
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 31 October 2022
Sec. Medicinal and Pharmaceutical Chemistry
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.1035949
This article is part of the Research TopicMedicinal Chemistry of Active Pharmaceutical Ingredients of Drug ProductsView all 8 articles
 Xiaowei Peng1†
Xiaowei Peng1† Xuhua He1†
Xuhua He1† Junrong Tang1
Junrong Tang1 Jianying Xiang1
Jianying Xiang1 Jia Deng1
Jia Deng1 Huan Kan1,2*
Huan Kan1,2* Yingjun Zhang3
Yingjun Zhang3 Guiliang Zhang4
Guiliang Zhang4 Ping Zhao1,2
Ping Zhao1,2 Yun Liu1,2*
Yun Liu1,2*Camellia fascicularis is a unique plant rich in bioactive components. However, the isolation of the active substances in C. fascicularis leaves via sequential extraction with solvents of different polarity and the determination of their antioxidant and antitumor activities have not been reported. In this study, the total methanol extract of C. fascicularis leaves was sequentially extracted with different polar solvents, and the corresponding petroleum ether extract (PEE), ethyl acetate extract (EAE), and water extract (WE) were analyzed for their contents in active substances such as flavonoids, polyphenols, polysaccharides, and saponins. The antioxidant ability of the polar extracts was investigated by determining their reducing power and the radical scavenging rate on 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and hydroxyl radicals, and CCK-8 and Annexin-FITC/propidium iodide staining assays were conducted to investigate their inhibitory effects on HCCLM6 and HGC27 tumor cells. The results showed that PEE had a high saponin content of 197.35 ± 16.21 mg OAE/g, while EAE and WE exhibited a relatively higher polysaccharide content of 254.37 ± 1.99 and 373.27 ± 8.67 mg GE/g, respectively. The EAE demonstrated the greatest reducing power and the strongest clearing abilities on ABTS and DPPH radicals with respective EC50 values of 343.45 ± 20.12 and 14.07 ± 0.06 μg/ml. Moreover, the antitumor ability of the different polar extracts was dose-dependent, with WE showing the most potent inhibitory ability against HCCLM6 and HGC27 cells.
Camellia fascicularis, a genus of Camellia in the family Theaceae, is an endemic plant in Yunnan province, China. C. fascicularis, which is a rare species resource with unique golden petals also known as “the giant panda in the plant kingdom,” “the queen of the tea family,” and “the living fossil of plants,” was first discovered in Hekou County and only distributed in Gejiu, Maguan, and Hekou counties (Liu et al., 2018). C. fascicularis leaves (CFLs) possess high amino acid and mineral content and are considered as an edible plant resource with high nutritional and health values (Hu et al., 2021). Bioactive components such as polyphenols, flavonoids, and saponins with strong in vitro antioxidant activity were also found in CFLs (Liu et al., 2019). In addition, polyphenols from CFLs were reported to exert anti-inflammatory properties via suppressing NF-KB and MAPK signaling pathways (Gao et al., 2022). However, to the best of our knowledge, the isolation of the active substances in CFLs according to the polarity of the extraction solvent and their antioxidant and antitumor activities have not been reported.
Extraction and isolation of natural drugs are one of the main research goals of natural medicinal chemistry (Takahashi et al., 2014). Natural drugs with clinically proven efficacy or having biological activity identified via activity screening must be first extracted and isolated to obtain the corresponding active substances. In the extraction process, bioactive components with different polarities can be extracted following the similarity compatibility principle and used as targets to determine the suitability of the extraction solvent (Abarca-Vargas et al., 2016). The extraction of functional components from plant resources using different polar solvents, among which the most commonly used are water, acetonitrile, methanol, ethanol, ethyl acetate, and petroleum ether has been extensively investigated. For example, Chuen et al. (2016) investigated the impact of water, methanol, ethanol, acetone extractions on the total phenolic content of Davidsonia pruriens, and Chang et al. (2021) explored the differences in the nutrient composition of Sechium edule shoot extracts obtained separately by water, hexane, methanol, and ethyl acetate.
The main objectives of this investigation were to preliminarily extract the active substances of CFLs and to investigate their antioxidant and antitumor properties. The total methanol extract of CFLs was subjected to sequential extraction with solvents of different polarity, i.e., petroleum ether, ethyl acetate, and water, and the corresponding extracts were analyzed to determine their respective contents in active substances such as flavonoids, polyphenols, polysaccharides, and saponins. Moreover, the antioxidant and antitumor activities of the petroleum ether extract (PEE), ethyl acetate extract (EAE), and water extract (WE) were further compared by conducting 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and hydroxyl radical assays and reducing power and cell toxicity analyses. The present study will provide an experimental basis for the isolation and screening of active substances with antioxidant and antitumor effects from CFLs, as well as the production of natural pharmaceuticals.
The voucher specimen (52860) of Camellia fascicularis identified by taxonomist Min Tianlu was stored at herbarium of Kunming Institute of Botany, Chinese Academy of Sciences. CFLs were obtained in Dawei Mountain (103.95 N, 22.66 E) in Hekou County, Yunnan Province, China in December 2019, which was identified as Camellia fascicularis by Professor Xiang Jianying, a taxonomist at Southwest Forestry University. CFLs were hot-air dried at 45°C until constant weight, pulverized to powder, then screened with 60-mesh sieves. The powders were kept at 4°C for further analysis.
DPPH and ABTS were acquired from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Cell Counting Kit-8 (CCK-8) was acquired from Shenyang Wanlei Bio-Technology Co., Ltd. (Shenyang, China). All other chemicals were analytical grade.
The extraction procedures of CFLs were schematically depicted in Figure 1. First, 10.68 kg of CFLs was immersed in methanol at room temperature, and the total extracts were obtained by filtering, concentrating, and drying under negative pressure. Then, the total extract was fully dissolved in distilled water under ultrasonic action and isolated sequentially with equal amounts of petroleum ether and ethyl acetate. Finally, 122.0 g of PEE, 177.8 g of EAE, and 115.8 g of WE were obtained after concentration and freeze-drying (Hu et al., 2017).
The TFC of CFLs extracts was measured by Liao’s approach with slight modifications (Liao et al., 2021). Briefly, the extracts were reacted with NaNO2, Al(NO3)3, and NaOH in turn, and the absorption value of the mixtures was then determined using an ultraviolet–visible spectrophotometer (UV-2600, Shimadzu, Japan). A calibration curve (y = 7.5357x + 0.0117, R2 = 0.9999) was constructed using a rutin standard, and the TFC was defined by rutin equivalent (RE; mg) per dry weight of extracts (g).
