- 1Key Laboratory of Bioactive Substances and Resources Utilization of Chinese Herbal Medicines, Ministry of Education, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Key Laboratory of Biodiversity Science and Ecological Engineering, Ministry of Education, College of Life Sciences, Beijing Normal University, Beijing, China
- 3Hubei Province Key Laboratory of Traditional Chinese Medicine Resource and Chemistry, Hubei University of Chinese Medicine, Wuhan, Hubei, China
- 4Key Laboratory of Plant Molecular Physiology, Institute of Botany, Chinese Academy of Sciences, Beijing, China
- 5State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China
- 6Department of Pharmaceutical Sciences, Beijing Institute of Radiation Medicine, Beijing, China
Phytochemical investigation was carried out for the flowers of Epimedium acuminatum Franchet. by first conducting LC-MS analysis, leading to the identification of 32 compounds. Furthermore, guided by LC-MS profiling, three new 8-prenylated quercetin glycosides (3′-hydroxylikarisoside C, 3′-hydroxylepimedoside E, 3′-hydroxyldiphylloside B), one new anthocyanin (delphinidin-3-O-p-coumaroyl-sophoroside) and six known compounds were isolated from the flowers of E. acuminatum for the first time, and their structures were characterized based on spectroscopic methods including 1D and 2D NMR, and HRESIMS. Combining our discoveries and literature survey, a revised classification of Epimedium flavonols was proposed as Type A (8-prenylated kaempferol based), which was further subdivided into subtype icaritin and subtype demethylicaritin, and Type B (8-prenylated quercetin based), which was further subdivided into subtype 3′-hydroxylicaritin and subtype 3′-hydroxyldemethylicaritin. The structure-activity relationship (SAR) study was carried out by comparing testosterone production-promoting activities of all the new compounds along with nine related Epimedium flavonols, revealing that the new 8-prenylated quercetin glycosides (subtype 3′-hydroxyldemethylicaritin in Type B) exhibited lower testosterone production-promoting activities in rat primary Leydig cells than Epimedium flavonols of subtype demethylicaritin in Type A, but possessed higher activities than the Epimedium flavonols of subtype icaritin in Type A. These results suggested that either methylation at C-4′ position or hydroxylation at C-3′ position of ring B could significantly reduce the testosterone production-promoting activities of Epimedium flavonols.
Introduction
Epimedium L. is a medicinally important herbaceous genus in the family Berberidaceae containing more than 60 perennial plant species. The native occurrences of these plants are geographically discontinuous from North Africa (Algeria) to East Asia, with most of them endemic to China (Stearn, 2002; Ying, 2002). Due to their special nourishing effects on kidney and bones, the leaves and rhizomes of Epimedium plants have long been used in traditional Chinese medicine (GMP, 2019; CP, 2020). Meanwhile, bearing beautiful evergreen heart-shaped leaves, and magnificent “spider-like” flowers, Epimedium plants are popular as garden plants for groundcover and ornamental decoration in Europe, North America and Japan (usually known as barrenworts) (Ma et al., 2011; Lone et al., 2018). Among Epimedium species, the leaves and rhizomes of Epimedium acuminatum Franch. were recorded as “HERBA EPIMEDII ACUMINATI” and “EPIMEDII RHIZOMA ET RADIX,” respectively, in the Quality standard of Ethnic Chinese medicinal materials in Guizhou province (GMP, 2003; GMP, 2019). Besides, its wide distribution in the southeastern part of China and attractive purplish red flowers have endowed it with great cultivation prospects, and therefore was selected for current study.
It was first in ancient Chinese materia medica “Shennong Bencao Jing (Sheng Nong’s herbal classic)” that Epimedium herbs were documented with main effects of treating sexual dysfunction, tonifying kidney Yang, bones and strength. Nowadays, modern pharmacological studies have verified a specific class of 8-prenylated flavonols in Epimedium plants as the major bioactive components (Epimedium flavonols), which exhibit extensive bioactivities of regulating hormone production, modulating immunological function, anti-rheumatic, anti-osteoporosis, anti-cancer, anti-aging, etc (Wu et al., 2003; Ma et al., 2011).
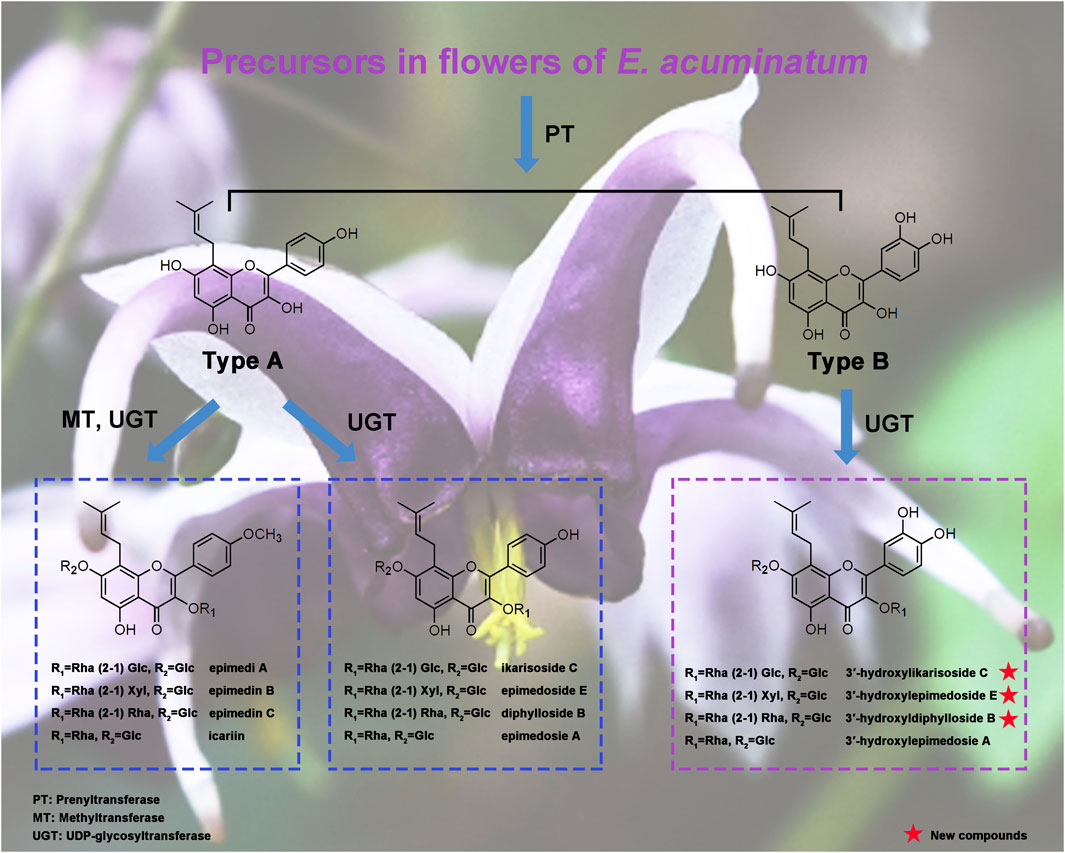
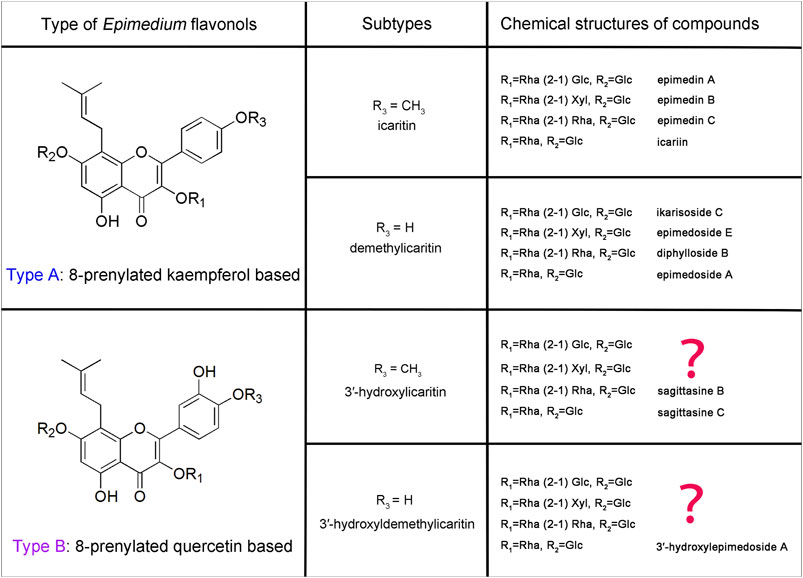
These Epimedium flavonols possess basic chemical structures with a prenyl group at C-8 position of ring A, and with the most common glycosylation pattern: a glucose at C-7, and a rhamnose initially at C-3 following the addition of glucose, xylose or rhamnose (1–2 linkage). However, the key difference is the presence or absence of hydroxyl group at C-3′ position of ring B. Depicted in Figure 1 are two key types of Epimedium flavonols, among which Type A Epimedium flavonols with the backbone of 8-prenylated kaempferol are widely found in Epimedium species, including subtypes of icaritin (4′-methoxyl) and demethylicaritin (4′-hydroxyl) (Jian et al., 2015; Zhou et al., 2021). By contrast, Type B of Epimedium flavonols with the backbone of 8-prenylated quercetin are rarely isolated or detected, including subtypes of 3′-hydroxylicaritin (4′-methoxyl) (Wang et al., 2007; Tu et al., 2011) and 3′-hydroxyldemethylicaritin (4′-hydroxyl) (Li et al., 2017), shown in Figure 1. Based on the common glycosylation pattern of Epimedium flavonols, it is rational to speculate the existence of a series of 8-prenylated quercetin glycosides in Epimedium species, and the bioactivities of which are worthy to be explored.
