- Guizhou Provincial Key Laboratory for Rare Animal and Economic Insects of the Mountainous Region, College of Biology and Environmental Engineering, Guiyang University, Guiyang, China
Microalgae are considered as the third-generation feedstock for biodiesel production, and lipid extraction plays a significant role in efficient production of biofuels. Numerous technologies including chemical, mechanical, and biological have been achieved but high efficiency and potential application on an industrial scale are still needed. This review discusses the factors that influence biodiesel quality and the relative green and sustainable solvents for lipid extraction.
Introduction
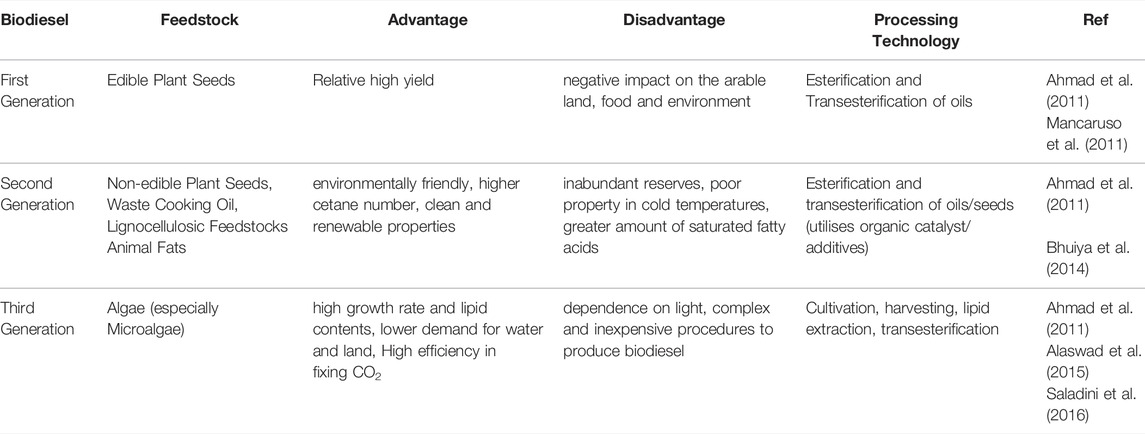
With the concern that fossil fuels have caused global warming and an energy crisis, there is a need to diminish the dependence on it and explore renewable energy. Biodiesel is considered as a potential alternative to petro-diesel as it is non-toxic, biodegradable, has an enhanced cetane number, higher flash point, is renewable, and is produced by transesterification of renewable feedstocks, resulting in monoalkyl esters from fatty acids (Meher et al., 2004; Hoekman et al., 2012; Fazal et al., 2013; Pan et al., 2022) In the past 2 decades, three generations of biodiesels have been investigated and each generation had advantages and disadvantages over different feedstocks. Table 1 provided the summary of the generations of biodiesels. High-efficiency converted algae for biodiesel urgently needs to be explored.
The Production of Biodiesel From Microalgae
Fuel Properties Parameters of Biodiesels
Generally, factors including the viscosity, oxidation stability, cetane number (CN), cold filter plugging point, flash point, saponification value (SV), energy density, and density of biodiesel are determined by the fatty acid composition, which plays a crucially important role in biodiesel qualities. How to optimize the parameters with technologies to enhance quality during the production process is a key question.
Fatty acids are comprised of unsaturated, namely mono-unsaturated (denoted as Cn:1) and polyunsaturated (Cn:2 or 3), and saturated (Cn:0) fatty acids. Viscosity increases along with the chain length and fatty acid saturability. Transesterification, also called alcoholysis, of the algae oil to the corresponding fatty ester (biodiesel) is the most promising approach to the high viscosity problem (Demirbas, 2009). Better oxidation stability, meanwhile, requires a high level of fatty acid saturation (Graboski and McCormick, 1998). CN increases with the enhancement in chain length and fatty acid saturation level (Içingür and Altiparmak, 2003; Knothe, 2005). The higher the saturation degree is, the poorer the cold filter plugging point is (Ramos et al., 2009). A shorter chain length provides a lower flash point and the density will be high when the polyunsaturation level is high (Karmakar et al., 2010). Fatty acid methyl esters with a carbon chain length from 12 to 20 are identified as biodiesel. The SV indicates the chain length of triglycerides and explains the content of free fatty acids, high levels of which can be reduced by acid catalysts (Srivastava and Prasad, 2000; Aransiola et al., 2010).
