- Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, China
A transition-metal-free method for the construction of 3-substituted or 3,4-disubstituted quinolines from readily available N,N-dimethyl enaminones and o-aminobenzyl alcohols is reported. The direct oxidative cyclocondensation reaction tolerates broad functional groups, allowing the efficient synthesis of various quinolines in moderate to excellent yields. The reaction involves a C (sp3)-O bond cleavage and a C=N bind and a C=C bond formation during the oxidative cyclization process, and the mechanism was proposed.
Introduction
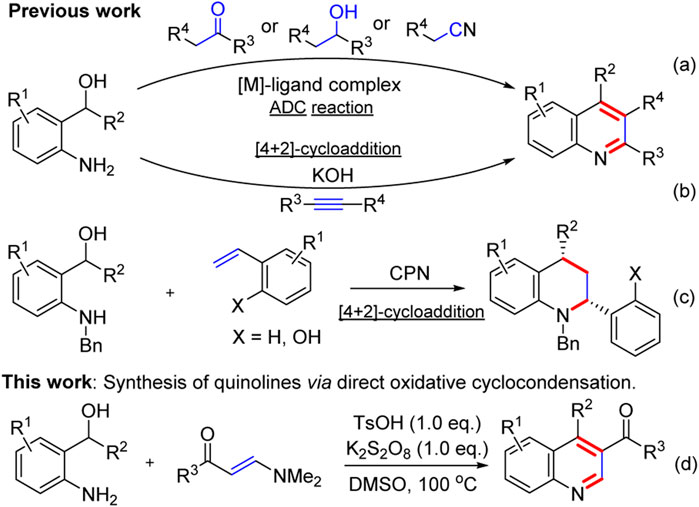
Quinolines represent an important class of heterocyclic compounds, which widely occur as a core structural motif in natural products (McCormick et al., 1996; Subbaraju et al., 2004; McCauley et al., 2020), pharmaceuticals (Gorka et al., 2013; Kokatla et al., 2013; Jentsch et al., 2018), functional materials (Tong et al., 2003; Kim et al., 2005; Zhang et al., 2014), organocatalysis or ligands (Biddle et al., 2007; Zhang and Sigman., 2007; Esteruelas et al., 2016), and valuable building blocks (Wan et al., 2016; Duan et al., 2018; Wang et al., 2019; Ankade et al., 2021). Due to their great importance, considerable efforts have been focused on the development of efficient synthetic methods to their structures and modifications over the past years. Classical methodologies (Bharate et al., 2015; Li et al., 2017; Harry et al., 2020), such as Camps, Combes, Conrad–Limpach, Doebner, Friedländer, Knorr, Pfitzinger, Pavorov, Skraup synthesis, and others, are known for the construction of quinoline rings; however, these reactions usually suffer from some limitations, such as harsh reaction conditions, tedious workup procedures, and special substrate designs (prefunctionalized anilines). Recently, many elegant strategies toward quinolone rings, such as using new building blocks (Jin et al., 2016; Tiwari et al., 2017; Wu et al., 2017; Trofimov et al., 2018) and multicomponent reactions (Chen et al., 2018; Wang et al., 2018; Zhao et al., 2019; Yang and Wan., 2020), have been developed to construct substituted quinolines. Despite these advances, the development of easy and efficient approaches for the construction of substituted quinolines remains to be explored.
Recently, o-aminobenzyl alcohols are versatile intermediates which have attracted increasing attention in organic synthesis owing to their high reactivity in the construction of N-heterocycles (Makarov et al., 2018; Wang et al., 2018; Xie et al., 2018; Yang and Gao., 2018), especially quinolines. In this regard, two strategies have been developed to construct the quinoline framework from o-aminobenzyl alcohols: acceptorless dehydrogenative coupling (ADC) reactions and [4 + 2]-cycloaddition reactions. The types of ADC reactions of o-aminobenzyl alcohols with ketones or secondary alcohols or nitriles to the construction of quinolines by the release of H2 and H2O as only by-products have been well-developed (Scheme 1). However, such attractive synthetic strategies required expensive transition-metal (TM) pincer complexes, such as Ir (Wang et al., 2016; Genc et al., 2019), Ru (Maji et al., 2018; Wan et al., 2019), Ni (Das et al., 2018; Das et al., 2018; Singh et al., 2018), Mn (Mastalir et al., 2016; Barman et al., 2018; Das et al., 2019), Cu (Tan et al., 2018), or Re (Wei et al., 2019) complexes. In addition, aza-ortho-quinone methides (aza-o-QMs), in situ generated from o-aminobenzyl alcohols as short-lived and highly reactive diene species, have been extensively investigated and applied in organic synthesis (Huang and Kang., 2017; Mei et al., 2017; Lee et al., 2019; Wang et al., 2021). In 2016, a KOH-promoted regioselective synthesis of quinolones via [4 + 2]-cycloaddition of aza-o-QMs with internal alkynes was disclosed by Verma and co-workers (Saunthwal et al., 2016) (Scheme 1b). In 2018, Shi and co-workers established chiral phosphoramide catalytic asymmetric [4 + 2]-cycloaddition of aza-o-QMs with o-hydroxystyrenes to afford chiral tetrahydroquinolines (Li et al., 2018) (Scheme 1c). This [4 + 2]-cycloaddition protocol enriched the partners of aza-o-QMs to construct quinolones. In spite of these powerful works, there is still a demand for new protocols for generation of quinolines from o-aminobenzyl alcohols. As our ongoing interest in quinoline synthesis (Lu et al., 2017; Zhou et al., 2018) and enaminone chemistry (Yu et al., 2011; Yu et al., 2013; Xu et al., 2016; Zhou et al., 2017; Fu et al., 2020; Chen et al., 2021; Huang and Yu., 2021; Yu et al., 2021; Zhang et al., 2021; Fu et al., 2022; Liu et al., 2022; Ying et al., 2022), herein, we report a transition-metal-free direct oxidative cyclocondensation strategy of o-aminobenzyl alcohols with N,N-dimethyl enaminones to synthesize 3-substituted or 3,4-disubstituted quinoline derivatives in moderate to excellent yields (Scheme 1d).