Until now, the leaves of Epimedium plants have been extensively examined and mainly found to produce Epimedium flavonols of icaritin subtype in Type A, and the rhizomes have been proven to principally accumulate Epimedium flavonols of demethylicaritin subtype in Type A (Jian et al., 2015; Zhou et al., 2021). By contrast, there has been few phytochemical researches focusing on flowers, leading to the limited understanding on the chemical constituents of Epimedium flowers (Yoshitama, 1984; Qin et al., 2022). Since flowers are taxonomically important organs and a potential source for medicinal use, the chemical constituents in different parts of E. acuminatum flowers (petals and inner sepals) were thoroughly explored by combining LC-MS and NMR data. Guided by a preliminary LC-MS profiling, three new 8-prenylated quercetin glycosides (subtype 3′-hydroxyldemethylicaritin in type B), one new anthocyanin and six known flavonols were isolated from flowers of E. acuminatum, structures of which were determined through 1D and 2D NMR, and HRESIMS. Based on our discoveries and literature survey, a revised classification for Epimedium flavonols was proposed. Finally, the testosterone production-promoting activities of all the new compounds were tested and compared with nine related Epimedium flavonols, and their structure-activity relationship (SAR) were analyzed, revealing that Epimedium flavonols of subtype demethylicaritin in Type A possessed the highest potency for stimulating the testosterone production, and either methylation at C-4′ position or hydroxylation at C-3′ position of ring B could decrease the bioactivity. Combining structural characterization and SAR data, our findings could provide fundamental rules for understanding structural variations in Epimedium flavonols, potential candidates for medicinal use, and assist the selection of substrates for identification of prenyltransferases (PTs), UDP-glycosyltransferases (UGTs) and methyltransferases (MTs) in Epimedium plants.
Results
LC-MS identification of compounds in petals and inner sepals of E. acuminatum
The ultraviolet-visible spectroscopy (UV-Vis spectra) showed the λmax at 520 nm for peaks 1, 3, 6, and 9 (Figure 2), which was very close to delphinidin. These peaks exhibited major fragment ions at m/z 303, indicating that they were delphinidin-derived anthocyanins. The UV-Vis spectra showed the λmax at 520 nm for peaks 2 and 5 (Figure 2), which was very close to petunidin. These compounds exhibited major fragment ions at m/z 317, indicating that they were petunidin-derived anthocyanins. The chemical structure of anthocyanins was tentatively identified by comparison with MS/MS data from authentic samples and references (Table 1). Peak 1 was identified as delphinidin-3,5-di-O-glucoside for producing molecular ions at m/z 627.1577 [M]+, and major fragment ions at m/z 465.1003 [M-Glc]+ and m/z 303.0490 [M-Glc-Glc]+. Peak 3 was identified as delphinidin-3-O-glucoside for giving molecular ions at m/z 465.1003 [M]+ with fragment ions of m/z 303.0490 [M-Glc]+, and was confirmed with authentic samples. Peaks 6 produced molecular ions at m/z 789.1882 [M]+ with only fragment ions at m/z 303.0490, indicating an unidentified delphinidin derivative, and was deduced to be delphinidin-3-O-caffeoyl-sophoroside. Peak 9 produced molecular ions at m/z 773.1935 [M]+, with only fragment ions at m/z 303.0490, indicating an unidentified delphinidin derivative (further isolated and determined with NMR data). Peak 2 was identified as petunidin-3,5-di-O-glucoside for showing molecular ions at m/z 641.1736 [M]+, and fragment ions at m/z 479.1200 [M-Glc]+ and m/z 317.1170 [M-Glc-Glc]+. Peak 5 was identified as petunidin-3-O-glucoside for giving molecular ions at m/z 479.1200 [M]+ and fragment ions at m/z 317.1170 [M-Glc]+.
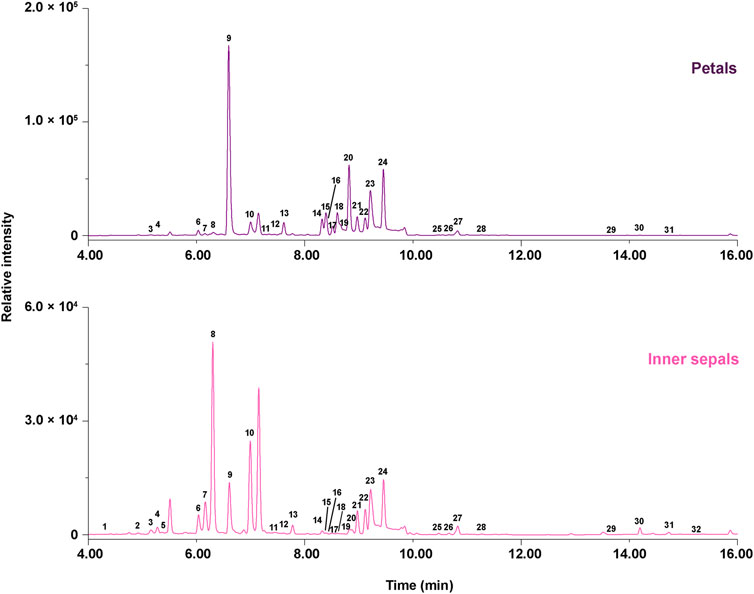
FIGURE 2. Ultra-high-performance liquid (UPLC) chromatograms at 280 nm for MeOH (0.1% acetic acid) extracts of petals and inner sepals of E. acuminatum. Upper panel: petals; Lower panel: inner sepals.

TABLE 1. Compounds identified in MeOH (0.1% acetic acid) extracts of petals and inner sepals from E. acuminatum.
