- Institute of Energy and Climate Research IEK 6, Nuclear Waste Management and Reactor Safety, Forschungszentrum Jülich, Jülich, Germany
Available data on the dependence of the equilibrium chemical potential of oxygen on degrees of doping, z, and non-stoichiometry, x, y, in U1-zLnzO2+0.5(x-y) fluorite solid solutions and data on the dependence of the lattice parameter, a, on the same variables are combined within a unified structural-thermodynamic model. The thermodynamic model fits experimental isotherms of the oxygen potential under the assumptions of a non-ideal mixing of the endmembers, UO2, UO2.5, UO1.5, LnO1.5, and Ln0.5U0.5O2, and of a significant reduction in the configurational entropy arising from short-range ordering (SRO) within cation-anion distributions. The structural model further investigates the SRO in terms of constraints on admissible values of cation coordination numbers and, building on these constraints, fits the lattice parameter as a function of z, y, and x. Linking together the thermodynamic and structural models allows predicting the lattice parameter as a function of z, T and the oxygen partial pressure. The model elucidates contrasting structural and thermodynamic changes due to the doping with LaO1.5, on the one hand, and with NdO1.5 and GdO1.5, on the other hand. An increased oxidation resistance in the case of Gd and Nd is attributed to strain effects caused by the lattice contraction due to the doping and to an increased thermodynamic cost of a further contraction required by the oxidation.
1 Introduction
One important aspect of a safety case for a geological repository for the high-level nuclear waste (e.g., UO2-based spent nuclear fuel, SNF) is to characterize the corrosion behaviour of and the consequent radionuclide release from the disposed wastes, after waste containers will be eventually breached due to corrosion. After unloading from a reactor (e.g., a light water reactor), the SNF still contains ∼95 wt% of uranium and ∼5 wt% of fission products and transuranium elements (TRU), Pu, Am, and Cm. Fission and activation products formed during irradiation of oxide fuels (e.g., UO2) could be classified into three categories, namely: 1) those forming metallic inclusions (e.g., Mo, Tc, Ru, Rh, and Pd), 2) those forming oxide precipitates (e.g., Rb, Cs, Ba, Zr, Nb, and Mo), and 3) those remaining as components of a solid solution with UO2 (e.g., Sr, Zr, Nb, lanthanides (Ln), and TRU) (Kleykamp, 1988; Bruno and Ewing, 2006; Carbol et al., 2012; Ewing, 2015).
In the repository system groundwater is the principal medium for the transport of radionuclides from the waste to the environment including corrosion of the waste. The unique chemical conditions are characterized by 1) generally reducing conditions due to a significant amount of hydrogen produced due to the anoxic corrosion of metallic waste containers, 2) the presence of locally formed oxidizing conditions caused by radiolytic products such as H2O2, and 3) complex groundwater chemistry. The radiolytic oxidants evoke locally oxidizing effects at the spent fuel surface leading to an oxidative dissolution of the waste, due to the oxidation of poorly soluble U(IV) to easily soluble U(VI) (Shoesmith, 2000; Eriksen et al., 2012; Shoesmith et al., 2013; Bosbach et al., 2020; Hansson et al., 2021).
During the last decades, a number of studies addressed the corrosion behaviour of SNF, leading to a good phenomenological understanding of the instant release of radionuclides and long-term corrosion rates (Kleykamp, 1985; Fanghänel et al., 2013; Ewing, 2015; Lemmens et al., 2017; Curti and Kulik, 2020). Here we focus on the question how the doping of uranium dioxide by a variety of fission and activation products affects the corrosion rate under repository conditions.
As fission yields of lanthanides, such as La, Ce, Pr, and Nd, due to the fission of 235U and 239Pu are high (Kleykamp, 1985), leading to significant amount of lanthanides dissolved in the UO2 matrix (up to 2–4 at%), UO2–LnO1.5 systems may serve as analogues of spent fuels illustrating certain aspects of burn-up on chemical stability (Ferry et al., 2005; Bruno and Ewing, 2006; Carbol et al., 2012). Effects of oxidative dissolution have been recently measured in aqueous (typically with H2O2 added) and in electrochemical systems both at corrosive (rest) potentials and at potentials promoting the conversion of U+4 to higher oxidation states. Such experiments applied to Gd-, Dy-, and Y-bearing samples have shown that the doping reduces dissolution yields and oxidative (anodic) currents relative to those measured for pure UO2 (Trummer et al., 2010; Razdan and Shoesmith, 2013; Casella et al., 2016; Kim et al., 2017; Liu et al., 2017; Barreiro Fidalgo and Jonsson, 2019). Similar reductions of oxidation rates have been measured for simulated fuels (SIMFUELs) that in addition to lanthanides contain a variety of other dopants (He et al., 2007; Nilsson and Jonsson, 2011; Razdan and Shoesmith, 2013; Liu et al., 2017).
Despite this effort, mechanisms of resistance to oxidation in Ln- or Y-doped systems and in chemically more complex simulated fuels remain unclear. In several studies the stabilizing effect was linked to the formation of Ln-VO clusters (VO denotes an oxygen vacancy), which are thought to reduce the number of vacant sites that could host oxygen anions (Razdan and Shoesmith, 2013; Kim et al., 2017; Liu et al., 2017). The formation of these clusters has been discussed in the frame of a point-defect model of Ln-doped UO2 by Park and Olander (Park and Olander, 1992). However, the fraction of such clusters in hyper-stoichiometric (oxidized) samples is predicted to be very small. Casella et al. (Casella et al., 2016) suggested that the stabilizing effect of Ln3+ could be related to its effectively negative charge that repels O-interstitials, Oi, and thus limits the fraction of interstitial sites able to accommodate an excess oxygen. Kim et al. (Kim et al., 2001) proposed that the decreased rates of oxidation in air measured on Gd-doped samples were due to a decreased fraction of U+4 (the fraction of cations able to be oxidized) caused by the ingrowth of Gd+3 and U+5. Furthermore, as most of the experiments have been done with dopants that promoted lattice contraction, a proposition was made that the stabilizing effect could be caused by a reduced rate of diffusion of oxygen anions into the interstitial sites promoted by the contraction (Kim et al., 2017). The study of Kim et al. (Kim et al., 2017) further proposed that variations in electrochemical reactivity of Gd-doped samples characterized by different types of non-stoichiometry could be related to different degrees of lattice contraction observed on Gd-doping in hypo-, hyper- and strictly-stochiometric samples. The largest effect of Gd on the oxidative anodic currents was noted in hyper-stoichiometric samples, those characterised by the strongest decrease in the lattice parameter with doping (Kim et al., 2017).
The similarity of stabilizing effects due to the doping observed in electrochemical oxidation (He et al., 2007; Razdan and Shoesmith, 2013; Kim et al., 2017; Liu et al., 2017), in oxidative dissolution (Trummer et al., 2010; Casella et al., 2016; Barreiro Fidalgo and Jonsson, 2019) and in air oxidation experiments (Kim et al., 2001) suggests that all these phenomena might be linked to a common thermodynamic factor.
The resistance of the UO2±δ solid solution to oxidation in air, where δ denotes deviation from stoichiometry, can be equated to the thermodynamic force (free energy) required for an insertion of an extra oxygen anion into its structure. This force at the thermodynamic equilibrium is proportional to the chemical potential of oxygen gas that is required for attaining a certain degree of hypo- or hyper-stoichiometry. Indeed, a large experimental effort has been invested in studying effects of Ln-doping on the chemical potential of oxygen in equilibrium with a given degree of hypo- or hyper-stoichiometry at a fixed temperature (Hagemark and Broli, 1967; Tetenbaum and Hunt, 1968; Javed, 1972; Saito, 1974; Une and Oguma, 1983a; Nakamura and Fujino, 1987; Lindemer and Sutton, 1988; Yoshida et al., 2011; Lee et al., 2016a; McMurray and Silva, 2016). These experiments invariably show that the larger the doping, the higher is the chemical potential, or, equivalently, the partial pressure of oxygen, at which a given degree of non-stoichiometry can be attained. Studies based on point-defect theory (Park and Olander, 1992), CALPHAD methodology (Saunders and Miodownik, 1998; Hillert, 2001) and on dual solution Gibbs energy minimization method (Karpov et al., 2001; Kulik et al., 2013) provided thermodynamically sound models able to predict the equilibrium partial pressure of oxygen at a given degree of doping and/or non-stoichiometry (Guéneau et al., 2011; McMurray et al., 2013; Degueldre et al., 2014; McMurray et al., 2015; Lee et al., 2016a; McMurray and Silva, 2016; Curti and Kulik, 2020). However, little effort has been so far invested in correlating these models with lattice parameter data. We argue here that a further understanding of mechanisms of oxidation resistance could be gained from a model that provides an accurate thermodynamic description of data on the dependence of the oxidation potential on the dopant type, on the degree of doping and non-stoichiometry, and simultaneously evaluates the dependence of the lattice parameter of UO2 on the same set of parameters. Previous research has shown that the lattice parameter, a, of fluorite solid solution changes markedly as a function of the type and degree of doping and the extent of non-stoichiometry (Ohmichi et al., 1981; Fukushima et al., 1983; Schreinemachers et al., 2014; Lee et al., 2016b; Schreinemachers et al., 2020). Combining this abundant structural information with thermodynamic data may provide means for understanding the role of the structure in the oxidation resistance of doped UO2.
