compounds [B (56), C (57), F (58), I (59), and M (60)] were also isolated from A. ochraceus (Liu et al., 2018; Hu et al., 2021). (56) possesses the pyranoindole ring, with molecular similarity with avrainvillamide (61), sclerotiamide (62), and stephacidin A (63) (Sugie et al., 2001; Qian-Cutrone et al., 2002; Cui et al., 2009). Possible biosynthetic rules have been suggested: deoxybrevianamide E was first catalyzed to (63) then to (56), followed by branching to notoamide A or (62) (Kato et al., 2007). (57) and M (60) are prenylated indole diketopiperazine alkaloids, which contain diketopiperazine and isoprenoid moieties or structures derived thereof. Isopentenylation usually gives compound biological and pharmacological properties distinct from their non-prenylated precursors (Li, 2010). Brevianamide B (56) and C (57) showed moderate cytotoxicity against HeLa and L1210. It is worth mentioning that (57) can induce G2/M-cell cycle arrest at a concentration of 6.3 mg/ml (Kato et al., 2007). (63) showed cytotoxic activity against various human tumor cell lines, while bisindole diketopiperazine alkaloid stephacidin B (64), the dimer of (63), exhibited more potent antitumor activities (Qian-Cutrone et al., 2002). Taichunamide D (65), as an N-methylsulfonyl derivative of 6-epi-stephacidin A first isolated from A. taichungensis, was also found from A. ochraceus (Liu et al., 2018).
Speramides A (66) and B (67), featured by the fusion of a pyrrolidine ring to bicyclo-[2,2,2] diazaoctane subunit, were derived from the precursor (55). Since the structure of (66) has a similarity to (63), it is proposed that (63) could be converted to (66) through oxidation and rearrangement. Evaluation of their bioactivity demonstrated that (66) had moderate antimicrobial activities against Pseudomonas aeruginosa (Williams et al., 1990; Chang et al., 2016).
Asperochramides A-D (68-71) is another group of indole diketopiperazine alkaloids isolated from A. ochraceus (Liu et al., 2018). (68) and (69) are a pair of epimers assigned with the same planar structure. The significant difference between (68) and (70) is the cyclization of 2-isopentenyl and the replacement of 1-isopentenyl by hydroxyl. Removing the two isopentenyl forms (71). Bio-activities studies demonstrated that (68) has anti-inflammatory potential (Liu et al., 2018).
Most of the diketopiperazine alkaloids isolated from A. ochraceus are directives of precursor condensing of tryptophan and a proline. However, epiamauromine (72), which was stereochemically different from amauromine (Takase et al., 1985) characterized by condensation of two prenylated tryptophan, and N-methylepiamauromine (73) were isolated from A. ochraceus (de Guzman et al., 1992). Cycloechinulin (74) is formed by condensation of tryptophan and an alanine (de Guzman et al., 1992). And tetrapeptide diketopiperazine waspergillamide B (75) is a conjugate of p-aminobenzoic acid, Gly, hydroxy-Val, and hydroxy-Leu residues. Nitro-substituted diketopiperazines are rare compounds with excellent activity (Quezada et al., 2017; Frank et al., 2019).
Circumdatins
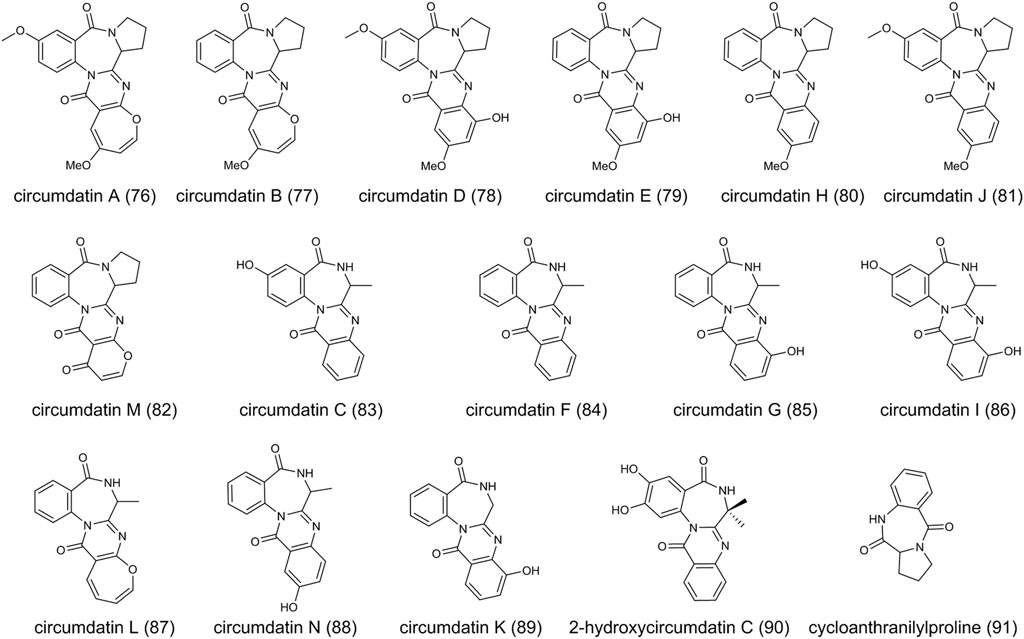
The circumdatins are a group of benzodiazepine alkaloids, first discovered in 1999 from a terrestrial isolate of the fungus A. ochraceus (Rahbæk and Breinholt, 1999). Structurally, the core chemical structure of benzodiazepines is the fusion of a benzene ring and a diazepine ring (Bhathiwal et al., 2022). Until now, thirteen compounds in the circumdatin family have been reported (circumdatins A-N). Biologically, circumdatin derivatives are generated from an amino acid and two anthranilic acids. Structural diversity of circumdatins depends on the type of amino acid and the different substituents. For example, circumdatins A (76) (Rahbæk and Breinholt, 1999; Ookura et al., 2008), B (77) (Rahbæk and Breinholt, 1999; Ookura et al., 2008), D (78) (Rahbæk and Breinholt, 1999), E (79) (Rahbæk and Breinholt, 1999), H (80) (Lopez-Gresa et al., 2005), J (81) (Zhichkin et al., 2010) and M (82) (Wang et al., 2019) contain proline, circumdatins C (83) (Rahbæk and Breinholt, 1999), F (84) (Rahbæk and Breinholt, 1999), G (85) (Dai et al., 2001), I (86) (Zhang et al., 2008), L (87) (Peng et al., 2013) and N (88) (Hu et al., 2021) contain alanine and circumdatin K (89) (Peng et al., 2013) contains glycine molecular structure, respectively (Figure 6). Most of the compounds were isolated from the genus Aspergillus, e.g., A. ochraceus, A. westerdijkiae, A. ostianus, and A. petrakii, while the (86) was isolated from the genus Exophiala (Zhang et al., 2008). In addition, a derivative 2-hydroxycircumdatin C (90) has been found in endophytic fungus A. ochraceus (Cui et al., 2009). As reported, A. ochraceus consistently produces circumdatins. Cycloanthranilylproline (91), isolated from A. ochraceus, is a kind of benzodiazepine alkaloid while not included in the circumdatin family (Nakatani et al., 2004; Frank et al., 2019). (91) derives from a proline and one molecule of anthranilic acid, and contains the fusion of a benzene ring and a diazepine ring.
Circumdatins demonstrated inhibitory activity similar to other inhibitors of the mammalian mitochondrial respiratory chain (Fontana et al., 2001). For example, the IC50 value of (80) against NADH oxidase is around 1.5 μM, indicating its potential to develop new tools for insect control (Lopez-Gresa et al., 2005). (83), (85), and (86) exhibited an ultraviolet-A protecting activity, which was better than the sunscreen agent oxybenzone (Zhang et al., 2008). (90) showed great DPPH radical-scavenging activity, which was more potent than the well-known butylated hydroxytoluene with an IC50 value of 9.9 mm (Cui et al., 2009). (78) demonstrated potential as an agent for neuroprotective effects by attenuating LPS-induced pro-Inflammatory responses (Zhang et al., 2020). Several compounds have been evaluated for their cytotoxicity, while no evidence provides to prove their cytotoxicity.
The biosynthesis of circumdatin remains to be explored in A. ochraceus and other fungi due to its structural complexity. However, the biosynthetic gene cluster of anthramycin and sibiromycin, which belong to the benzodiazepine family, have been identified clearly (Hu et al., 2007; Li et al., 2009), indicating NRPS might be involved in the biosynthesis of circumdatins.
