Corrigendum: Inhibition of SARS-CoV-2 by Targeting Conserved Viral RNA Structures and Sequences
- Department of Medicinal Chemistry, University of Kansas, Lawrence, KS, United States
The ongoing COVID-19/Severe Acute Respiratory Syndrome CoV-2 (SARS-CoV-2) pandemic has become a significant threat to public health and has hugely impacted societies globally. Targeting conserved SARS-CoV-2 RNA structures and sequences essential for viral genome translation is a novel approach to inhibit viral infection and progression. This new pharmacological modality compasses two classes of RNA-targeting molecules: 1) synthetic small molecules that recognize secondary or tertiary RNA structures and 2) antisense oligonucleotides (ASOs) that recognize the RNA primary sequence. These molecules can also serve as a “bait” fragment in RNA degrading chimeras to eliminate the viral RNA genome. This new type of chimeric RNA degrader is recently named ribonuclease targeting chimera or RIBOTAC. This review paper summarizes the sequence conservation in SARS-CoV-2 and the current development of RNA-targeting molecules to combat this virus. These RNA-binding molecules will also serve as an emerging class of antiviral drug candidates that might pivot to address future viral outbreaks.
Introduction
SARS-CoV-2’s Life Cycle and “Druggable” Targets
SARS-CoV-2 belongs to the betacoronavirus genus and is an enveloped ssRNA (+) virus with a genome length of about 30,000 nucleotides (RefSeq NC_045512) (Wu et al., 2020). The viral genome is 5’ capped and 3’ polyadenylated (Robson et al., 2020) so that it is recognized and treated as an mRNA by the host cell ribosome. Two-thirds of the viral genome at the 5’-end have two long open reading frames (ORFs), ORF1a and ORF1ab, encoding two replicase-associated polyprotein precursors, pp1a and pp1ab (Figure 1). These polyprotein precursors are cleaved by viral proteases into 16 non-structural proteins (nsps) (Kim et al., 2020), some of which have essential viral functions (Figure 1). For example, an RNA-dependent RNA polymerase (RdRP) complex consisting of nsp12 in pp1ab and nsps7 and 8 in pp1a is required for viral transcription and replication (Hillen et al., 2020). RdRP is the core enzyme in the viral “replication-transcription complex” (RTC) (Fung and Liu, 2019). The RTC then promotes 3’→5’ replication of the (–) viral genome to form a full-length double-stranded (ds) RNA in the endoplasmic reticulum (ER) membrane invaginations (Knoops et al., 2008). This dsRNA then serves as a template for transcribing the genomic and subgenomic RNAs by RTC-mediated transcription in the 5’→3’ direction (Wu and Brian, 2010) (Figure 1). RNA transcription for each coronavirus structural protein is accomplished through a “discontinuous” mechanism. The RTC binds to the leader transcriptional regulatory sequences (TRS-L) in the 5’ UTR and then “hops” onto the body TRS (TRS-B) sequence. These TRS-B sequences locate at the 5’-end of each structural gene for transcription (Zúñiga et al., 2004; Sola et al., 2015). After completing structural protein synthesis and genomic RNA replication, new coronavirus particles are assembled at the host ER and released through the Golgi apparatus to complete the viral life cycle (Sawicki et al., 2007).
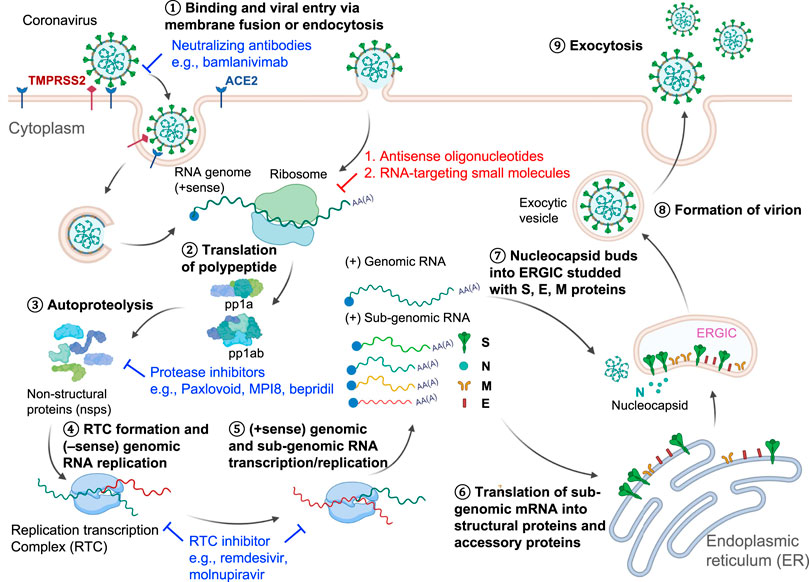
FIGURE 1. SARS-CoV-2 Life cycle and viral targets for antiviral development. ① The virus enters the host cell through endocytosis using spike protein-angiotensin-converting enzyme 2 (ACE2) interaction. ② The host ribosome then translates the positive-sense RNA genome. ③ The long polypeptide precursor is subsequently cleaved by the viral proteases into non-structural proteins (nsp), which will assemble the replication-transcription complex (RTC) for ④ viral RNA genome replication in the 3’→5’ direction and ⑤ transcription in the 5’→3’ direction for the whole genome and sub-genomic sequences. ⑥ The host ribosome further translates the sub-genomic sequences that encode the nucleocapsid proteins. ⑦–⑧ Newly synthesized nucleocapsid components are assembled in the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) to form the infectious virions, which are ⑨ released from the cell by maturation in the budding process. Some anti-SARS-CoV-2 agents illustrated in this figure include spike protein neutralizing antibody bamlanivimab, main protease inhibitors PF-07321332, MPI8, and bepridil, and RdRP inhibitors remdesivir and molnupiravir.
Current drug development pipelines have tackled different steps in the life cycle of SARS-CoV-2 (Figure 1). Spike protein-targeting antibodies (e.g., bamlanivimab) can effectively neutralize the virus and prevent viral entry (Gottlieb et al., 2021). RNA-targeting antisense oligonucleotides (ASO) or small molecules will degrade the viral RNA genome or hinder RNA translation (Li et al., 2021b, Li et al., 2021a.; Lulla et al., 2021; Rosenke et al., 2021; Sun et al., 2021a; Zhang et al., 2021; Zhu et al., 2021). The SARS-CoV-2 main protease (Mpro) is also an attractive drug target. PF-07321332 (Paxlovoid) was developed as an oral drug targeting Mpro and is being tested in a Phase 3 clinical trial (ClinicalTrials.gov Identifier: NCT04960202) (Owen et al., 2021). Other reported Mpro inhibitors such as an FDA-approved drug, bepridil, and a peptoid MPI8 were demonstrated to have efficacy in virus-infected cells (Ma et al., 2021; Vatansever et al., 2021). RdRP inhibitors remdesivir and molnupiravir, which impede the RNA replication/transcription processes, both showed clinical improvement in the COVID-19 patients (Wang et al., 2020; Fischer et al., 2021). In this review, we focused on the RNA-targeting approach, an emerging antiviral pharmacological modality that is complementary to traditional protein-targeting methods. An advantage of ASO-based drug development is the ability to rapidly generate drug candidates, which recognize the primary sequences of viral RNAs. The off-targets of the ASOs can also be quickly identified through experiments or predictive algorithms based on the primary sequences (Hagedorn et al., 2018; Yoshida et al., 2019). Compared to the ASO-based drug discovery, RNA-targeting small molecules are a relatively underdeveloped field. To date, only one non-ribosomal RNA binding molecule, risdiplam, has been approved by the FDA (Jaklevic, 2020). We envision that the chemical space, potency, off-targets for RNA-binding small molecules will be further investigated as therapeutics to antivirals and other human diseases (Hargrove, 2020; Meyer et al., 2020; Ursu et al., 2020). RNA-targeting molecules will probably synergically inhibit viral replication when combined with protein-targeting drugs in cocktail therapies.
Conserved RNA Sequences and Structures in SARS-CoV-2
The mutation rate of SARS-CoV-2 is estimated at 1 × 10−3 substitutions per base (30 nucleotides/genome) per year under neutral genetic drift conditions (van Dorp et al., 2020), or 1 × 10−5–1×10−4 substitutions per base (0.3–3 nucleotides/genome) in each transmission events from population phylodynamic studies (Van Egeren et al., 2021). This rate is much slower than some other RNA viruses, such as influenza A virus (Manzanares-Meza and Medina-Contreras, 2020) and human immunodeficiency virus (HIV) (Van Egeren et al., 2021). As of December 2, 2021, five circulating variants of SARS-CoV-2 are classified as variants of concern (VOC) in the World Health Organization (https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/), including B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.617.2 (Delta), and B.1.1.529 (Omicron) variants. Among these variants, the spike protein (S) harbors most of the nucleotide mutations compared to the original genomic sequence isolated from Wuhan, China, in December 2019 (Figure 2). Some mutations occur beyond the protein-coding region. For example, a prevalent mutation C241U (c.–63C>U) exits in the 5’ untranslated region (UTR) of all four VOCs (Figure 3A).
