- 1Inner Mongolian Key Laboratory of Environmental Chemistry, College of Chemistry and Environmental Science, Inner Mongolia Normal University, Hohhot, China
- 2School of Pharmacy, Inner Mongolia Medical University, Hohhot, China
- 3College of Geographical Science, Inner Mongolia Normal University, Hohhot, China
In this work, highly fluorescent copper nanomaterials were synthesized by using ascorbic acid as a ligand. The excitation wavelength of copper nanomaterials is 367 nm, and the emission wavelength is 420 nm. The size range is 5–6 nm. Nitrite can selectively quench the fluorescence of copper nanomaterials. Therefore, copper nanomaterials can be used to selectively detect nitrite ions. The linear equation is F = −32.94 c (NO2−) + 8,455, and the correlation coefficient is 0.9435. At the same time, we found that the fluorescence intensity of copper nanomaterials has a good correlation with temperature (20–60°C), which shows that they have great potential in the application of nanothermometers.
Introduction
Nitrite, as a food additive and soil raw material, widely exists in food and environment. But excessive nitrite in actual water will cause serious harm to public health and environment (Davis and Compton, 2000; Kroupova et al., 2008; Wang et al., 2009; Zhao and Li, 2020; Jahan et al., 2021). When the concentration of nitrite is higher than 4.5 mg/ml (Iii and Finke, 1999), it will damage the nervous system, spleen, and kidney of human beings and even leads to a high risk of cancer. Therefore, accurate detection of nitrite in drinking water sources, wastewater treatment systems, the food industry, and environment is of great significance.
In recent years, copper nanoparticles with high fluorescence have been widely used in biomedicine (Zhang et al., 2018), bioimaging (Satyvaldiev et al., 2018), environmental detection (Zhong et al., 2015; Li et al., 2018), catalysis (Wang et al., 2019), and other fields due to their excellent photoelectric properties, great biocompatibility, good stability, and low toxicity. Wang’s group reported that copper nanoclusters were synthesized using glutathione as stabilizer and ascorbic acid as reductant, and they detected nitrite ions by utilizing the quenching fluorescence effect (Zhou et al., 2017). The method has been successfully applied to the detection of nitrite ions in actual water samples. Li’s group reported that copper nanomaterials were synthesized using cysteine (Li et al., 2015; Ashraf et al., 2019; Aziz et al., 2019; Ashraf et al., 2020; Ashraf et al., 2021; Iftikhar et al., 2021). Based on the aggregation induction (AIE) of copper nanoclusters induced by sulfur ions, they established a fluorescence analysis method for the determination of hydrogen sulfide. It was applied to the detection of practical samples. Current analytical challenges faced by researchers in carrying out nitrite detection and temperature sensing are that the methods used are not suitable for online monitoring and in-site detection as they require fine instruments and complicated sample pretreatment. Therefore, fluorescence sensing has emerged as a tempting analytical method, as it does not require expensive instruments and involves a simple pretreatment procedure. Herein, copper nanomaterials were selected because the synthesis cost of copper nanomaterials is low and the fluorescence signal is good.
In this study, highly fluorescent copper nanomaterials were synthesized with ascorbic acid as a protective and reducing agent. Nitrite ions can effectively quench the fluorescence of copper nanomaterials and realize the detection of nitrite ions. As the synthesized copper nanomaterials have good temperature sensitivity, they can also be used as temperature sensors. The fluorescence intensity of copper nanomaterials is linear at different temperatures. Therefore, the synthesized copper nanomaterials have great potential in ion sensing, detection, and drug delivery.
Instrument, Reagent, and Experimental Section
Instrumentation
Fluorescence spectrogram was obtained by using an F-4600 fluorescence spectrophotometer (Hitachi High Tech. Company). Transmission electron microscope (TEM) signals were obtained by a JEOL-2100F transmission electron microscope (Japan) operating at a voltage of 200 kV. TEM sample characterization was carried out by dropping the sample in a carbon-coated copper mesh dispersion solution and drying at room temperature. An ESCALAB-250-XI X-ray photoelectron spectroscopy (XPS) analyzer was used (Thermo Fischer Scientific).
Chemical Reagent
Ascorbic acid (C6H8O6); Cu(NO3)2·3H2O; ZnSO4·7H2O; Pb(NO3)2 (analytically pure, Tianjin Wind Ship Chemical Reagent Technology Co., Ltd.); Bi(NO3)3·3H2O; AgNO3 (analytically pure, Beijing Fuchen Chemical Reagents Co., Ltd.); FeCl2·4H2O (analytically pure, Beijing Shle Chemical Plant); MnSO4·H2O (analytically pure, Beijing Chaoyang District Chemical Four Plant); Ni(NO3)2·6H2O (analytically pure, Beijing 5671 Chemical Plant); Al(NO3)3·9H2O (analytically pure, Tian Jin Bo Di Chemical Co., Ltd.); sodium citrate (analytically pure, Tian Jin Public and Private Joint Chemical Reagent First Plant); Cd(NO3)2 (analytically pure, Beijing Chemical Co., Ltd.); CoCl2•6H2O (analytically pure, Shang Hai Public and Private Joint Venture Factory); NaF (Beijing Public and Private Chemical Plant); NaCl, Na2S2O3·5H2O, and KCl (Tian Jin North Union Fine Chemicals Development Co., Ltd.); AgNO3 (Beijing Fuzhou Chemical Reagents Co., Ltd.); NaNO2 (Tianjin Public and Private Chemical Reagents First Plant); NaBr and NH4Cl (Tian Jin People Chemical Plant); Na2SiO3.9H2O (Beijing Yizhuang Middle School Chemical Plant); and water used in all experiments is ultra-pure water with a resistance of 18.25 MΩ.
