
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 04 November 2022
Sec. Theoretical and Computational Chemistry
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.1035902
A correction has been applied to this article in:
Corrigendum: Reaction mechanism of atomic layer deposition of zirconium oxide using zirconium precursors bearing amino ligands and water
 Rui Xu1
Rui Xu1 Zhongchao Zhou1
Zhongchao Zhou1 Jing Li1
Jing Li1 Xu Zhang1
Xu Zhang1 Yuanyuan Zhu1
Yuanyuan Zhu1 Hongping Xiao1*
Hongping Xiao1* Lina Xu1*
Lina Xu1* Yihong Ding1
Yihong Ding1 Aidong Li2
Aidong Li2 Guoyong Fang1*
Guoyong Fang1*As a unique nanofabrication technology, atomic layer deposition (ALD) has been widely used for the preparation of various materials in the fields of microelectronics, energy and catalysis. As a high-κ gate dielectric to replace SiO2, zirconium oxide (ZrO2) has been prepared through the ALD method for microelectronic devices. In this work, through density functional theory calculations, the possible reaction pathways of ZrO2 ALD using tetrakis(dimethylamino)zirconium (TDMAZ) and water as the precursors were explored. The whole ZrO2 ALD reaction could be divided into two sequential reactions, TDMAZ and H2O reactions. In the TDMAZ reaction on the hydroxylated surface, the dimethylamino group of TDMAZ could be directly eliminated by substitution and ligand exchange reactions with the hydroxyl group on the surface to form dimethylamine (HN(CH3)2). In the H2O reaction with the aminated surface, the reaction process is much more complex than the TDMAZ reaction. These reactions mainly include ligand exchange reactions between the dimethylamino group of TDMAZ and H2O and coupling reactions for the formation of the bridged products and the by-product of H2O or HN(CH3)2. These insights into surface reaction mechanism of ZrO2 ALD can provide theoretical guidance for the precursor design and improving ALD preparation of other oxides and zirconium compounds, which are based ALD reaction mechanism.
As an excellent nanofabrication technology, atomic layer deposition (ALD) can prepare large-area, uniform and conformal thin films at the atomic level (Klaus et al., 1997; Ritala et al., 2000; Hausmann et al., 2002; Chen et al., 2011). Meanwhile, the compositions and structures of thin films can also be controlled through varying the number of ALD cycles and precursors. ALD is a type of chemical vapor deposition (CVD) technique, namely, atomic layer chemical vapor deposition (ALCVD). It can divide the whole CVD reaction into several separate surface reactions. It can have the features of self-limitation and take full advantage of the gas-solid surface reactions. Currently, ALD has been widely used in the fields of microelectronics, nanotechnology, catalysis and energy, etc. (Zaera, 2008; Rolison et al., 2009; Marichy et al., 2012; O'Neill et al., 2015; Palmstrom et al., 2015; Asundi et al., 2019).
As the core of microelectronics technology, the development of large-scale integrated circuits obeys Moore’s law. Since the beginning of the 21st century, the thickness of SiO2 gate dielectrics in MOSFET devices has continuously decreased. However, the tunneling effect of electrons leads to significant leakage and power consumption and seriously affects the stability and reliability of MOSFET devices. Currently, using high-κ gate dielectrics to replace SiO2 is an effective method for solving the problem. Because of the high dielectric constant and thermodynamic stability, zirconium oxide (ZrO2) has been used as gate dielectrics via the ALD method for MOSFET devices (Gaskell et al., 2007; Dezelah IV et al., 2008; Kaipio et al., 2014; Jung et al., 2015; Kanomata et al., 2016; Mahuli et al., 2021; Xu et al., 2021).
In general, the prerequisite and key to the success of ALD technology require suitable precursors. For ZrO2 ALD, the zirconium precursors include these linked by alkyl, halide and alkoxy ligands, such as Zr(Cp)2, ZrCl4 and Zr(OEt2)4 (Williams et al., 2002; Yoshii et al., 2002; Niinistö et al., 2005; Knapas and Ritala, 2008). Subsequently, the zirconium precursor bearing amino ligands is also a candidate for ZrO2 ALD. Because of good volatility, thermal stability and high reactivity, tetrakis(dimethylamino)zirconium (TDMAZ, Zr(NMe2)4) has been studied (Provine et al., 2016). Different precursors have different effects on the overall ALD reaction. Experimentally, thermal ALD of ZrO2 can be performed using Zr(NMe2)4 as the zirconium source and H2O as the oxygen source. It can be written as two separate reactions as follows:
where an asterisk designates a surface species.
To obtain more insight into the ALD reaction mechanism of various materials, many theoretical calculations have been performed (Elliott, 2012; Hu et al., 2015; Elliott et al., 2016). These works include density functional theory (DFT) calculations, molecular dynamics and Monte Carlo simulations. For example, the ALD mechanism of oxides and nitrides, such as SiO2, Si3N4, Al2O3, TiO2, ZrO2 and HfO2, have been widely explored (Mukhopadhyay et al., 2008; Ren et al., 2008; Han et al., 2012; Huang et al., 2013; Huang et al., 2014). To date, only a few mechanisms of ZrO2 ALD using precursors with halide and alkyl ligands, such as ZrCl4 and ZrCp2Me2, have been studied (Brodskii et al., 2002; Jeloaica et al., 2003; Jeloaica et al., 2005; Ren et al., 2011; Zhou et al., 2013). However, the investigation of more effective zirconium precursors bearing amino ligands and their roles and reaction mechanism for ZrO2 ALD is still lacking.
Herein, we investigated the reactions of Zr(NMe2)4 and H2O on surfaces to gain more insight into the reaction mechanism of ZrO2 ALD using DFT. The whole reaction of ZrO2 ALD includes the TDMAZ half-reaction (A1 and A2) and H2O half-reaction (B1–B10), as shown in Figure 1. The results show that both TDMAZ and H2O can react with the hydroxyl and amino groups on the surface. These insights into the reaction mechanism of ZrO2 ALD can improve precursor design and ALD growth for other oxides and zirconium compounds and boost the further development of ALD chemistry.
To model the surface reaction of ZrO2 ALD, the cluster model Si63H48(OH)16 was adopted and shown in Figure 2. In general, silicon is used as a substrate material in microelectronic devices. The cluster model is based on the hydroxylated Si(001) surface with four layers of silicon atoms and sixteen hydroxyl (–OH) groups. Our previous and other works both proved that when the size of the surface is larger than the size of precursor molecules, the cluster and slab models can give similar results (Mukhopadhyay et al., 2008; Fang and Ma, 2013). The suspended bonds of the model are saturated with H atoms. To model the surface, the three layers of Si atoms at the bottom are fixed and sixteen Si atoms and hydroxyl groups on the surface are relaxed. The precursors include TDMAZ and H2O as shown in Figure 2.
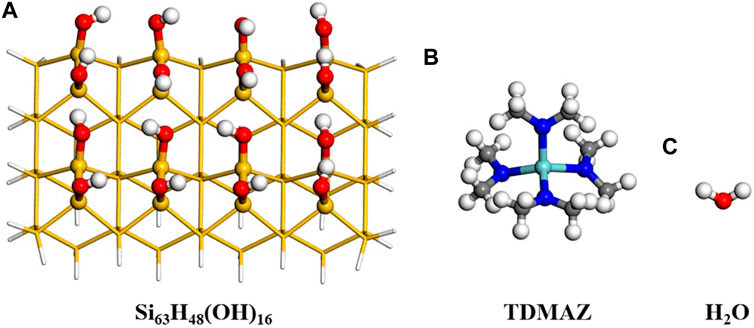
FIGURE 2. Surface model for Si63H48(OH)16, TDMAZ and H2O. The yellow, red, white, blue and light blue balls represent Si, O, H, N and Zr atoms, respectively.
