
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 14 December 2021
Sec. Chemical Physics and Physical Chemistry
Volume 9 - 2021 | https://doi.org/10.3389/fchem.2021.780935
 Qing Hou1,2,3*
Qing Hou1,2,3* John Buckeridge4*
John Buckeridge4* Aron Walsh5
Aron Walsh5 Zijuan Xie6
Zijuan Xie6 You Lu7
You Lu7 Thomas W. Keal7
Thomas W. Keal7 Jingcheng Guan3
Jingcheng Guan3 Scott M. Woodley3
Scott M. Woodley3 C. Richard A. Catlow3,8*
C. Richard A. Catlow3,8* Alexey A. Sokol3*
Alexey A. Sokol3*Cu impurities are reported to have significant effects on the electrical and optical properties of bulk ZnO. In this work, we study the defect properties of Cu in ZnO using hybrid quantum mechanical/molecular mechanical (QM/MM)–embedded cluster calculations based on a multi-region approach that allows us to model defects at the true dilute limit, with polarization effects described in an accurate and consistent manner. We compute the electronic structure, energetics, and geometries of Cu impurities, including substitutional and interstitial configurations, and analyze their effects on the electronic structure. Under ambient conditions, CuZn is the dominant defect in the d9 state and remains electronically passive. We find that, however, as we approach typical vacuum conditions, the interstitial Cu defect becomes significant and can act as an electron trap.
ZnO, with a wide bandgap of 3.44 eV (Reynolds et al., 1999), is one of the most widely studied transparent semiconductors, with applications in solar cells (Keis et al., 2002), light-emitting diodes (Tsukazaki et al., 2005a; Tsukazaki et al., 2005b), photocatalysts (Barick et al., 2010), and piezoelectric devices (Wang and Song, 2006). To optimize its performance in applications, it is important to understand and control the properties of impurities in ZnO. The Cu/ZnO system is a very important industrial methanol catalyst (Waugh, 1992; Baltes et al., 2008). Substitution of copper into ZnO (CuZnq) (here and elsewhere in this article, we denote the effective charge of the defect, q, with respect to the lattice site explicitly with the superscript) has been reported to improve the photocatalytic activity (Mohan et al., 2012), ferromagnetism (Xing et al., 2008), and gas sensitivity (Gong et al., 2006) of ZnO. By admittance spectroscopy experiments, CuZnq is found to possess an (0/−) acceptor level at 0.17 eV below the bottom of the conduction band (CBM) in ZnO (McCluskey et al., 2015). Doping with copper has been proposed as a route for producing stable p-type ZnO (Özgür et al., 2005), which, however, has not been successful to date and which partly inspired our current investigation. Moreover, although there are many investigations both experimental and computational on the effects of CuZn on the electrical and optical properties of ZnO, information about Cu interstitials (Cuiq) in ZnO is limited.
In this article, we report the properties of Cu in both substitutional and interstitial forms in ZnO using a hybrid quantum mechanical/molecular mechanical (QM/MM)–embedded cluster approach. For the description of point defects in crystals, the commonly used implementation of density functional theory (DFT) with periodic boundary conditions suffers from finite-size effects (Freysoldt et al., 2014). In contrast, the QM/MM method defines the vacuum reference level unambiguously and describes accurately the short- and long-range polarization effects of a charged defect in a host material. The approach allows us to compare the energetics of different defect configurations and charge states on an equal footing. We focus here on isolated defects, but copper impurities may also form complexes in a variety of ways, an investigation into which is underway and will be reported elsewhere.
In this work, the QM region containing 86 atoms is treated with DFT using a triple zeta plus polarization Gaussian basis set for oxygens (Def2-TZVPP) (Weigend and Ahlrichs, 2005) and a double zeta plus polarization set for Zn cations (cc-pVDZ-PP) (Peterson, 2003; Figgen et al., 2005) (relevant input files can be found in the git repository “https://github.com/qhou1/chemshell.git”). To reduce the computational load, we have removed f functions from the oxygen basis set and some of the highly diffuse functions from the cation basis sets, which do not contribute to the bonding in these ionic solids.
