- 1Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, Suzhou, China
- 2School of Life Sciences, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, China
With the development of technologies based on gold nanoparticles (AuNPs), bare AuNPs cannot meet the increasing requirements of biomedical applications. Modifications with different functional ligands are usually needed. DNA is not only the main genetic material, but also a good biological material, which has excellent biocompatibility, facile design, and accurate identification. DNA is a perfect ligand candidate for AuNPs, which can make up for the shortcoming of bare AuNPs. DNA-modified AuNPs (DNA-AuNPs) have exciting features and bright prospects in many fields, which have been intensively investigated in the past decade. In this review, we summarize the various approaches for the immobilization of DNA strands on the surface of AuNPs. Representative studies for biomedical applications based on DNA-AuNPs are also discussed. Finally, we present the challenges and future directions.
1 Introduction
Gold nanoparticles (AuNPs) are tiny particles of gold with a size of 1–100 nm (Cao et al., 2022). AuNPs have attracted extensive attention due to their special physical and chemical properties (Zha et al., 2021), which are different from the counterpart bulk materials and small molecules. As early as the fourth century, AuNPs were introduced into the production of glass to make the well-known Craggs cup, which can display red or green depending on how it is exposed to the light. In 1857, Faraday discovered that some fine particles could be obtained by treating aqueous solutions of chloroauric acid with a two-phase system dissolved in phosphorus and carbon disulfide (Faraday, 1997). The water suspension of these fine particles displays beautiful wine red, which completely differs from the block gold. Because of technical restrictions and the incompleteness of the AuNPs studies at the time, this is the first published article regarding the preparation of AuNPs.
With the development of nanotechnology, novel physicochemical properties of AuNPs are being discovered, e.g., high extinction coefficients in the visible region (Deng et al., 2014), fluorescence quenching effect (Zhang et al., 2017), huge specific surface area (Wall et al., 2017), facile synthesis (Zhang et al., 2022a), and strong localized surface plasmon resonance (LSPR) absorption (Ghosh and Pal, 2007). These properties make AuNPs have wide applications in the biomedical field (Miao and Tang, 2020a; Chai et al., 2021). For example, because the LSPR of AuNPs is highly sensitive to the size (Lee and El-Sayed, 2006; Tan et al., 2017), capping molecule (Chen et al., 2017), morphology (Burda et al., 2005; Liu et al., 2014), medium refractive index (Underwood and Mulvaney, 1994), and distribution state (Ma et al., 2018; Ding et al., 2019), they have been applied to different colorimetric biosensors (Ma et al., 2017; Ma et al., 2018). Additionally, because of the excellent fluorescence quenching ability, AuNPs have been used to develop various fluorescent biosensors for biomolecular analysis (Liu et al., 2018a; Miao et al., 2018). Moreover, AuNPs have several advantages over other nanomaterials that make them particularly status in the biomedical field (Zhang et al., 2019a; Ma et al., 2020). First, the synthesis of AuNPs is extremely simple and does not require expensive equipment (Frens, 1973; Brust et al., 1994). Second, raw materials for synthesizing AuNPs are easily available and cheap, making the application of AuNPs in the biomedical field extremely cost-effective (Wang et al., 2015). Third, the size, shape, aggregation state, and surface modification of AuNPs can be finely tuned, which is crucial in constructing biosensors (Brust et al., 1994). Finally, the biocompatibility of AuNPs is usually better than that of many other nanomaterials. These characteristics allow researchers to develop novel tools for the biomedical field (Miao et al., 2017; Zhang et al., 2022a). Nevertheless, naked AuNPs cannot meet the requirements of multifunctional biological applications. Certain limitations exist, such as poor stability in high-salt solutions, weak specificity, and sensitivity.
Deoxyribonucleic acid (DNA) is an organic compound with complicated molecular structures; it exists in the eukaryotic nucleus as a vital component of chromosomes. Since the 1980’s, DNA has become known as the vehicle for the organism’s genetic information. Afterward, Seeman and Sleiman. (2017) proposed that DNA is not only a genetic material but also performs some special functions due to its precise base pairing. Since then, DNA self-assembly technology based on base complementary pairing has developed rapidly (Jiang et al., 2021a; Miao and Tang, 2021). With the development of DNA technology, novel DNA functions have been found (Heuer-Jungemann and Liedl, 2019; Miao and Tang, 2020b; Miao and Tang, 2020c). Owing to the outstanding properties of DNA (Miao and Tang, 2021), such as excellent biocompatibility (Liu et al., 2022a), facile design (Chai et al., 2022a), and accurate identification (Duskova et al., 2020; Li et al., 2021), it has been applied in various fields (Aizitiaili et al., 2021; Jiang et al., 2021b; Mao et al., 2022).
DNA can perfectly make up for the shortcoming of bare AuNPs. Researchers have anchored various DNA probes on the surface of AuNPs, and studies have shown that the surface of AuNPs can form strong covalent bonds with thiol groups, readily. Afterward, the surface modification of AuNPs is usually based on the formation of Au–S bonds. DNA-modified AuNPs (DNA-AuNPs) are highly stable and have diverse functionalization options (Zhou et al., 2022a; Chen et al., 2022). Moreover, modified nanomaterials exhibit properties that differ from those of both the AuNPs and DNA from which they derive. DNA-AuNPs have two highlights: 1) they provide novel physical and chemical properties to each other (e.g., plasmonic (Liu et al., 2022b), catalytic (Yang et al., 2018), scattering (Wang et al., 2022), and quenching (Li et al., 2020)), which are paramount in the contexts of material design and probe design; 2) the nanomaterials can be employed as a scaffold for probe-assembling and concentration into a dense arrangement that gives rise to many of their functional properties and reaction efficiency due to the DNA modification of AuNPs (Xie et al., 2019), which offers more possibilities for their application in the biomedical field (Tang et al., 2018).
2 DNA modification of AuNPs
The DNA-AuNPs’ application in the biomedical field has emerged since 1996 with the landmark work for attaching thiol DNA to AuNPs, which was proposed by Mirkin et al. (1996) This proposed strategy made numerous applications of DNA-AuNPs possible, including the preparation of biosensing (Pan et al., 2019), bioimaging (Sun et al., 2019a; Zhou et al., 2022b), disease diagnosis (Lin et al., 2019), and drug delivery (Zheng et al., 2019). In brief, the simple and efficient modification methods of DNA-based AuNPs play a crucial role in all these applications (Degliangeli et al., 2014; Peng et al., 2022). With the exploration of the physicochemical properties of AuNPs, several modification methods that provide more convenient conditions for the DNA-AuNPs have been reported. To date, although several reviews have been presented to introduce the synthesis methods and application of AuNPs in biosensing and bioimaging, none of them has focused on the modification methods of AuNPs. Hence, we present a review of the modification methods of AuNPs in recent years to summarize and comment on their development and advance.
2.1 The salt-aging method
DNAs are strongly negatively charged and cannot pack densely without electrostatic screening. Mirkin et al. (1996) developed a method to prepare DNA-AuNPs that introduced the concept of salt-aging, which allows for high-density packing of thiol DNA on the AuNPs’ surface. This method remains the preferred one for the preparation of DNA-AuNPs. As shown in Figure 1, an excess amount of DNA and a high concentration of NaCl must be present, and NaCl is gradually added to increase DNA loading over 1–2 days. According to the literature, increasing the sodium ion concentration of the reaction solution to >0.15 M (up to ∼2.0 M with surfactants) screens the repulsive interactions between neighboring strands, thereby promoting higher densities as DNA assembles on the surface of AuNPs. Higher salt concentrations generally result in higher DNA densities until steric constraints prohibit further adsorption. The high loading of DNA on the AuNPs’ surface derives from 1) the substitutionally labile coordination sphere of citrate ions adsorbed onto the surfaces of the particle precursors used to prepare the DNA-AuNPs, 2) the thiol–Au chemistry used to surface immobilize the DNA, and 3) the salt-aging procedures used to decrease electrostatic interactions between neighboring DNA. In summary, the monolayer of DNA formed by this method is particularly stable because of the relatively strong Au–S interaction (compared with the Au–citrate interaction).
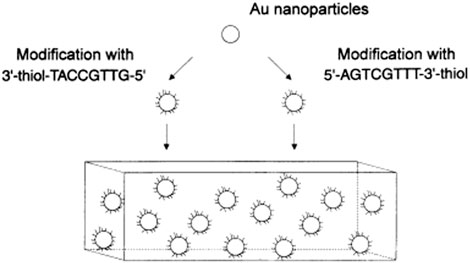
FIGURE 1. Illustration of the salt-aging method for DNA-AuNPs. Reprinted with permission from ref 50.
