- 1Faculty of Pharmacy, Fujian Medical University, Fuzhou, China
- 2College of Food Science, Fujian Agriculture and Forestry University, Fuzhou, China
- 3Wuyi University, Wuyishan, China
In this paper, we developed a quick, economical and sensitive colorimetric strategy for copper ions (Cu2+) quantification via the redox response of MnO2 nanosheets with glutathione (GSH). This reaction consumed MnO2 nanosheets, which acted as a catalyst for the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) to a blue product (oxTMB). In the presence of Cu2+, the GSH was catalyzed to GSSG (oxidized glutathione), and the solution changed from colorless to deep blue. Under the optimum conditions, the absorption signal of the oxidized product (oxTMB) became proportional to Cu2+ concentration in the range from 10 to 300 nM with a detection limit of 6.9 nM. This detection system showed high specificity for Cu2+. Moreover, the system has been efficaciously implemented for Cu2+ detection in actual tap water samples. The layered-nanostructures of MnO2 nanosheets make it possess high chemical and thermal stability. TMB can be quickly oxidized within 10 min by the catalyzing of MnO2 nanosheets with high oxidase-like activity. There is no need of expensive reagents, additional H2O2 and complicated modification processes during the colorimetric assay. Therefore, the strategy primarily based on MnO2 nanosheets is promising for real-time, rapid and highly sensitive detection of Cu2+ under practical conditions.
Introduction
Copper is an essential microelement for the human body and an important component of human proteins and enzymes. Lack of copper ions (Cu2+) will hinder the physiological activities of human body and easily cause various diseases (Scheiber et al., 2013; Chowdhury et al., 2018). On the other hand, copper is a heavy metal widely discovered in the environment, and excessive copper will produce severe toxic effects on humans, plants and microorganisms (Lee et al., 2016; Zhou et al., 2020). With the extensive utility of copper in industry and agriculture, it has become one of the main pollutants of social concern. Therefore, developing sensitive strategies to detect Cu2+ in environmental and biological samples is essential.
The detection techniques of Cu2+ have been greatly developed, such as atomic absorption spectroscopy (AAS) (Lima et al., 2012), inductively coupled plasma mass spectrometry (ICP-MS) (Dai et al., 2012; Khan et al., 2014), electrochemical techniques (Flavel et al., 2011; Zhu et al., 2017), fluorescence methods (Lan et al., 2010; Zhang et al., 2014; Zhang et al., 2020) and colorimetric assays (Ma et al., 2011; Weng et al., 2013; Le et al., 2014; Wang et al., 2017; Luo et al., 2020). Some of these methods suffer from the drawbacks of requirements for large instruments, special operators, complex sample pretreatment process, or poor reproducibility and reliability, which limits their on-side rapid detection and practical application (Du et al., 2014; Wang et al., 2019a). Among them, colorimetric analysis is concerned because of its simple and quick operation, low cost, visualization and no need for professional operators, which makes it widely applied.
Colorimetric assays are generally based on peroxidase or some nanomaterials as the mimic enzyme to initiate chromogenic reactions that produce colored compounds (Jangi et al., 2020; Wang et al., 2020; Xia et al., 2021). Many chromogenic reactions are carried out via catalyzing the peroxidase substrates, usually TMB or 2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (Tang et al., 2016). These reactions generally need additional hydrogen peroxide as the oxidant, which complicates the operation and increase the background signal. Strict time-controlling is necessary to improve the signal to noise ratio. There is also some other special color reactions, such as the etching of gold nanorods showing various color changes (Lin et al., 2016), the pink-blue transition caused by the aggregation of gold nanoparticles (Deng et al., 2013), and other methods generating colored compounds (Yin et al., 2015). Several colorimetric assays based on the nanomaterials of gold, silver and platinum nanoparticle have been developed for sensitive detection of Cu2+ (Ma et al., 2011; Le et al., 2014; Wang et al., 2017). The scarcity, high cost and easily poisoned of these precious noble metals, have hindered their large-scale use in practical colorimetric system (Kuo et al., 2014; Asif et al., 2017b). As a result, the creation of more economical and environment-friendly materials for colorimetric detection of Cu2+ is of significant importance.
In current years, some non-precious metals oxides-based nanosheets and nanosphere has been synthesized for electrochemical detection of various pollutants, such as MnO2, CuO and graphene (Asif et al., 2017a; Asif et al., 2017b; Asif et al., 2018; Asif et al., 2019a; Aziz et al., 2019). MnO2 nanosheets (NSs) have obtained high-level interest as a brand new type of two-dimensional (2D) nanomaterial (Chen et al., 2019). MnO2 nanosheet is a kind of graphene-like nanomaterial, which can be used as a DNA nanocarrier to construct ratiometric fluorescence biosensors to detect miRNA and living cell imaging (Wang et al., 2019a). Due to its large particular sensing area, high chemical durability and inherent oxidative enzyme mimic activity, MnO2 NSs are widely utilized in biosensors. The strong oxidation ability of MnO2 NSs makes it great potential to constitute a colorimetric strategy for practical applications. Also, MnO2 has excellent ion exchange and redox capabilities that can be widely used in supercapacitors, batteries and catalysis. It has been identified as the most promising electrode material for electrochemical energy storage systems, depending on its high density, high purity and sufficient electrochemical activity (Guo et al., 2019). some MnO2 NSs based material has been synthesized for photoelectrochemical detection of Cu2+ (Hammami et al., 2021), electrochemiluminescence detection of glutathione (Gao et al., 2016), fluorescence detection of Fe2+ (Jiang et al., 2022), ascorbic acid (Xu et al., 2017), and glutathione (Wang et al., 2016), colorimetric detect of glutathione (Ge et al., 2019), acetylcholinesterase (Yan et al., 2017) and chlorothalonil (Sheng et al., 2020). Nevertheless, the colorimetric assays based on MnO2 NSs for Cu2+ detection are very rare.