For electron exchange and correlation, we have employed the BB1k functional (Zhao et al., 2004), which has been fitted to both thermochemical and kinetic data including 42% exact exchange, and the PBE0 functional (Adamo and Barone, 1999), which is frequently used in plane-wave basis calculations including 25% exact exchange for comparison. In order to embed the QM cluster within a polar environment, the MM region containing 10,460 atoms is treated with a previously derived interatomic potential (Catlow et al., 2008).
The hybrid QM/MM-embedded cluster approach used is implemented in the ChemShell (Sherwood et al., 2003) package. The QM/MM energy is obtained in an additive approach as a sum of QM and MM terms with the interaction energy between the two regions accounted for the QM term whose Hamiltonian includes the embedding potential. The GAMESS-UK (Guest et al., 2005) code is employed in the QM calculations, while the GULP package has been used to calculate the MM contributions.
The formation energies of the Cu substitution with charge q are calculated according to the following reactions:
under Zn-rich/O-poor condition and
under Zn-poor/O-rich condition.
The formation energies of the Cu interstitial are calculated according to the following reaction:
under Zn-rich/O-poor condition and
Under Zn-poor/O-rich condition.
The chemical potentials of O2 molecular and single Zn atoms are calculated using GAMESS-UK with the corresponding basis set and density functional; the standard state energy of ZnO is derived from the experimental heat of formation (Haynes, 2014).
We first present the local geometries as well as the defect formation of the Cu interstitial Cuiq and Cu substitutional CuZnq; we next calculate the self-consistent Fermi energies and charge carrier and defect concentrations of Cu-doped ZnO. Finally, we report on the equilibrium between CuZnq and Cuiq.
As noted, Cu impurities have been widely studied due to their possible influence on the optical properties of ZnO. Moreover, by using the emission channeling technique, 60–70% of the Cu atoms are found to occupy the substitutional Zn site with root-mean-square displacements from the site of 0.16–0.17 Å (Wahl et al., 2004).
In our calculation, for the singly negatively charged state of CuZn−, the four nearest O neighbors of Cu are displaced outward by 0.11–0.14 Å (using the BB1k functional) as shown in Figure 1A. After an electron is removed from this negatively charged system, the neutrally charged state CuZn is formed. As shown by the spin density in Figure 1B, the resulting d9 Cu impurity drives a Jahn–Teller distortion, with the axial neighbor O relaxing inward by 0.06 Å and the other three non-axial O ions relaxing outward by 0.03 Å. In the +1 charge state, four neighbor O ions all relax inward by 0.04–0.07 Å around the d10 Cu ion (Figure 1C).
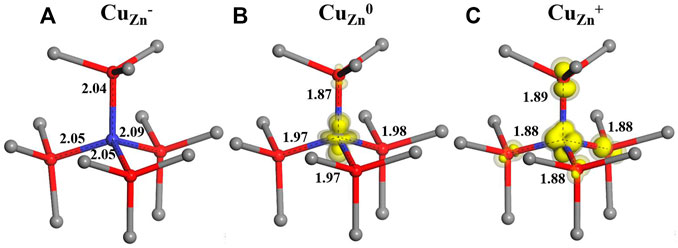
FIGURE 1. Calculated local structures for three charge states ( (A)-1, (B)0 and (C) +1) of the CuZn defect in ZnO, with zinc ions represented by grey spheres, oxygen ions by red spheres and copper ions by blue spheres.
The Cu impurities could also be present as interstitials in ZnO, in two possible positions: octahedral and tetrahedral sites. As discussed in previous studies by Janotti and Van de Walle (Janotti and Van de Walle, 2007) and Sokol et al. (Sokol et al., 2007), the Zn interstitial is expected to be more stable at the octahedral site than at the tetrahedral site. Hence, here, we only consider the interstitial at the octahedral site.
The calculated configurations are illustrated in Figure 2B. The Cu+ interstitial has a lower coordination by electron-rich O2- ions and forms a trigonal pyramid with the closest Cui–O separation distance of 1.98 Å and the two other distances of 2.01 and 2.05 Å (BB1k structures are shown in Figure 2A). On ionization, this nearly symmetric configuration is broken, with the Cu2+ ion moving toward one of the lattice oxygens (1.97, 1.98, and 2.11 Å). The next nearest O ions move now toward the interstitial Cu (by 0.16, 0.50, and 0.38 Å) but do not approach close enough to coordinate to this ion directly by a dative bond.