The TPC of CFLs extracts was measured according to Emanet’s method with slight variations (Emanet et al., 2022). Briefly, the extracts were reacted with Folin–Ciocalteu reagent and Na2CO3 in turn, and the absorption value of the mixtures was then measured. A calibration curve (y = 82.3714x + 0.0392, R2 = 0.9998) was constructed with gallic acid as standard, and the TPC was defined by gallic acid equivalent (GAE; mg) per dry weight of extracts (g).
The PC of the CFLs extracts was determined using Wang’s method with minor modifications (Wang et al., 2018). First, the extracts were refluxed in 95% ethanol by 6 h for the removal of oligosaccharides and monosaccharides. After that, 1 ml residue solution was reacted with 1 ml of 5% phenol solution and 5 ml concentrated sulfuric acid in turn, and the absorption value of the mixtures was determined at 490 nm. A calibration curve (y = 9.5961x + 0.00002, R2 = 0.9996) was obtained using a glucose standard, and the PC was defined as glucose equivalent (GE; mg) per dry weight of extracts (g).
Akbari’s method with slight modifications (Akbari et al., 2019) was used to determine the TSC of the CFLs extracts. Briefly, the extracts were reacted with a vanillin–acetic acid solution, perchloric acid, and acetic acid in turn, and the absorption value of the mixtures was then measured. An oleanolic acid standard was used to obtain a calibration curve (y = 7.5895x − 0.0084, R2 = 0.9993), and the TSC was defined as oleanolic acid equivalent (OAE; mg) per dry weight of extracts (g).
A literature procedure slightly modified was employed for evaluating the clearing effect of CFLs extracts on DPPH radical (Zhu et al., 2022). Specifically, 0.2 ml of CFLs extracts in varying concentrations (10–60 μg/ml) was combined into 0.2 ml of a DPPH radical ethanol solution. The mixed solutions were then stored for 30 min at 37°C, then the absorption value at 517 nm was detected using a microplate reader (SpectraMax190, Molecular Devices, United States).
Swat’s method with minor modifications was applied to evaluate the clearing ability of CFLs extracts on ABTS radicals (Swat et al., 2019). Briefly, 0.02 ml of CFLs extracts in varying concentrations (300–1300 μg/ml) was combined into 0.38 ml of an ABTS radical cation solution. The mixed solutions were stored for 5 min, and the absorption value at 734 nm was detected.
Mao’s method with minor modifications was employed for investigating the clearing effect of CFLs extracts on hydroxyl radical (Mao et al., 2013). In brief, 0.2 ml of CFLs extracts in varying concentrations (1000–11000 μg/ml) was reacted with an FeSO4 aqueous solution, a salicylic acid ethanol solution, and H2O2 in turn, and the absorption value of the mixtures at 510 nm was then measured.
Bao’s method with minor variations was adopted to study the reducing power of the CFLs extracts (Bao et al., 2020). Specifically, 0.1 ml of CFLs extracts in varying concentrations (300–1300 μg/ml) was reacted with phosphate buffer, potassium ferricyanide, and trichloroacetic acid in turn, and the absorption value of the mixtures at 700 nm was then determined.
Human hepatocellular carcinoma HCCLM6 and Human gastric cancer cell line HGC27 cells were obtained from Kunming Cell Bank (CAS, China) and preserved in Dulbecco’s modified Eagle medium (Gibco, United States). The media was added in penicillin (100 μg/ml) and 10% (v/v) fetal bovine serum at 37°C in an incubator (BB15, Thermo, United States) with a supply of 5% CO2 (Wang et al., 2019).
To evaluate the cytotoxicity of the CFLs extracts against HCCLM6 and HGC27 cells, the cell viability was quantified using CCK-8 (Zhang et al., 2020). In a 96-well microplate, cells were plated with 1 × 105/ml density and cultured for 24 h at 37°C. The cells were treated with 10 μL different concentrations of CFLs extracts DMSO solution (0, 50, 100, 150, 200, and 250 μg/ml) for 24 h. Then, each well was mixed with 10 µL CCK-8 solution and incubation was maintained for 1 h. The absorption value of the solution in well was determined using a microplate reader at 450 nm.
The cell apoptosis was detected following Dai’s method with minor modifications (Dai et al., 2021). Briefly, HCCLM6 and HGC27 cells in log growth phase were transferred to 6-well plates at a density of 1 × 105/ml and incubated at 37°C for 24 h in 5% CO2. Then, CFLs extracts in different concentrations (0, 50, 150 and 250 μg/ml) was applied and incubation was continued for 24 h. After digestion, centrifugation, and cleaning twice, the cells were stained for 15 min in the dark with Annexin V-FITC (5 μL) and propidium iodide (PI) (5 μL). A flow cytometry (FACSCanto II, BD, United States) was performed to identify the apoptotic cells.
Origin 2018 and GraphPad Prism eight were used for mapping, and all measurements were performed in triplicates. Statistical differences among groups were performed using Student’s t-test.
As shown in Table 1, TFC, TPC, PC, and TSC differed significantly between the different polar extracts (p < 0.05). Thus, TFC in three extracts ranged from 91.24 ± 13.14 to 115.38 ± 6.86 mg RE/g, with the content being significantly higher in WE and EAE than in PEE. TPC in the three extracts was significantly different, EAE had the highest TPC of 164.18 ± 12.05 mg GAE/g, WE possessed the TPC of 132.77 ± 5.81 mg GAE/g and PEE had the lowest TPC of 55.48 ± 5.02 mg GAE/g. Meanwhile, PC was the highest in WE followed by EAE and PEE with 373.27 ± 8.67, 254.37 ± 1.99, and 134.42 ± 2.44 mg GE/g, respectively. In contrast, TSC was the lowest in EAE at 100.95 ± 12.39 mg OAE/g and the highest in PEE at 197.35 ± 16.21 mg OAE/g.
The antioxidant properties of plant resources have become one of the most important indicators for evaluating their potential use in medicine (Li et al., 2020). In this work, the antioxidant capacities of the three CFLs extracts were determined according to DPPH, ABTS, hydroxyl radicals and reducing power assays using vitamin C (Vc) as a positive control.
In ethanol solution, DPPH radicals have a high absorption peak at 517 nm. Mixing of a DPPH ethanol solution with antioxidants results in a color lightening, and the fading degree is positively correlated with the ability of the antioxidants to scavenge free radicals (Wang et al., 2015). As shown in Figure 2A, the three CFLs extracts exhibited a certain ability to scavenge DPPH radicals. The clearance of three extracts improved as concentration (10–60 μg/ml) increased, where EAE exceeded Vc at 30–60 μg/ml and WE was close to Vc at 40–60 μg/ml, indicating that EAE and WE possessed efficient DPPH radical clearing capacity. The clearance of PEE was significantly weaker than that of EAE and WE (p < 0.05), which may be due to its lower content in flavonoids, polyphenols, and polysaccharides. The 50% maximal effect concentration (EC50) of DPPH radical scavenging by PEE, EAE, and WE was 57.68 ± 4.50, 14.07 ± 0.06, and 14.76 ± 3.78 μg/ml, respectively. In general, EAE exhibited the highest potential for scavenging DPPH radicals, followed by WE and PEE. This is consistent with the results of Hu’s DPPH assay on Astragalus chinensis (Hu et al., 2017).