2.2 Bromide as robust backfiller on the AuNPs
Unintended DNA absorption is difficult to be avoided, which reduces the DNA hybridization effect and weakens the colloidal stability of the conjugate. Hence, another backfiller that absorbs weaker than the thiol but stronger than certain bases may allow precise control of DNA conformation for a better modification effect. According to Martin et al. (Mirkin et al., 1996), halides containing F−, Cl−, Br−, and I− have been tested for exploring more controllable backfillers (Figure 2). By examining various sizes of AuNPs, it was found that Br− improved not only colloidal stability but also hybridization efficiency (Liu et al., 2018b). Additionally, NaBr was used as a backfiller to experiment on AuNPs with sizes from 5 to 80 nm and different DNA sequences to realize excellent results. In this regard, Br− can be considered as a backfiller that can more precisely control the DNA conformation on AuNPs. With this strategy, a novel and excellent assisted material for salt-aging method has emerged.
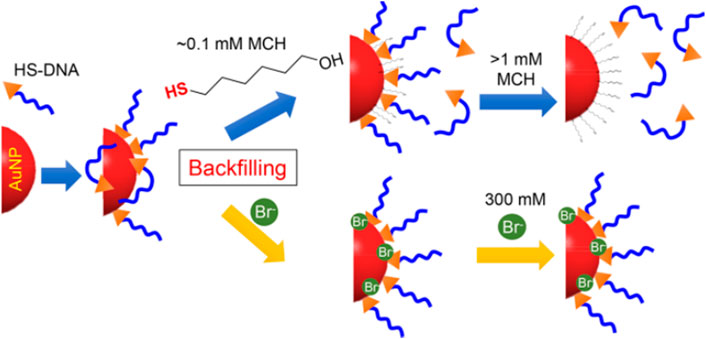
FIGURE 2. Illustration of bromide as a robust backfiller on the AuNPs for synthesizing DNA-AuNPs. Reprinted with permission from ref 58.
2.3 The vacuum centrifugation method
AuNPs conjugated with thiol DNA represent an attractive and alternative platform for applications in biosensors, medical diagnostics, and biological analyses. However, the DNA-AuNPs used in the above methods are time-consuming and need a 2 days incubation process to achieve a high DNA loading density. Considering the time-consuming feature of classic approaches, alternative methods that can shorten the time required while the quality of the DNA-AuNPs is preserved or improved are needed. An approach reported by Brust et al. (Kanaras et al., 2003) needs 20 h for the entire modified process. This method employs vacuum centrifugation instead of the aging step, which shortens the labeling time. In this method, the thiol DNA is incubated with AuNPs overnight, and the solution is diluted to a final volume of 1 ml containing NaCl (0.1 M) and sodium phosphate buffer (5 mM, pH 7.0) solutions. After further incubation for 2 h, the volume was slowly reduced to 150 µl by vacuum centrifugation over 3–4 h at 40°C. This is an important process that can ensure a gradual and simultaneous increase in ionic strength and DNA concentration, which can extremely stabilize DNA-AuNPs. This approach has great significance in developing the fast and reproducible synthesis of stable DNA-AuNPs.
2.4 BSPP coating approach
A novel strategy was developed by Alivisatos et al. (Claridge et al., 2005), which utilized a polymeric layer (bis (p-sulfonatophenyl)phenylphosphine dehydrate dipotassium salt, BSPP) to stabilize AuNPs before adding thiol DNA. The modification time is reduced to 12 h. In this strategy, BSPP is mixed with AuNPs to allow phosphine ligands to replace the citrate ligands. AuNPs with a diameter of 5 or 10 nm, which can form a complex with BSPP did not aggregate in aqueous buffers and could be subjected to repeated cycles of NaCl precipitation. Meanwhile, the thiol groups were incorporated into DNA, which can penetrate the ligand “shells” and react directly with the surface of AuNPs. Under the BSPP protection, the reaction efficiency was improved and the labeling time was decreased.
2.5 The modification method assisted by dATP
With BSPP protection, the salt-dependent aggregation of AuNPs was controlled, which decreased the labeling time but was not intended for preparing DNA-AuNPs with a high DNA loading density. Another approach for DNA-AuNPs construction was then proposed by Hsing et al. (Zhao et al., 2009) This strategy enabled rapid immobilization of high-density DNA on stable AuNPs in a salt environment. The strategy relied on fast and reversible binding of mononucleotide to AuNPs’ surfaces, which was used to quickly stabilize AuNPs in a salt solution by forming a mononucleotide layer on the AuNPs’ surface. The adsorption/desorption of this layer is thermally tunable. Figure 3 shows that the modified process starts with the reversible adsorption of dATP on the AuNPs for nanoparticle stabilization in salt solutions, followed by a temperature-facilitated ligand exchange reaction between the incoming thiol DNA and the protection dATP on the AuNPs’ surface. The synthesis of DNA-AuNPs using this approach could be achieved within several hours and DNA-AuNPs have similar or better characteristic attributes compared with those synthesized using conventional methods. The ability to modify AuNPs with specific DNAs is an efficient and improved application in molecular diagnostics and biomedical fields.
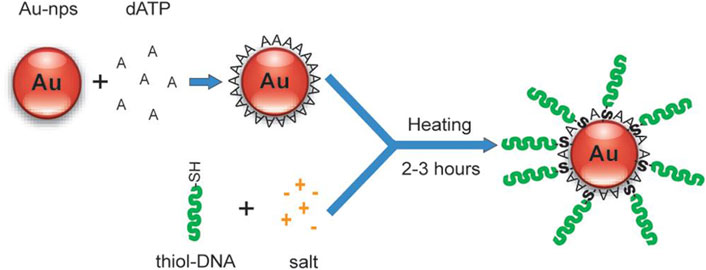
FIGURE 3. Mononucleotide-assisted conjugation of thiol DNA to AuNPs. Reprinted with permission from ref 61.
2.6 The synthesis method based on tween 80
Another simple and convenient method was presented by Wu et al. (Xu et al., 2011). This method exploited the stabilizing effect of Tween 80 on AuNPs in a salt solution and a heating-facilitated ligand exchange between Tween 80 and thiol DNA. Before the addition of thiol DNA, Tween 80 was added and adsorbed onto the AuNPs’ surfaces, which could effectively stabilize AuNPs in salt solutions by forming a protection layer on the AuNPs’ surfaces. Under the protection of Tween 80, DNA-AuNPs could be rapidly formed in a salt solution followed by a mild heating-facilitated ligand exchanged between Tween 80 and thiol DNA (Figure 4). Meanwhile, with this method, DNA-AuNPs can be completed within 2–3 h, and has similar or better quality in terms of DNA loading density and stability than the classic methods.
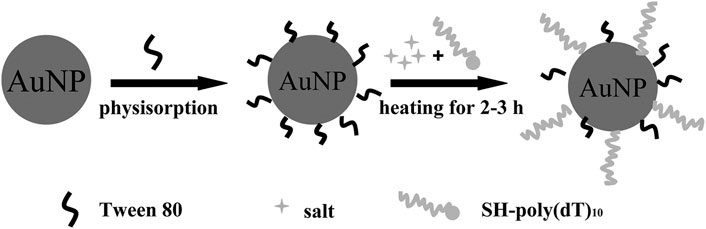
FIGURE 4. Schematic representation of the principle of Tween 80-mediated conjugation of thiol DNA to AuNP. Reprinted with permission from ref 62.
2.7 The freezing method
Nanoparticle solutions cannot be frozen, which causes irreversible aggregation. This is because salt and nanoparticles are excluded by the growing ice crystals that cause the local salt concentration to increase and screen the electrostatic repulsion between the nanoparticles (Deville, 2017). However, the nanoparticles are modified by some macromolecules, and the irreversible aggregation might be reversible because of the steric stabilization between the nanoparticles and macromolecules. According to this theory, a novel and easy-to-operate method was proposed by Liu et al. (Liu and Liu, 2017) (Figure 5). According to the report, after mixing the appropriate concentration of DNA with AuNPs, the mixture was placed in a laboratory freezer for 2 h or less time, followed by thawing at room temperature. The thiol DNA can be modified on the surface of AuNPs with high DNA density. This proposed method also applies to various sizes of AuNPs. Meanwhile, this method has the following merits. First, extra reagents are not required. Second, higher DNA density with higher colloidal stability compared with other methods. Although the other methods have driven the development of this field in so many years, this method brought novel features that will enable more applications in the biomedical field.
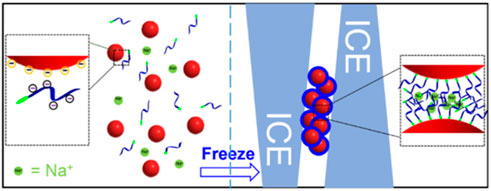
FIGURE 5. The principle of the freezing method that can attach thiol DNA onto the surface of AuNPs. Reprinted with permission from ref 63.
2.8 The two-step method based on mercaptoethanol
Afterward, a novel approach for thiol DNA-AuNPs was proposed by Herne et al. (Herne and Tarlov, 1997). They used alkanethiol self-assembly methods to fabricate DNA-AuNPs with known and reproducible probe coverages, and showed high hybridization activity. Figure 6 shows that the surface coverage of thiol-derivatized DNA on the surface of AuNPs can be precisely controlled by forming mixed monolayers of the thiol-derivatized DNA and MCH and containing the spacer thiol that was carefully chosen to minimize non-specific absorption of single-stranded DNAs. This strategy not only provides a novel method for synthesizing DNA-AuNPs but also shortens the labeling time; only 1 h is required for the entire modified process.
