
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Chem. , 13 April 2022
Sec. Analytical Chemistry
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.875241
This article is part of the Research Topic Reviews in Chemistry View all 14 articles
All forms of life have absolute request for metal elements, because metal elements are instrumental in various fundamental processes. Fluorescent probes have been widely used due to their ease of operation, good selectivity, high spatial and temporal resolution, and high sensitivity. In this paper, the research progress of various metal ion (Fe3+,Fe2+,Cu2+,Zn2+,Hg2+,Pb2+,Cd2+) fluorescent probes in recent years has been reviewed, and the fluorescence probes prepared with different structures and materials in different environments are introduced. It is of great significance to improve the sensing performance on metal ions. This research has a wide prospect in the application fields of fluorescence sensing, quantitative analysis, biomedicine and so on. This paper discusses about the development and applications of metal fluorescent probes in future.
Metal cations and anions play an important role in the versatile physiological and pathological processes, including metabolism, osmotic regulation, catalysis and so on. It is well known that normal biological events can be adversely affected by maladjustment on the levels of certain ions in organisms (Park et al., 2020). It is because of these ions has certain pathophysiological significance, so we explore how these ions detection in biological systems of sensitive and selective is important.
In order to detect and quantify ions, researchers are committed to developing appropriate chemical sensors. Fluorescence in combination with appropriate probes is a good way to measure metal ions, because fluorescence has certain advantages, such as being faster, less destructive and more sensitive, which can present information about the location and quantity of the target (Li et al., 2017). Fluorescence probe method means that the photophysical properties of probe molecules change obviously before and after the specific binding between probe and analyte, so as to detect the change of fluorescence signal, realizing the detection of different molecular or ion content in the organism or the environment (Xu et al., 2016). In general, for the fluorometric determination of cations or anions, the sensor must consist of two components: a fluorescent carrier and an ionic carrier, which may be independent species or covalently linked on a molecule.
Fluorescent probes can be combined with bioluminescence imaging technology to achieve in vivo detection at the cellular level or animal level, which is considered to be the most potential tool for studying different components in the organisms. Fluorescent probe for metal ions, therefore, carries on the synthesis and design gradually become the research hot spot. It is of great significance in chemistry, biology, clinical medicine and agriculture to search for novel organic molecular recognition carriers with high selectivity and to design a novel fluorescent probe for metal ion detection (Xu and Xu, 2016). To have an extensive overview of present studies, we summarized popular fluorescent probes from literatures, classified them according to the types of ions being detected, and presented their structures and strategies. In addition, we focus on numerous strategies to improve the selectivity of fluorescent probes, including metal-organic backbones, central hydrophilic external hydrophobic strategies, etc., which will provide additional insights for biomedicine.
In this review, the latest research progress of fluorescent probes for iron, copper, zinc, mercury, lead, chromium and other metal ions are summarized (Figure 1), and the development trend and application prospect of this field are also discussed. Basic information on the fluorescent probes for the detection of various ions is listed in Table 1.
Fe3+ is considered among the most important metal ions in biological systems and exerts an unparalleled role in many biological processes one of the most vital metal ions in biological systems, such as RNA and DNA synthesis, metabolism and so on. To develop new fluorescent probes having low levels of cellular toxicity, good biocompatibility and high solubility in water is becoming more and more important and urgent.
Lytton and co-workers (Lytton et al., 1992) put forward the earliest reported about an iron fluorescent sensor, which is on the basis of the iron carrier desferrioxamine B (DFO) model, connect to the fluorescent carrier 7-nitrobenz-2-oxa-1, 3-diazole (NBD). By detecting and monitoring Fe3+ performance in solution, the NBD-DFO model proves its advantage in monitoring iron under various conditions of iron imbalance diseases. For the past few years, various fluorescent probes developed for selective detection of Fe3+ can be divided into four categories according to the fluorescence signal processing: Turn-Off, Turn-on, ratiometric and chemodosimeters.
For the exploration of the iron ion, nano fluorescent probe plays an important role. Among them, one of the most well-studied materials is semiconductor nanoparticles, also known as quantum dots. In recent years, carbon dots (CDs) are widely used in fluorescent biological imaging and sensing (Zhu et al., 2013). However, the low quantum yield (QY) of CDs severely restricts its development and application. Chemically doped heteroatoms can effectively adjust electron density to improve the QY value of CDs. Therefore, the photochemical and physicochemical properties of carbon dots (CDs) can be efficiently regulated by chemical doping heteroatoms (Guo et al., 2015).
Shi et al. (Shi et al., 2016) synthesized N, P-CDs multi heteroatom (nitrogen and phosphorus co-doped carbon nanodots) fluorescence sensor, which has low cytotoxicity and high photostability, and measured its sensitivity to Fe3+. Liu et al. (Liu Z. et al., 2020) synthesized the non-toxic nitrogen-doped fluorescent carbon dots (N-CDs) that can detect ferric ions, at the same time can be used for fluorescent probe of Vero cell biological imaging (Figure 2A). Du et al. (Du et al., 2021) demonstrated that off-on switch fluorescent probe based on yellow emission carbon dots (y-CDs) provides a highly sensitive and selective method for the detection of ferric ions. Pu et al. (Pu et al., 2020) proposed a static quenching mechanism about the phenylalanine carbon dots (Phe-CDs), which kept its outstanding fluorescence intensity despite its extreme pH values. Atchudan et al. (Atchudan et al., 2021) reported that they synthesized fluorescent carbon dots (KN-CDs) by hydrothermal carbonization method as a good fluorescence sensor of Fe3+ based on the closing sensor of Fe (III) ions. Ju et al. (Ju and Chen, 2014) synthesized environmentally friendly nitrogen-doped graphene quantum dots (N-GQDs) for the unlabeled Fe3+ ions detection in various actual water (Figure 2B). Qi et al. (Qi et al., 2019) designed and demonstrated the promise of nitrogen-doped carbon quantum dots (N-CQDs) as probes for iron ions. Zhang et al. (Zhang Y. et al., 2021) obtained B1N2CQDs (core-shell carbon quantum dots), which has been proved that its detection of Fe3+ can be applied to both endocellular scenarios in biological system and river water samples due to its high stability and fluorescence quantum yield. Xu et al. (Xu Q. et al., 2015) synthesized Sulfur-doped carbon dots (S-doped C-dots), which can also be used to detect iron ions efficiently (Figure 2C).
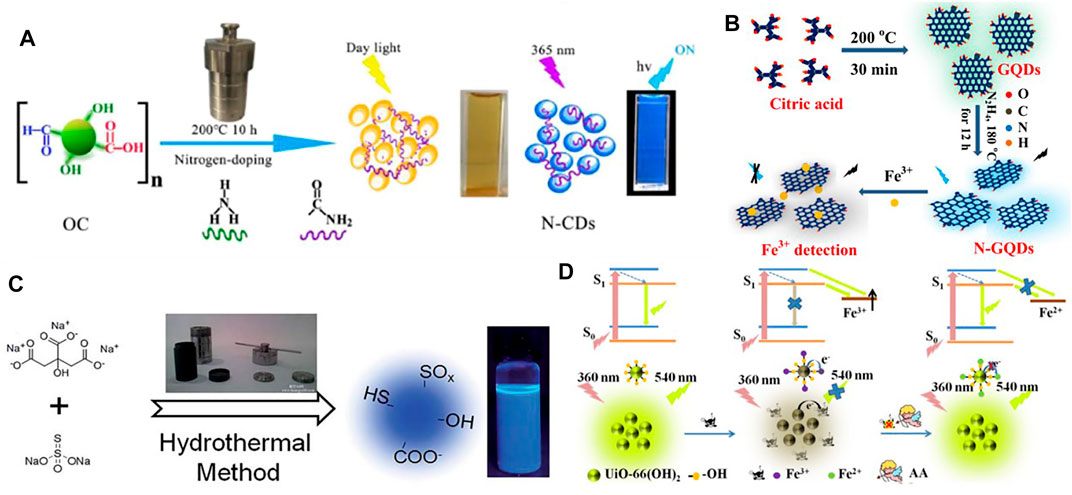
FIGURE 2. (A) The schematic diagram of preparation of fluorescent N-CDs via hydrothermal treatment (Liu Z. et al., 2020); (B) Schematic representation of the procedure for synthesizing N-GQDs and the detection of Fe3+ (Ju and Chen, 2014); (C) Scheme of the synthesis of S-doped C-dots with blue luminescence (Xu Q. et al., 2015); (D) Schematic illustration the “on-off-on” fluorescent detection of Fe3+, AA using UiO-66-(OH)2 as sensor (Wang H. et al., 2021).
Qu et al. (Qu et al., 2013) took advantage of dopamine for light source synthetic photoluminescence carbon nanoparticles (CNPs), which can be used as a very powerful fluorescence sensitive stage and has been successfully applied to iron ion detection in some water samples. Based on hydroxy functional metal organic skeleton (MOF) UiO-66-(OH)2, Wang et al. (Wang H. et al., 2021) also proposed a “on—off—on” fluorescent switch nano prober (Figure 2D). Dong et al. (Dong et al., 2021) synthesized the NIR PL of GSH-capped gold nanoclusters (GSH-AuNCs), which exhibits excellent sensing performance.
Xu and co-workers (Xu H. et al., 2015) obtained a owns two one-dimensional channel unparalleled of three-dimensional TB - BTB (benzene-1, 3, 5-tribenzoate) framework for high sensitivity detection of Fe3+. Using an EuL3 (L = 4'-(4-carboxyphenyl)-2, 2':6', 2 "-tripyridine) fluorescence sensor, Zheng and co-workers (Zheng et al., 2013) designed a portable daily life iron ion test paper. Azmi et al. (Al-Azmi and John, 2021) produced a tricyanofuran hydrazone (TCFH) optical probe for better recognition of Fe (III) ions in the hydro environment. Zhang (Zhang et al., 2018) reported a new fluorescence and specific color probe containing both deoxycholic acid and rhodamine molecules and used the spectrum to achieve the measurement of iron ions.
In order to achieve better sensing performance and water-solubility of organic molecular probes, Liu et al. (Liu J. et al., 2020) proposed a method with great potential and development space. They linked the inner hydrophobic molecule tribromophenol with the outer hydrophobic molecule coumarin on the basis of the original iron fluorescence probe (Figure 3A). Besides, in view of the water-soluble dilemma of organic small molecules, Sun et al. (Sun et al., 2020) also adopted a similar structural strategy, and developed a unique vanilloid fluorescent probe for the assay of iron ions by increasing the nucleophilic substitution reaction of LaOBr (Figure 3B). Designing composite materials is of great significance to the change of the water solubility of organic small molecules, and it can also improve the probe’s sensitivity to ions.