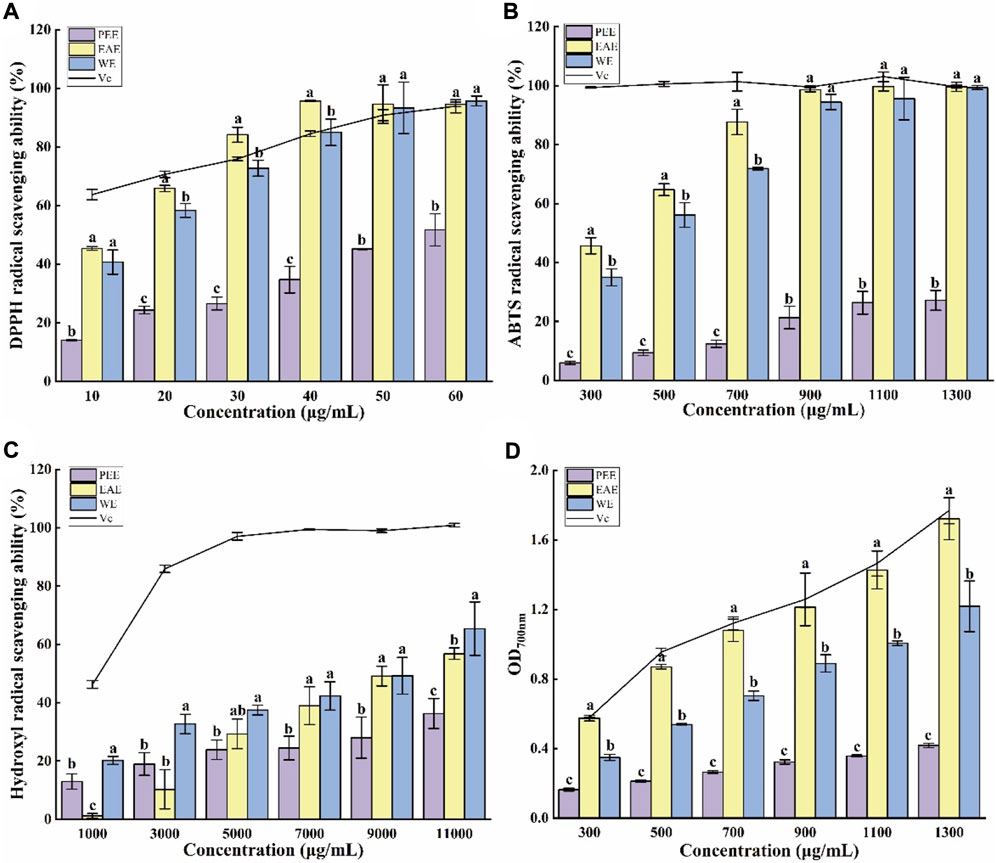
FIGURE 2. Antioxidant capacities of CFLs extracts. (A) DPPH radical scavenging ability, (B) ABTS radical scavenging ability, (C) hydroxyl radical scavenging ability, and (D) reducing power. Letters (a‐c) indicate statistically significant differences between the extracts (p < 0.05).
The blue-green color of an ABTS radical solution becomes lighter after reacting with antioxidants, with a more intense lightening indicating a stronger antioxidant capacity (Luo et al., 2010). Figure 2B shows the scavenging effect of three CFLs extracts on ABTS radicals. All three extracts demonstrated strong scavenging ability at 300–1300 μg/ml with dose-dependent effects. The ABTS scavenging rate of EAE and WE was significantly higher than that of PEE, with respective EC50 values of 343.45 ± 20.12, 432.72 ± 12.33 and, 2354.92 ± 288.03 μg/ml.
Hydroxyl radicals are active and harmful species that can induce aging and several diseases in the human body. Therefore, their elimination is extremely significant for human health (Su and Li, 2019). As shown in Figure 2C, WE exhibited the strongest scavenging capacity for hydroxyl radicals among the three CFLs extracts, which contrasts with the findings of the DPPH and ABTS radical scavenging tests. This may be due to the high PC in WE. The EC50 values of the hydroxyl radical scavenging by PEE, EAE, and WE were 15294.24 ± 88.14, 9262.31 ± 235.76, and 7913.80 ± 89.55 μg/ml, respectively. Similarly, it was also reported that the hydroxyl radical scavenging ability of EAE from Andrographis paniculata was higher than that of PEE (Saranya et al., 2010).
The reducing power intimately associated with the antioxidant capacity and can be used as an indication of the latter, and high absorbance represented strong reducing power (Liu et al., 2014). As shown in Figure 2D, the absorption value of the mixtures increased with the concentration, suggesting that the antioxidant capacity also increased gradually. The absorption value of EAE at 1300 μg/ml was 1.72 ± 0.12, which was close to the values of 1.77 ± 0.08 obtained for Vc and significantly higher than the value of 1.22 ± 0.15 for WE and 0.42 ± 0.01 for PEE (p < 0.05). Hu et al. (2017) also found that the reducing power of EAE from Astragalus chinensis was higher than that of WE and PEE.
To investigate the antitumor activity of CFL extracts, the cytotoxic effects of the three extracts were assessed on two different cell lines, i.e., HCCLM6 and HGC27cells. As shown in Figure 3A, the three extracts significantly inhibited (p < 0.05) the growth of HCCLM6 cells with dose-dependent properties. The viability of HCCLM6 cells treated with PEE, EAE, and WE was below 50% (41.07 ± 3.60%, 47.39 ± 1.60%, and 39.76 ± 2.72%, respectively) at 200 μg/ml. The EC50 values of PEE, EAE, and WE were respective 167.87 ± 6.25, 205.09 ± 3.48, and 162.12 ± 5.88 μg/ml. Zhang et al. (2012) have found that the EC50 of Celastrus orbiculatus extracts against HCCLM6 cell was 282.71 μg/ml, implying that PEE, EAE and WE possessed higher inhibitory effect on HCCLM6 cell than Celastrus orbiculatus extracts. As shown in Figure 3B, the CFLs extracts also exerted a significant inhibitory effect on HGC27 cells (p < 0.05). At 250 μg/ml, the viability of HGC27 cells treated with PEE, EAE, and WE was 31.96 ± 3.26%, 39.67 ± 0.78%, and 27.92 ± 2.79%, respectively. The respective EC50 values of PEE, EAE, and WE were 167.44 ± 8.09, 200.76 ± 11.34, and 147.42 ± 12.42 μg/ml, which were higher than the EC50 values of betulonic acid (34.13 μg/ml) against HGC27 cell (Chu et al., 2022). Notably, WE showed the smallest EC50 against HCCLM6 and HGC27 cells, indicating its strongest inhibitory activity. Overall, the CFLs extracts displayed significant toxic effects on HCCLM6 and HGC27 cells, among which the inhibitory effect of WE was the strongest. This is probably due to the fact that WE was richer in polysaccharides with certain antitumor effects. Antitumor activity has been reported as one of the typical properties of plant polysaccharides (Dong et al., 2016).
