- 1College of Basic Medical Sciences, College of Pharmacy, Academy of Integrative Medicine, Dalian Medical University, Dalian, China
- 2Second Affiliated Hospital of Dalian Medical University, Dalian, China
- 3State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, China
- 4Department of Chemistry, University of Bath, Bath, United Kingdom
- 5School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, China
β-Glucosidase (β-Glc) is an enzyme capable of the selective hydrolysis of the β-glycosidic bond of glycosides and glycans containing glucose. β-Glc expressed by intestinal microbiota has attracted increasing levels of interest, due to their important roles for the metabolism of exogenous substances in the gut. Using the 2-((6-hydroxy-2,3-dihydro-1H-xanthen-4-yl)methylene)malononitrile fluorophore (DXM-OH, λem 636 nm) and the recognition group β-Glucose, an enzymatic activatable turn-on fluorescent probe (DXM-Glc) was developed for the selective and sensitive sensing of β-Glc. In addition, DXM-Glc could be used to sense endogenous β-Glc in living fungal cells. Using DXM-Glc, Pichia terricola M2 was identified as a functional intestinal fungus with β-Glc expression. P. terricola M2 could transform the flavone glycoside Icariin to Icariside Ⅱ efficiently, which confirmed the metabolism of glycosides in the gut mediated by fungi. Furthermore, Icariside Ⅱ could inhibit the proliferation of human endometrial cancer cells (RL 95-2 and ishikawa) significantly, suggesting the metabolic activation of Icariin by intestinal fungi in vivo. Therefore, DXM-Glc as a probe for β-Glc provided a novel technique for the investigation of the metabolism of bioactive substances by intestinal microbiota.
Introduction
β-Glucosidase (β-d-glucopyranoside glucohydrolase, β-Glc) [E.C.3.2.1.21] is a well-known functional enzyme for the hydrolysis of the glycosidic bond of carbohydrate moieties. As a biocatalyst, β-Glc was been widely used in industrial preparations, exhibiting the advantages of improved conversion rate, mild reaction conditions, while being environmental-friendly, and enabling the simple purification of products(Hong, et al., 2012; Hati, et al., 2015; Zhong, et al., 2016; Abdella, et al., 2018).
Moreover, gut bacteria (e.g. Streptococcus thermophilus, L. acidophilus, L. delbrueckii, L. casei, L. plantarum, L. fermentum) express β-Glc, and participate in the metabolism of glycosides and carbohydrates from foods and pharmaceuticals(Donkor et al., 2008; Shen, et al., 2013). The glycosides from plants or foods exhibit poor intestinal absorption, however, the corresponding aglycones which are the metabolites generated by intestinal microbiota are more permeable and bioactive(Rekha et al., 2011). The aglycone metabolites are the main forms transported across the epithelial membrane and are likely to be the active forms in vivo(Quan et al., 2012a; Quan et al., 2012b). Therefore, intestinal bacteria or fungi with active β-Glc play an important role in the absorption, and metabolic activation of glycosides and carbohydrates. As such, the characterization and further exploration of intestinal β-Glc requires more investigation.
On the basis of the special catalytic characteristics of β-Glc, several fluorescent carbon dots have been developed as fluorescent biosensors for the sensitive determination of Glucosidase, including α-Glc and β-Glc(Kalaiyarasan, et al., 2019; Kong, et al., 2020; Liu, et al., 2020; Wang, et al., 2020). While, enzymatic fluorescent probes have been widely used to sense biological enzymes, exhibiting the advantages of high sensitivity, high selectivity and facilitate in vivo/in vitro imaging(Feng, et al., 2021; Li, et al., 2020; Liu, et al., 2021; Ning, et al., 2019; Tian, et al., 2021; Yan, et al., 2022; Zhang, et al., 2021). Using the excited-state intramolecular proton transfer (ESIPT) phenomenon, flavonol derivatives have been used as fluorescent indicators for β-Glc activity(Reszka, et al., 2020). However, an enzymatic activatable long wavelength fluorescent probe is still desirable for the sensing and determination of intestinal β-Glc.
In this study, a turn-on fluorescent probe (DXM-Glc) has been developed determining β-Glc activity sensitively and selectively. When combined with cultures of intestinal fungi, visual identification of target fungus expressing β-Glc was successfully achieved. Furthermore, using the identified intestinal fungus, Icarisid Ⅱ was released as a metabolite of Icariin, which exhibited significant cytotoxicity toward endometrial cancer cells.
Materials and Methods
Materials and Apparatus
Chromatographic methanol for HPLC was purchased from sigma-aldrich (MERCK, United States). All of the chemical reagents and solvents for the synthesis were obtained from Tianjin Kemiou Chemical Reagent Co., Ltd (Tianjin, P.R. China). The agar, glucose, Na2HPO4, and NaH2PO4 were produced by Dalian Meilun Biotechnology Co., Ltd (China). β-Glucosidase (β-Glc), α-Glucosidase (α-Glc), Lipase, Human serum albumin (HSA), β-Galactosidase (GAL), and β-N-Acetylglucosaminidase (NAG) were obtained from sigma-aldrich (MERCK, United States). Recombinant human carboxylesterases (CES1b, CES1c, and CES2) were purchased from Corning Incorporated Life Sciences.
NMR spectra were measured using Bruker-600 with tetramethylsilane (TMS) as the internal standard (Bruker, United States). HRESIMS data were acquired on an AB SciexX500r TOF mass spectrometer (AB Science, United States). Fluorescence microscopic imaging was conducted using a Leica Confocal Microscope (Leica Microsystems, Germany). The bioassay solutions in 96-well plates were analyzed using a BioTek Synergy H1 microplate reader (BioTek, United States). HPLC-UV analysis was performed using a Waters e2695 (Waters, United States).
Synthesis of Fluorescent Probe DXM-Glc
DXM-Glc was synthesized according to the route shown in Supplementary Scheme S1.
