- 1Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Institute of Traditional Chinese Medicine & Natural Products, College of Pharmacy, Jinan University, Guangzhou, China
- 2Department of Neurosurgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
- 3Guangdong Clinical Translational Center for Targeted Drug, Department of Pharmacology, School of Medicine, Jinan University, Guangzhou, China
Four new alkaloids (1–4) belonging to rare examples of bis-amide matrine-type were isolated from the seeds of sophora alopecuroides. Their structures including absolute configuration were determined by extensive spectroscopic analysis, electronic circular dichroism (ECD) interpretation, and X-ray diffraction crystallography. Chemically, bis-amide matrine-type alkaloids can provide new molecular template for structural modification. Compounds 3–4 displayed obvious anti-inflammatory effects based on the inhibition of two key pro-inflammatory cytokines [tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)] in a dose-dependent manner, with IC50 values from 35.6 to 45.8 μm.
Introduction
Sophora alopecuroides L. which belongs to the family of Leguminosae, is a salt-tolerant perennial herb plant and distributed in arid desert grassland of northwest China (Wang et al., 2012; Deng et al., 2019). The seeds of S. alopecuroides (Chinese name: Ku-Dou-Zi) has been regarded as a well-known traditional Chinese medicine for the treatment of fever, rheumatism, bacterial infection and inflammatory diseases (Huang et al., 2016). Previous phytochemical investigations on S. alopecuroides revealed alkaloids as one of principal active chemical constituents. Among them, matrine-type alkaloids exhibit diverse bioactivities such as antiviral (Zou et al., 2020), anti-insect (Huang et al., 2017), anti-tumor (Li et al., 2020), and promising anti-inflammatory activities (Huang et al., 2016; Li et al., 2020).
Chemically, matrine-type alkaloids are considered as ideal lead compounds for further structure modifications because of special chemical structure, widespread biological activities, high safety threshold, as well as available commercial sources. In order to improve the activities and amplify their applicants, many matrine-type derivatives have been synthesized and reported in the recent years (Huang et al., 2017; Cai et al., 2018; Cheng et al., 2018; Lv et al., 2018; Cheng et al., 2020; Xu et al., 2020). Interestingly, we have noticed that almost all structural modifications were concentrated in the variations of D ring, such as introducing substituents to C-13 and C-14 sites, opening D ring, fusing D ring and further molecular simplification. However, the amide bond located at D ring is a critical part that can responsible for many biological activities according to the molecular docking analysis (Peng et al., 2020). It is necessary to search more matrine-type template for the development of structural modification strategy.
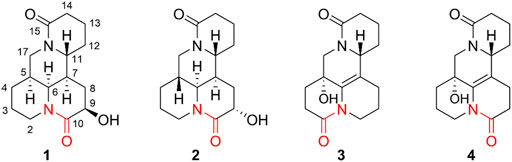
As a part of continuous systematic search for structurally unique and biologically meaningful natural products from the sophora species (Fan et al., 2019; Luo et al., 2021; Zhang et al., 2018a; Zhang et al., 2018b; Zhang et al., 2016; Zhang et al., 2017), four new matrine-type alkaloids (1–4) were obtained and identified (Figure 1). It is worth mentioning that compounds 1–4 are uncommon examples of bis-amide matrine with the second amide group at C-2 or C-10. To data, hundreds of matrine-type alkaloids have been reported but only few cases such as 2-oxymatrine, 10-oxy-5,6-dehydromatrine, and 10-oxysophoridine that possessed the bis-amide bond at C-2 or C-10 (Zhang et al., 2016; Zhang et al., 2018a). Additionally, all isolates were evaluated their anti-inflammatory activities in vitro based on production of two key pro-inflammatory cytokines (TNF-α and IL-6) in lipopolysaccharides (LPS)-stimulated RAW264.7 cells. Herein, the isolation, strucutre elucidation, and anti-inflammatory activites of those isolates are discussed.
