- Key Laboratory for Advanced Materials, Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, Institute of Fine Chemicals, East China University of Science and Technology, Shanghai, China
Dynamic fluorophore 9,14-diphenyl-9,14-dihydrodibenzo[a,c]phenazine (DPAC) affords a new platform to produce diverse emission outputs. In this paper, a novel DPAC-containing crown ether macrocycle D-6 is synthesized and characterized. Host-guest interactions of D-6 with different ammonium guests produced a variety of fluorescence with hypsochromic shifts up to 130 nm, which are found to be affected by choice of solvent or guest and host/guest stoichiometry. Formation of supramolecular complexes were confirmed by UV-vis titration, 1H NMR and HRMS spectroscopy.
Introduction
Supramolecular chemistry (Lehn, 2005; Stoddart, 2012; Yan et al., 2012; Yeung and Yam, 2015; Kolesnichenko and Anslyn, 2017; Liu et al., 2017; Zhou et al., 2017; Gu et al., 2018; Jana et al., 2018; Xia et al., 2020; Gu and Lehn, 2021; Shen et al., 2021; Zhang et al., 2021; Zhang et al., 2022a; Zhang et al., 2022b; Huang et al., 2022) is undergoing tremendous speed of development, being important tools to modulate optical properties of chemical systems. Multicolor emission has been extensively investigated over the past decade due to its considerable application prospects in displays (Nie et al., 2022; Zou et al., 2022), illumination (Lee et al., 2016; Zhang et al., 2019a; Gong et al., 2019), molecular/ion recognition (Wang et al., 2012; Li et al., 2017; Li et al., 2018a; Zhang et al., 2019b; Chen et al., 2019; Sun et al., 2020), and biosensing (Zhou et al., 2019; Dong et al., 2020; Yan et al., 2021; Du and Wei, 2022). Doping (Nie et al., 2022) or hybridizing (Cui et al., 2017) of different fluorophores are effective methods to generate multicolor emission, these systems usually requires more than a single excitation wavelength or stimulation methods to achieve multicolor emissions. However, many chemical systems exhibiting multicolor emission have been constructed in the presence of only one chromophore by the modulation of host-guest interaction (Zhang et al., 2016; Li et al., 2018a; Wang et al., 2020a; Wang et al., 2020b; Sun et al., 2020; Wu et al., 2020; Zhang et al., 2022c; Yu et al., 2022), pH (Li et al., 2018b; Bai et al., 2019; Radunz et al., 2019; Liu et al., 2021), hydrogen bonding (Wu et al., 2019; Tao et al., 2020), metal coordination (Lee et al., 2017), and other methods (Feng et al., 2015; Huang et al., 2015; Shi et al., 2018; Wang et al., 2019; Naren et al., 2020; Guo et al., 2021; Wang et al., 2022a; Wang et al., 2022b; Qiu et al., 2022; Zong et al., 2022). Although progresses have been made in the study of single-chromophore multicolor emission, it is still of great value to develop new and controllable multicolor emission systems for a wider range of application scenarios.
N,N′—diphenyl—dihydrodibenzo [a,c] phenazines (DPAC) possesses unique photophysical properties including the double fluorescence emission, large stokes shift and remarkable responsiveness to various environmental stimuli (Zhang et al., 2015; Zhang et al., 2020). In solution, the unique saddle-shaped structures of DPAC units undergo dynamic light-induced planarization processes upon photoexcitation and emit orange-red fluorescence. When such vibrational motions of the molecules are restricted, e.g., in the solid state, only the intrinsic blue fluorescence could be detected. The described vibration-induced emission (VIE) behavior of the DPAC chromophore has provided a new platform for chemists to build multicolor emission systems by meticulous control of its molecular geometry (Huang et al., 2015; Shi et al., 2018; Zhang et al., 2018). For example, in an inspiring work of Tian and Chou (Chen et al., 2017), a number of DPAC-based macrocycles with various sizes were systematically investigated. The different degrees of constraint of the DPAC units resulted in various emissions from 490 nm to 625 nm, showing the great potential of these chemically locked DPAC containing macrocycles in both fundamental studies and optical applications.
Herein, we designed and synthesized a large-size DPAC-based crown ether macrocycle D-6 whose dynamic DPAC chromophore was covalently locked by a conformational flexible hexaethylene glycol chain (see Scheme 1 for the structure of D-6). The electron-rich cavity of this crown ether was able to supramolecularly combine electron-deficient molecules/ions through host-guest interactions and subsequently increase the constrain of the DPAC wings. Relying on this understanding, we managed to produce multicolor fluorescent signals from orange to blue by: 1) respectively mixing the macrocycle with different ammonium guests G1-G5 (Scheme 1); 2) titration of an ammonium guest G5 to the emissive macrocycle D-6. White light emission was also obtained in this work in a specific stoichiometry of D-6 and G5.
