- 1Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Provincial Center for Research and Development of Natural Products, Yunnan Characteristic Plant Extraction Laboratory, School of Pharmacy, Yunnan University, Kunming, China
- 2Henan Engineering Research Center of Funiu Mountain’s Medical Resources Utilization and Molecular Medicine, School of Medical Sciences, Pingdingshan University, Pingdingshan, China
Lignans are widely present in traditional medicinal plants. Many natural arylnaphthalene lactone lignans (NALLs) isolated from the genera Justicia, Haplophyllum, and Phyllanthus possess interesting biological activities. Herein, we report a general strategy for the total synthesis of this kind of lignans. Features of this new approach are an aryl–alkyl Suzuki cross-coupling to introduce the dioxinone unit, a cation-induced cyclization to construct the aryl dihydronaphthalene, and base-mediated oxidative aromatization to furnish the arylnaphthalene core. By incorporating these key transformations, the total syntheses of justicidins B and E and taiwanin C covered type I and type II NALLs were accomplished.
1 Introduction
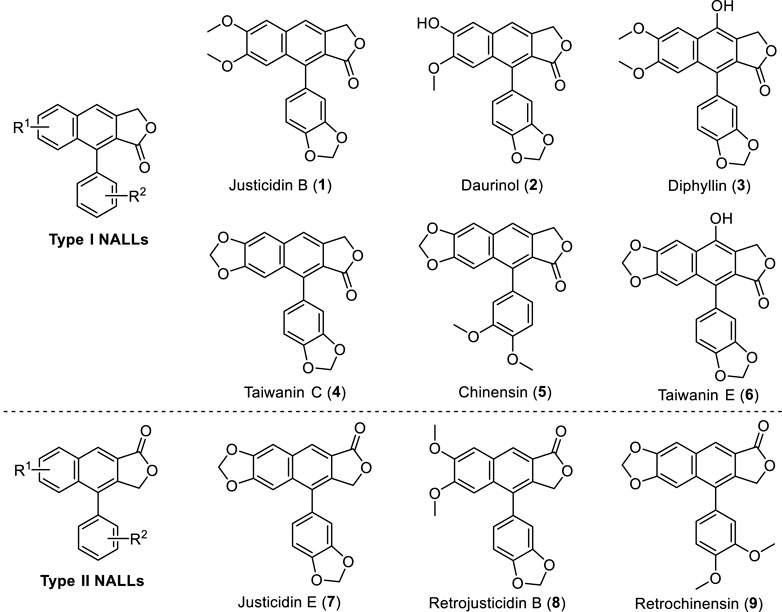
Natural arylnaphthalene lactone lignans (NALLs) are widely isolated from the plant family Acanthaceae (Day et al., 1999; Shen et al., 2004; Zhang et al., 2007; Jin et al., 2014; Jiang et al., 2017; Jin et al., 2017; Lv et al., 2021; Liu et al., 2022), Euphorbiaceae (Anjaneyulu et al., 1981; Wu et al., 2006) and Rutaceae (Gözler et al., 1984; Sheriha et al., 1984; Hesse et al., 1992; Ulubelen et al., 1994; Gözler et al., 1996), especially from the genera Justicia, Haplophyllum, and Phyllanthus. Many of these lignans possess a broad range of biological activities, including antimicrobial (Kawazoe et al., 2001), antifungal (Ashraf et al., 1995), anti-cancer (Wang et al., 2019), antiplatelet (Chen et al., 1996; Weng et al., 2004), antiprotozoal (Gertsch et al., 2003), antimetastatic (Hajdu et al., 2014), antiviral (Sagar et al., 2004; Yeo et al., 2005; Janmanchi et al., 2010), cytotoxic (Day et al., 2002; Chang et al., 2003; Susplugas et al., 2005; Vasilev et al., 2006), and neuroprotective activities (Chun et al., 2017) in cell-based assays or animal models. For instance, justicidin B exhibits powerful antimicrobial activity (El-Gendy et al., 2008) and inhibitory activity against the Sindbis virus (Charlton, 1998). Meanwhile, taiwanin C exhibits important antiplatelet activity (Daron et al., 2022) and was found to be a potent COX inhibitor (Ban et al., 2002). Some representative natural arylnaphthalene lactone lignans (1–9) are shown in Figure 1.
Because of their important pharmacological properties, NALLs have attracted attention from the organic synthetic community since the pioneering synthetic work on these lignans in 1895 by Michael et al. (1895). Synthetic efforts have resulted in many impressive approaches toward these highly substituted 1-arylnaphthalenes and culminated in the total synthesis of a series of arylnaphthalene lactone-type lignans (Chen et al., 2018; Zhao et al., 2018; Park et al., 2020). Methodologies for the construction of 1-arylnaphthalenes could be roughly classified into five categories: Diels–Alder type cycloaddition (Brown et al., 1964; Holmes et al., 1971; Klemm et al., 1971; Takano et al., 1985; Stevenson et al., 1989; Padwa et al., 1996; Xiong et al., 2012; Kudoh et al., 2013; Kocsis et al., 2014; Park et al., 2014; Meng et al., 2016), benzannulation (Ogiku et al., 1995; Flanagan et al., 2002; Nishii et al., 2005; Ishikawa et al., 2021; Moriguchi et al., 2021), Garratt–Braverman-type cyclization (Mondal et al., 2011; Mondal et al., 2012), transition metal-mediated cyclization (Murakami et al., 1998; Mizufune et al., 2001; Sato et al., 2004; Sato et al., 2007; Gudla et al., 2011; Patel et al., 2013; Wong et al., 2014; Kao et al., 2015; Naresh et al., 2015; Xiao et al., 2018), and other type of annulations (Ogiku et al., 1990; Kamal et al., 1994; Ogiku et al., 1995; Harrowven et al., 2001; Foley et al., 2010; He et al., 2014; Hayat et al., 2015; Yamamoto et al., 2015).
