- 1State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, State-Local Joint Laboratory for Comprehensive Utilization of Biomass, Center for R&D of Fine Chemicals, Guizhou University, Guiyang, China
- 2Guizhou Industry Polytechnic College, Guiyang, China
- 3Guizhou Institute of Products Quality Inspection and Testing, Guiyang, China
- 4School of Chemical and Environmental Engineering, Technical University of Crete, University Campus, Chania, Greece
- 5Faculty of Engineering and Natural Sciences, Tampere University, Tampere, Finland
The complexity and recalcitrance of the lignin structure is a major barrier to its efficient utilization and commercial production of high-value products. In recent years, the “bio-funneling” transformation ability of microorganisms has provided a significant opportunity for lignin conversion and integrated biorefinery. Based on the chemical structure of lignin, this mini-review introduces the recent advances of lignin depolymerization by bacterial strains and the application of microbial lignin degradation in lipids production. Furthermore, the current challenges, future trends and perspectives for microbe-based lignin conversion to lipids are discussed.
Introduction
The energy conversion of lignocellulosic biomass can effectively alleviate the pressure of energy crisis and environmental deterioration (Li et al., 2014; Liu et al., 2015; Li et al., 2017; Zhang and Wang, 2020). However, the sustainable and profitable development of the lignocellulose-based biorefinery industry relies on the holistic utilization of all carbon components, including cellulose, hemicellulose, and lignin (Li et al., 2015; Li H. et al., 2019; Zhao et al., 2019; Garlapati et al., 2020; Li et al., 2020a; Li et al., 2020b). Lignin is a three-dimensional biopolymer composed of three different phenylpropanoid monomers randomly polymerized by C-C and C-O bonds (Supanchaiyamat et al., 2019; Ali et al., 2021; Abu-Omar et al., 2021). Because of its high carbon-oxygen ratio and rich aromatics, it is the most promising material for generating products, such as biofuels and other high-value chemicals (Wang H. et al., 2019; Wang et al., 2021). However, the lack of efficient degradation and resource utilization technologies for lignin is the main bottleneck restricting the sustainability and cost-competitiveness of lignocellulose biorefinery (Sethupathy et al., 2022). The pulp and paper industry generates approximately 50 million tons of lignin annually with ca. 98%–99% being combusted for energy generation (Zevallos Torres et al., 2020).
Considering sustainability and industrialization, lignin-based biorefinery faces many challenges (Den et al., 2018; Yaguchi et al., 2020). The heterogeneous structure of lignin leads to its insolubility and diverse aromatics after depolymerization, which increases the difficulty of industrial transformation to target products. The diversity and complexity of linkages among the monomers increase the complexity of degradation. In addition, the degradation of lignin produces toxic inhibitors that require strong tolerance for microbial population.
The effective utilization of lignin has many significant advantages (Huang et al., 2022; Yao et al., 2022; Zhou et al., 2022). Firstly, it can improve the resource utilization efficiency and profitability of lignocellulosic biomass. Secondly, selective utilization of lignin can avoid inhibition issues resulting from lignin degradation products in the fermentation process and non-productive binding of enzymes. Thirdly, appropriate utilization of lignin could mitigate the potential environmental pollution related to huge wastewater effluents from the pulp and paper industry.
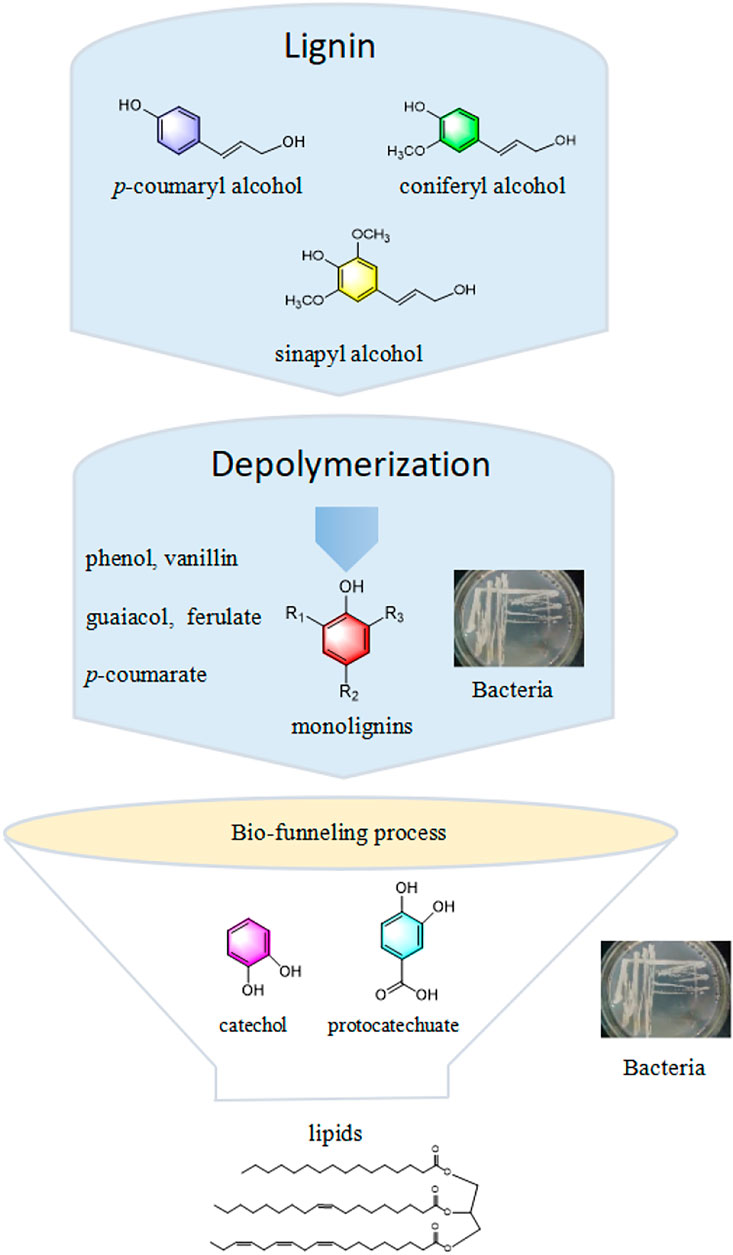
In this regard, bio-valorization is considered potentially advantageous due to its mild condition, eco-friendliness, and specificity in converting lignin into biofuels and chemicals (Singhania et al., 2022). Generally, lignin-derived bio-conversion mainly involves the microbial depolymerization of lignin into a broad spectrum of low-molecular-weight aromatics through oxidative enzymes secreted by microorganisms. These aromatics are then converted into value-added products, especially lipids, through microbial metabolism (Liu et al., 2019) (Figure 1). Conventional biodegradation achieved by fungi (e.g., brown- or white-rot fungi) has some drawbacks in terms of a long pretreatment period and poor environmental adaptability. In contrast, bacteria exhibit rapid reproduction, excellent adaptability to diverse environment, and easier genome editing, making them potential candidates for lignin-degrading strains in the future (Liu D. et al., 2018; Radhika et al., 2022). Therefore, the bio-funneling pathway process has attracted great attention due to its ability to overcome the heterogeneity of lignin-derivatives. The synthesis of lipids from lignin via the bio-funneling pathway using Rhodococcus strains has been widely reported in the literature (Li C. et al., 2019). Microorganism pool heterogeneous substrates into intermediates, such as protocatechuate or catechol, through a pathway known as the bio-funneling process. These intermediates further undergo central carbon metabolism to synthesize lipids by the cyclic cleavage, β-ketoadipate pathway (Salvachúa et al., 2015). To date, there are limited published reviews that have specifically addressed the use of bacteria for the production of lipids from lignin. This paper reviews current research advances on the conversion of lignin to lipids via biological pathways and possible strategies to improve lignin biotransformation, demonstrating an effective value chain from lignin to lipids in an eco-friendly and sustainable manner.