Synthesis of CuNPs
The specific method for synthesizing copper nanomaterials is as follows: first, 0.5 ml of 1 mmol/L copper nitrate solution and 3 ml of 10 mmol/L ascorbic acid solution were mixed and stirred for 20 min. Then 10 mmol/L sodium hydroxide solution was used to adjust the pH of the mixed solution to 6. After that, the solution was transferred to a 60°C constant temperature water bath for 5 h. Finally, after centrifugation, the supernatant of copper nanomaterials was preserved at 4°C for later use.
Detection of NO2−
Of the prepared copper nanoparticles, 450 ml was placed in 1.5-ml centrifuge tubes, and then 50 ml of nitrite solution was added at different concentrations to the centrifuge tubes. The fluorescence intensity was measured at 365 nm after 40 min reaction at room temperature.
Copper Nanomaterials for Temperature Sensing
The copper nanomaterial solution was placed in 1.5-ml centrifuge tubes. The tubes were heated at different temperatures for 20 min, and then the fluorescence intensities of the solutions were measured with a fluorescence spectrophotometer.
Results and Discussion
Characterization of Copper Nanomaterials
Figure 1A shows the excitation and emission spectra of copper nanomaterials. It demonstrates that the synthesized copper nanomaterials have a strong fluorescence signal, and the maximum emission wavelength is 420 nm. The copper nanomaterials were dropped on the nickel mesh of the carbon support. The samples were dried and tested by TEM and energy dispersive spectroscopy (EDS). The morphology of the prepared copper nanomaterials was characterized by TEM (Figure 1B). As shown in Figure 1B, the copper nanomaterials are uniformly dispersed and have a smaller particle size. Figure 1C presents a high-resolution transmission electron microscopy (HRTEM) image. The crystal lattice of d = 0.3348 nm corresponding to the face-centered cubic structure (110) of copper can be clearly