
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Chem. , 23 March 2022
Sec. Medicinal and Pharmaceutical Chemistry
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.869860
Pyridinones have been adopted as an important block in medicinal chemistry that could serve as hydrogen bond donors and acceptors. With the help of feasible synthesis routes via established condensation reactions, the physicochemical properties of such a scaffold could be manipulated by adjustment of polarity, lipophilicity, and hydrogen bonding, and eventually lead to its wide application in fragment-based drug design, biomolecular mimetics, and kinase hinge-binding motifs. In addition, most pyridinone derivatives exhibit various biological activities ranging from antitumor, antimicrobial, anti-inflammatory, and anticoagulant to cardiotonic effects. This review focuses on recent contributions of pyridinone cores to medicinal chemistry, and addresses the structural features and structure–activity relationships (SARs) of each drug-like molecule. These advancements contribute to an in-depth understanding of the potential of this biologically enriched scaffold and expedite the development of its new applications in drug discovery.
Privileged structure-guided scaffold re-evolution/refining is a primary strategy to identify structurally novel chemotypes by modifying the central core structure and the side chain of the existing active compounds, or to exploit undescribed bioactivites by making full use of readily derivatized motifs with well-established synthetic protocols (Song et al., 2013a; Song et al. 2013b; Song et al. 2014). Pyridinones are an interesting class of six-membered heterocyclic scaffolds with a nitrogen, an oxygen, and five carbon atoms, which have also been employed as bioisosteres for amides, pyridines, pyranones, pyrimidines, pyrazines, and phenol rings. According to the relative position between the nitrogen heteroatom and carbonyl moiety (Katrizky et al., 2010), two isomeric forms including 2- and 4-(1H)-pyridinones exist as skeletal components (Figure 1). In addition, a study on isomerization between the pyridinone and the corresponding hydroxypyridine indicates that the former form is favored, especially in physiological conditions.
Despite the small size of pyridinone, it could provide five derivatizable positions possessing four hydrogen bond acceptors and a hydrogen bond donor. Thus, it can form additional interactions with the therapeutic targets, cross the cell membrane easily, and increase the water solubility of molecules. In recent years, pyridinone derivatives have attracted extensive attention due to their versatile pharmaceutical, agricultural, and industrial applications (Zhang and Pike 2021). Protein kinases owning a highly conserved three-dimensional structure in their catalytic domains have become a druggable target class (Santos et al., 2017). Unsubstituted pyridinone could serve as a valid peptide bond isostere and form either a single or multiple H-bond interactions with the kinase hinge region to gain appropriate binding affinity, and have been explored in various kinase programs such as Met kinase, mitogen-activated protein kinase-interacting kinase, orotate phosphoribosyltransferase, monoamine oxidase B, mitogen-activated protein kinase, and Bruton’s tyrosine kinase. Pyridinone-containing compounds show a broad spectrum of pharmacological properties such as antitumor, antimicrobial, anti-inflammatory, antimalarial, antidepressant, and cardiotonic effects. The scaffold has become a hot point in medicinal chemistry, and an increasing number of FDA-approved drugs have reinforced its significance. As depicted in Figure 2, milrinone is a powerful cardiac stimulant, pirfenidone is an agent for idiopathic pulmonary fibrosis (IPF), gimeracil and tazemetostat are considered as antineoplastic drugs, ciclopirox is an antifungal agent, deferiprone is a well-known iron chelator, and doravirine is a valuable anti-HIV drug (Shipley et al., 1996; Niewerth et al., 2003; Cho and Kopp 2010; Harada et al., 2017; Sanchez et al., 2019). It could draw a conclusion that the pattern of substituents on pyridinone exercises considerable influence over the pharmacological properties and its therapeutic applications.
In the past decades, two main synthetic approaches have been reported to form the ring of pyridinone: one is to synthesize from pyridine or related six-membered ring forms via the introduction of carbonyl groups, and the other is by the cyclic condensation of two compounds (Figure 3).
Lin et al. (2003) used pyridine as substrates, and hydrogen peroxide and nitric acid as reagents to get 4-nitropyridine-N-oxide, followed by acetylization, and elimination reactions to generate pyridinone (Figure 4A). Another routine for preparing pyridinone (Demuner et al., 2009) is to primarily get deacetylated pyranone and then treat it with ammonia solution (Figure 4B).
Bai et al. (2018) reported a one-pot synthesis of 2-(1H)-pyridinone directly from dimethyl 3-oxopentanedioate, N,N-dimethylformamide dimethyl acetal (DMF-DMA), and primary amine by using L-proline as a catalyst. The reaction could proceed efficiently and ecofriendly with broad functional group tolerance (Figure 4C). Ethyl acetoacetate in a medium of aqueous ammonia is readily converted into imino ester (McElroy and DeShong 2003), followed by condensation with diethyl malonate to afford ethyl 4-hydroxy-2-pyridinone-3-carboxylate (Figure 4D). Abaszadeh et al. (2009) reported treatment of (chlorocarbonyl)phenyl ketene with enaminones in boiling toluene afforded 4-hydroxy-3,4,6-trisubstituted-2(1H)-pyridinone conveniently and rapidly (Figure 4E). As Guillemont et al. (2009) published, ethyl 3-aminocrotonate was condensed with the activated malonate derivative to afford 4-hydroxy-2-pyridinone conveniently in 75% yield in a single step without added base (Figure 4F). Ethyl 3-oxoglutarate has been reported (Platts et al., 2011) to directly react with triethylorthoformate to obtain ethyl 4-hydroxy-2-pyridinone-5-carboxylate in the presence of ammonia (Figure 4G).
The construction of the pyridinone moiety was carried out first between phenylacetonitrile and malonyl chloride in 56% yield. Then, electrophilic addition of dichlorocarbene species to the intermediate gave the desired product. The moiety can be formylated to afford pyridinone derivatives (Brondel et al., 2006) which are very significant intermediate to further prepare other pyridinones (Figure 4H).
Chun et al. (2009) successively reported a tandem one-pot conversion of nitriles with ethyl bromoacetate in THF to get various 2-pyridinone derivatives via the Blaise reaction intermediate, which was demonstrated to be a very efficient and operationally convenient method (Figure 4I). The possible mechanism involves vinyl zinc bromide obtained through Michael addition reaction of the Blaise reaction intermediate, and the propiolate was isomerized to the α-vinylated zinc bromide complex, followed by rearrangement and intramolecular ring-closing reaction to yield the 2-pyridinone structure (Figure 4J).
Despite its importance as a special scaffold in drug research, reviews on the medicinal application of pyridinone-containing derivatives are still rare. Consequently, the following subsections aim to present recent advances in bioactivity of pyridinone scaffolds, discuss the diversity of pharmacological and biological action, and exhibit the selected data to demonstrate structure–activity relationships (SARs) and the proposed mechanisms with their targets.
Cancer, known as malignant tumor, is a multifactor-causing disease (Atkins and Gershell 2002). Tumorigenesis is accompanied by many physiological and biochemical phenomena, for example, the uncontrolled cell growth and apoptosis, tumor angiogenesis, lower pH value, hypoxia, and metastasis (Jemal et al., 2013).
Novel pyridinone-containing molecules have attracted considerable attention for their broad-spectrum of antiproliferative activity against various human tumor cell lines. The useful scaffold can specifically arrest some targets including protein tyrosine kinases, Met kinase, histone deacetylase (HDAC), isocitrate dehydrogenase (IDH), cyclin, mitogen-activated protein kinase (MAPK), and ribonucleotide reductase. Accordingly, we classified the published derivatives into the following types based on different targets to control or prevent cancer.
Protein phosphorylation (Le Quesne et al., 2010) is a significant signal transduction pathway modulated by protein kinases (PKs) and phosphatases which is essential for living cells. PKs are regulatory enzymes that transfer the γ phosphate from ATP to the amino acid residues of protein substrate, while phosphatases perform the opposite process (Bryan and Rajapaksa 2018). Alafeefy et al. (2012) reported a series of pyridinone–quinazoline derivatives (42) as cytotoxic agents targeting protein tyrosine kinases (PTK). The analogs were synthesized by condensation of substituted quinazoline and malononitrile or ethyl cyanoacetate, the former of which were prepared from 2-amino-5-methylbenzoic acid. The synthesized compounds were screened for preliminary anticancer activity using three tumor cell lines, MCF-7, HeLa, and HepG2 in vitro. The results in Figure 5A demonstrated that all the pyridinone–quinazoline derivatives displayed >70% inhibition with improved IC50 values ranging from 9 to 15 μM and displayed suitable drug-like characteristics according to Lipinski’s rule of five. Most of the active compounds had a considerable number of hydrogen bond donors and acceptor groups comparable to model tyrphostin AG99. Among them, 42a and 42b were as potent as doxorubicin in anticancer activity and exhibited good bioavailability. Such observation rendered the series of pyridinone–quinazoline analogs deserving deeper research and development.
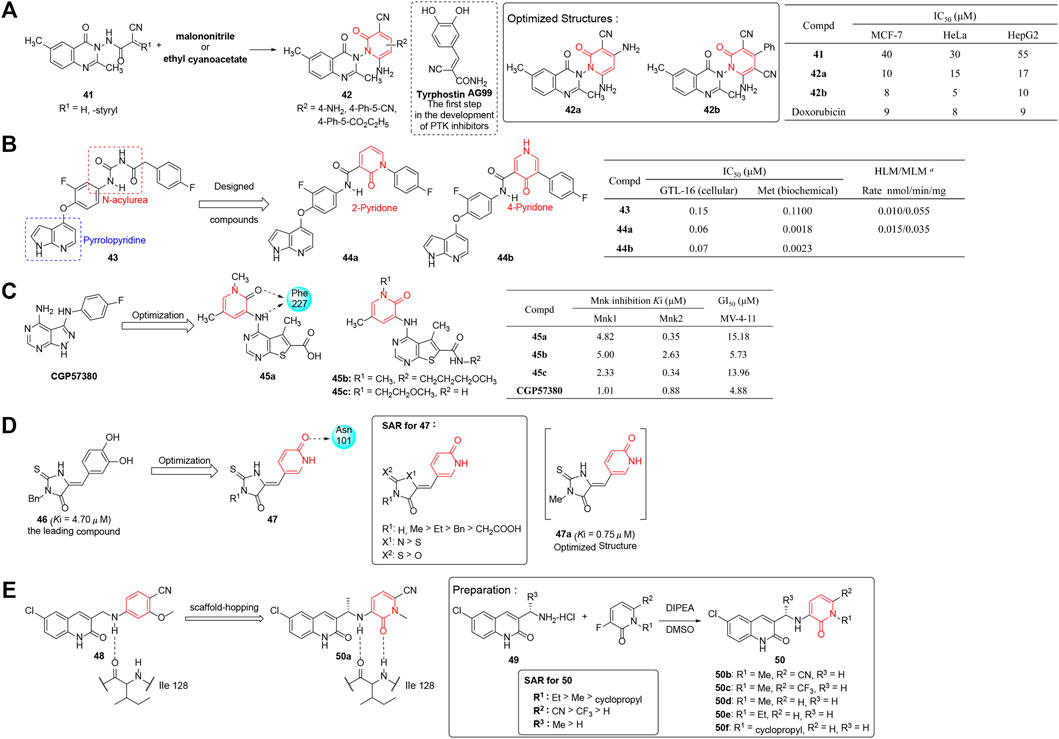
FIGURE 5. (A) Quinazoline-conjugated pyridinones were designed in the discovery of new PTK inhibitors (Alafeefy et al., 2012). (B) Pyrrolopyridine-pyridinone-based inhibitors of Met kinase (Kim et al., 2008). a Compounds at 3 μM incubated in 10 mg of protein (human (HLM) and mouse (MLM) liver microsome) for 10 min. (C) Pyridinone–thienopyrimidine derivatives were designed as potent and highly selective MNK inhibitor (Yu et al., 2015). (D) Synthesis of pyridinone–thiohydantoin derivatives and their SARs against IDH1 (R132H). (E) Synthesis and SAR of pyridinone-quinolinones as IDH1 (R132H) inhibitors.
Kim et al. (2008) synthesized a series of pyridinone-pyrrolopyridine-based inhibitors of Met kinase. With 43 as the lead compound, the pyrrolopyridine moiety was retained as the basic skeletal structure, and modification was mainly focused on replacing the acylurea moiety by pyridinone ring acting as a conformationally constraint role and increasing the carbonyl oxygen hydrogen bonds with the backbone NH of Asp1222 (Figure 5B). In this study, twenty-five derivatives were synthesized and evaluated for their antiproliferative activities by using the GTL-16 gastric carcinoma cell line and Met kinase assay. The result showed that compounds 44a and 44b enhanced the potency markedly (IC50 = 0.06 and 0.07 μM, respectively) and possessed excellent metabolic and pharmacokinetic parameters. The compounds also demonstrated good in vivo efficacy in the xenograft model. In addition, X-ray crystallography demonstrated 44a bound to the ATP-binding pocket of the Met kinase domain.
Yu et al. (2015) reported various pyridinone–thienopyrimidine derivatives which were designed based on the ChemBridge database targeting mitogen-activated protein kinase (MAPK)-interacting kinases (MNKs) whose phosphorylation process played a key role in oncogenic transformation. Compared with CGP57380, a known MNK inhibitor with unconquered kinase selectivity, serving as a positive reference substance, most compounds displayed moderate to potent MNK inhibitory activity with high selectivity toward MNK2. In particular, three compounds, 45a, 45b, and 45c, exhibited high activity in both MNK inhibition potency and antiproliferative effects (Figure 5C). Cellular mechanistic studies revealed the compounds were capable of downregulating the phosphorylated eIF4E, Mcl-1, and cyclin D1, and causing PARP cleavage. Interestingly, according to the docking with ATP binding pocket of MNK2, this fraction of pyridinone could generate multiple hydrophobic interactions and hydrogen bonds with Lys113, Arg125, and Phe159. This suggested the exploration of such structural diversity would provide potent and highly selective MNK inhibitors.
Isocitrate dehydrogenase (IDH) is one of the key enzymes in the tricarboxylic acid (TCA) cycle which is involved in catalyzing oxidative decarboxylation of isocitric acid to α-ketoglutaric acid. Recent research (Yan et al., 2009; Paschka et al., 2010; Borger et al., 2012) revealed that frequent hematologic and somatic cell variation of IDH1 have been related to certain cancers, including myelodysplastic syndromes (MDS), gliomas (grade II and III), acute myeloid leukemia (AML), intrahepatic cholangiocarcinoma, and some other sarcomas. Therefore, inhibitors of mutant IDH1 are counted on to provide a therapeutic benefit in related cancers.
Based on the modification of 3-benzyl-5-(3,4-dihydroxybenzylidene)-2-thiohydantoin (46, Ki = 4.7 μM) which was published previously (Liu et al., 2014), Wu et al. (2015) prepared a series of pyridinone–thiohydantoin derivatives as inhibitors of mutant IDH1. The inhibitory value (Ki) against mutant IDH1 (R132H) was tested, and results revealed that most compounds possessed improved inhibitory activities (Ki = 0.42–9.2 μM). In addition, most inhibitors could reduce the concentrations of D2HG, decrease hypermethyled phenotype of histones, and suppress the self-renewal ability of stem-like cancer cells in cell biology studies. The partial SAR showed that 2-thiohydantoin ring was the most favorable core, and a small substituent was more favored at the 3-position (Figure 5D). Remarkably, X-ray crystallography revealed 47a binded well to IDH1, among which the pyridinone moiety had favorable interactions with Asn101 and Gly97. In addition, studies showed a strong H-bond acceptor (pyridinone) instead of an H-bond donor (hydroxylbenzenyl) was essential for good inhibitory activity. This provided a platform for the design of structure-based inhibitors targeting IDH1 mutated cancers in more depth.
