- 1Fujian Key Laboratory of Drug Target Discovery and Structural and Functional Research, The School of Pharmacy, Fujian Medical University, Fuzhou, China
- 2School of Chemistry and Materials Science, University of Science and Technology of China, Hefei, China
In this study, to examine the possibility of using cage-like complexants to design nonmetallic superalkalis, a series of X@36adz (X = H, B, C, N, O, F, and Si) complexes have been constructed and investigated by embedding nonmetallic atoms into the 36adamanzane (36adz) complexant. Although X atoms possess very high ionization energies, these resulting X@36adz complexes possess low adiabatic ionization energies (AIEs) of 0.78–5.28 eV. In particular, the adiabatic ionization energies (AIEs) of X@36adz (X = H, B, C, N, and Si) are even lower than the ionization energy (3.89 eV) of Cs atoms, and thus, can be classified as novel nonmetallic superalkalis. Moreover, due to the existence of diffuse excess electrons in B@36adz, this complex not only possesses pretty low AIE of 2.16 eV but also exhibits a remarkably large first hyperpolarizability (β0) of 1.35 × 106 au, indicating that it can also be considered as a new kind of nonlinear optical molecule. As a result, this study provides an effective approach to achieve new metal-free species with an excellent reducing capability by utilizing the cage-like organic complexants as building blocks.
Introduction
Reducing agents with low ionization energies (IEs) play a crucial role in chemical synthesis. As is well-known, alkali metal atoms possess the lowest ionization energies (5.39–3.89 eV) (Lide, 2003) among all the elements in the periodic table. However, it is reported that a class of extraordinary compounds possesses even lower IEs than those of alkali metal atoms. Such species were termed “superalkalis” by Gutsev and Boldyrev (1982). Initially, superalkalis were designed by decorating an electronegative central atom with alkali-metal ligands, such as FLi2, OLi3, and NLi4 following the formula MLk+1 (L is an alkali-metal atom and M is an electronegative atom of valency k). In MLk+1, one more alkali metal atom will bring an extra valence electron for the electronic shell of M according to the octet rule. Consequently, such an MLk+1 complex has a great tendency to lose the extra valence electron and thus possess strong reducibility (Sun and Wu, 2019).
Owing to their excellent reducing ability, superalkalis can be used to synthesize unusual charge-transfer salts (Zintl and Morawietz, 1938; Jansen, 1976) with the counterpart possessing relatively low electron affinity and activate stable CO2 and N2 molecules (Park and Meloni, 2017; Zhao et al., 2017; Park and Meloni, 2018; Sun et al., 2019; Sikorska and Gaston, 2020) to produce high-value products (Zhang et al., 2021a; Zhang et al., 2021b). In particular, as a special subset of superatom (Reveles et al., 2009; Luo and Castleman, 2014), superalkalis can behave as alkali metal atoms and maintain their structural and electronic integrities when assembled into extended nanostructures (Reber et al., 2007). Hence, they offer an exciting prospect of serving as building blocks for nanomaterials with highly tunable properties (Jena and Sun 2018), such as supersalts (Giri et al., 2014), hydrogen storage materials (Merino et al., 2012), noble-gas-trapping agents (Pan et al., 2013), superbases (Srivastava and Misra, 2015), and nonlinear optical materials (Sun et al., 2014a; Sun et al., 2014b; Sun et al., 2016b; Sun et al., 2016c; Sun et al., 2018a).
In view of the great importance of superalkalis in chemistry, various superalkalis have been theoretically (Tong et al., 2009; Tong et al., 2011, Tong et al., 2012a,Tong et al., 2012b; Hou et al., 2013; Liu et al., 2014; Sun et al., 2013; Sun et al., 2016a; Giri et al., 2016; Zhao et al., 2017; Sun et al., 2018b; Sun et al., 2019; Park and Meloni, 2018; Sun and Wu, 2019; Tkachenko et al., 2019; Sikorska and Gaston, 2020) and experimentally (Lievens et al., 1999; Yokoyama et al., 2000, 2001; Hou and Wang, 2020) characterized in the past decades. To date, conventional mononuclear MLk+1 superalkalis have been expanded to dinuclear (Tong et al., 2009; Tong et al., 2011) and polynuclear (Tong et al., 2012a; Tong et al., 2012b; Liu et al., 2014) superalkalis, aromatic superalkalis (Sun et al., 2013), Zintl-ion-based superalkalis (Giri et al., 2016; Sun et al., 2018b), hyperalkalis (Sun et al., 2016a), alkali-metal complexes (Tkachenko et al., 2019), and so on. More importantly, some alkali-metal-free superalkalis (Hou et al., 2014; Liu et al., 2016), particularly nonmetallic superalkalis (Hou et al., 2013; Srivastava, 2019a; Srivastava, 2019b), have been proposed in recent years. For example, Hou et al. (2013) designed a class of M2H2n+1+ (M = F, O, N, C for n = 1, 2, 3, 4, respectively) superalkali cations by using hydrogen atoms as ligands. Following a similar rule, the other two series of nonmetallic superalkali cations, namely, FnHn+1+ (n = 1–10) and CxH4x+1+ (x = 1–5), have been proposed by Srivastava (2019a, 2019b). These achievements demonstrate that the potential of designing superalkalis of new type is limitless and thereby motivate us to create more diverse superalkali species by using different rules and ligands to further enrich the superalkali family.