seen. Figure 1D indicates that the particle size distribution of the copper nanomaterials is uniform, and the average diameter of the copper nanomaterials is in the range of 5.0 ± 0.1 nm. The EDS image and the elemental contents in the copper nanomaterials are presented in Figure 1E. The percentage of copper was 67.72%. The results indicated that the synthesized copper nanomaterials have the characteristics of good fluorescence, uniform dispersion, and small particle size, which indicates that highlyfluorescent copper nanomaterials have been successfully synthesized.
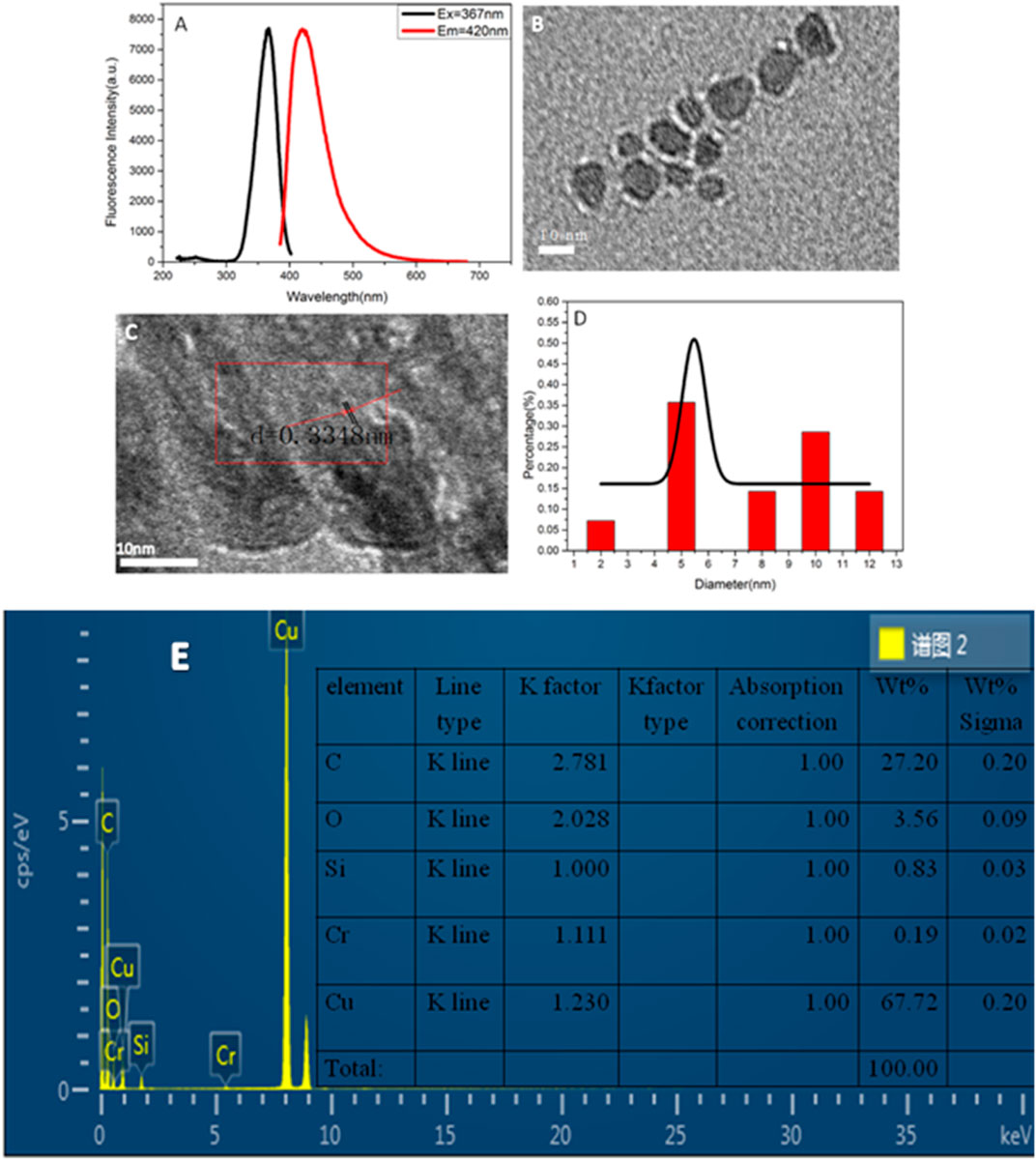
FIGURE 1. (A) Fluorescence spectra of copper nanomaterials; (B) TEM image of copper nanomaterials; (C) HRTEM image of copper nanomaterials; (D) size distribution histogram of copper nanomaterials; (E) ED image of CuNPs.
The chemical and surface properties of the copper nanoparticles were analyzed by ultraviolet-visible (UV) spectroscopy and Fourier transform infrared (FT-IR) spectroscopy. Figure 2A shows the ultraviolet absorption spectra of CuNPs and ascorbic acid. There is no obvious absorption peak of ascorbic acid but a small ultraviolet absorption peak of CuNPs near 340 nm, which corresponds to the fluorescence excitation wavelength of copper nanomaterials. Figure 2B exhibits the infrared spectra of the copper nanomaterials and ascorbic acid, which can broaden the characteristic peaks of CuNPs. The shrinkage vibration peak of 2,524 cm−1 belonging to -OH has disappeared in the copper nanomaterials, which proves that glutathione molecules bond to the surface of copper nanomaterials through Cu-O. The characteristic peaks of ascorbic acid did not appear, which proves that the ligand exchange was complete.
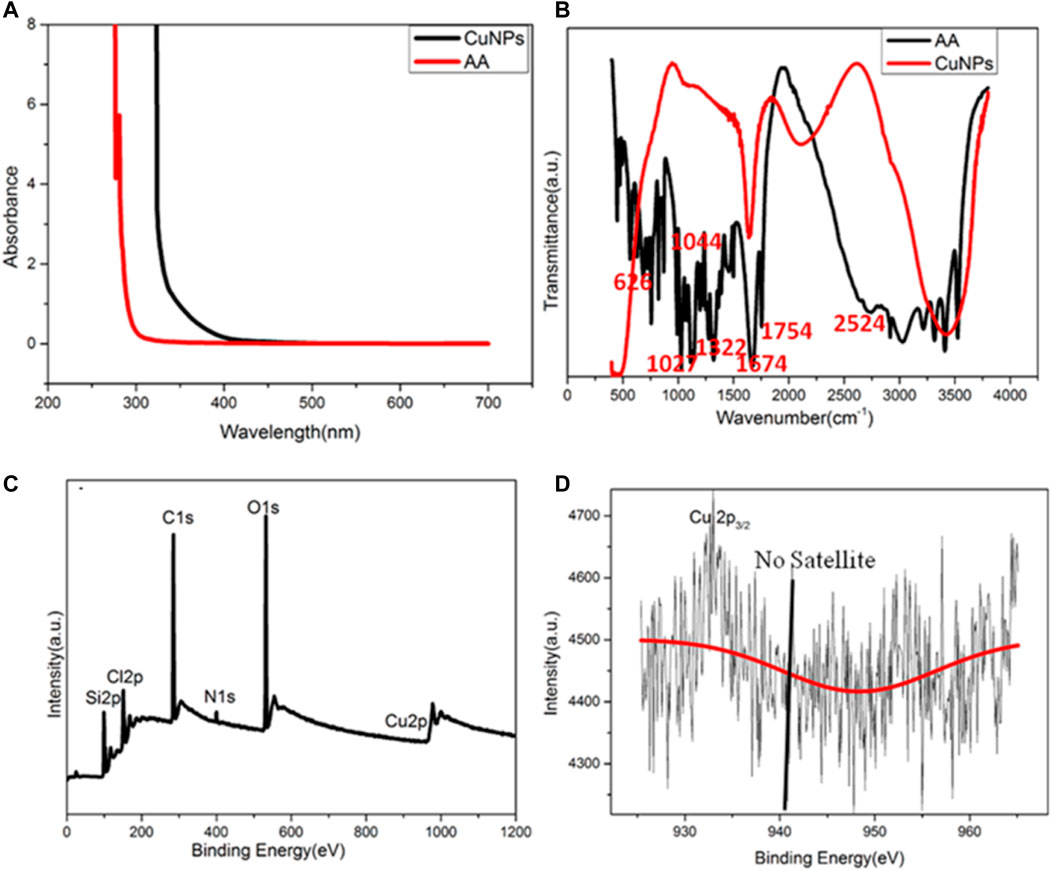
FIGURE 2. (A) UV–vis spectra of CuNPs and AA; (B) FT-IR spectra of CuNPs and AA; (C) XPS of CuNPs thus obtained; (D) amplified XPS of Cu 2p electrons.