Inspired by these well-designed processes and our previous efforts on cation-induced cyclization (Chen et al., 2017; Chen et al., 2019; Wei et al., 2021; Chen et al., 2022; Li et al., 2022), we recently developed an intramolecular cation-induced reaction to synthesize the highly substituted 1-aryl dihydronaphthalene unit, an advanced precursor of natural arylnaphthalene lactone lignans. In this paper, we report a general and flexible strategy toward the synthesis of justicidin E (type II NALLs), justicidin B, and taiwanin C (type I NALLs) based on this efficient cation-induced cyclization.
2 Results and discussion
2.1 Retrosynthetic analysis
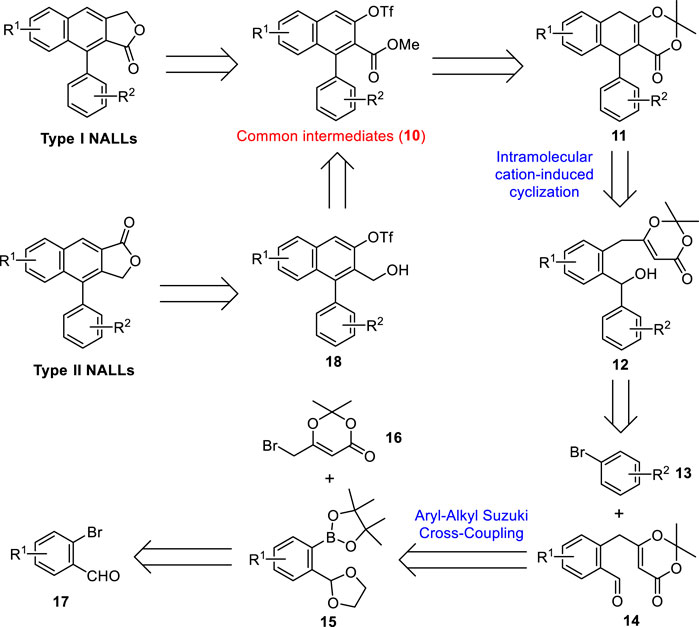
Our retrosynthetic analysis for both type I and type II NALLs is shown in Scheme 1. Type I NALLs could be achieved by a Stille cross-coupling between common intermediates (10) and tributylstannyl methanol followed by lactonization (Zhang et al., 2019). Type II NALLs could be accessed via carbonylative lactonization (Crisp et al., 1995) of triflate 18, which could be obtained via a reduction from common intermediates (10). Ring opening of dioxinone 11 followed by subsequent base-mediated oxidation (Zhao et al., 2020) and triflation would lead to methyl ester 10. Dihydronaphthalene 11 could be accessed through the intramolecular cation-induced cyclization of alcohol 12, which could be prepared by a selective nucleophilic addition of aryl lithium generated in situ from aryl bromide 13 to aldehyde 14. Aldehyde 14 was expected to be formed by an aryl–alkyl Suzuki cross-coupling between pinacolyl borate 15 and commercially available alkyl bromide 16 followed by a deprotection of the ketal moiety. Borate 15 could be obtained from commercially available bromide 17 via functional group protection, halogen–lithium exchange reaction, and borylation.
2.2 Total synthesis of justicidin B
We chose justicidin B, a type I NALL, as the first target of our synthetic journey. Our synthesis began with the preparation of pinacolyl borate 15a (Scheme 2). Treatment of commercially available bromo-aldehyde 17a with ethylene glycol provided its acetal, after subsequent halogen–lithium exchange by exposing it with n-butyllithium followed by borylation (Nagaki et al., 2012) provided 15a in 85% yield.
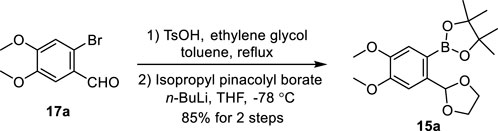
SCHEME 2. Gram-scale synthesis of pinacolyl borate 15a. Bu: butyl, THF: tetrahydrofuran, and Ts: p-toluenesulfonyl.
With pinacolyl borate in hand, we next explored aryl–alkyl Suzuki cross-coupling between borate 15a and commercially available alkyl bromide 16 (Table 1). Although numerous conditions for Suzuki cross-coupling reactions between alkyl halide and aryl boric acid or borate have been developed, using alkyl bromide 16 as a coupling partner to accomplish this cross-coupling reaction is still challenging due to the thermosensitive and base-sensitive dioxinone unit present in substrate 16 (Reber et al., 2009; Katsuki et al., 2017).

TABLE 1. Optimization for the aryl–alkyl Suzuki cross-couplinga.
In order to optimize the yield of this cross-coupling reaction, a systematic screening of reaction conditions was conducted (Table 1). Initially, we used the regular catalyst Pd(PPh3)4 employed in Suzuki cross-coupling (Miyaura et al., 1979). Not surprisingly, Pd(PPh3)4 was completely ineffective for the desired cross-coupling (Table 1, entry 1). Reactions were then conducted at a 0.2-mmol scale with several commercially available palladium catalysts (10 mol%) in the presence of PPh3 (20 mol%) and K3PO4 in 1,4-dioxane (Table 1, entries 2–5). We found that Pd(dba)2 served as an efficient Pd source for this coupling process (Table 1, entry 5). Next, the bases were screened, and the yield of the desired product 19a was not increased with a number of bases (Table 1, entries 5–9). A number of ligands were then used. We found that a ligand has a significant impact on the efficiency of this cross-coupling reaction (Table 1, entries 9–13). When the S-Phos ligand was used, the desired product 19a could be obtained with 51% yield (Table 1, entry 9). With the catalytic system in hand, we next screened the solvents, and DME gave the best results (Table 1, entries 13–18). Finally, the optimum reaction conditions for this coupling reaction (Table 1, entry 18) were established.