All species in ZrO2 ALD reactions were optimized using DFT with the M06-2X functional. The M06-2X functional is one of the most suitable functionals to describe the interactions between the precursors and the surface (Zhao and Truhlar, 2008a; Zhao and Truhlar, 2008b). This functional was also tested using the precursor reaction on the hydroxylated surface in previous work (Fang and Ma, 2013). By comparing different density functionals (M06-2X, PBEPBE and B3LYP) with the MP2 method, it was found that the M06-2X functional is appropriate for ALD surface reactions (Fang and Ma, 2013). Meanwhile, the dispersion correction for weak interactions was performed using Grimme dispersion method with the original D3 damping function (GD3) (Grimme et al., 2010; Grimme et al., 2011). To balance the computational accuracy and cost, the 6-311G(d,p) basis set was used for the relaxed atoms and adsorbates on the surface and the LANL2DZ basis set was used for the Zr atom. Other atoms of the substrate at the bottom were described using the 6-31G basis set. All stationary points and transition states were verified using frequency and intrinsic reaction coordinate (IRC) calculations. The Gibbs free energies were also calculated from the partition functions, as well as the enthalpy and entropy terms at different temperatures (298.15 and 473.15 K) and pressures (1 atm and 0.2 Torr) (Baletto and Ferrando, 2005; Levine, 2008; Li and Truhlar, 2014). Notably, the precursor molecules in the gas phase have three motions of rotation, translation, and vibration. When the precursors are adsorbed on the surface, the rotation and translation motions are lost and new vibrations are produced. In other words, the entropy of the surface has no contribution from translation and rotation, and only has the contribution from the vibrations (Zhou et al., 2022). All optimization, frequency and IRC calculations were performed with the Gaussian 09 program (Frisch et al., 2013). The corresponding thermodynamic properties were calculated by shermo program (Lu and Chen, 2021).
When the precursor tetrakis(dimethylammonium)zirconium (TDMAZ) approaches the hydroxylated surface, it can undergo two steps (A1 and A2) of the substitution and elimination of amino ligands. The Gibbs free energy profiles of the elimination reaction (A1) of the first amino ligand are shown in Figure 3. First, TDMAZ can be adsorbed on the hydroxylated surface to form intermediate Im1A1 with the adsorption energy (Eads) of 31.9 kcal/mol. Then, it can undergo a four-membered ring (4MR) transition state (TSA1) with very low activation energy (Ea) and the imaginary frequency of 373 cm−1. In TSA1, the Zr atom of the precursor can attack the O atom of the hydroxyl group on the surface. At the same time, the H atom of the hydroxyl group can be transferred to the N atom of the amino ligand of the precursor. Later, the intermediates Im2A1 and dimethylamine (HNMe2) can be generated. Eventually, the product PA1 (–OZr(NMe2)3) can be formed and the HNMe2 molecule can be released from the surface, in which the desorption energy (Edes) of dimethylamine is 21.4 kcal/mol. The bond length changes at the reaction center are listed in Table 1. In the reaction process, the lengths of the O–H and Zr–N bonds increase from 0.980 to 2.072 Å in Im1A1 to 2.536 and 2.368 Å in Im2A1, respectively. The lengths of the Zr–O and H–N bonds decrease from 2.481 to 2.119 Å in Im1A1 to 2.076 and 1.019 Å in Im2A1, respectively. All these indicate that O–H and Zr–N bonds are broken and Zr–O and H–N bonds are formed.
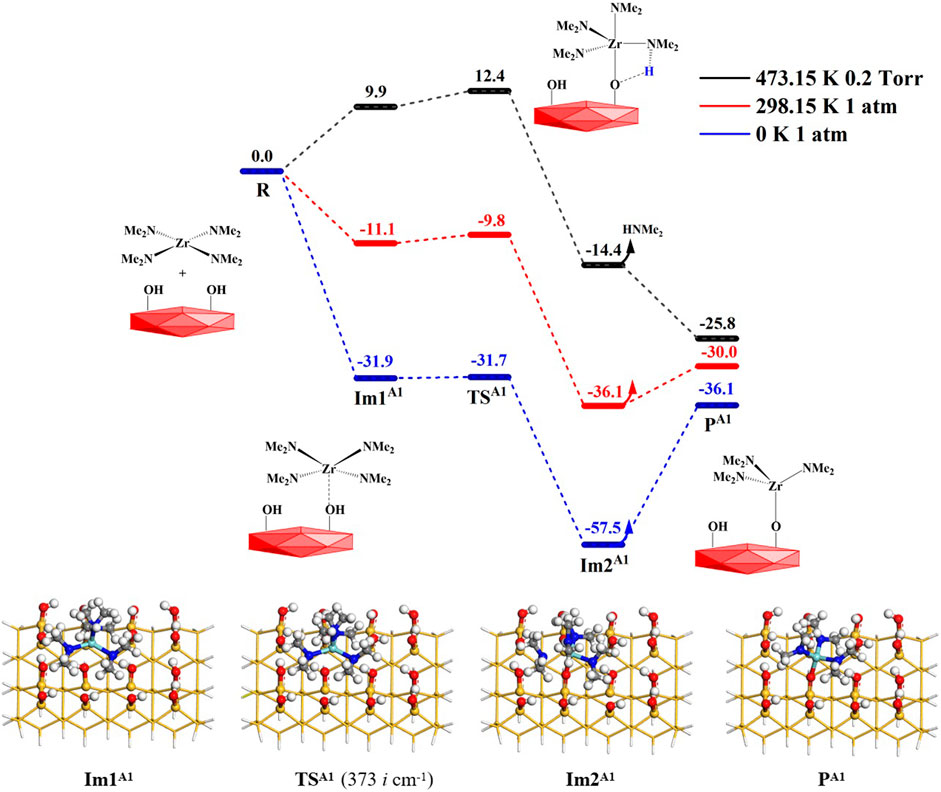
FIGURE 3. Gibbs free energy profiles (ΔG, kcal/mol) of the A1 section of the TDMAZ reaction on the hydroxylated surface.
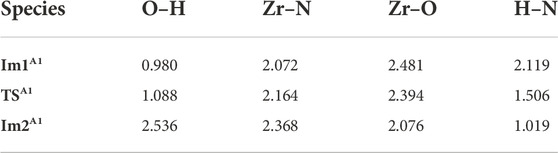
TABLE 1. Bond lengths (Å) at the reaction center in the A1 section of the TDMAZ reaction on the hydroxylated surface.
In general, the temperatures and pressures have a certain effect on the ALD surface reaction. As shown in Figure 3, at 298.15 K and 1 atm, the Gibbs activation energy (Ga) of the A1 reaction is very low at only 1.3 kcal/mol, which indicates that the reaction can easily occur at room temperature. The desorption of dimethylamine is also easy and requires a low desorption energy of about 6.0 kcal/mol. As a whole, the A1 reaction is exoergic by 30.0 kcal/mol. At 473.15 K and 0.2 Torr, the free energies of the intermediates, Im1A1 and Im2A1, and transition state TSA1 further increase because of temperature and entropy effects. The Gibbs activation energy of the A1 reaction increases to 12.4 kcal/mol. All of these indicate that the A1 reaction of TDMAZ on the hydroxylated surface is thermodynamically and kinetically favorable at the experimental condition of 473.15 K and 0.2 Torr.
The product PA1 (–OZr(NMe2)3) of the A1 section can react further with adjacent hydroxyl groups. In the A2 step, the elimination reaction of the second amino ligand of TDMAZ, there are four available hydroxyl groups on the hydroxylated surface in different directions, a, b, c and d, to form Im1A2, shown in Figure 4. Similar to the A1 step, the A2 reaction pathway also goes through a 4MR transition state TSA2 to obtain the bridged product PA2 (–OZr(NMe2)2O–) and release the small molecule dimethylamine. The corresponding Gibbs activation energies are 7.5, 6.6, 5.9 and 8.7 kcal/mol in the four directions relative to the product PA1 (–OZr(NMe2)3), indicating that the elimination of the second amino ligand of TDMAZ can occur easily. This is different from the first amino ligand elimination. In A1 reaction, the precursor adsorption leads to the reduction of the entropy and requires higher energy barrier of the first amino ligand elimination at certain temperature. In A2 reaction, the precursor has been anchored on the surface and the change of the entropy has little effect on the reaction barrier. As a result, the A2 reaction of the second amino ligand is also exoergic by about 20 kcal/mol. The Gibbs free energy of intermediate Im1A2−a in the a direction is lower than that of transition state TSA2−a, which is caused by the harmonic frequency overestimating the thermal correction of intermediate Im1A2−a, which is more stable than TSA2−a in terms of electronic energy.
