- 1The Laboratory for Phytochemistry and Botanical Pesticides, College of Agriculture, Jiangxi Agricultural University, Nanchang, China
- 2Jiangxi Province Key Laboratory of Tuberous Plant Biology, Jiangxi Agricultural University, Nanchang, China
- 3Key Laboratory of Crop Physiology, Ecology and Genetic Breeding, Ministry of Education/Jiangxi Province, Jiangxi Agricultural University, Nanchang, China
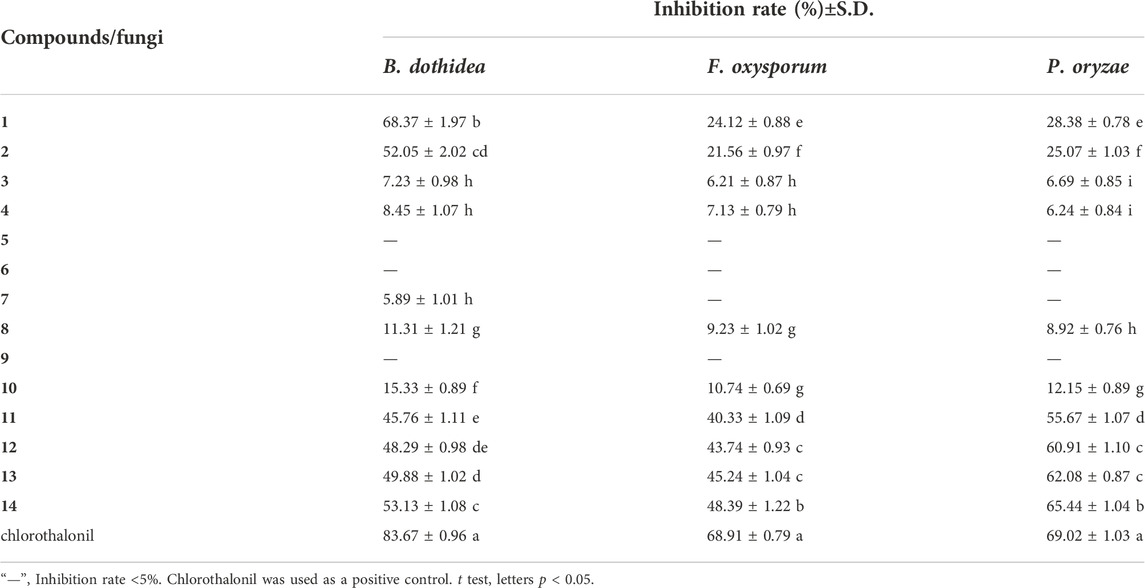
Two novel amides, named clauphenamides A and B, and twelve other known compounds were isolated from the twigs and leaves of Clausena lansium Lour. Skeels (Rutaceae). Their structures were elucidated on the basis of extensive spectroscopic analysis and comparison with data reported in the literature. Clauphenamide A (1) featured in the unit of N-2-(4,8-dimethoxyfuro [2,3-b]quinolin-7-yl)vinyl, and clauphenamide B (2) was a unprecedented N-phenethyl cinnamide dimer. Other known compounds belong to pyrrolidone amides (3 and 4), furacoumarins (7–10), simple coumarins (11–14), lignan (5) and sesquiterpene (6). Compounds 5, 6, 10 and 12 were separated from the genus (Clausena) for the first time, while 13 was isolated in the species (C. lansium) for the first time. The antifungal activities of the isolated compounds were assayed. As a result, at the concentration of 100 μg/ml, compared with the control (chlorothalonil, inhibition rate of 83.67%), compounds 1 and 2 were found to exhibit moderate antifungal activity against B. dothidea with inhibition rates of 68.39% and 52.05%, respectively. Compounds 11–14 also exhibited moderate activity against B. dothidea and F. oxysporum, with inhibition rates greater than 40%. In addition, compared with the control (chlorothalonil, inhibition rate of 69.02%), compounds 11–14 showed strong antifungal activity to P. oryzae, with inhibition rates greater than 55%. Among them, compound 14 has the strongest antifungal activity against P. oryzae, and the inhibition rate (65.44%) is close to that of the control chlorothalonil. Additionally, the structure-activity relationships of the separated compounds are also discussed preliminarily in this paper.
Introduction
Clausena lansium Lour. Skeels (Rutaceae), native to southern China and now distributed throughout the subtropical and tropical regions, is one of approximately 30 members of the genus Clausena (Rutaceae) (Editorial Committee, 1997; Peng et al., 2019). This plant is famous for its good medicinal value and delicious fruit. Previous phytochemical investigation on C. lansium has revealed that the chemical constituents of C. lansium are diverse, including alkaloids (Peng et al., 2018), coumarins (Peng et al., 2021), amides (Peng et al., 2020), sesquiterpenes (Liu et al., 2021), sesquiterpene glycosides (Peng et al., 2019), aromatic glycosides (Peng et al., 2019) and so on, endowing diverse pharmacological activities such as the antitumor, antifungal, antioxidant, hypoglycemic, nematicidal, hepatoprotectiv, neuroprotective, antiobesity, antimicrobial, and anti-inflammatory for this plant (Shen et al., 2012; Deng et al., 2014a; 2014b; Liu et al., 2014; Shen et al., 2014; Song et al., 2014; Xu et al., 2014; Du et al., 2015; Huang et al., 2017; Fan et al., 2018; Yan et al., 2018).
Amides are important active components in C. lansium, which are divided into cyclic amides (Yang et al., 1988), phenylpropionamides (Lin, 1989; Milner et al., 1996) and other amides (Peng et al., 2020). More than twenty amides have been isolated from C. lansium since 1988 (Yang et al., 1988; Liu et al., 1996; Lin, 1989; Milner et al., 1996), which exhibit a variety of biological activities, such as hepatoprotective, hypolipidemia, antispasmodic (Moshi et al., 2005), anti HIV (Sunthitikawinsakul et al., 2003), immunomodulatory (Manosroi et al., 2005), antiviral (Adebajo et al., 2009), antimalaria (Okokon et al., 2012), and cytotoxic (Sripisut et al., 2012) activities. Besides, amides from C. lansium also show the potential for development and utilization in pesticide activities, including insecticidal (Cheng et al., 2010; Han et al., 2013; Ramkumar et al., 2015), antifungal (Ng et al., 2003; Li et al., 2014; Yan et al., 2018) and phytocidal (Peng et al., 2021) effects.
Coumarins are another important active ingredient in C. lansium, mainly furacoumarins (Ito et al., 1998; Peng et al., 2021), which exhibit hypoglycemic (Zhang et al., 2012), anti-tumor (Prasad et al., 2010), antibacterial (Tada et al., 2002), herbicidal (Peng et al., 2021) and other activities.
