
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 12 August 2021
Sec. Analytical Chemistry
Volume 9 - 2021 | https://doi.org/10.3389/fchem.2021.695940
This article is part of the Research TopicAnalytical Methods for Elucidating Harmful Exposures Related to VapingView all 24 articles
 Cory Holder1*
Cory Holder1* Aaron Adams1,2
Aaron Adams1,2 Claire Allison1,2
Claire Allison1,2 Olivia Cote1,2
Olivia Cote1,2 Rachel Lippens1,2
Rachel Lippens1,2 Benjamin C Blount1
Benjamin C Blount1 Lanqing Wang1*
Lanqing Wang1*In August 2019, the Centers for Disease Control and Prevention (CDC) received the first reports of lung injuries that were eventually termed e-cigarette, or vaping, product use–associated lung injury (EVALI). As part of the investigation, CDC laboratories rapidly developed assays for analyzing substances in bronchoalveolar lavage (BAL) fluid collected from EVALI case patients. This report describes the development and validation of a high-throughput isotope dilution UHPLC-MS/MS method for measuring a major oxidative stress biomarker, 8-iso-prostaglandin F2α (8-isoprostane), in BAL fluid samples. The method showed good sensitivity, 17.6 pg/ml LOD, and requires only 50 μl of sample volume. The method had high throughput with an analytical run time of 11 min. The within-day and between-day coefficient of variation (CV) were below 2%. Accuracy, calculated from spiked recovery, at three spiking levels, ranged from 95.5–101.8%. This novel UHPLC-MS/MS method characterizes oxidative stress in lung epithelial tissue and thus helps to elucidate potential pathologic processes.
The Centers for Disease Control and Prevention (CDC) received initial case reports of a range of pulmonary illnesses requiring hospitalization in otherwise healthy users of e-cigarette, or vaping, products (EVPs) in August 2019. The number of hospitalized cases eventually rose to 2,807 with 68 confirmed deaths. (C (2020). Outbreak of L, 2020) The disease was initially attributed to an “unknown chemical exposure” and was eventually found to be strongly linked to inhaled Vitamin E acetate in vaping products. (Schier et al., 2019) EVALI patients were generally healthy before onset of symptoms, which included inflammation. (Krishnasamy et al., 2020) Inflammation can lead to oxidative stress (OS) which could contribute to the acute lung injury observed in EVALI case patients. (Imai et al., 2008) OS is characterized as an imbalance between reactive oxygen species (ROS) and anti-oxidants. ROS are unstable chemical compounds generated endogenously by immune responses, mitochondrial metabolism, and exposure to environmental toxicants such as tobacco smoke. (Ray et al., 2012) Elevated 8-iso-prostaglandin F2α (8-isoprostane) concentrations are indicative of OS and have been linked to the pathophysiology of many diseases including neurodegenerative diseases, lung diseases, and cancers. (Montuschi et al., 1998), (Malli et al., 2013), (Cracowski et al., 2002), (Baraldi et al., 2003), (Montuschi et al., 2000) To analyze 8-isoprostane, bronchoalveolar lavage (BAL) fluid was obtained via bronchoscopy by spraying normal saline onto the lung epithelial lining and then applying mild suction to retrieve a fraction of that saline along with components from the lung epithelial lining fluid. This report highlights the rapid development and validation of a novel isotope dilution UHPLC-MS/MS method measuring 8-isoprostane in BAL fluid. Application of this method allows for a useful measure of OS in the lung epithelial lining and thus provides insights about the pathophysiology of EVALI. (Blount et al., 2020).