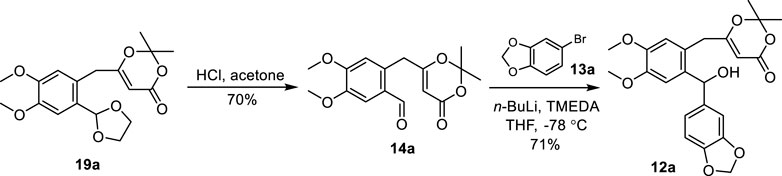
Next, the acetal protecting group of compound 19a was removed with HCl in acetone to produce aldehyde 14a (Scheme 3). The treatment of 13a with n-BuLi followed by the addition of aldehyde 14a unfortunately failed to yield the desired benzhydrol 12a. To promote the desired reaction, a number of additives were used including hexamethylphosphoric acid triamide (HMPA), N,N-dimethyl propylene urea (DMPU), and N,N,N′,N′-tetramethylethylenediamine (TMEDA). The addition of TMEDA provided benzhydrol 12a at 71% yield.
With benzhydrol 12a in hand, we next focused on the proposed cation-induced cyclization (Table 2). A number of Brønsted acids and Lewis acids (Table 2, entries 1–6) were used. Although the cyclization could be promoted by Brønsted acids, BF3·Et2O provided the best yield (Table 2, entry 6). The yield of the targeted product could be further improved when the reaction was conducted at a lower temperature (Table 2, entry 8). This cation-induced cyclization could be scaled up to 2.1 mmol (Table 2, entry 8, 0.90 g, and 68% yield).
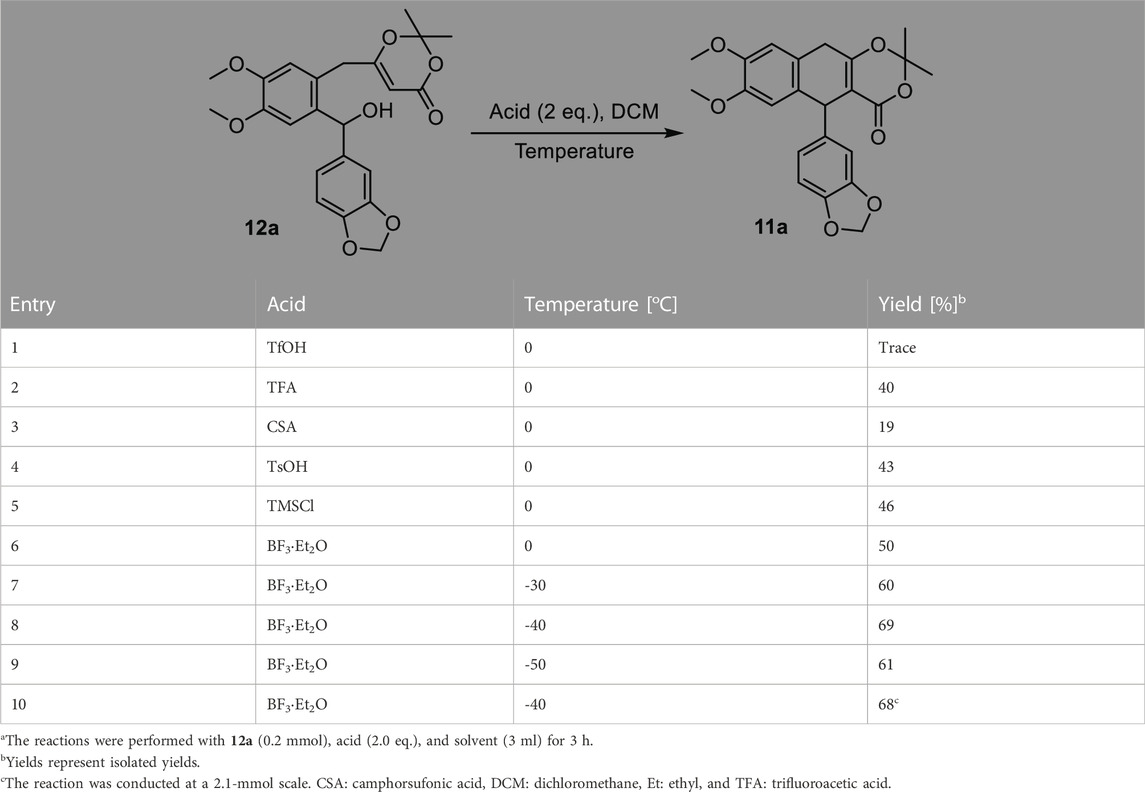
TABLE 2. Optimization for the intramolecular cation-induced cyclizationa.
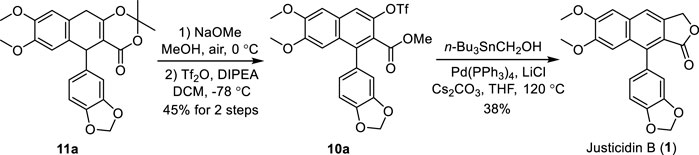
Having established the procedure for advanced intermediate 11a, research focus was then directed toward the total synthesis of justicidin B 1). The treatment of 11a with sodium methoxide in MeOH under air followed by the addition of Tf2O and DIPEA in DCM produced the first common intermediate 10a in 45% yield (Scheme 4). It is noteworthy that an oxidative (by air) aromatization occurred under strong basic conditions. Next, a Pd-catalyzed Stille cross-coupling of triflate 10a with tributylstannyl methanol in the presence of Pd(PPh3)4, Cs2CO3, and LiCl followed by spontaneous lactonization provided natural justicidin B (Zhang et al., 2019). The NMR spectra of our synthetic sample were in full agreement with those reported in the literature (Okigawa et al., 1970; Borges et al., 2018).
2.3 Total synthesis of taiwanin C and justicidin E
To demonstrate the generality and flexibility of our strategy, the total syntheses of naturally occurring arylnaphthalene lignans taiwanin C (type I) and justicidin E (type II) were conducted accordingly. Treatment of commercially available piperonyl bromide 17b with ethylene glycol in the presence of TsOH followed by a halogen–lithium exchange and borylation afforded the pinacolyl borate 15b in 74% yield (Scheme 5). Suzuki cross-coupling of bromide 16 with 15b under the optimum reaction conditions afforded the corresponding dioxinone 19b. Deprotection of the acetal of 19b with HCl in acetone followed by a selective 1,2-addition with the 3,4-methylenedioxyphenyllithium, which was generated in situ from the halogen–lithium exchange between bromide 13a and n-BuLi, yielded the benzhydrol 12b in 59% for two steps.