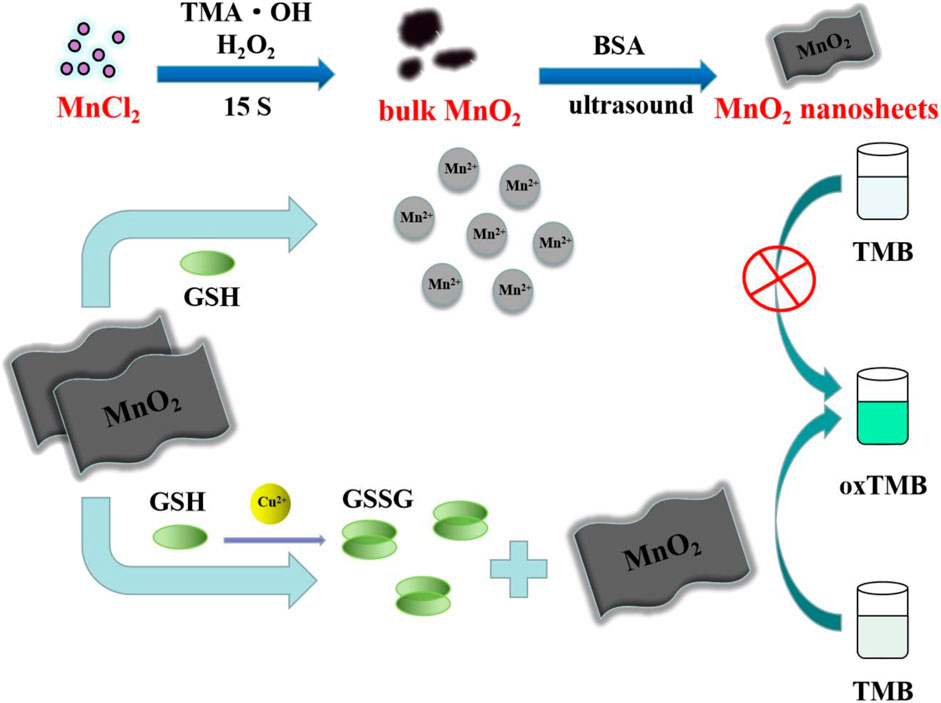
Herein, based on the catalytic oxidation activity of MnO2 NSs, an easy, speedy and economic colorimetric assay is established for the sensitive determination of Cu2+. TMB can be quickly oxidized by MnO2 NSs to form blue oxTMB without the need for H2O2. After interaction with reduced glutathione (GSH), the MnO2 NSs are dissolved, which reduce the formation amount of blue oxTMB, so the mixture remains colorless. When Cu2+ ions are introduced into the reaction mixture, Cu2+ catalyzes the formation of GSSG (oxidized glutathione) from GSH and thus inhibits the decomposition of MnO2 NSs. As a result, the color of the mixture changes from colorless to blue. According to these results, a simple colorimetric method for Cu2+ was established. Moreover, this method has high sensitivity and selectivity for the determination of Cu2+. The applicability of the assay in practical samples was also investigated.
Methods and Materials
Materials and Reagents
Tetramethylammonium hydroxide (TMAOH), cysteine (Cys), ascorbic acid (AA), uric acid (UA) were purchased from Aladdin Reagent Co., Ltd (Shanghai, China). Bovine serum albumin (BSA) and l-glutathione (GSH) were received from Sigma-Aldrich (St. Louis, Missouri, United States ). Manganese chloride tetrahydrate (MnCl2.4H2O), hydrogen peroxide (H2O2, 30 wt%), sodium hydroxide (NaOH), copper sulfate (CuSO4.5H2O), acetic acid (HAc), and ethanol were obtained from Sinopharm Chemical Reagent Co. (Shanghai, China). TMB substrate solution was obtained from Beyotime Biotechnology (Shanghai, China). All of the reagents used were analytical grades and were used directly (without further treatment). All the solutions were prepared from ultrapure water produced by a Milli-Q system.
Apparatus and Instrumentation
UV-Vis absorption spectra had been measured on the Cary-300 UV-Vis spectrophotometer (Agilent, United States ). X-ray photoelectron spectroscopy (XPS) spectra were measured on the ESCALAB MKⅡX-ray photoelectron spectrometer (Thermo Fisher Scientific, United States ). Morphology of MnO2 NSs was analyzed by Nova NanoSEM 230 field-emission scanning electron microscopy (FEI, United States ). Transmission electron microscope (TEM) photographs of MnO2 NSs were obtained on the JEM-2100 transmission electron microscope (JEOL, Japan). The level of metal ions in practical water samples was evaluated by an X SERIES II inductively coupled plasma mass spectroscopy (ICP-MS) (Thermo Fisher Scientific, United States ).
Synthesis of MnO2 NSs
MnO2 NSs were prepared according to the previous literature (Liu et al., 2017). Briefly, the mixture composed of 4.4 mL (25 wt%) TMAOH, 2 mL (3 wt%) H2O2 and 15 mL ultrapure water was quickly added into 10 mL MnCl2.4H2O solution (0.3 M) within 15 s. Then, the resulting dark brown suspension was stirred vigorously overnight at room temperature. After that, the prepared bulk manganese dioxide was centrifuged at 8,000 rpm for 10 min and washed three times with a large amount of distilled water and alcohol. The washed precipitate and 20 mg of BSA were dispersed in 20 mL ultrapure water and then treated with ultrasound for 10 h. The suspension was then centrifuged at 4,000 rpm for 15 min to remove the undispersed pellet. The supernatant was placed in the dark at 4°C for further use.
Colorimetric Detection of Cu2+
Copper sulfate was dissolved in ultrapure water to prepare various concentrations of Cu2+ solutions. At first, 14 μL of 0.5 mM GSH solution was mixed with 40 μL different concentrations of Cu2+ solutions and reacted for 5 min. Then, 15 μL MnO2 NSs (1.3 mg/mL) and 200 μL citric acid buffer (0.01 M, pH = 5.0) were added to the mixture and maintained 10 min at 45°C to ensure complete reaction. Subsequently, 100 μL of TMB substrate solution was introduced into each tube for another 10 min. Finally, the absorbance of the reaction solution in the wavelength range of 500–800 nm was determined. All reactions were carried out at 45°C.
Selectivity Investigation of Cu2+ Assay
In order to study whether other ions might interfere with the detection of Cu2+, some other metal ions were applied to the system with ten times concentrations of that of Cu2+. These ions including Fe3+, Ni2+, Ca2+, Mg2+, K+, Zn2+, Mn2+, Pb2+, Na+, Hg2+, and Ag+. 1.0 μM Cu2+ was used for the test and the concentration of other metal ions was 10 μM.
Detection of Cu2+ in Actual Samples
To investigate the application ability of the colorimetric method in the practical samples, the collected tap water and waste water were naturally settled overnight for experiments. The supernatant obtained was treated with the 0.45 μm filtration membrane to take away particulate impurity. Then different concentrations of Cu2+ were introduced to the filtrate for further detection. The procedure described above was used for Cu2+ detection in the tap water and waste water samples.