Caravella et al. (2020) synthesized a series of pyridinone quinolinones as mutant-specific inhibitors of IDH1. As shown in Figure 5E, the derivatives were designed on the basis of a previously reported ligand (48), by using a scaffold hopping approach. In consideration of the crystal structure of 48 in complex with IDH1 (R132H), which led to suboptimal potency and low solubility, the phenyl ring was changed to pyridinone to improve properties by increasing a hydrogen bond acceptor. In that study, crystal structures of 50 were synthesized through nucleophile substitution reaction, and further evaluated for their IDH1 (R132H) inhibition activity and solubility. Based on structure optimization and overall SAR that showed substitutions at position 7 of the quinolinone ring could improve potency, compound 50a was discovered as the clinical candidate for its potent and specific inhibition of mIDH1 in hematologic malignancies, solid tumors, and gliomas (NCT02719574 and NCT03684811). In particular, 50a possesses perfect ADME/PK properties and lowers 2-hydroxyglutarate levels in in vivo IDH1 xenograft models.
Organometallic complexes (Jamieson and Lippard 1999; Louie and Meade 1999; Hambley 2007; Meggers 2009; Dörr and Meggers 2014) are well-known and clinically proven chemotherapeutic agents, of which the representative drugs are cisplatin, carboplatin, and oxaliplatin. On entry into cells, the agents will react with DNA or protein and realize their antitumor properties (Hartinger and Dyson 2009). In recent years, a number of metal complexes were investigated for treatment of a broader range of tumors, with less side effects and higher therapeutic index.
Mendoza-Ferri et al. (2009a) discovered a number of ruthenium(II)–arene complexes which were derived from 51 to investigate the inhibitory action of the metal centers, the spacer length, the halide ligand, and the arene ligand on tumor cells (Mendoza-Ferri et al., 2009b). The analogs were linked by pyridinone-based ligands and the activities were measured using the MTT assay against the colon and ovarian cancer cell lines. The SAR summarized in Figure 6A demonstrated that the number and nature of metal centers significantly influenced the in vitro antineoplastic potency, due to the binuclear complex with Ru possessing increased solubility and lipophilicity. However, the type of halide made little difference owing to predominant formation of the same aquation product. Notably, in addition to lipophilicity, the spacer length relevant for the capability of hitting biomolecular targets also caused a pronounced role in the mode of action and altered the antitumor activity to a meaningful extent.
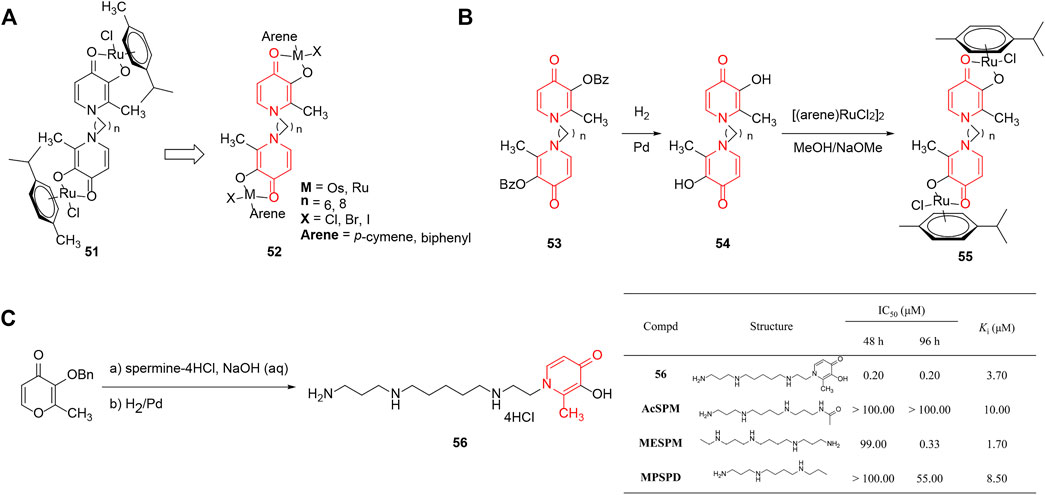
FIGURE 6. (A) SAR of ruthenium(II)–arene complexes linked by pyridinone-based ligands. (B) Synthesis and SAR of di-nuclear pyridinone-derived ruthenium complexes. (C) Cell growth inhibition and competition transport for pyridinone-containing polyamines (Bergeron et al., 2003).
The same group (Mendoza-Ferri et al., 2009a) reported that di-nuclear pyridinone-derived ruthenium complexes which displayed affinity toward transferrin and DNA were potential anticancer drugs. The complexes were generated by the reaction between bis(3-hydroxy-2-methyl-4-pyridinon-1-yl) alkanes and [(arene)RuCl2]2 in good yields, and were further evaluated for cytotoxicity against several lines. The data showed that chain length influenced the in vitro activity seriously, which could be explained by the association with lipophilicity (Figure 6B). Moreover, the most cytotoxic compound 55a exhibited the same level of IC50 values with cisplatin and oxoplatin, and showed no cross-resistance to oxoplatin. Bioanalytical characterization of diaqua species hydrolyzed by the ruthenium complexes exhibited affinity toward transferrin and DNA, suggesting that both proteins and nucleotides were potential targets.
Polyamines could act as vectors that deliver the bidentate chelator into the cell and serve as antineoplastic devices (Yan et al., 2005). Bergeron et al. (2003) designed 1-(12-amino-4,9-diazadodecyl)-2-methyl-3-hydroxy-4(1H)-pyridinone (56) and synthesized the conjugate by using 3-O-benzylmaltol and spermine as raw materials. As depicted in Figure 6C, compound 56 exhibited much better inhibitory activity (at least 230 times) than several other representative polyamines against leukemia cell, and was concentrated in the cell by more than 1900-fold against a gradient due to its efficient diffusion across the membranes. A subsequent study discovered that the chelators forming a complex with Fe(III) could inhibit tumor growth significantly through suppression of ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (AdoMetDC) and upregulating spermidine-spermine N1-acetyltransferase (SSAT). In addition, the conjugates could serve as efficient vectors for the intracellular transportation of other metal chelators, and this supplied an attractive system for further exploration of the impact of polyamine delivery.
The mammalian switch/sucrose non-fermentable (SWI/SNF) complex (Zeng and Zhou 2002; Hewings et al., 2012; Fedorov et al., 2015) is associated with chromatin remodeling and is highlighted as a potential anticancer target because the inhibition of remodeling complexes results in the arrest of leukemic cells in G1 and their differentiation. Bromodomain-containing protein 9 (BRD9), a SWI/SNF subunit, participates in the proliferation of acute myeloid leukemia (AML) cells. Therefore, BRD9 is proposed to be a new target for therapeutic strategies for AML (Vidler et al., 2012; Hohmann et al., 2016). In this regard, Martin et al. (2016) selected pyridinone scaffold compounds through Glide docking in high-concentration screening (HiCoS) library and filtering on the basis of molecular weight and lipophilicity. 57 was identified as an initial hit compound which could inhibit BDR9 with IC50 of 9.4 μM. Docking analysis of 57 in BDR9 elucidated the pyridinone moiety made important interactions with Asn100, Tyr57, and Tyr106, and the phenyl core had a π-interaction with Ile53. In order to improve the efficacy of 57, substituents were added to the phenyl group, and most of the derivatives showed potent inhibitory activity at nanomolar concentrations. The study revealed that the additional dimethoxyphenyl linker induced a favorable interaction with Phe47 and realized optimal T-stacking with Phe44, and the partial SAR is shown in Figure 7A. Being available to the scientific community as potent, selective, cell-permeable, and non-cytotoxic BRD9 inhibitors, such molecular probes provided a useful tool to broaden the field of chromatin regulators in medical oncology.
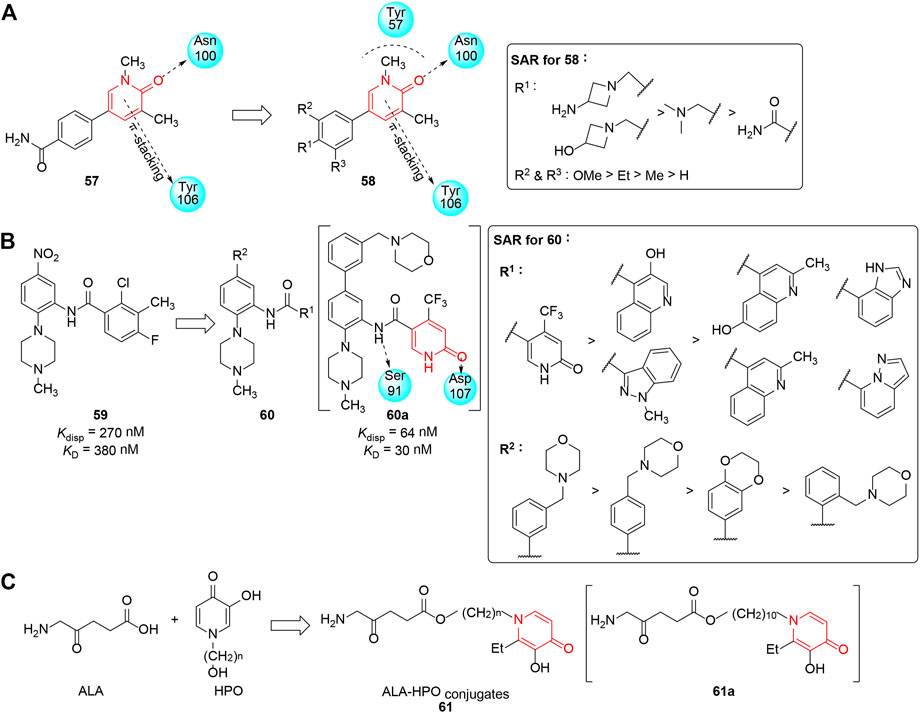
FIGURE 7. (A) SAR of the aromatic pyridinones as BRD9 inhibitors. (B) Pyridinones with inhibitory activity against WDR5–MLL1 interaction. (C) ALA-HPO conjugates as phototoxic agents.
Mixed-lineage leukemia 1 (MLL1) is a histone H3K4 methyltransferase, and plays an important role in gene regulation, development, and differentiation (Karatas et al., 2013). As an essential component of MLL1, WD repeat-containing protein 5 (WDR5) is critical for its stability and activity through combination with histone H3, and its chromosomal translocation has a close connection with mixed lineage leukemia (Wysocka et al., 2005; Ang et al., 2011; Bailey et al., 2015). Thus, inhibition of WDR5 might be a novel oncological therapeutic strategy. Getlik et al. (2016) developed N-phenyl-6-oxo-1,6-dihydropyridine-3-carboxamides as new class of WDR5 antagonists, which were designed on the basis of a previous discovery of inhibitor 59 by the same group (Chen et al., 2021). Two series of heteroaryl substituents at the C-5 position of the phenyl ring and C-1 position of amide were synthesized, respectively, and the WDR5-MLL1 Kdisp values of optimized compounds revealed suitable moieties such as pyridinone could significantly improve the overall properties in addition to potency. Functionally, the potency of compounds was assessed using fluorescein-labeled 9-Ala-FAM peptide, and a partial SAR revealed that the introduction of morpholine-substituted phenyl rings at C-5 was crucial for the improvement of binding affinity and solubility (Figure 7B). In particular, compound 60a exhibited excellent in vitro efficacy (Kdisp = 64 nM) and good pharmacokinetic profiles (via IP administration); it also decreased the survival time of human AML cells with N-terminal C/EBPα mutations (IC50 ≈ 5 μM). This allowed 60a to not only serve as a potent and selective chemical probe for disclosing the interaction between WDR5 and MLL1 but also be a candidate for the treatment of MLL1-driven acute myeloid leukemias.
Photodynamic therapy (PDT) is an effective targeted therapy (Vrouenraets et al., 2003; Agostinis et al., 2011) that utilizes photosensitizer, light, and oxygen to treat malignant and non-malignant tumor. As a traditional photosensitizer, the clinical potential of 5-aminolaevulinic acid (ALA), a precursor of protoporphyrin IX (PpIX), is hindered by its relatively poor bioavailability due to the hydrophilic nature (Brackett and Gollnick 2011). Considering the excellent cell membrane permeability of 3-hydroxypyridin-4-one chelators (HPOs), Battah et al. (2017) conjugated ALA with HPO iron chelators via ester bonds, which would hydrolyze enzymatically under cells’ internal environment and release the two agents following cellular uptake. Compared with the administration of ALA alone, the newly synthesized ALA-HPO conjugate 61 significantly increased phototoxicity and elevated phototherapeutic metabolite formation in all tested cell lines, especially following exposure to light (Figure 7C). Considering the elevation of cellular PpIX levels, compound 61a, containing a linker of (CH2)10, was found to be the most phototoxic compound with comparable efficacy to the ALA hexyl ester. Further investigation demonstrated that the main mechanism of ALA-HPO conjugates adopt a passive diffusion pathway, while the uptake of ALA was an active transportation coupled with ATP. This study demonstrates that ALA-HPOs provided a promising therapy for many types of cancer.
In the late years, diseases induced by viral infection have become increasingly prevalent and widely spread in the world. However, many disadvantages such as multidrug resistance, severe adverse effects, and high cost of some antiviral drugs have caused great disturbance to patients (Drew 2010; Bobrowski et al., 2020). These restrictions have emphasized the urgent demand to develop new drugs for antiviral treatments. A growing body of research has focused on pyridinone-containing compounds, which are proven to be a significant class of leading scaffolds to discover novel effective therapeutics (Zhou et al., 2018).
Polymerase acidic (PA) polypeptide chain which is an essential part of the heterotrimeric influenza RNA-dependent RNA polymerase (RdRP) plays a major role in the replication of influenza viruses (Xiao et al., 2006; Dias et al., 2009; Venkataraman et al., 2018). Thus, the endonuclease of the PA subunit becomes a promising target for the treatment of influenza infection. Parhi et al. utilized high-throughput X-ray crystallography fragment screening to discover effective endonuclease inhibitors as potential influenza drugs, and 5-chloro-3-hydroxypyridin-2(1H)-one was identified as the chelating ligand (Parhi et al., 2013). A series of such derivatives were then designed and synthesized in two steps, where halogenated 2,3-dimethoxypyridines were first treated with arylboronic acid under Suzuki-coupling conditions, and then further demethylation was performed to yield the desired aryl substituted 3-hydroxypyridin-2(1H)-ones (Figure 8A). Their inhibition ability for influenza A endonuclease was evaluated through a high-throughput fluorescence assay, and corresponding SAR indicated the substitutions of diphenyl groups at both the adjacent 5- and 6-positions induced a significant enhancement on potency. Interestingly, 63a and 63b, the two more potent analogs in this series, showed dramatically different binding modes that made interactions with the two active site metal ions (M1 and M2) using only two chelating groups, offering a reliable basis on developing a new class of anti-influenza drugs.