Materials and Methods
General Experimental Procedures
The UV spectra, optical rotations, and IR spectra were determined on a JASCO V-550 UV/VIS spectrophotometer in MeOH solutions, an Autopol JASCO P-1020 polarimeter with concentrations unit reported in g/100 ml, a JASCO FT/IR-480 plus FT-IR spectrometer with KBr disks and peaks reported in cm−1 (JASCO corporation, Tokyo, Japan), respectively. ECD spectrum were carried out on a JASCO J-810 spectrometer. NMR experiments including 1D and 2D spectra were run on a Bruker Avance 600 NMR (600 MHz for 1H/150 MHz for 13C) spectrometer using standard Bruker pulse sequences (Bruker-Biospin, United States). HRESIMS data were measured from an Agilent 6210 LC/MSD Q-TOF mass instrument (Agilent Technologies, CA, United States). Analysis and preparation of crude samples were performed on HPLC using Shimadzu 6AD series with a PDA detector (Shimadzu corporation, Tokyo, Janpan). Al2O3 thin layer chromatography (TLC) analysis was done on aluminium oxide 60 F 254 basic plates (Merck, China). Column chromatography (CC) was undertaken with D-101 macroporous resin (Diaion, Shanghai, China), simon alumina N (size 200–300 mesh, Aldrich, China), Sephadex LH-20 (size 25–100 mm, Fluka, Buchs, Switzerland), CHP20P MCI gel (size 75–150 μm, Sigma-Aldrich company Ltd. China), and ODS silica gel (size 50 mm; YMC, Tokyo, Japan).
Plant Material
The dry seeds of S. alopecuroides L. were collected from the area (GPS coordinates, 37°98′−38°22′ N, 106°20′−106°66′ E) of Wuzhong City, Ningxia Hui Autonomous Region, People’s Republic of China on August 2014. A voucher specimen (accession no. SA-2014–08–28) was authenticated by Prof. Guang-Xiong Zhou (Jinan University) and available for inspection at the Institute of Traditional Chinese Medicine and Natural Products, Jinan University, Guangzhou, P. R. China.
Extraction and Isolation
The dry seeds of S. alopecuroides (∼30 kg) were powdered and percolated thrice with 95% EtOH and the pooled extracts were concentrated to give the total extract (∼1.9 kg). The crude residue was suspended with 0.1 M aqueous HCl until acidified to pH 2–3. After removal of the nonalkaloid components with CHCl3, the remaining acidic solvent was subsequently adjusted to pH 9–10 basified with saturated NH3 solution in water and partitioned with CHCl3 five times to yield total alkaloids (0.8 kg, extraction coefficient: 2.67%). The CHCl3 layer alkaloid was fractionated via D−101 macroporous resin CC eluting with EtOH−H2O (from 10:90 to 95:5, v:v) to yield five major fractions (Fr.1−5), based on the ratio of ethanol and water. Fr. 1 was dissolved in deionized water, and extracted twice with ethyl acetate (EtOAc) to remove the fat-soluble part. Subsequently, Fr. 1A (50.1 g) was subjected to Al2O3-based chromatography column (200–300 mesh) eluted with CH2Cl2 containing increasing amount of MeOH (100:0 to 0:100, v/v) added 1% Et2NH to produce seven portions (Fr.1Aa–Fr.1Ag), based on Al2O3-based TLC analysis. Fr.1Af (18.0 g) was separated on a RP-MCI column chromatography with a gradient of CH3OH/H2O/Et2NH (5:95:0.01 to 60:40:0.01) to generated five fractions (Fr.1Af.1−5). Fr.1Af.1 was applied to a Sephadex LH−20 column (CH3OH/H2O, 2:1, v/v) to obtain three subfractions (Fr.1Af.1.1–Fr.1Af.1.3). Fr.1Af.1.2 was purified by ODS CC (CH3OH/H2O, 5:95→40:60, v/v) and further separated by preparative HPLC with CH3CN/H2O/Et2NH (15:85:0.01, v/v/v) to give compounds 1 (19.4 mg, tR = 21.4 min), 2 (11.1 mg, tR = 23.8 min), 3 (23.2 mg, tR = 17.8 min), 4 (24.9 mg, tR = 16.9 min).
(+)-10-Oxy-9β-hydroxymatrine (1): colorless crystals in MeOH; mp 147–148°C [α]25D +84.0 (c 0.01, CH3OH); UV (CH3OH) λmax (log ε) 205 (3.52) nm; IR (KBr) νmax 3,252, 2,927, 2,867, 1,627, 1,597, 1,408, 1,058 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 279.1698 [M + H]+ (calcd for C15H23N2O3, 279.1703).