Bio-Depolymerization of Lignin to Aromatic Compounds
Because of its natural aromatic skeleton, lignin has great potential for conversion into aromatics as biofuels or value-added chemicals, which plays an important role in improving carbon efficiency (Liao et al., 2020; Weng et al., 2021). Natural lignin mainly consists of three phenylpropanoid units, p-coumaryl alcohol (H-type unit), coniferyl alcohol (G-type unit), and sinapyl alcohol (S-type unit) (Becker and Wittmann, 2019), with a different number of methoxylation degrees on the aromatic rings (Mayr et al., 2021). The lignin monomers are conjugated by different bonds to form polymers with high resistance to chemical and biochemical depolymerization. Ether bonds, particularly β-O-4 ether linkages, predominate in lignin (Paananen et al., 2020; Szalaty et al., 2020).
Lignin depolymerization, the process of converting macro-molecular polymers into low-molecular-weight monomers or oligomers, is a key step in lignin valorization (Ragauskas et al., 2014). In nature, lignin degradation is induced by biological factors, such as fungi, bacteria, and abiotic factors (Radhika et al., 2021). Fungi are the most effective lignin-degrading microorganisms, mainly including white rot, brown rot, and soft rot, among which white rot has the strongest degradation ability (Ponnusamy et al., 2019; Salvachua et al., 2020). Nevertheless, the harsh growth conditions and complex genetic system of fungi greatly limit their application in industry. In addition to fungi, bacteria have also been reported to have the ability to degrade lignin. Although the degradation performance is not as good as that of fungi, bacteria demonstrate strong environmental adaptability (Xu et al., 2021). Screening for bacteria with strong lignin depolymerization capacity and identification of secreted enzymes are crucial for effective lignin utilization (Xu et al., 2019). The direct use of industrial enzymes for depolymerization can avoid severe and complex processes (e.g., high temperature and pressure, hazardous and expensive chemicals, and catalysts). Particularly, oxidases (i.e., laccases and peroxidases) are potential candidates for lignin depolymerization (Hamalainen et al., 2018).
Bacteria Involved in the Depolymerization of Lignin
In the presence of oxygen or under anaerobic conditions, bacteria use lignin as a carbon source and then secrete oxidative enzymes to depolymerize lignin (Weng et al., 2021). Rhodococcus, Proteobacteria, and pseudomonas are the main bacteria that can degrade lignin effectively (Lee et al., 2019). Substrate sources and screening methods are key factors in obtaining bacteria with excellent lignin-degradation performance (Xu et al., 2019). Lignin-degrading bacteria are commonly found in lignin-rich environments, such as pulp and paper wastewater, eroded bamboo slips, soil, rotten wood, compost, and even termite gut (Xu R. et al., 2018). The commonly used screening method is to obtain the bacteria with ligninolytic capacity by using lignin or lignin-derivatives as the sole carbon source, and a suitable secondary screening model is then constructed to differentiate the degradability of lignin. For instance, dyes are often used to indicate the degradation activity of lignin due to its close structure to lignin and decolorization visualization (Xu Z. et al., 2018). Pseudomonas putida NX-1, Klebsiella pneumoniae NX-1, and Ochrobactrum tritici with lignin degradation capability were screened from the leaf molds using Kraft lignin as the sole carbon source, and then their capabilities to degrade lignin were verified by simulating dye decolorization and measuring decomposition enzyme activity efficiently (Xu Z. et al., 2018). The results showed that Pseudomonas putida NX-1 was the most capable for degrading lignin and was able to secrete laccase, lignin peroxidase (LiP), and Mn-peroxidase (MnP) efficiently. The researchers obtained two strains of lignin-degrading bacteria from the rainforest by the laccase activity against ABTS and their capabilities to degrade Kraft lignin and lignin model dimer guaiacylglycerol-β-guaiacyl ether with abundant lignin-linkage (Huang et al., 2013). Bacillus amyloliquefaciens SL-7 obtained from tobacco straw demonstrated better secretion of ligninolytic enzymes (MnP, LiP, and laccase) and achieved 28.55% of lignin degradation after 15 days with a comparable degradation rate to fungi, which could be an excellent strain for lignin degradation by overcoming the disadvantage of low bacterial activity (Mei et al., 2020).