The Influence of Reaction Factors on Biodiesel Derived From Microalgae
The effect of water content mainly refers to the handled dry and original wet algal biomass (Atadashi et al., 2012) to produce biodiesel. The presence of water plays a crucial part in triglyceride hydrolyzing to free fatty acid (FFA) resulting in soap and emulsions formation, hence the water content control is lower than 0.05% (w/w) (Sanford et al., 2009). Another dimension, a high water content of up to about 98%, generates the hydrated shell around algal cells affecting energy as well as mass transfer (Martinez Guerra et al., 2018), furthermore, posing difficulty in the extraction of lipids.
Although homogeneous acid and base catalysts exhibit high efficiency and universality, the separation is tough and requires further neutralization. Although homogenous base-catalyzed reaction is 400 times quicker than the acid-catalyzed reaction, acidic catalysts are normally used for the feedstocks with high contents of FFAs and water (Aransiola et al., 2010), while the alkaline ones are very sensitive to them, affecting the introduction to the laboratory and the industrial popularly (Frascari et al., 2008). A heterogeneous catalyst is easily separated, reducing the cost of catalyst recovery (Tran et al., 2017; Zhang et al., 2019), and the inexpensive basic catalyst including calcium oxide, calcium hydroxide, and magnesium oxide also reduce the environmental impact (Zhang et al., 2010).
Biodiesel can be synthesized from algae through a traditional two-step method (oil extracted from the algae and then transesterified into biodiesel) or an in-situ approach (extraction of oil, esterification of FFAs, and transesterification of triglycerides occur simultaneously) (Sara et al., 2016; Martinez-Guerra et al., 2018; Al-Ameri and Al-Zuhair, 2019). The former requires a long time, a large reactor, and an even higher investment, while the latter offers an efficient method, which simplifies the production process, minimizes the dosage of solvents, and can give improved biodiesel yield.
The Ionic Liquids for Enhancing Lipid Recovery for Biodiesel Preparation
Ionic liquids (ILs) are widely known as green organic solvents and are non-aqueous salt composed of organic cations and organic or inorganic anions melting at low temperatures (<100°C). ILs are suitable for lipid extraction owing to the major advantages:
1)eco-friendly in nature (Zhao et al., 2019); 2)non-volatile and non-flammable (Vekariya, 2017; Harris et al., 2018); 3) good thermal and chemical stability (Khiratkar et al., 2018); 4)synthetic flexibility (Kim et al., 2012); and 5)immiscibility with organic solvents (Li et al., 2013).
The plausible mechanism of the transesterification of microalgal lipids with alcohol using sulfonic ILs catalyst is shown in Figure 1.
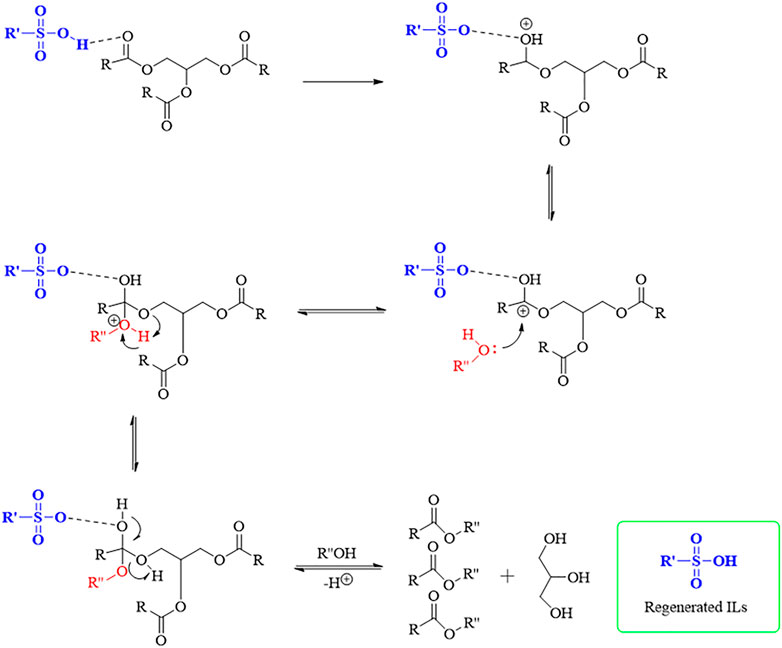
FIGURE 1. Plausible mechanism of transesterification of microalgal lipids with alcohol using sulfonic ILs catalyst [Here, R denotes the alkyl chain of the triglycerides, R′ denotes the struture of the ILs expert the -SO3H group and R’’denotes the alkyl group of alcohol].