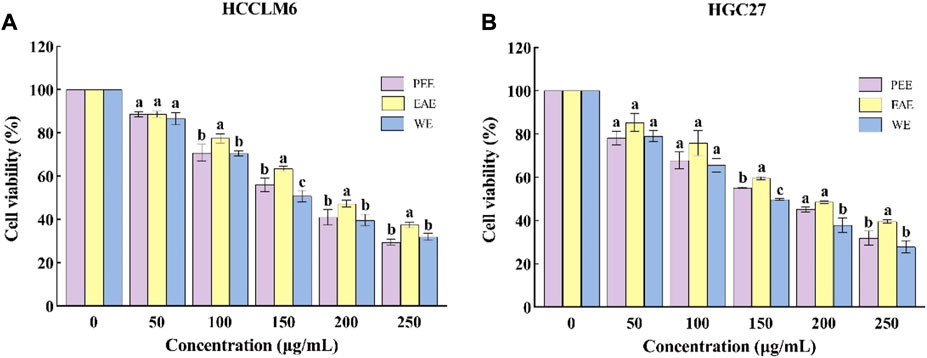
FIGURE 3. Cytotoxic activity of CFLs extracts. (A) Cytotoxic activity of CFLs extracts on HCCLM6 cells and (B) cytotoxic activity of CFLs extracts on HGC27 cells. Letters (a–c) represent statistically significant differences between the extracts (p < 0.05).
Exposure of phosphatidylserine to the cell surface is the early feature of apoptosis, which can be identified with annexin V labeled by the fluorescent dye FITC. After the membrane rupture, necrotic cells also bind to annexin V. Thus, cells can be simultaneously labeled with PI (Henry et al., 2013). In the top–right quadrant (Q2) of the flow cytometry plots shown in Figures 4, 5, late apoptotic cells were positive with PI and Annexin V-FITC. Meanwhile, in the bottom–right quadrant (Q4), early apoptotic cells were positive for Annexin V-FITC and negative for PI. As shown in the figures, treatment of HCCLM6 and HGC27 cells with increasing concentrations of PEE, EAE, and WE resulted in enhanced Annexin V-FITC staining, indicating an increase in early apoptotic cells. As shown in Figures 4B–D and Figures 5B–D, the percentages of apoptotic HCCLM6 cells treated with PEE, EAE, and WE were 51.70 ± 2.31%, 69.40 ± 3.05%, and 67.95 ± 2.85% at 250 μg/ml, respectively, and the percentages of apoptotic HGC27 cells were 36.06 ± 1.47%, 36.92 ± 2.13%, and 37.55 ± 1.85%, respectively. These findings suggested that the toxic effect of CFLs extracts on HCCLM6 cells was higher than on HGC27 cells and exhibited a dose-dependent relationship, furthermore, their antitumor effects were mainly achieved by promoting early apoptosis of cancer cells.
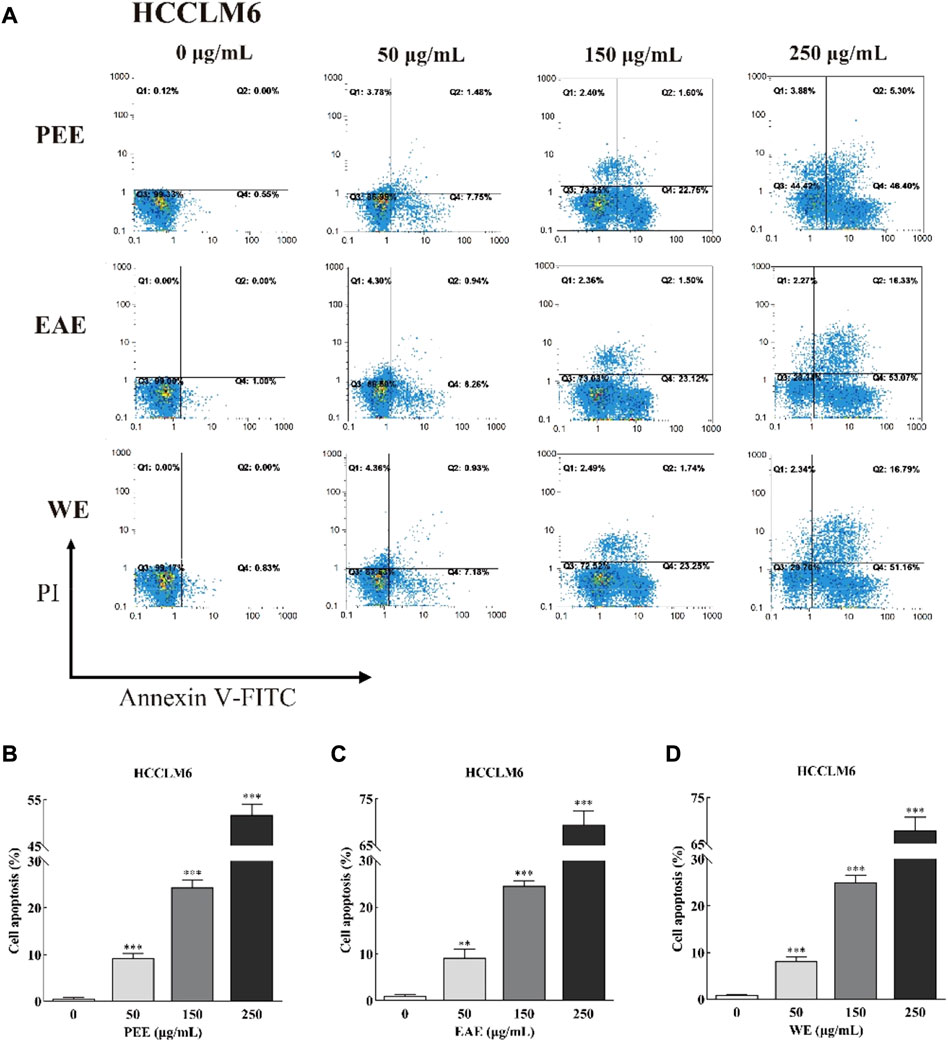
FIGURE 4. (A) Flow cytometry plots and (B–D) early and late cell apoptosis rate of HCCLM6 treated with CFLs extracts. **, p < 0.01; ***, p < 0.001 compared with the control group.
