- 1Department of Biotechnology, University of Engineering and Management, Kolkata, India
- 2Department of Food Technology and Bio-Chemical Engineering, Jadavpur University, Kolkata, India
- 3Malda Polytechnic, West Bengal State Council of Technical Education, Government of West Bengal, Malda, India
- 4AMH Energy Pvt. Ltd., Kolkata, India
- 5Department of Biotechnology, Maulana Abul Kalam Azad University of Technology, Haringhata, India
- 6School of Health Sciences, University Sains Malaysia, Kelantan, Malaysia
- 7Centre of Excellence, Khallikote University, Berhampur, India
- 8Research Division, Association for Biodiversity Conservation and Research (ABC), Balasore, India
Bacterial biofilms are responsible for the development of various chronic wound-related and implant-mediated infections and confer protection to the pathogenic bacteria against antimicrobial drugs and host immune responses. Hence, biofilm-mediated chronic infections have created a tremendous burden upon healthcare systems worldwide. The development of biofilms upon the surface of medical implants has resulted in the failure of various implant-based surgeries and therapies. Although different conventional chemical and physical agents are used as antimicrobials, they fail to kill the sessile forms of bacterial pathogens due to the resistance exerted by the exopolysaccharide (EPS) matrices of the biofilm. One of the major techniques used in addressing such a problem is to directly check the biofilm formation by the use of novel antibiofilm materials, local drug delivery, and device-associated surface modifications, but the success of these techniques is still limited. The immense expansion in the field of nanoscience and nanotechnology has resulted in the development of novel nanomaterials as biocidal agents that can be either easily integrated within biomaterials to prevent the colonization of microbial cells or directly approach the pathogen overcoming the biofilm matrix. The antibiofilm efficacies of these nanomaterials are accomplished by the generation of oxidative stresses and through alterations of the genetic expressions. Microorganism-assisted synthesis of nanomaterials paved the path to success in such therapeutic approaches and is found to be more acceptable for its “greener” approach. Metallic nanoparticles functionalized with microbial enzymes, silver–platinum nanohybrids (AgPtNHs), bacterial nanowires, superparamagnetic iron oxide (Fe3O4), and nanoparticles synthesized by both magnetotactic and non-magnetotactic bacteria showed are some of the examples of such agents used to attack the EPS.
Introduction
Global mortality and morbidity is maximally associated with infectious diseases and is one of the profound causes for the development of antibiotic resistance. It has been observed that after 1980, pharmaceutical companies stopped manufacturing novel antibiotics due to the lack of returns with respect to the investment, high cost associated with the development of drugs, and prolonged time requirement and for the rapid development of resistances (Whitchurch et al., 2002). The development of phenotypic resistances results in the amplification of resistances associated with genes toward diverse types of disinfectants and antibiotics. Biofilms are the group of organized colonies of microbial species comprising fungi, bacteria, and yeasts that develop a syntrophic association with their adherence to the biotic and abiotic surfaces by self-encapsulating extracellular polymeric substances (EPSs) (Costerton et al., 1995). The microcolonies existing within the EPS interact via the mechanism of quorum sensing (QS) that specifically helps in the development of the biofilm and the expression of virulence (Pircalabioru and Chifiriuc, 2020). The phenotypic and genotypic expressions of the sessile cells differ from the planktonic forms and are majorly associated with the development of resistances against antibiotics. Antibiotic resistance is actually imparted by the EPS, which prevents the penetration of the antibiotics and also induces the multidrug efflux pumps within the biofilm and thus results in the development of persister cells (Mah and O’Toole, 2001; Lewis, 2005). The metastasis of the sessile cells from the mature biofilm results in the transmission and dissemination of biofilm-associated infections (Nikolaev and Plakunov, 2007; Dongari-Bagtzoglou, 2008). The various infections that are associated with the biofilm on various biomedical surfaces are considered to be dangerous in healthcare sectors in comparison to the planktonic forms (Allegranzi et al., 2011; Zarb et al., 2012).This has resulted in the urgency to develop alternate therapeutic strategies to combat biofilm-associated infections, precisely through disintegration of the EPS matrix.
The field of nanotechnology involves scientific and engineering technologies that aim to synthesize various materials of nano-dimensions that have wide applications in the fields of bioprospecting, pharmaceuticals, human activities, and biomedical applications. The development of nanomaterials is a new and promising strategy for acting as therapeutic agents against various types of biofilm-associated pathogenic infections that are associated with implants and medical devices (Pircalabioru and Chifiriuc, 2020). Various types of nanomaterials have been associated to combat against various biofilms due to their prevailing properties which are microbiostatic, microbiocidal, and antipathogenic in nature and because they can be used for the purpose of delivering synthetic drugs and natural compounds (Grumezescu and Chifiriuc, 2014). Most of the nanoparticles (NPs) are metallic in nature and comprise metallic oxides, metal-based polymeric composites, polymers, chitosan-based nanomaterials, peptides, combinations of nanoparticle-associated antibiotics, and nanomaterials which have efficacy of antimicrobial agents without bringing about any damage to the host (Pati et al., 2020; Pati et al., 2021).
Although a number of reports are available on the antibiofilm activities of nanomaterials, like carbon nanotubes (Kang et al., 2007), oxygen-deficient zinc oxide (ZnO) nanowires (Elbourne et al., 2020), and core–shell nanofiber membranes loaded with silver nanoparticles (Alharbi et al., 2018), a very scanty number of reports are available on the nanomaterials formed from a microbial source. Since application of microbiogenic nanomaterials that can be used for the disruption of the biofilm matrix may be a significant strategy to combat biofilm-mediated infections, the present study presents an overview of nanomaterials synthesized from various microbial sources, their characteristic features, and their antibiofilm nature with a critical elucidation of their mode of action.
Microbial Synthesis of Nanomaterials
Nanomaterials, with dimensions lower than 100 nm, have attracted the interest of scientists due to their quantum size effect with the variation of electronic properties. Nanomaterials can have one, two, or three dimensions in the nanoscale, as exemplified by nanotubes, nanorods, nanoflowers, nanowires, nanofibers, fullerenes, dendrimers, and quantum dots (Tripathi and Chung, 2019). The microbiogenically synthesized nanostructures are preferred for their affectivity, convenience in production, and environment-friendliness (Ghosh et al., 2021). The microbial synthesis of NPs possesses various types of advantages in comparison to the other methods that include synthesis of nanomaterials with definite morphology, size, and chemical compositions. First of all, the synthesis can be performed under relatively mild physicochemical conditions. The convenience in handling the microbial cells results in the easy scaling up of the process and the ability to bring about the tuning of the characteristics of the nanomaterials by manipulating various cultivation parameters of the cultivation process (Prasad et al., 2016).
Bacteria-Mediated Synthesis of Nanomaterials
For the last few decades, bacterial cells have been used for the purpose of synthesizing various types of inorganic nanomaterials that include gold, silver, selenium, and silver NPs possessing diverse useful properties (Wang et al., 2010). It is the colloidal properties of the gold nanoparticles that determine their antioxidant nature.
Abinaya et al. (2019) synthesized selenium nanowires (Se NWs) using microbial exopolymer (MEP) from Bacillus licheniformis, which was found to be effective in the management of biofilms. The synthesis of AuNPs occurs via the ligands that are produced by the microbial species to prevent the formation of complexes (Reith et al., 2009). The cells of Rhodococcus sp. were used for the development of monodispersed AuNPs (Ahmad et al., 2003). It has been further observed that Deinococcus radiodurans was able to synthesize AuNPs in the presence of high radiation that resulted in the change of Au (III) to Au (I) and finally to Au (0) comprising various types of capping groups which help in stabilizing the AuNPs (Li et al., 2016). Different types of biochemical processes are responsible for the synthesis of NPs. The intracellular mechanism of metal bioreduction is accomplished via the interactions of intracellular enzymes and positively charged groups that help in the gripping of metallic ions from the medium, causing subsequent reduction inside the cell
Transferring electrons to metal ions reduces them to metals in nanodimensions. For example,
MNPs are thus formed on the surface of the cytoplasmic cell membrane due to the bioreduction of the metal ions by enzymes present on the cytoplasmic membrane and within the cytoplasm. In some cases, nucleation of MNPs was found to occur on the cell surface via enzymes and sugars in the cell wall, and later, metal nuclei were transported into the cell where they aggregated to larger sized particles. The process is initiated by the transfer of electrons from NADH by extracellular enzyme NADH reductase (Iravani, 2014).
In addition to the nitrate reductase enzymes, the carbonyl groups, such as –NH2, –OH, –SH, and –COOH, of some proteins and enzymes could stabilize the MNPs by binding to the NP surfaces by providing binding sites for metal ions, followed by the reduction of the metal ions outside the cells on the cell wall or in the periplasmic space. Bacteriogenic NPs can also be synthesized by metabolites of bacteria (Fang et al., 2019).
