- 1National Center for International Research of Bio-Targeting Theranostics, Guangxi Key Laboratory of Bio-Targeting Theranostics, Collaborative Innovation Center for Targeting Tumor Diagnosis and Therapy, Guangxi Talent Highland of Bio-Targeting Theranostics, Guangxi Medical University, Nanning, China
- 2Orthopedic Surgery Department, Institute of Arthritis Research in Integrative Medicine, Shanghai Academy of Traditional Chinese Medicine, Guanghua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Department of Medical Ultrasound and Central Laboratory, Shanghai Tenth People’s Hospital, Ultrasound Research and Education Institute, Tongji University School of Medicine, Shanghai, China
With the developments of nanobiotechnology and nanomedicine, non-invasive thermal ablation with fewer side effects than traditional tumor treatment methods has received extensive attention in tumor treatment. Non-invasive thermal ablation has the advantages of non-invasiveness and fewer side effects compared with traditional treatment methods. However, the clinical efficiency and biological safety are low, which limits their clinical application. Transition-metal based nanomaterials as contrast agents have aroused increasing interest due to its unique optical properties, low toxicity, and high potentials in tumor diagnosis. Transition-metal based nanomaterials have high conversion efficiency of converting light energy into heat energy, good near-infrared absorption characteristics, which also can targetedly deliver those loaded drugs to tumor tissue, thereby improving the therapeutic effect and reducing the damage to the surrounding normal tissues and organs. This article mainly reviews the synthesis of transition-metal based nanomaterials in recent years, and discussed their applications in tumor thermal ablation and diagnosis, hopefully guiding the development of new transition metal-based nanomaterials in enhancing thermal ablation.
Introduction
Population growth and aging have led to high mortality and morbidity rates of cancer, becoming one of the main factors endangering physical and mental health (Bray et al., 2018). Tumors in humans result from the accumulation of mutations in cells’ DNA genes, disrupting the mechanisms that regulate cell division and cell death, resulting in the uncontrolled proliferation of dysfunctional cells (Alexandrov et al., 2013). Despite significant success in anti-tumor research, traditional treatment methods have limitations such as various adverse reactions, poor specificity and inducible drug resistance. Therefore, it is imperative to find more effective tumor treatment and diagnosis methods to reduce the economic burden of patients caused by cancer and improve their life quality. Recently, nanobiotechnology in the field of treating and diagnosing tumors have raised concerns, which is equipped with higher efficacy and safety than traditional treatment methods, holding great potentials in clinical transition (Shi et al., 2017).
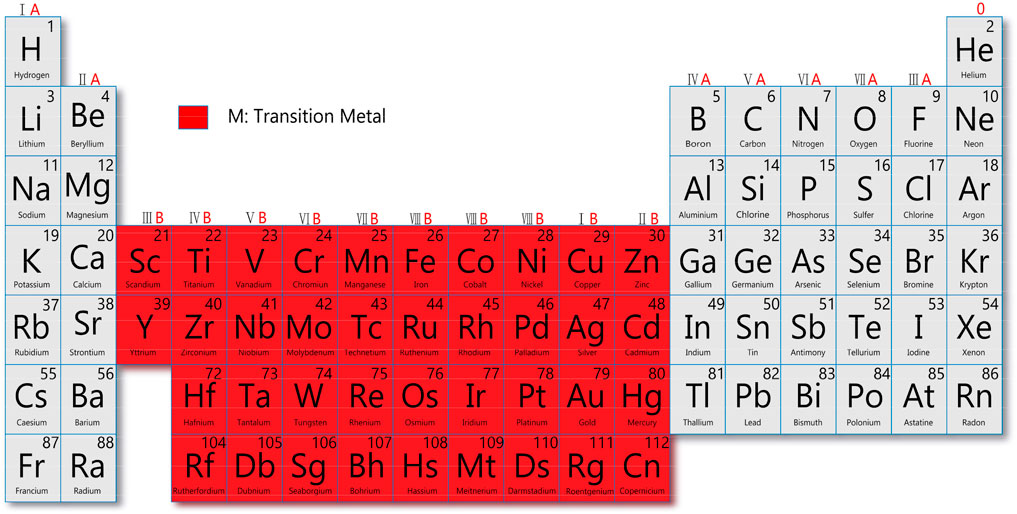
Transition metals belong to groups 3–12 in the periodic table and are d-block elements with filled electron orbits, which can combine with other elements to form complex structures. Transition-metal based nanomaterials especially after combining metal atoms with surrounding anions or molecules (Xu M. et al., 2021) have received much attention due to their high photothermal conversion efficiency, low cytotoxicity, good photothermal stability, abundant elemental composition, and good biocompatibility (Zhao et al., 2018). They are more suitable as photo-thermal energy converters than molecular optical absorbers due to their plasmonic properties and high optical and thermal stabilities (Lapotko, 2009), making them promising candidates for tumor therapy and diagnosis.
Traditional tumor treatments such as chemotherapy, radiotherapy, and surgery still serve as the first-line treatment methods. However, they cause significant trauma to the body and fail to effectively prevent tumor recurrence and metastasis (Wang et al., 2020). Non-invasive thermal tumor ablation can selectively destroy multiple tumor foci, resulting in coagulative necrosis of tumor tissues, which is regarded to be a more attractive and logical treatment approach (Goldberg et al., 2000). In addition, tumor tissues are more sensitive to temperature than normal tissues (Chu and Dupuy, 2014). Currently, non-invasive thermal ablation techniques include photothermal ablation (PTA) (Zhang et al., 2019), radiofrequency ablation (RFA) (Zhang et al., 2016a; Zhang et al., 2016b; Fang et al., 2019), magnetothermal ablation (MHA), and high-intensity focused ultrasound (HIFU) (Zhang et al., 2014; Zhang et al., 2021). Recent researches have investigated the application of transition-metal based nanomaterials in nanomedicine (Chimene et al., 2015). Desirable characteristics such as high near-infrared (NIR) absorption and good thermal conductivity enable transition-metal based nanomaterials to be widely used in biosensors (Wang Y. H. et al., 2017), multimodal imaging, drug delivery (Jahangirian et al., 2019), PTA (Liu Y. et al., 2019), and MHA (Chang et al., 2021). These appealing properties adequately guarantee the high efficacy of non-invasive thermal ablation against tumors via increasing the ablation temperature and destroying tumor cells (Urbanova and Pumera, 2019).
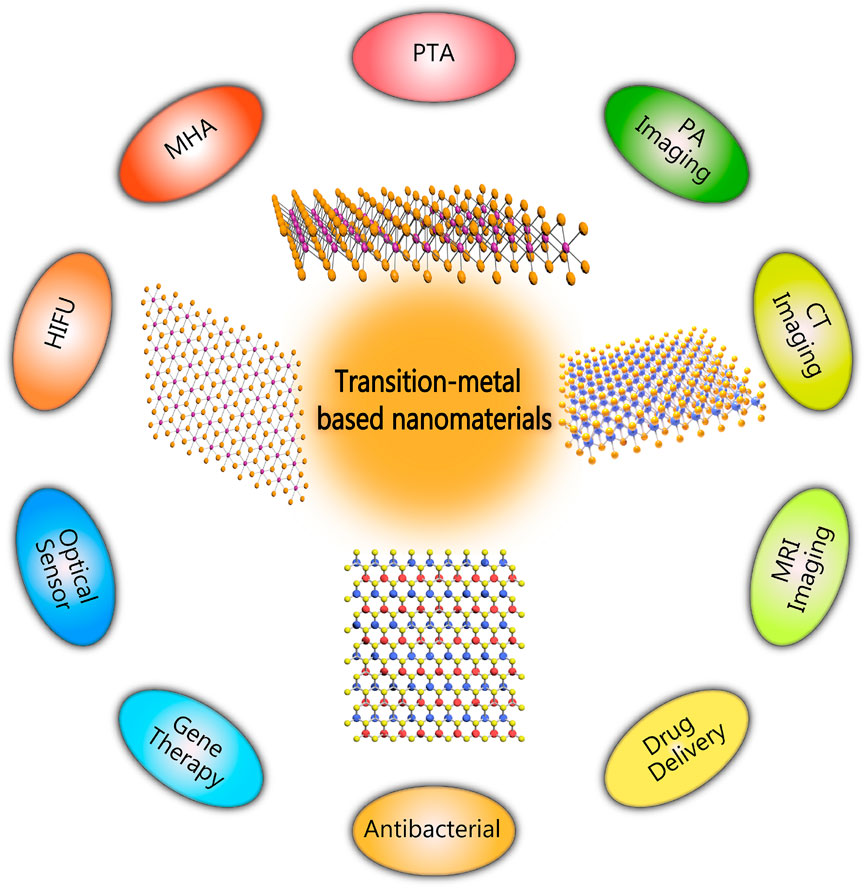
This article mainly reviewed recent progress of transition-metal based nanomaterials in non-invasive thermal ablation of tumors, such as HIFU, PTA, and MHA (Figure 1). Their potential in improving energy conversion efficiency is emphasized, and their applications in imaging diagnosis was introduced. Finally, the prospects and development directions of transition-metal based nanomaterials were discussed.
Transition-Metal Based Nanomaterials and Their Properties
Despite the successful application of cisplatin and aurein in clinics, transition-metal based nanomaterials have become a promising vehicle to deliver these drugs and have attracted much attention in the diagnosis and treatment of cancer (Luo et al., 2021). According to the definition by the International Union of Pure and Applied Chemistry, transition metals have atoms with incomplete d subshell, common cation, or free atom (Figure 2).