The UV-Vis spectra showed the λmax at around 240−280 nm and 350−360 nm for peaks 11−32 (Figure 2), indicating that these peaks corresponded to flavonols. Non-prenylated flavonols constituted the first set of flavanols found in the petals and inner sepals of E. acuminatum: peak 11 was identified as myricetin-3-O-glucoside isomer with molecular ions at m/z 481.0978 [M+H]+ and fragment ions at m/z 319.0431 [M+H-Glc]+; peak 12 was identified as a quercetin-3-O-rhamnose isomer giving molecular ions at m/z 449.1130 [M+H]+ and fragment ions at m/z 303.0490 [M+H-Rha]+; peak 13 were identified as kaempferol-3-O-rhamnose isomer producing molecular ions at m/z 433.1120 and fragment ions at m/z 287.0540 [M+H-Rha]+. By comparison with reference standards, peak 14, 15, and 19 were identified as hyperoside, isoquercitrin and astragalin respectively, and their molecular and fragment ions were shown in Table 1.
Peaks 21−24 and 25−28 (Figure 2) constituted the second set of flavanols (Type A), producing fragment ions at m/z 355 and 369, which contain a backbone of 8-prenylated kaempferol. With fragment ions at m/z 355 (demethylicaritin), peak 21 was identified as ikarisoside C for giving molecular ions at m/z 825.2831 [M+H]+, and fragment ions at m/z 663.2256 [M+H-Glc]+ and m/z 517.1721 [M+H-Glc-Rha]+; similarly, peak 22, 23, and 24 were identified as epimedoside E, diphylloside B, and epimedoside A with molecular ions at m/z 795.2740 [M+H]+, m/z 809.2872 [M+H]+, and m/z 663.2256 [M+H]+ (Table 1). Furthermore, these compounds corresponding to peaks 21–24 were directly isolated and structurally confirmed with NMR data. With fragment ions at m/z 369 (icaritin), peak 25 was identified as epimedin A for producing molecular ions at m/z 839.3002 [M+H]+, m/z 677.2462 [M+H-Glc]+, m/z 531.1848 [M+H-Glc-Rha]+, and m/z 369.1346 [M+H-Rha-Rha-Glc]+; similarly, peak 26, 27 and 28 were identified as epimedin B, epimedin C and icariin, separately, and were confirmed with the authentic samples (Table 1).
Peaks 16−18, and 20 (Figure 2) constituted the third set of flavanols (Type B) with fragment ions at m/z 371, indicating a backbone of 8-prenylated quercetin (3′-hydroxyldemethylicaritin). Peaks 16−18, and 20 gave molecular ions at m/z 679.2239 [M+H-Glc]+ and fragment ions at m/z 533.1674 [M+H-Glc-Rha]+ (Table 1), and these compounds were directly isolated and structurally confirmed with NMR data (see Section 2.2 and Section 5.4).
Structural elucidation
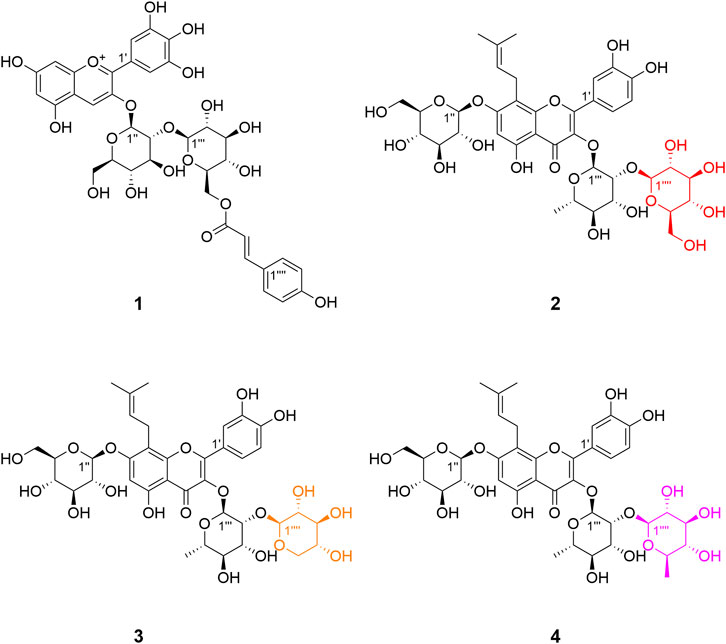
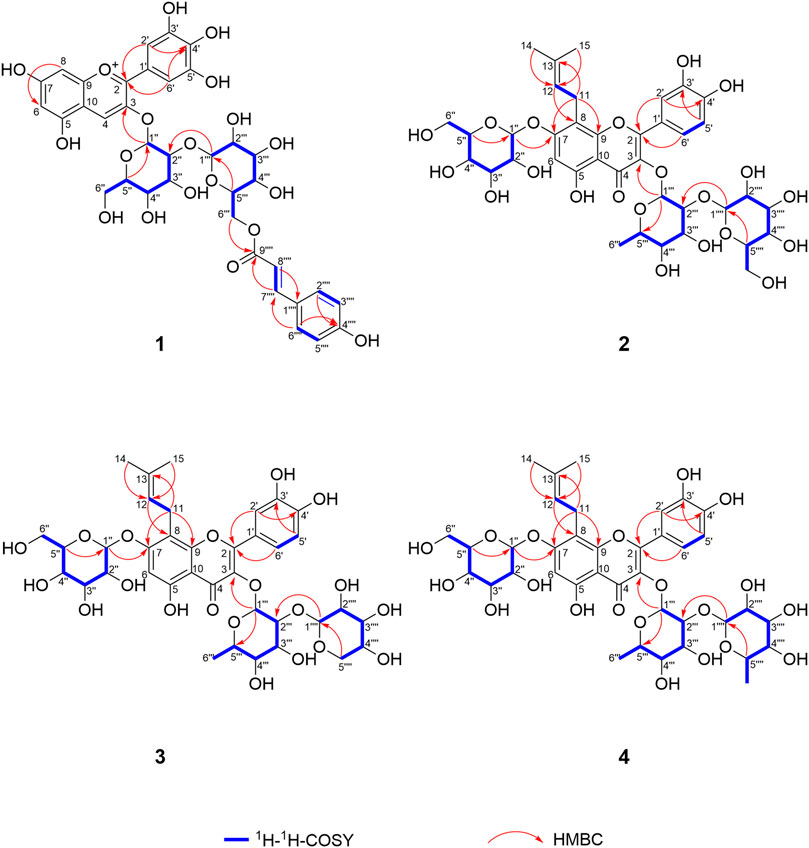
Delphinidin 3-O-p-coumaroyl-sophoroside (1, peak 9) was isolated as dark purplish red powder, the HRESIMS data (m/z 773.1940 [M]+, calcd for C36H37O19 + 773.1929) gave a molecular formula of C36H37O19+. The presence of major fragment ion at 303.0482 [M]+, in combination with the 1H NMR and 13C NMR data with characteristic proton chemical shifts shown in Table 2, demonstrated a skeleton of delphinidin. The presence of two sugar moieties was deduced from anomeric hydrogen signals at δH 4.80 (1H, d, J = 7.6 Hz) and δH 5.34 (1H, d, J = 7.5 Hz), and both were detected to be d-glucose from acid hydrolysis experiments. The β-configuration of glucose moieties was assumed by the observed coupling constants (J = 7.6 and 7.5 Hz) (Agrawal, 1992). The linkage of Glc A to C-3 of delphinidin, and the Glc A (2→1) Glc B was confirmed by HMBC correlations of H-1″ (δH 5.34) of Glc A and H-1‴ (δH 4.80) of Glc B with C-3 (δC 145.0) and C-2″ (δC 84.1) of Glc A, respectively. Additionally, the aromatic signals of the 1,4-substituted benzene ring (δH 6.72, 2H, d, J = 8.6 Hz, and δH 7.07, 2H, d, J = 8.6 Hz), and an ester group (δC 168.6), together with two olefinic protons signals exhibiting large coupling constants (δH 5.71, 1H, d, J = 16.0 Hz and δH 7.09 1H, d, J = 16.0 Hz) indicated a p-coumaric acid in trans-configuration, which was further confirmed with HMBC correlations from H-2‴′ (δH 7.07) to C-7‴′ (δC 146.3), and from H-7‴′ (δH 7.09) to C-9‴′ (δC 168.6). The acylation of Glc B with p-coumaric acid was deduced from HMBC correlations of methylene protons (δH 4.00, H-6a″ and 4.14, H-6b‴) with C-9‴′ (δC 168.6). Therefore, the structure of 1 was determined as shown (Figures 3, 4).