Here we develop structural-thermodynamic models for U1-zLnzO2+0.5(x-y) solid solutions (Ln = {La, Pr, Nd, Gd}), in which the non-stoichiometry, 0.5(x-y), originates due to the presence of a fraction x of UO2.5 (U = U+5) and/or a fraction y of AO1.5 (A = Ln+3, U+3) components. The aim is to predict the non-stoichiometry, the equilibrium cation species fractions and the lattice parameter, a, as functions of the composition, z, the temperature T, and the partial pressure of oxygen,
An important practical aspect of the present study is the development of a model that relates the equilibrium lattice parameter, a, to z, x, and y and simultaneously to T and
2 Methods and Models
2.1 Thermodynamic Model
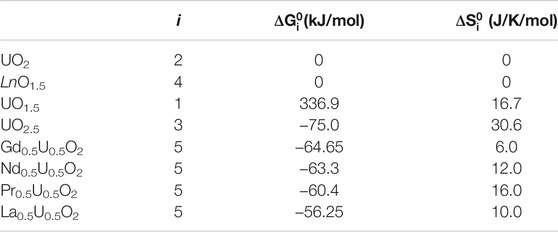
The thermodynamic model assumes a system of a constant chemical composition at a given temperature. The composition of the solid phase is defined by the fractions z and 1–z of the chemical endmembers with the compositions of LnO1.5 and UO2, respectively. We also assume that a system includes a large fixed amount of O2 gas with its partial pressure maintained at a given value. The endmember UO2 is assumed to exist in three different structural forms, namely, the stoichiometric UO2, the hypo-stoichiometric UO1.5 associated with ¼ moles of O2 gas and the hyper-stoichiometric UO2.5 associated with a negative amount of ¼ moles of O2. The negative sign implies that when excess O is inserted into UO2, an equivalent quantity (in moles) of O2 gas annihilates in the system. The equal sum of the endmembers LnO1.5 and UO2 reacts producing the fifth endmember, Ln0.5U0.5O2–1/8 O2 (Figure 1), which is also associated with a negative amount of O2 gas. When not explicitly indicated, the list of the endmembers will be associated with low case indices in the order 1- UO1.5, 2- UO2, 3- UO2.5, 4- LnO1.5, and 5- Ln0.5U0.5O2. This set of endmembers is sufficient to describe variations in stoichiometry of fluorite solid solutions at not too large fractions of LnO1.5. Fractions larger than z = 0.5 may require the consideration of the U1/3Ln2/3O2 endmember to describe stoichiometric states and the UO3 endmember to describe hyper-stoichiometry. Both these endmembers contain U+6. As the final aim of this study is to characterize the oxidation resistance of SNF, in which the content of LnO1.5 does not exceed few atomic percent, we have chosen to limit the set of U species to U+3, U+4, and U+5. Thus, the present model should be applied with caution when z > 0.5. The gas phase component associated with an endmember will be often omitted for brevity. For example, the endmember UO2.5 means a UO2.5 coexisting with a negative amount of ¼ moles of O2 gas. This recognition is important in the following definition of the standard Gibbs free energies of the endmembers. The free energy of each endmember is composed of two contributions, one arising from a solid-state transformation (insertion/deletion of 0.5 mole of O into/from the UO2 structure) and one arising from a creation (or an annihilation) of an equivalent amount of O2 gas. The latter contribution is proportional to the chemical potential of O2. The sum of these contributions is modelled relative to an equivalent combination of the free energies of LnO1.5 and stoichiometric UO2. The free energies of the latter endmembers are set equal zero. As the standard free energies of the other endmembers explicitly depend on the chemical potential, a change in the chemical potential, or a change in the partial pressure of O2, induces a change in endmember fractions. When the pressure is low (the chemical potential of O2 is strongly negative), UO1.5 + ¼ O2 endmember is stabilised over UO2. When the chemical potential is close to zero, UO2.5 – ¼ O2 is stabilised over UO2, consistently with the thermodynamic instability of UO2 in air. A shift of the endmember fractions towards the formation of the UO2.5 and Ln0.5U0.5O2 implies an oxidation of a fraction of U+4 to U+5, while a relative increase in the fraction of UO1.5 implies a reduction of a fraction of U+4 to U+3. The equilibrium endmember fractions are obtained via the minimization of the Gibbs free energy, which includes an additive sum of endmember contributions, a non-ideal term arising from the interactions between the endmembers and an entropic term. The equilibrium calculations are done here using a specially written FORTRAN code.
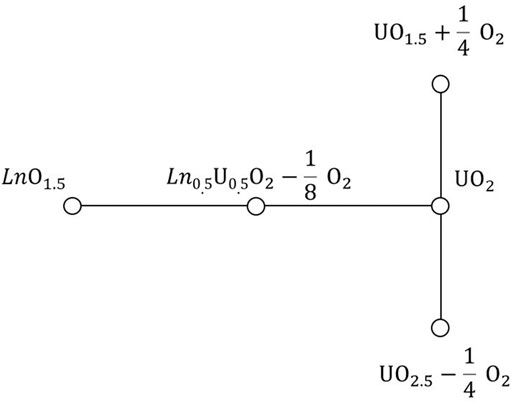
FIGURE 1. Relationship between endmembers of the thermodynamic model. Note, that all Ln-free endmembers have the same chemical composition of UO2.
2.1.1 A Model of UO2+0.5(x-y)
Pure UO2 fluorite is described here as a combination of two binary solid solution models spanning between the endmembers UO2 and UO1.5, in the first case, and UO2 and UO2.5, in the second case. U+6 states, i.e. the UO3 endmember, are not included within the present effort. The free energy of stoichiometric UO2 is set equal to zero, while the free energies of UO2.5 – ¼ O2 and UO1.5 + ¼ O2 endmembers are set equal to the effects of the reactions
and
For example, the effect of Eq. 1 splits into the effect of addition of 0.5 mole of O into the solid and the effect of annihilation of ¼ moles of O2 gas prepared at a given temperature T with a given partial pressure
Similarly, the effect of Eq. 2 splits into the effect of removing of 0.5 mole of O from the solid and the effect of creating of ¼ moles of O2 gas
The parameters
where
The reference Gibbs free energies for hypo-stoichiometric, UO2-0.5y, and hyper-stoichiometric solutions, UO2+0.5x, are written as follows
and
respectively, where y and x are the mole fractions of the components UO1.5 and UO2.5. Naturally, only the solid part of an endmember contributes to the structural formula, however, an equivalent positive or negative mole fraction of O2 gas is always associated with it. Thus, the quantities in Eqs. 6, 7 depend on the chemical potential of O2. The excess free energies are given as follows
and
where
Modelling the configurational entropy of anions requires consideration of effects of local order. Following (Bukaemskiy et al., 2021; Vinograd and Bukaemskiy, 2021) we assume a type of a short-range ordering in which two vacancies cannot approach each other closer than the fourth nearest anion-anion distance. Such a distribution can be emulated by restricting the location of vacancies to a simple cubic sublattice of the anion lattice in which the nearest distance is equal to the fourth nearest distance of the original anion lattice. This model implies that the mixing of vacancies and oxygen anions occurs on 1/8-th of available anion sites, while the concentration of vacancies within the sublattice is 8 times larger than the average concentration of vacancies of 0.25y. These assumptions lead to the equation
which is valid for 0 < y < 0.5. A more detailed derivation of Eq. 10 and of other entropy equations that appear further in the text is given in the Supplementary Materials.