Lignin-Depolymerizing Bacterial Enzymes
Ligninolytic enzymes, which are crucial for lignin valorization, are mainly divided into two groups, i.e., lignin modifying enzymes (LMEs) and lignin-degrading auxiliary enzymes (LDAs) (Iram et al., 2021). LMEs, including laccase and peroxidase (e.g., lignin peroxidase, manganese peroxidase, multifunctional peroxidases, etc.), are predominant oxidases that can directly depolymerize lignin. Although LDAs cannot directly depolymerize lignin, they can assist LMEs in the degradation of lignin (Reshmy et al., 2022). Unlike fungal lignin-depolymerizing oxidases, bacterial lignin depolymerization is dominated by laccase and DyP (Dye decolorizing peroxidase) (de Gonzalo et al., 2016). More importantly, bacterial enzymes possess higher thermal stability and robustness as compared to fungal enzymes.
Most enzymes operate under mild conditions and even acidic conditions, but most technical lignin is undissolvable under acidic conditions. Finnish biotechnology company MetGen Oy designs and supplies a genetically engineered commercial enzyme MetZyme® LIGNOTM which can function at higher pH of 10–11 and elevated temperatures with a certain reduction in lignin molecular weight and increased solubility (Hamalainen et al., 2018). This enzyme-mediated scheme provides high value for lignin valorization commercially. Extreme enzymes and extremophiles are very attractive in the future because of their ability to adapt to harsh processes and perform well in the bio-conversion process, potentially bringing enzymatic lignin decomposition closer to industrial applications (Zhu et al., 2022). Zhu et al. (2017) isolated a salt-tolerant and basophilic strain of Bacillus ligniniphilus L1 from abyssal sediments, and then identified 15 aromatics during the bio-conversion of alkaline lignin, exhibiting significant lignin-degrading capability and adaptability to extreme environments.
Bio-Conversion of Lignin to Lipids
As attractive feedstocks for biofuel production, lipids can be synthesized from lignin-based aromatic building blocks by oleaginous microorganisms with nearly 20% of lipids accumulation out of their dry cell weight (DCW) (Reshmy et al., 2022). Prominent oleaginous bacteria can exhibit excellent lipid accumulation capacities, such as Acinetobacter calcoaceticus (lipid accumulation up to 27%–38% of their DCW), Rhodococcus opacus (25%), and Bacillus alcalophilus (18%–24%) (Iram et al., 2021). Biocatalytic processes that integrate upstream depolymerization and bacterial aromatic metabolic pathways (bio-funneling process) can overcome the lignin inherent heterogeneity (Linger et al., 2014). Rhodococcus species, the oleaginous microorganisms, have efficient metabolism and tolerability of aromatics derived from lignin and their relative adaptability in genetic manipulation makes them promising for industrial applications (Xiong et al., 2016; Shields-Menard et al., 2017).
In general, the bio-conversion of lignin to lipids after depolymerization generally involves three stages (Chen and Wan, 2017; Lee et al., 2019): 1) conversion of lignin-degraded oligomers or monomers to catechol or protocatechuate via the bio-funneling pathway, 2) ring-opening pathways of the aromatic skeleton and β-ketoadipate pathway to produce acetyl-CoA, and 3) synthesis of lipids by oleaginous microorganisms under deficient conditions (e.g., nitrogen limitation). Microbially involved lignin bioconversion processes often pose a barrier to industrial applications aiming at high yield and high efficiency in lignin use (Ponnusamy et al., 2019).
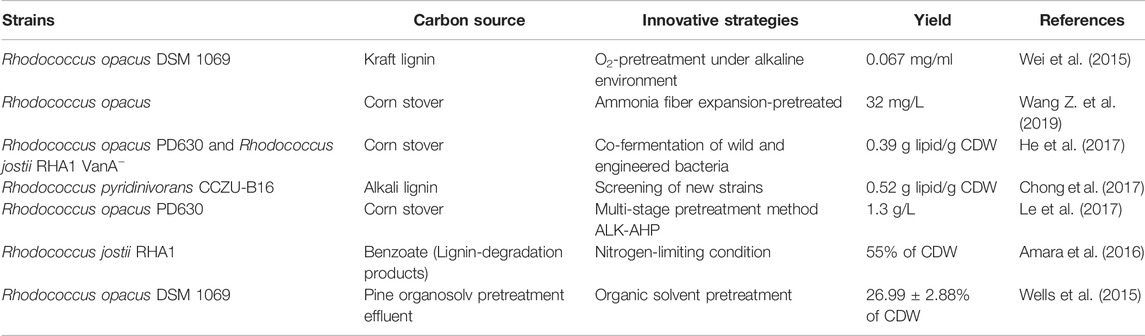
Bio-conversion of lignin to lipids has been effectively explored in terms of improved technology and development of new processes, such as pretreatment, co-culture, and other promising enhancement technologies, etc. A list of bacteria strains together with innovative strategies to produce lipids is shown in Table 1.
Pretreatment
Pretreatment is a crucial step in the integrated biorefinery (Zhang et al., 2021). Monitoring the lipid synthesis from lignin mediated by oleaginous microorganisms, it has been revealed that low-molecule-weight lignin-derived monomers were more favorable for lipid accumulation than macro-molecule of lignin. Therefore, integrating depolymerization processes that can reduce the molecular weight of lignin and break its inherent recalcitrance, with microbial synthesis processes could be applied to improve the efficiency of lipid accumulation (Li X. et al., 2019).