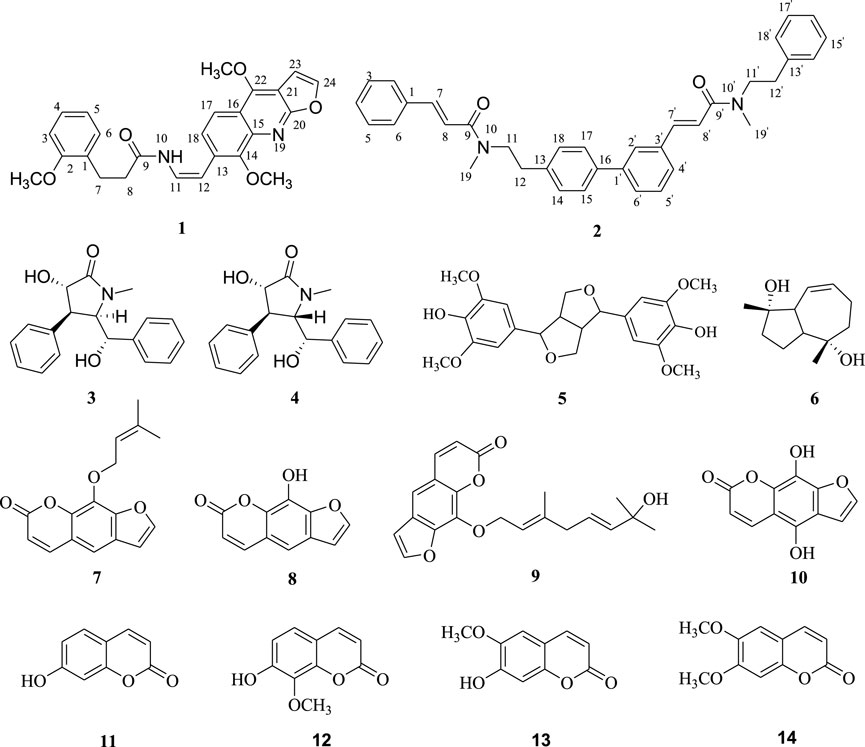
In the early stage, we carried out a detailed investigation on the chemical substances of C. lansium, and isolated various chemical components, including alkaloids (Peng et al., 2018), coumarins (Peng et al., 2021), sesquiterpenes (Liu et al., 2021), sesquiterpene glycosides (Peng et al., 2019), aromatic glycosides (Peng et al., 2019), amides (Song et al., 2014; Peng et al., 2020) and so on. As part of our continuous efforts to find new bioactive natural products, especially amides, alkaloids and coumarins, from C. lansium, a continuing chemical investigation on the twigs and leaves of C. lansium was carried out in the current work, leading to the isolation of four amides (1–4), eight coumarins (7–14), one lignan (5) and one sesquiterpene (6) (Figure 1). Compoud 1 was one unique amide with the unit of N-2-(4,8-dimethoxyfuro [2,3-b]quinolin-7-yl)vinyl, and 2 was a unprecedented N-phenethyl cinnamide dimer. Compounds 5, 6, 10 and 12 were separated from Clausena for the first time, while 13 was isolated in C. lansium for the first time. All compounds were evaluated for their antifungal activities against Botryosphaeria dothidea (Moug.) Ces. and De Not., Fusarium oxysporum and Pyricularia oryzae Cav. via a mycelial growth inhibition assay. In this paper, we described the isolation, identification, and antifungal activities screening and structure-activity relationships of the above mentioned chemical composition from C. lansium.
Materials and methods
General experimental procedures
UV spectra were obtained using a polarimeter (Horiba SEPA-300) (Horiba, Tokyo, Japan). An FT-IR spectrometer (Tensor 27 with KBr pellets) (BioRad, Hercules, CA, United States) was used to record IR spectra of compounds. NMR spectra were recorded with an instrument (Bruker Avance AV-400) (Switzerland, Bruker A.G.) at room temperature. HRESIMS data were obtained with a spectrometer (a Bruker Daltonics Inc. micro-TOF-Q). Reverse-phase medium-pressure liquid chromatography (RP-MPLC) was performed on a Buchi RP-MPLC instrument (Buchi Labortechnik AG, Flawil, Switzerland) with a YMC gel ODS column (50 μm, YMC Co., Ltd., Kyoto, Japan). Semipreparative high-performance Liquid Chromatography (HPLC) was performed on an Agilent 1260 instrument (Agilent, Palo Alto, CA, United States) with a UV detection and a column (Agilent Eclipse, XDB-C18, 5 μm, 9.4 × 250 mm). Column chromatography (CC) was carried out on silica gel (100–200 mesh, 200–300 mesh) (Qingdao Marine Chemical, Inc., Qingdao, China) and Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). TLC was performed with glass-precoated silica gel GF254 plates (Qingdao Marine Chemical Factory, China). The organic solvents were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Plant material
The twigs and leaves of C. lansium were collected from Qingyuan county (23°70′ N, 113°03′ E), Guangdong Province, China, in October 2015, and identified by Prof. Zhou Xin-xin, South China Botanical Garden, Chiese Academy of Sciences, Guangdong, China. A voucher specimen (no. 2015912) has been deposited in the Laboratory for Phytochemistry and Plant-derived Pesticides, College of Agriculture, Jiangxi Agri-cultural University.
Extraction and isolation
The air-dried twigs and leaves of C. lansium (11 kg) were crushed into a powder and extracted with refluxing 95% methanol (3 × 20 L, 6 h each time). The methanol extract was suspended in water (5 L) and partitioned with petroleum ether (PE), ethyl acetate (EtOAc) and n-butyl alcohol (n-BuOH) (3 × 5 L, each) to afford PE extract (210 g), EtOAc extract (890 g), and n-BuOH extract (130 g), respectively. The EtOAc part was chromatographed on a silica gel column (100–200 mesh) and eluted with a gradient mixture of PE-Acetone (1: 0 to 0: 1) to provide six major fractions: (Fr. A-F).
Fr. D (16.4 g) was subjected to RP-MPLC (MeOH/H2O, 20–100%) to give 9 fractions (Fr.D1−Fr.D9). Fr. D2 (57.2 mg) was chromatographed on silica gel column (200–300 mesh) eluted with a isocratic system of PE–Acetone (4:1) to give a mixture (33.1 mg) of compounds 3 and 4. The mixture was isolated and purified by HPLC (MeOH–H2O 73:27) to yield 3 (6 mg, tR = 23.3 min) and 4 (7 mg, tR = 26.5 min). Fr. D3 (43.1 mg) was further fractionated by Sephadex LH-20 column chromatography with MeOH and CH2Cl2 (1:1) to obtain three subfractions (Fr.D3.1 to Fr. D3.3), compounds 1 (5 mg, tR = 20.6 min) and 2 (6 mg, tR = 23.2 min) were obtained from Fr. D3.2 and Fr. D3.3 by Semipreparative HPLC (MeOH–H2O 75:25 and 76:24), respectively. Fr. D4 (57.8 mg) was chromatographed on silica gel column (200–300 mesh) eluted with a gradient system of PE–Acetone (5:1–3:1) to give five subfractions (Fr.D4.1 to Fr. D4.5). Then Fr. D4.2-Fr.D4.4 were repeatedly purified by Semipreparative HPLC (C₂H₃N-H2O 65:35–75:25). Finally, 8 (7 mg, C₂H₃N- H2O 60:40, tR = 21.2 min), 10 (6 mg, C₂H₃N-H2O 70:30, tR = 25.4 min) and 14 (5 mg, C₂H₃N-H2O 72:28, tR = 20.3 min) were obtained from Fr. D4.2, Fr. D4.3 and Fr. D4.4, respectively.