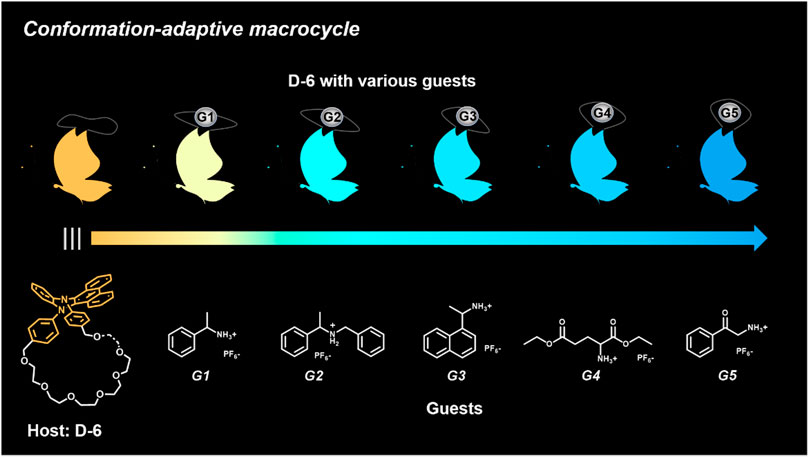
SCHEME 1. Chemical structures of conformation-adaptive macrocycle host D-6 and ammonium guests G1-G5, and schematic representations of their combinations exhibiting diverse emission from orange to blue when equimolar D-6 and different guests were mixed, respectively.
Experiment section
Synthesis of DPAC-crown ether ring (D-6) and guests
The macrocycle D-6 was synthesized in three steps from N, N′- diphenyl dihydrodibenzo [a, c] phenazine (the synthetic route is shown in Supplementary Scheme S1). Compound 3 were prepared referring to the method described in the literature (Zhang et al., 2015). In the final step, compound D-6 was produced with a 40% yield by the Williamson etherification reaction of compound 3 and hexaethylene glycol di (p-toluenesulfonate) under the templation of sodium hydride. 1H NMR, 13C NMR, and high-resolution mass spectrometry (HRMS) were used to confirm the chemical structure of D-6 (Supplementary Figures S13–S15). The five ammonium hexafluorophosphates G1-G5 involved in this paper were obtained by protonation and ion exchange of commercially available amines, or direct ion exchange of commercially available ammonium hydrochloride salts, respectively (see Supplementary Material for experimental details).
Materials and methods
The 1H NMR and 13C NMR data were measured by AV-400 NMR spectrometer made by Brucker Company, in which the internal standard reference was tetramethylsilane (TMS), and the detection temperature was room temperature (25°C, 298 K) unless otherwise specified. High resolution mass spectrometry (HRMS) was performed by Waters LCT Premier XE mass spectrometer, in which electrospray ionization (ESI) was used for ionization. The UV/Vis absorption spectra data were documented by a Shimadzu UV-2600 UV-Vis spectrophotometer and the fluorescent spectra were acquired by a Shimadzu RF6000 spectro fluorophotometer.
Results and discussion
It should be noted that D-6 is not the first DPAC-involving crown ether we investigate. In a previous work of Qu group (Yang et al., 2021), a smaller sized DPAC-ring with pentaethylene glycol backbone was inserted a dibenzylammonium guest to show the adaptive emission of the DPAC-ring (in contrast, the effect of dibenzylammonium salt on D-6 is detailed in Supplementary Figure S12). There, the host-guest interaction only caused a small spectral shift of 13 nm (from 490 nm to 477 nm) in acetonitrile with a small visual variation from light blue to blue. In comparison, the emission of the present macrocycle D-6 in acetonitrile reaches 584 nm (Figure 1A), 94 nm longer than the previously reported macrocycle, indicating a smaller constraint of D-6 in the guest-free state. Different solvents including toluene, dichloromethane, tetrahydrofuran, and acetonitrile were tested here and no significant disparity was generated (Figure 1A). Surprisingly, when D-6 in these solvents were respectively added G5, a drastic blue shift of 130 nm was detected only when dichloromethane was utilized as the solvent (Figure 1B), achieving a 10-fold dynamic variation in emission wavelength comparing to the previous work. Due to this huge variation which is beneficial to generate multicolor emissions, dichloromethane was chosen as the main solvent in the present work. And in every case, a volume fraction of 5% methanol was added to the solutions of ammonium guests in order to better dissolve the guests.
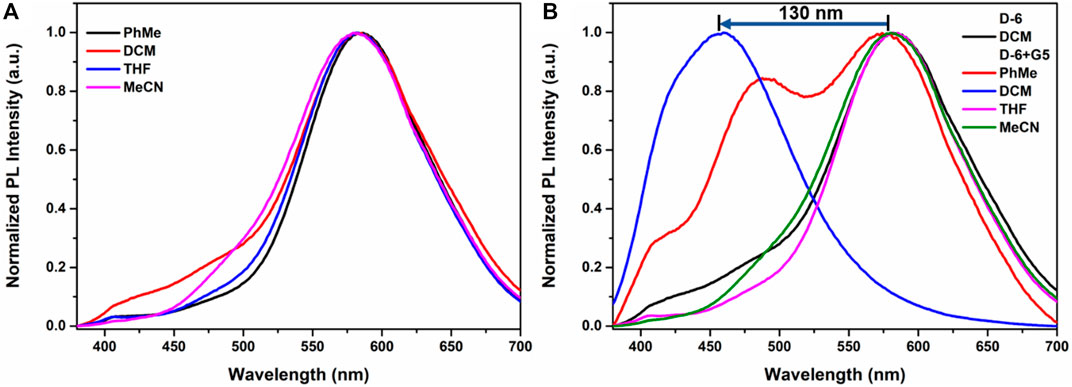
FIGURE 1. (A) Emission spectra of D-6 in different solvents. (B) Emission spectra of the mixtures of 1 eq. D-6 and 1 eq. G5 in different solvents.