The calculated formation energies of both CuZn and Cui are plotted in Figure 3. The (0/−) transition level of CuZnq is found to lie at 3.54 eV (PBE0) above the valence band maximum (VBM), which is in agreement with previous calculations by Lany and Zunger (Lany and Zunger, 2009), who reported 3.46 eV using generalized gradient approximation (GGA)+U with an additional hole-state correction for the Cu d state, and close to the calculations by Lyons et al. (Lyons et al., 2017), who reported 3.27 eV using the Heyd–Scuseria–Ernzerhof (HSE) hybrid functional. Our results contrast with Yan et al. (Yan et al., 2006), who reported 0.7 eV using local density approximation (LDA), and Gallino and Valentin (Gallino and Di Valentin, 2011), who reported 2.48 eV using B3LYP. The computed ε(0/−) using BB1k is, however, 4.40 eV. The (+/0) transition level of CuZnq is found to lie at 1.14 eV (BB1k) and 1.07 eV (PBE0) above the VBM, which yields a deep donor level, which is shallower than that of Lany and Zunger (Lany and Zunger, 2009), who reported 0.37 eV, and Lyons et al. (Lyons et al., 2017), who reported 0.46 eV.
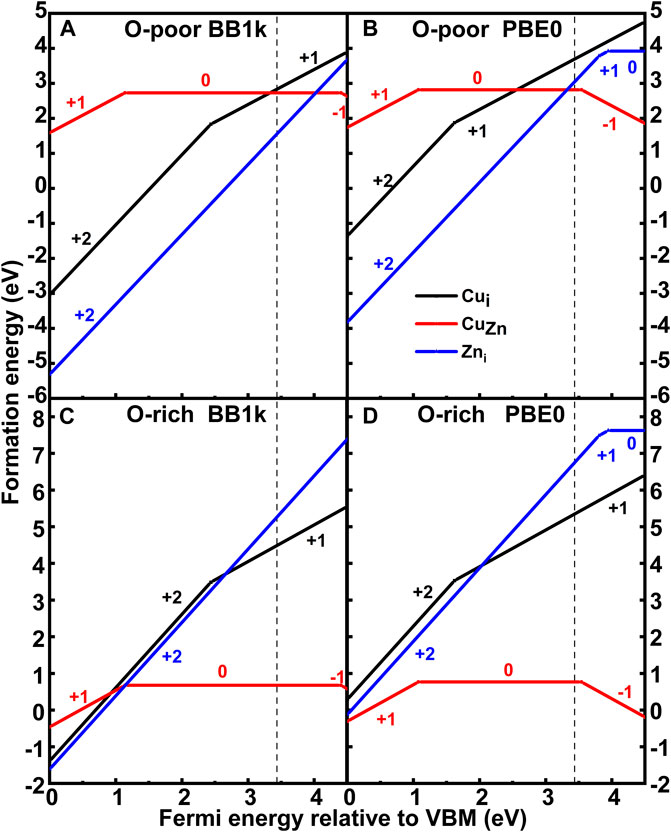
FIGURE 3. Formation energy of CuZn and Cui in ZnO as a function of the Fermi level relative to the valence band maximum (VBM) under O-poor ((A) BB1k and (B) PBE0) and O-rich ((C) BB1k and (D) PBE0) conditions.
In O-poor conditions, we observe that the Cuiq is more stable than CuZnq using BB1k until the Fermi level is very close to the CB from the calculated formation energies. In O-rich conditions, when the Fermi level is near the VBM, the interstitial Cu is still more stable than substitutional Cu. While for PBE0 results, the substitutional Cu becomes more stable than the interstitial Cu for the Fermi level greater than 2.56 eV under the Zn-rich condition. Under O-rich conditions, the substitutional Cu is the most stable defect type in the d9 state as a donor.
From the computed formation energies, the self-consistent Fermi energy and equilibrium defect and carrier concentrations can be determined. Here, we use the code “SC-FERMI” (Buckeridge, 2019). We focus on the results obtained using the BB1k functional, which reproduces the localization of holes on the oxygen sublattice more accurately than that on other functionals we have tested.
The concentration of each defect
where NX is the density of sites in which the defect may form, gXq is the degeneracy of the charge state, EF is the self-consistent Fermi energy, and k is the Boltzmann constant.