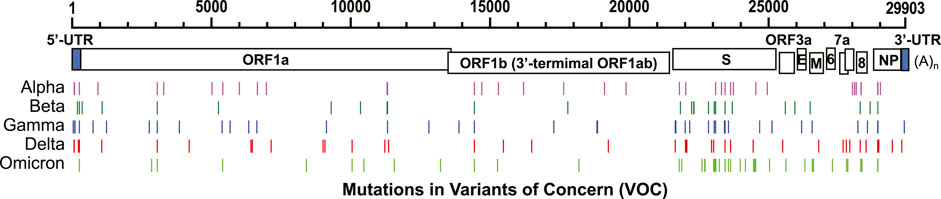
FIGURE 2. Nucleotide mutations in five VOCs compared to the original SARS-CoV-2 sequence discovered in Wuhan, China (RefSeq NC_045512). GenBank accession numbers: B.1.1.7 (Alpha): MZ344997, B.1.351 (Beta): MW598419, P.1 (Gamma): MZ169911, and B.1.617.2 (Delta): MZ359841. GISAID accession number: B.1.1.529 (Omicron): EPI_ISL_6795188.
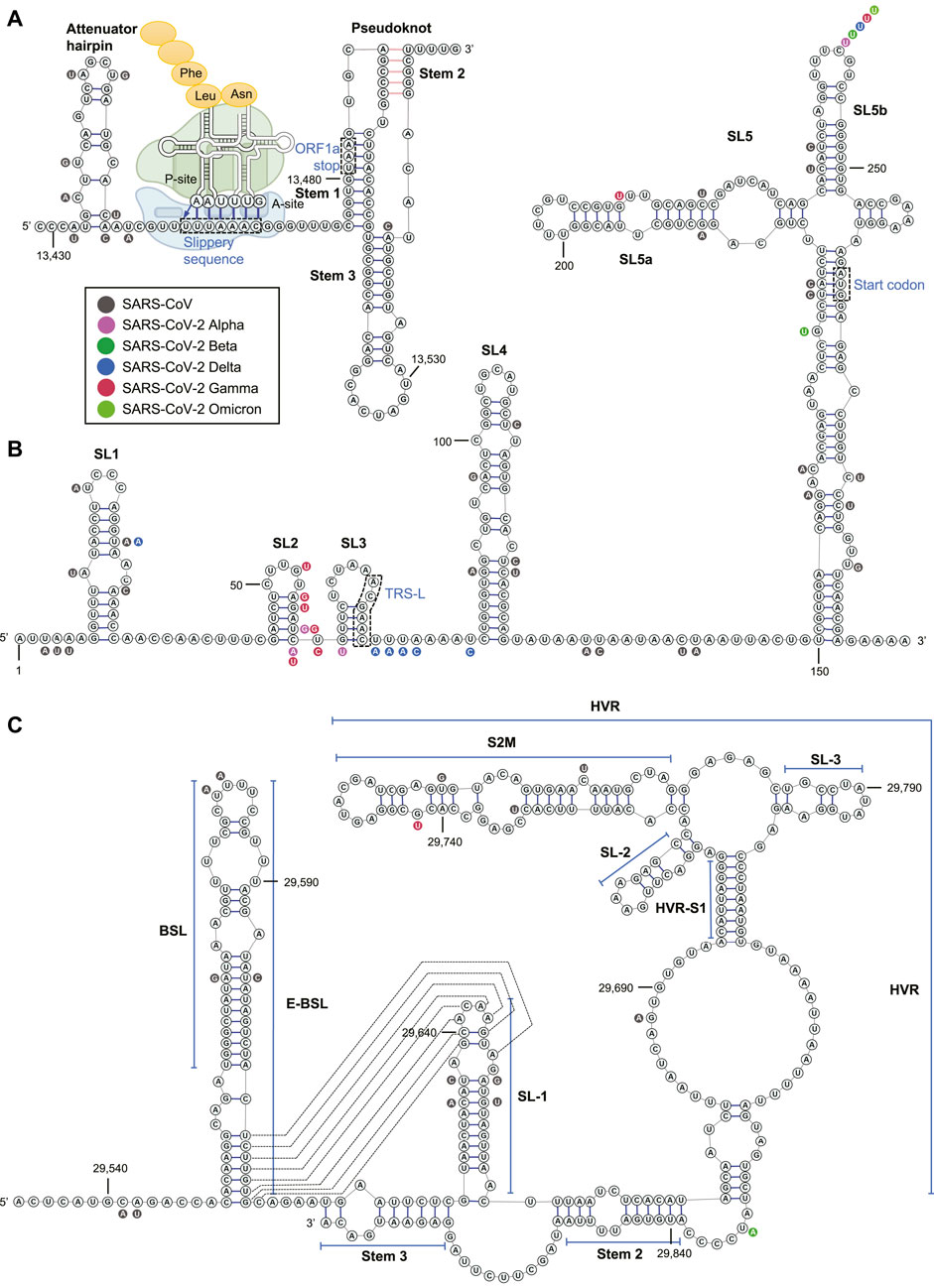
FIGURE 3. The RNA structure and nucleotide conservation of the (A) PFS element in SARS-CoV-2, (B) 5’ UTR, and (C) 3’ UTR. The ribosome acts on the slippery sequence to produce a –1 PFS is illustrated in (A).
By phylogenetic comparison of SARS-CoV-2, SARS-CoV, and SARS-related bat coronavirus sequences (Ceraolo and Giorgi, 2020), an earlier bioinformatics work from the Das group in 2020 identified 30 RNA regions as SARS-related conserved sequences and predicted 106 regions as SARS-CoV-2 conserved structures (Rangan et al., 2020). Shortly afterward, the RNA structures of SARS-CoV-2 were interrogated by chemical probing (Lan et al., 2020; Manfredonia et al., 2020; Sanders et al., 2020; Zhao et al., 2020; Sun et al., 2021a; Huston et al., 2021), psoralen crosslinking (Ziv et al., 2020), and NMR spectroscopy experiments (Wacker et al., 2020). Among all SARS-CoV-2 RNA structures identified, the 5’ and 3’ UTRs and a region named programmed –1 frameshift (PFS) element (13,459–13,546) in the ORF1a/ab have been intensively studied for their structures, functions, and druggability.
SARS-CoV-2 PFS Element
ORF1a is the 5’-terminal fraction of ORF1ab and has an in-frame stop codon at nucleotide 13,481. The correct translation of ORF1b (3’-terminal ORF1ab), which encodes the viral RdRP (nsp12), requires a PFS that shifts the ORF by –1 nucleotide via a “slippery sequence” to circumvent the ORF1a stop codon (Hagemeijer et al., 2012) (Figure 3A). Although the PFS element was not shown as a conserved structure in Das’ bioinformatics algorithm (Rangan et al., 2020), this region has demonstrated high-degree conservation among SARS-CoV and four VOC of CoV-2 (Figure 3A). The PFS element contains an attenuator hairpin (a negative regulator of the PFS), a slippery sequence (U_UUA_AAC motif), and a pseudoknot structure in betacoronavirus (Hagemeijer et al., 2012; Rangan et al., 2020) (Figure 3A). Once the ribosome recognizes the pseudoknotted structure, tRNAs in the ribosomal P- and A-sites re-bind to the –1 reading frame at the slippery sequence, and the ribosome starts to translate within the new reading frame (Bhatt et al., 2021) (Figure 3A). Without PFS, viral RNA translation would halt at the stop codon (13,481–13,483) within the pseudoknot (Figure 3A). It was demonstrated that the PFS element sequence alone could recapitulate the PFS activity without a protein cofactor in SARS-CoV (Baranov et al., 2005). The pseudoknotted structure was observed in NMR (Liphardt et al., 1999), chemical probing (Huston et al., 2021), cryo-EM (complexed with an elongating ribosome) (Bhatt et al., 2021), and X-ray crystallography (Roman et al., 2021).
SARS-CoV-2 UTRs
In the 5’ UTR (1–265), there are five stem-loops identified, SL1–5 (Figure 3B). SL1 was demonstrated to bind to nsp1 protein and cooperate in recruiting the human ribosome (Vankadari et al., 2020). SL5, which includes the genome start codon, is a four-helix junction essential for viral packaging (Escors et al., 2003) (Figure 3C). It is proposed that the structures of SL1, SL2, and SL4, but not the exact nucleotide sequences, play a more critical role in betacoronavirus function (Yang and Leibowitz, 2015).