Acetonitrile (HPLC grade), methanol (HPLC grade), formic acid (≥ 99.5%), ammonium hydroxide (certified ACS plus), and phosphate-buffered saline (PBS) without calcium and magnesium were purchased from Fisher Scientific (NJ, United States). Water (HPLC grade) was purchased from JT Baker (NJ, United States). Synthetic 8-Iso-prostaglandin F2α (8-isoprostane; CAS# 27415–26–5), 8-iso-prostaglandin F2α-d4 (>99%) (8-isoprostane d4; CAS# 211105–40–7), ent-8-iso-15(S)-PGF2α (≥98%) (CAS# 214748–66–0); ent-8-iso-PGF2α (≥98%) (CAS# 159812–83–6), 8-iso-PGE1 (≥98%) (CAS# 21003–46–3); PGE1 (≥98%) (CAS# 745–65–3); 8-iso-15(R)-PGF2α (≥98%) (CAS# 214748–65–9); PGF2α (≥98%) (CAS# 551–11–1); and 15(R)-PGF2α (≥98%) (CAS# 37658–84–7) were obtained from Cayman Chemical Company (MI, United States). Potassium phosphate monobasic crystals (Reagent ACS) was obtained from Acros (NJ, United States). Potassium phosphate buffer with a pH of 6.1 was prepared using a Mettler Toledo S220 pH meter (Greifensee, Switzerland).
The initial 8-isoprostane stock solution was prepared by adding 7.05 mg of the dry powder (99% purity) into a 200 ml volumetric flask and diluting with methanol in HPLC water (v/v 1:1) to obtain a native spiking solution concentration of 34.9 μg/ml. Working solutions were prepared for standards from serial dilutions of initial native and ISTD stock solutions with methanol and HPLC water (v/v 1:1). Standards were prepared at 10 concentrations ranging from 0 to 1,410 pg/ml by serial dilution of working solutions with methanol and HPLC water (v/v 1:9) and stored in 1.5 ml amber glass vials at −70°C. The materials for the standard solutions were all prepared gravimetrically, and mass results were reported on the conventional basis for weighing in air. These standard solutions were only used as external calibration standards for each analytical run.
The isotopically labeled internal standard (ISTD), 8-isoprostane d4, was dissolved in methyl acetate (100 μg/ml), then 0.5 ml of the initial solution was added to a 100-ml volumetric flask and diluted with methanol in HPLC water (v/v 1:1) resulting in a working solution with a concentration of 500 ng/ml. The final ISTD solution was made by adding 60 ml of the ISTD working solution to a 2,000 ml volumetric flask and diluting with methanol in HPLC water (v/v 1:9), bringing the final concentration to 15 ng/ml. The ISTD solution was dispensed into 2 ml cryovials, stored at −70°C, and thawed before sample preparation.
Non-EVALI BAL fluids used in this study were acquired from Discovery Life Sciences (Huntsville, AL, United States). They were shipped frozen on dry ice and then stored in −70°C freezers until analyzed.
Individual BAL fluids were screened and three BAL fluid samples with no detectable 8-isoprostane levels were selected to make a blank pool for accuracy, precision, and stability testing. Native 8-isoprostane was dissolved into methanol and water (v/v 1:9) to make spiking solutions. These solutions were spiked into the pooled BAL fluid to achieve six final pools with concentrations ranging from 0–2,000 pg/ml. The spiked pools were used for all method validation experiments. One of the BAL fluid samples with no detectable 8-isoprostane was spiked to create a series of four individual BAL fluid spiked levels with concentrations from 0–2,000 pg/ml. This spiked individual sample was used for LOD testing.
Because the available quantity of BAL fluid was not sufficient to perform all experiments, blanks and QC pools were prepared using phosphate-buffered saline (PBS). The PBS solution was spiked with native 8-isoprostane to form low, medium, and high QC concentrations of 200, 500, and 2,000 pg/ml, respectively. The saline QCs were processed in every analytical run and monitored for accuracy and precision.
An analytical run consisted of a blank, three quality controls (low, medium, and high), and up to 44 unknown BAL fluid samples, and each 96-well plate could hold two analytical runs. Ten external calibration standards were run in duplicate for each analytical run.