Results and Discussion
The Principle of Colorimetric Determination of Cu2+
The strategy of Cu2+ colorimetric assay based on MnO2 NSs is described in Scheme 1. Due to its intrinsic oxidase-like activity, the synthesized MnO2 NSs can catalyze the formation of blue oxidation product (oxTMB) from colorless substrate TMB, which possesses an absorption peak at 650 nm. No additional H2O2 is added during this process. As an antioxidant, GSH can react with MnO2 NSs in the way shown in Eq. 1, resulting in the reduction and decomposition of MnO2 NSs. The released Mn2+ loses the catalytic ability, thus the chromogenic reaction of TMB is inhibited. In the presence of Cu2+, Cu2+ can catalyze GSH to GSSG (Tang et al., 2016), resulting in less decomposition of MnO2 NSs and more generation of oxTMB. As a result, the color of the solution turns blue. Cu2+ concentration is linearly related to the absorption value of TMB oxidation products. Thus, a sensitive “turn on” Cu2+ detection scheme is established.
Feasibility of the Cu2+ Colorimetric Assay
Some control experiments were performed to confirm the feasibility of the colorimetric assay. As shown in Figure 1A, the characteristic peak (380 nm) of MnO2 NSs gradually decreased with the increasing GSH concentration, indicating that MnO2 NSs have been decomposed by GSH. Without GSH, the TMB oxidation process can be promoted by MnO2 NSs, generating colored solution and significant absorption signal (Figure 1B, curve a). After interaction with GSH, the MnO2 NSs was decomposed, and TMB oxidization was inhibited, which resulted in colorless solution and a great decrease of absorption signal (Figure 1B, curve b). Through the catalysis of Cu2+, GSH was oxidized to GSSG, which inhibited MnO2 NSs dissociation. Thus the absorption signal was enhanced (Figure 1B, curve c). According to these results, we concluded that Cu2+ could be quantified by measuring the absorption values of TMB oxidation products at 650 nm.
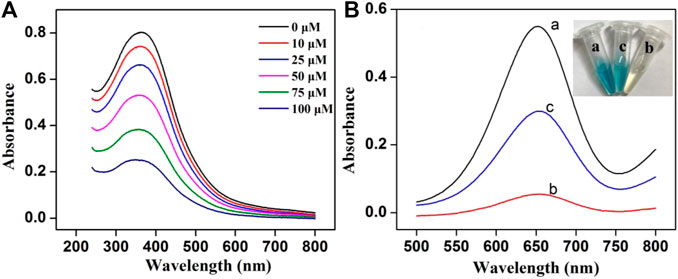
FIGURE 1. (A) The UV-vis absorption spectra of MnO2 NSs (162.5 μg/mL) with different concentrations of GSH (B) Absorption spectra of the system under different conditions: (a) MnO2 + TMB, (b) MnO2 + GSH + TMB and (c) MnO2 + Cu2+ + GSH + TMB (Inset: the photograph of the solution color).
Characterization of MnO2 NSs
After interaction with TMAOH, Mn2+ was oxidized to Mn4+ by H2O2 to form bulk MnO2. By treating it with BSA under ultrasonication, MnO2 NSs with good dispersibility, biocompatibility and high peroxidase activity were obtained, as previously described (Liu et al., 2012). The morphology of MnO2 NSs was analyzed by scanning electron microscopy (SEM). As displayed in Supplementary Figure S1, the obtained MnO2 NSs presented an obvious sheet-like morphology and well dispersed in water. As the TEM image (Figure 2A) shown, the obtained MnO2 NSs exhibit characteristic 2-D layered architecture with partial folds/wrinkles, which contribute to the large specific surface area and good dispersibility.
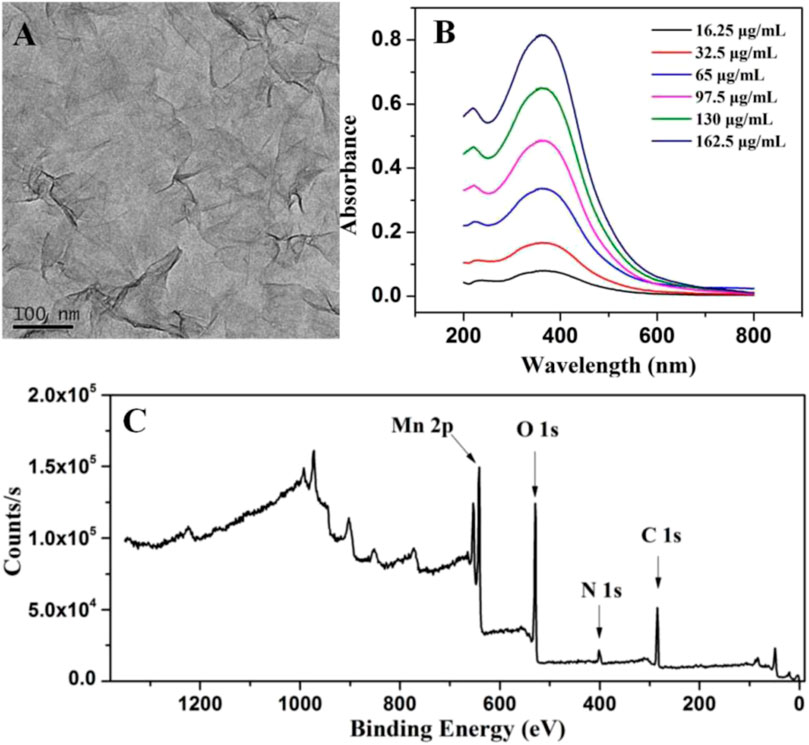
FIGURE 2. (A) TEM photograph of MnO2 NSs (B) Absorption spectra of different concentrations of MnO2 NSs (C) XPS characterization of MnO2 NSs.
The UV-Vis absorption spectra in Figure 2B shows that MnO2 NSs have a wide absorption spectra in the 250–500 nm range with a maximum absorption band at 380 nm, which can be ascribed to a d-d jump of Mn4+. This is in agreement with the optical properties of MnO2 NSs reported previously (Liu et al., 2012). Furthermore, with the increase of MnO2 NSs, the absorption intensity at 380 nm increased.