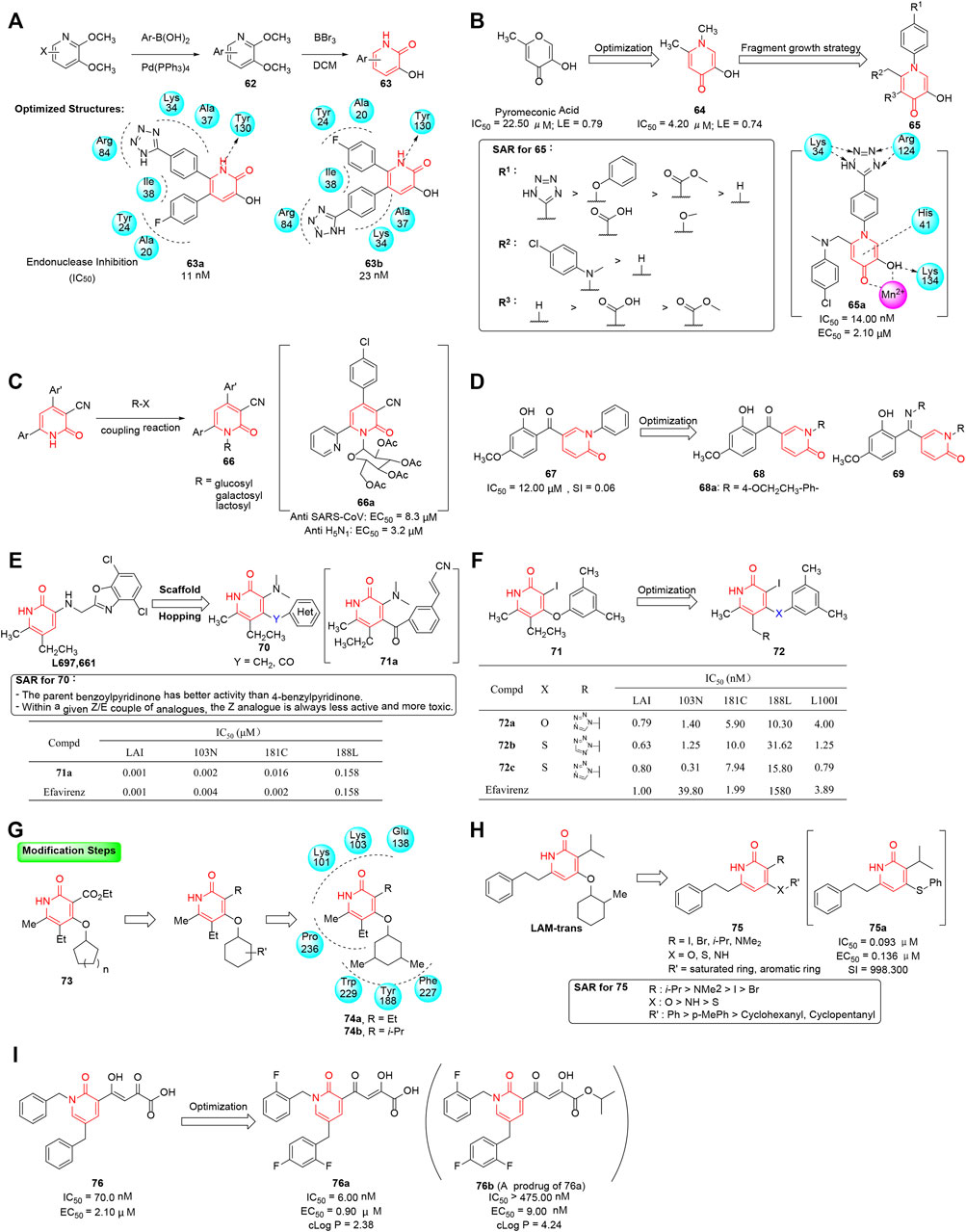
FIGURE 8. (A) Synthesis of phenyl substituted 3-hydroxypyridin-2(1H)-ones as endonuclease inhibitors. (B) From hit to lead for inhibitors of influenza PA endonuclease. (C) Antiviral activity of 2-ONN-based nucleoside analogs. (D) 2-pyridinone derivatives as inhibitors against HBV DNA replication and hepatitis B e-antigen (HBeAg) separately. (E) Design of pyridin-2(1H)-ones as NNRTIs by using a scaffold hopping approach (Benjahad et al., 2004). (F) Biological activity of IOPY and ISPY analogs (Benjahad et al., 2007). (G) Optimization of pyridinone-based NNRTIs. (H) SAR of pyridin-2(1H)-ones as inhibitors of HIV reverse transcriptase. (I) Optimization of 1,5-dibenzyl-2-pyridinones as InSTIs.
Further crystallographic and biochemical studies have demonstrated that a dinuclear metal active site which employed Mn2+ and Mg2+ cations existed in the endonuclease functional site of the PA subunit. Using metal-binding pharmacophore (MBP) library, the efficient fragments targeting the metal active spot could be found more time- and cost-effectively (Jacobsen et al., 2011), yielding a number of active hits and ultimately leading to the discovery of potent lead molecules. In this regard, Credille et al. (2016) screened pyromeconic acid as a moderate inhibitor of endonuclease activity (IC50 = 22.50 μM; ligand efficiency = 0.79). Further optimization was conducted by conversion of the pyrone ring to an N-methylpyridinone (64) resulting in a marked increase in potency (IC50 = 4.20 μM, ligand efficiency = 0.74), due to more aromatic character and greater electron density on the donating oxygen atoms. By applying “fragment growth” and “fragment merging” strategies, substituents were added at 4′-position of the N-inserted phenyl ring or 6-position of pyridinone and resulted in a marked increase in potency (Figure 8B). In particular, compound 65a (IC50 = 14.00 nM, EC50 = 2.10 μM) showed 4- to 5-fold more potent than L-742,001 (EC50 = 9.00 μM) which was one of the most potent inhibitors of influenza PA endonuclease reported in the viral challenge assay (Stevaert et al., 2013). Docking analysis of 65a in the PA subunit active site showed that 6-position phenylaminomethyl moiety could make an interaction with hydrophobic pocket, and the N-phenyltetrazole moiety was able to form a hydrogen bond with Arg124 and Lys34.
Nucleoside analogs are well-known potent antiviral agents (De Clercq 2004). Abou-Elkhair et al. (2014) reported a scaffold of 2-oxonicotinonitrile (2-ONN)-based acetylated nucleosides. The hybrids were designed on the basis of many published biologically active 2-ONN derivatives. The 2-ONN glucosides were synthesized by coupling 2-ONNs with bromosugars and evaluating the in vitro antivirus activity against influenza A virus (H5N1 and H3N4 serotype) and severe acute respiratory syndrome coronavirus (SARS-CoV). The selected acetylated 2-ONN glucoside 66a showed best antiviral activity (Figure 8C).
Hepatitis B virus (HBV) can cause both acute and chronic infections, leading to liver damage, cirrhosis, and hepatocellular carcinoma (HCC) with high mortality (Robinson 1994; Bhattacharya and Thio 2010). Among all DNA viruses, HBV is the most variable one because of its high mutation rate, replicative capability, and virion production. α-interferon is the major therapeutic option for the treatment of HBV infection till now, but the low success rate and serious side effects limited its application (Lavanchy 2004). Unlike all reported anti-HBV agents, 5-(2-hydroxy-4-methoxybenzoyl)-1-phenylpyridin-2(1H)-one (67) was remarkable for its unique chemical scaffold and was screened as a modest anti-HBV agent in an in-house library by Lv et al. (2010). With 67 as the starting point, a series of 2-pyridinone analogs were synthesized and evaluated for inhibitory activity against HBV-DNA replication, and preliminary SARs showed that the N-aryl derivatives exhibited better anti-HBV activity than the N-alkyl derivatives (Figure 8D). Among them, compound 68a exhibited the best inhibitory activity against HBV DNA replication (IC50 = 0.12 μM) and high selectivity (selectivity index: CC50/IC50 = 467). In order to investigate the mechanism of action, a genetic algorithm similarity program (GASP) was employed to construct a pharmacophore model of HBV inhibitors. The binding sites included three hydrophobic points, four HBA points, and one HBD point, and provided possibility for further structural modification.
Human immunodeficiency virus (HIV), identified in 1981 as the etiological agent of acquired immunodeficiency syndrome (AIDS), could cause high morbidity and mortality owing to the significant risk of opportunistic infections and malignancy, and become the leading pandemic disease (Ruelas and Greene 2013; Maeda et al., 2019). Because of a large amount of the viruses produced by an infected individual every day, HIV undergoes rapid genetic variation and generates diverse HIV subtypes, which in turn presents moving targets and complicates the development of effective drugs and vaccines (De Cock et al., 2012; Mbonye and Karn 2014).
Reverse transcriptase (RT), a crucial enzyme regulating the replicative stage of HIV (Kashuba et al., 2012), has been recognized as an attractive target for AIDS treatment. Accordingly, non-nucleoside reverse transcriptase inhibitors (NNRTIs) have been developed with potent anti-HIV-1 activity and favorable pharmacokinetic properties (Daar 2008), and thus gained approval for clinical use (nevirapine, delavirdine, efavirenz, etravirine, and rilpivirine). However, the continued emergence of resistant strains promoted the development of new NNRTIs to inhibit the existing mutants effectively (De Clercq 2001; Daeyaert et al., 2004; Sweeney and Klumpp 2008). Among the structurally diverse NNRTIs, pyridinone scaffolds demonstrated high potency against HIV-1 wild-type and drug-resistant strains.
Benjahad et al. (2004) published a series of articles introducing the discovery of pyridin-2(1H)-ones as potent NNRTIs via scaffold hopping of Merck pyridinone l697,661, a wild-type HIV inhibitor. Considering the pharmacophore of the known ligand (l697,661) and its binding mode with the hydrophobic pocket of reverse transcriptase, the benzo[d]oxazole moiety was replaced with various heterocyclic aryl rings, and two aromatic rings were linked by the methylene/carbonyl group. In that study, 102 new pyridinone analogs (70) were synthesized, and then further evaluated for inhibitory activity against wild-type HIV (LAI cell line) and the K103N, Y181C, and Y188L mutant strains. Among them, thirty-three compounds have been proven to be active at nanomolar range concentrations, and SAR showed that tiny substituent groups at the N-3 motif positively influenced the activity (Figure 8E). In particular, 3′-acrylonitrile-substituted analog 71a showed the most potent inhibitory activity against four HIV mutant strains, equipotent to that of efavirenz. X-ray crystal structure analysis showed the pyridinone motif engaged in a crucial N-1-H hydrogen-bond with K101, and thus provided a good explanation for the strong binding and good activity against different HIV strains.
On the basis of previous observations, 3-iodo-4-phenoxypyridinone (71) was characterized by its potent activity against wild-type HIV-1 reverse transcriptase (Benjahad et al., 2007). The promising biological results inspired further systematic optimization of 3-iodopyridin-2(1H)-ones; thus, a 96-member library of 3-iodo-4-phenoxypyridinone (IOPY) and 3-iodo-4-arylthiopyridinones (ISPY) analogs was prepared and evaluated for its in vitro activity against wild-type HIV-1 (LAI cell line), K103N, Y181C, and Y188L mutant strains. Due to the geometry/conformation differences between the ISPY and IOPY, even minor structural modifications brought considerable changes in activity. Further biological study was performed to reveal that azole substituents at C5-methyl position exhibited better potency than other functionalized groups. Notably, pyridinone derivatives (72a-c) showed excellent activity against a panel of strains comparable to the known efavirenz (Figure 8F). Finally, the presented data would serve as a valuable guide to further optimization of the 3-iodopyridinone (IOPY/ISPY) family.
Le Van et al. (2009) reported a series of 4-cycloalkyloxypyridin-2(1H)-one derivatives (73) with anti-HIV-1 activity. Molecular modeling of derivatives into the allosteric site showed the pyridinone ring was pinned between Leu100 and Val106, allowing the lactam NH to form a hydrogen bond with Lys101, which guaranteed high antiviral activity. In addition, the impact of different ring sizes and substituents of the C4-oxycycloalkyl group in pyridinone was evaluated, and further modulations focused on position 3 of the pyridinone moiety were performed mainly on the basis of molecular modeling studies (Figure 8G). After thorough SAR analysis, compounds 74a (EC50 = 0.001–0.359 μM) and 74b (EC50 = 0.001–0.642 μM) were discovered as most potent molecules against wild-type HIV-1 and several mutant strains, and showed comparable or even superior effect to that of efavirenz (EC50 = 0.001–0.525 μM).
Cao et al. (2015) reported a series of novel pyridinone derivatives which were designed based on the known anti-HIV agent LAM-trans. Preliminary SAR of the lead compound showed that modifications on positions 3, 4, and 6 of the pyridinone ring were crucial to the antiviral activity (Debnath et al., 2013). Thus, different substituents which were expected to interact with important amino acid residues were introduced at C3 and C4 of the scaffold on account of the molecular docking study. These pyridinone derivatives were synthesized and further evaluated for inhibitory activities by using TZM-bl cell lines. From the results, a partial SAR summarized in Figure 8H confirmed that introduction of the isopropyl moiety on the C-3 would be a great benefit to improve the anti-HIV activity. Notably, 75a exhibited the best profile against HIV-1 and could be flexibly docked into the binding site of the reverse transcriptase. Because of their excellent antiviral activity, the 6-phenethyl pyridinone family was worthy of further study and exploitation taking the biological and structural data as helpful guidance.
HIV-1 integrase (Trono et al., 2010) is an essential enzyme for retrovirus replication and has been established as an attractive antiviral target. Raltegravir, elvitegravir, and dolutegravir are representative marketed HIV-1 integrase strand transfer inhibitors (InSTIs) for their proven efficacy and excellent tolerability (Hare et al., 2010; Smith et al., 2020). In this regard, Seo et al. (2011) designed 1,5-dibenzyl-2-pyridinone (76) as an InSTI (IC50 = 70 nM), strategically assembled on a pyridinone scaffold. The lead optimization studies were undertaken by introducing various substituents (alkyls, chloro, methoxy, and mixed halo/alkyl) on the phenyl rings, and the related effects on the enzymology involving strand transfer (ST) step were investigated. A focused structure–activity investigation signified the derivatives that possessed fluoro substitution had more compelling ST inhibitory IC50 values (<10 nM), which was perhaps induced by hydrophobic and electrostatic interactions (Figure 8I). Notably, compound 76a emerged from these studies with the most potent inhibition activity and good stability in human liver microsomes. It was also found to possess a remarkable drug interaction profile with cytochrome P450 and human UGTs, as it did not exhibit any inhibition or activation of these isozymes. Because of the poor cellular permeability of 76a, its isopropyl ester prodrug, compound 76b, was designed with less polarity and notable therapeutic or selectivity index, and further biological studies are in progress.
Orotate phosphoribosyltransferase (OPRT), an important enzyme catalyzing the synthesis of pyrimidine nucleotides, has been developed as the target for various therapeutic purposes such as tuberculosis (TB), malaria, toxoplasmosis, and tumor (Javaid et al., 1999; Temmink et al., 2007; Abdo et al., 2010; Breda et al., 2012a). Inhibition of mycobacterial cell wall synthesis by the attenuation of M. tuberculosis OPRT (MtOPRT) enzyme would be a novel target for TB treatment and prophylaxis. Breda et al. (2012b) reported a series of analogs of orotate (OA) which was a substrate of MtOPRT. Structurally, the commercially available inhibitor 77c showed submicromolar inhibitory effect on MtOPRT enzyme activity and fit well with the enzyme’s binding pocket according to isothermal titration calorimetry (ITC) data. It was noteworthy that electron withdrawing groups attached on the pyridine-2(1H)-one rings contributed to inhibitory effect by increasing the acidity of the hydrogen bound to the nitrogen and thus improving the putative interactions with the active site. However, 77c was subjected to further optimization due to its unfavorable entropic contribution, detrimental to further pharmaceutical developability. Under the guidance of ITC data, an aromatic ring substituent was added to generate 77d (Figure 9A), and resulted in entropic optimization which was mainly reflected on a thermodynamic discrimination profile characteristic of high affinity to MtOPRT:PRPP. These derivatives represented lead compounds for further development targeting MtOPRT with increased biological activity and suitable properties for drug development.