3′-hydroxylikarisoside C (2, peak 16) was obtained as yellow powder, HRESIMS data (m/z 841.2773 [M+H]+, calcd for C38H49O21 + 841.2766) gave a molecular formula of C38H48O21. The 1H NMR data (Table 3) displayed characteristic signals of an isopentane group at δH 1.61 (3H, s), 1.71 (3H, s), 5.18 (1H, m), 3.42 (1H, m), and 3.58 (1H, m). The presence of three sugar moieties was deduced from anomeric hydrogen signals at 4.99 (1H, d, J = 7.6 Hz), δH 4.24 (1H, d, J = 7.9 Hz), and 5.50 (1H, d, J = 1.6 Hz), which was further confirmed to be d-glucose and l-rhamnose from acid hydrolysis. The large coupling constant observed with the anomeric hydrogens (J = 7.9 and 7.6 Hz) indicated a β-configuration for glucose moieties, whereas the α-configuration of rhamnose was deduced from the chemical shifts of C-3‴ (δC 70.2) and C-5‴ (δC 70.5) (Agrawal, 1992) (Table 3). The NMR data of two were highly similar to those of ikarisoside C, except for the existence of a 1,3,4-trisubstuted benzene ring, deduced from the ABX coupling system at δH 7.32 (1H, dd, J = 8.3, 2.2 Hz), 6.89 (1H, d, J = 8.3 Hz), and 7.41 (1H, d, J = 2.2 Hz) in 2, rather than an AABB coupling system observed for ikarisoside C, revealing the presence of a hydroxyl group at C-3′ in 2. HMBC correlations of H-1‴ (δH 5.50) and H-1″ (δH 4.99) with C-3 (δC 134.5) and C-7 (δC 160.4), respectively, supported that the 8-prenylated quercetin was glycosylated with Rha at C-3 and Glc A at C-7. The Rha (2→1) Glc B linkage was confirmed with the HMBC correlation from H-1‴′ (δH 4.24) of Glc B to C-2‴ (δC 81.5) of Rha. Therefore, the structure of 2 was identified as shown (Figures 3, 4).
3′-hydroxylepimedoside E (3, peak 17) was isolated as yellow powder, HRESIMS data (m/z 811.2677 [M+H]+, calcd for C37H47O20 + 811.2661) gave a molecular formula of C37H46O20. The NMR data were similar to those of epimedoside E, except for an ABX coupling system at δH 7.32 (1H, dd, J = 8.4, 2.2 Hz), 6.89 (1H, d, J = 8.4 Hz) and 7.41 (1H, d, J = 2.2 Hz) in 3, instead of a AABB coupling system in epimedoside E, supporting the existence of a hydroxyl group at C-3′ in 3. The three sugar moieties were confirmed to be d-glucose, l-rhamnose and d-xylose from acid hydrolysis. The large coupling constant observed with the anomeric hydrogen (J = 6.7 Hz) indicated a β-configuration for the glucose moiety, whereas the α-configuration for rhamnose and β-configuration for xylose was deduced from chemical shifts of C-3‴ (δC 70.3) and C-5‴ (δC 70.4) for rhamnose, and C-3‴′ (δC 76.2) and C-5‴′ (δC 65.7) for xylose, respectively (Agrawal, 1992) (Table 3). HMBC correlations of H-1‴ (δH 5.32) and H-1″ (δH 4.98) with C-3 (δC 134.2) and C-7 (δC 160.4), respectively, supported that the 8-prenylated quercetin was glycosylated with Rha at C-3 and Glc at C-7. The Rha (2→1) Xyl linkage was confirmed with the HMBC correlation from H-1‴′ (δH 4.16) of Xyl to C-2‴ (δC 80.1) of Rha. Therefore, the structure of 3 was elucidated as shown (Figures 3, 4).
3′-hydroxyldiphylloside B (4, peak 18) was obtained as yellow powder, HRESIMS data (m/z 825.2832 [M+H]+, calcd for C38H49O20 + 825.2817) gave a molecular formula of C38H48O20. The NMR data were similar to those of diphylloside B, except for an ABX coupling system at δH 7.31 (1H, dd, J = 8.4, 2.2 Hz), 6.87 (1H, d, J = 8.4 Hz) and 7.36 (1H, d, J = 2.2 Hz) in 4, instead of a AABB coupling system in diphylloside B, supporting the existence of a hydroxyl group at C-3′ in 4. The presence of three sugar moieties was deduced from anomeric hydrogen signals at δH 4.99 (1H, d, J = 7.7 Hz), 4.88 (1H, d, J = 1.7 Hz), and 5.39 (1H, d, J = 1.6 Hz), which was further confirmed to be d-glucose and l-rhamnose from acid hydrolysis. The large coupling constant observed with the anomeric hydrogen (J = 7.7 Hz) indicated a β-configuration for glucose moiety, whereas the α-configuration of rhamnose moieties was deduced from the chemical shifts of C-3‴ (δC 70.3) and C-5‴ (δC 70.7) of Rha A, and C-3‴′ (δC 70.5) and C-5‴′ (δC 68.8) of Rha B (Agrawal, 1992) (Table 3). HMBC correlations of H-1‴ (δH 5.39) and H-1″ (δH 4.99) with C-3 (δC 134.1) and C-7 (δC 160.5), respectively, supported that the 8-prenylated quercetin was glycosylated with Rha A at C-3 and Glc at C-7. The Rha A (2→1) Rha B linkage was confirmed from the HMBC correlation from H-1‴′ (δH 4.88) of Rha B to C-2‴ (δC 75.7) of Rha A. Therefore, the structure of 4 was identified as shown (Figures 3, 4).
Effects of different Epimedium flavonols on testosterone production in rat leydig cells
Epimedium flavonols was known to enhance the sexual abilities, and it has been widely reported that testosterone plays an important modulatory role in the control of sexual activities. Therefore, the new compounds 1–4 and other nine structurally related Epimedium flavonols were evaluated for their testosterone production-promoting activities in rat primary Leydig cells with forskolin as a positive control (Table 4), and the corresponding peaks in the LC-MS analysis of endogenous extract were labelled in Table 4. Cell viability was analyzed through MTT assay at a dosage of 5 μmol L−1, 1 μmol L−1 and 0.2 μmol L−1, and at the treatment conditions, the culture cells was all more than 90% of viability (Figure 5). At a dosage of 5 μmol L−1, all the tested compounds significantly increased the testosterone production over the untreated condition (1.12−1.56 fold) (Figure 5). The testosterone production-promoting effects of different types of Epimedium flavonols was in the order: subtype demethylicaritin in Type A (1.29−1.56 fold) > subtype 3′-hydroxyldemethylicaritin in type B (1.15−1.31 fold), including the compounds 2−4 > subtype icaritin in Type A (1.12−1.18 fold). Significant difference (p = 0.015) was found between subtype demethylicaritin in Type A and subtype icaritin in Type A, but was not detected between subtype demethylicaritin in Type A and subtype 3′-hydroxyldemethylicaritin in Type B (p = 0.132), and between subtype 3′-hydroxyldemethylicaritin in Type B and subtype icaritin in Type A (p = 0.067), see Figure 5.