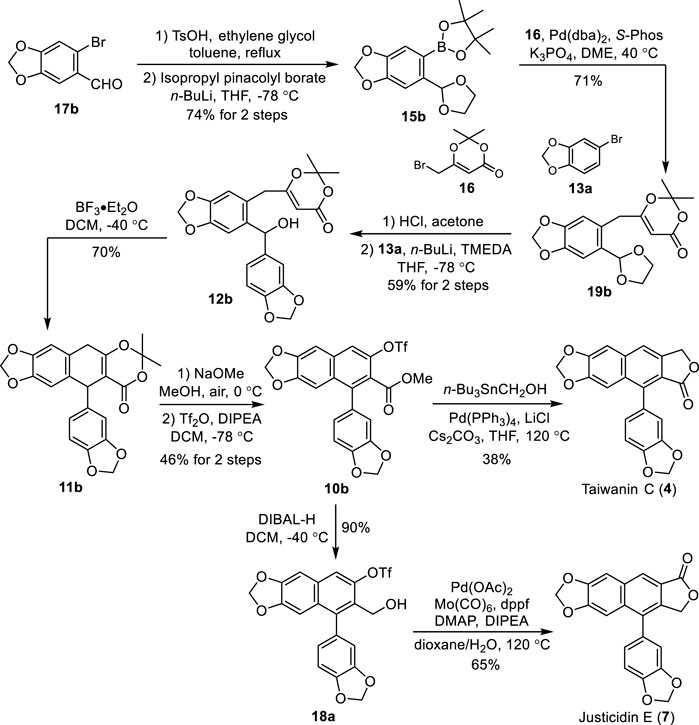
SCHEME 5. Total synthesis of taiwanin C (4) and justicidin E (7). DMAP: 4-dimethylaminopyridine, dppf: 1,1′-bis(diphenylphosphino)ferrocene.
Aryl dihydronaphthalene 11b was obtained successfully in 70% yield through our intramolecular cation-induced cyclization from benzhydrol 12b. The treatment of 11b with NaOMe in MeOH under air followed by triflation with Tf2O afforded the common intermediate 10b in 46% yield for two steps. Reaction of 10b with tributylstannyl methanol in the presence of Pd(PPh3)4, Cs2CO3, and LiCl produced the natural taiwanin C 4). Reduction of 10b with DIBAL-H provided the alcohol 18a in 90% yield. Natural justicidin E (7) was furnished in 38% isolated yield via an improved Pd-catalyzed carbonylative lactonization of triflate 18a with Co(CO)6. The NMR spectra of these two synthetic samples agree well with the reported literature (Anjaneyulu et al., 1981; Subbaraju et al., 1996; Flanagan et al., 2002).
3 Conclusion
We have developed a general and flexible strategy for the synthesis of justicidin B, taiwanin C, and justicidin E from commercially available materials. Key transformations to the success of the synthesis were an aryl–alkyl Suzuki cross-coupling, an intramolecular cation-induced cyclization, and a base-mediated oxidative aromatization. Our new approach paves the way toward the synthesis of biologically active natural arylnaphthalene lactone lignans and could be used for the preparation of their analogues.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HZ conceived the synthetic design. WC and HZ supervised the project. KW, YS, YX, WH, YM, and YL conducted the experimental work and data analysis. WC and HZ wrote the manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of China (U1702286, 21901224, 22261054, and 22271247), the Program for Changjiang Scholars and Innovative Research Team in University (IRT17R94), Ling-Jun Scholars of Yunnan Province (202005AB160003), YunLing Scholar Programs, Yunnan Fundamental Research Projects (202201AT070141 and 2019FI018), the Talent Plan of Yunnan Province (YNWR-QNBJ-2018-025), the Project of Yunnan Characteristic Plant Screening and R&D Service CXO Platform (2022YKZY001), and the National Key Research and Development Program of China (2019YFE0109200).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1103554/full#supplementary-material
References
Anjaneyulu, A. S. R., Ramaiah, P. A., Row, L. R., Venkateswarlu, R., Pelter, A., and Ward, R. S. (1981). New lignans from the heartwood of Cleistanthus collinus. Tetrahedron 37, 3641–3652. doi:10.1016/S0040-4020(01)98893-3
Atta-ur-Rahman, , Ashraf, M., Choudhary, M. I., Habib-ur-Rehman, , and Kazmi, M. H. (1995). Antifungal aryltetralin lignans from leaves of Podophyllum hexandrum. Phytochemistry 40 (2), 427–431. doi:10.1016/0031-9422(95)00195-D
Ban, H. S., Lee, S., Kim, Y. P., Yamaki, K., Shin, K. H., and Ohuchi, K. (2002). Inhibition of prostaglandin E2 production by taiwanin C isolated from the root of Acanthopanax chiisanensis and the mechanism of action. Biochem. Pharmacol. 64, 1345–1354. doi:10.1016/s0006-2952(02)01348-5
Borges, L. D. C., Negrão-Neto, R., Pamplona, S., Fernandes, L., Barros, M., Fontes-Júnior, E., et al. (2018). Anti-inflammatory and antinociceptive studies of hydroalcoholic extract from the leaves of Phyllanthus brasiliensis (Aubl.) Poir. and isolation of 5-O-β-D-glucopyranosyljusticidin B and six other lignans. Molecules 23, 941. doi:10.3390/molecules23040941
Brown, D., and Stevenson, R. (1964). Action of N, N-dicyclohexylcarbodiimide on phenylpropiolic acids: Synthesis of dehydro-otobain. Tetrahedron Lett. 5, 3213–3216. doi:10.1016/0040-4039(64)83136-1
Chang, W. L., Chiu, L. W., Lai, J. H., and Lin, H. C. (2003). Immunosuppressive flavones and lignans from Bupleurum scorzonerifolium. Phytochemistry 64, 1375–1379. doi:10.1016/j.phytochem.2003.08.002
Charlton, J. L. (1998). Antiviral activity of lignans. J. Nat. Prod. 61, 1447–1451. doi:10.1021/np980136z
Chen, C. C., Hsin, W. C., Ko, F. N., Huang, Y. L., Ou, J. C., and Teng, C. M. (1996). Antiplatelet arylnaphthalide lignans from Justicia procumbens. J. Nat. Prod. 59, 1149–1150. doi:10.1021/np960443+
Chen, W., Hu, D., Feng, Z., Lin, M., and Han, M. (2018). Progress in synthesis of α-arylnaphthalene lignan lactones. Chem. Bull. 81, 303–311. doi:10.14159/j.cnki.0441-3776.2018.04.003