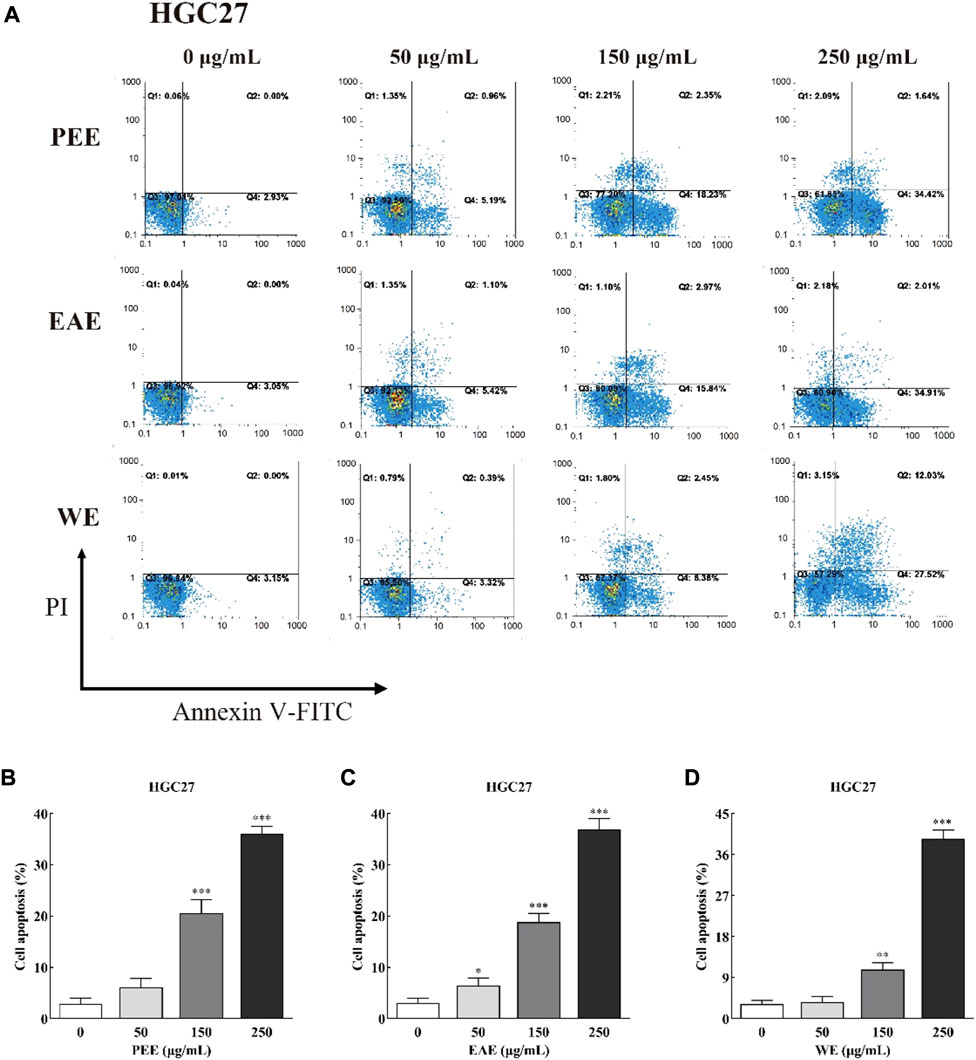
FIGURE 5. (A) Flow cytometry plots and (B–D) early and late apoptosis rate of HGC27 treated with CFLs extracts. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with the control group.
The relationship between the active substances and in vitro antioxidant and antitumor activities was assessed using Pearson’s correlation coefficients. As shown in Figure 6, the EC50 values of DPPH and ABTS radicals scavenged by CFLs extracts presented extremely significant correlations to TPC (p < 0.001) with Pearson’s correlation coefficients of −0.950 and −0.949, respectively. Meanwhile, the Pearson’s correlation coefficient between the EC50 of hydroxyl radical and PC was −0.938, implying an extremely significant positive correlation between the hydroxyl radical clearing ability and PC. The reducing power tended to increase with TPC, with a correlation coefficient of 0.972 (p < 0.001), suggesting an extremely significant positive correlation. Moreover, the EC50 of hydroxyl radical presented a highly significant correlation (p < 0.01) to TPC with a Pearson’s correlation coefficient of −0.888. Rocchetti et al. (2020) confirmed the correlation between phenolic compounds and the scavenging ability for DPPH and ABTS radicals using Pearson’s correlation analysis. In this study, highly significant correlations were observed between PC and EC50 of DPPH and ABTS radicals with Pearson’s correlation coefficients of −0.854 (p < 0.01) and −0.836 (p < 0.01), respectively. The Pearson’s correlation coefficient between the EC50 of HCCLM6 cell toxicity and TSC was −0.813 (p < 0.01), indicating a highly significant correlation between both factors.
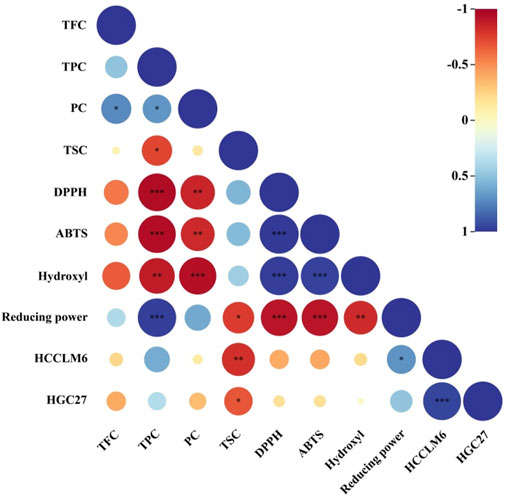
FIGURE 6. Heat map of Pearson’s correlation coefficient between active substances and in vitro antioxidant and antitumor activity. * Significant (p < 0.05), ** highly significant (p < 0.01), *** extremely significant (p < 0.001).
The initial separation of bioactive components can be performed according to their distribution coefficient in polar solvents. Accordingly, as one of commonly applied polar solvents for most bioactive substances (Paula et al., 2003), methanol was found suitable for extracting the main active substances such as flavonoids, polyphenols, polysaccharides, and saponins from CFLs in this work.