In the study using Rhodococcus opacus DSM 1069 to convert Kraft lignin to lipids, Wei et al. (2015) found that bacteria acted poorly when directly using Kraft lignin as substrate. After O2-pretreatment of Kraft lignin under an alkaline environment, the bacterium was able to use Kraft lignin and accumulate lipids with a maximum yield of 0.067 mg/ml. As compared to single pretreatment, combined pretreatment produced higher lipid concentration (12.8%–75.6%) because lignin-degrading bacteria released more monomers for use to achieve an optimal lipid yield of 1.83 g/L in fermentation. This indicates that combined pretreatment together with batch fermentation could be a promising strategy for efficient bio-conversion of lignin to lipids (Liu Z.-H. et al., 2018).
Co-Culture
In general, most industrial biosynthesis processes prefer to use a single engineered microorganism to facilitate the production control. But in a single microbe, there is a competition for cellular resources in different metabolic pathways (Borchert et al., 2022). The application of microbial co-culture systems can reduce these catabolic limitations, improve streams towards desired chemicals, or enhance microbial resistance to toxicity (Singh et al., 2019). Although multiple microbial cultures can increase the complexity of the bio-funnelling pathway towards a single product, the selection of compatible partners with synergistic functions could be challenging in the co-culture system (Zuniga et al., 2020). Further investigations are needed to elucidate the interactions between microorganisms and the dynamics of community structure in the system. Given the complementary nature of different bacteria in lignin depolymerization and assimilation, the use of bacterial communities can significantly improve the efficiency of the biological upgrading of lignin for lipids accumulation.
Li X. et al. (2019) set up a wild and engineered Rhodococcus co-culture system, which presented excellent capabilities to degrade lignin and accumulate lipids. After selective depletion of glucose by Rhodococcus strains, nearly half of the lignin was then depolymerized into monomers for cell growth and lipid synthesis. The highest lignin degradation rate was 23.2% for the single strain fermentation and 33.6% for the three co-cultured strains.
Some bacteria were found to promote the growth of microalgae (Subashchandrabose et al., 2011). The microalgal growth-promoting bacterium Azospirillum brasilense increased the total intracellular lipid content after co-immobilization with three strains of microalgae (de-Bashan et al., 2002). Introducing these engineered microbial communities with enhanced capacity provides another new concept for the production of lipids from lignin.
Other Promising Enhancement Technologies
Most of the lignin-derived aromatic compounds are inhibitory to microorganisms, resulting in reduction of the yield and tighter of lignocellulosic-based products (Singh et al., 2019). Thus, it is imperative to develop robust, tolerant and productive strains for effective biorefinery (Zhang and Bao, 2022). Comprehensive lipidomic research applying adaptive evolution using phenol-only carbon source for Rhodococcus opacus PD630 revealed a correlation between the strain’s lipid metabolism and phenol tolerance by affecting the constituent of mycolic acid and phospholipid membranes (Henson et al., 2018). Pelleted culture has been extensively studied for its high yield and ease of product collection, the low viscosity of the medium, and thus low energy consumption (Nair et al., 2016). Xu et al. (2022) reported for the first time the spontaneous formation of pellets by bacteria during fermentation with an alkaline treatment solution of maize stover as the carbon source, even in the absence of added chemical coagulants. It was found that the lipid content of the pellets was higher than that of the suspended biomass at low nitrogen concentrations. Moreover, this pellet form of microorganism has the potential for lipid production, suggesting a new strategy for the development of the biofuel industry. Liu et al. (2021) designed the “Plug-In Processes of Lignin” based on advanced pretreatment techniques to achieve a synergy of lignin biochemical conversion and carbohydrate production by reducing molecular weight and enhancing hydrophilic radicals to achieve profitable and sustainable biorefinery.
Conclusion
The efficient resource utilization of lignin can significantly improve the economic feasibility of lignocellulosic biorefinery towards sustainability and circularity. Tremendous progress has been made in recent decades in lignin valorization, however, more efforts are still desired to produce high-value compounds from lignin efficiently and economically. Lignin depolymerization plays a crucial role in bioconversion. Microbially mediated lignin depolymerization has become a hot spot of research because of its low energy consumption and eco-friendliness. Lignin-degrading bacteria could be a breakthrough in commercial utilization of lignin due to strong tolerance under extreme conditions, fast reaction rate, and ease of genetic manipulation. Despite some recent breakthroughs in the biotransformation of lignin to lipids, nitrogen optimization for exogenous protein expression and lipid accumulation, and improvement of production efficiency are main challenges in future research work. To address the challenges of lignin biotransformation into lipids, the industrial production of lipids from lignin by bacteria can be achieved by introducing emerging technologies, including predominant pretreatment techniques, adaptive evolutions, and genetic engineering. In the future, synergistic pathways could be elaborated for the production of lipids from the metabolism of sugars and aromatic substances. An effective value chain for depolymerization and bioconversion of lignin to lipids should be established in order to provide promising prospects for the production of biofuels.
Author Contributions
HW and XP wrote the original draft. HL conceptualized and edited the review, and acquired the funding. AG edited the manuscript. CH conceptualized and edited the review.