Fr. E (21.3 g) was subjected to RP-MPLC (MeOH/H2O, 30–100%) to give 6 fractions (Fr.E1−Fr.E6). Fr. E2 (91 mg) was further fractionated on silica gel column (200–300 mesh) eluted with a gradient system of PE–Acetone (5:1–3:1) to yield four subfractions (Fr.E2.1 to Fr. D2.4). Compounds 11 (6 mg, tR = 23.2 min) and 13 (9 mg, tR = 24.5 min) were isolated from Fr. E2.2 and Fr. E2.3 by Semipreparative HPLC (C₂H₃N–H2O 65:35 and 70:30), respectively. Similarly, Fr. E3 (82 mg) was subjected to silica gel column (200–300 mesh) eluted with a gradient system of PE–Acetone (5:1–3:1) to give three subfractions (Fr.E3.1 to Fr. D3.3). Fr. E3.2 was purified by repeated column chromatography (200–300 mesh, PE–Acetone 4:1–3:1) and Semipreparative HPLC (C₂H₃N–H2O 68:32) to obtain compound 5 (8 mg, tR = 23.6 min). Fr. E4 (75 mg) was separated by column chromatography (PE–Acetone 4:1–3:1) to give four subfractions (Fr.E4.1 to Fr. D4.4), then Fr. D4.2 was purified by Semipreparative HPLC (C₂H₃N–H2O 64:36) to give 12 (5 mg, tR = 21.3 min).
Fr. F (9.7 g) was subjected to RP-MPLC (MeOH/H2O, 30–100%) to give five fractions (Fr.F1−Fr.F5). Fr.F2 (39 mg) was subjected to silica gel column (200–300 mesh) eluted with a Isocratic system of PE–Acetone (4:1), and then was purified by Semipreparative HPLC (C₂H₃N–H2O 72:28) to give 6 (7 mg, tR = 18.7 min). Fr.F3 (110 mg) and Fr.F4 (99 mg) were repeated chromatographed on silica gel column (200–300 mesh) eluted with a isocratic system of PE–Acetone (4:1) to give 7 (34 mg) and 9 (14 mg), respectively.
Spectroscopic data
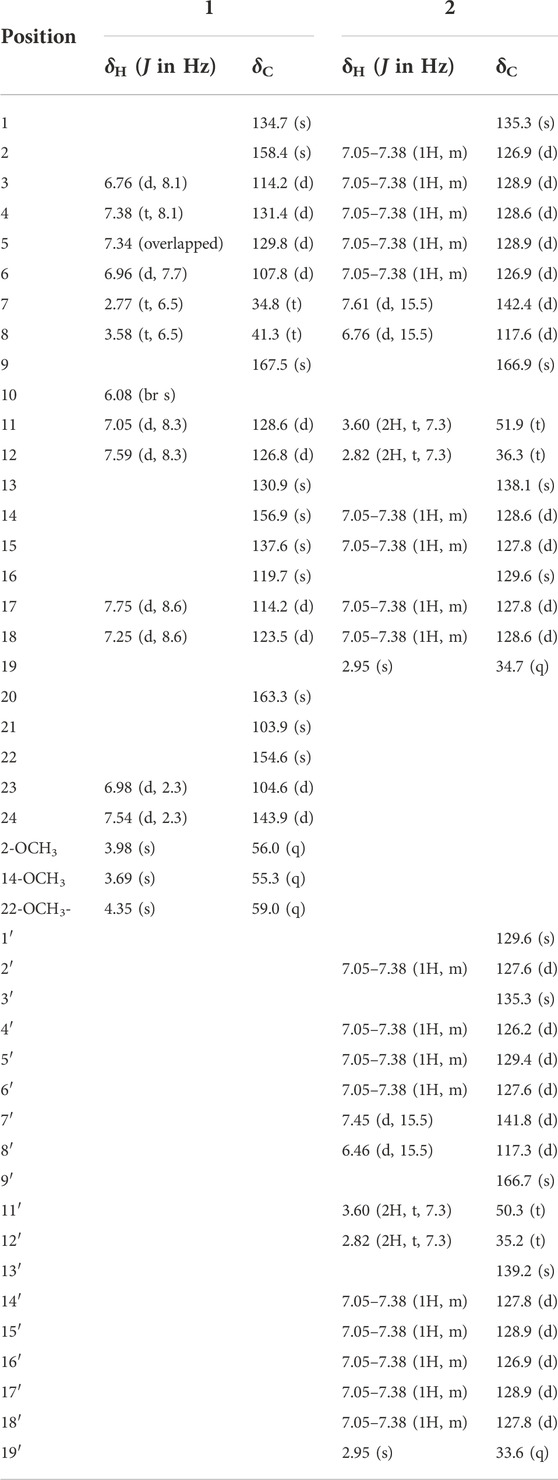
Clauphenamide A (1): yellowish needles; UV (MeOH): λmax nm: 221, 266, 304; IRνmax 3243, 2925, 1641, 1615, 1576, 1493 cm−1; 1H and 13C NMR spectroscopic data see Table 1; positive ion HRESIMS m/z 455.1584 [M + Na]+ (calcd. For C25H24N2O5Na, 455.1582).
Clauphenamide B (2): yellowish plates; UV (MeOH): λmax nm: 216, 221, 280; IRνmax 2911, 1637, 1605, 1572, 1491 cm−1; 1H and 13C NMR spectroscopic data see Table 1; positive ion HRESIMS m/z 551.2676 [M + Na]+ (calcd. For C36H36N2O2Na, 551.2675).
Compound 3: white solid, ESI-MS (positive ion) m/z 617 [2M + Na]+. 1H NMR (400 MHz, pyridine-d5) δH 7.47–7.02 (m, 10H, aromatic H), 5.23 (d, J = 2.3 Hz, 1H, H-7), 4.83 (d, J = 10.7 Hz, 1H, H-3), 4.55 (dd, J = 8.6, 2.3 Hz, 1H, H-5), 4.05 (dd, J = 10.7, 8.6 Hz, 1H, H-4), 3.34 (s, 3H, H-6); 13C NMR (100 MHz, pyridine-d5) δC 175.8 (s, C-2), 142.4 (s, C-1″), 137.4 (s, C-1′), 129.7 (d, C-3′, 5′), 128.6 (d, C-3″, 5″), 128.2(d, C-2′, 6′), 127.8 (d, C-2″, 6″), 127.5 (d, C-4′), 127.0 (d, C-4″), 73.2 (d, C-7), 70.4 (d,C-3), 66.7 (d, C-5), 51.3 (d, C-4), 31.1 (q, C-6).
