- School of Biotechnology and Health Sciences, Wuyi University, Jiangmen, China
Silibinin and capsaicin both are natural product molecules with diverse biological activities. In this article, we investigated the anti-inflammatory effects of silibinin combined with capsaicin in lipopolysaccharide (LPS)-induced RAW264.7 cells. The results showed that silibinin combined with capsaicin strongly inhibited LPS-induced nitric oxide (NO), tumor necrosis factor-α (TNF-α), Interleukin-6 (IL-6), and COX-2. Moreover, silibinin combined with capsaicin potently inhibited nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways. The results of the present study indicate that silibinin combined with capsaicin effectively inhibits inflammation.
Introduction
Inflammation is confirmed to be related to several progressive diseases (Irmscher et al., 2019; Bai et al., 2021), such as metabolic disorders (Bai et al., 2021), cancer, obesity (Kase et al., 2020), and cardiovascular disease. Thus, to eliminate or prevent inflammation is very necessary to maintain body health. Inflammatory cells are activated in the inflammatory process and release high levels of pro-inflammatory cytokine (Luo et al., 2019; Huang et al., 2022), including interleukin (IL)-1β, IL-6, nitric oxide (NO), and tumor necrosis factor-α (TNF-α), causing tissue damage and involve in the development of various diseases (Zhao et al., 2021). NO is an important pro-inflammatory mediator that is regulated by cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (Akanda et al., 2018). The secretion of pro-inflammatory mediators, such as COX-2, iNOS, IL-1β, IL-6, and TNF-α, is mediated by transcription factor nuclear factor-κB (NF-κB) (Wang et al., 2022). The NF-κB protein is composed of two subunits (P50 and P65) and bound to the inhibitor of NF-κB (IκB) to an inactive state (Yu et al., 2020). When cells are stimulated by endogenous and exogenous, IκB causes phosphorylation and degradation, resulting in the release of NF-κB and subsequently phosphorylation of p65 (Cai et al., 2021). Then, NF-κB is translocated to the nucleus from the cytoplasm and regulate the target gene (Chen et al., 2019). Moreover, mitogen-activated protein kinase (MAPK) signaling pathways are another important protein to regulate the inflammation process (Lee H. -J. et al., 2018; Zhao et al., 2021). MAPK pathway, composed of p38, ERK, and JNK, can also be activated by various stimuli. MAPK pathway is closely related to the NF-κB activation (Zhao et al., 2021). Thus, inhibiting NF-κB and MAPK pathways is the effective strategy to suppress the inflammatory response (Zhou et al., 2018; Moon et al., 2019).
Silibinin, the major active constituent of Silybum marianum, is one kind of polyphenolic flavonoid that has been used as medicine for a long time and is well known for its hepatoprotective and anti-carcinogenic effects (Hussain et al., 2020; Fallah et al., 2021). Moreover, silibinin, has been reported to have effective anti-inflammatory effects (Chen et al., 2020). Silibinin could down-regulate pro-inflammatory mediators in various inflammation-related disease models by the suppression of signaling pathway activations, such as NF-κB, MAPK, and STAT-3 (Rengasamy et al., 2019; Villegas-Aguilar et al., 2020). On the other hand, capsaicin is the active ingredient in chili peppers (Li et al., 2020) that has been used in lots of pharmacological researches to reveal its various physiological processes, such as cardiovascular (Wang et al., 2021), respiratory (Hernández-Pérez et al., 2020), and inflammation (Wang et al., 2021). Now, capsaicin has been verified to attenuate inflammation in some inflammation models (Hernández-Pérez et al., 2020).
Up to now, combination therapy has been considered as an effective strategy to attenuate inflammation (Bruni et al., 2018). The combination of different anti-inflammation agent results in the enhancement of pharmacological activity by acting on multi-target t(Hong et al., 2021). A combination of silibinin with brostallicin could increase the anti-apoptotic protein Bcl-2 and decrease in caspase 3 activity (Pook et al., 2006). Silibinin in combination with Pu-erh tea extract could relieve non-alcoholic fatty liver disease (Hu et al., 2017). In this study, the enhanced anti-inflammatory effects of the silibinin and capsaicin combination were investigated in LPS-induced RAW264.7 cells.