BAL fluid was thawed and centrifuged at 1,500 rpm for 12 min at 4°C, and the supernatant was transferred to 2 ml Nalgene cryovials. Prior to aliquoting, each BAL fluid sample was vortexed for approximately 10 s to homogenize the sample. A Hamilton Starlet system was utilized for the automated liquid transfer of internal standard, phosphate buffer, water, and methanol. Liquid transfers were performed using 50, 300, and 1,000 µl black conductive pipette tips from Hamilton in which 40 μl of the isotopically labeled internal standard working solution (15 ng/ml), 160 μl of buffer solution (0.5 M phosphate buffer, pH 6.1), and 1,150 μl of HPLC water, respectively, were dispensed into glass test tubes (12 × 75 mm). Due to variations in sample consistency, a manual transfer of 50 μl of BAL fluid was performed using 250 μl Ranin Precision Tips. A sample volume of 50 μl represents a 20 fold dilution and an appropriate correction factor was applied to the measured concentration. Finally, 400 µl of methanol was added to each sample tube. The entire contents in the glass tube were transferred to a 96-well weak anion exchange SPE plate using the Hamilton Starlet system. SPE cleanup was done using the Strata-X-AW 33 µm Polymeric Weak Anion, 60 mg/ 96-well plate from Phenomenex (Torrance, CA, United States). A Biotage Pressure + 96 positive pressure manifold (Biotage, Charlotte, NC, United States) using nitrogen gas generated in-house with a NM20ZA Peak Generator was used to apply positive pressure to the SPE plate. The SPE plate was washed with 1.8 ml of HPLC water, followed by a 1.8 ml solution of methanol in HPLC water (v/v 1:3), and finally 1.8 ml of acetonitrile. Samples were then eluted using methanol and collected in an Advantage Series SiliGuard coated 2 ml 96 deep square well collection plate with a tapered V-bottom (Analytical Sales and Services Inc., Flanders, NJ, United States), evaporated under nitrogen flow at 37°C, reconstituted with 50 μl of 25% methanol in water, vortexed lightly for approximately 2 min, and subsequently injected into the LC-MS/MS.
The LC-MS/MS instrument parameters were kept the same as our previously published CLIA urinary assay. (Holder et al., 2020) In brief, chromatographic separation was achieved using a Waters ACQUITY reversed-phase column (150 mm × 2.1 mm, particle size 1.8 μm, C18) and a Waters ACQUITY reversed-phase pre-column (5 mm × 1 mm, particle size 1.7 μm, C18) (Milford, MA, United States) on an ultra high performance liquid chromatographic system from Shimadzu Corp. (Columbia, MD, United States) A gradient program was performed with a combination of 0.15% formic acid in water (mobile phase A) and acetonitrile in 0.15% formic acid in water (v/v 1:1) (mobile phase B). The combined chromatographic flow rate was 0.65 ml/min, and acetonitrile was infused, post-column, at 0.15 ml/min. Tandem mass spectrometry analysis was performed using an AB SCIEX 6500 triple quadrupole with a Turbo IonSpray source (Foster City, CA) with a Peak Scientific (Scotland, United Kingdom) Table-N2 gas generator. Quantitation was achieved by monitoring the native compound transition, 353.3 to 193 m/z (quantitative) and 353.3 to 291 m/z (qualitative), with the corresponding isotopically labelled internal standard transition, 357.3 to 197 m/z. The total cycle time for this method was 11 min.
Accuracy for this assay was assessed through spike-and-recovery analyses of blank and spiked BAL fluid with known concentrations. For determining accuracy of both the pools and the individual samples, A pool of BAL fluid samples and an individual BAL fluid sample (BAL fluid 1 and BAL fluid 2, respectively, in Table 1) was spiked with three different concentration levels of native 8-isoprostane (200, 500, and 2,000 pg/ml) and compared the spiked concentrations to the initial measurement. Each sample was prepared in triplicate and measured using two different runs spanning 2 days, resulting in a total of 12 samples per spiking level. The concentration from each triplicate sample was then averaged to get the mean concentration of 8-isoprostane for that spiking level. The mean concentration values were used to calulate percent recovery (equation shown in Table1).