The total methanol extract of CFLs was then performed as sequential extraction, affording PEE, EAE, and WE. Among them, EAE had the highest TPC of 164.18 ± 12.05 mg GAE/g, WE had the maximum PC content of 373.27 ± 8.67 mg GE/g, and PEE had the largest TSC of 197.35 ± 16.21 mg OAE/g. Plant extracts are known to possess antioxidant properties (Kalidindi et al., 2015; Nurcholis et al., 2021; Saravanakumar et al., 2021) that can be exploited to control the damage exerted by the strong oxidation effect of free radicals, which are atoms, molecules, ions, or groups bearing an unpaired electron produced by enzymatic reactions in human metabolism. Studies have shown that mushroom polysaccharides exhibited strong scavenging ability against ABTS and hydroxyl radicals in a dose-dependent manner (Li et al., 2012). In addition, Rusu et al. (2019) have found that the water-acetone extract of hazelnut (Corylus avellana L.) showed significant in vitro antioxidant capacity due to its richness in 11 polyphenols including epicatechin, catechin, syringic acid, gallic acid, and protocatechuic acid, etc. Wang et al. (2019) have discovered that the ethanol-water extracts from Olea europaea leaves contained phenolic compounds, such as luteolin and apigenin, which exhibited dose-dependent scavenging activities against DPPH and superoxide radicals. Here, the three CFLs extracts were assessed for their in vitro antioxidant activities. EAE exhibited the strongest antioxidant capacity, which was consistent with the study on the antioxidant capacity of Ilex latifolia Thunb extracts (Hu et al., 2014). This may be attributed to the high solubility of polyphenols in ethyl acetate. Khanam et al. (2015) found that polyphenols were present in the ethyl acetate extract of Eurycoma longifolia but not in the petroleum ether extract. Moreover, Hassan et al. (2015) revealed that the ethyl acetate extracts of E. longifolia contained high content in polyphenols and exhibited strong antioxidant ability.
Natural medicine, especially Chinese medicine, plays a critical role in the treatment of cancer (Song et al., 2018; Zhang et al., 2018). Plant leaves have been proven to be an important source of natural antitumor drugs. For instance, aqueous extracts of Carica papaya leaves were found to inhibit the viability of K562 tumor cells (Otsuki et al., 2010). Al-Nemari et al. (2022) demonstrated that the leave extracts of Annona squamosa could effectively inhibit MCF-7 and MDA-MB-231 tumor cells. Moreover, acidic polysaccharides from Gynostemma pentaphyllum have been found to possess significant inhibitory activities against SPC-A-1 and MGC-803 cells (Yu et al., 2020). He et al. (2021) also found that Panax quinquefolius saponins possessed an inhibitory effect on DU145 cell viability. The present study demonstrates that the cell viability of HCCLM6 and HGC27 was significantly decreased after being treated with WE, which may be due to its high PC (Saidan et al., 2015). In fact, the antitumor activity of plant polysaccharides is one of their most prominent features (Zhang et al., 2007). Luo et al. (2014) revealed that water extracts of Coriolus versicolor had obvious inhibitory properties against breast cancer cells 4T1. In this study, we found that CFLs extracts inhibited cancer cell viability by promoting HCCLM6 and HGC27 cells early apoptosis. Similarly, Se-POP-3 has been shown to inhibit the proliferation of HepG2 and MCF-7 cells by promoting their early apoptosis (Zhang et al., 2020).
In the present work, significant deviations were observed in the active substances content of CFLs extracts obtained via sequential extraction, with EAE containing the highest TPC and WE and PEE having the maximum PC and TSC, respectively. PEE, EAE, and WE exhibited significant in vitro antioxidant capacity, with EAE showing the strongest scavenging ability for DPPH and ABTS radicals and the highest reducing power. Moreover, all three extracts especially WE, could remarkably inhibit the viability of HCCLM6 and HGC27 cells. Finally, the three extracts suppressed the growth of HCCLM6 and HGC27 cells via promoting the early apoptosis. Based on the results of this work, EAE and WE should be subjected to further purification to obtain high-purity compounds and to investigate their potential use in the treatment of free radical-related diseases. Moreover, the potential of WE as an antitumor drug merits further investigation. This work provides a reference for the utilization of C. fascicularis as a natural medicine.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
XP and XH; Writing-original draft, Data curation. JT, JX, and GZ; Investigation, Resources. JD and HK; Methodology, Supervision. YZ; Conceptualization, Methodology, Supervision. PZ; Methodology, Validation. YL; Conceptualization, Methodology, Resources, Writing-review and editing, Funding acquisition.
This work was supported by Yunnan Agricultural Basic Research Joint Special Project (202101BD070001–045), Yunnan Fundamental Research Projects (202201AT070050), and Botanic Gardens Conservation International Project (2063076).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abarca-Vargas, R., Malacara, C. F. P., and Petricevich, V. L. (2016). Characterization of chemical compounds with antioxidant and cytotoxic activities in Bougainvillea x buttiana Holttum and Standl, (var. Rose) extracts. Antioxidants 5 (4), 45–55. doi:10.3390/antiox5040045
Akbari, S., Abdurahman, N. H., Yunus, R. M., and Fayaz, F. (2019). Microwave-assisted extraction of saponin, phenolic and flavonoid compounds from Trigonella foenum-graecum seed based on two level factorial design. J. Appl. Res. Med. Aromat. Plants 14, 100212. doi:10.1016/j.jarmap.2019.100212
Al-Nemari, R., Bacha, A. B., Al-Senaidy, A., Almutairi, M. H., Arafah, M., Al-Saran, H., et al. (2022). Cytotoxic effects of Annona squamosa leaves against breast cancer cells via apoptotic signaling proteins. J. King Saud Univ. - Sci. 34 (4), 102013. doi:10.1016/j.jksus.2022.102013
Bao, Y. J., Ren, X. P., Zhu, Y. X., Zhang, Y. W., Peng, Z. Q., and Zhou, G. H. (2020). Comparison of lipid radical scavenging capacity of spice extract in situ in roast beef with DPPH and peroxy radical scavenging capacities in vitro models. LWT-Food Sci. Technol. 130 (4), 109626. doi:10.1016/j.lwt.2020.109626
Chang, K. A., Ley, S. L., Lee, M. Y., Yaw, H. Y., Lee, S. W., Chew, L. Y., et al. (2021). Determination of nutritional constituents, antioxidant properties, and α-amylase inhibitory activity of Sechium edule (chayote) shoot from different extraction solvents and cooking methods. LWT-Food Sci. Technol. 151, 112177. doi:10.1016/j.lwt.2021.112177