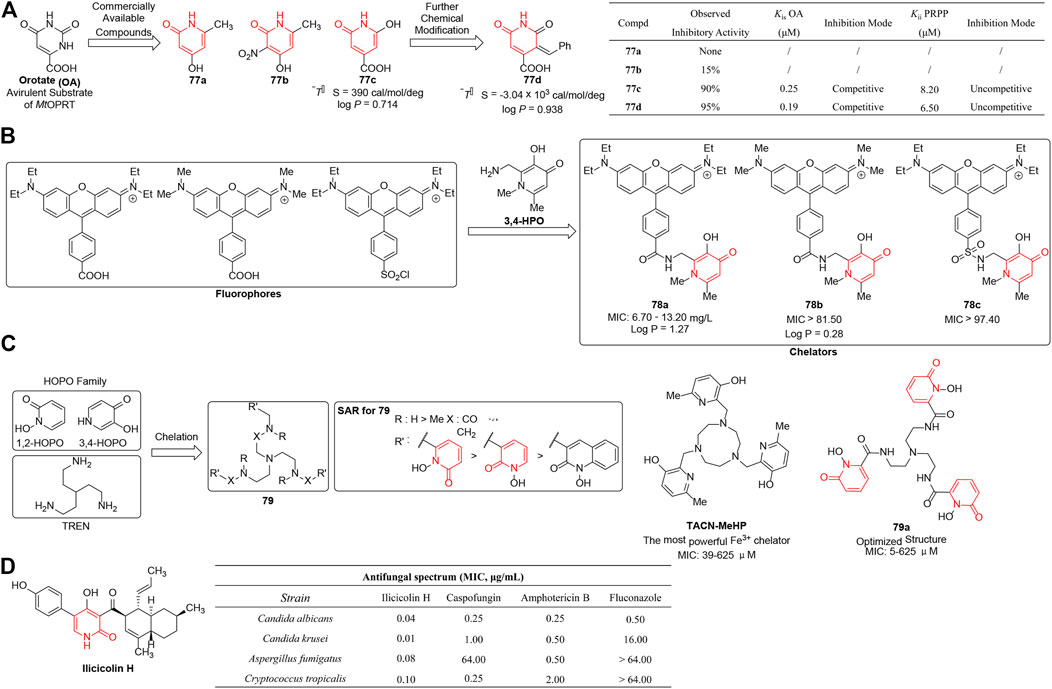
FIGURE 9. (A) 1,2-dihydropyridine-bearing OPRT inhibitors (Breda et al., 2012a). (B) 3,4-HPO chelators as antibacterial agents. (C) Antibacterial SARs of 1,2-HOPO chelators. (D) Antifungal activity of ilicicolin H (Breda et al., 2012b).
As an essential micronutrient needed for microbial metabolism, iron becomes an ideal primary target for metal withdrawal (Breda et al., 2012a). High-affinity iron-selective chelators have a therapeutic benefit in the treatment of infectious diseases by bacterial iron deprivation. Novais et al. (2018) reported a series of fluorescent 3-hydroxy-4-pyridinones (3,4-HPO) chelators as antibacterial agents which were obtained by coupling of the fluorophore to different 3,4-HPO ligands (Figure 9B). Disappointingly, most of the synthesized chelators exhibited inhibitory activity at very high concentrations (MIC: 70 mg/L to 180 mg/L). SAR revealed that the substituents on the nitrogen atom of the fluorophore affected the antibacterial activities a lot, and 78a was discovered to significantly improve bacteriostasis against Gram-positive strains ranging from 6.7 to 13.2 mg/L. The authors suggested that variable biological activity was probably associated with different lipophilicity which have a powerful influence on the permeability of the cell membrane.
Workman et al. (2020) assumed 1,2-HOPO chelators might have the safety advantages over 3,4-HOPO isomers due to lower pKa of the hydroxyl group and poorer permeability. In 2020, they reported a range of novel 1,2-HOPO chelators which were anchored on a tris(2-aminoethyl)amine (TREN) core (79) and the effect of different chelators on microbial growth. The results revealed that most compounds existed as powerful metal chelators and stunted the growth of bacteria by Fe3+ starvation. Among all derivatives, compound 79a was the most potent agent (MIC = 5–625 μM), almost comparable to that of TACN-MeHP (MIC = 39–625 μM) which was known as the most powerful Fe3+ chelator. As the SAR shown in Figure 9C, the nature and position of the linkers between 1,2-HOPO and TREN core strongly influenced the growth of microorganisms by iron(III) starvation, and both amide and amine linkages ensured optimal activity.
Singh et al. (2012) reported the discovery of ilicicolin H as a novel antifungal agent by screening natural product extracts isolated from Gliocladium roseum. The biological assay revealed that the compound exhibited potent inhibition against a wide range of fungi which was even better than those of the reference comparators including caspofungin, amphotercin B, and fluconazole (Figure 9D). In addition, ilicicolin H took effect by depressing mitochondrial cytochrome bc1 reductase (IC50 = 2–3 ng/ml) which represented an unique mode of action, and the in vivo efficacy of the inhibitor was modest due to the limitation by high plasma protein binding. Further structural modification and biological data suggested that hydroxyl-pyridinone was very crucial for antifungal activity.
Alzheimer’s disease (AD) is a neurodegenerative disorder (Hodson 2018) which would lead to typical symptoms like memory loss and cognitive impairment. However, the multifactorial and complex etiologies including amyloid-β (Aβ) deposits, tau protein hyperphosphorylation, metal ion dyshomeostasis, oxidative stress, and neurotransmitter system dysfunction have limited the therapeutic effects (León et al., 2013). On account of this, the multi-target-directed ligand (MTDL) strategy has been developed and provides promising therapeutic effects.
Zhang et al. (2019) designed and synthesized a series of (3-hydroxypyridin-4-one)-coumarin hybrids (80) targeting iron ion and monoamine oxidase B (MAO-B) cooperatively for the treatment of AD. The biological evaluation showed most compounds exhibited good iron-chelating effects (pFe3+ ≈ 18) as well as moderate to excellent anti-MAO-B activities. As summarized in Figure 10A, the SAR was revealed and potency was enhanced by modification at C-7 of the coumarin ring. In particular, compound 80a possessed the most promising MAO-B inhibitory activity (IC50 = 14.7 nM) and good U251 cell protective effect which contributed to the amelioration of cognitive dysfunction in a Morris water maze test model. Moreover, molecular docking analysis elucidated 80a could bind to both the entrance and the substrate cavity of MAO-B, indicating that the development of agents with multi-target-directed targets was a useful strategy for treating AD.
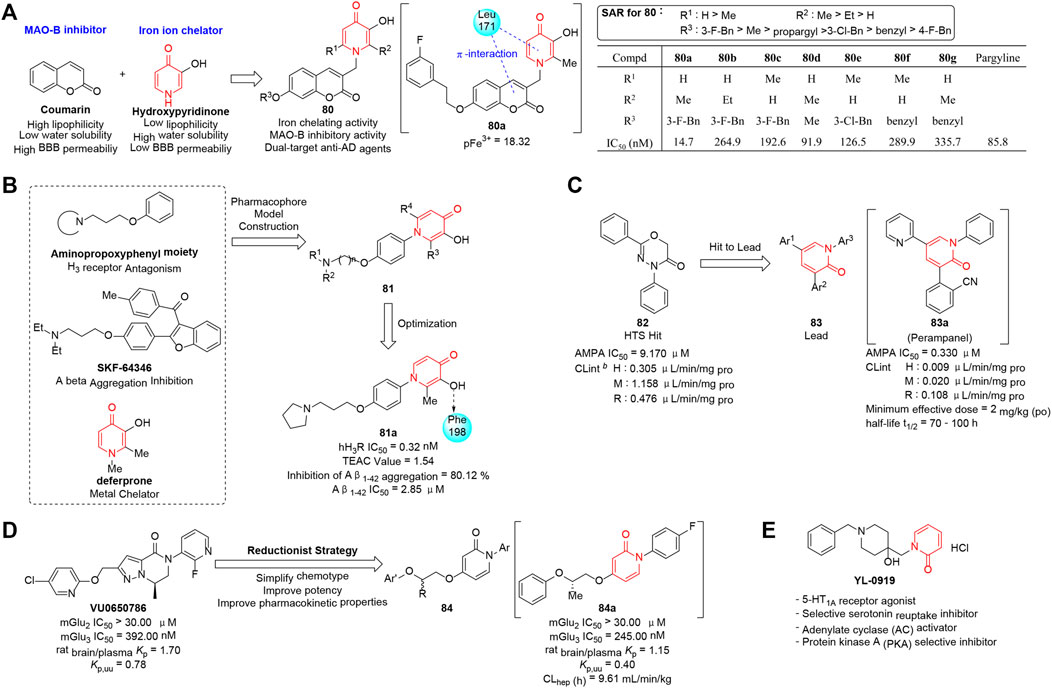
FIGURE 10. (A) Strategy and SAR of dual-target anti-AD agents (Zhang et al., 2019). (B) Discovery of pyridinone-based anti-AD agents. (C) Optimization of pyridinone-based AMPA-type glutamate receptor antagonists. (D) N-aryl phenoxyethoxypyridinones as highly selective mGlu3 NAMs. (E) Structure of YL-0919.
As quadruple functional agents for the therapy of AD, a series of novel 1-phenyl-3-hydroxy-4-pyridinone derivatives (81) targeting H3 receptor, Aβ peptide, metal ion, and radical simultaneously were reported by Hu’s group (2016). As shown in Figure 10B, compound 81 were designed by combining deferiprone (DFP) and aminopropoxyphenyl into one molecule through rational pharmacophore model construction. Biological evaluation indicated that 81a exhibited the most potent H3 receptor antagonistic activity (IC50 = 0.32 nM), efficient radical scavenging activity (Trolox-equivalent antioxidant capacity value = 1.54), excellent Aβ self-aggregation inhibitory activity (IC50 = 2.85 μM), and good chelating properties with copper and iron. The four functions mentioned earlier promoted 81a to be a potential candidate for the AD treatment. Moreover, an in vivo study demonstrated 81a possessed appropriate pharmacokinetic profiles and the proper ability to permeate the blood–brain barrier (BBB) efficiently. This work provided an attractive strategy to develop potential multifunctional agents with various mechanisms.
Glutamate (Niswender and Conn 2010), the primary excitatory neurotransmitter in the central nervous system, is associated with a series of neurological diseases including epilepsy which is triggered by high concentration of glutamate in the brain. Therefore, glutamate receptor antagonists are explored to block the corresponding gating. Hibi et al. (2012) discovered 82 as an initial hit by applying the high-throughput screening strategy, and then chemical modification was carried out to improve the stability. Thus, 82 was transformed to 1,3,5-triaryl-1H-pyridin-2-one derivatives (83) that could non-competitively inhibit the activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) which is known as one type of ionotropic glutamate receptors (iGluRs). As shown in Figure 10C, an activity-guided SAR optimization study was performed by manipulating individual aryl groups at 1,3,5-positions of pyridinone ring, and 2-(2-oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydropyridin-3-yl)benzonitrile (83a, perampanel) was revealed be an extremely potent non-competitive AMPA receptor antagonist (IC50 = 60 nM). In addition, 83a possessed powerful in vivo activity in preclinical AMPA-induced seizure models (ED50 = 0.47–1.60 mg/kg, minimum effective dose of 2 mg/kg po) and favorable pharmacokinetic characteristics in early clinical studies (CLint = 0.009 μL min−1 mg−1). It is gratifying that the compound has been approved by the FDA for treating patients with epilepsy in 2012.
G-protein-coupled metabotropic glutamate receptors (mGluRs) have emerged as validated therapeutic targets with potential for modulating prefrontal cortex (PFC) glutamate transmission. Engers et al. (2017) reported a series of pyrimidinone and pyridinone derivatives (84) as mGlu3 negative allosteric modulators (NAMs). The basic design of the scaffold was originated from VU0650786 which emerged as a valuable mGlu3 NAM in vivo probe with a complex molecular structure, cumbersome multistep synthetic procedure, and unsatisfactory physicochemical properties. To optimize the scaffold, a more flexible linker and an N-arylpyrimidine or N-arylpyridine head-piece were introduced through a reductionist strategy, and 12 analogs were synthesized and tested for their potency (Figure 10D). Finally, 84a was identified as the most effective mGlu3 NAM (IC50 = 245 nM) with excellent CNS penetration (rat brain/plasma Kp = 1.2) and displayed highly selective in vivo antidepressant activity (inactive at mGlu1,4,6,7,8) in a mouse tail suspension test (MED = 3 mg/kg).
5-HT1A receptor (Brummelte et al., 2017; Cortes-Altamirano et al., 2018; Żmudzka et al., 2018), an inhibitory G-protein-coupled receptor in the CNS, is implicated in a number of behavioral traits associated with anxiety and depression-like disorders. Improvement of 5-HT levels in the synaptic gap by regulating activity of 5-HT1A receptor and suppressing 5-HT reuptake provides promising therapeutic targets for the treatment of such mood disorders (Kaufman et al., 2016; Brummelte et al., 2017). Li’s group (2014) reported a novel antidepressant candidate YL-0919 with both 5-HT reuptake inhibitory effect and 5-HT1A receptor emotional activity (Figure 10E). The antidepressant effect and mechanism were evaluated by the animal model test and cAMP assays, and the results demonstrated that YL-0919 could remarkably reverse the depressive-like behaviors in a dose-dependent manner through the activation of the AC-cAMP-PKA signal-transduction passway in the frontal cortex. Like vilazodone, an FDA-approved drug sharing the same mechanisms as YL-0919, drugs with dual pharmacological effects on both serotonin transporter and 5-HT1A receptor have become the principal focus of research and development of novel antidepressant.
Tumor necrosis factor-α (TNF-α) is an important pro-inflammatory cytokine (Dingar et al., 2010), and its elevated level correlates with a broad range of inflammatory diseases such as rheumatoid arthritis (RA), psoriasis, and Crohn’s disease. Among a number of stress-activated protein kinases associated with TNF-α, p38α is validated as a potential therapeutic target (Wolańska et al., 2010; Ma et al., 2016; Slobodnyuk et al., 2019) that could act effectively on the cytokine expression and subsequently alleviate the inflammation. Selness et al. (2011) developed N-aryl pyridinones as selective p38α inhibitor chemotypes, which were identified from the previous discovery of the initial lead SC-25028 screened from a high-throughput full file screen. Structural modification was based on the key binding contacts between SC-25028 and p38α, and a series of N-aryl derivatives (85) were synthesized and evaluated for inhibitory activity, stability, and pharmacokinetic profiles (Figure 11A). The cascade assay findings proved that most analogs demonstrated robust inhibitory activity against p38α at nanomolar concentrations. In particular, compound 85a showed remarkable cellular activity (IC50 = 12 nM) with satisfactory metabolic stability, and exhibited potency in both acute and chronic models of inflammation.
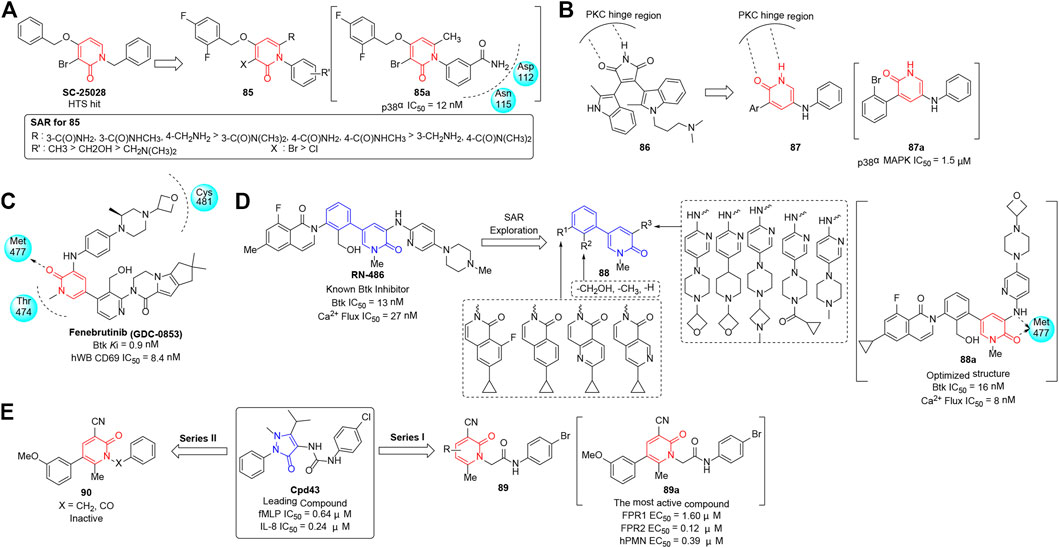
FIGURE 11. (A) N-aryl pyridinones with selective p38α inhibitory activity. (B) Pyridin-2(1H)-one analogs with p38α MAPK inhibitory activity. (C) Structure of fenebrutinib and its binding mode to Btk (PDB code 5VFI). (D) Optimization and PK properties study for pyridinone-based Btk inhibitors. (E) Two series of pyridinone-based FPR agonists.