More recently, Tkachenko et al. (2019) reported the record low ionization potentials (1.70–1.52 eV) of alkali metal complexes with crown ethers and cryptands and defined them as superalkali species. In fact, such alkali metal complexes were previously named as electrides, a special kind of ionic solids with trapped electrons serving as anions (Dye, 2009). Hence, this work first built a bridge between superalkalis and electrides. However, it is known that crown ethers and cryptands are prone to be cleaved at the C-O bonds (Redko et al., 2002). Fortunately, analogous complexants, such as adamanzane (adz) (Redko et al., 2002) and aza-cage (aza222) (Kim et al., 1999) with only C-N linkages and no amine hydrogens are considerably stable to synthesize the crystalline salts, including alkalides (Kim et al., 1999; Redko et al., 2002) and electrides (Redko et al., 2005) at room temperature. Hence, it is highly expected that such complexants could also be used as excellent building blocks to design and synthesize new superalkalis.
To verify this hypothesis, the 36adamanzane (36adz) has been chosen as a representative to design a series of X@36adz (X = H, B, C, N, O, F, and Si) by encapsulating nonmetallic atoms into the cavity of this cage-like complexant in this work (see Figure 1). The 36adz complexant is composed of tricyclic tetra-amines with aliphatic chains (Springborg, 2003), which has been used to synthesize a stable alkalide [H@36adz]+Na− (Redko et al., 2002). In this complexant, all the lone pairs of 4 N atoms direct toward the center of the cage (see Supplementary Figure S1). Under the repulsion of the lone pairs of N atoms, the outmost valence electrons of X are destabilized to different degrees, leading to the obvious rise of HOMO level of X@36adz as compared with the isolated 36adz complexant. As a result, these proposed complexes exhibit extraordinarily low AIE values of 0.78–5.28 eV although X atoms possess very high ionization energies (IEs) of 8.15–17.42 eV (Lide, 2003). In particular, the B@36adz complex also has the potential to serve as new nonlinear optical (NLO) molecule with a remarkably large first hyperppolarizability of 1.35 × 106 au because the valence electron of boron atom is pushed out of cage to form diffuse excess electrons. We hope that this work will not only provide new nonmetallic members for the superatom family, but will also open the door to design strong reducing matters by embedding nonmetallic atoms into the various cage-like complexants.
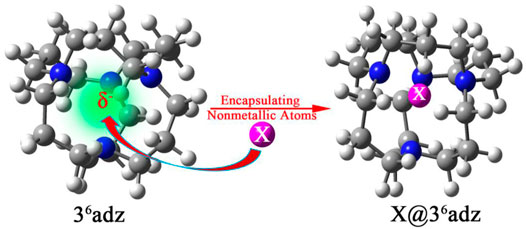
FIGURE 1. The schematic design strategy of X@36adz (X = H, B, C, N, O, F, and Si) based on the cage-like 36adz complexant.
Computational Details
In this work, all the calculations were carried out by using the coulomb-attenuated hybrid exchange-correlation functional (CAM-B3LYP) (Tawada et al., 2004; Yanai et al., 2004), which has been reported to be capable of providing not only the molecular geometries close to the experimentally observed structures but also the (hyper)polarizabilities close to those of the coupled cluster calculations (Limacher et al., 2009). Hence, this method has been widely used to calculate the (hyper)polarizabilities of NLO molecules in the previous works (Sun et al., 2014a; Sun et al., 2014b, Sun et al., 2016c). Also, a method test has also been carried out by sampling B@36adz (see Supplementary Table S1) to verify the reliability of this method in calculating the properties of such systems. From Supplementary Table S1, it is found that CAM-B3LYP gives approximately equal VIE and β0 to those obtained by several other functionals, which indicates that this method is reliable for these studied systems. Hence, all the optimized geometric structures of the studied species with real frequencies were obtained under the CAM-B3LYP/6-31+G(d) level. Based on the optimized structures, the single-point energies, nature population analysis (NPA) charges, and static electric properties were calculated at the CAM-B3LYP/6-311++G (d, p) level.
In this work, the vertical ionization energies (VIEs) of X@36adz (X = H, B, C, N, O, F, and Si) were calculated as the energy difference between the optimized neutral complex and the cation in the geometry of the neutral complex, while their adiabatic ionization energies (AIEs) are defined as the energy difference between the neutral and cationic complex at their respective optimized structures. In addition, the TD-M06-2X calculations were performed to obtain the transition energies and oscillator strengths of the crucial excited states as well as the difference of the dipole moments between the ground state and crucial excited state of X@36adz by using the 6-311++G (d, p) basis set. Herein, the dipole moments (µ0), polarizabilities (α0), and first hyperpolarizabilities (β0) are defined as follows,
where
All the above calculations were performed by using the GAUSSIAN 16 program package (Frisch et al., 2016). The dimensional plots of the molecular structures were generated with the GaussView program (Dennington et al., 2016).