Compound 4: white solid, ESI-MS (positive ion) m/z 320 [M + Na]+, 617 [2M + Na]+. 1H NMR (400 MHz, MeOD) δH 7.30 (d, J = 7.4 Hz, 2H, H-aromatic), 7.13 (t, J = 7.4 Hz, 2H, H- aromatic), 7.04 (m, 4H, H-aromatic), 6.79 (d, J = 7.4 Hz, 2H, H-aromatic), 5.21 (d, J = 2.1 Hz, 1H, H-7), 4.08 (d, J = 6.0 Hz, 1H, H-3), 3.97 (m, 1H, H-5), 3.21 (t, J = 6.0 Hz, 1H, H-4), 3.04 (s, 3H, H-6). 13CNMR (100 MHz, MeOD) δC 175.8 (s, C-2), 142.7 (s, C-1″), 141.4 (s, C-1′), 129.3 (d,C-aromatic), 129.1 (d, C-aromatic), 128.3 (d, C-aromatic), 127.3 (d, C-aromatic),127.1 (d, C-aromatic), 78.9 (d, C-7), 70.9 (d, C-3), 70.1 (d, C-5), 48.2 (d, C-4), 28.7(q, C-6).
Compound 5: colorless oil, ESI-MS (negative ion) m/z 418 [M]−. 1H NMR (400 MHz, CD3OD) δH 6.51 (4H, s, H-2, 2′, 6, 6′), 4.54 (2H, d, J = 3.9 Hz, H-7, 7′), 4.10 (2H, m, Ha-9, 9′), 3.72 (2H, dd, J = 9.2, 2.8 Hz, Hb-9, 9′), 3.67 (12H, s, H-OCH3), 2.97 (2H, br. s, H-8, 8′). 13C NMR (100 MHz, CD3OD) δC 149.2 (s, C-3, 3′, 5, 5′), 135.8 (s, C-4, 4′), 133.1 (s, C-1, 1′), 104.3 (d, C-2, 2′, 6, 6′), 87.5 (d, C-7, 7′), 72.6 (t, C-9, 9′), 56.6 (q, C-OCH3), 55.3 (d,C-8, 8′).
Compound 6: white powder. ESI-MS (positive ion) m/z 219 [M + Na]+. 1H NMR (400 MHz, CDCl3) δH 5.76 (dt, J = 7.2, 5.4 Hz, 1H, H-8), 5.71 (d, J = 11.3 Hz, 1H, H-9), 2.24 (m, 2H, H-7a, 10), 2.00 (m, 2H, H-7b, 4), 1.69 (m, 6H, H-2, 3, 6), 1.23 (s, 3H, H-12), 1.16 (s, 3H, H-11). 13C NMR (100 MHz, CDCl3) δC 131.6 (d, C-8), 130.3 (d, C-9), 80.1 (s, C-1), 75.1 (s, C-5), 51.2 (d, C-10), 50.4 (d, C-4), 42.5 (t, C-6), 40.2 (t, C-2), 23.6 (t, C-7), 22.5 (q, C-11), 21.7 (t, C-3), 21.6 (q, C-12).
Compound 7: yellow solid. ESI-MS (positive ion) m/z 293 [M + Na]+. 1H NMR (400 MHz, pyridine-d5) δH 7.97 (d, J = 2.1 Hz, 1H, H-2′), 7.86 (d, J = 9.6, 1H, H-4), 7.38 (s, 1H, H-5), 6.90 (d, J = 2.1 Hz, 1H, H-3′), 6.46 (d, J = 9.6 Hz, 1H, H-3), 5.61 (t, J = 7.1 Hz, 1H, H-2″), 5.09 (d, J = 7.1 Hz, 2H, H-1″), 1.65 (s, 6H, H-4″, 5″). 13C NMR (100 MHz, pyridine-d5) δC 160.5 (s, C-2), 148.6 (d, C-7), 147.6 (d, C-2′), 145.1 (d, C-4), 144.2 (s, C-8a), 139.2 (s, C-3″), 131.9 (s, C-8), 126.4 (s, C-6), 120.6 (d, C-2″), 117.1 (s, C-4a), 114.7 (d, C-3), 114.3 (d, C-5), 107.3 (d, C-3′), 70.4 (t, C-1″), 25.7 (q, C-4″), 18.2 (q, C-5″).
Compound 8: yellow solid, ESI-MS (positive ion) m/z 225 [M+ Na]+, 427 [2M + Na]+. 1H NMR (400 MHz, pyridine-d5) δH 8.00(d, J = 1.7 Hz, 1H, H-2′), 7.82 (d, J = 9.6 Hz, 1H, H-4), 7.23 (s, 1H, H-5), 6.90 (d, J = 1.7 Hz, 1H, H-3′), 6.44 (d, J = 9.6 Hz, 1H, H-3). 13C NMR (100 MHz, pyridine-d5) δC 161.2 (s, C-2), 147.4 (d, C-2′), 147.1 (s, C-7), 145.6 (d, C-4), 141.1 (s, C-8a), 132.6 (s, C-8), 126.2 (s, C-6), 117.2 (s, C-4a), 114.7 (d, C-3), 110.1 (d, C-5), 107.7 (d, C-3′).
Compound 9: yellow solid. ESI-MS (positive ion) m/z 377 [M + Na]+. 1H NMR (400 MHz, CDCl3) δH 7.73 (d, J = 9.7 Hz, 1H, H-4), 7.64 (d, J = 2.0 Hz, 1H, H-2′), 7.32 (s, 1H, H-5), 6.77 (d, J = 2.0 Hz, 1H, H-3′), 6.29 (d, J = 9.7 Hz, 1H, H-3), 5.49 (m, 3H, H-2″, 5″, 6″), 4.93 (d, J = 7.0 Hz, 2H, H-1″), 2.60 (d, J = 6.6 Hz, 2H, H-4″), 1.58 (s, 3H, H-8″), 1.23 (s, 6H, H-9″, 10″). 13C NMR (100 MHz, CDCl3) δC 160.5 (s, C-2), 148.5 (s, C-7), 146.6 (d, C-2′), 144.4 (d, C-4), 143.9 (s, C-8a), 141.8 (s, C-3″), 140.3 (d, C-6″), 131.4 (s, C-8), 125.7 (s, C-6), 123.7 (d, C-5″), 120.2 (d, C-2″), 116.5 (s, C-4a), 114.5 (d, C-3), 113.3 (d, C-5), 106.6 (d, C-3′), 70.6 (s, C-7″), 70.1 (t, C-1″), 42.0 (t, C-4″), 29.5 (q, C-9″, 10″), 16.6 (q, C-8″).
Compound 10: yellow solid. ESI-MS (positive ion) m/z 241 [M + Na]+. 1H NMR (400 MHz, pyridine-d5) δH 8.09 (d, J = 2.1 Hz, 1H, H-2′), 7.58 (d, J = 9.7 Hz, 1H, H-4), 6.69 (d, J = 2.1 Hz, 1H, H-3′), 6.53 (d, J = 9.7 Hz, 1H, H-3). 13C NMR (100 MHz, pyridine-d5) δC 161.2 (s, C-2), 148.3 (d, C-2′), 146.2 (s, C-7), 143.3 (d, C-4), 141.9 (s, C-5), 133.1 (s, C-8a), 127.7 (s, C-8), 116.6 (s, C-4a), 116.3 (s, C-6), 115.3 (d, C-3), 107.3 (d, C-3′).