Properties of Transition Metal Dichalcogenides
Transition Metal Dichalcogenides (TMDCs), denoted as MX2, are composed of transition metals; M denotes a group 4–7 element (such as Mo, W, Ta, Nb, and Mn), and X denotes chalcogens (such as S and Se) (Yun et al., 2020). The different structural compositions of TMDCs give them different properties, such as metals (NbS2, VSe2), semiconductors (MoS2, WS2), insulators (HfS), semi-metals (WTe2, TiSe2), and even superconductors (NbSe2, TaS2) (Zhang et al., 2018), for applications in different biological fields.
TMDCs have one layer of metal atoms sandwiched between two layers of chalcogen atoms. The van der Waals forces between the transition metals and sulfur atoms are weak. targeted delivery (Li et al., 2017). The high photothermal conversion efficiency (62.5%) of TMDC nanoparticles in the NIR region (650–900 nm) with strong absorption is of great significance for photoacoustic imaging (PA) and non-invasive tumor thermal ablation (Murugan et al., 2019). TMDCs have excellent optical and electrical properties, useful in various biosensors for detecting environmental pollution and bioactive molecules (Rohaizad et al., 2021). In addition, it was found that TMDCs can directly act on the cell wall of bacteria and destroy the vast majority of drug-resistant bacteria, potentially replacing antibiotics in the future (Debnath et al., 2021).
Characteristics of Transition Metal Oxides
Transition metal oxide (TMO) nanomaterials are used in cancer treatment and diagnosis due to their unique composition, structure, and physicochemical properties (Wen et al., 2020). Transition metals are filled with electrons in the s orbital, while the d orbital is vacant. Hence, TMO nanomaterials have high dielectric constants, wide bandgaps, electronic transition, and excellent electrical properties (Jia et al., 2020), which is appropriate for engineering biosensors.
In terms of composition, TMOs can serve as oxidants because they contain oxygen. This allows TMOs to be reduced and decomposed into transition metal ions in an acidic, hypoxic tumor microenvironment with high levels of glutathione (Yang G. et al., 2021). Therefore, transition metal ions can be used for biological imaging, such as Mn ions-mediated magnetic resonance imaging (MRI) (Zheng et al., 2021). Oxygen in metal oxides can enhance the efficacy of photodynamic therapy (Lin T. et al., 2018) and sonodynamic therapy (SDT) (Xu Q. et al., 2021) of tumors.
Characteristics of MXenes
MXenes are expressed as Mn+1XnTx, where M is a transition metal, and X is a carbon or nitrogen site; the maximum value of n is 4. Tx represents a functional group (VahidMohammadi et al., 2021). The functional group’s hydroxyl (OH), oxygen, or fluorine hydrophilicity is different from that of other transition-metal based nanomaterials. The advantages of MXenes, such as good biodegradability and biocompatibility, make it easier for use in nanomedicine (Lin H. et al., 2018). In addition, MXenes nanomaterials can be used as a delivery platform for anticancer drugs due to their large surface area, low toxicity, and targeting (Shukla, 2020). Photoacoustic imaging can acquire the unique optical properties of MXenes, and the excellent photothermal conversion efficiency determine that they can be used as a nanoreagent for PTT (Fu et al., 2021).
Non-Invasive Thermal Ablation Assisted by Transition-Metal Based Nanomaterials
Recently, thermal ablation therapy has emerged as a novel non-invasive treatment for localized solid malignancies by generating high temperature at the injury site, leading to protein denaturation, irreversible coagulative necrosis of tumor tissue, and rapid cell death (Wang M. et al., 2019). Noninvasive thermal tumor ablation modalities have been used clinically including PTA, MHA, and HIFU (Figure 3).
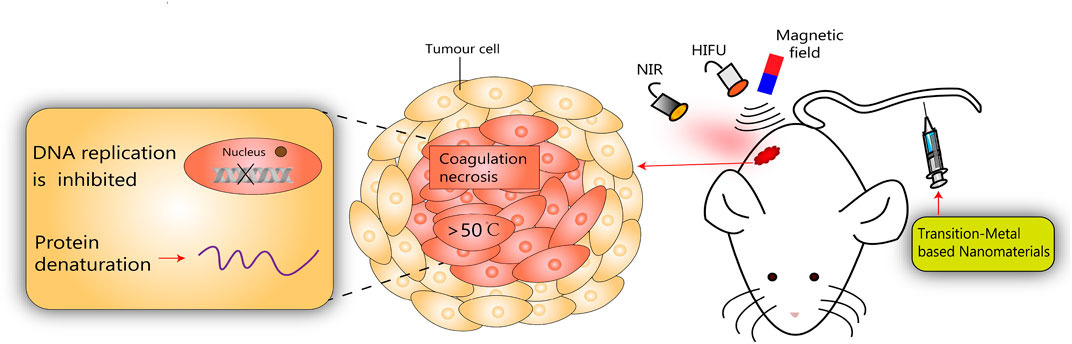
FIGURE 3. Schematic diagram of transition-metal based nanomaterials for noninvasive oncology thermal ablation.
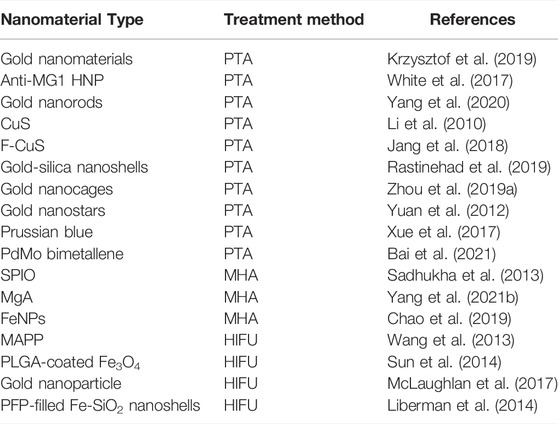
There are many similarities between the various thermal ablation methods. In a given lesion, the energy lost from heat plus the energy deposition from local tissue interactions equals the degree of coagulation necrosis (Goldberg et al., 2000). In recent years, attention has been paid to treat tumors using transition-metal based nanomaterials, and significant achievements have been made in auxiliary imaging diagnosis and cancer treatment. According to clinical demands, the rational design of transition-metal based nanomaterials can be endowed with special properties that increase the efficacy of thermal ablation of tumors. Some typical transition-metal based nanomaterials for non-invasive thermal ablation have been summarized in Table 1.
Transition Metal-Based Nanomaterials-Assisted Photothermal Ablation
PTA treatment involves using optical absorbers, such as copper sulfide nanoparticles, gold nanostructures, and carbon nanomaterials, to generate heat under NIR laser irradiation. The resulting local hyperthermia can destroy diseased tissue cells, with little damage to natural tissues (Goncalves et al., 2020). PTA has significant advantages over traditional treatments, such as less invasiveness, strong cancer cell specificity, and rapid recovery (Ban et al., 2017). Transition-metal based nanomaterials as selective photothermal absorbers can improve the efficiency of PTA and reduce damage to surrounding tissues (Jaque et al., 2014). The non-invasive performance can be further improved by enhancing the photothermal conversion efficiency of photothermal treatment agents (PTTAs) (Doughty et al., 2019). PTTAs absorb light or energy and convert it into heat, inducing local hyperthermia that promotes tumor ablation (Yang et al., 2015). Compared with dye sensitizer molecules, transition-metal based nanoparticles have larger absorption cross-sections due to their strong surface plasmon resonance effect and higher photothermal conversion efficiency than other photothermal agents (Jain et al., 2006).
Gold nanomaterials (GNPs) are one of the widely used PTTAs due to their excellent photothermal conversion and ability to convert absorbed NIR light into heat to induce high local hyperthermia, low toxicity, and biocompatibility (Cheng et al., 2014; Xing et al., 2016; Gupta and Malviya, 2021). GNPs absorb incident photons and convert them into heat energy to increase the temperature locally, leading to cell death. GNPs can achieve high light absorption efficiency at lower radiant energy, ensuring high-efficient PTA (Krzysztof et al., 2019). White et al. constructed anti-MG1 conjugated hybrid magnetic gold nanoparticles. Their strong NIR absorption peak at 800 nm make it possible to target NIR PTA of tumors and proved to have a catalytic role in PTA, greatly improving tumor ablation efficiency (White et al., 2017). Gold nanorods (GNRs) are considered to be ideal photothermal sensors due to their inherently high biocompatibility, tunable localized surface plasmon resonance peaks, and versatile surface functionalization (Song et al., 2015). Yang et al. (2020) validated the excellent tumor ablation ability of GNRs under 980 nm illumination in a mouse xenograft model and demonstrated their photothermal therapy potential for tumors in the NIR window. CuS nanoparticles, nanobiomaterials for PTA of cancer, have the advantages of unique optical properties, low production cost and cytotoxicity, and small size (Goel et al., 2014). Li et al. (2010) synthesized CuS nanoparticles that can convert light into thermal energy and validated their strong absorption ability in the NIR region. In addition, CuS in NIR laser irradiation can greatly improve PTAs efficiency. Jang et al. (2018) constructed Fucoidan-coated copper sulfide nanoparticles to improve PTA rate effectively and verified their stable photothermal efficiency by measuring the UV-Vis absorption spectra before and after laser irradiation.
Transition Metal-Based Nanomaterials-Assisted Magnetothermal Ablation
As a non-invasive local treatment strategy, MHA has received extensive attention in recent decades.