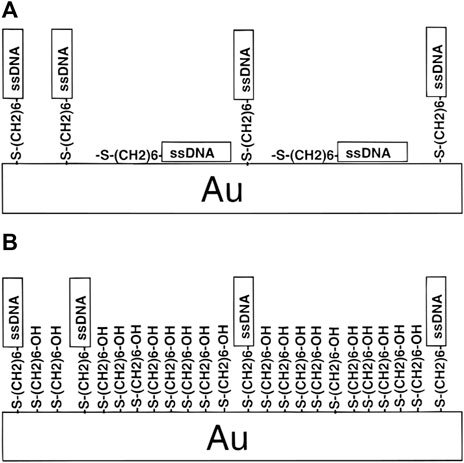
FIGURE 6. Schematic of the two-step method based on mercaptoethanol. Reprinted with permission from ref 64. (A) HS-ssDNA on AuNPs and (B) both HS-ssDNA and MCH adsorbed on AuNPs.
2.9 pH method
In the above methods, the assisted molecules must be gradually added to the complex of DNA and AuNPs over 1–2 h every 1–2 days, which is somewhat time-consuming. Hence, an approach for fast, convenient, and universal DNA loading is required. Liu et al. (Zhang et al., 2012) developed a novel method using a pH 3.0 citrate buffer to complete the attachment process in a few minutes (Figure 7). The only required reagent is a low-pH citrate buffer. This approach is universal for various sizes of AuNPs. Another important feature of this approach is to achieve quantitative DNA density and the attachment of multiple DNAs at designated ratios. This work indicated that pH provides a novel and fast method to tune the interaction between DNA and AuNPs with an excellent effect.
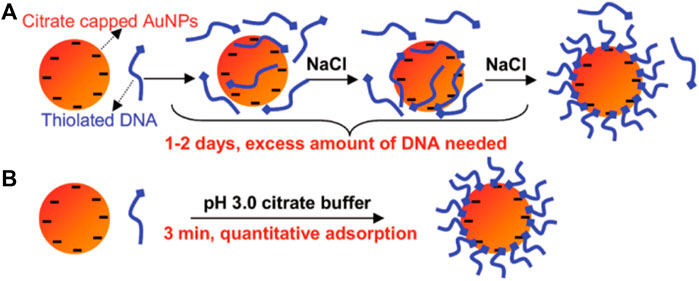
FIGURE 7. Scheme showing the adjusted pH method. Reprinted with permission from ref 65. (A) Traditional method and (B) low PH-assisted method.
2.10 Polyadenosinic acid-assisted method
The above methods are mainly based on strong Au–S chemistry to self-assemble thiol DNA at AuNPs However, precisely controlling the orientation and conformation of surface-tethered DNA and finely tuning its hybridization ability remain challenging. Fan et al. (Pei et al., 2012) developed a novel strategy for DNA-AuNPs free of DNA modifications (Figure 8). They demonstrated that poly adenine (ploy A) can serve as an effective anchoring block for preferential binding with the AuNPs’ surface, and the appended recognition block adopts an upright conformation that favors DNA hybridization. The lateral spacing and surface density of DNA on AuNPs can also be systematically modulated by adjusting the length of the poly A block. Significantly, this method results in DNA-AuNPs with high and tunable hybridization ability, which forms the basis of rapid plasmonic DNA biosensors.
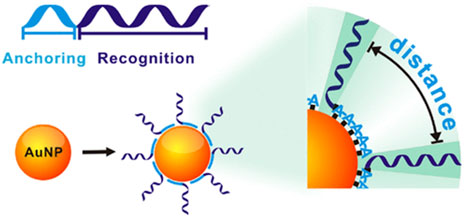
FIGURE 8. The principle of the ploy A-assisted method that can control on AuNPs by varying the length of poly A blocks. Reprinted with permission from ref 66.
3 Biomedical applications of DNA-AuNPs
AuNPs are widely used in the biomedical field due to their numerous outstanding properties. Furthermore, because of its programmable and sequence-specific interactions, DNA has emerged as a versatile and precise tool for biosensor (Chai and Miao, 2019), bioimaging (Meng et al., 2019), and drug delivery (Jiang et al., 2021b). DNA-AuNPs can overcome the shortcomings of naked AuNPs and endow AuNPs with promising applications in biomedical, such as the DNA-AuNPs being highly negatively charged, which improves the stability of AuNPs in complex samples. In summary, the DNA-AuNPs provide novel applications.
3.1 Biosensor
Biosensors are crucial analytical tools in the biomedical field, which uses the unique biophysical properties of materials to detect biomolecules. Biosensors include colorimetric biosensors (Hao et al., 2018; Chou et al., 2019), fluorescent biosensors (Liang et al., 2017; Jiang and Miao, 2020), and electrochemical biosensors (Jiang et al., 2021a; Chai et al., 2022b), which are differentiated by the output signal. DNA-AuNPs possess similar chemical and physical properties as naked AuNPs, which are endowed with better stability (Zhong et al., 2022) and functional diversity (Zhang et al., 2022b). Recently, many successful strategies for biosensors based on DNA-AuNPs have been proposed (Wang et al., 2020), and unexpected results have been achieved (Zhang et al., 2020).
3.1.1 Colorimetric biosensor based on DNA-AuNPs
Colorimetric biosensors (Zhou et al., 2022c) measure the concentration of molecules by comparing the color changes in the solution of colored substrates. The Lambert–Beer law (Rose, 1952) explains that the extinction coefficient of substrates is directly related to the detection sensitivity. Hence, AuNPs are perfect candidates for colorimetric biosensors because they have an extremely high extinction coefficient (e.g., 2.7 × 108 M−1 for 13 nm AuNPs), more than 1,000 times higher than those of organic dyes (Liu et al., 2007). As the modification methods of AuNPs mature, the explosive development of colorimetric biosensors has been reported for various species, including metal ions (Xie, 2018), nucleic acids (Karami et al., 2021; Zhu et al., 2021), proteins (Chen et al., 2016), and even cells (Zhang et al., 2014).
For metal ion detection, Diao et al. (2020) developed a sensitive biosensor based on DNA-AuNPs for Pb2+ detection (Figure 9A). They used the LSPR of DNA-AuNPs to determine Pb2+ concentration. This colorimetric strategy with high selectivity had a detection limit of 8.0 nM, demonstrating excellent sensing performance. Furthermore, Ma et al. (2019) employed DNA-AuNPs as the signal output for multiple miRNA diagnostics (Figure 9B). They developed a novel logic gate based on DNA-AuNPs for triple miRNAs analysis. The key to achieving a DNA-AuNPs-based colorimetric biosensor is controlling the nanoparticle dispersion and aggregation, which can be realized by crosslinking using a linker DNA. To achieve accurate target capture, the triple miRNA inputs can initiate toehold-mediated strand displacement and generate linker DNA of two DNA-AuNPs, and the researchers specially designed a Y-shaped probe by hybridization of three DNA strands. Afterward, the LSPR absorption of AuNPs is used as the output. This method was successfully demonstrated in serum samples, which have remarkable potential applications in early cancer diagnosis and therapy.
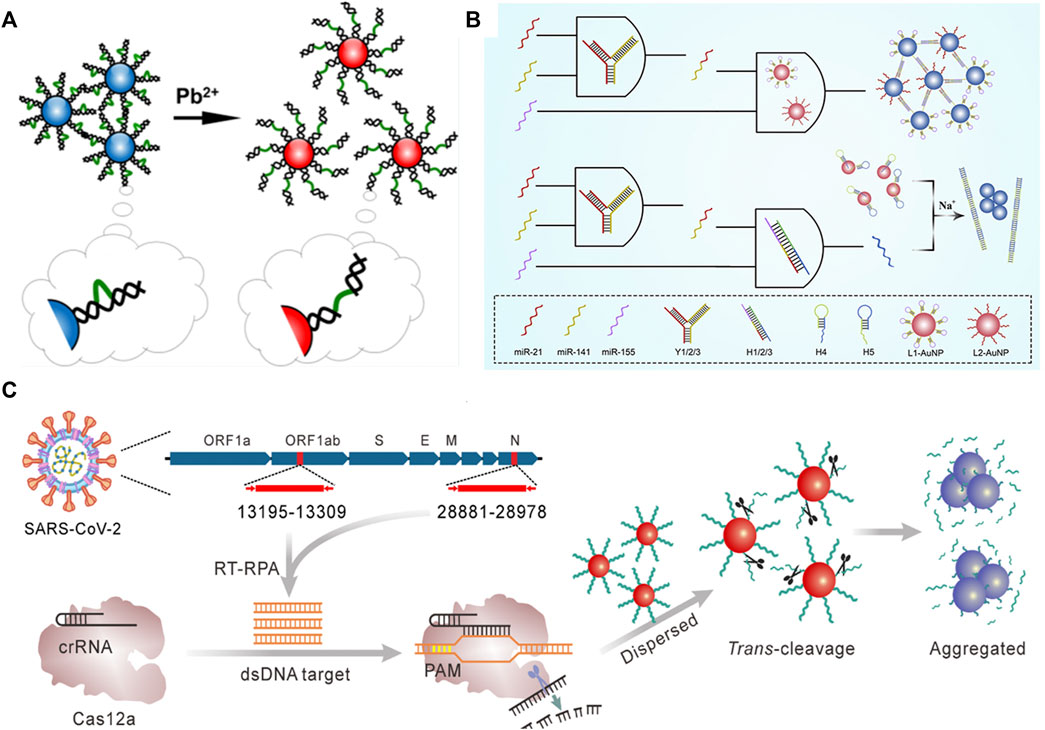
FIGURE 9. Illustration of the colorimetric biosensor detecting (A) Pb2+ (B) multiple miRNAs, and (C) gene. Reprinted with permission from refs 86–88.