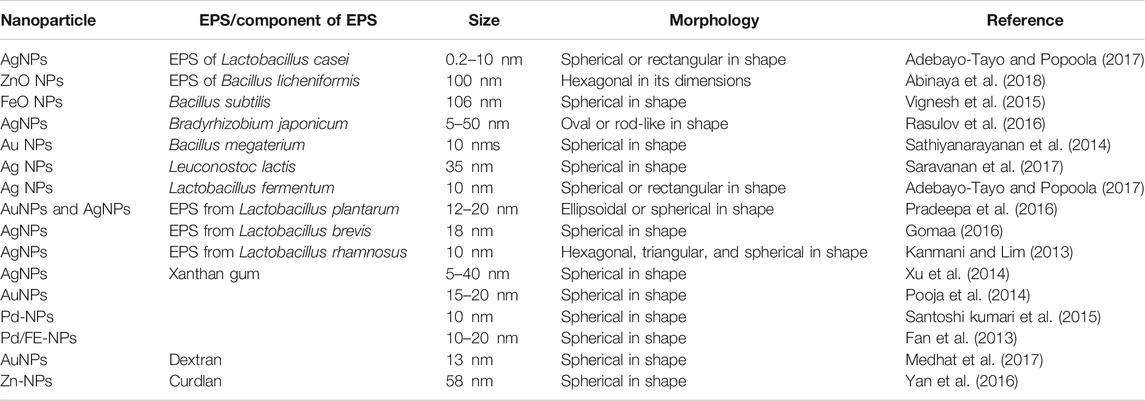
In the intracellular mechanism of metal bioreduction, interactions of intracellular enzymes and positively charged groups help in the gripping of metallic ions from the medium and the subsequent reduction inside the cell (Ovais et al., 2018).A number of physicochemical processes like complexation, nucleation, biosorption, stabilization, and growth are involved in the mechanism of nanoparticle synthesis. Various biomolecules that are associated with the bacterial cells like carbohydrates and proteins help in the stabilization of the NPs. It has been further observed that some groups of bacterial species like Gluconacetobacter help in the synthesis of nanocellulose. In comparison to nanofibrillated cellulose and nanocrystalline cellulose, bacterial nanocellulose possesses high purity, crystallinity, and large mechanical strength (Golmohammadi et al., 2017). The development of nanocellulose, a type of nanobiomaterial, has immense importance due to its biomedical applications (Morales-Narváez et al., 2015; Pourreza et al., 2015). The EPSs of the bacterial species comprise various functional groups that play an important role in the synthesis and the stabilization of the nanoparticles (Table 1) (Emam and Ahmed, 2016). The mucoadhesion properties of the EPSs result in the synthesis of NPs, thus resulting in the development of low surface energy, neutrality, and decrease in the low specificity recognition of the receptor capping, thereby making the NPs serve a wider applicability (Kanmani and Lim, 2013). The nanowires of bacterial origin are the groups of conductive proteinaceous pilus-like structures that are usually involved in the mechanism of electron transport by the involvement of the anaerobic dissimilatory metal-reducing groups of bacteria like Shewanella and Geobacter (Simonte et al., 2017) and aerobic bacterial species like P. aeruginosa (Simonte et al., 2017). Various types of bacterial species like Acetobacter xylinum, Pseudomonas aeruginosa, Escherichia coli, and many more can be used for the purpose of synthesizing PtNPs possessing a high potency of antibacterial and antibiofilm activities (Bloch et al., 2021).
Fungi-Associated Nanomaterial Synthesis
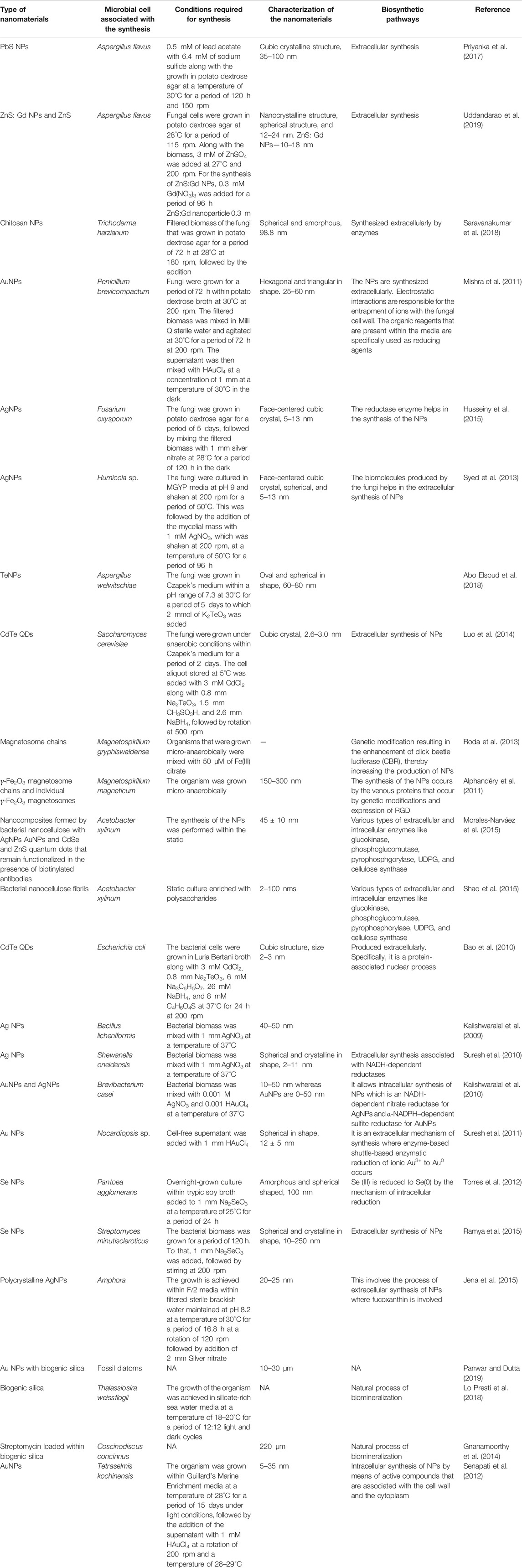
In recent times, fungi have been considered to be an important point of focus for synthesizing various types of nanomaterials and thus the development of the term myco-nanotechnology. Yeasts are found to be one of the most important types of fungi that play a significant role in synthesizing nanomaterials (Hulkoti and Taranath, 2014). Studies have shown the production of various water-soluble, biocompatible calcium telluride quantum dots by Saccharomyces cerevisiae having excellent physical characteristics. Such properties include flexibility of size under the influence of change of temperature and culture time and ability of photoluminescence, and these made them useful for various types of bio-labeling applications (Luo et al., 2014). S. cerevisiae also possesses the ability of synthesizing various types of Au–Ag alloy NPs that can be used for the purpose of various electrochemical sensor fabrications (Zheng et al., 2010). Fungi, as a whole, are considered to be one of the predominant sources for synthesizing nanomaterials due to their higher tolerance toward metals, higher ability of metal uptake and metal-binding capabilities, convenient way of culturing, and higher rates of synthesis of extracellular reductase enzymes (Syed et al., 2013) and other types of secondary metabolites (Dhillon et al., 2012). The fungal biomolecules help in the synthesis and stabilization of NPs (Syed et al., 2013).
Microalgae-Associated Synthesis of Nanomaterials
The use of microalgae that are groups of photosynthetic microbial cells in the synthesis of nanomaterials has gained a lot of importance in the field of nanotechnology (Dahoumane et al., 2016). Synthesis of NPs using algal cells takes place by the accumulation of cations within the matrix of the cell, thereby bringing about reduction (Dahoumane et al., 2017). The mechanism of biosynthesis involves exposure of the salt to cell cultures or the biomass of algae, thereby synthesizing the NPs. In algal organisms like seaweeds, the reduction of the metallic salt is achieved by the biomolecules associated with the cell wall, thereby resulting in the synthesis of NPs.
Mechanism of Biogenic Synthesis of Nanoparticles for Nanomaterials
A number of biogenically synthesized nanoparticles are reported from various bacterial, fungal, and algal species, which are found to have varied structure, size, and shape and are generally extracellularly synthesized. Later, they may be conjugated to other compounds to form nanomaterials (Table 2).
Role of Nanomaterials in Disintegration of Biofilm
About 80% of the microbial infections that occur within the body are associated with biofilms, and hence, it has resulted in a serious concern among healthcare personnel. Various studies showed that nosocomial organisms like S. epidermidis, S. aureus, and P. aeruginosa possess the ability to form biofilms very rapidly on the surfaces of medical devices. It has been observed that a biofilm is constituted of three layers: the initial layer remains adhered to the surface of the biomaterial, the next layer comprises the microcolonies, and the outer layer comprises planktonic organisms that remain free on the outer surface and possess the ability to get dispersed to the surroundings (Kunin, 1989; Costerton et al., 1999; Reid, 1999; Bernier et al., 2003)
Extracellular Polymeric Substance of Biofilm Matrix
In biofilms, the consortia of sessile microbial colonies remain adhered to a biotic or abiotic surface within the self-secreted extracellular polymeric substance (EPS), which comprises exopolysaccharides, proteins, lipids, nucleic acid, and various other types of biomolecules (Flemming et al., 2016; Lahiri et al., 2021c). EPS helps in the bacterial adhesion upon the surface and acts as cementing material between the cells, allowing them to remain in very close association, thereby allowing interactions between the cells (Dragoš and Kovács, 2017; Koo et al., 2017; Lahiri et al., 2021a).Various types of polymers that are of secondary origin like colloids and humic substances also remain embedded within the biofilm.