Pyrazines
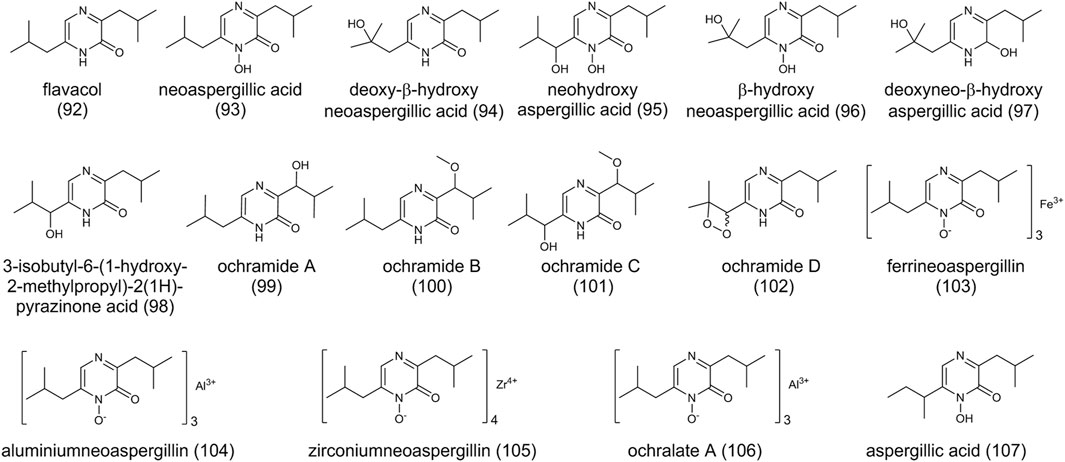
Pyrazines occur frequently in nature and are produced by plants, animals, and microorganisms (Ong et al., 2017). Several pyrazine compounds have been identified in A. ochraceus (Figure 7) and they are screened by bio-activities studies. Flavacol (92), neoaspergillic acid (93), and deoxy-β-hydroxyneoaspergillic acid (94) were first identified as pyrazine metabolites in 1972 (Yamazaki et al., 1972). Subsequently, neohydroxyaspergillic acid (95), β-hydroxyneoaspergillic acid (96), deoxyneo-β-hydroxyaspergillic (97), and 3-isobutyl-6-(1-hydroxy-2-methylpropyl)-2(1H)-pyrazinone acid (98) were also found in A. ochraceus (Maebayashi et al., 1978). More recently, ochramides A-D (99-102) were isolated from the fermentation broth of a marine coral-derived strain in a nutrient-limited medium (Peng et al., 2018). All of these compounds are derived from two leucine molecules with different modifications. It is suggested the first step of modification on the side chain is hydroxylation on the α position, followed by dehydration and rehydration on the β position. Three molecules of neoaspergillin chelate with one atom of iron, one atom of aluminum, or one atom zirconium to form ferrineoaspergillin (103), aluminiumneoaspergillin (104), and zirconiumneoaspergillin (105), respectively. N-hydroxy-ochramide B chelating with aluminum forms ochralate A (106).
It is reported the pyrazine compounds are biosynthesized from NRPS. The identification of a dihydropyrazine N,N′-dioxide metabolite proposes a noncanonical NRPS pathway for pyrazine derivatives through genome mining of Pseudomonas (Kretsch et al., 2018). A gene cluster containing an NRPS-like encoding gene in A. flavus is responsible for the synthesis of aspergillic acid (107) by NRPS-like gene inactivation experiment (Lebar et al., 2018). Structurally, (107) and (93) are closely related isomers. Many fungi from Aspergillus spp. can produce (93) and its hydroxylated analogs, and the genome mining shows they all harbor the homologs BGC of (107), indicating the same biosynthesis pathway between (107) and (93) (Lebar et al., 2019).
Bis-Indolyl Benzenoids
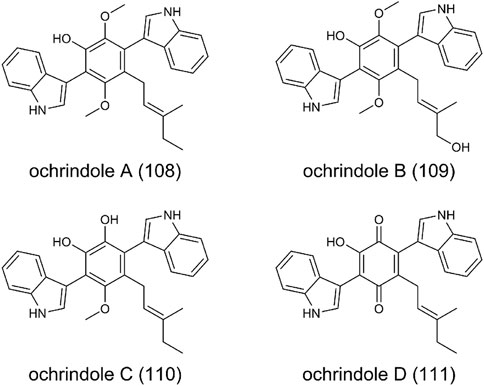
Ochrindoles A-D (108-111) were isolated from the sclerotia of A. ochraceus (de Guzman et al., 1994) (Figure 8). Structurally, (108-110) are bis-indolyl benzenoids, and (111) is bis-indolyl quinone. Members of these bis-indolyl structures have been reported from fungal metabolites, such as terriquinones from A. terreus (Balibar et al., 2007), kumbicins from A. kumbius (Lacey et al., 2016), asterriquinol from A. sclerotiorum (Whyte et al., 2000). These compounds typically contain prenyl groups at various positions on the indole moieties or the central benzenoid ring. The biosynthesis of bis-indolyl benzenoids and quinone has been extensively investigated. Briefly, an aminotransferase converts l-tryptophan to the indole pyruvic acid, and two molecules of indole pyruvic acid are dimerized by a single-module NRPS to form the bis-indolyl benzenoid skeleton. Then, the oxidoreductase, prenyltransferase, methytransferase successively play the catalytic function to form the corresponding products. Ochrindoles showed moderate activity against the corn earworm Helicoverpa zea, fungivorous beetle Carpophilus bemipterus, as well as bacterial Bacillus subtilis (de Guzman et al., 1994).
Nitrobenzoyl Sesquiterpenoids
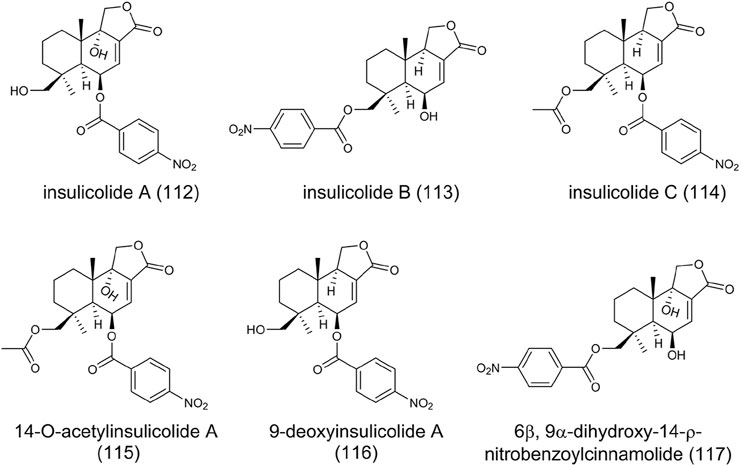
Sesquiterpenoids are abundant in nature, while nitrobenzoyl sesquiterpenoids are rare from natural sources. Until now, only several nitrobenzoyl sesquiterpenoids have been identified from marine-derived fungi A. ochraceus and A. insulicola (Belofsky et al., 1998; Fang et al., 2014; Tan et al., 2018). Insulicolides A-C (112-114), 14-O-acetylinsulicolide A (115), 9-deoxyinsulicolide A (116), and 6β, 9α-dihydroxy-14-ρ-nitrobenzoylcinnamolide (117) were isolated and characterized from marine-derived A. ochraceus (Figure 9). The significant inhibitory activities against the growth of renal carcinoma cells indicate these compounds possess antitumor potential (Tan et al., 2018).