The hyper-stoichiometric solution is characterized by the presence of oxygen interstitials, which are assumed to occupy octahedral sites. One of recent simulation studies provided arguments in favour of a model containing mono- and di-interstitials, with the proportion of di-interstitials increased at larger values of x (Palomares et al., 2019). Two types of di-interstitial cluster, the Willis cluster (Willis, 1978) and the split di-interstitial (Andersson et al., 2009) have been proposed based on neutron diffraction experiments and ab initio calculations. On the other hand, structural studies of compositions close to U4O9 stoichiometry (x = 0.5) suggested the presence of cubooctahedral clusters composed of interstitials and lattice anions shifted from their ideal positions (Bevan et al., 1986). We assume here that the appearance of cubooctahedral clusters signifies a transition from a defect fluorite type solid solution to a solid solution based of a U4O9-type structure and do not make an attempt of modelling the latter. We assume that the distribution of interstitials in hyper-stoichiometric defect fluorite could be simulated assuming the presence of mono- and di-interstitials only. To emulate the presence of just two types of clusters (i.e., the absence of higher-order clusters) we assume the existence of a sublattice within the ideal FCC lattice of interstitials, which is composed of an ordered arrangement of isolated (non-overlapping) pairs of initially vacant interstitial sites. Such an arrangement implies that only ¼ of totally available vacant sites can be occupied by O-interstitials. The concentration of interstitials within such a sublattice increases in four times from the value of 0.5x to the value of 2x. We further assume that the occupation of pairs of vacant sites within the sublattice occurs randomly. These assumptions lead to the following equation for the entropy of mixing
which is valid in hyper-stoichiometric domain of 0 < x < 0.5. Accidentally, this equation is similar to Eq. 10. This circumstance appears important as the thermodynamic data on UO2+0.5(x-y) show a nearly symmetric relationship between hypo- and hyper-stoichiometric branches of
The splitting of the model into the independent “hypo” and “hyper” domains is based on the assumptions that the reactions
and
do not occur separately from each other and that both are strongly shifted to the left. Practically, we assume that oxygen vacancies and oxygen interstitials annihilate each other, and the same assumption is made for U+3 and U+5 cations. These assumptions are reasonable at not too high temperatures considering large enthalpy effects of ∼4 and ∼1.5 eV computed for these reactions ab initio (Nerikar et al., 2009; Cooper et al., 2018). The present model is thus not intended for modelling of UO2 at very high temperatures (∼2000 K and above), where these assumptions become invalid. A consequence of these assumptions is that the chemical potential of O2 in equilibrium with pure UO2 cannot be defined, i.e. pure UO2 coexists with a range of
2.1.2 Ln-Doped UO2-Based Solid Solutions
Ln+3 can be charge balanced in the fluorite solid solution according to two different schemes
and
The first scheme implies the creation of oxygen vacancies. The second scheme requires an oxidation of one U+4 cation per one Ln+3. These schemes are put into the correspondence with LnO1.5 and Ln0.5U0.5O2 endmembers, respectively, where the latter implies the simultaneous addition of equal fractions of Ln+3 and U+5. The free energy of Ln0.5U0.5O2 endmember is defined here via the reaction
as follows
According to the previously made assumption, U+5 cannot occur together with U+3, as these cations annihilate each other via inverse reaction (Eq. 13). Thus, the Ln0.5U0.5O2 and UO1.5 endmembers cannot occur together. Due to this circumstance, doped hypo-stoichiometric solid solutions at a given fraction z of LnO1.5 could be conveniently split into two types, I and II, defined by the inequalities 0.5 > y > z and z > y > 0, respectively. The relationship of 0.5 > y > z implies that the fraction of vacancies is larger than this occurring due to the sole presence of the LnO1.5 endmember. Thus, UO1.5 endmember must necessarily be present creating an additional fraction of vacancies. This also implies that in such a solid solution Ln occurs only as LnO1.5. On the other hand, the relationship of z > y > 0 implies that the fraction of vacancies is smaller than this occurring due to the sole presence of LnO1.5, thus, a certain fraction of LnO1.5 is to be converted into Ln0.5U0.5O2. This second case is further split into the sub-cases IIa and IIb depending on z < 0.5 and z > 0.5. First, we consider the case I of 0.5 > y > z.
2.1.3 Hypo-Stoichiometric Solid Solution, z < y < 0.5, Type I
The reference free energy is spanned by the three endmembers UO2, UO1.5, and LnO1.5 according to the following equation
The excess free energy is given by
where
The configurational entropy of this solid solution has two contributions. The already familiar contribution due to the mixing of vacancies and lattice O2- anions is given by Eq. 10. The contribution due to the mixing of Ln+3 with U+4, and U+3 requires a special consideration. In a solid solution, in which the radii of III- and IV-valent cations have similar sizes, the III-valent cations are expected to be associated with vacancies (Solomon et al., 2014). This association is modelled here by requesting the coordination number of III-valent cations to be 7. The consequence is that Ln+3 can mix only with 7-fold U cations, the restriction that directly affects the configurational entropy. One can further note that the total fraction of 7-fold cations in such a solid solution is 2y. The structural formula becomes
A more detailed derivation of this equation is given in Supplementary Materials.
The total Gibbs free energy of the model is then given as
which is a function of z, T,
2.1.4 Hypo-Stoichiometric Solid Solution, 0 < y < z, z < 0.5, Type IIa
In this case the endmember UO1.5 is absent, thus U+5 formation is allowed, and, thus, two Ln-bearing endmembers can co-exist. The reference and the excess free energies are given by the equations
and
where
As in the case I, we assume that III-valent cations, i.e. Ln+3, are associated to vacancies. However, a part of Ln cations is involved in building the Ln0.5U0.5O2 endmember, which is intrinsically stoichiometric. Thus, it is natural to expect that a fraction z–y of Ln cations remains in 8-fold coordination. As the total fraction of 7-fold cations is 2y, a fraction of y U+4 cations need to be transferred into the 7-fold coordination. The structural formula takes the form
The total Gibbs free energy of the model is then given as
This model is valid for z < 0.5. When z = 0.5, all
2.1.5 Hypo-Stoichiometric Solid Solution, 2z – 1 < y < 0.5, 0.5 < z < 0.75, Type IIb
When z > 0.5, the fraction of
Other equations remain same as in the case IIa.
2.1.6 Hyper-Stoichiometric Solution, Type III
Assuming that vacancies are absent at hyper-stoichiometric compositions, Ln+3 can occur only as Ln0.5U0.5O2. Consequently, there are two fractions of U+5, a fraction of U+5, that is needed to balance Ln+3, and a fraction of U+5 that is needed to balance O-interstitials. The reference Gibbs free energy and the excess Gibbs free energy are given as follows
where
Considering that Ln0.5U0.5O2 is intrinsically stoichiometric, all Ln+3 cations and the equivalent fraction of U+5 cations are assumed to be in 8-fold coordination. The other U+5 cations that balance the interstitials may formally be prescribed coordination numbers larger than 8, because O-interstitials are expected to be in a close association to these cations due to the attraction of differently charged species. For simplicity we assign to all these atoms the 9-fold coordination. The structural formula of doped hyper-stoichiometric solid solution is
As Ln+3 cations are assumed to preserve the coordination of 8, they can be mixed with U atoms only over the fraction 1 – x of 8-fold coordinated sites, thus the configurational entropy of cations is given by the equation
The total Gibbs free energy of the model is then given as
which is a function of z, T,
2.2 Structural Model
Previous research has shown that the lattice parameter of UO2 solid solutions varies as a function of the composition and non-stoichiometry, and that these variations could be predicted based on an ionic packing model (Ohmichi et al., 1981; Fukushima et al., 1983; Lee et al., 2016b; Bukaemskiy et al., 2021). This model utilizes a geometrical relationship between the lattice parameter, a, and the sum of the averaged radii of cations,
which is determined by Figure 2.
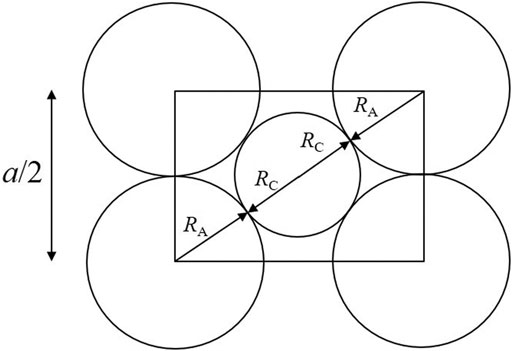
FIGURE 2. Geometric relationship used in the ion-packing model. The rectangle corresponds to the cross section of a cube composed of eight anions along its face diagonal. The spheres representing cations and anions are constrained to touch along the body diagonal. An additional condition (not used in the ion-packing model) could be set by requiring the spheres of anions to touch themselves along [100].
The radii of cation/anions are further evaluated as sums all of cation/anion radii weighted by the cation/anion fractions that contribute to the structural formula, where the anions include the lattice oxygen O2- and the oxygen vacancy
where
Following the study of Bukaemskiy et al. (Bukaemskiy et al., 2021) we assume that a vacancy has a defined radius that is larger than the ionic radius of oxygen. Such a model is consistent with the requirement that the average cation coordination number in hypo-stochiometric samples,
The radii of Ln, of O2- and of a vacancy are adopted from our previous study on ZrO2—LnO1.5 systems (Bukaemskiy et al., 2021). (Some radii in this set were slightly varied reflecting specific properties of UO2-based systems). The average radii can be trivially computed from the structural formulas corresponding to the models introduced above provided that the parameters x, y, and z are known. The relevant structural formulas and the equations to calculate average cation radii are given in Table 1.