Experimental
Materials
Silibinin (purity ≥98% by HPLC) and capsaicin (purity ≥98% by HPLC) were purchased from Shanghai Yuanye Biological Technology Co., Ltd. (Shanghai, China). Fetal bovine serum (FBS) and Dulbecco’s Modified Eagle’s Medium (DMEM) were purchased from Gibco (Grand Island, NY, United States). Lipopolysaccharide (LPS, from Escherichia coli O111:B4) and 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide (MTT) dye were obtained from Sigma-Aldrich (St. Louis, MO, United States). Antibodies against COX-2 (12282S), p-JNK (9251S), p-ERK (9101S), ERK (9102S), p-p38 (9211S), p38 (9212S), and p65 (8242S) were purchased from Cell Signaling Technology (Danvers, MA, United States). The IL-6 (mouse) ELISA Kit and murine TNF-α Pre-Coated ELISA kit were purchased from Cayman Chemical Co., Ltd. (Ann Arbor, MI, United States). The Griess reagent (modified) was obtained from Sigma-Aldrich. NE-PER Nuclear Extraction Kit was obtained from Thermo Fisher Scientific Inc. (Waltham, MA, United States).
Cell Culture
RAW264.7 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, United States). The cells were cultured in DMEM containing with 10% FBS and 1% P/S in an incubator maintained at 37°C under humidified atmospheric conditions consisting of 5% CO2.
Cell Viability Assay
RAW264.7 cells (5,000 cells/well) were seeded in 96-well plates and cultivated for 24 h. After silibinin and capsaicin with different concentrations were added for 2 h, the cells were treated with or without LPS (1 μg/ml) for 24 h. To discard the medium, MTT reagent was added, and incubated for 3 h. Formazan crystals were dissolved in 100 μL of DMSO, and then absorbance at 490 nm was measured.
NO Assay
After being plated into 96-well plates and incubated for 24 h, the cells were treated with silibinin and/or capsaicin for 2 h and subsequently co-treated with LPS (1 μg/ml) for 24 h. The medium was mixed with the Griess reagent (1: 1) and placed in the dark for 15 min. Then, the absorbance was measured at 540 nm.
Synergistic Effect Analysis
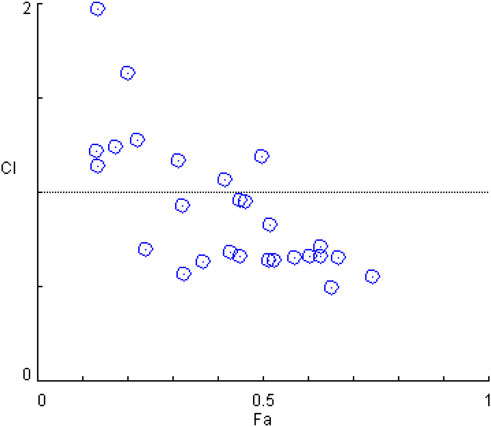
The synergistic effect between silibinin and capsaicin was analyzed based on NO assay data by the CompuSyn software 2.0. The NO assay data of silibinin and capsaicin alone or the combination were entered into the CompuSyn software, then combination index (CI) values could be produced in the default mode. The CI values revealed the additive, synergism, or antagonism of the combinations of the two drugs at different doses.
ELISA Assay
After being plated into 24-well plates for 24 h and the cells were treated with silibinin and/or capsaicin for 2 h and subsequently induced with LPS (1 μg/ml) for 24 h. Then, the supernatant was detected for TNF-α and IL-6 levels according to the manufacturer’s instructions.
Western Blot Analysis
After RAW264.7 cells (100,000 cells/dish) were cultured in a 100 mm dish for 24 h, cells were treated with silibinin and/or capsaicin for 2 h, and then treated with LPS (1 μg/ml). Then cells were collected, lysed with lysis buffer for 10 min at 4°C, and centrifuged for 10 min at 12,000 g to obtain supernatant. After determining the protein concentrations, supernatant was boiled with loading buffer for 10 min. Then the sample was separated by SDSPAGE (8%) and transfer to the nitrocellulose membrane, followed by the block for 1 h in non-fat milk (5%), incubated with the primary antibodies (COX-2, NF-κB p65, p-p38, p38, p-ERK, ERK, p-JNK, β-actin, and Lamin B) overnight at 4°C, and incubated with the secondary antibody for 1 h. Then, the sample was visualized by enhanced chemiluminescence (ECL) detection kits.
Statistical Analysis
Data were expressed as the mean ± SEM. We used the SPSS software to perform the statistical analysis. Data were analyzed using the one-way ANOVA. The results were considered statistically significant when p < 0.05.
Results and Discussion
Cytotoxicity Assay of Silibinin and Capsaicin
The cytotoxicity assay of silibinin and capsaicin was firstly evaluated using the MTT assay and the results are shown in Figure 1. It could be seen that silibinin (4–32 μM) and capsaicin (0.25–16 μM) showed no cytotoxicity to RAW264.7 cells both with or without the stimulation of LPS.