Compound 11: yellow solid. ESI-MS (positive ion) m/z 185 [M + Na]+. 1H NMR (400 MHz, CDCl3) δH 7.67 (d, J = 9.5 Hz, 1H, H-4), 7.42 (d, J = 8.0 Hz, 1H, H-5), 7.06 (m, 1H, H-6), 7.00 (m, 1H, H-8), 6.27 (d, J = 9.5 Hz, 1H, H-3). 13C NMR (100 MHz, CDCl3) δC 163.2 (s, C-7), 161.5 (s, C-2), 157.1 (s, C-9), 144.4 (d, C-4), 130.3 (d, C-5), 114.2 (d, C-6), 112.4 (d, C-3), 112.2 (s, C-10), 103.7 (d, C-8).
Compound 12: white solid. ESI-MS (positive ion) m/z 215 [M + Na]+. 1H NMR (400 MHz, acetone-d6) δH 7.86 (d, J = 9.6 Hz, 1H, H-4), 7.26 (d, J = 8.4 Hz, 1H, H-5), 6.88 (d, J = 8.4 Hz, 1H, H-6), 6.18 (d, J = 9.6 Hz, 1H, H-3), 3.92 (s, 3H, OCH3). 13C NMR (100 MHz, acetone-d6) δC 160.9 (s, C-2), 154.5 (s, C-7), 149.2 (s, C-9), 145.3 (d, C-4), 135.4 (s, C-8), 124.5 (d, C-5), 113.7 (d, C-6), 113.0 (s, C-10), 112.7 (d, C-3), 61.5 (q, OCH3).
Compound 13: yellow solid. ESI-MS (positive ion) m/z 215 [M + Na]+. 1H NMR (400 MHz, acetone-d6) δH 8.83 (s, 1H, OH), 7.84 (d, J = 9.5 Hz, 1H, H-4), 7.21 (s, 1H, H-5), 6.80 (s, 1H, H-8), 6.17 (d, J = 9.5 Hz, 1H, H-3), 3.90 (s, 3H, OCH3). 13C NMR (100 MHz, acetone-d6) δC 161.3 (s, C-2), 151.9 (s, C-8a), 151.2 (s, C-7), 146.1 (s, C-6), 144.7 (d, C-4), 113.3 (d, C-3), 112.2 (s, C-4a), 109.9 (d, C-5), 103.6 (d, C-8), 56.8 (q, OCH3).
Compound 14: yellow solid. ESI-MS (positive ion) m/z 229 [M + Na]+. 1H-NMR (400 MHz, CD3OD) δH: 7.86 (1H, d, J = 9.5 Hz, H-3), 7.12 (1H, s, H-5), 6.78 (1H, s, H-8), 6.20 (1H, d, J = 9.5 Hz, H-4), 3.88 (3H, s, 7-OCH3), 3.67 (3H, s, 6-OCH3); 13C-NMR (100 MHz, CD3OD) δC: 162.2 (s, C-2), 153.1 (s, C-7), 151.7 (s, C-9), 146.5 (s, C-6), 143.7 (d, C-4), 112.6 (d, C-3), 112.2 (s, C-10), 109.2 (d, C-5), 102.4 (d, C-8), 56.1 (q, 7-OCH3), 53.1 (q, 6-OCH3).
Antifungal assay
The inhibitory effects of compounds 1–14 on three fungi (B. dothidea, F. oxysporum and P. oryzae) were evaluated using a mycelial growth inhibition assay (Liu et al., 2009; Huang, et al., 2016). Briefly, dissolve the tested compound in acetone to form a compound solution with a concentration of 100ug/ml, and each compound solution was added to the PDA medium at approximate 50°C to give a culture dish with the toxic medium. After that, a 6-mm in diameter PDA disk with phytopathogen mycelium was transferred to the center of each culture dish, and the side with mycelia was downward. The dish containing an equal amount of acetone acted as solvent control. Each experiment was performed three times. All dishes were placed in a constant temperature incubator and cultured at 27°C. After 4 days, based on the cross method, the diameter of the pathogen growth circle was measured, and the mycelial growth inhibition rate was calculated according to the following formula:
Results and Discussion
Structure elucidation
Compound 1 was isolated as yellowish solid, its molecular formula was deduced to be C25H24N2O5 from its HR-ESIMS data (positive ions) (m/z 455.1584 [M + Na]+, calcd. 455.1582), indicative of 15 degrees of unsaturation. 1 gave IR absorption bands of -NH group at 3243 cm−1 and of an amide at 1641 cm−1. The 1H NMR spectrum of 1 (Table 1) contained signal at δH 6.08 (br s) assigned to the NH group, 7.54 and 6.98 (each, 1H, d, J = 2.3 Hz) assigned to H-24/H-23 on furan, 7.75 and 7.25 (each, 1H, d, J = 8.6 Hz) assigned to H-17/H-18 on benzene ring, 7.59 and 7.05 (each 1H, d, J = 8.3 Hz) assigned to H-12/H-11 on cis-olefin. Besides, the 1H NMR spectrum of 1 (Table 1) also revealed three methoxys (δH 4.35, 3H, s; 3.98, 3H, s and 3.69, 3H, s), two methylenes (δH 3.58, 2H, dd, J = 13.0, 6.5 Hz and δH 2.77, 2H, t, J = 6.5 Hz) and four aromatic protons of one disubstituted benzene ring (δH 7.38, 1H, t, J = 8.1 Hz; δH 7.34, 1H, overlapped; δH 6.96, 1H, d, J = 7.7 Hz and δH 6.76, 1H, d, J = 8.1 Hz). The 13C NMR spectrum (Table 1) revealed resonances for 25 carbons, attributable to three methoxys (δC, 55.3, 56.0 and 59.0), one amide carbonyl (δC, 167.5), two methylenes (δC, 41.3 and 34.8), ten sp2 methines (δC, 104.6, 107.8, 2 × 114.2, 123.5, 126.8, 128.6, 129.8, 131.4, 143,9), nine sp2 carbons (δC, 103.9, 119.7, 130.9, 134.7, 137.6, 154.6, 156.9, 158.4, 163.3). The HSQC spectrum supported this assignment and allowed the association of all these carbons with the directly attached protons.
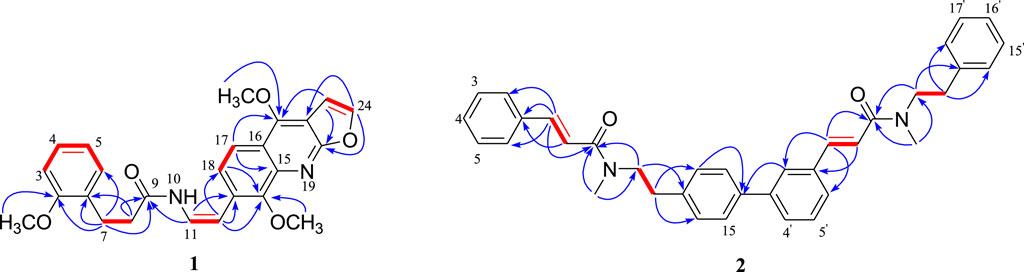
According to the correlations of the 1H–1H COSY spectrum, the 1H NMR multiplets could be classified into five spin systems (the red part in Figure 2). In order to establish the planar structure of 1, the HMBC correlations (Figure 2) were used to connect these fragments and locate quaternary carbons.