TABLE 1. Structural formulas of the solid solution models and the expressions to calculate average cation radii.
The thermodynamic model predicts the equilibrium x and y parameters as functions of z, T, and
3 Results and Discussion
3.1 Thermodynamic Model
Figure 3 shows the results of the model fit to
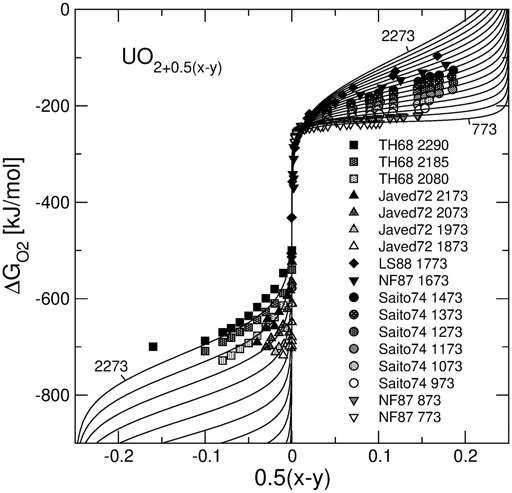
FIGURE 3. Oxygen chemical potential plotted vs. non-stoichiometry parameter of UO2+0.5(x-y). Solid lines are the isotherms predicted with the model. The temperature range is from 773 to 2273 K. The experimental data are from Tetenbaum and Hunt (Tetenbaum and Hunt, 1968), Javed (Javed, 1972), Lindemer and Sutton (Lindemer and Sutton, 1988), Nakamura and Fujino (Nakamura and Fujino, 1987) and Saito (Saito, 1974).
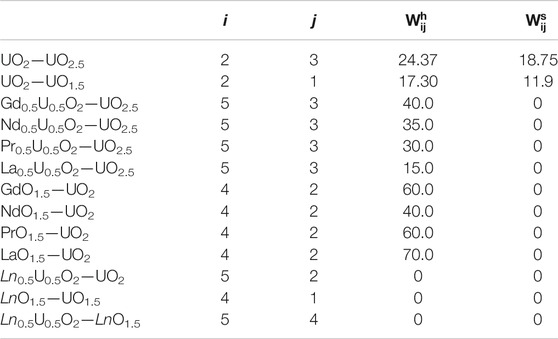
TABLE 3. Margules interaction parameters in kJ/mol determined via a model fit to the thermodynamic data.
Figure 4 shows ∆
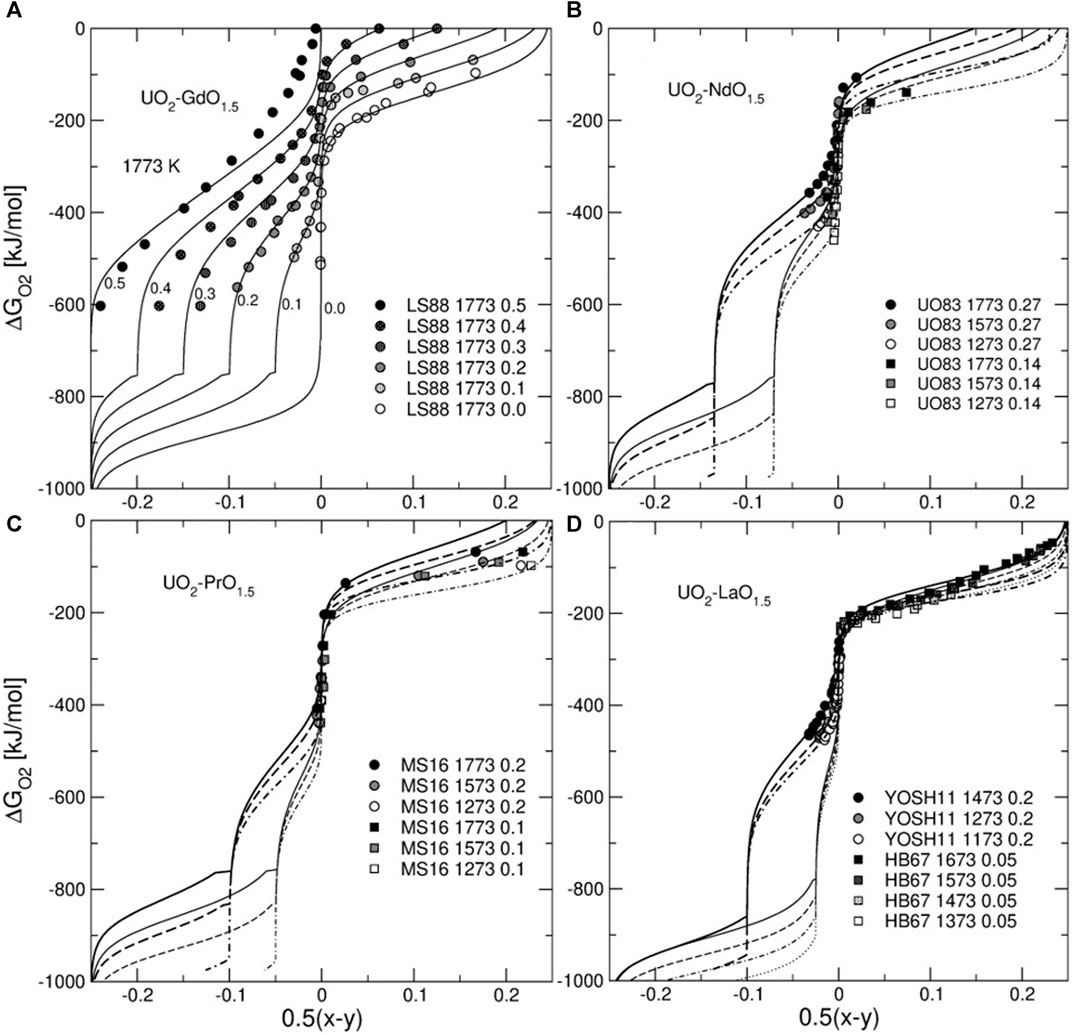
FIGURE 4. Oxygen chemical potential plotted vs. non-stoichiometry parameter of U1-zLnzO2+0.5(x-y). Lines are the isotherms predicted with the model. The temperature ranges in (b), (c), and (d) span the synthesis temperature interval given in the legend (the solid and the dotted lines correspond to the highest and lowest temperatures, respectively). The experimental data are from Lindemer and Sutton (Lindemer and Sutton, 1988), Une and Oguma (Une and Oguma, 1983a), McMurray and Silva (McMurray and Silva, 2016), Yoshida et al. (Yoshida et al., 2011) and Hagemark and Broli (Hagemark and Broli, 1967). Note an increase in the magnitude of the composition effect on the chemical potential of O2 in the direction from La to Gd.
3.2 Structural Model
Figure 5 displays the dependence of the lattice parameter in stoichiometric UO2—LnO1.5 systems. Linear relationships were assumed to determine (refine) values of cation radii in 8-fold coordination. Most of the values remain close to Shannon’s ionic radii (Shannon, 1976). The size of the oxygen anion of 1.3736 Å was adopted from Bukaemskiy et al. (Bukaemskiy et al., 2021) as fitted to data on ZrO2-based systems. The radii of cations in the 7-fold coordination are also from (Bukaemskiy et al., 2021). The effective size of the vacancy of 1.54 Å is slightly increased relative to the value of 1.53 ± 0.02 (Bukaemskiy et al., 2021) to give a better description of data on hypo-stoichiometric UO2-based solids solutions (Hill, 1962; Wadier, 1973), which are discussed below.