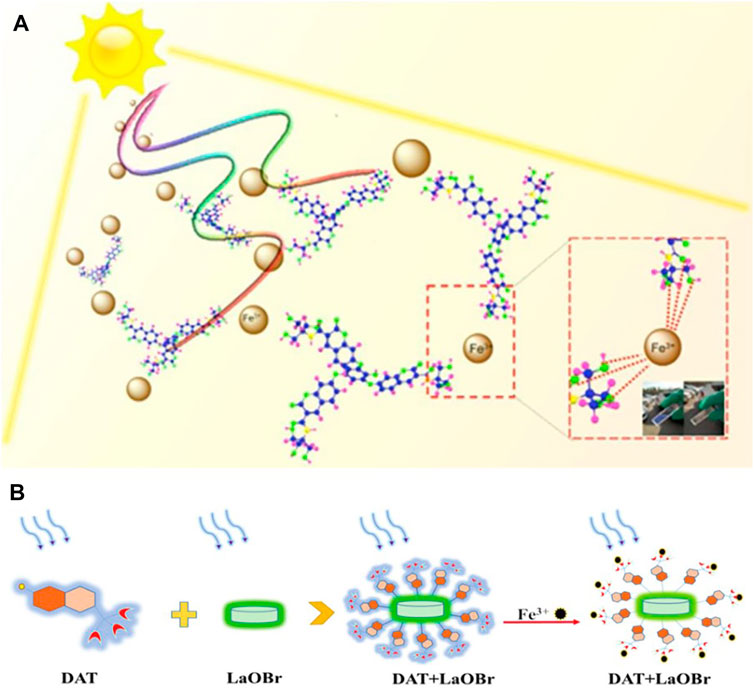
FIGURE 3. (A) The trimeric phenolic coumarin (TPC) designed through the internal hydrophobic-external hydrophilic strategy achieves greatly enhanced Fe3+ sensing performance, and can be used for naked eye detection with sunlight excitation (Liu J. et al., 2020); (B) A schematic diagram showing the preparation of the LaOBr/DAT [N-(2-hydroxy-1,1-bis (hydroxymethyl) ethyl)-7-hydroxycoumarin-3-carboxamide] composite and the mechanism of Fe3+sensing (Sun et al., 2020).
High levels of trivalent iron ions can cause many diseases, including cancer, organ disorders of the heart, liver and pancreas, hepatitis, Parkinson’s disease, Alzheimer’s disease, etc. Therefore, the development of fluorescent probes for iron ions has greatly facilitated biomedical imaging in human cells and has contributed to disease diagnosis.
In last several years, lots of researchers have designed different methods of novel iron (III) fluorescence sensors, and successfully synthesized sensors for monitoring the concentration of environmental iron and studying iron migration in various microbial species. Most sensors are derived from the principle of fluorescence quenching, so future probes should be preferentially designed for absorption at longer wavelength to avoid the internal filtering effect of iron (III) absorption in the UV-vis region.
Although the iron in the cells has two forms of Fe2+ and Fe3+, it mainly exists with the form of Fe2+ because of the reductive microenvironment of cells (Ma et al., 2021). On the one hand, it is difficult to establish a highly specific probe for the detection of Fe2+, because the different oxidation states of Fe2+ and Fe3+ vary with each other. On the other hand, due to the strong force between paramagnetic Fe2+ and fluorophore, fluorescence quenching is usually induced, resulting in part of the constructed probes being “on-off” type, which is not easy to observe due to the influence of spontaneous fluorescence of organisms (Wei et al., 2020). Currently, the Fe2+ fluorescent probes mainly include N-oxides, nitroxyl radicals, endoperoxides, bionic ligands, heavy metals, imines and so on.
In order to achieve high-efficiency sensing of Fe2+, Yan et al. (Yan, 2017) proposed a dual-correspondence luminescence probe with optimized metal-Organic Framework (MOF) as the main body. The special feature of this probe is the use of various luminescent substances, such as lanthanide ions, carbon quantum dots, etc. Xu et al. (Xu and Yan, 2015) also synthesized a layer similar to MOF [MIL-124, or Ga2 (OH)4 (C9O6H4)], and further designed and developed a probe to detect Fe2+ ions. For Fe2+ detection, Yang et al. (Yang et al., 2021) reported on the aggregation-induced emission (AIE) based probe named QM-Fe, and verified the feasibility of Fe2+ detection in viable cells. Zhang et al. (Zhang D. et al., 2021) gained a new spiropyran-based fluorescent probe to detect Fe2+ ion. The results show that the addition of Fe2+ with the probe solution improved the magnetic intensity of fluorescence by 6-fold. Wei et al. (Wei et al., 2020) developed a “on-off” fluorescent probe based on a carbon point (CDs), which has been efficiently used to detect Fe2+ in BSA solution, tap water and living cells (Figure 4A). In order to realize real-time detection of unstable ferric divalent ions in living systems, Long et al. (Long et al., 2018) designed a fluorescent probe of coumarin by using a unique cyclization reaction. Gao et al. (Gao et al., 2020) constructed a practical and novel Fe2+ fluorescent probe via a unique strategy with Fe2+-induced reducing reaction, which can act in different monitoring of the environment, such as living cells. Khatun et al. (Khatun et al., 2020) developed a kind of “Turn-On” probe P-Fe (II), which is used to measure the exact amount of ferrous ion (Fe2+) in cosmetics or living cells (Figure 4B). Liang et al. (Liang et al., 2021) developed a new camphor-based fluorimetic probe (CBO) with a natural monoterpene ketone camphor as a base material for the detection of Fe2+ and it can be easily observed by fluorescence of Fe2+ ions in some animals and in plant cells Fe2+ imaging (Figure 4C).
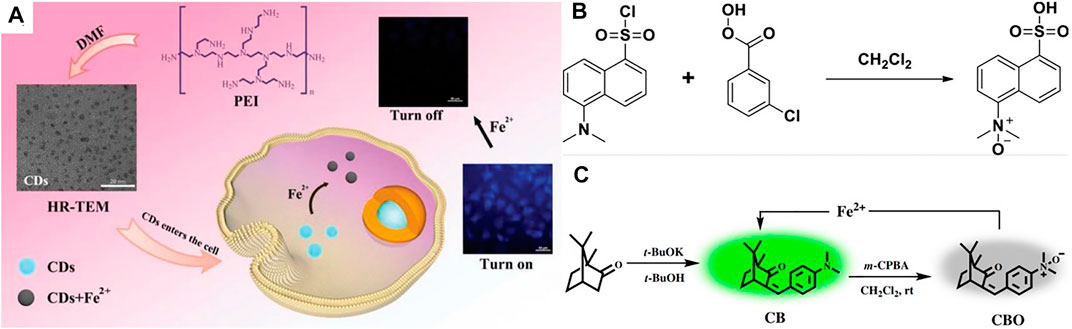
FIGURE 4. (A) One-pot hydrothermal synthesis of fluorescent CDs as a highly efficient “on-off” fluorescent probe for the rapid detection of intracellular Fe2+ (Zhang X. et al., 2020); (B) The synthesis route of probe FeP1 (Khatun et al., 2020); (C) The synthesis route of probe CBO (Liang et al., 2021).
By stimulating the two-photon microscope fluorescence imaging at 680 nm, Yang et al. (Yang et al., 2019) reported for the first time a novel intramolecular charge transfer (ICT) based two-photon, near-infrared (NIR) -enabled fluorescent probe for detecting Fe2+, and applied to the actual living cells. In view of the test organisms or the ferrous ions in aqueous environment, Zhang et al. (Zhang X. et al., 2020) on the basis of nitrogen oxides reduction reaction, invented and created a new kind of “off—on” fluorescent probes the NT—Fe (4-Amino-1, 8-naphthalimide), which greatly improved the efficiency of Fe2+ detection in zebrafish.
During recent years, although there are not numerous reports on the detection of Fe2+ by the methods of fluorescent probes, some progress has been made. It can be roughly divided into “off-on” type Fe2+ fluorescent probes, “on-off” type Fe2+ fluorescent probes, ratio type and other types of Fe2+ fluorescent probes. These in-depth exploration and research will be of great significance for in-depth understanding of the specific functions of Fe2+ in organisms and the mechanism of action on diseases.
As we all know, copper ion (Cu2+) is another heavy metal ion found in very high concentrations in our people’s body. As a cofactor of many enzymes, copper ion is inseparable from various enzymatic catalysis and electron transfer processes, and it occupies a unique and key position in various physiological processes. Therefore, if copper homeostasis is out of whack, it can lead to a lot of neurodegenerative diseases that we don’t expect. In order to achieve an optimal measurement performance for copper ions, small polymer fluorescent probes have been extensively used in the procedure of microscopic image analysis due to their certain uniqueness.
So far, methods about the Cu2+ ions’ detection using small molecular fluorescent probes have generally included the UV-toO-NIR regions of anthracene, danyl,pyrene, quinazoline, cyanine dyes, naphthalimide, quinoline, rhodamine, fluorescein, BODIPY and so on (Sivaraman et al., 2018). However, many significant properties of fluorescent probes used in living cells and in vivo still need to be improved, such as slow response speed, high detection limit and poor selectivity (Kar et al., 2013).
Recently, Zhou et al. (Zhou et al., 2021) designed an open red fluorescent probe that can be used well and efficiently to detect copper ions (Cu2+) in some food samples and live zebrafish. Besides, Copper (Cu) has also been found to be indispensable and extremely important in the process of oxygen-containing photosynthesis in biological systems. Li et al. (Li et al., 2021) developed a novel “turn on” NIR probe DCM-Cu whose basic is DCM for the detection of copper ion (II). Due to its wonderful sensitivity and low cytotoxicity toward Cu2+ with a Stokes shift of 140 nm, the probe has been widely used by scholars. What’s more, by covalently linking carboxylate-modified red fluorescent cadmium telluride (CdTe) quantum dots (QDs) with fluorescent blue carbon nanodots functionalized with amino groups (CDs), Wang et al. (Wang et al., 2016) developed a ratio fluorescence nanosensor with good performance for Cu2+ detection (Figure 5A). In addition, Zhu et al. (Zhu et al., 2012)also adopted a ratio fluorescence detection method for copper ions, in particular, the CdSe@C quantum dots (QDs) with different fluorophore dual-emission were selected. This probe ion recognition higher and better stability (Figure 5B). Lin et al. (Lin et al., 2014) successfully designed a new type of high-fluorescence metal-organic structure (MOFs) for the determination of copper ion content in water in the environment. This strategy utilized a branched chain poly-ethylamine encapsulated carbon quantum dot material, thus improving the fluorescence quantum yield and detection effect. By using mesoporous silica (MS) spheres as the material of nanometer reactors, Zong et al. (Zong et al., 2014) improved the fluorescence probe of carbon point (CDs), and made a contribution to the detection of copper ions by synthesizing CDs as fluorescence probe by “off-on” method. Wang et al. (Wang L. et al., 2020) implemented the chemical probe formation of the copper (II) complex L- Cu2+ as a dual-channel recognition probe, allowing one to identify dramatic changes in color with the naked eye. Yao et al. (Yao et al., 2013) designed a ratiometric fluorescent probe based on hybridized dual-emission quantum dots (QDs) and demonstrated that it can visualize real-time monitoring of copper ions in natural environmental water sources, greatly improving efficiency. Ye et al. (Ye et al., 2015) first reported the use of a cadmium pamoate metal-organic framework as a bifunctional fluorescent sensor for the detection of trace amounts of 2, 4, 6-Trinitrophenol (TNP) and Cu2+, a study that greatly improved the sensitivity of the fluorescent probe for the recognition of copper ions. For the detection of copper ions in actual samples, Tang et al. (Tang et al., 2020) developed a pyridine amide (BTPPA) with aggregation-induced emission (AIE) properties as a probe, which achieved by aggregating switching strategies, through “on-off-on” variation of emissions.