Because magnetic nanoparticles can absorb magnetic field energy, MHA can prevent unnecessary heating of surrounding healthy tissues, making it a promising tumor treatment modality (Thiesen and Jordan, 2008; Wang F. et al., 2017). The need for a magnetocaloric agent to induce heating effect, and the absence of tissue penetration limit in the used magnetic field, allows precise ablation of deep tumors (Yan et al., 2005). When the resistivity of the conductor is small (as in metals), the eddy currents induced by the alternating magnetic field (AMF) will be strong, and the resulting heat generated will be large. Therefore, transition-metal based nanomaterials make a strong, promising magnetic material, useful for magnetocaloric ablation (Muranaka et al., 2007).
The heat generated by iron peroxide nanoparticles (SPIO) in an oscillating radio frequency field is due to the hysteresis loss or Brownian rotation of the nanoparticles and depends on the oscillating magnetic field frequency. Under an applied electric field, the dipole interaction between adjacent particles increases the anisotropy; the concentration of nanoparticles can also lead to better heating performance (Chandrasekharan et al., 2020). Sadhukha et al. (2013) developed inhalable superparamagnetic iron oxide nanoparticles that were chelated with target-specific epidermal growth factor receptor for realizing targeted SPIOs accumulation in tumor, increasing thermal energy production and monitoring tumor ablation under MHT.
Many currently used magnetic nanoparticles, such as iron oxide, require strong AMFs for effective heating, while safer and more effective magnetocaloric formulations are required for tumor ablation therapy (Albarqi et al., 2019). Magnesium alloy (MgA) with excellent in vivo biocompatibility, biodegradability, and low elastic modulus, was widely used in clinical practice (Lin et al., 2019). Yang N. et al. (2021) verified that the MgA rods under magnetorheological fluid showed a significant temperature increase and MgA with a strong vortex thermal effect could effectively improve tumor PTA under a low magnetic field. Chao et al. (2019) found that pure iron nanoparticles (FeNPs) modified with polymers such as polyethylene glycol (PEG), stable in aqueous solution, can be used as an ultra-efficient magnetic material with sufficient heating under low-power AMF to achieve effective MHA. In addition, local injection of FeNPs-based nanomaterials for MHA can generate immune memory effect, inhibit tumor metastasis and prevent tumor recurrence.
Transition Metal-Based Nanomaterial-Assisted High-Intensity Focused Ultrasound Ablation
HIFU that has been widely used in clinical practice can significantly reduce damages to the surrounding tissue with precise transfer of heat energy to the tumor site (Bachu et al., 2021). This needle-free, non-ionizing and thermal ablation tool has become a common method for non-invasive ablation of various solid tumors, harvesting inspiring clinical results (Wang M. et al., 2019). HIFU absorbs high acoustic energy and focuses it on the selected area, which raises the temperature in the tissue to above 60–65°C, resulting in protein denaturation and irreversible tumor tissue coagulation and necrosis (Diederich and Hynynen, 1999).
However, high-efficiency, high-power ultrasound treatment can burn surrounding skin and healthy tissue, leading to adverse effects. The application of ultrasound absorbers can enhance HIFU therapy (Dibaji et al., 2014). The photothermal conversion properties and high stability of Au nanomaterials (AuNPs) make them suitable for HIFU (Qian et al., 2017). In addition, AuNPs enhance the therapeutic effect of HIFU by increasing the temperature and sound energy absorption rate (Sadeghi-Goughari et al., 2019). Wang et al. (2013) synthesized MSNC@Au-PFH-PEG, abb. as MAPP, using AuNPs as the capping layer. They verified that the high thermal conductivity and thermal efficiency of finely anchored AuNPs significantly increase thermal energy accumulation to enhance the effect of tumor thermal ablation. In addition, MAPP is used in enhanced ultrasound imaging. Under the guidance, the ablation effect and accuracy of high-frequency ultrasound can be significantly improved.
The clinical use of HIFU is increasing, but damage to the skin and adjacent healthy tissues cannot be ignored. Superparamagnetic iron oxide nanoparticles (SPIONs) have great prospects in the biomedical field, especially as ultrasound absorbers, owing to their reliable sources and excellent superparamagnetic properties (Wei et al., 2021). Devarakonda et al. (as cited in Sun et al., 2014) improved the thermal ablation effect of tumors by combining the advantages of SPIONs and polymers to construct PLGA-coated Fe3O4 microcapsules to enable energy deposition and enhance the absorption of ultrasonic waves. In addition, PLGA-coated Fe3O4 microcapsules were used as MR-guided contrast agents for improving the accuracy of tumor localization while minimizing damage to surrounding normal tissues.
Application of Transition-Metal Based Nanomaterials in Imaging Diagnosis
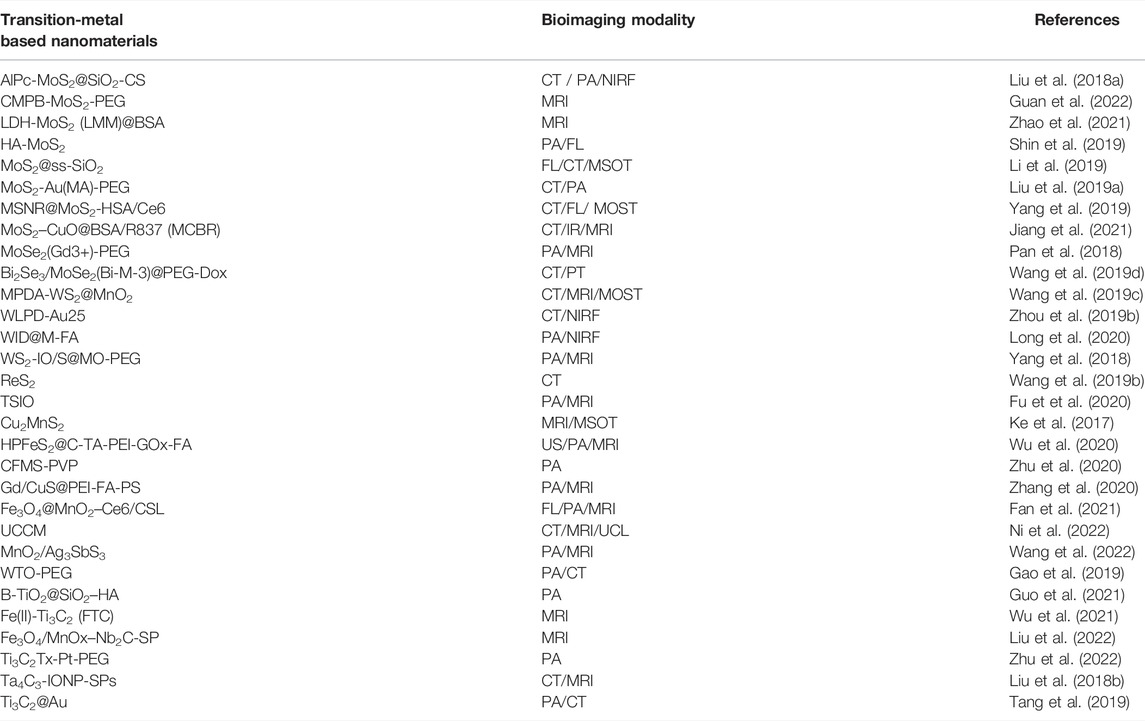
Because some tumors have no special symptoms in the early stages or are difficult to locate in the deep part of the body, most tumors are advanced or have metastases when diagnosed, with a low probability of cure. Therefore, early detection and diagnosis of cancer can give patients a chance for early cure and long-term survival. Currently used clinical imaging techniques, such as photoluminescence imaging, MRI, computed tomography (CT), positron emission tomography, ultrasound imaging, or optical imaging, hold great promise in cancer diagnosis (Huang et al., 2012). However, conventional contrast agents suffer from numerous problems, such as rapid bleaching, unsuitability for multicolor imaging, affected by the local chemical environment, interference from their background fluorescence, low brightness, and poor photostability (Sharma et al., 2006), limiting successful diagnosis. Depending on their good electrical conductivity, magnetic properties, biocompatibility, and non-toxicity (Sundaram et al., 2020), transition-metal-based nanomaterials can serve as contrast agents to build a nanomedicine platform for multimodal imaging-guided non-invasive thermal ablation such as photoacoustic imaging (Huang et al., 2018). The birth of this nanoplatform offers more opportunities for cancer patients to survive. We summarized some bioimaging applications of transition-metal based nanomaterials in Table 2.
Bioimaging Applications of Transition Metal Dichalcogenides
We mainly introduce the latest TMDC composite nanomaterials for bioimaging applications. Tungsten disulfide (WS2), Molybdenum disulfide (MoS2), and titanium disulfide (TiS2) received more attentions in bioimaging with the advantages of catalytic performance, photoluminescence, light absorption, and high wear resistance (Rohaizad et al., 2021). Liu L. et al. (2018) synthesized a novel chitosan (CS)-controlled aluminum chloride phthalocyanine (AlPc)-supported MoS2 nanocomposite (AlPcMoS2@SiO2-CS) with high HU value of 12HU Lg−1. This nanocomposite can solve the defects of short circulating half-life and non-specific distribution of traditional CT contrast agents and overcome poor tissue penetration and low sensitivity of PA (Liu et al., 2015). With the 4T1 mouse tumor-bearing model, they intravenously injected AlPcMoS2@SiO2-CS into mice and recognized a strong PA signal at tumor. Therefore, AlPc-MoS2@SiO2-CS can perform CT/PA dual-modal imaging, which is a non-invasive method for tumors.