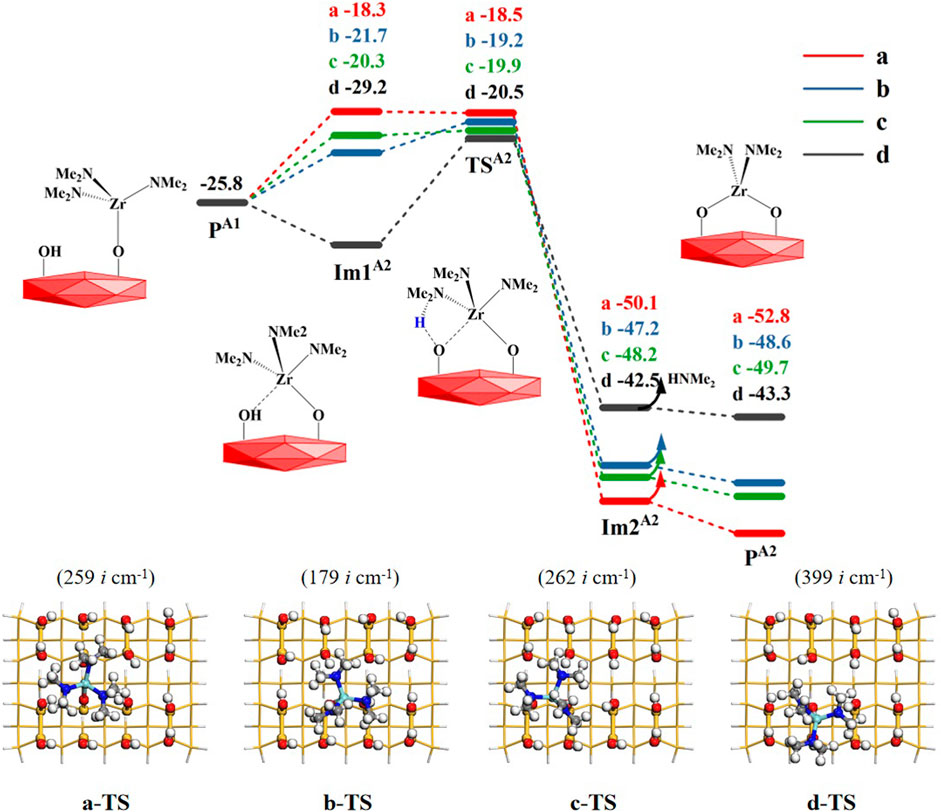
FIGURE 4. Gibbs free energy profiles (ΔG, kcal/mol) of the A2 section of the TDMAZ reaction with the hydroxylated surface at 473.15 K and 0.2 Torr. Symbols a, b, c and d represent four different directions of –OZr(NMe2)3 with adjacent hydroxyl groups.
According to Table 2, the atomic distances of Zr, O, H and N in the reaction center have similar changes, indicating the breakage of O–H and Zr–N bonds and the formation of Zr–O and H–N bonds. As a whole, all the bond changes in the four directions are similar to each other. Because the product PA2−a has the lowest energy and the most stable structure, the product in the a direction is used as the initial structure for the next reaction.
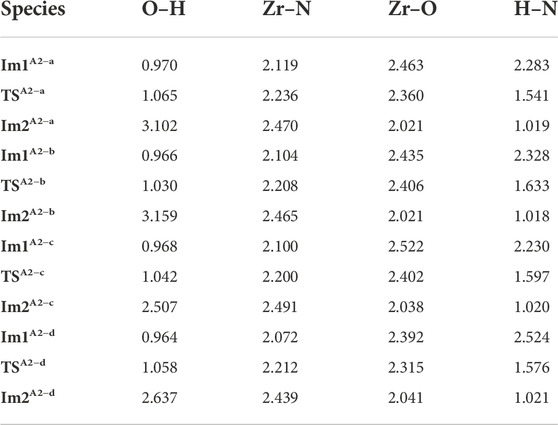
TABLE 2. Bond lengths (Å) at the reaction center in the A2 section of the TDMAZ reaction on the hydroxylated surface.
After the TDMAZ reaction with the hydroxylated surface, H2O can be pumped into the reactor and react with the aminated surface. The H2O reaction on the surface is more complex than the TDMAZ reaction with the hydroxylated surface and involves 10 reaction pathways (B1 to B10) to eliminate dimethylamine and water molecules, as shown in Figure 1.
As shown in Figure 5, a H2O molecule and the surface PA2(–OZr(NMe2)2O–) can undergo ligand exchange reaction B1 with the PA2 (–OZr(NMe2)2O–) surface. It can sequentially pass through the intermediate Im1B1, the 4MR transition state TSB1 and intermediate Im2B1. Eventually, the HNMe2 molecule can be released and the product PB1 (–OZr(NMe2)(OH)O–) can be formed. The B1 reaction is exoergic by 20.5 kcal/mol. The Ga of the reaction is 14.8 kcal/mol. From Supplementary Table S1, it can be seen that the distance between H and O atoms increases from 0.962 Å in Im1B1 to 2.282 Å in Im2B1, the distance between Zr and N atoms increases from 2.085 to 2.436 Å, whereas the distance between H and N atoms decreases from 2.890 to 1.021 Å, the distance between O and Zr atoms decreases from 2.393 to 2.015 Å. All these indicate that H–O and Zr–N bonds are cleaved and H–N and O–Zr bonds are formed in the B2 reaction.
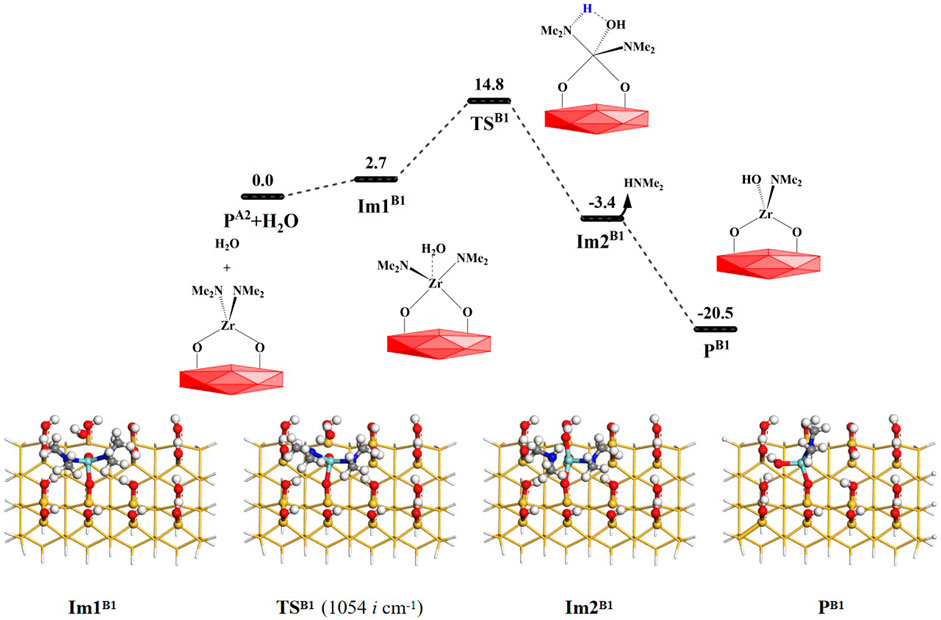
FIGURE 5. Gibbs free energy (ΔG, kcal/mol) profiles of the B1 section of the H2O reaction with the aminated surface.