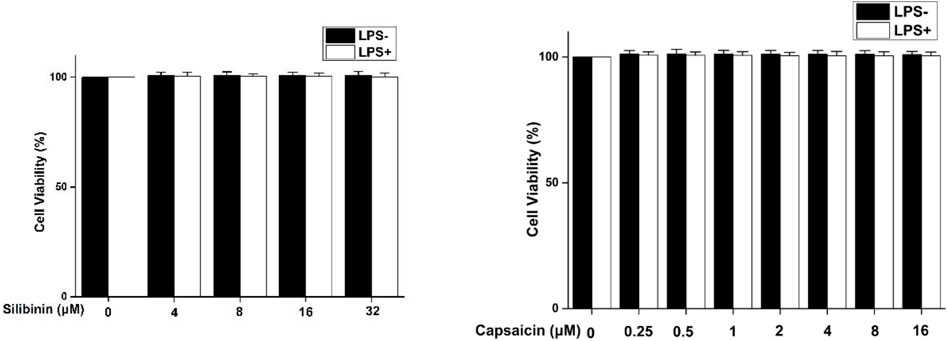
FIGURE 1. Cytotoxicity of silibinin and capsaicin on RAW264.7 cells. RAW264.7 cells were seeded in 96-well plates and cultivated for 24 h. After silibinin and capsaicin with different concentrations were added for 2 h, the cells were treated with or without LPS (1 μg/ml) for 24 h. To discard the medium, the MTT reagent was added , and incubated for 3 h. Formazan crystals were dissolved in 100 μL of DMSO, and then absorbance at 490 nm was measured.
Effects of Silibinin and Capsaicin on LPS-Induced NO
NO, secreted at inflammatory sites, is the important cellular mediato (Sugimoto et al., 2019). The effect of silibinin and capsaicin on the LPS-induced NO production was assayed. The results (Figure 2) showed that silibinin (4–32 μM) and capsaicin (0.25–16 μM) could effectively inhibit NO production induced by LPS (Figures 2A,B). The silibinin and capsaicin combination presented stronger inhibitory than silibinin or capsaicin alone (Figures 2C–F), suggesting that there was a synergistic effect between silibinin and capsaicin.
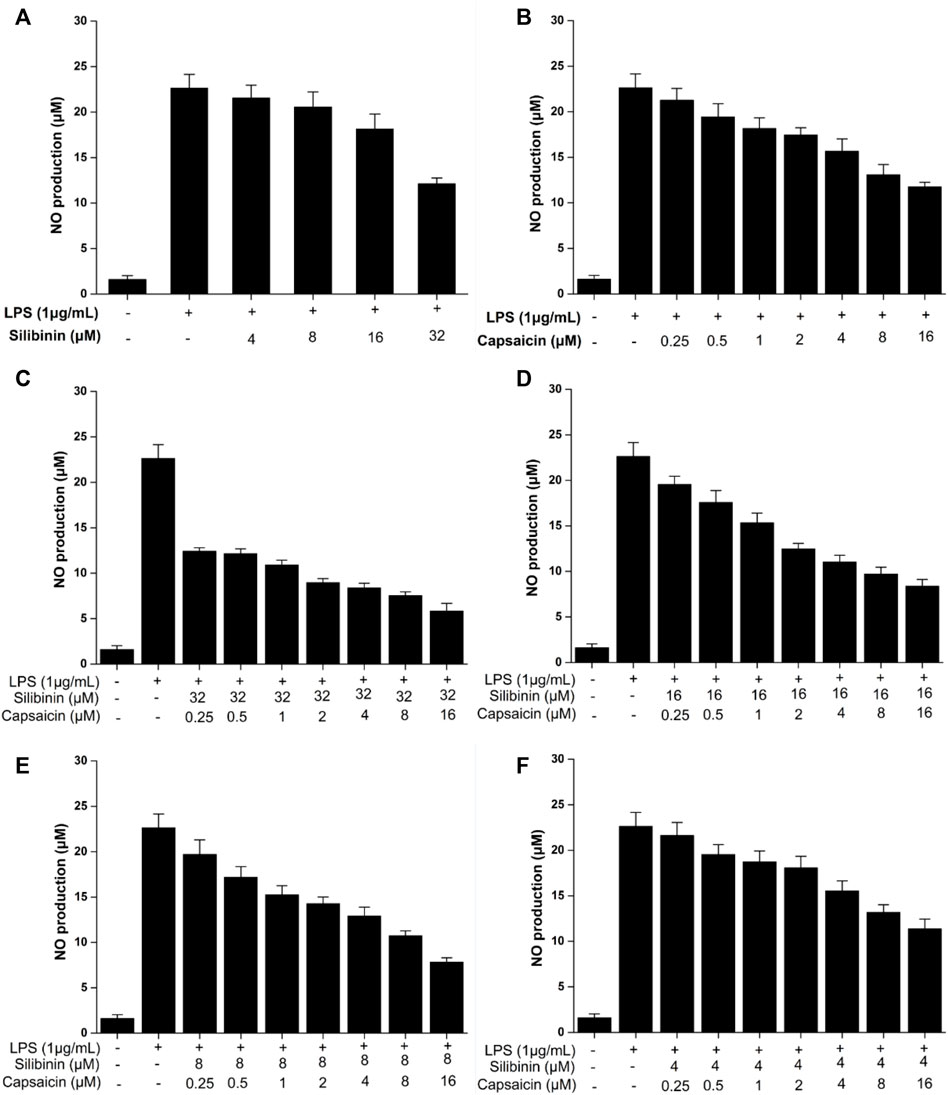
FIGURE 2. Inhibitory effect of silibinin (4–32 μM) on the LPS-induced NO (A). Inhibitory effect of capsaicin (0.25–16 μM) on the LPS-induced NO (B). Inhibitory effect of silibinin and capsaicin combination at different concentration ratios on the LPS-induced NO (C–F). After being plated into 96-well plates and incubated for 24 h, the cells were treated with silibinin and/or capsaicin for 2 h and subsequently co-treated with LPS (1 μg/ml) for 24 h. The medium wasmixed with the Griess reagent (1:1) and placed in the dark for 15 min. Then the absorbance was measured at 540 nm.