In the 3’ UTR, three main secondary structures were elucidated by chemical probing: bulged stem-loop (BSL), SL-1, and the highly variable region (HVR) (Figure 3C). Bioinformatics analysis and reverse genetics suggested the pseudoknotted structure formation at the base stem of BSL and the SL-1 loop in SARS-CoV (Goebel et al., 2004) (Figure 3B). The equilibrium between the double stem-loop and pseudoknot was proposed to be a molecular switch in SARS-CoV RNA transcription (Yang and Leibowitz, 2015). This equilibrium model is also supported by quantitative covariation analysis (Rfam: RF11065) (Mathews et al., 2004). However, the pseudoknot was not observed as a stable structure at 37°C in NMR experiments in a model betacoronavirus, mouse hepatitis virus (MHV) (Stammler et al., 2011). Chemical probing experiments also suggested the unfavorable formation of pseudoknot (Zhao et al., 2020; Huston et al., 2021).
The HVR in the 3' UTR is not essential to betacoronavirus. The HVR can be deleted without affecting viral propagation in cell culture, albeit the HVR-deleted MHV strain has lower pathogenicity in mice (Goebel et al., 2007). Nevertheless, some sub-region of the HVR is highly conserved among betacoronavirus, such as the stable S2M (Rangan et al., 2020) (Figure 3C). The Stem 3 region duplexed by a sequence at the 3’-end of the viral genome and that between BSL and SL-1 (Figure 3C) was shown to be essential for the MHV viability (Goebel et al., 2007; Liu et al., 2013) and phylogenetically conserved (Züst et al., 2008), although chemical probing result suggested that the formation of Stem 3 is not favorable (Zhao et al., 2020). It was demonstrated by psoralen crosslinking that the 3’-end of the genome in the Stem 3 region can bind to the viral 5’ UTR and cyclize the SARS-CoV-2 genome (Ziv et al., 2020).
Among the 106 predicted conserved structured RNA regions by the Das group (Rangan et al., 2020), two locate in the 5’ UTR: SL2–4 and SL5 (SARS-CoV-2-conserved structure-8 and -16), and one reside in the 3’ UTR: BSL-SL-1 region (SARS-CoV-2-conserved structure-33) (structure numbers provided in Rangan et al., 2020).
Viral RNA-Targeting Strategies
RNA-Binding Small Molecules Targeting the SARS-CoV-2 RNA Genome
De novo design of nucleic acid ligands has been pursued for more than 35 years. The field was first pioneered by the Dervan group in optimizing DNA-binding molecules (Dervan, 1986), and then by the Disney group to identify selective RNA-binding molecules. In the recent 15 years, Disney and others have established that “the right” synthetic small molecules can indeed bind to RNA structures, but not the primary sequences, with a high degree of selectivity (Fedorova et al., 2018; Warner et al., 2018; Hargrove, 2020; Ursu et al., 2020).
Viruses make use of their RNA structures to hijack host cell functions and promote viral life cycle progression. These viral RNA structures have been chosen as druggable targets in small-molecule drug development. For example, HIV-1 uses trans-activator protein (Tat) to interact with a highly structured transactivation response (TAR) hairpin in its RNA to enhance the viral transcription (Sophie et al., 1990; Schulze-Gahmen and Hurley, 2018). Peptoid inhibitors targeting the TAR-Tat interaction have been shown to inhibit HIV-1 replication in vitro and in vivo (Hamy et al., 1997).
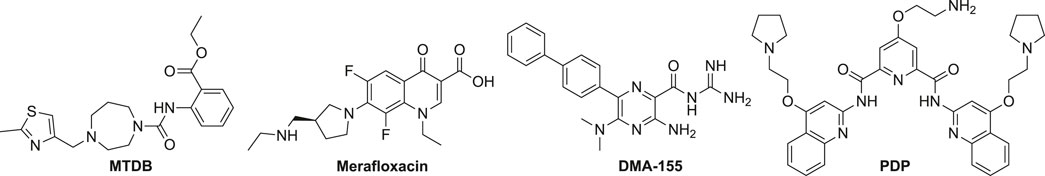
The discovery of RNA-targeting anti-SARS-CoV or CoV-2 small molecules primarily focused on the PFS element. MTDB was first identified by virtual screening and 3-dimensional (3D) modeling. MTBD can potently bind to the pseudoknot in the SARS-CoV PFS element and inhibit the PFS function in a dual luciferase system (Park et al., 2011) (Figure 4). The dual luciferase assay is widely used in discovering and validating PFS regulators. In this assay, the PFS element was placed in the junction of a Renilla/firefly fusion luciferase, and the fusion luciferase could only be produced when the PFS occurred (Harger and Dinman, 2003). It was demonstrated by small-angle X-ray scattering analysis and reverse genetics that the conformation and function of the pseudoknot in the PFS element between SARS-CoV and SARS-CoV-2 are highly similar (Kelly et al., 2020). Indeed, MTDB can also reduce the SARS-CoV-2 PFS activity by 60% (Kelly et al., 2020).
A mCherry/GFP dual fluorescent protein assay was used in a high-content imaging screen, which identified a novel small-molecule PFS inhibitor, merafloxacin (Figure 4). Merafloxacin had a half-maximal inhibitory concentration (IC50) in the dual fluorescent protein reporter cells at 19 μM and SARS-CoV-2-infected cells at 2.4 μM (Sun et al., 2021b). Merafloxacin belongs to the fluoroquinolone class known to interact with bacterial DNA and gyrase/topoisomerases (Aldred et al., 2014). Merafloxacin had a similar inhibitory effect to the reporter cells with mutated PFS elements, further suggesting that merafloxacin recognizes shape but not the primary sequence of the RNA (Sun et al., 2021b). Comparing MTDB and merafloxacin side-by-side, it was demonstrated that merafloxacin was a more potent inhibitor against PFS in SARS-CoV-2-infected Vero E6 cells (Bhatt et al., 2021).
Amiloride analogs (e.g., DMA-155, Figure 4) targeting the SARS-CoV-2 5’ UTR also exhibited antiviral activity in SARS-CoV-2-infected cells (Zafferani et al., 2020). NMR studies uncovered that SL4, SL5a, and SL6 could all bind to the amilorides (Zafferani et al., 2020). An RNA sequence (RG-1) having a high propensity to form a G-quadruplex (G4) in the SARS-CoV-2 genome was validated in the coding sequence of nucleocapsid phosphoprotein (N) in cells (Zhao et al., 2021). PDP was demonstrated to stabilize RG-1 G4 and reduce the protein levels of the viral N protein by inhibiting its translation both in vitro and in vivo (Zhao et al., 2021).
Several RNA-binding proteins (RBPs) in the host cells (e.g., IGF2BP1, hnRNP A1, and TIA1) were predicted to bind to the SARS-CoV-2 RNA genome (Sun et al., 2021a). Some FDA-approved small-molecule drugs, such as nilotinib, sorafenib, and deguelin, were demonstrated to interfere with essential RBP-viral RNA interactions and reduce the viral titer (Sun et al., 2021a). Strictly speaking, the targets of these drugs are host factors rather than viral RNA structures.
RNA-Binding ASOs Targeting the SARS-CoV-2 RNA Genome
Pharmacological Mechanisms of ASOs
ASOs are RNA or DNA sequences with 15–25 natural or modified nucleotides (Dhuri et al., 2020), which hybridize specifically via Watson-Crick base-pairing to a target RNA and modulate RNA splicing or gene expression (Roberts et al., 2020). ASOs generally act through two mechanisms in human cells: 1) cleaving of the target RNA via ASO-induced ribonuclease (RNase) H1 activity and 2) masking the target RNA from interaction with the human RBPs or the ribosome.
The ASOs used to induce RNase H1 activation are also termed “gapmers”. Gapmers usually contain a central DNA sequence (> 6 nucleotides) that hybridizes with the target RNA (Papargyri et al., 2020). RNase H1 is a ubiquitous ribonuclease found in the nucleus and the cytoplasm of all human cells (Crooke, 2017). RNase H1 specifically recognizes and hydrolyzes the RNA strand of the RNA-DNA heteroduplexes formed between the DNA block in the gapmer and the target RNA. Therefore, gapmers can be used to reduce the unwanted RNA level (i.e., gene knockdown) in a catalytic manner (Meng et al., 2015; Crooke, 2017). The DNA block in a gapmer is usually flanked (capped) by a short sequence of modified nucleotides to prevent exonuclease degradation.
“Masking” ASOs are commonly used as a steric block in the target RNA and, thereby, to modulate RNA splicing and suppress translation. The FDA has approved several ASOs acting through this mechanism to treat a variety of human diseases (Roberts et al., 2020; Tang et al., 2021a). For example, fomivirsen was the first FDA-approved ASO drug to treat cytomegalovirus (CMV) retinitis (approved in 1998; withdrawn in 2006 for lack of medical need) (Stein and Castanotto, 2017). Fomivirsen binds to the immediate early region 2 in the human CMV mRNA, halting the RNA translation of (IE)-2 protein which is crucial for viral replication (Geary et al., 2002). ASOs are also widely used for modulating RNA splicing in rare genetic diseases, such as Duchenne muscular dystrophy (DMD) and spinal muscular atrophy (SMA) (Tang et al., 2021b).