XPS was used to characterize the valence state of copper in CuNPs. The full spectrum indicated that CuNPs consist of C, O, N, Si, and Cu (Figure 2C). Figure 2D shows that no satellite peaks appear, which means that no Cu2+ exists. The strong peak of 932.3 eV belongs to zero-valent copper’s 2p3/2, and this is consistent with previous literature reports (Koski et al., 2012). However, it should be noted that the binding energy of 2p3/2 of Cu(0) is similar to that of Cu(I). Due to the charge transfer of the Cu-O bond, the valence state of Cu nanomaterials may be between 0 and +1.
Optimization of Experimental Synthesis Conditions
The synthesis time, temperature, molar ratio, and pH were optimized. By adjusting the molar ratio of copper nitrate to ascorbic acid, the appropriate drug dosage for the synthesis of copper nanomaterials was determined. As shown in Figure 3A, when the molar ratio of copper nitrate to ascorbic acid is 1:60, the fluorescence intensity of copper nanomaterials is higher. Therefore, the best molar ratio is 1:60. Figure 3B shows the effect of synthesis temperature on fluorescence intensities. When the temperature was changed from 30 to 90°C, the fluorescence intensities of CuNPs first increased and then decreased. The fluorescence intensity of CuNPs was the highest at 60°C. Therefore, the optimal synthesis temperature of copper nanomaterials is 60°C. Figures 3C,D show the optimum time and pH data for the synthesis of copper nanomaterials. With the increase in time, the fluorescence intensity of copper nanomaterials increases. When the synthesis time is set to 5 h, the fluorescence intensity is the highest (Figure 3C). Therefore, the optimal synthesis time of copper nanomaterials is 5 h. As shown in Figure 3D, the fluorescence intensity of copper nanomaterials is the highest at pH 6. Therefore, we chose pH 6 as the best condition. Here, we synthesized CuNPs under the above optimum conditions.
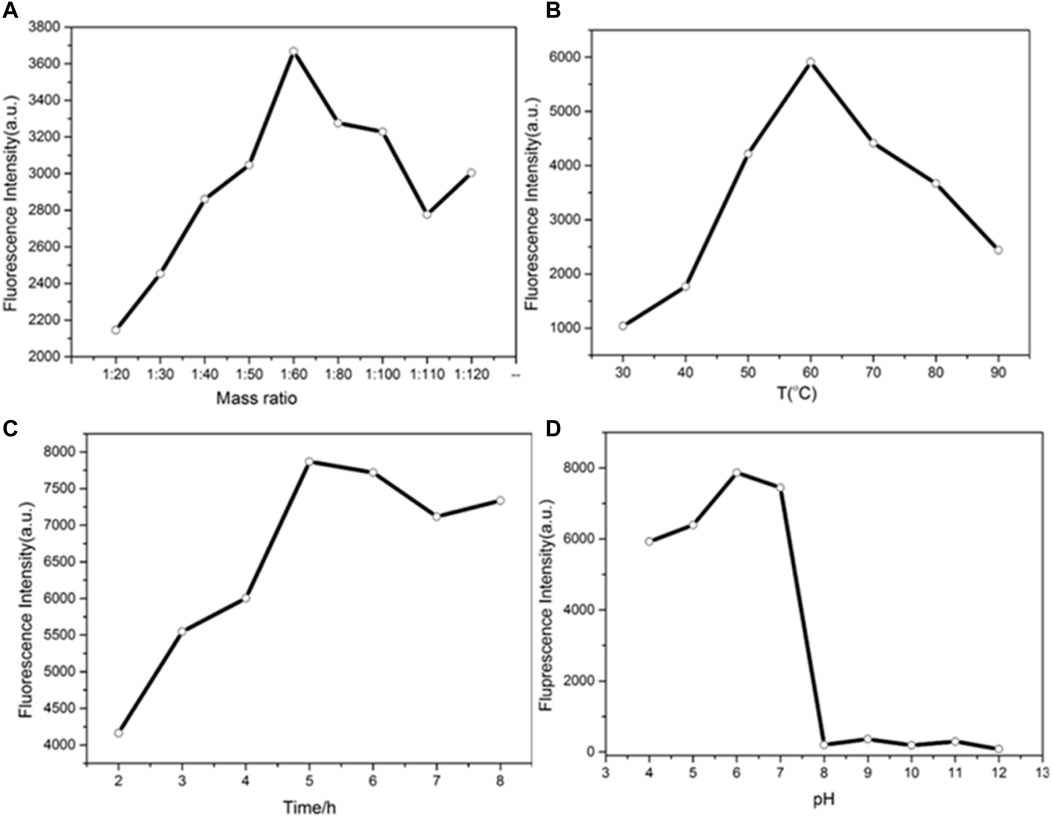
FIGURE 3. (A) Determination of the mass ratio of the synthesized CuNPs; (B) temperature of the synthesized CuNPs; (C) time of CuNP synthesis; (D) pH of CuNP synthesis.