Chen, W., Ma, Y., He, W., Wu, Y., Huang, Y., Zhang, Y., et al. (2022). Structure units oriented approach towards collective synthesis of sarpagine-ajmaline-koumine type alkaloids. Nat. Commun. 13, 908. doi:10.1038/s41467-022-28535-x
Chen, W., Tian, H., Tan, W., Liu, X., Yang, X., and Zhang, H. (2019). Total synthesis of (−)-vindoline. Tetrahedron 75, 1751–1759. doi:10.1016/j.tet.2018.11.046
Chen, W., Yang, X.-D., Tan, W.-Y., Zhang, X.-Y., Liao, X.-L., and Zhang, H. (2017). Total synthesis of (−)-Vindorosine. Angew. Chem. Int. Ed. 56, 12327–12331. doi:10.1002/anie.201707249
Chun, Y. S., Kim, J., Chung, S., Khorombi, E., Naidoo, D., Nthambeleni, R., et al. (2017). Protective roles of Monsonia angustifolia and its active compounds in experimental models of Alzheimer’s disease. J. Agric. Food Chem. 65, 3133–3140. doi:10.1021/acs.jafc.6b04451
Crisp, G. T., and Meyer, A. G. (1995). Synthesis of α, β-unsaturated lactams by palladium-catalysed intramolecular carbonylative coupling. Tetrahedron 51, 5585–5596. doi:10.1016/0040-4020(95)00219-X
Daron, É. C. A. S., Negri, W. T., Borges, A., Lescano, C. H., Antunes, E., and Laurentiz, R. S. D. (2022). Design, synthesis, and in vitro antiplatelet aggregation activities of taiwanin C. Nat. Prod. Res., 1–7. doi:10.1080/14786419.2022.2036145
Day, S.-H., Chiu, N.-Y., Won, S.-J., and Lin, C.-N. (1999). Cytotoxic lignans of Justicia ciliate. J. Nat. Prod. 62, 1056–1058. doi:10.1021/np9900167
Day, S. H., Lin, Y. C., Tsai, M. L., Tsao, L. T., Ko, H. H., Chung, M. I., et al. (2002). Potent cytotoxic lignans from Justicia procumbens and their effects on nitric oxide and tumor necrosis factor-α production in mouse macrophages. J. Nat. Prod. 65, 379–381. doi:10.1021/np0101651
El-Gendy, M. M. A., Hawas, U. W., and Jaspars, M. (2008). Novel bioactive metabolites from a marine derived bacterium Nocardia sp. ALAA 2000 J. Antibiot. 61, 379–386. doi:10.1038/ja.2008.53
Flanagan, S. R., Harrowven, D. C., and Bradley, M. (2002). A new benzannulation reaction and its application in the multiple parallel synthesis of arylnaphthalene lignans. Tetrahedron 58, 5989–6001. doi:10.1016/S0040-4020(02)00616-6
Foley, P., Eghbali, N., and Anastas, P. T. (2010). Advances in the methodology of a multicomponent synthesis of arylnaphthalene lactones. Green Chem. 12, 888–892. doi:10.1039/B913685A
Gertsch, J., Tobler, R. T., Brun, R., Sticher, O., and Heilmann, J. (2003). Antifungal, antiprotozoal, cytotoxic and piscicidal properties of Justicidin B and a new arylnaphthalide lignan from Phyllanthus piscatorum. Planta Med. 69, 420–424. doi:10.1055/s-2003-39706
Gözler, B., Rentsch, D., Gözler, T., Ünver, N., and Hesse, M. (1996). Lignans, alkaloids and coumarins from Haplophyllum vulcanicum. Phytochemistry 42, 695–699. doi:10.1016/0031-9422(96)00062-3
Gözler, T., Gözler, B., Patra, A., Leet, J. E., Freyer, A. J., and Shamma, M. (1984). Konyanin: A new lignan from Hapuophyllum vulcaniclm. Tetrahedron 40, 1145–1150. doi:10.1016/S0040-4020(01)99319-6
Gudla, V., and Balamurugan, R. (2011). Synthesis of arylnaphthalene lignan scaffold by gold-catalyzed intramolecular sequential electrophilic addition and benzannulation. J. Org. Chem. 76, 9919–9933. doi:10.1021/jo201918d
Hajdu, Z., Haskó, J., Krizbai, I. A., Wilhelm, I., Jedlinszki, N., Fazakas, C., et al. (2014). Evaluation of lignans from Heliopsis helianthoides var. scabra for their potential antimetastatic effects in the brain. J. Nat. Prod. 77, 2641–2650. doi:10.1021/np500508y
Harrowven, D. C., Bradley, M., Castro, J. L., and Flanagan, S. R. (2001). Total syntheses of justicidin B and retrojusticidin B using a tandem Horner–Emmons–Claisen condensation sequence. Tetrahedron Lett. 42, 6973–6975. doi:10.1016/S0040-4039(01)01436-8
Hayat, F., Kang, L., Lee, C. Y., and Shin, D. (2015). Synthesis of arylnaphthalene lignan lactone using benzoin condensation, intramolecular thermal cyclization and Suzuki coupling. Tetrahedron 71, 2945–2950. doi:10.1016/j.tet.2015.03.023
He, Y., Zhang, X., and Fan, X. (2014). Synthesis of naphthalene amino esters and arylnaphthalene lactone lignans through tandem reactions of 2-alkynylbenzonitriles. Chem. Commun. 50, 5641–5643. doi:10.1039/C4CC01738B
Hesse, M., Gozler, B., Arar, G., and Gozler, T. (1992). Isodaurinol, an arylnaphthalene lignan from Haplophyllum cappadocicum. Phytochemistry 31, 2473–2475. doi:10.1016/0031-9422(92)83302-F
Holmes, T. L., and Steveson, R. (1971). Arylnaphthalene lignans. Synthesis of justicidin E, taiwanin C, dehydrodimethylconidendrin, and dehydrodimethylretrodendrin. J. Org. Chem. 36, 3450–3453. doi:10.1021/jo00821a037