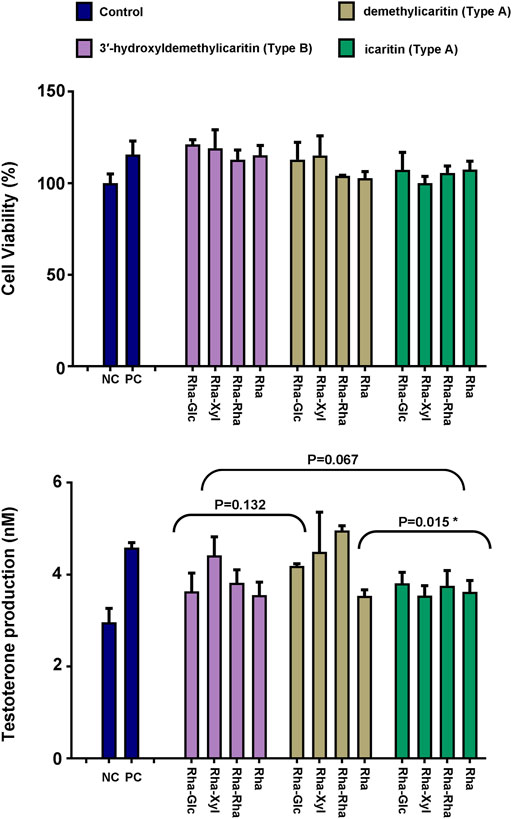
FIGURE 5. Effect of 12 different key Epimedium flavonols on testosterone production in rat Leydig cells. Cell viability was analyzed by MTT assay. Results were depicted as mean +/− SD, *p < 0.05.
Discussion
Classification and distribution pattern of Epimedium flavonols
In our LC-MS analyses conducted separately on petals and inner sepals from flowers of E. acuminatum, a total of 32 compounds were identified, including 22 flavonols, six anthocyanins, three phenolic acids and one alkaloid. Among the 22 flavonols, 12 were found to be Type A Epimedium flavonols, four were identified as Type B Epimedium flavonols, and the remaining six were identified as non-prenylated flavonols. For Type A Epimedium flavonols, both of the subtypes (icaritin and demethylicaritin) were detected, whereas only flavonols of 3′-hydroxyldemethylicaritin subtype was observed for Type B. The petals and inner sepals of E. acuminatum were highly similar in chemical constituents, except that petunidin derivates and delphinidin-3,5-di-O-glucoside were only present in the inner sepals. The content of Epimedium flavonols in the petals was higher than that in the inner sepals. The LC-MS profiling led to the isolation of one new anthocyanin (1), three new 8-prenylated quercetin glycosides (Type B) (2−4), and six known flavonols (5−10) from the flowers of E. acuminatum.
The new 8-prenylated quercetin glycosides (2−4), in combination with the previous discovered 3′-hydroxylepimedoside A (Li et al., 2017), exhibited common patterns of glycosylation, indicating that 8-prenylated quercetin glycosides should also be a key form of Epimedium flavonols, which has not been previously elucidated. Therefore, we proposed that the key classification of 8-prenylated flavonols in Epimedium plants (Figure 1) should be based on the initial backbone of kampferol (Type A) or quercetin (Type B). In Epimedium plants, Type A Epimedium flavonols were distributed more extensively than Type B. Subsequently, Type A and Type B Epimedium flavonols could be further divided into two subtypes based on the presence or absence of methylation on C-4′ of ring B (Figure 1). Clearly, the new 8-prenylated quercetin glycosides (2−4) isolated in our study completed the Epimedium flavonols of subtype 3′-hydroxyldemethylicaritin in Type B, and Epimedium flavonols of subtype 3′-hydroxylicaritin in Type B still remains incomplete. Besides, for plant flavonoids, it is common for methylation to occur on the C-3′ position of ring B (eg. isorhamnetin, syringetin, laricitrin, petunidin) (Koirala et al., 2016). However, for Epimedium flavonols, it seems a lot easier for methylation to occur on the C-4′ position rather than the C-3′ position of ring B, suggesting the uniqueness of O-methyltransferases in Epimedium plants. Additionally, previous studies had deduced dihydroicaritin derivates in Epimedium species (Yu et al., 2016; Zhou et al., 2021) from LC-MS data, but their fragmentation patterns and retention time were identical with the new 8-prenylated quercetin glycosides isolated in this study, suggesting that the dihydroicaritin derivates reported in previous LC-MS studies of Epimedium should be corrected to the 3′-hydroxyldemethylicaritin derivates reported here. This correction could further be correlated with our literature survey, in which dihydroicaritin derivates has never been isolated from Epimedium species.
The relative content of the 12 key Epimedium flavonols was measured and found to be different in petals, inner sepals, rhizomes, and leaves of E. acuminatum (Figure 6; Table 5). In general, the relative content of Type A Epimedium flavonols was higher than Type B. The relative content of Epimedium flavonols of subtype icaritin in Type A (4′-methoxyl) in different tissues was in the order: Leaves > rhizomes >> petals > inner sepals, whereas the highest content of subtype demethylicaritin in Type A (4′-hydroxyl) was found in rhizomes, followed by petals, inner sepals, and then leaves. Epimedium flavonols of subtype 3′-hydroxyldemethylicaritin in Type B (4′-hydroxyl) was highest in petals, and only trace amount was detected in inner sepals, rhizomes, and leaves, which should be reason why this subtype of Epimedium flavonols has remained elusive for long. On the whole, the different accumulation pattern of hydroxylated and methylated Epimedium flavonols in different tissues should indicate different biological functions in the corresponding tissues, which are worthy to be investigated. According to studies on biological functions of hydroxylated and methylated flavonoids in plants, hydroxylated flavonoids might contribute to the formation of colors and tastes of flowers which could facilitates pollination, whereas methylation improves chemical stability and membrane permeability, and maybe also the toxicity of flavonoids, which might help to resist microorganism and herbivores (Lahtinen et al., 2004; Alseekh et al., 2020).
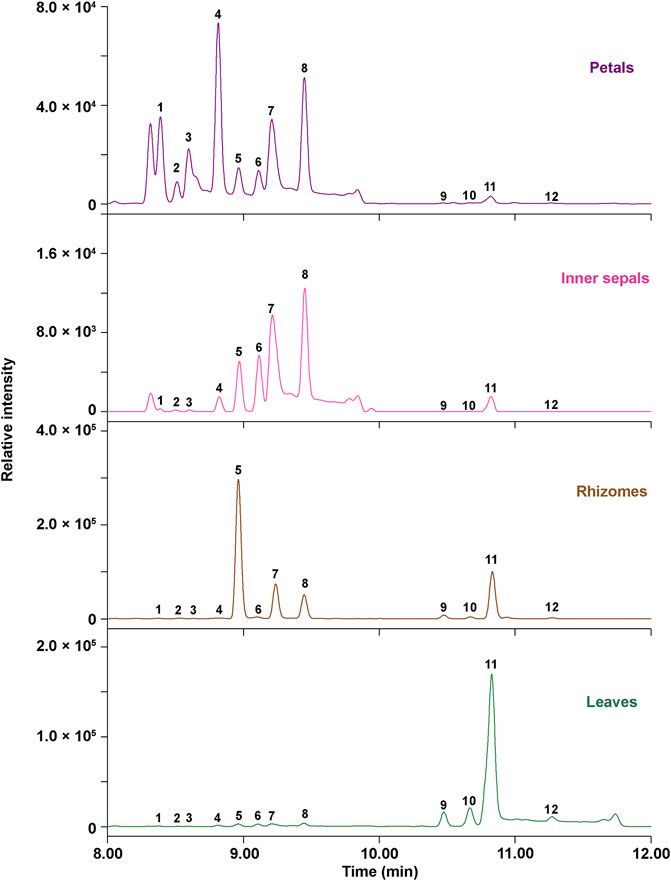
FIGURE 6. Ultra-high-performance liquid (UPLC) chromatograms at 360 nm for relative quantification of 12 key Epimedium flavonols in different tissues of E. acuminatum.