Stability of CuNPs
The stability of the synthesized CuNPs was evaluated by comparing the fluorescence intensities of CuNPs stored for different periods and at different conditions (Figure 4). Figure 4A shows that the fluorescence intensity of the synthesized CuNPs measured at 5 h and 1 month after storage remains basically unchanged. It is concluded that CuNPs can be preserved for 1 month at 4°C. The fluorescence intensities of CuNPs were almost unchanged when the CuNPs were exposed to a xenon lamp for 100 min (Figure 4B). Figure 4C depicts the effect of different concentrations of NaCl solutions on the stability of CuNPs. It was found that the fluorescence intensity of copper nanomaterials was independent of the concentration of NaCl solution (up to 1 mol/L). These results indicate that the nanomaterials have good storage and fluorescence stability.
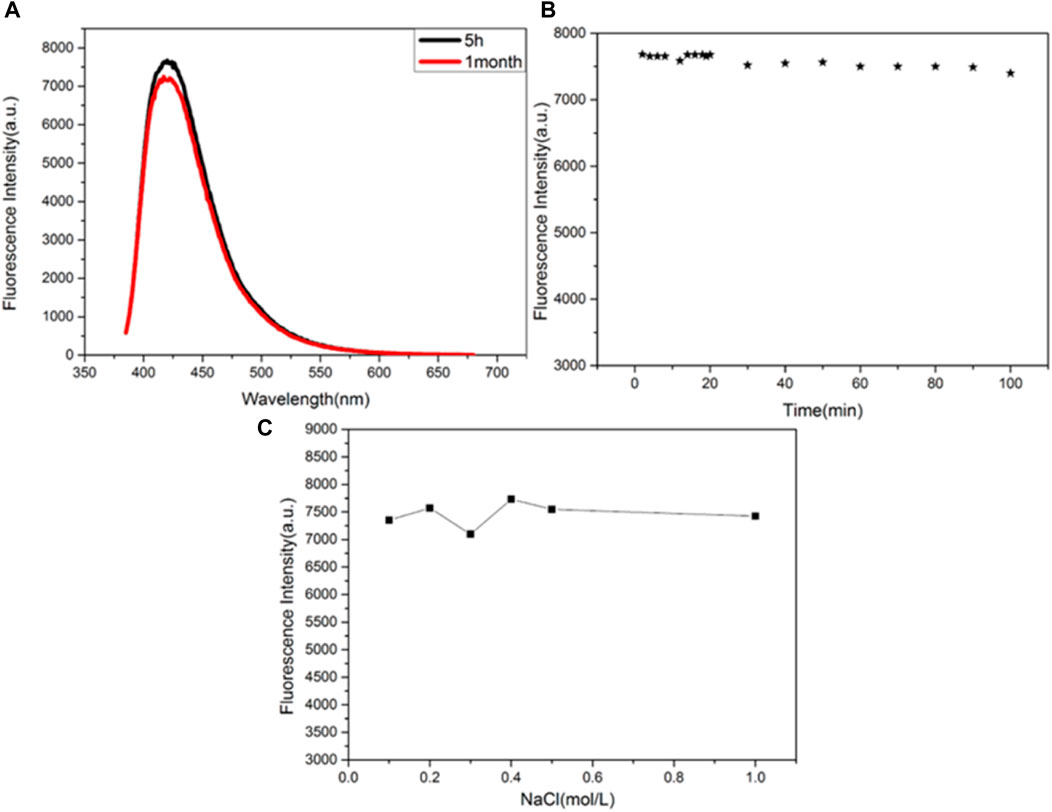
FIGURE 4. (A) Storage time; (B) irradiation time; (C) effects of the concentration of NaCl solution on the fluorescence intensity of the CuNPs.
Selectivity
To further investigate the selectivity of this method, contrast experiments were performed using 18 metal cations (K+, Na+, Ca2+, Hg+, Cr3+, Fe3+, Pb2+, and Bi3+) and 10 inorganic anions (F−, Cl−, Br−, I−, S2−, and NO2−) as the interference for detection of nitrite ions at the concentration of interference are 100 μmol/L. As shown in Figure 5, only nitrite ions show a strong quenching effect on the fluorescence of CuNPs. While Cd2+, Fe3+, and Hg+ have little effect on the fluorescence of CuNPs, other ions have no obvious effect on it. All these results indicate that this method possesses good selectivity for nitrite ion detection.