Ishikawa, S., Masuyama, Y., Adachi, T., Shimonishi, T., Morimoto, S., and Tanabe, Y. (2021). Synthesis of naphthaleman family utilizing regiocontrolled benzannulation: Unique molecules composed of multisubstituted naphthalenes. ACS Omega 6, 32682–32694. doi:10.1021/acsomega.1c04413
Janmanchi, D., Tseng, Y. P., Wang, K. C., Huang, R. L., Lin, C. H., and Yeh, S. F. (2010). Synthesis and the biological evaluation of arylnaphthalene lignans as anti-hepatitis B virus agents. Bioorg. Med. Chem. 18, 1213–1226. doi:10.1016/j.bmc.2009.12.038
Jiang, J., Dong, H., Wang, T., Zhao, R., Mu, Y., Geng, Y., et al. (2017). A strategy for preparative separation of 10 lignans from Justicia procumbens L. by high-speed counter-current chromatography. Molecules 22, 2024. doi:10.3390/molecules22122024
Jin, H., Yang, S., and Dong, J.-X. (2017). New lignan glycosides from Justicia procumbens. J. Asian Nat. Prod. Res. 19, 1–8. doi:10.1080/10286020.2016.1241771
Jin, H., Yin, H.-L., Liu, S.-J., Chen, L., Tian, Y., Li, B., et al. (2014). Cytotoxic activity of lignans from Justicia procumbens. Fitoterapia 94, 70–76. doi:10.1016/j.fitote.2014.01.025
Kamal, A., Daneshtalab, M., and Micetich, R. G. (1994). A rapid entry into podophyllotoxin congeners: Synthesis of justicidin B. Tetrahedron Lett. 35, 3879–3882. doi:10.1016/S0040-4039(00)76691-3
Kao, T. T., Lin, C. C., and Shia, K. S. (2015). The total synthesis of retrojusticidin B, justicidin E, and helioxanthin. J. Org. Chem. 80, 6708–6714. doi:10.1021/acs.joc.5b00866
Katsuki, N., Isshiki, S., Fukatsu, D., Okamura, J., Kuramochi, K., Kawabata, T., et al. (2017). Total synthesis of dendrochrysanene through a frame rearrangement. J. Org. Chem. 82, 11573–11584. doi:10.1021/acs.joc.7b02223
Kawazoe, K., Yutani, A., Tamemoto, K., Yuasa, S., Shibata, H., Higuti, T., et al. (2001). Phenylnaphthalene compounds from the subterranean part of Vitex rotundifolia and their antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Nat. Prod. 64, 588–591. doi:10.1021/np000307b
Klemm, L. H., Klemm, R. A., Santhanam, P. S., and White, D. V. (1971). Intramolecular Diels-Alder reactions. VI. Synthesis of 3-hydroxymethyl-2-naphthoic acid lactones. J. Org. Chem. 36, 2169–2172. doi:10.1021/jo00814a029
Kocsis, L. S., and Brummond, K. M. (2014). Intramolecular dehydro-Diels–Alder reaction affords selective entry to arylnaphthalene or aryldihydronaphthalene lignans. Org. Lett. 16, 4158–4161. doi:10.1021/ol501853y
Kudoh, T., Shishido, A., Ikeda, K., Saito, S., and Ishikawa, T. (2013). Concise synthesis of arylnaphthalene lignans by regioselective intramolecular anionic Diels–Alder reactions of 1, 7-diaryl-1, 6-diynes. Synlett 24, 1509–1512. doi:10.1055/s-0033-1339184
Li, R., Wei, K., Chen, W., Li, L., and Zhang, H. (2022). Carbon–sulfur bond formation: Tandem process for the synthesis of functionalized isothiazoles. Org. Lett. 24, 339–343. doi:10.1021/acs.orglett.1c03994
Liu, B., Zhang, T., Xie, Z., Hong, Z., Lu, Y., Long, Y., et al. (2022). Effective components and mechanism analysis of anti-platelet aggregation effect of Justicia procumbens L. J. Ethnopharmacol. 294, 115392. doi:10.1016/j.jep.2022.115392
Lv, J.-P., Yang, S., Dong, J.-X., and Jin, H. (2021). New cyclopeptide alkaloids from the whole plant of Justicia procumbens L. Nat. Prod. Res. 35, 4032–4040. doi:10.1080/14786419.2020.1758090
Meng, J., Du, L., and Guo, L. (2016). Synthesis of arylnaphthalene lignan lactones. Chin. J. Org. Chem. 36, 2723–2728. doi:10.6023/cjoc201603007
Michael, A., and Bucher, J. E. (1895). Ueber die einwirkung von essigsäureanhydrid auf säuren der acetylenreihe. Ber. Dtsch. Chem. Ges. 28, 2511–2512. doi:10.1002/cber.18950280337
Miyaura, N., and Suzuki, A. (1979) Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J. Chem. Soc. Chem. Commun., 866–867. doi:10.1039/C39790000866
Mizufune, H., Nakamura, M., and Mitsudera, H. (2001). The first regiospecific synthesis of helioxanthin by novel palladium-catalyzed benzannulation reaction of α, β-bisbenzylidene-γ-lactone. Tetrahedron Lett. 42, 437–439. doi:10.1016/S0040-4039(00)01982-1
Mondal, S., Maji, M., and Basak, A. (2011). A Garratt–Braverman route to aryl naphthalene lignans. Tetrahedron Lett. 52, 1183–1186. doi:10.1016/j.tetlet.2011.01.011
Mondal, S., Mitra, T., Mukherjee, R., Addy, P. S., and Basak, A. (2012). Garratt–Braverman cyclization, a powerful tool for C–C bond formation. Synlett 23, 2582–2602. doi:10.1055/s-0032-1317321