Results and Discussion
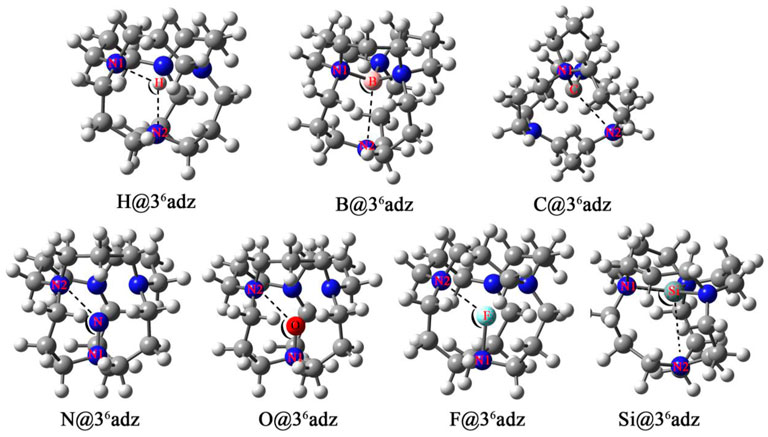
Initially, seven X@36adz (X = H, B, C, N, O, F, and Si) compounds have been constructed by encapsulating one X atom into a 36adz cage. After optimization, the geometric structures of X@36adz are illustrated in Figure 2, while the corresponding cations are plotted in Supplementary Figure S2. Moreover, selected structural parameters of these resulting X@36adz compounds are summarized in Table 1.
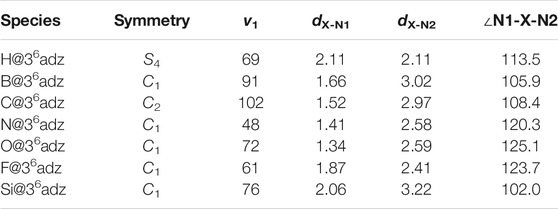
TABLE 1. Symmetry point group, the lowest vibrational frequencies v1 (in cm−1), the bond lengths of X-N1 and X-N2 bonds (dX-N1 and dX-N2, in Å), ∠N1-X-N2 angle (in deg) of X@36adz (X = H, B, C, N, O, F, and Si) compounds.
As shown in Figure 1, 36adz is a cage-like complexant with S4 symmetry. From Figure 2, it is observed that the geometric integrity of 36adz cage is well-preserved in these X@36adz compounds. However, the geometric symmetries of these compounds are lowered to C1 and C2, except for H@36adz, which maintains the S4 symmetry of 36adz. To be specific, the encapsulated hydrogen atom located at the central position of 36adz in H@36adz, yields the newly formed N-H bonds of 2.11 Å and ∠N1-H-N2 of 113.5°. As for B@36adz, the boron atom tends to bind with 3 N atoms of the complexant, forming 3 N-B bonds of 1.63 Å ∼ 1.66 Å, while the distance between the uncombined N and B atoms is as long as 3.02 Å. The C@36adz complex possesses a C2-symmetric structure, where the introduced carbon atom prefers to bind with 2 N atoms of 36adz by forming two N-C bonds of 1.52 Å. Differently, the more electronegative N, O, and F atoms are linked to only 1 N atom of the cage complexant via N-N, N-O, and N-F bonds of 1.41, 1.34, and 1.87 Å, respectively, generating the very similar structures of X@36adz (X = N, O, and F). Similar to B@36adz, the introduced silicon atom tends to bind with 3 N atoms of complexant via 3 N-Si bonds of 2.06–2.35 Å in Si@36adz.
By turning to the cations of X@36adz, it is found that only the optimized structure of [B@36adz]+ cation almost coincides with the geometry of the corresponding neutral one (see Supplementary Figure S2). For instance, the critical geometric parameters of dB-N1, dB-N2, and ∠N1-B-N2 are hardly changed after one electron is lost from B@36adz. However, for the rest of X@36adz (X = H, C, N, O, F, and Si), quite different geometries of cationic and neutral complexes were found. For instance, the H+ is attached to 1 N atom of the complexant in the resulting [H@36adz]+, while the doped N atom turns to combine with 2 N atoms of 36adz in [N@36adz]+ and Si atom almost moves to the center of the cage in [Si@36adz]+. The geometry of C@36adz is distorted from C2 symmetry to C1 with the changes of 0.29 Å for the C-N2 bond and 7.3° for ∠N1-C-N2. As for [F@36adz]+, the N-F bond is shortened from 1.87 Å to 1.38 Å because the introduced F atom further loses 0.333e (see Supplementary Table S1) and thus tends to bind more tightly with the N atom of the complexant. Also, as shown in Table 2, the difference in the geometry can also be reflected by the difference of 0.29–3.06 eV between the vertical ionization energies (VIEs) and adiabatic ionization energies (AIEs) of these X@36adz (X = H, C, N, O, F, and Si) species.
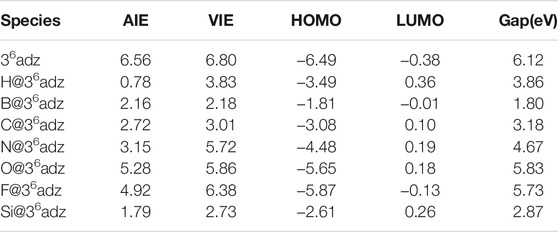
TABLE 2. Adiabatic ionization energies (AIEs, in eV), vertical ionization energies (VIEs, in eV), HOMO and LUMO energy levels (in eV), and the HOMO–LUMO gaps of 36adz and X@36adz (X = H, B, C, N, O, F, and Si) compounds.