UV-vis spectroscopy and fluorescence spectroscopy were utilized to study the photophysical properties of D-6. All the spectra were recorded at room temperature. The maximum UV absorbance of D-6 is approximately 352 nm which is attributed to the DPAC chromophore rather than the crown ether moiety (Supplementary Figure S1A), in line with the earlier studies on DPAC systems showing no apparent absorption data above 400 nm. Upon excitation of 360 nm UV light, the solution of D-6 emitted orange fluorescence at 584 nm as shown in Figure 1A, suggesting a weak constraint of DPAC wings.
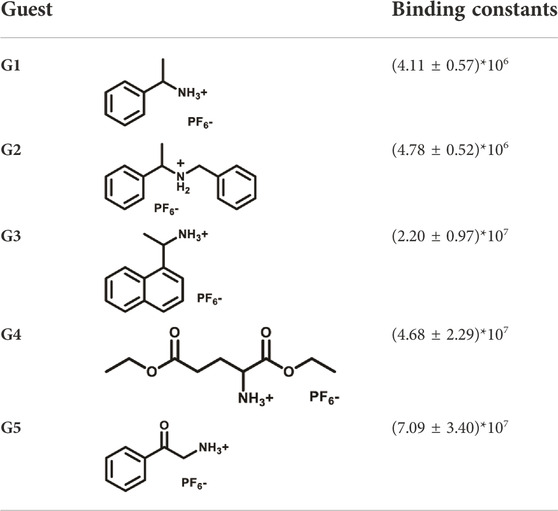
The responsiveness of D-6 to the supramolecular guests G1-G5 were then studied. First, UV-vis titrations were carried out to investigate the supramolecular complexations and to determine the binding ratios of the host macrocycle and the guests (Supplementary Figures S2–S6). All the absorbances underwent gradual decreases when the guests were added to the solutions of D-6. Meanwhile, hypsochromic shifts of ∼5 nm could be observed in the cases of G3-G5, indicating stronger combinations of D-6 with them. In all cases, when the molar ratios of guest cations and the host were 1:1, the Job’s Plot curves reached the maximum values, revealing that all the guests were hosted by D-6 macrocycle in the ratio of 1:1. Meanwhile, the quantum yields and fluorescence lifetimes of D-6 and the host-guest complexes of different guests were also measured as detailed in Supplementary Table S1.
Fluorescence spectra of the host-guest mixtures were then recorded at room temperature to examine the impact of supramolecular complexation on the fluorescence characteristics of D-6 macrocycle. Different degrees of variations, both visually and spectrally, were observed when the solutions of D-6 in dichloromethane were added equimolar ammonium salts respectively. The emission spectra of D-6 before and after the addition of guests were transformed into CIE coordinates: D-6 (0.45, 0.45), G1 (0.34, 0.40), G2 (0.22, 0.33), G3 (0.20, 0.30), G4 (0.20, 0.27), and G5 (0.17, 0.19) (Figure 2B and Supplementary Figure S12). The emission of D-6 and G1 was pale yellow with two peaks at 490 nm and 571 nm (red curve in Figure 2A), probably due to their insufficient host-guest complexation. In comparison, addition of all the other four guests G2-G5 brought huge hypsochromic shifts in emission wavelength (100, 101, 106, and 130 nm for G2, G3, G4, and G5, respectively), demonstrating the large impact of host-guest interactions. In particular, the addition of G5 to D-6 produced the largest shift of 130 nm from 584 nm (orange) to 454 nm (dark blue). The large variations of emission color in response to different guests were most likely caused by the conformational adaptation of D-6. The originally relaxed ethylene glycol backbone underwent a stronger resistance in tension after the insertion of the guests. Simultaneously, the wings of the DPAC unit were constrained to perform light-induced structural planarization, resulting in the changes of fluorescence. Potentially, the D-6 macrocycle could be used as a supramolecular fluorescent probe to distinguish different ammonium salts.