The electron (n0) and hole (p0) carrier concentrations can be determined by integrating the density of states weighed by the appropriate Fermi–Dirac function:
where
The computed self-consistent EF and equilibrium carrier and equilibrium concentrations of Cu impurities with native defects in ZnO as a function of T are shown in Figure 4. The range of temperatures is 0–1500 K, which encompasses common synthesis temperatures of ZnO and a majority of device operational temperatures.
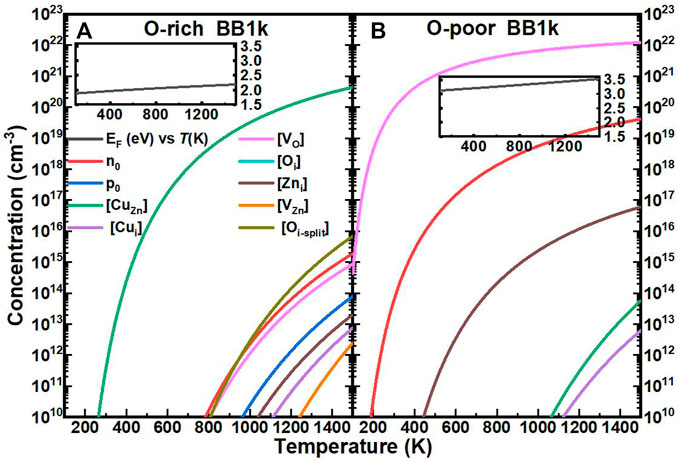
FIGURE 4. Calculated self-consistent Fermi energy (EF, relative to the CBM, black line) and equilibrium concentrations of electrons (n0, red line), holes (p0, blue line), Cu substitutionals ([CuZn], green line), Cu interstitials ([Cui], purple line), oxygen vacancies ([VO], magenta line), oxygen interstitials ([Oi], cyan line), zinc interstitials ([Zni], brown line), zinc vacancies ([VZn], orange line), and oxygen split interstitials ([Oi], dark yellow line)in ZnO as a function of temperature, determined using the BB1k hybrid density functional, under O-rich (A) and O-poor (B) conditions.
In O-rich conditions, the EF remains deep in the bandgap, between 1.9 and 2.2 eV above the VBM as T is increased, as shown in the inset of Figure 4A. The carrier concentrations remain below 1016 cm−3 for T ≤ 1500 K, with the Cu interstitial concentration [Cui] three-order of magnitude below. The [CuZn] in the neutrally charged state is the dominant defect in this range of EF, with the concentration above 1018 cm−3 for T > 600 K, which is close to the experimental result of ∼1018 cm−3 at room temperature (Kanai, 1991).
In O-poor conditions, due to the lower formation energies, EF moves closer to the CB and even above the CBM as shown in the inset of Figure 4B. From our analysis, ZnO is found to be n-type with electron concentrations n0 of 1016 cm−3 for T > 453 K (Hou, 2021) (details of the properties of native defects in ZnO will be published in the future).
We next investigated the equilibrium defect concentrations of Cu impurities in ZnO with fixed EF as a function of T (Figures 5, 6). We mainly considered four conditions of EF at: (A) 0.1 eV above the VBM, (B) 0.1 eV below the CBM, (C) 0.1 eV above the CBM, and (D) 1 eV above the CBM. We note here that the concentrations are not available when the formation energies of the defects are negative based on Eq. 7. In O-rich conditions, the [CuZn] in the neutrally charged state is the dominant defect for all four conditions. In O-poor conditions, when the EF is near to the CBM, the [Cui+] is close to [CuZn0], but both are in relatively low concentrations.