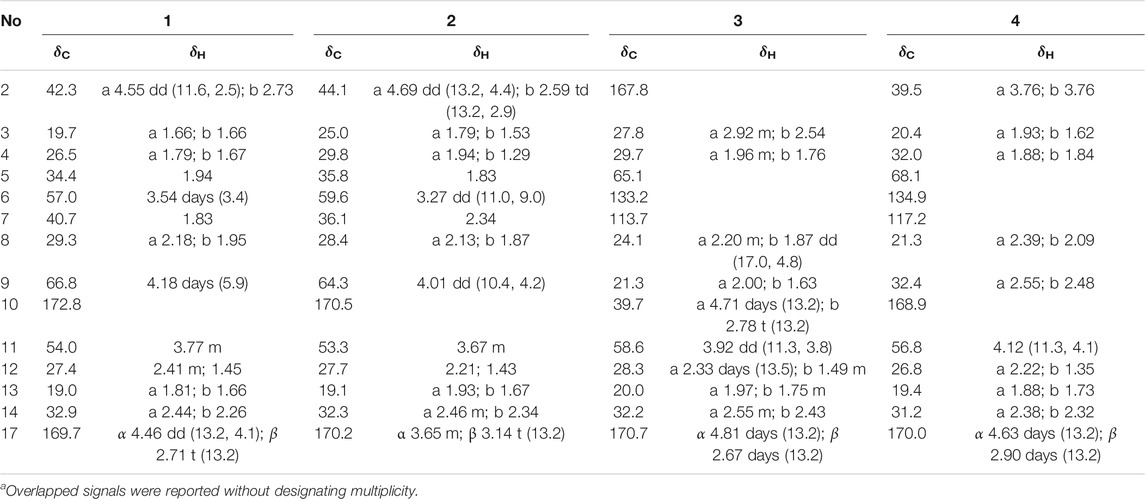
TABLE 1. 1H (600 MHz) and13C NMR (150 MHz) data of compounds one to four in CDCl3 (δ in ppm, J in Hz)a.
(−)-10-Oxy-9α-hydroxysophoridine (2): pale yellow oil in MeOH [α]25D −29.2 (c 0.01, CH3OH); UV (CH3OH) λmax (log ε) 206 (3.33) nm; ECD (CH3OH) λmax (Δε) 228 (+1.9) nm; IR (KBr) νmax 3,412, 3,256, 2,932, 2,867, 1,625, 1,603, 1,409, and 1,186 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 279.1702 [M + H]+ (calcd for C15H23N2O2, 279.1703).
(−)-2-Oxy-5-hydroxy-6,7-dehydromatrine (3): colorless block crystals in MeOH; mp 135–136°C [α]25D −8.9 (c 0.01, CH3OH); UV (CH3OH) λmax (log ε) 201 (4.08), 242 (3.11) nm; IR (KBr) νmax 3,408, 2,925, 2,765, 1,626, 1,459, and 1,098 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 277.1541 [M + H]+ (calcd for C15H21N2O3, 277.1547).
(−)-10-Oxy-5-hydroxy-6,7-dehydromatrine (4): pale yellow oil in MeOH [α]25D −12.2 (c 0.01, CH3OH); UV (CH3OH) λmax (log ε) 206 (3.25) nm; ECD (CH3OH) λmax (Δε) 209 (−1.9), 228 (+2.5), 257 (−4.8) nm; IR (KBr) νmax 3,408, 2,933, 2,803, 1,623, 1,441, 1,193 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 277.1546 [M + H]+ (calcd for C15H21N2O3, 277.1547).
Single X-Ray Diffraction Data Analysis
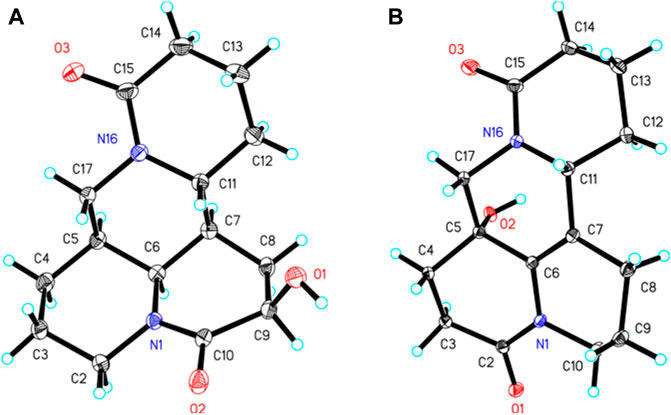
The single-crystal X-ray diffraction data of one and three were acquired on an Agilent diffractometer with Cu Kα radiation. The structures were solved by the SHELXT structure solution program and refined with full-matrix least-squares minimization on F2 using SHELXL via OLEX2 software package. Crystallographic data for one and three have been deposited in the Cambridge Crystallographic Data Centre (free of charge at https://www.ccdc.cam.ac.uk/) under deposition numbers CCDC are 2055031 and 2095514, respectively.