Mechanisms Associated With the Disruption of Biofilm
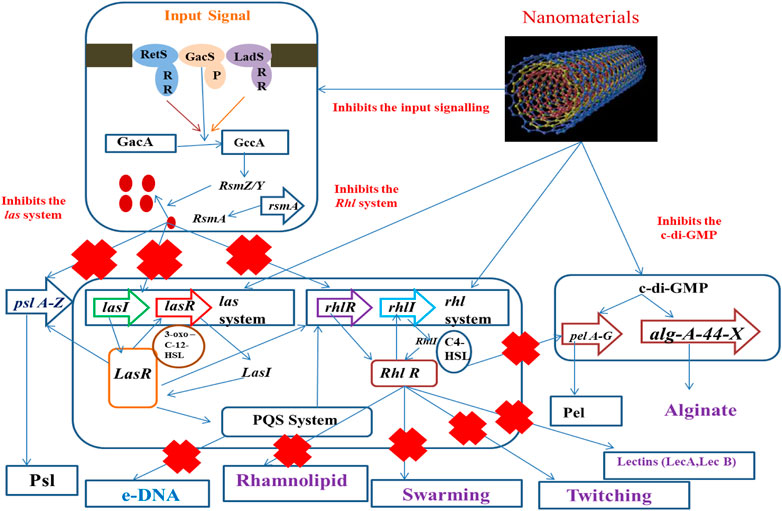
Now, most biofilm eradication approaches involve the development of antibiofilm agents, aimed at preventing the early stages of biofilm construction or acting as biofilm dispersal agents, intended to cause disruption of the mature biofilm. They may follow any of the potential ways like checking of quorum sensing, destruction of eDNA, and affecting swarming and twitching (Figure 1) or can directly damage the biofilm-forming cell.
Nanomaterials With Direct Antibiofilm Property
Nanomaterials formed of microbiogenic nanoparticles like nanosilver or nanogold particles efficiently block the active sites and thus hinder the mechanism of quorum sensing (Lahiri et al., 2021b). They can inhibit the metabolic events for the EPS production, thereby hampering the formation of biofilm (Samanta et al., 2017).
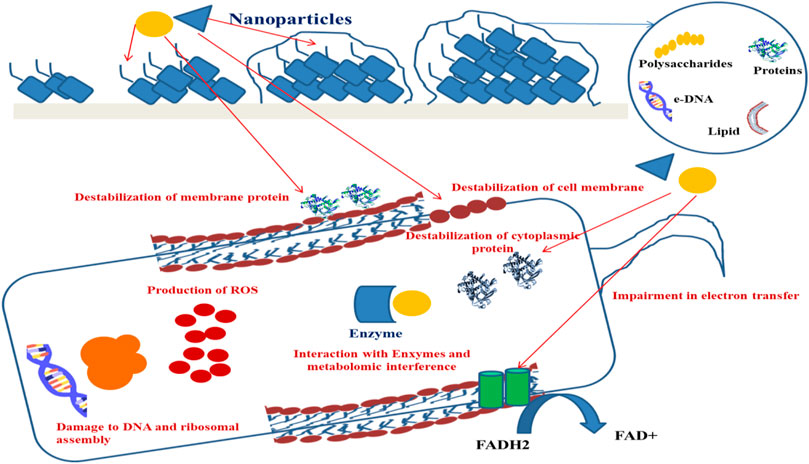
The interaction between NPs and biofilm can be accomplished through the allocation of nanoparticles of the nanomaterials at the vicinity of the biofilm matrix, followed by their attachment. NPs, due to electrostatic interaction, can now bring about mechanical damage to the bacterial cell or can develop oxidative stress followed by the production of reactive oxygen species (ROS) and, as a result of metallic cation release, can interrupt the normal structure and functions of proteins (Shkodenko et al., 2020).
Alteration in Biofilm-Forming Signaling Pathways
Alteration in the signaling cascade involves the prevention of the production of EPS that provides an alternate mechanism for preventing the adhesion of bacteria, thus hindering the formation of biofilms. Thus, it forms a very important target for the next-generation therapeutics (Sintim et al., 2010). In recent times, various studies have been conducted by the utilization of anti-QS agents that prevent the adherence of bacterial species by surface modifications (Kratochvil et al., 2015). Surface immobilization by the use of QS-inhibiting agents also prevents the development of biofilms (Brackman et al., 2016; Kim et al., 2017)
Strategies of Development of Antibiofilm Agent Targeting Extracellular DNA
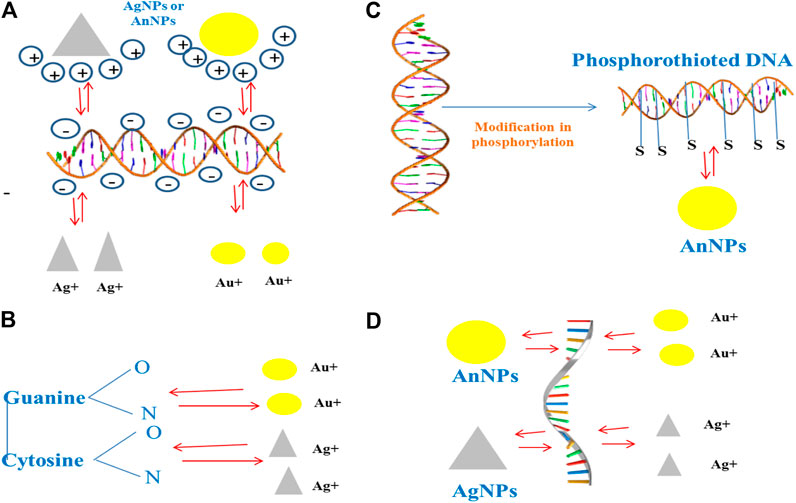
Extracellular DNA (eDNA) forms an important structural component in stabilizing the biofilm architecture and develops resistance to drugs. It has been observed that substances like amphiphilic cargo and enzymes bring about the cleavage of eDNA, thereby bringing about effective degradation of biofilm (Swartjes et al., 2013). eDNA is polyanionic in nature and shows interactions with AuNPs and AgNPs, which are the positively charged molecules. Studies have shown that AuNPs exhibit covalent as well as noncovalent interactions with the backbone of polyanionic eDNA (Carnerero et al., 2017). The gold and silver ions that come from the respective NPs interact with the nitrogen and oxygen atoms that are associated with the nitrogen bases that are present within the DNA background with the Van der Waals and hydrophobic forces, but the electrostatic forces are dominant over the Van der Waals force (Koo et al., 2015; Jiang and Ran, 2018; Radzig et al., 2019). The degradation of DNA was achieved by the interaction along with the binding of gold ions, and this was an important reason to bring about the damage of DNA in place of the ROSs that are being produced by AuNPs. It has been further observed that damage caused by ROS-mediated oxidation induces the repair mechanism of DNA within bacteria. However, the mutant group bacterial strains showed impaired DNA repair mechanisms and are more vulnerable with respect to the wild-type strains to the gold ions (Radzig et al., 2019). Studies have showed that phosphorothionation brings about modification within bacterial DNA, protecting it from various types of unfavorable environmental conditions (Howard et al., 2013). AuNPs can easily react with these DNA and bring about the change in their chemistry, thus resulting in disintegration of the DNA (Figure 2).
Mechanism of Extracellular Polymeric Substance Disruption With Biogenic Nanoparticles
The extracellular matrix provides strength to the indwelling sessile microbial species within the biofilm by virtue of various biomacromolecules known as EPSs, thereby contributing toward shortened antimicrobial susceptibility. So far, EPS targeting for biofilm control has remained underexploited due to lower penetration capabilities of various antibiofilm agents such as antibiotics, biofilm degrading enzymes, and bioactive compounds. Nanoparticles (NPs) have emerged either as EPS matrix disruptors or as carriers of EPS matrix disruptors, and several approaches have recently been proposed (Fulaz et al., 2019). NPs have also been observed to interfere with the cell–cell communication signaling cascade, thus acting as quorum sensing inhibitors (Naik and Kowshik, 2014; Miller et al., 2015; Singh et al., 2015; Al-Shabib et al., 2016; Srinivasan et al., 2017).
EPSs (polysaccharide skeleton, proteins, and DNA) or bacterial cells are, in general, negatively charged, hence providing an efficient way of interaction with positively charged NPs (Flemming et al., 2000; Regiel-Futyra et al., 2017). It has been observed that cationic NPs can penetrate and diffuse well within the matrix as compared to neutral or anionic NPs (Li et al., 2015). It has also been observed that hydrophilic NPs have poorer localization effects within the bacterial cells than hydrophobic NPs due to the formation of stable EPS-hydrophobic components with the NPs (Mitzel et al., 2016). EPS comprises proteins (TasA, TapB, BslA, SipW, CdrA, and lectins), eDNA, and polysaccharides (Pel, Psl, PIA, alginate, and cellulose) that are responsible for adhesion, water retention, aggregation, cohesion, redox reactions, and enzymatic activity and provide structural integrity and a protective barrier to the biofilms. The most important NP–biofilm interactions involve electrostatic, hydrophobic, and steric forces. Electrostatic interactions are mainly responsible for the initial adhesion to surfaces (biofilm matrix or bacterial cells) (Flemming and Wingender, 2010; Habimana et al., 2014). Hydrophobic interactions play a major role in the biofilm formation and its regulation (Renner and Weibel, 2011; Flemming et al., 2016). Steric interactions are needed for colloidal stabilization of the NPs, preventing their self-aggregation (Huangfu et al., 2019).