As shown in Figure 6, the H2O molecule can further react with the product PB1 (–OZr(NMe2)(OH)O–). Similar to B1, the B2 reaction is also a ligand exchange reaction between the hydroxyl group and the amino ligand. First, the water molecule can interact with the product PB1 (–OZr(NMe2)(OH)O–) surface to form the intermediate Im1B2. Subsequently, it can go through a 4MR transition state TSB2 to form the intermediate Im2B2. Finally, the HNMe2 can be released and the product PB2 (–OZr(OH)2O–) can be obtained. The B2 reaction is exoergic by 17.8 kcal/mol and the Gibbs activation energy is 11.8 kcal/mol. Supplementary Table S2 lists the changes in the bond lengths at the reaction center of the B2 reaction. Similar to the B1 reaction, H–O and Zr–N bonds are gradually broken and H–N and O–Zr bonds are gradually formed during the process of the B2 reaction. As a whole, B1 and B2 reactions are both exoergic and require low energy barriers, indicating that H2O and the aminated surface can easily react with each other.
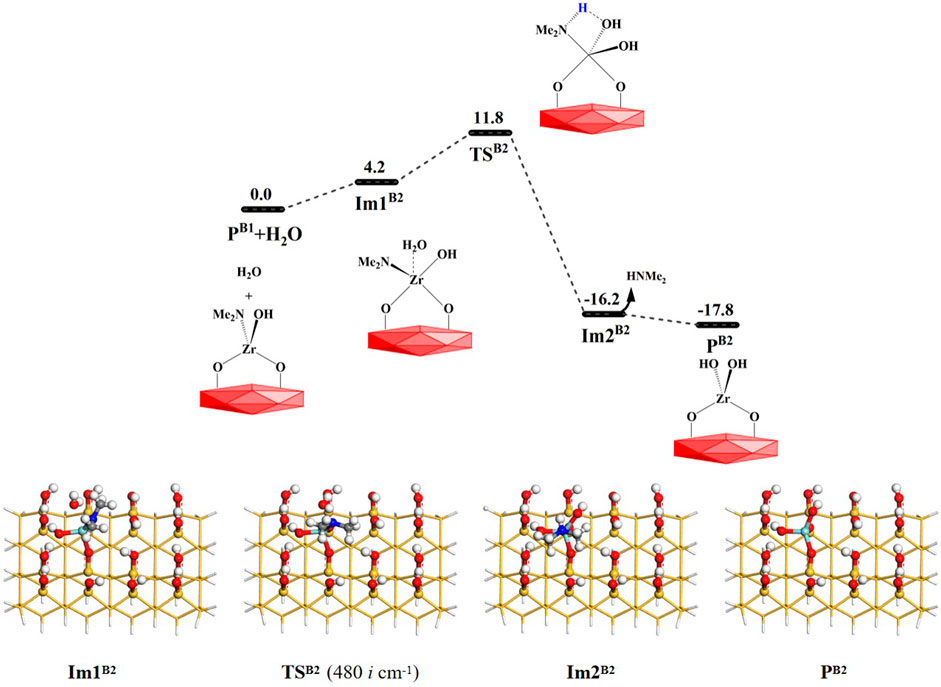
FIGURE 6. Gibbs free energy (ΔG, kcal/mol) profiles of the B2 section of the H2O reaction with the aminated surface.
As mentioned above, the TDMAZ reaction on the hydroxylated surface can form another product PA1 (–OZr(NMe2)3), which can also react directly with a water molecule. As shown in Figure 7, H2O can react with the product PA1 (–OZr(NMe2)3) via the B3 pathway. A water molecule can be adsorbed on the –OZr(NMe2)3 surface to form an intermediate Im1B3. It can undergo a 4MR transition state TSB3 with the Gibbs activation energy of 14.2 kcal/mol and an intermediate Im2B3. Finally, HNMe2 can be released and the product PB3 (–OZr(NMe2)2(OH) can be formed, releasing the small molecule. The B3 reaction is also exoergic by 13.5 kcal/mol. As listed in Supplementary Table S3, the lengths of the H–O and Zr–N bonds gradually increase and the lengths of the H–N and O–Zr bonds gradually decrease. All these indicate that the H–O and Zr–N bonds at the reaction center are broken and the H–N and O–Zr bonds are formed during the B3 reaction.
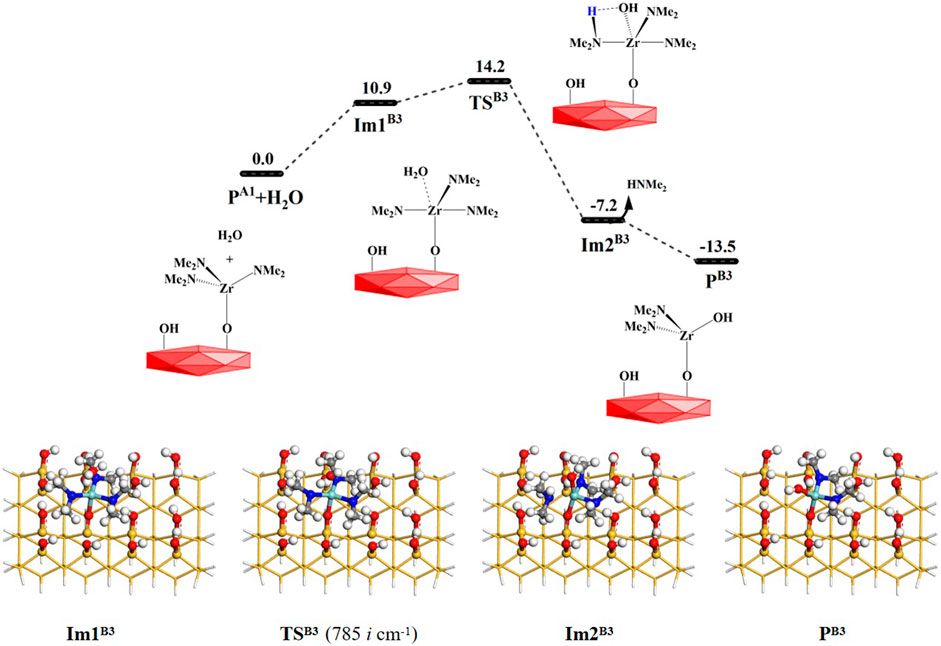
FIGURE 7. Gibbs free energy (ΔG, kcal/mol) profiles of the B3 section of the H2O reaction with the aminated surface.
Similarly, other water molecules can further attack the Zr atom and react with the aminated surface. As shown in Figure 1, the reaction processes of the B4 and B5 pathways are similar to that of the B3 reaction. They can form the intermediates Im2B4 and Im2B5 and undergo 4MR transition states TSB4 and TSB5, respectively. Lastly, HNMe2 can be released and the products PB4 and PB5 can be obtained, as shown in Figures 8, 9. The B4 and B5 reactions are exoergic by 27.0 and 12.8 kcal/mol and the corresponding Gibbs activation energies are 12.4 and 12.3 kcal/mol, respectively. However, different from the desorption of HNMe2 in the B4 reaction, the release of HNMe2 in the B5 reaction requires a low energy of 2.3 kcal/mol. Supplementary Tables S4, S5 list the changes in the bond lengths at the reaction centers, which indicate that the H–O and Zr–N bonds are cleaved and the H–N and O–Zr bonds are formed.