Biosensors based on DNA-AuNPs can not only detect multiple nucleic acids but also achieve the detection of genes by the naked eye. As shown in Figure 9C, Zhang et al. (2021) reported a facile and cost-effective biosensor for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). In this regard, reverse transcription recombinase polymerase amplification coupled with CRISPR-Cas12a colorimetric assay was proposed for SARS-CoV-2 detection. The researchers used DNA-AuNPs as a universal colorimetric readout, which can specifically target the ORF1ab and N regions of the SARS-CoV-2 genome. Notably, this strategy coupled with the isothermal amplification and Cas12a activation process can significantly improve the specificity.
3.1.2 Fluorescence biosensor based on DNA-AuNPs
Fluorescent is known as an extremely sensitive technique that can even achieve single molecule detection sensitivity and plays a crucial role in biomedical diagnostics and biotechnology. However, many fluorescent assays face several challenges including the photostability and autofluorescence of biological samples. Recently, nanoparticle-based fluorescent biosensors have served as alternatives to traditional fluorescence dye-based assays. AuNPs have been demonstrated as highly efficient energy acceptors because of the continuous electron–hole pair excitation in the surface of AuNPs. Because of this property of AuNPs, novel fluorescent biosensors based on DNA-AuNPs have been developed for various molecule detection.
Due to the design flexibility of DNA and excellent signal amplification ability, DNA-AuNPs have been widely applied to intracellular fluorescence imaging. Gao et al. (2021) demonstrated a novel fluorescent biosensor for miRNA-21 detection (Figure 10A). In this strategy, DNA-AuNPs were encapsulated within a dissociable zeolitic imidazolate framework-8 (ZIF-8) to facilitate endocytosis and ensure sufficient internal cofactors (Zn2+) to realize a self-driven pattern in the acidic environment of the cell lysosome. This strategy based on DNA-AuNPs presents satisfactory sensitivity and specificity. Importantly, the sensing platform could distinguish the variations of targets in living cancer cells with extremely high efficiency and exceptional precision, also offering a powerful tool for intracellular imaging.
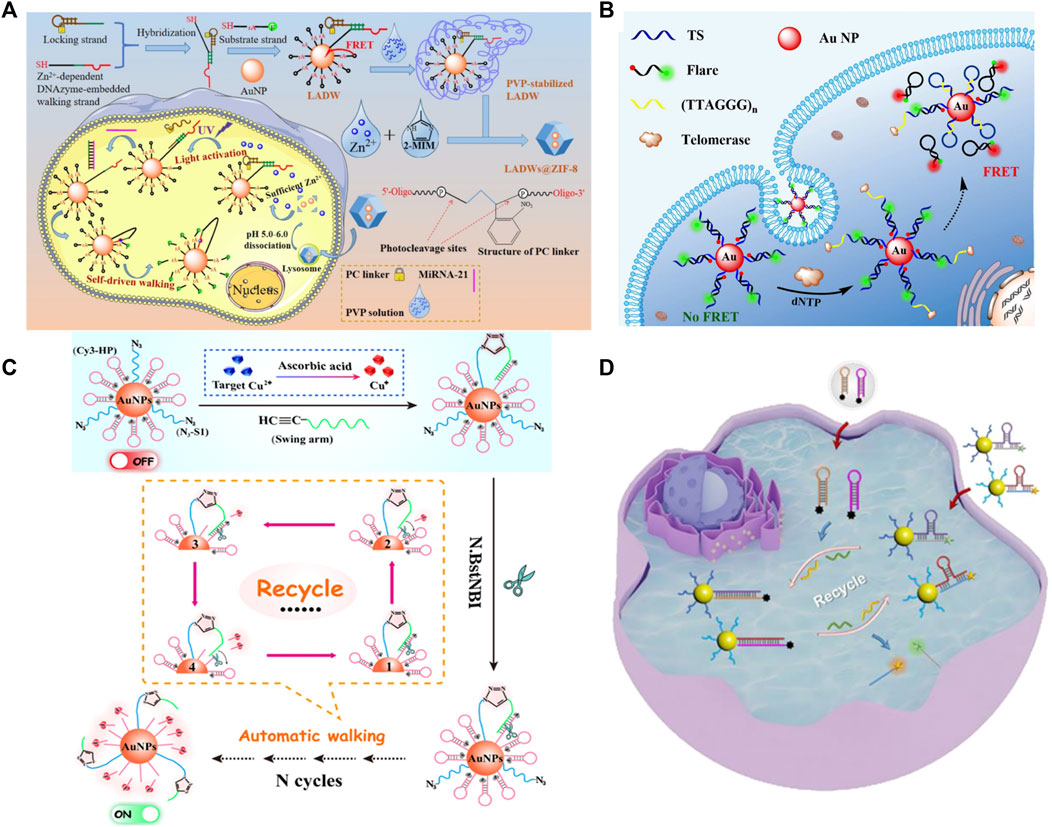
FIGURE 10. Illustration of a fluorescent biosensor based on DNA modified for detecting (A) miRNA, (B) telomerase activity, Cu2+, (C) multiple targets, and (D) multiple miRNAs in situ imaging. Reprinted with permission from refs 89–92.
Moreover, DNA-AuNPs can be used for enzyme activity detection. Yang et al. (2017) designed a fluorescent biosensor for intracellular telomerase detection. This strategy demonstrated a probe termed FRET nanoflare based on DNA-AuNPs to detect the activity of telomerase in living cells with extremely high specificity (Figure 10B). This approach can detect telomerase in cell lysate with the limit of detection (LOD) down to ∼33 Hela cells. Furthermore, in situ imaging and monitoring of intracellular telomerase is satisfactory.
Biosensors based on DNA-AuNPs are also used to detect intracellular metal ions by a series of chemical reactions. A highly selective and sensitive “OFF–ON” fluorescent biosensor designed for intracellular Cu2+ detection has been reported (Wang et al., 2021) (Figure 10C). This strategy depends on integrating the high selectivity of the Cu(I)-catalyzed click reaction with the ultrahigh sensitivity of a spherical nucleic acid-based three-dimensional (3D) DNA walker. Accordingly, the ingenious integration of an efficient click reaction and smart 3D DNA walker endows the constructed fluorescent biosensor with superior selectivity and ultrahigh sensitivity.
Biosensors based on DNA-AuNPs have a brilliant performance in single-target detection; they can also be applied to detect multiple targets. Xu et al. (2022) employed DNA-AuNPs as fluorophore/mass dual-encoded nanoprobes, which can execute target-triggered hairpin self-assembly to enable in situ amplified imaging and follow-up mass spectrometry (MS) quantification of intracellular multiple miRNAs (Figure 10D). Fluorescence imaging and MS quantification of miRNA-21 and miRNA-141 have also been accomplished in different cell lines, highlighting its potential in cell subtyping. This strategy creates a new approach for multiple intracellular miRNA detection and determining multiple biomarkers in the biomedical field.
3.1.3 Electrochemical biosensor based on DNA-AuNPs
Electrochemical biosensors are widely used for biomolecule detection because they are portable, simple, easy to use, and cost-effective. Because of their excellent conductivity, high surface areas, and catalytic properties, AuNPs as ideal candidates for electrochemical-based detection strategies offer new opportunities for highly sensitive biomarker detection. Miao and Tang. (2019) employed DNA-AuNPs for circulating tumor cell (CTC) detection, which provides a novel strategy for the sensitive and accurate quantification of CTC (Figure 11A). Owing to the large specific surface area of AuNPs, numerous walker strands are easily modified on AuNPs. The aptamer sequence can especially recognize the transmembrane receptor protein of CTC, and the AuNPs can be enriched on the surface of cells. The aptamer shows extremely excellent sensitivity and because multiple walker stands are modified on a single AuNP, hybridization with several tracks on the electrode occurs simultaneously for following nicking endonuclease-catalyzed cleaving, which provides excellent signal amplification. This strategy based on DNA-AuNPs can achieve the resolution of a single-cell level with excellent selectivity. The ability to distinguish CTC in blood samples also demonstrates its promising use in cancer diagnosis.