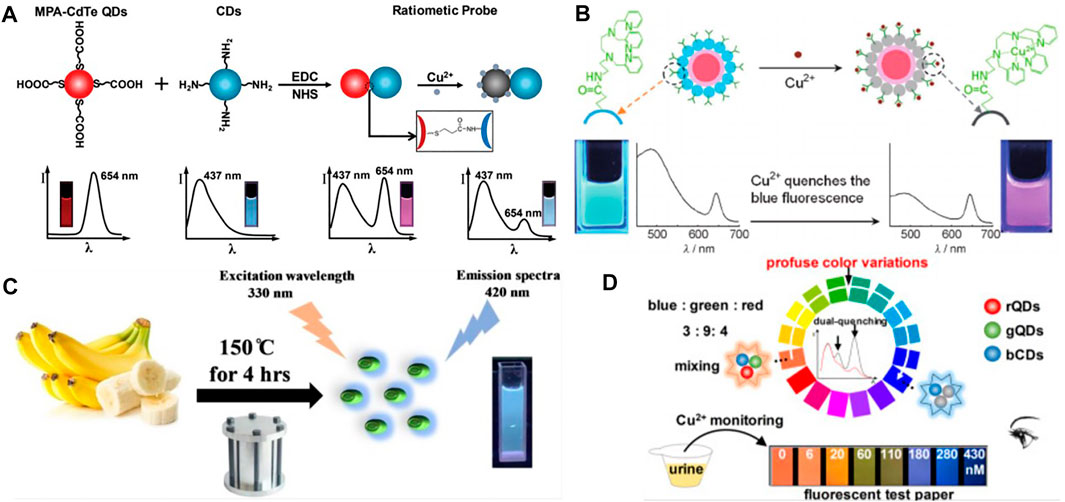
FIGURE 5. (A) Schematic illustration of the formation of the dual-emission ratiometric fluorescence probe and the visual detection principle for copper ions (Wang et al., 2016); (B) Dual-emission fluorescent sensing of Cu2+ ions based on a CdSe@C-TPEA nanohybrid (Zhu et al., 2012); (C) Schematic representation of NS-CQDs synthesis synthesizing NS-CQDs from banana juice (Chaudhary et al., 2020); (D) Schematic illustration of the visual detection principle for Cu2+ using the tricolor probe (Cai et al., 2018).
Fluorescent probes for the detection of Cu(II) in water samples are also accessible from natural sources, such as Chaudhary et al. (Chaudhary et al., 2020)’s synthesized and published highly fluorescent N,S co-doped CQD (NS-CQD) at an excitation wavelength of 330 nm with an enhanced quantum yield (32%) (Figure 5C). For monitoring and identifying Cu2+, Wang et al. (Wang Z.-G. et al., 2020) developed a novel colorimetric/fluorescent probe (7-(diethylamino)-2-oxo-2H-chromen-3-yl)methylene)-4-(dimethylamino) benzohydrazide (HL). Furthermore, in order to improve the continuity of the process when detecting copper ions, Mohammadi et al. (Mohammadi and Ghasemi, 2020) developed a novel pyrimidine-based chemosensor (PyrCS) and was able to achieve an intuitive vivid colorimetric response for observation in a specific pH range. Song et al. (Song et al., 2018) designed a dual emission ratio fluorescence sensing membrane for copper ion detection. This double emission film managed to fabricate the chitosan, graphite carbonitride (G-C3N4) and Gold nanoclusters (Au NCs). The film has high sensitivity and portability, which opens up a new way for the detection of copper ions in the environment. Ranee et al. (Ranee et al., 2018) synthesized quinoline based novel fluorescent probes for the selectivity and sensitivity of detecting Cu2+ ions. Kumar et al. (Gujuluva Gangatharan et al., 2018) synthesized an easily available and portable “off-off” colorimetric and fluorescent probe with excellent results in the application of trace Cu2+ ions in real water samples. Cai et al. (Cai et al., 2018) reported a fluorescent test paper probe consisting of three different emitting quantum dots including blue (bCDs), green (gQDs), and red (rQDs), which enables a simple and rapid detection of Cu2+ ions in human urine by observing the color of the filter paper (Figure 5D). Abnormal levels of copper ions in living organisms can also cause many neurological related diseases, and therefore it is of great importance for biomedicine to have a variety of copper ion fluorescent probes for use in living cells.
Zinc is the next most prevalent of the transition metal ions in the human body. In small amounts, Zn2+ is beneficial to people’s health, but at higher concentrations, it appears toxic. Zinc imbalances have been linked to serious neurological diseases, such as Alzheimer’s and Parkinson’s (Xu et al., 2012). So far, people have developed a variety of Zn2+ fluorescent sensors, and successfully applied in living cells, hippocampal slices, and Zn2+ imaging in zebrafish, especially Lippard and Nagano. In more recent years, zinc ion fluorometric probes have been arranged under different columns of fluorescent architecture, including unquinoline, rhodamine, naphthalene, coumarin, naphthalimide, pyrene, luciferin, derivatives of phenol and several other fluorophore groups (Wang F. et al., 2021).
Walkup et al. (Walkup et al., 2000) first prepared a novel, highly affinity, selective, membrane permeable Zn2+ fluorescence sensor. Roy et al. (Roy et al., 2007) investigated a DFP based sensor for the first time, which can be used as a fluorescent probe for the detection of zinc ions and applied under certain physiological conditions. Peng et al. (Peng et al., 2015) designed and synthesized a chromophore-based up-conversion nanoparticle (UCNPs) nano system as a fluorescent probe for Zn2+ by combining chromophore groups and lanthanide-doped UCNPs together. This method was demonstrated to significantly improve the efficiency of divalent Zn ion detection in specific animals, such as zebrafish. Kim et al. (Kim et al., 2013) reported a unique fluorogenic Zn2+ chemosensor based on a cap-typed tripodal Schiffbase. Zhou et al. (Zhou et al., 2010) designed a hydrazone-pyrene-based fluorescent probe with simplicity and efficiency in order to improve the selectivity of the fluorescent probe for Zn ions. Then they further expanded the application scenario of the probe and verified the feasibility of detecting Zn2+ ions in pancreatic β-cells, which made a contribution to the biomedical field. Hagimori et al. (Hagimori, 2013) reported that novel fluorescent probes based on pyridine-pyridone possess low molecular weight for zinc ion detection. Price et al. (Price et al., 2018) reported AQA-F, a fluorescent probe that can be used to detect zinc ions in prostate and prostate cancer cell lines in vitro. Zhang (Zhang et al., 2012) developed a pyrazoline based fluorescent probe and verified that this probe showed 40-fold enhanced fluorescence for Zn2+ compared to other metal ions, i.e., good selectivity for Zn ions, and that it could be well applied in living neuronal cells.
Zhang et al. (Zhang et al., 2016) reported two near-infrared fluorescent probes based on the fluorophore platform corresponding to Rhodol functionalized by dimethylamine Zn (II) binding groups, and this probe enables the detection of Zn (II) ions produced by intracellular metalloproteins. In addition to this, Fang et al. (Fang et al., 2016) similarly synthesized and studied two NIR fluorescent probes (A and B), but chose different material bases. One is a semicyano structure attached to dimethylamine (DPA) and the other is a dimethylamine derivative with pyridine substituted by pyrazine, which are very effective in detecting zinc ions in living cells. Among the various near-infrared fluorescence (NIR) probes, Zhang et al. (Zhang Y. et al., 2020) synthesized a near-infrared fluorescence (NIR) probe NR-Zn consisting of a dicyanoisophorone derivative and a dimethylamine molecule in a structure (Figure 6A). So far, this probe has proved to be successfully applicable for zinc ion recognition in Hela cells. Kang et al. (Kang et al., 2019) designed a sophisticated fluorescent probe test paper in the acyl hydrazine linkage mode that allows copper ion detection based on color change according to a 365 nm UV lamp (Figure 6B). In some specific scenarios, such as the detection of Zn2+ ions in CH3CN/HEPES solution (1/1, 10.0 mu M, pH = 7.0), a Schiff base fluorescent probe (L) designed by Chang et al. (Chang et al., 2020) can efficiently achieve this purpose. Wang et al. (Wang et al., 2018) devised and synthesized a novel coumarin based dual chemosensor (probe 1), which was observed by fluorescent cell imaging as a bio-imaging fluorescent sensor for detecting Zn2+ in human cancer cells (Figure 6C).
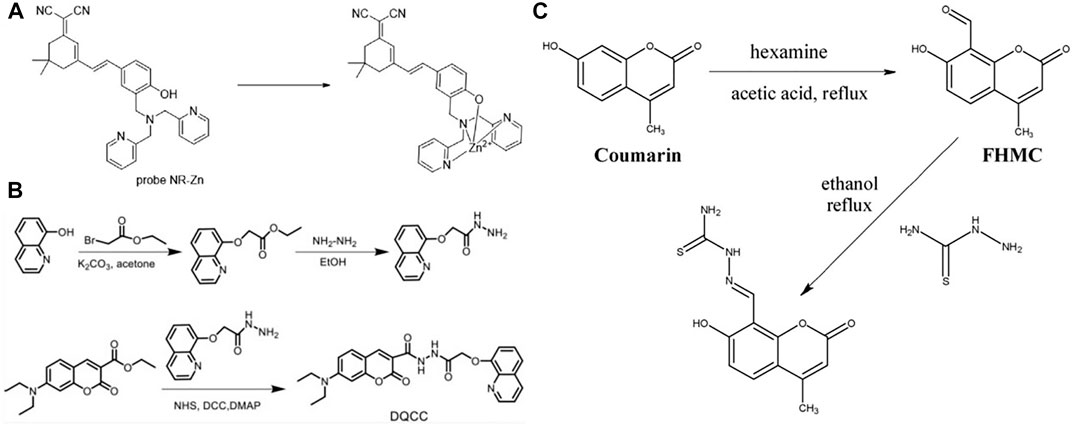
FIGURE 6. (A) The structure of the probe NR-Zn and its interaction mode with Zn (II) ion (Zhang Y. et al., 2020); (B) The synthesis route of DQCC (Kang et al., 2019); (C) The synthesis route of probe 1 (Wang et al., 2018).
Among all kinds of heavy metal ions, lead, cadmium and mercury ions do great harm to the environment and human beings because of their dangerous properties. Since its high toxicity directly or indirectly affect human health, it has been widely concerned in the world. These three heavy metal ions are not biodegradable and can therefore accumulate in the environment, leading to food and water contamination. Heavy metal ions and proteins (or enzymes) can have a strong interaction in the human body, so that the protein inactivity, resulting in chronic poisoning (He and Lu, 2001). People exposed to even very low amount of lead, cadmium and mercury ions can lead to diseases of various systems of the human body. Therefore, a reliable, and convenient method to detect heavy metal ions, especially Hg2+, Pb2+ and Cd2+, has generated a lot of interest in recent years, which is of great significance not only in environmental research, but also in food research and industry and agriculture (Kim et al., 2012).