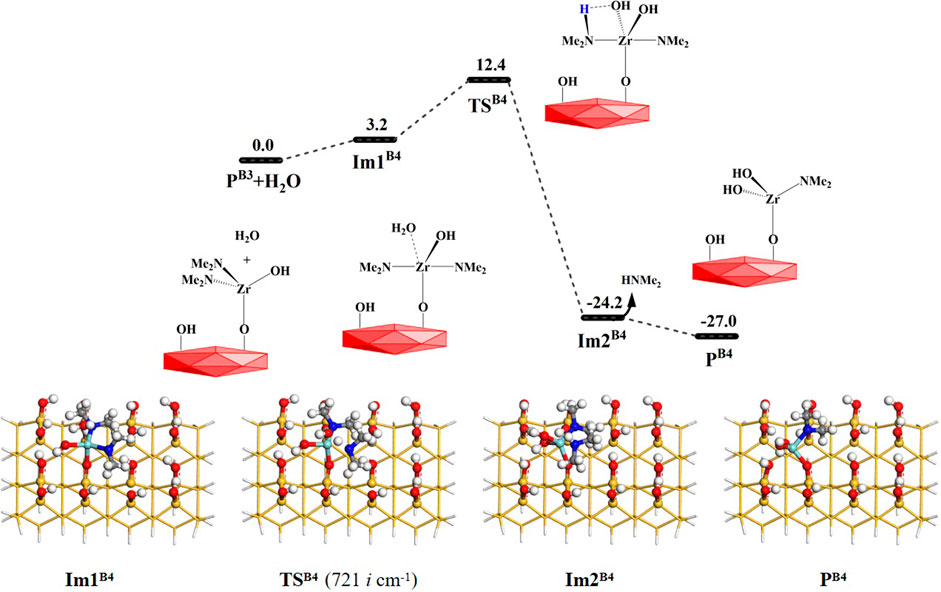
FIGURE 8. Gibbs free energy (ΔG, kcal/mol) profiles of the B4 section of the H2O reaction with the aminated surface.
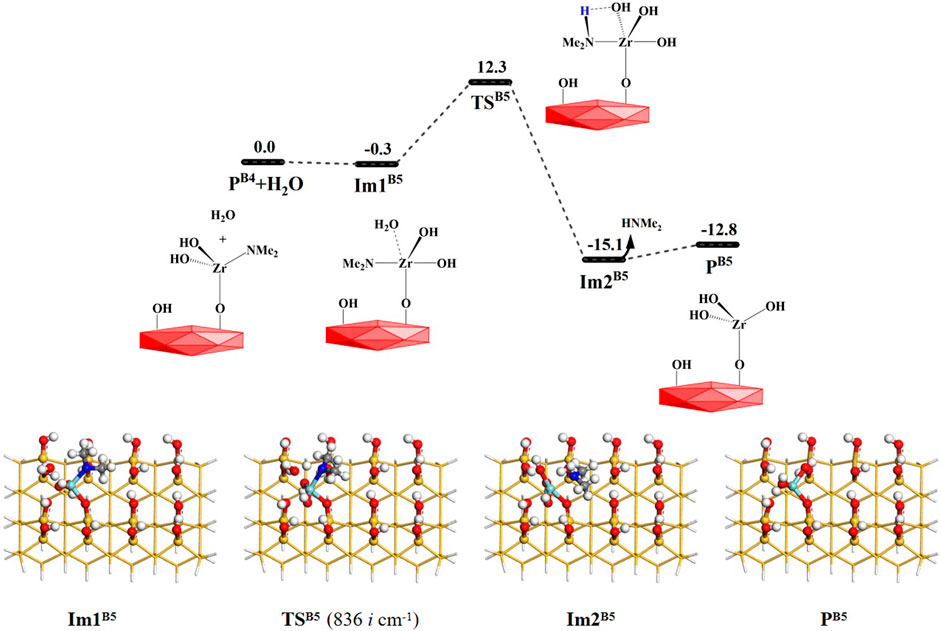
FIGURE 9. Gibbs free energy (ΔG, kcal/mol) profiles of the B5 section of the H2O reaction with the aminated surface.
From Figure 1, it can be seen that amino ligands of the intermediate products PB3 and PB4 can react not only with water but also with the adjacent hydroxyl groups on the surface to eliminate the amino ligands and form dimethylamine and bridged products, namely, the coupling reactions between surface amino hydroxyl groups. As shown in Figure 10, the Zr atom of the product PB3 (–Zr(NMe2)2OH) can attack the O atom on the adjacent hydroxyl group to form an intermediate Im1B6 with lower energy. Then, the H atom of the adjacent hydroxyl group on the surface can be transferred to the N atom of the amino ligand to form dimethylamine. It can go through a lower-energy 4MR transition state TSB6 with the Gibbs activation energy of 3.2 kcal/mol and an intermediate Im2B6. Finally, HNMe2 is released and the intermediate product PB6 is generated. As a whole, the B6 reaction is exoergic by 34.0 kcal/mol. As listed in Supplementary Table S6, the lengths of the H–O and Zr–N bonds gradually increase and the lengths of the H–N and O–Zr bonds gradually decrease in the reaction center. The bond lengths of Zr–N and O–H increase from 2.127 and 0.976 Å in Im1B6 to 2.465 and 2.898 Å in Im2B6, and the bond lengths of N–H and Zr–O decrease from 2.012 and 2.478 Å to 1.020 and 1.987 Å, respectively.
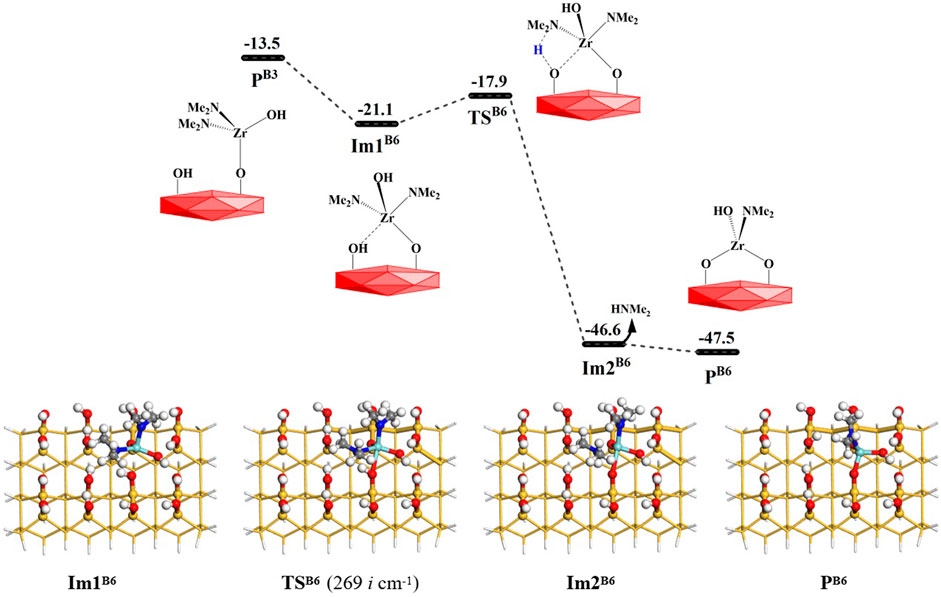
FIGURE 10. Gibbs free energy (ΔG, kcal/mol) profiles of the B6 section of the H2O reaction with the aminated surface.
Similar to the B6 reaction, the Zr atom of the intermediate product PB4 (–OZr(NMe2)(OH)2) can attack the O atom on the surrounding hydroxyl group during the B7 reaction. It can go through an intermediate Im1B7 and a hydrogen-transfer transition state TSB7, in which the H atom on the hydroxyl group can be transferred to the N atom of the dimethylamino group. Lastly, the HNMe2 molecule can be released and intermediate product PB7 (–OZr(OH)2O–) can be formed. The B7 reaction is exoergic by 24.8 kcal/mol and requires a very low Gibbs activation energy of 2.4 kcal/mol. From Figure 11, it can be seen that the Gibbs free energy of intermediate Im1B7 is higher than that of TSB7, but the electronic energy of Im1B7 is lower than that of TSB7, which results from the overestimation of the thermodynamic correction of Im1B7 by the harmonic frequency. According to the data in Supplementary Table S7, the tendencies of the breakage of H–O and Zr–N bonds and the formation of H–N and O–Zr bonds are also shown.

FIGURE 11. Gibbs free energy (ΔG, kcal/mol) profiles of the B7 section of the H2O reaction with the aminated surface.