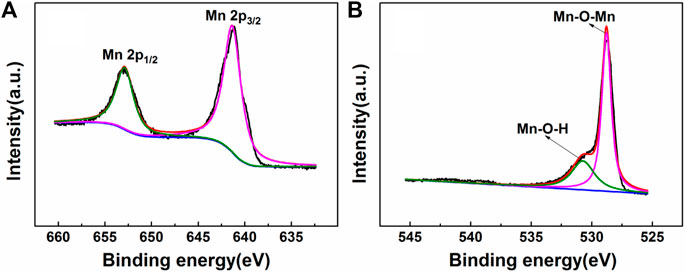
Chemical and elemental compositions of MnO2 NSs were analyzed by XPS. The wide scan spectrum results are represented in Figure 2C. Four main peaks centered at 647.2, 528.7, 401.6 and 284.7 eV can be ascribed to Mn 2p, O 1s, N 1s and C 1s (Liu et al., 2012; Asif et al., 2019b). Figure 3 shows the high-resolution XPS spectra of Mn 2p and O 1s. From Figure 3A, two strong characteristic peaks located at 652.9 and 641.2 eV emerge in the Mn 2p1/2 and Mn 2p3/2 core-level spectra, corresponding to the presence of Mn4+ moieties (Iftikhar et al., 2021). In Figure 3B, the peak at 528.7 eV is caused by O 1s. The O 1 s spectrum contains two sub energy states in Figure 3B and these peaks can be assigned to Mn–O–Mn and Mn–O–H (Liu et al., 2012; Asif et al., 2022; Aziz et al., 2022).This indicate that the acquisition of pure MnO2 and the oxidation valence of Mn is +4 (Saha and Pal, 2014). These results indicate the successful synthesis of MnO2 NSs.
Optimization of the Experimental Conditions
In order to improve the sensitivity of the Cu2+ detection system, we further optimized some important experimental conditions, including pH, reaction temperature, the dosage of TMB substrate solution and GSH concentration. At first, the optimal pH of the reaction system was studied. HAc-NaAc buffer solutions with different pH were added to the reaction system. Supplementary Figure S2 shows the absorption values at different pH in the presence and absence of Cu2+. In the presence of 250 nM Cu2+, the absorption increment increases sharply in the pH range of 3.5–5.0. While the pH reaches 5.5, the increment of absorption decreases instead. As continues to raise the pH, the absorption increment returns to a slow upward trend. It is determined that the absorption increment is maximal when the pH value is 5.0. Therefore, the pH was selected at 5.0 for further test.
We also studied the influence of temperature on Cu2+ sensing systems. The absorption values at different temperatures in the presence and absence of Cu2+ are shown in Supplementary Figure S3. In the range of 25–45°C, the increase of temperature is beneficial to the reaction, and the absorbance difference of the final solution presents a steady rise trend. When the temperature reaches 45°C, the absorbance difference reached the maximum. Once the temperature is above 45°C, the temperature has a negative impact on this system. In order to obtain good experimental results, the colorimetric Cu2+ detection was carried out at the temperature of 45°C.
Subsequently, it was found that the amount of TMB substrate solution was related to the absorption intensity of the reaction solution at 650 nm. As Supplementary Figure S4 shown, the absorption value increases gradually with the increase of TMB substrate solution and remains stable when the amount of TMB is more than 100 μL. Therefore, the dosage of TMB substrate solution was selected as 100 μL.
The concentration of GSH used to decompose MnO2 NSs is critical. In order to achieve higher detection sensitivity, it was also optimized. As shown in Supplementary Figure S5A, the absorption value of the solution gradually decreases with the increase of GSH concentration in the reaction system. The solution color also changes from blue to colorless. These results indicated that MnO2 NSs were decomposed into Mn2+ by GSH, which inhibited the production of blue oxTMB. Meanwhile, there was a good linear relationship between the absorbance and the concentration of GSH from 0 to 18.75 μM (Supplementary Figure S5B). Considering the sensitivity and practical conditions of the system, the colorimetric assay was performed by selecting 17.5 μM GSH.
Sensitivity, Reproducibility and Stability Investigation of Cu2+ Assay
The sensitivity of the colorimetric assay was verified by adding different concentrations of Cu2+ to the solution at the optimal conditions. The recorded UV-vis absorption spectra of the system and the calibration curve established by absorption values of different concentrations of Cu2+ at 650 nm are presented in Figure 4. The results showed that with the increase of Cu2+ concentration, absorption value exhibited a rising trend and the color of the solution became deeper (Figure 4A). The absorption value has a good linear relationship with Cu2+ concentration from 10 to 300 nM, and the correlation coefficient is 0.997 (Figure 4B). Cu2+ detection limit (LOD) was estimated to about 6.9 nM (3σ). Because of the hazardous effects of high levels of Cu2+, the US Environmental Protection Agency stipulates that the maximum level of Cu2+ allowed in drinking water was about 20 μM (U.S.E.P. Agency, 2012). Thus, this colorimetric assay is suitable for the detection of low concentration Cu2+ in the environment.
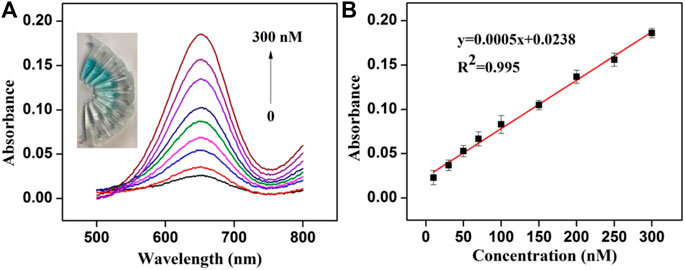
FIGURE 4. (A) Absorption spectra obtained for colorimetric detection of Cu2+ (Inset: the photograph of the color change of the solution) (B) Linear relationship between Cu2+concentration (10–300 nM) and the absorption value.
Compared with other reported methods in Table 1, this strategy could achieve a lower detection limit and a wider detection range. Besides, the characteristic of the novel colorimetric assay, such as simple operation (no additional H2O2 was required), low toxicity, and low cost (without the need of precious metal or nucleic acid), makes it great potential in high sensitivity and rapid test of Cu2+ in drinking water and environment.