FIGURE 11. Schematic of electrochemical biosensors (A) based on DNA walker and (B) nitrogen-doped graphene sheets (NGS) for detecting cells.
Dou et al. (2019) developed a novel platform for living cancer cells, which highly dispersed AuNPs on nitrogen-doped graphene sheets (NGS). The sensing platform could improve the electrocatalytic ability to detect nitric oxide (NO) released from live cancer cells (Figure 11B). Because of the synergistic enhancement of the catalytic activities of AuNPs and NGS as well as the high dispersion of AuNPs, such a nanocomposite shows significant electro-oxidation capability toward NO, leading to a highly sensitive with an extremely low detection limit for NO in vitro.
3.2 Bioimaging and therapy based on DNA-AuNPs
The imaging technique of cancer cells is of great significance for early cancer diagnosis and targeted therapy. DNA-AuNPs have attracted considerable attention because of their high loading capacity and excellent biocompatibility. Bioimaging and therapy for disease cells based on DNA-AuNPs is promising new single-entity techniques that possess significant advantages in early cancer diagnosis and treatment.
3.2.1 Bioimaging
DNA-AuNPs are ideal candidates for bioimaging for in situ detection and monitoring of various molecules because AuNPs have excellent fluorescence effects and biocompatibility. Yang et al. (2018) developed an RNA-cleaving DNAzyme-based TP imaging probe for Zn2+ detection in living cells, which uses DNA-AuNPs for the imaging of intracellular metal ions. As shown in Figure 12A, they modified the Zn2+-specific DNAzyme with a fluorophore, which can be quenched by both AuNPs and the molecular quencher, and recognized intracellular Zn2+ in the surface of AuNPs. This strategy can be generally applied to detect other molecules in biological systems under TP imaging with highly sensitive and low phototoxicity.
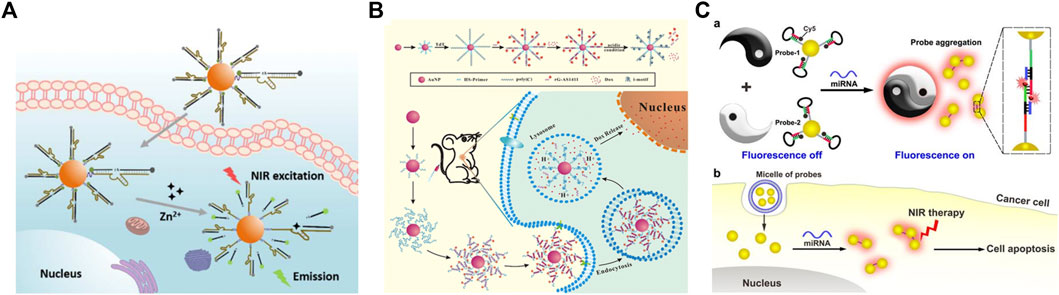
FIGURE 12. Schematic of the application of DNA-modified AuNPs in (A) bioimaging (B) drug delivery, and (C) photothermal therapy. Reprinted with permission from refs 95, 98, 99.
Other works based on DNA-AuNPs were reported for miRNA in situ imaging in living cells. For example, a novel strategy for miRNA detection was developed by Ye et al. (2021). In this strategy, a near-infrared (NIR)-photoactivatable system, which was powered by endogenous adenosine triphosphate, was developed for in situ miRNA imaging with spatial and temporal resolutions. Chen et al. (2021) also developed a specific and sensitive platform for cancer-related miRNA in living cells, which is desirable for cancer diagnosis and treatment.
3.2.2 Drug delivery
Because of the non-toxic and biocompatible characteristics of DNA-AuNPs, which have attracted increasing attention in the drug carriers, Sun et al. (Sun et al., 2019b) developed a novel carrier based on DNA-AuNPs for tumor-targeted delivery and site-specific release of the anticancer drug as an efficient method to overcome the side effects of traditional cancer therapy (Figure 12B). In this strategy, terminal deoxynucleotidyl transferase-catalyzed DNA extension reaction is used to prepare a thick DNA layer on the surface of AuNPs by extending long poly C) sequences from DNA primers immobilized on AuNPs. The poly C) extension products can then hybridize with G-rich oligonucleotides to give CG-rich DNA duplexes for loading the anticancer drug doxorubicin and multiple aptamers. The Dox-loaded DNA carrier can be efficiently internalized in cancer cells by recognizing multiple aptamers with the protein on the surface of cancer cells and achieving the burst release of drugs in acidic organelles due to the i-motif formation-induced DNA duplex destruction. This pH-responsive drug carrier was demonstrated to be promising for highly efficient delivery of Dox and selective killing of cancer cells in both in vitro and in vivo experiments, showing great potential in drug delivery.
3.2.3 Photothermal therapy
Photothermal therapy is another application based on DNA-AuNPs for the therapy of cancer cells. Qian et al. (2016) developed a binary system for miRNA-21-targeted imaging and photothermal treatment in single cells. The binary system comprises a pair of thiol DNA probes, which are modified on AuNPs and encapsulated in the liposome for cell delivery (Figure 12C). The DNA-AuNPs were functionalized with Cy5-marked molecular beacon, which could be opened upon miRNA-21-triggered hybridization and turn on the fluorescence of Cy5 for localizing miRNA-21. The miRNA-induced aggregation shifts the absorption of AuNPs to NIR, which can be observed under dark-field microscopy and further used for the following photothermal therapy. Hence, MCF-7 breast cancer cells can be selectively killed via NIR irradiation. The proposed strategy provides a novel application of DNA-AuNPs for intracellular miRNA analysis and targeted treatment against cancer. In addition, Chen et al. (2020) proposed a furin-instructed intracellular gold nanoparticle aggregation strategy and a furin-responsive gold nanoparticle platform (AuNP@1) is designed for effective PTT of cancer both in vitro and in vivo. And Zhang et al. (2019b) developed a novel immunological AuNP through intracellular generation and exocytosis for combinatorial PTT and immunotherapy, and significantly improved overall survival of mice.
4 Conclusion and perspective
In this study, we have discussed various modification methods of DNA-AuNPs designed and successfully applied in the biomedical field. With the emergence of novel modification methods of DNA-AuNPs, more convenient conditions for applying them have emerged. These nanomaterials have paved the way for developing highly sensitive and efficient tools for the biomedical field. Although many applications based on DNA-AuNPs have been successfully established, they are still facing several challenges. First, the modification methods of DNA-AuNPs combined with new technologies are fairly insufficient. Second, the variation of AuNPs in size, shape, concentration, and dispersion buffer could cause significant variation in the modification effect, yielding poor reproducibility of the platforms based on DNA-AuNPs; hence, uniform modification methods of DNA-AuNPs are urgently needed. Third, most DNA-AuNP-based assays are just at the laboratory level and clinical applications have not been realized. They need to be combined with more clinical applications for disease diagnosis and therapy.
Author contributions
XM: writing—original draft; literature collection. XL: literature collection; processing of pictures. GL: ideas; writing—review and editing. JJ: writing—review and editing; formulation of overarching goals and aims.