Crystal Data for (−)-10-oxy-9β-hydroxymatrine (1): C15H24N2O4 (M = 296.36 g/mol): orthorhombic, space group P212121 (no. 19), a = 8.4437 (2) Å, b = 10.5310 (3) Å, c = 33.5834 (7) Å, V = 2,986.26 (13) Å3, Z = 8, T = 149.99 (10) K, μ(Cu Kα) = 0.784 mm−1, Dcalc = 1.318 g/cm3, 13,927 reflections measured (5.262° ≤ 2Θ ≤ 147.692°), 5,895 unique (Rint = 0.0312, Rsigma = 0.0387) which were used in all calculations. The final R1 was 0.0385 (I > 2σ(I)) and wR2 was 0.0912 (all data). Flack parameter −0.09 (10). Hooft parameter: −0.06 (10). CCDC number is 2055031.
Crystal Data for (−)-2-oxy-5-hydroxy-6,7-dehydromatrine (3): C15H22N2O4 (M = 294.34 g/mol): orthorhombic, space group P212121 (no. 19), a = 7.41520 (10) Å, b = 10.77360 (10) Å, c = 17.6626 (2) Å, V = 1,411.04 (3) Å3, Z = 4, T = 150.00 (10) K, μ(CuKα) = 0.829 mm−1, Dcalc = 1.386 g/cm3, 9,266 reflections measured (9.616° ≤ 2Θ ≤ 147.622°), 2,781 unique (Rint = 0.0197, Rsigma = 0.0155) which were used in all calculations. The final R1 was 0.0285 (I > 2σ(I)) and wR2 was 0.0714 (all data). Flack parameter −0.01 (6). Hooft parameter: −0.05 (5). CCDC number is 2095514.
Electronic Circular Dichroism Calculations
Conformational analysis was initially performed using sybyl-X-2.1.1 program. Conformers occurring within a 10 kcal/mol energy window from the global minimum were chosen for geometry optimization and energy calculation using DFT with the B3LYP functional and the 6–311++G (d,p) basis set with the Gaussian09 program. Calculated results were completed using TD-DFT with the CPCM model in MeOH based on the optimized geometries. Finally, the Boltzmann-averaged ECD spectra of the two compounds were obtained and visualized with SpecDis 1.61 and drawn using the Origin Pro nine program. The half bandwidth (σ) and UV correction values applied in SpecDis for the final calculated ECD spectra are 0.30 eV and −5 nm, 0.30 eV and −6 nm for compounds 2 and 4, respectively, which are within the reasonable range. Their optimized conformation geometries, thermodynamic parameters, and populations of all conformations (2 and 4) are provided in supporting information.
In vitro Cytotoxicity Assay
The cytotoxicity of those isolated alkaloids on RAW 264.7 cells were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) assay. Various concentrations of test alkaloids were used to treat the cells by the methods that published previously (He et al., 2019; Zhang et al., 2021).
In vitro Anti-inflammatory Assay
The anti-inflammatory activities of 1–4 on LPS-stimulated TNF-α and IL-6 expression in RAW 264.7 cells were assessed using enzyme-linked immunosorbent assay (ELISA) as published previously (He et al., 2019). Dexamethasone and the medium without LPS were selected as the positive or negative control group.
Results and Discussion
Compound 1 was isolated as colorless block crystals from CH3OH, mp 147–148°C [α]25D +84.0 (c 0.01, CH3OH). The molecular formula was deduced to be C15H22N2O3 on the basis of the HRESIMS protonated molecular ion peak [M + H]₊ at m/z 278.1630, suggesting six indices of hydrogen deficiency. The UV spectrum showed maximum absorption at 206 nm was typical for non-conjugated amide of matrine-type backbone. The IR spectrum displayed a hydroxy group (3,202 cm−1) and two lactam functionalities (1,627 and 1,597 cm−1). The 1H NMR spectrum (Table 1) exhibited signals for [δH 4.70 dd (J = 13.2, 4.6 Hz), 2.69 m] and [δH 4.53 days (J = 13.0 Hz), 2.66 m], ascribed to two sets diagnostic methylene protons adjacent to N-atom of amide bond. The 13C NMR spectrum (Table 1) revealed 15 carbon resonances categorized by DEPT experiments as two carbonyls (δC 172.4 and 170.3), five methines (δC 74.5, 54.6, 54.3, 42.0, and 31.5), and eight methylenes (δC 46.7, 32.4, 32.1, 28.4, 27.9, 25.3, 20.0, 18.9). The above spectroscopic data as well as biogenetic considerations indicated that compound 1 was a derivative of matrine (Zhang et al., 2018b).