As an important subtribe of mitogen-activated protein kinases (MAPKs), p38α MAPK has been proven to contribute to pain hypersensitivity (Jensen and Finnerup 2014) associated with tactile inputs in the spinal dorsal horn (SDH) or medullary dorsal horn (MDH). Therefore, inhibitors of p38α MAPK are proposed to alleviate chronic pain syndromes including neuropathic and inflammatory pain (Liu et al., 2017; He et al., 2018; Canovas and Nebreda 2021). Previously, (2-methyl-indol)-BIM-1 (86) was recognized as a potent protein kinase C (PKC) inhibitor by Grant’s group. Visseq et al. (2020) reported a novel series of 3,5-disubstituted pyridin-2(1H)-ones (87), which were designed based on the structure of 86 whose central core formed H-bond interactions with the hinge region of PKC (Figure 11B). Antiallodynic activity was evaluated for the synthesized compounds using Complete Freund’s Adjuvant (CFA) models, and most of the derivatives prevented the development of mechanical allodynia. SAR showed that the 3-position of the pyridinone moiety tolerated bulky groups, while substitution at the 2-position by a bromine atom or an ethoxycarbonyl group was favorable. Finally, compound 87a was suggested as a candidate p38α MAPK inhibitor (IC50 = 1.5 μM) in terms of the rapid response ability to reverse facial neuropathic allodynia in rats.
Bruton’s tyrosine kinase (Btk), a Tec family tyrosine kinase, is a critical effector molecule in hematopoietic cells and plays multiple roles in B-cell development (Crawford et al., 2018). Overexpression of Btk may lead to B-cell lymphoproliferative disorders (Pal Singh et al., 2018), such as hematologic malignancies and autoimmune diseases including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and multiple sclerosis (MS). Therefore, the kinase has been identified as an emerging therapeutic target for the treatment of cancer and immune system dysfunction (Pal Singh et al., 2018). Decades of research have advanced several Btk inhibitors for clinical evaluation; among them, fenebrutinib (GDC-0853), structurally based on an amino pyridone hinge scaffold (Johnson et al., 2016), is a notable one that binds to the ATP-binding site of Btk without interacting with Cys481 (Figure 11C). In addition, fenebrutinib (Young et al., 2015) possesses high selectivity, potential efficacy, and favorable pharmacokinetic (PK) and pharmacodynamic (PD) profiles, and is currently under phase 2 investigation for patients with RA (NCT02833350).
RN486, a novel reversible Btk inhibitor discovered by Roche, exhibited perfect activity and high selectivity, and displayed satisfactory inhibition of arthritis in animal models. As illustrated in Figure 11D, Zhao et al. (2015) took advantage of 5-phenylpyridin-2(1H)-one skeleton which was derived from RN486 as a privileged scaffold, and modified it on R1, R2, and R3, thus investigating a novel series of reversible Btk inhibitors (88) in 2015. The bioactivity was evaluated by measuring the in vitro enzymatic and cellular (Ramos cell) inhibition, and the SAR showed that the hydroxyl group on R2 was crucial for good potency. Moreover, derivative 88a exhibited excellent PK properties and perfect in vivo efficacy of inhibiting arthritis in the collagen-induced arthritis (CIA) model.
Formyl peptide receptor (FPR), a target for specific pro-resolving mediators (SPMs), acts in dual effects by performing pro-resolving and anti-inflammatory activities, and contributes to the resolution of inflammation (Stama et al., 2017). Previously, Cpd43 (Odobasic et al., 2018), a mixed FPR1/FPR2 agonist, was recognized as an effective therapeutic agent for rheumatoid arthritis developed by Amgen. Crocetti et al. (2020) expanded the imidazolone of Cpd43 to pyridinone scaffold and reported a series of derivatives (89) containing a 4-bromophenylacetamide fragment which was proven to be crucial for the potency. They evaluated the FPR emotional activity of the synthesized compounds by measuring intracellular Ca2+ flux in transfected cells and revealed that the majority of analogs exhibited effective mixed FPR agonist activity in the submicromolar/micromolar range. A partial SAR was revealed in Series I (89), where the position and types of substituents at pyridinone rings had a great influence on the properties of FPR excitatory, while the replacement of 4-bromophenylacetamide chain in Series II (90) led to completely inactive compounds (Figure 11E). In particular, 89a was validated as the most active structure, with EC50 value of 1.60 μM for FPR1 and 0.12 μM for FPR2. It could also directly induce neutrophil chemotaxis and inhibit WKYMVm-induced chemotaxis via desensitization. In vivo evaluation of 89a exhibited similar efficacy with ibuprofen and a longer lasting effect in a rat model of rheumatoid arthritis.
Dysregulation of blood coagulation results in various thromboembolic disorders with inappropriate formation of fibrin, subsequently causing tissue ischemia (Chapin and Hajjar 2015). Serine proteases including factor Xa (FXa), factor XIa (FXIa), factor VII (FVII)/tissue factor (TF), and thrombin play a pivotal role in the amplification of thrombin production and are considered potential targets for anticoagulant therapy (Melnikova 2009; Bagoly et al., 2012). The pyridinone template has been proven to be an appropriate peptidomimetic template in the design of thrombin inhibitors and tissue factor/factor VIIa inhibitors due to its ability to mimic the hydrogen bond array of the backbone of peptide inhibitors and provides a good fit of the inhibitor in the enzyme active site. With the intention to improve the receptor binding affinity and selectivity, Kranjc et al. (2005) reported a series of novel thrombin inhibitors (91) containing a pyridinone core and (±)-4,5,6,7-tetrahydro-2H-indazol-5-ylmethanamine as P1 arginine side-chain mimetics. Each compound’s in vitro inhibitory potency was measured by amidolytic enzyme assay, and a partial SAR shown in Figure 12A indicated that the binding affinity was closely related to the absolute configuration. Finally, (+)-enantiomer 92 (thrombin Ki = 0.047 μM) was optimal and the X-ray co-crystal structure of 92 bound to human thrombin revealed its bicyclic ring fit well into the S1 pocket, while the central aromatic P2 core and the sulfonyl group exhibited only weak influence on binding of the inhibitor to the thrombin active site.
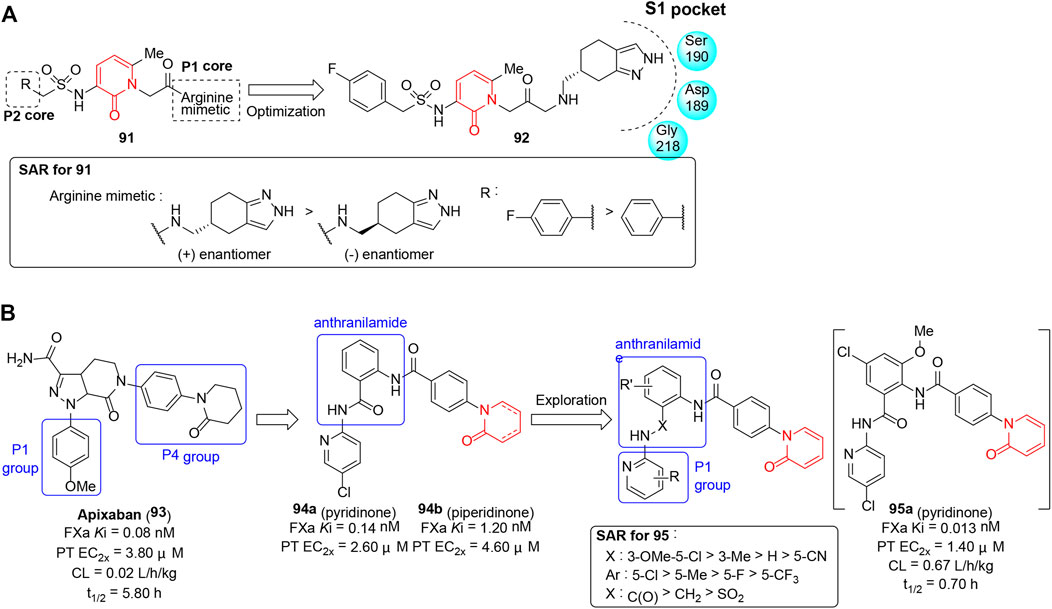
FIGURE 12. (A) SAR of pyridinone as thrombin inhibitors. (B) Pyridinones with FXa inhibitory activity.
Corte et al. (2008) reported anthranilamide-pyridinone hybrids (95) as selective FXa inhibitors. Initially, hits (94) were discovered by introducing the anthranilamide scaffold into the clinical drug apixaban and incorporating the phenyl piperidinone or pyridinone in P4 group (Figure 12B). SAR revealed incorporation of the unsaturated version, the phenyl pyridinone which formed an edge to face interaction with W215, and was appropriately sandwiched between Y99 and F174; it promoted 94a as a potent fXa inhibitor with similar in vitro activity to apixaban. Further structural optimization which focused on the exploration of P1 group and anthranilamide scaffold generated 95a as a novel fXa inhibitor that showed the best profiles in terms of potency, selectivity, and oral bioavailability. However, 95a exhibited a shorter in vivo half-life than apixaban in a dog pharmacokinetics study due to its higher clearance.
Plasmodium malaria (Lubell et al., 2014) is a prevalent and devastating parasitic disease due to its high virulence and drug resistance. As a consequence, it is an urgent requirement for the development of new antimalarial drugs with novel chemotypes. Cytochrome bc1 (Barton et al., 2010; Fisher et al., 2020) has played a prominent part in mitochondrial respiratory chain of Plasmodium falciparum. Hence, specific impairment of mitochondrion by binding to the site of cytochrome bc1 could provide an attractive option for antimalarial drugs. In this regard, Leon et al. (2008) reported the synthesis and biological evaluation of a series of 4(1H)-pyridinone hybrids (96) which were derived from anti-coccidial drug clopidol containing the same core developed by GSK. Structurally, the desired inhibitors were afforded via incorporation of lipophilic side chains into clopidol, yielding a number of derivatives with significant improvement in potency (Figure 13A). Candidate compound 96a exhibited 500-fold increased in vitro activity compared to clopidol and selectively inhibited respiration by acting upon the cytbc1 complex. Although the poor solubility and oral bioavailability of derivatives did not meet the requirements for therapeutic use, the research has encouraged the further exploration of this series as potential antimalarials.
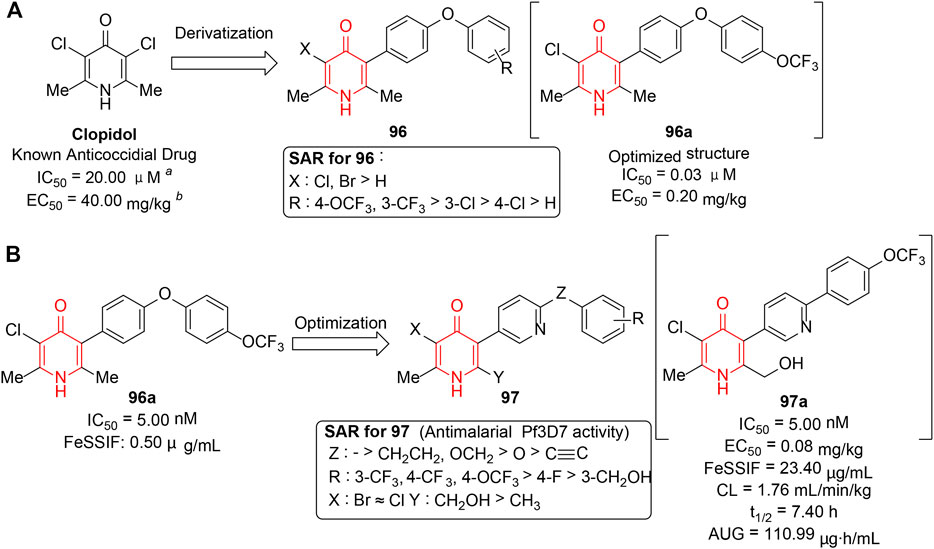
FIGURE 13. (A) Design of 4(1H)-pyridinone hybrids based on a known active ligand. (B) Antimalarial SARs of 5-pyridinyl-4(1H)-pyridinone derivatives.
In 2018, the same group (Bueno et al., 2018) performed the follow-up exploration starting from the previously developed antagonists 96. A series of novel “hybrid” polar 4(1H)-pyridinone derivatives (97) were obtained by introducing pyridine rings into lipophilic side chains or attaching polar moieties such as hydroxymethyl group to the 4(1H)-pyridinone cores. The results revealed that most new compounds displayed improved pharmacokinetic profiles including physicochemical properties and oral bioavailabilities, and maintained excellent potency as 96a in vitro and in vivo. The selected results for partial SAR are summarized in Figure 13B, which showed that 1) rigid linkers between two aromatic rings exhibited better activity than the flexible ones; 2) as for phenyl substituents, electron-withdrawing groups such as CF3 or OCF3 enhanced activity; and 3) the effect of the type of halogens at C3-pyridinone could be negligible. Eventually, 97a was suggested as the most efficacious antimalarial, with a long half-life and a high AUC. The novel compounds widened the scope of the antimalarial 4(1H)-pyridinones available, thus opening new ways for the chemical exploration of this exciting family of antimalarials.
Heart failure (Bueno et al., 2018) is considered to be the major cause of death in patients with cardiac disease. Among therapeutic targets strengthening myocardial contraction, phosphodiesterase-3 (PDE3) is a validated one that could increase calcium influx in cardiac myocytes and trigger positive inotropic effects, and agents such as milrinone and amrinone have been approved for clinical use (Thompson et al., 2007). Pietrangelo et al. (2010) reported a series of 2(1H)-pyridinone (99) as analogs of milrinone, in which the pyridyl moiety was replaced with an ester or amide group based on the docking study between PDE3 crystal structure and published antagonisms (98a and 98b). In order to evaluate the efficiency of 99, the degree of calcium channel activation during the plasma membrane depolarization in H9C2 cardiomyocytes was evaluated through the calcium imaging assay, and partial SARs revealed amide derivatives exhibited more potent activity than esters (Figure 14). The authors attributed this to the better resistance of amides under hydrolysis conditions, while the esters were probably cleaved to afford the inactive acid. As a result, among milrinone analogs, only amide derivatives could support intracellular [Ca2+] influx following chemical depolarization.
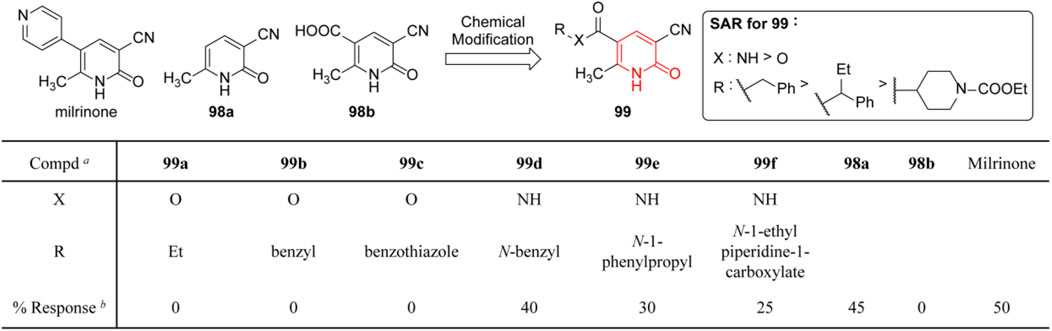
FIGURE 14. Cardiotonic SARs of 3-cyano-6-methyl-2(1H)-pyridinone (Pietrangelo et al., 2010). a Percentage of differentiated H9C2 that prolonged the KCl-dependent [Ca2+]i transient in the presence of new compounds and milrinone. b Concentration of 10 μM.