Moriguchi, K., Sasaki, R., Morita, J. I., Kamakura, Y., Tanaka, D., and Tanabe, Y. (2021). Ipso-Type regiocontrolled benzannulation for the synthesis of uniquely substituted α-arylnaphthalenes: Application to the first total synthesis of chaihunaphthone. ACS Omega 6, 18135–18156. doi:10.1021/acsomega.1c02000
Murakami, M., Hoshino, Y., Ito, H., and Ito, Y. (1998). Palladium-catalyzed coupling reactions of N-methoxy-N-methylcarbamoyl chloride for the synthesis of N-methoxy-N-methylamides. Chem. Lett. 27, 163–164. doi:10.1246/cl.1998.163
Nagaki, A., Moriwaki, Y., and Yoshida, J. I. (2012). Flow synthesis of arylboronic esters bearing electrophilic functional groups and space integration with Suzuki–Miyaura coupling without intentionally added base. Chem. Commun. 48, 11211–11213. doi:10.1039/C2CC36197C
Naresh, G., Kant, R., and Narender, T. (2015). Silver(I)-catalyzed regioselective construction of highly substituted alpha-naphthols and its application toward expeditious synthesis of lignan natural products. Org. Lett. 17, 3446–3449. doi:10.1021/acs.orglett.5b01477
Nishii, Y., Yoshida, T., Asano, H., Wakasugi, K., Morita, J. I., Aso, Y., et al. (2005). Regiocontrolled benzannulation of diaryl (gem-dichlorocyclopropyl) methanols for the synthesis of unsymmetrically substituted α-arylnaphthalenes: Application to total synthesis of natural lignan lactones. J. Org. Chem. 70, 2667–2678. doi:10.1021/jo047751u
Ogiku, T., Seki, M., Takahashi, M., Ohmizu, H., and Iwasaki, T. (1990). A new two-step synthesis of 1-arylnaphthalene lignans from cyanohydrins. Tetrahedron Lett. 31, 5487–5490. doi:10.1016/S0040-4039(00)97879-1
Ogiku, T., Yoshida, S. I., Ohmizu, H., and Iwasaki, T. (1995). Efficient syntheses of 1-arylnaphthalene lignan lactones and related compounds from cyanohydrins. J. Org. Chem. 60, 4585–4590. doi:10.1021/jo00119a041
Okigawa, M., Maeda, T., and Kawano, N. (1970). The isolation and structure of three new lignans from Justicia procumbens Linn. var. leucantha Honda. Tetrahedron 26, 4301–4305. doi:10.1016/S0040-4020(01)93074-1
Padwa, A., Cochran, J. E., and Kappe, C. O. (1996). Tandem Pummerer-Diels-Alder reaction sequence. A novel cascade process for the preparation of 1-arylnaphthalene lignans. J. Org. Chem. 61, 3706–3714. doi:10.1021/jo960295s
Park, J. E., Lee, J., Seo, S. Y., and Shin, D. (2014). Regioselective route for arylnaphthalene lactones: Convenient synthesis of taiwanin C, justicidin E, and daurinol. Tetrahedron Lett. 55, 818–820. doi:10.1016/j.tetlet.2013.12.014
Park, S., Kim, J.-H., Kim, S.-H., and Shin, D. (2020). Transition metal-mediated annulation approaches for synthesis of arylnaphthalene lignan lactones. Front. Chem. 8, 628. doi:10.3389/fchem.2020.00628
Patel, R. M., and Argade, N. P. (2013). Palladium-promoted 2+2+2 cocyclization of arynes and unsymmetrical conjugated dienes: Synthesis of justicidin B and retrojusticidin B. Org. Lett. 15, 14–17. doi:10.1021/ol3028658
Reber, K. P., Tilley, S. D., and Sorensen, E. J. (2009). Bond formations by intermolecular and intramolecular trappings of acylketenes and their applications in natural product synthesis. Chem. Soc. Rev. 38, 3022–3034. doi:10.1039/B912599J
Sagar, K. S., Chang, C. C., Wang, W. K., Lin, J. Y., and Lee, S. S. (2004). Preparation and anti-HIV activities of retrojusticidin B analogs and azalignans. Bioorg. Med. Chem. 12, 4045–4054. doi:10.1016/j.bmc.2004.05.036
Sato, Y., Tamura, T., Kinbara, A., and Mori, M. (2007). Synthesis of biaryls via palladium-catalyzed 2+2+2 cocyclization of arynes and diynes: Application to the synthesis of arylnaphthalene lignans. Adv. Synth. Catal. 349, 647–661. doi:10.1002/adsc.200600587
Sato, Y., Tamura, T., and Mori, M. (2004). Arylnaphthalene lignans through Pd-catalyzed 2+2+2 cocyclization of arynes and diynes: Total synthesis of taiwanins C and E. Angew. Chem. Int. Ed. 43, 2436–2440. doi:10.1002/anie.200453809
Shen, C.-C., Ni, C.-L., Huang, Y.-L., Huang, R.-L., and Chen, C. (2004). Furanolabdane diterpenes from Hypoestes purpurea, J. Nat. Prod. 67, 1947–1949. doi:10.1021/np0497402
Sheriha, G. M., and Abou Amer, K. M. (1984). Lignans of Haplophyllum tuberculatum. Phytochemistry 23, 151–153. doi:10.1016/0031-9422(84)83096-4
Stevenson, R., and Weber, J. V. (1989). Improved methods of synthesis of lignan arylnaphthalene lactones via arylpropargyl arylpropiolate esters. J. Nat. Prod. 52, 367–375. doi:10.1021/np50062a024
Subbaraju, G. V., and Pillai, K. R. (1996). Lignans from Justicia diffusa willd. Indian J. Chem. B 35, 1233–1234.