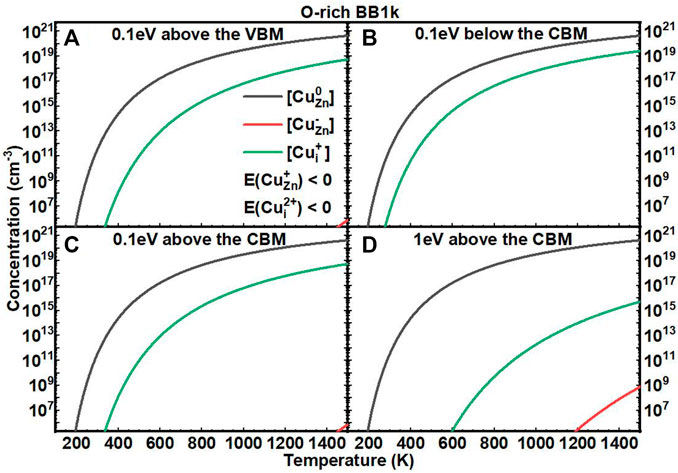
FIGURE 5. Calculated equilibrium concentrations of Cu substitutional in the 0 charge state ([CuZn0], black line), Cu substitutional in the −1 charge state ([CuZn−], red line), Cu substitutional in the +1 charge state ([CuZn+], blue line), Cu interstitial in the +1 charge state ([CuZn+], green line), and Cu interstitial in the +2 charge state ([CuZn2+], purple line) in ZnO as a function of temperature at a fixed fermi level ((A) 0.1eV above the VBM, (B) 0.1eV below the CBM, (C) 0.1eV above the CBM, (D) 1eV above the CBM), determined using the BB1k hybrid density functional, under O-rich conditions.
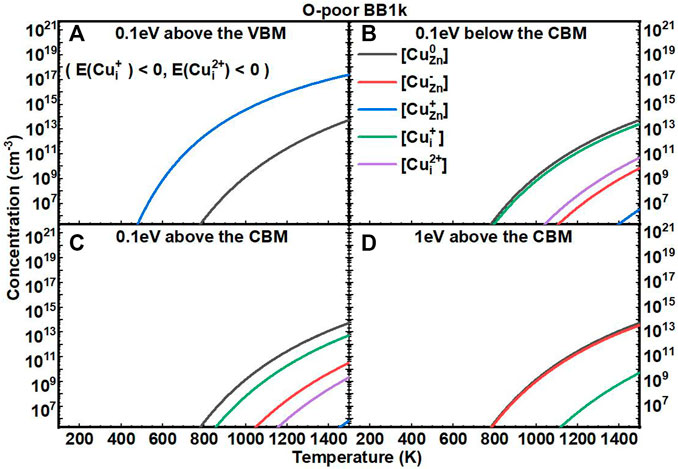
FIGURE 6. Calculated equilibrium concentrations of Cu substitutional in the 0 charge state ([CuZn0], black line), Cu substitutional in the −1 charge state ([CuZn−], red line), Cu substitutional in the +1 charge state ([CuZn+], blue line), Cu interstitial in the +1 charge state ([CuZn+], green line), and Cu interstitial in the +2 charge state ([CuZn2+], purple line) in ZnO as a function of temperature at a fixed fermi level ((A) 0.1eV above the VBM, (B) 0.1eV below the CBM, (C) 0.1eV above the CBM, (D) 1eV above the CBM), determined using the BB1k hybrid density functional, under O-poor conditions.
To compare our results to the experiment, it is important to relate the theoretically defined O-rich and O-poor condition to the oxygen chemical potential under different temperature and partial pressure conditions. The chemical potential of oxygen gas at varying oxygen partial pressures at a given temperature is expressed by:
By setting the zero state of
where H is the enthalpy, and S is the entropy. Based on the data from thermochemical tables (Stull and Prophet, 1971),
Our procedure then follows the method by Reuter and Scheffler (Reuter and Scheffler, 2001). The formation energies of Cu impurities in ZnO as a function of the O partial pressure from 10–22 to 1 atm at 300 and 1000 K are shown in Figure 7. At 300 K, the CuZn in the neutrally charged state remains as the dominant defect for O partial pressures from 10–22 to 1 atm, which is consistent with the defect concentration results. At high temperatures of 1000 K, the Cui+ becomes the dominant defect under very low O partial pressures, with the crossover from CuZn0 to Cui+ at 6.5*10–9 atm.
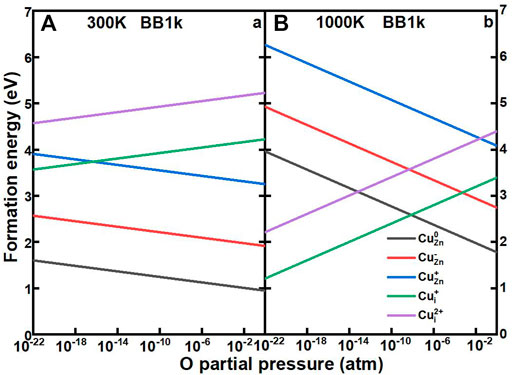
FIGURE 7. Calculated formation energies of CuZn0 (black line), CuZn− (red line), CuZn+ (blue line), CuZn+ (green line), and CuZn2+ (purple line) in ZnO as a function of oxygen partial pressures, determined using the BB1k hybrid density functional, under 300K (A) and 1000 K (B)>.