More interestingly, as shown in Table 2, extraordinarily low AIE values of 0.78–5.28 eV were found for all the studied X@36adz (X = H, B, C, N, O, F, and Si) complexes, although X atoms possess very high ionization energies (IEs) of 8.15–17.42 eV (Lide, 2003). Such low AIE values of X@36adz are not only lower than that of 6.56 eV for the 36adz complexant but also significantly lower than that of 5.39 eV (Lide, 2003) for lithium atom. In particular, the AIE values of H@36adz (0.78 eV), B@36adz (2.16 eV), C@36adz (2.72 eV), N@36adz (3.15 eV), and Si@36adz (1.79 eV) are even lower than the IE of 3.89 eV (Lide, 2003) for Cs atoms. Hence, these compounds should be classified as novel nonmetallic superalkalis.
How to understand the low IE values of such X@36adz complexes? We can find some clues from the frontier molecular orbital analysis. From Figure 3, a clear inverse correlation between the VIE values and HOMO levels of these studied compounds can be observed, that is, the higher the HOMO level is, the lower the VIE is. This is reasonable considering the fact that the valence electrons on the higher HOMOs are easier to be ionized. To be specific, all the HOMO energies (−1.81 ∼ −5.87 eV) of X@36adz are much higher than that of −6.49 eV for 36adz, because of the repulsion between the lone pairs of N atoms and the outmost valence electrons of X, resulting in the lower VIEs (2.18–6.38 eV) than that (6.80 eV) of 36adz. In particular, B@36adz exhibits the highest HOMO level of −1.81 eV, and thus possesses the lowest VIE of 2.18 eV among these X@36adz complexes. This is because that the valence electron of embedded boron atom is pushed out of the cage by the lone pairs of N atoms of the complexant, forming a electride-like molecule [B+@36adz](e‒) with obvious diffuse electrons in the HOMO of B@36adz (see Supplementary Figure S3). Thus, the existence of diffuse excess electrons in its high-lying HOMO level results in the high reducibility of this B@36adz complex.
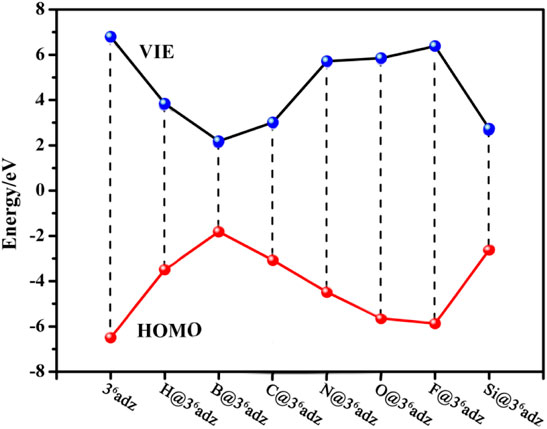
FIGURE 3. The relationship between the VIE values and HOMO levels of X@36adz (X = H, B, C, N, O, F, and Si) compounds.
Differently, as shown in Supplementary Figure S3, the valence electrons are accommodated into the HOMOs mainly composed of the 1s atomic orbital of embedded hydrogen atom in H@36adz, and the np orbitals of C and Si atoms in X@36adz (X = C and Si), which show obvious antibonding character with respect to the central atom-complexant interaction. Such antibonding HOMOs destabilize the neutral structures of X@36adz (X = H, C, and Si) and result in their low VIE values (Gutsev and Boldyrev, 1987; Tkachenko et al., 2019). Hence, these 3 species also have quite low VIE values of 2.73–3.83 eV. However, it should be mentioned that the VIEs of 5.72–6.38 eV for X@36adz (X = N, O, and F) are larger than that of 5.39 eV for Li atom, although their HOMOs also possess obvious antibonding character. This is attributed to the larger elertonegativities of N, O, and F atoms than H, C, and Si atoms, which hinders the ionization of the valence electrons on their np orbitals in the HOMOs of X@36adz (X = N, O, and F).
On the other hand, the difference between the VIE and AIE values are also related to the different electron distribution in the HOMOs of X@36adz. To be specific, the geometric structure of B@36adz is hardly changed after its diffuse excess electron of HOMO is lost, resulting in its nearly equal VIE (2.18 eV) and AIE (2.16 eV) values. However, the destabilization of antibonding HOMOs for the neutral X@36adz (X = H, C, and Si) complexes drives the embedded X atom to lose nearly one valence electron (0.667 e ∼ 0.867 e, as shown in Supplementary Table S1), forming relatively stable [X@36adz]+ cations. After losing one electron, the formed X+ ion changes its interaction mode with the cage complexant, which leads to the large structural distortion and considerable difference between the VIE and AIE values of X@36adz (X = H, C, and Si). Note that the AIE of H@36adz is as low as 0.78 eV because the formed [H@36adz]+ is very stable and has been identified in various synthesized ionic compounds, such as [H@36adz]+X‒ (X = Cl, Br, I, and Na) (Kim et al., 1994; Springborg et al., 1996; Redko et al., 2002).