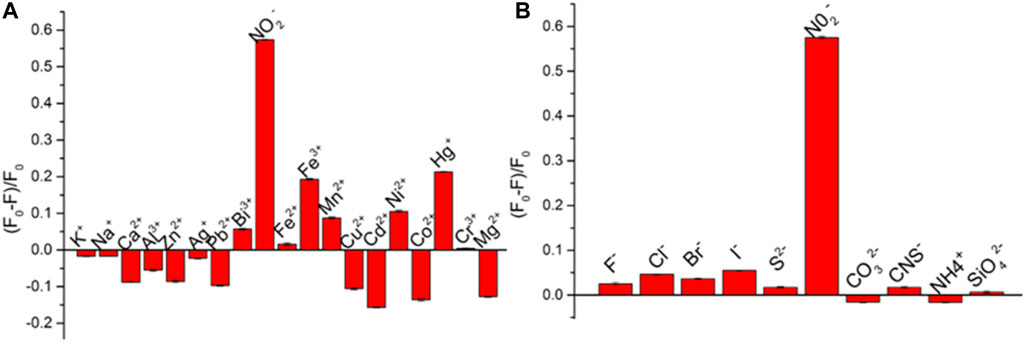
FIGURE 5. Selectivity of CuNPs toward I− sensing over other common metal ions (A) and anions (B). The concentration of all metal ions is 100 μmol/L.
Detection of NO2−
Based on the quenching effect of nitrite ions on the fluorescence of CuNPs, we established a fluorescence quenching sensor for accurate detection of nitrite ions. Figure 6A indicates that the fluorescence intensities of copper nanomaterials decrease with the increasing concentration of nitrite ions. Figure 6B shows a good linear relationship between the quenching fluorescence intensities of CuNPs and nitrite concentration in the range of 10 μ M–180 μ M. The linear equation is F = −32.94 c (NO2−) + 8,455, and the correlation coefficient is 0.9435. Figure 6C shows the TEM image of CuNPs after adding NO2−. It demonstrates that the fluorescence quenching is caused by the aggregation of nanomaterials because of the interaction between the surface ligand (ascorbic acid) of CuNPs and NO2−. The results are basically consistent with previous reports. Nitrite is a kind of food additive and is used for its coloring and antiseptic properties. It is widely used in cooked meat and canned animal food and is also used as an enema agent. Nitrite is not only a carcinogen but can also cause food poisoning when ingested at 0.2–0.5 g and can cause death at 3 g. Accurate detection of the nitrite content in food online is a key problem.
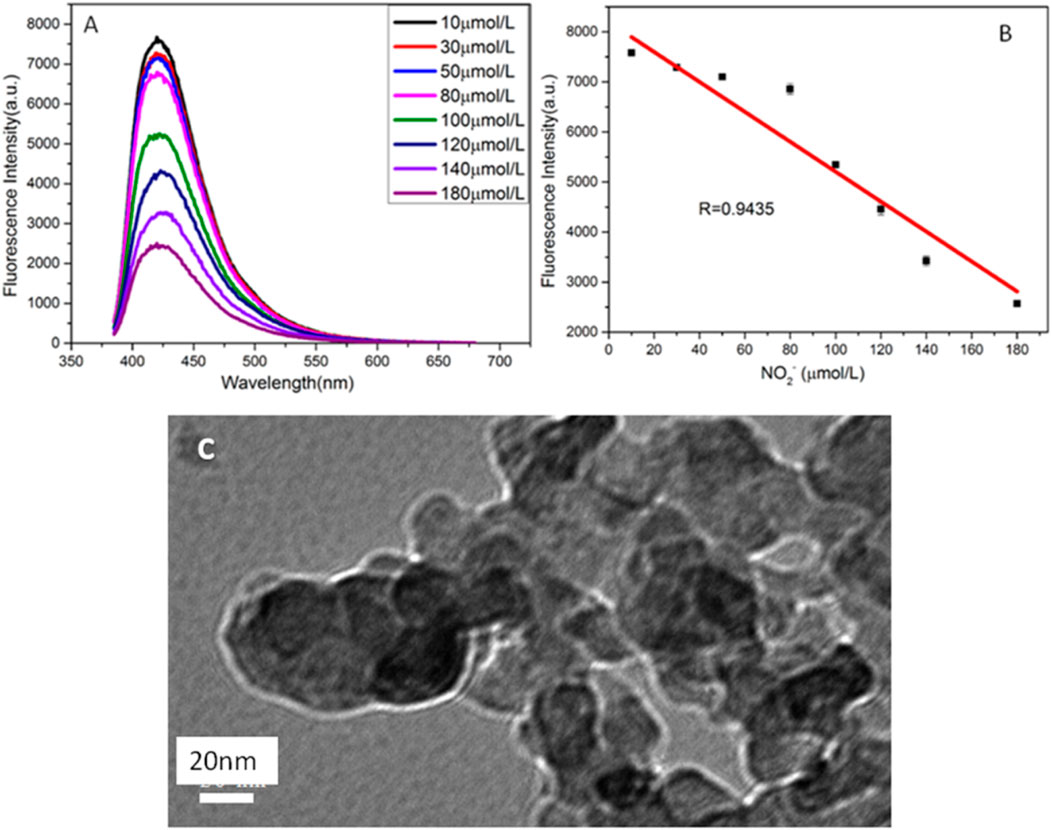
FIGURE 6. (A) Fluorescence spectra of CuNPs obtained with different concentrations of NO2− (from top to bottom: 10, 30, 50, 80, 100, 120, 1,400, and 180 μmol/L, respectively); (B) relationship between F and the concentration of NO2−; (C) TEM of copper nanomaterials after adding NO2−.