Results and discussion
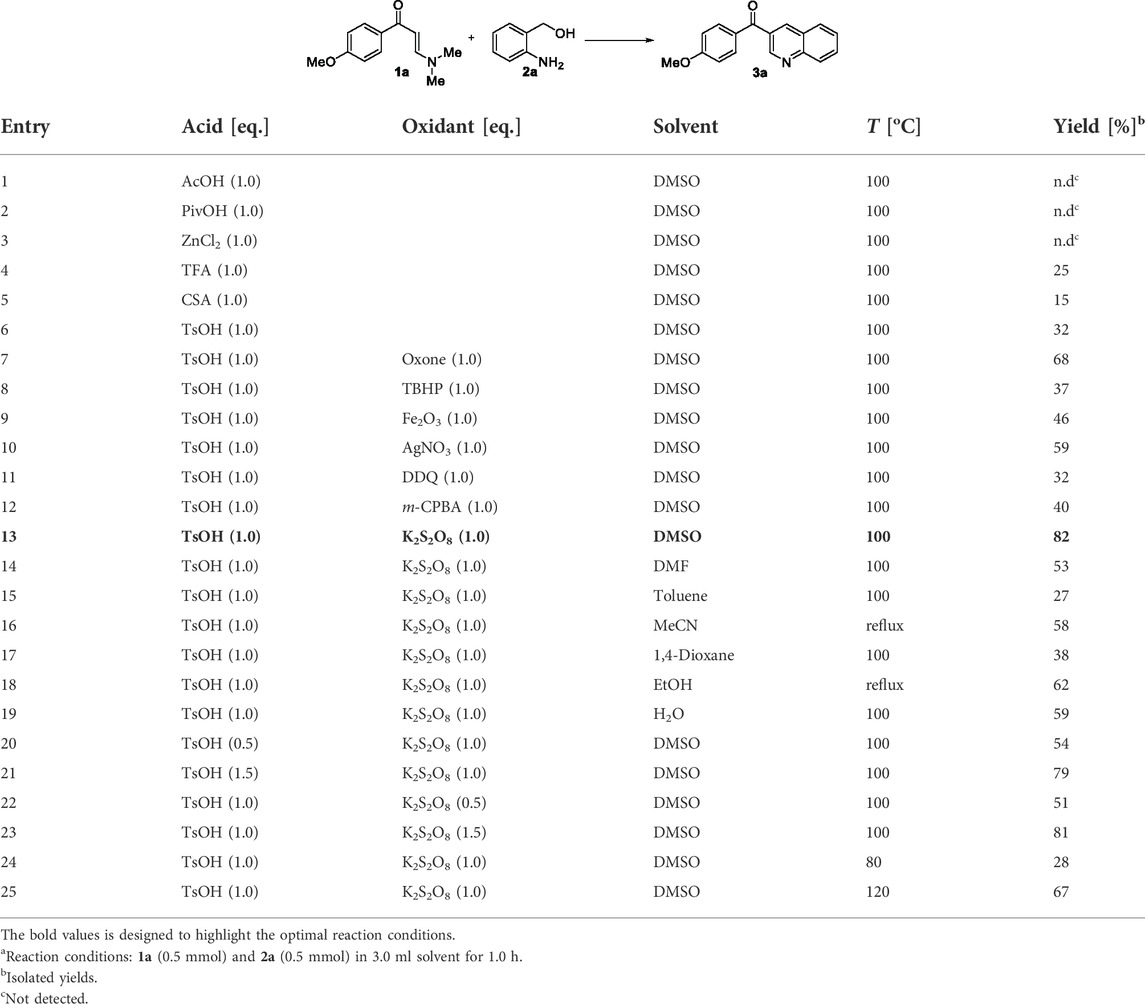
Our investigation started with the reaction of readily available N,N-dimethyl enaminone 1a with o-aminobenzyl alcohol 2a as model substrates in (Table 1). We carried out the model reaction in the presence of AcOH in DMSO at 100°C, but the desired product 3a was not obtained (entry 1). Various acids were screened, such as pivalic acid (PivOH), ZnCl2, trifluoroacetic acid (TFA), 10-camphorsulfonic acid (CSA), and p-toluenesulfonic acid (TsOH), which suggested that TsOH was the most suitable acid for this reaction in 32% yield. A series of oxidants show positive effects for the reaction (entries 7–13). To our delight, K2S2O8 was found to be the most effective one to give the desired quinolone 3a for greatly increasing the yield to 82% (entry 13). Further experiments showed that DMSO was the first choice for solvents; other solvents, such as DMF, toluene, MeCN, 1,4-dioxane, EtOH, and water, were inferior (entries 14–19). With respect to the acid and oxidant loading, 1.0 equiv of TsOH and 1.0 equiv of K2S2O8 were found to be optimal (entries 20–23). The reaction temperature was also screened, and the results showed that 100°C was still with giving the best yield (entries 24–25).