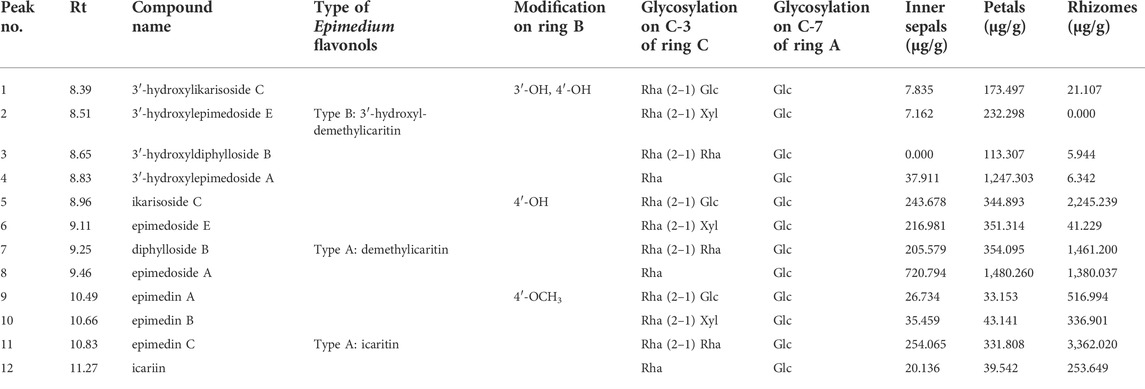
TABLE 5. Relative quantification of 12 key Epimedium flavonols in the MeOH (containing 0.1% acetic acid) extract of petals, inner sepals, rhizomes, and leaves from E. acuminatum.
Different types of Epimedium flavonols exhibit different testosterone production-promoting activities
To investigate the SAR of different types of Epimedium flavonols on testosterone production-promoting activities, the new compounds (1−4) and nine structurally related compounds were tested, which could be divided into three major types of Epimedium flavonols based on their chemical structures except for the new compound 1 (Table 4). As a result, all the tested Epimedium flavonols and the new compound 1 were found to increase testosterone production over the control. Furthermore, most of the compounds did not significantly affect the cell proliferation except for the new compound 1, whose impact on the increase of testosterone production may be partly due to the enhancement of cell proliferation. Based on the bioactivities and chemical structures of tested compounds, it can be concluded that either methylation at C-4′ position or hydroxylation at C-3′ position of ring B could decrease the biological activity (Figure 5; Table 4). In addition, the comparison among compounds within each subtypes suggested a trend that the addition of sugar moieties to the C-3 position of ring C would increase the biological activity, which needs to be further investigated.
Conclusion
Phytochemical investigation on petals and inner sepals from flowers of E. acuminatum was first carried out with LC-MS analysis, leading to identification of 32 compounds. Subsequently, the LC-MS profiling guided the discovery of three new 8-prenylated quercetin glycosides, one new anthocyanin and six known compounds. In combination with previous phytochemical studies on Epimedium plants, we proposed Epimedium flavonols to be classified into Type A (8-prenylated kaempferol based), including icaritin and demethylicaritin subtypes, and Type B (8-prenylated quercetin based), including 3′-hydroxylicaritin and 3′-hydroxyldemethylicaritin subtypes. The SAR study was conducted by comparing testosterone production-promoting activities of new compounds with nine related Epimedium flavonols, suggesting that either methylation at C-4′ position or hydroxylation at C-3′ position of ring B could decrease the biological activity.
Methods and material
Plant materials
Plants were grown in the Germplasm Repository (Guizhou province, China) at latitude 32.06 and longitude 131.31. Fresh petals, inner sepals, leaves, and rhizomes of E. acuminatum were collected, and quick-frozen in liquid nitrogen in the spring of 2021. The samples were later freeze-dried and stored at −20°C until extraction. Voucher specimens were deposited at the Herbarium of the Institute of Medicinal Plant Development, Beijing, China (IMPLAD), and were identified by Professor Baolin Guo (IMPLAD).
General experimental procedures
High-resolution mass spectrometry was conducted on a Waters Acquity UPLC I-Class Plus-Xevo G2 XS Q-ToF (Waters Corporation, Milford, United States), equipped with an ESI source. The separation was carried out with a RP-C18 column (150 mm × 3.0 mm) with particle size of 1.8 µm (Agilent Technologies, Santa Clara, United States). The NMR spectra were measured on a Bruker Avance III 400 MHz and a 500 MHz spectrometer (Bruker, Rheinstetten, Germany), referenced to the solvent signals of CD3OD and DMSO-d6. Semipreparative HPLC was conducted on a Lumtech K-501 equipped with a K-2501 UV detector. A 250 mm × 50 mm, 10 μm, ODS column and a 250 mm × 10 mm, 5 μm, ODS-A column (YMC, Kyoto, Japan) were applied for obtaining desired fractions and further purification, respectively. Macroporous adsorption resin D101 (Meilun biotechnology, Dalian, China), and Sephadex LH-20 (40−70 μm) (Pharmacia Biotech AB, Uppsala, Sweden) were used for column chromatography. Chromatographic grade methanol and acetonitrile (Thermo Fisher Scientific, Waltham, United States) were used for preparative HPLC. Analytical grade methanol and acetic acid (Tianjin SaiFuRui Technology, Tianjin, China) was used for extraction and column chromatography. Reference standards including delphinindin-3,5-di-O-glucoside, delphinidin-3-O-glucoside, petunidin-3-O-glucoside, neochlorogenic acid, chlorogenic acid, and astragalin were purchased from Sigma-Aldrich Co., Ltd. (Burlington, United States), and reference standards of hyperoside, isoquercitrin, astragalin, magnoline, epimedins A−C, icariin and 2″-O-rhamnosylicariside II were purchased from Chengdu PUSH-biotechnology Co., Ltd. (Chengdu, China). Other reference standards used in this study were isolated and their structures were confirmed through a comparison of NMR and MS data with the literature.
LC-ESI/Q-TOF/MS analyses
The samples of petals, inner sepals, leaves and rhizomes from E. acuminatum, separately, were extracted with MeOH (containing 0.1% acetic acid) at 4°C and were centrifuged for 10 min at 10,000 rpm. The supernatants were collected, dried with vaccum centrifuge concentrator (CV100-DNA, Aijimu, Beijing, China), and stored at −20°C until analysis. The dried extracts were re-dissolved in MeOH right before analysis and were examined on a liquid chromatography quadrupole time-of-flight mass spectrometer, Q-TOF-MS (Waters Xevo G2-XS QTOF) (Waters Corporation, Milford, United States) with an ESI source consisting of ACQUITY UPLC I-Class instrument (Waters Corporation, Milford, United States). The separation was carried out with a RP-C18 column (150 mm × 3.0 mm, 1.8 µm) at 40°C. The elution program consisted 0.1% formic acid (A) and acetonitrile (B) as the mobile phases, and a gradient elution profile was applied (flow rate of 0.4 ml min−1): 0−1 min (5% B), 1−8 min (5%–30% B), 8−12 min (30%–40% B), 12−16 min (40%–95% B), 16−17 min (95%–100% B), 17−21 min (100% B), 21−22 min (100% B−5% B), 22−25 min (5% B). The mass spectrometer operated in positive ion mode. ESI source parameters were as follows: capillary voltages 0.5 kV; desolvation gas flow 1000 L Hr−1 (N2). MS spectra were obtained over the range of m/z 100−1,200.
The compounds in sample extracts were identified by comparing with the retention times, characteristics of UV-Vis spectra of peaks and the mass spectrometric information of reference standards using software MassLynx v4.1. The relative content of 12 key Epimedium flavonols was calculated from peak areas of samples based on the intensity of the corresponding standard compounds.
Extraction and isolation
A mixture of dried petals and inner sepals of E. acuminatum (100 g) were extracted in ultrasonic bath (1 h) for three times with 80% MeOH (containing 1% acetic acid) at room temperature in darkness. The mixture was filtered through a Buchner funnel and the pooled filtrates were concentrated under reduced pressure at 45°C. The extract was then partitioned against petroleum ether, and concentrated to obtain the crude anthocyanin-flavonol containing MeOH-aqueous extract.