Steroids
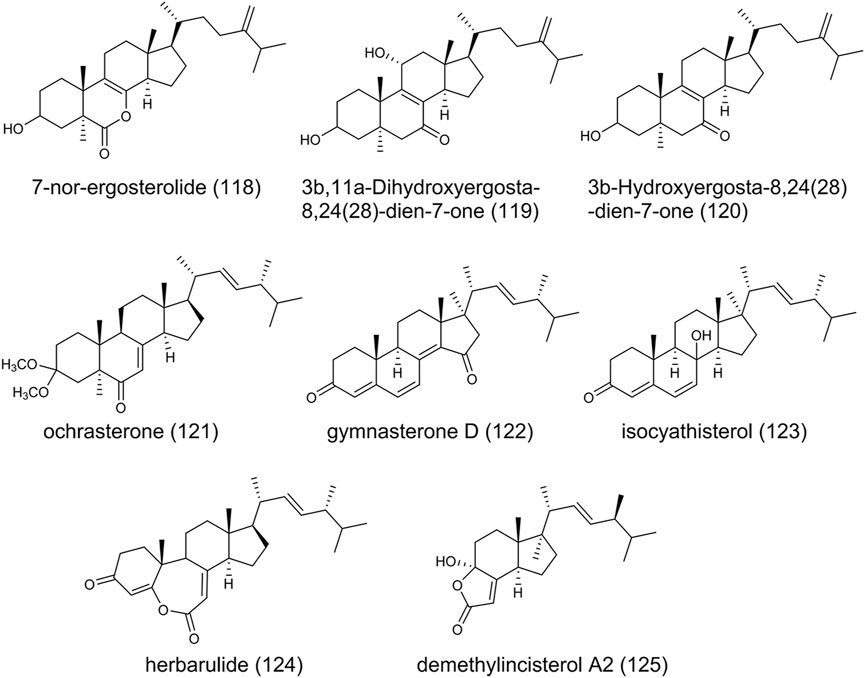
Steroids function as components of cell membranes or signaling molecules in living cells, with four rings arranged in a specific molecular configuration. As shown in Figure 10, 7-nor-ergosterolide (118), featured by a γ, δ-unsaturated pentalactone B-ring system, is the first 7-norsteroid of naturally occurring and isolated from A. ochraceus. In addition, 3β,11α-Dihydroxyergosta-8,24(28)-dien-7-one (119) and 3β-Hydroxyergosta-8,24(28)-dien-7-one (120) were identified from the same fungal strain and exhibited selective cytotoxic activity against tumor cell lines (Cui et al., 2010). Recently, a new ergostane-type sterol derivative ochrasterone (121), gymnasterone D (122), isocyathisterol (123), herbarulide (124), and demethylincisterol A2 (125) have been obtained (Hu et al., 2021; Tong et al., 2022). (123) was first discovered from A. ustus with weak antibacterial activity (Liu et al., 2014). Previously, in a bioactivity-guided search for new compounds in a marine sponge Homaxinella sp., the degraded (125) displayed significant cytotoxicity when it was tested against a panel of five human solid tumor cell lines (Mansoor et al., 2005).
Others
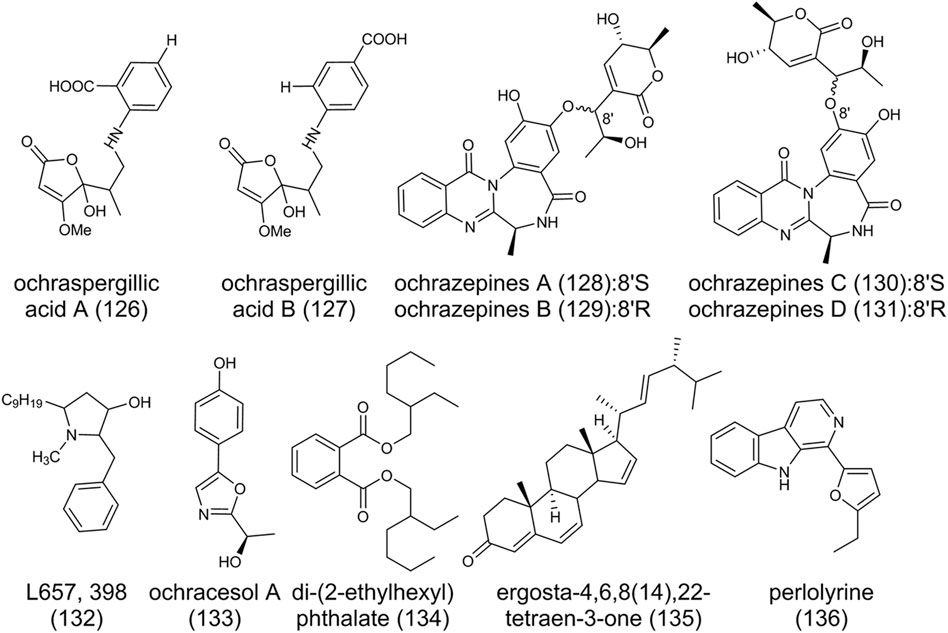
As shown in Figure 11, alkaloids ochraspergillic acids A (126), B (127), the adducts of dihydropenicillic acid (31) and ο- or ρ-aminobenzoic acid, were produced when A. ochraceus co-culture with Bacillus subtilis (Frank et al., 2019). Feeding experiments by adding either anthranilic acid or l-tryptophan to a solid rice medium also demonstrated the production of (126), indicating anthranilic acid and l-tryptophan are building blocks of ochraspergillic acids. Ochrazepines A-D (128-131) are dimerized from 2-hydroxycircumdatin C (90) and aspyrone (10) through a nucleophilic addition to epoxide (Fan et al., 2019). Semi-synthesis by nucleophilic addition reactions confirmed the speculation that (10) possibly underwent a SN1-like process to form the more stable allyl carbon positive ion, immediately followed by reaction with the oxygen anions of (90) to yield two pairs of epimers (128)/(129) and (130)/(131), respectively. The change of bioactivity of these compounds due to conjugation indicated the formation of hybrids provides more natural products for bioactivity studies. L657,398 (132), with broad antifungal activity, is a pyrollidine isolated from the mycelium of A. ochraceus in liquid fermentation (Schwartz et al., 1988). Ochracesol A (133), which contains an oxazole ring, exhibited anti-PD activities on SH-SY5Y cells (Hu et al., 2021). Di-(2-ethylhexyl) phthalate (134), ergosta-4,6,8 (14),22-tetraen-3-one (135), and a beta-carboline alkaloid perlolyrine (136) have also been found in A. ochraceus (Hu et al., 2021).
Discussion
Common fungi, especially A. ochraceus, are regularly underestimated for their biosynthetic potential, which deserves our recognition. Here, we comprehensively review the known SMs produced by A. ochraceus, and discuss their bioactivities and biosynthetic pathway. A. ochraceus produces a range of polyketides, nonribosomal peptides, diketopiperazine, terpenes, and other alkaloids. Except for the mycotoxins OTA, (6) and (7), these compounds possess antimicrobial, antiviral, anti-insect, antitumor, antioxidant, and anti-inflammatory activities. Thus, A. ochraceus strains could be valuable sources of compounds in the areas of medicine and agriculture.
In terms of fungi, a large number of natural products have been isolated from A. ochraceus until now. Nonetheless, several strategies have been used for enhancing the chemical diversity of microorganisms. Different media used in the cultivation of A. ochraceus leads to the production of different compounds. For example, when several inorganic salts or organic supplements are added to the solid rice medium culture, A. ochraceus is found to produce different metabolites, resulting in discovering the novel compounds; and this strategy verifies the OSMAC (One Strain MAny Compounds) theory (Frank et al., 2019). Grimm-Allen iron-limited medium allows A. ochraceus to secrete the extracellular siderophores (Jalal et al., 1984). Furthermore, the cultivation of two different microbial strains (A. ochraceus and B. subtilis) together leads to the induction of (126) and (127), which are not previously observed in the independent culture of each strain (Frank et al., 2019). Genetically, the changes in the cultural environment alter the gene expression profiles, hence activating silent SM gene clusters (Keller, 2019). For example, a total of 64 backbone SM genes, which are responsible for the biosynthesis of the chemical skeleton, are differentially expressed when A. nidulans undergoes a fungal-fungal cocultivation, leading to the activation of 14 aspernidine derivatives (Wang et al., 2022b).
Although a large number of compounds have been discovered in A. ochraceus, some of them are derived from the same biosynthetic pathway, e.g., (8) and (9) (Harris and Mantle, 2001). In fact, the number of genes encoding biosynthetic enzymes clearly outnumbers the identified compounds in A. ochraceus (Gonçalves et al., 2021). Finally, we remind the scientific community not to discredit the ability of A. ochraceus to produce natural products and encourage them to explore unexpected natural products through manipulating nutritional or environmental factors.
Author Contributions
GW, EL, WW, JZ and XG wrote the manuscript. LC and FX revised the manuscript.