Temperature Sensing
A temperature-sensitive sensor based on copper nanomaterials was studied. As shown in Figure 7A, the fluorescence intensity of copper nanomaterials varies with temperature, from 20°C to 60°C. There is a good linear relationship between fluorescence intensity and temperature. The calibration equation is F = − 42.62T + 7,200, and the correlation coefficient is 0.9645 (as shown in Figure 7B). Therefore, the synthesized copper nanomaterial shows great potential in the application of nanothermometers.
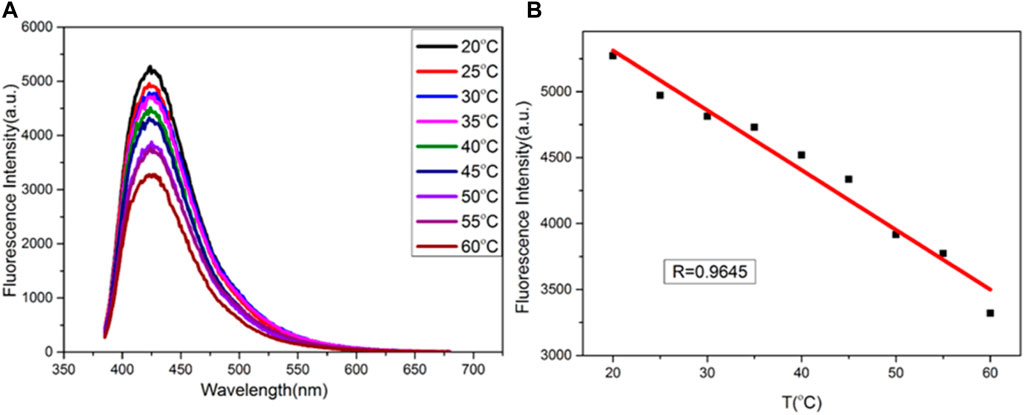
FIGURE 7. (A) Fluorescence spectra of CuNPs obtained at different temperatures (from top to bottom: 20, 25, 30, 35, 40, 45, 50, 55, and 60°C, respectively); (B) relationship between F and temperature.
Conclusion
In summary, we synthesized a new kind of copper nanomaterial using ascorbic acid as both protectant and reductant. Nitrite ions can selectively quench the fluorescence of copper nanomaterials. Based on the fluorescence quenching mechanism, a sensor for detecting nitrite ions was established. We also found that there is a linear relationship between fluorescence signal and temperature in the temperature range of 20–60°C. Therefore, our new copper nanomaterials show great potential in the application of nanothermometers.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
NW: synthesis; LG: characterization; and JA: drafting and writing the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos 21864020 and 51503106); the Natural Science Foundation of Inner Mongolia (Grant No. 2018MS02012); the Collaborative Innovation Center for Water Environmental Security of Inner Mongolia Autonomous Region, China (Grant No. XTCX003); and the Inner Mongolia Innovation Guide Project and Research Project of Higher School, Department of Education of Inner Mongolia Autonomous Region (Grant No. NJZC16047).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ashraf, G., Asif, M., Aziz, A., Dao, A. Q., Zhang, T., Iftikhar, T., et al. (2020). Facet-energy Inspired Metal Oxide Extended Hexapods Decorated with Graphene Quantum Dots: Sensitive Detection of Bisphenol A in Live Cells. Nanoscale 12 (16), 9014–9023. doi:10.1039/c9nr10944g
Ashraf, G., Asif, M., Aziz, A., Iftikhar, T., and Liu, H. (2021). Rice-Spikelet-like Copper Oxide Decorated with Platinum Stranded in the CNT Network for Electrochemical In Vitro Detection of Serotonin. ACS Appl. Mater. Inter. 13 (5), 6023–6033. doi:10.1021/acsami.0c20645
Ashraf, G., Asif, M., Aziz, A., Wang, Z., Qiu, X., Huang, Q., et al. (2019). Nanocomposites Consisting of Copper and Copper Oxide Incorporated into MoS4 Nanostructures for Sensitive Voltammetric Determination of Bisphenol A. Microchim Acta 186 (6), 337. doi:10.1007/s00604-019-3406-9
Aziz, A., Asif, M., Ashraf, G., Azeem, M., Majeed, I., Ajmal, M., et al. (2019). Advancements in Electrochemical Sensing of Hydrogen Peroxide, Glucose and Dopamine by Using 2D Nanoarchitectures of Layered Double Hydroxides or Metal Dichalcogenides. A Review. Microchim Acta 186 (10), 671. doi:10.1007/s00604-019-3776-z
Davis, J., and Compton, R. G. (2000). Sonoelectrochemically Enhanced Nitrite Detection. Analytica Chim. Acta 404, 241–247. doi:10.1016/s0003-2670(99)00724-2
Iftikhar, T., Xu, Y., Aziz, A., Ashraf, G., Li, G., Asif, M., et al. (2021). Tuning Electrocatalytic Aptitude by Incorporating α-MnO2 Nanorods in Cu-MOF/rGO/CuO Hybrids: Electrochemical Sensing of Resorcinol for Practical Applications. ACS Appl. Mater. Inter. 13 (27), 31462–31473. doi:10.1021/acsami.1c07067