Bulk ZnO contains significant concentrations of intrinsic defects, to which are attributed the intrinsic n-type conductivity in ZnO (Harrison, 1954; Thomas, 1957; Hagemark, 1976). In the presence of the Cu dopants, there can be interchange of electrons or holes between Cu substitutional with extrinsic defects and Cu interstitial with intrinsic defect Zn vacancy or interstitial, for e.g.,
The corresponding processes and their reaction energies ΔEf (in eV) are listed in Table 1, with the electron in the CB and the hole in the VB. In general, CuZn0 remains energetically preferable. Cu will not migrate directly from the substitutional to the interstitial site under ambient conditions, owing to the high reaction energy at 6.21 and 6.15 eV. However, under O-poor/Zn-rich conditions, in the presence of the native defect Zn interstitial, Cu will spontaneously migrate to the interstitial site with a reaction energy of −0.45 eV; the Cui can trap electrons from CB.
We have investigated the copper dopants in both substitutional and interstitial forms in ZnO from embedded cluster calculations. By computing defect formation energies, we find that the Cu substitutional in the neutrally charged state is the dominant defect under O-rich conditions, which acts as a deep donor, while under Zn-rich conditions Cu interstitial becomes more stable than the Cu substitutional, which is consistent with the results of the equilibrium carrier and effect concentrations as a function of temperature. The Cu will not migrate directly from the substitutional to the interstitial site under ambient conditions, but under Zn-rich conditions Cu will spontaneously migrate to the interstitial site and trap electrons in the presence of Zn interstitial.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
All authors contributed to the components of the science and techniques used in the work and to the development and revision of the manuscript.
QH thanks the China Scholarship Council-UCL Joint Research Scholarship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors acknowledge the use of the YOUNG, ARCHER, and ARCHER2 UK National Supercomputing Service (http://www.archer2.ac.uk) via membership of UK’s HEC Materials Chemistry Consortium, which is funded by EPSRC (EP/R029431 and EP/T022213).
Adamo, C., and Barone, V. (1999). Toward Reliable Density Functional Methods Without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 110 (13), 6158–6170. doi:10.1063/1.478522
Baltes, C., Vukojevic, S., and Schuth, F. (2008). Correlations Between Synthesis, Precursor, and Catalyst Structure and Activity of a Large Set of CuO/ZnO/Al2O3 Catalysts for Methanol Synthesis. J. Catal. 258 (2), 334–344. doi:10.1016/j.jcat.2008.07.004
Barick, K., Singh, S., Aslam, M., Bahadur, D. J. M., and Materials, M. (2010). Porosity and Photocatalytic Studies of Transition Metal Doped ZnO Nanoclusters. 134(1-3), 195–202. doi:10.1016/j.micromeso.2010.05.026
Buckeridge, J. (2019). Equilibrium point Defect and Charge Carrier Concentrations in a Material Determined Through Calculation of the Self-Consistent Fermi Energy. Comp. Phys. Commun. 244, 329–342. doi:10.1016/j.cpc.2019.06.017
Catlow, C. R. A., French, S. A., Sokol, A. A., Al-Sunaidi, A. A., and Woodley, S. M. (2008). Zinc Oxide: A Case Study in Contemporary Computational Solid State Chemistry. J. Comput. Chem. 29 (13), 2234–2249. doi:10.1002/jcc.21051
Figgen, D., Rauhut, G., Dolg, M., and Stoll, H. (2005). Energy-consistent Pseudopotentials for Group 11 and 12 Atoms: Adjustment to Multi-Configuration Dirac-Hartree-Fock Data. Chem. Phys. 311 (1-2), 227–244. doi:10.1016/j.chemphys.2004.10.005
Freysoldt, C., Grabowski, B., Hickel, T., Neugebauer, J., Kresse, G., Janotti, A., et al. (2014). First-principles Calculations for Point Defects in Solids. Rev. Mod. Phys. 86 (1), 253–305. doi:10.1103/revmodphys.86.253