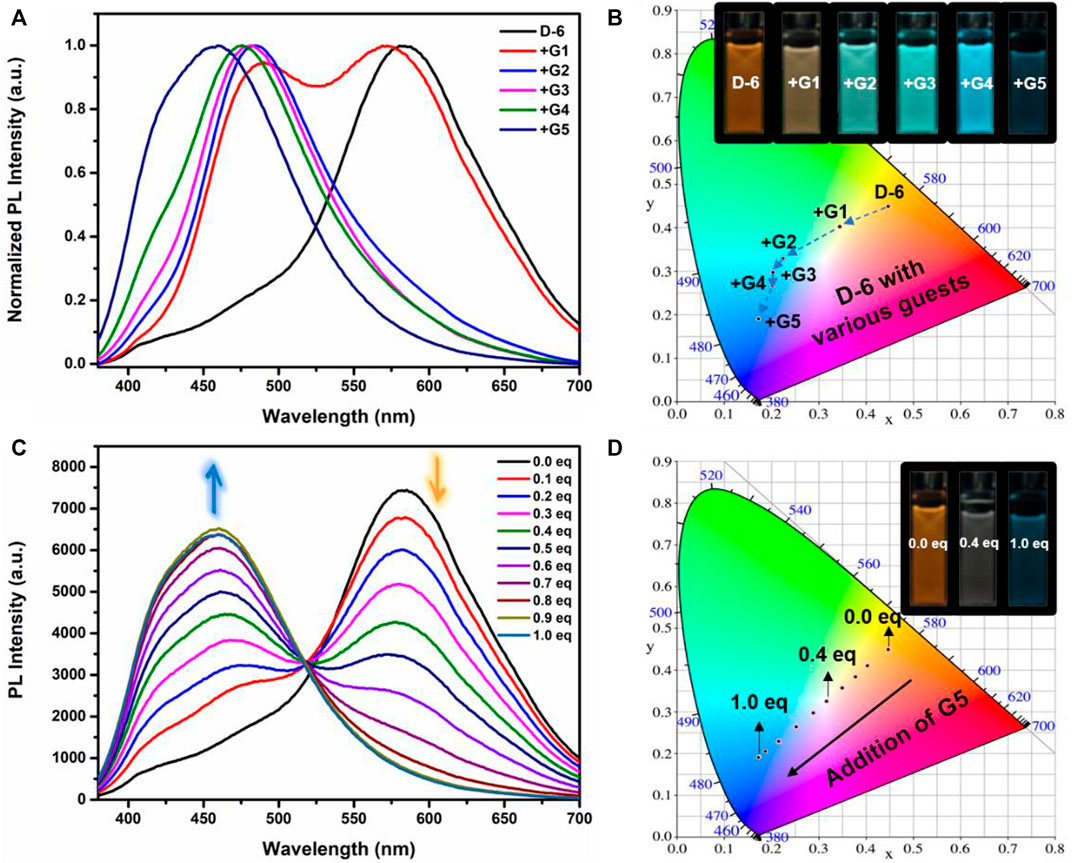
FIGURE 2. (A) Normalized fluorescence emission spectra of D-6 alone (black) and in the presence of various guests [in dichloromethane; (D-6) = 10 μM; λex = 360 nm]. (B) Chromaticity coordinates (CIE) of D-6 and the host-guest complexes in dichloromethane. Inset: images of D-6 and D-6⊃Guests upon irradiation with 360 nm UV light. (C) Fluorescence curves upon titration of 0.1 eq. G5 to the solution of D-6 [in dichloromethane; λex = 360 nm; (D-6) = 10 μM]. (D) CIE diagram of D-6 solutions containing various quantities of G5 (every 0.1 eq.). Inset: images of solutions of D-6 containing 0, 0.4, and 1.0 eq. G5 under irradiation with 360 nm UV light.
Fluorometric titration of G5 to D-6 was then carried out. As is clearly shown in Figure 2C, upon gradient addition of 0.1 eq. G5, the main emission peak of D-6 at 584 nm gradually decreased while a peak around 470 nm arose and increased simultaneously. Eventually, the new peak stopped to increase when 1 eq. guest was added. Additionally, noticeable visual changes could be observed after each 0.1 equivalent G5 was added. The fluorescence spectra discussed above were also translated to CIE coordinates as following: 0 eq. (0.44, 0.44), 0.1 eq. (0.40, 0.41), 0.2 eq. (0.38, 0.38), 0.3 eq. (0.35, 0.36), 0.4 eq. (0.32, 0.33), 0.5 eq. (0.29, 0.3), 0.6 eq. (0.25, 0.26), 0.7 eq. (0.21, 0.23), 0.8 eq. (0.17, 0.19), 0.9 eq. (0.17, 0.19), 1.0 eq. (0.17, 0.19). From the CIE chromaticity diagram (Figure 2D), a linear variation in color was accomplished, including the white light intermediate spot at (0.32, 0.33). Thus, multicolor emissions are efficiently obtained in this system by simple addition of the supramolecular guest to the host.
The formation of the host-guest complexes was further confirmed by 400 MHz 1H NMR (see Supplementary Figures S16–S22 for the NMR spectra of the mixtures of D-6 and G1-G4, respectively). The spectra of D-6 (blue), G5 (red), and their equimolar mixture (green) were shown in Figure 3. After the mixing of G5 and D-6, the methylene proton Hf of G5 is observed to significantly upshifted by 0.48 ppm. Similarly, all the phenyl protons of G5 shifted to the higher field (0.37 ppm for Ha,e, 0.24 ppm for Hb,d, and 0.13 ppm for Hc). Meanwhile, all of the protons of D-6 underwent displacements, especially H5,6, H9, and H14,15 (−0.11 ppm for H5,6, −0.12 ppm for H9, and ∼−0.09 ppm for H14,15). All the changes could be explained by the insertion of G5 into the cavity of D-6 and the consequent formation of the host-guest structure. Similar spectral variations were also found in the other four cases. In addition, the molecular ion peaks found for D-6 and its visitors in the HRMS data also support the complexation of them (Supplementary Figures S17–S25).
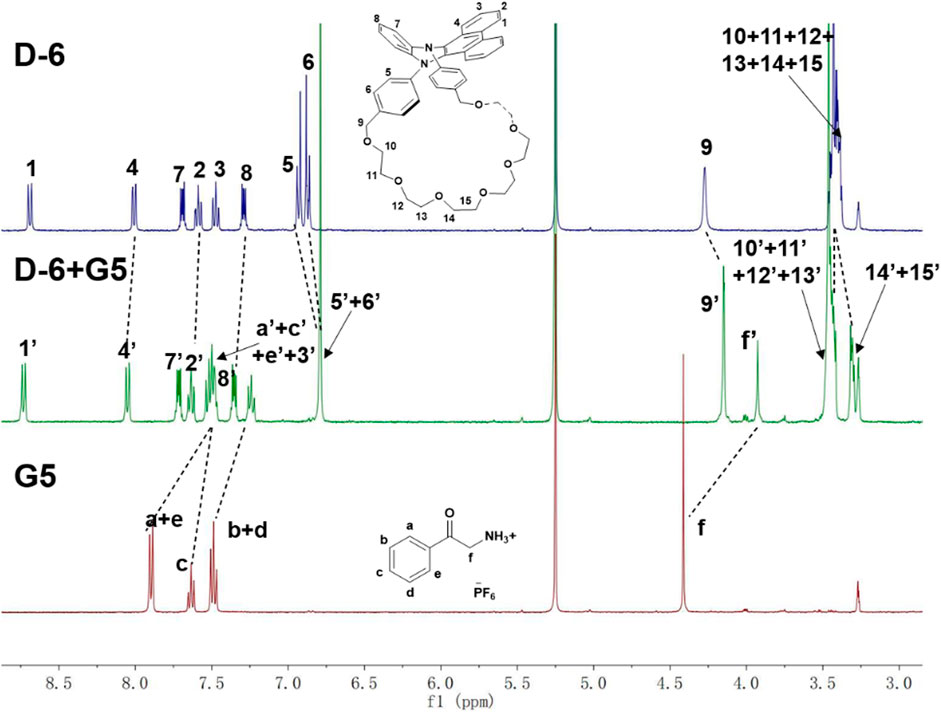
FIGURE 3. The 400 MHz 1H NMR of D-6 (blue), G5 (red) and their equimolar mixture D-6 + G5 (green) in dichloromethane-d2/methanol-d4 (v/v = 95:5).