In a study conducted by Cremonini et al. (2016), selenium nanoparticles (SeNPs) of bacterial origin were reported to stop biofilm formation and disassemble mature glycocalyx of P. aeruginosa and Candida spp. The Stenotrophomonas maltophilia [Sm-SeNPs(-)] and Bacillus mycoides [Bm-SeNPs(+)] had stronger antimicrobial effects than synthetic selenium nanoparticles (Ch-SeNPs) (Cremonini et al., 2016). Thus, biogenic SeNPs appear to be reliable candidates for safe medical applications alone. In another work, biogenic AgNPs synthesized using Desertifilum sp. (D-SNPs) were able to inhibit biofilms of MRSA, resulting in imbalance in CAT, GSH, GPx, and ATPase levels and subsequently forming apoptotic bodies and causing cell wall damage in addition to denaturation of MRSA cellular proteins and genotoxicity (Hamida et al., 2020).
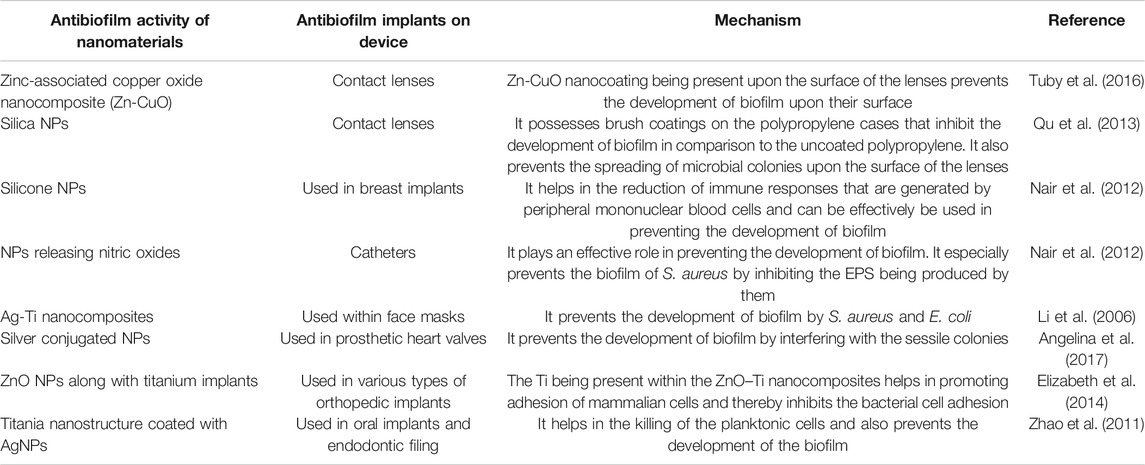
Owing to the high surface-to-volume ratio, NPs possess an efficient transport phenomenon within the biofilm matrix. The size of NPs controls the initial penetration within the matrix, and the NP surface properties, namely, charge and functional groups control the mode of interaction with the matrix components. The presence of organic molecules (proteins, lipids, nucleic acids, carbohydrates, metabolites, etc.) within the biofilm matrix has been reported to be responsible for the formation of a biomolecular corona-like coating on the surface of NPs due to the phenomenon of adsorption on the NP surface (Mu et al., 2014; Docter et al., 2015; Ikuma et al., 2015; Ke et al., 2017; Stan et al., 2018). The physicochemical properties of NPs involve characteristics like size, shape, hydrophobicity, surface charge, curvature, and functionalization that are responsible for the altered interaction between NPs and biofilm matrices or microbial cells (Canesi and Corsi, 2016; Mi et al., 2018). For example, adsorption of NPs on the microbial cell surface has been observed to cause cellular membrane puncture, along with generation of reactive oxygen species (ROS), inhibiting mitochondrial activity, protein, and DNA synthesis (Hajipour et al., 2012; Joo and Aggarwal, 2018). Copper NPs synthesized by P. aeruginosa were found to increase the velocity of wound healing (Tiwari et al., 2014), whereas silver NPs from P. chrysogenum were found to be effective against the biofilm-producing bacteria S. aureus, P. aeruginosa, E. coli, and B.cereus (Akila et al., 2014). Nanomaterials can be successfully applied to remove or check device-associated biofilm formation (Table 3, Figure 3).
Conclusion
The biofilm matrix, also sometimes known as “the dark matter,” is a complex material which creates a barrier shielding the indwelling cells from antimicrobial therapy, immune responses, and environmental challenges and hence prevents eradication strategies. Due to the outstanding challenges presented by the biofilm matrix, a multidisciplinary approach is needed to tackle this problem. Nanotechnology is a plausible solution for antimicrobial and delivery system methodologies for enhanced penetration and targeted delivery of antimicrobials within the biofilm matrix. EPS-targeting strategies involve matrix disruption and enhancing the susceptibility of the biofilm toward antimicrobial therapy.
One of the ways for the synthesis of biogenic NPs involves microbial cells as a reducing, stabilizing, and capping agent in an eco-friendly, sustainable, nontoxic, and inexpensive way. Many researchers have studied the role of bacteria (both Gram-positive and Gram-negative), fungi, or algae in the production of NPs. These methods have resulted in the replacement of various toxic physicochemical methods. However, a few of the questions such as alterations in EPS composition during different environmental/growth conditions, non-commercialization of NP-based antibiofilm technologies, ultimate fate of antibiofilm NPs in vivo, and release of NPs into the environment still remain to be answered. Future research work should highlight the complete biofilm eradication by focusing on both the EPS matrix and the microbial cells, minimizing toxicity and resistance development while enhancing the therapeutic effect with the help of nanostructures formed from microbial sources.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
We acknowledge Universiti Sains Malaysia and Fundamental Research Grant Scheme (203/PPSK/6171258) awarded by Ministry of Higher Education, Malaysia for financial support related to the APC.
Conflict of Interest
Author SG was employed by the company AMH Energy Pvt. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abinaya, M., Vaseeharan, B., Divya, M., Sharmili, A., Govindarajan, M., Alharbi, N. S., et al. (2018). Bacterial Exopolysaccharide (EPS)-coated ZnO Nanoparticles Showed High Antibiofilm Activity and Larvicidal Toxicity against Malaria and Zika Virus Vectors. J. Trace Elem. Med. Biol. 45, 93–103. doi:10.1016/j.jtemb.2017.10.002
Abinaya, M., Vaseeharan, B., Rekha, R., Shanthini, S., Govindarajan, M., Alharbi, N. S., et al. (2019). Microbial Exopolymer-Capped Selenium Nanowires - Towards New Antibacterial, Antibiofilm and Arbovirus Vector Larvicides?. J. Photochem. Photobiol. B: Biol. 192, 55–67. doi:10.1016/j.jphotobiol.2019.01.009
Abo Elsoud, M. M., Al-Hagar, O. E. A., Abdelkhalek, E. S., and Sidkey, N. M. (2018). Synthesis and Investigations on Tellurium Myconanoparticles. Biotechnol. Rep. 18, e00247. doi:10.1016/j.btre.2018.e00247
Adebayo-Tayo, B. C., and Popoola, A. O. (2017). Biogenic Synthesis and Antimicrobial Activity of Silver Nanoparticle Using Exopolysaccharides from Lactic Acid Bacteria. Int. J. Nano Dimens. 8, 61–69. doi:10.22034/ijnd.2017.24377
Ahmad, A., Senapati, S., Khan, M. I., Kumar, R., Ramani, R., Srinivas, V., et al. (2003). Intracellular Synthesis of Gold Nanoparticles by a Novel Alkalotolerant actinomycete,Rhodococcusspecies. Nanotechnology 14, 824–828. doi:10.1088/0957-4484/14/7/323
Akila, S., Nanda, A., and Salai, R. G. (2014). In-Vivo Wound Healing Activity of Silver Nanoparticles: An Investigation. Int. J. Sci. Res. 3, 1208–1212.