Chemical Modification in ASOs
Several chemical modifications of ASOs have been developed to improve their stability and cellular uptake (Crooke, 2017). For example, replacing the natural phosphodiester bridge with a phosphorothioate group in the ASO would significantly increase its half-life in vivo due to high serum protein binding and nuclease resistance (Temsamani et al., 1993). Phosphorothioate linkage in ASOs retains the RNase H1 recognition and is usually used throughout gapmers (Lulla et al., 2021). Alkylation of the 2’-OH in the ribose with a methoxyethyl group (MOE) in the ASO would enhance the hybridization stability and lessen the nonspecific binding (Dhuri et al., 2020). It is estimated that each MOE substitution increases the melting temperature (Tm) by 2 °C (Freier and Altmann, 1997). Locked nucleic acid (LNA) is a class of modified ribose where the 2’-OH is linked to the 4’-CH via a constrained methylene bridge (Singh et al., 1998). The constrained LNA maintains a preferable conformation in RNA binding and, therefore, would significantly increase the hybridization stability in ASOs (+2–4°C in Tm per LNA substitution) (Koshkin et al., 1998). One or more LNAs can be used in ASOs, and the ASOs with interspersed combination of LNA and DNA nucleotides are also termed “mixmers” (Bernardo et al., 2012). A popular ASO form in clinical use is based on a phosphorodiamidate morpholino oligomer (PMO) skeleton. PMOs have morpholine subunits instead of ribose/deoxyribose and are linked by the phosphorodiamidate group (Dhuri et al., 2020). PMOs have various advantages, including reduced nonspecific binding imparted by the neutral charge and complete nuclease resistance (Dhuri et al., 2020).
Anti-SARS-CoV-2 ASOs
By using 3D antisense modeling, a PMO named PRF3p was optimized to target the Stem 3 region in the PFS element (Li et al., 2021b) (Figure 5). The PRF3p binding disrupted the pseudoknotted structure in the PFS element and inhibited the frameshift, eventually leading to a knockdown of the genes encoded by the ORF1b in the virus-infected 293T cells (Li et al., 2021b). Gapmers S2D, S3D-1, S2D-2, and Slp-2 targeting PFS elements were reported to have efficacy in Huh-7 inoculated with SARS-CoV-2 with a luciferase reporter (Zhang et al., 2021).
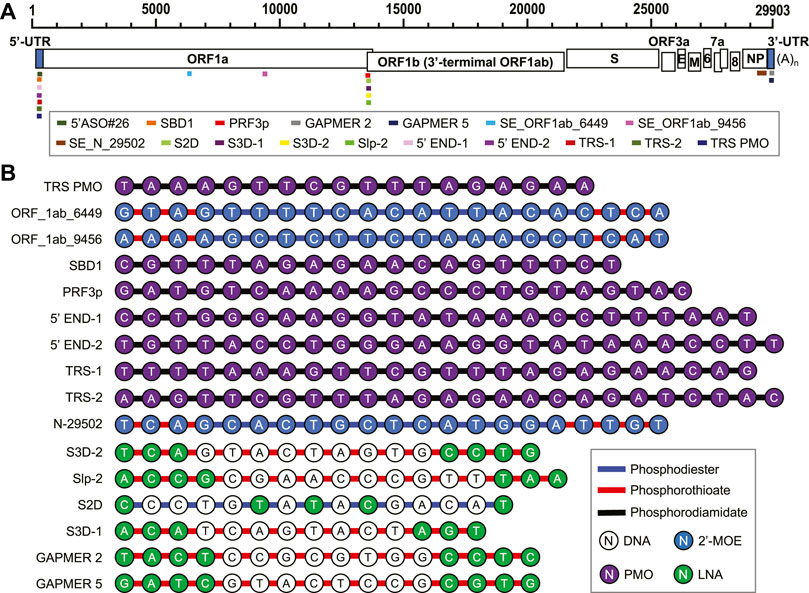
FIGURE 5. (A) Antiviral ASO binding sites in the SARS-CoV-2 genome. (B) Chemical composition of the anti-SARS-CoV-2 ASOs.
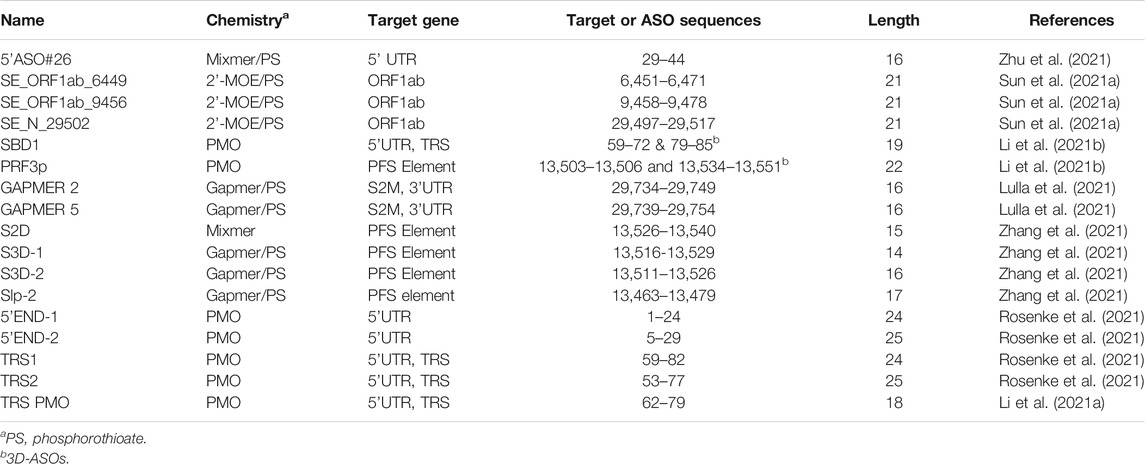
A PMO named SBD1 was designed to target the conserved TRS-L region in the SARS-CoV 5’ UTR (Figure 5), and thereby inhibited the “discontinuous” transcription (Li et al., 2021b). The suppression of sub-genomic RNA transcription ultimately led to the reduction of viral structural protein levels and virus titer (Li et al., 2021). Two PMOs, 5’END-1 and 5’END-2, targeted the viral 5’ UTR and were shown to inhibit the translation pre-initiation complex (Rosenke et al., 2021). The 5’-end of ORF1a is also a region for ASO-binding to have antiviral effects. Two 2’-MOE/phosphorothioate-modified ASOs targeting this region, SE_ORF1ab_6449 and SE_ORF1ab_9456, were reported to effectively inhibit SARS-CoV infection in Vero E6 cells (Figure 5) (Sun et al., 2021a). Gapmers 2 and 5 targeting the conserved S2M sequences in the 3’ UTR were demonstrated to have efficacy in degrading the viral RNA genome (Figure 5) (Lulla et al., 2021). The current development of ASO-based anti-SARS-CoV-2 agents is summarized in Table 1.
RNA-Degrading Chimeras
The RNA-degrading chimeras follow a well-established precedent from the protein field, namely, the proteolysis targeting chimera or PROTAC. PROTACs bind to their target protein using a guide arm as “bait”. The effector arm of PROTACs recruits an endogenous E3 ubiquitin ligase resulting in polyubiquitination and subsequent proteasomal degradation of the target protein (Schapira et al., 2019). The Disney group first extended this chimeric degrader concept to the RNA field by creating a ribonuclease targeting chimera (RIBOTAC) (Costales et al., 2018). RIBOTACs have been developed as a new class of chimeric molecules that use a guide arm to bind to the RNA sequence of interest. The effector arm of RIBOTAC would recruit the endogenous ribonuclease (RNase) L, causing degradation of the target RNA without affecting the host transcriptome (Costales et al., 2018; Disney, 2019; Costales et al., 2020; Liu et al., 2020; Meyer et al., 2020).