Conventional Ionic Liquids
The research explored the lipid extraction effect of [Bmim][MeSO4] from Chlorella vulgaris combined with ultrasound pre-treatment and drew a comparison to the traditional Soxhlet method and Bligh and Dyer’s method. Results demonstrated that IL exhibited 2-fold and 1.6-fold higher lipid extraction than the classic approaches (Kim et al., 2013). Similarly, Choi compared the lipid extraction yield of Chlorella vulgaris by the mixture of ILs, with the assistance of organic solvents. They confirmed that lipid extraction yield was enhanced using IL mixtures, which was ascribed to the synergistic effects with different anions (Choi et al., 2014). The introduction of ILs on wet algal biomass has proven to be the easiest and most efficient method of lipid extraction (Orr and Rehmann, 2016). Furthermore, the poly-ILs catalyst with a large surface area and abundant mesopores have also been investigated for biodiesel preparation (Bian et al., 2020). The combination of magnetic nanoparticles (MNPs) and ILs was used to separate microalgae from the aqueous phase with 99% efficiency and 99% lipids extraction efficiency under ILs/hexane, respectively (Egesa and Plucinski 2022).
Due to ILs’ unrealistic application at an industrial scale due to costs and environmental impact, limited articles are available in the literature on the synthesis of biodiesel (Motlagh et al., 2019). ILs have been confirmed to not be harmful for humans, but the preparation routes involve processes that require expensive, toxic, and volatile reagents (Harris et al., 2018; Singh and Savoy, 2020).
Deep Eutectic Solvents
DESs are generally comprised of organic salts (such as choline chloride, choline acetate, quaternary ammonium salt, or phosphonium salt) and hydrogen-bond donors (HBD) (such as amides, amines, alcohols, and carboxylic acids) that are stable in hydrogen bond interactions, with a melting point lower than that of anionic and cationic counterparts (Zhang et al., 2010; Durand et al., 2013). As a novel class of renewable solvents, DESs emerge with several benefits including low-cost synthesis, non-toxicity, low volatility, and high biodegradability (Zhang et al., 2010; Radošević et al., 2015).
The investigation reported that the cell wall of Chlorella sp. and Chlorococcum sp. contains α-cellulose, hemicellulose, protein, lipid, and ash (Loos and Meindl, 1982). tThe combination of DESs and α-cellulose, hemicellulose, affords new hydrogen bonds that could damage the microalgae cells to enhance the lipid extraction. Three different DESs, aqueous choline chloride-oxalic acid (aCh-O), aqueous choline chloride-ethylene glycol (aCh-EG), and aqueous urea-acetamide (aU-A), were applied to pretreated Chlorella sp. and the lipid recovery rate of biomass was evaluated. Results demonstrated that the lipid recovery rate was enhanced from 52.0% of a blank control group to 80.9, 66.9, and 75.3% of the biomass treated by aCh-O, aCh-EG, and aU-A, respectively (Lu et al., 2016). There a consistent conclusion obtained when DESs are treated on wet and unbroken (water content is 65–67%) with Chlorella sp. and Chlorococcum sp. (GN38) through one-step and two-step methods (Pan et al., 2017).
Conclusion and Perspectives
The review discusses the factors that influence biodiesel quality and conversion of microalgal. It is necessary to adjust these technical parameters with analysis to ensure the feasibility of biodiesel production. The main aims of green solvents for extraction should be eco-friendliness, less dosage of solvent, increasing the quality of the product without byproducts, and saving energy. The efficient DESs with suitable organic salts and HBD to extract lipid are in demand. Microalgae research and development are expansive and synthesis technology for biodiesel from microalgae still requires much investigation. The life cycle analysis of the existing processes will be beneficial for commercial application.
Author Contributions
XL and DY jointly conceived the article and discussed the outline. XL wrote the manuscript. DY and HL have made preliminary revisions to the manuscript. CL and XL coordinated the entire content of the manuscript and made detailed revisions.