Gallino, F., and Di Valentin, C. (2011). Copper Impurities in Bulk ZnO: A Hybrid Density Functional Study. J. Chem. Phys. 134 (14), 144506. doi:10.1063/1.3575198
Gong, H., Hu, J. Q., Wang, J. H., Ong, C. H., Zhu, F. R., and Chemical, A. B. (2006). Nano-crystalline Cu-Doped ZnO Thin Film Gas Sensor for CO. Sensors Actuators B: Chem. 115 (1), 247–251. doi:10.1016/j.snb.2005.09.008
Guest*, M. F., Bush, I. J., Van Dam, H. J., Sherwood, P., Thomas, J. M., Van Lenthe, J. H., et al. (2005). The GAMESS-UK Electronic Structure Package: Algorithms, Developments and Applications. Mol. Phys. 103 (6-8), 719–747. doi:10.1080/00268970512331340592
Hagemark, K. (1976). Defect Structure of Zn-Doped ZnO. J. Solid State. Chem. 16 (3-4), 293–299. doi:10.1016/0022-4596(76)90044-x
Harrison, S. E. (1954). Conductivity and Hall Effect of ZnO at Low Temperatures. Phys. Rev. 93 (1), 52–62. doi:10.1103/physrev.93.52
Hou, Q. (2021). Theory of Defects in N-type Transparent Conducting Oxides. London: UCL (University College London).
Janotti, A., and Van de Walle, C. G. (2007). Native Point Defects in ZnO. Phys. Rev. B 76 (16), 165202. doi:10.1103/physrevb.76.165202
Kanai, Y. (1991). Admittance Spectroscopy of Cu-Doped ZnO Crystals. Jpn. J. Appl. Phys. 30 (4R), 703. doi:10.1143/jjap.30.703
Keis, K., Magnusson, E., Lindström, H., Lindquist, S.-E., and Hagfeldt, A. (2002). A 5% Efficient Photoelectrochemical Solar Cell Based on Nanostructured ZnO Electrodes. Solar Energ. Mater. solar Cell 73 (1), 51–58. doi:10.1016/s0927-0248(01)00110-6
Lany, S., and Zunger, A. J. P. R. B. (2009). Polaronic Hole Localization and Multiple Hole Binding of Acceptors in Oxide Wide-gap Semiconductors. 80(8), 085202. doi:10.1103/physrevb.80.085202
Lyons, J. L., Alkauskas, A., Janotti, A., and Van de Walle, C. G. (2017). Deep Donor State of the Copper Acceptor as a Source of green Luminescence in ZnO. Appl. Phys. Lett. 111 (4), 042101. doi:10.1063/1.4995404
McCluskey, M. D., Corolewski, C. D., Lv, J., Tarun, M. C., Teklemichael, S. T., Walter, E. D., et al. (2015). Acceptors in ZnO. J. Appl. Phys. 117 (11), 112802. doi:10.1063/1.4913827
Mohan, R., Krishnamoorthy, K., and Kim, S.-J. (2012). Enhanced Photocatalytic Activity of Cu-Doped ZnO Nanorods. Solid State. Commun. 152 (5), 375–380. doi:10.1016/j.ssc.2011.12.008
Özgür, Ü., Alivov, Y. I., Liu, C., Teke, A., Reshchikov, M., Doğan, S., et al. (2005). A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 98 (4), 11. doi:10.1063/1.1992666
Peterson, K. A. (2003). Systematically Convergent Basis Sets with Relativistic Pseudopotentials. I. Correlation Consistent Basis Sets for the Post-d Group 13-15 Elements. J. Chem. Phys. 119 (21), 11099–11112. doi:10.1063/1.1622923
Reuter, K., and Scheffler, M. (2001). Composition, Structure, and Stability of RuO2 (110) as a Function of Oxygen Pressure. Phys. Rev. B 65 (3), 035406. doi:10.1103/physrevb.65.035406
Reynolds, D. C., Look, D. C., Jogai, B., Litton, C. W., Cantwell, G., and Harsch, W. C. (1999). Valence-band Ordering in ZnO. Phys. Rev. B 60 (4), 2340–2344. doi:10.1103/physrevb.60.2340
Sherwood, P., de Vries, A. H., Guest, M. F., Schreckenbach, G., Catlow, C. R. A., French, S. A., et al. (2003). QUASI: A General Purpose Implementation of the QM/MM Approach and its Application to Problems in Catalysis. J. Mol. Struct. THEOCHEM 632 (1-3), 1–28. doi:10.1016/s0166-1280(03)00285-9
Sokol, A. A., French, S. A., Bromley, S. T., Catlow, C. R. A., van Dam, H. J. J., and Sherwood, P. (2007). Point Defects in ZnO. Faraday Discuss. 134, 267–282. doi:10.1039/b607406e
Stull, D., and Prophet, H. (1971). JANAF Thermochemical Tables. 2nd Edn., 37. Washington, DC: US National Bureau of Standards.