By processing the spectra of UV-vis titrations (respective addition of G1-G5 into the solutions of D-6) using nonlinear regression methods (Thordarson, 2011), a number of corresponding binding constants were obtained (Table 1). The large magnitudes of binding constants further clarify the formation of supramolecular complexes. It is already known from the fluorescence spectra of D-6 along and with the five guests that the degrees of hypsochromic shifts increase in the order of G1-G5. Interestingly, the binding constants are perfectly in line with this sequence, showing the binding constant dependance of color change. The differences of binding constants were considered to be partially due to the different electron deficiency of the ammoniums. Comparing to G1-G3, the carbonyl groups of G4 and G5 increase the electron deficiency of their ammonium sites and consequently bring higher affinity in the complexation with the electron rich crown ether cavity. However, the relationship of the chemical inputs and the emission colors in the present work can hardly be contributed to only this reason. The long hexaethylene glycol backbone gives access to the guests with different sizes while the strong topological flexibility of the backbone gives possibility to the conformational adaptation of the DPAC unit and the whole macrocycle. Relationally, the different rigidities of the guests could also affect the binding geometry and the binding constants.
Conclusion
In conclusion, relying on the light induced structural planarization of DPAC derivatives, we synthesized a new emissive macrocycle D-6 as a conformation-adaptive supramolecular host. By the respective incorporation of various ammonium guests, multicolor emission from orange to white to deep blue was accomplished. Solvent and host/guest stoichiometry were found to be effectors that influence the optical outputs. The supramolecular host-guest complexation was confirmed by UV-vis titration, 1H NMR, and HRMS data. The guest-dependent emission of D-6 shown in this work is potential to distinguish different ammoniums, which would be continuously studied by our group in future. The use of supramolecular chemistry to modulate emission wavelengths over a broad range afford an effective way to obtain multicolor emission within a less complicated system.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization, C-SM and C-XZ; methodology, CM and CY; validation, CM and CY; formal analysis, CM, S-WZ, and CY; investigation, CM and CY; data curation, CM; writing—original draft preparation, CM; writing—review and editing, RG; supervision, RG; project administration, RG; funding acquisition, RG.
Funding
This work was supported by the National Natural Science Foundation of China (grants 22205064) and Shanghai Pujiang Program (grant 22PJ1402200).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1087610/full#supplementary-material
References
Bai, J., Ma, Y., Yuan, G., Chen, X., Mei, J., Zhang, L., et al. (2019). Solvent-controlled and solvent-dependent strategies for the synthesis of multicolor carbon dots for pH sensing and cell imaging. J. Mat. Chem. C 7, 9709–9718. doi:10.1039/c9tc02422k
Chen, W., Chen, C. L., Zhang, Z., Chen, Y. A., Chao, W. C., Su, J., et al. (2017). Snapshotting the excited-state planarization of chemically locked N, N'-Disubstituted dihydrodibenzo[a, c]phenazines. J. Am. Chem. Soc. 139, 1636–1644. doi:10.1021/jacs.6b11789
Chen, W., Guo, C., He, Q., Chi, X., Lynch, V. M., Zhang, Z., et al. (2019). Molecular cursor caliper: A fluorescent sensor for dicarboxylate dianions. J. Am. Chem. Soc. 141, 14798–14806. doi:10.1021/jacs.9b07170
Cui, Q., Xu, J., Shen, G., Zhang, C., Li, L., and Antonietti, M. (2017). Hybridizing carbon nitride colloids with a shell of water-soluble conjugated polymers for tunable full-color emission and synergistic cell imaging. ACS Appl. Mat. Interfaces 9, 43966–43974. doi:10.1021/acsami.7b13212
Dong, Z., Wang, Y., Wang, C., Meng, H., Li, Y., and Wang, C. (2020). Cationic peptidopolysaccharide with an intrinsic AIE effect for combating bacteria and multicolor imaging. Adv. Healthc. Mat. 9, e2000419. doi:10.1002/adhm.202000419
Du, J., and Wei, L. (2022). Multicolor photoactivatable Raman probes for subcellular imaging and tracking by cyclopropenone caging. J. Am. Chem. Soc. 144, 777–786. doi:10.1021/jacs.1c09689