Iii, J. D. A., and Finke, R. G. (1999). A Review of Modern Transition-Metal Nanoclusters: Their Synthesis, Characterization, and Applications in Catalysis. J. Mole. Catal. Chem. 145, 1–44. doi:10.1016/S1381-1169(99)00098-9
Jahan, I., Erci, F., and Isildak, I. (2021). Facile Microwave-Mediated green Synthesis of Non-toxic Copper Nanoparticles Using Citrus Sinensis Aqueous Fruit Extract and Their Antibacterial Potentials. J. Drug Deliv. Sci. Technol. 61, 102172. doi:10.1016/j.jddst.2020.102172
Koski, K. J., Cha, J. J., Reed, B. W., Wessells, C. D., Kong, D., and Cui, Y. (2012). High-Density Chemical Intercalation of Zero-Valent Copper into Bi2Se3 Nanoribbons. J. Am. Chem. Soc. 134, 7584–7587. doi:10.1021/ja300368x
Kroupova, H., Machova, J., Piackova, V., Blahova, J., Dobsikova, R., Novotny, L., et al. (2008). Effects of Subchronic Nitrite Exposure on Rainbow trout (Oncorhynchus mykiss). Ecotoxicology Environ. Saf. 71, 813–820. doi:10.1016/j.ecoenv.2008.01.015
Li, S., Tang, T., Chai, F., Qu, F., Wei, T., Wang, C., et al. (2018). Facile Synthesis of Bimetallic Ag-Cu Nanoparticles for Colorimetric Detection of Mercury Ion and Catalysis. Sens. Actua. Chem. 325, 255–260. doi:10.1016/j.snb.2017.08.159
Li, Z., Guo, S., and Lu, C. (2015). A Highly Selective Fluorescent Probe for Sulfide Ions Based on Aggregation of Cu Nanocluster Induced Emission Enhancement. Analyst 140, 2719–2725. doi:10.1039/c5an00017c
Satyvaldiev, A. S., Zhasnakunov, Z. K., Omurzak, E., Doolotkeldieva, T. D., Bobusheva, S. T., Orozmatova, G. T., et al. (2018). Copper Nanoparticles: Synthesis and Biological Activity. IOP Conf. Ser. Mater. Sci. Eng. 302, 012075. doi:10.1088/1757-899x/302/1/012075
Wang, N., Ga, L., and Ai, J. (2019). Synthesis of Novel Fluorescent Copper Nanomaterials and Their Application in Detection of Iodide Ions and Catalysis. Anal. Methods 11, 44–48. doi:10.1039/c8ay01871e
Wang, P., Mai, Z., Dai, Z., Li, Y., and Zou, X. (2009). Construction of Au Nanoparticles on Choline Chloride Modified Glassy Carbon Electrode for Sensitive Detection of Nitrite. Biosens. Bioelectron. 24, 3242–3247. doi:10.1016/j.bios.2009.04.006
Zhang, D., Hu, J., Yang, X.-y., Wu, Y., Su, W., and Zhang, C.-Y. (2018). Target-initiated Synthesis of Fluorescent Copper Nanoparticles for the Sensitive and Label-free Detection of Bleomycin. Nanoscale 10, 11134–11142. doi:10.1039/c8nr02780c
Zhao, Z., and Li, Y. (2020). Developing Fluorescent Copper Nanoclusters: Synthesis, Properties, and Applications. Colloids Surf. B-Biointerfaces 195, 111244. doi:10.1016/j.colsurfb.2020.111244
Zhong, Y., Wang, Q., He, Y., Ge, Y., and Song, G. (2015). A Novel Fluorescence and Naked Eye Sensor for Iodide in Urine Based on the Iodide Induced Oxidative Etching and Aggregation of Cu Nanoclusters. Sensors Actuators B: Chem. 209, 147–153. doi:10.1016/j.snb.2014.11.060
Keywords: CuNPs, nitrite, temperature detection, nanomaterial, fluorescence
Citation: Wang N, Ga L, Ai J and Wang Y (2022) Fluorescent Copper Nanomaterials for Sensing NO2− and Temperature. Front. Chem. 9:805205. doi: 10.3389/fchem.2021.805205
Received: 30 October 2021; Accepted: 16 December 2021;
Published: 25 January 2022.
Edited by:
Muhammad Asif, Wuhan Institute of Technology, ChinaReviewed by:
Ayesha Aziz, Huazhong University of Science and Technology, ChinaMuhammad Wajid Ullah, Jiangsu University, China
Copyright © 2022 Wang, Ga, Ai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Ai, aW1hY2FqMDFAMTYzLmNvbQ==
 Ning Wang1
Ning Wang1 Jun Ai
Jun Ai