The detection of mercury ions is of great importance due to their high toxicity and wide distribution in the environment, through which they can enter the food chain and then have an impact on human life and health (Staudinger and Borisov, 2015). Therefore, there is an urgent need for simple, inexpensive and reliable mercury detection methods with high selectivity and sensitivity. In recent years, fluorescence probes used to detect mercury ions are classified according to the changes of fluorescence signal, including signal attenuation fluorescence probes, signal enhancement fluorescence probes and ratio fluorescence probes (Wang Y. et al., 2021). The applications of mercury ion probes can be broadly divided into two areas: environmental and biological.
In purpose of detecting ions in the environment, Lu et al. (Lu et al., 2012) reported for the first time a water-soluble fluorescent carbon nanoparticles (CPs) and used to detect Hg2+ in natural environmental lakes, a method that is environmentally friendly, economical and simple with greater universality. In addition, in order to detect Hg2+ in real lake water, Zhang et al. (Zhang and Chen, 2014) also tried another method, where they obtained nitrogen-doped carbon quantum dots (N-CQDs) using folic acid as the carbon and nitrogen sources, which proved to be highly luminescent with a detection limit of 0.23 μM. Wang et al. (Wang S. et al., 2021) synthesized thioctic acid-carbon dots (SCDs), which was used as an “off-on” type fluorescent probe in the detection of Hg2+. Xu et al. (Xu et al., 2018) expected to identify Hg2+ by changes in fluorescence spectra and fabricated a colorimetric long-wavelength type fluorescent probe Hg-P to obtain higher selectivity. Tao et al. (Tao et al., 2020) synthesized a fluorescent probe based on a simple coumarin derivative, which could recognize mercury Hg2+ selectively in aqueous solution.
In the field of biology, in order to identify Hg2+ in living cells, fluorescent probes with less cytotoxicity and better biocompatibility are needed, therefore Li et al. (Li et al., 2015) designed nitrogen-sulphur co-doped carbon dots (N,S/C-dots) and demonstrated their high fluorescence quantum yield (FLQY, 25%) and good practical application. Wu et al. (Wu and Tong, 2019) synthesized a nitrogen-sulfur co-doped carbon dots (N,S-CDs) as a fluorescent probe with good luminescence properties and high fluorescence quantum yield (16.1%), and achieved good results in Hg2+ ion recognition detection in HepG2 cells (Figure 7A). Li et al. (Liu et al., 2018) have done some research on nano sensors by developing a turn-on nanoprobe, which was prepared based on the principle of fluorescence resonance energy transfer (FRET) between long-chain inducer-functionalized upconversion nanoparticles (UCNPs) and short-chain inducer-functionalized gold nanoparticles (GNPs) (Figure 7B).
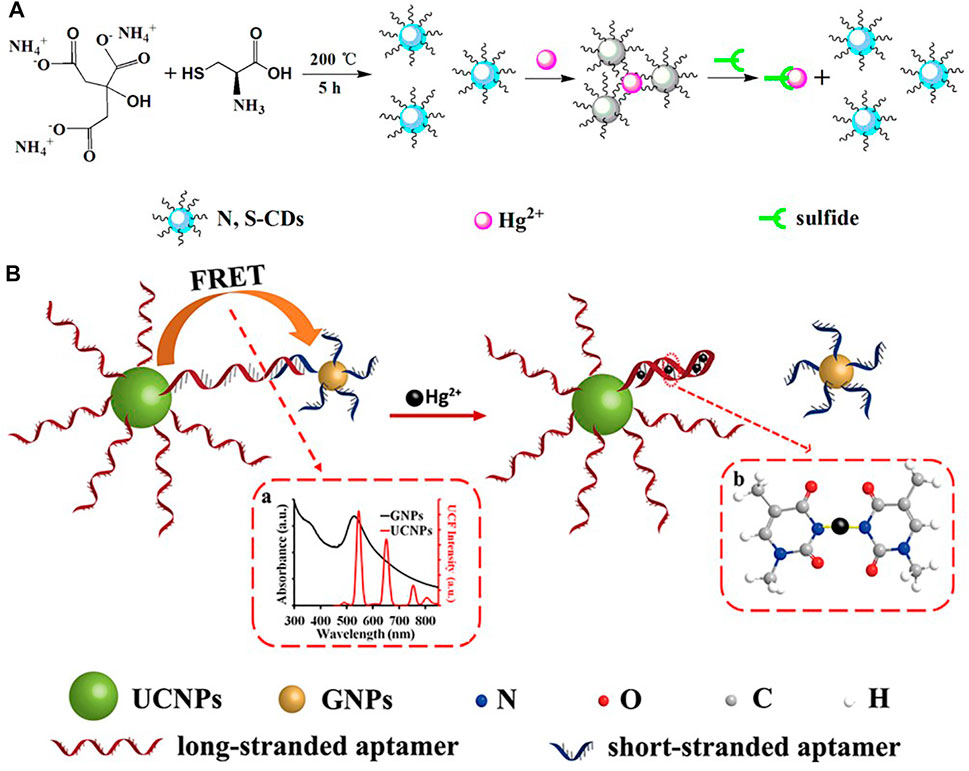
FIGURE 7. (A) Schematic Illustration of the Strategy for Hg2+ Ion and Sulfide Detection (Wu and Tong, 2019); (B) Schematic description of the UCNPs-aptamers-GNPs FRET sensor for Hg2+ (Liu et al., 2018).
Pan et al. (Pan et al., 2018) reported the first reaction-based fluorescent probe (ATC-Hg) that detects Hg (II), which was designed based on the “covalent assembly” principle and is now used to enable the detection of Hg ions in E. coli. Chen et al. (Chen et al., 2019) synthesized a reactive fluorescent probe PIC based on peramidine group, this probe is very high in selectivity due to the fluorescence can be enhanced by 42-fold and is a promising method for the determination of mercury ions. Zhou et al. (Zhou et al., 2017) designed and synthesized a novel small molecule ratiometric fluorescent probe P-Hg using the ESIPT/ICT mechanism, which can be better used to identify Hg2+. This probe can be applied to capture mercury ions both in the natural environment and in biological systems.
Given the diversity of both environmental conditions and biological environments, the field of innovative fluorescent probes for the selective detection of Hg2+ faces challenges such as endogenous active substance interference, leaky cells, and photostability. Fluorescent probes are generally constructed from organic molecular framework with poor stability. Hg2+, as a kind of toxic heavy metal ion, seriously disrupts the natural physiological activities of living organisms. It is of great significance to design powerful probe tools to study the effects of Hg2+ on the health of organisms (Wang Y. et al., 2021).
Lead is a highly toxic substance used in batteries, gasoline and paints. Lead contamination is a chronic concern that creates a lasting threat to our human health and the environment in which we live. Even very small amounts of lead can cause serious damage to various neurological and reproductive systems in our bodies, and can even cause hypertension, lower IQ and slower reactions. According to a large study of scholars, fluorescence and colorimetric sensors were well used, and roughly divided into several categories according to its receptors, including chemical sensor based on nanoparticles, polymer, small molecules, naphthalimide and nanoparticles (Kim et al., 2012).
A number of nanoparticle-based sensing systems have been investigated. Song et al. (Song et al., 2019) designed a fluorescent ion probe for achieving the measurement of lead ions in aquatic solutions using covalent binding fluorescence of 1, 8-naphthylamine dyes with cellulose nanocrystals (CNCs) (Figure 8A). Zhang et al. (Zhang J. et al., 2021) designed a new dual-functional oligonucleotide (OND) probe for the trace Pb (II) detection, in which the 5' end is a single fluorescent moiety using HEX labeling (Figure 8B). Chen et al. (Chen et al., 2020) developed a fluorometric nanoprobe for the determination of Pb (II) based on up-converted nanoparticles (UCNPs) and on magnetic Fe3O4-modified (MNPs) gold nanoparticles (GNPs) (Figure 8C).
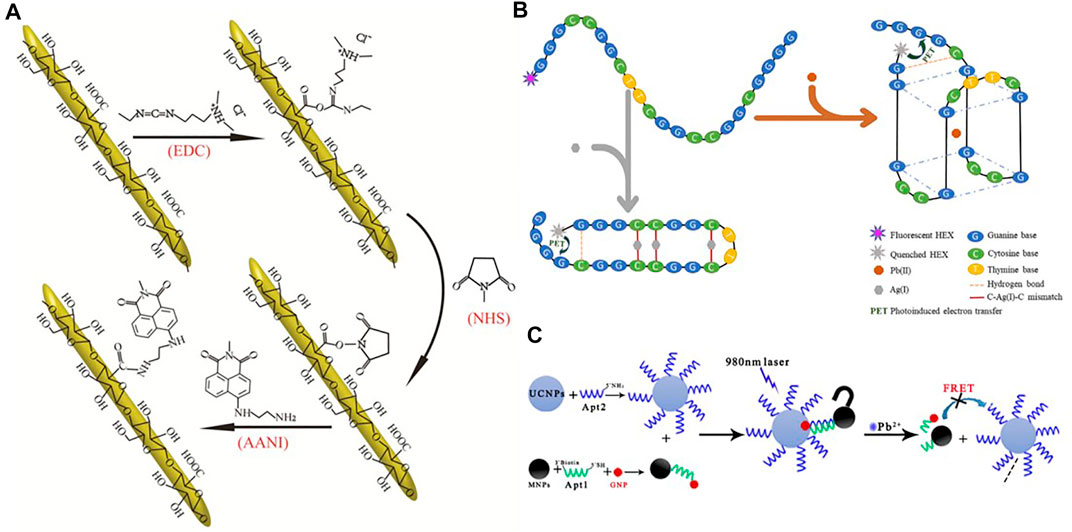
FIGURE 8. (A) Schematic synthesis route of the covalent immobilization of 1,8-naphthalimide dye on the surface of CNC (Song et al., 2019); (B) Schematic diagram for illustrating the simultaneous detection of Pb (II) and Ag (I) ions utilizing single-labelled florescent OND probe (Zhang J. et al., 2021); (C) a Schematic presentation of fluorescent nanoprobe based on fluorescence resonance energy transfer (FRET) between UCNPs and GNPs-MNPs for detection of Pb2+ (Chen et al., 2020).
Several sensing systems based on polymers and small molecule particles have been developed. Chini et al. (Chini et al., 2019) reported a polymodal sensing method consisting of a highly fluorescent dansyl-labeled copolymer P (MMA-co-Dansyl-Ala-HEMA) (DCP) and a small molecule diketopyrrolopyrrole (DPP) for the assay of the heavy metal lead (Pb2+). Liu et al. (Liu J. et al., 2013) reported a highly selective Pb2+ fluorescent probe that is consisted of a BODIPY fluorescent moiety and a polyamide receptor. Anand et al. (Anand et al., 2015) synthesized a de novo probe 5-[(anthracene-9-methylene) amino] quinolin-10-ol (ANQ) on the anthracene platform. What’s more, Liu et al.(Liu et al., 2015) reported a fluorescent turn-on probe of an oxadiazole derivative (OXD) that is based on the Schiff base molecule and was used to detect lead ions.
Fluorescent sensors based on naphthalimide are also an essential structure. Jiang et al. (Jiang et al., 2021) prepared a chemical fluorescent probe by attaching thiocarbamate to a naphthylamine derivative and applied it for Pb2+ recognition in specific chemical reagents. Un et al. (Un et al., 2014) successfully developed a good performance naphthylamino fluorescent probe named NPA with Pb2+ recognition.