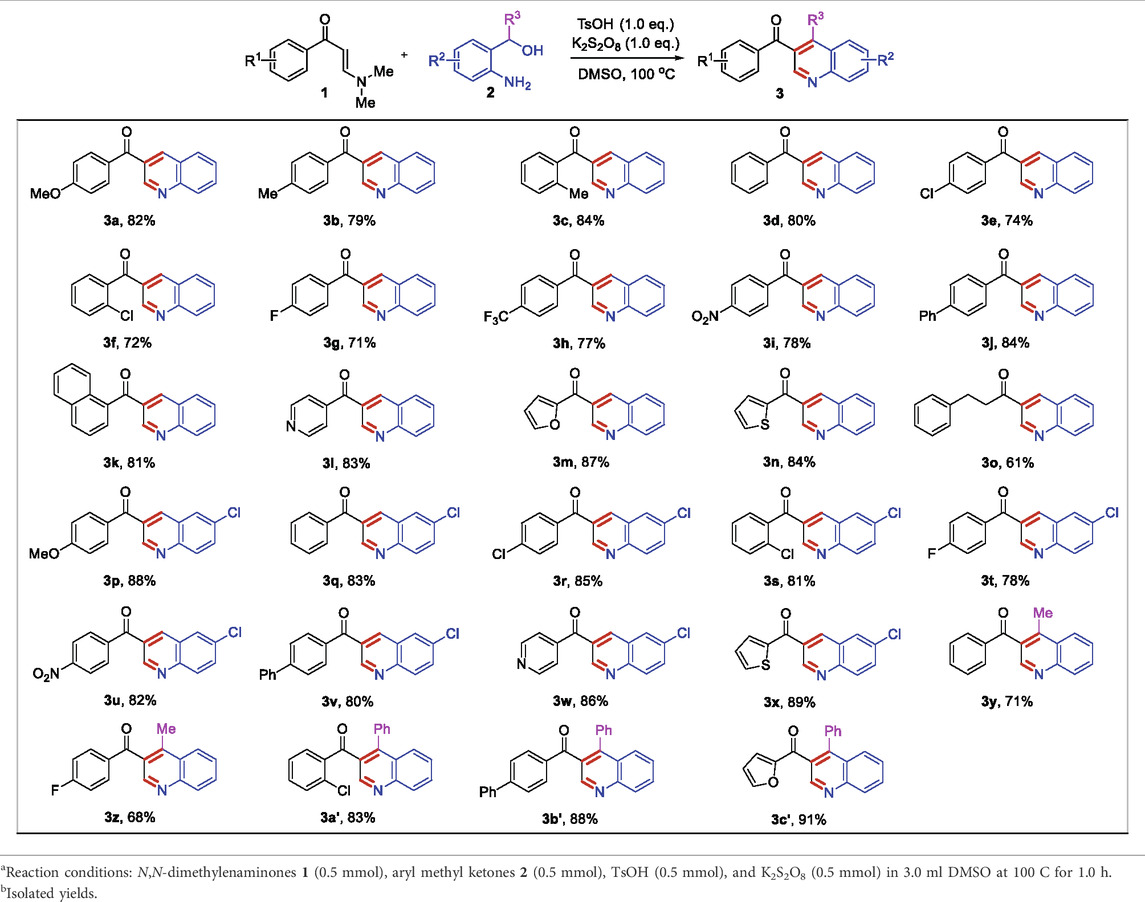
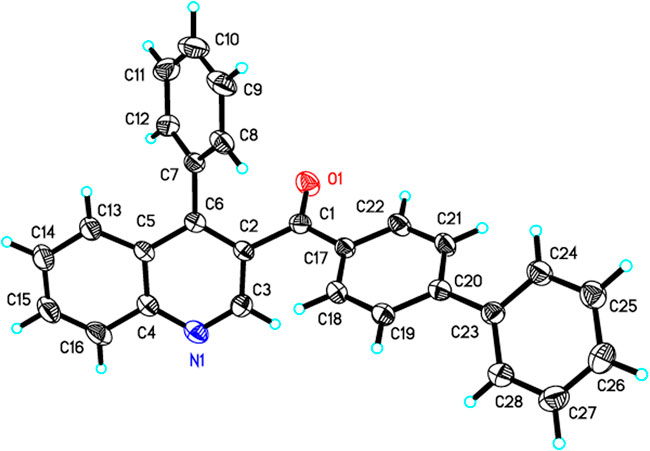
Under the optimized reaction conditions, we next investigated the substrate scope of this direct oxidative cyclocondensation reaction (Table 2). A wide range of N,N-dimethyl enaminones 1 bearing different substituents could be used in this transformation. For example, N,N-dimethyl enaminones bearing electron-rich (4-OMe, 4-Me, and 2-Me), electron-neutral (4-H), halogenated (4-Cl, 2-Cl, and 4-F), and electron-deficient (4-CF3 and 4-NO2) groups at the aryl ring were tolerated, affording the corresponding 3-substituted quinoline products in good to excellent yields (71–84%, 3a−3i). Subsequently, 4-biphenyl and 1-naphthyl N,N-dimethyl enaminones were also well compatible with the reaction, giving the expected product in excellent yields (81–84%, 3j−3k). Furthermore, various heteroaryl N,N-dimethyl enaminones, including 4-pyridyl, 2-furanyl, and 2-thienyl, were well tolerated in this reaction, affording the corresponding products in excellent yields (83–87%, 3l−3n). The phenylethyl enamamine worked well for the reaction, furnishing the corresponding quinoline product 3o in 61% yield. The o-aminobenzyl alcohol scope was also examined. Bearing halogenation (5-Cl) was well tolerated on the phenyl ring of the o-aminobenzyl alcohols, furnishing the corresponding 3-substituted quinoline products in good to excellent yields (78–89%, 3p−3x). Notably, 1-(o-aminobenzyl) ethanol and o-aminobenzhydrol were also employed, affording 3,4-disubstituted quinolines in moderate to excellent yields (68–91%, 3y−3c’). Moreover, the structure of 3j was unambiguously confirmed by X-ray crystallographic analysis (CCDC 1846910, Figure 1).