Overactive bladder (OAB) is a symptom syndrome of urinary urgency which is generally accompanied by frequency and nocturia (Abrams et al., 2003). Prostaglandin EP3 receptor (Chen and Kuo 2019) could regulate the excitability of bladder smooth muscle and is considered a potential target for OAB. Jin et al. (2010) reported a series of 3-oxazolidinedione-6-aryl-pyridinones (102) as selective EP3 receptor antagonists, generated by the optimization of 100 and 101 which were identified through the high-throughput screening (HTS) method. A thorough investigation on 101 indicated the lead compound exhibited excellent pharmacokinetic profiles and robust potency in several overactive bladder models (Figure 15). But the unsubstituted naphthyl moiety of 101 could be oxidized to generate reactive metabolites in glutathione trapping studies. To address such potential bioactivation liability, a substituted aryl group was introduced to the lead compound, resulting in the discovery of compound 102a with improved stability and excellent efficacy. In addition, these highly potent, selective, and orally bioavailable compounds were beneficial tool compounds for developing and validating potential therapeutic benefits arising from selective EP3 inhibition.
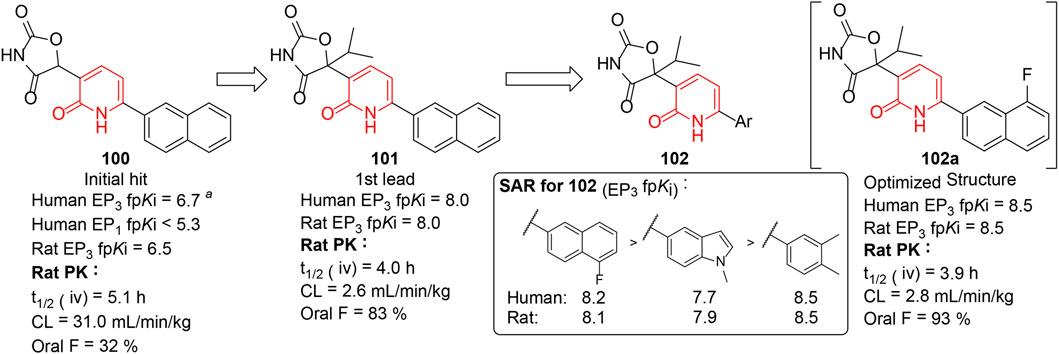
FIGURE 15. EP3 inhibitory activities of 3-oxazolidinedione-6-aryl-pyridinones (Jin et al., 2010). a Value of functional pKi(fpKi) was obtained from a EP3 fluorometric imaging plate reader (FLIPR) assay.
In summary, we described significant roles that pyridinone scaffolds played in medicinal chemistry owing to their wide range of pharmacological properties. Most of the drug discovery projects presented in this work benefited from the chemical diversifiability and convenient synthesis method of target groups. Structurally, N1, C3, C5, and C6 of the pyridinone scaffold could be easily functionalized via C-H or N-H activation and produces corresponding pyridinone-containing compounds. These compounds exhibit various biological activities in low concentrations (often submicromolar), such as cytotoxic, antibacterial, anti-inflammatory, antiviral, antimalarial, anticoagulant, and psychotropic activities, and some have known SAR and mechanisms of action (their effect on certain enzymes, receptors, etc.), which makes them potentially useful in pharmaceutic research. In addition, the pyridinone moiety serves as key binders between two pharmacophores, and improves pharmacokinetics by altering the solubility and selectively interacting with the enzyme’s binding site. The ongoing studies covering various fields of medicinal chemistry have also revealed several small pyridinone analogs with notable biological activity, and some of them are under clinical trials and possess brilliant application prospects. These advancements contribute to an in-depth understanding of the potential of this biologically enriched scaffold and pave the way to apply the prospective novel pyridinone-based derivatives for further rational development in drug discovery.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This work was supported by grants from the Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province (Grant No. ARRLKF20-06).
Abaszadeh, M., Sheibani, H., and Saidi, K. (2009). The Reaction of (Chlorocarbonyl)phenyl Ketene with Enaminones: A Novel Synthesis of Some 5-Acyl-4-Hydroxy-2-(1h)-Pyridinones and 7-Hydroxy-5-Oxo-1,4-Diazepin Derivative. J. Heterocyclic Chem. 46, 96–99. doi:10.1002/jhet.14
Abdo, M., Zhang, Y., Schramm, V. L., and Knapp, S. (2010). Electrophilic Aromatic Selenylation: New OPRT Inhibitors. Org. Lett. 12, 2982–2985. doi:10.1021/ol1010032
Abou-Elkhair, R. A. I., Moustafa, A. H., Haikal, A. Z., and Ibraheem, A. M. (2014). Synthesis and Biological Evaluation of 2-oxonicotinonitriles and 2-oxonicotinonitrile Based Nucleoside Analogues. Eur. J. Med. Chem. 74, 388–397. doi:10.1016/j.ejmech.2013.12.055
Abrams, P., Cardozo, L., Fall, M., Griffiths, D., Rosier, P., Ulmsten, U., et al. (2003). The Standardisation of Terminology in Lower Urinary Tract Function: Report from the Standardisation Sub-committee of the International Continence Society. Urology 61, 37–49. doi:10.1016/s0090-4295(02)02243-4
Agostinis, P., Berg, K., Cengel, K. A., Foster, T. H., Girotti, A. W., Gollnick, S. O., et al. (2011). Photodynamic Therapy of Cancer: an Update. CA: A Cancer J. Clinicians 61, 250–281. doi:10.3322/caac.20114
Alafeefy, A. M., Alqasoumi, S. I., Ashour, A. E., Masand, V., Al-Jaber, N. A., Ben Hadda, T., et al. (2012). Quinazoline-tyrphostin as a New Class of Antitumor Agents, Molecular Properties Prediction, Synthesis and Biological Testing. Eur. J. Med. Chem. 53, 133–140. doi:10.1016/j.ejmech.2012.03.044
Ang, Y.-S., Tsai, S.-Y., Lee, D.-F., Monk, J., Su, J., Ratnakumar, K., et al. (2011). Wdr5 Mediates Self-Renewal and Reprogramming via the Embryonic Stem Cell Core Transcriptional Network. Cell 145, 183–197. doi:10.1016/j.cell.2011.03.003
Atkins, J. H., and Gershell, L. J. (2002). Selective Anticancer Drugs. Nat. Rev. Drug Discov. 1, 491–492. doi:10.1038/nrd842
Bagoly, Z., Koncz, Z., Hársfalvi, J., and Muszbek, L. (2012). Factor XIII, Clot Structure, Thrombosis. Thromb. Res. 129, 382–387. doi:10.1016/j.thromres.2011.11.040
Bai, F., Hu, D., Liu, Y., and Wei, L. (2018). One-Pot and Multicomponent Synthesis of N-Substituted-4-Hydroxyl-2-Pyridones. Chin. J. Org. Chem. 38, 2054–2059. doi:10.6023/cjoc201801015
Bailey, J. K., Fields, A. T., Cheng, K., Lee, A., Wagenaar, E., Lagrois, R., et al. (2015). WD Repeat-Containing Protein 5 (WDR5) Localizes to the Midbody and Regulates Abscission. J. Biol. Chem. 290, 8987–9001. doi:10.1074/jbc.M114.623611
Barton, V., Fisher, N., Biagini, G. A., Ward, S. A., and O’Neill, P. M. (2010). Inhibiting Plasmodium Cytochrome Bc1: a Complex Issue. Curr. Opin. Chem. Biol. 14, 440–446. doi:10.1016/j.cbpa.2010.05.005
Battah, S., Hider, R. C., MacRobert, A. J., Dobbin, P. S., and Zhou, T. (2017). Hydroxypyridinone and 5-Aminolaevulinic Acid Conjugates for Photodynamic Therapy. J. Med. Chem. 60, 3498–3510. doi:10.1021/acs.jmedchem.7b00346
Benjahad, A., Courté, K., Guillemont, J., Mabire, D., Coupa, S., Poncelet, A., et al. (2004). 4-benzyl- and 4-Benzoyl-3-Dimethylaminopyridin-2(1h)-Ones, a New Family of Potent Anti-HIV Agents: Optimization and In Vitro Evaluation against Clinically Important HIV Mutant Strains. J. Med. Chem. 47, 5501–5514. doi:10.1021/jm0407658
Benjahad, A., Oumouch, S., Guillemont, J., Pasquier, E., Mabire, D., Andries, K., et al. (2007). Structure-activity Relationship in the 3-Iodo-4-Phenoxypyridinone (IOPY) Series: The Nature of the C-3 Substituent on Anti-HIV Activity. Bioorg. Med. Chem. Lett. 17, 712–716. doi:10.1016/j.bmcl.2006.10.082
Bergeron, R. J., McManis, J. S., Franklin, A. M., Yao, H., and Weimar, W. R. (2003). Polyamine−Iron Chelator Conjugate. J. Med. Chem. 46, 5478–5483. doi:10.1021/jm0302694
Bhattacharya, D., and Thio, C. L. (2010). Review of Hepatitis B Therapeutics. Clin. Infect. Dis. 51, 1201–1208. doi:10.1086/656624
Bobrowski, T., Melo-Filho, C. C., Korn, D., Alves, V. M., Popov, K. I., Auerbach, S., et al. (2020). Learning from History: Do Not Flatten the Curve of Antiviral Research!. Drug Discov. Today 25, 1604–1613. doi:10.1016/j.drudis.2020.07.008
Borger, D. R., Tanabe, K. K., Fan, K. C., Lopez, H. U., Fantin, V. R., Straley, K. S., et al. (2012). Frequent Mutation of Isocitrate Dehydrogenase (IDH)1 and IDH2 in Cholangiocarcinoma Identified through Broad-Based Tumor Genotyping. Oncologist 17, 72–79. doi:10.1634/theoncologist.2011-0386
Brackett, C. M., and Gollnick, S. O. (2011). Photodynamic Therapy Enhancement of Anti-tumor Immunity. Photochem. Photobiol. Sci. 10, 649–652. doi:10.1039/c0pp00354a
Breda, A., Machado, P., Rosado, L. A., Souto, A. A., Santos, D. S., and Basso, L. A. (2012). Pyrimidin-2(1H)-ones Based Inhibitors of Mycobacterium tuberculosis Orotate Phosphoribosyltransferase. Eur. J. Med. Chem. 54, 113–122. doi:10.1016/j.ejmech.2012.04.031
Breda, A., Rosado, L. A., Lorenzini, D. M., Basso, L. A., and Santos, D. S. (2012). Molecular, Kinetic and Thermodynamic Characterization of Mycobacterium Tuberculosisorotate Phosphoribosyltransferase. Mol. Biosyst. 8, 572–586. doi:10.1039/c1mb05402c
Brondel, N., Renoux, B., and Gesson, J.-P. (2006). New Strategy for the Synthesis of Phosphatase Inhibitors TMC-69-6H and Analogs. Tetrahedron Lett. 47, 9305–9308. doi:10.1016/j.tetlet.2006.10.102
Brummelte, S., Mc Glanaghy, E., Bonnin, A., and Oberlander, T. F. (2017). Developmental Changes in Serotonin Signaling: Implications for Early Brain Function, Behavior and Adaptation. Neuroscience 342, 212–231. doi:10.1016/j.neuroscience.2016.02.037
Bryan, M. C., and Rajapaksa, N. S. (2018). Kinase Inhibitors for the Treatment of Immunological Disorders: Recent Advances. J. Med. Chem. 61, 9030–9058. doi:10.1021/acs.jmedchem.8b00667
Bueno, J. M., Calderon, F., Chicharro, J., De la Rosa, J. C., Díaz, B., Fernández, J., et al. (2018). Synthesis and Structure-Activity Relationships of the Novel Antimalarials 5-Pyridinyl-4(1h)-Pyridones. J. Med. Chem. 61, 3422–3435. doi:10.1021/acs.jmedchem.7b01256
Canovas, B., and Nebreda, A. R. (2021). Diversity and Versatility of P38 Kinase Signalling in Health and Disease. Nat. Rev. Mol. Cel Biol 22, 346–366. doi:10.1038/s41580-020-00322-w
Cao, Y., Zhang, Y., Wu, S., Yang, Q., Sun, X., Zhao, J., et al. (2015). Synthesis and Biological Evaluation of Pyridinone Analogues as Novel Potent HIV-1 NNRTIs. Bioorg. Med. Chem. 23, 149–159. doi:10.1016/j.bmc.2014.11.012
Caravella, J. A., Lin, J., Diebold, R. B., Campbell, A.-M., Ericsson, A., Gustafson, G., et al. (2020). Structure-Based Design and Identification of FT-2102 (Olutasidenib), a Potent Mutant-Selective IDH1 Inhibitor. J. Med. Chem. 63, 1612–1623. doi:10.1021/acs.jmedchem.9b01423
Chapin, J. C., and Hajjar, K. A. (2015). Fibrinolysis and the Control of Blood Coagulation. Blood Rev. 29, 17–24. doi:10.1016/j.blre.2014.09.003
Chen, L. C., and Kuo, H. C. (2019). Pathophysiology of Refractory Overactive Bladder. Lower Urinary Tract Symptoms 11, 177–181. doi:10.1111/luts.12262
Chen, W., Chen, X., Li, D., Zhou, J., Jiang, Z., You, Q., et al. (2021). Discovery of DDO-2213 as a Potent and Orally Bioavailable Inhibitor of the WDR5-Mixed Lineage Leukemia 1 Protein-Protein Interaction for the Treatment of MLL Fusion Leukemia. J. Med. Chem. 64, 8221–8245. doi:10.1021/acs.jmedchem.1c00091
Cho, M. E., and Kopp, J. B. (2010). Pirfenidone: an Anti-fibrotic Therapy for Progressive Kidney Disease. Expert Opin. Investig. Drugs 19, 275–283. doi:10.1517/13543780903501539
Chun, Y. S., Ryu, K. Y., Ko, Y. O., Hong, J. Y., Hong, J., Shin, H., et al. (2009). One-pot Synthesis of 2-pyridones via Chemo- and Regioselective Tandem Blaise Reaction of Nitriles with Propiolates. J. Org. Chem. 74, 7556–7558. doi:10.1021/jo901642t
Clercq, E. D. (2004). Antivirals and Antiviral Strategies. Nat. Rev. Microbiol. 2, 704–720. doi:10.1038/nrmicro975
Corte, J. R., Fang, T., Pinto, D. J. P., Han, W., Hu, Z., Jiang, X.-J., et al. (2008). Structure-activity Relationships of Anthranilamide-Based Factor Xa Inhibitors Containing Piperidinone and Pyridinone P4 Moieties. Bioorg. Med. Chem. Lett. 18, 2845–2849. doi:10.1016/j.bmcl.2008.03.092
Cortes-Altamirano, J. L., Olmos-Hernandez, A., Jaime, H. B., Carrillo-Mora, P., Bandala, C., Reyes-Long, S., et al. (2018). Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 Receptors and Their Role in the Modulation of Pain Response in the Central Nervous System. Cn 16, 210–221. doi:10.2174/1570159x15666170911121027
Crawford, J. J., Johnson, A. R., Misner, D. L., Belmont, L. D., Castanedo, G., Choy, R., et al. (2018). Discovery of GDC-0853: A Potent, Selective, and Noncovalent Bruton's Tyrosine Kinase Inhibitor in Early Clinical Development. J. Med. Chem. 61, 2227–2245. doi:10.1021/acs.jmedchem.7b01712
Credille, C. V., Chen, Y., and Cohen, S. M. (2016). Fragment-Based Identification of Influenza Endonuclease Inhibitors. J. Med. Chem. 59, 6444–6454. doi:10.1021/acs.jmedchem.6b00628
Crocetti, L., Vergelli, C., Guerrini, G., Cantini, N., Kirpotina, L. N., Schepetkin, I. A., et al. (2020). Novel Formyl Peptide Receptor (FPR) Agonists with Pyridinone and Pyrimidindione Scaffolds that Are Potentially Useful for the Treatment of Rheumatoid Arthritis. Bioorg. Chem. 100, 103880. doi:10.1016/j.bioorg.2020.103880
Daar, E. S. (2008). Emerging Resistance Profiles of Newly Approved Antiretroviral Drugs. Top. HIV Med. 16, 110–116.