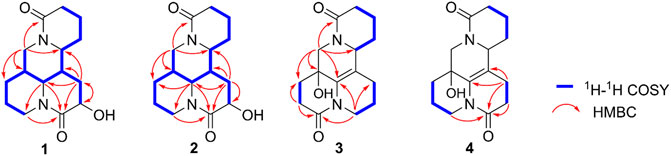
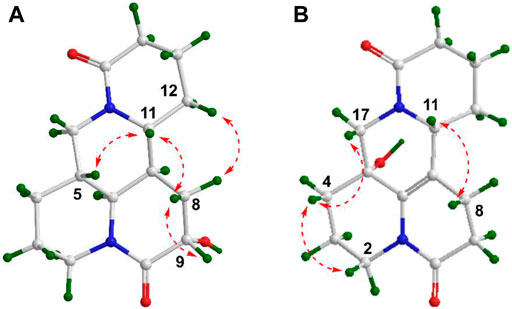
The planar structure and relative configuration of one was ascertained by comprehensive analysis of 2D NMR experiment (including 1H–1H COSY, HSQC, HMBC, and NOESY spectra). The characteristic HMBC correlations from H2-17 to C-4/C-5/C-6/C-11/C-17, from H-6 to C-2/C-5/C-7/C-10/C-11, from H2-8 to C-6/C-7/C-9/C-10/C-11, and from H-9 to C-7/C-8/C-10, together with 1H−1H COSY correlations of H-6/H-7/H-8/H-9 in B ring (Figure 2), implied one more amide bond occurring at C-10 and a hydroxy group locating on C-9 of matrine-based skeleton. Furthermore, the coupling constant between H-9 and H2-8 (3J = 5.6 Hz) suggested H-9 occupied the equatorial position (α) in six-member ring, supported by the absence of H-9/H-11 cross-peak signal in the NOESY spectrum. Finally, the absolute configuration of (5S,6S,7,9,11R)-1 was unequivocally established by a single crystal X-ray diffraction (Figure 3) using Cu Kα radiation [Flack parameter: 0.09 (13)]. Thus, the structure of one was deduced as shown in Figure 1, and named as (+)-10-oxy-9β-hydroxymatrine.
Compound 2 [α]25D −29.2 (c 0.01, CH3OH), possessed a molecular formula of C15H22N2O3 (calcd for C15H23N2O3, 279.1703) via the HRESIMS ion peak at m/z 279.1704 [M + H]+ and 13C NMR data. Its spectroscopic values showed that two is structurally similar to sophoridine (Zhang et al., 2018a), while the obviously differences were that an additional amide carbon (δC 170.5) and a more O-bearing methine [δC 64.3, δH 4.01 dd (J = 10.4, 4.2 Hz)] are observed. The key HMBC cross-peaks of H2-8 to C-6/C-7/C-9/C-10/C-11 and H-6 to C-2/C-4/C-8/C-10, together with sequential COSY correlations of H-6/H-7/H2-8/H-9 in B ring (Figure 2) lead to the full structural assignment of 2, as shown in Figure 1. The configuration of OH-9 was deduced as equatorial orientation (α), which was determined by the large coupling constant of H-8α/H-9 (JH-9/H-8α = 10.4 Hz) and NOE cross-peaks (Figure 4) of H-9 (δH 4.01)/H-8a (δH 2.13)/H-11 (δH 3.67). Consequently, the absolute structure of two was corroborated by comparing the experiment and calculated CD curve (Figure 5). Compound 2 was thereby deduced and named as (−)-10-oxy-9α-hydroxysophridine.
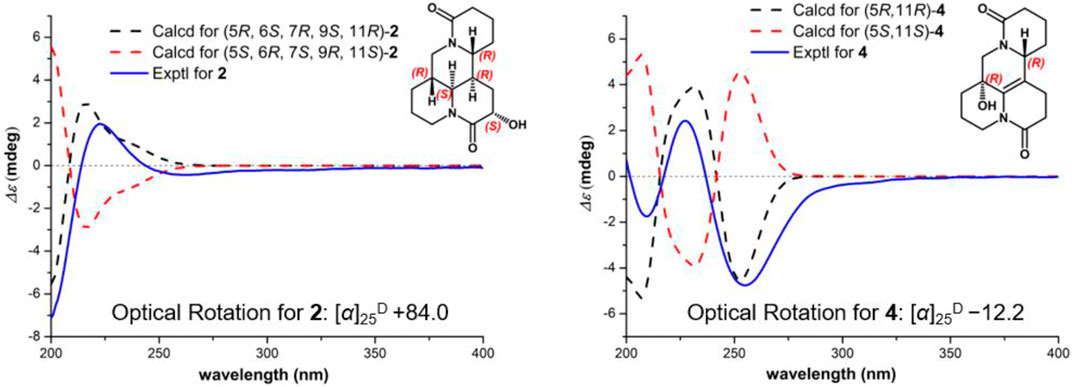
FIGURE 5. Experimental ECD and calculated spectra of 2 and 4 at the B3LYP/6–31+G (d) level [2: σ = 0.30 eV, UV shift = −5 nm; 4: σ = 0.30 eV, UV shift = −6 nm].