RNase L plays an essential role in an innate immune response pathway, namely the oligoadenylate synthetase (OAS)-RNase L pathway. In a viral infection, OAS senses dsRNA and synthesizes 2',5'-linked oligoadenylates (2–5A) that activate RNase L by dimerization (Naik et al., 1998). RNase L cleaves single-stranded (ss) RNA preferentially on UA, UG, and UU sites (Floyd-Smith et al., 1981; Wreschner et al., 1981), leading to global RNA degradation, arrest of protein synthesis, and apoptosis (Li et al., 2004). A small-molecule RNase L dimerizer (i.e., activator) was previously discovered (Kd = 18 µM to RNase L monomer), presenting a modest antiviral effect as a single agent against human parainfluenza virus in cells (Thakur et al., 2007). The structure of this RNase L dimerizer was further modified to serve as an RNase L recruiter fragment in RIBOTAC (Costales et al., 2018; Costales et al., 2020; Haniff et al., 2020; Liu et al., 2020). Recently, the Disney group discovered a series of compounds that bound to the attenuator hairpin in the PFS element and used them as the guide arm for the RIBOTAC modality (Haniff et al., 2020). One of the small-molecule RIBOTACs, C5-RIBOTAC, has been shown to reduce SARS-CoV-2 RNA levels in a cellular model (Figure 6A) (Haniff et al., 2020).
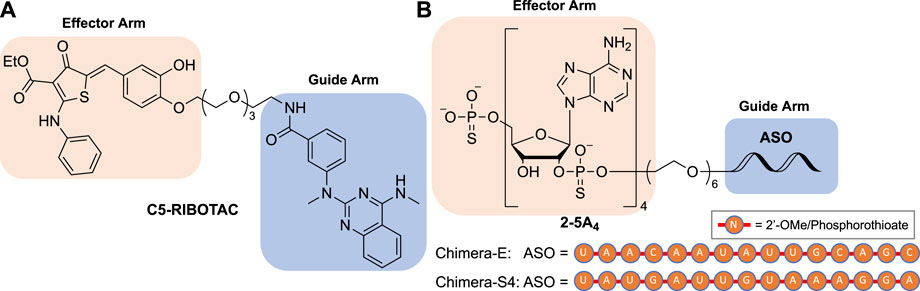
FIGURE 6. Structures of (A) small molecule-based and (B) ASO-based RIBOTACs targeting the SARS-CoV-2 RNA genome.
Following the first small-molecule RIBOTAC report, another type of nucleic acid-based RIBOTAC targeting SARS-CoV-2 demonstrating efficacy in virus-infected cells was also disclosed (Su et al., 2021). This type of RIBOTACs target the spike or envelope protein coding RNA using a 15-nucleotide complementary antisense oligonucleotide (ASO) as the guide arm and a 2',5'-linked tetraadenylate (2–5A4) as an RNase L recruiter (Figure 6B). These RIBOTACs have been shown to reduce viral titer in virus-infected Vero E6 cells (Su et al., 2021).
Discussion
Molecules targeting conserved viral RNA sequences and structures are a newly emerged pharmacological modality that can significantly expand our antiviral arsenal. ASOs that recognize primary viral RNA sequences can be rapidly designed and optimized in early drug discovery. The major obstacles to the clinical use of ASOs are the unfavorable cellular uptake and distribution (Moschos et al., 2011; Geary et al., 2015). Recently, administration by inhalation has shown promising results in ASO delivery in lung tissues (Crosby et al., 2017; Berber et al., 2021), which will probably be useful for the treatment of respiratory viruses, such as SARS-CoV-2. Other technologies in ASO delivery have been advanced in the field, such as liposome-enclosed ASOs (Garbuzenko et al., 2010) and ASOs conjugated with cell-penetrating peptides (CPPs) (McClorey and Banerjee, 2018). These technologies have the potential to further improve the pharmacokinetics of ASOs as antivirals.
Targeting RNA structures will broaden the spectrum of the small-molecule “druggability”. Compared to traditional protein targets in viruses, such as RdRP and proteases, a completely different target specificity will be obtained for RNA ligands. As illustrated in the SARS-CoV-2 5’ UTR, the RNA structures but not the exact sequences are conserved across betacoronavirus strains (Yang and Leibowitz, 2015). Such structural conservation will likely make the structure-recognizing small molecules cross-active within the viral genus. Despite the above promising features, the in vivo activity and toxicity profile of RNA-targeting small molecules as antivirals are still obscure. Major efforts are required to address these issues before RNA-targeting molecules can be used as antiviral drugs in clinics.
Conclusion
Fueled by the current advances in RNA-binding small molecules, ASOs, and RNA-degrading chimeras, RNA-targeting strategies have already been demonstrated the use in inhibiting SARS-CoV-2. With further advances in structure modeling for RNAs and understanding of the RNA-ligand interactions, the RNA-targeting drug discovery platforms have the potential to quickly generate antiviral candidates to address future viral outbreaks.
Author Contributions
SH, ZT, JZ, and JW wrote the manuscript. ZT analyzed the sequencing data.
Funding
This review was supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health under award number P20GM113117 and the University of Kansas (KU) One University Open Access Author Fund sponsored jointly by the KU Provost, KU Vice Chancellor for Research & Graduate Studies, and KUMC Vice Chancellor for Research and managed jointly by the Libraries at the Medical Center and KU – Lawrence.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aldred, K. J., Kerns, R. J., and Osheroff, N. (2014). Mechanism of Quinolone Action and Resistance. Biochemistry 53, 1565–1574. doi:10.1021/bi5000564
Baranov, P. V., Henderson, C. M., Anderson, C. B., Gesteland, R. F., Atkins, J. F., and Howard, M. T. (2005). Programmed Ribosomal Frameshifting in Decoding the SARS-CoV Genome. Virology 332, 498–510. doi:10.1016/j.virol.2004.11.038
Berber, B., Aydin, C., Kocabas, F., Guney-Esken, G., Yilancioglu, K., Karadag-Alpaslan, M., et al. (2021). Gene Editing and RNAi Approaches for COVID-19 Diagnostics and Therapeutics. Gene Ther. 28, 290–305. doi:10.1038/s41434-020-00209-7
Bernardo, B. C., Gao, X.-M., Winbanks, C. E., Boey, E. J. H., Tham, Y. K., Kiriazis, H., et al. (2012). Therapeutic Inhibition of the miR-34 Family Attenuates Pathological Cardiac Remodeling and Improves Heart Function. Proc. Natl. Acad. Sci. 109, 17615–17620. doi:10.1073/pnas.1206432109
Bhatt, P. R., Scaiola, A., Loughran, G., Leibundgut, M., Kratzel, A., Meurs, R., et al. (2021). Structural Basis of Ribosomal Frameshifting during Translation of the SARS-CoV-2 RNA Genome. Science 372, 1306–1313. doi:10.1126/science.abf3546
Ceraolo, C., and Giorgi, F. M. (2020). Genomic Variance of the 2019‐nCoV Coronavirus. J. Med. Virol. 92, 522–528. doi:10.1002/jmv.25700
Costales, M. G., Aikawa, H., Li, Y., Childs-Disney, J. L., Abegg, D., Hoch, D. G., et al. (2020). Small-molecule Targeted Recruitment of a Nuclease to Cleave an Oncogenic RNA in a Mouse Model of Metastatic Cancer. Proc. Natl. Acad. Sci. USA 117, 2406–2411. doi:10.1073/pnas.1914286117
Costales, M. G., Matsumoto, Y., Velagapudi, S. P., and Disney, M. D. (2018). Small Molecule Targeted Recruitment of a Nuclease to RNA. J. Am. Chem. Soc. 140, 6741–6744. doi:10.1021/jacs.8b01233
Crooke, S. T. (2017). Molecular Mechanisms of Antisense Oligonucleotides. Nucleic Acid Ther. 27, 70–77. doi:10.1089/nat.2016.0656
Crosby, J. R., Zhao, C., Jiang, C., Bai, D., Katz, M., Greenlee, S., et al. (2017). Inhaled ENaC Antisense Oligonucleotide Ameliorates Cystic Fibrosis-like Lung Disease in Mice. J. Cystic Fibrosis 16, 671–680. doi:10.1016/j.jcf.2017.05.003