Precision within a run and between runs was determined by using duplicate samples from two BAL fluid pools spiked with native 8-isoprostane at concentrations 200 and 2,000 pg/ml. These results were obtained from five analytical runs over the span of 3 days. The coefficient of variation (CV) was calculated to evaluate both within-run and between-run variation (Table 2).
Stability testing was done with two spiked BAL fluid pools of concentrations 500 and 2,000 pg/ml. Six samples from each pool were aliquoted and tested for their initial concentrations. The three test conditions were designed to simulate common sample preparation scenarios, freeze-thaw stability, benchtop stability, and processed sample stability. To test freeze-thaw stability, two samples from each pool were frozen at −70°C and then thawed a room temperature three times each. Testing benchtop stability was done by leaving two samples from each pool out at room temperature for 24 h. To test processed sample stability, samples were left in a collection plate for 24 h in an autosampler set to 4°C, before being reinjected. Results from the initial measurements were then compared to the measurements following the stability testing.
We looked at potential chromatographic interferences in 18 individual BAL fluid samples and none were observed. Representative chromatograms of a real BAL fluid sample, with a calculated concentration of 437 pg/ml, are shown in Figure 1. Additional chromatograms of extracted saline blanks, saline spikes and spiked BAL fluid pools are shown in the Supplemental Figure S1. Multiple ion transitions (quantitative, qualitative, and isotopically labelled internal standard) were monitored to ensure the method was selective to a single compound, 8-isoprostane.
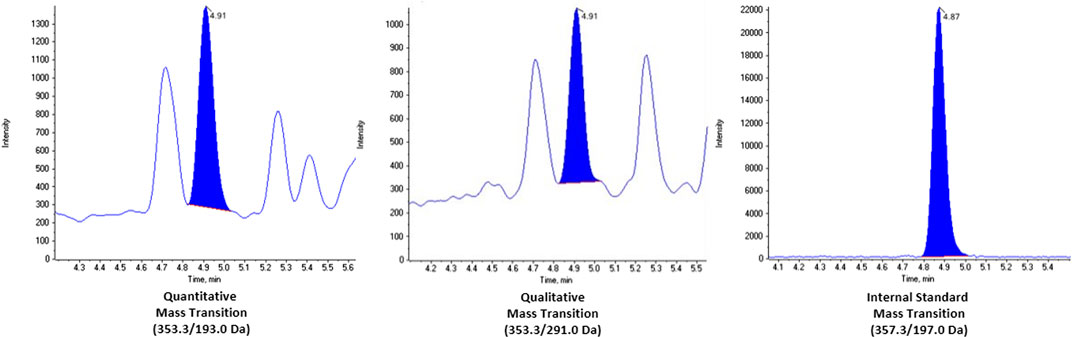
FIGURE 1. Chromatograms of real BAL fluid sample with concentration 437 pg/ml. The mass transitions for each channel are as follows: quantitative (353.3/193.0 Da), qualitative (353.3/291.0 da), and internal standard (353.3/197.0 Da).
Limit of detection (LOD) was determined by analyzing four BAL fluid pools with known 8-isoprostane concentrations (0–2,000 pg/ml) in triplicate on 5 separate runs spanning 3 days. The standard deviation of each pool was plotted against the mean concentrations, and found the Y-intercept, which represents the standard deviation at zero-spike (S0). The LOD was defined as 3 times S0. (Taylor, 1987)
Accuracy was calculated by comparing the obtained mean concentration with the native 8-isoprostane spiking level, with values that ranged from 95.5–101.8%. The mean recovery was determined to be 97.8% with a standard deviation of 2.5% (Table 1). Our previous assay of 8-isoprostane in urine resulted in recoveries ranging from 92.7 to 106.7% with a mean recovery of 99.7%, indicating that the results from our BAL fluid assay are consistent from analysis of a different physiological matrix. (Holder et al., 2020) We compared the ISTD responses between unextracted calibration samples and extracted samples to evaluate sample recovery and calculated an average recovery of 55% for all extracted samples.