MRI is a non-invasive imaging technique that can distinguish image contrast difference between normal and diseased tissues (Iima and Bihan, 2016). Nowadays, people use Prussian blue containing ferric ions as a T2-weighted MRI agent to guide MHA (Zhu et al., 2015). Shao et al. (as cited in Guan et al., 2022) synthesized a transition metal composite nanosheet (CMPB-MoS2-PEG) composed of Cu/Mn ion-doped Pb and MoS2 for MRI of tumors and can aggregate at tumor sites for a long time (Figure 4A). Zhao et al. (2021) used layered double hydroxide and bovine serum albumin (BSA)-modified MoS2 to obtain a composite transition-metal based nanomaterials for T1-weighted MRI. Utilizing the characteristics of the tumor microenvironment, hypoxia, weak acid, reducing (Watson et al., 2021), intravenous injection of LMM@BSA clay sheets into H29 cell tumor-bearing mice showed the significantly-enhanced in vivo MR imaging brightness.
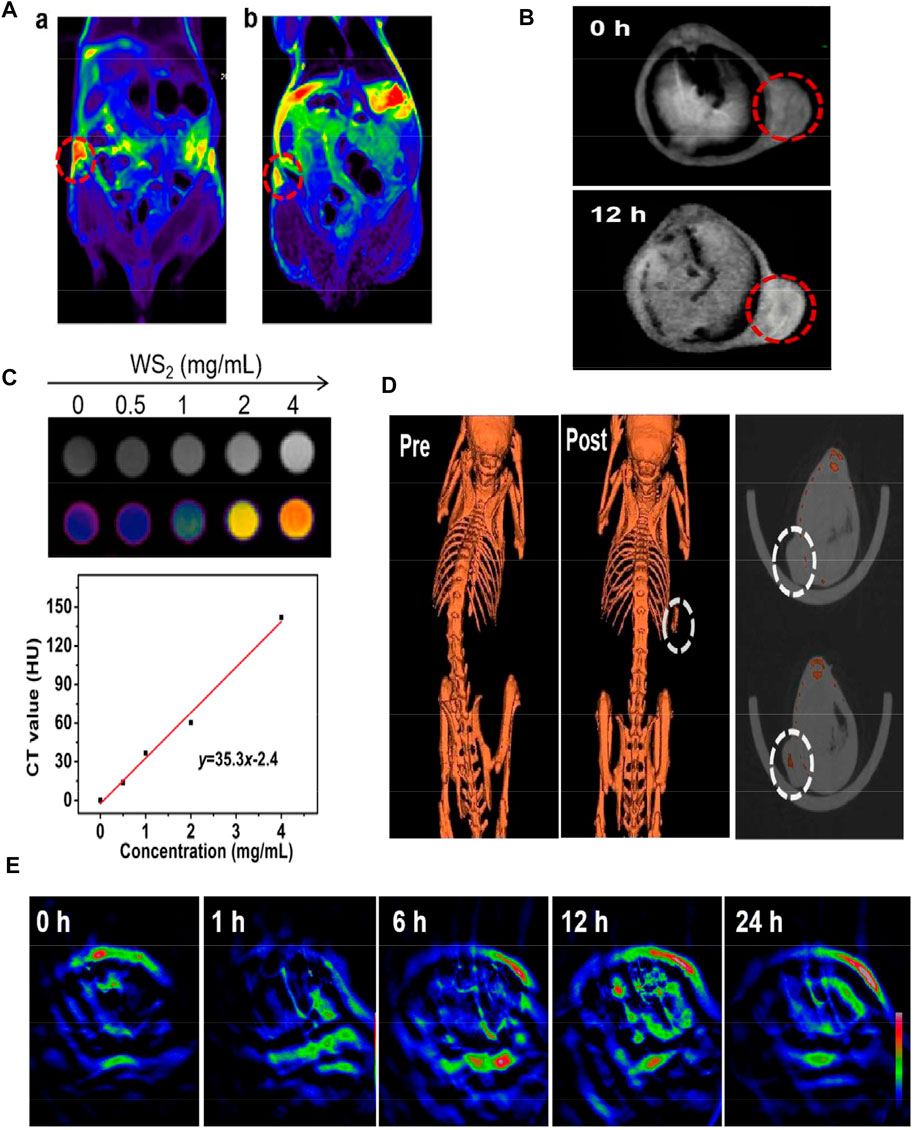
FIGURE 4. (A) T2-weighted MRI before and after injection of CMPB-MoS2-PEG. (B) T1-weighted MRI before and after injection of MPDA-WS2@MnO2. (C) The CT value (Hounsfield units, Hu) increased linearly with the increase of the concentration. (D) CT imaging of tumor on mice before and after injection of MPDA-WS2@MnO2. (E) MOST imaging of tumor on mice before and after injection of MPDA-WS2@MnO2. Reprinted (adapted) with permission from Guan et al. (2022).J Colloid Interface Sci. 2022, 608 (Pt 1),344–354. Copyright 2021 Elsevier Inc. Reprinted (adapted) with permission from Wang et al. (2019c). biomaterials. 2019,220,119405.Copyright 2019 Elsevier Ltd.
Multispectral photoacoustic tomography is an emerging imaging technique that combines the advantages of optical and ultrasound imaging with high resolution and sensitivity (Ke et al., 2017). Wang et al. (2019c) synthesized mesoporous dopamine (MPDA) and manganese dioxide (MnO2) onto WS2 to obtain MPDA-WS2@MnO2 nanoparticles. In 4T1 tumor-bearing mice, CT signal increased significantly from 52 HU Lg−1 to 419 HU Lg−1, 30 min after administration, while the PA signal and T1-weighted signal doubled after 12 h (Figure 4B). This CT/MOST/MR multi-modal imaging makes transition-metal based nanomaterials more accurate and reliable in biological imaging (Figures 4C–E). Fu et al. (2020) anchored iron oxide (IO) on titanium disulfide (TiS2) nanosheets to obtain TISO nanoplatforms as T2-weighted contrast agents for MRI, increasing the T2 relaxation rate (R2) by 8.9 times. In addition, the NIR window (NIR-II) irradiation increased the PA imaging amplitude by 1.58 times, and a clearer MR/PA image was obtained.
Transition Metal Oxides in Bioimaging Applications
Compared with TMDCs, TMOs have redox and cation exchange capabilities (Jia et al., 2020). Currently, their research is still at a relatively nascent stage, so we will elaborate on the application of MnO2 and TiO2 in bioimaging, the hottest research in recent years. Fan et al. (2021) successfully prepared Fe3O4@MnO2–Ce6/CSL nanodiagnostic platform by loading terpenoid (CSL)/photosensitizer Ce6 on the surface to grow Fe3O4 MnO2 nanoparticles. In Bel-7402 tumor-bearing nude mice, the Mn and Fe ions released by the nanomaterials were found to enhance the magnetic resonance T1 signal and weaken the T2 signal. Moreover, the release of Ce6 can achieve fluorescence imaging (FL) and a strong PA signal. Thus, this triple imaging platform of MRI/FL/PA can clearly locate the tumor. Gao et al. (2019) showed that the HU value of WTO nanoparticles obtained by PEGylation of W-doped TiO2 was 7.9 HU Lg−1 higher than the 6.3 HU Lg−1 using the traditional contrast agent (iopromide), so the CT signal become stronger. In addition, the strong absorption in the NIR-II window makes it possible to use glycated WTO for dual-mode imaging of CT/PA.
MXenes in Bioimaging Applications
MXenes are 2D carbides, nitrides, and carbonitrides representing transition metals. The most distinctive feature of MXenes is their ability to target tumor cells with minimal cytotoxicity to nonmalignant cells (Szuplewska et al., 2020). They are also highly conductive and magnetic, making them a diagnostic tool for cancer (VahidMohammadi et al., 2021). Wu et al. (2021) anchored ferrous ions on the Ti3C2 nanolayer to obtain a multifunctional nanoplatform of Fe(II)-Ti3C2 (FTC). They found that the MRI imaging time in MKN45 tumor-bearing nude mice was as long as 24 h, creating a time window for treating tumors. Liu et al. (2022) successfully synthesized Fe3O4/MnOx–Nb2C-SP composite MXenes nanoplatform by loading magnetic Fe3O4/MnOx on niobium carbide (Nb2C) ultrathin nanosheets and modifying them with soybean phospholipid. The platform injected 4T1 tumor-bearing mice intravenously, and the results showed that the T1 signal intensity increased by 43.21%, while the T2 signal intensity decreased by 31.43%, achieving T1/T2 contrast-enhanced MRI imaging.
Conclusion and Outlook
Nanotechnology is a new research field that has developed rapidly in recent years. It has had a huge impact in multiple research fields while simultaneously providing significant challenges and opportunities. A large number of studies have shown that transition-metal based nanomaterials can not only effectively improve the tumor treatment effect of various ablation techniques through various mechanisms, but also provide the possibility for early diagnosis and treatment of tumors, and more importantly, inhibit tumor metastasis and prevent tumor recurrence. Transition-metal based nanomaterials are important in medicine and pharmaceutical sciences, mainly because of their combined catalytic and redox properties and coordination abilities. In this paper, the characteristics and properties of transition-metal based nanomaterials are reviewed. Transition-metal based nanomaterials provide accurate and efficient tumor thermal ablation, while biological imaging can be used for early and differential diagnosis of diseases.
Despite the attractive results of transition-metal based nanomaterials, there are still some issues that need to be addressed. First, the current research on transition-metal based nanomaterials is limited, and the systematic biosafety is not clear enough; secondly, these nanomaterials lack sufficient targeting ability and may cause damages to normal human tissues or cells. Although transition-metal based nanomaterials have many problems to be solved, they still provide unlimited potential and great chances for cancer treatment and diagnosis, and their research progress opened up new fields in diagnosing and treating many diseases.