Finally, considering the diffuse excess electron in the HOMO of B@36adz, it is highly expected that this superalkali also exhibits considerable nonlinear optical (NLO) response. Thus, the static electric properties of these studied X@36adz compounds and 36adz complexant were calculated and listed in Table 3. It is observed that B@36adz has the largest dipole moment (3.326 au) and polarizability (1,599 au) among these X@36adz complexes because of the existence of diffuse electrons in the HOMO of this superalkali. In particular, the first hyperpolarizability (β0) of B@36adz is as large as 1.35 × 106 au, which is significantly larger than those of the reported superalkalis and superalkali-based NLO materials, such as the aromatic organometallic superalkali Au3(Py)3 (3.74 × 104 au) (Parida et al., 2018), superalkali-based alkalide Li3O+(calix [4]pyrrole)M‒ (M = Li, Na, and K) (1.18 × 104–3.33 × 104 au) (Sun et al., 2014a), and superalkali-based electride Li3O@Al12N12 (8.73 × 105 au) (Sun et al., 2016b), indicating that this proposed superalkali species can indeed be considered as a new kind of NLO molecule of high performance.
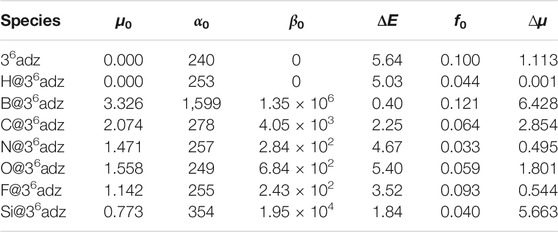
TABLE 3. Calculated dipole moments (µ0, in au), polarizabilities (α0, in au), first hyperpolarizabilities (β0, in au), transition energies (ΔE, in eV), oscillator strength (f0), and the difference in the dipole moments (∆µ, in Debye) between the ground and crucial excited states of 36adz and X@36adz (X = H, B, C, N, O, F, and Si) compounds.
To understand the eminently large β0 value of B@36adz, we focus our attention on the simple two-level model (Oudar, 1977; Oudar and Chemla, 1977),
where ΔE, f0, and Δµ are the transition energy, oscillator strength, and the difference in the dipole moment between the ground state and crucial excited state, respectively. According to this two-level expression, β0 is proportional to f0 and ∆µ, while is inversely proportional to the cube of ΔE, and therefore, the transition energy is considered to be the decisive factor in the first hyperpolarizability (Sun et al., 2014a,b, 2016b,c). Hence, the ΔE, f0, and Δµ values of the crucial excited states with the largest oscillator strength of 36adz and X@36adz are summarized in Table 3. It is noted that B@36adz possesses extremely smaller ΔE and much larger f0 and ∆µ values than those of other X@36adz (X = H, C, N, O, F, and Si) compounds, which rationalizes its largest β0 value among these studied X@36adz species. In addition, the proposed C@36adz and Si@36adz superalkalis also show considerable β0 values of 4.05 × 103 au and 1.95 × 104 au, respectively, because of their relatively smaller ΔE values and larger Δµ values.
Conclusion
By using 36adamanzane (36adz) as a complexant, a series of X@36adz (X = H, B, C, N, O, F, and Si) compounds were constructed and studied based on the density functional theory. It is interesting to find that the X@36adz (X = H, B, C, N, and Si) complexes possess lower AIE values than the IE of Cs atoms though the X atoms and 36adz possess very high IE values. Thereby, they can be regarded as a new kind of nonmetallic superalkalis. In particular, different from other complexes, the low IE of B@36adz is derived from the diffuse excess electron formed by the repulsion between the valence electron of the embedded boron atom and lone pairs of N atoms of the complexant. Due to the existence of diffuse electrons, this superalkali also possesses a remarkably large β0 of 1.35 × 106 au, which can serve as a new kind of NLO molecule. Hence, it is highly hoped that the theoretical design and characterization of these nonmetallic superalkali species could provide meaningful references to further design novel reducing matters or NLO materials by using such cage-like molecules as complexants.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the Natural Science Foundation of Fujian Province (2021J01682) and the National Natural Science Foundation of China (21603032).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the National Supercomputing Center in Shenzhen for providing computational resources.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.853160/full#supplementary-material
References
Dennington, R. D., Keith, T. A., and Millam, J. M. (2016). GaussView, Version 6. Shawnee Mission, KS: Semichem Inc.
Dye, J. L. (2009). Electrides: Early Examples of Quantum Confinementfinement. Acc. Chem. Res. 42, 1564–1572. doi:10.1021/ar9000857
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., et al. (2016). Gaussian 16, Revision A.03. Wallingford CT, USA: Gaussian, Inc.