Synergistic Effect Analysis
The synergistic effect of silibinin and capsaicin was analyzed based on the results of NO assay data. The CI value is a quantitative parameter on the degree of drug interaction, including synergism, additive effect, and antagonism. Cl < 1 indicated that there was synergistic effect, and the lower CI means the higher synergistic effect. Figure 3 presented the CI values calculated by the CompuSyn software 2.0. The Silibinin and capsaicin combination had CI values ranging from 0.50 to 3.80. Among them, silibinin (8 μM) and capsaicin (16 μM) combination had the lowest CI value of 0.50, meaning that silibinin (8 μM) and capsaicin (16 μM) combination had the best synergistic effect. Then, the mechanistic studies of The silibinin and capsaicin combination were investigated using the optimal dose ratio.
Effect of Silibinin and Capsaicin on TNF-α and IL-6
The secretion of pro-inflammatory cytokines, such as TNF-α and IL-6, is important to relieve exogenous stimuli and repair the injured tissues (Yang et al., 2020). So, the effect of silibinin and capsaicin on TNF-α and IL-6 was detected using ELISA assay and the results are shown in Figure 4. Treatment with silibinin and capsaicin alone could effectively inhibit the LPS-induced TNF-α and IL-6. While, the silibinin and capsaicin combination showed a stronger inhibitory effect on TNF-α and IL-6 than silibinin and capsaicin alone.
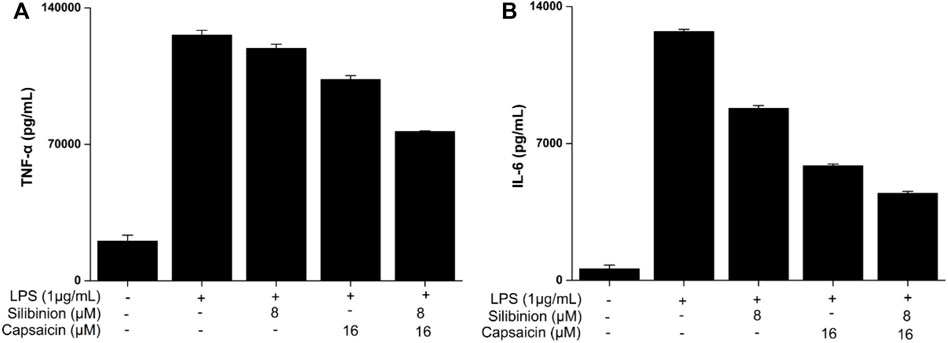
FIGURE 4. Effect of silibinin and capsaicin on LPS-induced TNF-α (A) and IL-6 (B). After being plated into 24-well plates for 24 h and the cells were treated with silibinin and/or capsaicin for 2 h and subsequently induced with LPS (1 μg/ml) for 24 h. Then, the supernatant was detected for TNF-α and IL-6 levels according to the manufacturer’s instructions.
Effect of Silibinin and Capsaicin on LPS-Induced COX-2
COX-2 has been reported to play an important role in the inflammatory process (Wang et al., 2019). Based on the significant inhibition of NO by silibinin in combination with capsaicin or alone, their effect on COX-2 was assayed. As shown in Figure 5, LPS treatment obviously induced and increased COX-2 expression. Treatment with silibinin and capsaicin alone could inhibit the COX-2 expression with a reduction of 12.5 and 76.9%, respectively. The Silibinin and capsaicin combination obviously suppressed the COX-2 expression with a reduction of 76.9%. The results showed that capsaicin (16 μM) had stronger effect than silibinin (8 μM), while the combination did present higher inhibition that capsaicin. That is to say that the combination of silibinin and capsaicin had little enhancement on the reduction of COX-2.