In addition, the hydroxyl groups on the intermediate products PB3, PB4 and PB5 can also combine with the adjacent hydroxyl groups to form water molecules, namely the coupling reactions between surface hydroxyl groups. These coupling reactions correspond to B8, B9 and B10 reactions, shown in Figure 1. The structure of the Zr reaction center can change from a tetrahedral structure to a bridged structure.
In the B8 reaction, the Zr atom of PA3 can attack the O atom of the adjacent hydroxyl group on the surface to form the intermediate Im1B8, which is similar to the B6 reaction. Subsequently, the H atom of the hydroxyl group on the surface can react with the adjacent hydroxyl group to form a water molecule. Considering that the steric hindrance of the hydroxyl group is smaller than that of the dimethylamino group, the hydroxyl group on the Zr atom can react with the adjacent hydroxyl group not only from above but also from the side, as shown in Figure 12. The corresponding Gibbs activation energy is 4.6 or 3.3 kcal/mol, respectively. As a whole, the B8 reaction is exoergic by 13.5 kcal/mol. As listed in Supplementary Table S8, the changes in the bond lengths of the reaction center show the cleavage of old O–H and Zr–O bonds and the formation of new O–H and Zr–O bonds.
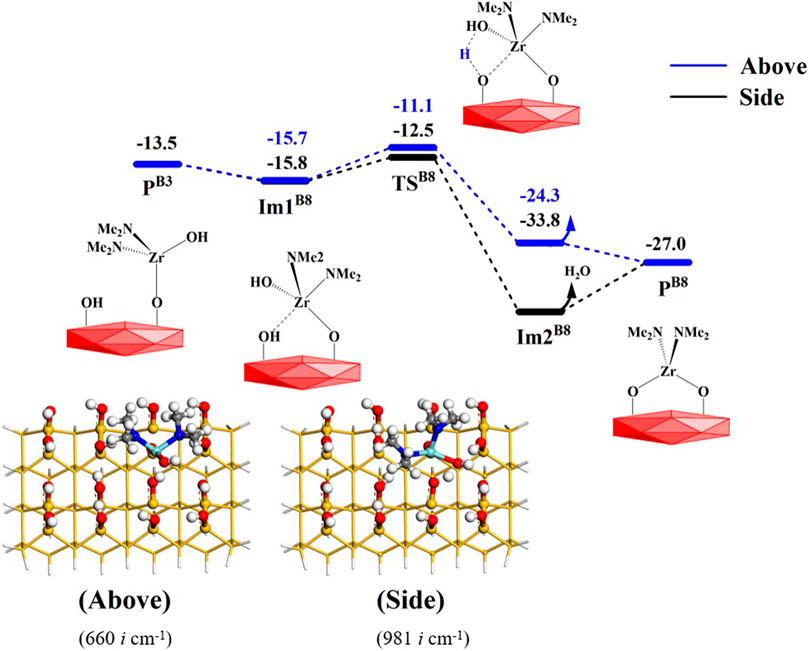
FIGURE 12. Gibbs free energy (ΔG, kcal/mol) profiles of the B8 section of the H2O reaction with the aminated surface.
As shown in Figure 13, the B9 reaction process is similar to the B8 section. The Zr atom of intermediate PB4 can attack the adjacent hydroxyl group to form intermediate ImB9. Subsequently, the hydroxyl group on the Zr atom can react with the adjacent hydroxyl group through the transition state TSB9 and the intermediate Im2B9. Finally, the product PB8 (–OZr(NMe2)(OH)O–) can be obtained. The Gibbs free energy activation energy is about 5.0 kcal/mol. The desorption energy of H2O release is about 14.0 kcal/mol. The whole B9 process is exoergic by 7.0 kcal/mol. From Supplementary Table S9, it can be seen that with the elimination of the hydroxyl group, the lengths of Zr–O′ and O–H bonds increase from 2.004 and 0.970 Å to 2.284 and 2.882 Å, and the lengths of Zr–O and O′–H bonds decrease from 2.398 and 2.491 Å to 2.030 and 0.980 Å, respectively. All these indicate that the Zr–O bond is formed and the –OH group is eliminated.
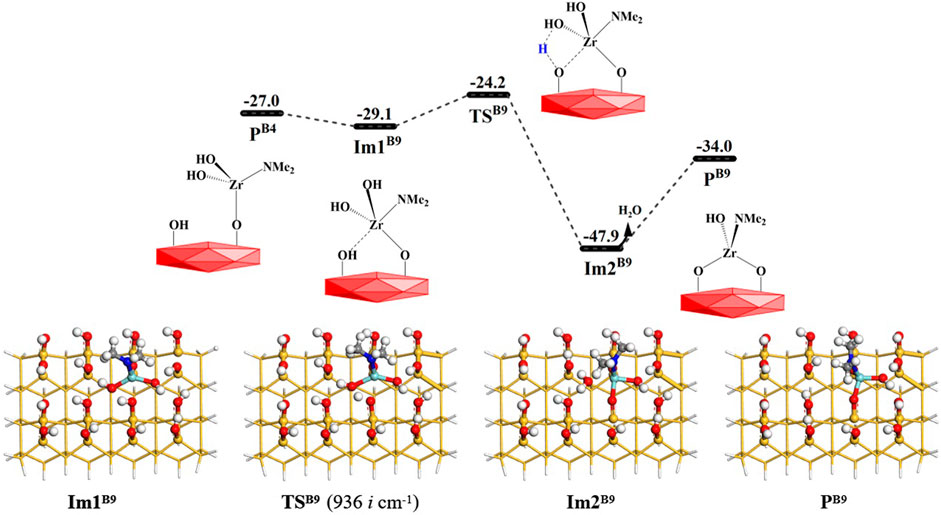
FIGURE 13. Gibbs free energy (ΔG, kcal/mol) profiles of the B9 section of the H2O reaction with the aminated surface.
As shown in Figure 14, the intermediate product PB5 (–OZr(OH)3) of the B5 reaction can also eliminate a hydroxyl group on the Zr atom by bridged reaction B10. The hydroxyl group on PB5 can react with the H atom on the adjacent hydroxyl group via the intermediate ImB10 and the 4MR transition state TSB10 to release H2O molecules and form the final product PB10 (–OZr(OH)2O). The Gibbs activation energy in the B10 reaction is 7.9 kcal/mol and the desorption energy of the water molecule is 7.1 kcal/mol. Supplementary Table S10 lists the bond lengths at the reaction center, which indicates the breakage of old bonds and the formation of new bonds in the B10 section. In comparison with B6–B10, the Gibbs activation energies of the dimethylamino elimination reactions are lower than those of hydroxyl elimination reactions, which indicates that the elimination reactions of dimethylamine occur relatively easily and are kinetically more favorable.
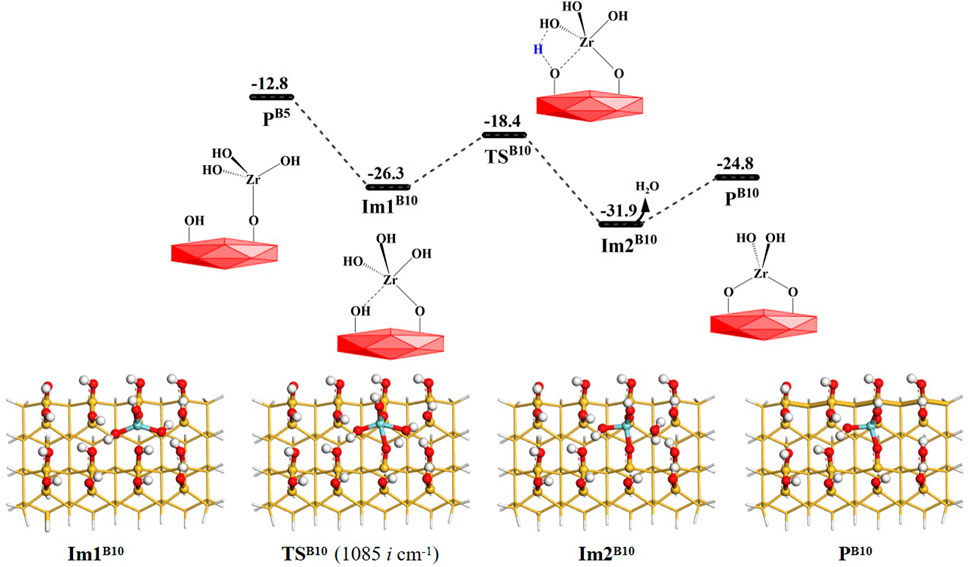
FIGURE 14. Gibbs free energy (ΔG, kcal/mol) profiles of the B10 section of the H2O reaction with the aminated surface.