Other sensors based on receptors are still available for the detection of lead ions. Recently, Bi and co-workers (Bi et al., 2017) rationally designed and developed a unique near-infrared fluorescent probe (NIR-PbP) and verified the capability of probing Pb (II) ions in both solution and live cells. Mei et al. (Mei et al., 2020) designed a newly available fluorescent probe L for Pb2+ based phenanthroline derivative. Khandare et al. (Khandare et al., 2014) developed a fluorescent sensor based on aggregation-induced emission (AIE) using the intense affinity of lead ions for phosphate residues. Zhao et al. (Zhao et al., 2016) developed a fluorescent biosensor to detect Pb2+ that utilized a water-soluble derivative of cationic perylene (compound 1) with the characteristics of simplicity, label-free and high speed.
Cd ions are also toxic to cells, while there are few fluorescent tools to study Cd2+ toxicity. Due to the very similar binding properties of Cd2+ and Zn2+, this poses a great challenge and difficulty for the development of Cd2+ probes. The first HK-2 intracellular fluorescent probe for Cd2+ was a Liu Cd-1 consisting of fluorescein and thiocarbamate (Carter et al., 2014). Nowadays, various methods have been well developed for the determination of Cd2+, such as simultaneous radiation X-ray spectrometry, synchrotron X-ray spectrometry and atomic absorption chromatography. In recent years, researches on Cd2+ ion fluorescent probes include the following categories: Quinolone-based, coumarin-, benzothiazole based, rhodamine-based, dansyl based, and diarylethylene based Cd2+ fluorescent sensor, There are also other small organic molecules based Cd2+ sensor and nanosensor-based Cd2+ fluorescent sensor. In addition, fluorescence and colorimetric nano Cd2+ sensors, such as metal-organic framework (MOF), quantum dots (QD), nanoclusters (NCs) and nanoparticles (NPs), have also made good progress in recent years (Shi et al., 2021).
First, due to the ease of synthesis and precise control of the active site (Abdollahi et al., 2020), the synthesis and design of metal-organic framework (MOF) have been applied to the design of neutral architectures for anion recognition (Esrafili et al., 2020) and the selective capture of heavy metal ions (Esrafili et al., 2021). To detect Cd2+ in alkaline solutions, Hao et al. (Hao and Yan, 2015) reported the one of the first fluorescent probes for Cd2+ on the basis of lanthanide structure of functionalized metal-organic framework (MOF), and this sensor has the advantage of a high sensitivity. Liu et al. (Liu Q. et al., 2013) designed a kind of new fluorescent probe DQCd2 for Cd2+ based on 4-piperidinylquinoline by utilizing the ratiometric reaction in phosphate buffered saline solution (PBS) buffer, and its emission intensity was significantly enhanced. Tsukamoto et al. (Tsukamoto et al., 2016) a highly practical naphthalenyl Cd2+ fluorescent probe, which allows a good selectivity and is suitable for a range of pH values since this method has almost no background reaction. Furthermore, Shim et al. (Shim and Tae, 2011) also developed a rhodamine-hydroxylamine platform-based fluorescent probe consisting of a picolinic and a cycloalkene-binding unit (Figure 9A). Xin et al. (Xin et al., 2013) synthesized the first fluorescent probe on the basis of difluoroborane dibenzoyl for the determination of Cd2+. Liu et al. (Liu et al., 2010) developed DBITA, a ratiofluorescent sensor that can be used for the recognition of Cd2+ in live cells or aqueous media, with a Cd2+-induced emission redshift of 53 nm (Figure 9B). Sun et al. (Sun et al., 2016) reported a ratio-measured and reversible fluorescent probe for the recognition of Cd2+ in living cells that was based on a 6-(dimethylamino) quinaldine derivative. For Cd2+ in living human cells, Jiang et al. (Jiang et al., 2014) developed a novel fluorescence probe (L) for C-3-symmetric Schiff alkaloids on the basis of 8-hydroxy-2-methylquinoline. Liu et al. (Liu et al., 2014) synthesized a well-performing organic salt probe in the determination of Cd2+ based on bis-1, 3, 4-oxadiazole derivatives and BAPTA. Liu et al. (Liu et al., 2012) developed and synthesized a novel two-photon excited cadmium fluorescent probe (named TPCd) based on o-phenylenediamine derivatives and Prodan (6-acetyl-2-methoxynaphthalene) derivatives by a two-photon approach, and demonstrated that Cd2+ detection in organisms is feasible and versatile. Therefore, this is a very meaningful study.
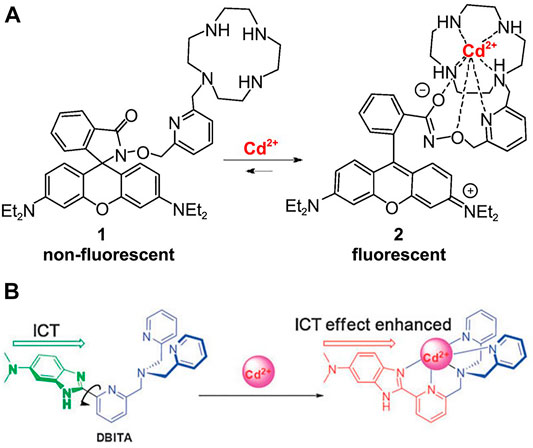
FIGURE 9. (A) Binding of Rhodamine-Cyclen Probe 1 with Cd2+ (Shim and Tae, 2011); (B) Proposed Cd2+ binding mode of DBITA (Liu et al., 2010).
In summary, the identification and detection of biologically and environmentally critical species is already an important area of research in the field of the chemosensor. Fluorometry in combination with suitable probes is the preferred and excellent method of measuring these analytes due to the fast, nondestructive and sensitive nature of fluorometry measurements, and important progress has been realized in the definition and composition of fluorescent chemosensors predicated on various platforms. More and more researchers are engaged in this field, and have accumulated a lot of theoretical and practical experience in the development, synthesis and application of probes, accelerating further development of new fluorescent probes.
In this paper, the research progress of fluorescent probes for metal ion detection is reviewed. These fluorescent probes are mainly divided into organic small molecule probes and nano fluorescent probes. For different metal ions, different materials are selected to design and synthesize fluorescent probes, such as quantum dots, coumarin derivatives, benzene derivatives, etc. The development of sensing mechanisms plays an important role in improving the sensing performance of metal ions. For example, the solar excited naked eye Fe3+ detection is realized by improving the luminescence efficiency based on the central hydrophobic/external hydrophilic strategy (Sun et al., 2020). So far, the main efforts have been devoted to the study of new sensing mechanisms, strategies to expand the range of detected metal ions, and methods to improve sensitivity and selectivity. However, there are still limitations and unparalleled challenges in the practical application of fluorescent probes. The variation of pH, temperature and probe behavior in different environments, as well as the accompanying fluorescence burst effect in some fluorescent sensors, have raised high requirements for the design of fluorescent probes. Therefore, in future, lots of aspects need to be further improved, including compatibility of probes in organisms and accuracy of fluorescent probe sensing, etc., so they can be applicable to more complex detection environments. We believe that fluorescent probes will become a very powerful tool in the biomedical field and make great contributions to biology in future.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication. All authors conducted literature compilation and discussion. LL and JW wrote this article. BD, SX, and CL checked and reviewed this article.
This work was supported by the Major Basic Research Projects of Shandong Natural Science Foundation (ZR2020ZD36) and the National Natural Science Foundation of China (82073475).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdollahi, N., Akbar Razavi, S. A., Morsali, A., and Hu, M.-L. (2020). High Capacity Hg(II) and Pb(II) Removal Using MOF-Based Nanocomposite: Cooperative Effects of Pore Functionalization and Surface-Charge Modulation. J. Hazard. Mater. 387, 121667. doi:10.1016/j.jhazmat.2019.121667
Al-Azmi, A., and John, E. (2021). Synthesis and Characterization of Novel Tricyanofuran Hydrazone Probe: Solvatochromism, Density‐functional Theory Calculation and Selective Fluorescence, and Colorimetric Determination of Iron (III). Luminescence 36 (5), 1220–1230. doi:10.1002/bio.4047
Anand, T., Sivaraman, G., Mahesh, A., and Chellappa, D. (2015). Aminoquinoline Based Highly Sensitive Fluorescent Sensor for Lead(II) and Aluminum(III) and its Application in Live Cell Imaging. Analytica Chim. Acta 853, 596–601. doi:10.1016/j.aca.2014.11.011
Atchudan, R., Edison, T. N. J. I., Perumal, S., Vinodh, R., Sundramoorthy, A. K., Babu, R. S., et al. (2021). Leftover Kiwi Fruit Peel-Derived Carbon Dots as a Highly Selective Fluorescent Sensor for Detection of Ferric Ion. Chemosensors 9 (7), 166. doi:10.3390/chemosensors9070166
Bi, J., Fang, M., Wang, J., Xia, S., Zhang, Y., Zhang, J., et al. (2017). Near-infrared Fluorescent Probe for Sensitive Detection of Pb(II) Ions in Living Cells. Inorg. Chim. Acta 468, 140–145. doi:10.1016/j.ica.2017.06.044