In general, to a solution of DXM-OH (55.2 mg, 0.20 mmol) in 10 ml of dry DCM, 2,3,4,6-tetra-O-acetyl-α-d-glucosyl bromide (164.4 mg, 0.4 mmol), Cs2CO3 (163 mg, 0.5 mmol) were added. The reaction mixture was stirred at room temperature overnight, filtered, and evaporated. The residue was dissolved in 10 ml of MeOH, and CH3ONa (108 mg, 2 mmol) was added. The mixture was stirred at room temperature for 1 h, neutralized with 1M HCl, filtered, and evaporated. The residue was purified by HPLC to afford 20.5 mg DXM-Glc as dark red powder. Yield: 23.4% in two steps. The chemical structure of DXM-Glc was determined by 1H, 13C NMR and HR-MS data (Supplementary Figures S1-S3).
1H NMR (600 MHz, DMSO-d6) δH 8.17 (s, 1H), 7.46 (d, J = 8.5 Hz, 1H), 7.36 (s, 1H), 7.25 (s, 1H), 6.96 (d, J = 7.1 Hz, 1H), 5.41 (d, J = 4.7 Hz, 1H), 5.15 (d, J = 4.0 Hz, 1H), 5.08 (d, J = 4.8 Hz, 1H), 5.04 (d, J = 7.5 Hz, 1H), 4.59 (s, 1H), 3.69 (d, J = 8.6 Hz, 1H), 3.49 (d, J = 5.7 Hz, 1H), 3.25 (d, J = 5.2 Hz, 1H), 3.19 (d, J = 4.8 Hz, 1H), 3.12 (s, 2H), 2.75 (s, 2H), 2.63 (s, 2H), 1.82–1.69 (m, 2H). 13C NMR (150 MHz, DMSO-d6) δC 160.22, 159.51, 153.74, 150.53, 132.39, 128.88, 126.82, 118.07, 116.63, 116.11, 114.61, 109.94, 103.66, 100.39, 77.55, 76.96, 73.61, 69.98, 68.11, 61.04, 28.57, 24.80, 20.44. HRMS cacld for [M + H]+ 439.1500, found m/z 439.1508.
Hydrolysis of DXM-Glc Catalyzed by β-Glucosidase
In a phosphate buffer solution (pH 7.4, 100 mM), β-Glc (100 μg/ml) and DXM-Glc (10 μM, DMSO <1%, v/v) were co-incubated at 37°C for 30 min. Then, dimethyl sulfoxide (33%, v/v) was added to inactivate the β-Glc activity and terminate the enzymatic reaction. When the denatured proteins were precipitated by centrifugation at 20,000 ×g for 20 min, the fluorescence intensity corresponding to the production of DXM-OH was measured using a Microplate reader with an excitation wavelength of 600 nm and emission wavelength of 636 nm.
The fluorescence response of DXM-Glc (10 μM) toward β-Glc with different concentrations (0, 2, 5, 10, 20, 40, 60, 80, 100 μg/ml) has been recorded using an excitation laser at 600 nm. The linear relationship between the fluorescence intensity and β-Glc concentrations was calculated using linear regression equation. The limit of detection (LOD) was calculated using 3σ/slope.
Interference by Various Species on the Fluorescence Emission of DXM-Glc
Various ions including K+, Na+, Mg2+, Ca2+, Ba2+, Zn2+, Cu2+, Ni2+, Sn2+, Mn2+, CO32-, SO42- and amino acids (200 μM) were co-incubated with DXM-Glc in phosphate buffer at 37°C for 30 min, respectively. Then, the fluorescence intensity was measured using a Microplate reader (λex 600 nm/λem 636 nm).
The selectivity of DXM-Glc toward β-Glc was evaluated in the presence of other biological enzymes β-Glc, α-Glc, HSA, GAL, NAG, CES1b, CES1c, and CES2 (100 μg/ml). The fluorescence responses of DXM-Glc toward these enzymes were measured using Microplate reader (λex 600 nm/λem 636 nm).
Visual Sensing of β-Glc With Intestinal Fungus
The fungi strains were cultured in martin broth modified (MTB: glucose 200 g/L, yeast extract 2 g/L, KH2PO4 1 g/L, Mg2SO4 0.5 g/L) at 32°C, 160 r/min. 36 h later, the probe DXM-Glc was added with a final concentration of 20 μM and incubated for 4 h. Then, the fluorescence intensity was measured using Microplate reader (λex 600 nm/λem 636 nm). Similarly, after incubation, Pichia terricola M2 cells were washed with PBS and diluted in saline. The suspensions were dropped on glass slides and imaged using a laser confocal microscope (λex 561/λem 600–660 nm).
Biotransformation of Icariin by Pichia terricola M2
Pichia terricola M2 was cultured in MTB medium at 32°C, 160 r/min. Icariin was added with a final concentration of 10 mg/ml. Five days later, the fungal cells were collected from the fermentation broth by centrifugation (5,000 g, 10 min). Then, the supernatant was inactivated by acetonitrile for HPLC analysis, which was used to confirm the production of Icariside II. Icariside II was purified by extraction of the fermentation broth using ethyl acetate, and the metabolite was then purified using preparative HPLC.
Icariin, yellow powder. 1H-NMR (DMSO-d6, 600 MHz) δH 12.57 (1H, s), 7.89 (2H, d, J = 9.0 Hz), 7.13 (2H, d, J = 9.0 Hz), 6.63 (1H, s), 5.35 (1H, s), 5.28 (1H, s), 5.16 (1H, t, J = 7.2 Hz), 5.12 (1H, s), 5.05 (1H, d, J = 4.2 Hz), 5.00 (1H, d, J = 7.2 Hz), 4.98 (1H, d, J = 4.8 Hz), 4.73 (1H, d, J = 3.6 Hz), 4.66 (1H, d, J = 3.6 Hz), 4.62 (1H, t, J = 3.6 Hz), 4.00 (1H, s), 3.85 (3H, s), 3.71 (1H, m), 3.57 (1H, dd, J = 14.4, 6.6 Hz), 3.46 (4H, m), 3.30 (4H, m), 3.14 (2H, m), 3.08 (1H, m), 1.69 (3H, s), 1.60 (3H, s), 0.79 (3H, d, J = 6.6 Hz). 13C-NMR (DMSO-d6, 150 MHz) δC 178.30, 161.42, 160.52, 159.09, 157.33, 153.02, 134.65, 131.12, 130.57, 122.27, 122.14, 114.09, 108.30, 105.60, 101.99, 100.54, 98.13, 77.19, 76.61, 73.36, 71.11, 70.71, 70.31, 70.08, 69.66, 60.63, 55.51, 25.47, 21.42, 17.87, 17.46. HR-MS: m/z 677.2430, [M + H]+, calcd for C33H41O15, 677.2440 (Supplementary Figures S4-S6).