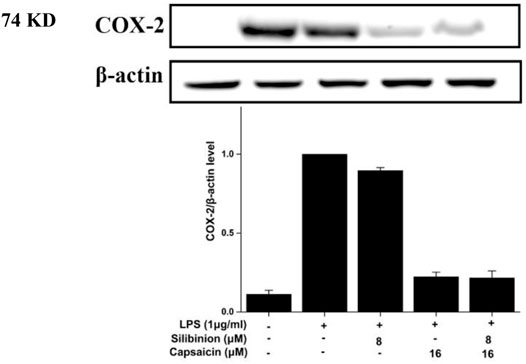
FIGURE 5. Effect of silibinin and capsaicin on the LPS-induced COX-2. After separated by SDSPAGE (8%), transfer to the nitrocellulose membrane, and block for 1 h in non-fat milk (5%), the sample was incubated with the primary antibodies COX-2 overnight at 4°C, and subsequently incubation with the secondary antibody for 1 h.
Effect of Silibinin and Capsaicin on the LPS-Induced NF-κB Pathway
NF-κB, an essential transcription factor, was involved in proinflammatory responses. When stimulated, NF-κB (p65) was released from its bound complex and translocated into the nucleus from the cytosol, then regulating pro-inflammatory genes production (Lee S. -B. et al., 2018). As observed from Figure 6, silibinin and capsaicin pre-treatment alone could down-regulate the activation of LPS-induced NF-κB (p65) by 44.8 and 51.7%, respectively. Simultaneously, the silibinin and capsaicin combination obviously down-regulated the NF-κB activation by 62.1%, which was higher than that of silibinin and capsaicin alone, revealing the synergistic effects of the two compounds. Chen et al.‘s research revealed that silibinin could enhance the inhibitory effect of thymol on LPS-induced NF-κB (p65) activation (Chen et al., 2020). Our results revealed that silibinin also improved the inhibitory effect of capsaicin on LPS-induced NF-κB (p65) activation.
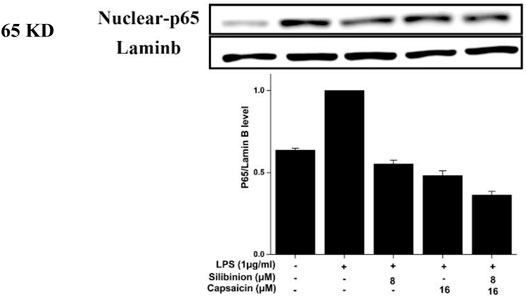
FIGURE 6. Effect of silibinin and capsaicin on the LPS-induced NF-κB pathway. After separated by SDSPAGE (8%), transfer to the nitrocellulose membrane, and block for 1 h in non-fat milk (5%), the sample incubated with the primary antibodies p65 overnight at 4°C, and subsequently incubation with the secondary antibody for 1 h.
Effect of Silibinin and Capsaicin on LPS-Induced MAPK Pathway
Finally, the effect of silibinin and capsaicin on the LPS-induced MAPK pathway was assayed by evaluate the change of p-p38 and p-ERK. As can be seen in Figure 7, LPS treatment caused obvious over expressions on p-p38 that could be reduced by the treatment of silibinin (7.8% reduction) and capsaicin (9.4% reduction) alone. The Silibinin and capsaicin combination could effectively down-regulate the p-p38 expression (31.3% reduction). In contrast, silibinin, capsaicin, and silibinin in combination with capsaicin reduced p-ERK expression by 3.3, 4.9, and 62.3%, respectively. Previous reports indicated that silibinin and capsaicin could inhibit inflammation by suppressing the MAPK pathway activation. Our results also revealed that silibinin and capsaicin had a synergistic effect on the suppression of the LPS-induced MAPK pathway.
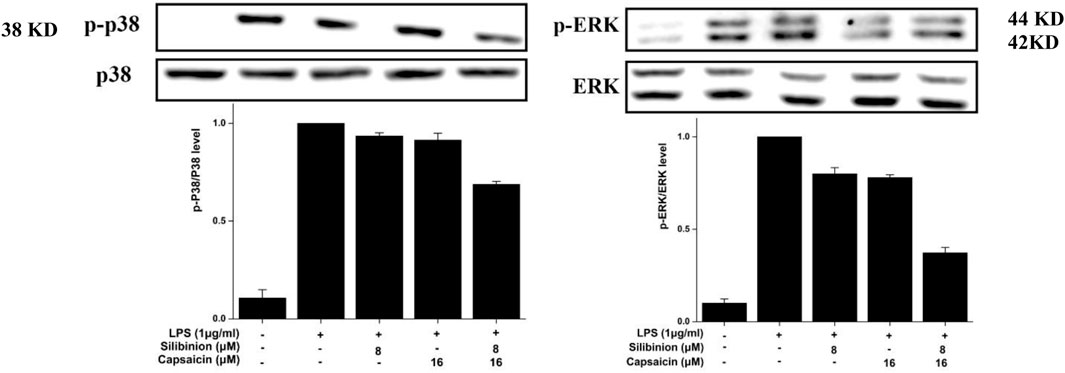
FIGURE 7. Effect of silibinin and capsaicin on the LPS-induced MAPK pathway. After separated by SDSPAGE (8%), transfer to the nitrocellulose membrane, and block for 1 h in non-fat milk (5%), the sample was incubated with the primary antibodies p-p38, p38, p-ERK, and ERK overnight at 4 °C, respectively, and subsequently incubation with the secondary antibody for 1 h.