Funding
This work was financially supported by the scientific research funds of Guiyang University (GYU-KY-(2022)), the Guizhou Provincial Key Laboratory for Rare Animal and Economic Insects of the Mountainous Region ((2018)5102), and the National Natural Science Foundation of China (22065004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, A. L., Yasin, N. H. M., Derek, C. J. C., and Lim, J. K. (2011). Microalgae as a Sustainable Energy Source for Biodiesel Production: A Review. Renew. Sustain. Energy Rev. 15, 584–593. doi:10.1016/j.rser.2010.09.018
Al-Ameri, M., and Al-Zuhair, S. (2019). Using Switchable Solvents for Enhanced, Simultaneous Microalgae Oil Extraction-Reaction for Biodiesel Production. Biochem. Eng. J. 141, 217–224. doi:10.1016/j.bej.2018.10.017
Alaswad, A., Dassisti, M., Prescott, T., and Olabi, A. G. (2015). Technologies and Developments of Third Generation Biofuel Production. Renew. Sustain. Energy Rev. 51, 1446–1460. doi:10.1016/j.rser.2015.07.058
Aransiola, E., Betiku, E., Layokun, S., and Solomon, B. (2010). Production of Biodiesel by Transesterification of Refined Soybean Oil. Int. J. Biol. Chem. Sci. 4 (2), 58132. doi:10.4314/ijbcs.v4i2.58132
Atadashi, I. M., Aroua, M. K., Abdul Aziz, A. R., and Sulaiman, N. M. N. (2012). The Effects of Water on Biodiesel Production and Refining Technologies: a Review. Renew. Sustain. Energy Rev. 16, 3456–3470. doi:10.1016/j.rser.2012.03.004
Bhuiya, M. M. K., Rasul, M. G., Khan, M. M. K., Ashwath, N., Azad, A. K., and Hazrat, M. A. (2014). Second Generation Biodiesel: Potential Alternative To-Edible Oil-Derived Biodiesel. Energy Procedia 61, 1969–1972. doi:10.1016/j.egypro.2014.12.054
Bian, Y., Zhang, J., Liu, C., and Zhao, D. (2020). Synthesis of Cross-Linked Poly Acidic Ionic Liquids and its Application in Biodiesel Production. Catal. Lett. 150, 969–978. doi:10.1007/s10562-019-02988-0
Choi, S.-A., Oh, Y.-K., Jeong, M.-J., Kim, S. W., Lee, J.-S., and Park, J.-Y. (2014). Effects of Ionic Liquid Mixtures on Lipid Extraction from Chlorella Vulgaris. Renew. Energy 65, 169–174. doi:10.1016/j.renene.2013.08.015
Demirbas, A. (2009). Production of Biodiesel from Algae Oils. Energy Source. Part A 31, 163–168. doi:10.1080/15567030802093955
Durand, E., Lecomte, J., and Villeneuve, P. (2013). Deep Eutectic Solvents: Synthesis, Application, and Focus on Lipase‐catalyzed Reactions. Eur. J. Lipid Sci. Technol. 115 (4), 379–385. doi:10.1002/ejlt.201200416
Egesa, D., and Plucinski, P. (2022). Efficient Extraction of Lipids from Magnetically Separated Microalgae Using Ionic Liquids and Their Transesterification to Biodiesel. Biomass Convers. bior. doi:10.1007/s13399-022-02377-5
Fazal, M. A., Haseeb, A. S. M. A., and Masjuki, H. H. (2013). Investigation of Friction and Wear Characteristics of Palm Biodiesel. Energy Convers. Manag. 67, 251–256. doi:10.1016/j.enconman.2012.12.002
Frascari, D., Zuccaro, M., Pinelli, D., and Paglianti, A. (2008). A Pilot-Scale Study of Alkali-Catalyzed Sunflower Oil Transesterification with Static Mixing and with Mechanical Agitation. Energy fuels. 22, 1493–1501. doi:10.1021/ef700584h
Graboski, M. S., and McCormick, R. L. (1998). Combustion of Fat and Vegetable Oil Derived Fuels in Diesel Engines. Prog. Energy Combust. Sci. 24, 125–164. doi:10.1016/s0360-1285(97)00034-8
Harris, J., Viner, K., Champagne, P., and Jessop, P. G. (2018). Advances in Microalgal Lipid Extraction for Biofuel Production: a Review. Biofuels, Bioprod. Bioref. 12 (6), 1118–1135. doi:10.1002/bbb.1923