Author Contributions
QP: Data curation; resources; writing-original draft. ZQ: Data curation; resources; validation. KZ: conception and design; supervision; validation. All authors contributed to the article and approved the submitted version.
Funding
We acknowledge the support from National Natural Science Foundation of China (Grant No. 82022033), the Fundamental Research Funds for the Central Universities (22120210561), Shanghai Rising-Star Program (Grant No. 19QA1406800) and Shanghai Talent Development Fund (Grant No. 2019040) and Shanghai Young Top-Notch Talent, Scientific and Technological Innovation Major Base of Guangxi (No. 2018-15Z04), Guangxi Key Research and Development Project (No. AB20117001), Guangxi science and technology bases and talent special project (No. AD17129062).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JC declared a past co-authorship with the author KZ to the handling editor.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or any claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are very appreciated for the help from Prof. Yongxiang Zhao (Guangxi Medical University) in language polishing.
References
Albarqi, H. A., Wong, L. H., Schumann, C., Sabei, F. Y., Korzun, T., and Li, X. (2019). Biocompatible Nanoclusters with High Heating Efficiency for Systemically Delivered Magnetic Hyperthermia. ACS Nano 13, 6383–6395. doi:10.1021/acsnano.8b06542
Alexandrov, L. B., Nik-Zainal, S., Wedge, D. C., Aparicio, S. A., Behjati, S., Biankin, A. V., et al. (2013). Signatures of Mutational Processes in Human Cancer. Nature 500, 415–421. doi:10.1038/nature12477
Bachu, V. S., Kedda, J., Suk, I., Green, J. J., and Tyler, B. (2021). High-Intensity Focused Ultrasound: A Review of Mechanisms and Clinical Applications. Ann. Biomed. Eng. 49, 1975–1991. doi:10.1007/s10439-021-02833910.1007/s10439-021-02833-9
Bai, L., Yi, W., Wang, Y., Tian, Y., Zhou, B., Yi, T., et al. (2021). A PdMo Bimetallene with Precise Wavelength Adjustment and Catalysis for Synergistic Photothermal Ablation and Hydrogen Therapy of Cancer at Different Depths. J. Mater. Chem. B 9, 6441–6459. doi:10.1039/d1tb01284c
Ban, Q., Bai, T., Duan, X., and Kong, J. (2017). Noninvasive Photothermal Cancer Therapy Nanoplatforms via Integrating Nanomaterials and Functional Polymers. Biomater. Sci. 5, 190–210. doi:10.1039/c6bm00600k
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 68, 394–424. doi:10.3322/caac.21492
Chandrasekharan, P., Tay, Z. W., Hensley, D., Zhou, X. Y., Fung, B. K., Colson, C., et al. (2020). Using Magnetic Particle Imaging Systems to Localize and Guide Magnetic Hyperthermia Treatment: Tracers, Hardware, and Future Medical Applications. Theranostics 10, 2965–2981. doi:10.7150/thno.40858
Chang, M., Hou, Z., Wang, M., Li, C., and Lin, J. (2021). Recent Advances in Hyperthermia Therapy-Based Synergistic Immunotherapy. Adv. Mater. 33, 2004788. doi:10.1002/adma.202004788
Chao, Y., Chen, G., Liang, C., Xu, J., Dong, Z., Han, X., et al. (2019). Iron Nanoparticles for Low-Power Local Magnetic Hyperthermia in Combination with Immune Checkpoint Blockade for Systemic Antitumor Therapy. Nano Lett. 19, 4287–4296. doi:10.1021/acs.nanolett.9b00579
Cheng, K., Kothapalli, S. R., Liu, H., Koh, A. L., Jokerst, J. V., Jiang, H., et al. (2014). Construction and Validation of Nano Gold Tripods for Molecular Imaging of Living Subjects. J. Am. Chem. Soc. 136, 3560–3571. doi:10.1021/ja412001e
Chimene, D., Alge, D. L., and Gaharwar, A. K. (2015). Two-Dimensional Nanomaterials for Biomedical Applications: Emerging Trends and Future Prospects. Adv. Mater. 27, 7261–7284. doi:10.1002/adma.201502422
Chu, K. F., and Dupuy, D. E. (2014). Thermal Ablation of Tumours: Biological Mechanisms and Advances in Therapy. Nat. Rev. Cancer 14, 199–208. doi:10.1038/nrc3672
Debnath, A., Saha, S., Li, D. O., Chu, X. S., Ulissi, Z. W., Green, A. A., et al. (2021). Elimination of Multidrug-Resistant Bacteria by Transition Metal Dichalcogenides Encapsulated by Synthetic Single-Stranded DNA. ACS Appl. Mater. Inter. 13, 8082–8094. doi:10.1021/acsami.0c22941
Dibaji, S. A. R., Al-Rjoub, M. F., Myers, M. R., and Banerjee, R. K. (2014). Enhanced Heat Transfer and Thermal Dose Using Magnetic Nanoparticles during HIFU Thermal Ablation—An In-Vitro Study. Nanotechnol. Eng. Med. 4, 041003. doi:10.1115/1.4027340
Diederich, C., and Hynynen, K. (1999). Ultrasound Technology for Hyperthermia. Ultrasound Med. Biol. 25, 871–887. doi:10.1016/s0301-5629(99)00048-4
Doughty, A. C. V., Hoover, A. R., Layton, E., Murray, C. K., Howard, E. W., and Chen, W. R. (2019). Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 12, 779. doi:10.3390/ma12050779
Fan, S., Zhang, Y., Tan, H., Xue, C., He, Y., Wei, X., et al. (2021). Manganese/iron-Based Nanoprobes for Photodynamic/Chemotherapy Combination Therapy of Tumor Guided by Multimodal Imaging. Nanoscale 13, 5383–5399. doi:10.1039/d0nr08831e
Fang, Y., Li, H., Yin, H., Xu, S., Ren, W., Ding, S., et al. (2019). Radiofrequency-Sensitive Longitudinal Relaxation Tuning Strategy Enabling the Visualization of Radiofrequency Ablation Intensified by Magnetic Composite. ACS Appl. Mater. Inter. 11, 11251–11261. doi:10.1021/acsami.9b02401
Fu, B., Sun, J., Wang, C., Shang, C., Xu, L., Li, J., et al. (2021). MXenes: Synthesis, Optical Properties, and Applications in Ultrafast Photonics. Small 17, 2006054. doi:10.1002/smll.202006054
Fu, Q., Li, Z., Ye, J., Li, Z., Fu, F., Lin, S. L., et al. (2020). Magnetic Targeted Near-Infrared II PA/MR Imaging Guided Photothermal Therapy to Trigger Cancer Immunotherapy. Theranostics 10, 4997–5010. doi:10.7150/thno.43604
Gao, K., Tu, W., Yu, X., Ahmad, F., Zhang, X., Wu, W., et al. (2019). W-doped TiO2 Nanoparticles with Strong Absorption in the NIR-II Window for Photoacoustic/CT Dual-Modal Imaging and Synergistic Thermoradiotherapy of Tumors. Theranostics 9, 5214–5226. doi:10.7150/thno.33574
Goel, S., Chen, F., and Cai, W. (2014). Synthesis and Biomedical Applications of Copper Sulfide Nanoparticles: From Sensors to Theranostics. Small 10, 631–645. doi:10.1002/smll.201301174
Goldberg, S. N., Gazelle, G. S., and Mueller, P. R. (2000). Thermal Ablation Therapy for Focal Malignancy: A Unified Approach to Underlying Principles, Techniques, and Diagnostic Imaging Guidance. AJR Am. J. Roentgenol 174, 323–331. doi:10.2214/ajr.174.2.1740323
Goncalves, A. S. C., Rodrigues, C. F., Moreira, A. F., and Correia, I. J. (2020). Strategies to Improve the Photothermal Capacity of Gold-Based Nanomedicines. Acta Biomater. 116, 105–137. doi:10.1016/j.actbio.2020.09.008
Guan, S., Liu, X., Fu, Y., Li, C., Wang, J., Mei, Q., et al. (2022). A Biodegradable "Nano-Donut" for Magnetic Resonance Imaging and Enhanced Chemo/Photothermal/Chemodynamic Therapy through Responsive Catalysis in Tumor Microenvironment. J. Colloid Interf. Sci 608, 344–354. doi:10.1016/j.jcis.2021.09.186
Guo, X., Wen, C., Xu, Q., Ruan, C., Shen, X. C., and Liang, H. (2021). A Full-Spectrum Responsive B-TiO2@SiO2-HA Nanotheranostic System for NIR-II Photoacoustic Imaging-Guided Cancer Phototherapy. J. Mater. Chem. B 9, 2042–2053. doi:10.1039/d0tb02952a
Gupta, N., and Malviya, R. (2021). Understanding and Advancement in Gold Nanoparticle Targeted Photothermal Therapy of Cancer. Biochim. Biophys. Acta Rev. Cancer 1875, 188532. doi:10.1016/j.bbcan.2021.188532