As outlined in our previous assay, the calibration curve was prepared by spiking 10 known standard levels of 8-isoprostane in water, with concentrations ranging from 8.8 to 1,410 pg/ml. (Holder et al., 2020) Each standard level was run in duplicate for each analytical run. Strong linearity was observed with an R2 of 0.9999.
The within run precision ranged from 1.36–1.95%, and the between run precision ranged from 1.54–1.92% (Table 2). Thus our BAL fluid method was more precise than our previously published urine assay, possibly because BAL fluid is a cleaner matrix. Furthermore, our BAL fluid method had substantially better precision than a typical EIA assay (e.g., Cayman EIA within run: 9.5%, between run: 20.2%). (Cayman Chemical 8-Isopros, 1635)
Fully resolving 8-isoprostane from all interfering peaks is critical to achieving a reliable and repeatable measurement since this analyte belongs to a class of compounds, F2-isoprostanes, consisting of 64 isomers. Immunoassays are known to suffer from cross-reactivity which could explain the reported poor agreement between LC-MS and EIA for 8-isoprostane, and while GC-MS can be extremely sensitive, extensive sample preparation using harsh derivatizing agents is a necessity, making the GC-MS approach less desirable. (Klawitter et al., 2011; Smith et al., 2011) It is important to note that some published LC-MS/MS methods are not highly selective and measure a sum of F2-isoprostanes and not 8-isoprostane specifically. (Taylor et al., 2008) To evaluate possible interference with 8-isoprostane, we examined the following eicosanoids with similar mass transitions: ent-8-iso-15(S)-PGF2α; ent-8-iso-PGF2α; 8-iso-PGE1; PGE1; 8-iso-15(R)-PGF2α; PGF2α; and 15(R)-PGF2α. Our UHPLC-MS/MS method fully resolves the analyte from all interfering peaks and can reliably be used to quantify 8-isoprostane in BAL fluid and other matrixes.
Percent differences of the initial measurements and post-test measurements ranged from -0.8–2.1% across all three test conditions and both pools (Table 3). These results indicate that 8-isoprostane is stable under all three conditions tested. Long term stability was not assessed due to the rapid method development timeline dictated by the emergency response.
While working in a high throughput laboratory, samples may undergo many freeze-thaw cycles and or be left in an autosampler over the weekend. The results of these tests show that 8-isoprostane levels did not change in samples that underwent the tested conditions, and thus can be used for future analyses. Furthermore, being a stable and robust analyte further supports the use of 8-Isoprostane as a key biomarker of oxidative stress usable in high throughput studies.
The method detection limit for 8-isoprostane (8.8 pg/ml) was calculated as 3 times S0. (Taylor, 1987) We ultimately set the LOD to 17.6 pg/ml for the EVALI samples as we used half the sample volume for analysis due to limited supply. Applications of this method that require an LOQ can use 10 S0 (29.3 pg/ml). The calibration range for this method is 8.8 pg/ml to 1,410 pg/ml; our lowest calibrator is lower than our set LOD and a typical deviation is less than 5% of the target concentration. However, Malli et al. measured 8-isoprostane, using EIA, in serum and BAL fluids of patients with either sarcoidosis or idiopathic pulmonary fibrosis (IPF) and found similar concentrations in serum and BAL. They reported median (25–75% interquartile range) concentrations of 8-isoprostane in sarcoidosis patients [serum: 132.8 (92.27–194.9) pg/ml; BAL: 220.6 (133.6–403.3) pg/ml] and IPF patients [serum: 77.25 (52.42–162.5) pg/ml; BAL: 74.87 (62.23–115.1) pg/ml]. (Malli et al., 2013) Bastani and others applied their LC-MS/MS method measuring 8-isoprostane in plasma, urine, full blood, and erythrocytes. (Bastani et al., 2009) To our knowledge there are no other LC-MS methods for measuring 8-isoprostane in BAL fluid, however, our calibration curve is appropriate for reported BAL fluid 8-isoprostane concentrations using EIA. It is difficult to compare the results of LC-MS methods to EIA methods due to the differences in selectivity, so LODs between the two methods cannot be compared. (Klawitter et al., 2011; Smith et al., 2011) Additionally, the volume of BAL fluid obtained from a patient can vary according to technique used to obtain it. Thus, we only report qualitative results for the BAL fluid samples in this study. (De Jesús et al., 2020; Morel Espinosa et al., 2021) Of the 18 samples that were tested using this method, 14 of them had a concentration above the 17.6 pg/ml LOD.