Dervan, P. B. (1986). Design of Sequence-specific DNA-Binding Molecules. Science 232, 464–471. doi:10.1126/science.2421408
Dhuri, K., Bechtold, C., Quijano, E., Pham, H., Gupta, A., Vikram, A., et al. (2020). Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 9, 2004–1373. doi:10.3390/jcm9062004
Disney, M. D. (2019). Targeting RNA with Small Molecules to Capture Opportunities at the Intersection of Chemistry, Biology, and Medicine. J. Am. Chem. Soc. 141, 6776–6790. doi:10.1021/jacs.8b13419
Escors, D., Izeta, A., Capiscol, C., and Enjuanes, L. (2003). Transmissible Gastroenteritis Coronavirus Packaging Signal Is Located at the 5′ End of the Virus Genome. J. Virol. 77, 7890–7902. doi:10.1128/jvi.77.14.7890-7902.2003
Fedorova, O., Jagdmann, G. E., Adams, R. L., Yuan, L., Van Zandt, M. C., and Pyle, A. M. (2018). Small Molecules that Target Group II Introns Are Potent Antifungal Agents. Nat. Chem. Biol. 14, 1073–1078. doi:10.1038/s41589-018-0142-0
Fischer, W., Eron, J. J., Holman, W., Cohen, M. S., Fang, L., Szewczyk, L. J., et al. (2021). Molnupiravir, an Oral Antiviral Treatment for COVID-19. medRxiv. doi:10.1101/2021.06.17.21258639
Floyd-Smith, G., Slattery, E., and Lengyel, P. (1981). Interferon Action: RNA Cleavage Pattern of a (2′-5′)Oligoadenylate-dependent Endonuclease. Science 212, 1030–1032. doi:10.1126/science.6165080
Freier, S., and Altmann, K. H. (1997). The Ups and downs of Nucleic Acid Duplex Stability: Structure-Stability Studies on Chemically-Modified DNA:RNA Duplexes. Nucleic Acids Res. 25, 4429–4443. doi:10.1093/nar/25.22.4429
Fung, T. S., and Liu, D. X. (2019). Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol. 73, 529–557. doi:10.1146/annurev-micro-020518-115759
Garbuzenko, O. B., Saad, M., Pozharov, V. P., Reuhl, K. R., Mainelis, G., and Minko, T. (2010). Inhibition of Lung Tumor Growth by Complex Pulmonary Delivery of Drugs with Oligonucleotides as Suppressors of Cellular Resistance. Proc. Natl. Acad. Sci. 107, 10737–10742. doi:10.1073/pnas.1004604107
Geary, R. S., Henry, S. P., and Grillone, L. R. (2002). Fomivirsen. Clin. Pharmacokinet. 41, 255–260. doi:10.2165/00003088-200241040-00002
Geary, R. S., Norris, D., Yu, R., and Bennett, C. F. (2015). Pharmacokinetics, Biodistribution and Cell Uptake of Antisense Oligonucleotides. Adv. Drug Deliv. Rev. 87, 46–51. doi:10.1016/j.addr.2015.01.008
Goebel, S. J., Hsue, B., Dombrowski, T. F., and Masters, P. S. (2004). Characterization of the RNA Components of a Putative Molecular Switch in the 3′ Untranslated Region of the Murine Coronavirus Genome. J. Virol. 78, 669–682. doi:10.1128/jvi.78.2.669-682.2004
Goebel, S. J., Miller, T. B., Bennett, C. J., Bernard, K. A., and Masters, P. S. (2007). A Hypervariable Region within the 3′ Cis -Acting Element of the Murine Coronavirus Genome Is Nonessential for RNA Synthesis but Affects Pathogenesis. J. Virol. 81, 1274–1287. doi:10.1128/JVI.00803-06
Gottlieb, R. L., Nirula, A., Chen, P., Boscia, J., Heller, B., Morris, J., et al. (2021). Effect of Bamlanivimab as Monotherapy or in Combination with Etesevimab on Viral Load in Patients with Mild to Moderate COVID-19. JAMA 325, 632–644. doi:10.1001/jama.2021.0202
Hagedorn, P. H., Pontoppidan, M., Bisgaard, T. S., Berrera, M., Dieckmann, A., Ebeling, M., et al. (2018). Identifying and Avoiding Off-Target Effects of RNase H-dependent Antisense Oligonucleotides in Mice. Nucleic Acids Res. 46, 5366–5380. doi:10.1093/nar/gky397
Hagemeijer, M., Rottier, P., and Haan, C. (2012). Biogenesis and Dynamics of the Coronavirus Replicative Structures. Viruses 4, 3245–3269. doi:10.3390/v4113245
Hamy, F., Felder, E. R., Heizmann, G., Lazdins, J., Aboul-ela, F., Varani, G., et al. (1997). An Inhibitor of the Tat/TAR RNA Interaction that Effectively Suppresses HIV-1 Replication. Proc. Natl. Acad. Sci. 94, 3548–3553. doi:10.1073/pnas.94.8.3548
Haniff, H. S., Tong, Y., Liu, X., Chen, J. L., Suresh, B. M., Andrews, R. J., et al. (2020). Targeting the SARS-CoV-2 RNA Genome with Small Molecule Binders and Ribonuclease Targeting Chimera (RIBOTAC) Degraders. ACS Cent. Sci. 6, 1713–1721. doi:10.1021/acscentsci.0c00984
Harger, J. W., and Dinman, J. D. (2003). An In Vivo Dual-Luciferase Assay System for Studying Translational Recoding in the Yeast Saccharomyces cerevisiae. RNA 9, 1019–1024. doi:10.1261/rna.5930803
Hargrove, A. E. (2020). Small Molecule-RNA Targeting: Starting with the Fundamentals. Chem. Commun. 56, 14744–14756. doi:10.1039/D0CC06796B
Hillen, H. S., Kokic, G., Farnung, L., Dienemann, C., Tegunov, D., and Cramer, P. (2020). Structure of Replicating SARS-CoV-2 Polymerase. Nature 584, 154–156. doi:10.1038/s41586-020-2368-8
Huston, N. C., Wan, H., Strine, M. S., de Cesaris Araujo Tavares, R., Wilen, C. B., and Pyle, A. M. (2021). Comprehensive In Vivo Secondary Structure of the SARS-CoV-2 Genome Reveals Novel Regulatory Motifs and Mechanisms. Mol. Cel 81, 584–598. e5. doi:10.1016/j.molcel.2020.12.041
Jaklevic, M. C. (2020). Oral Drug Approved for Spinal Muscular Atrophy. JAMA 324, 1026. doi:10.1001/jama.2020.16783
Kelly, J. A., Olson, A. N., Neupane, K., Munshi, S., San Emeterio, J., Pollack, L., et al. (2020). Structural and Functional Conservation of the Programmed −1 Ribosomal Frameshift Signal of SARS Coronavirus 2 (SARS-CoV-2). J. Biol. Chem. 295, 10741–10748. doi:10.1074/jbc.AC120.013449
Kim, D., Lee, J.-Y., Yang, J.-S., Kim, J. W., Kim, V. N., and Chang, H. (2020). The Architecture of SARS-CoV-2 Transcriptome. Cell 181, 914–921. e10. doi:10.1016/j.cell.2020.04.011
Knoops, K., Kikkert, M., Worm, S. H. E. v. d., Zevenhoven-Dobbe, J. C., van der Meer, Y., Koster, A. J., et al. (2008). SARS-coronavirus Replication Is Supported by a Reticulovesicular Network of Modified Endoplasmic Reticulum. Plos Biol. 6, e226. doi:10.1371/journal.pbio.0060226
Koshkin, A. A., Singh, S. K., Nielsen, P., Rajwanshi, V. K., Kumar, R., Meldgaard, M., et al. (1998). LNA (Locked Nucleic Acids): Synthesis of the Adenine, Cytosine, Guanine, 5-methylcytosine, Thymine and Uracil Bicyclonucleoside Monomers, Oligomerisation, and Unprecedented Nucleic Acid Recognition. Tetrahedron 54, 3607–3630. doi:10.1016/S0040-4020(98)00094-5
Lan, T. C. T., Allan, M. F., Malsick, L. E., Khandwala, S., Nyeo, S. S. Y., Sun, Y., et al. (2020). Insights into the Secondary Structural Ensembles of the Full SARS-CoV-2 RNA Genome in Infected Cells. bioRxiv. doi:10.1101/2020.06.29.178343
Li, C., Callahan, A. J., Simon, M. D., Totaro, K. A., Mijalis, A. J., Phadke, K.-S., et al. (2021a). Fully Automated Fast-Flow Synthesis of Antisense Phosphorodiamidate Morpholino Oligomers. Nat. Commun. 12, 4396. doi:10.1038/s41467-021-24598-4
Li, G., Xiang, Y., Sabapathy, K., and Silverman, R. H. (2004). An Apoptotic Signaling Pathway in the Interferon Antiviral Response Mediated by RNase L and C-Jun NH2-terminal Kinase. J. Biol. Chem. 279, 1123–1131. doi:10.1074/jbc.M305893200