Huang, K., Li, Z., Lin, J., Han, G., and Huang, P. (2018). Two-Dimensional Transition Metal Carbides and Nitrides (MXenes) for Biomedical Applications. Chem. Soc. Rev. 47, 5109–5124. doi:10.1039/c7cs00838d
Huang, Y., He, S., Cao, W., Cai, K., and Liang, X. J. (2012). Biomedical Nanomaterials for Imaging-Guided Cancer Therapy. Nanoscale 4, 6135–6149. doi:10.1039/c2nr31715j
Iima, M., and Bihan, D. L. (2016). Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology 278, 13–32. doi:10.1148/radiol.2015150244
Jahangirian, H., Kalantari, K., Izadiyan, Z., Rafiee-Moghaddam, R., Shameli, K., and Webster, T. J. (2019). A Review of Small Molecules and Drug Delivery Applications Using Gold and Iron Nanoparticles. Int. J. Nanomedicine 14, 1633–1657. doi:10.2147/IJN.S184723
Jain, P. K., Lee, K. S., El-Sayed, I. H., and El-Sayed, M. A. (2006). Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 110, 7238–7248. doi:10.1021/jp057170o
Jang, B., Moorthy, M. S., Manivasagan, P., Song, K., Lee, K. D., Kwak, M., et al. (2018). Fucoidan-Coated CuS Nanoparticles for Chemo-And Photothermal Therapy against Cancer. Oncotarget 9, 12649–12661. doi:10.18632/oncotarget.23898
Jaque, D., Martinez Maestro, L., del Rosal, B., Haro-Gonzalez, P., Benayas, A., Plaza, J. L., et al. (2014). Nanoparticles for Photothermal Therapies. Nanoscale 6, 9494–9530. doi:10.1039/c4nr00708e
Jia, Y., Yi, X., Li, Z., Zhang, L., Yu, B., Zhang, J., et al. (2020). Recent Advance in Biosensing Applications Based on Two Dimensional Transition Metal Oxide Nanomaterials. Talanta 219, 121308. doi:10.1016/j.talanta.2020.121308
Jiang, F., Ding, B., Liang, S., Zhao, Y., Cheng, Z., Xing, B., et al. (2021). Intelligent MoS2-CuO Heterostructures with Multiplexed Imaging and Remarkably Enhanced Antitumor Efficacy via Synergetic Photothermal Therapy/ Chemodynamic Therapy/ Immunotherapy. Biomaterials 268, 120545. doi:10.1016/j.biomaterials.2020.120545
Ke, K., Yang, W., Xie, X., Liu, R., Wang, L. L., Lin, W. W., et al. (2017). Copper Manganese Sulfide Nanoplates: A New Two-Dimensional Theranostic Nanoplatform for MRI/MSOT Dual-Modal Imaging-Guided Photothermal Therapy in the Second Near-Infrared Window. Theranostics 7, 4763–4776. doi:10.7150/thno.21694
Krzysztof, S., Gorzkiewicz, M. G., and Klajnert-Maculewicz, B (2019). Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 16, 1–23. doi:10.1021/acs.molpharmaceut.8b00810
Lapotko, D. (2009). Optical Excitation and Detection of Vapor Bubbles Around Plasmonic Nanoparticles. Opt. Express 17, 2538–2556. doi:10.1364/oe.17.002538
Li, P., Liu, L., Lu, Q., Yang, S., Yang, L., Cheng, Y., et al. (2019). Ultrasmall MoS2 Nanodots-Doped Biodegradable SiO2 Nanoparticles for Clearable FL/CT/MSOT Imaging-Guided PTT/PDT Combination Tumor Therapy. ACS Appl. Mater. Inter. 11, 5771–5781. doi:10.1021/acsami.8b18924
Li, X., Shan, J., Zhang, W., Su, S., Yuwen, L., and Wang, L. (2017). Recent Advances in Synthesis and Biomedical Applications of Two-Dimensional Transition Metal Dichalcogenide Nanosheets. Small 13, 1602660. doi:10.1002/smll.201602660
Li, Y., Lu, W., Huang, Q., Huang, M., Li, C., and Chen, W. (2010). Copper Sulfide Nanoparticles for Photothermal Ablation of Tumor Cells. Nanomedicine 5, 1161–1171. doi:10.2217/nnm.10.85
Liberman, A., Wu, Z., Barback, C. V., Viveros, R. D., Wang, J., Ellies, L. G., et al. (2014). Hollow Iron-Silica Nanoshells for Enhanced High Intensity Focused Ultrasound. J. Surg. Res. 190, 391–398. doi:10.1016/j.jss.2014.05.009
Lin, H., Chen, Y., and Shi, J. (2018a). Insights into 2D MXenes for Versatile Biomedical Applications: Current Advances and Challenges Ahead. Adv. Sci. 5, 1800518. doi:10.1002/advs.201800518
Lin, T., Zhao, X., Zhao, S., Yu, H., Cao, W., Chen, W., et al. (2018b). O2-Generating MnO2 Nanoparticles for Enhanced Photodynamic Therapy of Bladder Cancer by Ameliorating Hypoxia. Theranostics 8, 990–1004. doi:10.7150/thno.22465
Lin, Z., Zhao, Y., Chu, P. K., Wang, L., Pan, H., Zheng, Y., et al. (2019). A Functionalized TiO2/Mg2TiO4 Nano-Layer on Biodegradable Magnesium Implant Enables Superior Bone-Implant Integration and Bacterial Disinfection. Biomaterials 219, 119372. doi:10.1016/j.biomaterials.2019.119372
Liu, J., Zheng, X., Yan, L., Zhou, L., Tian, G., Yin, W., et al. (2015). Bismuth Sulfide Nanorods as a Precision Nanomedicine for In Vivo Multimodal Imaging-Guided Photothermal Therapy of Tumor. ACS Nano 9, 696–707. doi:10.1021/nn506137n
Liu, L., Wang, J., You, Q., Sun, Q., Song, Y., Wang, Y., et al. (2018a). NIRF/PA/CT Multi-Modality Imaging Guided Combined Photothermal and Photodynamic Therapy Based on Tumor Microenvironment-Responsive Nanocomposites. J. Mater. Chem. B 6, 4239–4250. doi:10.1039/c8tb00859k
Liu, T., Shen, S., Huang, Y., Zhang, X., Lai, Z., Tran, T. H., et al. (2019a). Controllable Growth of Au Nanostructures onto MoS2 Nanosheets for Dual-Modal Imaging and Photothermal-Radiation Combined Therapy. Nanoscale 11, 22788–22795. doi:10.1039/c9nr06513j
Liu, Y., Bhattarai, P., Dai, Z., and Chen, X. (2019b). Photothermal Therapy and Photoacoustic Imaging via Nanotheranostics in Fighting Cancer. Chem. Soc. Rev. 48, 2053–2108. doi:10.1039/c8cs00618k
Liu, Z., Lin, H., Zhao, M., Dai, C., Zhang, S., Peng, W., et al. (2018b). 2D Superparamagnetic Tantalum Carbide Composite MXenes for Efficient Breast-Cancer Theranostics. Theranostics 8, 1648–1664. doi:10.7150/thno.23369
Liu, Z., Zhao, M., Yu, L., Peng, W., Chen, Y., and Zhang, S. (2022). Redox Chemistry-Enabled Stepwise Surface Dual Nanoparticle Engineering of 2D MXenes for Tumor-Sensitive T1 and T2 MRI-Guided Photonic Breast-Cancer Hyperthermia in the NIR-II Biowindow. Biomater. Sci. 10, 1562–1574. doi:10.1039/d1bm01957k
Long, Y., Wu, X., Li, Z., Fan, J., Hua, X., and Liu, B. (2020). PEGylated WS2 Nanodrug System with Erythrocyte Membrane Coating for Chemo/Photothermal Therapy of Cervical Cancer. Biomater. Sci. 8, 5088–5105. doi:10.1039/d0bm00972e
Luo, Y., Fu, Y., Huang, Z., and Li, M. (2021). Transition Metals and Metal Complexes in Autophagy and Diseases. J. Cel Physiol 236, 7144–7158. doi:10.1002/jcp.30359
McLaughlan, J. R., Cowell, D. M. J., and Freear, S. (2017). Gold Nanoparticle Nucleated Cavitation for Enhanced High Intensity Focused Ultrasound Therapy. Phys. Med. Biol. 63, 015004. doi:10.1088/1361-6560/aa97e9
Muranaka, H., Horiguchi, T., Usui, S., Ueda, Y., Nakamura, O., and Ikeda, F. (2007). Dependence of RF Heating on Sar and Implant Position in a 1.5T Mr System. Magn. Reson. Med. Sci. 6, 199–209. doi:10.2463/mrms.6.199
Murugan, C., Sharma, V., Murugan, R. K., Malaimegu, G., and Sundaramurthy, A. (2019). Two-Dimensional Cancer Theranostic Nanomaterials: Synthesis, Surface Functionalization and Applications in Photothermal Therapy. J. Control. Release 299, 1–20. doi:10.1016/j.jconrel.2019.02.015
Ni, J., Xu, H., Zhong, Y., Zhou, Y., and Hu, S. (2022). Activatable UCL/CT/MR-Enhanced In Vivo Imaging-Guided Radiotherapy and Photothermal Therapy. J. Mater. Chem. B 10, 549–561. doi:10.1039/d1tb02006d
Pan, J., Zhu, X., Chen, X., Zhao, Y., and Liu, J. (2018). Gd(3+)-Doped MoSe2 Nanosheets Used as a Theranostic Agent for Bimodal Imaging and Highly Efficient Photothermal Cancer Therapy. Biomater. Sci. 6, 372–387. doi:10.1039/c7bm00894e