Hoekman, S. K., Broch, A., Robbins, C., Ceniceros, E., and Natarajan, M. (2012). Review of Biodiesel Composition, Properties, and Specifications. Renew. Sustain. Energy Rev. 16, 143–169. doi:10.1016/j.rser.2011.07.143
Içingür, Y., and Altiparmak, D. (2003). Effect of Fuel Cetane Number and Injection Pressure on a DI Diesel Engine Performance and Emissions. Energy Convers. Manag. 44, 389–397. doi:10.1016/S0196-8904(02)00063-8
Karmakar, A., Karmakar, S., and Mukherjee, S. (2010). Properties of Various Plants and Animals Feedstocks for Biodiesel Production. Bioresour. Technol. 101, 7201–7210. doi:10.1016/j.biortech.2010.04.079
Khiratkar, A. G., Balinge, K. R., Patle, D. S., Krishnamurthy, M., Cheralathan, K. K., and Bhagat, P. R. (2018). Transesterification of castor Oil Using Benzimidazolium Based Brønsted Acid Ionic Liquid Catalyst. Fuel 231, 458–467. doi:10.1016/j.fuel.2018.05.127
Kim, Y.-H., Choi, Y.-K., Park, J., Lee, S., Yang, Y.-H., Kim, H. J., et al. (2012). Ionic Liquid-Mediated Extraction of Lipids from Algal Biomass. Bioresour. Technol. 109, 312–315. doi:10.1016/j.biortech.2011.04.064
Kim, Y.-H., Park, S., Kim, M. H., Choi, Y.-K., Yang, Y.-H., Kim, H. J., et al. (2013). Ultrasound-assisted Extraction of Lipids from Chlorella Vulgaris Using [Bmim][MeSO4]. Biomass Bioenergy 56, 99–103. doi:10.1016/j.biombioe.2013.04.022
Knothe, G. (2005). Dependence of Biodiesel Fuel Properties on the Structure of Fatty Acid Alkyl Esters. Fuel Process. Technol. 86, 1059–1070. doi:10.1016/j.fuproc.2004.11.002
Li, H., He, X., Zhang, Q., Chang, F., Xue, W., Zhang, Y., et al. (2013). Polymeric Ionic Hybrid as Solid Acid Catalyst for the Selective Conversion of Fructose and Glucose to 5-hydroxymethylfurfural. Energy Technol. 1, 151–156. doi:10.1002/ente.201200041
Loos, E., and Meindl, D. (1982). Composition of the Cell Wall of Chlorella Fusca. Planta 156 (3), 270–273. doi:10.1007/BF00393735
Lu, W., Alam, M. A., Pan, Y., Wu, J., Wang, Z., and Yuan, Z. (2016). A New Approach of Microalgal Biomass Pretreatment Using Deep Eutectic Solvents for Enhanced Lipid Recovery for Biodiesel Production. Bioresour. Technol. 218, 123–128. doi:10.1016/j.biortech.2016.05.120
Mancaruso, E., Sequino, L., and Vaglieco, B. M. (2011). First and Second Generation Biodiesels Spray Characterization in a Diesel Engine. Fuel 90 (9), 2870–2883. doi:10.1016/j.fuel.2011.04.028
Martinez-Guerra, E., Howlader, M. S., Shields-Menard, S., French, W. T., and Gude, V. G. (2018). Optimization of Wet Microalgal FAME Production from Nannochloropsis Sp. Under the Synergistic Microwave and Ultrasound Effect. Int. J. Energy Res. 42, 1934–1949. doi:10.1002/er.3989
Meher, L., Vidyasagar, D., and Naik, S. (2004). Technical Aspects of Biodiesel Production by Trans- Esterification—A Review. Renew. Sustain. Energy Rev. 10, 248–268. doi:10.1016/j.rser.2004.09.002
Orr, V. C. A., and Rehmann, L. (2016). Ionic Liquids for the Fractionation of Microalgae Biomass. Curr. Opin. Green Sustain. Chem. 2, 22–27. doi:10.1016/j.cogsc.2016.09.006
Pan, H., Xia, Q., Li, H., Wang, Y., Shen, Z., Wang, Y., et al. (2022). Direct Production of Biodiesel from Crude Euphorbia Lathyris L. Oil Catalyzed by Multifunctional Mesoporous Composite Materials. Fuel 309, 122172. doi:10.1016/j.fuel.2021.122172