Funding
The authors acknowledge the financial support from the Natural Science Foundation of Shandong Province (ZR2022QH207 to JJ), the 2020 Talent Project of Shandong First Medical University (921001003002 to JJ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aizitiaili, M., Jiang, Y., Jiang, L., Yuan, X., Jin, K., Chen, H., et al. (2021). Programmable engineering of DNA-AuNP encoders integrated multimodal coupled analysis for precision discrimination of multiple metal ions. Nano Lett. 21 (5), 2141–2148. doi:10.1021/acs.nanolett.0c04887
Brust, M., Walker, M., Bethell, D., Schiffrin, D. J., and Whyman, R. (1994). Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc. Chem. Commun. 7, 801–802. doi:10.1039/c39940000801
Burda, C., Chen, X., Narayanan, R., and El-Sayed, M. A. (2005). Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 105 (4), 1025–1102. doi:10.1021/cr030063a
Cao, J., Lv, P., Shu, Y., and Wang, J. (2022). Aptamer/AuNPs encoders endow precise identification and discrimination of lipoprotein subclasses. Biosens. Bioelectron. X. 196, 113743. doi:10.1016/j.bios.2021.113743
Chai, H., Ma, X., Sun, H., and Miao, P. (2022). DNA–MnO2 nanoconjugates investigation and application for electrochemical polymerase chain reaction. Anal. Chem. 94 (11), 4565–4569. doi:10.1021/acs.analchem.1c04844
Chai, H., and Miao, P. (2019). Bipedal DNA walker based electrochemical genosensing strategy. Anal. Chem. 91 (8), 4953–4957. doi:10.1021/acs.analchem.9b01118
Chai, H., Tang, Y., Guo, Z., and Miao, P. (2022). Ratiometric electrochemical switch for circulating tumor DNA through recycling activation of blocked DNAzymes. Anal. Chem. 94 (6), 2779–2784. doi:10.1021/acs.analchem.1c04037
Chai, H., Wang, M., Tang, L., and Miao, P. (2021). Ultrasensitive electrochemical detection of miRNA coupling tetrahedral DNA modified gold nanoparticles tags and catalyzed hairpin assembly. Anal. Chim. Acta X. 1165, 338543. doi:10.1016/j.aca.2021.338543
Chen, J. C., Ma, Y. C., Du, W., Dai, T. Y., Wang, Y. F., Jiang, W., et al. (2020). Furin-instructed intracellular gold nanoparticle aggregation for tumor photothermal therapy. Adv. Funct. Mat. 30 (50), 2001566. doi:10.1002/adfm.202001566
Chen, M., Duan, R., Xu, S., Duan, Z., Yuan, Q., Xia, F., et al. (2021). Photoactivated DNA walker based on DNA nanoflares for signal-amplified microRNA imaging in single living cells. Anal. Chem. 93 (48), 16264–16272. doi:10.1021/acs.analchem.1c04505
Chen, S., Zhao, J., Xu, C., Zhang, L., Shi, B., Tian, J., et al. (2022). Intracellular multicomponent synchronous DNA-walking strategy for the simultaneous quantification of tumor-associated proteins in a single cell. Anal. Chem. 94, 15847–15855. doi:10.1021/acs.analchem.2c03771
Chen, Y., Xianyu, Y., and Jiang, X. (2017). Surface modification of gold nanoparticles with small molecules for biochemical analysis. Acc. Chem. Res. 50 (2), 310–319. doi:10.1021/acs.accounts.6b00506
Chen, Z., Tan, L., Hu, L., Zhang, Y., Wang, S., and Lv, F. (2016). Real colorimetric thrombin aptasensor by masking surfaces of catalytically active gold nanoparticles. ACS Appl. Mat. Interfaces 8 (1), 102–108. doi:10.1021/acsami.5b08975
Chou, Y.-C., Shih, C.-I., Chiang, C.-C., Hsu, C.-H., and Yeh, Y.-C. (2019). Reagent-free DOPA-dioxygenase colorimetric biosensor for selective detection of L-DOPA. Sensors Actuators B Chem. 297, 126717. doi:10.1016/j.snb.2019.126717
Claridge, S. A., Goh, S. L., Fréchet, J. M. J., Williams, S. C., Micheel, C. M., and Alivisatos, A. P. (2005). Directed assembly of discrete gold nanoparticle groupings using branched DNA scaffolds. Chem. Mat. 17 (7), 1628–1635. doi:10.1021/cm0484089
Degliangeli, F., Kshirsagar, P., Brunetti, V., Pompa, P. P., and Fiammengo, R. (2014). Absolute and direct microRNA quantification using DNA–gold nanoparticle probes. J. Am. Chem. Soc. 136 (6), 2264–2267. doi:10.1021/ja412152x
Deng, J., Yu, P., Wang, Y., Yang, L., and Mao, L. (2014). Visualization and quantification of neurochemicals with gold nanoparticles: Opportunities and challenges. Adv. Mat. 26 (40), 6933–6943. doi:10.1002/adma.201305619
Deville, S. (2017). Freezing colloids: Observations, principles, control, and use: Applications in materials science, life science, earth science, food science, and engineering. Springer International.
Diao, W., Wang, G., Wang, L., Zhang, L., Ding, S., Takarada, T., et al. (2020). Opposite effects of flexible single-stranded DNA regions and rigid loops in DNAzyme on colloidal nanoparticle stability for “turn-on” plasmonic detection of lead ions. ACS Appl. Bio Mat. 3 (10), 7003–7010. doi:10.1021/acsabm.0c00873
Ding, Y., Liu, Y., Chen, Y., Huang, Y., and Gao, Y. (2019). Photochemical vapor generation for colorimetric speciation of inorganic selenium. Anal. Chem. 91 (5), 3508–3515. doi:10.1021/acs.analchem.8b05117
Dou, B., Li, J., Jiang, B., Yuan, R., and Xiang, Y. (2019). DNA-templated in situ synthesis of highly dispersed AuNPs on nitrogen-doped graphene for real-time electrochemical monitoring of nitric Oxide released from live cancer cells. Anal. Chem. 91 (3), 2273–2278. doi:10.1021/acs.analchem.8b04863
Duskova, K., Lejault, P., Benchimol, É., Guillot, R., Britton, S., Granzhan, A., et al. (2020). DNA junction ligands trigger DNA damage and are synthetic lethal with DNA repair inhibitors in cancer cells. J. Am. Chem. Soc. 142 (1), 424–435. doi:10.1021/jacs.9b11150
Faraday, M. (1997). X. The bakerian lecture. —experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. Lond B Biol. Sci. 147, 145–181. doi:10.1098/rstl.1857.0011
Frens, G. (1973). Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 241 (105), 20–22. doi:10.1038/physci241020a0
Gao, J. L., Liu, Y. H., Zheng, B., Liu, J. X., Fang, W. K., Liu, D., et al. (2021). Light-activated and self-driven autonomous DNA nanomachine enabling fluorescence imaging of microRNA in living cells with exceptional precision and efficiency. ACS Appl. Mat. Interfaces 13 (27), 31485–31494. doi:10.1021/acsami.1c07333
Ghosh, S. K., and Pal, T. (2007). Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 107 (11), 4797–4862. doi:10.1021/cr0680282
Hao, N., Lu, J., Zhou, Z., Hua, R., and Wang, K. (2018). A pH-resolved colorimetric biosensor for simultaneous multiple target detection. ACS Sens. 3 (10), 2159–2165. doi:10.1021/acssensors.8b00717
Herne, T. M., and Tarlov, M. J. (1997). Characterization of DNA probes immobilized on gold surfaces. J. Am. Chem. Soc. 119 (38), 8916–8920. doi:10.1021/ja9719586
Heuer-Jungemann, A., and Liedl, T. (2019). From DNA tiles to functional DNA materials. Trends Chem. 1 (9), 799–814. doi:10.1016/j.trechm.2019.07.006
Jiang, Y., Chai, H., Feng, N., and Miao, P. (2021). Highly sensitive electrochemical sensor for an miR-200c assay based on ligation-assisted DNA strand displacements. ACS Sustain. Chem. Eng. 9 (28), 9257–9263. doi:10.1021/acssuschemeng.1c01454
Jiang, Y., and Miao, P. (2020). DNA dumbbell and chameleon silver nanoclusters for miRNA logic operations. Research 2020, 1–8. doi:10.34133/2020/1091605
Jiang, Y., Sun, M., Ouyang, N., Tang, Y., and Miao, P. (2021). Synergistic chemo-thermal therapy of cancer by DNA-templated silver nanoclusters and polydopamine nanoparticles. ACS Appl. Mat. Interfaces 13 (18), 21653–21660. doi:10.1021/acsami.1c04608
Kanaras, A. G., Wang, Z., Bates, A. D., Cosstick, R., and Brust, M. (2003). Towards multistep nanostructure synthesis: Programmed enzymatic self-assembly of DNA/gold systems. Angew. Chem. Int. Ed. 42 (2), 191–194. doi:10.1002/anie.200390075
Karami, A., Hasani, M., Azizi Jalilian, F., and Ezati, R. (2021). Hairpin-spherical nucleic acids for diagnosing COVID-19: A simple method to generalize the conventional PCR for molecular assays. Anal. Chem. 93 (26), 9250–9257. doi:10.1021/acs.analchem.1c01515