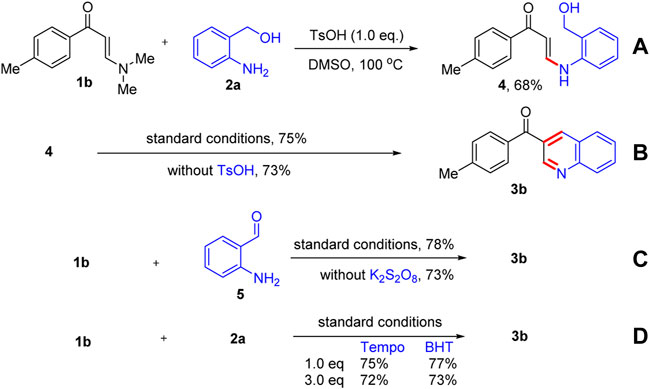
To further understand the reaction mechanism, some control experiments were carried out, and the results are presented in Scheme 2. When N,N-dimethyl enaminone 1b was reacted with o-aminobenzyl alcohol 2a in the absence of K2S2O8, the N-aryl enaminone intermediate product 4 was obtained in 68% yield by a transamination process (Scheme 2). Next, product 3b was obtained in 75% yield by the intramolecular cyclization reaction of intermediate 4 under optimized reaction conditions. However, the intramolecular cyclization reaction could also proceed smoothly without the addition of TsOH, affording product 3b in 73% yield (Scheme 2). When N,N-dimethyl enaminone 1b was reacted with 2-aminobenzaldehyde 5 under the standard conditions or in the absence of K2S2O8, product 3b was, respectively, isolated in 78 and 73% yields (Scheme 2). Additionally, the reaction was unaffected completely by adding the radical inhibitors Tempo and BHT (Scheme 2). These results revealed that N-aryl enaminone 4 and 2-aminobenzaldehyde 5 were important intermediates for this reaction, and the reaction was not a free-radical process.
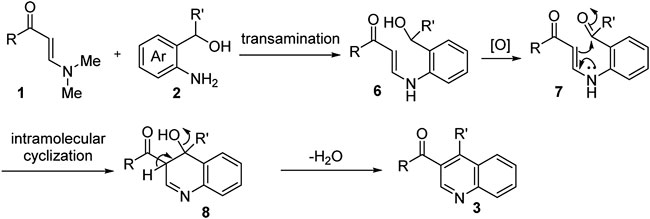
Based on the above results and previous studies (Zhou et al., 2018), a possible mechanism for this transformation is proposed (Scheme 3). N,N-dimethyl enaminones 1 reacted with o-aminobenzyl alcohols 2 promoted by TsOH to furnish the N-aryl enaminone intermediate 6 via a transamination process. Next, intermediate 6 underwent K2S2O8-assisted oxidation to form the ketone intermediate 7, which was then converted into intermediate 8 through intramolecular cyclization reaction. Finally, quinolone products 3 were obtained via elimination of a molecule of H2O and oxidative aromatization.
Conclusion
In conclusion, we have developed a concise protocol for the synthesis of 3-substituted or 3,4-disubstituted quinolines with moderate to excellent yields using readily available N,N-dimethyl enaminones and o-aminobenzyl alcohols promoted by TsOH/K2S2O8. This direct oxidative cyclocondensation reaction enriched the quinoline synthesis method from o-aminobenzyl alcohols.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author.