Icariside II, yellow powder. 1H-NMR (DMSO-d6, 600 MHz) δH 12.52 (1H, s), 10.85 (1H, s), 7.85 (2H, d, J = 9.0 Hz), 7.11 (2H, d, J = 9.0 Hz), 6.31 (1H, s), 5.26 (1H, s), 5.15 (1H, t, J = 6.6 Hz), 4.97 (1H, d, J = 4.2 Hz), 4.71 (1H, d, J = 4.2 Hz), 4.64 (1H, d, J = 5.4 Hz), 3.98 (1H, br s), 3.85 (3H, s), 3.47 (1H, m), 3.41 (1H, dd, J = 14.4, 6.6 Hz), 3.33 (1H, m), 3.13 (1H, m), 3.06 (1H, m), 1.67 (3H, s), 1.02 (3H, s), 0.78 (3H, d, J = 6.0 Hz). 13C-NMR (DMSO-d6, 150 MHz) δC 177.99, 161.68, 161.28, 158.85, 156.75, 153.79, 134.43, 131.03, 130.42, 122.41, 122.27, 114.06, 105.95, 104.17, 101.96, 98.35, 71.11, 70.65, 70.30, 70.07, 55.49, 25.43, 21.17, 17.79, 17.46. HR-MS: m/z 515.1909, [M + H]+, calcd for C27H31O10, 515.1912 (Supplementary Figures S7-S9).
Inhibitory Effect of Icariside II on Human Endometrial Cancer Cells
First, serum-free medium was added to the real-time label free cell analyzer to remove the background value, and cells (3 ×104) were inoculated into a 16-well culture plate of the cell analyzer. Then, a certain concentration of Icariin II was added respectively (0, 10, 20, 40 μM). After mixing, the sample was placed in the real-time unmarked cell analyzer, and the cell proliferation curve was obtained after 48 h.
Cell viability was determined using a CCK-8 assay. In brief, 3 × 103 cells were seeded into 96-well culture plates allowed to adhere overnight, and then the cells were changed to fresh medium containing various concentrations of Icariside II dissolved in DMSO (final concentration, 0.1%). After incubation for 24, 48, and 72 h, CCK-8 was added, and the absorbance was measured at 450 nm by EnSpire® Multimode Plate Reade (Perkin Elmer, United States). Cell viability in the vehicle control groups was considered 100%. Each assay was carried out in triplicate.
To analyze the effects of Icariside II on colony formation, single cells (3 × 103 per well) were seeded in 6-well plate containing 2 ml growth medium with 10% FBS and cultured for 24 h. Then, the medium was removed, and cells were treated with various concentrations of Icariside II (0, 10, 20, 40 μM). After 24 h, cells were washed with PBS and supplemented with fresh growth medium, cells were routinely incubated for about 10 days until colonies were large enough to be visualized. Then colonies were stained with 0.1% crystal violet and counted.
Statistical Analysis
The measurements of fluorescence intensities and cell viabilities were repeated at least three times. Data are represented as the mean ± standard deviation (SD). Analysis of variance and Student’s t-test were used to compare the values of the test and control samples. p < 0.05 was the statistically significant difference. Graphpad Prism 8 software was used for all statistical analysis.
Results and Discussion
DXM-Glc as an Off-On Fluorescent Probe for β-Glc
β-Glc is known to mediate the cleavage of β-O-glycosidic bond of glycosides and glycans. The stereo configuration of the glycoside was specifically recognized by β-Glc. According to the catalytic characteristics of β-Glc, a fluorescent substrate was developed with a glucose group grafted through a β-O-glycosidic bond. 2-((6-hydroxy-2,3-dihydro-1H-xanthen-4-yl)methylene)malononitrile (DXM-OH) was chosen as an intramolecular charge transfer (ICT) fluorophore which exhibited good photostability and excellent biocompatibility (Liu, et al., 2018; Pang, et al., 2020; Sun, et al., 2019). Therefore, using DXM-OH, a novel fluorescent probe (DXM-Glc) was developed for sensing of β-Glc (Figure 1A). Using HPLC analysis, the hydrolysis of DXM-Glc mediated by β-Glc was confirmed and the production of DXM-OH was observed (Supplementary Figure S10). Herein, the absorption spectra of both DXM-Glc and DXM-OH have been measured, and a red-shift was observed for DXM-OH in comparison with DXM-Glc (Figure 1B). DXM-Glc was non fluorescent, due to reduced intramolecular charge transfer (ICT), while DXM-OH exhibited strong fluorescence, which could be used detect β-Glc sensitively and without interference from biological samples (Figure 1C).