Specifically, the 3-phenylpropionyl was deduced from the HMBC correlations of H-7 with the sp2 methine (C-6) and the amide carbonyl (C-9), and H-8 with the sp2 quaternary carbon (C-1). After careful analysis of the remaining 1H and 13C NMR signals of 1, it was inferred that there was also a structural fragment similar to skimmianine (4,7,8-trimethoxyfuro [2,3-b]quinoline) (Liu et al., 1991) in compound 1 in addition to a pair of cis double bonds. Comparison of the NMR spectroscopic data of this fragment with those of the known skimmianine established that the fragment had a very similar structure to the latter, but with a double bond replacing 13-methoxy. To confirm the location of the double bond, HSQC and HMBC experiments were conducted, in the HMBC spectrum (Figure 2), the correlations of H-12 with C-14 and C-18, and H-11 with C-13 supported the connection of the double bond to C-13. Ulteriorly, the HMBC correlation (Figure 2) of H-11 with C-9 showed that the 3-(2-methoxyphenyl)propanamido and the fragment similar to skimmianina were connected by the double bond. In addition, the HMBC (Figure 2) correlations of H-7 and H-(2-OCH3) with C-2 revealed that one methoxy was attached to C-2. Thus, the structure of clauphenamide A (1), with the unit of N-2-(4,8-dimethoxyfuro [2,3-b]quinolin-7-yl)vinyl, was determined as shown in Figure 1.
Compound 2, a yellowish plates, was found to have a molecular formula of C36H36N2O2, which was deduced from the HRESIMS data (positive ions, m/z 551.2676 [M + Na]+, calcd. 551.2675). The 1H NMR spectrum of 2 (Table 1) contained two pairs of trans-olefinic protons signals (δH 7.61, 6.76, each 1H, d, J = 15.5 Hz and δH 7.45, 6.46, each 1H, d, J = 15.5 Hz), two pairs of methylenes coupled to each other (δH 3.60, 4H, d, J = 7.3 Hz and δH 2.82, 4H, t, J = 7.3 Hz) and two N-methyls (δH 2.95, 6H, s) (Lin, 1989). Thus, 2 was assigned as a phenylpropionamide (lansiumamide C) (Lin, 1989) dimer. The HMBC correlations of the H-2′and C-7′/C-16 implied that C-1′ was connected to C-16. So, the structure of clauphenamide B (2), the first phenylpropionamide dimer, was proposed as shown in Figure 1.
By comparing the spectral data of the 12 known compounds with those reported in the literature, their structures were identified as (-)-clausenamide (3) (Yang, et al., 1988), neoclausenamide (4) (Yang, et al., 1988), syringaresinol (5) (Ouyang et al., 2007), radicol (6) (Zhao et al., 2008), imperatorin (7) (Dien et al., 2012), 8-hydroxyfurocoumarin (8) (Kumar et al., 1995), (E,E)-8-(7-hydroxy-3,7-dimethylocta-2,5-dienyloxy)psoralen (9) (Ito et al., 1998), 5,8-dihydroxypsoralen (10) (Marumoto and Miyazawa, 2012), umbelliferone (11) (Baba et al., 1987), 7-hydroxy-8-methoxycoumarin (12) (Alexander et al., 1987), scopoletin (13) (Liu et al., 2011), scoparone (14) (Chen et al., 2010).
Antifungal activity
The antifungal activities of compounds 1–14 were evaluated (Table 2) by a mycelial growth inhibition assay. At the concentration of 100 μg/ml, compared with the control (chlorothalonil, inhibition rate of 83.67%), compounds 1 and 2 were found to exhibit moderate activity against B. dothidea with inhibition rate values of 68.39% and 52.05%, respectively. Compounds 11–14 showed antifungal activities to varying degrees against B. dothidea, F. oxysporum and P. oryzae, with inhibition rates greater than 40%. In addition, compared with the control (chlorothalonil, inhibition rate of 69.02%), compounds 11–14 showed strong antifungal activity to P. oryzae, with inhibition rates greater than 55%. Among them, compound 14 has the strongest antifungal activity against P. oryzae, and the inhibition rate (65.44%) is close to that of the control chlorothalonil. Compounds 5–10 showed weak antifungal activity against three kinds of fungi, and the inhibition rate was less than 15%.
Structure-activity relationship
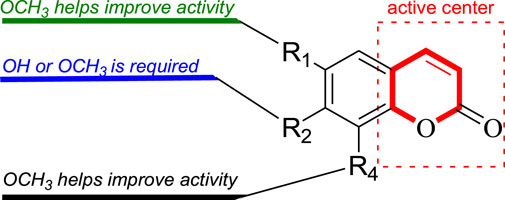
In this study, Fourteen different types of compounds showed antifungal activities to varying degrees against B. dothidea, F. oxysporum and P. oryzae. Comparison of coumarins (7–14) suggests that the inhibitory activities of simple coumarins on three fungi are generally higher than that of furacoumarins. Further comparison of simple coumarins (11–14) reveals that the substitutions at positions 7 and 6 seem to be helpful to improve the antifungal activities of coumarins against three fungi, and methoxylations at positions 7 and 6 have better inhibitory effect on three fungi than hydroxylation. The structure-activity relationship of simple coumarins could be drawn as shown in Figure 3.
Conclusion
In our present research, fourteen compounds were isolated from the twigs and leaves of C. lansium, among which compounds 1 and 2 were two novel amides. Compounds 5, 6, 10 and 12 were separated from the genus (Clausena) for the first time, while 13 was isolated in the species (C. lansium) for the first time. All isolated compounds were evaluated for their antifungal activities against B. dothidea, F. oxysporum and P. oryzae. As a result, clauphenamide A (1) and clauphenamide B (2) displayed moderate activity against B. dothidea. Umbelliferone (11), 7-hydroxy-8-methoxycoumarin (12), scopoletin (13), and scoparone (14) also exhibited moderate activity against B. dothidea and F. oxysporum. In addition, 11–14 showed strong antifungal activity against P. oryzae, among them, 14 has the strongest antifungal effect against P. oryzae and its inhibition rate is close to that of the control (chlorothalonil). Preliminary structure-activity relationship analysis revealed that: (1) Simple coumarins generally have higher antifungal effects than furacoumarins; (2) Methoxylations at positions 7 and 6 of simple coumarins have better inhibitory effects on fungi than hydroxylation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
WP: conceived, designed the experiments, and revised the manuscript; XF and SX: experimental design and the draft writing; DC, MY, and MX collected the plant material; QZ, YH, and HW carried out the experiments and data analyses; All authors have read and approved the published version of the manuscript.