Conclusion
In summary, we investigated the enhanced anti-inflammatory effects of the silibinin and capsaicin combination in LPS-induced RAW264.7 cells and the results showed that the silibinin and capsaicin combination had the best synergistic effect at a concentration ratio of 8–16 μM. The combination could effectively inhibit LPS- induced over the production of NO, TNF-α, IL-6, and COX-2. Their effective anti-inflammatory effect was related with the inhibiton of NF-κB and MAPK activities (Figure 8). Thus, it was concluded that the silibinin and capsaicin combination had a good anti-inflammatory effect and was expected to be used in the prevention and treatment of inflammation-related diseases.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
YZ, and JC contributed to cells. XW, XZ, CH, YK, JinL, JiaL, YH, and XZ contributed to the characterization and analysis. CL supervised the work and prepared the manuscript.
Funding
This work was financially supported by the Department of Education of Guangdong Province (Nos. 333 2019KZDXM035, 2021ZDZX4041, 2021KTSCX135, and 2021KCXTD044) and Jiangmen City Science and Technology Basic Research Project (Grant No. 2020030101030005457).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.934541/full#supplementary-material
References
Akanda, M., Kim, I.-S., Ahn, D., Tae, H.-J., Nam, H.-H., Choo, B.-K., et al. (2018). Anti-Inflammatory and Gastroprotective Roles of Rabdosia Inflexa through Downregulation of Pro-inflammatory Cytokines and MAPK/NF-κB Signaling Pathways. Int. J. Mol. Sci. 19, 584. doi:10.3390/ijms19020584
Bai, J., Zhang, Y., Tang, C., Hou, Y., Ai, X., Chen, X., et al. (2021). Gallic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Inflammation-Related Diseases. Biomed. Pharmacother. 133, 110985. doi:10.1016/j.biopha.2020.110985
Bruni, N., Della Pepa, C., Oliaro-Bosso, S., Pessione, E., Gastaldi, D., and Dosio, F. (2018). Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 23, 2478. doi:10.3390/molecules23102478
Cai, Y., Zhang, Y., Chen, H., Sun, X.-H., Zhang, P., Zhang, L., et al. (2021). MicroRNA-17-3p Suppresses NF-κB-Mediated Endothelial Inflammation by Targeting NIK and IKKβ Binding Protein. Acta Pharmacol. Sin. 42, 2046–2057. doi:10.1038/s41401-021-00611-w
Chen, J., Li, D.-L., Xie, L.-N., Ma, Y.-R., Wu, P.-P., Li, C., et al. (2020). Synergistic Anti-inflammatory Effects of Silibinin and Thymol Combination on LPS-Induced RAW264.7 Cells by Inhibition of NF-κB and MAPK Activation. Phytomedicine 78, 153309. doi:10.1016/j.phymed.2020.153309
Chen, K.-L., Li, L., Li, C.-M., Wang, Y.-R., Yang, F.-X., Kuang, M.-Q., et al. (20192019). SIRT7 Regulates Lipopolysaccharide-Induced Inflammatory Injury by Suppressing the NF-κB Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 1–15. doi:10.1155/2019/3187972
Fallah, M., Davoodvandi, A., Nikmanzar, S., Aghili, S., Mirazimi, S. M. A., Aschner, M., et al. (2021). Silymarin (Milk Thistle Extract) as a Therapeutic Agent in Gastrointestinal Cancer. Biomed. Pharmacother. 142, 112024. doi:10.1016/j.biopha.2021.112024
Hernández‐Pérez, T., Gómez‐García, M. d. R., Valverde, M. E., and Paredes‐López, O. (2020). Capsicum Annuum (Hot Pepper): An Ancient Latin‐American Crop with Outstanding Bioactive Compounds and Nutraceutical Potential. A Review. Compr. Rev. Food Sci. Food Saf. 19, 2972–2993. doi:10.1111/1541-4337.12634