Qian, X., Han, X., and Chen, Y. (2017). Insights into the Unique Functionality of Inorganic Micro/Nanoparticles for Versatile Ultrasound Theranostics. Biomaterials 142, 13–30. doi:10.1016/j.biomaterials.2017.07.016
Rastinehad, A. R., Anastos, H., Wajswol, E., Winoker, J. S., Sfakianos, J. P., Doppalapudi, S. K., et al. (2019). Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study. Proc. Natl. Acad. Sci. U S A. 116, 18590–18596. doi:10.1073/pnas.1906929116
Rohaizad, N., Mayorga-Martinez, C. C., Fojtu, M., Latiff, N. M., and Pumera, M. (2021). Two-Dimensional Materials in Biomedical, Biosensing and Sensing Applications. Chem. Soc. Rev. 50, 619–657. doi:10.1039/d0cs00150c
Sadeghi-Goughari, M., Jeon, S., and Kwon, H. J. (2019). Enhancing Thermal Effect of Focused Ultrasound Therapy Using Gold Nanoparticles. IEEE Trans. Nanobioscience 18, 661–668. doi:10.1109/TNB.2019.2937327
Sadhukha, T., Wiedmann, T. S., and Panyam, J. (2013). Inhalable Magnetic Nanoparticles for Targeted Hyperthermia in Lung Cancer Therapy. Biomaterials 34, 5163–5171. doi:10.1016/j.biomaterials.2013.03.061
Sharma, P., Brown, S., Walter, G., Santra, S., and Moudgil, B. (2006). Nanoparticles for Bioimaging. Adv. Colloid Interf. Sci 123-126, 471–485. doi:10.1016/j.cis.2006.05.026
Shi, J., Kantoff, P. W., Wooster, R., and Farokhzad, O. C. (2017). Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 17, 20–37. doi:10.1038/nrc.2016.108
Shin, M. H., Park, E. Y., Han, S., Jung, H. S., Keum, D. H., Lee, G. H., et al. (2019). Multimodal Cancer Theranosis Using Hyaluronate-Conjugated Molybdenum Disulfide. Adv. Healthc. Mater. 8, 1801036. doi:10.1002/adhm.201801036
Shukla, V. (2020). The Tunable Electric and Magnetic Properties of 2D MXenes and Their Potential Applications. Mater. Adv. 1, 3104–3121. doi:10.1039/d0ma00548g
Song, J., Yang, X., Jacobson, O., Huang, P., Sun, X., Lin, L., et al. (2015). Ultrasmall Gold Nanorod Vesicles with Enhanced Tumor Accumulation and Fast Excretion from the Body for Cancer Therapy. Adv. Mater. 27, 4910–4917. doi:10.1002/adma.201502486
Sun, Y., Zheng, Y., Li, P., Wang, D., Niu, C., Gong, Y., et al. (2014). Evaluation of Superparamagnetic Iron Oxide-Polymer Composite Microcapsules for Magnetic Resonance-Guided High-Intensity Focused Ultrasound Cancer Surgery. BMC Cancer 14, 800. doi:10.1186/1471-2407-14-800
Sundaram, A., Ponraj, J. S., Wang, C., Peng, W. K., Manavalan, R. K., Dhanabalan, S. C., et al. (2020). Engineering of 2D Transition Metal Carbides and Nitrides MXenes for Cancer Therapeutics and Diagnostics. J. Mater. Chem. B 8, 4990–5013. doi:10.1039/d0tb00251h
Szuplewska, A., Kulpinska, D., Dybko, A., Chudy, M., Jastrzebska, A. M., Olszyna, A., et al. (2020). Future Applications of MXenes in Biotechnology, Nanomedicine, and Sensors. Trends Biotechnol. 38, 264–279. doi:10.1016/j.tibtech.2019.09.001
Tang, W., Dong, Z., Zhang, R., Yi, X., Yang, K., Jin, M., et al. (2019). Multifunctional Two-Dimensional Core-Shell MXene@Gold Nanocomposites for Enhanced Photo-Radio Combined Therapy in the Second Biological Window. ACS Nano 13, 284–294. doi:10.1021/acsnano.8b05982
Thiesen, B., and Jordan, A. (2008). Clinical Applications of Magnetic Nanoparticles for Hyperthermia. Int. J. Hyperthermia 24, 467–474. doi:10.1080/02656730802104757
Urbanova, V., and Pumera, M. (2019). Biomedical and Bioimaging Applications of 2D Pnictogens and Transition Metal Dichalcogenides. Nanoscale 11, 15770–15782. doi:10.1039/c9nr04658e
VahidMohammadi, A., Rosen, J., and Gogotsi, Y. (2021). The World of Two-Dimensional Carbides and Nitrides (MXenes). Science 372, 1165. doi:10.1126/science.abf1581
Wang, F., Yang, Y., Ling, Y., Liu, J., Cai, X., Zhou, X., et al. (2017a). Injectable and Thermally Contractible Hydroxypropyl Methyl Cellulose/Fe3O4 for Magnetic Hyperthermia Ablation of Tumors. Biomaterials 128, 84–93. doi:10.1016/j.biomaterials.2017.03.004
Wang, M., Chang, M., Chen, Q., Wang, D., Li, C., Hou, Z., et al. (2020). Au2Pt-PEG-Ce6 Nanoformulation with Dual Nanozyme Activities for Synergistic Chemodynamic Therapy / Phototherapy. Biomaterials 252, 120093. doi:10.1016/j.biomaterials.2020.120093
Wang, M., Lei, Y., and Zhou, Y. (2019a). High-Intensity Focused Ultrasound (HIFU) Ablation by the Frequency Chirps: Enhanced Thermal Field and Cavitation at the Focus. Ultrasonics 91, 134–149. doi:10.1016/j.ultras.2018.08.017
Wang, Q., Qu, B., Li, J., Liu, Y., Dong, J., Peng, X., et al. (2022). Multifunctional MnO2/Ag3SbS3 Nanotheranostic Agent for Single-Laser-Triggered Tumor Synergistic Therapy in the NIR-II Biowindow. ACS Appl. Mater. Inter. 14, 4980–4994. doi:10.1021/acsami.1c21752
Wang, X., Chen, H., Zheng, Y., Ma, M., Chen, Y., Zhang, K., et al. (2013). Au-Nanoparticle Coated Mesoporous Silica Nanocapsule-Based Multifunctional Platform for Ultrasound Mediated Imaging, Cytoclasis and Tumor Ablation. Biomaterials 34, 2057–2068. doi:10.1016/j.biomaterials.2012.11.044
Wang, X., Wang, J., Pan, J., Zhao, F., Kan, D., Cheng, R., et al. (2019b). Rhenium Sulfide Nanoparticles as a Biosafe Spectral CT Contrast Agent for Gastrointestinal Tract Imaging and Tumor Theranostics In Vivo. ACS Appl. Mater. Inter. 11, 33650–33658. doi:10.1021/acsami.9b10479
Wang, Y. H., Huang, K. J., and Wu, X. (2017b). Recent Advances in Transition-Metal Dichalcogenides Based Electrochemical Biosensors: A Review. Biosens. Bioelectron. 97, 305–316. doi:10.1016/j.bios.2017.06.011
Wang, Y., Song, S., Lu, T., Cheng, Y., Song, Y., Wang, S., et al. (2019c). Oxygen-Supplementing Mesoporous Polydopamine Nanosponges with WS2 QDs-Embedded for CT/MSOT/MR Imaging and Thermoradiotherapy of Hypoxic Cancer. Biomaterials 220, 119405. doi:10.1016/j.biomaterials.2019.119405
Wang, Y., Zhao, J., Chen, Z., Zhang, F., Wang, Q., Guo, W., et al. (2019d). Construct of MoSe2/Bi2Se3 Nanoheterostructure: Multimodal CT/PT Imaging-Guided PTT/PDT/Chemotherapy for Cancer Treating. Biomaterials 217, 119282. doi:10.1016/j.biomaterials.2019.119282
Watson, M. J., Vignali, P. D. A., Mullett, S. J., Overacre-Delgoffe, A. E., Peralta, R. M., Grebinoski, S., et al. (2021). Metabolic Support of Tumour-Infiltrating Regulatory T Cells by Lactic Acid. Nature 591, 645–651. doi:10.1038/s41586-020-03045-2
Wei, H., Hu, Y., Wang, J., Gao, X., Qian, X., and Tang, M. (2021). Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications. Int. J. Nanomedicine 16, 60976113. doi:10.2147/IJN.S321984
Wen, J., Yang, K., and Sun, S. (2020). MnO2-Based Nanosystems for Cancer Therapy. Chem. Commun. 56, 70657079. doi:10.1039/d0cc02782k
White, S. B., Kim, D. H., Guo, Y., Li, W., Yang, Y., Chen, J., et al. (2017). Biofunctionalized Hybrid Magnetic Gold Nanoparticles as Catalysts for Photothermal Ablation of Colorectal Liver Metastases. Radiology 285, 809819. doi:10.1148/radiol.2017161497
Wu, F., Zhang, Q., Zhang, M., Sun, B., She, Z., Ge, M., et al. (2020). Hollow Porous Carbon Coated FeS2-Based Nanocatalysts for Multimodal Imaging-Guided Photothermal, Starvation, and Triple-Enhanced Chemodynamic Therapy of Cancer. ACS Appl. Mater. Inter. 12, 10142–10155. doi:10.1021/acsami.0c00170
Wu, Y., Song, X., Xu, W., Sun, K. Y., Wang, Z., Lv, Z., et al. (2021). NIR-activated Multimodal Photothermal/Chemodynamic/Magnetic Resonance Imaging Nanoplatform for Anticancer Therapy by Fe(II) Ions Doped MXenes (Fe-Ti3 C2 ). Small 17, 2101705. doi:10.1002/smll.202101705