Funding
This research was funded by Scientific Research Project of Tianjin Municipal Education Commission (2017KJ179), the Tackling-plan Project of Henan Department of Science and Technology (222102310518), Special Fund Project of Zhengzhou Basic and Applied Basic Research (ZZSZX202003) and Beijing Natural Science Foundation (6191001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aracri, F. M., Cavalcanti, R. M. F., and Guimaraes, L. H. S. (2019). Extracellular Tannase from Aspergillus ochraceus: Influence of the Culture Conditions on Biofilm Formation, Enzyme Production, and Application. J. Microbiol. Biotechnol. 29 (11), 1749–1759. doi:10.4014/jmb.1903.03060
Awad, G., Mathieu, F., Coppel, Y., and Lebrihi, A. (2005). Characterization and Regulation of New Secondary Metabolites from Aspergillus ochraceus M18 Obtained by UV Mutagenesis. Can. J. Microbiol. 51 (1), 59–67. doi:10.1139/w04-117
Balibar, C. J., Howard-Jones, A. R., and Walsh, C. T. (2007). Terrequinone A Biosynthesis through L-Tryptophan Oxidation, Dimerization and Bisprenylation. Nat. Chem. Biol. 3 (9), 584–592. doi:10.1038/nchembio.2007.20
Belofsky, G. N., Jensen, P. R., Renner, M. K., and Fenical, W. (1998). New Cytotoxic Sesquiterpenoid Nitrobenzoyl Esters from a Marine Isolate of the Fungus Aspergillus versicolor. Tetrahedron 54 (9), 1715–1724. doi:10.1016/s0040-4020(97)10396-9
Bhathiwal, A. S., Bendi, A., and Tiwari, A. (2022). A Study on Synthesis of Benzodiazepine Scaffolds Using Biologically Active Chalcones as Precursors. J. Mol. Struct. 1258, 132649. doi:10.1016/j.molstruc.2022.132649
Bilen Ozyurek, S., Avcioglu, N. H., and Seyis Bilkay, I. (2021). Mycoremediation Potential of Aspergillus ochraceus NRRL 3174. Arch. Microbiol. 203 (10), 5937–5950. doi:10.1007/s00203-021-02490-5
Chang, C.-F., Myokei, R., Sakurai, A., Takahashi, N., and Tamura, S. (1969). Aspochracin, a New Insecticidal Metabolite of Aspergillus ochraceus. Agric. Biol. Chem. 33 (10), 1501–1506. doi:10.1271/bbb1961.33.1501
Chang, Y.-W., Yuan, C.-M., Zhang, J., Liu, S., Cao, P., Hua, H.-M., et al. (2016). Speramides A–B, Two New Prenylated Indole Alkaloids from the Freshwater-Derived Fungus Aspergillus ochraceus KM007. Tetrahedron Lett. 57 (45), 4952–4955. doi:10.1016/j.tetlet.2016.09.071
Chowdhury, P. S., Gupta, P., and Kumar, P. (2009). First Asymmetric Total Synthesis of Aspinolide A. Tetrahedron Lett. 50 (50), 7018–7020. doi:10.1016/j.tetlet.2009.09.151
Cole, R. J., Moore, J. H., Davis, N. D., Kirksey, J. W., and Diener, U. L. (1971). 4-Hydroxymellein. New Metabolite of Aspergillus ochraceus. J. Agric. Food Chem. 19 (5), 909–911. doi:10.1021/jf60177a003
Creppy, E., Chakor, K., Fisher, M., and Dirheimer, G. (1990). The Mycotoxin Ochratoxin A Is a Substrate for Phenylalanine Hydroxylase in Isolated Rat Hepatocytes and In Vivo. Arch. Toxicol. 64 (4), 279–284. doi:10.1007/bf01972987
Cui, C. M., Li, X. M., Li, C. S., Sun, H. F., Gan, S. S., and Wang, B. G. (2009). Benzodiazepine Alkaloids from Marine-Derived Endophytic Fungus Aspergillus ochraceus. Helv. Chim. Acta 92 (7), 1366–1370. doi:10.1002/hlca.200900084
Cui, C. M., Li, X. M., Meng, L., Li, C. S., Huang, C. G., and Wang, B. G. (2010). 7-Nor-ergosterolide, a Pentalactone-Containing Norsteroid and Related Steroids from the Marine-Derived Endophytic Aspergillus ochraceus EN-31. J. Nat. Prod. 73 (11), 1780–1784. doi:10.1021/np100386q
Dai, J., Carte, B. K., Sidebottom, P. J., Sek Yew, A. L., Ng, S., Huang, Y., et al. (2001). Circumdatin G, a New Alkaloid from the Fungus Aspergillus ochraceus. J. Nat. Prod. 64 (1), 125–126. doi:10.1021/np000381u
Dai, J., Park, G., Wright, M. W., Adams, M., Akman, S. A., and Manderville, R. A. (2002). Detection and Characterization of a Glutathione Conjugate of Ochratoxin A. Chem. Res. Toxicol. 15 (12), 1581–1588. doi:10.1021/tx0255929
Day, A. M., and Quinn, J. (2019). Stress-Activated Protein Kinases in Human Fungal Pathogens. Front. Cell. Infect. Microbiol. 9, 261. doi:10.3389/fcimb.2019.00261
de Guzman, F. S., Bruss, D. R., Rippentrop, J. M., Gloer, K. B., Gloer, J. B., Wicklow, D. T., et al. (1994). Ochrindoles AD: New Bis-Indolyl Benzenoids from the Sclerotia of Aspergillus ochraceus NRRL 3519. J. Nat. Prod. 57 (5), 634–639. doi:10.1021/np50107a011
de Guzman, F. S., Gloer, J. B., Wicklow, D. T., and Dowd, P. F. (1992). New Diketopiperazine Metabolites from the Sclerotia of Aspergillus ochraceus. J. Nat. Prod. 55 (7), 931–939. doi:10.1021/np50085a013
Durand, N., Fontana, A., Meile, J.-C., Suàrez-Quiroz, M.-L., Schorr-Galindo, S., and Montet, D. (2019). Differentiation and Quantification of the Ochratoxin A Producers Aspergillus ochraceus and Aspergillus westerdijkiae Using PCR-DGGE. J. Basic Microbiol. 59 (2), 158–165. doi:10.1002/jobm.201800172
El-Khonezy, M. I., Elgammal, E. W., Ahmed, E. F., and Abd-Elaziz, A. M. (2021). Detergent Stable Thiol-Dependant Alkaline Protease Produced from the Endophytic Fungus Aspergillus ochraceus BT21: Purification and Kinetics. Biocatal. Agric. Biotechnol. 35, 102046. doi:10.1016/j.bcab.2021.102046
Fan, Y., Zhou, Y., Du, Y., Wang, Y., Fu, P., and Zhu, W. (2019). Circumdatin-aspyrone Conjugates from the Coral-Associated Aspergillus ochraceus LCJ11-102. Mar. Drugs 17 (7), 400. doi:10.3390/md17070400
Fang, W., Lin, X., Zhou, X., Wan, J., Lu, X., Yang, B., et al. (2014). Cytotoxic and Antiviral Nitrobenzoyl Sesquiterpenoids from the Marine-Derived Fungus Aspergillus ochraceus Jcma1F17. Med. Chem. Commun. 5 (6), 701–705. doi:10.1039/c3md00371j
Faucet-Marquis, V., Pont, F., Størmer, F. C., Rizk, T., Castegnaro, M., and Pfohl-Leszkowicz, A. (2006). Evidence of a New Dechlorinated Ochratoxin A Derivative Formed in Opossum Kidney Cell Cultures after Pretreatment by Modulators of Glutathione Pathways: Correlation with DNA-Adduct Formation. Mol. Nutr. Food Res. 50 (6), 530–542. doi:10.1002/mnfr.200500219
Ferrara, M., Gallo, A., Cervini, C., Gambacorta, L., Solfrizzo, M., Baker, S. E., et al. (2021). Evidence of the Involvement of a Cyclase Gene in the Biosynthesis of Ochratoxin A in Aspergillus carbonarius. Toxins 13 (12), 892. doi:10.3390/toxins13120892
Ferrara, M., Gallo, A., Perrone, G., Magistà, D., and Baker, S. E. (2020). Comparative Genomic Analysis of Ochratoxin A Biosynthetic Cluster in Producing Fungi: New Evidence of a Cyclase Gene Involvement. Front. Microbiol. 11, 581309. doi:10.3389/fmicb.2020.581309
Fontana, A., Cimino, G., Gavagnin, M., González, M. C., and Estornell, E. (2001). Novel Inhibitors of Mitochondrial Respiratory Chain: Endoperoxides from the Marine Tunicate Stolonica Socialis. J. Med. Chem. 44 (14), 2362–2365. doi:10.1021/jm0011373
Frank, M., Özkaya, F., Müller, W., Hamacher, A., Kassack, M., Lin, W., et al. (2019). Cryptic Secondary Metabolites from the Sponge-Associated Fungus Aspergillus ochraceus. Mar. Drugs 17 (2), 99. doi:10.3390/md17020099
Frisvad, J. C., and Larsen, T. O. (2015). Chemodiversity in the Genus Aspergillus. Appl. Microbiol. Biotechnol. 99 (19), 7859–7877. doi:10.1007/s00253-015-6839-z
Fuchser, J., Grabley, S., Noltemeyer, M., Philipps, S., Thiericke, R., and Zeeck, A. (1994). Secondary Metabolites by Chemical Screening, 28. Aspinonene, a New Multifunctional Fungal Metabolite. Liebigs Ann. Chem. 1994 (8), 831–835. doi:10.1002/jlac.199419940812
Fuchser, J., Thiericke, R., and Zeeck, A. (1995). Biosynthesis of Aspinonene, a Branched Pentaketide Produced by Aspergillus ochraceus, Related to Aspyrone. J. Chem. Soc. Perkin Transactions 1 (13), 1663–1666. doi:10.1039/p19950001663
Fuchser, J., and Zeeck, A. (1997). Aspinolides and Aspinonene/aspyrone Co-metabolites, New Pentaketides Produced by Aspergillus ochraceus. Liebigs Ann./Recl. 1, 87–95. doi:10.1002/jlac.199719970114
Garnerin, T., Dassonville-Klimpt, A., and Sonnet, P. (2017). “Fungal Hydroxamate Siderophores: Biosynthesis, Chemical Synthesis and Potential Medical Applications,” in Antimicrobial Research: Novel Bioknowledge and Educational Programs. Editor A. Méndez-Vilas. Badajoz, Spain: Formatex Research Center.