Al-Shabib, N. A., Husain, F. M., Ahmed, F., Khan, R. A., Ahmad, I., Alsharaeh, E., et al. (2016). Biogenic Synthesis of Zinc Oxide Nanostructures from Nigella Sativa Seed: Prospective Role as Food Packaging Material Inhibiting Broad-Spectrum Quorum Sensing and Biofilm. Sci. Rep. 6, 36761. doi:10.1038/srep36761
Alharbi, H. F., Luqman, M., and Khan, S. T. (2018). Antibiofilm Activity of Synthesized Electrospun Core-Shell Nanofiber Composites of PLA and PVA with Silver Nanoparticles. Mater. Res. Express 5, 095001. doi:10.1088/2053-1591/aad4df
Allegranzi, B., Nejad, S. B., Combescure, C., Graafmans, W., Attar, H., Donaldson, L., et al. (2011). Burden of Endemic Health-Care-Associated Infection in Developing Countries: Systematic Review and Meta-Analysis. The Lancet 377, 228–241. doi:10.1016/S0140-6736(10)61458-4
Alphandéry, E., Faure, S., Seksek, O., Guyot, F., and Chebbi, I. (2011). Chains of Magnetosomes Extracted from AMB-1 Magnetotactic Bacteria for Application in Alternative Magnetic Field Cancer Therapy. ACS Nano 5, 6279–6296. doi:10.1021/nn201290k
Angelina, J. T. T., Ganesan, S., Panicker, T. M. R., Narayani, R., Paul Korath, M., and Jagadeesan, K. (2017). Pulsed Laser Deposition of Silver Nanoparticles on Prosthetic Heart Valve Material to Prevent Bacterial Infection. Mater. Tech. 32, 148–155. doi:10.1080/10667857.2016.1160503
Bao, H., Lu, Z., Cui, X., Qiao, Y., Guo, J., Anderson, J. M., et al. (2010). Extracellular Microbial Synthesis of Biocompatible CdTe Quantum Dots. Acta Biomater. 6, 3534–3541. doi:10.1016/j.actbio.2010.03.030
Bernier, S. P., Silo-Suh, L., Woods, D. E., Ohman, D. E., and Sokol, P. A. (2003). Comparative Analysis of Plant and Animal Models for Characterization of Burkholderia Cepacia Virulence. Iai 71, 5306–5313. doi:10.1128/iai.71.9.5306-5313.2003
Bloch, K., Pardesi, K., Satriano, C., and Ghosh, S. (2021). Bacteriogenic Platinum Nanoparticles for Application in Nanomedicine. Front. Chem. 9, 624344. doi:10.3389/fchem.2021.624344
Brackman, G., Breyne, K., De Rycke, R., Vermote, A., Van Nieuwerburgh, F., Meyer, E., et al. (2016). The Quorum Sensing Inhibitor Hamamelitannin Increases Antibiotic Susceptibility of Staphylococcus aureus Biofilms by Affecting Peptidoglycan Biosynthesis and eDNA Release. Sci. Rep. 6, 20321. doi:10.1038/srep20321
Canesi, L., and Corsi, I. (2016). Effects of Nanomaterials on marine Invertebrates. Sci. Total Environ. 565, 933–940. doi:10.1016/j.scitotenv.2016.01.085
Carnerero, J. M., Jimenez‐Ruiz, A., Castillo, P. M., and Prado‐Gotor, R. (2017). Covalent and Non‐Covalent DNA-Gold‐Nanoparticle Interactions: New Avenues of Research. ChemPhysChem 18, 17–33. doi:10.1002/cphc.201601077
Costerton, J. W., Lewandowski, Z., Caldwell, D. E., Korber, D. R., and Lappin-Scott, H. M. (1995). Microbial Biofilms. Annu. Rev. Microbiol. 49, 711–745. doi:10.1146/annurev.mi.49.100195.003431
Costerton, J. W., Stewart, P. S., and Greenberg, E. P. (1999). Bacterial Biofilms: A Common Cause of Persistent Infections. Science 284, 1318–1322. doi:10.1126/science.284.5418.1318
Cremonini, E., Zonaro, E., Donini, M., Lampis, S., Boaretti, M., Dusi, S., et al. (2016). Biogenic Selenium Nanoparticles: Characterization, Antimicrobial Activity and Effects on Human Dendritic Cells and Fibroblasts. Microb. Biotechnol. 9, 758–771. doi:10.1111/1751-7915.12374
Dahoumane, S. A., Mechouet, M., Wijesekera, K., Filipe, C. D. M., Sicard, C., Bazylinski, D. A., et al. (2017). Algae-mediated Biosynthesis of Inorganic Nanomaterials as a Promising Route in Nanobiotechnology - a Review. Green. Chem. 19, 552–587. doi:10.1039/C6GC02346K
Dahoumane, S. ., Mechouet, M., Alvarez, F. ., Agathos, S., and Jeffryes, C. (2016). Microalgae: An Outstanding Tool in Nanotechnology. Bionatura 1, 196–201. doi:10.21931/rb/2016.01.04.7
Dhillon, G. S., Brar, S. K., Kaur, S., and Verma, M. (2012). Green Approach for Nanoparticle Biosynthesis by Fungi: Current Trends and Applications. Crit. Rev. Biotechnol. 32, 49–73. doi:10.3109/07388551.2010.550568
Docter, D., Westmeier, D., Markiewicz, M., Stolte, S., Knauer, S. K., and Stauber, R. H. (2015). The Nanoparticle Biomolecule corona: Lessons Learned - challenge Accepted?. Chem. Soc. Rev. 44, 6094–6121. doi:10.1039/C5CS00217F
Dongari-Bagtzoglou, A. (2008). Pathogenesis of Mucosal Biofilm Infections: Challenges and Progress. Expert Rev. Anti-infective Ther. 6, 201–208. doi:10.1586/14787210.6.2.201
Dragoš, A., and Kovács, Á. T. (2017). The Peculiar Functions of the Bacterial Extracellular Matrix. Trends Microbiol. 25, 257–266. doi:10.1016/j.tim.2016.12.010
Elbourne, A., Cheeseman, S., Wainer, P., Kim, J., Medvedev, A. E., Boyce, K. J., et al. (2020). Significant Enhancement of Antimicrobial Activity in Oxygen-Deficient Zinc Oxide Nanowires. ACS Appl. Bio Mater. 3, 2997–3004. doi:10.1021/acsabm.0c00065
Elizabeth, E., Baranwal, G., Krishnan, A. G., Menon, D., and Nair, M. (2014). ZnO Nanoparticle Incorporated Nanostructured Metallic Titanium for Increased Mesenchymal Stem Cell Response and Antibacterial Activity. Nanotechnology 25, 115101. doi:10.1088/0957-4484/25/11/115101
Emam, H. E., and Ahmed, H. B. (2016). Polysaccharides Templates for Assembly of Nanosilver. Carbohydr. Polym. 135, 300–307. doi:10.1016/j.carbpol.2015.08.095
Fan, G., Cang, L., Qin, W., Zhou, C., Gomes, H. I., and Zhou, D. (2013). Surfactants-enhanced Electrokinetic Transport of Xanthan Gum Stabilized nanoPd/Fe for the Remediation of PCBs Contaminated Soils. Sep. Purif. Tech. 114, 64–72. doi:10.1016/j.seppur.2013.04.030
Fang, X., Wang, Y., Wang, Z., Jiang, Z., and Dong, M. (2019). Microorganism Assisted Synthesized Nanoparticles for Catalytic Applications. Energies 12, 190. doi:10.3390/en12010190
Flemming, H.-C., Wingender, J., Mayer, C., Körstgens, V., and Borchard, W. (2000). “Cohesiveness in Biofilm Matrix Polymers,” in In Community Structure And Co-operation in Biofilms Society for General Microbiology Symposia. Editors D. G. Allison, H. M. Lappin-Scott, M. Wilson, and P. Gilbert (Cambridge: Cambridge University Press), 87–106. doi:10.1017/CBO9780511754814.007
Flemming, H.-C., Wingender, J., Szewzyk, U., Steinberg, P., Rice, S. A., and Kjelleberg, S. (2016). Biofilms: an Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 14, 563–575. doi:10.1038/nrmicro.2016.94
Flemming, H.-C., and Wingender, J. (2010). The Biofilm Matrix. Nat. Rev. Microbiol. 8, 623–633. doi:10.1038/nrmicro2415
Fulaz, S., Vitale, S., Quinn, L., and Casey, E. (2019). Nanoparticle-Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 27, 915–926. doi:10.1016/j.tim.2019.07.004
Ghosh, S., Lahiri, D., Nag, M., Dey, A., Sarkar, T., Pathak, S. K., et al. (2021). Bacterial biopolymer: its role in pathogenesis to effective biomaterials. Polymers 13, 1–28. doi:10.3390/polym13081242
Gnanamoorthy, P., Anandhan, S., and Prabu, V. A. (2014). Natural Nanoporous Silica Frustules from marine Diatom as a Biocarrier for Drug Delivery. J. Porous Mater. 21, 789–796. doi:10.1007/s10934-014-9827-2
Golmohammadi, H., Morales-Narváez, E., Naghdi, T., and Merkoçi, A. (2017). Nanocellulose in Sensing and Biosensing. Chem. Mater. 29, 5426–5446. doi:10.1021/acs.chemmater.7b01170
Gomaa, E. Z. (2016). Exopolysaccharide-mediated Silver Nanoparticles Produced by Lactobacillus Brevis NM101-1 as Antibiotic Adjuvant. Microbiology 85, 207–219. doi:10.1134/S0026261716020077
Grumezescu, A., and Chifiriuc, C. (2014). Editorial (Thematic Issue: Prevention of Microbial Biofilms - the Contribution of Micro and Nanostructured Materials). Cmc 21, 3311. doi:10.2174/0929867321666140304101314