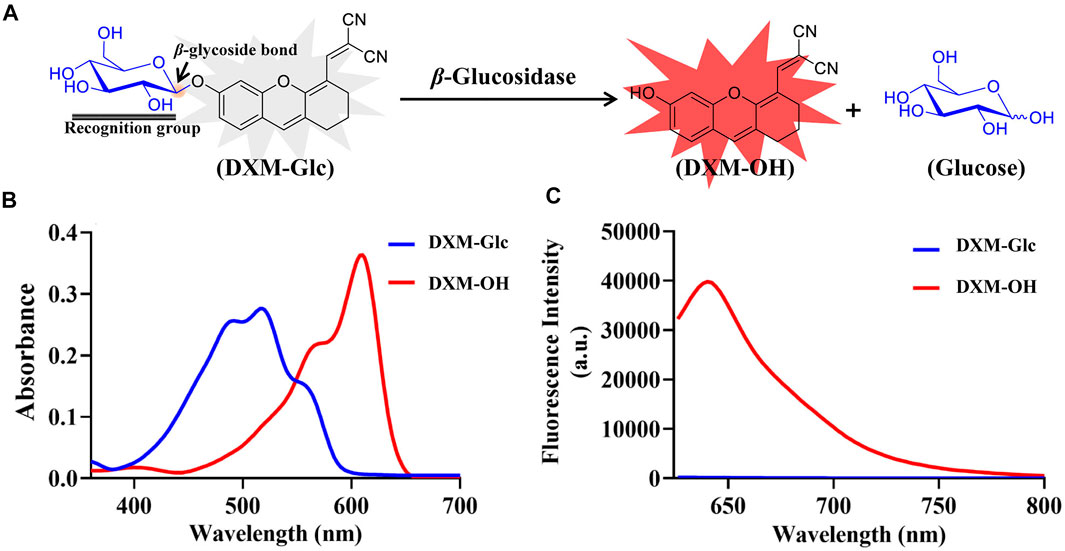
FIGURE 1. Design of fluorescent probe for β-Glc. (A) Illustration for the hydrolysis of DXM-Glc mediated by β-Glc. (B) Absorbance spectra of DXM-Glc and DXM-OH. (C) Fluorescence spectra of DXM-Glc and DXM-OH (λex 600 nm).
For the development of a fluorescent probe for β-Glc, various validations were performed for the enzymatic reaction between DXM-Glc and β-Glc. Firstly, a series of fluorescence spectra were acquired for the enzymatic hydrolysis of DXM-Glc with different amounts of β-Glc (Figure 2A). The fluorescence intensity and β-Glc concentrations exhibited a good linear relationship (Figure 2B), which indicated the potential application for accurate determination of β-Glc activity. The limit of detection (LOD) was calculated to be 0.02 mU/mL (0.2 μg/ml) using 3σ/slope, indicating excellent sensitivity of DXM-Glc toward β-Glc. As a designed fluorescent probe for β-Glc, the substrate specificity of DXM-Glc was evaluated in presence of various species. Common ions and amino acids exhibited no interference for the fluorescence intensity of DXM-Glc, indicating the suitability of the system for application for the assay of biological samples (Supplementary Figure S12). More importantly, DXM-Glc exhibited good selectivity toward β-Glc in comparison with various biological enzymes, especial other glycosidases, such as α-Glc, GAL and NAG (Figure 2C). Thus, DXM-Glc was determined to be a reliable substrate for β-Glc. In addition, the enzymatic hydrolysis of DXM-Glc mediated by β-Glc exhibited Michaelis-Menten kinetics, with Km = 45.97 μM and Vmax = 2.19 nmol/min/mg. Therefore, DXM-Glc was a highly sensitive and selective fluorescent probe for β-Glc in a biological environment.
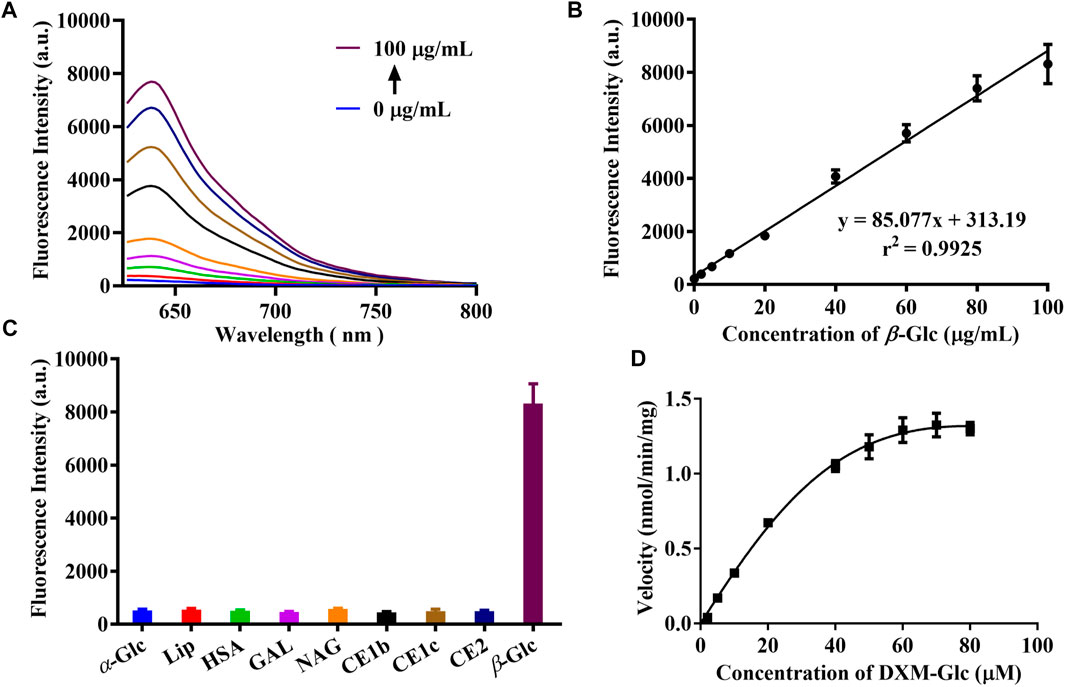
FIGURE 2. (A) The fluorescence response of DXM-Glc toward β-Glc with different concentrations (0–100 μg/ml). (B) The linear relationship between the fluorescence intensity and β-Glc concentrations. (C) The fluorescence response of DXM-Glc toward different enzymes. (D) The hydrolysis kinetics of DXM-Glc mediated by β-Glc.