Feng, H.-T., Xiong, J.-B., Zheng, Y.-S., Pan, B., Zhang, C., Wang, L., et al. (2015). Multicolor emissions by the synergism of intra/intermolecular slipped π–π stackings of tetraphenylethylene-DiBODIPY conjugate. Chem. Mat. 27, 7812–7819. doi:10.1021/acs.chemmater.5b03765
Gong, T., Li, Y., Lei, B., Zhang, X., Liu, Y., and Zhang, H. (2019). Solid-state silicon nanoparticles with color-tunable photoluminescence and multifunctional applications. J. Mat. Chem. C 7, 5962–5969. doi:10.1039/c9tc00938h
Gu, R., Flidrova, K., and Lehn, J. M. (2018). Dynamic covalent metathesis in the C═C/C═N exchange between knoevenagel compounds and imines. J. Am. Chem. Soc. 140, 5560–5568. doi:10.1021/jacs.8b01849
Gu, R., and Lehn, J. M. (2021). Constitutional dynamic selection at low Reynolds number in a triple dynamic system: Covalent dynamic adaptation driven by double supramolecular self-assembly. J. Am. Chem. Soc. 143, 14136–14146. doi:10.1021/jacs.1c04446
Guo, Y., Wu, A., Qiu, C., Ge, F., Jiang, Y., Gong, Y., et al. (2021). Force-induced molecular isomerization for the construction of multicolor luminescent segmented molecular crystals. Adv. Opt. Mater. 10, 2101794. doi:10.1002/adom.202101794
Huang, B., Liu, X., Yang, G., Tian, J., Liu, Z., Zhu, Y., et al. (2022). A near-infrared organoplatinum(II) metallacycle conjugated with heptamethine cyanine for trimodal cancer therapy. CCS Chem. 4, 2090–2101. doi:10.31635/ccschem.021.202100950
Huang, W., Sun, L., Zheng, Z., Su, J., and Tian, H. (2015). Colour-tunable fluorescence of single molecules based on the vibration induced emission of phenazine. Chem. Commun. 51, 4462–4464. doi:10.1039/c4cc09613d
Jana, A., Bahring, S., Ishida, M., Goeb, S., Canevet, D., Salle, M., et al. (2018). Functionalised tetrathiafulvalene- (TTF-) macrocycles: Recent trends in applied supramolecular chemistry. Chem. Soc. Rev. 47, 5614–5645. doi:10.1039/c8cs00035b
Kolesnichenko, I. V., and Anslyn, E. V. (2017). Practical applications of supramolecular chemistry. Chem. Soc. Rev. 46, 2385–2390. doi:10.1039/c7cs00078b
Lee, S. H., Shin, N., Kwak, S. W., Hyun, K., Woo, W. H., Lee, J. H., et al. (2017). Intriguing indium-salen complexes as multicolor luminophores. Inorg. Chem. 56, 2621–2626. doi:10.1021/acs.inorgchem.6b02797
Lee, S., Lee, Y., Kim, D. Y., and Panin, G. N. (2016). Multicolor emission from poly(p-phenylene)/nanoporous ZnMnO organic-inorganic hybrid light-emitting diode. ACS Appl. Mat. Interfaces 8, 35435–35439. doi:10.1021/acsami.6b11539
Lehn, J.-M. (2005). Dynamers: Dynamic molecular and supramolecular polymers. Prog. Polym. Sci. 30, 814–831. doi:10.1016/j.progpolymsci.2005.06.002
Li, D., Lu, F., Wang, J., Hu, W., Cao, X. M., Ma, X., et al. (2018a). Amorphous metal-free room-temperature phosphorescent small molecules with multicolor photoluminescence via a host-guest and dual-emission strategy. J. Am. Chem. Soc. 140, 1916–1923. doi:10.1021/jacs.7b12800
Li, M., Yuan, Y., and Chen, Y. (2018b). Acid-induced multicolor fluorescence of pyridazine derivative. ACS Appl. Mat. Interfaces 10, 1237–1243. doi:10.1021/acsami.7b16050
Li, Y., Yu, X., and Yu, T. (2017). Eu3+based mesoporous hybrid material with tunable multicolor emission modulated by fluoride ion: Application for selective sensing toward fluoride ion. J. Mat. Chem. C 5, 5411–5419. doi:10.1039/c7tc01240c
Liu, X., Qin, Y., Zhu, J., Zhao, X., Cheng, T., Jiang, Y., et al. (2021). Acid-induced tunable white light emission based on triphenylamine derivatives. Chin. Chem. Lett. 32, 1537–1540. doi:10.1016/j.cclet.2020.10.012
Liu, Z., Nalluri, S. K. M., and Stoddart, J. F. (2017). Surveying macrocyclic chemistry: From flexible crown ethers to rigid cyclophanes. Chem. Soc. Rev. 46, 2459–2478. doi:10.1039/c7cs00185a
Naren, G., Li, S., and Andreasson, J. (2020). A simplicity-guided cocktail approach toward multicolor fluorescent systems. Chem. Commun. 56, 3377–3380. doi:10.1039/c9cc10040g
Nie, J., Zhou, B., Fang, S., Zhong, H., Li, H., and Shi, Y. (2022). Efficient multicolor and white photoluminescence in erbium- and holmium-incorporated Cs2NaInCl6:Sb3+ double perovskites. Chem. Mat. 34, 6288–6295. doi:10.1021/acs.chemmater.2c00333
Qiu, S., Zhang, Z., Wu, Y., Tong, F., Chen, K., Liu, G., et al. (2022). Vibratile dihydrophenazines with controllable luminescence enabled by precise regulation of π-conjugated wings. CCS Chem. 4, 2344–2353. doi:10.31635/ccschem.021.202101193