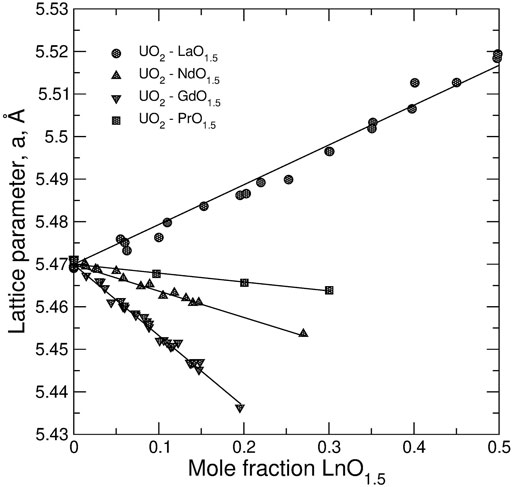
FIGURE 5. The dependence of the lattice parameter in stoichiometric UO2–LnO1.5 systems on the composition. The data are fitted to linear equations to determine the radii of
The hyper-stoichiometric domain (0 < x < 0.3) is successfully described with a model in which U+5 cations that are needed for charge balancing the interstitials are assumed to be 9-fold coordinated with the ionic radius of 0.976 Å. This is reasonable assumption considering the lack of data. The interstitial oxygen is assumed to have no direct influence on the lattice parameter. Indeed, the size of an interstitial cannot be simply reflected within the ion-packing concept, because its structural position does not comply with a regular position of a cation or an anion. Thus, its effect is mapped onto the effective radius of
Figure 6 shows the predicted dependence of the lattice parameter in UO2—PrO1.5 and UO2—NdO1.5 on the composition and on non-stoichiometry. An increase in the hyper-stoichiometry causes a linear decrease of the lattice parameter. Clearly, the composition dependence of the lattice parameter is significantly less pronounced in the case of Pr—U system than in the case of Nd—U system. This is because the half sum of the radii of 8-fold Pr+3 and U+5, is almost equal to the radius of U+4 cation.
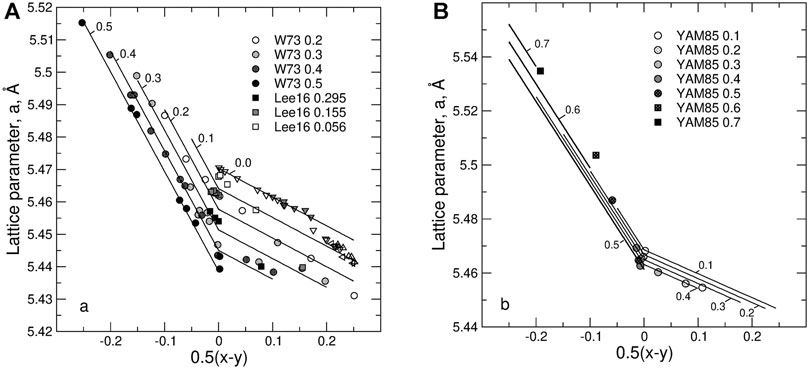
FIGURE 6. The dependence of the lattice parameter on non-stoichiometry, 0.5(x-y), and z in (A) U1-zNdzO2+0.5(x-y) and (B) U1-zPrzO2+0.5(x-y) systems. Solid lines are compositional isopleths predicted with the model. The experimental data (circles and squares) are from Wadier (Wadier, 1973) (circles), Lee et al. (Lee et al., 2016b) (squares), Yamashita et al. (Yamashita et al., 1985) (circles and squares are the samples synthesized at 1623 K), Lynds et al. (Lynds et al., 1963) (open down triangles), Belbeoch (Belbeoch et al., 1964) (shaded down triangles), Matsui and Naito (Matsui and Naito, 1975) (open up triangles), Schaner (Schaner, 1960) (left triangles), Grønvold (Grønvold, 1955) (shaded up triangles). The data on pure UO2 obviously fall on two trends. The first trend that extends from the stoichiometric UO2 up to x ∼ 0.30 is assumed to represent the fluorite-type solid solution. The trend extending to larger x values is assumed to represent U4O9-type structure. Thus, only the data from the first trend were used in the fit.
3.3 Structural-Thermodynamic Model
The thermodynamic relationships allow the prediction of x and y, as functions of z, T, and
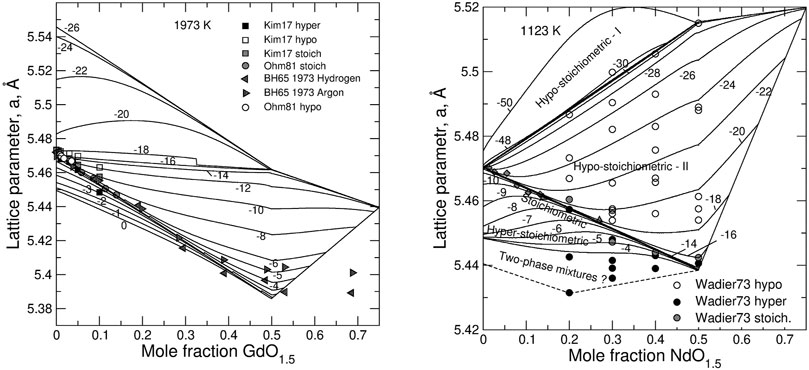
FIGURE 7. Relationship between the lattice parameter and the degree of doping, z, in GdO1.5—UO2 (left) and NdO1.5–UO2 (right) systems. Solid lines are calculated with the model isopleths of constant
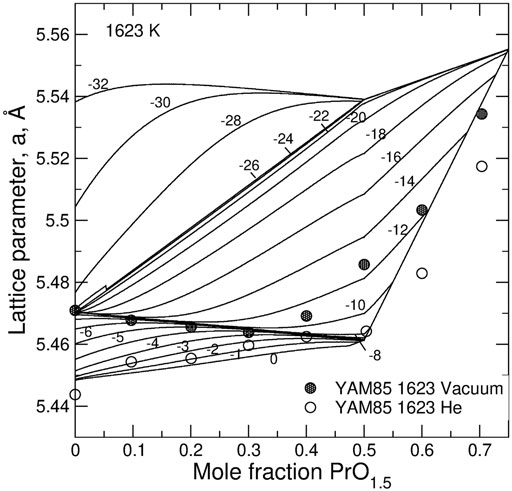
FIGURE 8. Relationship between the lattice parameter and the degree of doping, z, and
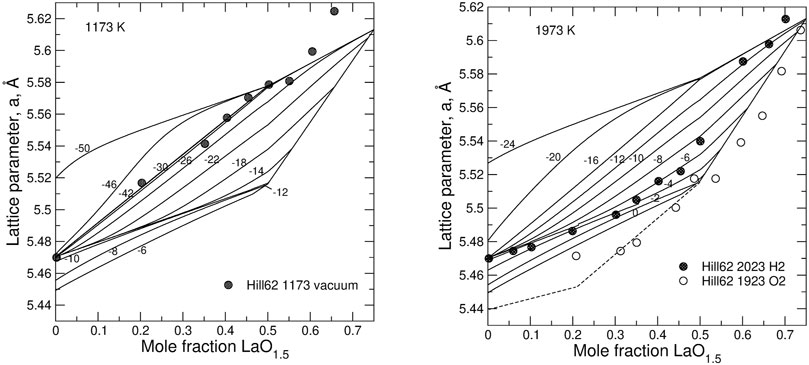
FIGURE 9. Relationship between the lattice parameter and the degree of doping, z, in LaO1.5–UO2 system predicted from the model. The experimental data series from Hill (Hill, 1962) are interpreted to be synthesized at –42 <
Our hypothesis is that the stabilizing effect of Ln-doping in the case of Nd, Gd (and smaller Ln) is primarily caused by the lattice contraction due to the doping and by the increasing strain energy cost of an additional contraction (additional overlap of oxygen anions along [100]) required by the oxidation. At the same time, an increase in the oxygen chemical potential with the extent of hyper-stoichiometry observed in both undoped and doped systems (Figures 3, 4) can be equally well rationalized. As the oxidation from UO2 to UO2+0.5x (x < 0.5) causes an unfavourable decrease in the lattice parameter, the oxidation (i.e. an increase in x) is a self-inhibiting process. This self-inhibition effect could be responsible for the decrease in the U+6 yields after repeating exposures to H2O2 observed recently in oxidative dissolution experiments (Maier et al., 2020).
Further development of the model would require extending it to Ln-dopants with the cation radii smaller than this of Gd+3, to Y and to di-valent dopants. An explicit introducing of U1/3Ln2/3O2 and UO3 endmembers would probably cure apparent problems of the present model at z > 0.5.
4 Conclusion
The data on the dependence of the oxygen potential of doped UO2 solid solutions on the composition, temperature and non-stoichiometry are linked to the data on the dependence of the lattice parameter on composition and non-stoichiometry within the same model frame. This brings up a possibility of relating changes in the lattice parameter to T and
Good fit of the model to available structural data on the dependence of the lattice parameter, a, on z, x, and y shows that the model assumptions of short-range ordering, such as the vacancy-vacancy avoidance, the avoidance of di-interstitial clusters, the association of vacancies to III-valent cations and the association of U+5 to interstitial O2- are reasonable. These assumptions have an important effect on the structural model by limiting the spectrum of cation coordination numbers and thus allowing for the application of the ion-packing concept.
The SRO also imposes a profound effect on the thermodynamic model causing a significant reduction in the configurational entropy relative to the ideal mixing model. This model feature is essential for achieving a good fit to
The entropy decreases 1) due to the ordering of vacancies and interstitials, 2) due to the cation-anion association effects and 3) due to the neglect of the configurational effect from U+4/U+3 and U+4/U+5 mixing. The achieved consistency between the structural and thermodynamic descriptions provides an argument in favour of the validity of these assumptions. Further experimental and computational studies aiming at testing these assumptions in other similar systems would be desirable.