Cai, Y., You, J., You, Z., Dong, F., Du, S., and Zhang, L. (2018). Profuse Color-Evolution-Based Fluorescent Test Paper Sensor for Rapid and Visual Monitoring of Endogenous Cu2+ in Human Urine. Biosens. Bioelectron. 99, 332–337. doi:10.1016/j.bios.2017.07.072
Carter, K. P., Young, A. M., and Palmer, A. E. (2014). Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 114 (8), 4564–4601. doi:10.1021/cr400546e
Chang, Y., Li, B., Mei, H., Xu, K., Xie, X., and Yang, L. (2020). A Novel Reversible Fluorescent Probe for Zinc(II) Ion and Bioimaging in Living Cells. Supramolecular Chem. 32 (7), 393–402. doi:10.1080/10610278.2020.1749627
Chaudhary, N., Gupta, P. K., Eremin, S., and Solanki, P. R. (2020). One-step green Approach to Synthesize Highly Fluorescent Carbon Quantum Dots from Banana Juice for Selective Detection of Copper Ions. J. Environ. Chem. Eng. 8 (3), 103720. doi:10.1016/j.jece.2020.103720
Chen, C.-G., Vijay, N., Thirumalaivasan, N., Velmathi, S., and Wu, S.-P. (2019). Coumarin-based Hg2+ Fluorescent Probe: Fluorescence Turn-On Detection for Hg2+ Bioimaging in Living Cells and Zebrafish. Spectrochimica Acta A: Mol. Biomol. Spectrosc. 219, 135–140. doi:10.1016/j.saa.2019.04.048
Chen, M., Hassan, M., Li, H., and Chen, Q. (2020). Fluorometric Determination of Lead(II) by Using Aptamer-Functionalized Upconversion Nanoparticles and Magnetite-Modified Gold Nanoparticles. Microchim. Acta 187 (1), 9. doi:10.1007/s00604-019-4030-4
Chini, M. K., Kumar, V., Maiti, B., De, P., and Satapathi, S. (2019). A Dual "Turn-on/Turn-Off" "FRET" Sensor for Highly Sensitive and Selective Detection of lead and Methylene Blue Based on Fluorescent Dansyl Tagged Copolymer and Small Molecule Diketopyrrolopyrrole. Polym. Test. 79, 105997. doi:10.1016/j.polymertesting.2019.105997
Dong, W., Yu, J., Gong, X., Liang, W., Fan, L., and Dong, C. (2021). A Turn-Off-On Near-Infrared Photoluminescence Sensor for Sequential Detection of Fe3+ and Ascorbic Acid Based on Glutathione-Capped Gold Nanoclusters. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 247, 119085. doi:10.1016/j.saa.2020.119085
Du, J., Yang, Y., Shao, T., Qi, S., Zhang, P., Zhuo, S., et al. (2021). Yellow Emission Carbon Dots for Highly Selective and Sensitive OFF-ON Sensing of Ferric and Pyrophosphate Ions in Living Cells. J. Colloid Interf. Sci. 587, 376–384. doi:10.1016/j.jcis.2020.11.108
Esrafili, L., Firuzabadi, F. D., Morsali, A., and Hu, M.-L. (2021). Reuse of Predesigned Dual-Functional Metal Organic Frameworks (DF-MOFs) after Heavy Metal Removal. J. Hazard. Mater. 403, 123696. doi:10.1016/j.jhazmat.2020.123696
Esrafili, L., Morsali, A., Hu, M.-L., Azhdari Tehrani, A., Carlucci, L., Mercandelli, P., et al. (2020). Size-Selective Urea-Containing Metal-Organic Frameworks as Receptors for Anions. Inorg. Chem. 59 (22), 16421–16429. doi:10.1021/acs.inorgchem.0c02215
Fang, M. X., Xia, S., Bi, J. H., Wigstrom, T. P., Valenzano, L., Wang, J. B., et al. (2016). Detecting Zn(II) Ions in Live Cells with Near-Infrared Fluorescent Probes. Molecules 24 (8), 1592. doi:10.3390/molecules24081592
Gao, G., Wang, X., Wang, Z., Jin, X., Ou, L., Zhou, J., et al. (2020). A Simple and Effective Dansyl Acid Based "Turn-On" Fluorescent Probe for Detecting Labile Ferrous Iron in Physiological saline and Live Cells. Talanta 215, 120908. doi:10.1016/j.talanta.2020.120908
Gujuluva Gangatharan, V. K., Mookkandi Palsamy, K., Gandhi, S., Jamespandi, A., Kandasamy, A., Arunachalam, T., et al. (2018). Reversible NIR Fluorescent Probes for Cu2+ Ions Detection and its Living Cell Imaging. Sensors Actuators B: Chem. 255, 3235–3247. doi:10.1016/j.snb.2017.09.150
Guo, R., Zhou, S., Li, Y., Li, X., Fan, L., and Voelcker, N. H. (2015). Rhodamine-Functionalized Graphene Quantum Dots for Detection of Fe3+ in Cancer Stem Cells. ACS Appl. Mater. Inter. 7 (43), 23958–23966. doi:10.1021/acsami.5b06523
Hagimori, M. (2013). Development of Zn2+ Selective Fluorescent Probes for Biological Applications. Yakugaku Zasshi 133 (10), 1087–1092. doi:10.1248/yakushi.13-00192
Hao, J.-N., and Yan, B. (2015). A Water-Stable Lanthanide-Functionalized MOF as a Highly Selective and Sensitive Fluorescent Probe for Cd2+. Chem. Commun. 51 (36), 7737–7740. doi:10.1039/C5CC01430A
He, W., and Lu, J. (2001). Distribution of Cd and Pb in a Wetland Ecosystem. Sc. China Ser. B-chem. 44 (1), 178–184. doi:10.1007/BF02884825
Jiang, C., Yang, l., Li, P., Liu, Y., Li, S., Fu, Y., et al. (2021). A Simple and Rapid Fluorescent Approach for Pb2+ Determination and Application in Water Samples and Living Cells. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 263, 120168. doi:10.1016/j.saa.2021.120168
Jiang, X.-J., Li, M., Lu, H.-L., Xu, L.-H., Xu, H., Zang, S.-Q., et al. (2014). A Highly Sensitive C3-Symmetric Schiff-Base Fluorescent Probe for Cd2+. Inorg. Chem. 53 (24), 12665–12667. doi:10.1021/ic501279y
Ju, J., and Chen, W. (2014). Synthesis of Highly Fluorescent Nitrogen-Doped Graphene Quantum Dots for Sensitive, Label-free Detection of Fe (III) in Aqueous media. Biosens. Bioelectron. 58, 219–225. doi:10.1016/j.bios.2014.02.061
Kang, T., Wang, H., Wang, X., and Feng, L. (2019). A Facile Zn(II) Probe Based on Intramolecular Charge Transfer with Fluorescence Red-Shift. Microchemical J. 148, 442–448. doi:10.1016/j.microc.2019.05.035
Kar, C., Adhikari, M. D., Ramesh, A., and Das, G. (2013). NIR- and FRET-Based Sensing of Cu2+ and S2- in Physiological Conditions and in Live Cells. Inorg. Chem. 52 (2), 743–752. doi:10.1021/ic301872q
Khandare, D. G., Joshi, H., Banerjee, M., Majik, M. S., and Chatterjee, A. (2014). An Aggregation-Induced Emission Based "Turn-On" Fluorescent Chemodosimeter for the Selective Detection of Pb2+ Ions. RSC Adv. 4 (87), 47076–47080. doi:10.1039/C4RA09451D
Khatun, S., Biswas, S., Binoy, A., Podder, A., Mishra, N., and Bhuniya, S. (2020). Highly Chemoselective Turn-On Fluorescent Probe for Ferrous (Fe2+) Ion Detection in Cosmetics and Live Cells. J. Photochem. Photobiol. B: Biol. 209, 111943. doi:10.1016/j.jphotobiol.2020.111943
Kim, H. N., Ren, W. X., Kim, J. S., and Yoon, J. (2012). Fluorescent and Colorimetric Sensors for Detection of lead, Cadmium, and Mercury Ions. Chem. Soc. Rev. 41 (8), 3210–3244. doi:10.1039/C1CS15245A
Kim, K. B., Kim, H., Song, E. J., Kim, S., Noh, I., and Kim, C. (2013). A Cap-type Schiff Base Acting as a Fluorescence Sensor for Zinc(II) and a Colorimetric Sensor for Iron(II), Copper(II), and Zinc(II) in Aqueous media. Dalton Trans. 42 (47), 16569–16577. doi:10.1039/c3dt51916c
Li, J., Yim, D., Jang, W.-D., and Yoon, J. (2017). Recent Progress in the Design and Applications of Fluorescence Probes Containing crown Ethers. Chem. Soc. Rev. 46 (9), 2437–2458. doi:10.1039/c6cs00619a
Li, L., Yu, B., and You, T. (2015). Nitrogen and Sulfur Co-doped Carbon Dots for Highly Selective and Sensitive Detection of Hg (Ⅱ) Ions. Biosens. Bioelectron. 74, 263–269. doi:10.1016/j.bios.2015.06.050
Li, Z., Xu, Y., Xu, H., Cui, M., Liu, T., Ren, X., et al. (2021). A Dicyanomethylene-4h-Pyran-Based Fluorescence Probe with High Selectivity and Sensitivity for Detecting Copper (II) and its Bioimaging in Living Cells and Tissue. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 244, 118819. doi:10.1016/j.saa.2020.118819
Liang, Y., Zhang, Y., Li, M., Meng, Z., Gao, Y., Yin, J., et al. (2021). A Highly Effective "Turn-On" Camphor-Based Fluorescent Probe for Rapid and Sensitive Detection and its Biological Imaging of Fe2+. Anal. Bioanal. Chem. 413 (25), 6267–6277. doi:10.1007/s00216-021-03581-4
Lin, X., Gao, G., Zheng, L., Chi, Y., and Chen, G. (2014). Encapsulation of Strongly Fluorescent Carbon Quantum Dots in Metal-Organic Frameworks for Enhancing Chemical Sensing. Anal. Chem. 86 (2), 1223–1228. doi:10.1021/ac403536a
Liu, J., Guo, Y., Dong, B., Sun, J., Lyu, J., Sun, L., et al. (2020). Water-soluble Coumarin Oligomer Based Ultra-sensitive Iron Ion Probe and Applications. Sensors Actuators B: Chem. 320, 128361. doi:10.1016/j.snb.2020.128361
Liu, J., Wu, K., Li, S., Song, T., Han, Y., and Li, X. (2013). A Highly Sensitive and Selective Fluorescent Chemosensor for Pb2+ Ions in an Aqueous Solution. Dalton Trans. 42 (11), 3854–3859. doi:10.1039/c2dt32531d
Liu, Q., Feng, L., Yuan, C., Zhang, L., Shuang, S., Dong, C., et al. (2014). A Highly Selective Fluorescent Probe for Cadmium Ions in Aqueous Solution and Living Cells. Chem. Commun. 50 (19), 2498–2501. doi:10.1039/C3CC48668K
Liu, Q.Q., Li, G.-P., Zhu, D.-J., Xue, L., and Jiang, H. (2013). Design of Quinoline-Based Fluorescent Probe for the Ratiometric Detection of Cadmium in Aqueous media. Chin. Chem. Lett. 24 (6), 479–482. doi:10.1016/j.cclet.2013.04.002
Liu, W., Wu, G., Gu, X., Yuan, X., Li, J., and Wang, H. (2015). Synthesis of Schiff Base-Based 1,2,4-Oxadiazole Derivative as Fluorescence Turn-On Sensor for High Selectivity of Pb2+. J. Fluoresc. 25 (3), 557–561. doi:10.1007/s10895-015-1534-0