Susplugas, S., Hung, N. V., Bignon, J., Thoison, O., Kruczynski, A., Sévenet, T., et al. (2005). Cytotoxic arylnaphthalene lignans from a Vietnamese acanthaceae, Justicia patentiflora. J. Nat. Prod. 68, 734–738. doi:10.1021/np050028u
Takano, S., Otaki, S., and Ogasawara, K. (1985). A new route to 1-phenylnaphthalenes by cycloaddition: A simple and selective synthesis of some naphthalene lignan lactones. Tetrahedron Lett. 26, 1659–1660. doi:10.1016/S0040-4039(00)98577-0
Ulubelen, A., Meriçli, A. H., Meriçli, F., and Kaya, Ü. (1994). An alkaloid and lignans from Haplophyllum telephioides. Phytochemistry 35, 1600–1601. doi:10.1016/S0031-9422(00)86905-8
Vasilev, N., Elfahmi,, , Bos, R., Kayser, O., Momekov, G., and Konstantinov, S. (2006). Production of Justicidin B, a cytotoxic arylnaphthalene lignan from genetically transformed root cultures of Linum leonii. Nat. Prod. 69, 1014–1017. doi:10.1021/np060022k
Wang, S.-H., Wu, H.-C., Badrealam, K. F., Kuo, Y.-H., Chao, Y.-P., Hsu, H.-H., et al. (2019). Taiwanin E induces cell cycle arrest and apoptosis in arecoline/4-NQO-Induced oral cancer cells through modulation of the ERK signaling pathway. Front. Oncol. 9, 1309. doi:10.3389/fonc.2019.01309
Wei, K., Sun, Y. C., Li, R., Zhao, J. F., Chen, W., and Zhang, H. (2021). Synthesis of highly functionalized pyridines: A metal-free cascade process. Org. Lett. 23, 6669–6673. doi:10.1021/acs.orglett.1c02234
Weng, J. R., Ko, H. H., Yeh, T. L., Lin, H. C., and Lin, C. N. (2004). Two new arylnaphthalide lignans and antiplatelet constituents from Justicia procumbens. Arch. Pharm. 337, 207–212. doi:10.1002/ardp.200300841
Wong, Y.-C., Kao, T.-T., Huang, J.-K., Jhang, Y.-W., Chou, M.-C., and Shia, K.-S. (2014). Manganese(III)-catalyzed oxidative cyclization of aryl 1-cyanoalk-5-ynyl ketone systems: A convenient and general approach to cyclopenta[b]naphthalene derivatives. Adv. Synth. Catal. 356, 3025–3038. doi:10.1002/adsc.201400257
Wu, S.-J., and Wu, T.-S. (2006). Cytotoxic arylnaphthalene lignans from Phyllanthus oligospermus. Chem. Pharm. Bull. 54, 1223–1225. doi:10.1248/cpb.54.1223
Xiao, J., Cong, X.-W., Yang, G.-Z., Wang, Y.-W., and Peng, Y. (2018). Divergent asymmetric syntheses of podophyllotoxin and related family members via stereoselective reductive Ni-catalysis. Org. Lett. 20, 1651–1654. doi:10.1021/acs.orglett.8b00408
Xiong, L., Bi, M. G., Wu, S., and Tong, Y. F. (2012). Total synthesis of 6′-hydroxyjusticidin A. J. Asian Nat. Prod. Res. 14, 322–326. doi:10.1080/10286020.2011.653561
Yamamoto, Y., Mori, S., and Shibuya, M. (2015). A combined transitionmetal-catalyzed and photopromoted process: Synthesis of 2, 3-fused 4-phenylnaphthalen-1-yl carboxylates from 1, 7-diaryl-1, 6-diynes. Chem. Eur. J. 21, 9093–9100. doi:10.1002/chem.201500978
Yeo, H., Li, Y., Fu, L., Zhu, J. L., Gullen, E. A., Dutschman, G. E., et al. (2005). Synthesis and antiviral activity of helioxanthin analogues. J. Med. Chem. 48, 534–546. doi:10.1021/jm034265a
Zhang, L., Zhang, Y., Li, W., and Qi, X. (2019). Total synthesis of (−)-alstofolinine A through a furan oxidation/rearrangement and indole nucleophilic cyclization cascade. Angew. Chem. Int. Ed. 58, 4988–4991. doi:10.1002/anie.201900156
Zhang, Y., Bao, F., Hu, J., Liang, S., Zhang, Y., Du, G., et al. (2007). Antibacterial lignans and triterpenoids from Rostellularia procumbens. Planta Med. 73, 1596–1599. doi:10.1055/s-2007-99374h
Zhao, C., Rakesh, K. P., Mumtaz, S., Moku, B., Asiri, A. M., Marwani, H. M., et al. (2018). Arylnaphthalene lactone analogues: Synthesis and development as excellent biological candidates for future drug discovery. RSC Adv. 8, 9487–9502. doi:10.1039/c7ra13754k
Keywords: total synthesis, natural products, arylnaphthalene lactone lignans, Suzuki cross-coupling, cation-induced cyclization
Citation: Wei K, Sun Y, Xu Y, Hu W, Ma Y, Lu Y, Chen W and Zhang H (2022) Total synthesis of justicidin B, justicidin E, and taiwanin C: A general and flexible approach toward the synthesis of natural arylnaphthalene lactone lignans. Front. Chem. 10:1103554. doi: 10.3389/fchem.2022.1103554
Received: 20 November 2022; Accepted: 09 December 2022;
Published: 22 December 2022.
Edited by:
Anton V. Dolzhenko, Monash University, AustraliaCopyright © 2022 Wei, Sun, Xu, Hu, Ma, Lu, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Chen, d2VuY2hlbkB5bnUuZWR1LmNu; Hongbin Zhang, emhhbmdoYkB5bnUuZWR1LmNu
†These authors have contributed equally to this work
 Kai Wei
Kai Wei Yucui Sun
Yucui Sun Yiren Xu
Yiren Xu Wen Hu
Wen Hu Ying Ma
Ying Ma Yi Lu
Yi Lu Wen Chen
Wen Chen Hongbin Zhang
Hongbin Zhang