Author contributions
FY designed the project. KR, ZC, PZ, DL, and YS performed the experiments. FY supervised the work and prepared the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was financially supported by the National Natural Science Foundation of China (21961018), the Natural Science Foundation of Yunnan Province (201901T070302 and 202202AG050008), and the Plan of Funding Outstanding Young Talents of Yunnan Province.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.1008568/full#supplementary-material
References
Ankade, S. B., Shabade, A. B., Soni, V., and Punji, B. (2021). Unactivated alkyl halides in transition-metal-catalyzed C−H bond alkylation. ACS Catal. 11, 3268–3292. doi:10.1021/acscatal.0c05580
Barman, M. K., Jana, A., and Majia, B. (2018). Phosphine-free NNN-manganese complex catalyzed alkylation of ketones with primary alcohols and Friedla¨nder quinoline synthesis. Adv. Synth. Catal. 360, 3233–3238. doi:10.1002/adsc.201800380
Bharate, J. B., Vishwakarma, R. A., and Bharate, S. B. (2015). Metal-free domino one-pot protocols for quinoline synthesis. RSC Adv. 5, 42020–42053. doi:10.1039/c5ra07798b
Biddle, M. M., Lin, M., and Scheidt, K. A. (2007). Catalytic enantioselective synthesis of flavanones and chromanones. J. Am. Chem. Soc. 129, 3830–3831. doi:10.1021/ja070394v
Chen, L., Huang, R., Kong, L.-B., Lin, J., and Yan, S.-J. (2018). Facile route to the synthesis of 1, 3-diazahetero-cycle-fused [1, 2-a]quinoline derivatives via cascade reactions. ACS Omega 3, 1126–1136. doi:10.1021/acsomega.7b01856
Chen, X.-B., Huang, S.-T., Li, J., Yang, Q., Yang, L., and Yu, F. (2021). Highly regioselective and chemoselective [3+3] annulation of enaminones with ortho-fluoronitrobenzenenes: Divergent synthesis of aposafranones and their N-oxides. Org. Lett. 23, 3032–3037. doi:10.1021/acs.orglett.1c00710
Das, J., Singh, K., Vellakkaran, M., and Banerjee, D. (2018). Nickel-catalyzed hydrogen-borrowing strategy for α-alkylation of ketones with alcohols: A new route to branched gem-bis(alkyl) ketones. Org. Lett. 20, 5587–5591. doi:10.1021/acs.orglett.8b02256
Das, K., Mondal, A., Pal, D., and Srimani, D. (2019). Sustainable synthesis of quinazoline and 2-aminoquinoline via dehydrogenative coupling of 2-aminobenzyl alcohol and nitrile catalyzed by phosphine-free manganese pincer complex. Org. Lett. 21, 3223–3227. doi:10.1021/acs.orglett.9b00939
Das, S., Maiti, D., and Sarkar, S. D. (2018). Synthesis of polysubstituted quinolines from α-2-aminoaryl alcohols via Nickel-catalyzed dehydrogenative coupling. J. Org. Chem. 83, 2309–2316. doi:10.1021/acs.joc.7b03198
Duan, Y., Liu, Y., and Wan, J.-P. (2018). Copper-catalyzed one-pot N-acylation and C5−H halogenation of 8-aminoquinolines: The dual role of acyl halides. J. Org. Chem. 83, 3403–3408. doi:10.1021/acs.joc.8b00068
Esteruelas, M. A., Larramona, C., and Onate, E. (2016). Osmium-mediated direct C−H bond activation at the 8-position of quinolines. Organometallics 35, 1597–1600. doi:10.1021/acs.organomet.6b00264
Fu, L., Xu, W., Pu, M., Wu, Y., Liu, Y., and Wan, J.-P. (2022). Rh-Catalyzed [4 + 2] annulation with a removable monodentate structure toward iminopyranes and pyranones by C–H annulation. Org. Lett. 24, 3003–3008. doi:10.1021/acs.orglett.2c00912
Fu, L., Xu, Z., Wan, J.-P., and Liu, Y. (2020). The domino chromone annulation and a transient halogenation-mediated C–H alkenylation toward 3-vinyl chromones. Org. Lett. 22, 9518–9523. doi:10.1021/acs.orglett.0c03548
Genc, S., Arslan, B., Gulcemal, S., Gunnaz, S., Cetinkaya, B., and Gulcemal, D. (2019). Iridium(I)-catalyzed C-C and C-N bond formation reactions via the borrowing hydrogen strategy. J. Org. Chem. 84, 6286–6297. doi:10.1021/acs.joc.9b00632
Gorka, A. P., Dios, A., and Roepe, P. D. (2013). Quinoline drug−heme interactions and implications for antimalarial cytostatic versus cytocidal activities. J. Med. Chem. 56, 5231–5246. doi:10.1021/jm400282d
Harry, N. A., Ujwaldev, S. M., and Anilkumar, G. (2020). Recent advances and prospects in the metal-free synthesis of quinolines. Org. Biomol. Chem. 18, 9775–9790. doi:10.1039/d0ob02000a
Huang, H., and Kang, J. Y. (2017). Organocatalytic phosphonylation of in situ formed o-quinone methides. Org. Lett. 19, 5988–5991. doi:10.1021/acs.orglett.7b03019
Huang, J., and Yu, F. (2021)., 53. Synthesis-Stuttgart, 587–610. doi:10.1055/s-0040-1707328Recent advances in organic synthesis based on N, N-dimethyl enaminonesSynthesis.