Lee, K. S., and El-Sayed, M. A. (2006). Gold and silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. J. Phys. Chem. B 110 (39), 19220–19225. doi:10.1021/jp062536y
Li, D., Luo, Z., An, H., Yang, E., Wu, M., Huang, Z., et al. (2020). Poly-adenine regulated DNA density on AuNPs to construct efficient DNA walker for microRNA-21 detection. Talanta 217, 121056. doi:10.1016/j.talanta.2020.121056
Li, Q., Tong, Z., Cao, Y., and Gu, H. (2021). DNAs catalyzing DNA nanoconstruction. Chem 7 (10), 2556–2568. doi:10.1016/j.chempr.2021.08.008
Liang, L., Lan, F., Ge, S., Yu, J., Ren, N., and Yan, M. (2017). Metal-enhanced ratiometric fluorescence/naked eye bimodal biosensor for lead ions analysis with bifunctional nanocomposite probes. Anal. Chem. 89 (6), 3597–3605. doi:10.1021/acs.analchem.6b04978
Lin, M., Yi, X., Huang, F., Ma, X., Zuo, X., and Xia, F. (2019). Photoactivated nanoflares for mRNA detection in single living cells. Anal. Chem. 91 (3), 2021–2027. doi:10.1021/acs.analchem.8b04434
Liu, B., and Liu, J. (2017). Freezing directed construction of bio/nano interfaces: Reagentless conjugation, denser spherical nucleic acids, and better nanoflares. J. Am. Chem. Soc. 139 (28), 9471–9474. doi:10.1021/jacs.7b04885
Liu, B., Wu, P., Huang, Z., Ma, L., and Liu, J. (2018). Bromide as a robust backfiller on gold for precise control of DNA conformation and high stability of spherical nucleic acids. J. Am. Chem. Soc. 140 (13), 4499–4502. doi:10.1021/jacs.8b01510
Liu, C., Gou, S., Bi, Y., Gao, Q., Sun, J., Hu, S., et al. (2022). Smart DNA-gold nanoparticle hybrid hydrogel film based portable, cost-effective and storable biosensing system for the colorimetric detection of lead (II) and uranyl ions. Biosens. Bioelectron. X. 210, 114290. doi:10.1016/j.bios.2022.114290
Liu, F., Wong, M. M., Chiu, S. K., Lin, H., Ho, J. C., and Pang, S. W. (2014). Effects of nanoparticle size and cell type on high sensitivity cell detection using a localized surface plasmon resonance biosensor. Biosens. Bioelectron. X. 55, 141–148. doi:10.1016/j.bios.2013.11.075
Liu, J.-W., Wang, Y.-M., Zhang, C.-H., Duan, L.-Y., Li, Z., Yu, R.-Q., et al. (2018). Tumor-targeted graphitic carbon nitride nanoassembly for activatable two-photon fluorescence imaging. Anal. Chem. 90 (7), 4649–4656. doi:10.1021/acs.analchem.7b05192
Liu, J., Ye, L. Y., Zhang, Y., Yang, H., Zhou, L., Luo, E., et al. (2022). Nonenzymatic target-driven DNA nanomachine for monitoring malathion contamination in living cells and bioaccumulation in foods. Anal. Chem. 94 (14), 5667–5673. doi:10.1021/acs.analchem.2c00315
Liu, X., Atwater, M., Wang, J., and Huo, Q. (2007). Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surfaces B Biointerfaces 58 (1), 3–7. doi:10.1016/j.colsurfb.2006.08.005
Ma, P.-Q., Huang, Q., Li, H.-D., Yin, B.-C., and Ye, B.-C. (2020). Multimachine communication network that mimics the adaptive immune response. J. Am. Chem. Soc. 142 (8), 3851–3861. doi:10.1021/jacs.9b11545
Ma, X., Chen, X., Tang, Y., Yan, R., and Miao, P. (2019). Triple-input molecular AND logic gates for sensitive detection of multiple miRNAs. ACS Appl. Mat. Interfaces 11 (44), 41157–41164. doi:10.1021/acsami.9b16812
Ma, X., Gao, L., Tang, Y., and Miao, P. (2018). Gold nanoparticles-based DNA logic gate for miRNA inputs analysis coupling strand displacement reaction and hybridization chain reaction. Part. Part. Syst. Charact. 35 (2), 1700326. doi:10.1002/ppsc.201700326
Ma, X., Guo, Z., Mao, Z., Tang, Y., and Miao, P. (2017). Colorimetric theophylline aggregation assay using an RNA aptamer and non-crosslinking gold nanoparticles. Microchim. Acta 185 (1), 33. doi:10.1007/s00604-017-2606-4
Mao, X., Liu, M., Li, Q., Fan, C., and Zuo, X. (2022). DNA-based molecular machines. JACS Au 2 (11), 2381–2399.
Meng, L., Ma, W., Lin, S., Shi, S., Li, Y., and Lin, Y. (2019). Tetrahedral DNA nanostructure-delivered DNAzyme for gene silencing to suppress cell growth. ACS Appl. Mat. Interfaces 11 (7), 6850–6857. doi:10.1021/acsami.8b22444
Miao, P., and Tang, Y. (2021). Cascade strand displacement and bipedal walking based DNA logic system for miRNA diagnostics. ACS Cent. Sci. 7 (6), 1036–1044. doi:10.1021/acscentsci.1c00277
Miao, P., and Tang, Y. (2020). DNA walking and rolling nanomachine for electrochemical detection of miRNA. Small 16 (47), 2004518. doi:10.1002/smll.202004518
Miao, P., and Tang, Y. (2020). Dumbbell hybridization chain reaction based electrochemical biosensor for ultrasensitive detection of exosomal miRNA. Anal. Chem. 92 (17), 12026–12032. doi:10.1021/acs.analchem.0c02654
Miao, P., and Tang, Y. (2019). Gold nanoparticles-based multipedal DNA walker for ratiometric detection of circulating tumor cell. Anal. Chem. 91 (23), 15187–15192. doi:10.1021/acs.analchem.9b04000
Miao, P., and Tang, Y. (2020). Two-dimensional hybridization chain reaction strategy for highly sensitive analysis of Intracellular mRNA. Anal. Chem. 92 (18), 12700–12709. doi:10.1021/acs.analchem.0c03181
Miao, P., Tang, Y., and Wang, L. (2017). DNA modified Fe3O4@Au magnetic nanoparticles as selective probes for simultaneous detection of heavy metal ions. ACS Appl. Mat. Interfaces 9 (4), 3940–3947. doi:10.1021/acsami.6b14247
Miao, X., Cheng, Z., Ma, H., Li, Z., Xue, N., and Wang, P. (2018). Label-free platform for microRNA detection based on the fluorescence quenching of positively charged gold nanoparticles to silver nanoclusters. Anal. Chem. 90 (2), 1098–1103. doi:10.1021/acs.analchem.7b01991
Mirkin, C. A., Letsinger, R. L., Mucic, R. C., and Storhoff, J. J. (1996). A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382 (6592), 607–609. doi:10.1038/382607a0
Pan, W., Liu, X., Wan, X., Li, J., Li, Y., Lu, F., et al. (2019). Rapid preparation of Au–Se–peptide nanoprobe based on a freezing method for bioimaging. Anal. Chem. 91 (24), 15982–15987. doi:10.1021/acs.analchem.9b04616
Pei, H., Li, F., Wan, Y., Wei, M., Liu, H., Su, Y., et al. (2012). Designed diblock oligonucleotide for the synthesis of spatially isolated and highly hybridizable functionalization of DNA-gold nanoparticle nanoconjugates. J. Am. Chem. Soc. 134 (29), 11876–11879. doi:10.1021/ja304118z
Peng, B., Liu, Z., and Jiang, Y. (2022). Aggregation of DNA-grafted nanoparticles in water: The critical role of sequence-dependent conformation of DNA coating. J. Phys. Chem. B 126 (4), 847–857. doi:10.1021/acs.jpcb.1c09450
Qian, R. C., Cao, Y., and Long, Y. T. (2016). Binary system for microRNA-targeted imaging in single cells and photothermal cancer therapy. Anal. Chem. 88 (17), 8640–8647. doi:10.1021/acs.analchem.6b01804
Rose, H. E. (1952). Breakdown of the lambert-beer law. Nature 169 (4294), 287–288. doi:10.1038/169287a0
Seeman, N. C., and Sleiman, H. F. (2017). DNA nanotechnology. Nat. Rev. Mat. 3 (1), 17068. doi:10.1038/natrevmats.2017.68
Sun, G.-Y., Du, Y.-C., Cui, Y.-X., Wang, J., Li, X.-Y., Tang, A.-N., et al. (2019). Terminal deoxynucleotidyl transferase-catalyzed preparation of pH-responsive DNA nanocarriers for tumor-targeted drug delivery and therapy. ACS Appl. Mat. Interfaces 11 (16), 14684–14692. doi:10.1021/acsami.9b05358
Sun, J., Zhang, X., Li, T., Xie, J., Shao, B., Xue, D., et al. (2019). Ultrasensitive on-site detection of biological active ricin in complex food matrices based on immunomagnetic enrichment and fluorescence switch-on nanoprobe. Anal. Chem. 91 (10), 6454–6461. doi:10.1021/acs.analchem.8b04458
Tan, L., Chen, Z., Zhang, C., Wei, X., Lou, T., and Zhao, Y. (2017). Colorimetric detection of Hg2+ based on the growth of aptamer-coated AuNPs: The effect of prolonging aptamer strands. Small 13 (14), 1603370. doi:10.1002/smll.201603370
Tang, W., Wang, Z., Yu, J., Zhang, F., and He, P. (2018). Internal calibration potentiometric aptasensors for simultaneous detection of Hg2+, Cd2+, and As3+ based on a screen-printed carbon electrodes array. Anal. Chem. 90 (14), 8337–8344. doi:10.1021/acs.analchem.7b04150