We have developed and validated a partially automated, selective, and robust UHPLC-MS/MS method for quantifying 8-isoprostane in bronchoalveolar lavage (BAL) fluid. This method is easily adaptable for high-throughput work flow and will be applied to BAL fluid samples collected from EVALI case patients in support of CDC’s 2019 EVALI response. Although EVALI has been strongly linked to inhaled vitamin E acetate from EVPs (Blount et al., 2020), the pathology of how vitamin E acetate causes lung injury remains uncharacterized and may involve OS.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
CH and LW designed the experiments. CH, AA, CA, OC, and RL conducted experiments. CH, LW, and BB reviewed data. BB lead CDC’s 2019 Lung Injury Laboratory Task Force and secured funding. All authors contributed to drafting and editing this manuscript.
The CDC funded all research activity described in this manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the United States. Department of Health and Human Services or the Centers for Disease Control and Prevention.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the Centers for Disease Control and Prevention for providing funding for this project. We thank Matt Karwowski, the CDC’s Lung Injury Response Laboratory Working Group, state health departments, EVALI clinicians, and EVALI patients for coordinating and providing BAL fluid samples. We also thank Erin L. Wade, Stephen Arnstein and Brian Crow for their technical support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.695940/full#supplementary-material
Baraldi, E., Ghiro, L., Piovan, V., Carraro, S., Ciabattoni, G., Barnes, P. J., et al. (2003). Increased Exhaled 8-Isoprostane in Childhood Asthma. CHEST 124 (1), 25–31. doi:10.1378/chest.124.1.25
Bastani, N. E., Gundersen, T. E., and Blomhoff, R. (2009). Determination of 8-epi PGF2α concentrations as a biomarker of oxidative stress using triple-stage liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 23, 2885–2890. doi:10.1002/rcm.4197
Blount, B. C., Karwowski, M. P., Shields, P. G., Morel-Espinosa, M., Valentin-Blasini, L., Gardner, M., et al. (2020). Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 382 (8), 697–705. . Epub 2019 Dec 20. PMID: 31860793; PMCID: PMC7032996. doi:10.1056/NEJMoa1916433
Cayman Chemical 8-Isoprostane ELISA Kit. Item No. 516351. 2016. Cayman Chemicals. Available at: https://www.caymanchem.com/pdfs/516351.pdf (Accessed April 14, 2021).
CDC (2020). Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. Atlanta, Georgia: Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#what-we-know (Accessed April 14, 2021).