Radunz, S., Andresen, E., Wurth, C., Koerdt, A., Tschiche, H. R., and Resch-Genger, U. (2019). Simple self-referenced luminescent pH sensors based on upconversion nanocrystals and pH-sensitive fluorescent BODIPY dyes. Anal. Chem. 91, 7756–7764. doi:10.1021/acs.analchem.9b01174
Shen, Y. R., Gao, X., Cui, Z., and Jin, G. X. (2021). Rational design and synthesis of interlocked [2]Catenanes featuring half-sandwich cp*Rh/Ir units and pyrene-based ligands. Chin. J. Chem. 39, 3303–3308. doi:10.1002/cjoc.202100546
Shi, L., Song, W., Lian, C., Chen, W., Mei, J., Su, J., et al. (2018). Dual-emitting dihydrophenazines for highly sensitive and ratiometric thermometry over a wide temperature range. Adv. Opt. Mater. 6, 1800190. doi:10.1002/adom.201800190
Stoddart, M. E. B. a. J. F., and Stoddart, J. F. (2012). Dynamic imine chemistry. Chem. Soc. Rev. 41, 2003–2004. doi:10.1039/c2cs15305j
Sun, G., Pan, J., Wu, Y., Liu, Y., Chen, W., Zhang, Z., et al. (2020). Supramolecular assembly-driven color-tuning and white-light emission based on crown-ether-functionalized dihydrophenazine. ACS Appl. Mat. Interfaces 12, 10875–10882. doi:10.1021/acsami.0c00780
Tao, L., Luo, Z.-W., Lan, K., Wang, P., Guan, Y., Shen, Z., et al. (2020). Stimuli-responsive luminescent supramolecular polymers based on hydrogen bonding: Molecular fabrication, phase structure, and controllable-rewritable behavior. Polym. Chem. 11, 6288–6294. doi:10.1039/d0py00907e
Thordarson, P. (2011). Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 40, 1305–1323. doi:10.1039/C0CS00062K
Wang, J., Huang, Z., Ma, X., and Tian, H. (2020a). Visible-light-excited room-temperature phosphorescence in water by cucurbit[8]uril-mediated supramolecular assembly. Angew. Chem. Int. Ed. 59, 9928–9933. doi:10.1002/anie.201914513
Wang, J., Wang, N., Wu, G., Wang, S., and Li, X. (2019). Multicolor emission from non-conjugated polymers based on a single switchable boron chromophore. Angew. Chem. Int. Ed. 58, 3082–3086. doi:10.1002/anie.201812210
Wang, Q., Lin, B., Chen, M., Zhao, C., Tian, H., and Qu, D. H. (2022a). A dynamic assembly-induced emissive system for advanced information encryption with time-dependent security. Nat. Commun. 13, 4185. doi:10.1038/s41467-022-31978-x
Wang, Q., Zhang, Q., Zhang, Q. W., Li, X., Zhao, C. X., Xu, T. Y., et al. (2020b). Color-tunable single-fluorophore supramolecular system with assembly-encoded emission. Nat. Commun. 11, 158. doi:10.1038/s41467-019-13994-6
Wang, X., Hu, J., Liu, T., Zhang, G., and Liu, S. (2012). Highly sensitive and selective fluorometric off–on K+ probe constructed via host–guest molecular recognition and aggregation-induced emission. J. Mat. Chem. 22, 8622. doi:10.1039/c2jm16510d
Wang, Y., Zhou, Q., He, X., Zhang, Y., Tan, H., Xu, J., et al. (2022b). Dithienylethene metallodendrimers with high photochromic efficiency. Chin. Chem. Lett. 33, 1613–1618. doi:10.1016/j.cclet.2021.09.048
Wu, H., Wang, Y., Jones, L. O., Liu, W., Song, B., Cui, Y., et al. (2020). Ring-in-Ring(s) complexes exhibiting tunable multicolor photoluminescence. J. Am. Chem. Soc. 142, 16849–16860. doi:10.1021/jacs.0c07745
Wu, S., Li, W., Sun, Y., Zhang, X., Zhuang, J., Hu, H., et al. (2019). Synthesis of dual-emissive carbon dots with a unique solvatochromism phenomenon. J. Colloid Interface Sci. 555, 607–614. doi:10.1016/j.jcis.2019.07.089
Xia, D., Wang, P., Ji, X., Khashab, N. M., Sessler, J. L., and Huang, F. (2020). Functional supramolecular polymeric networks: The marriage of covalent polymers and macrocycle-based host-guest interactions. Chem. Rev. 120, 6070–6123. doi:10.1021/acs.chemrev.9b00839
Yan, H., Huo, F., Yue, Y., Chao, J., and Yin, C. (2021). Rapid reaction, slow dissociation aggregation, and synergetic multicolor emission for imaging the restriction and regulation of biosynthesis of cys and GSH. J. Am. Chem. Soc. 143, 318–325. doi:10.1021/jacs.0c10840
Yan, X., Wang, F., Zheng, B., and Huang, F. (2012). Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 41, 6042–6065. doi:10.1039/c2cs35091b
Yang, S., Zhao, C.-X., Crespi, S., Li, X., Zhang, Q., Zhang, Z.-Y., et al. (2021). Reversibly modulating a conformation-adaptive fluorophore in [2]catenane. Chem 7, 1544–1556. doi:10.1016/j.chempr.2021.02.019