Underwood, S., and Mulvaney, P. (1994). Effect of the solution refractive index on the color of gold colloids. Langmuir 10 (10), 3427–3430. doi:10.1021/la00022a011
Wall, M. A., Harmsen, S., Pal, S., Zhang, L., Arianna, G., Lombardi, J. R., et al. (2017). Surfactant-free shape control of gold nanoparticles enabled by unified theoretical framework of nanocrystal synthesis. Adv. Mat. 29 (21). doi:10.1002/adma.201605622
Wang, F., Curry, D. E., and Liu, J. (2015). Driving adsorbed gold nanoparticle assembly by merging lipid gel/fluid interfaces. Langmuir 31 (49), 13271–13274. doi:10.1021/acs.langmuir.5b03606
Wang, J., Qu, X., Xu, C., Zhang, Z., Qi, G., and Jin, Y. (2022). Thermoplasmonic regulation of the mitochondrial metabolic state for promoting directed differentiation of dental pulp stem cells. Anal. Chem. 94 (27), 9564–9571. doi:10.1021/acs.analchem.2c00288
Wang, Y., Hu, N., Liu, C., Nie, C., He, M., Zhang, J., et al. (2020). An RNase H-powered DNA walking machine for sensitive detection of RNase H and the screening of related inhibitors. Nanoscale 12 (3), 1673–1679. doi:10.1039/c9nr07550j
Wang, Z., Jia, N., Zhou, X., Han, J., and Bu, H. (2021). Cu(I)-catalyzed click reaction-triggered 3D DNA walker for constructing an "OFF-ON" fluorescent biosensor for Cu(2+) detection. ACS Appl. Bio Mat. 4 (4), 3571–3578. doi:10.1021/acsabm.1c00073
Xie, N., Liu, S., Fang, H., Yang, Y., Quan, K., Li, J., et al. (2019). Three-dimensional molecular transfer from DNA nanocages to inner gold nanoparticle surfaces. ACS Nano 13 (4), 4174–4182. doi:10.1021/acsnano.8b09147
Xie, Y. (2018). K+ colorimetric detection in the nanomolar range based on K+-aptamer quadruplex-NPs association. Sensors Actuators B Chem. 269, 22–26. doi:10.1016/j.snb.2018.04.166
Xu, H., Zhang, Z., Wang, Y., Zhang, X., Zhu, J. J., and Min, Q. (2022). Sense and validate: Fluorophore/mass dual-encoded nanoprobes for fluorescence imaging and MS quantification of intracellular multiple microRNAs. Anal. Chem. 94 (16), 6329–6337. doi:10.1021/acs.analchem.2c00513
Xu, S., Yuan, H., Xu, A., Wang, J., and Wu, L. (2011). Rapid synthesis of stable and functional conjugates of DNA/gold nanoparticles mediated by Tween 80. Langmuir 27 (22), 13629–13634. doi:10.1021/la203632c
Yang, C., Yin, X., Huan, S. Y., Chen, L., Hu, X. X., Xiong, M. Y., et al. (2018). Two-photon DNAzyme-gold nanoparticle probe for imaging intracellular metal ions. Anal. Chem. 90 (5), 3118–3123. doi:10.1021/acs.analchem.7b04171
Yang, X. J., Zhang, K., Zhang, T. T., Xu, J. J., and Chen, H. Y. (2017). Reliable forster resonance energy transfer probe based on structure-switching DNA for ratiometric sensing of telomerase in living cells. Anal. Chem. 89 (7), 4216–4222. doi:10.1021/acs.analchem.7b00267
Ye, M., Kong, Y., Zhang, C., Lv, Y., Cheng, S., Hou, D., et al. (2021). Near-infrared light controllable DNA walker driven by endogenous adenosine triphosphate for in situ spatiotemporal imaging of intracellular microRNA. ACS Nano 15 (9), 14253–14262. doi:10.1021/acsnano.1c02229
Zha, Y., Li, Y., Hu, P., Lu, S., Ren, H., Liu, Z., et al. (2021). Duplex-specific nuclease-triggered fluorescence immunoassay based on dual-functionalized auNP for acetochlor, metolachlor, and propisochlor. Anal. Chem. 93 (41), 13886–13892. doi:10.1021/acs.analchem.1c02736
Zhang, D., Wu, T. T., Qin, X. Y., Qiao, Q., Shang, L. H., Song, Q. L., et al. (2019). Intracellularly generated immunological gold nanoparticles for combinatorial photothermal therapy and immunotherapy against tumor. Nano Lett. 19 (9), 6635–6646. doi:10.1021/acs.nanolett.9b02903
Zhang, S., Chen, C., Xue, C., Chang, D., Xu, H., Salena, B. J., et al. (2020). Ribbon of DNA lattice on gold nanoparticles for selective drug delivery to cancer cells. Angew. Chem. Int. Ed. Engl. 59 (34), 14692–14700. doi:10.1002/ange.202005624
Zhang, W. S., Pan, J., Li, F., Zhu, M., Xu, M., Zhu, H., et al. (2021). Reverse transcription recombinase polymerase amplification coupled with CRISPR-Cas12a for facile and highly sensitive colorimetric SARS-CoV-2 detection. Anal. Chem. 93 (8), 4126–4133. doi:10.1021/acs.analchem.1c00013
Zhang, X., Liu, S., Song, X., Wang, H., Wang, J., Wang, Y., et al. (2019). Robust and universal SERS sensing platform for multiplexed detection of alzheimer’s disease core biomarkers using PAapt-AuNPs conjugates. ACS Sens. 4 (8), 2140–2149. doi:10.1021/acssensors.9b00974
Zhang, X., Servos, M. R., and Liu, J. (2012). Instantaneous and quantitative functionalization of gold nanoparticles with thiolated DNA using a pH-assisted and surfactant-free route. J. Am. Chem. Soc. 134 (17), 7266–7269. doi:10.1021/ja3014055
Zhang, X., Wei, X., Qi, J., Shen, J., Xu, J., Gong, G., et al. (2022). Simultaneous detection of bladder cancer exosomal MicroRNAs based on inorganic nanoflare and DNAzyme walker. Anal. Chem. 94 (11), 4787–4793. doi:10.1021/acs.analchem.1c05588
Zhang, X., Xiao, K., Cheng, L., Chen, H., Liu, B., Zhang, S., et al. (2014). Visual and highly sensitive detection of cancer cells by a colorimetric aptasensor based on cell-triggered cyclic enzymatic signal amplification. Anal. Chem. 86 (11), 5567–5572. doi:10.1021/ac501068k
Zhang, X., Zhang, J., Zhang, F., and Yu, S. (2017). Probing the binding affinity of plasma proteins adsorbed on au nanoparticles. NANOSCALE 9 (14), 4787–4792. doi:10.1039/c7nr01523b
Zhang, Z., Ma, X., Zhu, J., Yan, R., and Miao, P. (2022). Roll-to-Roll DNA nanomachine for ultrasensitive electrochemical determination of miRNA. Langmuir 38 (36), 11130–11135. doi:10.1021/acs.langmuir.2c02102
Zhao, W., Lin, L., and Hsing, I. M. (2009). Rapid synthesis of DNA-functionalized gold nanoparticles in salt solution using mononucleotide-mediated conjugation. Bioconjug. Chem. 20 (6), 1218–1222. doi:10.1021/bc900080p
Zheng, X.-S., Zong, C., Wang, X., and Ren, B. (2019). Cell-penetrating peptide conjugated SERS nanosensor for in situ intracellular pH imaging of single living cells during cell cycle. Anal. Chem. 91 (13), 8383–8389. doi:10.1021/acs.analchem.9b01191
Zhong, W., Wu, J., Huang, Y., Xing, C., and Lu, C. (2022). Target-activated, light-actuated three-dimensional DNA walker nanomachine for amplified miRNA detection. Langmuir 38 (3), 1151–1157. doi:10.1021/acs.langmuir.1c02834
Zhou, H., Jiang, Y., Zhao, W., and Zhang, S. (2022). Light-activated nanodevice for on-demand imaging of miRNA in living cells via logic assembly. ACS Appl. Mat. Interfaces 14 (11), 13070–13078. doi:10.1021/acsami.2c00376
Zhou, Y., Li, Z., Wu, T., Ning, J., Hu, G., and Nie, Y. (2022). A DNA–Au nanomachine activated by dual types of biomarkers for multi-site imaging and gene silencing. ChemComm. Pub Date: 2022-11-01. doi:10.1039/D2CC05017J
Zhou, Y., Lv, S., Wang, X.-y., Kong, L., and Bi, S. (2022). Biometric photoelectrochemical–visual multimodal biosensor based on 3D hollow HCdS@Au nanospheres coupled with target-induced ion exchange reaction for antigen detection. Anal. Chem. 94 (41), 14492–14501. doi:10.1021/acs.analchem.2c03885
Keywords: DNA modification, biosensor, DNA hybridization, gold nanoparticles, therapy
Citation: Ma X, Li X, Luo G and Jiao J (2022) DNA-functionalized gold nanoparticles: Modification, characterization, and biomedical applications. Front. Chem. 10:1095488. doi: 10.3389/fchem.2022.1095488
Received: 11 November 2022; Accepted: 29 November 2022;
Published: 13 December 2022.
Edited by:
Xiaoli Zhu, Tongji University, ChinaReviewed by:
Guangfeng Wang, Anhui Normal University, ChinaChengmin Jiang, American Society for Biochemistry and Molecular Biology, United States
Copyright © 2022 Ma, Li, Luo and Jiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gangyin Luo, bHVvZ3lAc2liZXQuYWMuY24=; Jin Jiao, amlhb2ppbkBzZGZtdS5lZHUuY24=
 Xiaoyi Ma
Xiaoyi Ma Xiaoqiang Li1
Xiaoqiang Li1 Jin Jiao
Jin Jiao