Ghibaudo, G., and Peano, A. (2010). Chronic Monolateral Otomycosis in a Dog Caused by Aspergillus ochraceus. Vet. Dermatol. 21 (5), 522–526. doi:10.1111/j.1365-3164.2010.00884.x
Ghosh, S., and Rao, R. V. (2007). Total Synthesis of Aspinolide B: a Ring-Closing Metathesis Approach. Tetrahedron Lett. 48 (39), 6937–6940. doi:10.1016/j.tetlet.2007.07.176
Gil-Serna, J., Vázquez, C., and Patiño, B. (2020). The Genomic Regions that Contain Ochratoxin a Biosynthetic Genes Widely Differ in Aspergillus Section Circumdati Species. Toxins 12 (12), 754. doi:10.3390/toxins12120754
Gillman, I. G., Clark, T. N., and Manderville, R. A. (1999). Oxidation of Ochratoxin A by an Fe− Porphyrin System: Model for Enzymatic Activation and DNA Cleavage. Chem. Res. Toxicol. 12 (11), 1066–1076. doi:10.1021/tx9901074
Gonçalves, M. F. M., Hilário, S., Tacão, M., Van de Peer, Y., Alves, A., and Esteves, A. C. (2021). Genome and Metabolome MS-based Mining of a Marine Strain of Aspergillus affinis. J. Fungi (Basel). 7 (12), 1091. doi:10.3390/jof7121091
Hakamifard, A., Hashemi, M., Fakhim, H., Aboutalebian, S., Hajiahmadi, S., and Mohammadi, R. (2021). Fatal Disseminated Aspergillosis in an Immunocompetent Patient with COVID-19 Due to Aspergillus ochraceus. J. Med. Mycol. 31 (2), 101124. doi:10.1016/j.mycmed.2021.101124
Hallock, Y. F., Clardy, J., Kenfield, D. S., and Strobel, G. (1988). De-O-methyldiaporthin, a Phytotoxin from Drechslera Siccans. Phytochemistry 27 (10), 3123–3125. doi:10.1016/0031-9422(88)80012-8
Hansen, C. E., Dueland, S., Drevon, C. A., and Størmer, F. (1982). Metabolism of Ochratoxin A by Primary Cultures of Rat Hepatocytes. Appl. Environ. Microbiol. 43 (6), 1267–1271. doi:10.1128/aem.43.6.1267-1271.1982
Harris, J. P., and Mantle, P. G. (2001). Biosynthesis of Diaporthin and Orthosporin by Aspergillus ochraceus. Phytochemistry 57 (2), 165–169. doi:10.1016/s0031-9422(01)00004-8
Hassanzad, M., Mortezaee, V., Bongomin, F., Poorabdollah, M., Sharifynia, S., Maleki, M., et al. (2019). Successful Control of Exacerbation of Allergic Bronchopulmonary Aspergillosis Due to Aspergillus terreus in a Cystic Fibrosis Patient with Short-Term Adjunctive Therapy with Voriconazole: A Case Report. J. de Mycol. Medicale 29 (2), 189–192. doi:10.1016/j.mycmed.2019.02.001
Houbraken, J., Kocsubé, S., Visagie, C. M., Yilmaz, N., Wang, X. C., Meijer, M., et al. (2020). Classification of Aspergillus, Penicillium, Talaromyces and Related Genera (Eurotiales): An Overview of Families, Genera, Subgenera, Sections, Series and Species. Stud. Mycol. 95, 5–169. doi:10.1016/j.simyco.2020.05.002
Hu, J., Li, Z., Gao, J., He, H., Dai, H., Xia, X., et al. (2019). New Diketopiperazines from a Marine-Derived Fungus Strain Aspergillus versicolor MF180151. Mar. Drugs 17 (5), 262. doi:10.3390/md17050262
Hu, L., Tian, S., Wu, R., Tong, Z., Jiang, W., Hu, P., et al. (2021). Identification of Anti-parkinson's Disease Lead Compounds from Aspergillus ochraceus Targeting Adenosin Receptors A2A. ChemistryOpen 10 (6), 630–638. doi:10.1002/open.202100022
Hu, Y., Phelan, V., Ntai, I., Farnet, C. M., Zazopoulos, E., and Bachmann, B. O. (2007). Benzodiazepine Biosynthesis in Streptomyces Refuineus. Chem. Biol. 14 (6), 870–701. doi:10.1016/j.chembiol.2007.07.006
Huff, W. E., and Hamilton, P. B. (1979). Mycotoxins–their Biosynthesis in Fungi: Ochratoxins–Metabolites of Combined Pathways. J. Food Prot. 42 (10), 815–820. doi:10.4315/0362-028x-42.10.815
Huffman, J., Gerber, R., and Du, L. (2010). Recent Advancements in the Biosynthetic Mechanisms for Polyketide‐derived Mycotoxins. Biopolymers 93 (9), 764–776. doi:10.1002/bip.21483
Hussain, H., Jabeen, F., Krohn, K., Al-Harrasi, A., Ahmad, M., Mabood, F., et al. (2015). Antimicrobial Activity of Two Mellein Derivatives Isolated from an Endophytic Fungus. Med. Chem. Res. 24 (5), 2111–2114. doi:10.1007/s00044-014-1250-3
IARC (1993). “Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins,” in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (Lyon (FR): International Agency for Research on Cancer), 56, 489–521.
Jalal, M., Mocharla, R., Barnes, C., Hossain, M. B., Powell, D., Eng-Wilmot, D. L., et al. (1984). Extracellular Siderophores from Aspergillus Ochraceous. J. Bacteriol. 158 (2), 683–688. doi:10.1128/jb.158.2.683-688.1984
Jathanna, H. M., and Rao, C. V. (2022). Using Aspergillus ochraceus, a Native Fungus, to Convert Biodiesel-Derived Crude Glycerol to Single Cell Oil for Commercial Applications. Waste Biomass Valorization 13, 2831–2845. doi:10.1007/s12649-022-01695-z
Jia, B., Ma, Y.-M., Liu, B., Chen, P., Hu, Y., and Zhang, R. (2019). Synthesis, Antimicrobial Activity, Structure-Activity Relationship and Molecular Docking Studies of Indole Diketopiperazine Alkaloids. Front. Chem. 7, 837. doi:10.3389/fchem.2019.00837
Kamiya, K., Arai, M., Setiawan, A., and Kobayashi, M. (2017). Anti-dormant Mycobacterial Activity of Viomellein and Xanthomegnin, Naphthoquinone Dimers Produced by Marine-Derived Aspergillus Sp. Nat. Prod. Commun. 12 (4), 1934578X1701200. doi:10.1177/1934578X1701200428
Kandemir, H., Ilkit, M., and Çürük, A. (2015). Xanthomegnin Detection Does Not Discriminate between Trichophyton Rubrum and T. Mentagrophytes Complexes. J. Microbiol. Methods 111, 122–126. doi:10.1016/j.mimet.2015.02.009
Kanisawa, M., and Suzuki, S. (1978). Induction of Renal and Hepatic Tumors in Mice by Ochratoxin A, a Mycotoxin. Gan 69 (4), 599–600.