Jentsch, N. G., Hart, A. P., Hume, J. D., Sun, J., McNeely, K. A., Lama, C., et al. (2018). Synthesis and evaluation of aryl quinolines as HIV-1 integrase multimerization inhibitors. ACS Med. Chem. Lett. 9, 1007–1012. doi:10.1021/acsmedchemlett.8b00269
Jin, H., Tian, B., Song, X., Xie, J., Rudolph, M., Rominger, F., et al. (2016). Gold-catalyzed synthesis of quinolinesfrom propargyl silyl ethersand anthranilsthrough the umpolung of a gold carbene carbon. Angew. Chem. Int. Ed. 55, 12688–12692. doi:10.1002/anie.201606043
Kim, J. I., Shin, I.-S., Kim, H., and Lee, J.-K. (2005). Efficient electrogenerated chemiluminescence from cyclometalated Iridium(III) complexes. J. Am. Chem. Soc. 127, 1614–1615. doi:10.1021/ja043721x
Kokatla, H. P., Sil, D., Malladi, S. S., Balakrishna, R., Hermanson, A. R., Fox, L. M., et al. (2013). Exquisite selectivity for human toll-like receptor 8 in substituted furo[2, 3-c]quinolines. J. Med. Chem. 56, 6871–6885. doi:10.1021/jm400694d
Lee, A., Zhu, J. L., Feoktistova, T., Brueckner, A. C., Cheong, P. H.-Y., and Scheidt, K. A. (2019). Carbene-catalyzed enantioselective decarboxylative annulations to access dihydrobenzoxazinonesand quinolones. Angew. Chem. Int. Ed. 58, 5941–5945. doi:10.1002/anie.201900600
Li, L.-Z., Wang, C.-S., Guo, W.-F., Mei, G.-J., and Shi, F. (2018). Catalytic asymmetric [4+2] cycloaddition of in situ generated o-quinone methide imines with o-hydroxystyrenes: Diastereo- and enantioselective construction of tetrahydroquinoline frameworks. J. Org. Chem. 83, 614–623. doi:10.1021/acs.joc.7b02533
Li, Y., Cao, X., Liu, Y., and Wan, J.-P. (2017). Regioselective three-component synthesis of 2, 3-disubstituted quinolines via the enaminone modified Povarov reaction. Org. Biomol. Chem. 15, 9585–9589. doi:10.1039/c7ob02411h
Liu, D., Lu, X., Zhang, Q., Zhao, Y., Zhang, B., Sun, Y., et al. (2022). Facile approach to multifunctionalized 5-alkylidene-3-pyrrolin-2-ones via regioselective oxidative cyclization of 2, 4-pentanediones with primary amines and sodium sulfonates. Org. Chem. Front. 9, 4078–4084. doi:10.1039/d2qo00473a
Lu, L., Zhou, P., Hu, B., Li, X., Huang, R., and Yu, F. (2017). An improved Pfitzinger reaction: Eco-efficient synthesis of quinaldine-4-carboxylates by TMSCl-mediated. Tetrahedron Lett. 58, 3658–3661. doi:10.1016/j.tetlet.2017.08.014
Maji, M., Chakrabarti, K., Paul, B., Roy, B. C., and Kundua, S. (2018). Ruthenium(II)-NNN-pincer-complex-catalyzed reactions between various alcohols and amines for sustainable C-N and C-C bond formation. Adv. Synth. Catal. 360, 722–729. doi:10.1002/adsc.201701117
Makarov, A. S., Uchuskin, M. G., and Gevorgyan, V. (2018). Intramolecular palladium-catalyzed oxidative amination of furans: Synthesis of functionalized indoles. J. Org. Chem. 83, 14010–14021. doi:10.1021/acs.joc.8b02470
Mastalir, M., Glatz, M., Pittenauer, E., Allmaier, G., and Kirchner, K. (2016). Sustainable synthesis of quinolines and pyrimidines catalyzed by manganese PNP pincer complexes. J. Am. Chem. Soc. 138, 15543–15546. doi:10.1021/jacs.6b10433
McCauley, E. P., Smith, G. C., and Crews, P. (2020). Unraveling structures containing highly conjugated pyrrolo[4, 3, 2-de]quinoline cores that are deficient in diagnostic proton NMR signals. J. Nat. Prod. 83, 174–178. doi:10.1021/acs.jnatprod.9b01111
McCormick, J. L., McKee, T. C., Cardellina, J. H., and Boyd, M. R. (1996). HIV inhibitory natural products. 26. Quinoline alkaloids from Euodia roxburghiana. J. Nat. Prod. 59, 469–471. doi:10.1021/np960250m
Mei, G.-J., Zhu, Z.-Q., Zhao, J.-J., Bian, C.-Y., Chen, J., Chen, R.-W., et al. (2017). Brønsted acid-catalyzed stereoselective [4+3] cycloadditions of ortho-hydroxybenzyl alcohols with N, N-cyclic azomethine imines. Chem. Commun. 53, 2768–2771. doi:10.1039/c6cc09775h
Saunthwal, R. K., Patel, M., and Verma, A. K. (2016). Metal- and protection-free [4+2] cycloadditions of alkynes with azadienes: Assembly of functionalized quinolines. Org. Lett. 18, 2200–2203. doi:10.1021/acs.orglett.6b00817