Chu, Z. W., Luo, Y. Y., Ni, T. Y., Zhu, M., Feng, X. Y., Liu, Y. Q., et al. (2022). Betulonic acid, as one of the active components of the Celastrus orbiculatus extract, inhibits the invasion and metastasis of gastric cancer cells by mediating cytoskeleton rearrangement in vitro. Molecules 27 (3), 1025. doi:10.3390/molecules27031025
Chuen, T. L. K., Vuong, Q. V., Hirun, S., Bowyer, M. C., Predebon, M. J., Goldsmith, C. D., et al. (2016). Antioxidant and anti-proliferative properties of Davidson’s plum (Davidsonia pruriens F. Muell) phenolic-enriched extracts as affected by different extraction solvents. J. Herb. Med. 6 (4), 187–192. doi:10.1016/j.hermed.2016.08.005
Dai, S. S., Zhang, W. H., Dou, Y. W., Liu, H. M., Chen, X., Shi, J. H., et al. (2021). Towards a better understanding of the relationships between the structure and antitumour activity of Gastrodia elata polysaccharides by asymmetrical flow field-flow fractionation. Food Res. Int. 149, 110673. doi:10.1016/j.foodres.2021.110673
Dong, X. D., Feng, Y. Y., Liu, Y. N., Ji, H. Y., Yu, S. S., Liu, A. J., et al. (2016). A novel polysaccharide from Castanea mollissima Blume: Preparation, characteristics and antitumor activities in vitro and in vivo. Carbohydr. Polym. 7 (1), 116323–116414. doi:10.1016/j.carbpol.2020.116323
Emanet, M., Sen, O., Pignatelli, F., Lavarello, C., Petretto, A., and Ciofani, G. (2022). Hazelnut extract-loaded nanostructured lipid carriers and evaluation of their antioxidant properties. Front. Bioeng. Biotechnol. 10, 953867953867. doi:10.3389/fbioe.2022.953867
Gao, M. Z., Peng, X. W., Tang, J. R., Deng, J., Wang, F., Zhang, Y., et al. (2022). Anti-inflammatory effects of Camellia fascicularis polyphenols via attenuation of NF-κB and MAPK pathways in LPS-induced THP-1 macrophages. J. Inflamm. Res. 15, 851–864. doi:10.2147/JIR.S349981
Hassan, W., Zulkifli, R., Ahmad, F., and Yunus, M. (2015). Antioxidant and tyrosinase inhibition activities of Eurycoma longifolia and Swietenia macrophylla. J. Appl. Pharm. Sci. 5 (8), 006–010. doi:10.7324/japs.2015.50802
He, S., Lyu, F. Q., Lou, L. X., Liu, L., Li, S. L., Jakowitsch, J., et al. (2021). Anti-tumor activities of Panax quinquefolius saponins and potential biomarkers in prostate cancer. J. Ginseng Res. 45, 273–286. doi:10.1016/j.jgr.2019.12.007
Henry, C. M., Hollville, E., and Martin, S. J. (2013). Measuring apoptosis by microscopy and flow cytometry. Methods 61 (2), 90–97. doi:10.1016/j.ymeth.2013.01.008
Hu, T., He, X. W., and Jiang, J. G. (2014). Functional analyses on antioxidant, anti-inflammatory, and antiproliferative effects of extracts and compounds from Ilex latifolia Thunb, a Chinese bitter tea. J. Agric. food Chem. 62 (34), 8608–8615. doi:10.1021/jf501670v
Hu, T., Liu, Q. M., He, X. W., Huang, F., Zhang, M. W., and Jiang, J. G. (2017). Identification of bioactives from Astragalus chinensis L.f. and their antioxidant, anti-inflammatory and anti-proliferative effects. J. Food Sci. Technol. 54 (13), 4315–4323. doi:10.1007/s13197-017-2902-3
Hu, X., Tang, J. R., Zhang, G. L., Deng, J., Kan, H., Zhang, Y. J., et al. (2021). Optimization of extraction process and antioxidant activities of saponins from Camellia fascicularis leaves. Food Meas. 15 (1), 1889–1898. doi:10.1007/s11694-020-00754-0
Kalidindi, N., Thimmaiah, N. V., Jagadeesh, N. V., Nandeep, R., Swetha, S., and Kalidindi, B. (2015). Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa Linn. Leaves. J. Food Drug Anal. 23 (4), 795–802. doi:10.1016/j.jfda.2015.04.012
Khanam, Z., Wen, C. S., and Bhat, I. U. H. (2015). Phytochemical screening and antimicrobial activity of root and stem extracts of wild Eurycoma longifolia Jack (Tongkat Ali). J. King Saud Univ. - Sci. 27 (1), 23–30. doi:10.1016/j.jksus.2014.04.006
Li, X. Y., Wang, Z. Y., Wang, L., Walid, E., and Zheng, H. (2012). In vitro antioxidant and anti-proliferation activities of polysaccharides from various extracts of different mushrooms. Int. J. Mol. Sci. 13, 5801–5817. doi:10.3390/ijms13055801
Li, X., Wang, P., Zhu, J. Q., Li, J. J., Ji, Z. Y., Kang, Q. Z., et al. (2020). Comparative study on the bioactive components and in vitro biological activities of three green seedlings. Food Chem. x. 321, 126716. doi:10.1016/j.foodchem.2020.126716
Liao, J. Q., Guo, Z. R., and Yu, G. C. (2021). Process intensification and kinetic studies of ultrasound-assisted extraction of flavonoids from peanut shells. Ultrason. Sonochem. 76, 105661. doi:10.1016/j.ultsonch.2021.105661
Liu, Y., Du, Y. Q., Wang, J. H., Zha, X. Q., and Zhang, J. B. (2014). Structural analysis and antioxidant activities of polysaccharide isolated from Jinqian mushroom. Int. J. Biol. Macromol. 64, 63–68. doi:10.1016/j.ijbiomac.2013.11.029
Liu, Y., Luo, X. L., Lan, Z. Q., Tang, J. R., Zhao, P., and Kan, H. (2018). Ultrasonic-assisted extraction and antioxidant capacities of flavonoids from Camellia fascicularis leaves. CyTA - J. Food 16 (1), 105–112. doi:10.1080/19476337.2017.1343867
Liu, Y., Kan, H., Fang, F. Y., Tang, J. R., Zhang, Y. J., and Zhao, P. (2019). Microwave-assisted extraction and antioxidant activities of polyphenols from Camellia fascicularis leaves. Curr. Top. Nutraceutical Res. 17 (2), 164–171. doi:10.37290/ctnr2641-452x.17:164-171
Luo, A. X., He, X. J., Zhou, S. D., Fan, Y. J., Luo, A. S., and Chun, Z. (2010). Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 79 (4), 1014–1019. doi:10.1016/j.carbpol.2009.10.033
Luo, K. W., Yue, G. G. L., Ko, C. H., Lee, J. K. M., Gao, S., Li, L. M., et al. (2014). In vivo and in vitro anti-tumor and anti-metastasis effects of Coriolus versicolor aqueous extract on mouse mammary 4T1 carcinoma. Phytomedicine 21, 1078–1087. doi:10.1016/j.phymed.2014.04.020
Mao, J. W., Yin, J., Ge, Q., Jiang, Z. L., and Gong, J. Y. (2013). In vitro antioxidant activities of polysaccharides extracted from Moso Bamboo-Leaf. Int. J. Biol. Macromol. 55, 1–5. doi:10.1016/j.ijbiomac.2012.12.027
Nurcholis, W., Putri, D. N. S., Husnawat, H., Aisyah, S. L., and Priosoeryanto, B. P. (2021). Total flavonoid content and antioxidant activity of ethanol and ethyl acetate extracts from accessions of Amomum compactum fruits. Ann. Agric. Sci. 66, 58–62. doi:10.1016/j.aoas.2021.04.001
Otsuki, N., Dang, N. H., Kumagai, E., Kondo, A., Iwata, S., and Morimoto, C. (2010). Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J. Ethnopharmacol. 127 (3), 760–767. doi:10.1016/j.jep.2009.11.024
Paula, E. M., Mathangi, D. C., and Namasivayam, A. (2003). Free radical changes in methanol toxicity. Indian J. Physiol. Pharmacol. 47 (2), 207–211.