Visual Identification of β-Glc Activity in Intestinal Fungi
As mentioned above, intestinal bacteria are involved in the metabolism of glycosides. However, limited research on the β-Glc of intestinal fungi has been reported. Herein, we identified β-Glc active fungi using the developed fluorescent probe. 36 intestinal fungi strains were cultured with DXM-Glc and the fluorescence intensity of fungal culture medium was measured, so as to determine the β-Glc activity. As shown in Figure 3A, the C3 position of the heat map indicated the strongest fluorescence intensity along with the β-Glc, which corresponded to the intestinal fungus Pichia terricola M2. Although it has been reported that β-Glc produced by Pichia genus (Zhang, et al., 2020), this research represents the first exploration of β-Glc in Pichia terricola species. In order to monitor intracellular β-Glc, we evaluated the activity visually using the fluorescence imaging of Pichia terricola M2 stained using DXM-Glc. Compared with the blank group, strong fluorescence was observed for Pichia terricola M2 cells (Figure 3B). In addition, when the β-Glc inhibitor miglitol was co-incubated with DXM-Glc (Zamoner, et al., 2019), the Pichia terricola M2 cells displayed weaker fluorescence, indicating that the fluorescence sensing of Pichia terricola M2 by DXM-Glc was β-Glc dependent. Therefore, the fluorescent probe DXM-Glc could be used to visually identify intestinal fungus exhibiting β-Glc activity. Based on the excellent cell permeability, DXM-Glc could be used as a practical tool for imaging endogenous β-Glc in living fungal cells. In addition, the identified intestinal fungus Pichia terricola M2 is an important resource for investigating the metabolism of glycosides in the gut.
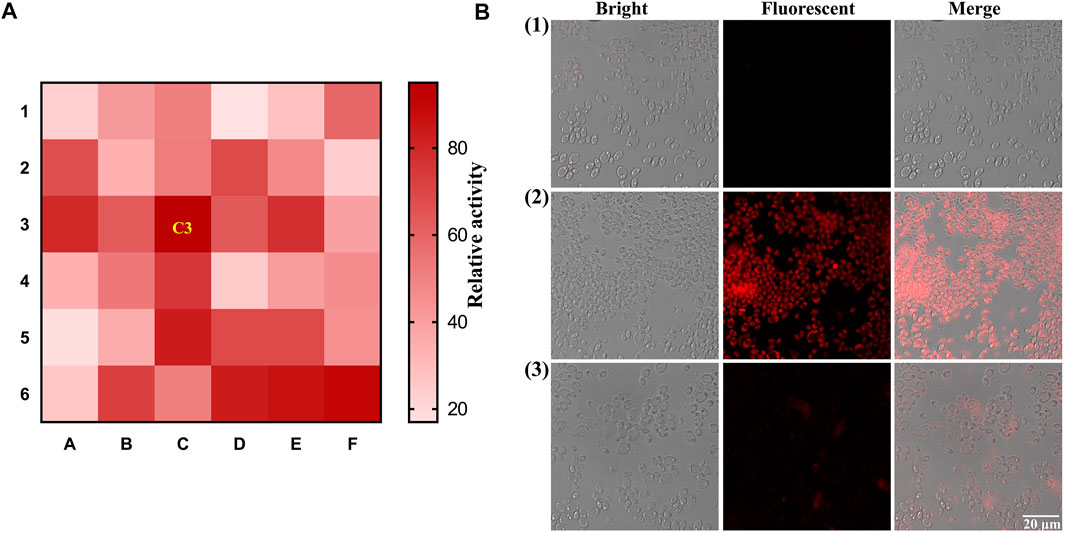
FIGURE 3. Visual identification of intestinal fungi with strong activity of β-Glc. (A) The heat-map for the screening of intestinal fungal β-Glc. (B) Fluorescence imaging of Pichia terricola M2 stained by DXM-Glc using Confocal laser scanning microscope. (1) Blank group. (2) Pichia terricola M2 stained by DXM-Glc. (3) Pichia terricola M2 stained by DXM-Glc in the presence of miglitol.
Metabolism of Icariin by Intestinal Fungus Pichia terricola M2
Icariin (ICA), a natural flavonoid glycoside containing glucose and rhamnose, is the major constituent of herba Epimedii (>0.05%). ICA exhibits various biological activities, including anti-inflammatory, antidepressant, antioxidative, antiatherosclerosis, anticancer, and insulin resistance(Sun, et al., 2020). Icariside II (ICA II, Baohuoside I) is one of the metabolites of ICA, which is a loss of the glucosyl moiety at the C-7 position of ICA (Figure 4A). Recently, metabolic and pharmacokinetic studies have revealed that ICA is metabolized by intestinal microbiota in vivo and absorbed as ICA II(Cheng, et al., 2016). Though both ICA and ICA II exhibited many common pharmacological effects, ICA II exhibits stronger biological activity when compared with ICA. Therefore, it was proposed that ICA II is the activated form of ICA in vivo.
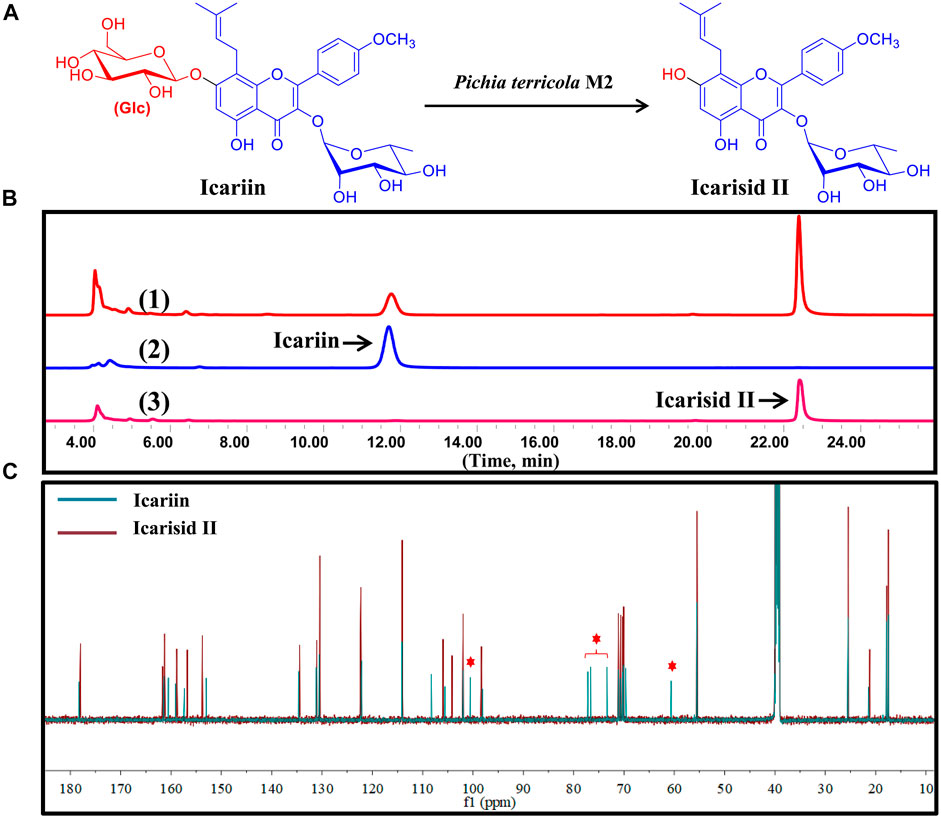
FIGURE 4. Icariin was transformed to Icarisid Ⅱ by intestinal fungus Pichia terricola M2. (A) Illustration for the biotransformation of Icariin by Pichia terricola M2. (B) HPLC chromatograms for the analysis of biotransformation of Icariin by Pichia terricola M2. (1) Co-incubation of Icariin and Pichia terricola M2. (2) Icariin reference. (3) Icarisid Ⅱ reference. (C) Comparison of the 13C NMR spectra of Icariin and Icarisid Ⅱ.