Kato, H., Yoshida, T., Tokue, T., Nojiri, Y., Hirota, H., Ohta, T., et al. (2007). Notoamides A–D: Prenylated Indole Alkaloids Isolated from a Marine‐derived Fungus, Aspergillus Sp. Angew. Chem. Int. Ed. 46 (13), 2254–2256. doi:10.1002/anie.200604381
Keller, N. P. (2019). Fungal Secondary Metabolism: Regulation, Function and Drug Discovery. Nat. Rev. Microbiol. 17 (3), 167–180. doi:10.1038/s41579-018-0121-1
Kimura, Y., Nakahara, S., and Fujioka, S. (1996). Aspyrone, a Nematicidal Compound Isolated from the Fungus, Aspergillus melleus. Biosci. Biotechnol. Biochem. 60 (8), 1375–1376. doi:10.1271/bbb.60.1375
Koehler, P., Cornely, O. A., Böttiger, B. W., Dusse, F., Eichenauer, D. A., Fuchs, F., et al. (2020). COVID-19 Associated Pulmonary Aspergillosis. Mycoses 63 (6), 528–534. doi:10.1111/myc.13096
Komarevtsev, S. K., Timorshina, S. N., Leontieva, M. R., Shabunin, S. V., Lobakova, E. S., and Osmolovskiy, A. A. (2021). Effect of Immobilization of the Micromycete Aspergillus ochraceus VKM-F4104d in Polymeric Carriers on the Production of the Fibrinolytic Protease Activator of Blood Plasma Protein C. Appl. Biochem. Microbiol. 57 (4), 475–480. doi:10.1134/S0003683821030078
Kretsch, A. M., Morgan, G. L., Tyrrell, J., Mevers, E., Vallet-Gély, I., and Li, B. (2018). Discovery of (Dihydro) Pyrazine N-Oxides via Genome Mining in Pseudomonas. Org. Lett. 20 (16), 4791–4795. doi:10.1021/acs.orglett.8b01944
Lacey, H. J., Vuong, D., Pitt, J. I., Lacey, E., and Piggott, A. M. (2016). Kumbicins A–D: Bis-Indolyl Benzenoids and Benzoquinones from an Australian Soil Fungus, Aspergillus Kumbius. Aust. J. Chem. 69 (2), 152–160. doi:10.1071/ch15488
Lebar, M. D., Cary, J. W., Majumdar, R., Carter-Wientjes, C. H., Mack, B. M., Wei, Q., et al. (2018). Identification and Functional Analysis of the Aspergillic Acid Gene Cluster in Aspergillus flavus. Fungal Genet. Biol. 116, 14–23. doi:10.1016/j.fgb.2018.04.009
Lebar, M., Mack, B., Carter-Wientjes, C., and Gilbert, M. (2019). The Aspergillic Acid Biosynthetic Gene Cluster Predicts Neoaspergillic Acid Production in Aspergillus Section Circumdati. World Mycotoxin J. 12 (3), 213–222. doi:10.3920/wmj2018.2397
Li, Q., Shi, L., Liu, Y., Guan, S., Zhang, S., Cai, B., et al. (2021). Improved 11α-Hydroxycanrenone Production by Modification of Cytochrome P450 Monooxygenase Gene in Aspergillus ochraceus. Acta Pharm. 71 (1), 99–114. doi:10.2478/acph-2021-0004
Li, S.-M. (2010). Prenylated Indole Derivatives from Fungi: Structure Diversity, Biological Activities, Biosynthesis and Chemoenzymatic Synthesis. Nat. Prod. Rep. 27 (1), 57–78. doi:10.1039/b909987p
Li, S., Marquardt, R., and Frohlich, A. (2000). Identification of Ochratoxins and Some of Their Metabolites in Bile and Urine of Rats. Food Chem. Toxicol. 38 (2-3), 141–152. doi:10.1016/s0278-6915(99)00153-2
Li, W., Khullar, A., Chou, S., Sacramo, A., and Gerratana, B. (2009). Biosynthesis of Sibiromycin, a Potent Antitumor Antibiotic. Appl. Environ. Microbiol. 75 (9), 2869–2878. doi:10.1128/aem.02326-08
Liu, J., Gu, B., Yang, L., Yang, F., and Lin, H. (2018). New Anti-inflammatory Cyclopeptides from a Sponge-Derived Fungus Aspergillus Violaceofuscus. Front. Chem. 6, 226. doi:10.3389/fchem.2018.00226
Liu, X.-H., Miao, F.-P., Liang, X.-R., and Ji, N.-Y. (2014). Ergosteroid Derivatives from an Algicolous Strain of Aspergillus ustus. Nat. Prod. Res. 28 (15), 1182–1186. doi:10.1080/14786419.2014.923996
Lopez-Gresa, M. P., Gonzalez, M. C., Primo, J., Moya, P., Romero, V., and Estornell, E. (2005). Circumdatin H, a New Inhibitor of Mitochondrial NADH Oxidase, from Aspergillus ochraceus. J. Antibiot. (Tokyo). 58 (6), 416–419. doi:10.1038/ja.2005.54
Ma, Y.-M., Liang, X.-A., Kong, Y., and Jia, B. (2016). Structural Diversity and Biological Activities of Indole Diketopiperazine Alkaloids from Fungi. J. Agric. Food Chem. 64 (35), 6659–6671. doi:10.1021/acs.jafc.6b01772
Macheleidt, J., Mattern, D. J., Fischer, J., Netzker, T., Weber, J., Schroeckh, V., et al. (2016). Regulation and Role of Fungal Secondary Metabolites. Annu. Rev. Genet. 50, 371–392. doi:10.1146/annurev-genet-120215-035203
Maebayashi, Y., Sumita, M., Fukushima, K., and Yamazaki, M. (1978). Isolation and Structure of Red Pigment from Aspergillus ochraceus Wilh. Chem. Pharm. Bull. 26 (4), 1320–1322. doi:10.1248/cpb.26.1320
Malir, F., Ostry, V., and Novotna, E. (2013a). Toxicity of the Mycotoxin Ochratoxin A in the Light of Recent Data. Toxin Rev. 32 (2), 19–33. doi:10.3109/15569543.2013.782504
Malir, F., Ostry, V., Pfohl-Leszkowicz, A., Malir, J., and Toman, J. (2016). Ochratoxin A: 50 Years of Research. Toxins (Basel) 8 (7), 191. doi:10.3390/toxins8070191
Malir, F., Ostry, V., Pfohl-Leszkowicz, A., and Novotna, E. (2013b). Ochratoxin A: Developmental and Reproductive Toxicity-An Overview. Birth Defects Res. B 98 (6), 493–502. doi:10.1002/bdrb.21091
Mansoor, T. A., Hong, J., Lee, C.-O., Bae, S.-J., Im, K. S., and Jung, J. H. (2005). Cytotoxic Sterol Derivatives from a Marine Sponge Homaxinella Sp. J. Nat. Prod. 68 (3), 331–336. doi:10.1021/np0496690
Mdachi, S. (2016). Naturally Occurring Mellein-type 3, 4-dihydroisocoumarins and Related Lactones: Synthetic Approaches-A Review. Tanz. J. Sci. 42 (1), 24–63. doi:10.4314/TJS.V42I1
Moore, J. H., Davis, N. D., and Diener, U. L. (1972). Mellein and 4-hydroxymellein Production by Aspergillus ochraceus Wilhelm. Appl. Microbiol. 23 (6), 1067–1072. doi:10.1128/am.23.6.1067-1072.1972
Motohashi, K., Inaba, S., Takagi, M., and Shin-Ya, K. (2009). JBIR-15, a New Aspochracin Derivative, Isolated from a Sponge-Derived Fungus, Aspergillus sclerotiorum Huber Sp080903f04. Biosci. Biotech. Bioch. 73 (8), 1898–1900. doi:10.1271/bbb.90228
Nakatani, S., Yamamoto, Y., Hayashi, M., Komiyama, K., and Ishibashi, M. (2004). Cycloanthranilylproline-derived Constituents from a Myxomycete Fuligo candida. Chem. Pharm. Bull. 52 (3), 368–370. doi:10.1248/cpb.52.368
Nicolaisen, M., Sandal, T., Frisvad, J. C., and Rossen, L. (1996). 2D-PAGE Examination of mRNA Populations from Penicillium freii Mutants Deficient in Xanthomegnin Biosynthesis. Microbiol. Res. 151 (3), 285–290. doi:10.1016/s0944-5013(96)80026-7
Ong, K. T., Liu, Z.-Q., and Tay, M. G. (2017). Review on the Synthesis of Pyrazine and its Derivatives. Borneo J. Resour. Sci. Technol. 7 (2), 60–75. doi:10.33736/bjrst.591.2017