Yeung, M. C., and Yam, V. W. (2015). Luminescent cation sensors: From host-guest chemistry, supramolecular chemistry to reaction-based mechanisms. Chem. Soc. Rev. 44, 4192–4202. doi:10.1039/c4cs00391h
Yu, C., Wang, X., Zhao, C.-X., Yang, S., Gan, J., Wang, Z., et al. (2022). Optically probing molecular shuttling motion of [2]rotaxane by a conformation-adaptive fluorophore. Chin. Chem. Lett. 33, 4904–4907. doi:10.1016/j.cclet.2022.03.004
Zhang, B., De Alwis Watuthanthrige, N., Wanasinghe, S. V., Averick, S., and Konkolewicz, D. (2021). Complementary dynamic chemistries for multifunctional polymeric materials. Adv. Funct. Mater. 32, 2108431. doi:10.1002/adfm.202108431
Zhang, J., Ren, B., Deng, S., Huang, J., Jiang, L., Zhou, D., et al. (2019a). Voltage-dependent multicolor electroluminescent device based on halide perovskite and chalcogenide quantum-dots emitters. Adv. Funct. Mat. 30, 1907074. doi:10.1002/adfm.201907074
Zhang, Q., Crespi, S., Toyoda, R., Costil, R., Browne, W. R., Qu, D. H., et al. (2022b). Stereodivergent chirality transfer by noncovalent control of disulfide bonds. J. Am. Chem. Soc. 144, 4376–4382. doi:10.1021/jacs.1c10000
Zhang, Q., Qu, D. H., Feringa, B. L., and Tian, H. (2022a). Disulfide-mediated reversible polymerization toward intrinsically dynamic smart materials. J. Am. Chem. Soc. 144, 2022–2033. doi:10.1021/jacs.1c10359
Zhang, Q. W., Li, D., Li, X., White, P. B., Mecinovic, J., Ma, X., et al. (2016). Multicolor photoluminescence including white-light emission by a single host-guest complex. J. Am. Chem. Soc. 138, 13541–13550. doi:10.1021/jacs.6b04776
Zhang, Y., Zhang, C., Chen, Y., Yu, J., Chen, L., Zhang, H., et al. (2022c). Photo-Controlled reversible multicolor room-temperature phosphorescent solid supramolecular pseudopolyrotaxane. Adv. Opt. Mater. 10, 2102169. doi:10.1002/adom.202102169
Zhang, Z., Chen, C. L., Chen, Y. A., Wei, Y. C., Su, J., Tian, H., et al. (2018). Tuning the conformation and color of conjugated polyheterocyclic skeletons by installing ortho-methyl groups. Angew. Chem. Int. Ed. 57, 9880–9884. doi:10.1002/anie.201806385
Zhang, Z., Li, H., Li, Y., and Yu, X. (2019b). Full-color emission of a Eu(3+)-based mesoporous hybrid material modulated by Zn(2+) ions: Emission color changes for Zn(2+) sensing via an ion exchange approach. Dalton Trans. 48, 10547–10556. doi:10.1039/c9dt01668f
Zhang, Z., Sun, G., Chen, W., Su, J., and Tian, H. (2020). The endeavor of vibration-induced emission (VIE) for dynamic emissions. Chem. Sci. 11, 7525–7537. doi:10.1039/d0sc01591a
Zhang, Z., Wu, Y. S., Tang, K. C., Chen, C. L., Ho, J. W., Su, J., et al. (2015). Excited-state conformational/electronic responses of saddle-shaped N, N'-Disubstituted-Dihydrodibenzo[a, c]phenazines: Wide-tuning emission from red to deep blue and white light combination. J. Am. Chem. Soc. 137, 8509–8520. doi:10.1021/jacs.5b03491
Zhou, J., Yu, G., and Huang, F. (2017). Supramolecular chemotherapy based on host-guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 46, 7021–7053. doi:10.1039/c6cs00898d
Zhou, W., Chen, Y., Yu, Q., Li, P., Chen, X., and Liu, Y. (2019). Photo-responsive cyclodextrin/anthracene/Eu(3+) supramolecular assembly for a tunable photochromic multicolor cell label and fluorescent ink. Chem. Sci. 10, 3346–3352. doi:10.1039/c9sc00026g
Zong, Z., Zhang, Q., Qiu, S. H., Wang, Q., Zhao, C., Zhao, C. X., et al. (2022). Dynamic timing control over multicolor molecular emission by temporal chemical locking. Angew. Chem. Int. Ed. Engl. 61, e202116414. doi:10.1002/anie.202116414
Keywords: vibration-induced emission, host-guest interactions, multicolor emission, conformational adaptivity, supramolecular chemistry
Citation: Ma C-S, Yu C, Zhao C-X, Zhou S-W and Gu R (2022) Multicolor emission based on a N, N′—Disubstituted dihydrodibenzo [a, c] phenazine crown ether macrocycle. Front. Chem. 10:1087610. doi: 10.3389/fchem.2022.1087610
Received: 02 November 2022; Accepted: 21 November 2022;
Published: 05 December 2022.
Edited by:
Qi Zhang, University of Groningen, NetherlandsReviewed by:
Lin Xu, East China Normal University, ChinaFan Xu, Eindhoven University of Technology, Netherlands
Copyright © 2022 Ma, Yu, Zhao, Zhou and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruirui Gu, Z3VydWlydWlAZWN1c3QuZWR1LmNu
 Chang-Shun Ma
Chang-Shun Ma Ruirui Gu
Ruirui Gu