The developed models are thermodynamically simple and transparent. An introduction of a new chemical component, such as LnO1.5, requires just two parameters to define the standard thermodynamic properties of a Ln0.5U0.5O2 endmember and two Margules parameters to model the interactions Ln0.5U0.5O2—UO2.5 and LnO1.5—UO2. This offers a great advantage over models based on the Compound Energy Formalism (Saunders and Miodownik, 1998; Hillert, 2001) that require a larger number of adjustable parameters. The present model requires the Gibbs free energy minimization with respect to just one parameter, y or x, in each model domain.
The small number of thermodynamic parameters provides a possibility of identifying main factors that are responsible for the dependence of the oxidation potential on the doping and on the extent of oxidation. In hyper-stoichiometric region these are the Margules parameters for the interactions between Ln0.5U0.5O2 and UO2.5 and between UO2 and UO2.5. As the first parameter correlates with da/dz slope, the strain associated with the lattice contraction appears to be the likely cause of the stabilization of doped samples against the oxidation.
The proposed mechanism of the resistance of Ln-doped UO2 to the thermodynamically controlled oxidation may be equally applicable to more complex UO2-based simulated spent fuel, as it is known that the lattice parameter of simulated fuel (e.g. UO2 + Zr, Ce, Pr, Nd, and Y) decreases with the simulated burn-up (Une and Oguma, 1983b).
The thermodynamic and structural models developed here provide a possible explanation for the observed corrosion resistance of Ln doped UO2 compared to pure UO2 under conditions expected in a deep geological repository for spent nuclear fuel. However, with respect to the corrosion of SNF under disposal conditions other effects (e.g., radiation effects, He build up, effects of other non-Ln fission products) need to be taken into account.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
The authors confirm contribution to the paper as follows: study conception and design: VV, AB, DB, GM, and GD; data collection: VV, AB; modelling: VV, AB; analysis and interpretation of results: VV, AB, DB, GM, and GD; draft manuscript preparation: VV, AB, GD, GM, and DB. All authors reviewed the results and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge discussions with Dr. D. A. Kulik (PSI) and Dr. E. Curti (PSI) which helped to develop the thermodynamic formalism.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.705024/full#supplementary-material
References
Anderson, J. S., Sawyer, J. O., Worner, H. W., Willis, G. M., and Bannister, M. J. (1960). Decomposition of Uranium Dioxide at its Melting Point. Nature 185 (4717), 915–916. doi:10.1038/185915b0
Andersson, D. A., Lezama, J., Uberuaga, B. P., Deo, C., and Conradson, S. D. (2009). Cooperativity Among Defect Sites in AO2+x and A4O9 (A=U, Np, Pu): Density Functional Calculations. Phys. Rev. B 79 (2), 024110. doi:10.1103/PhysRevB.79.024110
Baena, A., Cardinaels, T., Govers, K., Pakarinen, J., Binnemans, K., and Verwerft, M. (2015). Lattice Contraction and Lattice Deformation of UO2 and ThO2 Doped with Gd2 O3. J. Nucl. Mater. 467, 135–143. doi:10.1016/j.jnucmat.2015.09.018
Barreiro Fidalgo, A., and Jonsson, M. (2019). Radiation Induced Dissolution of (U,Gd)O2 Pellets in Aqueous Solution - A Comparison to Standard UO2 Pellets. J. Nucl. Mater. 514, 216–223. doi:10.1016/j.jnucmat.2018.11.037
Beals, R. J., and Handwerk, J. H. (1965). Solid Solutions in the System Urania-Rare-Earth Oxides: I, UO2-GdO1.5,. J. Am. Ceram. Soc. 48 (5), 271–274. doi:10.1111/j.1151-2916.1965.tb14735.x
Belbeoch, M. B., Laredo, M. E., and Perio, P. (1964). Examen par rayons X, après trempe, d'oxydes d'uranium de type UO2. J. Nucl. Mater. 13 (1), 100–106. doi:10.1016/0022-3115(64)90072-8
Bevan, D. J. M., Grey, I. E., and Willis, B. T. M. (1986). The crystal Structure of β-U4O9−y. J. Solid State. Chem. 61 (1), 1–7. doi:10.1016/0022-4596(86)90002-2
Bosbach, D., Brandt, F., Bukaemskiy, A., Deissmann, G., Kegler, P., Klinkenberg, M., et al. (2020). Research for the Safe Management of Nuclear Waste at Forschungszentrum Jülich: Materials Chemistry and Solid Solution Aspects. Adv. Eng. Mater. 22 (6), 1901417. doi:10.1002/adem.201901417
Bruno, J., and Ewing, R. C. (2006). Spent Nuclear Fuel. Elements 2 (6), 343–349. doi:10.2113/gselements.2.6.343
Bukaemskiy, A. A., Vinograd, V. L., and Kowalski, P. M. (2021). Ion Distribution Models for Defect Fluorite ZrO2 - AO1.5 (A = Ln, Y) Solid Solutions: I. Relationship between Lattice Parameter and Composition. Acta Materialia 202, 99–111. doi:10.1016/j.actamat.2020.10.045
Carbol, P., Wegen, D. H., Wiss, T., and Fors, P. (2012). “Spent Fuel as Waste Material,” in Comprehensive Nuclear Materials. Editor R. Konings (Amsterdam (Netherlands): Elsevier), 389–420. doi:10.1016/b978-0-08-056033-5.00106-3
Cardinaels, T., Hertog, J., Vos, B., de Tollenaere, L., Delafoy, C., and Verwerft, M. (2012). Dopant Solubility and Lattice Contraction in Gadolinia and Gadolinia-Chromia Doped UO2 Fuels. J. Nucl. Mater. 424 (1), 289–300. doi:10.1016/j.jnucmat.2012.02.014
Casella, A., Hanson, B., and Miller, W. (2016). The Effect of Fuel Chemistry on UO2 Dissolution. J. Nucl. Mater. 476, 45–55. doi:10.1016/j.jnucmat.2016.04.025
Cooper, M. W. D., Murphy, S. T., and Andersson, D. A. (2018). The Defect Chemistry of UO2±x from Atomistic Simulations. J. Nucl. Mater. 504, 251–260. doi:10.1016/j.jnucmat.2018.02.034
Curti, E., and Kulik, D. A. (2020). Oxygen Potential Calculations for Conventional and Cr-Doped UO2 Fuels Based on Solid Solution Thermodynamics. J. Nucl. Mater. 534, 152140. doi:10.1016/j.jnucmat.2020.152140
Degueldre, C., Pin, S., Poonoosamy, J., and Kulik, D. A. (2014). Redox State of Plutonium in Irradiated Mixed Oxide Fuels. J. Phys. Chem. Sol. 75 (3), 358–365. doi:10.1016/j.jpcs.2013.11.001
Eriksen, T. E., Shoesmith, D. W., and Jonsson, M. (2012). Radiation Induced Dissolution of UO2 Based Nuclear Fuel - A Critical Review of Predictive Modelling Approaches. J. Nucl. Mater. 420 (1), 409–423. doi:10.1016/j.jnucmat.2011.10.027
Ewing, R. C. (2015). Long-term Storage of Spent Nuclear Fuel. Nat. Mater 14 (3), 252–257. doi:10.1038/nmat4226
Fanghänel, T., Rondinella, V. V., Glatz, J.-P., Wiss, T., Wegen, D. H., Gouder, T., et al. (2013). Reducing Uncertainties Affecting the Assessment of the Long-Term Corrosion Behavior of Spent Nuclear Fuel. Inorg. Chem. 52 (7), 3491–3509. doi:10.1021/ic302012c
Ferry, C., Poinssot, C., Broudic, V., Cappelaere, C., Desgranges, L., Garcia, P., et al. (2005). Synthesis on the Spent Fuel Long Term Evolution. France: CEA.