Giri, S., Behera, S., and Jena, P. (2014). Superalkalis and Superhalogens as Building Blocks of Supersalts. J. Phys. Chem. A. 118 (3), 638–645. doi:10.1021/jp4115095
Giri, S., Reddy, G. N., and Jena, P. (2016). Organo-Zintl Clusters [P7R4]: A New Class of Superalkalis. J. Phys. Chem. Lett. 7, 800–805. doi:10.1021/acs.jpclett.5b02892
Gutsev, G. L., and Boldyrev, A. I. (1982). DVM Xα Calculations on the Electronic Structure of "superalkali" Cations. Chem. Phys. Lett. 92, 262–266. doi:10.1016/0009-2614(82)80272-8
Gutsev, G. L., and Boldyrev, A. I. (1987). The Electronic Structure of Superhalogens and Superalkalies. Russ. Chem. Rev. 56 (6), 519–531. doi:10.1070/rc1987v056n06abeh003287
Hou, G.-L., and Wang, X.-B. (2020). Potassium Iodide Cluster Based Superhalogens and Superalkalis: Theoretical Calculations and Experimental Confirmation. Chem. Phys. Lett. 741, 137094. doi:10.1016/j.cplett.2020.137094
Hou, N., Li, Y., Wu, D., and Li, Z.-R. (2013). Do Nonmetallic Superalkali Cations Exist? Chem. Phys. Lett. 575, 32–35. doi:10.1016/j.cplett.2013.05.014
Hou, N., Wu, D., Li, Y., and Li, Z.-R. (2014). Lower the Electron Affinity by Halogenation: an Unusual Strategy to Design Superalkali Cations. J. Am. Chem. Soc. 136 (7), 2921–2927. doi:10.1021/ja411755t
Jansen, M. (1976). Na3NO3-kein Orthonitrit. Angew. Chem. Int. Ed. 88, 411. doi:10.1002/ange.19760881205
Jena, P., and Sun, Q. (2018). Super Atomic Clusters: Design Rules and Potential for Building Blocks of Materials. Chem. Rev. 118 (11), 5755–5870. doi:10.1021/acs.chemrev.7b00524
Kim, J., Ichimura, A. S., Huang, R. H., Redko, M., Phillips, R. C., and Jackson, J. E. (1999). Crystalline Salts of Na and K (Alkalides) that Are Stable at Room Temperature. J. Am. Chem. Soc. 121, 10666–10667. doi:10.1021/ja992667v
Kim, R. D. H., Wilson, M., and Haseltine, H. (1994). Simple Preparations of Tricyclic Orthoamides and Macrocyclic Triamines. Synth. Commun. 24 (21), 3109–3114. doi:10.1080/00397919408011324
Lievens, P., Thoen, P., Bouckaert, S., Bouwen, W., Vanhoutte, F., Weidele, H., et al. (1999). Ionization Potentials of LinO (2≤n≤70) Clusters: Experiment and Theory. J. Chem. Phys. 110 (21), 10316. doi:10.1063/1.478965
Limacher, P. A., Mikkelsen, K. V., and Lüthi, H. P. (2009). On the Accurate Calculation of Polarizabilities and Second Hyperpolarizabilities of Polyacetylene Oligomer Chains Using the CAM-B3lyp Density Functional. J. Chem. Phys. 130 (19), 194114. doi:10.1063/1.3139023
Liu, J. Y., Wu, D., Sun, W. M., Li, Y., and Li, Z. R. (2014). Trivalent Acid Radical-Centered YLi4+ (Y = PO4, AsO4, VO4) Cations: New Polynuclear Species Designed to Enrich the Superalkali Family. Dalton Trans. 43 (48), 18066–18073. doi:10.1039/c4dt02347a
Liu, J. Y., Xi, Y. J., Li, Y., Li, S. Y., Wu, D., and Li, Z. R. (2016). Does Alkaline-Earth-Metal-Based Superalkali Exist? J. Phys. Chem. A. 120 (51), 10281–10288. doi:10.1021/acs.jpca.6b10555
Luo, Z., and Castleman, A. W. (2014). Special and General Superatoms. Acc. Chem. Res. 47 (10), 2931–2940. doi:10.1021/ar5001583
Merino, G., Chattaraj, P. M., and Pan, S. (2012). Hydrogen Trapping Potential of Some Li-Doped Star-like Clusters and Super-alkali Systems. Phys. Chem. Chem. Phys. 14, 10345–10350. doi:10.1039/c2cp40794a
Oudar, J. L., and Chemla, D. S. (1977). Hyperpolarizabilities of the Nitroanilines and Their Relations to the Excited State Dipole Moment. J. Chem. Phys. 66 (6), 2664. doi:10.1063/1.434213
Oudar, J. L. (1977). Optical Nonlinearities of Conjugated Molecules. Stilbene Derivatives and Highly Polar Aromatic Compounds. J. Chem. Phys. 67 (2), 446. doi:10.1063/1.434888
Pan, S., Contreras, M., Romero, J., Reyes, A., Chattaraj, P. K., and Merino, G. (2013). C5Li7+ and O2Li5+ as Noble-Gas-Trapping Agents. Chem. Eur. J. 19 (7), 2322–2329. doi:10.1002/chem.201203245