Hong, S., Dia, V. P., and Zhong, Q. (2021). Synergistic Anti-inflammatory Activity of Apigenin and Curcumin Co-encapsulated in Caseins Assessed with Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Int. J. Biol. Macromol. 193, 702–712. doi:10.1016/j.ijbiomac.2021.10.153
Hu, W.-Y., Ma, X.-H., Zhou, W.-Y., Li, X.-X., Sun, T.-T., and Sun, H. (2017). Preventive Effect of Silibinin in Combination with Pu-Erh Tea Extract on Non-alcoholic Fatty Liver Disease in Ob/ob Mice. Food Funct. 8, 1105–1115. doi:10.1039/c6fo01591c
Huang, P., Hong, J., Mi, J., Sun, B., Zhang, J., Li, C., et al. (2022). Polyphenols Extracted from Enteromorpha Clathrata Alleviates Inflammation in Lipopolysaccharide-Induced RAW 264.7 Cells by Inhibiting the MAPKs/NF-κB Signaling Pathways. J. Ethnopharmacol. 286, 114897. doi:10.1016/j.jep.2021.114897
Hussain, A., Bourguet-Kondracki, M.-L., Hussain, F., Rauf, A., Ibrahim, M., Khalid, M., et al. (2020). The Potential Role of Dietary Plant Ingredients against Mammary Cancer: a Comprehensive Review. Crit. Rev. Food Sci. Nutr. 62, 2580–2605. doi:10.1080/10408398.2020.1855413
Irmscher, S., Brix, S. R., Zipfel, S. L. H., Halder, L. D., Mutlutürk, S., Wulf, S., et al. (2019). Serum FHR1 Binding to Necrotic-type Cells Activates Monocytic Inflammasome and Marks Necrotic Sites in Vasculopathies. Nat. Commun. 10, 2961. doi:10.1038/s41467-019-10766-0
Kase, N. G., Gretz Friedman, E., Brodman, M., Kang, C., Gallagher, E. J., and Leroith, D. (2020). The Midlife Transition and the Risk of Cardiovascular Disease and Cancer Part I: Magnitude and Mechanisms. Am. J. Obstetrics Gynecol. 223, 820–833. doi:10.1016/j.ajog.2020.05.051
Lee, H.-J., Ko, H.-J., Song, D.-K., and Jung, Y.-J. (2018). Lysophosphatidylcholine Promotes Phagosome Maturation and Regulates Inflammatory Mediator Production through the Protein Kinase A-Phosphatidylinositol 3 Kinase-P38 Mitogen-Activated Protein Kinase Signaling Pathway during Mycobacterium tuberculosis Infection in Mouse Macrophages. Front. Immunol. 9, 920. doi:10.3389/fimmu.2018.00920
Lee, S.-B., Shin, J.-S., Han, H.-S., Lee, H.-H., Park, J. C., and Lee, K.-T. (2018). Kaempferol 7-O-β-D-Glucoside Isolated from the Leaves of Cudrania Tricuspidata Inhibits LPS-Induced Expression of Pro-inflammatory Mediators through Inactivation of NF-κB, AP-1, and JAK-STAT in RAW 264.7 Macrophages. Chemico-biological Interact. 284, 101–111. doi:10.3389/fimmu.2018.0092010.1016/j.cbi.2018.02.022
Li, R., Lan, Y., Chen, C., Cao, Y., Huang, Q., Ho, C.-T., et al. (2020). Anti-obesity Effects of Capsaicin and the Underlying Mechanisms: a Review. Food Funct. 11, 7356–7370. doi:10.1039/d0fo01467b
Luo, Z., Zheng, B., Jiang, B., Xue, X., Xue, E., and Zhou, Y. (2019). Peiminine Inhibits the IL-1β Induced Inflammatory Response in Mouse Articular Chondrocytes and Ameliorates Murine Osteoarthritis. Food Funct. 10, 2198–2208. doi:10.1039/C9FO00307J
Moon, S. W., Ahn, C.-B., Oh, Y., and Je, J.-Y. (2019). Lotus (Nelumbo nucifera) Seed Protein Isolate Exerts Anti-inflammatory and Antioxidant Effects in LPS-Stimulated RAW264.7 Macrophages via Inhibiting NF-κB and MAPK Pathways, and Upregulating Catalase Activity. Int. J. Biol. Macromol. 134, 791–797. doi:10.1016/j.ijbiomac.2019.05.094
Pook, S.-H., Toh, C.-K., and Mahendran, R. (2006). Combination of Thiol Antioxidant Silibinin with Brostallicin Is Associated with Increase in the Anti-apoptotic Protein Bcl-2 and Decrease in Caspase 3 Activity. Cancer Lett. 238, 146–152. doi:10.1016/j.canlet.2005.07.002