Compound 3, colorless block crystals, showed an [M + H]+ ion peak at m/z 277.1541 (calcd for C15H21N2O3, 401.1571) in the HRESIMS spectrum, consistent with the molecular formula of C15H20N2O3. The 1H and 13C NMR data of 3 (Table 1) were similar to those of 5-hydroxy-6,7-dehydro-matrine (Zhang et al., 2018b). The most notable difference was that the methylene at C-10 was replaced by another lactam group (δC 172.8), which was verified by the key 1H–1H COSY correlation of H-2/H-3, and HMBC cross-peaks from H2-3 to C-2/C-4, from H2-10 to C-2/C-6/C-8, and from H2-17 to C-4/C-5/C-6/C-11/C-17 (Figure 2). Moreover, the planar structure and absolute configuration of three were determined by X-ray crystallography (Figure 3) with an excellent Flack parameter [−0.01 (6)]. Hence, the complete structure of three was established and named as (−)-2-oxy-5-hydroxy-6,7-dehydromatrine.
Compound 4 [α]25D −12.2 (c 0.01, CH3OH), was obtained simultaneously with three in the same preparation liquid phase condition. Comprehensive analysis of its spectroscopic data indicated that four possessed the same molecular formula and almost identical 1D NMR resonances (Table 1) as that of 3, except for the second amide group exchanged from A ring to B ring. In the 2D NMR spectra of 4, the 1H–1H COSY correlations of H2-2/H2-3/H2-4 and H2-8/H2-9, as well as HMBCs from H2-2 to C-10, and from H2-8 to C-6/C-7/C-9 suggested the second amide group is located on B ring (Figure 2). Additionally, good consistency between the experimental CD curve and calculated ECD curve (Figure 5) allowed the assignment of (5R,11R) absolute configuration. Thus, compound 4 was elucidated and named as (−)-10-oxy-5-hydroxy-6,7-dehydromatrine.
At present, the structural modifications of matrine-type alkaloids have mainly focused on the variations of D ring due to the amide group at C-15 is an active reaction site. (Cai et al., 2018; Cai et al., 2020). Compounds 1–4 possessing rare bis-amide matrine-type motif could provide new molecular template and ideas for synthetic chemists. For example, new derivatives could be designed by the introduction of a protecting group on the D ring and subsequent structural modification via the second amide bond at C-2 or C-10 position on the A or B ring. So that the key part of D ring responsible for biological activity can be retained, and some unexpected good results may emerge.
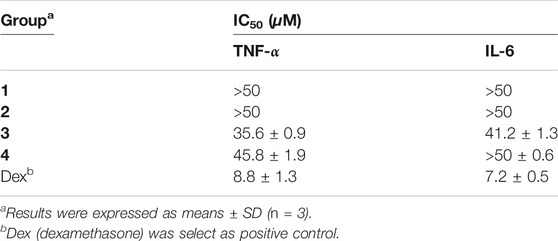
IL-6 and TNF-α are essential mediators in inflammation processes. Owing to the extracts of S. alopecuroides possessing remarkable clinical effects on various kinds of inflammation, all isolated alkaloids (1–4) were evaluated for their LPS-stimulated TNF-α and IL-6 production in RAW 264.7 cells using ELISA. Firstly, a MTT cytotoxicity assay was used to examine the cell viability of murine RAW 264.7 cells. The results displayed that all alkaloids at the concentration ranges from 3.125 to 50 μM were non-toxic on RAW 264.7 cells after 24 h treatment. Therefore, the tested alkaloids were applied to those concentration range to perform the next experiment. The production of IL-6 and TNF-α in the culture medium of the LPS-treated group both increased significantly (p < 0.001) in comparison with the control group after 24 h. However, co-incubation with compounds 3–4 suppressed the secretion of IL-6 and TNF-α in a dose-dependent manner, suggesting that compounds 3–4 are inhibitor of the initial phase of the inflammatory cascade initiated by LPS stimulation. As shown in Table 2, compounds 3–4 can inhibit the expression of those two pro-inflammatory mediator secretions (TNF-α and IL-6) with IC50 values from 35.6 to 45.8 μm, while compounds 1–2 were inactive. In light of the structures and the anti-inflammatory activity, the existence of unsaturated double bond of Δ6(7) may favor this inhibitory effect.