Parida, R., Reddy, G. N., Ganguly, A., Roymahapatra, G., Chakraborty, A., and Giri, S. (2018). On the Making of Aromatic Organometallic Superalkali Complexes. Chem. Commun. 54 (31), 3903–3906. doi:10.1039/c8cc01170b
Park, H., and Meloni, G. (2018). Activation of Dinitrogen with a Superalkali Species, Li3F2. ChemPhysChem 19 (3), 256–260. doi:10.1002/cphc.201701232
Park, H., and Meloni, G. (2017). Reduction of Carbon Dioxide with a Superalkali. Dalton Trans. 46 (35), 11942–11949. doi:10.1039/c7dt02331f
Reber, A. C., Khanna, S. N., and Castleman, A. W. (2007). Superatom Compounds, Clusters, and Assemblies: Ultra Alkali Motifs and Architectures. J. Am. Chem. Soc. 129, 10189–10194. doi:10.1021/ja071647n
Redko, M. Y., Jackson, J. E., Huang, R. H., and Dye, J. L. (2005). Design and Synthesis of a Thermally Stable Organic Electride. J. Am. Chem. Soc. 127, 12416–12422. doi:10.1021/ja053216f
Redko, M. Y., Vlassa, M., Jackson, J. E., Misiolek, A. W., Huang, R. H., and Dye, J. L. (2002). Inverse Sodium Hydride”: A Crystalline Salt that Contains H and Na-. J. Am. Chem. Soc. 124, 5928–5929. doi:10.1021/ja025655+
Reveles, J. U., Clayborne, P. A., Reber, A. C., Khanna, S. N., Pradhan, K., Sen, P., et al. (2009). Designer Magnetic Superatoms. Nat. Chem. 1 (4), 310–315. doi:10.1038/nchem.249
Sikorska, C., and Gaston, N. (2020). N4Mg6M (M = Li, Na, K) Superalkalis for CO2 Activation. J. Chem. Phys. 153, 144301. doi:10.1063/5.0025545
Springborg, J. (2003). Adamanzanes–Bi- and Tricyclic Tetraamines and Their Coordination Compounds. Dalton Trans. (9), 1653–1665. doi:10.1039/b300510k
Springborg, J., Pretzmann, U., and Olsen, C. E. (1996). An Inert Proton Coordinated inside the Tetrahedral Cage [36]Adamanzane. Synthesis of the inside Monoprotonated Amine 1,5,9,13-Tetraazatricyclo[7.7.3.35,13]docosane. Acta Chem. Scand. A. 50, 294–298. doi:10.3891/acta.chem.scand.50-0294
Srivastava, A. K. (2019a). Ab Initio investigations on Non-metallic Chain-Shaped FnHn+1+ Series of Superalkali Cations. Chem. Phys. Lett. 721, 7–11. doi:10.1016/j.cplett.2019.02.021
Srivastava, A. K. (2019b). CxH4x+1+ (X = 1–5): a Unique Series of Organic Superalkali Cations. Mol. Phys., 1–7.
Srivastava, A. K., and Misra, N. (2015). Superalkali-hydroxides as Strong Bases and Superbases. New J. Chem. 39 (9), 6787–6790. doi:10.1039/c5nj01259g
Sun, W.-M., Li, C.-Y., Kang, J., Wu, D., Li, Y., Ni, B.-L., et al. (2018a). Superatom Compounds under Oriented External Electric Fields: Simultaneously Enhanced Bond Energies and Nonlinear Optical Responses. J. Phys. Chem. C 122 (14), 7867–7876. doi:10.1021/acs.jpcc.8b01896
Sun, W.-M., Li, X.-H., Li, Y., Liu, J.-Y., Wu, D., Li, C.-Y., et al. (2016a). On the Feasibility of Designing Hyperalkali Cations Using Superalkali Clusters as Ligands. J. Chem. Phys. 145 (19), 194303. doi:10.1063/1.4967461
Sun, W.-M., Li, X.-H., Wu, D., Li, Y., He, H.-M., Li, Z.-R., et al. (2016b). A Theoretical Study on Superalkali-Doped Nanocages: Unique Inorganic Electrides with High Stability, Deep-Ultraviolet Transparency, and Considerable Nonlinear Optical Response. Dalton Trans. 45 (17), 7500–7509. doi:10.1039/c6dt00342g
Sun, W.-M., Li, Y., Wu, D., and Li, Z.-R. (2013). Designing Aromatic Superatoms. J. Phys. Chem. C 117 (46), 24618–24624. doi:10.1021/jp408810e
Sun, W.-M., Wu, D., Kang, J., Li, C.-Y., Chen, J.-H., Li, Y., et al. (2018b). Decorating Zintl Polyanions with Alkali Metal Cations: A Novel Strategy to Design Superatom Cations with Low Electron Affinity. J. Alloys Compd. 740 (5), 400–405. doi:10.1016/j.jallcom.2017.12.075
Sun, W.-M., Zhang, X.-L., Pan, K.-Y., Chen, J.-H., Wu, D., Li, C.-Y., et al. (2019). On the Possibility of Using the Jellium Model as a Guide to Design Bimetallic Superalkali Cations. Chem. Eur. J. 25 (17), 4358–4366. doi:10.1002/chem.201806194
Sun, W. M., Fan, L. T., Li, Y., Liu, J. Y., Wu, D., and Li, Z. R. (2014a). On the Potential Application of Superalkali Clusters in Designing Novel Alkalides with Large Nonlinear Optical Properties. Inorg. Chem. 53 (12), 6170–6178. doi:10.1021/ic500655s