Rocchetti, G., Pagnossa, J. P., Blasi, F., Cossignani, L., Piccoli, R. H., Zengin, G., et al. (2020). Phenolic profiling and in vitro bioactivity of Moringa oleifera leaves as affected by different extraction solvents. Food Res. Int. 127, 108712. doi:10.1016/j.foodres.2019.108712
Rusu, M. E., Fizesan, I., Pop, A., Gheldiu, A. N., Mocan, A., Crisan, G., et al. (2019). Enhanced recovery of antioxidant compounds from hazelnut (Corylus avellana L.) involucre based on extraction optimization: Phytochemical profile and biological activities. Antioxidants 8, 460. doi:10.3390/antiox8100460
Saidan, N. H., Hamil, M. S. R., Memon, A. H., Abdelbari, M. M., Hamdan, M. R., Mohd, K. S., et al. (2015). Selected metabolites profiling of orthosiphon stamineus benth leaves extracts combined with chemometrics analysis and correlation with biological activities. BMC Complement. Altern. Med. 15 (1), 350. doi:10.1186/s12906-015-0884-0
Saranya, P., Geetha, A., and Narmadha Selvamathy, S. M. K. (2010). The antioxidant and H+K+ ATPase inhibitory effect of Andrographis paniculata and andrographolide-in vitro and in vivo studies. Pharmacologyonline 1, 356–376.
Saravanakumar, K., Park, S., Anand, M. A. V., Sathiyaseelan, A., Vishnupriya, V., Kim, S., et al. (2021). Chemical composition, antioxidant, and anti-diabetic activities of ethyl acetate fraction of Stachys riederi var. japonica (Miq.) in streptozotocin-induced type 2 diabetic mice. Food Chem. Toxicol. 155, 112374. doi:10.1016/j.fct.2021.112374
Song, J. R., Lan, J. J., Chen, C., Hu, S. C., Song, J. L., Liu, W. L., et al. (2018). Design, synthesis and bioactivity investigation of tetrandrine derivatives as potential anti-cancer agents. MedChemComm 9 (7), 1131–1141. doi:10.1039/c8md00125a
Su, Y., and Li, L. (2019). Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 229 (3), 115407. doi:10.1016/j.carbpol.2019.115407
Swat, M., Rybicka, I., and Gliszczyńska-Świglo, A. (2019). Characterization of fulvic acid beverages by mineral profile and antioxidant capacity. Foods 8 (12), 605. doi:10.3390/foods8120605
Takahashi, J. A., Gomes, D. C., Lyra, F. H., Santos, G. F., and Martins, L. R. (2014). The remarkable structural diversity achieved in ent-kaurane diterpenes by fungal biotransformations. Molecules 19, 1856–1886. doi:10.3390/molecules19021856
Wang, J., Zhao, Y. M., Li, W., Wang, Z. B., and Shen, L. X. (2015). Optimization of polysaccharides extraction from Tricholoma mongolicum Imai and their antioxidant and antiproliferative activities. Carbohydr. Polym. 131, 322–330. doi:10.1016/j.carbpol.2015.06.009
Wang, Y. G., Xu, Y., Ma, X. Q., Liu, X. F., Yang, M. J., Fan, W. G., et al. (2018). Extraction, purification, characterization and antioxidant activities of polysaccharides from Zizyphus jujuba cv. Linzexiaozao. Int. J. Biol. Macromol. 118, 2138–2148. doi:10.1016/j.ijbiomac.2018.07.059
Wang, B. X., Qu, J. P., Feng, S. L., Chen, T., Yuan, M., Huang, Y., et al. (2019). Seasonal variations in the chemical composition of Liangshan olive leaves and their antioxidant and anticancer activities. Foods 8 (12), 657. doi:10.3390/foods8120657
Yu, S. S., Yu, J., Dong, X. D., Li, S., and Liu, A. J. (2020). Structural characteristics and anti-tumor/-oxidant activity in vitro of an acidic polysaccharide from Gynostemma pentaphyllum. Int. J. Biol. Macromol. 161, 721–728. doi:10.1016/j.ijbiomac.2020.05.274
Zhang, M., Cui, S. W., Cheung, P. C. K., and Wang, Q. (2007). Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 18 (1), 4–19. doi:10.1016/j.tifs.2006.07.013
Zhang, H., Qian, Y. Y., Liu, Y. Q., Li, G. Q., Cui, P. F., Zhu, Y. D., et al. (2012). Celastrus orbiculatus extract induces mitochondrial-mediated apoptosis in human hepatocellular carcinoma cells. J. Traditional Chin. Med. 32 (4), 621–626. doi:10.1016/S0254-6272(13)60081-3
Zhang, Q. W., Lin, L. G., and Ye, W. C. (2018). Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med. 13 (3), 20. doi:10.1186/s13020-018-0177-x
Zhang, Y. S., Zhang, Z. M., Liu, H., Wang, D., Wang, J. H., Deng, Z. W., et al. (2020). Physicochemical characterization and antitumor activity in vitro of a selenium polysaccharide from Pleurotus ostreatus. Int. J. Biol. Macromol. 165, 2934–2946. doi:10.1016/j.ijbiomac.2020.10.168
Keywords: Camellia fascicularis, extracts, active substances, antioxidant, antitumor
Citation: Peng X, He X, Tang J, Xiang J, Deng J, Kan H, Zhang Y, Zhang G, Zhao P and Liu Y (2022) Evaluation of the in vitro antioxidant and antitumor activity of extracts from Camellia fascicularis leaves. Front. Chem. 10:1035949. doi: 10.3389/fchem.2022.1035949
Received: 03 September 2022; Accepted: 12 October 2022;
Published: 31 October 2022.
Edited by:
Mohd Imran, Northern Border University, Saudi ArabiaReviewed by:
Rameshkumar Santhanam, University of Malaysia Terengganu, MalaysiaCopyright © 2022 Peng, He, Tang, Xiang, Deng, Kan, Zhang, Zhang, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Kan, a2FuaHVhbkBzd2Z1LmVkdS5jbg==; Yun Liu, bGl1eXVuQHN3ZnUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.