Ookura, R., Kito, K., Ooi, T., Namikoshi, M., and Kusumi, T. (2008). Structure Revision of Circumdatins A and B, Benzodiazepine Alkaloids Produced by Marine Fungus Aspergillus ostianus, by X-Ray Crystallography. J. Org. Chem. 73 (11), 4245–4247. doi:10.1021/jo800348d
Ostry, V., Malir, F., and Ruprich, J. (2013). Producers and Important Dietary Sources of Ochratoxin A and Citrinin. Toxins 5 (9), 1574–1586. doi:10.3390/toxins5091574
Palmer, C. M., and Alper, H. S. (2019). Expanding the Chemical Palette of Industrial Microbes: Metabolic Engineering for Type III PKS‐derived Polyketides. Biotechnol. J. 14 (1), 1700463. doi:10.1002/biot.201700463
Peng, J., Zhang, X.-Y., Tu, Z.-C., Xu, X.-Y., and Qi, S.-H. (2013). Alkaloids from the Deep-Sea-Derived Fungus Aspergillus westerdijkiae DFFSCS013. J. Nat. Prod. 76 (5), 983–987. doi:10.1021/np400132m
Peng, X., Wang, Y., Zhu, T., and Zhu, W. (2018). Pyrazinone Derivatives from the Coral-Derived Aspergillus ochraceus LCJ11-102 under High Iodide Salt. Arch. Pharm. Res. 41 (2), 184–191. doi:10.1007/s12272-017-0928-8
Pfohl-Leszkowicz, A., and Manderville, R. A. (2007). Ochratoxin A: An Overview on Toxicity and Carcinogenicity in Animals and Humans. Mol. Nutr. Food Res. 51 (1), 61–99. doi:10.1002/mnfr.200600137
Pilli, R. A., Victor, M. M., and de Meijere, A. (2000). First Total Synthesis of Aspinolide B, a New Pentaketide Produced by Aspergillus Ochraceus. J. Org. Chem. 65 (19), 5910–5916. doi:10.1021/jo000327i
Qian-Cutrone, J., Huang, S., Shu, Y. Z., Vyas, D., Fairchild, C., Menendez, A., et al. (2002). Stephacidin A and B: Two Structurally Novel, Selective Inhibitors of the Testosterone-dependent Prostate Lncap Cells. J. Am. Chem. Soc. 124 (49), 14556–14557. doi:10.1021/ja028538n
Quezada, M., Shang, Z., Kalansuriya, P., Salim, A. A., Lacey, E., and Capon, R. J. (2017). Waspergillamide A, a Nitro Depsi-Tetrapeptide Diketopiperazine from an Australian Mud Dauber Wasp-Associated Aspergillus sp.(CMB-W031). J. Nat. Prod. 80 (4), 1192–1195. doi:10.1021/acs.jnatprod.6b01062
Rahbæk, L., and Breinholt, J. (1999). Circumdatins D, E, and F: Further Fungal Benzodiazepine Analogues from Aspergillus ochraceus. J. Nat. Prod. 62 (6), 904–905. doi:10.1021/np980495u
Romero-Borbón, E., Grajales-Hernández, D., Armendáriz-Ruiz, M., Ramírez-Velasco, L., Rodríguez-González, J. A., Cira-Chávez, L. A., et al. (2018). Type C Feruloyl Esterase from Aspergillus ochraceus: A Butanol Specific Biocatalyst for the Synthesis of Hydroxycinnamates in a Ternary Solvent System. Electron. J. Biotechnol. 35, 1–9. doi:10.1016/j.ejbt.2018.06.004
Romsdahl, J., and Wang, C. C. C. (2019). Recent Advances in the Genome Mining of Aspergillus Secondary Metabolites (Covering 2012–2018). MedChemComm 10 (6), 840–866. doi:10.1039/C9MD00054B
Roy, S., Saha, B., and Gupta Bhattacharya, S. (2021). Identifying Novel Allergens from a Common Indoor Mould Aspergillus ochraceus. J. Proteomics 238, 104156. doi:10.1016/j.jprot.2021.104156
Ruhland, M., Engelhardt, G., and Wallnöter, P. (1996). Production of 14 C-Ochratoxin A by Penicillium verrucosum Sp. 1761 in Liquid Culture. Mycotoxin Res. 12 (1), 7–13. doi:10.1007/bf03192075
Schwartz, R. E., Liesch, J., Hensens, O., Zitano, L., Honeycutt, S., Garrity, G., et al. (1988). L-657, 398, a Novel Antifungal Agent: Fermentation, Isolation, Structural Elucidation and Biological Properties. J. Antibiot. 41 (12), 1774–1779. doi:10.7164/antibiotics.41.1774
Stack, M. E., Eppley, R. M., Dreifuss, P. A., and Pohland, A. E. (1977). Isolation and Identification of Xanthomegnin, Viomellein, Rubrosulphin, and Viopurpurin as Metabolites of Penicillium viridicatum. Appl. Environ. Microbiol. 33 (2), 351–355. doi:10.1128/aem.33.2.351-355.1977
Stack, M. E., and Mislivec, P. B. (1978). Production of Xanthomegnin and Viomellein by Isolates of Aspergillus ochraceus, Penicillium cyclopium, and Penicillium viridicatum. Appl. Environ. Microbiol. 36 (4), 552–554. doi:10.1128/aem.36.4.552-554.1978
Stevenson, L. J., Owen, J. G., and Ackerley, D. F. (2019). Metagenome Driven Discovery of Nonribosomal Peptides. Acs Chem. Biol. 14 (10), 2115–2126. doi:10.1021/acschembio.9b00618
Steyn, P., Holzapfel, C., and Ferreira, N. (1970). The Biosynthesis of the Ochratoxins, Metabolites of Aspergillus ochraceus. Phytochemistry 9 (9), 1977–1983. doi:10.1016/s0031-9422(00)85349-2
Størmer, F., Hansen, C. E., Pedersen, J., Hvistendahl, G., and Aasen, A. J. (1981). Formation of (4R)-And (4S)-4-Hydroxyochratoxin A from Ochratoxin A by Liver Microsomes from Various Species. Appl. Environ. Microbiol. 42 (6), 1051–1056. doi:10.1128/aem.42.6.1051-1056.1981
Størmer, F., Støren, O., Hansen, C. E., Pedersen, J., and Aasen, A. (1983). Formation of (4R)-And (4S)-4-Hydroxyochratoxin A and 10-hydroxyochratoxin A from Ochratoxin A by Rabbit Liver Microsomes. Appl. Environ. Microbiol. 45 (4), 1183–1187. doi:10.1128/aem.45.4.1183-1187.1983
Sugie, Y., Hirai, H., Inagaki, T., Ishiguro, M., Kim, Y.-J., Kojima, Y., et al. (2001). A New Antibiotic CJ-17, 665 from Aspergillus ochraceus. J. Antibiot. (Tokyo). 54 (11), 911–916. doi:10.7164/antibiotics.54.911
Takase, S., Kawai, Y., Uchida, I., Tanaka, H., and Aoki, H. (1985). Structure of Amauromine, a New Hypotensive Vasodilator Produced by Sp. Tetrahedron 41 (15), 3037–3048. doi:10.1016/s0040-4020(01)96656-6
Tan, Y., Yang, B., Lin, X., Luo, X., Pang, X., Tang, L., et al. (2018). Nitrobenzoyl Sesquiterpenoids with Cytotoxic Activities from a Marine-Derived Aspergillus ochraceus Fungus. J. Nat. Prod. 81 (1), 92–97. doi:10.1021/acs.jnatprod.7b00698
Tong, Z., Xiao, X., Lu, Y., Zhang, Y., Hu, P., Jiang, W., et al. (2022). New Metabolites from Aspergillus ochraceus with Antioxidative Activity and Neuroprotective Potential on H2O2 Insult SH-Sy5y Cells. Molecules 27 (1), 52. doi:10.3390/molecules27010052
van der Merwe, K. J., Steyn, P., and Fourie, L. (1965a). Mycotoxins. Part II. The Constitution of Ochratoxins A, B, and C, Metabolites of Aspergillus ochraceus Wilh. J. Chem. Soc. Perkin 1, 7083–7088. doi:10.1039/jr9650007083
van der Merwe, K. J., Steyn, P. S., Fourie, L., Scott, D. B., and Theron, J. J. (1965b). Ochratoxin A, a Toxic Metabolite Produced by Aspergillus ochraceus Wilh. Nature 205 (976), 1112–1113. doi:10.1038/2051112a0