Sun, W. M., Li, Y., Li, X. H., Wu, D., He, H. M., Li, C. Y., et al. (2016c). Stability and Nonlinear Optical Response of Alkalides that Contain a Completely Encapsulated Superalkali Cluster. Chemphyschem 17 (17), 2672–2678. doi:10.1002/cphc.201600389
Sun, W. M., Wu, D., Li, Y., and Li, Z. R. (2014b). Theoretical Study on Superalkali (Li3) in Ammonia: Novel Alkalides with Considerably Large First Hyperpolarizabilities. Dalton Trans. 43 (2), 486–494. doi:10.1039/c3dt51559a
Sun, W. M., and Wu, D. (2019). Recent Progress on the Design, Characterization, and Application of Superalkalis. Chem. Eur. J. 25 (41), 9568–9579. doi:10.1002/chem.201901460
Tawada, Y., Tsuneda, T., Yanagisawa, S., Yanai, T., and Hirao, K. (2004). A Long-Range-Corrected Time-dependent Density Functional Theory. J. Chem. Phys. 120, 8425–8433. doi:10.1063/1.1688752
Tkachenko, N. V., Sun, Z. M., and Boldyrev, A. I. (2019). Record Low Ionization Potentials of Alkali Metal Complexes with Crown Ethers and Cryptands. Chemphyschem 20 (16), 2060–2062. doi:10.1002/cphc.201900422
Tong, J., Li, Y., Wu, D., Li, Z.-R., and Huang, X.-R. (2011). Ab Initio Investigation on a New Class of Binuclear Superalkali Cations M2Li2k+1+ (F2Li3+, O2Li5+, N2Li7+, and C2Li9+). J. Phys. Chem. A. 115 (10), 2041–2046. doi:10.1021/jp110417z
Tong, J., Li, Y., Wu, D., Li, Z. R., and Huang, X. R. (2009). Low Ionization Potentials of Binuclear Superalkali B2Li11. J. Chem. Phys. 131 (16), 164307. doi:10.1063/1.3254835
Tong, J., Li, Y., Wu, D., and Wu, Z.-J. (2012a). Theoretical Study on Polynuclear Superalkali Cations with Various Functional Groups as the Central Core. Inorg. Chem. 51 (11), 6081–6088. doi:10.1021/ic202675j
Tong, J., Wu, Z., Li, Y., and Wu, D. (2012b). Prediction and Characterization of Novel Polynuclear Superalkali Cations. Dalton Trans. 42, 577–584. doi:10.1039/c2dt31429k
Yanai, T., Tew, D. P., and Handy, N. C. (2004). A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3lyp). Chem. Phys. Lett. 393 (1-3), 51–57. doi:10.1016/j.cplett.2004.06.011
Yokoyama, K., Haketa, N., Tanaka, H., Furukawa, K., and Kudo, H. (2000). Ionization Energies of Hyperlithiated Li2F Molecule and LinFn-1 (N= 3, 4) Clusters. Chem. Phys. Lett. 330, 339–346. doi:10.1016/s0009-2614(00)01109-x
Yokoyama, K., Tanaka, H., and Kudo, H. (2001). Structure of Hyperlithiated Li3O and Evidence for Electronomers. J. Phys. Chem. A. 105, 4312–4315. doi:10.1021/jp0037450
Zhang, X.-L., Ye, Y.-L., Zhang, L., Li, X.-H., Yu, D., Chen, J.-H., et al. (2021a). Designing an Alkali-metal-like Superatom Ca3B for Ambient Nitrogen Reduction to Ammonia. Phys. Chem. Chem. Phys. 23 (34), 18908–18915. doi:10.1039/d1cp01533h
Zhang, X.-L., Zhang, L., Ye, Y.-L., Li, X.-H., Ni, B.-L., Li, Y., et al. (2021b). On the Role of Alkali-metal-like Superatom Al12P in Reduction and Conversion of Carbon Dioxide. Chem. Eur. J. 27 (3), 1039–1045. doi:10.1002/chem.202003733
Zhao, T., Wang, Q., and Jena, P. (2017). Rational Design of Super-alkalis and Their Role in CO2 Activation. Nanoscale 9 (15), 4891–4897. doi:10.1039/c7nr00227k
Keywords: superalkali, adamanzane, superatom, nonlinear optics, reducing matters
Citation: Ye Y-L, Pan K-Y, Ni B-L and Sun W-M (2022) Designing Special Nonmetallic Superalkalis Based on a Cage-like Adamanzane Complexant. Front. Chem. 10:853160. doi: 10.3389/fchem.2022.853160
Received: 12 January 2022; Accepted: 25 February 2022;
Published: 14 March 2022.
Edited by:
Sugata Chowdhury, National Institute of Standards and Technology (NIST), United StatesReviewed by:
Gourhari Jana, University of California, Irvine, United StatesSantanab Giri, Haldia Institute of Technology, India
Copyright © 2022 Ye, Pan, Ni and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Ming Sun, c3Vud21AZmptdS5lZHUuY24=
 Ya-Ling Ye1
Ya-Ling Ye1 Wei-Ming Sun
Wei-Ming Sun