Singh, K., Vellakkaran, M., and Banerjee, D. (2018). A nitrogen-ligated nickel-catalyst enables selective intermolecular cyclisation of β- and γ-amino alcohols with ketones: Access to five and sixmembered N-heterocycles. Green Chem. 20, 2250–2256. doi:10.1039/c8gc00318a
Subbaraju, G. V., Kavitha, J., Rajasekhar, D., and Jimenez, J. I. (2004). Jusbetonin, the first indolo[3, 2-b]quinoline alkaloid glycoside, from Justicia betonica. J. Nat. Prod. 67, 461–462. doi:10.1021/np030392y
Tan, D.-W., Li, H.-X., Zhu, D.-L., Li, H.-Y., Young, D. J., Yao, J.-L., et al. (2018). Ligand-controlled copper(I)-catalyzed cross-coupling of secondary and primary alcohols to α-alkylated ketones, pyridines, and quinolines. Org. Lett. 20, 608–611. doi:10.1021/acs.orglett.7b03726
Tiwari, D. K., Phanindrudu, M., Wakade, S. B., Nanubolu, J. B., and Tiwari, D. K. (2017). Functionalization of saturated ketones with anthranils via Cu-catalyzed sequential dehydrogenation/aza-Michael addition/annulation cascade reactions in one-pot. Chem. Commun. 53, 5302–5305. doi:10.1039/c7cc01195d
Tong, H., Wang, L., Jing, X., and Wang, F. (2003). Turn-on” conjugated polymer fluorescent chemosensor for fluoride ion. Macromolecules 36, 2584–2586. doi:10.1021/ma0258612
Trofimov, B. A., Belyaeva, K. V., Nikitina, L. P., Mal’kina, A. G., Afonin, A. V., Ushakov, I. A., et al. (2018). Transition metal-free one-pot double C–H functionalization of quinolines with disubstituted electron-deficient acetylenes. Chem. Commun. 54, 5863–5866. doi:10.1039/c8cc03269f
Wan, J.-P., Li, Y., and Liu, Y. (2016). Annulation based on 8-aminoquinoline assisted C–H activation: An emerging tool in N-heterocycle construction. Org. Chem. Front. 3, 768–772. doi:10.1039/c6qo00077k
Wan, X.-M., Liu, Z.-L., Liu, W.-Q., Cao, X.-N., Zhu, X., Zhao, X.-M., et al. (2019). NNN pincer Ru(II)-catalyzed dehydrogenative coupling of 2-aminoarylmethanols with nitriles for the construction of quinazolines. Tetrahedron 75, 2697–2705. doi:10.1016/j.tet.2019.03.046
Wang, B.-Q., Zhang, C.-H., Tian, X.-X., Lin, J., and Yan, S.-J. (2018). Cascade reaction of isatins with 1, 1-enediamines: Synthesis of multisubstituted quinoline-4-carboxamides. Org. Lett. 20, 660–663. doi:10.1021/acs.orglett.7b03803
Wang, H.-Q., Ma, W., Sun, A., Sun, X.-Y., Jiang, C., Zhang, Y.-C., et al. (2021). (4 + 2) cyclization of aza-o-quinone methides with azlactones: Construction of biologically important dihydroquinolinone frameworks. Org. Biomol. Chem. 19, 1334–1343. doi:10.1039/d0ob02388d
Wang, J., Zhou, R., Zhuang, S., and Wu, A. (2018). Acid-mediated four-component tandem cyclization: Access to multifused 1, 3-benzoxazine frameworks. Tetrahedron 74, 7283–7289. doi:10.1016/j.tet.2018.10.059
Wang, R., Fan, H., Zhao, W., and Li, F. (2016). Acceptorless dehydrogenative cyclization of o-aminobenzyl alcohols with ketones to quinolines in water catalyzed by water soluble metal-ligand bifunctional catalyst [Cp*(6, 6′-(OH)2bpy)(H2O)] [OTf]2. Org. Lett. 18, 3558–3561. doi:10.1021/acs.orglett.6b01518
Wang, Y., Dong, B., Wang, Z., Cong, X., and Bi, X. (2019). Silver-catalyzed reduction of quinolines in water. Org. Lett. 21, 3631–3634. doi:10.1021/acs.orglett.9b01055
Wei, D., Dorcet, V., Darcel, C., and Sortais, J.-B. (2019). Synthesis of quinolines through acceptorless dehydrogenative coupling catalyzed by Rhenium PN(H)P complexes. ChemSusChem 12, 3078–3082. doi:10.1002/cssc.201802636
Wu, X., Geng, X., Zhao, P., Zhang, J., Gong, X., Wu, Y.-D., et al. (2017). I2-Promoted Povarov-type reaction using 1, 4-dithane-2, 5-diol as an ethylene surrogate: Formal [4 + 2] synthesis of quinolines. Org. Lett. 19, 1550–1553. doi:10.1021/acs.orglett.7b00361
Xie, F., Chen, Q.-H., Xie, R., Jiang, H.-F., and Zhang, M. (2018). MOF-derived nanocobalt for oxidative functionalization of cyclic amines to quinazolinones with 2-aminoarylmethanols. ACS Catal. 8, 5869–5874. doi:10.1021/acscatal.8b01366
Xu, H., Zhou, B., Zhou, P., Zhou, J., Shen, Y., Yu, F., et al. (2016). Insights into the unexpected chemoselectivity in brønsted acid catalyzed cyclization of isatins with enaminones: Convenient synthesis of pyrrolo[3, 4-c]quinolin-1-ones and spirooxindoles. Chem. Commun. 52, 8002–8005. doi:10.1039/c6cc02659a