The crude extract (82.0 g) was firstly passed through a macroporous adsorption resin D101 (un-polarity) with distilled water to remove most of the polysaccharides, and then the solvent was changed to 50% EtOH to elute anthocyanins and desired flavonols. The 50% EtOH residue (5.93 g) was first separated by semipreparative HPLC with a 250 mm × 50 mm, 10 μm, ODS column using solvents CH3CN (A) and 0.5% acetic acid (B). The elution profile consisted of 15% A for 25 min, and 20% A for 30 min to obtain three desired fractions (Fr.1−3).
Fr.1 (182.5 mg) was further separated by passing through Sephadex LH-20 (eluted with 40% MeOH) to yield compound 1 (105.0 mg).
Fr.2 (176.6 mg) was first separated by passing through Sephadex LH-20 with MeOH to obtain three subfractions (Fr.2A−2C). Fr.2A (53.1 mg) was further purified by semipreparative HPLC with a 250 mm × 10 mm, 5 μm, ODS-A column (YMC, Kyoto, Japan) using solvents CH3CN (A) and 0.025% formic acid (B). The elution profile consisted of a linear gradient from 17% to 21% A in 20 min, isocratic elution for the next 30 min, followed by 100% A for 15 min at a flow rate of 2.0 ml/min to afford compound 2 (9.0 mg, tR = 40.6 min), compound 3 (8.6 mg, tR = 43.3 min) and compound 4 (8.0 mg, tR = 46.6 min).
Fr.2B (61.3 mg) and 20.5 mg of Fr.2C (50.8 mg) was separated using the same elution profile as Fr.2A, yielding compound 5 (22.5 mg, tR = 48.9 min) and 6 (8.0 mg, tR = 38.2 min), respectively. 80.9 mg of Fr.3 (243.2 mg) was subjected to semipreparative HPLC with a 250 mm × 10 mm, 5 μm, ODS-A column (YMC, Kyoto, Japan) using solvents [CH3CN: 0.025% formic acid, (23:77, v/v)] at 2.0 ml/min to provide compound 7 (6.2 mg, tR = 35.4 min), 8 (6.3 mg, tR = 38.1 min), 9 (5.6 mg, tR = 41.6 min), and 10 (17.6 mg, tR = 43.5 min).
Delphinidin-3-O-p-coumaroyl-sophoroside (1): dark purplish red powder. HRESIMS m/z 773.1924 [M]+ (calcd for C36H37O19 + 773.1929). 1H and 13C NMR (CD3OD-DCl) data, see Table 2.
3′-hydroxylikarisoside C (2): yellow powder. HRESIMS m/z 841.2773 [M+H]+ (calcd for C38H49O21 841.2766). 1H and 13C NMR (DMSO-d6) data, see Table 3.
3′-hydroxylepimedoside E (3): yellow powder. HRESIMS m/z 811.2742 [M+H]+ (calcd for C38H49O21 811.2661). 1H and 13C NMR (DMSO-d6) data, see Table 3.
3′-hydroxyldiphylloside B (4): yellow powder. HRESIMS m/z 825.2897 [M+H]+ (calcd for C38H49O21 825.2817). 1H and 13C NMR (DMSO-d6) data, see Table 3.
Six known flavonols were characterized as 3′-hydroxyl-epimedoside A (5) (Li et al., 2017), hyperoside (6) (Liu et al., 2015), ikarisoside C (7) (Fukai & Nomura, 1988), epimedoside E (8) (Mizuno et al., 1991), diphylloside B (9) (Mizuno et al., 1988), and epimedoside A (10) (Xu, 1982) through the comparison of NMR and MS data with the literature.
Acid hydrolysis
Standard sugars and compounds 1–4 (each 1.0 mg) were dissolved in 6 mol L−1 CF3COOH (1 ml) and heated at 90°C for 2 h and cooled to room temperature. The hydrolysate was extracted with CHCl3 for three times, and the aqueous layer was concentrated to obtain the residue containing sugars. The obtained residues were dissolved in pyridine (200 μl), and L-cysteine methyl ester hydrochloride (1 mg) was added and heated at 60°C for 1 h. Subsequently, o-tolyl isothiocyanate (10 μl) was added and heated at 60°C for another 1 h (Mitaine-Offer et al., 2010). After the reaction, the supernatants were filtrated and subjected to UPLC analysis (Vanquish Flex UHPLC system equipped with CAD detector) (Thermo Fisher Scientific, Waltham, United States) using a 100 mm × 2.1 mm, 1.8 μm, HSS T3 column (Waters Corporation, Milford, United States). The elution program consisted of a linear gradient of CH3CN in water (containing 0.1% formic acid, v/v) from 20% to 30% for 8 min (flow rate: 0.6 ml/min). The atomization temperature and wave filtering time were set at 35°C and 1 s, respectively. For compound 2 and 4, derivatives of l-rhamnose and d-glucose were detected. However, for compound 3, d-xylose was also observed. For compound 1, only derivative of d-glucose was detected [tR 4.43 min for d-glucose, tR 6.31 min for l-rhamnose, and tR 4.79 for d-xylose].
Cellular viability and testosterone assays
The rat primary Leydig cells were prepared and cultured as previously described with modifications (Sharma et al., 2006; Chang et al., 2011). Briefly, these cells were initially plated at a density of 1 × 106/ml with 2 ml culture medium in 6-well plates at the conditions: 37°C with 5% CO2. During the period of cell culture, the morphological changes of cells were monitored with an inverted microscope (Nikon Eclipse Ti2, Japan). To measure the purity of rat primary Leydig cells, 3β-HSD staining method was used (Chiao et al., 2002). For cellular viability assays, tested rat primary Leydig cells were plated at a density of 8,000 live cells per well in 96-well culture plates, which were placed in a 37°C, 5% CO2 incubator for 24 h before the activity assay. Before the assay, all the wells in culture plates were replaced with fresh 100 μl of culture medium, and the cells were treated with different tested compounds in a 37°C, 5% CO2 incubator for 72 h.
The cellular viability was evaluated using the MTT proliferation assay. In the MTT proliferation assay, the positive control samples were set with the treatment of forskolin (5 μmol L−1, 1 μmol L−1, and 0.2 μmol L−1), and the negative control samples were set without any compounds. For each experimental group, three replicates were set. During the assay, the diluted MTT solution was added to each well and incubated for 4 h. The supernatant was discarded, 100 μl of DMSO was added to each well, and the absorbance was measured at 570 nm by a microplate reader (Synergy HT, BioTek Instruments, Vermont, United States). Testosterone secreted into the culture medium was measured using testosterone ELISA kits according to the manufacturer’s instructions (Nanjing Jiancheng Biological Technology, Nanjing, China).
Statistical analysis
Statistical analysis of the measurement of testosterone was performed via a two-tailed student’s t-test with two-sample equal variance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Hubei Experimental Animal Research Center.
Author contributions
BG, HZ, and GS: conceptualization, investigation, reviewing of the paper. YZ: investigation, experiments, data analysis, writing of the original draft. ZL, CZ, ZX, EL, GL, JL, and BM: experiments, data analysis, reviewing of the paper. CZ, CX, and YW: investigation. All authors contributed to the article and approved the submitted manuscript version.
Funding
This work was financially supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-031).
Acknowledgments
We are grateful to Yanjiao Luo, Yue Zhang, Xiang Liu, Zhongbao Feng, and Xincun Wang for help in sample collection, and we are also grateful to Zhengbing Gu, Yisheng Chang, Mingwei Ma, Yanduo Wang, Qi Li, Yue Tan, Peng Liu, and Tian Qin for help in experiments and data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1014110/full#supplementary-material
References
Agrawal, P. K. (1992). NMR Spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 31, 3307–3330. doi:10.1016/0031-9422(92)83678-R
Alseekh, S., Perez de Souza, L. P., Benina, M., and Fernie, A. R. (2020). The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 174, 112347. doi:10.1016/j.phytochem.2020.112347
Chang, Y. F., Lee-Chang, J. S., Panneerdoss, S., MacLean, J. A., and Rao, M. K. (2011). Isolation of Sertoli, Leydig, and spermatogenic cells from the mouse testis. Biotechniques 51, 341–344. doi:10.2144/000113764
Chiao, Y. C., Cho, W. L., and Wang, P. S. (2002). Inhibition of testosterone production by propylthiouracil in rat Leydig Cells1. Biol. Reprod. 67, 416–422. doi:10.1095/biolreprod67.2.416
Chinese Pharmacopoeia Commission (2020). Chinese pharmacopoeia, 2020 ed, Part I. Beijing: China Medical Science and Technology Press.