The reproducibility of the colorimetric sensor was evaluated by intra-assay. Five parallel measurements have been performed to detect 100 nM Cu2+ at the same conditions. The obtained relative standard deviation (RSD) was calculated to be 2.9%. The long-term stability of the colorimetric sensor was also investigated. The MnO2 NSs were stored in dark under 4 °C when it is not used. After stored for more than 1 month, the Cu2+ induced absorption response could retain 96% of its original value. These results indicate that the colorimetric sensor shows good repeatability and stability.
Interference Study
To investigate the specificity of colorimetric determination of Cu2+, series interference experiments were also carried out. Various metal ions including Fe3+, Ni2+, Ca2+, Mg2+, K+, Zn2+, Mn2+, Pb2+, Na+, Hg2+, Ag+ and common compounds with redox properties were studied. Under the same conditions, 1.0 μM Cu2+ or other metal ions with ten times the concentration of Cu2+ were added to the system to collect the absorption signal. Although Ag+ and Hg2+ could give rise to measurable absorbance signals (Supplementary Figure S6) in the concentration of 10 μM, the interference is small when they are at the same concentration as Cu2+ (1.0 μM) (Supplementary Figure S7), and they will not significantly affect the detection of Cu2+ at this concentration in the environmental system. In addition, KBr (1.25 mM) and KCl (12.5 mM) were used as masking agents to eliminate the interference of Hg2+ and Ag+. It could be seen in Figure 5 that with the coexistence of KBr and KCl, the colorimetric assay showed high specificity for Cu2+. At the same time, other metal ions with concentration ten times of Cu2+ made no difference in the sensitivity of Cu2+ analysis. Common redox substances, such as 100 μM of Cys, AA, UA and 1.0 mg/mL of BSA had no significant effect on the detection of Cu2+ (Supplementary Figure S7). It is worth noting that the selectivity can be observed with the naked eye. Hence, the specific detection of Cu2+ can be achieved by the established colorimetric assay.
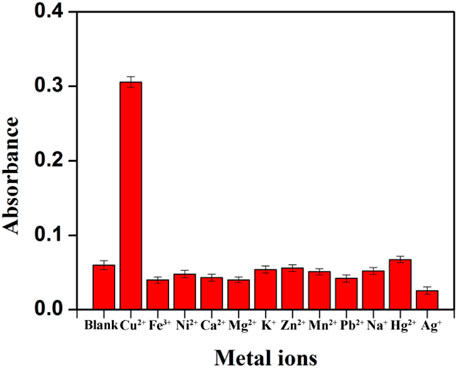
FIGURE 5. Study on the selectivity of the established Cu2+ assay after the addition of masking agents (The concentration of Cu2+ was 1.0 μM, other metal ions concentration was ten-fold of Cu2+).
Detection of Cu2+ in Real Samples
To verify the applicability of this method in practical samples and complex sample matrix, Cu2+ spiked tap water (collected from the laboratory) and waste water (collected from a point source suspected to discharge metal ions by Longyan Rare Earths LTD.) were prepared for the test. The practical samples were settled overnight at room temperature, and then the impurities were removed by filtration with a 0.45 μm filter membrane. For comparison, ICP-MS was also used to evaluate the Cu2+ level in these samples. The initial concentration of Cu2+ detected by ICP-MS in tap water and waste water is 3.5 and 235.2 nM, respectively. The analytical performances of the proposed colorimetric assay for the detection of Cu2+ from waste water is in good agreement with the results obtained by ICP-MS with RSD value of 1.74% (Supplementary Table S1). This result indicated the good accuracy of the proposed colorimetric assay for the analysis of Cu2+ in real samples. After dilution, series of Cu2+ with different concentrations were added into the pretreated water samples and detected by this colorimetric method. The detection results are listed in Supplementary Table S1. The recovery of standard Cu2+ spiked samples is between 95.2 and 105.7%, with RSD< 3.24% (n = 3). The results illustrate that this colorimetric method is promising for applying efficient quantitative detection of Cu2+ in actual samples with high sensitivity.
Conclusion
To summarize, we developed a novel colorimetric method to detect Cu2+ rapidly and effectively. The basic principle of the method is that Cu2+ can catalyze GSH to GSSG, thus inhibiting the decomposition of the single-layer MnO2 NSs into Mn2+ by GSH to achieve Cu2+ detection. Under the optimal experimental conditions, the colorimetric system exhibits highly sensitive, broad linear range, low detection limit and rapid analysis. In addition, the experimental results showed that this method is expected to be used for the determination of Cu2+ in practical conditions. Compared to natural enzymes, the layered nanostructures of 2D MnO2 NSs makes themselves possess higher chemical and thermal stability. It should be noted that the TMB can be quickly oxidized by MnO2 NSs with high oxidase-like activity within 10 min. Importantly, the colorimetric assay does not require any expensive reagents, additional H2O2 or complicated modification processes and can complete the detection under mild conditions in a short time, which is expected to be applied to the detection of Cu2+ in other practical situations. It is worth mentioning that combining with Cu-contained nanomaterials, this colorimetric method can provide a novel general sensing strategy for the indirect detection of a variety of analytes.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
ST and YW designed this study. QL carried out the experiment, data acquisition, and data analysis. JH provided assistance for data acquisition and manuscript editing. ST and QL drafted the manuscript. WC provided contribution suggestions of the manuscript. FA, HX, HS, and WY helped to revise the manuscript. All authors contributed to the manuscript and approved the final version.