Liu, Y., Dong, X., Sun, J., Zhong, C., Li, B., You, X., et al. (2012). Two-photon Fluorescent Probe for Cadmium Imaging in Cells. Analyst 137 (8), 1837–1845. doi:10.1039/c2an16254g
Liu, Y., Ouyang, Q., Li, H., Chen, M., Zhang, Z., and Chen, Q. (2018). Turn-On Fluoresence Sensor for Hg2+ in Food Based on FRET between Aptamers-Functionalized Upconversion Nanoparticles and Gold Nanoparticles. J. Agric. Food Chem. 66 (24), 6188–6195. doi:10.1021/acs.jafc.8b00546
Liu, Z,Z., Chen, M., Guo, Y., Zhou, J., Shi, Q., and Sun, R. (2020). Oxidized Nanocellulose Facilitates Preparing Photoluminescent Nitrogen-Doped Fluorescent Carbon Dots for Fe3+ Ions Detection and Bioimaging. Chem. Eng. J. 384, 123260. doi:10.1016/j.cej.2019.123260
Liu, Z., Zhang, C., He, W., Yang, Z., Gao, X., and Guo, Z. (2010). A Highly Sensitive Ratiometric Fluorescent Probe for Cd2+ Detection in Aqueous Solution and Living Cells. Chem. Commun. 46 (33), 6138–6140. doi:10.1039/c0cc00662a
Long, L., Wang, N., Han, Y., Huang, M., Yuan, X., Cao, S., et al. (2018). A Coumarin-Based Fluorescent Probe for Monitoring Labile Ferrous Iron in Living Systems. Analyst 143 (11), 2555–2562. doi:10.1039/C8AN00556G
Lu, W., Qin, X., Liu, S., Chang, G., Zhang, Y., Luo, Y., et al. (2012). Economical, Green Synthesis of Fluorescent Carbon Nanoparticles and Their Use as Probes for Sensitive and Selective Detection of Mercury(II) Ions. Anal. Chem. 84 (12), 5351–5357. doi:10.1021/ac3007939
Lytton, S. D., Mester, B., Libman, J., Shanzer, A., and Ioav Cabantchik, Z. (1992). Monitoring of Iron(III) Removal from Biological Sources Using a Fluorescent Siderophore. Anal. Biochem. 205 (2), 326–333. doi:10.1016/0003-2697(92)90443-B
Ma, S., Yu, Q., Lu, L., Li, L., Liu, W., Wu, Z., et al. (2021). Recent Progress in Fluorescent Probes for the Detection of Ferrous Ion. Chin. J. Org. Chem. 41 (1), 229–240. doi:10.6023/cjoc202006003
Mei, H., Yang, M., Liu, X., Tian, Y., and Xu, K. (2020). A Novel Phenanthroline-Based Fluorescent Probe for Pb(II). Chin. J. Org. Chem. 40 (8), 2508–2512. doi:10.6023/cjoc202004037
Mohammadi, A., and Ghasemi, Z. (2020). A Simple Pyrimidine Based Colorimetric and Fluorescent Chemosensor for Sequential Detection of Copper (II) and Cyanide Ions and its Application in Real Samples. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 228, 117730. doi:10.1016/j.saa.2019.117730
Pan, S.-L., Li, K., Li, L.-L., Li, M.-Y., Shi, L., Liu, Y.-H., et al. (2018). A Reaction-Based Ratiometric Fluorescent Sensor for the Detection of Hg(II) Ions in Both Cells and Bacteria. Chem. Commun. 54 (39), 4955–4958. doi:10.1039/C8CC01031E
Park, S.-H., Kwon, N., Lee, J.-H., Yoon, J., and Shin, I. (2020). Synthetic Ratiometric Fluorescent Probes for Detection of Ions. Chem. Soc. Rev. 49 (1), 143–179. doi:10.1039/c9cs00243j
Peng, J., Xu, W., Teoh, C. L., Han, S., Kim, B., Samanta, A., et al. (2015). High-Efficiency In Vitro and In Vivo Detection of Zn2+ by Dye-Assembled Upconversion Nanoparticles. J. Am. Chem. Soc. 137 (6), 2336–2342. doi:10.1021/ja5115248
Price, T. W., Firth, G., Eling, C. J., Kinnon, M., Long, N. J., Sturge, J., et al. (2018). A 18F Radiolabelled Zn(ii) Sensing Fluorescent Probe. Chem. Commun. 54 (26), 3227–3230. doi:10.1039/c8cc00687c
Pu, Z.-F., Wen, Q.-L., Yang, Y.-J., Cui, X.-M., Ling, J., Liu, P., et al. (2020). Fluorescent Carbon Quantum Dots Synthesized Using Phenylalanine and Citric Acid for Selective Detection of Fe3+ Ions. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 229, 117944. doi:10.1016/j.saa.2019.117944
Qi, H., Teng, M., Liu, M., Liu, S., Li, J., Yu, H., et al. (2019). Biomass-derived Nitrogen-Doped Carbon Quantum Dots: Highly Selective Fluorescent Probe for Detecting Fe3+ Ions and Tetracyclines. J. Colloid Interf. Sci. 539, 332–341. doi:10.1016/j.jcis.2018.12.047
Qu, K., Wang, J., Ren, J., and Qu, X. (2013). Carbon Dots Prepared by Hydrothermal Treatment of Dopamine as an Effective Fluorescent Sensing Platform for the Label-free Detection of Iron(III) Ions and Dopamine. Chem. Eur. J. 19 (22), 7243–7249. doi:10.1002/chem.201300042
Ranee, S. J., Sivaraman, G., Pushpalatha, A. M., and Muthusubramanian, S. (2018). Quinoline Based Sensors for Bivalent Copper Ions in Living Cells. Sensors Actuators B: Chem. 255, 630–637. doi:10.1016/j.snb.2017.08.111
Roy, P., Dhara, K., Manassero, M., Ratha, J., and Banerjee, P. (2007). Selective Fluorescence Zinc Ion Sensing and Binding Behavior of 4-Methyl-2,6-Bis(((phenylmethyl)imino)methyl)phenol: Biological Application. Inorg. Chem. 46 (16), 6405–6412. doi:10.1021/ic700420w
Shi, B., Su, Y., Zhang, L., Huang, M., Liu, R., and Zhao, S. (2016). Nitrogen and Phosphorus Co-doped Carbon Nanodots as a Novel Fluorescent Probe for Highly Sensitive Detection of Fe3+ in Human Serum and Living Cells. ACS Appl. Mater. Inter. 8 (17), 10717–10725. doi:10.1021/acsami.6b01325
Shi, C.-t., Huang, Z.-y., Wu, A.-b., Hu, Y.-x., Wang, N.-c., Zhang, Y., et al. (2021). Recent Progress in Cadmium Fluorescent and Colorimetric Probes. RSC Adv. 11 (47), 29632–29660. doi:10.1039/D1RA05048F
Shim, S.-Y., and Tae, J.-S. (2011). Rhodamine Cyclen-Based Fluorescent Chemosensor for the Detection of Cd2+. Bull. Korean Chem. Soc. 32 (8), 2928–2932. doi:10.5012/bkcs.2011.32.8.2928
Sivaraman, G., Iniya, M., Anand, T., Kotla, N. G., Sunnapu, O., Singaravadivel, S., et al. (2018). Chemically Diverse Small Molecule Fluorescent Chemosensors for Copper Ion. Coord. Chem. Rev. 357, 50–104. doi:10.1016/j.ccr.2017.11.020
Song, R., Zhang, Q., Chu, Y., Zhang, L., Dai, H., and Wu, W. (2019). Fluorescent Cellulose Nanocrystals for the Detection of lead Ions in Complete Aqueous Solution. Cellulose 26 (18), 9553–9565. doi:10.1007/s10570-019-02760-y
Song, S., Wang, C., Zhao, Y., Hu, T., Zhou, X., Zhao, T., et al. (2018). Gold-Cluster-Based Dual-Emission Nanocomposite Film as Ratiometric Fluorescent Sensing Paper for Specific Metal Ion. Part. Part. Syst. Charact. 35 (4), 1700471. doi:10.1002/ppsc.201700471
Staudinger, C., and Borisov, S. M. (2015). Long-wavelength Analyte-Sensitive Luminescent Probes and Optical (Bio)sensors. Methods Appl. Fluoresc. 3 (4), 042005. doi:10.1088/2050-6120/3/4/042005
Sun, L., Liu, J., Xu, S., Dong, B., Lv, J., Hu, S., et al. (2020). High Fluorescence LaOBr/coumarin Organic-Inorganic Composite Nanomaterials for Ultra-sensitive Fe3+ Sensing, Fluorescence Imaging and Water-Based Ink Anti-counterfeiting Applications. J. Mater. Chem. C 8 (39), 13733–13742. doi:10.1039/D0TC03354E
Sun, Z.-G., Li, Z., Yuan, D.-d., Gao, J.-F., Lin, L., Lin, J., et al. (2016). A Quinoline-Based Ratiometric and Reversible Fluorescent Probe for Cadmium Imaging in Living Cells. Chem. Pharm. Bull. 64 (1), 27–33. doi:10.1248/cpb.c15-00579
Tang, L., Xia, J., Zhong, K., Tang, Y., Gao, X., and Li, J. (2020). A Simple AIE-Active Fluorogen for Relay Recognition of Cu2+ and Pyrophosphate through Aggregation-Switching Strategy. Dyes Pigm. 178, 108379. doi:10.1016/j.dyepig.2020.108379
Tao, P., Chen, D., Xu, Z., Song, Y., Li, H., and Xian, C. (2020). A Fluorescent Probe for the Dual Detection of Mercury Ions and Thiols Based on a Simple Coumarin Derivative. Coloration Technol. 136 (1), 75–86. doi:10.1111/cote.12447
Tsukamoto, K., Shimabukuro, S., Mabuchi, M., and Maeda, H. (2016). A Naphthalimide-Based Cd2+Fluorescent Probe with Carbamoylmethyl Groups Working as Chelators and PET-Promoters under Neutral Conditions. Chem. Eur. J. 22 (25), 8579–8585. doi:10.1002/chem.201600556
Un, H.-I., Huang, C.-B., Huang, J., Huang, C., Jia, T., and Xu, L. (2014). A Naphthalimide-Based Fluorescence "Turn-On" Probe for the Detection of Pb2+in Aqueous Solution and Living Cells. Chem. Asian J. 9 (12), 3397–3402. doi:10.1002/asia.201402946
Walkup, G. K., Burdette, S. C., Lippard, S. J., and Tsien, R. Y. (2000). A New Cell-Permeable Fluorescent Probe for Zn2+. J. Am. Chem. Soc. 122 (23), 5644–5645. doi:10.1021/ja000868p
Wang, F., Wang, K., Kong, Q., Wang, J., Xi, D., Gu, B., et al. (2021). Recent Studies Focusing on the Development of Fluorescence Probes for Zinc Ion. Coord. Chem. Rev. 429, 213636. doi:10.1016/j.ccr.2020.213636
Wang, H., Wang, X., Kong, R.-M., Xia, L., and Qu, F. (2021). Metal-organic Framework as a Multi-Component Sensor for Detection of Fe3+, Ascorbic Acid and Acid Phosphatase. Chin. Chem. Lett. 32 (1), 198–202. doi:10.1016/j.cclet.2020.10.017
Wang, L., Li, W., Zhi, W., Huang, Y., Han, J., Wang, Y., et al. (2018). A New Coumarin Schiff Based Fluorescent-Colorimetric Chemosensor for Dual Monitoring of Zn2+ and Fe3+ in Different Solutions: An Application to Bio-Imaging. Sensors Actuators B: Chem. 260, 243–254. doi:10.1016/j.snb.2017.12.200
Wang, L., Wei, Z.-L., Chen, Z.-Z., Liu, C., Dong, W.-K., and Ding, Y.-J. (2020). A Chemical Probe Capable for Fluorescent and Colorimetric Detection to Cu2+ and CN− Based on Coordination and Nucleophilic Addition Mechanism. Microchemical J. 155, 104801. doi:10.1016/j.microc.2020.104801