Conclusions
The systematic investigation of the seeds of S. alopecuroides led to the isolation of four uncommon bis-amide matrine-type alkaloids. Their special chemical structure can provide a new perspective for developing novel modificatory strategies based on A or B ring. The biological assay revealed two new compounds displayed obvious anti-inflammatory activity. This study not only enriched the structural diversity of matrine-type alkaloids, but also provided an attractive molecular candidate for pharmaceutical chemists.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
DL performed the extract, isolation, and structural elucidation of all chemical structures, and wrote the preliminary manuscript. ZT and WY contributed to the pharmacological assay. CF, NC, and ZW assisted in acquiring the experimental data. YZ, GW, and YL supervised and guided all experiments, rewrote and reviewed the manuscript. All authors have read and approved the published version of manuscript.
Funding
This project was supported financially by grants from the National Natural Science Foundation of China (nos. 81803376, 82074116, 81973190), the Guangdong Basic and Applied Basic Research Foundation (no. 2020B1515020033), the Natural Science Foundation of Guangdong Province (no. 2018B030311020), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01Y036), Guangdong Basic and Applied Basic Research Foundation-Regional Joint Fund (Youth Fund Project, no. 2020A1515110415).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thanked the High Performance Public Computing Service Platform of Jinan University for the help of theoretical ECD calculations.
Supplementary Material
The Supplementary Material for this article including original UV, CD, IR, HRESIMS, 1D/2D NMR spectra can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.740421/full#supplementary-material
References
Cai, X.-H., Guo, H., and Xie, B. (2018). Structural Modifications of Matrine-Type Alkaloids. Mini. Rev. Med. Chem. 18, 730–744. doi:10.2174/1389557516666161104150334
Cai, X. H., Zhang, H. Y., and Xie, B. (2020). Matrine-Family Alkaloids: Versatile Precursors for Bioactive Modifications. Min. Rev. Med. Chem. 16, 431–453. doi:10.2174/1573406415666190507121744
Cheng, X., He, H., Wang, W. X., Dong, F., Zhang, H., Ye, J., et al. (2020). Semi-Synthesis and Characterization of Some New Matrine Derivatives as Insecticidal Agents. Pest Manag. Sci. 76, 2711–2719. doi:10.1002/ps.5817
Cheng, X., Ye, J., He, H., Liu, Z., Xu, C., Wu, B., et al. (2018). Synthesis, Characterization and In Vitro Biological Evaluation of Two Matrine Derivatives. Sci. Rep. 8, 15686. doi:10.1038/s41598-018-33908-8
Deng, X. X., Wang, R. Z., Wu, X. L., Gao, Q. X., Han, L., Gao, X. J., et al. (2019). Sophora Alopecuroides L.: An Ethnopharmacological, Phytochemical, and Pharmacological Review. J. Ethnopharmacol. 248, 112172. doi:10.1016/j.jep.2019.112172
Fan, C. L., Zhang, Y. B., Chen, Y., Xie, P., Wang, G. C., Tian, H. Y., et al. (2019). Alopecuroides A–E, Matrine-Type Alkaloid Dimers from the Aerial Parts of Sophora Alopecuroides. J. Nat. Prod. 82, 3227–3232. doi:10.1021/acs.jnatprod.8b01081
He, L. J., Liu, J. S., Luo, D., Zheng, Y. R., Zhang, Y. B., Wang, G. C., et al. (2019). Quinolizidine Alkaloids from Sophora Tonkinensis and Their Anti-Inflammatory Activities. Fitoterapia 139, 104391. doi:10.1016/j.fitote.2019.104391
Huang, J. L., Lv, M., and Xu, H. (2017). Semisynthesis of Some Matrine Ether Derivatives as Insecticidal Agents. Rsc Adv. 7, 15997–16004. doi:10.1039/c7ra00954b
Huang, Y. X., Wang, G., Zhu, J. S., Zhang, R., and Zhang, J. (2016). Traditional Uses, Phytochemistry, and Pharmacological Properties of Sophora Alopecuroides L. Eur. J. Inflamm. 14, 128–132. doi:10.1177/1721727x16642779