Habimana, O., Semião, A. J. C., and Casey, E. (2014). The Role of Cell-Surface Interactions in Bacterial Initial Adhesion and Consequent Biofilm Formation on Nanofiltration/reverse Osmosis Membranes. J. Membr. Sci. 454, 82–96. doi:10.1016/j.memsci.2013.11.043
Hajipour, M. J., Fromm, K. M., Akbar Ashkarran, A., Jimenez de Aberasturi, D., Larramendi, I. R. d., Rojo, T., et al. (2012). Antibacterial Properties of Nanoparticles. Trends Biotechnol. 30, 499–511. doi:10.1016/j.tibtech.2012.06.004
Hamida, R. S., Ali, M. A., Goda, D. A., Khalil, M. I., and Al-Zaban, M. I. (2020). Novel Biogenic Silver Nanoparticle-Induced Reactive Oxygen Species Inhibit the Biofilm Formation and Virulence Activities of Methicillin-Resistant Staphylococcus aureus (MRSA) Strain. Front. Bioeng. Biotechnol. 8, 433. doi:10.3389/fbioe.2020.00433
Howard, S. T., Newman, K. L., McNulty, S., Brown-Elliott, B. A., Vasireddy, R., Bridge, L., et al. (2013). Insertion Site and Distribution of a Genomic Island Conferring DNA Phosphorothioation in the Mycobacterium Abscessus Complex. Microbiology 159, 2323–2332. doi:10.1099/mic.0.070318-0
Huangfu, X., Xu, Y., Liu, C., He, Q., Ma, J., Ma, C., et al. (2019). A Review on the Interactions between Engineered Nanoparticles with Extracellular and Intracellular Polymeric Substances from Wastewater Treatment Aggregates. Chemosphere 219, 766–783. doi:10.1016/j.chemosphere.2018.12.044
Hulkoti, N. I., and Taranath, T. C. (2014). Biosynthesis of Nanoparticles Using Microbes-A Review. Colloids Surf. B: Biointerfaces 121, 474–483. doi:10.1016/j.colsurfb.2014.05.027
Husseiny, S. M., Salah, T. A., and Anter, H. A. (2015). Biosynthesis of Size Controlled Silver Nanoparticles by Fusarium Oxysporum, Their Antibacterial and Antitumor Activities. Beni-Suef Univ. J. Basic Appl. Sci. 4, 225–231. doi:10.1016/j.bjbas.2015.07.004
Ikuma, K., Decho, A. W., and Lau, B. L. T. (2015). When Nanoparticles Meet Biofilmsâ€"interactions Guiding the Environmental Fate and Accumulation of Nanoparticles. Front. Microbiol. 6, 591. doi:10.3389/fmicb.2015.00591
Iravani, S. (2014). Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Scholarly Res. Notices 2014, 359316. doi:10.1155/2014/359316
Jena, J., Pradhan, N., Dash, B. P., Panda, P. K., and Mishra, B. K. (2015). Pigment Mediated Biogenic Synthesis of Silver Nanoparticles Using Diatom Amphora Sp. And its Antimicrobial Activity. J. Saudi Chem. Soc. 19, 661–666. doi:10.1016/j.jscs.2014.06.005
Jiang, W.-Y., and Ran, S.-Y. (2018). Two-stage DNA Compaction Induced by Silver Ions Suggests a Cooperative Binding Mechanism. J. Chem. Phys. 148, 205102. doi:10.1063/1.5025348
Joo, S. H., and Aggarwal, S. (2018). Factors Impacting the Interactions of Engineered Nanoparticles with Bacterial Cells and Biofilms: Mechanistic Insights and State of Knowledge. J. Environ. Manage. 225, 62–74. doi:10.1016/j.jenvman.2018.07.084
Kalishwaralal, K., Banumathi, E., Pandian, S. R. K., Deepak, V., Muniyandi, J., Eom, S. H., et al. (2009). Silver Nanoparticles Inhibit VEGF Induced Cell Proliferation and Migration in Bovine Retinal Endothelial Cells. Colloids Surf. B: Biointerfaces 73, 51–57. doi:10.1016/j.colsurfb.2009.04.025
Kalishwaralal, K., Deepak, V., Ram Kumar Pandian, S., Kottaisamy, M., BarathmaniKanth, S., Kartikeyan, B., et al. (2010). Biosynthesis of Silver and Gold Nanoparticles Using Brevibacterium Casei. Colloids Surf. B: Biointerfaces 77, 257–262. doi:10.1016/j.colsurfb.2010.02.007
Kang, S., Pinault, M., Pfefferle, L. D., and Elimelech, M. (2007). Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 23, 8670–8673. doi:10.1021/la701067r
Kanmani, P., and Lim, S. T. (2013). Synthesis and Structural Characterization of Silver Nanoparticles Using Bacterial Exopolysaccharide and its Antimicrobial Activity against Food and Multidrug Resistant Pathogens. Process Biochem. 48, 1099–1106. doi:10.1016/j.procbio.2013.05.011
Ke, P. C., Lin, S., Parak, W. J., Davis, T. P., and Caruso, F. (2017). A Decade of the Protein Corona. ACS Nano 11, 11773–11776. doi:10.1021/acsnano.7b08008
Kim, M. K., Zhao, A., Wang, A., Brown, Z. Z., Muir, T. W., Stone, H. A., et al. (2017). Surface-attached Molecules Control Staphylococcus aureus Quorum Sensing and Biofilm Development. Nat. Microbiol. 2, 17080. doi:10.1038/nmicrobiol.2017.80
Koo, H., Allan, R. N., Howlin, R. P., Stoodley, P., and Hall-Stoodley, L. (2017). Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 15, 740–755. doi:10.1038/nrmicro.2017.99
Koo, K. M., Sina, A. A. I., Carrascosa, L. G., Shiddiky, M. J. A., and Trau, M. (2015). DNA-bare Gold Affinity Interactions: Mechanism and Applications in Biosensing. Anal. Methods 7, 7042–7054. doi:10.1039/C5AY01479D
Kratochvil, M. J., Tal-Gan, Y., Yang, T., Blackwell, H. E., and Lynn, D. M. (2015). Nanoporous Superhydrophobic Coatings that Promote the Extended Release of Water-Labile Quorum Sensing Inhibitors and Enable Long-Term Modulation of Quorum Sensing inStaphylococcus Aureus. ACS Biomater. Sci. Eng. 1, 1039–1049. doi:10.1021/acsbiomaterials.5b00313
Kunin, C. (1989). Blockage of Urinary Catheters: Role of Microorganisms and Constituents of the Urine on Formation of Encrustations. J. Clin. Epidemiol. 42, 835–842. doi:10.1016/0895-4356(89)90096-6
Lahiri, D., Nag, M., Sarkar, T., Dutta, B., and Ray, R. R. (2021a). Antibiofilm Activity of α-Amylase from Bacillus Subtilis and Prediction of the Optimized Conditions for Biofilm Removal by Response Surface Methodology (RSM) and Artificial Neural Network (ANN). Appl. Biochem. Biotechnol. 193, 3509. doi:10.1007/s12010-021-03509-9
Lahiri, D., Nag, M., Sheikh, H. I., Sarkar, T., Edinur, H. A., Pati, S., et al. (2021b). Microbiologically-Synthesized Nanoparticles and Their Role in Silencing the Biofilm Signaling Cascade. Front. Microbiol. 12, 636588. doi:10.3389/fmicb.2021.636588
Lahiri, D., Nag, M., Banerjee, R., Mukherjee, D., Garai, S., Sarkar, T., et al. (2021c). Amylases: biofilm inducer or biofilm inhibitor? Front. Cell. Infect. Microbiol. 11, 660048. doi:10.3389/fcimb.2021.660048
Lewis, K. (2005). Persister Cells and the riddle of Biofilm Survival. Biochemistry (Moscow) 70, 267–274. doi:10.1007/s10541-005-0111-6
Li, J., Li, Q., Ma, X., Tian, B., Li, T., Yu, J., et al. (2016). Biosynthesis of Gold Nanoparticles by the Extreme Bacterium Deinococcus Radiodurans and an Evaluation of Their Antibacterial Properties. Int. J. Nanomedicine 11, 5931–5944. doi:10.2147/IJN.S119618
Li, X., Yeh, Y.-C., Giri, K., Mout, R., Landis, R. F., Prakash, Y. S., et al. (2015). Control of Nanoparticle Penetration into Biofilms through Surface Design. Chem. Commun. 51, 282–285. doi:10.1039/C4CC07737G
Li, Y., Leung, P., Yao, L., Song, Q. W., and Newton, E. (2006). Antimicrobial Effect of Surgical Masks Coated with Nanoparticles. J. Hosp. Infect. 62, 58–63. doi:10.1016/j.jhin.2005.04.015
Luo, Q.-Y., Lin, Y., Li, Y., Xiong, L.-H., Cui, R., Xie, Z.-X., et al. (2014). Nanomechanical Analysis of Yeast Cells in CdSe Quantum Dot Biosynthesis. Small 10, 699–704. doi:10.1002/smll.201301940
Mah, T.-F. C., and O’Toole, G. A. (2001). Mechanisms of Biofilm Resistance to Antimicrobial Agents. Trends Microbiol. 9, 34–39. doi:10.1016/S0966-842X(00)01913-2
Medhat, D., Hussein, J., El-Naggar, M. E., Attia, M. F., Anwar, M., Latif, Y. A., et al. (2017). Effect of Au-Dextran NPs as Anti-tumor Agent against EAC and Solid Tumor in Mice by Biochemical Evaluations and Histopathological Investigations. Biomed. Pharmacother. 91, 1006–1016. doi:10.1016/j.biopha.2017.05.043
Mi, G., Shi, D., Wang, M., and Webster, T. J. (2018). Reducing Bacterial Infections and Biofilm Formation Using Nanoparticles and Nanostructured Antibacterial Surfaces. Adv. Healthc. Mater. 7, 1800103. doi:10.1002/adhm.201800103