Wang, S., Chen, H., Xie, H., Wei, L., Xu, L., Zhang, L., et al. (2021). A Novel Thioctic Acid-Carbon Dots Fluorescence Sensor for the Detection of Hg2+ and Thiophanate Methyl via S-Hg Affinity. Food Chem. 346, 128923. doi:10.1016/j.foodchem.2020.128923
Wang, Y., Zhang, C., Chen, X., Yang, B., Yang, L., Jiang, C., et al. (2016). Ratiometric Fluorescent Paper Sensor Utilizing Hybrid Carbon Dots-Quantum Dots for the Visual Determination of Copper Ions. Nanoscale 8 (11), 5977–5984. doi:10.1039/C6NR00430J
Wang, Y., Zhang, L., Han, X., Zhang, L., Wang, X., and Chen, L. (2021). Fluorescent Probe for Mercury Ion Imaging Analysis: Strategies and Applications. Chem. Eng. J. 406, 127166. doi:10.1016/j.cej.2020.127166
Wang, Z.-G., Ding, X.-J., Huang, Y.-Y., Yan, X.-J., Ding, B., Li, Q.-Z., et al. (2020). The Development of Coumarin Schiff Base System Applied as Highly Selective Fluorescent/colorimetric Probes for Cu2+ and Tumor Biomarker Glutathione Detection. Dyes Pigm. 175, 108156. doi:10.1016/j.dyepig.2019.108156
Wei, S., Tan, L., Yin, X., Wang, R., Shan, X., Chen, Q., et al. (2020). A Sensitive "ON-OFF" Fluorescent Probe Based on Carbon Dots for Fe2+ Detection and Cell Imaging. Analyst 145 (6), 2357–2366. doi:10.1039/C9AN02309G
Wu, H., and Tong, C. (2019). Nitrogen- and Sulfur-Codoped Carbon Dots for Highly Selective and Sensitive Fluorescent Detection of Hg2+ Ions and Sulfide in Environmental Water Samples. J. Agric. Food Chem. 67 (10), 2794–2800. doi:10.1021/acs.jafc.8b07176
Xin, L., Chen, Y.-Z., Niu, L.-Y., Wu, L.-Z., Tung, C.-H., Tong, Q.-X., et al. (2013). A Selective Turn-On Fluorescent Probe for Cd2+ Based on a boron Difluoride β-dibenzoyl Dye and its Application in Living Cells. Org. Biomol. Chem. 11 (18), 3014–3019. doi:10.1039/c3ob40376a
Xu, H., Hu, H.-C., Cao, C.-S., and Zhao, B. (2015). Lanthanide Organic Framework as a Regenerable Luminescent Probe for Fe3+. Inorg. Chem. 54 (10), 4585–4587. doi:10.1021/acs.inorgchem.5b00113
Xu, J., Wang, Z., Liu, C., Xu, Z., Wang, N., Cong, X., et al. (2018). A Highly Selective Colorimetric and Long-Wavelength Fluorescent Probe for the Detection of Hg2+. Luminescence 33 (6), 1122–1127. doi:10.1002/bio.3518
Xu, Q., Pu, P., Zhao, J., Dong, C., Gao, C., Chen, Y., et al. (2015). Preparation of Highly Photoluminescent Sulfur-Doped Carbon Dots for Fe(III) Detection. J. Mater. Chem. A. 3 (2), 542–546. doi:10.1039/C4TA05483K
Xu, W., Zeng, Z., Jiang, J.-H., Chang, Y.-T., and Yuan, L. (2016). Discerning the Chemistry in Individual Organelles with Small-Molecule Fluorescent Probes. Angew. Chem. Int. Ed. 55 (44), 13658–13699. doi:10.1002/anie.201510721
Xu, X.-Y., and Yan, B. (2015). Eu(III)-Functionalized MIL-124 as Fluorescent Probe for Highly Selectively Sensing Ions and Organic Small Molecules Especially for Fe(III) and Fe(II). ACS Appl. Mater. Inter. 7 (1), 721–729. doi:10.1021/am5070409
Xu, Z., Liu, X., Pan, J., and Spring, D. R. (2012). Coumarin-derived Transformable Fluorescent Sensor for Zn2+. Chem. Commun. 48 (39), 4764–4766. doi:10.1039/c2cc30963g
Xu, Z., and Xu, L. (2016). Fluorescent Probes for the Selective Detection of Chemical Species inside Mitochondria. Chem. Commun. 52 (6), 1094–1119. doi:10.1039/c5cc09248e
Yan, B. (2017). Lanthanide-Functionalized Metal-Organic Framework Hybrid Systems to Create Multiple Luminescent Centers for Chemical Sensing. Acc. Chem. Res. 50 (11), 2789–2798. doi:10.1021/acs.accounts.7b00387
Yang, L., Chen, Q., Gan, S., Guo, Q., Zhang, J., Zhang, H., et al. (2021). An Activatable AIEgen Probe for In-Situ Monitoring and Long-Term Tracking of Ferrous Ions in Living Cells. Dyes Pigm. 190, 109271. doi:10.1016/j.dyepig.2021.109271
Yang, X., Wang, Y., Liu, R., Zhang, Y., Tang, J., Yang, E.-b., et al. (2019). A Novel ICT-Based Two Photon and NIR Fluorescent Probe for Labile Fe2+ Detection and Cell Imaging in Living Cells. Sensors Actuators B: Chem. 288, 217–224. doi:10.1016/j.snb.2019.02.123
Yao, J., Zhang, K., Zhu, H., Ma, F., Sun, M., Yu, H., et al. (2013). Efficient Ratiometric Fluorescence Probe Based on Dual-Emission Quantum Dots Hybrid for On-Site Determination of Copper Ions. Anal. Chem. 85 (13), 6461–6468. doi:10.1021/ac401011r
Ye, J., Zhao, L., Bogale, R. F., Gao, Y., Wang, X., Qian, X., et al. (2015). Highly Selective Detection of 2,4,6-Trinitrophenol and Cu2+Ions Based on a Fluorescent Cadmium-Pamoate Metal-Organic Framework. Chem. Eur. J. 21 (5), 2029–2037. doi:10.1002/chem.201405267
Zhang, D., Qi, Y., Li, Y., Song, Y., Xian, C., Li, H., et al. (2021). A New Spiropyran-Based Fluorescent Probe for Dual Sensing of Ferrous Ion and pH. J. Fluoresc. 31 (4), 1133–1141. doi:10.1007/s10895-021-02741-0
Zhang, J., Ma, X., Chen, W., Bai, Y., Xue, P., Chen, K., et al. (2021). Bifunctional Single-Labelled Oligonucleotide Probe for Detection of Trace Ag(I) and Pb(II) Based on cytosine-Ag(I)-cytosine Mismatches and G-Quadruplex. Analytica Chim. Acta 1151, 338258. doi:10.1016/j.aca.2021.338258
Zhang, R., and Chen, W. (2014). Nitrogen-doped Carbon Quantum Dots: Facile Synthesis and Application as a "Turn-Off" Fluorescent Probe for Detection of Hg2+ Ions. Biosens. Bioelectron. 55, 83–90. doi:10.1016/j.bios.2013.11.074
Zhang, S., Adhikari, R., Fang, M., Dorh, N., Li, C., Jaishi, M., et al. (2016). Near-Infrared Fluorescent Probes with Large Stokes Shifts for Sensing Zn(II) Ions in Living Cells. ACS Sens. 1 (12), 1408–1415. doi:10.1021/acssensors.6b00490
Zhang, X., Chen, Y., Cai, X., Liu, C., Jia, P., Li, Z., et al. (2020). A Highly Sensitive Rapid-Response Fluorescent Probe for Specifically Tracking Endogenous Labile Fe2+ in Living Cells and Zebrafish. Dyes Pigm. 174, 108065. doi:10.1016/j.dyepig.2019.108065
Zhang, Y., Qin, H., Huang, Y., Zhang, F., Liu, H., Liu, H., et al. (2021). Highly Fluorescent Nitrogen and boron Doped Carbon Quantum Dots for Selective and Sensitive Detection of Fe3+. J. Mater. Chem. B 9 (23), 4654–4662. doi:10.1039/D1TB00371B
Zhang, Y., Yuan, B., and Ma, D. (2020). Selective Recognition of Zn(II) Ions in Live Cells Based on Chelation Enhanced Near-Infrared Fluorescent Probe. Inorg. Chim. Acta 508, 119640. doi:10.1016/j.ica.2020.119640
Zhang, Z., Deng, C., Zou, Y., and Chen, L. (2018). A Novel Fluorescent and Colorimetric Probe for cascade Selective Detection of Fe(III) and Pyrophosphate Based on a Click Generated Cyclic Steroid-Rhodamine Conjugate. J. Photochem. Photobiol. A: Chem. 356, 7–17. doi:10.1016/j.jphotochem.2017.12.023
Zhang, Z., Wang, F.-W., Wang, S.-Q., Ge, F., Zhao, B.-X., and Miao, J.-Y. (2012). A Highly Sensitive Fluorescent Probe Based on Simple Pyrazoline for Zn2+ in Living Neuron Cells. Org. Biomol. Chem. 10 (43), 8640–8644. doi:10.1039/c2ob26375k
Zhao, X.-H., Gong, L., Wu, Y., Zhang, X.-B., and Xie, J. (2016). Cationic-perylene-G-quadruplex Complex Based Fluorescent Biosensor for Label-free Detection of Pb 2+. Talanta 149, 98–102. doi:10.1016/j.talanta.2015.11.038
Zheng, M., Tan, H., Xie, Z., Zhang, L., Jing, X., and Sun, Z. (2013). Fast Response and High Sensitivity Europium Metal Organic Framework Fluorescent Probe with Chelating Terpyridine Sites for Fe3+. ACS Appl. Mater. Inter. 5 (3), 1078–1083. doi:10.1021/am302862k
Zhou, Y., He, X., Chen, H., Wang, Y., Xiao, S., Zhang, N., et al. (2017). An ESIPT/ICT Modulation Based Ratiometric Fluorescent Probe for Sensitive and Selective Sensing Hg2+. Sensors Actuators B: Chem. 247, 626–631. doi:10.1016/j.snb.2017.03.085
Zhou, Y., Kim, H. N., and Yoon, J. (2010). A Selective 'Off-On' Fluorescent Sensor for Zn2+ Based on Hydrazone-Pyrene Derivative and its Application for Imaging of Intracellular Zn2+. Bioorg. Med. Chem. Lett. 20 (1), 125–128. doi:10.1016/j.bmcl.2009.11.028
Zhou, Z., Tang, H., Chen, S., Huang, Y., Zhu, X., Li, H., et al. (2021). A Turn-On Red-Emitting Fluorescent Probe for Determination of Copper(II) Ions in Food Samples and Living Zebrafish. Food Chem. 343, 128513. doi:10.1016/j.foodchem.2020.128513
Zhu, A., Qu, Q., Shao, X., Kong, B., and Tian, Y. (2012). Carbon-Dot-Based Dual-Emission Nanohybrid Produces a Ratiometric Fluorescent Sensor for In Vivo Imaging of Cellular Copper Ions. Angew. Chem. Int. Ed. 51 (29), 7185–7189. doi:10.1002/anie.201109089
Zhu, S., Meng, Q., Wang, L., Zhang, J., Song, Y., Jin, H., et al. (2013). Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. Int. Ed. 52 (14), 3953–3957. doi:10.1002/anie.201300519
Keywords: fluorescent probes, metal ions, cell imaging, sensors, quantum dots
Citation: Li L, Wang J, Xu S, Li C and Dong B (2022) Recent Progress in Fluorescent Probes For Metal Ion Detection. Front. Chem. 10:875241. doi: 10.3389/fchem.2022.875241
Received: 15 February 2022; Accepted: 28 March 2022;
Published: 13 April 2022.
Edited by:
Shusheng Zhang, Linyi University, ChinaCopyright © 2022 Li, Wang, Xu, Li and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biao Dong, ZG9uZ2JAamx1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.