Funding
This study was financially supported by the National Natural Science Foundation of China (21908033), Guizhou Provincial S&T Project [ZK(2022)011], and Fok Ying-Tong Education Foundation (161030).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer QZ declared a past co-authorship with the author HL to the handling editor.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu-Omar, M. M., Barta, K., Beckham, G. T., Luterbacher, J. S., Ralph, J., Rinaldi, R., et al. (2021). Guidelines for Performing Lignin-First Biorefining. Energy Environ. Sci. 14 (1), 262–292. doi:10.1039/d0ee02870c
Ali, S. S., Al-Tohamy, R., Koutra, E., El-Naggar, A. H., Kornaros, M., and Sun, J. (2021). Valorizing Lignin-like Dyes and Textile Dyeing Wastewater by a Newly Constructed Lipid-Producing and Lignin Modifying Oleaginous Yeast Consortium Valued for Biodiesel and Bioremediation. J. Hazard. Mater. 403, 123575. doi:10.1016/j.jhazmat.2020.123575
Amara, S., Seghezzi, N., Otani, H., Diaz-Salazar, C., Liu, J., and Eltis, L. D. (2016). Characterization of Key Triacylglycerol Biosynthesis Processes in Rhodococci. Sci. Rep. 6, 24985. doi:10.1038/srep24985
Becker, J., and Wittmann, C. (2019). A Field of Dreams: Lignin Valorization into Chemicals, Materials, Fuels, and Health-Care Products. Biotechnol. Adv. 37 (6), 107360. doi:10.1016/j.biotechadv.2019.02.016
Borchert, A. J., Henson, W. R., and Beckham, G. T. (2022). Challenges and Opportunities in Biological Funneling of Heterogeneous and Toxic Substrates beyond Lignin. Curr. Opin. Biotechnol. 73, 1–13. doi:10.1016/j.copbio.2021.06.007
Chen, Z., and Wan, C. (2017). Biological Valorization Strategies for Converting Lignin into Fuels and Chemicals. Renew. Sust. Energ. Rev. 73, 610–621. doi:10.1016/j.rser.2017.01.166
Chong, G.-G., Huang, X.-J., Di, J.-H., Xu, D.-Z., He, Y.-C., Pei, Y.-N., et al. (2017). Biodegradation of Alkali Lignin by a Newly Isolated Rhodococcus Pyridinivorans CCZU-B16. Bioproc. Biosyst. Eng. 41 (4), 501–510. doi:10.1007/s00449-017-1884-x
de Gonzalo, G., Colpa, D. I., Habib, M. H. M., and Fraaije, M. W. (2016). Bacterial Enzymes Involved in Lignin Degradation. J. Biotechnol. 236, 110–119. doi:10.1016/j.jbiotec.2016.08.011
de-Bashan, L. E., Bashan, Y., Moreno, M., Lebsky, V. K., and Bustillos, J. J. (2002). Increased Pigment and Lipid Content, Lipid Variety, and Cell and Population Size of the Microalgae chlorella spp. When Co-immobilized in Alginate Beads with the Microalgae-Growth-Promoting Bacterium Azospirillum Brasilense. Can. J. Microbiol. 48 (6), 514–521. doi:10.1139/w02-051
Den, W., Sharma, V. K., Lee, M., Nadadur, G., and Varma, R. S. (2018). Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 6, 141–163. doi:10.3389/fchem.2018.00141
Garlapati, V. K., Chandel, A. K., Kumar, S. P. J., Sharma, S., Sevda, S., Ingle, A. P., et al. (2020). Circular Economy Aspects of Lignin: Towards a Lignocellulose Biorefinery. Renew. Sust. Energ. Rev. 130, 109977. doi:10.1016/j.rser.2020.109977
Hämäläinen, V., Grönroos, T., Suonpää, A., Heikkilä, M. W., Romein, B., Ihalainen, P., et al. (2018). Enzymatic Processes to Unlock the Lignin Value. Front. Bioeng. Biotechnol. 6, 20. doi:10.3389/fbioe.2018.00020
He, Y., Li, X., Ben, H., Xue, X., and Yang, B. (2017). Lipid Production from Dilute Alkali Corn Stover Lignin by Rhodococcus Strains. ACS Sust. Chem. Eng. 5 (3), 2302–2311. doi:10.1021/acssuschemeng.6b02627
Henson, W. R., Hsu, F.-F., Dantas, G., Moon, T. S., and Foston, M. (2018). Lipid Metabolism of Phenol-Tolerant Rhodococcus Opacus Strains for Lignin Bioconversion. Biotechnol. Biofuels 11, 339. doi:10.1186/s13068-018-1337-z
Huang, C., Jiang, X., Shen, X., Hu, J., Tang, W., Wu, X., et al. (2022). Lignin-enzyme Interaction: A Roadblock for Efficient Enzymatic Hydrolysis of Lignocellulosics. Renew. Sust. Energ. Rev. 154. doi:10.1016/j.rser.2021.111822
Huang, X.-F., Santhanam, N., Badri, D. V., Hunter, W. J., Manter, D. K., Decker, S. R., et al. (2013). Isolation and Characterization of Lignin-Degrading Bacteria from Rainforest Soils. Biotechnol. Bioeng. 110 (6), 1616–1626. doi:10.1002/bit.24833
Iram, A., Berenjian, A., and Demirci, A. (2021). A Review on the Utilization of Lignin as a Fermentation Substrate to Produce Lignin-Modifying Enzymes and Other Value-Added Products. Molecules 26 (10), 2960. doi:10.3390/molecules26102960
Le, R. K., Wells Jr., T., Das, P., Meng, X., Stoklosa, R. J., Bhalla, A., et al. (2017). Conversion of Corn stover Alkaline Pre-treatment Waste Streams into Biodiesel via Rhodococci. RSC Adv. 7 (7), 4108–4115. doi:10.1039/c6ra28033a
Lee, S., Kang, M., Bae, J.-H., Sohn, J.-H., and Sung, B. H. (2019). Bacterial Valorization of Lignin: Strains, Enzymes, Conversion Pathways, Biosensors, and Perspectives. Front. Bioeng. Biotechnol. 7, 209. doi:10.3389/fbioe.2019.00209
Li, C., Chen, C., Wu, X., Tsang, C.-W., Mou, J., Yan, J., et al. (2019). Recent Advancement in Lignin Biorefinery: With Special Focus on Enzymatic Degradation and Valorization. Bioresour. Techn. 291, 121898. doi:10.1016/j.biortech.2019.121898
Li, H., Guo, H., Fang, Z., Aida, T. M., and Smith, R. L. (2020b). Cycloamination Strategies for Renewable N-Heterocycles. Green. Chem. 22, 582–611. doi:10.1039/c9gc03655e