In the present study, Pichia terricola M2 could catalyze the cleavage of the β-O-glycoside bond between glucose and flavone, producing ICA II as the metabolite (Figure 4A). This special enzymatic biotransformation of ICA was confirmed using HPLC analysis, where ICA II was found as the sole chromatographic peak (Figure 4B). Through a comparison of the 13C NMR spectra of ICA and ICA II confirmed removal of the glucose moiety (δC 60–105 ppm) (Figure 4C), which indicated the substrate specificity of β-Glc in Pichia terricola M2. Therefore, Pichia terricola M2 an intestinal fungus with β-Glc activity could transform ICA to ICA II efficiently, which not only indicated the intestinal metabolism of ICA in vivo, but also could be used to prepare ICA II as a bioactive substance for further pharmaceutical development.
Icarisid Ⅱ as a Cytotoxic Agent Against Human Endometrial Cancer Cells
As mentioned above, ICA Ⅱ is a metabolite of ICA mediated by Pichia terricola M2. It was proposed that ICA Ⅱ would exhibit improved membrane permeability enhancing bioavailability. As such, we evaluated the cytotoxicity of ICA Ⅱ toward two endometrial cancer cell lines. Therefore, we monitored the growth curves of RL95-2 and ishikawa for >60 h, to determine the effect of ICA Ⅱ on cancer cells. From these experiments it was clear that ICA Ⅱ could inhibit the growth of RL95-2 at 20 μM, and interfere with the growth of ishikawa at 10 μM (Figure 5A). Using a CCK8 assay for cancer cell viability, ICA Ⅱ also exhibited cytotoxicity against both RL95-2 and ishikawa cancer cells (Figure 5B). Furthermore, the biological effect of ICA Ⅱ on cell colony formation was evaluated in addition to the proliferation inhibitory effect. ICA Ⅱ significantly suppressed colony formation in a concentration dependent manner (Figure 5C). So, ICA Ⅱ as a metabolite of ICA exhibited significant cytotoxicity towards human endometrial cancer cells, which could be generated using intestinal fungus. These results confirm the metabolism of ICA in the gut by intestinal microbiota generating ICA Ⅱ, which could indicate a key biological function of ICA.
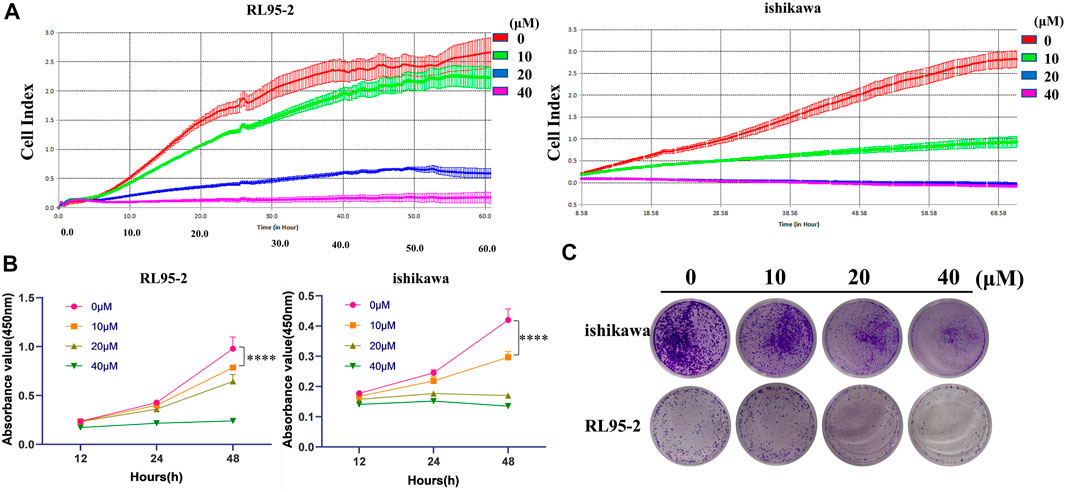
FIGURE 5. Icarisid Ⅱ displayed cytotoxic effect against human endometrial cancer cells. (A) Grow curves of cancer cells RL 95-2 and ishikawa in the presence of Icarisid Ⅱ. (B) Cell viability of RL 95-2 and ishikawa in the presence of Icarisid Ⅱ at 12, 24, and 48 h. (C) Inhibitory effect of Icarisid Ⅱ against cell colony formation of RL 95-2 and ishikawa.