Li, Y., Garcia, G., Arumugaswami, V., and Guo, F. (2021b). Structure-based Design of Antisense Oligonucleotides that Inhibit SARS-CoV-2 Replication. bioRxiv. doi:10.1101/2021.08.23.457434
Liphardt, J., Napthine, S., Kontos, H., and Brierley, I. (1999). Evidence for an RNA Pseudoknot Loop-helix Interaction Essential for Efficient −1 Ribosomal Frameshifting. J. Mol. Biol. 288, 321–335. doi:10.1006/jmbi.1999.2689
Liu, P., Yang, D., Carter, K., Masud, F., and Leibowitz, J. L. (2013). Functional Analysis of the Stem Loop S3 and S4 Structures in the Coronavirus 3′UTR. Virology 443, 40–47. doi:10.1016/j.virol.2013.04.021
Liu, X., Haniff, H. S., Childs-Disney, J. L., Shuster, A., Aikawa, H., Adibekian, A., et al. (2020). Targeted Degradation of the Oncogenic MicroRNA 17-92 Cluster by Structure-Targeting Ligands. J. Am. Chem. Soc. 142, 6970–6982. doi:10.1021/jacs.9b13159
Lulla, V., Wandel, M. P., Bandyra, K. J., Ulferts, R., Wu, M., Dendooven, T., et al. (2021). Targeting the Conserved Stem Loop 2 Motif in the SARS-CoV-2 Genome. J. Virol. 95, e0066321. doi:10.1128/JVI.00663-21
Ma, X. R., Alugubelli, Y. R., Ma, Y., Vatansever, E. C., Scott, D. A., Qiao, Y., et al. (2021). MPI8 Is Potent against SARS‐CoV‐2 by Inhibiting Dually and Selectively the SARS‐CoV‐2 Main Protease and the Host Cathepsin L. ChemMedChem. doi:10.1002/cmdc.202100456
Manfredonia, I., Nithin, C., Ponce-Salvatierra, A., Ghosh, P., Wirecki, T. K., Marinus, T., et al. (2020). Genome-wide Mapping of SARS-CoV-2 RNA Structures Identifies Therapeutically-Relevant Elements. Nucleic Acids Res. 48, 12436–12452. doi:10.1093/nar/gkaa1053
Manzanares-Meza, L. D., and Medina-Contreras, O. (2020). SARS-CoV-2 and Influenza: a Comparative Overview and Treatment Implications. Boletín Médico Del. Hosp. Infantil de México 77, 262–273. doi:10.24875/BMHIM.20000183
Mathews, D. H., Disney, M. D., Childs, J. L., Schroeder, S. J., Zuker, M., and Turner, D. H. (2004). Incorporating Chemical Modification Constraints into a Dynamic Programming Algorithm for Prediction of RNA Secondary Structure. Proc. Natl. Acad. Sci. 101, 7287–7292. doi:10.1073/pnas.0401799101
McClorey, G., and Banerjee, S. (2018). Cell-Penetrating Peptides to Enhance Delivery of Oligonucleotide-Based Therapeutics. Biomedicines 6, 51. doi:10.3390/biomedicines6020051
Meng, L., Ward, A. J., Chun, S., Bennett, C. F., Beaudet, A. L., and Rigo, F. (2015). Towards a Therapy for Angelman Syndrome by Targeting a Long Non-coding RNA. Nature 518, 409–412. doi:10.1038/nature13975
Meyer, S. M., Williams, C. C., Akahori, Y., Tanaka, T., Aikawa, H., Tong, Y., et al. (2020). Small Molecule Recognition of Disease-Relevant RNA Structures. Chem. Soc. Rev. 49, 7167–7199. doi:10.1039/d0cs00560f
Moschos, S. A., Frick, M., Taylor, B., Turnpenny, P., Graves, H., Spink, K. G., et al. (2011). Uptake, Efficacy, and Systemic Distribution of Naked, Inhaled Short Interfering RNA (siRNA) and Locked Nucleic Acid (LNA) Antisense. Mol. Ther. 19, 2163–2168. doi:10.1038/mt.2011.206
Naik, S., Paranjape, J. M., and Silverman, R. H. (1998). RNase L Dimerization in a Mammalian Two-Hybrid System in Response to 2',5'-oligoadenylates. Nucleic Acids Res. 26, 1522–1527. doi:10.1093/nar/26.6.1522
Owen, D. R., Allerton, C. M. N., Anderson, A. S., Aschenbrenner, L., Avery, M., Berritt, S., et al. (2021). An Oral SARS-CoV-2 M Pro Inhibitor Clinical Candidate for the Treatment of COVID-19. Science, eabl4784. doi:10.1126/science.abl4784
Papargyri, N., Pontoppidan, M., Andersen, M. R., Koch, T., and Hagedorn, P. H. (2020). Chemical Diversity of Locked Nucleic Acid-Modified Antisense Oligonucleotides Allows Optimization of Pharmaceutical Properties. Mol. Ther. - Nucleic Acids 19, 706–717. doi:10.1016/j.omtn.2019.12.011
Park, S.-J., Kim, Y.-G., and Park, H.-J. (2011). Identification of RNA Pseudoknot-Binding Ligand that Inhibits the −1 Ribosomal Frameshifting of SARS-Coronavirus by Structure-Based Virtual Screening. J. Am. Chem. Soc. 133, 10094–10100. doi:10.1021/ja1098325
Rangan, R., Zheludev, I. N., Hagey, R. J., Pham, E. A., Wayment-Steele, H. K., Glenn, J. S., et al. (2020). RNA Genome Conservation and Secondary Structure in SARS-CoV-2 and SARS-Related Viruses: a First Look. RNA 26, 937–959. doi:10.1261/rna.076141.120
Roberts, T. C., Langer, R., and Wood, M. J. A. (2020). Advances in Oligonucleotide Drug Delivery. Nat. Rev. Drug Discov. 19, 673–694. doi:10.1038/s41573-020-0075-7
Robson, F., Khan, K. S., Le, T. K., Paris, C., Demirbag, S., Barfuss, P., et al. (2020). Coronavirus RNA Proofreading: Molecular Basis and Therapeutic Targeting. Mol. Cel 79, 710–727. doi:10.1016/j.molcel.2020.07.027
Roman, C., Lewicka, A., Koirala, D., Li, N.-S., and Piccirilli, J. A. (2021). The SARS-CoV-2 Programmed −1 Ribosomal Frameshifting Element Crystal Structure Solved to 2.09 Å Using Chaperone-Assisted RNA Crystallography. ACS Chem. Biol. 16, 1469–1481. doi:10.1021/acschembio.1c00324
Rosenke, K., Leventhal, S., Moulton, H. M., Hatlevig, S., Hawman, D., Feldmann, H., et al. (2021). Inhibition of SARS-CoV-2 in Vero Cell Cultures by Peptide-Conjugated Morpholino Oligomers. J. Antimicrob. Chemother. 76, 413–417. doi:10.1093/jac/dkaa460
Roy, S., Delling, U., Chen, C. H., Rosen, C. A., and Sonenberg, N. (1990). A Bulge Structure in HIV-1 TAR RNA Is Required for Tat Binding and Tat-Mediated Trans-activation. Genes Dev. 4, 1365–1373. doi:10.1101/GAD.4.8.1365
Sanders, W., Fritch, E. J., Madden, E. A., Graham, R. L., Vincent, H. A., Heise, M. T., et al. (2020). Comparative Analysis of Coronavirus Genomic RNA Structure Reveals Conservation in SARS-like Coronaviruses. bioRxiv. doi:10.1101/2020.06.15.153197
Sawicki, S. G., Sawicki, D. L., and Siddell, S. G. (2007). A Contemporary View of Coronavirus Transcription. J. Virol. 81, 20–29. doi:10.1128/JVI.01358-06
Schapira, M., Calabrese, M. F., Bullock, A. N., and Crews, C. M. (2019). Targeted Protein Degradation: Expanding the Toolbox. Nat. Rev. Drug Discov. 18, 949–963. doi:10.1038/s41573-019-0047-y
Schulze-Gahmen, U., and Hurley, J. H. (2018). Structural Mechanism for HIV-1 TAR Loop Recognition by Tat and the Super Elongation Complex. Proc. Natl. Acad. Sci. USA 115, 12973–12978. doi:10.1073/pnas.1806438115
Singh, S. K., Koshkin, A. a., Wengel, J., and Nielsen, P. (1998). LNA (Locked Nucleic Acids): Synthesis and High-Affinity Nucleic Acid Recognition. Chem. Commun. 1998, 455–456. doi:10.1039/a708608c
Sola, I., Almazán, F., Zúñiga, S., and Enjuanes, L. (2015). Continuous and Discontinuous RNA Synthesis in Coronaviruses. Annu. Rev. Virol. 2, 265–288. doi:10.1146/annurev-virology-100114-055218
Stammler, S. N., Cao, S., Chen, S.-J., and Giedroc, D. P. (2011). A Conserved RNA Pseudoknot in a Putative Molecular Switch Domain of the 3′-untranslated Region of Coronaviruses Is Only Marginally Stable. RNA 17, 1747–1759. doi:10.1261/rna.2816711
Stein, C. A., and Castanotto, D. (2017). FDA-approved Oligonucleotide Therapies in 2017. Mol. Ther. 25, 1069–1075. doi:10.1016/j.ymthe.2017.03.023