Li, H., Li, Y., Fang, Z., and Smith, R. L. (2019). Efficient Catalytic Transfer Hydrogenation of Biomass-Based Furfural to Furfuryl Alcohol with Recycable Hf-Phenylphosphonate Nanohybrids. Catal. Today 319, 84–92. doi:10.1016/j.cattod.2018.04.056
Li, H., Saravanamurugan, S., Yang, S., and Riisager, A. (2015). Catalytic Alkylation of 2-methylfuran with Formalin Using Supported Acidic Ionic Liquids. ACS Sust. Chem. Eng. 3, 3274–3280. doi:10.1021/acssuschemeng.5b00850
Li, H., Wu, H., Yu, Z., Zhang, H., and Yang, S. (2020a). CO2 -Enabled Biomass Fractionation/Depolymerization: A Highly Versatile Pre‐Step for Downstream Processing. ChemSusChem 13 (14), 3565–3582. doi:10.1002/cssc.202000575
Li, H., Zhang, Q., Bhadury, P., and Yang, S. (2014). Furan-type Compounds from Carbohydrates via Heterogeneous Catalysis. Curr. Org. Chem. 18, 547–597. doi:10.2174/13852728113176660138
Li, H., Zhao, W., and Fang, Z. (2017). Hydrophobic Pd Nanocatalysts for One-Pot and High-Yield Production of Liquid Furanic Biofuels at Low Temperatures. Appl. Catal. B Environ. 215, 18–27. doi:10.1016/j.apcatb.2017.05.039
Li, X., He, Y., Zhang, L., Xu, Z., Ben, H., Gaffrey, M. J., et al. (2019). Discovery of Potential Pathways for Biological Conversion of poplar wood into Lipids by Co-fermentation of Rhodococci Strains. Biotechnol. Biofuels 12, 60–75. doi:10.1186/s13068-019-1395-x
Liao, Y., Koelewijn, S.-F., Van Den Bossche, G., Van Aelst, J., Van Den Bosch, S., Renders, T., et al. (2020). A Sustainable Wood Biorefinery for Low-Carbon Footprint Chemicals Production. Science 367 (6484), 1385–1390. doi:10.1126/science.aau1567
Linger, J. G., Vardon, D. R., Guarnieri, M. T., Karp, E. M., Hunsinger, G. B., Franden, M. A., et al. (2014). Lignin Valorization through Integrated Biological Funneling and Chemical Catalysis. Proc. Natl. Acad. Sci. U.S.A. 111 (33), 12013–12018. doi:10.1073/pnas.1410657111
Liu, D., Yan, X., Zhuo, S., Si, M., Liu, M., Wang, S., et al. (2018). Pandoraea sp. B-6 Assists the Deep Eutectic Solvent Pretreatment of Rice Straw via Promoting Lignin Depolymerization. Bioresour. Techn. 257, 62–68. doi:10.1016/j.biortech.2018.02.029
Liu, J., Li, H., Liu, Y.-C., Lu, Y.-M., He, J., Liu, X.-F., et al. (2015). Catalytic Conversion of Glucose to 5-hydroxymethylfurfural over Nano-Sized Mesoporous Al2O3-B2o3 Solid Acids. Catal. Commun. 62, 19–23. doi:10.1016/j.catcom.2015.01.008
Liu, Z.-H., Hao, N., Wang, Y.-Y., Dou, C., Lin, F., Shen, R., et al. (2021). Transforming Biorefinery Designs with 'Plug-In Processes of Lignin’ to Enable Economic Waste Valorization. Nat. Commun. 12 (1), 3912. doi:10.1038/s41467-021-23920-4
Liu, Z.-H., Le, R. K., Kosa, M., Yang, B., Yuan, J., and Ragauskas, A. J. (2019). Identifying and Creating Pathways to Improve Biological Lignin Valorization. Renew. Sust. Energ. Rev. 105, 349–362. doi:10.1016/j.rser.2019.02.009
Liu, Z.-H., Xie, S., Lin, F., Jin, M., and Yuan, J. S. (2018). Combinatorial Pretreatment and Fermentation Optimization Enabled a Record Yield on Lignin Bioconversion. Biotechnol. Biofuels 11, 21. doi:10.1186/s13068-018-1021-3
Mayr, S. A., Subagia, R., Weiss, R., Schwaiger, N., Weber, H. K., Leitner, J., et al. (2021). Oxidation of Various Kraft Lignins with a Bacterial Laccase Enzyme. Int. J. Mol. Sci. 22 (23), 13161. doi:10.3390/ijms222313161
Mei, J., Shen, X., Gang, L., Xu, H., Wu, F., and Sheng, L. (2020). A Novel Lignin Degradation Bacteria-Bacillus Amyloliquefaciens SL-7 Used to Degrade Straw Lignin Efficiently. Bioresour. Techn. 310, 123445. doi:10.1016/j.biortech.2020.123445
Nair, R. B., Lennartsson, P. R., and Taherzadeh, M. J. (2016). Mycelial Pellet Formation by Edible Ascomycete Filamentous Fungi, Neurospora Intermedia. AMB Expr. 6 (1), 31. doi:10.1186/s13568-016-0203-2
Paananen, H., Eronen, E., Mäkinen, M., Jänis, J., Suvanto, M., and Pakkanen, T. T. (2020). Base-catalyzed Oxidative Depolymerization of Softwood Kraft Lignin. Ind. Crops Prod. 152, 112473. doi:10.1016/j.indcrop.2020.112473
Ponnusamy, V. K., Nguyen, D. D., Dharmaraja, J., Shobana, S., Banu, J. R., Saratale, R. G., et al. (2019). A Review on Lignin Structure, Pretreatments, Fermentation Reactions and Biorefinery Potential. Bioresour. Techn. 271, 462–472. doi:10.1016/j.biortech.2018.09.070
Radhika, N. L., Sachdeva, S., and Kumar, M. (2022). Lignin Depolymerization and Biotransformation to Industrially Important Chemicals/Biofuels. Fuel 312, 122935. doi:10.1016/j.fuel.2021.122935
Radhika, N. L., Sachdeva, S., and Kumar, M. (2021). Microbe Assisted Depolymerization of Lignin Rich Waste and its Conversion to Gaseous Biofuel. J. Environ. Manage. 300, 113684. doi:10.1016/j.jenvman.2021.113684
Ragauskas, A. J., Beckham, G. T., Biddy, M. J., Chandra, R., Chen, F., Davis, M. F., et al. (2014). Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 344 (6185), 1246843. doi:10.1126/science.1246843
Reshmy, R., Athiyaman Balakumaran, P., Divakar, K., Philip, E., Madhavan, A., Pugazhendhi, A., et al. (2022). Microbial Valorization of Lignin: Prospects and Challenges. Bioresour. Techn. 344 (Pt A), 126240. doi:10.1016/j.biortech.2021.126240
Salvachúa, D., Karp, E. M., Nimlos, C. T., Vardon, D. R., and Beckham, G. T. (2015). Towards Lignin Consolidated Bioprocessing: Simultaneous Lignin Depolymerization and Product Generation by Bacteria. Green. Chem. 17 (11), 4951–4967.