Miller, K. P., Wang, L., Chen, Y.-P., Pellechia, P. J., Benicewicz, B. C., and Decho, A. W. (2015). Engineering Nanoparticles to Silence Bacterial Communication. Front. Microbiol. 6, 189. doi:10.3389/fmicb.2015.00189
Mishra, A., Tripathy, S. K., Wahab, R., Jeong, S.-H., Hwang, I., Yang, Y.-B., et al. (2011). Microbial Synthesis of Gold Nanoparticles Using the Fungus Penicillium brevicompactum and Their Cytotoxic Effects against Mouse mayo Blast Cancer C2C12 Cells. Appl. Microbiol. Biotechnol. 92, 617–630. doi:10.1007/s00253-011-3556-0
Mitzel, M. R., Sand, S., Whalen, J. K., and Tufenkji, N. (2016). Hydrophobicity of Biofilm Coatings Influences the Transport Dynamics of Polystyrene Nanoparticles in Biofilm-Coated Sand. Water Res. 92, 113–120. doi:10.1016/j.watres.2016.01.026
Morales-Narváez, E., Golmohammadi, H., Naghdi, T., Yousefi, H., Kostiv, U., Horák, D., et al. (2015). Nanopaper as an Optical Sensing Platform. ACS Nano 9, 7296–7305. doi:10.1021/acsnano.5b03097
Mu, Q., Jiang, G., Chen, L., Zhou, H., Fourches, D., Tropsha, A., et al. (2014). Chemical Basis of Interactions between Engineered Nanoparticles and Biological Systems. Chem. Rev. 114, 7740–7781. doi:10.1021/cr400295a
Naik, K., and Kowshik, M. (2014). Anti-Quorum Sensing Activity of AgCl-TiO2 Nanoparticles with Potential Use as Active Food Packaging Material. J. Appl. Microbiol. 117, 972–983. doi:10.1111/jam.12589
Nair, N., Pilakka-Kanthikeel, S., Saiyed, Z., Yndart, A., and Nair, M. (2012). Silicone Nanoparticles Do Not Induce Immune Responses by Naïve Human Peripheral Blood Mononuclear Cells. Plast. Reconstr. Surg. 130, 128e–137e. doi:10.1097/PRS.0b013e318254b359
Nikolaev, Y. A., and Plakunov, V. K. (2007). Biofilm-“City of Microbes” or an Analogue of Multicellular Organisms? Microbiology 76, 125–138. doi:10.1134/S0026261707020014
Ovais, M., Khalil, A., Ayaz, M., Ahmad, I., Nethi, S., and Mukherjee, S. (2018). Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Ijms 19, 4100. doi:10.3390/ijms19124100
Panwar, V., and Dutta, T. (2019). Diatom Biogenic Silica as a Felicitous Platform for Biochemical Engineering: Expanding Frontiers. ACS Appl. Bio Mater. 2, 2295–2316. doi:10.1021/acsabm.9b00050
Pati, S., Chatterji, A., Dash, B. P., Raveen Nelson, B., Sarkar, T., Shahimi, S., et al. (2020). Structural Characterization and Antioxidant Potential of Chitosan by γ-irradiation from the Carapace of Horseshoe Crab. Polymers 12, 2361. doi:10.3390/polym12102361
Pati, S., Sarkar, T., Sheikh, H. I., Bharadwaj, K. K., Mohapatra, P. K., Chatterji, A., et al. (2021). γ-Irradiated chitosan from Carcinoscorpius rotundicauda (Latreille, 1802) improves the shelf life of refrigerated aquatic products. Front. Mar. Sci. 8, 664961. doi:10.3389/fmars.2021.664961
Pircalabioru, G. G., and Chifiriuc, M.-C. (2020). Nanoparticulate Drug-Delivery Systems for Fighting Microbial Biofilms: from Bench to Bedside. Future Microbiol. 15, 679–698. doi:10.2217/fmb-2019-0251
Pooja, D., Panyaram, S., Kulhari, H., Rachamalla, S. S., and Sistla, R. (2014). Xanthan Gum Stabilized Gold Nanoparticles: Characterization, Biocompatibility, Stability and Cytotoxicity. Carbohydr. Polym. 110, 1–9. doi:10.1016/j.carbpol.2014.03.041
Pourreza, N., Golmohammadi, H., Naghdi, T., and Yousefi, H. (2015). Green In-Situ Synthesized Silver Nanoparticles Embedded in Bacterial Cellulose Nanopaper as a Bionanocomposite Plasmonic Sensor. Biosens. Bioelectron. 74, 353–359. doi:10.1016/j.bios.2015.06.041
Pradeepa,, , Vidya, S. M., Udaya Bhat, K., Huilgol, P., and Avadhani, K. (2016). Preparation of Gold Nanoparticles by Novel Bacterial Exopolysaccharide for Antibiotic Delivery. Life Sci. 153, 171–179. doi:10.1016/j.lfs.2016.04.022
Prasad, R., Pandey, R., and Barman, I. (2016). Engineering Tailored Nanoparticles with microbes:Quo Vadis? WIREs Nanomed Nanobiotechnol 8, 316–330. doi:10.1002/wnan.1363
Presti, M. L., Ragni, R., Vona, D., Leone, G., Cicco, S., and Farinola, G. M. (2018). In Vivo doped Biosilica from Living Thalassiosira Weissflogii Diatoms with a Triethoxysilyl Functionalized Red Emitting Fluorophore. MRS Adv. 3, 1509–1517. doi:10.1557/adv.2018.60
Priyanka, U., Gowda, A. K. ., Elisha, M. ., Teja B, S., N, N., and Mohan B, R. (2017). Biologically Synthesized PbS Nanoparticles for the Detection of Arsenic in Water. Int. Biodeterior. Biodegradation 119, 78–86. doi:10.1016/j.ibiod.2016.10.009
Qu, W., Hooymans, J. M. M., Qiu, J., de-Bont, N., Gelling, O.-J., van der Mei, H. C., et al. (2013). Nonadhesive, Silica Nanoparticles-Based brush-coated Contact Lens Cases-Compromising between Ease of Cleaning and Microbial Transmission to Contact Lenses. J. Biomed. Mater. Res. 101, 640–647. doi:10.1002/jbm.b.32866
Radzig, M., Koksharova, O., Khmel, I., Ivanov, V., Yorov, K., Kiwi, J., et al. (2019). Femtosecond Spectroscopy of Au Hot-Electron Injection into TiO2: Evidence for Au/TiO2 Plasmon Photocatalysis by Bactericidal Au Ions and Related Phenomena. Nanomaterials 9, 217. doi:10.3390/nano9020217
Ramya, S., Shanmugasundaram, T., and Balagurunathan, R. (2015). Biomedical Potential of Actinobacterially Synthesized Selenium Nanoparticles with Special Reference to Anti-biofilm, Anti-oxidant, Wound Healing, Cytotoxic and Anti-viral Activities. J. Trace Elem. Med. Biol. 32, 30–39. doi:10.1016/j.jtemb.2015.05.005
Rasulov, B., Rustamova, N., Yili, A., Zhao, H.-Q., and Aisa, H. A. (2016). Synthesis of Silver Nanoparticles on the Basis of Low and High Molar Mass Exopolysaccharides of Bradyrhizobium Japonicum 36 and its Antimicrobial Activity against Some Pathogens. Folia Microbiol. 61, 283–293. doi:10.1007/s12223-015-0436-5
Regiel-Futyra, A., Dąbrowski, J. M., Mazuryk, O., Śpiewak, K., Kyzioł, A., Pucelik, B., et al. (2017). Bioinorganic Antimicrobial Strategies in the Resistance Era. Coord. Chem. Rev. 351, 76–117. doi:10.1016/j.ccr.2017.05.005
Reid, G. (1999). Biofilms in Infectious Disease and on Medical Devices. Int. J. Antimicrob. Agents 11, 223–226. doi:10.1016/s0924-8579(99)00020-5
Reith, F., Etschmann, B., Grosse, C., Moors, H., Benotmane, M. A., Monsieurs, P., et al. (2009). Mechanisms of Gold Biomineralization in the Bacterium Cupriavidus Metallidurans. Proc. Natl. Acad. Sci. 106, 17757–17762. doi:10.1073/pnas.0904583106
Renner, L. D., and Weibel, D. B. (2011). Physicochemical Regulation of Biofilm Formation. MRS Bull. 36, 347–355. doi:10.1557/mrs.2011.65
Roda, A., Cevenini, L., Borg, S., Michelini, E., Calabretta, M. M., and Schüler, D. (2013). Bioengineered Bioluminescent Magnetotactic Bacteria as a Powerful Tool for Chip-Based Whole-Cell Biosensors. Lab. Chip 13, 4881–4889. doi:10.1039/c3lc50868d
Samanta, S., Singh, B. R., and Adholeya, A. (2017). Intracellular Synthesis of Gold Nanoparticles Using an Ectomycorrhizal Strain EM-1083 of Laccaria Fraterna and its Nanoanti-Quorum Sensing Potential against Pseudomonas aeruginosa. Indian J. Microbiol. 57, 448–460. doi:10.1007/s12088-017-0662-4
Santoshi kumari, A., Venkatesham, M., Ayodhya, D., and Veerabhadram, G. (2015). Green Synthesis, Characterization and Catalytic Activity of Palladium Nanoparticles by Xanthan Gum. Appl. Nanosci. 5, 315–320. doi:10.1007/s13204-014-0320-7
Saravanakumar, K., Chelliah, R., MubarakAli, D., Jeevithan, E., Oh, D.-H., Kathiresan, K., et al. (2018). Fungal Enzyme-Mediated Synthesis of Chitosan Nanoparticles and its Biocompatibility, Antioxidant and Bactericidal Properties. Int. J. Biol. Macromolecules 118, 1542–1549. doi:10.1016/j.ijbiomac.2018.06.198