Cracowski, J.-L., Durand, T., and Bessard, G. (2002). Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends Pharmacol. Sci. 23 (8), 360–366. doi:10.1016/s0165-6147(02)02053-9
De Jesús, V. R., Silva, L., Newman, C. A., and Blount, B. C. (2020). Novel methods for the analysis of toxicants in bronchoalveolar lavage fluid samples from e-cigarette, or vaping, product use associated lung injury (EVALI) cases: Terpenes. Rapid Commun. Mass. Spectrom. 34 (19), 0951–4198. doi:10.1002/rcm.8879
Holder, C., Adams, A., McGahee, E., Xia, B., Blount, B. C., and Wang, L. (2020). High-Throughput and Sensitive Analysis of Free and Total 8-Isoprostane in Urine with Isotope-Dilution Liquid Chromatography-Tandem Mass Spectrometry. ACS Omega 5 (19), 10919–10926. PMID: 32455212; PMCID: PMC7241033. doi:10.1021/acsomega.0c00661
Imai, Y., Kuba, K., Neely, G. G., Yaghubian-Malhami, R., Perkmann, T., van Loo, G., et al. (2008). Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133 (2), 235–249. PMID: 18423196; PMCID: PMC7112336. doi:10.1016/j.cell.2008.02.043
Klawitter, J., Haschke, M., Shokati, T., Klawitter, J., and Christians, U. (2011). Quantification of 15-F2t -isoprostane in human plasma and urine: results from enzyme-linked immunoassay and liquid chromatography/tandem mass spectrometry cannot be compared. Rapid Commun. Mass. Spectrom. 25 (4), 463–468. doi:10.1002/rcm.4871
Krishnasamy, V. P., Hallowell, B. D., Ko, J. Y., Board, A., Hartnett, K. P., Salvatore, P. P., et al. Update: Characteristics of a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury - United States, August 2019-January 2020. MMWR Morb. Mortal. Wkly. Rep. (2020). 69:90–94. doi:10.15585/mmwr.mm6903e2
Malli, F., Bardaka, F., Tsilioni, I., Karetsi, E., Gourgoulianis, K. I., and Daniil, Z. (2013). 8-isoprostane levels in serum and bronchoalveolar lavage in idiopathic pulmonary fibrosis and sarcoidosis. Food Chem. Toxicol. 61, 160–163. doi:10.1016/j.fct.2013.05.016
Montuschi, P., Collins, J. V., Ciabattoni, G., Lazzeri, N., Corradi, M., Kharitonov, S. A., et al. (2000). Exhaled 8-Isoprostane as anIn VivoBiomarker of Lung Oxidative Stress in Patients with COPD and Healthy Smokers. Am. J. Respir. Crit. Care Med. 162 (3), 1175–1177. doi:10.1164/ajrccm.162.3.2001063
Montuschi, P., Toni, G. C., Paredi, P., Pantelidis, P., du Bois, R. M., Kharitonov, S. A., et al. (1998). 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. AM. J. Respir. Crit. Care Med. 158 (5 Pt 1), 1524–1527. doi:10.1164/ajrccm.158.5.9803102
Morel Espinosa, M., Blount, B. C., and Valentin-Blasini, L. (2021). Liquid chromatography-tandem mass spectrometry method for measuring vitamin E acetate in bronchoalveolar lavage fluid. J. Chromatogr. B 1171, 122607. doi:10.1016/j.jchromb.2021.122607
Ray, P. D., Huang, B.-W., and Tsuji, Y. (2012). Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 24 (5), 981–990. doi:10.1016/j.cellsig.2012.01.008
Schier, J. G., Meiman, J. G., Layden, J., Mikosz, C. A., VanFrank, B., King, B. A., et al. Severe Pulmonary Disease Associated with Electronic-Cigarette-Product Use - Interim Guidance. MMWR Morb Mortal Wkly Rep. (2019). 68:787–790. doi:10.15585/mmwr.mm6836e2
Smith, K. A., Shepard, J., Wakil, A., and Kilpatrick, E. S. (2011). A comparison of methods for the measurement of 8-isoPGF2α: a marker of oxidative stress. Ann. Clin. Biochem. 48 (Pt 2), 147, 2017. PMID: 21292864. doi:10.1258/acb.2010.010151
Keywords: EVALI (e-cigarette or vaping product use-associated lung injury), 8-isoprostane, UHPLC-MS/MS, BAL fluid, oxidative stress
Citation: Holder C, Adams A, Allison C, Cote O, Lippens R, Blount BC and Wang L (2021) A Novel UHPLC-MS/MS Method for Measuring 8-iso-Prostaglandin F2α in Bronchoalveolar Lavage Fluid. Front. Chem. 9:695940. doi: 10.3389/fchem.2021.695940
Received: 15 April 2021; Accepted: 21 July 2021;
Published: 12 August 2021.
Edited by:
Alberto Salomone, University of Turin, ItalyReviewed by:
Michelle R Peace, Virginia Commonwealth University, United StatesCopyright © 2021 Holder, Adams, Allison, Cote, Lippens, Blount and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cory Holder, bXZvNUBjZGMuZ292; Lanqing Wang, bGZ3M0BjZGMuZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.