Daeyaert, F., de Jonge, M., Heeres, J., Koymans, L., Lewi, P., Vinkers, M. H., et al. (2004). A Pharmacophore Docking Algorithm and its Application to the Cross-Docking of 18 HIV-NNRTI's in Their Binding Pockets. Proteins 54, 526–533. doi:10.1002/prot.10599
De Clercq, E. (2001). New Developments in Anti-HIV Chemotherapy. Il Farmaco 56, 3–12. doi:10.1016/s0014-827x(01)01007-2
De Cock, K. M., Jaffe, H. W., and Curran, J. W. (2012). The Evolving Epidemiology of HIV/AIDS. Aids 26, 1205–1213. doi:10.1097/QAD.0b013e328354622a
Debnath, U., Verma, S., Jain, S., Katti, S. B., and Prabhakar, Y. S. (2013). Pyridones as NNRTIs against HIV-1 Mutants: 3D-QSAR and Protein Informatics. J. Comput. Aided Mol. Des. 27, 637–654. doi:10.1007/s10822-013-9667-1
Dias, A., Bouvier, D., Crépin, T., McCarthy, A. A., Hart, D. J., Baudin, F., et al. (2009). The Cap-Snatching Endonuclease of Influenza Virus Polymerase Resides in the PA Subunit. Nature 458, 914–918. doi:10.1038/nature07745
Dingar, D., Merlen, C., Grandy, S., Gillis, M.-A., Villeneuve, L. R., Mamarbachi, A. M., et al. (2010). Effect of Pressure Overload-Induced Hypertrophy on the Expression and Localization of P38 MAP Kinase Isoforms in the Mouse Heart. Cell Signal. 22, 1634–1644. doi:10.1016/j.cellsig.2010.06.002
Dörr, M., and Meggers, E. (2014). Metal Complexes as Structural Templates for Targeting Proteins. Curr. Opin. Chem. Biol. 19, 76–81. doi:10.1016/j.cbpa.2014.01.005
Drew, W. L. (2010). Cytomegalovirus Resistance Testing: Pitfalls and Problems for the Clinician. Clin. Infect. Dis. 50, 733–736. doi:10.1086/650463
Engers, J. L., Bollinger, K. A., Weiner, R. L., Rodriguez, A. L., Long, M. F., Breiner, M. M., et al. (2017). Design and Synthesis of N-Aryl Phenoxyethoxy Pyridinones as Highly Selective and CNS Penetrant mGlu3 NAMs. ACS Med. Chem. Lett. 8, 925–930. doi:10.1021/acsmedchemlett.7b00249
Fedorov, O., Castex, J., Tallant, C., Owen, D. R., Martin, S., Aldeghi, M., et al. (2015). Selective Targeting of the BRG/PB1 Bromodomains Impairs Embryonic and Trophoblast Stem Cell Maintenance. Sci. Adv. 1, e1500723. doi:10.1126/sciadv.1500723
Fisher, N., Meunier, B., and Biagini, G. A. (2020). The Cytochromebc1complex as an Antipathogenic Target. FEBS Lett. 594, 2935–2952. doi:10.1002/1873-3468.13868
Getlik, M., Smil, D., Zepeda-Velázquez, C., Bolshan, Y., Poda, G., Wu, H., et al. (2016). Structure-Based Optimization of a Small Molecule Antagonist of the Interaction between WD Repeat-Containing Protein 5 (WDR5) and Mixed-Lineage Leukemia 1 (MLL1). J. Med. Chem. 59, 2478–2496. doi:10.1021/acs.jmedchem.5b01630
Guillemont, J., Benjahad, A., Oumouch, S., Decrane, L., Palandjian, P., Vernier, D., et al. (2009). Synthesis and Biological Evaluation of C-5 Methyl Substituted 4-arylthio and 4-Aryloxy-3-Iodopyridin-2(1h)-One Type Anti-HIV Agents. J. Med. Chem. 52, 7473–7487. doi:10.1021/jm900802y
Hambley, T. W. (2007). Developing New Metal-Based Therapeutics: Challenges and Opportunities. Dalton Trans., 4929–4937. doi:10.1039/b706075k
Harada, K., Ferdous, T., Harada, T., Takenawa, T., and Ueyama, Y. (2017). Gimeracil Enhances the Antitumor Effect of Cisplatin in Oral Squamous Cell Carcinoma Cells In Vitro and In Vivo. Oncol. Lett. 14, 3349–3356. doi:10.3892/ol.2017.6602
Hare, S., Gupta, S. S., Valkov, E., Engelman, A., and Cherepanov, P. (2010). Retroviral Intasome Assembly and Inhibition of DNA Strand Transfer. Nature 464, 232–236. doi:10.1038/nature08784
Hartinger, C. G., and Dyson, P. J. (2009). Bioorganometallic Chemistry-From Teaching Paradigms to Medicinal Applications. Chem. Soc. Rev. 38, 391–401. doi:10.1039/b707077m
He, Y., She, H., Zhang, T., Xu, H., Cheng, L., Yepes, M., et al. (2018). p38 MAPK Inhibits Autophagy and Promotes Microglial Inflammatory Responses by Phosphorylating ULK1. J. Cel Biol 217, 315–328. doi:10.1083/jcb.201701049
Hewings, D. S., Rooney, T. P. C., Jennings, L. E., Hay, D. A., Schofield, C. J., Brennan, P. E., et al. (2012). Progress in the Development and Application of Small Molecule Inhibitors of Bromodomain-Acetyl-Lysine Interactions. J. Med. Chem. 55, 9393–9413. doi:10.1021/jm300915b
Hibi, S., Ueno, K., Nagato, S., Kawano, K., Ito, K., Norimine, Y., et al. (2012). Discovery of 2-(2-Oxo-1-Phenyl-5-Pyridin-2-Yl-1,2-Dihydropyridin-3-Yl)benzonitrile (Perampanel): A Novel, Noncompetitive α-Amino-3-hydroxy-5-methyl-4-isoxazolepropanoic Acid (AMPA) Receptor Antagonist. J. Med. Chem. 55, 10584–10600. doi:10.1021/jm301268u
Hohmann, A. F., Martin, L. J., Minder, J., Roe, J.-S., Shi, J., Steurer, S., et al. (2016). Abstract LB-206: A Bromodomain-Swap Allele Demonstrates that On-Target Chemical Inhibition of BRD9 Limits the Proliferation of Acute Myeloid Leukemia Cells. Cancer Res. 76. LB-206-LB-206. doi:10.1158/1538-7445.AM2016-LB-206
Jacinto Demuner, A., Moreira Valente, V. M., Almeida Barbosa, L. C., Rathi, A., Donohoe, T., and Thompson, A. (2009). Synthesis and Phytotoxic Activity of New Pyridones Derived from 4-Hydroxy-6-Methylpyridin-2(1h)-One. Molecules 14, 4973–4986. doi:10.3390/molecules14124973
Jacobsen, J. A., Fullagar, J. L., Miller, M. T., and Cohen, S. M. (2011). Identifying Chelators for Metalloprotein Inhibitors Using a Fragment-Based Approach. J. Med. Chem. 54, 591–602. doi:10.1021/jm101266s
Jamieson, E. R., and Lippard, S. J. (1999). Structure, Recognition, and Processing of Cisplatin−DNA Adducts. Chem. Rev. 99, 2467–2498. doi:10.1021/cr980421n
Javaid, Z. Z., el Kouni, M. H., and Iltzsch, M. H. (1999). Pyrimidine Nucleobase Ligands of Orotate Phosphoribosyltransferase from Toxoplasma Gondii. Biochem. Pharmacol. 58, 1457–1466. doi:10.1016/s0006-2952(99)00231-2
Jemal, A., Siegel, R., Xu, J. Q., and Ward, E. (2013). Cancer Statistics. Cancer 52, 1–24. doi:10.1002/caac.20073
Jensen, T. S., and Finnerup, N. B. (2014). Allodynia and Hyperalgesia in Neuropathic Pain: Clinical Manifestations and Mechanisms. Lancet Neurol. 13, 924–935. doi:10.1016/s1474-4422(14)70102-4
Jin, J., Morales-Ramos, Á., Eidam, P., Mecom, J., Li, Y., Brooks, C., et al. (2010). Novel 3-Oxazolidinedione-6-Aryl-Pyridinones as Potent, Selective, and Orally Active EP3 Receptor Antagonists. ACS Med. Chem. Lett. 1, 316–320. doi:10.1021/ml100077x
Johnson, A. R., Kohli, P. B., Katewa, A., Gogol, E., Belmont, L. D., Choy, R., et al. (2016). Battling Btk Mutants with Noncovalent Inhibitors that Overcome Cys481 and Thr474 Mutations. ACS Chem. Biol. 11, 2897–2907. doi:10.1021/acschembio.6b00480
Karatas, H., Townsend, E. C., Cao, F., Chen, Y., Bernard, D., Liu, L., et al. (2013). High-affinity, Small-Molecule Peptidomimetic Inhibitors of MLL1/WDR5 Protein-Protein Interaction. J. Am. Chem. Soc. 135, 669–682. doi:10.1021/ja306028q
Kashuba, A. D., Patterson, K. B., Dumond, J. B., and Cohen, M. S. (2012). Pre-exposure Prophylaxis for HIV Prevention: How to Predict success. The Lancet 379, 2409–2411. doi:10.1016/s0140-6736(11)61852-7
Katrizky, A., Ramsden, C. A., Joule, J. A., and Zhdankin, V. V. (2010). Handbook of Heterocyclic Chemistry. Third Edition, 41–42.
Kaufman, J., DeLorenzo, C., Choudhury, S., and Parsey, R. V. (2016). The 5-HT1A Receptor in Major Depressive Disorder. Eur. Neuropsychopharmacol. 26, 397–410. doi:10.1016/j.euroneuro.2015.12.039
Kim, K. S., Zhang, L., Schmidt, R., Cai, Z.-W., Wei, D., Williams, D. K., et al. (2008). Discovery of Pyrrolopyridine−Pyridone Based Inhibitors of Met Kinase: Synthesis, X-ray Crystallographic Analysis, and Biological Activities. J. Med. Chem. 51, 5330–5341. doi:10.1021/jm800476q
Kranjc, A., Mašič, L. P., Reven, S., Mikic, K., Preželj, A., Stegnar, M., et al. (2005). Novel Pyrazinone and Pyridinone Thrombin Inhibitors Incorporating Weakly Basic Heterobicyclic P1-Arginine Mimetics. Eur. J. Med. Chem. 40, 782–791. doi:10.1016/j.ejmech.2005.03.007
Lavanchy, D. (2004). Hepatitis B Virus Epidemiology, Disease burden, Treatment, and Current and Emerging Prevention and Control Measures. J. Viral Hepat. 11, 97–107. doi:10.1046/j.1365-2893.2003.00487.x
Le Quesne, J. P., Spriggs, K. A., Bushell, M., and Willis, A. E. (2010). Dysregulation of Protein Synthesis and Disease. J. Pathol. 220, 140–151. doi:10.1002/path.2627
Le Van, K., Cauvin, C., de Walque, S., Georges, B., Boland, S., Martinelli, V., et al. (2009). New Pyridinone Derivatives as Potent HIV-1 Nonnucleoside Reverse Transcriptase Inhibitors. J. Med. Chem. 52, 3636–3643. doi:10.1021/jm801438e
León, R., Garcia, A. G., and Marco-Contelles, J. (2013). Recent Advances in the Multitarget-Directed Ligands Approach for the Treatment of Alzheimer's Disease. Med. Res. Rev. 33, 139–189. doi:10.1002/med.20248
Lin, Z. G., Ren, W., Zhang, J. G., and Miao, P. (2003). Studies on Synthesis of 1-Amino-Y-Carboline Derivative. Chin. J. Med. Chem. 13, 267–269.