Funding
This work was supported by the National Natural Science Foundation of China (21874019, 21605019) and the Natural Science Foundation of Fujian Province (2020J01635, 2019J05046).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all members of laboratory for their technical support and academic discussions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.812503/full#supplementary-material
References
Asif, M., Aziz, A., Ashraf, G., Iftikhar, T., Sun, Y., Xiao, F., et al. (2022). Unveiling Microbiologically Influenced Corrosion Engineering to Transfigure Damages into Benefits: A Textile Sensor for H2O2 Detection in Clinical Cancer Tissues. Chem. Eng. J. 427, 131398. doi:10.1016/j.cej.2021.131398
Asif, M., Aziz, A., Ashraf, G., Wang, Z., Wang, J., Azeem, M., et al. (2018). Facet-Inspired Core-Shell Gold Nanoislands on Metal Oxide Octadecahedral Heterostructures: High Sensing Performance toward Sulfide in Biotic Fluids. ACS Appl. Mater. Inter. 10, 36675–36685. doi:10.1021/acsami.8b12186
Asif, M., Aziz, A., Wang, H., Wang, Z., Wang, W., Ajmal, M., et al. (2019a). Superlattice Stacking by Hybridizing Layered Double Hydroxide Nanosheets with Layers of Reduced Graphene Oxide for Electrochemical Simultaneous Determination of Dopamine, Uric Acid and Ascorbic Acid. Microchim. Acta 186 (2), 61. doi:10.1007/s00604-018-3158-y
Asif, M., Aziz, A., Wang, Z., Ashraf, G., Wang, J., Luo, H., et al. (2019b). Hierarchical CNTs@CuMn Layered Double Hydroxide Nanohybrid with Enhanced Electrochemical Performance in H2S Detection from Live Cells. Anal. Chem. 91 (6), 3912–3920. doi:10.1021/acs.analchem.8b04685
Asif, M., Haitao, W., Shuang, D., Aziz, A., Zhang, G., Xiao, F., et al. (2017b). Metal Oxide Intercalated Layered Double Hydroxide Nanosphere: With Enhanced Electrocatalyic Activity towards H2O2 for Biological Applications. Sensors Actuators B: Chem. 239, 243–252. doi:10.1016/j.snb.2016.08.010
Asif, M., Liu, H., Aziz, A., Wang, H., Wang, Z., Ajmal, M., et al. (2017a). Core-shell Iron Oxide-Layered Double Hydroxide: High Electrochemical Sensing Performance of H2O2 Biomarker in Live Cancer Cells with Plasma Therapeutics. Biosens. Bioelectron. 97, 352–359. doi:10.1016/j.bios.2017.05.057
Awad, F. S., AbouZied, K. M., Bakry, A. M., Abou El-Maaty, W. M., El-Wakil, A. M., and El-Shall, M. S. (2020). Highly Fluorescent Hematoporphyrin Modified Graphene Oxide for Selective Detection of Copper Ions in Aqueous Solutions. Analytica Chim. Acta 1140, 111–121. doi:10.1016/j.aca.2020.10.016
Aziz, A., Asif, M., Ashraf, G., Iftikhar, T., Hu, J., Xiao, F., et al. (2022). Boosting Electrocatalytic Activity of Carbon Fiber@Fusiform-like Copper-Nickel LDHs: Sensing of Nitrate as Biomarker for NOB Detection. J. Hazard. Mater. 422, 126907. doi:10.1016/j.jhazmat.2021.126907
Aziz, A., Asif, M., Azeem, M., Ashraf, G., Wang, Z., Xiao, F., et al. (2019). Self-Stacking of Exfoliated Charged Nanosheets of LDHs and Graphene as Biosensor with Real-Time Tracking of Dopamine from Live Cells. Analytica Chim. Acta 1047, 197–207. doi:10.1016/j.aca.2018.10.008
Chen, J., Meng, H., Tian, Y., Yang, R., Du, D., Li, Z., et al. (2019). Recent Advances in Functionalized MnO2 Nanosheets for Biosensing and Biomedicine Applications. Nanoscale Horiz. 4 (2), 321–338. doi:10.1039/c8nh00274f
Chowdhury, S., Rooj, B., Dutta, A., and Mandal, U. (2018). Review on Recent Advances in Metal Ions Sensing Using Different Fluorescent Probes. J. Fluoresc. 28 (4), 999–1021. doi:10.1007/s10895-018-2263-y
Dai, B., Cao, M., Fang, G., Liu, B., Dong, X., Pan, M., et al. (2012). Schiff Base-Chitosan Grafted Multiwalled Carbon Nanotubes as a Novel Solid-phase Extraction Adsorbent for Determination of Heavy Metal by ICP-MS. J. Hazard. Mater. 219-220, 103–110. doi:10.1016/j.jhazmat.2012.03.065
Deng, J., Jiang, Q., Wang, Y., Yang, L., Yu, P., and Mao, L. (2013). Real-Time Colorimetric Assay of Inorganic Pyrophosphatase Activity Based on Reversibly Competitive Coordination of Cu2+ between Cysteine and Pyrophosphate Ion. Anal. Chem. 85 (19), 9409–9415. doi:10.1021/ac402524e
Di Masi, S., Pennetta, A., Guerreiro, A., Canfarotta, F., De Benedetto, G. E., and Malitesta, C. (2020). Sensor Based on Electrosynthesised Imprinted Polymeric Film for Rapid and Trace Detection of Copper(II) Ions. Sensors Actuators B: Chem. 307, 127648. doi:10.1016/j.snb.2019.127648
Du, Y., Lim, B. J., Li, B., Jiang, Y. S., Sessler, J. L., and Ellington, A. D. (2014). Reagentless, Ratiometric Electrochemical DNA Sensors with Improved Robustness and Reproducibility. Anal. Chem. 86, 8010–8016. doi:10.1021/ac5025254
Flavel, B. S., Nambiar, M., and Shapter, J. G. (2011). Electrochemical Detection of Copper Using a Gly-Gly-His Modified Carbon Nanotube Biosensor. Silicon 3 (4), 163–171. doi:10.1007/s12633-011-9080-0
Gao, W., Liu, Z., Qi, L., Lai, J., Kitte, S. A., and Xu, G. (2016). Ultrasensitive Glutathione Detection Based on Lucigenin Cathodic Electrochemiluminescence in the Presence of MnO2 Nanosheets. Anal. Chem. 88 (15), 7654–7659. doi:10.1021/acs.analchem.6b01491