Finnis, M. W., Lozovoi, A. Y., and Alavi, A. (2005). The Oxidation of NiAl: What Can We Learn from Ab Initio Calculations?. Annu. Rev. Mater. Res. 35 (1), 167–207. doi:10.1146/annurev.matsci.35.101503.091652
Fukushima, S., Ohmichi, T., Maeda, A., and Handa, M. (1983). Thermal Conductivity of Near-Stoichiometric (U, Nd)O2, (U, Sm)O2 and (U, Eu)O2 Solid Solutions. J. Nucl. Mater. 114 (2), 312–325. doi:10.1016/0022-3115(83)90270-2
Fukushima, S., Ohmichi, T., Maeda, A., and Watanabe, H. (1982). The Effect of Gadolinium Content on the thermal Conductivity of Near-Stoichiometric (U,Gd)O2 Solid Solutions. J. Nucl. Mater. 105 (2), 201–210. doi:10.1016/0022-3115(82)90375-0
Grønvold, F. (1955). High-temperature X-ray Study of Uranium Oxides in the UO2·U3O8 Region. J. Inorg. Nucl. Chem. 1 (6), 357–370. doi:10.1016/0022-1902(55)80046-2
Guéneau, C., Dupin, N., Sundman, B., Martial, C., Dumas, J.-C., Gossé, S., et al. (2011). Thermodynamic Modelling of Advanced Oxide and Carbide Nuclear Fuels: Description of the U-Pu-O-C Systems. J. Nucl. Mater. 419 (1), 145–167. doi:10.1016/j.jnucmat.2011.07.033
Hagemark, K., and Broli, M. (1967). Equilibrium Oxygen Pressures over Solid Solutions of Urania-Yttria and Urania-Lanthana at 1100° to 14OO°C. J. Am. Ceram. Soc. 50 (11), 563–567. doi:10.1111/j.1151-2916.1967.tb14999.x
Hansson, N. L., Tam, P. L., Ekberg, C., and Spahiu, K. (2021). XPS Study of External α-radiolytic Oxidation of UO2 in the Presence of Argon or Hydrogen. J. Nucl. Mater. 543, 152604. doi:10.1016/j.jnucmat.2020.152604
He, H., Keech, P. G., Broczkowski, M. E., Noël, J. J., and Shoesmith, D. W. (2007). Characterization of the Influence of Fission Product Doping on the Anodic Reactivity of Uranium Dioxide. Can. J. Chem. 85, 702–713. doi:10.1139/V07-056
Hill, D. C. (1962). Phase Relations and Crystal Chemistry in the System Uranium Oxide-Lanthanum Oxide. J. Am. Ceram. Soc. 45 (6), 258–263. doi:10.1111/j.1151-2916.1962.tb11140.x
Hillert, M. (2001). The Compound Energy Formalism. J. Alloys Compd. 320 (2), 161–176. doi:10.1016/S0925-8388(00)01481-X
Hirai, M., and Ishimoto, S. (1991). Thermal Diffusivities and Thermal Conductivities of UO2-Gd2O3. J. Nucl. Sci. Technol. 28 (11), 995–1000. doi:10.1080/18811248.1991.9731462
Hong, S. J., and Virkar, A. V. (1995). Lattice Parameters and Densities of Rare-Earth Oxide Doped Ceria Electrolytes. J. Am. Ceram. Soc. 78 (2), 433–439. doi:10.1111/j.1151-2916.1995.tb08820.x
Javed, N. A. (1972). Thermodynamic Study of Hypostoichiometric Urania. J. Nucl. Mater. 43 (3), 219–224. doi:10.1016/0022-3115(72)90053-0
Karpov, I. K., Chudnenko, K. V., Kulik, D. A., Avchenko, O. V., and Bychinski, V. A. (2001). Minimization of Gibbs Free Energy in Geochemical Systems by Convex Programming. Geochem. Int. 39 (11).
Kim, J.-G., Ha, Y.-K., Park, S.-D., Jee, K.-Y., and Kim, W.-H. (2001). Effect of a Trivalent Dopant, Gd3+, on the Oxidation of Uranium Dioxide. J. Nucl. Mater. 297 (3), 327–331. doi:10.1016/S0022-3115(01)00639-0
Kim, J., Lee, J., Youn, Y.-S., Liu, N., Kim, J.-G., Ha, Y.-K., et al. (2017). The Combined Influence of Gadolinium Doping and Non-stoichiometry on the Structural and Electrochemical Properties of Uranium Dioxide. Electrochimica Acta 247, 942–948. doi:10.1016/j.electacta.2017.07.023
Kleykamp, H. (1988). The Chemical State of Fission Products in Oxide Fuels at Different Stages of the Nuclear Fuel Cycle. Nucl. Technol. 80 (3), 412–422. doi:10.13182/NT88-A34065
Kleykamp, H. (1985). The Chemical State of the Fission Products in Oxide Fuels. J. Nucl. Mater. 131 (2), 221–246. doi:10.1016/0022-3115(85)90460-X
Kowalski, P. M., and Li, Y. (2016). Relationship between the Thermodynamic Excess Properties of Mixing and the Elastic Moduli in the Monazite-type Ceramics. J. Eur. Ceram. Soc. 36 (8), 2093–2096. doi:10.1016/j.jeurceramsoc.2016.01.051
Kulik, D. A., Wagner, T., Dmytrieva, S. V., Kosakowski, G., Hingerl, F. F., Chudnenko, K. V., et al. (2013). GEM-selektor Geochemical Modeling Package: Revised Algorithm and GEMS3K Numerical Kernel for Coupled Simulation Codes. Comput. Geosci. 17, 1–24. doi:10.1007/s10596-012-9310-6
Lee, S. M., Knight, T. W., McMurray, J. W., and Besmann, T. M. (2016). Measurement of the Oxygen Partial Pressure and Thermodynamic Modeling of the U-Nd-O System. J. Nucl. Mater. 473, 272–282. doi:10.1016/j.jnucmat.2016.02.024
Lee, S. M., Knight, T. W., Voit, S. L., and Barabash, R. I. (2016). Lattice Parameter Behavior with Different Nd and O Concentrations in (U1−yNdy)O2±X Solid Solution. Nucl. Technol. 193 (2), 287–296. doi:10.13182/NT14-136
Lemmens, K., González-Robles, E., Kienzler, B., Curti, E., Serrano-Purroy, D., Sureda, R., et al. (2017). Instant Release of Fission Products in Leaching Experiments with High Burn-Up Nuclear Fuels in the Framework of the Euratom Project FIRST-Nuclides. J. Nucl. Mater. 484, 307–323. doi:10.1016/j.jnucmat.2016.10.048
Leyva, A. G., Vega, D., Trimarco, V., and Marchi, D. (2002). Homogeneity Characterisation of Sintered (U,Gd)O2 Pellets by X-ray Diffraction. J. Nucl. Mater. 303 (1), 29–33. doi:10.1016/S0022-3115(02)00819-X
Lindemer, T. B., and Sutton, A. L. (1988). Study of Nonstoichiometry of U1-zGdzO2+-X>. J. Am. Ceram. Soc. 71 (7), 553–561. doi:10.1111/j.1151-2916.1988.tb05919.x
Liu, N., He, H., Noël, J. J., and Shoesmith, D. W. (2017). The Electrochemical Study of Dy2O3 Doped UO2 in Slightly Alkaline Sodium Carbonate/bicarbonate and Phosphate Solutions. Electrochimica Acta 235, 654–663. doi:10.1016/j.electacta.2017.03.075
Lynds, L., Young, W. A., Mohl, J. S., and Libowitz, G. G. (1963). X-Ray and Density Study of Nonstoichiometry in Uranium Oxides. Adv. Chem. Nonstoichiometric Compd. 39, 58–65. doi:10.1021/ba-1964-0039.ch005
Maier, A. C., Kegler, P., Klinkenberg, M., Baena, A., Finkeldei, S., Brandt, F., et al. (2020). On the Change in UO2 Redox Reactivity as a Function of H2O2 Exposure. Dalton Trans. 49, 1241–1248. doi:10.1039/c9dt04395k
Marrocchelli, D., Bishop, S. R., and Kilner, J. (2013). Chemical Expansion and its Dependence on the Host Cation Radius. J. Mater. Chem. A. 1 (26), 7673–7680. doi:10.1039/C3TA11020F
Marrocchelli, D., Bishop, S. R., Tuller, H. L., and Yildiz, B. (2012). Understanding Chemical Expansion in Non-stoichiometric Oxides: Ceria and Zirconia Case Studies. Adv. Funct. Mater. 22 (9), 1958–1965. doi:10.1002/adfm.201102648
Matsui, T., and Naito, K. (1975). Phase Relation and Defect Structures of Nonstoichiometric U4O9±y and UO2+x at High Temperatures. J. Nucl. Mater. 56 (3), 327–335. doi:10.1016/0022-3115(75)90050-1
McMurray, J. W., Shin, D., and Besmann, T. M. (2015). Thermodynamic Assessment of the U-La-O System. J. Nucl. Mater. 456, 142–150. doi:10.1016/j.jnucmat.2014.09.031