Rengasamy, K. R. R., Khan, H., Gowrishankar, S., Lagoa, R. J. L., Mahomoodally, F. M., Khan, Z., et al. (2019). The Role of Flavonoids in Autoimmune Diseases: Therapeutic Updates. Pharmacol. Ther. 194, 107–131. doi:10.1016/j.pharmthera.2018.09.009
Sugimoto, M. A., Vago, J. P., Perretti, M., and Teixeira, M. M. (2019). Mediators of the Resolution of the Inflammatory Response. Trends Immunol. 40, 212–227. doi:10.1016/j.it.2019.01.007
Villegas-Aguilar, M. d. C., Fernández-Ochoa, Á., Cádiz-Gurrea, M. d. l. L., Pimentel-Moral, S., Lozano-Sánchez, J., Arráez-Román, D., et al. (2020). Pleiotropic Biological Effects of Dietary Phenolic Compounds and Their Metabolites on Energy Metabolism, Inflammation and Aging. Molecules 25, 596. doi:10.3390/molecules25030596
Wang, F., Xue, Y., Fu, L., Wang, Y., He, M., Zhao, L., et al. (2021). Extraction, Purification, Bioactivity and Pharmacological Effects of Capsaicin: A Review. Crit. Rev. Food Sci. Nutr., 1–29. doi:10.1080/10408398.2021.1884840
Wang, H. M.-D., Fu, L., Cheng, C. C., Gao, R., Lin, M. Y., Su, H. L., et al. (2019). Inhibition of LPS-Induced Oxidative Damages and Potential Anti-inflammatory Effects of Phyllanthus Emblica Extract via Down-Regulating NF-κB, COX-2, and iNOS in RAW 264.7 Cells. Antioxidants 8, 270. doi:10.3390/antiox8080270
Wang, Q., Huang, J., Zheng, Y., Guan, X., Lai, C., Gao, H., et al. (2022). Selenium-enriched Oolong Tea (Camellia Sinensis) Extract Exerts Anti-inflammatory Potential via Targeting NF-κB and MAPK Pathways in Macrophages. Food Sci. Hum. Wellness 11, 635–642. doi:10.1016/j.fshw.2021.12.020
Yang, H., Wu, L., Deng, H., Chen, Y., Zhou, H., Liu, M., et al. (2020). Anti-inflammatory Protein TSG-6 Secreted by Bone Marrow Mesenchymal Stem Cells Attenuates Neuropathic Pain by Inhibiting the TLR2/MyD88/NF-κB Signaling Pathway in Spinal Microglia. J. Neuroinflammation 17, 1–21. doi:10.1186/s12974-020-1731-x
Yu, H., Lin, L., Zhang, Z., Zhang, H., and Hu, H. (2020). Targeting NF-κB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Sig Transduct. Target Ther. 5, 1–23. doi:10.1038/s41392-020-00312-6
Zhao, H., Wu, L., Yan, G., Chen, Y., Zhou, M., Wu, Y., et al. (2021). Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Sig Transduct. Target Ther. 6, 1–46. doi:10.1038/s41392-021-00658-5
Zhou, M.-M., Zhang, W.-Y., Li, R.-J., Guo, C., Wei, S.-S., Tian, X.-M., et al. (2018). Anti-inflammatory Activity of Khayandirobilide A from Khaya Senegalensis via NF-κB, AP-1 and P38 MAPK/Nrf2/HO-1 Signaling Pathways in Lipopolysaccharide-Stimulated RAW 264.7 and BV-2 Cells. Phytomedicine 42, 152–163. doi:10.1016/j.phymed.2018.03.016
Keywords: anti-inflammatory, silibinin, capsaicin, NF-κB, MAPK
Citation: Zheng Y, Chen J, Wu X, Zhang X, Hu C, Kang Y, Lin J, Li J, Huang Y, Zhang X and Li C (2022) Enhanced Anti-Inflammatory Effects of Silibinin and Capsaicin Combination in Lipopolysaccharide-Induced RAW264.7 Cells by Inhibiting NF-κB and MAPK Activation. Front. Chem. 10:934541. doi: 10.3389/fchem.2022.934541
Received: 06 May 2022; Accepted: 23 May 2022;
Published: 30 June 2022.
Edited by:
Xi Zheng, Rutgers, The State University of New Jersey, United StatesReviewed by:
Zhong Liu, Jinan University, ChinaRuifang Zhang, Guangdong University of Technology, China
Copyright © 2022 Zheng, Chen, Wu, Zhang, Hu, Kang, Lin, Li, Huang, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Li, d3l1Y2hlbWxjQDEyNi5jb20=
†These authors have contributed equally to this work
 Yingying Zheng†
Yingying Zheng† Chunmei Hu
Chunmei Hu Jing Lin
Jing Lin Chen Li
Chen Li