Conclusions
β-Glc is an enzyme able to hydrolyze the β-glycosidic bond, and has been used as a biocatalyst in the industrial preparation of target materials. In addition, it is found in intestinal microbiota where it is involved in the metabolism of glycosides in foods or pharmaceutical substances, enhancing the absorption, bioavailability, and bioactivity of the glycosides. In this study, the conjugate of glucose and a fluorophore linked through a β-O-glycosidic bond has been developed as an enzymatic activatable fluorescent probe (DXM-Glc) for β-Glc. DXM-Glc exhibited high selectivity and sensitivity toward β-Glc. Using DXM-Glc, Pichia terricola M2 was identified as intestinal fungus expressing β-Glc, which could transform ICA to ICA Ⅱ efficiently. Furthermore, ICA Ⅱ was found to significantly inhibit the proliferation of human endometrial cancer cells. Therefore, the DXM-Glc probe can be used to evaluate the β-Glc activity of intestinal microbiota and monitor the metabolism of bioactive substances.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
GW: Methodology, Investigation, Writing–original draft. FY: Investigation, Data curation. YW: Methodology. YL: Investigation. JC: Resources. ZY: Methodology. LF: Data curation. TJ: Writing–review and editing. CW: Project administration, Funding acquisition. YK: Project administration, Writing–review and editing.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81872970), Liaoning Revitalization Talents Program (XLYC1907017), High-level Talents of Dalian (2020RJ09). Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University (2020ZD01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
TJ wishes to thank the Royal Society for a Wolfson Research Merit Award.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.919624/full#supplementary-material
References
Abdella, A., El-Baz, A. F., Ibrahim, I. A., Mahrous, E. E., and Yang, S.-T. (2018). Biotransformation of Soy Flour Isoflavones by Aspergillus niger NRRL 3122 β-glucosidase Enzyme. Nat. Prod. Res. 32 (20), 2382–2391. doi:10.1080/14786419.2017.1413569
Cheng, T., Yang, J., Zhang, T., Yang, Y. S., and Ding, Y. (2016). Optimized Biotransformation of Icariin into Icariside II by β-Glucosidase from Trichoderma Viride Using Central Composite Design Method. Biomed. Res. Int. 2016, 5936947. doi:10.1155/2016/5936947
Donkor, O. N., and Shah, N. P. (2008). Production of Beta-Glucosidase and Hydrolysis of Isoflavone Phytoestrogens by Lactobacillus Acidophilus, Bifidobacterium Lactis, and Lactobacillus Casei in Soymilk. J. Food Sci. 73 (1), M15–M20. doi:10.1111/j.1750-3841.2007.00547.x
Feng, L., Tian, Z., Zhang, M., He, X., Tian, X., Yu, Z., et al. (2021). Real-time Identification of Gut Microbiota with Aminopeptidase N Using an Activable NIR Fluorescent Probe. Chin. Chem. Lett. 32 (10), 3053–3056. doi:10.1016/j.cclet.2021.03.056
Hati, S., Vij, S., Singh, B. P., and Mandal, S. (2015). β -Glucosidase Activity and Bioconversion of Isoflavones during Fermentation of Soymilk. J. Sci. Food Agric. 95 (1), 216–220. doi:10.1002/jsfa.6743
Hong, H., Cui, C.-H., Kim, J.-K., Jin, F.-X., Kim, S.-C., and Im, W.-T. (2012). Enzymatic Biotransformation of Ginsenoside Rb1 and Gypenoside XVII into Ginsenosides Rd and F2 by Recombinant β-glucosidase from Flavobacterium Johnsoniae. J. Ginseng Res. 36 (4), 418–424. doi:10.5142/jgr.2012.36.4.418
Kalaiyarasan, G., Veerapandian, M., JebaMercy, G., Balamurugan, K., and Joseph, J. (2019). Amygdalin-Functionalized Carbon Quantum Dots for Probing β-Glucosidase Activity for Cancer Diagnosis and Therapeutics. ACS Biomater. Sci. Eng. 5 (6), 3089–3099. doi:10.1021/acsbiomaterials.9b00394
Kong, B., Yang, T., Hou, P., Li, C. H., Zou, H. Y., and Huang, C. Z. (2020). Enzyme‐triggered Fluorescence Turn‐off/turn‐on of Carbon Dots for Monitoring β‐glucosidase and its Inhibitor in Living Cells. Luminescence 35 (2), 222–230. doi:10.1002/bio.3717
Li, L., Feng, L., Zhang, M., He, X., Luan, S., Wang, C., et al. (2020). Visualization of Penicillin G Acylase in Bacteria and High-Throughput Screening of Natural Inhibitors Using a Ratiometric Fluorescent Probe. Chem. Commun. 56 (34), 4640–4643. doi:10.1039/d0cc00197j
Liu, J., Wu, F., Liu, C., Bao, H., and Fu, T. (2020). "Turn-on" Fluorometric Probe for α-glucosidase Activity Using Red Fluorescent Carbon Dots and 3,3',5,5'-tetramethylbenzidine. Mikrochim. Acta 187 (9), 498. doi:10.1007/s00604-020-04479-1
Liu, M., Lv, Y., Jie, X., Meng, Z., Wang, X., Huang, J., et al. (2018). A Super-sensitive Ratiometric Fluorescent Probe for Monitoring Intracellular Subtle pH Fluctuation. Sensors Actuators B Chem. 273, 167–175. doi:10.1016/j.snb.2018.06.048
Liu, T., Wang, Y., Feng, L., Tian, X., Cui, J., Yu, Z., et al. (2021). 2D Strategy for the Construction of an Enzyme-Activated NIR Fluorophore Suitable for the Visual Sensing and Profiling of Homologous Nitroreductases from Various Bacterial Species. ACS Sens. 6 (9), 3348–3356. doi:10.1021/acssensors.1c01216