Through DFT calculations, possible pathways for the ZrO2 ALD reaction of tetrakis(dimethylammonium)zirconium and water on the hydroxylated Si(100) surface were investigated in detail. The whole reaction mechanism includes two main reactions: TDMAZ reactions with the hydroxylated surface and water reactions with the aminated surface. In the TDMAZ reaction, the precursor can eliminate the dimethylamino group by a substitution reaction with the hydroxyl group on the surface. At the same time, the second dimethylamino group of the precursor can be eliminated with the help of other hydroxyl groups on the surface. Considering the configuration of the hydroxylated surface and the Zr–O bond length, only up to two dimethylamines can be eliminated on the Si surface, and the remaining dimethylamine needs to be eliminated by the H2O reaction. With increasing temperature, the release of a small molecule adsorbed on the surface takes place more readily. In the H2O reaction, the ligand exchange reactions and coupling reactions can alternately occur. In the ligand exchange reactions between the hydroxyl and amino groups, the Gibbs activation energies of the reaction are about 12 kcal/mol, which are the highest in the H2O reaction. In the coupling reactions, the hydroxyl or amino groups can react with the neighboring hydroxyl group with lower Gibbs activation energy. Moreover, the coupling reaction of the dimethylamino ligand with the hydroxyl group on the surface is easier than that between the hydroxyl groups on the surface. All these insights into ZrO2 ALD could guide the design of new precursors and ALD preparation of other oxides and zirconium compounds.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
RX: Investigation, Methodology, Writing. ZZ: Investigation. JL: Investigation. XZ: Investigation. YZ: Investigation. HX: Investigation, Resources, Writing. LX: Investigation, Resources, Writing. YD: Investigation. AL: Investigation. GF: Investigation, Resources, Writing.
This work was supported by National Natural Science Foundation of China (21873073 and 52073142), Zhejiang Provincial Natural Science Foundation (LY22B030007), Open Project (M30031 and M35047) of National Laboratory of Solid State Microstructures at Nanjing University and Hongzhiwei Technology.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1035902/full#supplementary-material
Asundi, A. S., Raiford, J. A., and Bent, S. F. (2019). Opportunities for atomic layer deposition in emerging energy technologies. ACS Energy Lett. 4, 908–925. doi:10.1021/acsenergylett.9b00249
Baletto, F., and Ferrando, R. (2005). Structural properties of nanoclusters: energetic, thermodynamic, and kinetic effects. Rev. Mod. Phys. 77, 371–423. doi:10.1103/RevModPhys.77.371
Brodskii, V., Rykova, E., Bagatur'yants, A., and Korkin, A. (2002). Modelling of ZrO2 deposition from ZrCl4 and H2O on the Si(100) surface: initial reactions and surface structures. Comput. Mat. Sci. 24, 278–283. doi:10.1016/s0927-0256(02)00192-1
Chen, Y. W., Prange, J. D., Duhnen, S., Park, Y., Gunji, M., Chidsey, C. E., et al. (2011). Atomic layer-deposited tunnel oxide stabilizes silicon photoanodes for water oxidation. Nat. Mat. 10, 539–544. doi:10.1038/nmat3047
Dezelah IV, C. L., Niinistö, J., Kukli, K., Munnik, F., Lu, J., Ritala, M., et al. (2008). The atomic layer deposition of HfO2 and ZrO2 using advanced metallocene precursors and H2O as the oxygen source. Chem. Vap. Depos. 14, 358–365. doi:10.1002/cvde.200806716
Elliott, S. D. (2012). Atomic-scale simulation of ALD chemistry. Semicond. Sci. Technol. 27, 074008. doi:10.1088/0268-1242/27/7/074008
Elliott, S. D., Dey, G., Maimaiti, Y., Ablat, H., Filatova, E. A., and Fomengia, G. N. (2016). Modeling mechanism and growth reactions for new nanofabrication processes by atomic layer deposition. Adv. Mat. 28, 5367–5380. doi:10.1002/adma.201504043
Fang, G., and Ma, J. (2013). Rapid atomic layer deposition of silica nanolaminates: synergistic catalysis of lewis/brønsted acid sites and interfacial interactions. Nanoscale 5, 11856–11869. doi:10.1039/c3nr02086j
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., et al. (2013). Gaussian 09, revision E.01. Wallingford CT: Gaussian Inc.
Gaskell, J. M., Jones, A. C., Chalker, P. R., Werner, M., Aspinall, H. C., Taylor, S., et al. (2007). Deposition of lanthanum zirconium oxide high-k films by liquid injection ALD and MOCVD. Chem. Vap. Depos. 13, 684–690. doi:10.1002/cvde.200706637
Grimme, S., Antony, J., Ehrlich, S., and Krieg, H. (2010). A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104. doi:10.1063/1.3382344
Grimme, S., Ehrlich, S., and Goerigk, L. (2011). Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465. doi:10.1002/jcc.21759
Han, B., Zhang, Q., Wu, J., Han, B., Karwacki, E. J., Derecskei, A., et al. (2012). On the mechanisms of SiO2 thin-film growth by the full atomic layer deposition process using bis(t-butylamino)silane on the hydroxylated SiO2(001) surface. J. Phys. Chem. C 116, 947–952. doi:10.1021/jp2094802
Hausmann, D., Becker, J., Wang, S., and Gordon, R. G. (2002). Rapid vapor deposition of highly conformal silica nanolaminates. Science 298, 402–406. doi:10.1126/science.1073552
Hu, X., Schuster, J., Schulz, S. E., and Gessner, T. (2015). Surface chemistry of copper metal and copper oxide atomic layer deposition from copper(ii)acetylacetonate: a combined first-principles and reactive molecular dynamics study. Phys. Chem. Chem. Phys. 17, 26892–26902. doi:10.1039/c5cp03707g
Huang, L., Han, B., Han, B., Derecskei-Kovacs, A., Xiao, M., Lei, X., et al. (2014). Density functional theory study on the full ALD process of silicon nitride thin film deposition via BDEAS or BTBAS and NH3. Phys. Chem. Chem. Phys. 16, 18501–18512. doi:10.1039/c4cp02741h
Huang, L., Han, B., Han, B., Derecskei-Kovacs, A., Xiao, M., Lei, X., et al. (2013). First-principles study of a full cycle of atomic layer deposition of SiO2 thin films with di(sec-butylamino)silane and ozone. J. Phys. Chem. C 117, 19454–19463. doi:10.1021/jp405541x
Jeloaica, L., Estève, A., Djafari Rouhani, M., and Estève, D. (2003). Density functional theory study of HfCl4, ZrCl4, and Al(CH3)3 decomposition on hydroxylated SiO2: initial stage of high-κ atomic layer deposition. Appl. Phys. Lett. 83, 542–544. doi:10.1063/1.1587261
Jeloaica, L., Esteve, A., Dkhissi, A., Esteve, D., and Djafari-Rouhani, M. (2005). Three-step mechanism of the water recombination reactions on SiO2/Si surface in the first stage of ZrO2 atomic layer deposition. Comput. Mat. Sci. 33, 59–65. doi:10.1016/j.commatsci.2004.12.060