Salvachúa, D., Werner, A. Z., Pardo, I., Michalska, M., Black, B. A., Donohoe, B. S., et al. (2020). Outer Membrane Vesicles Catabolize Lignin-Derived Aromatic Compounds in Pseudomonas Putida KT2440. Proc. Natl. Acad. Sci. U.S.A. 117 (17), 9302–9310. doi:10.1073/pnas.1921073117
Sethupathy, S., Murillo Morales, G., Gao, L., Wang, H., Yang, B., Jiang, J., et al. (2022). Lignin Valorization: Status, Challenges and Opportunities. Bioresour. Techn. 347, 126696. doi:10.1016/j.biortech.2022.126696
Shields-Menard, S. A., Amirsadeghi, M., Green, M., Womack, E., Sparks, D. L., Blake, J., et al. (2017). The Effects of Model Aromatic Lignin Compounds on Growth and Lipid Accumulation of Rhodococcus Rhodochrous. Int. Biodeterior. Biodegradation 121, 79–90. doi:10.1016/j.ibiod.2017.03.023
Singh, A., Bedore, S. R., Sharma, N. K., Lee, S. A., Eiteman, M. A., and Neidle, E. L. (2019). Removal of Aromatic Inhibitors Produced from Lignocellulosic Hydrolysates by Acinetobacter Baylyi ADP1 with Formation of Ethanol by Kluyveromyces Marxianus. Biotechnol. Biofuels 12, 91. doi:10.1186/s13068-019-1434-7
Singhania, R. R., Patel, A. K., Raj, T., Chen, C.-W., Ponnusamy, V. K., Tahir, N., et al. (2022). Lignin Valorisation via Enzymes: A Sustainable Approach. Fuel 311, 122608. doi:10.1016/j.fuel.2021.122608
Subashchandrabose, S. R., Ramakrishnan, B., Megharaj, M., Venkateswarlu, K., and Naidu, R. (2011). Consortia of Cyanobacteria/Microalgae and Bacteria: Biotechnological Potential. Biotechnol. Adv. 29 (6), 896–907. doi:10.1016/j.biotechadv.2011.07.009
Supanchaiyamat, N., Jetsrisuparb, K., Knijnenburg, J. T. N., Tsang, D. C. W., and Hunt, A. J. (2019). Lignin Materials for Adsorption: Current Trend, Perspectives and Opportunities. Bioresour. Techn. 272, 570–581. doi:10.1016/j.biortech.2018.09.139
Szalaty, T. J., Klapiszewski, Ł., and Jesionowski, T. (2020). Recent Developments in Modification of Lignin Using Ionic Liquids for the Fabrication of Advanced Materials-A Review. J. Mol. Liquids 301, 112417. doi:10.1016/j.molliq.2019.112417
Wang, H., Peng, X., Zhang, H., Yang, S., and Li, H. (2021). Microorganisms-Promoted Biodiesel Production from Biomass: A Review. Energy Convers. Manag. X 12, 100137. doi:10.1016/j.ecmx.2021.100137
Wang, H., Pu, Y., Ragauskas, A., and Yang, B. (2019). From Lignin to Valuable Products-Strategies, Challenges, and Prospects. Bioresour. Techn. 271, 449–461. doi:10.1016/j.biortech.2018.09.072
Wang, Z., Li, N., and Pan, X. (2019). Transformation of Ammonia Fiber Expansion (AFEX) Corn stover Lignin into Microbial Lipids by Rhodococcus Opacus. Fuel 240, 119–125. doi:10.1016/j.fuel.2018.11.081
Wei, Z., Zeng, G., Huang, F., Kosa, M., Huang, D., and Ragauskas, A. J. (2015). Bioconversion of Oxygen-Pretreated Kraft Lignin to Microbial Lipid with Oleaginous Rhodococcus Opacus DSM 1069. Green. Chem. 17 (5), 2784–2789. doi:10.1039/c5gc00422e
Wells, T., Wei, Z., and Ragauskas, A. (2015). Bioconversion of Lignocellulosic Pretreatment Effluent via Oleaginous Rhodococcus Opacus DSM 1069. Biomass Bioenergy 72, 200–205. doi:10.1016/j.biombioe.2014.11.004
Weng, C., Peng, X., and Han, Y. (2021). Depolymerization and Conversion of Lignin to Value-Added Bioproducts by Microbial and Enzymatic Catalysis. Biotechnol. Biofuels 14 (1), 84–105. doi:10.1186/s13068-021-01934-w
Xiong, X., Lian, J., Yu, X., Garcia-Perez, M., and Chen, S. (2016). Engineering Levoglucosan Metabolic Pathway in Rhodococcus Jostii RHA1 for Lipid Production. J. Ind. Microbiol. Biotechnol. 43 (11), 1551–1560. doi:10.1007/s10295-016-1832-9
Xu, B., Li, Q., Pu, Y., Xie, S., Ragauskas, A. J., Arreola-Vargas, J., et al. (2022). A Unique Bacterial Pelletized Cultivation Platform in Rhodococcus Opacus PD630 Enhanced Lipid Productivity and Simplified Harvest for Lignin Bioconversion. ACS Sust. Chem. Eng. 10 (3), 1083–1092. doi:10.1021/acssuschemeng.1c05239