Saravanan, C., Rajesh, R., Kaviarasan, T., Muthukumar, K., Kavitake, D., and Shetty, P. H. (2017). Synthesis of Silver Nanoparticles Using Bacterial Exopolysaccharide and its Application for Degradation of Azo-Dyes. Biotechnol. Rep. 15, 33–40. doi:10.1016/j.btre.2017.02.006
Sathiyanarayanan, G., Vignesh, V., Saibaba, G., Vinothkanna, A., Dineshkumar, K., Viswanathan, M. B., et al. (2014). Synthesis of Carbohydrate Polymer Encrusted Gold Nanoparticles Using Bacterial Exopolysaccharide: a Novel and Greener Approach. RSC Adv. 4, 22817–22827. doi:10.1039/C4RA01428F
Senapati, S., Syed, A., Moeez, S., Kumar, A., and Ahmad, A. (2012). Intracellular Synthesis of Gold Nanoparticles Using Alga Tetraselmis Kochinensis. Mater. Lett. 79, 116–118. doi:10.1016/j.matlet.2012.04.009
Shao, W., Liu, H., Liu, X., Sun, H., Wang, S., and Zhang, R. (2015). pH-Responsive Release Behavior and Anti-bacterial Activity of Bacterial Cellulose-Silver Nanocomposites. Int. J. Biol. Macromolecules 76, 209–217. doi:10.1016/j.ijbiomac.2015.02.048
Shkodenko, L., Kassirov, I., and Koshel, E. (2020). Metal Oxide Nanoparticles against Bacterial Biofilms: Perspectives and Limitations. Microorganisms 8, 1545. doi:10.3390/microorganisms8101545
Simonte, F., Sturm, G., Gescher, J., and Sturm-Richter, K. (2017). Extracellular Electron Transfer and Biosensors. Adv. Biochem. Eng. Biotechnol. 167, 15–38. doi:10.1007/10_2017_34
Singh, B. R., Singh, B. N., Singh, A., Khan, W., Naqvi, A. H., and Singh, H. B. (2015). Mycofabricated Biosilver Nanoparticles Interrupt Pseudomonas aeruginosa Quorum Sensing Systems. Sci. Rep. 5, 13719. doi:10.1038/srep13719
Sintim, H. O., Smith, J. A., Wang, J., Nakayama, S., and Yan, L. (2010). Paradigm Shift in Discovering Next-Generation Anti-infective Agents: Targeting Quorum Sensing, C-Di-GMP Signaling and Biofilm Formation in Bacteria with Small Molecules. Future Med. Chem. 2, 1005–1035. doi:10.4155/fmc.10.185
Srinivasan, R., Mohankumar, R., Kannappan, A., Karthick Raja, V., Archunan, G., Karutha Pandian, S., et al. (2017). Exploring the Anti-Quorum Sensing and Antibiofilm Efficacy of Phytol against Serratia marcescens Associated Acute Pyelonephritis Infection in Wistar Rats. Front. Cel. Infect. Microbiol. 7, 498. doi:10.3389/fcimb.2017.00498
Stan, M. S., Cinteza, L. O., Petrescu, L., Mernea, M. A., Calborean, O., Mihailescu, D. F., et al. (2018). Dynamic Analysis of the Interactions between Si/SiO2 Quantum Dots and Biomolecules for Improving Applications Based on Nano-Bio Interfaces. Sci. Rep. 8, 5289. doi:10.1038/s41598-018-23621-x
Suresh, A. K., Pelletier, D. A., Wang, W., Broich, M. L., Moon, J.-W., Gu, B., et al. (2011). Biofabrication of Discrete Spherical Gold Nanoparticles Using the Metal-Reducing Bacterium Shewanella Oneidensis. Acta Biomater. 7, 2148–2152. doi:10.1016/j.actbio.2011.01.023
Suresh, A. K., Pelletier, D. A., Wang, W., Moon, J.-W., Gu, B., Mortensen, N. P., et al. (2010). Silver Nanocrystallites: Biofabrication usingShewanella Oneidensis,and an Evaluation of Their Comparative Toxicity on Gram-Negative and Gram-Positive Bacteria. Environ. Sci. Technol. 44, 5210–5215. doi:10.1021/es903684r
Swartjes, J., Das, T., Sharifi, S., Subbiahdoss, G., Sharma, P. K., Krom, B. P., et al. (2013). A Functional DNase I Coating to Prevent Adhesion of Bacteria and the Formation of Biofilm. Adv. Funct. Mater. 23, 2843–2849. doi:10.1002/adfm.201202927
Syed, A., Saraswati, S., Kundu, G. C., and Ahmad, A. (2013). Biological Synthesis of Silver Nanoparticles Using the Fungus Humicola Sp. And Evaluation of Their Cytoxicity Using normal and Cancer Cell Lines. Spectrochimica Acta A: Mol. Biomol. Spectrosc. 114, 144–147. doi:10.1016/j.saa.2013.05.030
Tiwari, M., Narayanan, K., Thakar, M. B., Jagani, H. V., and Venkata Rao, J. (2014). Biosynthesis and Wound Healing Activity of Copper Nanoparticles. IET nanobiotechnol. 8, 230–237. doi:10.1049/iet-nbt.2013.0052
Torres, S. K., Campos, V. L., León, C. G., Rodríguez-Llamazares, S. M., Rojas, S. M., González, M., et al. (2012). Biosynthesis of Selenium Nanoparticles by Pantoea Agglomerans and Their Antioxidant Activity. J. Nanopart Res. 14, 1236. doi:10.1007/s11051-012-1236-3
Tripathi, R. M., and Chung, S. J. (2019). Biogenic Nanomaterials: Synthesis, Characterization, Growth Mechanism, and Biomedical Applications. J. Microbiol. Methods 157, 65–80. doi:10.1016/j.mimet.2018.12.008
Tuby, R., Gutfreund, S., Perelshtein, I., Mircus, G., Ehrenberg, M., Mimouni, M., et al. (2016). Fabrication of a Stable and Efficient Antibacterial Nanocoating of Zn-CuO on Contact Lenses. ChemNanoMat 2, 547–551. doi:10.1002/cnma.201600066
Uddandarao, P., Balakrishnan, R. M., Ashok, A., Swarup, S., and Sinha, P. (2019). Bioinspired ZnS:Gd Nanoparticles Synthesized from an Endophytic Fungi Aspergillus flavus for Fluorescence-Based Metal Detection. Biomimetics 4, 11. doi:10.3390/biomimetics4010011
Vignesh, V., Sathiyanarayanan, G., Sathishkumar, G., Parthiban, K., Sathish-Kumar, K., and Thirumurugan, R. (2015). Formulation of Iron Oxide Nanoparticles Using Exopolysaccharide: Evaluation of Their Antibacterial and Anticancer Activities. RSC Adv. 5, 27794–27804. doi:10.1039/C5RA03134F
Wang, T., Yang, L., Zhang, B., and Liu, J. (2010). Extracellular Biosynthesis and Transformation of Selenium Nanoparticles and Application in H2O2 Biosensor. Colloids Surf. B: Biointerfaces 80, 94–102. doi:10.1016/j.colsurfb.2010.05.041
Whitchurch, C. B., Tolker-Nielsen, T., Ragas, P. C., and Mattick, J. S. (2002). Extracellular DNA Required for Bacterial Biofilm Formation. Science 295, 1487. doi:10.1126/science.295.5559.1487
Xu, W., Jin, W., Lin, L., Zhang, C., Li, Z., Li, Y., et al. (2014). Green Synthesis of Xanthan Conformation-Based Silver Nanoparticles: Antibacterial and Catalytic Application. Carbohydr. Polym. 101, 961–967. doi:10.1016/j.carbpol.2013.10.032
Yan, J.-K., Wang, Y.-Y., Zhu, L., and Wu, J.-Y. (2016). Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Carboxylic Curdlan and Their Interaction with Bovine Serum Albumin. RSC Adv. 6, 77752–77759. doi:10.1039/C6RA15395J
Zarb, P., Coignard, B., Griskeviciene, J., Muller, A., Vankerckhoven, V., Weist, K., et al. (2012). The European Centre for Disease Prevention and Control (ECDC) pilot point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use. Euro Surveill. Bull. Eur. sur Les Mal. Transm. = Eur. Commun. Dis. Bull. 17, 20316. doi:10.2807/ese.17.46.20316-en
Zhao, L., Wang, H., Huo, K., Cui, L., Zhang, W., Ni, H., et al. (2011). Antibacterial Nano-Structured Titania Coating Incorporated with Silver Nanoparticles. Biomaterials 32, 5706–5716. doi:10.1016/j.biomaterials.2011.04.040
Keywords: microbial nanomaterials, antibiofilm, exopolysaccharide, medical devices, nanotechnology, bioprospecting
Citation: Nag M, Lahiri D, Sarkar T, Ghosh S, Dey A, Edinur HA, Pati S and Ray RR (2021) Microbial Fabrication of Nanomaterial and Its Role in Disintegration of Exopolymeric Matrices of Biofilm. Front. Chem. 9:690590. doi: 10.3389/fchem.2021.690590
Received: 03 April 2021; Accepted: 06 May 2021;
Published: 24 May 2021.
Edited by:
Sougata Ghosh, RK University, IndiaReviewed by:
Smaranika Pattnaik, Sambalpur University, IndiaLei Wang, Harbin Institute of Technology, China
Copyright © 2021 Nag, Lahiri, Sarkar, Ghosh, Dey, Edinur, Pati and Ray. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rina Rani Ray, cmF5cHVtaWNyb0BnbWFpbC5jb20=; Siddhartha Pati, cGF0aXNpZGRoYXJ0aGFAZ21haWwuY29t; Hisham Atan Edinur, ZWRpbnVyQHVzbS5teQ==
†These authors have contributed equally to this work
 Moupriya Nag
Moupriya Nag Dibyajit Lahiri
Dibyajit Lahiri Tanmay Sarkar
Tanmay Sarkar Sujay Ghosh4
Sujay Ghosh4 Hisham Atan Edinur
Hisham Atan Edinur Siddhartha Pati
Siddhartha Pati Rina Rani Ray
Rina Rani Ray