Ge, J., Cai, R., Chen, X., Wu, Q., Zhang, L., Jiang, Y., et al. (2019). Facile Approach to Prepare HSA-Templated MnO2 Nanosheets as Oxidase Mimic for Colorimetric Detection of Glutathione. Talanta 195, 40–45. doi:10.1016/j.talanta.2018.11.024
Guo, C., Liu, H., Li, J., Hou, Z., Liang, J., Zhou, J., et al. (2019). Ultrathin δ-MnO2 Nanosheets as Cathode for Aqueous Rechargeable Zinc Ion Battery. Electrochimica Acta 304, 370–377. doi:10.1016/j.electacta.2019.03.008
Hammami, A., Assaker, I. B., and Chtourou, R. (2021). Regenerative, Low-Cost and Switchable Photoelectrochemical Sensor for Detection of Cu2+ Using MnO2-GO Heterojunction. J. Solid State. Electrochem. doi:10.1007/s10008-021-05092-9
Hormozi Jangi, S. R., Akhond, M., and Absalan, G. (2020). A Novel Selective and Sensitive Multinanozyme Colorimetric Method for Glutathione Detection by Using an Indamine Polymer. Analytica Chim. Acta 1127, 1–8. doi:10.1016/j.aca.2020.06.012
Hu, J., Wang, L., Zhang, X., Yu, W., Gao, H.-W., Solin, N., et al. (2021). Selective Colorimetric Detection of Copper (II) by a Protein-Based Nanoprobe. Spectrochimica Acta A: Mol. Biomol. Spectrosc. 252, 119462. doi:10.1016/j.saa.2021.119462
Iftikhar, T., Xu, Y., Aziz, A., Ashraf, G., Li, G., Asif, M., et al. (2021). Tuning Electrocatalytic Aptitude by Incorporating α-MnO2 Nanorods in Cu-MOF/rGO/CuO Hybrids: Electrochemical Sensing of Resorcinol for Practical Applications. ACS Appl. Mater. Inter. 13 (27), 31462–31473. doi:10.1021/acsami.1c07067
Jiang, M., Xu, S., Yu, Y., Gao, Y., Yin, Z., Li, J., et al. (2022). Turn-on Fluorescence Ferrous Ions Detection Based on MnO2 Nanosheets Modified Upconverion Nanoparticles. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 264, 120275. doi:10.1016/j.saa.2021.120275
Jiang, P., Li, S., Han, M., Liu, Y., and Chen, Z. (2019). Biocompatible Ag2S Quantum Dots for Highly Sensitive Detection of Copper Ions. Analyst 144 (8), 2604–2610. doi:10.1039/c9an00096h
Khan, N., Jeong, I. S., Hwang, I. M., Kim, J. S., Choi, S. H., Nho, E. Y., et al. (2014). Analysis of Minor and Trace Elements in Milk and Yogurts by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS). Food Chem. 147, 220–224. doi:10.1016/j.foodchem.2013.09.147
Kuo, C.-C., Lan, W.-J., and Chen, C.-H. (2014). Redox Preparation of Mixed-Valence Cobalt Manganese Oxide Nanostructured Materials: Highly Efficient Noble Metal-free Electrocatalysts for Sensing Hydrogen Peroxide. Nanoscale 6, 334–341. doi:10.1039/c3nr03791f
Lan, G.-Y., Huang, C.-C., and Chang, H.-T. (2010). Silver Nanoclusters as Fluorescent Probes for Selective and Sensitive Detection of Copper Ions. Chem. Commun. 46 (8), 1257–1259. doi:10.1039/b920783j
Lee, S., Barin, G., Ackerman, C. M., Muchenditsi, A., Xu, J., Reimer, J. A., et al. (2016). Copper Capture in a Thioether-Functionalized Porous Polymer Applied to the Detection of Wilson's Disease. J. Am. Chem. Soc. 138, 7603–7609. doi:10.1021/jacs.6b02515
Li, L., Yuan, Z., Peng, X., Li, L., He, J., and Zhang, Y. (2014). Highly Selective Colorimetric Detection of Copper Ions Using Cysteamine Functionalized Gold Nanoparticles. J. Chin. Chem. Soc 61 (12), 1371–1376. doi:10.1002/jccs.201400188
Lima, G. F., Ohara, M. O., Clausen, D. N., Nascimento, D. R., Ribeiro, E. S., Segatelli, M. G., et al. (2012). Flow Injection On-Line Minicolumn Preconcentration and Determination of Trace Copper Ions Using an Alumina/Titanium Oxide Grafted Silica Matrix and FAAS. Microchim. Acta 178 (1-2), 61–70. doi:10.1007/s00604-012-0807-4
Lin, Y., Zhao, M., Guo, Y., Ma, X., Luo, F., Guo, L., et al. (2016). Multicolor Colormetric Biosensor for the Determination of Glucose Based on the Etching of Gold Nanorods. Sci. Rep. 6 (1), 37879. doi:10.1038/srep37879
Liu, J., Meng, L., Fei, Z., Dyson, P. J., Jing, X., and Liu, X. (2017). MnO2 Nanosheets as an Artificial Enzyme to Mimic Oxidase for Rapid and Sensitive Detection of Glutathione. Biosens. Bioelectron. 90, 69–74. doi:10.1016/j.bios.2016.11.046
Liu, X., Wang, Q., Zhao, H., Zhang, L., Su, Y., and Lv, Y. (2012). BSA-templated MnO2 Nanoparticles as Both Peroxidase and Oxidase Mimics. Analyst 137 (19), 4552–4558. doi:10.1039/c2an35700c
Luo, L., Su, Z., Zhuo, J., Huang, L., Nian, Y., Su, L., et al. (2020). Copper-Sensitized "Turn on" Peroxidase-like Activity of MMoO4 (M = Co, Ni) Flowers for Selective Detection of Aquatic Copper Ions. ACS Sust. Chem. Eng. 8 (33), 12568–12576. doi:10.1021/acssuschemeng.0c03822
Lv, J., Zhang, C., Wang, S., Li, M., and Guo, W. (2021). MOF-derived Porous ZnO-Co3O4 Nanocages as Peroxidase Mimics for Colorimetric Detection of Copper(ii) Ions in Serum. Analyst 146 (2), 605–611. doi:10.1039/d0an01383h
Ma, Y.-r., Niu, H.-y., Zhang, X.-l., and Cai, Y.-q. (2011). Colorimetric Detection of Copper Ions in Tap Water during the Synthesis of Silver/Dopamine Nanoparticles. Chem. Commun. 47 (47), 12643–12645. doi:10.1039/c1cc15048k
Saha, S., and Pal, A. (2014). Microporous Assembly of MnO2 Nanosheets for Malachite green Degradation. Sep. Purif. Tech. 134, 26–36. doi:10.1016/j.seppur.2014.07.021
Scheiber, I., Dringen, R., and Mercer, J. F. B. (2013). Copper: Effects of Deficiency and Overload. Met. Ions Life 13, 359–387. doi:10.1007/978-94-007-7500-8_11