Xu, C., Su, X., Wang, J., Zhang, F., Shen, G., Yuan, Y., et al. (2021). Characteristics and Functional Bacteria in a Microbial Consortium for rice Straw Lignin-Degrading. Bioresour. Techn. 331, 125066. doi:10.1016/j.biortech.2021.125066
Xu, R., Zhang, K., Liu, P., Han, H., Zhao, S., Kakade, A., et al. (2018). Lignin Depolymerization and Utilization by Bacteria. Bioresour. Techn. 269, 557–566. doi:10.1016/j.biortech.2018.08.118
Xu, Z., Lei, P., Zhai, R., Wen, Z., and Jin, M. (2019). Recent Advances in Lignin Valorization with Bacterial Cultures: Microorganisms, Metabolic Pathways, and Bio-Products. Biotechnol. Biofuels 12, 32–50. doi:10.1186/s13068-019-1376-0
Xu, Z., Qin, L., Cai, M., Hua, W., and Jin, M. (2018). Biodegradation of Kraft Lignin by Newly Isolated Klebsiella pneumoniae, Pseudomonas putida, and Ochrobactrum tritici Strains. Environ. Sci. Pollut. Res. 25 (14), 14171–14181. doi:10.1007/s11356-018-1633-y
Yaguchi, A., Franaszek, N., O'neill, K., Lee, S., Sitepu, I., Boundy-Mills, K., et al. (2020). Identification of Oleaginous Yeasts that Metabolize Aromatic Compounds. J. Ind. Microbiol. Biotechnol. 47 (9–10), 801–813. doi:10.1007/s10295-020-02269-5
Yao, L., Yang, H., Meng, X., and Ragauskas, A. J. (2022). Towards a Fundamental Understanding of the Role of Lignin in the Biorefinery Process. Front. Energ. Res. 9, 895. doi:10.3389/fenrg.2021.804086
Zevallos Torres, L. A., Lorenci Woiciechowski, A., De Andrade Tanobe, V. O., Karp, S. G., Guimarães Lorenci, L. C., Faulds, C., et al. (2020). Lignin as a Potential Source of High-Added Value Compounds: A Review. J. Clean. Prod. 263, 121499. doi:10.1016/j.jclepro.2020.121499
Zhang, C., and Wang, F. (2020). Catalytic Lignin Depolymerization to Aromatic Chemicals. Acc. Chem. Res. 53 (2), 470–484. doi:10.1021/acs.accounts.9b00573
Zhang, L., Song, Y., Wang, Q., and Zhang, X. (2021). Culturing Rhodotorula Glutinis in Fermentation-Friendly Deep Eutectic Solvent Extraction Liquor of Lignin for Producing Microbial Lipid. Bioresour. Techn. 337, 125475. doi:10.1016/j.biortech.2021.125475
Zhang, Y., and Bao, J. (2022). Tolerance of Trichosporon Cutaneum to Lignin Derived Phenolic Aldehydes Facilitate the Cell Growth and Cellulosic Lipid Accumulation. J. Biotechnol. 343, 32–37. doi:10.1016/j.jbiotec.2021.09.009
Zhao, W., Chi, X., Li, H., He, J., Long, J., Xu, Y., et al. (2019). Eco-Friendly Acetylcholine-Carboxylate Bio-Ionic Liquids for Controllable N-Methylation and N-Formylation Using Ambient CO2 at Low Temperatures. Green. Chem. 21 (3), 567–577. doi:10.1039/c8gc03549k
Zhou, M., Fakayode, O. A., Ahmed Yagoub, A. E., Ji, Q., and Zhou, C. (2022). Lignin Fractionation from Lignocellulosic Biomass Using Deep Eutectic Solvents and its Valorization. Renew. Sust. Energ. Rev. 156, 111986. doi:10.1016/j.rser.2021.111986
Zhu, D., Qaria, M. A., Zhu, B., Sun, J., and Yang, B. (2022). Extremophiles and Extremozymes in Lignin Bioprocessing. Renew. Sust. Energ. Rev. 157, 112069. doi:10.1016/j.rser.2021.112069
Zhu, D., Zhang, P., Xie, C., Zhang, W., Sun, J., Qian, W.-J., et al. (2017). Biodegradation of Alkaline Lignin by Bacillus ligniniphilus L1. Biotechnol. Biofuels 10, 44. doi:10.1186/s13068-017-0735-y
Keywords: lignin, bio-conversion, microbial depolymerization, lipids, bio-funneling
Citation: Wang H, Peng X, Li H, Giannis A and He C (2022) Recent Biotechnology Advances in Bio-Conversion of Lignin to Lipids by Bacterial Cultures. Front. Chem. 10:894593. doi: 10.3389/fchem.2022.894593
Received: 11 March 2022; Accepted: 28 March 2022;
Published: 12 April 2022.
Edited by:
Haian Xia, Nanjing Forestry University, ChinaCopyright © 2022 Wang, Peng, Li, Giannis and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hu Li, aGxpMTNAZ3p1LmVkdS5jbg==; Chao He, Y2hhby5oZUB0dW5pLmZp
 Huan Wang
Huan Wang Xiaodong Peng3
Xiaodong Peng3 Hu Li
Hu Li Chao He
Chao He