Vinokurova, N., Khmel'nitskaya, I., Baskunov, B., and Arinbasarov, M. (2003). Occurrence of Indole Alkaloids Among Secondary Metabolites of Soil Aspergillus Spp. Appl. Biochem. Micro. 39 (2), 192–196. doi:10.1023/A:1022598215599
Visagie, C. M., Varga, J., Houbraken, J., Meijer, M., Kocsubé, S., Yilmaz, N., et al. (2014). Ochratoxin Production and Taxonomy of the Yellow Aspergilli (Aspergillus Section Circumdati). Stud. Mycol. 78, 1–61. doi:10.1016/j.simyco.2014.07.001
Wang, F., Hu, Z., Li, C., Wu, X., and Cao, S. (2019). Circumdatin M, a New Benzodiazepine Alkaloid with a Unique Pyrimidone-4-Pyrone Moiety from a Hawaiian Marine Fungus Aspergillus Sp. FM242. Tetrahedron Lett. 60 (26), 1724–1726. doi:10.1016/j.tetlet.2019.05.061
Wang, G., Li, Y., Yang, B., Li, E., Wu, W., Si, P., et al. (2022a). AwAreA Regulates Morphological Development, Ochratoxin A Production, and Fungal Pathogenicity of Food Spoilage Fungus Aspergillus westerdijkiae Revealed by an Efficient Gene Targeting System. Front. Microbiol. 13, 857726. doi:10.3389/fmicb.2022.857726
Wang, G., Liu, Z., Lin, R., Li, E., Mao, Z., Ling, J., et al. (2016). Biosynthesis of Antibiotic Leucinostatins in Bio-Control Fungus Purpureocillium Lilacinum and Their Inhibition on Phytophthora Revealed by Genome Mining. PLoS Pathog. 12 (7), e1005685. doi:10.1371/journal.ppat.1005685
Wang, G., Ran, H., Fan, J., Keller, N. P., Liu, Z., Wu, F., et al. (2022b). Fungal-fungal Cocultivation Leads to Widespread Secondary Metabolite Alteration Requiring the Partial Loss-Of-Function VeA1 Protein. Sci. Adv. 8 (17), eabo6094. doi:10.1126/sciadv.abo6094
Wang, L., Wang, Y., Wang, Q., Liu, F., Selvaraj, J., Liu, L., et al. (2015). Functional Characterization of New Polyketide Synthase Genes Involved in Ochratoxin a Biosynthesis in Aspergillus Ochraceus Fc-1. Toxins 7 (8), 2723–2738. doi:10.3390/toxins7082723
Wang, M.-H., Zhang, X.-Y., Tan, X.-M., Niu, S.-B., Sun, B.-D., Yu, M., et al. (2020). Chetocochliodins A-I, Epipoly(thiodioxopiperazines) from Chaetomium Cochliodes. J. Nat. Prod. 83 (4), 805–813. doi:10.1021/acs.jnatprod.9b00239
Wang, X., Yang, X., Jia, X., Jin, P., Wang, Z., Lu, F., et al. (2020). Determination of Steroid Hydroxylation Specificity of an Industrial Strain Aspergillus ochraceus TCCC41060 by Cytochrome P450 Gene CYP68J5. Ann. Microbiol. 70 (1), 45. doi:10.1186/s13213-020-01577-6
Wang, Y., Wang, L., Wu, F., Liu, F., Wang, Q., Zhang, X., et al. (2018). A Consensus Ochratoxin A Biosynthetic Pathway: Insights from the Genome Sequence of Aspergillus ochraceus and a Comparative Genomic Analysis. Appl. Environ. Microbiol. 84 (19), e01009–18. doi:10.1128/AEM.01009-18
Whyte, A. C., Joshi, B. K., Gloer, J. B., Wicklow, D. T., and Dowd, P. F. (2000). New Cyclic Peptide and Bisindolyl Benzenoid Metabolites from the Sclerotia of Aspergillus sclerotiorum. J. Nat. Prod. 63 (7), 1006–1009. doi:10.1021/np000103v
Williams, R. M., Glinka, T., Kwast, E., Coffman, H., and Stille, J. K. (1990). Asymmetric, Stereocontrolled Total Synthesis of (-)-brevianamide B. J. Am. Chem. Soc. 112 (2), 808–821. doi:10.1021/ja00158a048
Xiao, H., Marquardt, R. R., Frohlich, A. A., and Ling, Y. Z. (1995). Synthesis and Structural Elucidation of Analogs of Ochratoxin A. J. Agric. Food Chem. 43 (2), 524–530. doi:10.1021/jf00050a050
Xu, X., Naseri, A., Houbraken, J., Akbari, F., Wang, X., Zhao, R., et al. (2021). Identification and In Vitro Antifungal Susceptibility of Causative Agents of Onychomycosis Due to Aspergillus Species in Mashhad, Iran. Sci. Rep. 11 (1), 6808. doi:10.1038/s41598-021-86038-z
Yamazaki, M., Maebayashi, Y., and Miyaki, K. (1972). Isolation of a New Type of Pyrazine Metabolite from Aspergillus ochraceus WILH. Chem. Pharm. Bull. 20 (10), 2274–2276. doi:10.1248/cpb.20.2274
Yamazaki, M., Maebayashi, Y., and Miyaki, K. (1971). The Isolation of Secalonic Acid A from Aspergillus ochraceus Cultured on Rice. Chem. Pharm. Bull. 19 (1), 199–201. doi:10.1248/cpb.19.199
Yu, R., Liu, J., Wang, Y., Wang, H., and Zhang, H. (2021). Aspergillus niger as a Secondary Metabolite Factory. Front. Chem. 9, 701022. doi:10.3389/fchem.2021.701022
Yurchenko, A. N., Trinh, P. T. H., Smetanina, O. F., Rasin, A. B., Popov, R. S., Dyshlovoy, S. A., et al. (2019). Biologically Active Metabolites from the Marine Sediment-Derived Fungus Aspergillus flocculosus. Mar. Drugs 17 (10), 579. doi:10.3390/md17100579
Zhang, C., Hu, L., Liu, D., Huang, J., and Lin, W. (2020). Circumdatin D Exerts Neuroprotective Effects by Attenuating LPS-Induced Pro-inflammatory Responses and Downregulating Acetylcholinesterase Activity In Vitro and In Vivo. Front. Pharmacol. 11, 760. doi:10.3389/fphar.2020.00760
Zhang, D., Yang, X., Kang, J. S., Choi, H. D., and Son, B. W. (2008). Circumdatin I, a New Ultraviolet-A Protecting Benzodiazepine Alkaloid from a Marine Isolate of the Fungus Exophiala. J. Antibiot. 61 (1), 40–42. doi:10.1038/ja.2008.108
Zhichkin, P. E., Jin, X., Zhang, H., Peterson, L. H., Ramirez, C., Snyder, T. M., et al. (2010). A Concise Synthesis of Enantiopure Circumdatins E, H and J. Org. Biomol. Chem. 8 (6), 1287–1289. doi:10.1039/b925494c
Zhu, X., Hua, Y., Li, X., Kong, X., Zhang, C., and Chen, Y. (2021). Isolation and Characterization of an Activator-dependent Protease from Aspergillus ochraceus Screened from Low Denatured Defatted Soybean Meal and the Proteolysis of Soy Proteins. LWT 150, 112026. doi:10.1016/j.lwt.2021.112026
Zou, Q., Wu, H., Huang, J., Wu, M., Li, M., Cheng, D., et al. (2017). Study on the Secondary Metabolites of Aspergillus ochraceus. J. Org. Chem. Res. 5 (1), 15–20.
Keywords: Aspergillus ochraceus, secondary metabolite, structure, bioactivity, biosynthesis
Citation: Chen L, Li E, Wu W, Wang G, Zhang J, Guo X and Xing F (2022) The Secondary Metabolites and Biosynthetic Diversity From Aspergillus ochraceus. Front. Chem. 10:938626. doi: 10.3389/fchem.2022.938626
Received: 07 May 2022; Accepted: 21 June 2022;
Published: 25 August 2022.
Edited by:
Tihomir Tomašič, University of Ljubljana, SloveniaReviewed by:
Bo Pang, Apertor Pharmaceuticals, Inc., United StatesQin Yang, Normal School of Pisa, Italy
Copyright © 2022 Chen, Li, Wu, Wang, Zhang, Guo and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Wang, d2FuZ2dhbmcwMkBjYWFzLmNu
†These authors have contributed equally to this work
 Lin Chen
Lin Chen Erfeng Li
Erfeng Li Wenqing Wu2†
Wenqing Wu2† Gang Wang
Gang Wang Fuguo Xing
Fuguo Xing