Funding
This project was funded by the National Natural Science Foundation of China (No. 32060102 and 31660094), NSFC-Jiangxi Province, China (No. 20181BAB204002), the earmarked fund for Innovation team of Jiangxi Agricultural University (JXAUCXTD002) and Jiangxi Sericultural Industry Technology System (No. JXARS-23). The authors, therefore, acknowledge with thanks above funds for technical and financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1104805/full#supplementary-material
References
Adebajo, A. C., Iwalewa, E. O., Obuotor, E. M., Ibikunle, G. F., Omisore, N. O., Adewunmi, C. O., et al. (2009). Pharmacological properties of the extract and some isolated compounds of Clausena lansium stem bark: Anti-trichomonal, antidiabetic, anti-inflammatory, hepatoprotective and antioxidant effects. J. Ethnopharmacol. 122, 10–19. doi:10.1016/j.jep.2008.11.015
Alexander, I. G., Viaran, J. M., and Noreen, B. O. (1987). Coumarins from two coleonema species. Phytochemistry 26, 257–260. doi:10.1016/S0031-9422(00)81523-X
Baba, K., Takeuch, i. K., Tabata, Y., Taniguchi, M., Kozawa, M., and Zasshi, Y. (1987). Chemical Studies on the Constituents of the Thymelaeceous Phants IV Structure of A New Spiro Biflavonoid Genkwanol A from the Root of Daphne Genkwa Sieb. et Zucc. Yakugaku Zasshi 107, 525–529. doi:10.1248/yakushi1947.107.7_525
Chen, J. X., Huang, S. H., Wang, Y., Shao, M., and Ye, W. C. (2010). Studies on the chemical constituents from Lobelia chinensis. J. Chin. Med. Mat. 33, 1721–1724. doi:10.13863/j.issn1001-4454.2010.11.033
Cheng, S. S., Chang, H. T., Lin, C. Y., Chen, P. S., Huang, C. G., Chen, W. J., et al. (2010). Insecticidal activities of leaf and twig essential oils from Clausena excavata against Aedes aegypti and Aedes albopictus larvae. Pest Manag. Sci. 65, 339–343. doi:10.1002/ps.1693
Deng, H. D., Mei, W. L., Guo, Z. K., Liu, S., Zuo, W. J., Dong, W. H., et al. (2014a). Monoterpenoid coumarins from the peels of Clausena lansium. Planta Med. 80, 955–958. doi:10.1055/s-0034-1382839
Deng, H. D., Mei, W. L., Wang, H., Guo, Z. K., Dong, W. H., Wang, H., et al. (2014b). Carbazole alkaloids from the peels of Clausena lansium. J. Asian Nat. Prod. Res. 16, 1024–1028. doi:10.1080/10286020.2014.930442
Dien, P. H., Nhan, N. T., Thuy, H. T. L., and Quang, D. N. (2012). Main constituents from the seeds of Vietnamese cnidium monnieri and cytotoxic activity. Nat. Prod. Res. 26, 2107–2111. doi:10.1080/14786419.2011.619186
Du, Y. Q., Liu, H., Li, C. J., Ma, J., Zhang, D., Li, L., et al. (2015). Bioactive carbazole alkaloids from the stemsof Clausena lansium. Fitoterapia 103, 122–128. doi:10.1016/j.fitote.2015.03.018
Editorial Committee (1997). Editorial committee of flora of China Chinese Academy of Sciences, 1997. Flora of China. Beijing, China: Science Press, 126. 43.
Fan, Y. J., Chen, H. Q., Mei, W. L., Kong, F. D., Li, F. X., Chen, P. W., et al. (2018). Nematicidal amidealkaloids from the seeds of Clausena lansium. Fitoterapia 128, 20–25. doi:10.1016/j.fitote.2018.04.023
Han, Y., Li, L. C., Hao, W. B., Tang, M., and Wan, S. Q. (2013). Larvicidal activity of lansiumamide B from the seeds of Clausena lansium against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 112, 511–516. doi:10.1007/s00436-012-3161-x
Huang, L., Li, D., Xu, Y. S., Feng, Z. L., Meng, F. C., Zhang, Q. W., et al. (2017). Clausoxamine, an alkaloid possessing a 1, 3-oxazine-4-one ring from the seeds of Clausena lansium and the antiobesity effect of lansiumamide B. RSC Adv. 7, 46900–46905. doi:10.1039/C7RA09793J
Huang, Y. F., Wang, H. C., Cheng, Q. Y., Wang, J., Zhang, C. Q., and Lu, H. X. (2016). Inhibitory activities of six fungicides against mycelial growth and conidial germination of alternaria alternate. Chin. J. Pest Sci. 18, 263–367. doi:10.16801/j.issn.1008-7303.2016.0035
Ito, C., Katsuno, S., and Furukawa, H. (1998). Structures of lansiumarin-A, -B, -C, three new furocoumarins from Clausena lansium. Chem. Pharm. Bull. 46, 341–343. doi:10.1248/cpb.46.341
Kumar, V., Vallipuram, K., Adebajo, A. C., and Reisch, J. (1995). Dihydroxy-3-formyl-1-(3'-methyl-2'-butenyl) carbazole from Clausena lansium. Phytochemistry 240, 71563–71565. doi:10.1016/0031-9422(95)00452-d
Li, L. C., Feng, X. J., Tang, M., Hao, W. B., Han, Y., Zhang, G. B., et al. (2014). Antibacterial activity of lansiumamide B to tobacco bacterial wilt (Ralstonia solanacearum). Microbiol. Res. 169, 522–526. doi:10.1016/j.micres.2013.12.003
Lin, J. H. (1989). Cinnamamide derivatives from Clausena lansium. Phytochemistry 28, 621–622. doi:10.1016/0031-9422(89)80063-9
Liu, H., Li, F., Li, C. J., Yang, J. Z., Li, L., Chen, N. H., et al. (2014). Bioactive furanocoumarins from stems of Clausena lansium. Phytochemistry 107, 141–147. doi:10.1016/j.phytochem.2014.08.002
Liu, M. C., Hu, D. Y., Song, B. A., Song, Y., and Lin-Hong, J. (2011). Chemical constituents from uncaria sinensis (oliv.) havil. Nat. Prod. Res. Dev. 23, 1058–1060. doi:10.16333/j.1001-6880.2011.06.013
Liu, S. L., Wei, L. X., Wang, D., and Gao, C. Y. (1991). Studies on the Chemical Constituents from the Peel of Zanthoxylum Schinifolium Sieb et Zucc. Acta Pharm. Sin. 26, 836–840. doi:10.16438/j.0513-4870.1991.11.007
Liu, X. Y., Fu, X. X., Li, Y. Y., Xiong, Z. H., Li, B. T., and Peng, W. W. (2021). The sesquiterpenes from the stem and leaf of Clausena lansium with their potential antibacterial activities. Nat. Prod. Res. 35, 4887–4893. doi:10.1080/14786419.2020.1741577
Liu, Y. X., Gong, Z. Y., and Wan, S. Q. (2009). Inhibitory effects of clausenamide alkaloid on seven fruit pathogenic fungi. Plant Prot. 35, 53–56. doi:10.1026//0049-8637.33.4.221
Manosroi, A., Saraphanchotiwitthaya, A., and Manosroi, J. (2005). Vivo immunomodulating activity of wood extracts from Clausena excavata burm. F. J. Ethnopharmacol. 102, 5–9. doi:10.1016/j.jep.2005.04.033