Xing, R., Liu, K., Jiao, T., Zhang, N., Ma, K., Zhang, R., et al. (2016). An Injectable Self-Assembling Collagen-Gold Hybrid Hydrogel for Combinatorial Antitumor Photothermal/Photodynamic Therapy. Adv. Mater. 28, 3669–3676. doi:10.1002/adma.201600284
Xu, M., Song, Y., Wang, J., and Li, N. (2021a). Anisotropic Transition Metal–Based Nanomaterials for Biomedical Applications. View 2, 20200154. doi:10.1002/viw.20200154
Xu, Q., Zhan, G., Zhang, Z., Yong, T., Yang, X., and Gan, L. (2021b). Manganese Porphyrin-Based Metal-Organic Framework for Synergistic Sonodynamic Therapy and Ferroptosis in Hypoxic Tumors. Theranostics 11, 19371952. doi:10.7150/thno.45511
Xue, P., Bao, J., Wu, Y., Zhang, Y., and Kang, Y. (2017). Porous Prussian Blue Nanocubes as Photothermal Ablation Agents for Efficient Cancer Therapy. J. Nanosci Nanotechnol 17, 168–174. doi:10.1166/jnn.2017.12408
Yan, S., Zhang, D., Gu, N., Zheng, J., Ding, A., Wang, Z., et al. (2005). Therapeutic Effect of Fe2O3 Nanoparticles Combined with Magnetic Fluid Hyperthermia on Cultured Liver Cancer Cells and Xenograft Liver Cancers. J. Nanosci Nanotechnol 5, 1185–1192. doi:10.1166/jnn.2005.219
Yang, G., Ji, J., and Liu, Z. (2021a). Multifunctional MnO2 Nanoparticles for Tumor Microenvironment Modulation and Cancer Therapy. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol 13, e1720. doi:10.1002/wnan.1720
Yang, G., Zhang, R., Liang, C., Zhao, H., Yi, X., Shen, S., et al. (2018). Manganese Dioxide Coated WS2 @Fe3O4 /sSiO2 Nanocomposites for pH-Responsive MR Imaging and Oxygen-Elevated Synergetic Therapy. Small 14, 1702664. doi:10.1002/smll.201702664
Yang, H., He, H., Tong, Z., Xia, H., Mao, Z., and Gao, C. (2020). The Impact of Size and Surface Ligand of Gold Nanorods on Liver Cancer Accumulation and Photothermal Therapy in the Second Near-Infrared Window. J. Colloid Interf. Sci 565, 186–196. doi:10.1016/j.jcis.2020.01.026
Yang, L., Tseng, Y. T., Suo, G., Chen, L., Yu, J., Chiu, W. J., et al. (2015). Photothermal Therapeutic Response of Cancer Cells to Aptamer-Gold Nanoparticle-Hybridized Graphene Oxide under NIR Illumination. ACS Appl. Mater. Inter. 7, 5097–5106. doi:10.1021/am508117e
Yang, L., Wang, J., Yang, S., Lu, Q., Li, P., and Li, N. (2019). Rod-shape MSN@MoS2 Nanoplatform for FL/MSOT/CT Imaging-Guided Photothermal and Photodynamic Therapy. Theranostics 9, 3992–4005. doi:10.7150/thno.32715
Yang, N., Gong, F., Cheng, L., Lei, H., Li, W., Sun, Z., et al. (2021b). Biodegradable Magnesium Alloy with Eddy Thermal Effect for Effective and Accurate Magnetic Hyperthermia Ablation of Tumors. Natl. Sci. Rev. 8, nwaa122. doi:10.1093/nsr/nwaa122
Yuan, H., Khoury, C. G., Wilson, C. M., Grant, G. A., Bennett, A. J., and Vo-Dinh, T. (2012). In Vivo Particle Tracking and Photothermal Ablation Using Plasmon-Resonant Gold Nanostars. Nanomedicine 8, 1355–1363. doi:10.1016/j.nano.2012.02.005
Yun, B., Zhu, H., Yuan, J., Sun, Q., and Li, Z. (2020). Synthesis, Modification and Bioapplications of Nanoscale Copper Chalcogenides. J. Mater. Chem. B 8, 4778–4812. doi:10.1039/d0tb00182a
Zhang, C., Sun, W., Wang, Y., Xu, F., Qu, J., Xia, J., et al. (2020). Gd-/CuS-Loaded Functional Nanogels for MR/PA Imaging-Guided Tumor-Targeted Photothermal Therapy. ACS Appl. Mater. Inter. 12, 9107–9117. doi:10.1021/acsami.9b23413
Zhang, K., Chen, H., Li, F., Wang, Q., Zheng, S., Xu, H., et al. (2014). A Continuous Tri-phase Transition Effect for HIFU-Mediated Intravenous Drug Delivery. Biomaterials 35, 5875–5885. doi:10.1016/j.biomaterials.2014.03.043
Zhang, K., Fang, Y., He, Y., Yin, H., Guan, X., Pu, Y., et al. (2019). Extravascular Gelation Shrinkage-Derived Internal Stress Enables Tumor Starvation Therapy with Suppressed Metastasis and Recurrence. Nat. Commun. 10, 5380. doi:10.1038/s41467-019-13115-3
Zhang, K., Li, P., Chen, H., Bo, X., Li, X., and Xu, H. (2016b). Continuous Cavitation Designed for Enhancing Radiofrequency Ablation via a Special Radiofrequency Solidoid Vaporization Process. ACS Nano 10, 2549–2558. doi:10.1021/acsnano.5b07486
Zhang, K., Li, P., He, Y., Bo, X., Li, X., Li, D., et al. (2016a). Synergistic Retention Strategy of RGD Active Targeting and Radiofrequency-Enhanced Permeability for Intensified RF & Chemotherapy Synergistic Tumor Treatment. Biomaterials 99, 34–46. doi:10.1016/j.biomaterials.2016.05.014
Zhang, X., Lai, Z., Ma, Q., and Zhang, H. (2018). Novel Structured Transition Metal Dichalcogenide Nanosheets. Chem. Soc. Rev. 47, 3301–3338. doi:10.1039/c8cs00094h
Zhang, Y., Guo, L., Kong, F., Duan, L., Li, H., Fang, C., et al. (2021). Nanobiotechnology-Enabled Energy Utilization Elevation for Augmenting Minimally-Invasive and Noninvasive Oncology Thermal Ablation. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol 13, e1733. doi:10.1002/wnan.1733
Zhao, J., Wu, H., Zhao, J., Yin, Y., Zhang, Z., Wang, S., et al. (2021). 2D LDH-MoS2 Clay Nanosheets: Synthesis, Catalasemimic Capacity, and Imaging-Guided Tumor Photo-Therapy. J. Nanobiotechnol 19, 36. doi:10.1186/s12951-020-00763-7
Zhao, W., Li, A., Zhang, A., Zheng, Y., and Liu, J. (2018). Recent Advances in Functional-Polymer-Decorated Transition Metal Nanomaterials for Bioimaging and Cancer Therapy. ChemMedChem 13, 2134–2149. doi:10.1002/cmdc.201800462
Zheng, Z., Jia, Z., Qu, C., Dai, R., Qin, Y., Rong, S., et al. (2021). Biodegradable Silica-Based Nanotheranostics for Precise MRI/NIR-II Fluorescence Imaging and Self-Reinforcing Antitumor Therapy. Small 17, 2006508. doi:10.1002/smll.202006508
Zhou, J., Wang, Q., Geng, S., Lou, R., Yin, Q., and Ye, W. (2019b). Construction and Evaluation of Tumor Nucleus-Targeting Nanocomposite for Cancer Dual-Mode Imaging - Guiding Photodynamic Therapy/Photothermal Therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 102, 541–551. doi:10.1016/j.msec.2019.04.088
Zhou, W., Chen, M., Liu, X., Zhang, W., Cai, F., Li, F., et al. (2019a). Selective Photothermal Ablation of Cancer Cells by Patterned Gold Nanocages Using Surface Acoustic Waves. Lab. Chip 19, 3387–3396. doi:10.1039/c9lc00344d
Zhu, W., Liu, K., Sun, X., Wang, X., Li, Y., Cheng, L., et al. (2015). Mn2+-Doped Prussian Blue Nanocubes for Bimodal Imaging and Photothermal Therapy with Enhanced Performance. ACS Appl. Mater. Inter. 7, 11575–11582. doi:10.1021/acsami.5b02510
Zhu, Y., Wang, Y., Williams, G. R., Fu, L., Wu, J., Wang, H., et al. (2020). Multicomponent Transition Metal Dichalcogenide Nanosheets for Imaging-Guided Photothermal and Chemodynamic Therapy. Adv. Sci. 7, 2000272. doi:10.1002/advs.202000272
Keywords: transition-metal based nanomaterials, non-invasive thermal ablation, nanomedicine, imaging diagnosis, cancer
Citation: Peng Q, Qian Z, Gao H and Zhang K (2022) Recent Advances in Transition-Metal Based Nanomaterials for Noninvasive Oncology Thermal Ablation and Imaging Diagnosis. Front. Chem. 10:899321. doi: 10.3389/fchem.2022.899321
Received: 18 March 2022; Accepted: 31 March 2022;
Published: 14 April 2022.
Edited by:
Zhongmin Tang, University of Wisconsin-Madison, United StatesCopyright © 2022 Peng, Qian, Gao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huali Gao, Zmx5Z2dnQDE2My5jb20=; Kun Zhang, emhhbmcxOTg2a3VuQDEyNi5jb20=
†These authors have contributed equally to this work
 Qiuxia Peng
Qiuxia Peng Zhangbo Qian
Zhangbo Qian Huali Gao
Huali Gao Kun Zhang
Kun Zhang