Taylor, F. H., Buckeridge, J., and Catlow, C. R. A. (2016). Defects and Oxide Ion Migration in the Solid Oxide Fuel Cell Cathode Material LaFeO3. Chem. Mater. 28 (22), 8210–8220. doi:10.1021/acs.chemmater.6b03048
Thomas, D. (1957). Interstitial Zinc in Zinc Oxide. J. Phys. Chem. Sol. 3 (3-4), 229–237. doi:10.1016/0022-3697(57)90027-6
Tsukazaki, A., Kubota, M., Ohtomo, A., Onuma, T., Ohtani, K., Ohno, H., et al. (2005a). Blue Light-Emitting Diode Based on ZnO. Jpn. J. Appl. Phys. 44 (5L), L643. doi:10.1143/jjap.44.l643
Tsukazaki, A., Ohtomo, A., Onuma, T., Ohtani, M., Makino, T., Sumiya, M., et al. (2005b). Repeated Temperature Modulation Epitaxy for P-type Doping and Light-Emitting Diode Based on ZnO. Nat. Mater. 4 (1), 42–46. doi:10.1038/nmat1284
Wahl, U., Rita, E., Correia, J., Alves, E., and Soares, J., (2004). Lattice Location and Stability of Implanted Cu in ZnO. 69 (1), 012102. doi:10.1103/physrevb.69.012102
Wang, Z. L., and Song, J. (2006). Piezoelectric Nanogenerators Based on Zinc Oxide Nanowire Arrays. Science 312 (5771), 242–246. doi:10.1126/science.1124005
Waugh, K. C. (1992). Methanol Synthesis. Catal. Today 15 (1), 51–75. doi:10.1016/0920-5861(92)80122-4
Weigend, F., and Ahlrichs, R. (2005). Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 7 (18), 3297–3305. doi:10.1039/b508541a
Xing, G. Z., Yi, J. B., Tao, J. G., Liu, T., Wong, L. M., Zhang, Z., et al. (2008). Comparative Study of Room-Temperature Ferromagnetism in Cu-Doped ZnO Nanowires Enhanced by Structural Inhomogeneity. Adv. Mater. 20 (18), 3521–3527. doi:10.1002/adma.200703149
Yan, Y., Al-Jassim, M. M., and Wei, S.-H. (2006). Doping of ZnO by Group-IB Elements. Appl. Phys. Lett. 89 (18), 181912. doi:10.1063/1.2378404
Keywords: zinc oxide, copper, hybrid QM/MM, dopant, defects
Citation: Hou Q, Buckeridge J, Walsh A, Xie Z, Lu Y, Keal TW, Guan J, Woodley SM, Catlow CRA and Sokol AA (2021) The Interplay of Interstitial and Substitutional Copper in Zinc Oxide. Front. Chem. 9:780935. doi: 10.3389/fchem.2021.780935
Received: 22 September 2021; Accepted: 23 November 2021;
Published: 14 December 2021.
Edited by:
Tim D Veal, University of Liverpool, United KingdomReviewed by:
Keith Butler, Rutherford Appleton Laboratory, United KingdomCopyright © 2021 Hou, Buckeridge, Walsh, Xie, Lu, Keal, Guan, Woodley, Catlow and Sokol. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Hou, cWluZy5ob3UuMTZAdWNsLmFjLnVr; John Buckeridge, ai5idWNrZXJpZGdlQGxzYnUuYWMudWs=; C. Richard A. Catlow, Yy5yLmEuY2F0bG93QHVjbC5hYy51aw==; Alexey A. Sokol, YS5zb2tvbEB1Y2wuYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.