Li, Y., Wang, G., Liu, J., and Ouyang, L. (2020). Quinolizidine Alkaloids Derivatives from Sophora Alopecuroides Linn: Bioactivities, Structure-Activity Relationships and Preliminary Molecular Mechanisms. Eur. J. Med. Chem. 188, 111972. doi:10.1016/j.ejmech.2019.111972
Luo, D., Wu, Z. N., Zhang, J. H., Lin, Q., Chen, N. H., Chen, S., et al. (2021). Sophaloseedlines A−G: Diverse Matrine-Based Alkaloids from Sophora Alopecuroides with Potential Anti-Hepatitis B Virus Activities. Chin. J. Chem. 39, 2555–2562. doi:10.1002/cjoc.202100279
Lv, M., Liu, G. C., Jia, M. H., and Xu, H. (2018). Synthesis of Matrinic Amide Derivatives Containing 1,3,4-thiadiazole Scaffold as Insecticidal/acaricidal Agents. Bioorg. Chem. 81, 88–92. doi:10.1016/j.bioorg.2018.07.034
Peng, W., Xu, Y., Han, D., Feng, F., Wang, Z., Gu, C., et al. (2020). Potential Mechanism Underlying the Effect of Matrine on COVID-19 Patients Revealed through Network Pharmacological Approaches and Molecular Docking Analysis. Arch. Physiol. Biochem. 1–8. doi:10.1080/13813455.2020.1817944
Wang, H. Q., Guo, S., Qian, D. W., Qian, Y. F., and Duan, J. A. (2012). Comparative Analysis of Quinolizidine Alkaloids from Different Parts of Sophora Alopecuroides Seeds by UPLC–MS/MS. J. Pharmaceut. Biomed. 67, 16–21. doi:10.1016/j.jpba.2012.04.024
Xu, J., Sun, Z., Hao, M., Lv, M., and Xu, H. (2020). Evaluation of Biological Activities, and Exploration on Mechanism of Action of Matrine–Cholesterol Derivatives. Bioorg. Chem. 94, 103439. doi:10.1016/j.bioorg.2019.103439
Zhang, B. Y., Liu, D., Ji, W. Y., Otsuki, K. H., Higai, K. J., Zhao, F., et al. (2021). Sacraoxides A–G, Bioactive Cembranoids from Gum Resin of Boswellia Sacra. Front. Chem. 9, 649287. doi:10.3389/fchem.2021.649287
Zhang, Y. B., Luo, D., Yang, L., Cheng, W., He, L. J., Kuang, G. K., et al. (2018a). Matrine-Type Alkaloids from the Roots of Sophora Flavescens and Their Antiviral Activities against the Hepatitis B Virus. J. Nat. Prod. 81, 2259–2265. doi:10.1021/acs.jnatprod.8b00576
Zhang, Y. B., Yang, L., Luo, D., Chen, N. H., Wu, Z. N., Ye, W. C., et al. (2018b). Sophalines E-I, Five Quinolizidine-Based Alkaloids with Antiviral Activities against the Hepatitis B Virus from the Seeds of Sophora Alopecuroides. Org. Lett. 20, 5942–5946. doi:10.1021/acs.orglett.8b02637
Zhang, Y. B., Zhan, L. Q., Li, G. Q., Wang, F., Wang, Y., Li, Y. L., et al. (2016). Dimeric Matrine-Type Alkaloids from the Roots of Sophora Flavescens and Their Anti-Hepatitis B Virus Activities. J. Org. Chem. 81, 6273–6280. doi:10.1021/acs.joc.6b00804
Zhang, Y. B., Zhang, X. L., Chen, N. H., Wu, Z. N., Ye, W. C., Li, Y. L., et al. (2017). Four Matrine-Based Alkaloids with Antiviral Activities against HBV from the Seeds of Sophora Alopecuroides. Org. Lett. 19, 424–427. doi:10.1021/acs.orglett.6b03685
Keywords: matrine-type alkaloids, water-soluble alkaloids, Sophora alopecuroides, structure modification, structure elucidation
Citation: Luo D, Tu Z, Yin W, Fan C, Chen N, Wu Z, Ding W, Li Y, Wang G and Zhang Y (2021) Uncommon Bis-Amide Matrine-type Alkaloids From Sophora alopecuroides With Anti-inflammatory Effects. Front. Chem. 9:740421. doi: 10.3389/fchem.2021.740421
Received: 13 July 2021; Accepted: 01 September 2021;
Published: 15 September 2021.
Edited by:
Laurent G. Désaubry, INSERM U1260 Nanomedicine régénératrice (RNM), FranceReviewed by:
Gennaro Pescitelli, University of Pisa, ItalyAhmed Elkamhawy, Mansoura University, Egypt
Copyright © 2021 Luo, Tu, Yin, Fan, Chen, Wu, Ding, Li, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaolan Li, dGxpeWxAam51LmVkdS5jbg==; Guocai Wang, dHdhbmdndW9jYWlAam51LmVkdS5jbg==; Yubo Zhang, eWJ6aGFuZzk5QDEyNi5jb20=
 Ding Luo
Ding Luo Zhenchao Tu1
Zhenchao Tu1 Yaolan Li
Yaolan Li Guocai Wang
Guocai Wang Yubo Zhang
Yubo Zhang