McMurray, J. W., Shin, D., Slone, B. W., and Besmann, T. M. (2013). Thermochemical Modeling of the U1−yGdyO2±x Phase. J. Nucl. Mater. 443 (1), 588–595. doi:10.1016/j.jnucmat.2013.08.005
McMurray, J. W., and Silva, C. M. (2016). Experimental Oxygen Potentials for U1−yPryO2±x and Thermodynamic Assessment of the U-Pr-O System. J. Nucl. Mater. 470, 111–118. doi:10.1016/j.jnucmat.2015.11.059
Nakamura, A., and Fujino, T. (1987). Thermodynamic Study of UO2+x by Solid State Emf Technique. J. Nucl. Mater. 149 (1), 80–100. doi:10.1016/0022-3115(87)90501-0
Nerikar, P., Watanabe, T., Tulenko, J. S., Phillpot, S. R., and Sinnott, S. B. (2009). Energetics of Intrinsic point Defects in Uranium Dioxide from Electronic-Structure Calculations. J. Nucl. Mater. 384 (1), 61–69. doi:10.1016/j.jnucmat.2008.10.003
Nilsson, S., and Jonsson, M. (2011). H2O2 and Radiation Induced Dissolution of UO2 and SIMFUEL Pellets. J. Nucl. Mater. 410 (1), 89–93. doi:10.1016/j.jnucmat.2011.01.020
Ohmichi, T., Fukushima, S., Maeda, A., and Watanabe, H. (1981). On the Relation between Lattice Parameter and O/M Ratio for Uranium Dioxide-Trivalent Rare Earth Oxide Solid Solution. J. Nucl. Mater. 102 (1), 40–46. doi:10.1016/0022-3115(81)90544-4
Palomares, R. I., McDonnell, M. T., Yang, L., Yao, T., Szymanowski, J. E. S., Neuefeind, J., et al. (2019). Oxygen point Defect Accumulation in Single-phase UO2+x. Phys. Rev. Mater. 3 (5), 053611. doi:10.1103/PhysRevMaterials.3.053611
Park, K., and Olander, D. R. (1992). Defect Models for the Oxygen Potentials of Gadolinium-And Europium-Doped Urania. J. Nucl. Mater. 187 (1), 89–96. doi:10.1016/0022-3115(92)90323-D
Prieur, D., Martel, L., Vigier, J.-F., Scheinost, A. C., Kvashnina, K. O., Somers, J., et al. (2018). Aliovalent Cation Substitution in UO2: Electronic and Local Structures of U1-yLayO2±x Solid Solutions. Inorg. Chem. 57 (3), 1535–1544. doi:10.1021/acs.inorgchem.7b02839
Razdan, M., and Shoesmith, D. W. (2013). Influence of Trivalent-Dopants on the Structural and Electrochemical Properties of Uranium Dioxide (UO2). J. Electrochem. Soc. 161 (3), H105–H113. doi:10.1149/2.047403jes
Saito, Y. (1974). Nonstoichiometry in Uranium Dioxide. J. Nucl. Mater. 51 (1), 112–125. doi:10.1016/0022-3115(74)90121-4
Saunders, N., and Miodownik, A. P. (1998). CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide. Oxford, New York: Pergamon.
Schaner, B. E. (1960). Metallographic Determination of the UO2-U4O9 Phase Diagram. J. Nucl. Mater. 2 (2), 110–120. doi:10.1016/0022-3115(60)90038-6
Schreinemachers, C., Bukaemskiy, A. A., Klinkenberg, M., Neumeier, S., Modolo, G., and Bosbach, D. (2014). Characterization of Uranium Neodymium Oxide Microspheres Synthesized by Internal Gelation. Prog. Nucl. Energ. 72, 17–21. doi:10.1016/j.pnucene.2013.07.016
Schreinemachers, C., Leinders, G., Modolo, G., Verwerft, M., Binnemans, K., and Cardinaels, T. (2020). Fabrication of Nd- and Ce-Doped Uranium Dioxide Microspheres via Internal Gelation. J. Nucl. Mater. 535, 152128. doi:10.1016/j.jnucmat.2020.152128
Shannon, R. D. (1976). Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. Sect A. 32(5):751–767. doi:10.1107/S0567739476001551
Shoesmith, D. W. (2000). Fuel Corrosion Processes under Waste Disposal Conditions. J. Nucl. Mater. 282 (1), 1–31. doi:10.1016/S0022-3115(00)00392-5
Shoesmith, D. W. (2013). “The Chemistry/electrochemistry of Spent Nuclear Fuel as a Wasteform,” in Uranium: Cradle to Grave. Editors P. Burns, and G. Simon (Canada: Mineralogical Society of Canada), 337–368.
Soldati, A. L., Gana Watkins, I., Fernández Zuvich, A., Napolitano, F., Troiani, H., Caneiro, A., et al. (2016). Synthesis and Characterization of Gd2O3 Doped UO2 Nanoparticles. J. Nucl. Mater. 479, 436–446. doi:10.1016/j.jnucmat.2016.07.033
Solomon, J. M., Alexandrov, V., Sadigh, B., Navrotsky, A., and Asta, M. (2014). Computational Study of the Energetics and Defect Clustering Tendencies for Y- and La-Doped UO2. Acta Materialia 78, 282–289. doi:10.1016/j.actamat.2014.06.052
Stadlbauer, E., Wichmann, U., Lott, U., and Keller, C. (1974). Thermodynamics and Phase Relationships of the Ternary Lanthanum-Uranium-Oxygen System. J. Solid State. Chem. 10 (4), 341–350. doi:10.1016/0022-4596(74)90043-7
Tetenbaum, M., and Hunt, P. D. (1968). High‐Temperature Thermodynamic Properties of Oxygen‐Deficient Urania. J. Chem. Phys. 49 (11), 4739–4744. doi:10.1063/1.1669953
Trummer, M., Dahlgren, B., and Jonsson, M. (2010). The Effect of Y2O3 on the Dynamics of Oxidative Dissolution of UO2. J. Nucl. Mater. 407 (3), 195–199. doi:10.1016/j.jnucmat.2010.10.014
Une, K., and Oguma, M. (1983). Oxygen Potentials of (U,Nd)O2 ± x Solid Solutions in the Temperature Range 1000–1500°C. J. Nucl. Mater. 118 (2), 189–194. doi:10.1016/0022-3115(83)90224-6
Une, K., and Oguma, M. (1983). Oxygen Potentials of UO2 Fuel Simulating High Burnup. J. Nucl. Sci. Technol. 20 (10), 844–851. doi:10.1080/18811248.1983.9733476
Vinograd, V. L., Brandt, F., Rozov, K., Klinkenberg, M., Refson, K., Winkler, B., et al. (2013). Solid-aqueous Equilibrium in the BaSO4-RaSO4-H2O System: First-Principles Calculations and a Thermodynamic Assessment. Geochimica et Cosmochimica Acta 122, 398–417. doi:10.1016/j.gca.2013.08.028
Vinograd, V. L., and Bukaemskiy, A. A. (2021). Ion Distribution Models for Defect Fluorite ZrO2 - AO1.5 (A = Ln, Y) Solid Solutions: II. Thermodynamics of Mixing and Ordering. Acta Materialia 202, 55–67. doi:10.1016/j.actamat.2020.10.046
Wadier, J. F. (1973). Phase Diagrams and Thermodynamic Properties of the Uranium-Neodymium-Oxygen System. Tech. rep. Rapport CEA-R-4507,Commisariat a l'energie atomique.
Willis, B. T. M. (1978). The Defect Structure of Hyper-Stoichiometric Uranium Dioxide. Acta Cryst. Sect A. 34 (1), 88–90. doi:10.1107/S0567739478000157
Yamashita, T., Fujino, T., and Tagawa, H. (1985). Phase Relations and crystal Chemistry in the Ternary PrO1.5-UO2-O2 System. J. Nucl. Mater. 132 (2), 192–201. doi:10.1016/0022-3115(85)90414-3
Keywords: UO2 fluorite, non-stoichiometry, oxidation resistance, lattice parameter, thermodynamic modelling
Citation: Vinograd VL, Bukaemskiy AA, Modolo G, Deissmann G and Bosbach D (2021) Thermodynamic and Structural Modelling of Non-Stoichiometric Ln-Doped UO2 Solid Solutions, Ln = {La, Pr, Nd, Gd}. Front. Chem. 9:705024. doi: 10.3389/fchem.2021.705024
Received: 04 May 2021; Accepted: 22 September 2021;
Published: 08 November 2021.
Edited by:
Maik Kurt Lang, The University of Tennessee, United StatesReviewed by:
Xiaolong Zhu, Merck (United States), United StatesAnna Shelyug, Institute of Solid State Chemistry, Russia
Copyright © 2021 Vinograd, Bukaemskiy, Modolo, Deissmann and Bosbach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: V. L. Vinograd, di52aW5vZ3JhZEBmei1qdWVsaWNoLmRl
 Victor L. Vinograd
Victor L. Vinograd Andrey A. Bukaemskiy
Andrey A. Bukaemskiy Guido Deissmann
Guido Deissmann