Su, X., Ma, W., Feng, D., Cheng, B., Wang, Q., Guo, Z., et al. (2021). Efficient Inhibition of SARS‐CoV‐2 Using Chimeric Antisense Oligonucleotides through RNase L Activation. Angew. Chem. Int. Ed. 60, 21662–21667. doi:10.1002/anie.202105942
Sun, L., Li, P., Ju, X., Rao, J., Huang, W., Ren, L., et al. (2021a). In Vivo structural Characterization of the SARS-CoV-2 RNA Genome Identifies Host Proteins Vulnerable to Repurposed Drugs. Cell 184, 1865–1883. e20. doi:10.1016/j.cell.2021.02.008
Sun, Y., Abriola, L., Niederer, R. O., Pedersen, S. F., Alfajaro, M. M., Silva Monteiro, V., et al. (2021b). Restriction of SARS-CoV-2 Replication by Targeting Programmed −1 Ribosomal Frameshifting. Proc. Natl. Acad. Sci. USA 118, e2023051118. doi:10.1073/pnas.2023051118
Tang, Z., Zhao, J., Pearson, Z. J., Boskovic, Z. V., and Wang, J. (2021a). RNA-targeting Splicing Modifiers: Drug Development and Screening Assays. Molecules 26, 2263. doi:10.3390/molecules26082263
Tang, Z., Zhao, J., Pearson, Z. J., Boskovic, Z. V., and Wang, J. (2021b). RNA-targeting Splicing Modifiers: Drug Development and Screening Assays. Molecules 26, 2263. doi:10.3390/molecules26082263
Temsamani, J., Tang, J.-Y., Padmapriya, A., Kubert, M., and Agrawal, S. (1993). Pharmacokinetics, Biodistribution, and Stability of Capped Oligodeoxynucleotide Phosphorothioates in Mice. Antisense Res. Dev. 3, 277–284. doi:10.1089/ard.1993.3.277
Thakur, C. S., Jha, B. K., Dong, B., Das Gupta, J., Silverman, K. M., Mao, H., et al. (2007). Small-molecule Activators of RNase L with Broad-Spectrum Antiviral Activity. Proc. Natl. Acad. Sci. 104, 9585–9590. doi:10.1073/pnas.0700590104
Ursu, A., Childs-Disney, J. L., Andrews, R. J., O’Leary, C. A., Meyer, S. M., Angelbello, A. J., et al. (2020). Design of Small Molecules Targeting RNA Structure from Sequence. Chem. Soc. Rev. 49, 7252–7270. doi:10.1039/d0cs00455c
van Dorp, L., Acman, M., Richard, D., Shaw, L. P., Ford, C. E., Ormond, L., et al. (2020). Emergence of Genomic Diversity and Recurrent Mutations in SARS-CoV-2. Infect. Genet. Evol. 83, 104351. doi:10.1016/j.meegid.2020.104351
Van Egeren, D., Novokhodko, A., Stoddard, M., Tran, U., Zetter, B., Rogers, M., et al. (2021). Risk of Rapid Evolutionary Escape from Biomedical Interventions Targeting SARS-CoV-2 Spike Protein. PLoS One 16, e0250780. doi:10.1371/journal.pone.0250780
Vankadari, N., Jeyasankar, N. N., and Lopes, W. J. (2020). Structure of the SARS-CoV-2 Nsp1/5′-Untranslated Region Complex and Implications for Potential Therapeutic Targets, a Vaccine, and Virulence. J. Phys. Chem. Lett. 11, 9659–9668. doi:10.1021/acs.jpclett.0c02818
Vatansever, E. C., Yang, K. S., Drelich, A. K., Kratch, K. C., Cho, C.-C., Kempaiah, K. R., et al. (2021). Bepridil Is Potent against SARS-CoV-2 In Vitro. Proc. Natl. Acad. Sci. USA 118, e2012201118. doi:10.1073/pnas.2012201118
Wacker, A., Weigand, J. E., Akabayov, S. R., Altincekic, N., Bains, J. K., Banijamali, E., et al. (2020). Secondary Structure Determination of Conserved SARS-CoV-2 RNA Elements by NMR Spectroscopy. Nucleic Acids Res. 48, 12415–12435. doi:10.1093/nar/gkaa1013
Wang, Y., Zhang, D., Du, G., Du, R., Zhao, J., Jin, Y., et al. (2020). Remdesivir in Adults with Severe COVID-19: a Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. The Lancet 395, 1569–1578. doi:10.1016/S0140-6736(20)31022-9
Warner, K. D., Hajdin, C. E., and Weeks, K. M. (2018). Principles for Targeting RNA with Drug-like Small Molecules. Nat. Rev. Drug Discov. 17, 547–558. doi:10.1038/nrd.2018.93
Wreschner, D. H., McCauley, J. W., Skehel, J. J., and Kerr, I. M. (1981). Interferon Action-Sequence Specificity of the ppp(A2′p)nA-dependent Ribonuclease. Nature 289, 414–417. doi:10.1038/289414a0
Wu, F., Zhao, S., Yu, B., Chen, Y.-M., Wang, W., Song, Z.-G., et al. (2020). A New Coronavirus Associated with Human Respiratory Disease in China. Nature 579, 265–269. doi:10.1038/s41586-020-2008-3
Wu, H.-Y., and Brian, D. A. (2010). Subgenomic Messenger RNA Amplification in Coronaviruses. Proc. Natl. Acad. Sci. 107, 12257–12262. doi:10.1073/pnas.1000378107
Yang, D., and Leibowitz, J. L. (2015). The Structure and Functions of Coronavirus Genomic 3′ and 5′ Ends. Virus. Res. 206, 120–133. doi:10.1016/j.virusres.2015.02.025
Yoshida, T., Naito, Y., Yasuhara, H., Sasaki, K., Kawaji, H., Kawai, J., et al. (2019). Evaluation of Off‐target Effects of Gapmer Antisense Oligonucleotides Using Human Cells. Genes Cells 24, 827–835. doi:10.1111/gtc.12730
Zafferani, M., Haddad, C., Luo, L., Davila-Calderon, J., Yuan-Chiu, L., et al. (2021). Amilorides inhibit SARS-CoV-2 replication in vitro by targeting RNA structures. Sci. Adv. 7, eabl6096. doi:10.1126/sciadv.abl6096
Zhang, K., Zheludev, I. N., Hagey, R. J., Haslecker, R., Hou, Y. J., Kretsch, R., et al. (2021). Cryo-EM and Antisense Targeting of the 28-kDa Frameshift Stimulation Element from the SARS-CoV-2 RNA Genome. Nat. Struct. Mol. Biol. 28, 747–754. doi:10.1038/s41594-021-00653-y
Zhao, C., Qin, G., Niu, J., Wang, Z., Wang, C., Ren, J., et al. (2021). Targeting RNA G‐Quadruplex in SARS‐CoV‐2: A Promising Therapeutic Target for COVID‐19? Angew. Chem. Int. Ed. 60, 432–438. doi:10.1002/ANIE.202011419
Zhao, J., Qiu, J., Aryal, S., Hackett, J., and Wang, J. (2020). The RNA Architecture of the SARS-CoV-2 3′-Untranslated Region. Viruses 12, 1473. doi:10.3390/v12121473
Zhu, C., Lee, J. Y., Woo, J. Z., Xu, L., Nguyenla, X., Yamashiro, L. H., et al. (2021). An Intranasal ASO Therapeutic Targeting SARS-CoV-2. bioRxiv. doi:10.1101/2021.05.17.444397
Ziv, O., Price, J., Shalamova, L., Kamenova, T., Goodfellow, I., Weber, F., et al. (2020). The Short- and Long-Range RNA-RNA Interactome of SARS-CoV-2. Mol. Cel 80, 1067–1077. doi:10.1016/j.molcel.2020.11.004
Zúñiga, S., Sola, I., Alonso, S., and Enjuanes, L. (2004). Sequence Motifs Involved in the Regulation of Discontinuous Coronavirus Subgenomic RNA Synthesis. J. Virol. 78, 980–994. doi:10.1128/jvi.78.2.980-994.2004
Keywords: SARS-CoV-2, antiviral, RNA-targeting, small molecule, antisense oligonucleotide, untranslated region, programmed frameshift, RIBOTAC
Citation: Hegde S, Tang Z, Zhao J and Wang J (2021) Inhibition of SARS-CoV-2 by Targeting Conserved Viral RNA Structures and Sequences. Front. Chem. 9:802766. doi: 10.3389/fchem.2021.802766
Received: 27 October 2021; Accepted: 29 November 2021;
Published: 23 December 2021.
Edited by:
Jun Wang, Rutgers, The State University of New Jersey, United StatesReviewed by:
Tao Liu, Peking University, ChinaLiqiang Chen, University of Minnesota Twin Cities, United States
Copyright © 2021 Hegde, Tang, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingxin Wang, d2FuZy5qaW5neGluQGt1LmVkdQ==
†These authors have contributed equally to this work.
 Shalakha Hegde
Shalakha Hegde Zhichao Tang
Zhichao Tang Junxing Zhao
Junxing Zhao Jingxin Wang
Jingxin Wang