Fukai, T., and Nomura, T. (1988). Seven prenylated flavonol glycosides from two Epimedium species. Phytochemistry 27, 259–266. doi:10.1016/0031-9422(88)80627-7
Guizhou Medical Products Administration, (2003). Quality standard of Ethnic Chinese medicinal materials in Guizhou province, 2003. Guiyang: Guizhou Technology Press.
Guizhou Medical Products Administration, (2019). Quality standard of Ethnic Chinese medicinal materials in Guizhou province, 2019. Beijing: China Medical Science Press.
Jian, Z. Y., Xu, G. F., Chen, H. Z., Wang, H. S., and Hu, X. Q. (2015). Study on the differences of major pharmaceutical ingredients in different parts and processed medicinal material of Epimedium Brevicornu Maxim. in Taihang mountain. Nutr. Hosp. 32, 913–917. doi:10.3305/nh.2015.32.2.8927
Koirala, N., Thuan, T., Ghimire, G. P., Thang, T., and Sohng, J. K. (2016). Methylation of flavonoids: Chemical structures, bioactivities, progress and perspectives for biotechnological production. Enzyme Microb. Technol. 86, 103–116. doi:10.1016/j.enzmictec.2016.02.003
Lahtinen, M., Salminen, J. P., Kapari, L., Lempa, K., Ossipov, V., Sinkkonen, J., et al. fnm (2004). Defensive effect of surface flavonoid aglycones of Betula pubescens leaves against first instar Epirrita autumnata larvae. J. Chem. Ecol. 30, 2257–2268. doi:10.1023/B:JOEC.0000048787.34388.dd
Li, F., Du, B. W., Lu, D. F., Wu, W. X., Wongkrajang, K., L, W., et al. (2017). Flavonoid glycosides isolated from Epimedium brevicornum and their estrogen biosynthesis-promoting effects. Sci. Rep. 7, 7760. doi:10.1038/s41598-017-08203-7
Liu, D. Y., Shi, X. F., Wang, Z. Y., Wang, X. D., Ma, Q. H., Fan, B., et al. (2015). Anticancer constituents of ethyl acetate extract from Euphorbia helioscopia. Chem. Nat. Compd. 51, 969–971. doi:10.1007/s10600-015-1465-7
Lone, S. A., Gupta, A. P., Manzoor, M. M., Goyal, P., Hassan, Q. P., and Gupta, S. (2018). “Epimedium elatum (morr & decne): A therapeutic medicinal plant from northwestern himalayas of India,” in Plant and human health, volume 1, Ethnobotany and Physiology. Editor M. Ozturk Switzerland: Springer Nature, 619–656. doi:10.1007/978-3-319-93997-1_17
Ma, H. P., He, X. R., Yang, Y., Li, M. X., Hao, D. J., and Jia, Z. P. (2011). The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 134, 519–541. doi:10.1016/j.jep.2011.01.001
Mitaine-Offer, A. C., Penez, N., Miyamoto, T., Delaude, C., Mirjolet, J. F., Duchamp, O., et al. (2010). Acylated triterpene saponins from the roots of Securidaca longepedunculata. Phytochemistry 71, 90–94. doi:10.1016/j.phytochem.2009.09.022
Mizuno, M., Iinuma, M., Tanaka, T., Sakakibara, N., Fujikawa, T., Hanioka, S., et al. (1988). Flavonol glycosides in the roots of Epimedium diphyllum. Phytochemistry 27, 3645–3647. doi:10.1016/0031-9422(88)80784-2
Mizuno, M., Kanie, Y., Iinuma, M., Tanaka, T., and Lang, F. A. (1991). Two flavonol glycosides, hexandrasides C and D from the underground parts of Vancouveria Hexandra. Phytochemistry 30, 2765–2768. doi:10.1016/0031-9422(91)85141-L
Qin, W. H., Yang, Y., Wang, Y. H., Zhang, X. M., and Liu, X. (2022). Transcriptomic and metabolomic analysis reveals the difference between large and small flower taxa of Herba Epimedii during flavonoid accumulation. Sci. Rep. 12, 2762. doi:10.1038/s41598-022-06761-z
Sharma, R. S., Pal, P. C., and Rajalakshmi, M. (2006). Isolation and culture of Leydig cells from adult rats. Indian J. Clin. biochem. 21, 27–33. doi:10.1007/BF02913063
Stearn, W. T. (2002). The genus Epimedium and other herbaceous Berberidaceae. Portland, Oregon: Timber Press.
Tu, F. J., Dai, Y., Yao, Z. H., Wang, X. L., Yao, X. S., and Qin, L. (2011). Flavonol glycosides from Epimedium pubescens. Chem. Pharm. Bull. 59, 1317–1321. doi:10.1248/cpb.59.1317
Wang, G. J., Tsai, T. H., and Lin, L. C. (2007). Prenylflavonol, acylated flavonol glycosides and related compounds from Epimedium sagittatum. Phytochemistry 68, 2455–2464. doi:10.1016/j.phytochem.2007.05.035
Wu, H., Lien, E. J., and Lien, L. L. (2003). Chemical and pharmacological investigations of Epimedium species. Prog. Drug Res. 60, 1–57. doi:10.1007/978-3-0348-8012-1_1
Xu, S. (1982). Isolation and identification of icariin and epimedoside A. Chin. Tradit. Herb. Drugs 13, 9–11.
Ying, T. S. (2002). Petal evolution and distribution patterns of Epimedium L. (Berberidaceae). Acta Phytotaxon. Sin. 6, 481–489. doi:10.1088/1009-1963/11/5/313
Yoshitama, K. (1984)., 97. Tokyo, 429–435. doi:10.1007/BF02489575Anthocyanins and their distribution in the genus EpimediumBot. Mag.
Yu, X. E., Qin, J. P., Li, J. C., Huang, W. Z., Wang, Z. Z., and Xiao, W. (2016). Rapid analysis on chemical constituents in Yinyanghuo Zonghuangtong capsule by UPLC/Q-TOF-MS/MS. China J. Chin. Mat. Med. 41, 4587–4597. doi:10.4268/cjcmm20162417
Keywords: Epimedium flavonols, phytochemical investigation, flowers, testosterone production, structure-activity relationship, Epimedium acuminatum
Citation: Zhang Y, Zhang C, Li Z, Zeng C, Xue Z, Li E, Li G, Li J, Shen G, Xu C, Wang Y, Ma B, Zhang H and Guo B (2022) New 8-prenylated quercetin glycosides from the flowers of Epimedium acuminatum and their testosterone production-promoting activities. Front. Chem. 10:1014110. doi: 10.3389/fchem.2022.1014110
Received: 08 August 2022; Accepted: 24 August 2022;
Published: 10 October 2022.
Edited by:
Zhengxi Hu, Huazhong University of Science and Technology, ChinaReviewed by:
Guangzhong Yang, South-Central University for Nationalities, ChinaBing You Yang, Heilongjiang University of Chinese Medicine, China
Copyright © 2022 Zhang, Zhang, Li, Zeng, Xue, Li, Li, Li, Shen, Xu, Wang, Ma, Zhang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Zhang, emhodWkxMkBpYmNhcy5hYy5jbg==; Baolin Guo, YmxndW9AaW1wbGFkLmFjLmNu
 Yixin Zhang
Yixin Zhang Cheng Zhang2
Cheng Zhang2 Zhen Xue
Zhen Xue Juan Li
Juan Li Guoan Shen
Guoan Shen Baiping Ma
Baiping Ma Hui Zhang
Hui Zhang