Ning, J., Liu, T., Dong, P., Wang, W., Ge, G., Wang, B., et al. (2019). Molecular Design Strategy to Construct the Near-Infrared Fluorescent Probe for Selectively Sensing Human Cytochrome P450 2J2. J. Am. Chem. Soc. 141 (2), 1126–1134. doi:10.1021/jacs.8b12136
Pang, X., Li, Y., Zhou, Z., Lu, Q., Xie, R., Wu, C., et al. (2020). Visualization of Endogenous β-galactosidase Activity in Living Cells and Zebrafish with a Turn-On Near-Infrared Fluorescent Probe. Talanta 217, 121098. doi:10.1016/j.talanta.2020.121098
Quan, L.-H., Min, J.-W., Jin, Y., Wang, C., Kim, Y.-J., and Yang, D.-C. (2012a). Enzymatic Biotransformation of Ginsenoside Rb1 to Compound K by Recombinant β-Glucosidase from Microbacterium Esteraromaticum. J. Agric. Food Chem. 60 (14), 3776–3781. doi:10.1021/jf300186a
Quan, L.-H., Min, J.-W., Yang, D.-U., Kim, Y.-J., and Yang, D.-C. (2012b). Enzymatic Biotransformation of Ginsenoside Rb1 to 20(S)-Rg3 by Recombinant β-glucosidase from Microbacterium Esteraromaticum. Appl. Microbiol. Biotechnol. 94 (2), 377–384. doi:10.1007/s00253-011-3861-7
Rekha, C. R., and Vijayalakshmi, G. (2011). Accelerated Fermentation of 'idli' Batter Using Soy Residue Okara. J. Food Sci. Technol. 48 (3), 329–334. doi:10.1007/s13197-011-0248-9
Reszka, M., Serdiuk, I. E., Kozakiewicz, K., Nowacki, A., Myszka, H., Bojarski, P., et al. (2020). Influence of a 4′-substituent on the Efficiency of Flavonol-Based Fluorescent Indicators of β-glycosidase Activity. Org. Biomol. Chem. 18 (38), 7635–7648. doi:10.1039/d0ob01505a
Shen, H., Leung, W.-I., Ruan, J.-Q., Li, S.-L., Lei, J.-C., Wang, Y.-T., et al. (2013). Biotransformation of Ginsenoside Rb1 via the Gypenoside Pathway by Human Gut Bacteria. Chin. Med. 8 (1), 22. doi:10.1186/1749-8546-8-22
Sun, X., Wang, M., Lu, Y., Fan, C., Lu, Y., and Lu, Z. (2019). The Construction of an Effective Far-Red Fluorescent and Colorimetric Platform Containing a Merocyanine Core for the Specific and Visual Detection of Thiophenol in Both Aqueous Medium and Living Cells. New J. Chem. 43 (35), 14139–14144. doi:10.1039/c9nj03020d
Sun, Z. G., Lang, Z. F., Mu, Y. D., Li, J., Xing, C. X., Yan, L., et al. (2020). Therapeutic Effect and Mechanism of Icariin Combined with Calcium Sensitive Receptor on Mouse Gastric Cancer Cells. J. Biol. Regul. Homeost. Agents 34 (5), 1831–1836. doi:10.23812/20-228-L
Tian, X., Liu, T., Ma, Y., Gao, J., Feng, L., Cui, J., et al. (2021). A Molecular‐Splicing Strategy for Constructing a Near‐Infrared Fluorescent Probe for UDP‐Glucuronosyltransferase 1A1. Angew. Chem. Int. Ed. 60 (46), 24566–24572. doi:10.1002/anie.202109479
Wang, M., Wang, M., Zhang, F., and Su, X. (2020). A Ratiometric Fluorescent Biosensor for the Sensitive Determination of α-glucosidase Activity and Acarbose Based on N-Doped Carbon Dots. Analyst 145 (17), 5808–5815. doi:10.1039/d0an01065k
Yan, F., Cui, J., Wang, C., Tian, X., Li, D., Wang, Y., et al. (2022). Real-time Quantification for Sulfite Using a Turn-On NIR Fluorescent Probe Equipped with a Portable Fluorescence Detector. Chin. Chem. Lett. doi:10.1016/j.cclet.2022.03.006
Zamoner, L. O. B., Aragão-Leoneti, V., and Carvalho, I. (2019). Iminosugars: Effects of Stereochemistry, Ring Size, and N-Substituents on Glucosidase Activities. Pharmaceuticals 12 (3), 108. doi:10.3390/ph12030108
Zhang, M., Tian, Z., Wang, J., Tian, X., Wang, C., Cui, J., et al. (2021). Visual Analysis and Inhibitor Screening of Leucine Aminopeptidase, a Key Virulence Factor for Pathogenic Bacteria-Associated Infection. ACS Sens. 6 (10), 3604–3610. doi:10.1021/acssensors.1c01161
Zhang, W., Zhuo, X., Hu, L., and Zhang, X. (2020). Effects of Crude β-Glucosidases from Issatchenkia Terricola, Pichia Kudriavzevii, Metschnikowia Pulcherrima on the Flavor Complexity and Characteristics of Wines. Microorganisms 8 (6), 953. doi:10.3390/microorganisms8060953
Keywords: β-glucosidase, fluorescent probe, Pichia terricola M2, iIcariside Ⅱ, endometrial cancer
Citation: Wang G, Yan F, Wang Y, Liu Y, Cui J, Yu Z, Feng L, James TD, Wang C and Kong Y (2022) Visual Sensing of β-Glucosidase From Intestinal Fungus in the Generation of Cytotoxic Icarisid II. Front. Chem. 10:919624. doi: 10.3389/fchem.2022.919624
Received: 13 April 2022; Accepted: 29 April 2022;
Published: 27 May 2022.
Edited by:
Cheuk-Fai Chow, The Education University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Qiuyu Gong, Independent researcher, Singapore, SingaporeLin Yuan, Hunan University, China
Copyright © 2022 Wang, Yan, Wang, Liu, Cui, Yu, Feng, James, Wang and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tony D. James, dC5kLmphbWVzQGJhdGguYWMudWs=; Chao Wang, d2FjaF9lZHVAc2luYS5jb20=; Ying Kong, eWluZ2tvbmdAZG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Gang Wang
Gang Wang Fei Yan1,2†
Fei Yan1,2† Yufei Wang
Yufei Wang Yingping Liu
Yingping Liu Lei Feng
Lei Feng Tony D. James
Tony D. James Chao Wang
Chao Wang Ying Kong
Ying Kong