Sheng, E., Lu, Y., Tan, Y., Xiao, Y., Li, Z., and Dai, Z. (2020). Oxidase-mimicking Activity of Ultrathin MnO2 Nanosheets in a Colorimetric Assay of Chlorothalonil in Food Samples. Food Chem. 331, 127090. doi:10.1016/j.foodchem.2020.127090
Su, S., Mo, Z., Tan, G., Wen, H., Chen, X., and Hakeem, D. A. (2021). PAA Modified Upconversion Nanoparticles for Highly Selective and Sensitive Detection of Cu2+ Ions. Front. Chem. 8, 619764. doi:10.3389/fchem.2020.619764
Tang, S., Wang, M., Li, Z., Tong, P., Chen, Q., Li, G., et al. (2017). A Novel Sensitive Colorimetric Sensor for Cu2+ Based on In Situ Formation of Fluorescent Quantum Dots with Photocatalytic Activity. Biosens. Bioelectron. 89, 866–870. doi:10.1016/j.bios.2016.09.105
Wang, H.-B., Chen, Y., Li, Y., and Liu, Y.-M. (2016). A Sensitive Fluorescence Sensor for Glutathione Detection Based on MnO2 Nanosheets-Copper Nanoclusters Composites. RSC Adv. 6 (83), 79526–79532. doi:10.1039/c6ra17850b
Wang, S., Wang, L., Xu, X., Li, X., and Jiang, W. (2019a). MnO2 Nanosheet-Mediated Ratiometric Fluorescence Biosensor for MicroRNA Detection and Imaging in Living Cells. Analytica Chim. Acta 1063, 152–158. doi:10.1016/j.aca.2019.02.049
Wang, X., Feng, S., He, D., and Jiang, P. (2020). Porous Manganese-Cobalt Oxide Microspheres with Tunable Oxidase Mimicking Activity for Sulfide Ion Colorimetric Detection. Chem. Commun. 56 (90), 14098–14101. doi:10.1039/d0cc06209j
Wang, X., Liu, G., Qi, Y., Yuan, Y., Gao, J., Luo, X., et al. (2019b). Embedded Au Nanoparticles-Based Ratiometric Electrochemical Sensing Strategy for Sensitive and Reliable Detection of Copper Ions. Anal. Chem. 91 (18), 12006–12013. doi:10.1021/acs.analchem.9b02945
Wang, Y.-F., Pan, N., and Peng, C.-F. (2017). A Highly Sensitive Colorimetric Method for Copper Ions Detection Based on Controlling the Peroxidase-like Activity of Au@Pt Nanocatalysts. Anal. Sci. 33 (3), 321–325. doi:10.2116/analsci.33.321
Weng, Z., Wang, H., Vongsvivut, J., Li, R., Glushenkov, A. M., He, J., et al. (2013). Self-Assembly of Core-Satellite Gold Nanoparticles for Colorimetric Detection of Copper Ions. Analytica Chim. Acta 803, 128–134. doi:10.1016/j.aca.2013.09.036
Xia, Y., Chen, T., Zhang, L., Zhang, X., Shi, W., Chen, G., et al. (2021). Colorimetric Detection of Exosomal MicroRNA through Switching the Visible-Light-Induced Oxidase Mimic Activity of Acridone Derivate. Biosens. Bioelectron. 173, 112834. doi:10.1016/j.bios.2020.112834
Xu, Y.-L., Niu, X.-Y., Chen, H.-L., Zhao, S.-G., and Chen, X.-G. (2017). Switch-on Fluorescence Sensor for Ascorbic Acid Detection Based on MoS2 Quantum Dots-MnO2 Nanosheets System and its Application in Fruit Samples. Chin. Chem. Lett. 28 (2), 338–344. doi:10.1016/j.cclet.2016.10.003
Yan, X., Song, Y., Wu, X., Zhu, C., Su, X., Du, D., et al. (2017). Oxidase-mimicking Activity of Ultrathin MnO2 nanosheets in Colorimetric Assay of Acetylcholinesterase Activity. Nanoscale 9 (6), 2317–2323. doi:10.1039/c6nr08473g
Ye, Z., Li, L., Zhao, F., Yang, Q., Wang, Y., Bohinc, K., et al. (2020). Dual-Emission Fluorescent Probe Templated by Spherical Polyelectrolyte Brush for Ratiometric Detection of Copper Ions. J. Mater. Sci. 55, 10168–10184. doi:10.1007/s10853-020-04757-6
Yin, K., Li, B., Wang, X., Zhang, W., and Chen, L. (2015). Ultrasensitive Colorimetric Detection of Cu2+ Ion Based on Catalytic Oxidation of L-Cysteine. Biosens. Bioelectron. 64, 81–87. doi:10.1016/j.bios.2014.08.058
Zhang, J., Chen, M.-Y., Bai, C.-B., Qiao, R., Wei, B., Zhang, L., et al. (2020). A Coumarin-Based Fluorescent Probe for Ratiometric Detection of Cu2+ and its Application in Bioimaging. Front. Chem. 8, 800. doi:10.3389/fchem.2020.00800
Zhang, S., Yu, T., Sun, M., Yu, H., Zhang, Z., Wang, S., et al. (2014). Highly Sensitive and Selective Fluorescence Detection of Copper (II) Ion Based on Multi-Ligand Metal Chelation. Talanta 126, 185–190. doi:10.1016/j.talanta.2014.03.076
Zhou, X., Wu, X., He, H., Liang, H., Yang, X., Nie, J., et al. (2020). Contrast-Enhancing Fluorescence Detection of Copper Ions by Functional Fluorescent Microgels. Sensors Actuators B: Chem. 320, 128328. doi:10.1016/j.snb.2020.128328
Keywords: colorimetric system, MnO2 mimetic enzyme, copper ions, rapid detection, water sample
Citation: Tang S, Liu Q, Hu J, Chen W, An F, Xu H, Song H and Wang Y-W (2021) A Simple Colorimetric Assay for Sensitive Cu2+ Detection Based on the Glutathione-Mediated Etching of MnO2 Nanosheets. Front. Chem. 9:812503. doi: 10.3389/fchem.2021.812503
Received: 10 November 2021; Accepted: 06 December 2021;
Published: 24 December 2021.
Edited by:
Fei Xiao, Huazhong University of Science and Technology, ChinaReviewed by:
Abdul Rahman Rahman, Zhejiang University, ChinaTayyaba Iftikhar, Huazhong University of Science and Technology, China
Copyright © 2021 Tang, Liu, Hu, Chen, An, Xu, Song and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shurong Tang, c3J0YW5nQGZqbXUuZWR1LmNu; Hongbo Song, c2doZ2JvZGVAMTYzLmNvbQ==; Yi-Wei Wang, eXd3YW5nZnp1QDE2My5jb20=
 Shurong Tang
Shurong Tang Qiao Liu2,3
Qiao Liu2,3 Wei Chen
Wei Chen