Marumoto, S., and Miyazawa, M. (2012). Structure-activity relationships for naturally occurring coumarins as beta-secretase inhibitor. Bioorg. Med. Chem. 20, 784–788. doi:10.1016/j.bmc.2011.12.002
Milner, P. H., Coates, N. J., Gilpin, M. L., Spear, S. R., and Eggleston, D. S. (1996). SB-204900, A novel oxirane carboxamide from Clausena lansium. J. Nat. Prod. 59, 400–402. doi:10.1021/np9600614
Moshi, M. J., Kagashe, G. A. B., and Mbwambo, Z. H. (2005). Plants used to treat epilepsy by Tanzanian traditional healers. J. Ethnopharmacol. 97, 327–336. doi:10.1016/j.jep.2004.11.015
Ng, T. B., Lam, S. K., and Fong, W. P. (2003). A homodimeric sporamin-type trypsin inhibitor with antiproliferative, HIV reverse transcriptase-inhibitory and antifungal activities from wampee (Clausena lansium) seeds. Biol. Chem. 384, 289–293. doi:10.1515/BC.2003.032
Okokon, J. E., Etebong, E. O., Udobang, J. A., and Essien, G. E. (2012). Antiplasmodial and analgesic activities of Clausena anisata. Asian pac. J. Trop. Med. 3, 214–219. doi:10.1016/s1995-7645(12)60027-3
Ouyang, M. A., Wein, Y. S., Zhang, Z. K., and Kuo, Y. H. (2007). Inhibitory activity against tobacco mosaic virus (TMV) replication of pinoresinol and syringaresinol lignans and their glycosides from the root of rhus javanica var. J. Agric. Food Chem. 55, 6460–6465. doi:10.1021/jf0709808
Peng, W. W., Fu, X. X., Li, Y. Y., Xiong, Z. H., Shi, X. G., Zhang, F., et al. (2019). Phytochemical study of stem and leaf of Clausena lansium. Molecules 24, 3124. doi:10.3390/molecules24173124
Peng, W. W., Fu, X. X., Xiong, Z. H., Wu, H. L., Chang, J. W., Huo, G. H., et al. (2020). Taxonomic significance and antitumor activity of alkaloids from Clausena lansium lour. Skeels (rutaceae). Biochem. Syst. Ecol. 90, 104046. doi:10.1016/j.bse.2020.104046
Peng, W. W., Fu, X. X., Xiong, Z. H., Xiang, M. L., Yang, Y. L., Wu, H. L., et al. (2021). Chemical components from the stems and leaves of Clausena lansium lour. Skeels and their potential herbicidal effects. Pest Manag. Sci. 77, 1355–1360. doi:10.1002/ps.6150
Peng, W. W., Zheng, L. X., Ji, C. J., Shi, X. G., Xing, Z. H., and Shangguan, X. C. (2018). Carbazole alkaloids isolated from the branch and leaf extracts of Clausena lansium. Chin. J. Nat. Med. 16, 509–512. doi:10.1016/S1875-5364(18)30087-6
Prasad, K. N., Xie, H., Hao, J., Yang, B., Qiu, S. X., Wei, X. Y., et al. (2010). Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee Clausena lansium (lour.) skeels peel. Food Chem. x. 118, 62–66. doi:10.1016/j.foodchem.2009.04.073
Ramkumar, G., Karthi, S., Muthusamy, R., Natarajan, D., and Shivakumar, M. (2015). Insecticidal and repellent activity of Clausena dentata (rutaceae) plant extracts against Aedes aegypti and Culex quinquefasciatus mosquitoes (Diptera: Culicidae). Parasitol. Res. 1145, 1139–1144. doi:10.1007/s00436-014-4288-8
Shen, D. Y., Chan, Y. Y., Hwang, T. L., Juang, S. H., Huang, S. C., Kuo, P. C., et al. (2014). Constituents of the roots of Clausena lansium and their potential antiinflammatory activity. J. Nat. Prod. 77, 1215–1223. doi:10.1021/np500088u
Shen, D. Y., Chao, C. H., Chan, H. H., Huang, G. J., Hwang, T. L., Lai, C. Y., et al. (2012). Bioactiveconstituents of Clausena lansium and A method for discrimination ofaldose enantiomers. Phytochemistry 82, 110–117. doi:10.1016/j.phytochem.2012.06.019
Song, W. W., Zeng, G. Z., Peng, W. W., Chen, K. X., and Tan, N. H. (2014). Cytotoxic amides and quinolones from Clausena lansium. Helv. Chim. Acta 97, 298–305. doi:10.1002/hlca.201300323
Sunthitikawinsakul, A., Kongkathip, N., Kongkathip, B., Phonnakhu, S., Daly, J. W., Spande, T. F., et al. (2003). Anti-HIV-Ⅰ limonoid: First isolation from Clausena excavata. Phytother. Res. 17, 1101–1103. doi:10.1002/ptr.1381
Tada, Y., Shikishima, Y., Takaishi, Y., ShibataHigutiHonda, H. T. G., Honda, G., Ito, M., et al. (2002). Coumarins and gamma-pyrone derivatives from prangos pabularia: Antibacterial activity and inhibition of cytokine release. Phytochemistry 59, 649–654. doi:10.1016/S0031-9422(02)00023-7
Xu, X. Y., Xie, H. H., and Wei, X. Y. (2014). Jasmonoid glucosides, sesquiterpenes and coumarins from the fruit of Clausena lansium. LWT - Food Sci. Technol. 59, 65–69. doi:10.1016/j.lwt.2014.04.033
Yan, H., Xiong, Z., Xie, N., Liu, S. Z., Zhang, L., L., Xu, F., et al. (2018). Bioassay-guided isolation of antifungal amides against Sclerotinia sclerotiorum from the seeds of Clausena lansium. Ind. Crops Prod. 121, 352–359. doi:10.1016/j.indcrop.2018.05.037
Yang, M. H., Chen, Y. Y., and Liang, H. (1988). Three novel cyclic amides from Clausena lansium. Phytochemistry 27, 445–450. doi:10.1016/0031-9422(88)83117-0
Zhang, R., Wan, S., and Zhao, D. (2012). Advances in chemical constituents and biological activities of Clausena lansium. Nat. Prod. Res. Devt. 24, 118–123. doi:10.16333/j.1001-6880.2012.01.025
Keywords: Clausena lansium, amide, coumarin, antifungal activity, structure-activity relationships
Citation: Fu X, Xiao S, Cao D, Yuan M, Xiang M, Zhou Q, Huang Y, Wei H and Peng W (2022) Antifungal active ingredient from the twigs and leaves of Clausena lansium Lour. Skeels (Rutaceae). Front. Chem. 10:1104805. doi: 10.3389/fchem.2022.1104805
Received: 22 November 2022; Accepted: 28 November 2022;
Published: 13 December 2022.
Edited by:
Yefeng Tang, Tsinghua University, ChinaReviewed by:
Jiwen Zhang, Northwest A&F University, ChinaYa Li, Lanzhou University, China
Chen Huabao, Sichuan Agricultural University, China
Copyright © 2022 Fu, Xiao, Cao, Yuan, Xiang, Zhou, Huang, Wei and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenwen Peng, d3dwZW5nQGp4YXUuZWR1LmNu
†These authors have contributed equally to this work
 Xiaoxiang Fu1†
Xiaoxiang Fu1† Qinghong Zhou
Qinghong Zhou Yingjin Huang
Yingjin Huang Wenwen Peng
Wenwen Peng