Pan, Y., Alam, M. A., Wang, Z., Huang, D., Hu, K., Chen, H., et al. (2017). One-step Production of Biodiesel from Wet and Unbroken Microalgae Biomass Using Deep Eutectic Solvent. Bioresour. Technol. 238, 157–163. doi:10.1016/j.biortech.2017.04.038
Radošević, K., CvjetkoBubalo, M., GaurinaSrček, V., Grgas, D., LandekaDragičević, T., and RadojčićRedovniković, I. (2015). Evaluation of Toxicity and Biodegradability of Choline Chloride Based Deep Eutectic Solvents. Ecotx. Environ. Safe. 112, 46–53. doi:10.1016/j.ecoenv.2014.09.034
Ramos, M. J., Fernández, C. M., Casas, A., Rodríguez, L., and Pérez, Á. (2009). Influence of Fatty Acid Composition of Raw Materials on Biodiesel Properties. Bioresour. Technol. 100, 261–268. doi:10.1016/j.biortech.2008.06.039
Rezaei Motlagh, S., Harun, R., Awang Biak, D., Hussain, S., Wan Ab Karim Ghani, W., Khezri, R., et al. (2019). Screening of Suitable Ionic Liquids as Green Solvents for Extraction of Eicosapentaenoic Acid (EPA) from Microalgae Biomass Using COSMO-RS Model. Molecules 24, 713. doi:10.3390/molecules24040713
Saladini, F., Patrizi, N., Pulselli, F. M., Marchettini, N., and Bastianoni, S. (2016). Guidelines for Emergy Evaluation of First, Second and Third Generation Biofuels. Renew. Sustain. Energy Rev. 66, 221–227. doi:10.1016/j.rser.2016.07.073
Sanford, S. D., White, J. M., Shah, P. S., Wee, C., Valverde, M. A., and Meier, G. R. (2009). Feedstock and Biodiesel characteristics report. Renew. Energy Group. Rep.
Sara, M., Brar, S. K., and Blais, J. F. (2016). Comparative Study between Microwave and Ultrasonication Aided In Situ Transesterification of Microbial Lipids. RSC Adv. 6, 56009–56017. doi:10.1039/c6ra10379k
Singh, S. K., and Savoy, A. W. (2020). Ionic Liquids Synthesis and Applications: an Overview. J. Mol. Liq. 297, 112038. doi:10.1016/j.molliq.2019.112038
Srivastava, A., and Prasad, R. (2000). Triglycerides-based Diesel Fuels. Renew. Sustain. Energy Rev. 4, 111–133. doi:10.1016/s1364-0321(99)00013-1
Tran, D.-T., Chang, J.-S., and Lee, D.-J. (2017). Recent Insights into Continuous-Flow Biodiesel Production via Catalytic and Non-catalytic Transesterification Processes. Appl. Energy 185, 376–409. doi:10.1016/j.apenergy.2016.11.006
Vekariya, R. L. (2017). A Review of Ionic Liquids: Applications towards Catalytic Organic Transformations. J. Mol. Liq. 227, 44–60. doi:10.1016/j.molliq.2016.11.123
Zhang, H., Li, H., Hu, Y., Venkateswara Rao, K. T., Xu, C., and Yang, S. (2019). Advances in Production of Bio-Based Ester Fuels with Heterogeneous Bifunctional Catalysts. Renew. Sustain. Energy Rev. 114, 109296. doi:10.1016/j.rser.2019.109296
Zhang, J., Chen, S., Yang, R., and Yan, Y. (2010). Biodiesel Production from Vegetable Oil Using Heterogenous Acid and Alkali Catalyst. Fuel 89, 2939–2944. doi:10.1016/j.fuel.2010.05.009
Keywords: microalgae, biodiesel, lipid extraction, deep eutectic solvents, green solvent
Citation: Liu X, Yu D, Luo H and Li C (2022) Green Solvents for Lipid Extraction From Microalgae to Produce Biodiesel. Front. Chem. 10:884274. doi: 10.3389/fchem.2022.884274
Received: 26 February 2022; Accepted: 11 April 2022;
Published: 18 May 2022.
Edited by:
Hu Li, Guizhou University, ChinaReviewed by:
Qiuyun Zhang, Anshun University, ChinaJian He, Jishou University, China
Hu Pan, Jiaxing University, China
Copyright © 2022 Liu, Yu, Luo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Can Li, bGljYW43OTAxMDhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xiaofang Liu
Xiaofang Liu Dayong Yu
Dayong Yu Hangyu Luo
Hangyu Luo Can Li
Can Li