Liu, J., Wang, G.-H., Duan, Y.-H., Dai, Y., Bao, Y., Hu, M., et al. (2017). Modulation of the Nur77-Bcl-2 Apoptotic Pathway by P38α MAPK. Oncotarget 8, 69731–69745. doi:10.18632/oncotarget.19227
Liu, Z., Yao, Y., Kogiso, M., Zheng, B., Deng, L., Qiu, J. J., et al. (2014). Inhibition of Cancer-Associated Mutant Isocitrate Dehydrogenases: Synthesis, Structure-Activity Relationship, and Selective Antitumor Activity. J. Med. Chem. 57, 8307–8318. doi:10.1021/jm500660f
Louie, A. Y., and Meade, T. J. (1999). Metal Complexes as Enzyme Inhibitors. Chem. Rev. 99, 2711–2734. doi:10.1021/cr9804285
Lubell, Y., Dondorp, A., Guérin, P. J., Drake, T., Meek, S., Ashley, E., et al. (2014). Artemisinin Resistance - Modelling the Potential Human and Economic Costs. Malar. J. 13, 452. doi:10.1186/1475-2875-13-452
Lv, Z., Sheng, C., Wang, T., Zhang, Y., Liu, J., Feng, J., et al. (2010). Design, Synthesis, and Antihepatitis B Virus Activities of Novel 2-pyridone Derivatives. J. Med. Chem. 53, 660–668. doi:10.1021/jm901237x
Ma, K., Zhang, H., and Baloch, Z. (2016). Pathogenetic and Therapeutic Applications of Tumor Necrosis Factor-α (TNF-α) in Major Depressive Disorder: A Systematic Review. Ijms 17, 733. doi:10.3390/ijms17050733
Maeda, K., Das, D., Kobayakawa, T., Tamamura, H., and Takeuchi, H. (2019). Discovery and Development of Anti-HIV Therapeutic Agents: Progress towards Improved HIV Medication. Ctmc 19, 1621–1649. doi:10.2174/1568026619666190712204603
Martin, L. J., Koegl, M., Bader, G., Cockcroft, X.-L., Fedorov, O., Fiegen, D., et al. (2016). Structure-Based Design of an In Vivo Active Selective BRD9 Inhibitor. J. Med. Chem. 59, 4462–4475. doi:10.1021/acs.jmedchem.5b01865
Mbonye, U., and Karn, J. (2014). Transcriptional Control of HIV Latency: Cellular Signaling Pathways, Epigenetics, Happenstance and the hope for a Cure. Virology 454-455, 328–339. doi:10.1016/j.virol.2014.02.008
McElroy, W. T., and DeShong, P. (2003). Siloxane-based Cross-Coupling of Bromopyridine Derivatives: Studies for the Synthesis of Streptonigrin and Lavendamycin. Org. Lett. 5, 4779–4782. doi:10.1021/ol0357503
Meggers, E. (2009). Targeting Proteins with Metal Complexes. Chem. Commun., 1001–1010. doi:10.1039/b813568a
Mendoza-Ferri, M. G., Hartinger, C. G., Mendoza, M. A., Groessl, M., Egger, A. E., Eichinger, R. E., et al. (2009a). Transferring the Concept of Multinuclearity to Ruthenium Complexes for Improvement of Anticancer Activity. J. Med. Chem. 52, 916–925. doi:10.1021/jm8013234
Mendoza-Ferri, M. G., Hartinger, C. G., Nazarov, A. A., Eichinger, R. E., Jakupec, M. A., Severin, K., et al. (2009b). Influence of the Arene Ligand, the Number and Type of Metal Centers, and the Leaving Group on the In Vitro Antitumor Activity of Polynuclear Organometallic Compounds. Organometallics 28, 6260–6265. doi:10.1021/om900715j
Niewerth, M., Kunze, D., Seibold, M., Schaller, M., Korting, H. C., and Hube, B. (2003). Ciclopirox Olamine Treatment Affects the Expression Pattern of Candida Albicans Genes Encoding Virulence Factors, Iron Metabolism Proteins, and Drug Resistance Factors. Antimicrob. Agents Chemother. 47, 1805–1817. doi:10.1128/AAC.47.6.1805-1817.2003
Niswender, C. M., and Conn, P. J. (2010). Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322. doi:10.1146/annurev.pharmtox.011008.145533
Novais, Â., Moniz, T., Rebelo, A. R., Silva, A. M. G., Rangel, M., and Peixe, L. (2018). New Fluorescent Rosamine Chelator Showing Promising Antibacterial Activity against Gram-Positive Bacteria. Bioorg. Chem. 79, 341–349. doi:10.1016/j.bioorg.2018.05.013
Odobasic, D., Jia, Y., Kao, W., Fan, H., Wei, X., Gu, R., et al. (2018). Formyl Peptide Receptor Activation Inhibits the Expansion of Effector T Cells and Synovial Fibroblasts and Attenuates Joint Injury in Models of Rheumatoid Arthritis. Int. Immunopharmacology 61, 140–149. doi:10.1016/j.intimp.2018.05.028
Pal Singh, S., Dammeijer, F., and Hendriks, R. W. (2018). Role of Bruton's Tyrosine Kinase in B Cells and Malignancies. Mol. Cancer 17, 57. doi:10.1186/s12943-018-0779-z
Parhi, A. K., Xiang, A., Bauman, J. D., Patel, D., Vijayan, R. S. K., Das, K., et al. (2013). Phenyl Substituted 3-Hydroxypyridin-2(1h)-Ones: Inhibitors of Influenza A Endonuclease. Bioorg. Med. Chem. 21, 6435–6446. doi:10.1016/j.bmc.2013.08.053
Paschka, P., Schlenk, R. F., Gaidzik, V. I., Habdank, M., Krönke, J., Bullinger, L., et al. (2010). IDH1andIDH2Mutations Are Frequent Genetic Alterations in Acute Myeloid Leukemia and Confer Adverse Prognosis in Cytogenetically Normal Acute Myeloid Leukemia WithNPM1Mutation WithoutFLT3Internal Tandem Duplication. Jco 28, 3636–3643. doi:10.1200/jco.2010.28.3762
Pietrangelo, T., Giampietro, L., De Filippis, B., La Rovere, R., Fulle, S., and Amoroso, R. (2010). Effect of Milrinone Analogues on Intracellular Calcium Increase in Single Living H9C2 Cardiac Cells. Eur. J. Med. Chem. 45, 4928–4933. doi:10.1016/j.ejmech.2010.08.001
Platts, M. Y., Barber, C. G., Chiva, J.-Y., Eastwood, R. L., Fenwick, D. R., Paradowski, K. A., et al. (2011). A Concise Synthesis of HIV Integrase Inhibitors Bearing the Dipyridone Acid Motif. Tetrahedron Lett. 52, 512–514. doi:10.1016/j.tetlet.2010.11.112
Qin, J.-J., Chen, H.-X., Zhao, N., Yuan, L., Zhang, Y.-Z., Yang, R.-F., et al. (2014). The Role of Activation of the 5-HT1A Receptor and Adenylate Cyclase in the Antidepressant-like Effect of YL-0919, a Dual 5-HT1A Agonist and Selective Serotonin Reuptake Inhibitor. Neurosci. Lett. 582, 104–108. doi:10.1016/j.neulet.2014.09.009
Robinson, M.D, W. S. (1994). Molecular Events in the Pathogenesis of Hepadnavirus-Associated Hepatocellular Carcinoma. Annu. Rev. Med. 45, 297–323. doi:10.1146/annurev.med.45.1.297
Ruelas, D. S., and Greene, W. C. (2013). An Integrated Overview of HIV-1 Latency. Cell 155, 519–529. doi:10.1016/j.cell.2013.09.044
Sanchez, R. I., Fillgrove, K. L., Yee, K. L., Liang, Y., Lu, B., Tatavarti, A., et al. (2019). Characterisation of the Absorption, Distribution, Metabolism, Excretion and Mass Balance of Doravirine, a Non-nucleoside Reverse Transcriptase Inhibitor in Humans. Xenobiotica 49, 422–432. doi:10.1080/00498254.2018.1451667
Santos, R., Ursu, O., Gaulton, A., Bento, A. P., Donadi, R. S., Bologa, C. G., et al. (2017). A Comprehensive Map of Molecular Drug Targets. Nat. Rev. Drug Discov. 16, 19–34. doi:10.1038/nrd.2016.230
Selness, S. R., Boehm, T. L., Walker, J. K., Devadas, B., Durley, R. C., Kurumbail, R., et al. (2011). Design, Synthesis and Activity of a Potent, Selective Series of N -aryl Pyridinone Inhibitors of P38 Kinase. Bioorg. Med. Chem. Lett. 21, 4059–4065. doi:10.1016/j.bmcl.2011.04.120
Seo, B. I., Uchil, V. R., Okello, M., Mishra, S., Ma, X.-H., Nishonov, M., et al. (2011). Discovery of a Potent HIV Integrase Inhibitor that Leads to a Prodrug with Significant Anti-HIV Activity. ACS Med. Chem. Lett. 2, 877–881. doi:10.1021/ml2001246
Sheng, R., Tang, L., Jiang, L., Hong, L., Shi, Y., Zhou, N., et al. (2016). Novel 1-Phenyl-3-Hydroxy-4-Pyridinone Derivatives as Multifunctional Agents for the Therapy of Alzheimer's Disease. ACS Chem. Neurosci. 7, 69–81. doi:10.1021/acschemneuro.5b00224
Shipley, J. B., Tolman, D., Hastillo, A., and Hess, M. L. (1996). Milrinone: Basic and Clinical Pharmacology and Acute and Chronic Management. Am. J. Med. Sci. 311, 286–291. doi:10.1097/00000441-199606000-00011
Singh, S. B., Liu, W., Li, X., Chen, T., Shafiee, A., Card, D., et al. (2012). Antifungal Spectrum, In Vivo Efficacy, and Structure-Activity Relationship of Ilicicolin H. ACS Med. Chem. Lett. 3, 814–817. doi:10.1021/ml300173e
Slobodnyuk, K., Radic, N., Ivanova, S., Llado, A., Trempolec, N., Zorzano, A., et al. (2019). Autophagy-induced Senescence Is Regulated by P38α Signaling. Cell Death Dis 10, 376. doi:10.1038/s41419-019-1607-0
Smith, S. J., Zhao, X. Z., Passos, D. O., Lyumkis, D., Burke, T. R., and Hughes, S. H. (2020). HIV-1 Integrase Inhibitors that Are Active against Drug-Resistant Integrase Mutants. Antimicrob. Agents Chemother. 64. doi:10.1128/aac.00611-20
Song, Y. n., Chen, W., Kang, D., Zhang, Q., Zhan, P., and Liu, X. (2014). "Old Friends in New Guise": Exploiting Privileged Structures for Scaffold Re-Evolution/Refining. Cchts 17, 536–553. doi:10.2174/1386207317666140122101631
Song, Y. n., Zhan, P., and Liu, X. (2013). Heterocycle-thioacetic Acid Motif: a Privileged Molecular Scaffold with Potent, Broad-Ranging Pharmacological Activities. Cpd 19, 7141–7154. doi:10.2174/13816128113199990505
Song, Y. n., Zhan, P., Zhang, Q., and Liu, X. (2013). Privileged Scaffolds or Promiscuous Binders: a Glance of Pyrrolo[2,1-F][1,2,4]triazines and Related Bridgehead Nitrogen Heterocycles in Medicinal Chemistry. Cpd 19, 1528–1548. doi:10.2174/1381612811319080020
Stama, M. L., Ślusarczyk, J., Lacivita, E., Kirpotina, L. N., Schepetkin, I. A., Chamera, K., et al. (2017). Novel Ureidopropanamide Based N-Formyl Peptide Receptor 2 (FPR2) Agonists with Potential Application for central Nervous System Disorders Characterized by Neuroinflammation. Eur. J. Med. Chem. 141, 703–720. doi:10.1016/j.ejmech.2017.09.023
Stevaert, A., Dallocchio, R., Dessi, A., Pala, N., Rogolino, D., Sechi, M., et al. (2013). Mutational Analysis of the Binding Pockets of the Diketo Acid Inhibitor L-742,001 in the Influenza Virus PA Endonuclease. J. Virol. 87, 10524–10538. doi:10.1128/jvi.00832-13
Sweeney, Z. K., and Klumpp, K. (2008). Improving Non-nucleoside Reverse Transcriptase Inhibitors for First-Line Treatment of HIV Infection: the Development Pipeline and Recent Clinical Data. Curr. Opin. Drug Discov. Devel 11, 458–470.
Temmink, O. H., de Bruin, M., Turksma, A. W., Cricca, S., Laan, A. C., and Peters, G. J. (2007). Activity and Substrate Specificity of Pyrimidine Phosphorylases and Their Role in Fluoropyrimidine Sensitivity in colon Cancer Cell Lines. Int. J. Biochem. Cel Biol. 39, 565–575. doi:10.1016/j.biocel.2006.10.009
Thompson, P., Manganiello, V., and Degerman, E. (2007). Re-Discovering PDE3 Inhibitors - New Opportunities for a Long Neglected Target. Ctmc 7, 421–436. doi:10.2174/156802607779941224
Trono, D., Van Lint, C., Rouzioux, C., Verdin, E., Barré-Sinoussi, F., Chun, T.-W., et al. (2010). HIV Persistence and the prospect of Long-Term Drug-free Remissions for HIV-Infected Individuals. Science 329, 174–180. doi:10.1126/science.1191047
Venkataraman, S., Prasad, B., and Selvarajan, R. (2018). RNA Dependent RNA Polymerases: Insights from Structure, Function and Evolution. Viruses 10, 76. Viruses 10. doi:10.3390/v10020076
Vidler, L. R., Brown, N., Knapp, S., and Hoelder, S. (2012). Druggability Analysis and Structural Classification of Bromodomain Acetyl-Lysine Binding Sites. J. Med. Chem. 55, 7346–7359. doi:10.1021/jm300346w
Visseq, A., Descheemaeker, A., Pinto-Pardo, N., Nauton, L., Théry, V., Giraud, F., et al. (2020). Pyridin-2(1H)one Derivatives: A Possible New Class of Therapeutics for Mechanical Allodynia. Eur. J. Med. Chem. 187, 111917. doi:10.1016/j.ejmech.2019.111917
Vrouenraets, M. B., Visser, G. W., Snow, G. B., and van Dongen, G. A. (2003). Basic Principles, Applications in Oncology and Improved Selectivity of Photodynamic Therapy. Anticancer Res. 23, 505–522.
Wolańska, M., Taudul, E., Bańkowska-Guszczyn, E., and Kinalski, M. (2010). Tumor Necrosis Factor in Uterine Leiomyomas at Various Stages of Tumor Growth. Ginekol Pol. 81, 431–434.
Workman, D. G., Hunter, M., Wang, S., Brandel, J., Hubscher, V., Dover, L. G., et al. (2020). The Influence of Linkages between 1-Hydroxy-2(1h)-Pyridinone Coordinating Groups and a Tris(2-Aminoethyl)amine Core in a Novel Series of Synthetic Hexadentate Iron(III) Chelators on Antimicrobial Activity. Bioorg. Chem. 95, 103465. doi:10.1016/j.bioorg.2019.103465
Wu, F., Jiang, H., Zheng, B., Kogiso, M., Yao, Y., Zhou, C., et al. (2015). Inhibition of Cancer-Associated Mutant Isocitrate Dehydrogenases by 2-Thiohydantoin Compounds. J. Med. Chem. 58, 6899–6908. doi:10.1021/acs.jmedchem.5b00684
Wysocka, J., Swigut, T., Milne, T. A., Dou, Y., Zhang, X., Burlingame, A. L., et al. (2005). WDR5 Associates with Histone H3 Methylated at K4 and Is Essential for H3 K4 Methylation and Vertebrate Development. Cell 121, 859–872. doi:10.1016/j.cell.2005.03.036
Xiao, M., Li, H., Wang, Y., Wang, X., Wang, W., Peng, J., et al. (2006). Characterization of the N-Terminal Domain of Classical Swine Fever Virus RNA-dependent RNA Polymerase. J. Gen. Virol. 87, 347–356. doi:10.1099/vir.0.81385-0
Yan, H., Parsons, D. W., Jin, G., McLendon, R., Rasheed, B. A., Yuan, W., et al. (2009). IDH1andIDH2Mutations in Gliomas. N. Engl. J. Med. 360, 765–773. doi:10.1056/NEJMoa0808710
Yan, Y. K., Melchart, M., Habtemariam, A., and Sadler, P. J. (2005). Organometallic Chemistry, Biology and Medicine: Ruthenium Arene Anticancer Complexes. Chem. Commun., 4764–4776. doi:10.1039/b508531b
Yeates, C. L., Batchelor, J. F., Capon, E. C., Cheesman, N. J., Fry, M., Hudson, A. T., et al. (2008). Synthesis and Structure-Activity Relationships of 4-pyridones as Potential Antimalarials. J. Med. Chem. 51, 2845–2852. doi:10.1021/jm0705760
Young, W. B., Barbosa, J., Blomgren, P., Bremer, M. C., Crawford, J. J., Dambach, D., et al. (2015). Potent and Selective Bruton's Tyrosine Kinase Inhibitors: Discovery of GDC-0834. Bioorg. Med. Chem. Lett. 25, 1333–1337. doi:10.1016/j.bmcl.2015.01.032
Yu, M., Li, P., K.C. Basnet, S., Kumarasiri, M., Diab, S., Teo, T., et al. (2015). Discovery of 4-(dihydropyridinon-3-Yl)amino-5-Methylthieno[2,3-D]pyrimidine Derivatives as Potent Mnk Inhibitors: Synthesis, Structure-Activity Relationship Analysis and Biological Evaluation. Eur. J. Med. Chem. 95, 116–126. doi:10.1016/j.ejmech.2015.03.032
Zeng, L., and Zhou, M.-M. (2002). Bromodomain: an Acetyl-Lysine Binding Domain. FEBS Lett. 513, 124–128. doi:10.1016/s0014-5793(01)03309-9
Zhang, C., Yang, K., Yu, S., Su, J., Yuan, S., Han, J., et al. (2019). Design, Synthesis and Biological Evaluation of Hydroxypyridinone-Coumarin Hybrids as Multimodal Monoamine Oxidase B Inhibitors and Iron Chelates against Alzheimer's Disease. Eur. J. Med. Chem. 180, 367–382. doi:10.1016/j.ejmech.2019.07.031
Zhang, Y., and Pike, A. (2021). Pyridones in Drug Discovery: Recent Advances. Bioorg. Med. Chem. Lett. 38, 127849. doi:10.1016/j.bmcl.2021.127849
Zhao, X., Xin, M., Huang, W., Ren, Y., Jin, Q., Tang, F., et al. (2015). Design, Synthesis and Evaluation of Novel 5-Phenylpyridin-2(1h)-One Derivatives as Potent Reversible Bruton's Tyrosine Kinase Inhibitors. Bioorg. Med. Chem. 23, 348–364. doi:10.1016/j.bmc.2014.11.006
Zhou, Z., Liu, T., Zhang, J., Zhan, P., and Liu, X. (2018). Influenza A Virus Polymerase: an Attractive Target for Next-Generation Anti-influenza Therapeutics. Drug Discov. Today 23, 503–518. doi:10.1016/j.drudis.2018.01.028
Keywords: drug-like molecules, medicinal chemistry, pyridinone, biological activity, structure–activity relationship (SAR)
Citation: Lin S, Liu C, Zhao X, Han X, Li X, Ye Y and Li Z (2022) Recent Advances of Pyridinone in Medicinal Chemistry. Front. Chem. 10:869860. doi: 10.3389/fchem.2022.869860
Received: 05 February 2022; Accepted: 02 March 2022;
Published: 23 March 2022.
Edited by:
Sergio F. Sousa, University of Porto, PortugalCopyright © 2022 Lin, Liu, Zhao, Han, Li, Ye and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shibo Lin, bGluMDc0MTAyOEB5ZWFoLm5ldA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.