Jung, J.-S., Lee, S.-K., Hong, C.-S., Shin, J.-H., Kim, J.-M., and Kang, J.-G. (2015). Atomic layer deposition of ZrO2 thin film on Si(100) using {η5:η1-Cp(CH2)3NMe}Zr(NMe2)2/O3 as precursors. Thin Solid Films 589, 831–837. doi:10.1016/j.tsf.2015.07.037
Kaipio, M., Blanquart, T., Banerjee, M., Xu, K., Niinistö, J., Longo, V., et al. (2014). Atomic layer deposition of TiO2 and ZrO2 thin films using heteroleptic guanidinate precursors. Chem. Vap. Depos. 20, 209–216. doi:10.1002/cvde.201407115
Kanomata, K., Tokoro, K., Imai, T., Pansila, P., Miura, M., Ahmmad, B., et al. (2016). Room-temperature atomic layer deposition of ZrO2 using tetrakis(ethylmethylamino)zirconium and plasma-excited humidified argon. Appl. Surf. Sci. 387, 497–502. doi:10.1016/j.apsusc.2016.06.122
Klaus, J. W., Sneh, O., and George, S. M. (1997). Growth of SiO2 at room temperature with the use of catalyzed sequential half-reactions. Science 278, 1934–1936. doi:10.1126/science.278.5345.1934
Knapas, K., and Ritala, M. (2008). In situ reaction mechanism studies on atomic layer deposition of ZrO2 from (CpMe)2Zr(OMe)Me and water or ozone. Chem. Mat. 20, 5698–5705. doi:10.1021/cm800460b
Li, Z. H., and Truhlar, D. G. (2014). Nanothermodynamics of metal nanoparticles. Chem. Sci. 5, 2605. doi:10.1039/c4sc00052h
Lu, T., and Chen, Q. (2021). Shermo: A general code for calculating molecular thermochemistry properties. Comput. Theor. Chem. 1200, 113249. doi:10.1016/j.comptc.2021.113249
Mahuli, N., Cavanagh, A. S., and George, S. M. (2021). Atomic layer deposition of hafnium and zirconium oxyfluoride thin films. J. Vac. Sci. Technol. A 39, 022403. doi:10.1116/6.0000731
Marichy, C., Bechelany, M., and Pinna, N. (2012). Atomic layer deposition of nanostructured materials for energy and environmental applications. Adv. Mat. 24, 1017–1032. doi:10.1002/adma.201104129
Mukhopadhyay, A. B., Musgrave, C. B., and Sanz, J. F. (2008). Atomic layer deposition of hafnium oxide from hafnium chloride and water. J. Am. Chem. Soc. 130, 11996–12006. doi:10.1021/ja801616u
Niinistö, J., Rahtu, A., Putkonen, M., Ritala, M., Leskelä, M., and Niinistö, L. (2005). In situ quadrupole mass spectrometry study of atomic-layer deposition of ZrO2 using Cp2Zr(CH3)2 and water. Langmuir 21, 7321–7325. doi:10.1021/la0500732
O'Neill, B. J., Jackson, D. H. K., Lee, J., Canlas, C., Stair, P. C., Marshall, C. L., et al. (2015). Catalyst design with atomic layer deposition. ACS Catal. 5, 1804–1825. doi:10.1021/cs501862h
Palmstrom, A. F., Santra, P. K., and Bent, S. F. (2015). Atomic layer deposition in nanostructured photovoltaics: tuning optical, electronic and surface properties. Nanoscale 7, 12266–12283. doi:10.1039/c5nr02080h
Provine, J., Schindler, P., Torgersen, J., Kim, H. J., Karnthaler, H.-P., and Prinz, F. B. (2016). Atomic layer deposition by reaction of molecular oxygen with tetrakisdimethylamido-metal precursors. J. Vac. Sci. Technol. A Vac. Surfaces Films 34, 01A138. doi:10.1116/1.4937991
Ren, J., Cui, C., Zhou, G., Liu, Y., Hu, Y., and Wang, B. (2011). A theoretical study on initial growth mechanism of ZrO2 film using cyclopentadienyl-type precursor. Thin Solid Films 519, 3716–3721. doi:10.1016/j.tsf.2011.01.278
Ren, J., Zhou, G., Hu, Y., Jiang, H., and Zhang, D. W. (2008). Surface reactions in atomic layer deposition of HfO2, ZrO2 and Al2O3 on hydroxylated and sulfur-passivated GaAs(100) surfaces: A comparative study by density functional theory. Appl. Surf. Sci. 254, 7115–7121. doi:10.1016/j.apsusc.2008.05.237
Ritala, M., Kukli, K., Rahtu, A., Raisanen, P. I., Leskela, M., Sajavaara, T., et al. (2000). Atomic layer deposition of oxide thin films with metal alkoxides as oxygen sources. Science 288, 319–321. doi:10.1126/science.288.5464.319
Rolison, D. R., Long, J. W., Lytle, J. C., Fischer, A. E., Rhodes, C. P., McEvoy, T. M., et al. (2009). Multifunctional 3D nanoarchitectures for energy storage and conversion. Chem. Soc. Rev. 38, 226–252. doi:10.1039/b801151f
Williams, P. A., Roberts, J. L., Jones, A. C., Chalker, P. R., Bickley, J. F., Steiner, A., et al. (2002). Novel mononuclear zirconium and hafnium alkoxides; improved precursors for the MOCVD of ZrO2 and HfO2. J. Mat. Chem. 12, 165–167. doi:10.1039/b109994a
Xu, W., Lemaire, P. C., Sharma, K., Gasvoda, R. J., Hausmann, D. M., and Agarwal, S. (2021). Mechanism for growth initiation on aminosilane-functionalized SiO2 during area-selective atomic layer deposition of ZrO2. J. Vac. Sci. Technol. A 39, 032402. doi:10.1116/6.0000699
Yoshii, N., Takahashi, N., Nakamura, T., and Yoshioka, M. (2002). Preparation of ZrO2 nano-films by an alternate reaction using ZrCl4 and O2 under atmospheric pressure. Electrochem. Solid-State Lett. 5, C85. doi:10.1149/1.1498016
Zaera, F. (2008). The surface chemistry of thin film atomic layer deposition (ALD) processes for electronic device manufacturing. J. Mat. Chem. 18, 3521–3526. doi:10.1039/b803832e
Zhao, Y., and Truhlar, D. G. (2008). Density functionals with broad applicability in chemistry. Acc. Chem. Res. 41, 157–167. doi:10.1021/ar700111a
Zhao, Y., and Truhlar, D. G. (2008). The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241. doi:10.1007/s00214-007-0310-x
Zhou, G., Ren, J., and Zhang, S. (2013). Initial growth mechanisms of ZrO2 and TiO2 thin films using cycloheptatrienyl–cyclopentadienyl heteroleptic precursors: A comparative study by density functional theory. Appl. Surf. Sci. 283, 968–974. doi:10.1016/j.apsusc.2013.07.054
Keywords: zirconium oxide, atomic layer deposition, reaction mechanism, tetrakis(dimethylamino)zirconium, density functional theory
Citation: Xu R, Zhou Z, Li J, Zhang X, Zhu Y, Xiao H, Xu L, Ding Y, Li A and Fang G (2022) Reaction mechanism of atomic layer deposition of zirconium oxide using zirconium precursors bearing amino ligands and water. Front. Chem. 10:1035902. doi: 10.3389/fchem.2022.1035902
Received: 03 September 2022; Accepted: 06 October 2022;
Published: 04 November 2022.
Edited by:
Sudip Pan, University of Marburg, GermanyReviewed by:
Jose Luis Cabellos, Polytechnic University of Tapachula, MexicoCopyright © 2022 Xu, Zhou, Li, Zhang, Zhu, Xiao, Xu, Ding, Li and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongping Xiao, aHBfeGlhb0B3enUuZWR1LmNu; Lina Xu, eHVsaW5hQHd6dS5lZHUuY24=; Guoyong Fang, ZmFuZ2d5QHd6dS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.