Yang, B., and Gao, S. (2018). Recent advances in the application of Diels–Alder reactions involving o-quinodimethanes, aza-o-quinone methides and o-quinone methides in natural product total synthesis. Chem. Soc. Rev. 47, 7926–7953. doi:10.1039/c8cs00274f
Yang, L., and Wan, J.-P. (2020). Ethyl lactate-involved three-component dehydrogenative reactions: Biomass feedstock in diversity-oriented quinoline synthesis. Green Chem. 22, 3074–3078. doi:10.1039/d0gc00738b
Ying, J., Liu, T., Liu, Y., and Wan, J.-P. (2022). Base-promoted annulative difluoromethylenation of enaminones with BrCF2CO2Et toward 2, 2-difluorinated 2, 3-dihydrofurans. Org. Lett. 24, 2404–2408. doi:10.1021/acs.orglett.2c00671
Yu, F., Huang, R., Ni, H., Fan, J., Yan, S., and Lin, J. (2013). Three-component stereoselective synthesis of spirooxindole derivatives. Green Chem. 15, 453–462. doi:10.1039/c2gc36552a
Yu, F., Yan, S., Hu, L., Wang, Y., and Lin, J. (2011). Cascade reaction of isatins with heterocyclic ketene aminals: Synthesis of imidazopyrrolo-quinoline derivatives. Org. Lett. 13, 4782–4785. doi:10.1021/ol201783d
Yu, Q., Liu, Y., and Wan, J.-P. (2021). Metal-free C(sp2)-H perfluoroalkylsulfonylation and configuration inversion: Stereoselective synthesis of α-perfluoroalkylsulfonyl E-enaminones. Chin. Chem. Lett. 32, 3514–3517. doi:10.1016/j.cclet.2021.04.037
Zhang, B., Liu, D., Sun, Y., Zhang, Y., Feng, J., and Yu, F. (2021). Preparation of thiazole-2-thiones through TBPB-promoted oxidative cascade cyclization of enaminones with elemental sulfur. Org. Lett. 23, 3076–3082. doi:10.1021/acs.orglett.1c00751
Zhang, Y., and Sigman, M. S. (2007). Palladium(II)-catalyzed enantioselective aerobic dialkoxylation of 2-propenyl phenols: A pronounced effect of copper additives on enantioselectivity. J. Am. Chem. Soc. 129, 3076–3077. doi:10.1021/ja070263u
Zhang, Z., Shi, Y., Pan, Y., Cheng, X., Zhang, L., Chen, J., et al. (2014). Quinoline derivative-functionalized carbon dots as a fluorescent nanosensor for sensing and intracellular imaging of Zn2+. J. Mat. Chem. B 2, 5020–5027. doi:10.1039/c4tb00677a
Zhao, P., Wu, X., Zhou, Y., Geng, X., Wang, C., Wu, Y.-D., et al. (2019). Direct synthesis of 2, 3-diaroyl quinolines and pyridazino[4, 5-b]quinolines via an I2-promoted one-pot multicomponent reaction. Org. Lett. 21, 2708–2711. doi:10.1021/acs.orglett.9b00685
Zhou, P., Hu, B., Li, L., Rao, K., Yang, J., and Yu, F. (2017). Mn(OAc)3-Promoted oxidative Csp3-P bond formation through Csp2-Csp2 and P-H bond cleavage: Access to β-ketophosphonates. J. Org. Chem. 82, 13268–13276. doi:10.1021/acs.joc.7b02391
Zhou, P., Hu, B., Rao, K., Li, L., Yang, J., Gao, C., et al. (2018). Chemoselective synthesis of N-aryl-enaminones and 3-aroyl quinolines via temperature and amount of catalyst synergistic control. Synlett 29, 519–524. doi:10.1055/s-0036-1590950
Zhou, P., Hu, B., Wang, Y., Zhang, Q., Li, X., Yan, S., et al. (2018). Convenient synthesis of quinoline-4-carboxylate derivatives through the Bi(OTf)3-catalyzed domino cyclization/esterification reaction of isatins with enaminones in alcohols. Eur. J. Org. Chem. 2018, 4527–4535. doi:10.1002/ejoc.201800734
Keywords: quinolines, N, N-dimethyl enaminones, o-aminobenzyl alcohols, oxidative cyclocondensation reaction, transition-metal-free
Citation: Rao K, Chai Z, Zhou P, Liu D, Sun Y and Yu F (2022) Transition-metal-free approach to quinolines via direct oxidative cyclocondensation reaction of N,N-dimethyl enaminones with o-aminobenzyl alcohols. Front. Chem. 10:1008568. doi: 10.3389/fchem.2022.1008568
Received: 01 August 2022; Accepted: 09 August 2022;
Published: 21 September 2022.
Edited by:
Wen Chen, Yunnan University, ChinaReviewed by:
Jie-Ping Wan, Jiangxi Normal University, ChinaShen Xianfu, Qujing Normal University, China
Copyright © 2022 Rao, Chai, Zhou, Liu, Sun and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuchao Yu, eXVmdWNoYW8wNUAxMjYuY29t
†These authors have contributed equally to this work
 Kairui Rao†
Kairui Rao† Fuchao Yu
Fuchao Yu